- 1Key Laboratory of Forest Genetics and Biotechnology of Ministry of Education, Co-innovation Center for Sustainable Forestry in Southern China, College of Forestry, Nanjing Forestry University, Nanjing, China
- 2Experimental Center of Subtropical Forestry, Chinese Academy of Forestry, Fenyi, China
Heat stress is one of the major abiotic stresses affecting plant growth and productivity. Cryptomeria fortunei (Chinese cedar) is an excellent timber and landscaping tree species in southern China thanks to its beautiful appearance, straight texture and ability to purify the air and improve the environment. In this study, we first screened 8 excellent C. fortunei families (#12, #21, #37, #38, #45, #46, #48, #54) in a second generation seed orchard. We then analyzed the electrolyte leakage (EL) and lethal temperature at 50% (LT50) values under heat stress, to identify the families with the best heat resistance (#48) and the lowest heat resistance (#45) and determine the physiological and morphological response of different threshold-resistance of C. fortune to heat stress. The relative conductivity of the C. fortunei families showed an increasing trend with increasing temperature, following an “S” curve, and the half-lethal temperature ranges between 39°C and 43.2°C. The activities of SOD and POD fluctuated in the early stage of stress but decreased after 37°C. We observed the changes in the cell ultrastructure at 43°C, and the mesophyll cell structure of #48 was less damaged than that of #45. Eight heat resistance gene, including CfAPX1, CfAPX2, CfHSP11, CfHSP21, CfHSP70, CfHSFA1a, CfHSFB2a and CfHSFB4, were all up-regulated in #45 and #48, and there were significant differences between #45 and #48 under different heat stress treatments. We found a significant difference in heat tolerance between #45 and #48, such that #48 shows higher heat tolerance capability and could be exploited in breeding programs. We conclude that the strongly heat-resistant family had a more stable physiological state and a wider range of heat stress adaptations.
1 Introduction
With the globe becoming warm, high temperature stress has become one of the major environmental stresses limiting plant growth, metabolism and productivity (Berry and Bjorkman, 1980; Hasanuzzaman et al., 2013). In recent years, the frequent occurrence of extremely high temperatures has posed a severe challenge to the ability of plants to withstand high temperatures (Borrell et al., 2020). In the published research on the effects of heat stress on plant physiology, there have been many studies on the relationship between heat tolerance and plant physiological indicators, but the types of plants studied have mainly included herbs and vegetables. In contrast, research on forest trees has been limited. Different plants have different heat-resistance mechanisms, and the same plant may also be affected by the synergistic effect of multiple heat-resistance mechanisms (Hasanuzzaman et al., 2010). Compared with agricultural crops, forest trees have more complex cultivation conditions, more resilience mechanisms and longer growth cycles (Bi and Zhuge, 2008). During their growth and development, trees are affected by various environmental factors, among which temperature, light and water are the most important abiotic factors influencing tree growth. When the damage caused by high-temperature stress exceeds a plant’s own regulatory capacity, the plant exhibits heat-damage symptoms (Nahar et al., 2015).
Heat stress usually means that the temperature exceeds the threshold level for a period of time, which is enough to cause irreversible damage to plant growth and is a serious threat to global crop production (Vetter et al., 1958). The cell membrane is the critical barrier between cells and the external environment. Therefore, heat stress can initially cause changes in cell membrane permeability, leading to the denaturation of membrane proteins, separation of cell membrane lipids, and disruption of normal cell physiological activities (Hemantaranjan et al., 2014). Changes in cell membrane permeability will cause the cell membrane to lose its ability to select substances, the electrolytes in the cytoplasm to extravasate, and the conductivity of cell tissue fluids to increase (Jiang et al., 2016). Chlorophyll synthesis is sensitive to heat stress and is a good indicator of heat stress damage (Gosavi et al., 2014). Heat stress can cause various changes in chloroplasts, such as changes in thylakoid structure, the loss of granal stacking, and granal swelling (Ashraf and Hafeez, 2004; Rodríguez et al., 2005). In addition, heat stress-mediated changes in chloroplast ultrastructure can be used to indicate the heat tolerance of plant varieties at the cellular level (Zou et al., 2017).
The protective enzymes in plants mainly include catalase (CAT), peroxidase (POD), superoxide dismutase (SOD) and ascorbate peroxidase (APX) (Hong et al., 2021; Hu et al., 2021). For instance, there was a significant negative correlation between the activities of SOD and POD in different bottle gourd seedlings and the heat damage index of bottle gourds (Tian et al., 2020). SOD mainly scavenges superoxide anions in plants and converts them into H2O2 and O2, thereby maintaining cellular homeostasis and protecting cells from oxidative damage (Talbi et al., 2015). One of the important physiological mechanisms of plants in response to heat stress is the accumulation of osmoregulatory factors. The main osmotic regulators in plant cells mainly include proline, soluble sugars, and soluble proteins (Kaplan et al., 2004).
The survival strategy of plants under heat stress conditions often involves altering gene expression during transcription/translation, resulting in the production of heat shock proteins (HSPs). During cell growth and development, HSPs participate in protein synthesis, folding, and target protein degradation, thereby maintaining the stability of the cell membrane system (Usman et al., 2015; Khan and Shahwar, 2020). In plant genomes, approximately 7% of the coding sequences are assigned to transcription factors (TFs). Compared with animals and yeast, many of them belong to large gene families, such as heat stress transcription factors (HSFs) (Fragkostefanakis et al., 2015). Mishra found that in tomato (Solanum lycopersicum), at each developmental stage, the plants with HsfA1a silencing were more sensitive to heat stress than the wild type (Mishra et al., 2002).
Cryptomeria fortunei Hooibrenk is an evergreen tree species of the Cupressaceae family. It is an excellent timber and garden tree species in southern China (Zhang et al., 2021). With increasing global warming, the living environment of C. fortunei is also worsening. Therefore, it is of great theoretical and practical significance to study the physiological mechanism and genes related to the heat tolerance of C. fortunei in response to heat stress for subsequent molecular genetics and breeding research. In this study, we preselected the most and least thermo sensitive families among the eight tested families. We then analyzed the changes in needle phenotype, antioxidant activity, and chlorophyll content and ultrastructure of the two families under heat stress. Finally, we used the transcriptome data of C. fortunei available in our laboratory to identify heat resistance-related genes and explore their expression levels in families of C. fortunei with different levels of heat resistance, which laid a foundation for molecular breeding of heat resistance in C. fortunei.
2 Materials and methods
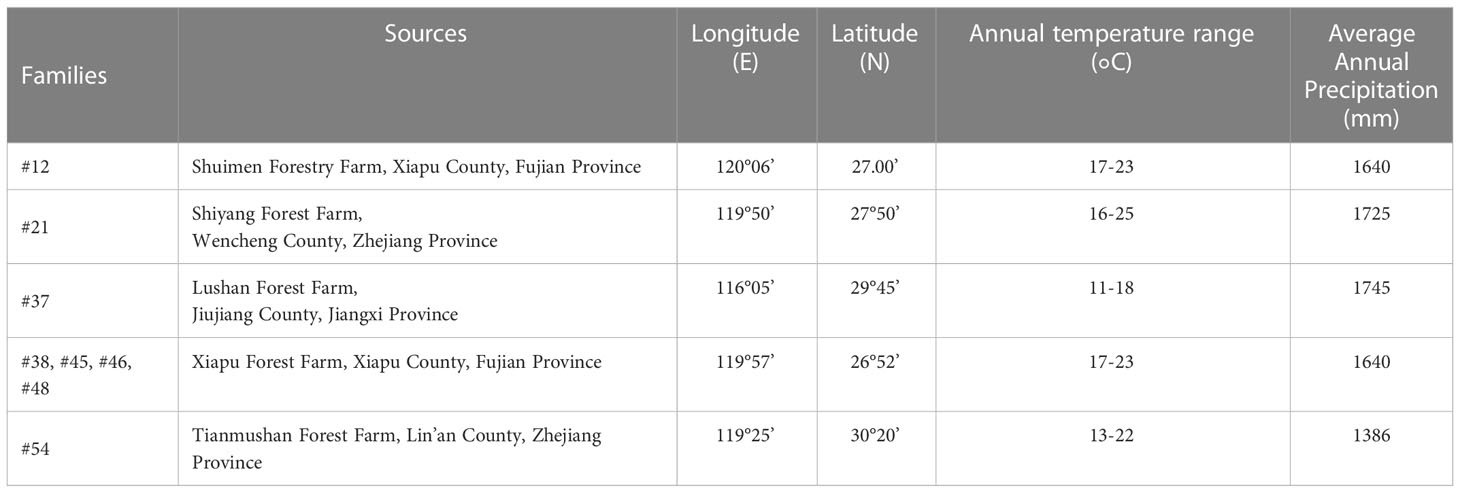
2.1 Plant materials
Our collection of C. fortunei is mainly derived from the eastern part of China (Table 1). The seedlings of eight elite C. fortunei families that were the offspring of the second orchard of C. fortunei in Xiapu forest farm, Xiapu County, Fujian Province, were selected. The material was harvested in 2018, and the seedlings were raised in 2019. In May 2021, we moved these to our laboratory in Nanjing (118°50’ E, 32°05’ N) for this study. To carry out heat stress, we pretreated 2-year-old healthy C. fortunei plants in the growth chamber under a 12/12-h photoperiod (day/night) at 25 °C/20 °C and 60% humidity and conducted a 4-week preliminary experiment.
