- 1Department of Humanities, University of Pavia, Pavia, Italy
- 2IRCCS Mondino Foundation, Pavia, Italy
- 3Department of Human and Social Sciences, University of Bergamo, Bergamo, Italy
- 4Department of Psychology, Hebrew University of Jerusalem, Jerusalem, Israel
- 5The Laboratory for Visual Neuroplasticity, Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, MA, United States
Introduction: The capacity to understand others’ emotions and react accordingly is a key social ability. However, it may be compromised in case of a profound sensory loss that limits the contribution of available contextual cues (e.g., facial expression, gestures, body posture) to interpret emotions expressed by others. In this study, we specifically investigated whether early blindness affects the capacity to interpret emotional vocalizations, whose valence may be difficult to recognize without a meaningful context.
Methods: We asked a group of early blind (N = 22) and sighted controls (N = 22) to evaluate the valence and the intensity of spontaneous fearful and joyful non-verbal vocalizations.
Results: Our data showed that emotional vocalizations presented alone (i.e., with no contextual information) are similarly ambiguous for blind and sighted individuals but are perceived as more intense by the former possibly reflecting their higher saliency when visual experience is unavailable.
Disussion: Our study contributes to a better understanding of how sensory experience shapes ememotion recognition.
Introduction
To successfully interact in social contexts we need to react to communication signals, including emotional signals expressed by others. Observers can perceive emotions from others’ facial, bodily, vocal, and verbal expressions, and this perception is further informed by contextual information. Among the available emotional cues, non-verbal vocalizations represent powerful primitive signals (e.g., Cowen et al., 2019) that prompt reactions (e.g., Warren et al., 2006), but whose valence may not be so obvious when no other contextual information is available (Atias et al., 2019). Indeed, Atias et al. (2019) showed that participants could not distinguish the valence of human non-verbal vocalizations occurring in real intense positive (e.g., reacting to the reunion with a loved one) and negative (e.g., reacting to an attacker invading one’s home) situations when these vocalizations were presented alone. However, when the affective vocalizations were matched with the corresponding visual contexts, participants immediately understood their valence correctly. As another example, Lavan et al. (2015) demonstrated that facial expressions drive the emotional interpretation of intense laughter and crying, further supporting the importance of information drawn from visual contexts.
Although we are usually presented with “contextualized” vocalizations, this may not be the case of a blind person who cannot rely on visual cues such as facial expressions or gestures in interpreting vocal emotional signals. Consistent experimental evidence suggests that blind people compensate for the lack of visual input and experience by increased sensitivity of their tactile (e.g., Cattaneo et al., 2010a; Bauer et al., 2015; Gurtubay-Antolin and Rodríguez-Fornells, 2017) and auditory senses (for a recent review, Sabourin et al., 2022). For instance, in the auditory domain blind individuals outperform sighted controls in the processing of pitch changes (e.g., Arnaud et al., 2018), localization (e.g., Battal et al., 2020), voice recognition (Hölig et al., 2014, 2015), as well as in auditorily presented sentence comprehension (Loiotile et al., 2020). Available findings on the processing of vocal emotions in blind individuals are mixed, with some studies reporting enhanced discrimination of emotional vocalizations in blind participants compared to the sighted (Klinge et al., 2010), others reporting no differences (Gamond et al., 2017) or even worse performance in the blind (e.g., Martins et al., 2019; Chen and Whitney, 2022; Sarzedas et al., 2024). Interestingly, blindness has also been associated with physiological differences in the way emotional vocalizations are processed: Klinge et al. (2010) reported increased amygdala activation to negatively valenced stimuli in early blind participants [see Klinge et al. (2012)], suggesting that emotional vocalizations may be perceived as more salient when visual input is lacking. Furthermore, recent electrophysiological evidence suggests that early blindness relates to facilitated brain processing of authenticity of vocal emotional expressions (Sarzedas et al., 2024) and prior evidence showed ERP attention effects in blind individuals across a broader range of different emotional prosodies compared to sighted individuals (Topalidis et al., 2020).
To shed light on whether a lack of visual input and experience is associated with an improved capacity to interpret emotional vocalizations, we presented the same intense emotional vocalizations [as previously used by Atias et al. (2019)] to a group of early blind individuals and a group of normally sighted controls. Participants were asked to evaluate the stimuli as emotionally positive or negative, as well as to rate their intensity.
