- 1School of Optometry, Université de Montréal, Montréal, QC, Canada
- 2Department of Psychology, Concordia University, Montréal, QC, Canada
- 3Department of Psychology, Université de Montréal, Montréal, QC, Canada
- 4School of Physical and Occupational Therapy, McGill University, Montréal, QC, Canada
Introduction: The occurrence of age-related vision changes is inevitable. However, some of these changes can become pathological. Research indicates that vision and hearing loss is correlated with age-related cognitive decline, and with a higher risk of developing dementia due to Alzheimer’s disease. Low vision rehabilitation could possibly be a protective factor against cognitive decline, as it provides the clients with compensatory strategies to overcome their visual deficits.
Objectives and hypothesis: The aim of this pilot study was to assess correlations between visual and cognitive functions in older adults referred for low vision rehabilitation. We hypothesized that more severe impairment of visual acuity and contrast sensitivity would be correlated with more advanced levels of cognitive impairment. The second objective was to examine which of these correlations would remain significant once established variables that influence cognition are statistically removed (e.g., age, education).
Methods: Thirty-eight older adults (age range: 66–97 years old) with a visual impairment (acuity <20/70) were recruited before the onset of their low vision rehabilitation. They underwent vision (reading acuity, reading speed, contrast sensitivity), hearing (audiogram, speech-in-noise perception) and cognitive (global cognition, memory, executive functions) testing, and demographic information was obtained.
Results and discussion: Correlations among global cognition and visual aid use, memory and reading speed, memory and contrast sensitivity, memory, and visual aid use, and between executive functions and contrast sensitivity were significant. Correlations between contrast sensitivity and memory, as well as between global cognition and visual aid use remained significant after controlling for age and education. The present study is relevant to clinicians who are assessing the cognitive status of older adults, such as neuropsychologists, because it highlights the importance of considering low vision when administering neuropsychological tests, especially to persons who have not yet received rehabilitation for their visual impairment.
Introduction
Changes in vision, such as the inevitable arrival of the need for reading glasses in middle age (presbycusis) are expected as a part of the normal aging process (Haegerstrom-Portnoy et al., 2002); however, there are eye diseases associated with aging that can further impair the visual abilities of older adults (Wong et al., 2014). Some visual functions change as a result of visual pathologies, such as age-related macular degeneration (AMD), glaucoma or diabetic retinopathy, all of which can cause low vision (Wittich and Gagné, 2016). Low vision (LV) has been described as a decline in visual functions that alters the ability to complete visual tasks, and that cannot be treated with corrective or contact lenses, or other surgical or medical interventions (Corn and Erin, 2010). The World Health Organization (2019) predicts that the number of individuals with AMD will increase 1.2-fold from 2020 (roughly 195 million) to 2030 (roughly 243 million). Interestingly, research indicates that visual decline due to AMD, and vision loss in general, are correlated with age-related cognitive decline, and persons with AMD are at higher risk of developing dementia due to Alzheimer’s disease (Rogers and Langa, 2010; Zhou et al., 2016; Nagarajan et al., 2022). The link between visual and cognitive functioning and aging has a long-standing knowledge base (Lindenberger and Baltes, 1994; Lindenberger et al., 2001); however, the majority of correlational explorations of vision and cognition have been conducted with population-based data sets (Hong et al., 2016) or have focused on specific sub-populations with cognitive or visual diagnoses [e.g., vision in patients with Alzheimer’s disease (AD; Kirby et al., 2010), or cognition in persons with AMD (Zhou et al., 2016)] that have received treatment or are being treated. Low vision rehabilitation is a service that aims at improving daily living by providing techniques to use remaining intact vision. We are unaware of any studies on the effect of low vision rehabilitation on cognition that have been able to include participants with low vision (e.g., visual acuity below 20/60) that had not (yet) received rehabilitation services. A recent longitudinal study focused on functional improvement in activities of daily living in veterans with low vision, with or without cognitive impairment, but the study did not assess cognition pre- and post-rehabilitation (Whitson et al., 2020). The present pilot study aimed to explore the relationship among sensory and cognitive variables, specifically in older adults who had not yet received low vision rehabilitation, thereby presenting an opportunity to better understand the relationship among their pre-rehabilitation sensory-cognitive abilities. This understanding is required for future studies that will explore the effect vision rehabilitation may have on cognition in older adults with low vision (Wittich et al., 2021).
As a result of the normal aging of the visual system, near visual acuity is expected to decrease somewhat, making the reading of small print more challenging (Pierscionek and Weale, 1995). Similarly, aging affects cognitive abilities, such as memory and processing speed (Park and Gutchess, 2016), and a decline in cognitive function is expected. In addition, cognitive pathologies, such as AD and vascular dementia, are two of the most predominant forms of dementia, causing significant changes in cognition (World Health Organization, 2017).
Several studies have linked changes in visual function (e.g., visual acuity, reading acuity) to changes in cognitive function (Rogers and Langa, 2010; Dupuis et al., 2014; Zhou et al., 2016; Davies-Kershaw et al., 2018; Zheng et al., 2018; Ehrlich et al., 2021). For example, cognitive impairment, as measured by on a score of lower than 28 on the Mini Mental State Examination (MMSE), is related to slower reading speed on the Minnesota Low Vision Reading Test (MNRead; Swenor et al., 2016). Similarly, impairment in visual acuity has been linked to lower scores on the Montreal Cognitive Assessment (MoCA; Dupuis et al., 2014), a screening tool designed to identify persons at risk for mild cognitive impairment. Additionally, individuals with mild cognitive impairment and AD have higher rates of reduced reading acuity than individuals with subjective cognitive decline (Wittich et al., 2019). In a meta-analysis, the presence of AMD has been linked to lower scores on various cognitive tests (i.e., Mini-Mental State Examination, Mini-Cog test, Trail Making Test A; Zhou et al., 2016).
Several studies have found vision-related abnormalities in individuals with AD. Visual deficits (e.g., difficulty reading, loss of contrast sensitivity) are commonly found in AD (Lee and Martin, 2004), and visual complaints are often reported by AD patients in the early stages of the disease and may precede the memory decline (Ikram et al., 2012). In addition, a decrease in the visual field and/or contrast sensitivity, as well as fixation problems, are common in AD patients (Ikram et al., 2012). A longitudinal study found that self-reported low vision was a risk factor for AD (Davies-Kershaw et al., 2018). Moreover, this relationship between AD and visual deficits is also present in the brain at the structural level, whereby the topological organization of higher-level visual networks is altered in individuals with AD and mild cognitive impairment (Deng et al., 2016).
Furthermore, several studies focusing on functional outcomes explored the link between changes in vision (e.g., visual acuity, contrast sensitivity) and changes in cognition (e.g., global cognition, executive functions, psychomotor speed). Longitudinal studies have demonstrated an association between low vision and cognitive decline over time, whereby the severity of the decline in visual acuity over 8 years, as measured by the ETDRS chart (Early Treatment of Diabetic Retinopathy Study), is linked to the severity of the cognitive decline at the 8-year follow-up, as measured by the MMSE (Zheng et al., 2018). Another study showed that low vision is associated with the transition from normal cognition to cognitive impairment or dementia, using Digit Symbol Substitution Test and the Modified MMSE), to measure cognition (Ehrlich et al., 2021). A longitudinal study assessing multiple aspects of visual functioning (e.g., contrast sensitivity, visual acuity, and stereo acuity) found that impairments in these measures of vision were associated with increased risks of a decrease in psychomotor speed, worse executive functioning, and general cognitive decline over 9 years (Swenor et al., 2019). A risk factor for cognitive decline and AD is uncorrected self-reported low vision (Rogers and Langa, 2010), while wearing glasses and having good visual acuity can be protective against cognitive decline (Spierer et al., 2016).
Many mechanisms have been postulated to explain this link between sensory and cognitive decline. First, the information degradation hypothesis states that neurobiological processes (e.g., AMD) weaken the strength of the perceptual signal, which, in turn, negatively affect perceptual and cognitive processing (Monge and Madden, 2016). In the case of AMD, the visual decline may cause a decrease in the stimulation, thereby resulting in cognitive decline. Second, the sensory deprivation hypothesis states that a persistent absence of adequate sensory input will create neuronal atrophy and will later result in cognitive decline (Clay et al., 2009). In the case of AMD, the reduced visual input due to visual decline may create neuronal atrophy, meaning that cognitive decline may result later because of AMD. Third, the cognitive compensation hypothesis states that cognitive functions could be used to compensate for the visual decline, therefore monopolizing the cognitive resources and causing cognitive decline (Roberts and Allen, 2016). In the case of AMD, this could mean that cognitive resources, such as attention, could be used to compensate for vision loss. Therefore, not all cognitive functions are available for higher-level tasks. For example, as attention is a key function in the encoding process, this compensation could result in less in-depth encoding, leading to memory decline.