2.2 Heat stress treatment
The shoots with consistent growth across the families of C. fortunei were collected and subjected to heat stress treatment at 25°C, 29°C, 33°C, 37°C, 39°C, 41°C, and 43°C. Starting from 25°C, the first 3 temperatures were increased at 4°C/h, and the temperature was increased at 2°C/h after 37°C. When the target temperature was reached, the treatment was performed for 9 hours. Six needles from three positions were taken after 12 h of treatment. The plants of families #45 and #48 were sampled after 0 h, 3 h, 6 h and 9 h of heat stress treatment (43°C). The plants of groups #45 and #48 were sampled after 0h, 3 h, 6 h and 9 hof heat stress treatment (43°C). These samples were wrapped in tin foil, placed in liquid nitrogen for rapid freezing, and then stored in an ultralow temperature refrigerator at -80°C. The experimental treatment was repeated three times, and three plants were treated each time.
2.3 Electrolyte leakage (EL) and lethal temperature at 50% (LT50)
EL was measured according to the method of Zhang with some modifications (Zhang et al., 2020). Here, 0.2 g of the treated sample needles was weighed; samples were placed in 10 ml of distilled water and shaken with a shaker at room temperature. After 4 hours of incubation, the conductivity was measured by a conductivity meter (DDS-307, Leici Instruments Co., Shanghai, China). The formula EL(%)=(E1−E0)/(E2−E0)×100 was used, wherein the conductivity of distilled water was E0 and that of the extract was E1. The sample was heated in a boiling water bath for 15 min. After cooling to room temperature, the ultimate conductivity was measured as E2.
In combination with the logistic equation, the relative conductivity was fitted with the logistic regression equation as Y=K (/1+ae-bt), where Y is EL, t is temperature, K is the maximum EL, and a and b are unknown parameters. The equation was processed so that the second derivative was equal to zero, and the values of a and b and the correlation coefficient R were obtained. The inflection point temperature of the relative conductivity curve corresponding to the processing temperature is the half lethal temperature, that is, LT50=lna/b (Janáček and Prášil, 1991; Huang et al., 2016).
2.4 Chlorophyll content
Needle samples (0.2 g) of #45 and #48 after heat stress treatment were weighed and placed in a mortar; calcium carbonate, quartz sand and 2-3 ml of 95% alcohol were added to grind the sample into a homogenate, and 7-8 ml of 95% alcohol was added until the tissue turned white. The supernatant was centrifuged at 500 rpm 3 times at room temperature for 10 minutes each time, and the OD665 (optical density at 665 nm) and OD649 (optical density at 649 nm) of the supernatant were measured with a spectrophotometer. The chlorophyll (a+b) content was measured according to the method described by Lichtenthaler and Wellburn (Lichtenthaler and Wellburn, 1983).
2.5 Determination of SOD and POD activity
The needles of #45 and #48 were collected as samples after heat stress treatment. We then weighed the needles (0.2 g), ground them in liquid nitrogen, and suspended them in 3.0 ml of a solution consisting of 50 mM phosphate buffer pH 7.8. The homogenate was centrifuged at 10000 rpm for 20 minutes at 4°C. The supernatant was taken to analyze SOD and POD activities. SOD activity analysis was based on the ability of SOD to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) (Luo et al., 1999). The activity of SOD was expressed as the activity units of the enzyme that inhibited 50% of the NBT. SOD activity was expressed as U g-1 FW protein. POD activity was measured by using guaiacol as the substrate (Thomas et al., 1982). One unit of POD activity was defined as a change of 0.01 in 1 min at 470 nm. POD activity was expressed as U g-1 FW min-1.
2.6 Ultrastructural observations
Needle samples # 45 and # 48 were collected and subjected to 37°C and 43°C for 24 hours, and samples at 25°C were collected as controls. After treatment, three mature needles were sampled. The needles were cut into a 3 mm segment size with a blade and placed in a 5 ml penicillin bottle; 2 ml of 3% glutaraldehyde solution was added, the air in the bottle was extracted with a syringe, which caused the needles to sink entirely to the bottom of the bottle. Samples were then stored in a 4°C refrigerator for 24 h. The specimens were rinsed with 0.1 mol L-1 phosphate buffer solution with a pH of 7.0 3 times for 30 minutes each time. The specimens were subsequently dehydratedin a series of graded alcohol solutions, namely, 15%, 30%, 50%, 70%, 80%, 90%, 95%, 100% solution, followed by acetone solution at room temperature. The samples were infiltrated and embedded with Poly Bed 812 epoxy resin, dried and stored for 8 hours. The samples were then sectioned using ultratome III ultramicrotome (LKB, Bromma, Stockholm, Sweden). The samples were subsequently stained in uranium dioxide acetate and lead citrate and then observed via the transmission electron microscope (TEM) (JEM-2100, JEOL Ltd., Tokyo, Japan). From the TEM images, ten chloroplasts were selected for observation. The shape of the chloroplast is approximated to be an ellipse, and the calculation formula of ellipse area is as follows: S=πab, where S is the elliptical area (µm2), a is the semimajor axis (µm), and b is the semiminor axis (µm).
2.7 Gene expression
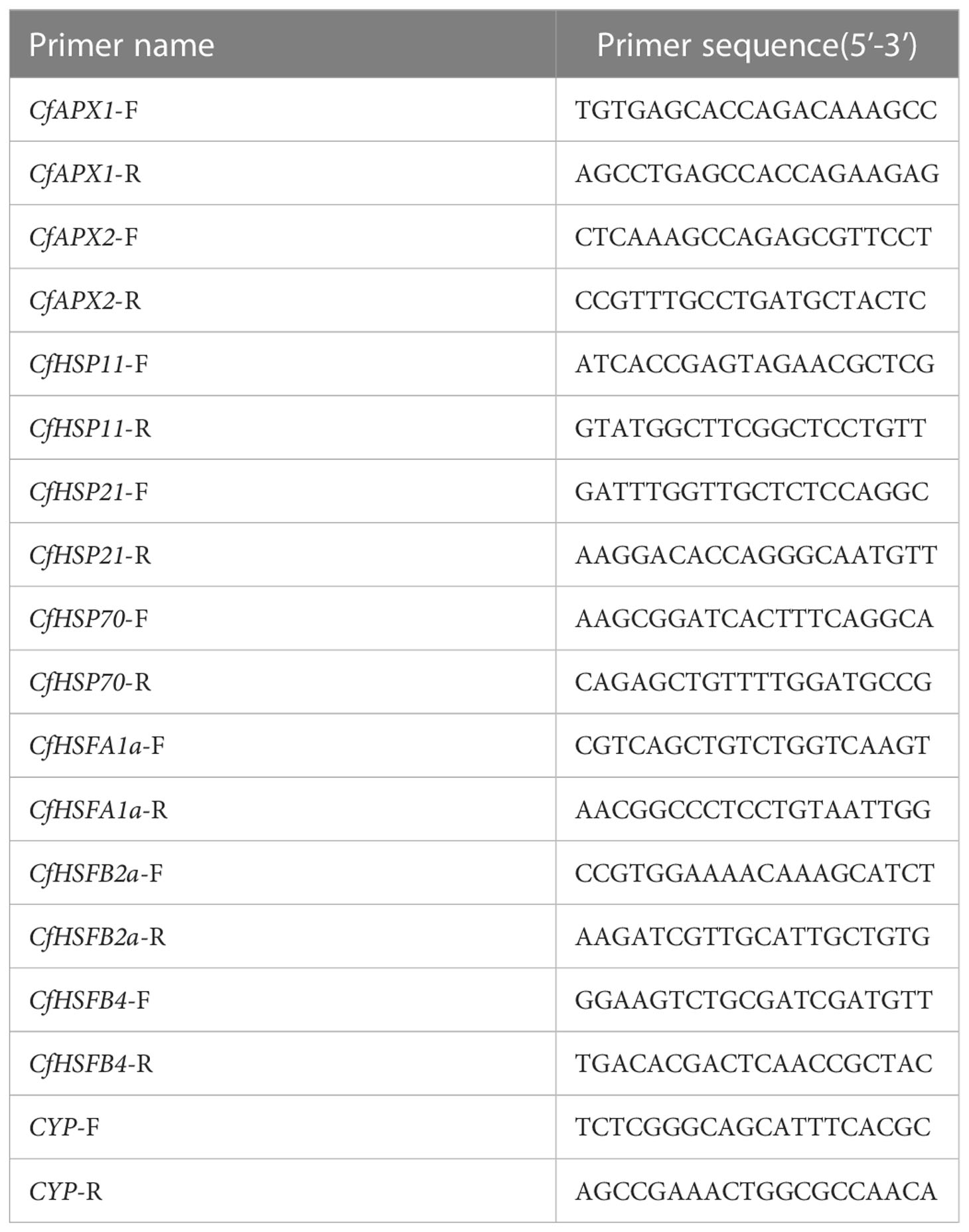
The total RNA extracted from the needles of C. fortunei treated for 0, 3, 6, and 9 h under heat stress at 43°C was used as the template. A TaKaRa Mini BEST Plant RNA Extraction Kit was used to extract total RNA (Spin-column) (Takara, Beijing, China). The RNA concentration was measured by spectrophotometer (NanoDrop, 2000; Thermo Scientific, Waltham, Massachusetts, USA). Reverse transcription was performed with a HiScript III RT SuperMix for qPCR (+gDNA wiper) (Vazyme, Nanjing, China) reverse transcription kit according to the manufacturer’s instructions. According to the existing C. fortunei transcriptome sequencing data in our laboratory, eight genes related to heat resistance were screened. Homologous sequences were identified by the NCBI online tool BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi ), and specific primers used were designed by Primer Premier 5.0. The CYP gene of C. fortunei was used as the internal reference gene. The reverse transcription cDNA template was diluted 10 times, and three replicates were evaluated. A ChamQ SYBR qPCR Master Mix (Low Rox Premixed) kit (Vazyme) was used for qRT-PCR analysis. All qPCRs were carried out on an Applied Biosystems 7500 real-time PCR system (ABI, Foster City, CA, USA). The expression levels were calculated using the 2-ΔΔCt method (Zhang et al., 2021). Table 2 lists all primers used to quantify the reverse transcription polymerase chain reaction (PCR). Experimental consumables, including 96 well 0.2 ml semi-skirt PCR plates, were obtained from NEST Biotechnology Co. Ltd (Wuxi, China).