Methods
Participants
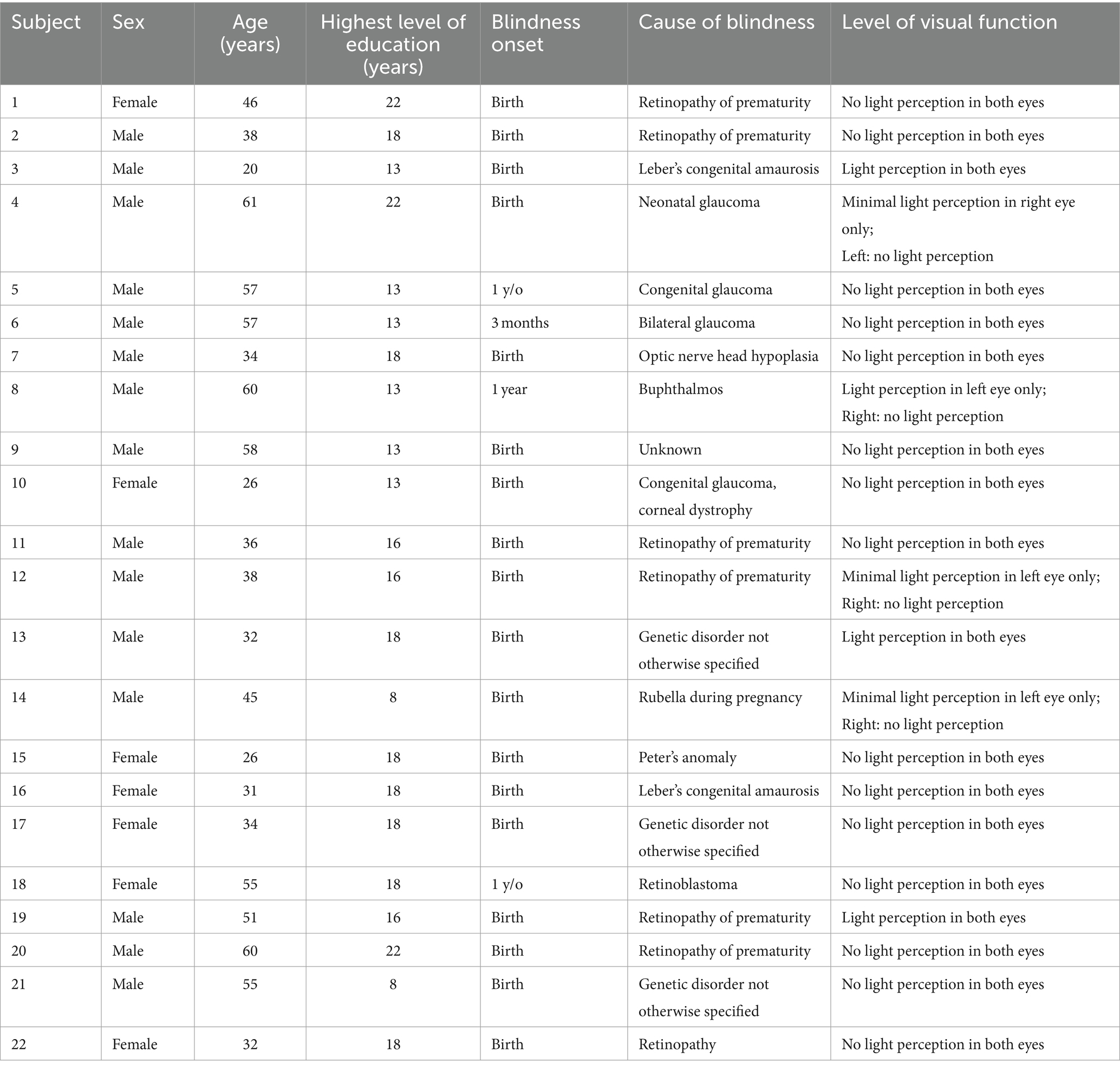
Twenty-two early blind individuals (seven females), mean age 43.3 years ±13.2 SD (age range 20–61 years), mean education level of 16 ± 3.92 years, and 22 normally sighted participants (seven females), mean age 43.0 ± 13.6 years (age range 23–61 years), mean education level of 16.2 ± 3.39 years, took part in the experiment. The two groups did not differ in terms of age, t(42) < 1, p = 0.96, and education level, t(42) < 1, p = 0.83. Characteristics of blind participants are reported in Table 1. None of the blind participants reported having any prior visual memories. All blind participants were experienced Braille readers, and they were independent travelers.