Another hypothesis is the common cause hypothesis, which states changes in sensory and cognitive ability are due to a single common cause, such as the aging brain (Kiely and Anstey, 2015). Other hypotheses have also been proposed to explain the relationship between concomitant changes in sensory and cognitive functions. Social participation has been proposed as a key factor in cognitive health. For example, individuals with vision loss might fear falls when going outside and prefer not to attend social event for this reason. This reduced participation might be followed by a reduction in cognitive capacities (Bourassa et al., 2015).
Recently, some anatomical studies have linked AMD and AD (Dentchev et al., 2003; Chang et al., 2014; Ratnayaka et al., 2015). The brain and the retina share many similarities, such as the same embryological origin, as well as some anatomical and physiological features (Ikram et al., 2012). Chang et al. (2014) presented evidence that the three well-known AD biomarkers—protein amyloid-ß, senile plaques, and neurofibrillary tangles, are also present in the retina of AD patients. Another study linked amyloid-ß deposits in the retina to AMD (Dentchev et al., 2003). These discoveries are an important step in understanding the critical link between AMD and AD.
According to the first three hypotheses, the degradation of the sensory input is proposed to be linked to cognitive decline. The first two hypotheses state that the degradation of the sensory input is causing the degradation of cognition. Therefore, focusing on ways to reinstate the visual input in AMD patients could theoretically preserve or potentially improve cognitive functioning. According to the third hypothesis, reducing the effort needed to see and read could reduce the cognitive demands for tasks involving reading, and more cognitive resources could be allocated to higher-level tasks. Therefore, in line with all three hypotheses, low vision rehabilitation should be a way to preserve or even improve cognitive functioning in patients with AMD. At present, little is known about any relationship among sensory and cognitive variables before the vision rehabilitation process in persons living with AMD.
In Quebec, individuals with vision loss have access to rehabilitation services and assistive devices provided by the government at no cost to the client (Government of Quebec, 2022). Eligible individuals can undergo low vision rehabilitation to maximize their residual vision and learn strategies to compensate for their vision loss (Gordon et al., 2015). These services can include adaptations of the client’s physical environment to ensure proper lighting and adaptive techniques for reading, instruction in the use of assistive devices to facilitate visual activities, and other considerations that might be appropriate for the client. Low vision rehabilitation has been linked to an improvement in quality of life and participation in activities of daily living (Binns et al., 2012). However, to our knowledge, there are currently no data available that evaluate the impact of low vision rehabilitation on cognition in older adults.
Pilot study objectives
The first objective of this pilot study was to assess correlations between the sensory and cognitive functions in older adults referred for low vision rehabilitation. We hypothesized that more severe impairment of visual acuity and contrast sensitivity would be correlated with more advanced levels of cognitive impairment (e.g., processing speed, executive functioning, and memory). Should such relationships exist, the second objective was to examine which of these correlations would remain significant once established variables that influence cognition are statistically removed (e.g., age, education).
Methodology
The Institutional Review Board approval for this study was obtained from the Centre de recherche interdisciplinaire en réadaptation du Montréal métropolitain (CRIR #1284-1217). Additional details about the larger protocol context, within which these pilot data were collected, have been published elsewhere (Wittich et al., 2021).
Participants
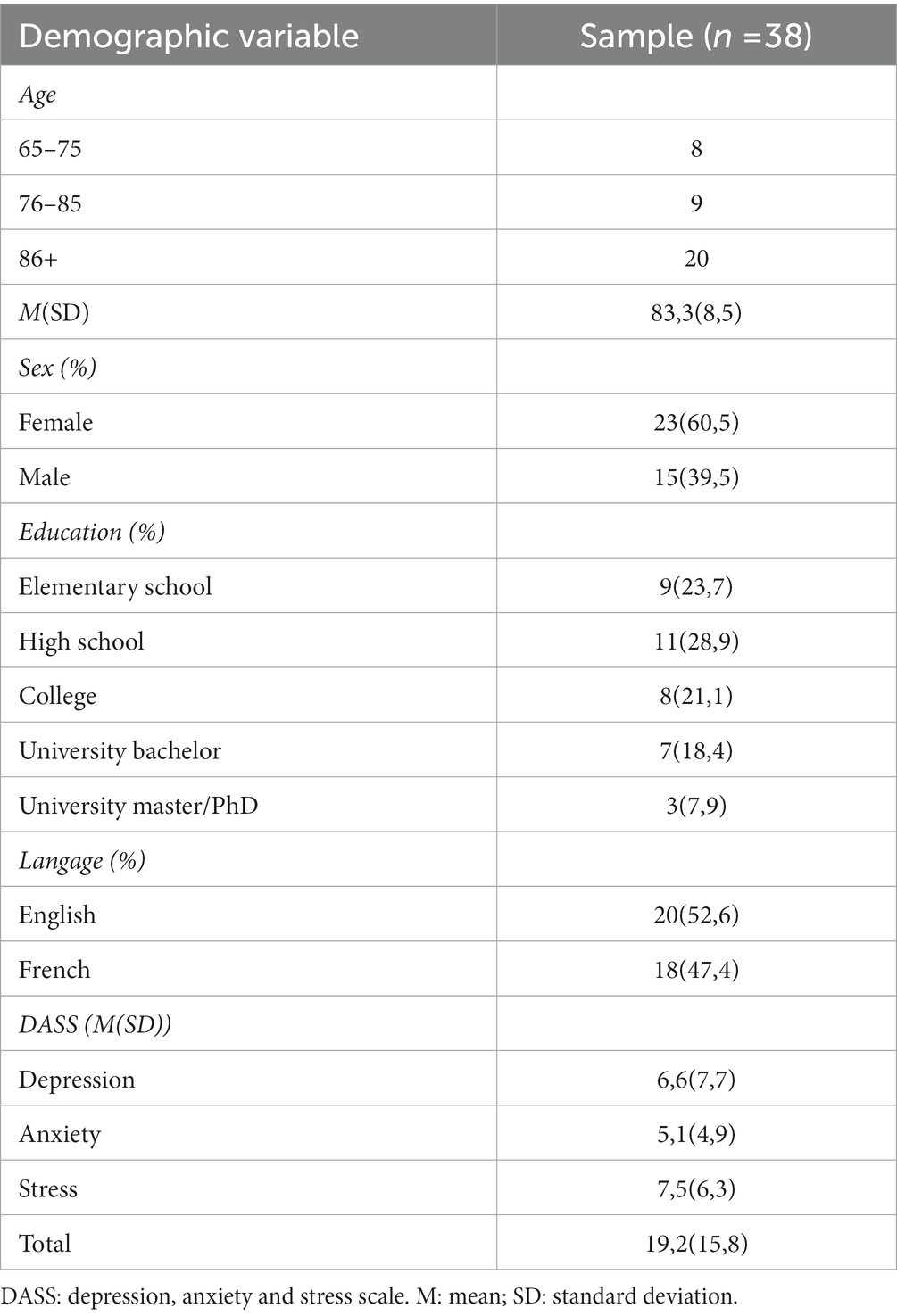
Low vision participants were recruited from the two rehabilitation centres in the Montreal region (Centre de Réadaptation Lethbridge-Layton-Mackay du CIUSSS du Centre-Ouest-de-l’Île-de-Montréal, and l’Institut Nazareth et Louis-Braille du CISSS de la Montérégie-Centre). Inclusion criteria were: being at least 65 years old, having a diagnosis of AMD as confirmed by the referring optometrist or ophthalmologist, and being eligible for low vision rehabilitation according to the following Quebec Health Ministry criteria (Government of Quebec, 2022): visual acuity in the better eye of < 20/70 (6/21; ETDRS or Feinbloom) with standard optical correction of lesser than four dioptres, or ≤ 20/60 (6/18) for individuals with a degenerative visual problem and perception of impairment. Exclusion criteria were having a neurological disorder as noted in the patient file or self-reported by the patient, a severe hearing loss (> 70 dB mean pure-tone average hearing level in the better ear), or a diagnosed form of dementia, as noted in the participants’ rehabilitation file or referral. The sample was composed of 38 participants (age range: 66–97 years old). Their demographic information can be found in Table 1.
Materials
All tests were selected because they are suited for individuals with vision impairment (i.e., they do not depend on vision), are available and validated in French and in English, and have acceptable psychometric properties. Most testing sessions took place in the participants’ home. A demographic questionnaire was used to collect basic information about the participants, such as their age, level of education and living circumstances. The questionnaire also contained items about their reading habits, both before and after the onset of their visual impairment. In addition, the Depression, Anxiety and Stress Scale (DASS 21; Lovibond and Lovibond, 1995) was used to assess the mental health of participants over the past week (Henry and Crawford, 2005) because it has good psychometric properties when used with older adults (Gloster et al., 2008).