2.8 Statistical analysis
GraphPad Prism 8 was used for statistical analysis and mapping. When p ≤ 0.05, the Pearson rank correlation method was used to calculate the bivariate correlation between traits and corresponding gene expression levels. The data were analyzed by one-way ANOVA, and Duncan’s test was conducted in accordance with p ≤ 0.05. These analyses were conducted using SPSS 26.0.
3 Results
3.1 Individual C. fortunei families show varying degrees of heat resistance
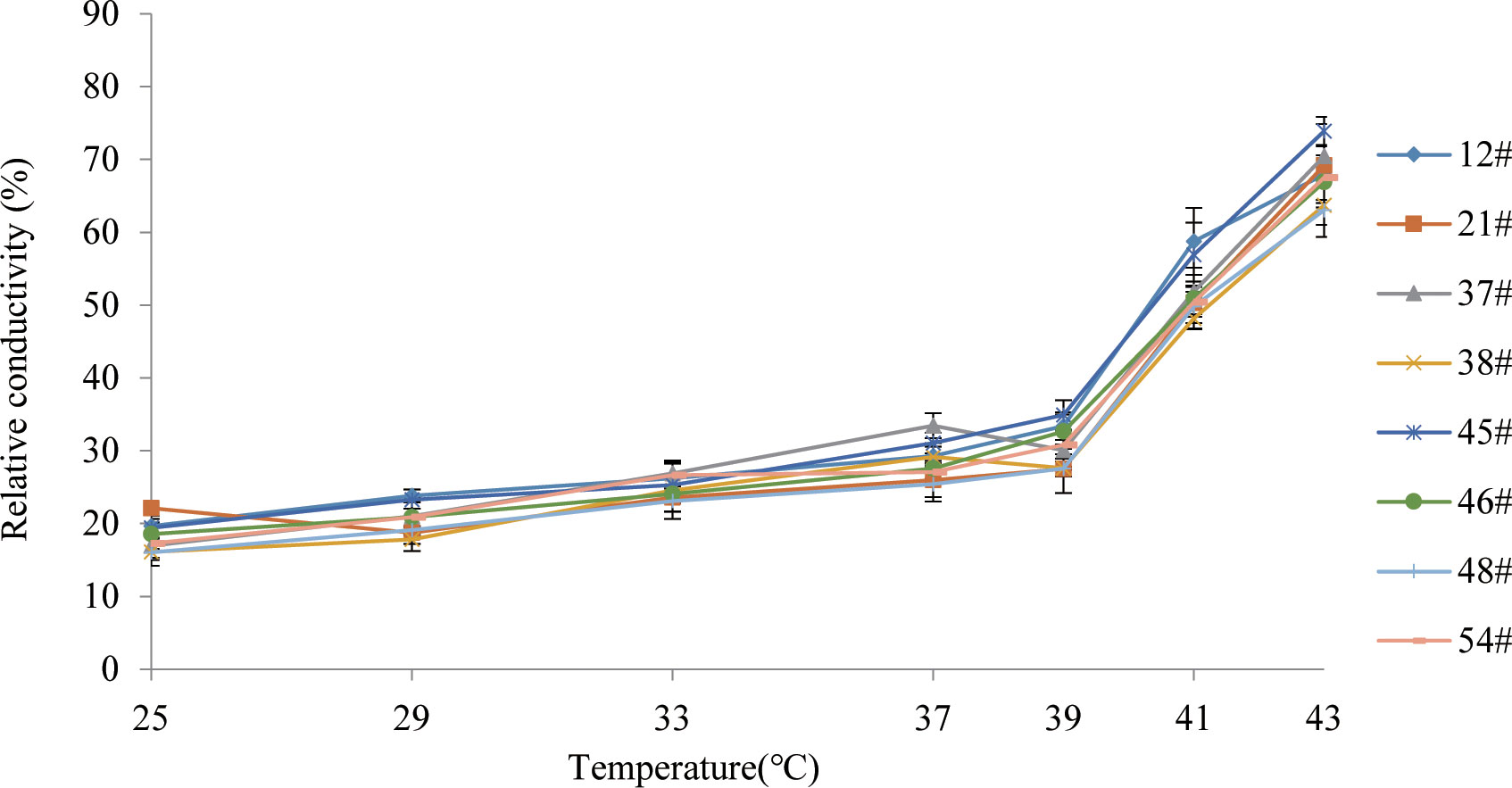
We aimed to preselect the most and least heat resistant C. fortunei families from our set of 8 by analyzing the EL and LT50 values in response to heat stress. The EL values of all C. fortunei families increased gradually with increasing temperature, showing a consistent “S”-shaped curve response (Figure 1). We found that the lowest LT50 was for #45, which was 39.9°C, and the highest LT50 was for #48, which was 43.1°C (Table 3). Subsequently, we selected these two families for further physiological index tests and related experiments to study the widest possible range of heat-resistant responses within C. fortunei.
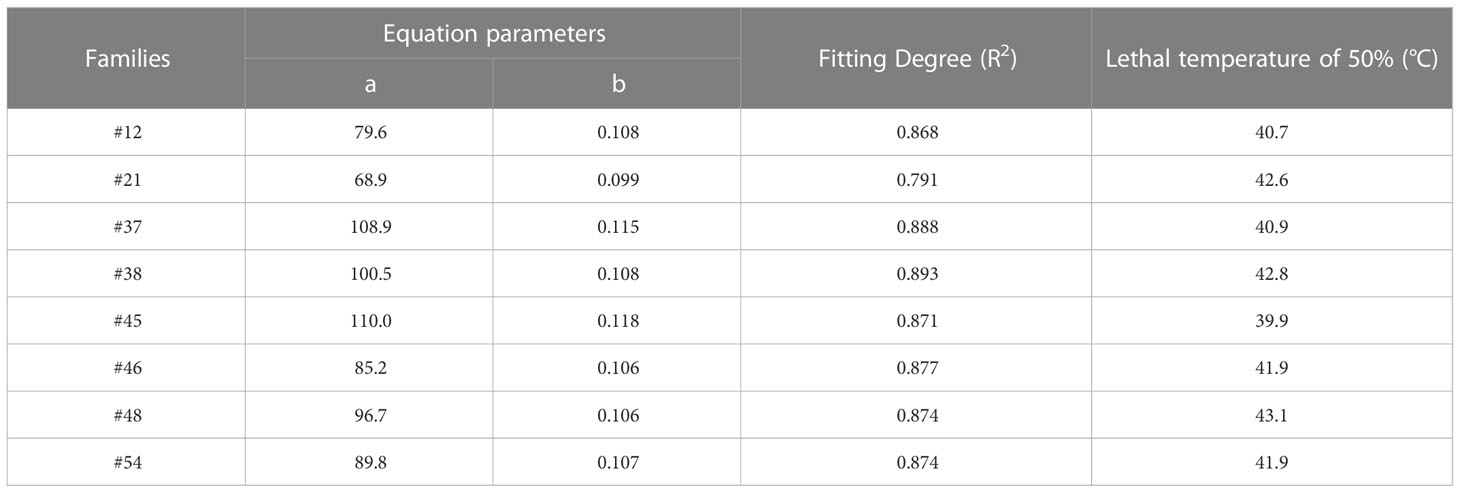
Table 3 Regression equation of electrolyte leakage and temperature and lethal temperature of 50% of C. fortunei.