Informed consent was obtained from all participants. The study was approved by the local ethical committee and was carried out in accordance with the Declaration of Helsinki.
Stimuli
Stimuli consisted of 40 spontaneous vocalizations (20 positive, 20 negative) identical to those used in the study of Atias et al. (2019). Stimuli were brief vocalization “bursts” that portrayed the first reaction of a vocalizer involved in real-life situations of intense joy or intense fear. Examples of the scenarios for fearful and joyful situations were pranks to believe a snake or a spider is in one’s home and the announcement of a new baby to grandparents, respectively. For each scenario, vocalizations were selected if they did not include any verbal information, were expressed by a single expresser, and if no auditory cue other than the expresser’s vocalization was present in the recording [see Supplementary Material of Atias et al. (2019) for additional information on the selection and editing of the vocalizations]. The duration of the vocalizations ranged from 400 to 2,600 ms (mean duration = 876 ms, SD = 390 ms; median duration = 847 ms).
Procedure
The experiment was conducted in person. Participants were seated comfortably and wore headphones. Sighted control participants were blindfolded during the entire experiment. The experiment consisted of an emotional evaluation task similar to that used in the study of Atias et al. (2019). Specifically, participants were instructed to evaluate the valence (“Is the emotion expressed positive or negative?) and the intensity (i.e., “How intense is the emotion expressed?”) of real-life vocalizations. Participants rated the valence and the intensity of the same 40 vocalizations in two different blocks using a 1–9 Likert scale (for the valence block: 1 = extremely negative, 9 = extremely positive; for the intensity block: 1 = not intense at all, 9 = extremely intense; presentation order was counterbalanced across participants). At the beginning of each block, the participants were told whether they had to evaluate the valence or the intensity of the presented stimuli. After listening to each vocalization, participants verbally expressed their evaluation, and the experimenter pressed the corresponding number key on the response keyboard. Stimuli presentation and data recording were implemented using E-prime 2.0 software. The experiment lasted approximately 30 min (including instructions and debriefing).
Statistical analysis
As with the previous study of Atias et al. (2019), valence ratings were transformed (by applying the formula: “X – 5”) such that positive values represent increasingly positive valence judgments (1 to 4) and negative values represent increasingly negative valence judgments (−1 to −4). To evaluate the possible effect of blindness on valence recognition, we performed a mixed repeated-measures ANOVA, with Valence (negative vs. positive) as a within-subjects factor and Group (blind vs. sighted) as a between-subjects factor. A similar ANOVA was performed on Intensity scores.
Results
Mean valence ratings from both positive (M = −1.22, SD = 1.37) and negative vocalizations (M = −1.48, SD = 1.41) were found to be in the negative valence range. This suggests that overall, participants tended to interpret all vocalizations as emotionally, negative (see Figure 1A). The mixed repeated-measures ANOVA on valence recognition revealed a significant main effect of Valence, F(1,42) = 9.01, p = 0.005, ηp2 = 0.18, indicating that participants identified positive vocalizations as slightly more positive than negative ones. Neither the main effect of Group, F(1,42) = 2.56, p = 0.12, nor the interaction Valence by Group, F(1,42) = 0.0007, p = 0.98, reached statistical significance.