Vision measures
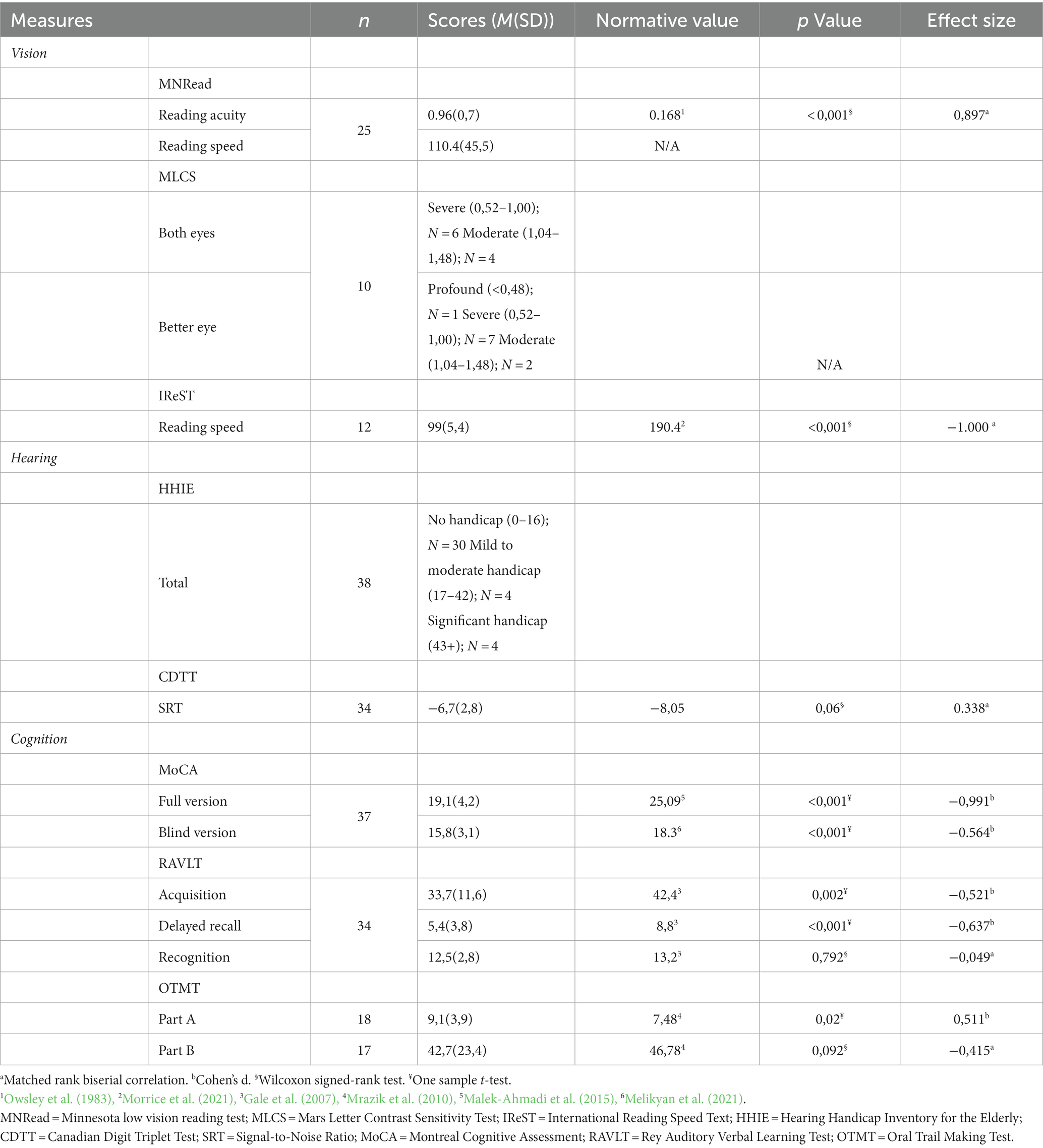
The Minnesota Low Vision Reading Test (MNRead) was used to assess the maximum reading speed (i.e., reading speed when there is no character size limit; in words per minute) and reading acuity (Mansfield et al., 1994), because it is specifically designed for low vision patients and is adapted for populations of all ages. The Mars Letter Contrast Sensitivity Test (MLCS) was used to assess contrast sensitivity, which requires participants to read letters where the contrast decreases with each letter (Arditi, 2005). Finally, the International Reading Speed Text (IReST) was used to assess reading speed (in words per minute). This test is commonly used with individuals with low vision and has been validated with younger and older adults (Trauzettel-Klosinski and Dietz, 2012; Morrice et al., 2020, 2021). Participants were encouraged to use any assistive devices that they may have to complete the task. The norms for Canadian older adults aged 60 years old and older from Morrice et al. (2021) were used. These norms only offer normative data for anglophone Canadians. Finally, participants responded to two items from the Canadian Longitudinal Study on Aging (Kirkland et al., 2015), asking “How do you characterize your eyesight (using glasses or corrected lenses if you use them)?” (excellent, very good, good, fair, poor) and “Besides glasses or contact lenses, do you use any aids or specialized equipment for persons who are blind or visually impaired” (yes/no). The second question had an optional follow-up item on which type of devices are used, to elucidate any potential effect of device access and/or use before vision rehabilitation began through professional services.
Cognitive measures
To assess the cognitive function of participants, four tests were administered. First, the MoCA (Nasreddine et al., 2005) was used to screen the overall cognition of the participants. It was scored in both its traditional way as well as in its blind format(Wittich et al., 2010), because many participants were unable to complete the visual items of the test. The score of the MoCA-Blind is obtained by eliminating the visual items. A score of 26 on the MoCA and 18 on the MoCA-Blind are the cut-off scores indicating that an individual may be at risk for mild cognitive impairment. The three different versions of the MoCA (7.1, 7.2, 7.3) were assigned randomly. The normative values used for the MoCA regular version were for older adults aged 80–89 years old because the mean age and most of our participants fall into this age range (Malek-Ahmadi et al., 2015).
The Rey Auditory Verbal Learning Test (RAVLT) was used to assess the verbal learning and the verbal memory of our participants (Strauss et al., 2006). This test evaluates memory encoding, storage, and retrieval (Strauss et al., 2006). Both, the learning, delayed recall, and recognition parts were used. The RAVLT is scored for acquisition (i.e., number of words recalled in the first five trials), delayed recall (i.e., the number of words recalled after the 20-min interval) and recognition (i.e., number of words correctly identified in a 50-word list), whereby higher scores indicate better performance. The normative values used were acquired by Gale et al. (2007) in a population of older adults aged 80 to 89 years old.
A third measure was the Oral Trail Making Test parts A & B (OTMT), the auditory version of the Trail Making Test. It is designed for populations for which drawing may be a problem, such as individuals with a visual impairment (Strauss et al., 2006). It is composed of two trials, a first trial where the participant is asked to count from 1 to 25 as fast as possible, and a second trial where they are asked to count from 1 to 13 by alternating between a number and a letter in both chronological and alphabetical order. This test assesses attention, speed, and mental flexibility. The outcome measure is the time, in seconds, participants took to complete the task, whereby lower scores indicate better performance (Strauss et al., 2006). The normative values for the OTMT used in the present pilot study were acquired in a sample of older adults aged 69–90 years old.
Hearing measures
As hearing loss is correlated with cognitive decline (Lin et al., 2013), and has been identified as the largest potentially modifiable risk factors in mid-life for dementia in late life (Livingston et al., 2020). Hearing assessment was included in the protocol to be further explored in the analyses. However, these data are only included here for descriptive purposes. First, the experimenter performed an otoscopy with the participant’s authorization. This is a visual examination of the ear canal to evaluate its integrity, using an otoscope. Both ears were examined before proceeding to the hearing test. The Canadian Digit Triplet Test (CDTT) was then used to assess speech perception in noise, using a signal-to-noise ratio threshold (Giguère et al., 2020). The outcome measure was the signal-to-noise ratio, indicating how much noise a person could tolerate while still being able to comprehend speech (more negative values indicate better tolerance of noise). Participants also complete the Hearing Handicap Inventory for the Elderly (Ventry and Weinstein, 1982) to explore self-reported situational and emotional hearing abilities.
Procedure
All eligible individuals were identified and contacted by the admissions office at each rehabilitation center according to the eligibility criteria. If interested, their contact information was sent to the research team. Informed written consent was obtained, and participants completed the questionnaires, hearing, reading and cognitive assessment components. Participants were scheduled for their first testing session before their global initial intake exam by a professional from the rehabilitation center. All the questionnaires were administered verbally. The testing session began with the demographic questionnaire and the DASS 21. Thereafter, the participants’ vision was assessed using the MNRead and the Mars Letter Contrast Sensitivity test. Then, the MoCA and the RAVLT part 1 were performed. During the 20-min break between the RAVLT part 1 (acquisition) and part 2 (delayed recall and recognition), the participants completed the CDTT. If the participant did not need a break at this point, or if their testing session progressed at a good speed, they additionally performed the IReST and/or the OTMT, depending on how much time remained during the 20-min break. Then the RAVLT delayed recall and the RAVLT recognition were performed. The sessions lasted approximately 2 h, and the participants were encouraged to take as many breaks as they needed. The testing sessions were conducted by two experimenters (e.g., student and research assistant) to facilitate the flow of the otherwise lengthy protocol. Participants were informed that they would be contacted at a later date for eventual follow-up as part of a larger protocol that examines cognitive outcomes of vision rehabilitation (Wittich et al., 2021).