3.2 Heat stress causes needle damage in C. fortunei families
Heat stress often leads to changes in leaf color in plants. After heat stress treatment at 37°C, compared with the control (25°C), the needles of #45 and #48 showed no significant difference in needle shape or color (Figures 2A, B), yet at ≥ 39°C, they showed different degrees of etiolation. The degree of conifer browning damage increased significantly with increasing heat stress. After treatment at 39°C, the needles of #45 were browned and dried, and at 43°C, most of the needles had dried (Figures 2C, D) . Furthermore, heat stress also caused the top needles to wilt and droop, making them thicker and easier to detach. In addition, compared with the #45 family, phenotypic changes in the #48 family under high-temperature stress were not obvious, and the degree of needle browning and drying was lower. This is consistent with the fact that the LT50 of the #48 family is higher than that of the #45 family (Table 3).

Figure 2 The phenotypes of the C fortune families changed significantly under heat stress. (A) #45 phenotype at 25°C, 37°C and 43°C; (B) #48 phenotype at 25 °C, 37 °C and 43 °C; (C) needle phenotype of #45; (D) needle phenotype of #48. (A, B) Bars=10 cm; (C, D) Bars=2 cm.
3.3 Chlorophyll contents of the C. fortunei families change under different heat stresses
Chlorophyll synthesis is sensitive to heat stress and is a good indicator of heat stress injury (Gosavi et al., 2014). Heat stress had an adverse effect on the chlorophyll content of seedlings of the C. fortunei families. We found that the chlorophyll a content of the #45 and #48 needles showed a decreasing trend with increasing temperature. Under the 25°C-39°C treatment, the change was relatively small, and the decrease was obvious after treatment at 39°C (Figure 3A). The chlorophyll a, b and chlorophyll (a+b) contents of #48 needles were less than those of #45 needles following heat stress treatment under 39°C but more than those of #45 needles at 39-43°C (Figures 3A–C). These results indicated that #48 changed slowly under heat stress and was more stable under heat stress than #45. However, the chlorophyll a/b ratio did not increase (Figure 3D).
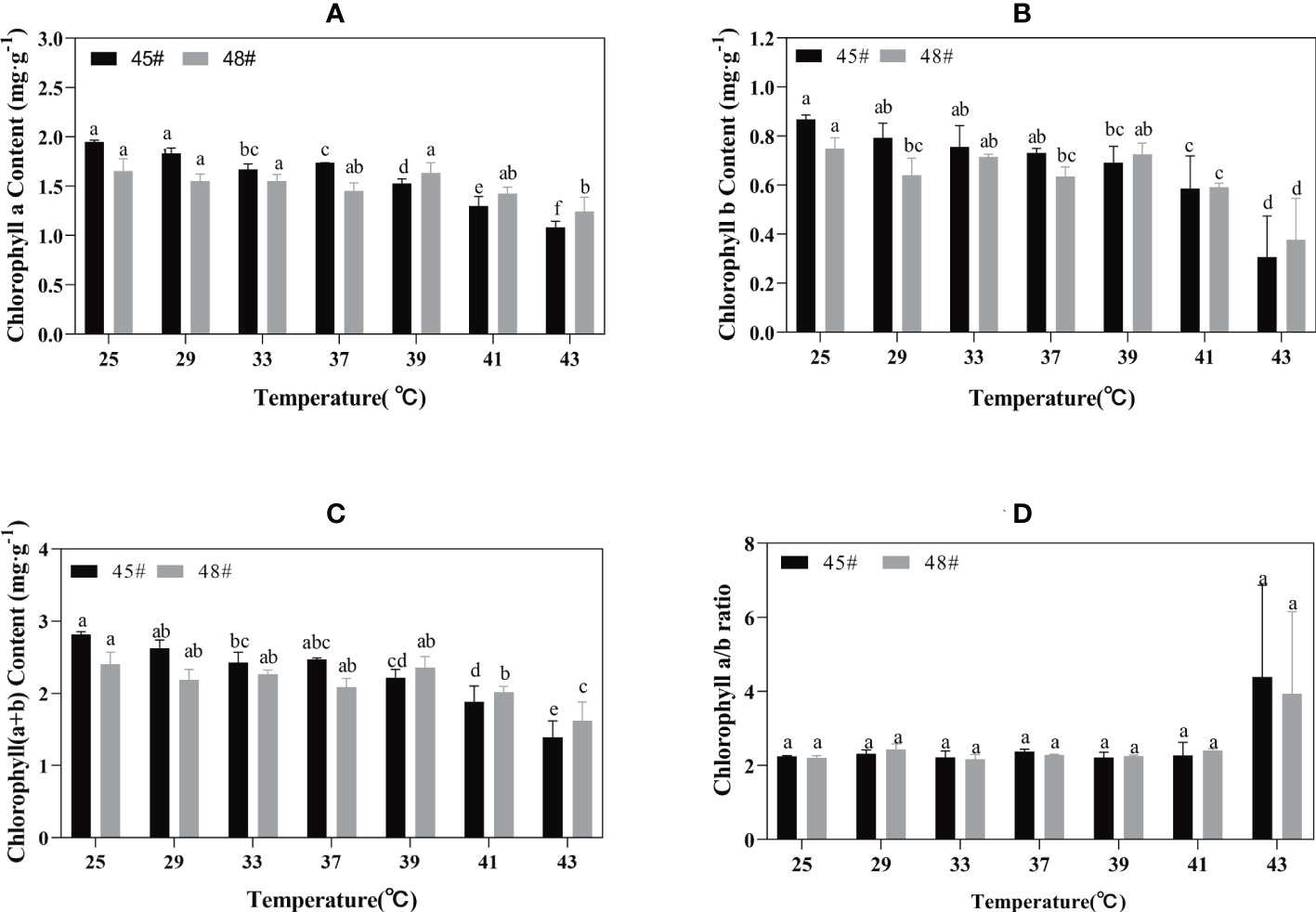
Figure 3 Chlorophyll changes in C. fortune families under heat stress. (A) Chlorophyll a content; (B) Chlorophyll b content; (C) Chlorophyll (a+b) content; (D) Chlorophyll a/b ratio. Each value in the graph is the mean ± standard error (n=3). The different patterns indicate different treatment temperatures, and the lowercase letters above each bar indicate significant differences (p < 0.05).
3.4 The antioxidant system of the C. fortunei families changes under different heat stresses
Heat stress treatment significantly affected the activities of POD and SOD in #45 and #48. During heat stress, the SOD activities of #45 and #48 needles were the highest at 37°C; compared with 25°C, the increase was 182.61% and 158.22%, respectively (Figure 4). With increasing temperature stress, the POD activities of #45 and #48 showed a trend of first increasing and then decreasing, similar to the changes in SOD activities. The POD activity also showed a downward trend after 37°C, and #45 at 43°C decreased by 68.99% compared with that at 37°C. After treatment at 39°C, both the SOD and POD activities of #48 were higher than those of #45 (Figure 4).
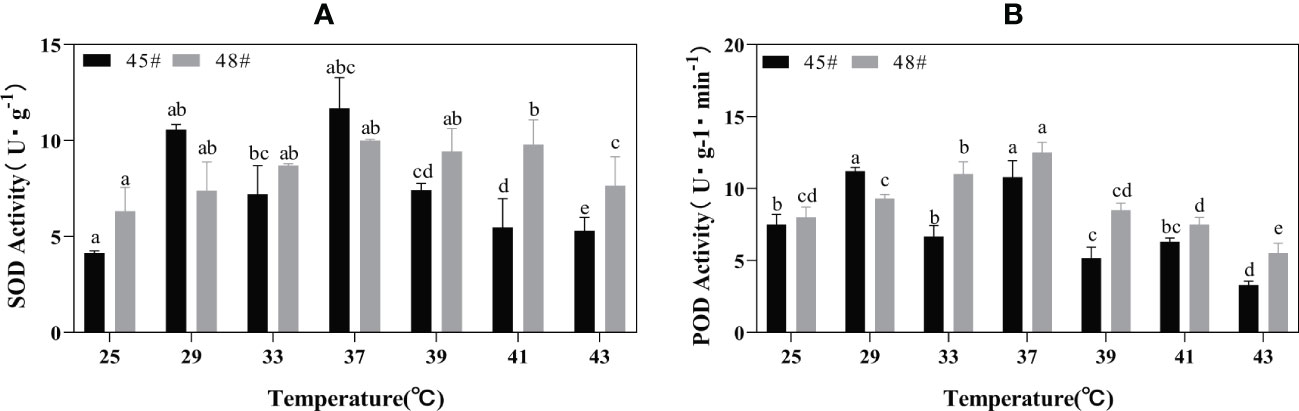
Figure 4 Changes in the POD and SOD contents of C fortunei families under heat stress. (A) SOD content; (B) POD content. Each value in the graph is the mean ± standard error (n=3). The different patterns indicate different treatment temperatures, and the lowercase letters above each bar indicate significant differences (p < 0.05).