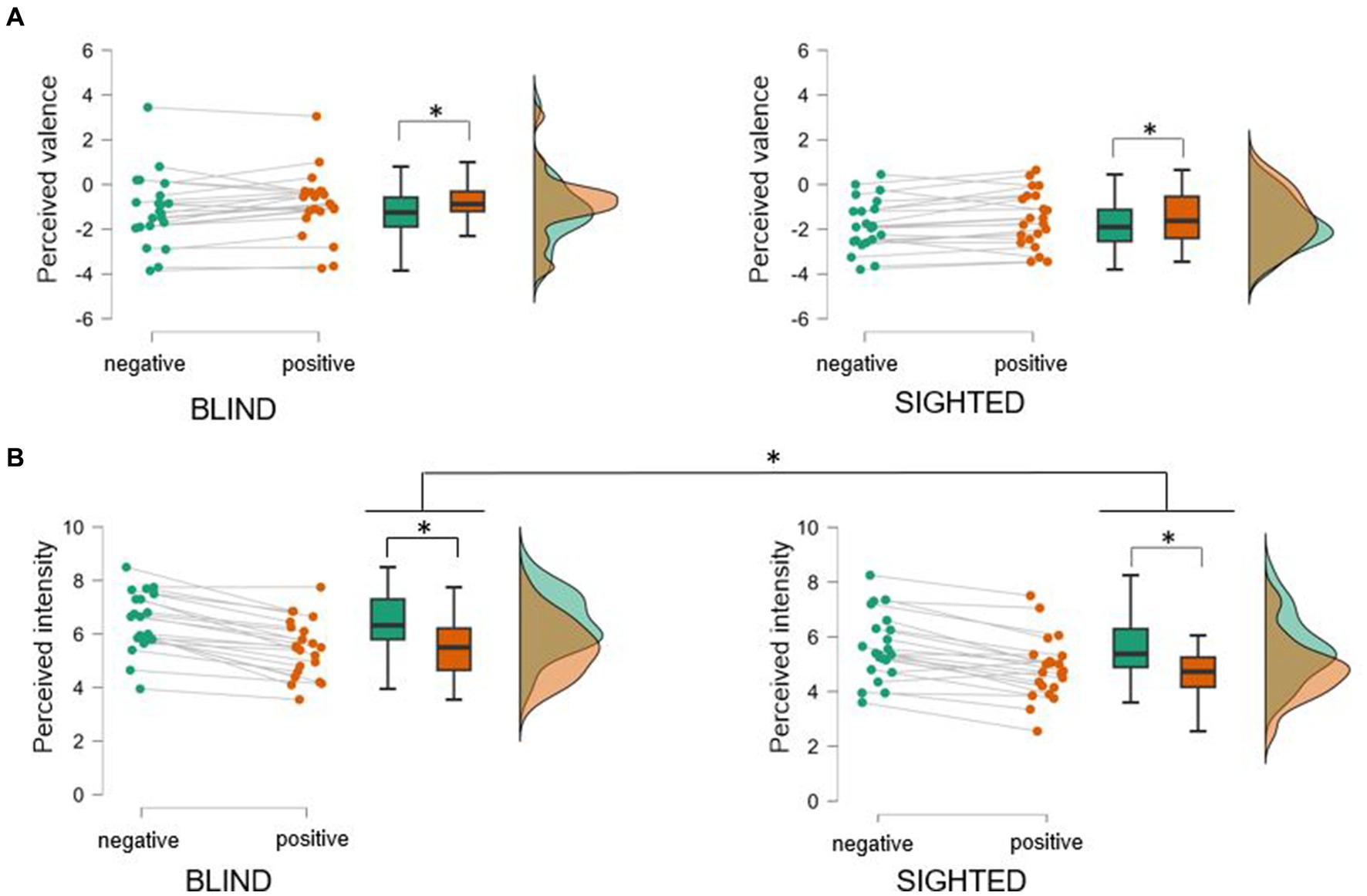
Figure 1. Perceived valence (A) and intensity (B) of real-life vocalizations evoked in intense positive and negative situations in early blind and sighted participants. Overall, both blind and sighted participants rated all the vocalizations as negative irrespective of their original valence (note that negative vocalizations were perceived as slightly more negative than positive ones). Both groups assigned moderate/high intensity to the vocalizations, with negative vocalizations beeing perceived more intense than positive onse. Critically, blind individuals rated the intensity of all vocalizations as higher compared to sighted controls. Asterisks indicate significant differences (ps < 0.05) between experimental conditions.
Regarding intensity scores, vocalizations were overall perceived at an intermediary level of intensity (just above the middle score of the intensity 1–9 Likert scale) (see Figure 1B). The mixed repeated-measures ANOVA on intensity scores revealed a main effect of Valence, F(1,42) = 103.51, p < 0.001, ηp2 = 0.71, indicating that negative vocalizations were rated more intense than positive. Furthermore, the factor of Group was significant, F(1,42) = 4.86, p = 0.033, ηp2 = 0.10, indicating that blind participants perceived the vocalizations to be more intense (M = 5.94, SD = 1.05) than sighted participants (M = 4.95, SD = 1.14). The interaction Valence by Group, F(1,42) = 0.915, p = 0.34, was not statistically significant.