Results
Prior to the analysis, the data were thoroughly screened for any missing or extreme values. When possible and/or suitable, the missing values were replaced by the mean of the sample or the mean of the participant (for questionnaires with multiple items). Two extreme values were removed from the data: one from the CDTT test, whereby the results indicated that the participant had an extremely severe hearing impairment that did not correspond to the communication experience with the participant and was, therefore, more likely a headphone malfunction. The second extreme value was a MoCA score of 4. Since the participant fell asleep during the MoCA, we judged that his result was not an adequate representation of his cognitive capacities. As for the other tests completed by this participant, they were included in the analysis as he was fully awake during this part of the testing, and his scores did not fall more than three SD from the group mean. Sample sizes for all available measures are provided in Table 2. Small samples (e.g., scores on the IReST) are explained by the participants’ inability to read because they had not yet received any vision rehabilitation. Missing data play a potentially important role in the assessment of cognition in persons with sensory impairment (Abraham et al., 2023); however, we did not observe any noteworthy patterns in missing cognitive measures, given that they were all chosen to accommodate the presence of visual impairment.
Descriptive analysis
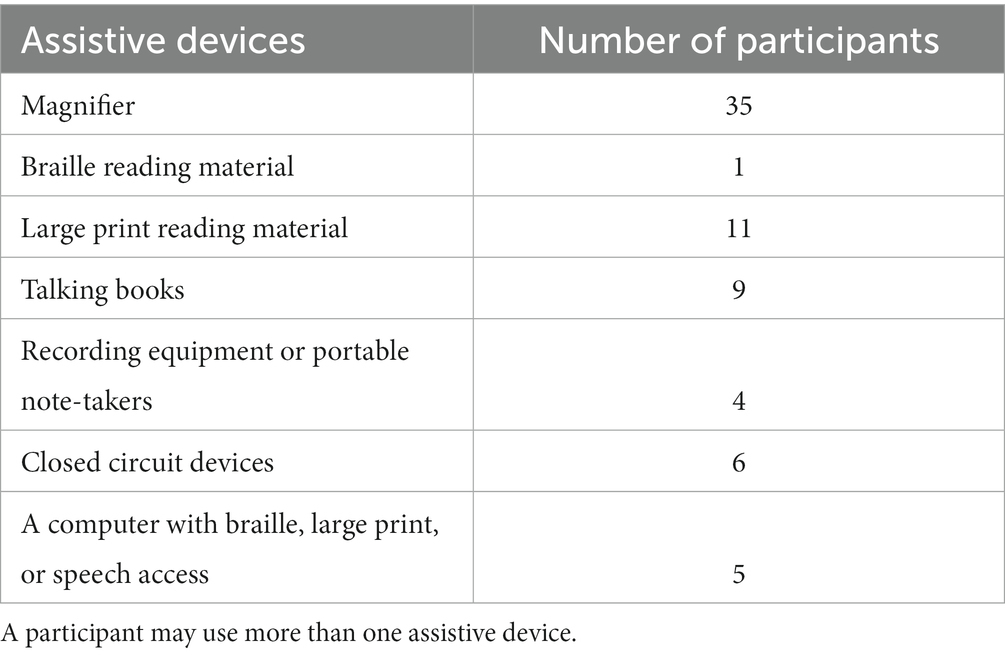
All participants were residents of Québec, Canada. The DASS 21 mean score was in the normal range (depression: 0–9, anxiety: 0–7, stress: 0–14). However, 15.7% of our sample showed signs of moderate depression, 13.1% showed signs of moderate anxiety, and 5.3% showed signs of moderate stress, according to the DASS-21. Most (35/38, 92%) participants were already using visual aids before the onset of their visual rehabilitation. The number of participants using various assistive devices prior to their rehabilitation is presented in Table 3. The demographic information of the participants is listed in Table 1.
The means and the standard deviations of all test scores can be found in Table 2. Regarding the MoCA—full version, 3 (7.9%) participants had a pass score of 26 or higher, whereas, for the MoCA—blind version, 13 (34.2%) participants passed with a score of 18 or higher. A chi-squared analysis was not possible since nobody passed the MoCA-B and also failed the complete MoCA.
The mean scores on the visual, hearing, and cognitive tests, as well as the results of the t-tests for the normative comparisons to existing standard values, are listed below in Table 2. If the normality assumption was not respected, a Wilcoxon signed-rank non-parametric test was performed.
Vision
As would be expected for participants awaiting low vison rehabilitation, our participants had significantly lower visual acuities and reading speeds than age-matched healthy older adults (see Table 2). For the MNRead, we used data from a study by Owsley et al. (1983) as normative values to compare our sample. As their data are separated according to decade, we took the mean of the 60s, 70s and 80s age groups as the normative value for our sample. For the IReST, we used data from Morrice et al. (2021) who produced norms for adults aged 60 years old and older. There are currently no average normative values for the Letter Mars Contrast Sensitivity. However, a breakdown of our sample in terms of contrast sensitivity impairment severity in Table 2 indicates that binocular contrast sensitivity was at least moderately impaired for all participants.
Cognition
As for the cognitive measures, the sample performed significantly worse in both the acquisition and delayed recall parts of the RAVLT (Gale et al., 2007), as well as in the first OTMT part A (Mrazik et al., 2010), and the MoCA-Blind (Melikyan et al., 2021) in comparison to the normative values. There was no significant difference between scores from our sample and the normative data for the RAVLT recognition and OTMT part B.
Correlation analyses
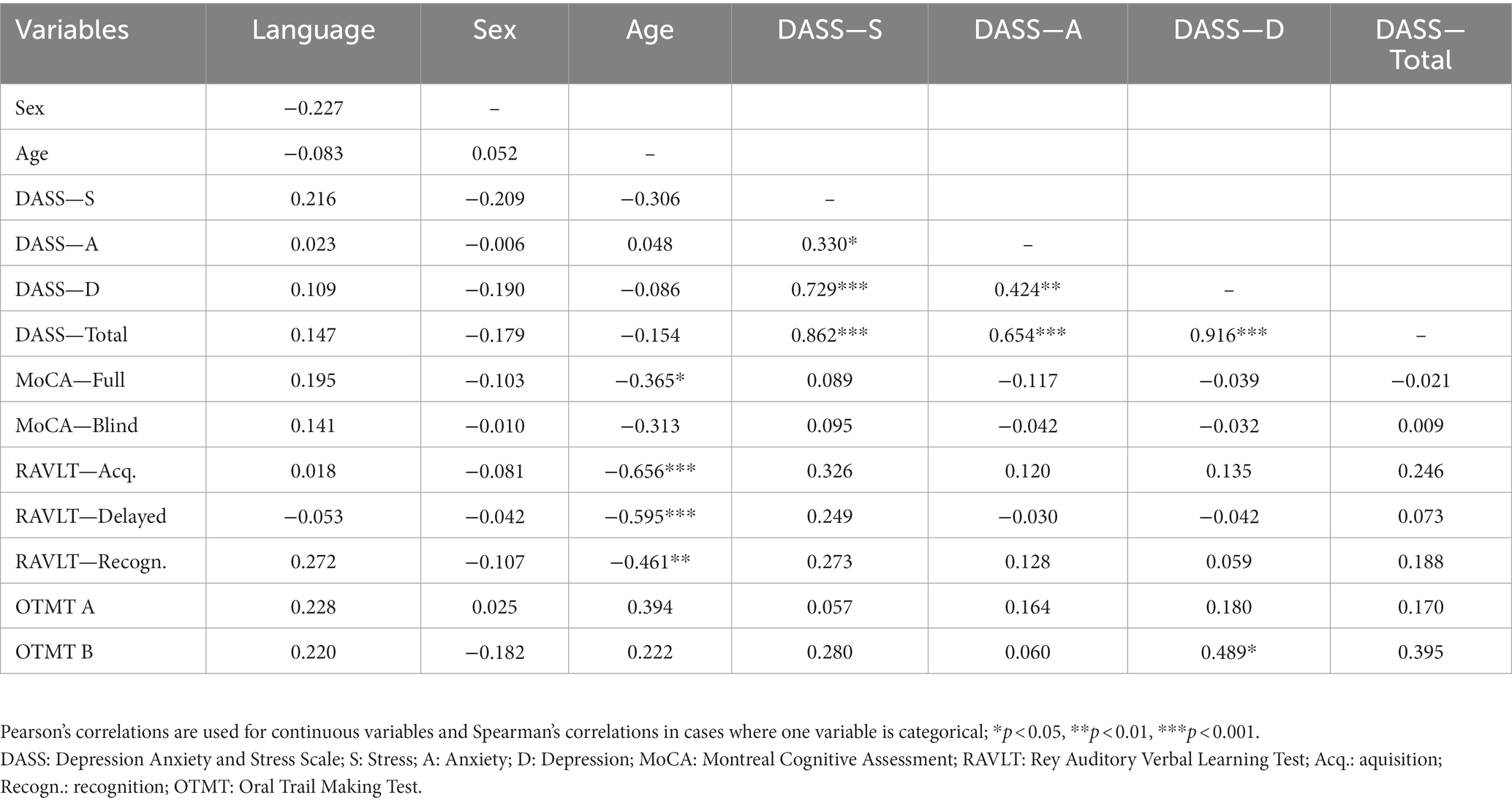
Correlations among cognition and demographic variables can be found in Table 4. Age was significantly negatively correlated with the full MoCA score (r = −0.365, p < 0.05), the RAVLT acquisition (r = −0.656, p < 0.001), the RAVLT delayed recall (r = −0.595, p < 0.001) and the RAVLT recognition (r = −0.461, p < 0.01). There was also a significant positive correlation between education and RAVLT – acquisition (r = 0.409, p < 0.05).