3.5 Effect of heat stress on the ultrastructure of mesophyll cells of C. fortunei
Under heat stress, the ultrastructure of mesophyll cells of the #45 and #48 families changed obviously. Compared with the control (25°C) (Figures 5A–D), we found that the ultrastructure of #48 was not significantly different under heat stress at 37°C, but the number of osmiophilic granules in its chloroplasts was significantly increased, and no starch granules were observed (Figures 5G, H). In addition, we also found that #45 showed similar changes, but #45 had more obvious changes in chloroplast structure than #48, and it could be clearly seen that the thylakoid sheet structure in the chloroplast was distorted and loose (Figures 5E, F). Both #45 and #48 mesophyll cells exhibited severe plasmolysis at 43°C, and some organelles moved toward the center of the cell (Figures 5I, K). In the cells of #45, the tonoplast membrane was ruptured, the chloroplast structure was further expanded, deformed or even ruptured, and the internal osmiophilic granules increased. The thylakoid sheets were loose and disordered (Figure 5J). In contrast, the cells of #48 were less damaged. We observed a relatively intact plasma membrane, but a damaged nucleus and mitochondrial structure, in which chromatin within the nucleus aggregated into darker clumps, and the chloroplast structure was ruptured, but its thylakoid sheets remained tightly packed (Figure 5L). According to the above results, the ultrastructure of mesophyll cells in both families responded to heat stress, and the #45 family was more susceptible to heat stress damage than the #48 family.
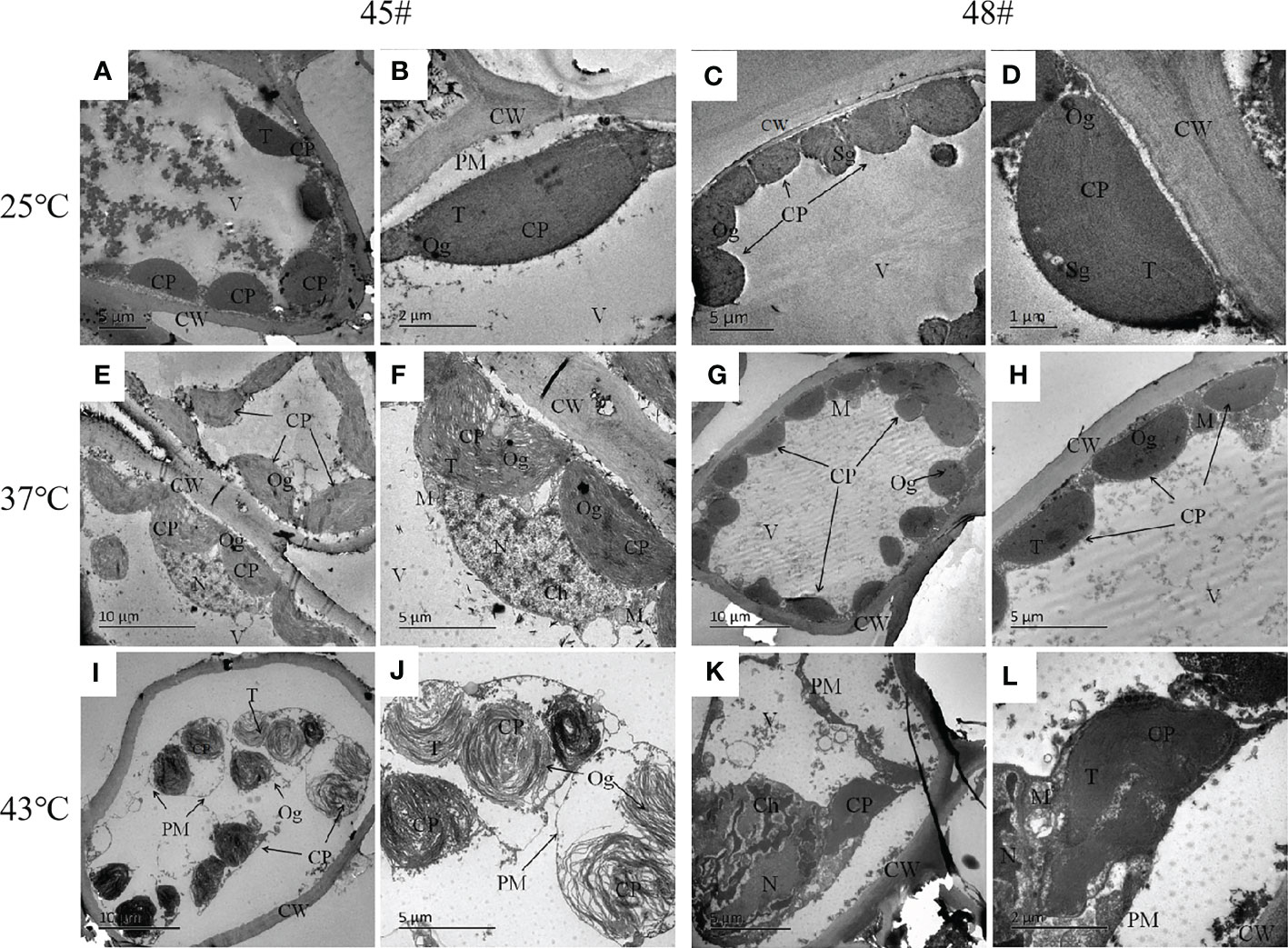
Figure 5 Changes in the ultrastructure of mesophyll cells in #45 and #48 under heat stress (A, B) #45 room-temperature treatment; (C, D) #48 room-temperature treatment; (E, F) #45 subjected to 37°C; (G, H) #48 subjected to 37°C; (I, J) #45 subjected to 43°C; (K, L) #48 subjected to 43°C. Cell wall, CW; Membrane, PM; Vacuole, V; Chloroplast: CP; Thylakoids, T; Starch granules, SG; Mitochondria, m; Nucleus, n; Chromatin, ch; Osmiophilic particles, Og.
3.6 Effect of heat stress on the chloroplast size of C. fortunei
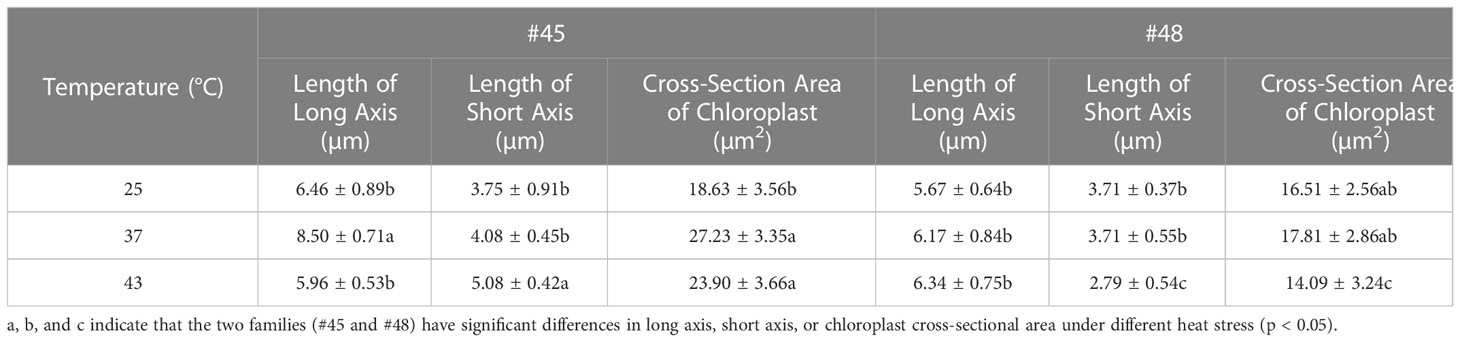
The photosynthesis efficiency depends not only on the number of chloroplasts in cells but also on the surface area of chloroplasts (Bondada et al., 1994). The sectional area of #45 and #48 chloroplasts first increased and then decreased with increasing heat stress (Table 4). The changes in the chloroplast cross-sectional area of #45 at 37°C were significantly increased compared with the control (25°C), but the changes in #48 at 37°C were not significant. We found that the chloroplast cross-sectional area of #48 changed significantly when treated at 43°C, and the chloroplast cross-sectional area decreased significantly. We therefore conclude that the effect of heat stress on #45 was greater than that on #48, which showed weaker heat tolerance and more severe damage.
3.7 Expression of heat resistance genes in C. fortunei families under heat stress
To gain more insights into the response of C. fortunei to heat stress, we evaluated eight heat-resistance-related genes screened from the existing transcriptome data under 43°C culture conditions (Figure 6). We found that the transcript levels were significantly different in #45 and #48. The relative expression of CfAPX1 under heat stress showed a trend of first increasing and then decreasing in both families, and its gene expression in #48 was higher than that in #45 (Figure 6A). Under heat stress, the relative expression of CfAPX2 showed a trend of first increasing and then decreasing in #45 and reached the highest level at 6 h, which was 6.0 times that of the control (0 h) (Figure 6B). The expression level of CfHSFB2a in #45 was higher than that in #48 at 0 h, 3 h and 6 h (Figure 6D). The relative expression of CfHSP70 in #45 reached the highest level at 3 h and decreased significantly thereafter. We observed that between the two families, the relative expression levels of genes in #48 and #45 were not significantly different under the 0h and 3 h treatments, while gene expression in #48 was significantly higher than that in #45 under the 6 h and 9 h treatments (Figures 6F–H).