Discussion
The capacity to understand others’ emotions and react accordingly is a key social ability that may be compromised in case of a profound sensory loss. Here we specifically investigated whether early blindness affects the capacity to interpret emotional vocalizations, whose valence may be difficult to recognize in the absence of a meaningful context (e.g., Atias et al., 2019). We found that blind and sighted participants found discriminating the valence of intense emotional vocalizations similarly difficult when no other contextual information was provided. Nonetheless, blind participants rated the affective vocalizations as more intense than sighted controls, possibly reflecting a higher perceived saliency.
The intensity of non-verbal vocalizations is characterized by specific acoustic profiles. Intense vocal stimuli are associated with increased duration, high fundamental frequency (pitch), and more high-frequency energy in the spectrum (bright timbre) (Anikin, 2020). These features inform about the significance and saliency of the stimuli, therefore attracting (automatic/bottom-up) attention and facilitating information prioritization and efficiency of appraisal (Belin and Zatorre, 2015; Anikin, 2020). For instance, screams have acoustic features dedicated to alarm signals but do not inform about the specific content meaning (fear vs. anger) of the emotion conveyed or the speaker’s identity (Anikin, 2020). Our findings indicate that blind participants are more sensitive than sighted individuals to these intensity-related acoustic characteristics, possibly because they represent a unique or primary source of sensory information during social interactions signaling danger or possible negative outcomes. Similar evidence was previously reported by Klinge et al. (2010, 2012) also showing that blindness is associated with greater amygdala sensitivity in response to emotional prosodic stimuli (and in particular negative ones) suggesting a neural mechanism for this observed behavioral compensation (Klinge et al., 2010, 2012). Interestingly, this finding may also be interpreted considering the higher interoceptive capacity observed in blind individuals (Radziun et al., 2023). High interoceptive ability is associated with increased sensitivity to the emotions of others (Terasawa et al., 2014) and with increased connectivity of the salience network (Chong et al., 2017). This may contribute to the perceived higher intensity of emotional stimuli by blind individuals, a hypothesis that deserves further investigation.
Our data show that blind participants performed similarly to the sighted controls in processing the valence of spontaneous intense vocal expressions. In particular, and regardless of visual status, participants rated both positive and negative vocalizations in the negative range of the valence scale, thus not reliably differentiating their valence, and replicating the previous findings of Atias et al. (2019). Hence, blindness does not seem to lead to an advantage in emotional recognition of vocal stimuli [see Gamond et al. (2017), Martins et al. (2019), Chen et al. (2022), and Sarzedas et al. (2024)], as with other domains such as spatial auditory localization or sentence comprehension (Battal et al., 2020; Loiotile et al., 2020). One possibility is that blind individuals do not develop better competencies in this domain because they may rely on other sources of contextual information such as verbal content [see Occelli et al. (2017)]. Indeed, in social interactions in everyday life, vocal emotional cues are usually accompanied by verbal semantic information. This may be more common for blind individuals who often rely on what other people say or on audio descriptions when accessing certain forms of media (e.g., television). Moreover, prior evidence also suggests that blindness may not affect social cognition abilities overall, which may develop via other sources [Ricciardi et al., 2009; for a recent meta-analysis, see Arioli et al. (2021)]. For instance, blind and sighted individuals similarly evaluate others’ trustworthiness based on the pitch of the voice (Oleszkiewicz et al., 2017) and similarly form social impressions of others [Ferrari et al., 2017; Arioli et al., 2024; see Bedny et al. (2009) and Arioli et al. (2021), for evidence on similar neural correlates of social cognition in the blind and sighted]. Overall, this suggests that blindness-related compensatory mechanisms in the auditory domain are restricted to specific auditory functions such as navigation/orientation, as well as spatial and language processing (Cattaneo et al., 2008, 2010b; Handjaras et al., 2016; Battal et al., 2020; Loiotile et al., 2020), rather than being generalized to the socioemotional domain. The advantage of blind individuals in language processing might be sufficient to ensure proper emotional and social competence or at least, socioemotional skills that are comparable to that of sighted people.