Cognition and vision
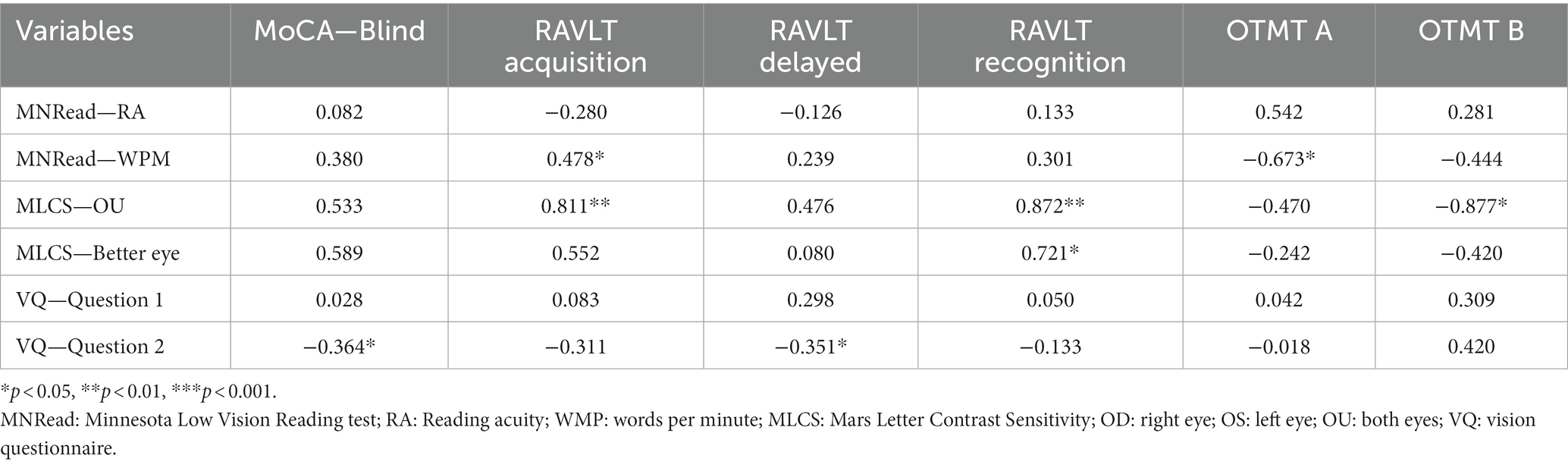
Pearson’s and Spearmen’s correlation coefficients between cognition and vision measures can be found in Table 5. For the vision tests, we used the MNRead (both reading acuity and reading speed), the Mars Letter Contrast sensitivity (only for both eyes and the better eye), and the vision questionnaire (both questions). For cognition, we used the MoCA (blind version only to eliminate the effect of vision), the RAVLT (acquisition, delayed recall and recognition to better understand memory processes) and the OTMT (both parts).
As for the vision and cognition correlations, the MNRead—reading speed was significantly and positively correlated with the RAVLT—acquisition score, as well as significantly and negatively correlated with OTMT part A. The binocular contrast sensitivity was positively correlated with the RAVLT—acquisition and the RAVLT—recognition, as well as negatively correlated with the OTMT part B. Additionally, contrast sensitivity in the better eye was positively correlated with the RAVLT—recognition. Finally, the second question of the vision questionnaire [“Besides glasses or contact lenses, do you use any aids or specialized equipment for persons who are blind or visually impaired?”] was negatively correlated with the MoCA-Blind and the RAVLT—recognition.
Linear regressions
For every significant correlation mentioned in the previous section, a linear regression was conducted with age and education as covariates to assess if the association between these variables would remain significant. Age and education were selected because these two aspects are important to control variables in cognitive research (Rabbitt et al., 1995).
Cognition and vision
For the MNRead—reading speed correlations, none of them remained significant after controlling for age and education.
The positive correlation between the binocular contrast sensitivity and the RAVLT—recognition remained significant (p = 0.018) after controlling for age and education. The other contrast sensitivity correlations did not remain significant after controlling for age and education. This result indicates that better binocular sensitivity is associated with memory, even after controlling for age and education.
For the vision questionnaire correlation, the negative correlations between the second question, the MoCA-Blind remained significant after controlling for age and education (MoCA—blind: p = 0.033). This indicates that older adults that are already using visual aids before their rehabilitation have better overall cognition, even after controlling for age and education. The correlation between the vision questionnaire—question 2 and the RAVLT—recognition did not remain significant after controlling for age and education.
Discussion
The purpose of the present pilot study was to characterize the sensory and cognitive status of adults with AMD referred for visual rehabilitation. Our first objective was to assess the correlations between sensory and cognitive functions in our participants before controlling for variables known to be important in the context of cognition. The second objective was to examine which of these correlations would remain significant once established variables that influence cognition are statistically removed (i.e., age and education).
First, the association between contrast sensitivity and word recognition, as measured by the RAVLT, remained significant after controlling for age and education. This result was surprising given our limited sample size, given the reduction in statistical power by controlling for additional variables. This finding emphasizes the importance of contrast sensitivity as a variable in sensory-cognitive relationships in aging, which has previously been highlighted in the scientific literature. Globally, lower contrast sensitivity has been associated with a higher risk of mild cognitive impairment and dementia in older women, as assessed by an expanded neuropsychological tests battery and a panel of clinical experts (Ward et al., 2018). Contrast sensitivity has also been identified as a measure that is likely to detect early AD-associated changes in groups with subjective cognitive decline, amnestic mild cognitive impairment, and AD (Risacher et al., 2013). Moreover, impairments in contrast sensitivity as measured by the Pelli–Robson chart (similar in its structure to the MLCS used in the present study) are associated with a decline in global cognition as measured by the Modified Mini-Mental State Examination (Swenor et al., 2019).
Furthermore, episodic memory is an important cognitive function in sensory-cognitive aging, as it tends to decline both in normal and pathological aging. Our result is also particularly interesting in that sense, as word recognition processes are usually the ones that are the most preserved in normal aging (Perlmutter, 1979). Compared to free recall and cued recall, recognition facilitates information retrieving by presenting all the encoded information within distractors. Therefore, the recognition process helps differentiate between difficulties in encoding in comparison to difficulties retrieving information. Our results replicate studies that indicate that contrast sensitivity could be sensitive to subtle changes in cognition, specifically in the encoding process, in non-demented older adults (Risacher et al., 2013; Ward et al., 2018; Swenor et al., 2019).
One other correlation remained significant after controlling for age and education: the association between the use of visual aids and global cognition, as measured by the blind version of the MoCA. Even though our data were collected before the beginning of the low vision rehabilitation process, many participants were proactive in looking for devices that could facilitate reading and were already using various visual aids, the most popular being a magnifier. Therefore, this result may indicate that individuals already using visual aids, even before the beginning of the formal rehabilitation process, may have better global cognition, as measured by the MoCA (blind version). However, as our data are correlational, this could also mean that individuals with better baseline global cognition are more proactive, seeking out the use of visual aids even before the beginning of the formal rehabilitation process. This is also in line with the current literature, as a recent study demonstrated that the use of visual aids demonstrated a potential effect on slowing cognitive decline in a long-term care facility environment (Kwan et al., 2022).
Finally, our results corroborate findings in the scientific literature regarding the association between low vision and cognitive decline. We found that older adults with low vision have significantly poorer cognitive functions (e.g., episodic memory, processing speed, global cognition) compared to normative values. In line with previous research (Zhou et al., 2016; Zheng et al., 2018; Lee et al., 2020), these results indicate that the occurrence of vision loss in non-demented individuals is associated with poorer global cognition, memory, and executive functions.