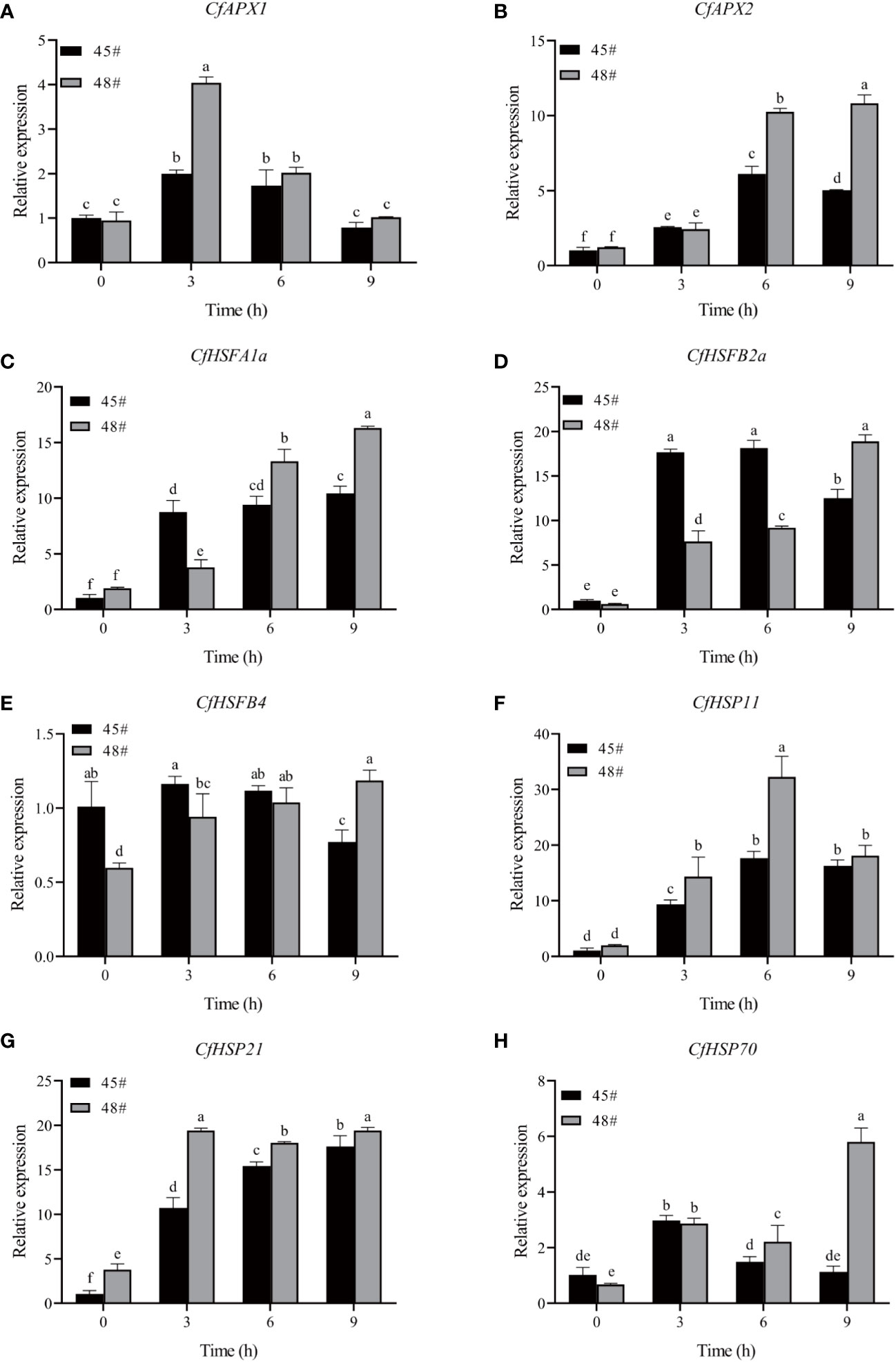
Figure 6 Effects of heat stress on gene expression in C. fortunei needles. The time course of heat in C. fortune shifted to 43°C. (A) CfAPX1; (B) CfAPX2; (C) CfHSP11; (D) CfHSFB2a; (E) CfHSFB4; (F) CfHSP11; (G) CfHSP21; (H) CfHSP70. Each value in the graph is the mean ± standard error (n=3).
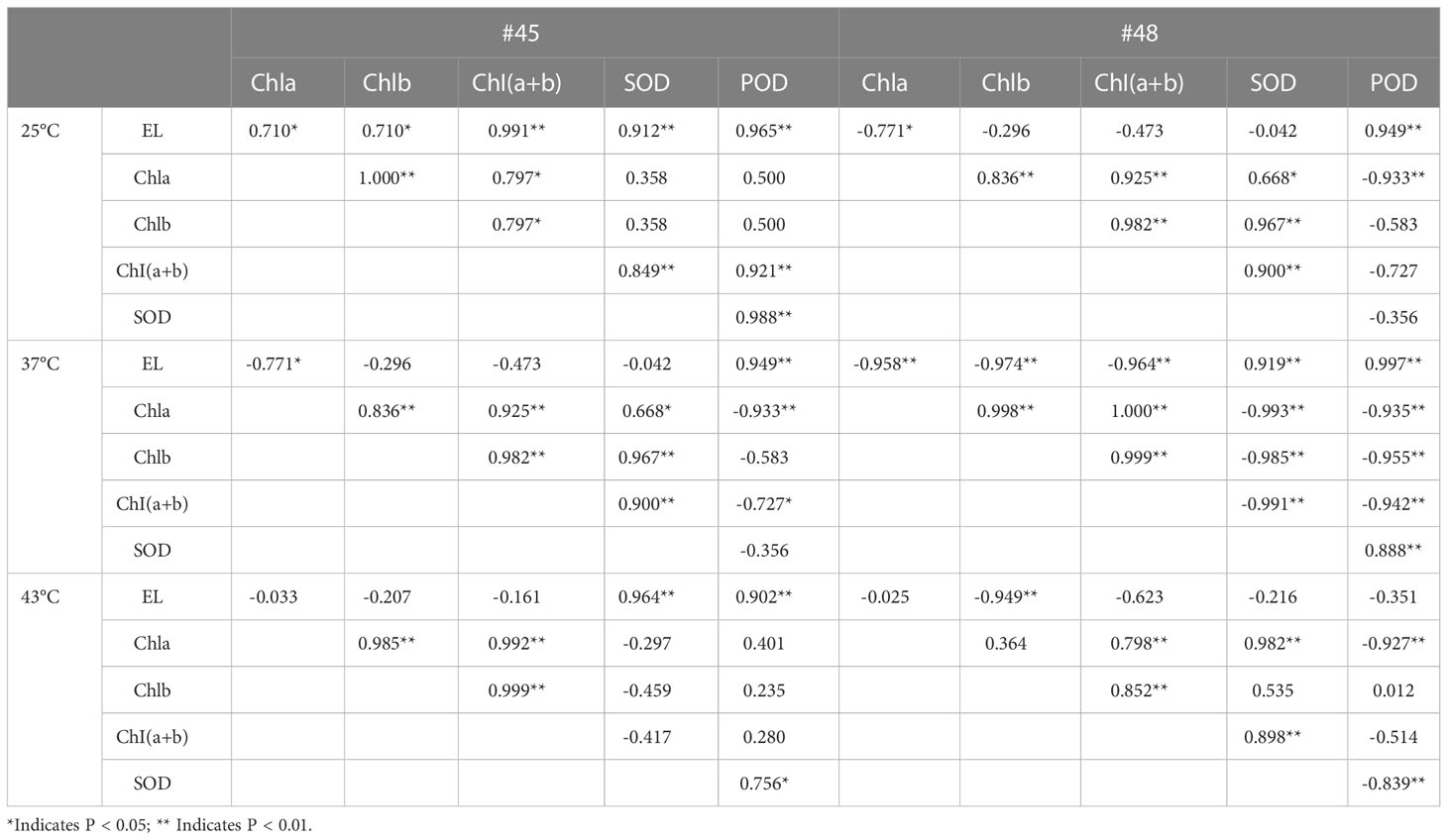
3.8 Correlation of various physiological indicators in C. fortunei families under heat stress
At 25°C, the EL of the #45 family was significantly positively correlated with five physiological parameters, and there was a positive correlation between these five physiological parameters (Table 5). At room temperature, the EL of #48 had no significant correlation with chlorophyll a; however, chlorophyll (a+b) content and SOD activity were negatively correlated. In addition, POD in #48 was significantly positively correlated with EL but negatively correlated with the other four physiological indicators.
Under 37 °C heat stress, the EL and POD of #45 and #48 were significantly positively correlated with each other and negatively correlated with the chlorophyll content (Table 5). In the #45 family, POD was negatively correlated with the other 4 physiological parameters. In addition, under 37 °C treatment, SOD in the #48 family was significantly negatively correlated with chlorophyll but extremely significantly positively correlated with POD and EL.
After heat stress at 43 °C, the POD activity in the #45 family was positively correlated with the five physiological indicators, while that in the #48 family was negatively correlated with the other four indicators except chlorophyll b, which was positively correlated and not significant. At the same time, after heat stress at 43 °C, the EL of the #48 family was negatively correlated with these five physiological parameters.