In interpreting our findings, some limitations should be acknowledged. The first limitation relates to the stimuli we used. Indeed, to make the task more sensitive to detect possible group differences, we intentionally employed ambiguous intense vocalizations (Atias et al., 2019) without providing any contextual information. The task may have been therefore too demanding hiding possible group differences. Prior evidence suggests that emotional stimuli may influence behavior only when their emotional content is relevant to the participants’ goal given the context in which they operate (e.g., Calbi et al., 2022; Mancini et al., 2022; Mirabella et al., 2023). Accordingly, future studies may test whether “contextualized” vocalizations elicit different reactions in blind and sighted participants, possibly also employing more nuanced vocalizations [see Cowen et al. (2019)] as well as physiological indexes of emotional arousal (e.g., skin conductance response, heart rate). A second limitation relates to the lack of control tasks in our study. In particular, one may object that the higher intensity rates reported by blind participants may represent an unspecific effect possibly extending to other auditory (non-emotional) stimuli or reflecting response biases, and this possibility should be controlled for in an additional task. However, in our study we specifically asked participants to evaluate the intensity of the emotion expressed and not “loudness” of the sounds, and prior studies have shown that early blind individuals exhibit similar sensitivity thresholds for (neutral) sounds’ intensity discrimination [Voss and Zatorre, 2012; for a review see Sabourin et al. (2022)].
In summary, although our findings are preliminary and deserve further investigation, our study represents the first attempt to explore the effect of early blindness on the perception of spontaneous emotional vocalizations.
Data availability statement
The dataset for this article is not publicly available due to concerns regarding participants’ anonymity. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Research Integrity and Ethics Committee of the University of Bergamo. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. MA: Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. DA: Methodology, Writing – review & editing. LM: Supervision, Validation, Writing – review & editing. ZC: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by a PRIN grant (201755TKFE) to ZC by Italian Ministry of Education University and Research.
Acknowledgments
We are extremely grateful to Unione Italiana dei Ciechi e degli Ipovedenti of Legnano, Milano and Pordenone for their help in recruiting and providing support to test blind participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anikin, A. (2020). The link between auditory salience and emotion intensity. Cogn. Emot. 34, 1246–1259. doi: 10.1080/02699931.2020.1736992
Arioli, M., Ferrari, C., Merabet, L. B., and Cattaneo, Z. (2024). Direct reciprocity and reputation shape trust decisions similarly in blind and sighted individuals. Conscious. Cogn. 120:103683. doi: 10.1016/j.concog.2024.103683
Arioli, M., Ricciardi, E., and Cattaneo, Z. (2021). Social cognition in the blind brain: a coordinate-based meta-analysis. Hum. Brain Mapp. 42, 1243–1256. doi: 10.1002/hbm.25289
Arnaud, L., Gracco, V., and Ménard, L. (2018). Enhanced perception of pitch changes in speech and music in early blind adults. Neuropsychologia 117, 261–270. doi: 10.1016/j.neuropsychologia.2018.06.009
Atias, D., and Aviezer, H. (2022). Real-life and posed vocalizations to lottery wins differ fundamentally in their perceived valence. Emotion 22, 1394–1399. doi: 10.1037/emo0000931
Atias, D., Todorov, A., Liraz, S., Eidinger, A., Dror, I., Maymon, Y., et al. (2019). Loud and unclear: intense real-life vocalizations during affective situations are perceptually ambiguous and contextually malleable. J. Exp. Psychol. Gen. 148, 1842–1848. doi: 10.1037/xge0000535
Battal, C., Occelli, V., Bertonati, G., Falagiarda, F., and Collignon, O. (2020). General enhancement of spatial hearing in congenitally blind people. Psychol. Sci. 31, 1129–1139. doi: 10.1177/0956797620935584
Bauer, C., Yazzolino, L., Hirsch, G., Cattaneo, Z., Vecchi, T., and Merabet, L. B. (2015). Neural correlates associated with superior tactile symmetry perception in the early blind. Cortex 63, 104–117. doi: 10.1016/j.cortex.2014.08.003