Mechanisms
The present results are in concordance with some postulated mechanisms explaining the link between sensory and cognitive processes. Our results could be explained by the information degradation hypothesis, which states that neurobiological processes, such as vision loss, diminish the strength of the perceptual signal, which negatively affect the perceptual processing and, therefore, cognitive processing (Monge and Madden, 2016). As this hypothesis does not postulate an association over time, it is possible to examine it using cross-sectional data like ours. It appears in our sample that, as compared to normative data, our low vision participants have significantly lower scores in global cognition, memory (acquisition and delayed recall), and executive functions (processing speed). Moreover, our results show that only 7.9% of our sample passed the MoCA, and 34.2% passed the MoCA-Blind. These results indicate that roughly 66% of our sample could be at risk for cognitive impairment, whereas the estimated prevalence of cognitive impairment in the Canadian population is 25% in adults 85 years old and older (Canadian Institute for Health Information, 2021).
The sensory deprivation hypothesis states that a persistent absence of adequate sensory input creates neuronal atrophy and later results in cognitive decline (Clay et al., 2009). The cognitive compensation hypothesis states that cognitive functions could be used to compensate for the visual decline, therefore monopolizing the cognitive resources and causing cognitive decline (Roberts and Allen, 2016). However, both these hypotheses require prospective data. Thus, we cannot speculate using our cross-sectional results. Our data indicate that individuals that are already using visual assistive devices before their visual rehabilitation have significantly better global cognition scores (both versions of the MoCA) and better delayed recall than those who do not already use assistive devices. As of now, our results align with only one of these proposed mechanisms, as our participants may have self-protected their cognition by initiating the rehabilitation process. Eventual prospective data are needed to assess the effect of time and rehabilitation services on cognitive functions, as planned in our larger protocol (Wittich et al., 2021).
Vision and demographic variables
In line with previous studies (Dupuis et al., 2014; Zhou et al., 2016), our results indicated that, as age increases, global cognition and memory scores were lower. There was no significant correlation between the DASS 21 and the cognitive scores. Likely, our participants predominantly fall within the normal range of the depression, anxiety, and stress scores on the DASS 21. A recent study showed that 14% of Canadian older adults from the Canadian Longitudinal Study on Aging had symptoms of depression as measured by the Center for Epidemiological Studies Depression Scale Short Version 10 (Kuspinar et al., 2020). This is in line with our results, where 15.7% of our sample have moderate symptoms of depression. Moreover, it is noteworthy that the older portion of the Canadian population tends to present less depression, anxiety, and stress symptoms, and this is even true during the COVID-19 pandemic (Nwachukwu et al., 2020). It has been reported that older adults with depressive symptoms have lower cognitive scores than non-depressed older adults (Ganguli et al., 2006), which may explain the absence of the correlation between DASS 21 scores and any of the cognitive measure in our sample of older adults with a low proportion of depressive symptoms.
The present study is relevant to clinicians screening or assessing the cognitive status of older adults, such as neuropsychologists, because it highlights the importance of considering low vision when administering neuropsychological tests, especially to persons who have not (yet) received rehabilitation for their visual impairment. Therefore, visual ability should be screened when cognition is assessed to have an accurate assessment of cognitive functioning (Campos et al., 2019). Furthermore, the present results are important for eye care professionals that are interacting with individuals with low vision because the success of service delivery may partially depend on considering the cognitive status of the patient. Finally, the present results are interesting for researchers in the field of sensory-cognitive aging, as they highlight the importance of contrast sensitivity and the usage of visual aids for cognitive functions. Our results are in concordance with previous literature on the subject that states an association between vision, hearing and cognitive functions (Lindenberger and Baltes, 1994; Baltes and Lindenberger, 1997; Lindenberger et al., 2001; Lang et al., 2002).
Limitations and future directions
The main limitation of this pilot study is its sample size because recruitment was interrupted due to the COVID-19 pandemic. Given the large number of measures, there is a risk of alpha error cumulation that could possibly have been controlled by adjusting the significance level (e.g., Bonferroni correction); however, such a correction would have been too strict to make the results interpretable. The protocol has now been modified to accommodate pandemic safety measures, but it remains unclear how easily pre-pandemic findings can be compared to data collected at a later point in time. Either way, more participants will be needed to strengthen the interpretation of the results. Another limitation is that the sample size is too small to control for the hearing status of our participants, even though hearing impairment has been identified as the largest potentially modifiable risk factor for dementia (Livingston et al., 2020). Moreover, only individuals with AMD were recruited for this project. Therefore, the results cannot be generalized to older adults with other ocular pathologies, such as glaucoma, diabetic retinopathy, optic neuropathy or atrophy that have each been linked to changes in cognition (Harrabi et al., 2015; Carelli et al., 2017; Lee et al., 2020). Finally, some variability in the data may be introduced by the methodological choice to test participants in their homes. Even though recruitment was facilitated because participants with visual impairment did not need to travel to a testing site, the testing conditions were not homogenous.
Conclusion
The results of this pilot study clearly emphasize the importance of assessing the sensory abilities of older adults with low vision together with evaluating their cognitive abilities. Our participants with AMD demonstrated lower cognitive abilities than what would be expected from their sighted counterparts, even when choosing testing materials that did not rely on functional vision. A larger sample and prospective data are needed to assess the potential effect of low vision rehabilitation on cognition in older adults with AMD. The next step will be to implement the full protocol of this research project (Wittich et al., 2021), thereby expanding our understanding of sensory-cognitive aging and the role sensory rehabilitation can play in preserving cognitive function.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Centre de recherche interdisciplinaire en réadaptation du Montréal métropolitain (CRIR #1284-1217). The patients/participants provided their written informed consent to participate in this study.
Author contributions
GA contributed to conceptualization, formal analysis, investigation, methodology, validation, writing the original draft, and writing—review and editing. NP, AtJ, AaJ, SJ, VB, and EK contributed to conceptualization funding acquisition, formal analysis and data interpretation, investigation, methodology, resources, writing—original draft, and writing, review, and editing. WW led the conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, writing, review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the Fonds de recherche du Québec—Santé (Programme DMLA #252235), in partnership with the Fondation Antoine-Turmel and the Réseau de recherche en santé de la vision, and the Canadian Institutes for Health Research #169713 with a patient-oriented project grant for early career investigators.
Acknowledgments
We would like to acknowledge the contribution of the rehabilitation centers that have partnered with us to make this project possible: Centre de Réadaptation Lethbridge-Layton-Mackay du CIUSSS du Centre-Ouest-de-l’Île-de-Montréal, and l’Institut Nazareth et Louis-Braille du CISSS de la Montérégie-Centre, both members of the Centre de recherche interdisciplinaire en réadaptation du Montréal métropolitain.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abraham, A. G., Hong, C., Deal, J. A., Bettcher, B. M., Pelak, V. S., Gross, A., et al. (2023). Are cognitive researchers ignoring their senses? The problem of sensory deficit in cognitive aging research. J. Am. Geriatr. Soc. doi: 10.1111/jgs.18229
Arditi, A. (2005). Improving the design of the letter contrast sensitivity test. Investig. Ophthalmol. Vis. Sci. 46, 2225–2229. doi: 10.1167/iovs.04-1198
Baltes, P., and Lindenberger, U. (1997). Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol. Aging 12, 12–21. doi: 10.1037/0882-7974.12.1.12
Binns, A. M., Bunce, C., Dickinson, C., Harper, R., Tudor-Edwards, R., Woodhouse, M., et al. (2012). How effective is low vision service provision? A systematic review. Surv. Ophthalmol. 57, 34–65. doi: 10.1016/j.survophthal.2011.06.006
Bourassa, K. J., Memel, M., Woolverton, C., and Sbarra, D. A. (2015). Social participation predicts cognitive functioning in aging adults over time: comparisons with physical health, depression, and physical activity. Aging Ment. Health 21, 133–146. doi: 10.1080/13607863.2015.1081152
Campos, J. L., Höbler, F., Bitton, E., Labreche, T., McGilton, K. S., and Wittich, W. (2019). Screening for vision impairments in individuals with dementia living in long-term care: a scoping review. J. Alzheimers Dis. 68, 1039–1049. doi: 10.3233/JAD-181129
Canadian Institute for Health Information (2021). Dementia in Canada: Summary. Available at: https://www.cihi.ca/en/dementia-in-canada/dementia-in-canada-summary (Accessed February 25, 2023).
Carelli, V., Morgia, C.la, Ross-Cisneros, F. N., and Sadun, A. A. (2017). Optic neuropathies: the tip of the neurodegeneration iceberg. Hum. Mol. Genet. 26, R139–R150. doi: 10.1093/HMG/DDX273
Chang, L. Y. L., Lowe, J., Ardiles, A., Lim, J., Grey, A. C., Robertson, K., et al. (2014). Alzheimer’s disease in the human eye. Clinical tests that identify ocular and visual information processing deficit as biomarkers. Alzheimers Dement. 10, 251–261. doi: 10.1016/J.JALZ.2013.06.004
Clay, O. J., Edwards, J. D., Ross, L. A., Okonkwo, O., Wadley, V. G., Roth, D. L., et al. (2009). Visual function and cognitive speed of processing mediate age-related decline in memory span and fluid intelligence. J. Aging Health 21, 547–566. doi: 10.1177/0898264309333326
Corn, A. L., and Erin, J. N. (2010). Foundations of Low Vision: Clinical and Functional Perspectives. 2nd. New York, NY: AFB Press.