4 Discussion
The cell membrane constitutes the primary heat stress target in plants. The lipid molecules on the biofilm transform from a colloidal state to a liquid crystal state under thermal stress, which increases the permeability of the lipid membrane and allows the penetration of intracellular electrolytes, which leads to an increase in EL (Lastra et al., 1982). There is a positive correlation between the rate of lipid peroxidation and EL: with increasing heat stress, the relative permeability of the plasma membrane and EL also increases (Lukatkin, 2003). In the present study, the EL of each family of C. fortunei gradually increased with the intensification of heat stress and exhibited an overall “S”-shaped curve. At 25°C-37°C, the EL increased slowly and then increased sharply after 37°C. The EL of C. fortunei family #48 was at a lower level during the whole stress process, indicating that #48 had less electrolyte extravasation, less damage to the cell membrane, and stronger heat resistance.
We found that heat stress led to a decrease in photosynthetic pigment content and impaired chlorophyll biosynthesis in plastids (Marchand et al., 2005; Dutta et al., 2009). Chlorophyll content can reflect the degree of heat stress of plants to a certain extent, thus reflecting the heat tolerance of plants (Bhusal et al., 2018). Studies have shown that heat stress leads to increased decomposition of chlorophyll in plants. Part of the reason may be that high temperature inhibits chlorophyll biosynthesis, resulting in a lower synthesis rate than the decomposition rate. Another reason may be that the content of reactive oxygen species in plants increases under heat stress, thus causing oxidative damage, which then accelerates the degradation of chlorophyll (Sun et al., 2006). The chlorophyll a and chlorophyll b contents in #45 after heat stress (43°C) decreased by 44.4% and 64.6%, respectively, compared with those of the control, while the contents of chlorophyll a and chlorophyll b in #48 decreased by 24.8% and 49.6% respectively, indicating that chlorophyll b in C. fortunei was more sensitive to heat stress than chlorophyll a. With the intensification of heat stress, the chlorophyll a content in the cedar family showed a downward trend, and the chlorophyll b and chlorophyll (a+b) contents changed in the same way. However, chlorophyll contents of #45 and #48 increased at 37 °C and 39 °C respectively. Therefore, we speculate that after adapting to high-temperature stress, C. fortunei scavenges reactive oxygen species to alleviate high-temperature damage through the initiation of various anti-high-temperature mechanisms, thus leading to the restoration of the chlorophyll content (Chang, 2013). The decrease in chlorophyll content at the beginning of stress may be due to the sudden high temperature affecting the normal physiological state of C. fortunei cells, and the accumulation of intracellular reactive oxygen species, which inhibits the biosynthesis of chlorophyll in cells and accelerates the decomposition of chlorophyll.
The enzyme reaction system formed during the long-term evolution of plants. To eliminate oxidative stress caused by heat stress and enhance plant protection, SOD and POD are key enzymes in the plant defense system (Larkindale and Vierling, 2008). These enzymes exist in various plant cells and can scavenge intracellular O2-, balance oxygen free radicals, and maintain their metabolic stability (Vidya et al., 2018). In our study, the decrease in SOD activity in #45 at 33°C may be due to the sudden high temperature disrupting the normal physiological metabolism of cedar trees, whereas the subsequent increase in activity is due to a self-protective response to a stressful environment after thermos table exercise. The changes in POD activity in the two cedar families in this study were similar to those in SOD activity, showing a trend of first increasing and then decreasing. Chloroplasts are believed to be the most sensitive organelles (Lütz et al., 2012). Without heat stress, the SOD activity of #48 was higher than that of #45. After heat stress at 39°C, the SOD activity of #48 was significantly higher than that of #45, indicating that #48 could more effectively remove ROS. In addition, correlation analysis showed that under 43°C heat stress, the SOD activity and chlorophyll content of #48 were significantly positively correlated, while the chlorophyll content of #45 during this period was not significantly negatively correlated with SOD activity. Free radicals may cause damage to the chloroplast membrane in plants (Zhang et al., 2021). This result indicated that the increase in the active oxygen content in plants from the #45 family under heat stress caused more serious oxidative damage, which accelerated the degradation of chlorophyll. This further illustrated that #45 has a lower heat resistance than #48.
We found that in the process of heat stress, the chloroplasts of plant leaves expanded and deformed, the thylakoid sheets were loose, the structure was disordered, the plastid globules increased, and the chloroplast envelope was ruptured and disintegrated. Among them, photosystem II on the thylakoid membrane was one of the most sensitive parts, and the damage from heat stress was irreversible (Sheng et al., 2006; Guo et al., 2018). In this study, with the intensification of heat stress, osmiophilic granules increased in the chloroplasts of both families, and the chloroplasts in the needles of the two families were damaged to different degrees under heat stress. Studies have shown that osmiophilic granules are the product of degradation of thylakoid membrane lipid aggregates, which reflects the increased degree of chloroplast damage under stress conditions (Zhou et al., 2018).
The expression of the APX gene was induced by high temperature, drought, low temperature, salt and other stresses. The product of this gene can efficiently remove H2O2 in plants, thereby maintaining cellular redox homeostasis and improving plant stress resistance (Krasensky and Jonak, 2012). Using wild-type and mutant Arabidopsis plants as materials, the results showed that APX1, APX2 and APX6 were significantly induced by heat stress, and APX3 and APX5 showed obvious inducible expression in response to heat stress (Li et al., 2019). The accumulation of HSPs is controlled by HSFs and plays a central role in the plant heat shock response and the acquisition of heat tolerance by plants or other organisms (Tian et al., 2021). For example, AtHSFa and AtHSFb in the Arabidopsis A1 subfamily can regulate the expression of related genes and the synthesis of HSP genes in the early stage of the plant response to heat stress, thus helping the plant resist to the damage caused by high temperature (Baniwal et al., 2004; Ikeda et al., 2011). In this study, during heat stress, the expression of each HSF gene in the #48 family was higher than that in the #45 family after 9 h of treatment. In general, the expression of heat resistance genes in the #48 family was higher under heat stress, which explained why the #48 family was more heat-tolerant than the #45 family at the molecular level, and the results were also consistent with previous research results.
5 Conclusions
We found that the LT50 values of #45 and #48 were the lowest at 39.9°C and the highest at 43.1°C, respectively, out of the eight C. fortunei families, which indicated that #45 had the weakest heat resistance and #48 had the strongest heat resistance. In addition, the chlorophyll content and the antioxidant system were closely related to heat tolerance. At the same time, we found that changes in mesophyll cell ultrastructure and chloroplast cross-sectional area were closely related heat stress parameters in C. fortunei. Heat stress also induced up-regulation of the CfAPX1, CfAPX2, CfHSP11, CfHSP21, CfHSP70, CfHSFA1a, CfHSFB2a and CfHSFB4 genes, and each gene was significantly different under different heat stress treatments in families #45 and #48. This study lays a foundation for studying the molecular mechanisms of the response and tolerance of C. fortunei to heat stress.
Data availability statement
The data presented in the study are deposited in the NCBI repository, accession number PRJNA644276.
Author contributions
JYX: Methodology, Validation, Writing-original draft. PZ: Methodology, Validation. JX: Conceptualization, Writing-review & editing, Funding acquisition project administration. JC: Methodology, Validation, Data curation, Writing-original draft, Resources. YZ: Methodology, Validation, Data curation. JY: Data curation, Resources. LZ: Validation, Data curation. HH: Validation, Data curation. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Fujian Seed Industry Innovation and Industrialization Project of Fujian Province (ZYCX-LY-202101), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Acknowledgments
We thank Nanjing Forestry University for providing the platform.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
C. fortunei, Cryptomeria fortunei; EL, electrolyte leakage; LT50, lethal temperature at 50%; NBT, nitroblue tetrazolium; TEM, transmission electron microscope; SOD, superoxide dismutase; POD, peroxidase; CAT, catalase; APX, ascorbate peroxidase; HSPs, heat shock proteins; PCR, polymerase chain reaction; TFs, transcription factors; HSFs, heat stress transcription factors; OD, optical density.