Bedny, M., Pascual-Leone, A., and Saxe, R. R. (2009). Growing up blind does not change the neural bases of theory of mind. Proc. Natl. Acad. Sci. 106, 11312–11317. doi: 10.1073/pnas.0900010106
Belin, P., and Zatorre, R. J. (2015). Neurobiology: sounding the alarm. Curr. Biol. 25, R805–R806. doi: 10.1016/j.cub.2015.07.027
Calbi, M., Montalti, M., Pederzani, C., Arcuri, E., Umiltà, M. A., Gallese, V., et al. (2022). Emotional body postures affect inhibitory control only when task-relevant. Front. Psychol. 13:1035328. doi: 10.3389/fpsyg.2022.1035328
Cattaneo, Z., Fantino, M., Silvanto, J., Tinti, C., Pascual-Leone, A., and Vecchi, T. (2010a). Symmetry perception in the blind. Acta Psychol. 34, 398–402. doi: 10.1016/j.actpsy.2010.04.002
Cattaneo, Z., Fantino, M., Tinti, C., Silvanto, J., and Vecchi, T. (2010b). Crossmodal interaction between the mental number line and peripersonal haptic space representation in sighted and blind individuals. Atten. Percept. Psychophys. 72, 885–890. doi: 10.3758/APP.72.4.885
Cattaneo, Z., Vecchi, T., Cornoldi, C., Mammarella, I., Bonino, D., Ricciardi, E., et al. (2008). Imagery and spatial processes in blindness and visual impairment. Neurosci. Biobehav. Rev. 32, 1346–1360. doi: 10.1016/j.neubiorev.2008.05.002
Chen, Z., and Whitney, D. (2022). Inferential emotion tracking (IET) reveals the critical role of context in emotion recognition. Emotion 22, 1185–1192. doi: 10.1037/emo0000934
Chong, J. S. X., Ng, G. J. P., Lee, S. C., and Zhou, J. (2017). Salience network connectivity in the insula is associated with individual differences in interoceptive accuracy. Brain Struct. Funct. 222, 1635–1644. doi: 10.1007/s00429-016-1297-7
Cowen, A. S., Elfenbein, H. A., Laukka, P., and Keltner, D. (2019). Mapping 24 emotions conveyed by brief human vocalization. Am. Psychol. 74, 698–712. doi: 10.1037/amp0000399
Ferrari, C., Vecchi, T., Merabet, L. B., and Cattaneo, Z. (2017). Blindness and social trust: the effect of early visual deprivation on judgments of trustworthiness. Conscious. Cogn. 55, 156–164. doi: 10.1016/j.concog.2017.08.005
Gamond, L., Vecchi, T., Ferrari, C., Merabet, L. B., and Cattaneo, Z. (2017). Emotion processing in early blind and sighted individuals. Neuropsychology 31, 516–524. doi: 10.1037/neu0000360
Gurtubay-Antolin, A., and Rodríguez-Fornells, A. (2017). Neurophysiological evidence for enhanced tactile acuity in early blindness in some but not all haptic tasks. NeuroImage 162, 23–31. doi: 10.1016/j.neuroimage.2017.08.054
Handjaras, G., Ricciardi, E., Leo, A., Lenci, A., Cecchetti, L., Cosottini, M., et al. (2016). How concepts are encoded in the human brain: a modality independent, category-based cortical organization of semantic knowledge. NeuroImage 135, 232–242. doi: 10.1016/j.neuroimage.2016.04.063
Hölig, C., Föcker, J., Best, A., Röder, B., and Büchel, C. (2014). Brain systems mediating voice identity processing in blind humans. Hum. Brain Mapp. 35, 4607–4619. doi: 10.1002/hbm.22498
Hölig, C., Föcker, J., Best, A., Röder, B., and Büchel, C. (2015). Crossmodal plasticity in the fusiform gyrus of late blind individuals during voice recognition. NeuroImage 103, 374–382. doi: 10.1016/j.neuroimage.2014.09.050
Klinge, C., Röder, B., and Büchel, C. (2010). Increased amygdala activation to emotional auditory stimuli in the blind. Brain 133, 1729–1736. doi: 10.1093/brain/awq102
Klinge, C., Röder, B., and Büchel, C. (2012). Does training or deprivation modulate amygdala activation? NeuroImage 59, 1765–1771. doi: 10.1016/j.neuroimage.2011.08.046
Lavan, N., Lima, C. F., Harvey, H., Scott, S. K., and McGettigan, C. (2015). I thought that i heard you laughing: contextual facial expressions modulate the perception of authentic laughter and crying. Cognit. Emot. 29, 935–944. doi: 10.1080/02699931.2014.957656
Loiotile, R., Lane, C., Omaki, A., and Bedny, M. (2020). Enhanced performance on a sentence comprehension task in congenitally blind adults. Lang Cogn Neurosci 35, 1010–1023. doi: 10.1080/23273798.2019.1706753
Martins, A. T., Faísca, L., Vieira, H., and Gonçalves, G. (2019). Emotional recognition and empathy both in deaf and blind adults. The Journal of Deaf Studies and Deaf Education. 24, 119–127.