Davies-Kershaw, H. R., Hackett, R. A., Cadar, D., Herbert, A., Orrell, M., and Steptoe, A. (2018). Vision impairment and risk of dementia: findings from the English longitudinal study of ageing. J. Am. Geriatr. Soc. 66, 1823–1829. doi: 10.1111/JGS.15456
Deng, Y., Shi, L., Lei, Y., and Wang, D. (2016). Altered topological organization of high-level visual networks in Alzheimer’s disease and mild cognitive impairment patients. Neurosci. Lett. 630, 147–153. doi: 10.1016/J.NEULET.2016.07.043
Dentchev, T., Milam, A. H., Lee, V. M.-Y., Trojanowski, J. Q., and Dunaief, J. L. (2003). Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol. Vis. 9, 184–190.
Dupuis, K., Pichora-Fuller, M. K., Chasteen, A. L., Marchuk, V., Singh, G., and Smith, S. L. (2014). Effects of hearing and vision impairments on the Montreal cognitive assessment. Aging Neuropsychol. Cognit. 22, 413–437. doi: 10.1080/13825585.2014.968084
Ehrlich, J. R., Swenor, B. K., Zhou, Y., and Langa, K. M. (2021). The longitudinal association of vision impairment with transitions to cognitive impairment and Dementia: Findings from The Aging, Demographics and Memory Study. J. Gerontol. A. Biol. Sci. Med. Sci. 76, 2187–93. doi: 10.1093/gerona/glab157/6290819
Gale, S. D., Baxter, L., Connor, D. J., Herring, A., and Comer, J. (2007). Sex differences on the Rey auditory verbal learning test and the brief visuospatial memory test–revised in the elderly: Normative data in 172 participants. J. Clin. Exp. Neuropsychol. 29, 561–567. doi: 10.1080/13803390600864760
Ganguli, M., Du, Y., Dodge, H. H., Ratcliff, G. G., and Chang, C. C. H. (2006). Depressive symptoms and cognitive decline in late life: a prospective epidemiological study. Arch. Gen. Psychiatry 63, 153–160. doi: 10.1001/ARCHPSYC.63.2.153
Giguère, C., Lagacé, J., Ellaham, N. N., Pichora-Fuller, M. K., Goy, H., Bégin, C., et al. (2020). Development of the Canadian digit triplet test in English and French. J. Acoust. Soc. Am. 147, EL252–EL258. doi: 10.1121/10.0000825
Gloster, A. T., Rhoades, H. M., Novy, D., Klotsche, J., Senior, A., Kunik, M., et al. (2008). Psychometric properties of the depression anxiety and stress Scale-21 in older primary care patients. J. Affect. Disord. 110, 248–259. doi: 10.1016/J.JAD.2008.01.023
Gordon, K., Bonfanti, A., Pearson, V., Markowitz, S. N., Jackson, M. L., and Small, L. (2015). Comprehensive vision rehabilitation. Can. J. Ophthalmol. 50, 85–86. doi: 10.1016/j.jcjo.2014.11.009
Government of Quebec (2022). Regulation respecting insured visual aids and related services. Available at: https://www.legisquebec.gouv.qc.ca/en/document/cr/A-29,%20r.%203 (Accessed February 25, 2023).
Haegerstrom-Portnoy, G., Schneck, M. E., Brabyn, J. A., and Lott, L. A. (2002). Development of refractive errors into old age. Optom. Vis. Sci. 79, 643–649. doi: 10.1097/00006324-200210000-00010
Harrabi, H., Kergoat, M. J., Rousseau, J., Boisjoly, H., Schmaltz, H., Moghadaszadeh, S., et al. (2015). Age-related eye disease and cognitive function. Invest. Ophthalmol. Vis. Sci. 56, 1217–1221. doi: 10.1167/IOVS.14-15370
Henry, J. D., and Crawford, J. R. (2005). The short-form version of the depression anxiety stress scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 44, 227–239. doi: 10.1348/014466505X29657
Hong, T., Mitchell, P., Burlutsky, G., Liew, G., and Wang, J. J. (2016). Visual impairment, hearing loss and cognitive function in an older population: longitudinal findings from the blue mountains eye study. PLoS One 11:e0147646. doi: 10.1371/journal.pone.0147646
Ikram, M. K., Cheung, C. Y., Wong, T. Y., and Chen, C. P. L. H. (2012). Retinal pathology as biomarker for cognitive impairment and Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 83, 917–922. doi: 10.1136/JNNP-2011-301628
Kiely, K. M., and Anstey, K. J. (2015). “Common Cause Theory in Aging,” in Encyclopedia of Geropsychology. ed. N. Pachana (Singapore: Springer) (2015). 1–11.
Kirby, E., Bandelow, S., and Hogervorst, E. (2010). Visual impairment in Alzheimer’s disease: a critical review. J. Alzheimers Dis. 21, 15–34. doi: 10.3233/JAD-2010-080785
Kirkland, S. A., Griffith, L. E., Menec, V., Wister, A., Payette, H., Wolfson, C., et al. (2015). Mining a unique Canadian resource: the Canadian longitudinal study on aging. Can. J. Aging 34, 366–377. doi: 10.1017/S071498081500029X
Kuspinar, A., Verschoor, C. P., Beauchamp, M. K., Dushoff, J., Ma, J., Amster, E., et al. (2020). Modifiable factors related to life-space mobility in community-dwelling older adults: results from the Canadian longitudinal study on aging. BMC Geriatr. 20, 1–12. doi: 10.1186/S12877-020-1431-5/FIGURES/2
Kwan, R. Y. C., Kwan, C. W., Kor, P. P. K., and Chi, I. (2022). Cognitive decline, sensory impairment, and the use of audio-visual aids by long-term care facility residents. BMC Geriatr. 22:216. doi: 10.1186/s12877-022-02895-x
Lang, R., Rieckmann, N., and Baltes, M. (2002). Adapting to aging losses. Do resources facilitate strategies of selection compensation and optimization in everyday functioning? Journals of gerontology B. Psychol. Sci. 57B, 501–509. doi: 10.1093/geronb/57.6.P501
Lee, A. G., and Martin, C. O. (2004). Neuro-ophthalmic findings in the visual variant of Alzheimer’s disease. Ophthalmology 111, 376–380. doi: 10.1016/S0161-6420(03)00732-2
Lee, S. S. Y., Wood, J. M., and Black, A. A. (2020). Impact of glaucoma on executive function and visual search. Ophthalmic Physiol. Opt. 40, 333–342. doi: 10.1111/OPO.12679
Lin, F., Yaffe, K., Xia, J., Xue, Q.-L., Harris, T. B., Purchase-Helzner, E., et al. (2013). Hearing loss and cognitive decline in older adults. JAMA Intern. Med. 173, 293–299. doi: 10.1001/jamainternmed.2013.1868
Lindenberger, U., and Baltes, P. (1994). Sensory functioning and intelligence in old age: a strong connection. Psychol. Aging 9, 339–355. doi: 10.1037/0882-7974.9.3.339
Lindenberger, U., Scherer, H., and Baltes, P. (2001). The strong connection between sensory and cognitive performance in old age: not due to sensory acuity reductions operating during cognitive assessment. Psychol. Aging 16, 196–205. doi: 10.1037/0882-7974.16.2.196
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
Lovibond, P. F., and Lovibond, S. H. (1995). The structure of negative emotional states: comparison of the depression anxiety stress scales (DASS) with the Beck depression and anxiety inventories. Behav. Res. Ther. 33, 335–343. doi: 10.1016/0005-7967(94)00075-u
Malek-Ahmadi, M., Powell, J. J., Belden, C. M., Oconnor, K., Evans, L., Coon, D. W., et al. (2015). Age- and education-adjusted normative data for the Montreal cognitive assessment (MoCA) in older adults age. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 22, 755–761. doi: 10.1080/13825585.2015.1041449
Mansfield, J. S., Legge, G. E., Luebker, A., and Cunningham, K. (1994). MNRead Acuity Charts; Continuous-text Reading-acuity Charts for Normal and Low Vision. Long Island City NY: Distributed by Lighthouse Low Vision Products.