Mancini, C., Falciati, L., Maioli, C., and Mirabella, G. (2022). Happy facial expressions impair inhibitory control with respect to fearful facial expressions but only when task-relevant. Emotion 22, 142–152. doi: 10.1037/emo0001058
Mirabella, G., Grassi, M., Mezzarobba, S., and Bernardis, P. (2023). Angry and happy expressions affect forward gait initiation only when task relevant. Emotion 23, 387–399. doi: 10.1037/emo0001112
Occelli, V., Lacey, S., Stephens, C., Merabet, L. B., and Sathian, K. (2017). Enhanced verbal abilities in the congenitally blind. Exp. Brain Res. 235, 1709–1718. doi: 10.1007/s00221-017-4931-6
Oleszkiewicz, A., Pisanski, K., Lachowicz-Tabaczek, K., and Sorokowska, A. (2017). Voice-based assessments of trustworthiness, competence, and warmth in blind and sighted adults. Psychon. Bull. Rev. 24, 856–862. doi: 10.3758/s13423-016-1146-y
Radziun, D., Korczyk, M., Crucianelli, L., Szwed, M., and Ehrsson, H. H. (2023). Heartbeat counting accuracy is enhanced in blind individuals. J. Exp. Psychol. Gen. 152, 2026–2039. doi: 10.1037/xge0001366
Ricciardi, E., Bonino, D., Sani, L., Vecchi, T., Guazzelli, M., Haxby, J. V., et al. (2009). Do we really need vision? How blind people “see” the actions of others. J. Neurosci. 29, 9719–9724. doi: 10.1523/JNEUROSCI.0274-09.2009
Sabourin, C. J., Merrikhi, Y., and Lomber, S. G. (2022). Do blind people hear better? Trends Cogn Sci. 26, 999–1012. doi: 10.1016/j.tics.2022.08.016
Sarzedas, J., Lima, C. F., Roberto, M. S., Scott, S. K., Pinheiro, A. P., and Conde, T. (2024). Blindness influences emotional authenticity perception in voices: Behavioral and ERP evidence. Cortex 172, 254–270. doi: 10.1016/j.cortex.2023.11.005
Terasawa, Y., Moriguchi, Y., Tochizawa, S., and Umeda, S. (2014). Interoceptive sensitivity predicts sensitivity to the emotions of others. Cognit. Emot. 28, 1435–1448. doi: 10.1080/02699931.2014.888988
Topalidis, P., Zinchenko, A., Gädeke, J. C., and Föcker, J. (2020). The role of spatial selective attention in the processing of affective prosodies in congenitally blind adults: an ERP study. Brain Res. 1739:146819. doi: 10.1016/j.brainres.2020.146819
Voss, P., and Zatorre, R. J. (2012). Occipital cortical thickness predicts performance on pitch and musical tasks in blind individuals. Cereb. Cortex 22, 2455–2465. doi: 10.1093/cercor/bhr311
Keywords: intense emotions, real-life vocalizations, blindness, compensatory mechanisms, arousal
Citation: Ferrari C, Arioli M, Atias D, Merabet LB and Cattaneo Z (2024) Perception and discrimination of real-life emotional vocalizations in early blind individuals. Front. Psychol. 15:1386676. doi: 10.3389/fpsyg.2024.1386676
Edited by:
Giovanni Mirabella, University of Brescia, ItalyReviewed by:
Julia Föcker, University of Lincoln, United KingdomDominika Radziun, Jagiellonian University, Poland
Copyright © 2024 Ferrari, Arioli, Atias, Merabet and Cattaneo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Arioli, maria.arioli@unibg.it
 Chiara Ferrari
Chiara Ferrari Maria Arioli
Maria Arioli Doron Atias4
Doron Atias4 Lotfi B. Merabet
Lotfi B. Merabet