Melikyan, Z. A., Malek-Ahmadi, M., O’Connor, K., Atri, A., Kawas, C. H., and Corrada, M. M. (2021). Norms and equivalences for MoCA-30, MoCA-22, and MMSE in the oldest-old. Aging Clin. Exp. Res. 33, 3303–3311. doi: 10.1007/S40520-021-01886-Z/FIGURES/1
Monge, Z. A., and Madden, D. J. (2016). Linking cognitive and visual perceptual decline in healthy aging: the information degradation hypothesis. Neurosci. Biobehav. Rev. 69, 166–173. doi: 10.1016/J.NEUBIOREV.2016.07.031
Morrice, E., Hughes, J., Stark, Z., Wittich, W., and Johnson, A. (2020). Validation of the international Reading speed texts in a Canadian sample. Optom. Vis. Sci. 97, 509–517. doi: 10.1097/OPX.0000000000001538
Morrice, E., Soldano, V., Addona, C., Murphy, C. E., and Johnson, A. P. (2021). Validation of the international Reading speed texts in a sample of older (60+) Canadian adults. Optom. Vis. Sci. 98, 971–975. doi: 10.1097/OPX.0000000000001746
Mrazik, M., Millis, S., and Drane, D. L. (2010). The oral trail making test: effects of age and concurrent validity. Arch. Clin. Neuropsychol. 25, 236–243. doi: 10.1093/arclin/acq006
Nagarajan, N., Assi, L., Varadaraj, V., Motaghi, M., Sun, Y., Couser, E., et al. (2022). Vision impairment and cognitive decline among older adults: a systematic review. BMJ Open 12:e047929. doi: 10.1136/bmjopen-2020-047929
Nasreddine, Z. S., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Nwachukwu, I., Nkire, N., Shalaby, R., Hrabok, M., Vuong, W., Gusnowski, A., et al. (2020). COVID-19 pandemic: age-related differences in measures of stress, anxiety and depression in Canada. Int. J. Environ. Res. Public Health 2020 17:6366. doi: 10.3390/IJERPH17176366
Owsley, C., Sekuler, R., and Siemsen, D. (1983). Contrast sensitivity throughout adulthood. Vis. Res. 23, 689–699. doi: 10.1016/0042-6989(83)90210-9
Park, D., and Gutchess, A. (2016). The cognitive neuroscience of aging and culture. Curr. Dir. Psychol. Sci. 15, 105–108. doi: 10.1111/j.0963-7214.2006.00416.x
Perlmutter, M. (1979). Age differences in adults’ free recall, cued recall, and recognition. J. Gerontol. 34, 533–539. doi: 10.1093/GERONJ/34.4.533
Pierscionek, B. K., and Weale, R. A. (1995). The optics of the eye-lens and lenticular senescence. A review. Doc. Ophthalmol. 89, 321–335. doi: 10.1007/BF01203708
Rabbitt, P., Donlan, C., Watson, P., McInnes, L., and Bent, N. (1995). Unique and interactive effects of depression, age, socioeconomic advantage, and gender on cognitive performance of normal healthy older people. Psychol. Aging 10, 307–313. doi: 10.1037//0882-7974.10.3.307
Ratnayaka, J. A., Serpell, L. C., and Lotery, A. J. (2015). Dementia of the eye: the role of amyloid beta in retinal degeneration. Eye 2015 8, 1013–1026. doi: 10.1038/eye.2015.100,29,1013,1026
Risacher, S. L., WuDunn, D., Pepin, S. M., MaGee, T. R., McDonald, B. C., Flashman, L. A., et al. (2013). Visual contrast sensitivity in Alzheimer’s disease, mild cognitive impairment, and older adults with cognitive complaints. Neurobiol. Aging 34, 1133–1144. doi: 10.1016/J.NEUROBIOLAGING.2012.08.007
Roberts, K. L., and Allen, H. A. (2016). Perception and cognition in the ageing brain: a brief review of the short- and long-term links between perceptual and cognitive decline. Front. Aging Neurosci. 8, 1–7. doi: 10.3389/fnagi.2016.00039
Rogers, M. A. M., and Langa, K. M. (2010). Untreated poor vision: a contributing factor to late-life dementia. Am. J. Epidemiol. 171, 728–735. doi: 10.1093/aje/kwp453
Spierer, O., Fischer, N., Barak, A., and Belkin, M. (2016). Correlation between vision and cognitive function in the elderly: a cross-sectional study. Medicine (United States) 95, e2423–e2425. doi: 10.1097/MD.0000000000002423
Strauss, E., Sherman, E., and Spreen, O. (2006). A compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford, England.
Swenor, B. K., Lesche, S., Varadaraj, V., and Ramulu, P. Y. (2016). Short and sustained reading speeds in AMD patients: an interaction with cognitive status. Invest. Ophthalmol. Vis. Sci. 57:1947.
Swenor, B. K., Wang, J., Varadaraj, V., Rosano, C., Yaffe, K., Albert, M., et al. (2019). Vision impairment and cognitive outcomes in older adults: the health ABC study. J. Gerontol. A Biol. Sci. Med. Sci. 74, 1454–1460. doi: 10.1093/gerona/gly244
Trauzettel-Klosinski, S., and Dietz, K. (2012). Standardized assessment of reading performance: the new international reading speed texts IReST. Invest. Ophthalmol. Vis. Sci. 53, 5452–5461. doi: 10.1167/iovs.11-8284
Ventry, I. M., and Weinstein, B. E. (1982). The hearing handicap inventory for the elderly: a new tool. Ear Hear. 3, 128–134. doi: 10.1097/00003446-198205000-00006
Ward, M. E., Gelfand, J. M., Lui, L. Y., Ou, Y., Green, A. J., Stone, K., et al. (2018). Reduced contrast sensitivity among older women is associated with increased risk of cognitive impairment. Ann. Neurol. 83, 730–738. doi: 10.1002/ANA.25196
Whitson, H. E., Woolson, S., Olsen, M., Massof, R., Ferguson, S. M., Muir, K. W., et al. (2020). Cognitive impairment among veterans in outpatient vision rehabilitation. Optom. Vis. Sci. 97, 462–469. doi: 10.1097/OPX.0000000000001522
Wittich, W., Al-Yawer, F., and Phillips, N. (2019). Visual acuity and contrast sensitivity at various stages of cognitive impairment in the COMPASS-ND study. Invest. Ophthalmol. Vis. Sci. 60:5913.
Wittich, W., and Gagné, J. (2016). “Perceptual aspects of gerotechnology” in Gerontechnology: Research, Practice, and Principles in the Field of Technology and Aging. ed. S. Kwon (New York NY: Springer Publishing Co), 13–34.
Wittich, W., Phillips, N., Nasreddine, Z. S., and Chertkow, H. (2010). Sensitivity and specificity of the Montreal cognitive assessment modified for individuals who are visually impaired. J. Vis. Impair Blind 104, 360–368. doi: 10.1177/0145482X1010400606
Wittich, W., Pichora-Fuller, M. K., Johnson, A., Joubert, S., Kehayia, E., Bachir, V., et al. (2021). Effect of reading rehabilitation for age-related macular degeneration on cognitive functioning: protocol for a nonrandomized pre-post intervention study. JMIR Res. Protoc. 10:e19931. doi: 10.2196/19931
Wong, W. L., Su, X., Li, X., Cheung, C. M. G., Klein, R., Cheng, C.-Y., et al. (2014). Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob. Health 2, e106–e116. doi: 10.1016/S2214-109X(13)70145-1
World Health Organization (2017). Global action plan on the public health response to dementia 2017–2025. Geneva, World Health Organization.
World Health Organization (2019). World Report on Vision. Available at https://www.who.int/publications-detail-redirect/9789241516570 (Accessed August 3, 2021).
Zheng, D. D., Swenor, B. K., Christ, S. L., West, S. K., Lam, B. L., and Lee, D. J. (2018). Longitudinal associations between visual impairment and cognitive functioning. JAMA Ophthalmol. 136, 989–995. doi: 10.1001/jamaophthalmol.2018.2493
Keywords: dementia, macular degeneration, aging, vision rehabilitation, visual impairment, low vision (re)habilitation
Citation: Aubin G, Phillips N, Jaiswal A, Johnson AP, Joubert S, Bachir V, Kehayia E and Wittich W (2023) Visual and cognitive functioning among older adults with low vision before vision rehabilitation: A pilot study. Front. Psychol. 14:1058951. doi: 10.3389/fpsyg.2023.1058951
Edited by:
Eirini Mavritsaki, Birmingham City University, United KingdomReviewed by:
Bayan Al Othman, University of Rochester, United StatesVera Heyl, Heidelberg University of Education, Germany
Copyright © 2023 Aubin, Phillips, Jaiswal, Johnson, Joubert, Bachir, Kehayia and Wittich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Walter Wittich, walter.wittich@umontreal.ca
 Gabrielle Aubin
Gabrielle Aubin Natalie Phillips
Natalie Phillips Atul Jaiswal
Atul Jaiswal Aaron Paul Johnson
Aaron Paul Johnson Sven Joubert
Sven Joubert Vanessa Bachir1
Vanessa Bachir1 Eva Kehayia
Eva Kehayia Walter Wittich
Walter Wittich