References
Ashraf, M., Hafeez, M. (2004). Thermotolerance of pearl millet and maize at early growth stages: Growth and nutrient relations. Biol. Plantarum. 48, 81–86. doi: 10.1023/B:BIOP.0000024279.44013.61
Baniwal, S. K., Bharti, K., Chan, K. Y., Fauth, M., Ganguli, A., Kotak, S., et al. (2004). Heat stress response in plants: a complex game with chaperones and more than twenty heat stress transcription factors. J. Biosci. 29, 471–487. doi: 10.1007/BF02712120
Berry, J., Bjorkman, O. (1980). Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 31, 491–543. doi: 10.1146/annurev.pp.31.060180.002423
Bhusal, N., Sharma, P., Sareen, S., Sarial, A. K. (2018). Mapping QTLs for chlorophyll content and chlorophyll fluorescence in wheat under heat stress. Biol. Plantarum. 62, 721–731. doi: 10.1007/s10535-018-0811-6
Bi, Y., Zhuge, Q. (2008). Progress in genetic engineering of forest trees under abiotic stresses. World Forestry Res. 21, 5. doi: 10.13348/j.cnki.sjlyyj.2008.05.006
Bondada, B. R., Oosterhuis, D. M., Wullschleger, S. D., Kim, K. S., Harris, W. M. (1994). Anatomical considerations related to photosynthesis in cotton (Gossypium hirsutum l.) leaves, bracts, and the capsule wall. J. Exp. Bot. 45, 111–118. doi: 10.1093/jxb/45.1.111
Borrell, J. S., Dodsworth, S., Forest, F., Pérez-Escobar, O. A., Lee, M. A., Mattana, E., et al. (2020). The climatic challenge: which plants will people use in the next century? Environ. Exp. Bot. 170, 103872. doi: 10.1016/j.envexpbot.2019.103872
Chang, R. J. (2013). Study on physiological and biochemical responses of two kinds of begonia semperflorens in leaf color under high temperature stress (Zhejiang A&F University). [dissertation/master's thesis]. [Lin’an (ZJ)]. Available at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201401&filename=1014101502.nh&uniplatform=NZKPT&v=ygfxUV4xHU9yXgtORfRkPPe6aqrNsyERsiEkctwmaNMaoI6WWRlyWTjN0bFQTJr
Dutta, S., Mohanty, S., Tripathy, B. C. (2009). Role of temperature stress on chloroplast biogenesis and protein import in pea. Plant Physiol. 150, 1050–1061. doi: 10.1104/pp.109.137265
Fragkostefanakis, S., Röth, S., Schleiff, E., Scharf, K. D. (2015). Prospects of engineering thermotolerance in crops throughmodulation of heat stress transcription factor and heatshock protein networks. Plant Cell Environ. 38, 1881–1895. doi: 10.1111/pce.12396
Gosavi, U., Jadhav, A. S., Kale, A. A., Gadakh, S. R. (2014). Effect of heat stress on proline, chlorophyll content, heat shock proteins and antioxidant enzyme activity in sorghum (Sorghum bicolor) at seedlings stage. Indian J. Biotechnol. 13, 356–363.
Guo, Y., Zhang, Z., Fu, Q., Guang, X., Zheng, J., Li., B. (2018). Effects of high temperature stress on leaf structures and physiological metabolism of Potentilla fruticosa l. Northern Horticulture. 23, 93–98. doi: 10.11937/bfyy.20173974
Hasanuzzaman, M., Hossain, M. A., Fujita, M. (2010). Physiological and biochemical mechanisms of nitric oxide induced abiotic stress tolerance in plants. Am. J. Plant Physiol. 5, 295–324. doi: 10.3923/ajpp.2010.295.324
Hasanuzzaman, M., Nahar, K., Alam, M. M., Roychowdhury, R., Fujita, M. (2013). Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 14, 9643–9684. doi: 10.3390/ijms14059643
Hemantaranjan, A., Bhanu, A. N., Singh, M. N., Yadav, D. K., Patel, P. K., Singh, R., et al. (2014). Heat stress responses and thermotolerance. Adv. Plants Agric. Res. 1, 62–70. doi: 10.15406/apar.2014.01.00012
Hong, Z., Liu, S., Hong, C., Lei, X. (2021). Resistance response of five afforestation tree species under drought stress. J. Nanjing For. Univ. Nat. Sci. Ed. 45, 111–119. doi: 10.12302/j.issn.1000-2006.202002019
Huang, H., Jian, Q., Jiang, Y., Duan, X., Qu, H. (2016). Enhanced chilling tolerance of banana fruit treated with malic acid prior to low-temperature storage. Postharvest Biol. Tec. 111, 209–213. doi: 1016j.postharvbio.2015.09.008
Hu, Q., Qian, R., Zhang, Y., Zhang, X., Ma., X., Zheng, J. (2021). Physiological and gene expression changes of Clematis crassifolia and Clematis cadmia in response to heat stress. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.624875
Ikeda, M., Mitsuda, N., Ohme-Takagi, M. (2011). Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible hsfs but positively regulate the acquired thermotolerance. Plant Physiol. 157, 1243–1254. doi: 10.1104/pp.111.179036
Janáček, J., Prášil, I. (1991). Quantification of plant frost injury by nonlinear fitting of an s-shaped function. Cryo Letters. 12, 47–52.
Jiang, Y., Qiu, Y., Hu, Y., Yu, D. (2016). Heterologous expression of AtWRKY57 confers drought tolerance in Oryza sativa. front. Plant Sci. 7. doi: 10.3389/fpls.2016.00145
Kaplan, F., Kopka, J., Haskell, D. W., Zhao, W., Gatzke, N., Sung, D. Y., et al. (2004). Exploring the temperature-stress metabolome of arabidopsis. Plant Physiol. 136, 4159–4168. doi: 10.1104/pp.104.052142
Khan, Z., Shahwar, D. (2020). “Role of heat shock proteins (HSPs) and heat stress tolerance in crop plants,” in Sustainable agriculture in the era of climate change. Eds. Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S. (Cham: Springer). doi: 10.1007/978-3-030-45669-6_9
Krasensky, J., Jonak, C. (2012). Drought, salt, and temperature stress-inducedmetabolic rearrangements and regulatory networks. J. Exp. Bot. 63, 1593–1608. doi: 10.1093/jxb/err460
Larkindale, J., Vierling, E. (2008). Core genome responses involved in acclimation to high temperature. Plant Physiol. 146, 748–761. doi: 10.1104/pp.107.112060
Lastra, O., Gómez, M., López-Gorgé, J., del Río, L. A. (1982). Catalase activity and isozyme pattern of the metalloenzyme system, superoxide dismutase, as a function of leaf development during growth of Pisum sativum l. plants. Physiologia Plantarum. 5, 209–213. doi: 10.1111/j.1399-3054.1982.tb02289.x
Lichtenthaler, H. K., Wellburn, A. R. (1983). Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc T. 11, 591–592. doi: 10.1042/bst0110591
Li, Z., Li, J., Bing, J., Zhang, C. (2019). The role analysis of APX gene family in the growth and developmental processes and in response to abiotic stresses in Arabidopsis thaliana. Hereditas (Beijing). 41 (6), 534–547. doi: 10.16288/j.yczz.19-026
Lukatkin, A. S. (2003). Contribution of oxidative stress to the development of cold-induced damage to leaves of chilling-sensitive plants: 3. Injury Cell membranes by chilling temperatures. Russ. J. Plant Physiol. 50, 243–246. doi: 10.1023/A:1022985500733
Luo, Y., Zhang, Y., Zhong, Y., Kong, Z. (1999). Toxicological study of two novel pesticides on earthworm Eisenia foetida. Chemosphere. 39, 2347–2356. doi: 10.1016/S0045-6535(99)00142-3
Lütz, C., Bergweiler, P., Di, P. L., Holzinger, A. (2012). “Cell organelle structure and function in alpine and polar plants are influenced by growth conditions and climate,” in Plants in alpine regions. Ed. Lütz, C. (Vienna: Springer). doi: 10.1007/978-3-7091-0136-0_5
Marchand, F. L., Mertens, S., Kockelbergh, F., Beyens, L., Nijs, I. (2005). Performance of high arctic tundra plants improved during but deteriorated after exposure to a simulated extreme temperature event. Global Change Biol. 11, 2078–2089. doi: 10.1111/j.1365-2486.2005.01046.x
Mishra, S. K., Tripp, J., Winkelhaus, S., Tschiersch, B., Theres, K., Nover, L., et al. (2002). In the complex family of heat stress transcription factors, HsfA1 has aunique role as master regulator of thermotolerance in tomato. Genes Dev. 16, 1555–1567. doi: 10.1101/gad.228802
Nahar, K., Hasanuzzaman, M., Ahamed, K. U., Hakeem, K. R., Ozturk, M., Fujita, M. (2015). “Plant responses and tolerance to high temperature stress: Role of exogenous phytoprotectants,” in Crop production and global environmental issues. Ed. Hakeem, K. (Cham: Springer). doi: 10.1007/978-3-319-23162-4_17
Rodríguez, M. G., Canales, E., Borrás-Hidalgo, O. (2005). Molecular aspects of abiotic stress in plants. Biotecnol. Apl. 22, 1–10.
Sheng, X., Li, J., Zhang, X., Wei, H., Cui, L. (2006). Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp. Bot. 56, 274–285. doi: 10.1016/j.envexpbot.2005.03.002
Sun, Y., Wei, J., Fan, A. (2006). Effects of salicylic acid on chlorophyll fluorescence and xanthophyll cycle in cucumber leaves under high temperature and strong light. Chin. J. Appl. Ecology. 3, 399–402. doi: 10.13287/j.1001-9332.2006.0082
Talbi, S., Romero-Puertas, M. C., Hernández, A., Terrón, L., Ferchichi, A., Sandalio, L. M. (2015). Drought tolerance in a saharian plant Oudneya africana: role of antioxidant defences. Environ. Exp. Bot. 111, 114–126. doi: 10.1016/j.envexpbot.2014.11.004
Thomas, R. L., Jen, J. J., Morr, C. V. (1982). Changes in soluble and bound peroxidase-IAA oxidase during tomato fruit development. J. Food Sci. 47, 158–161. doi: 10.1111/j.1365-2621.1982.tb11048.x
Tian, S., Chen, C., Zhang, Z. (2020). Physiological and biochemical response to heat stress and heat resistances evaluation of bottle gourds. Acta Agriculturae Shanghai. 36, 18–23. doi: 10.15955/j.issn1000-3924.2020.06.04
Tian, F., Hu, X., Yang, X., Yang, X., Chen, J., Lu, M., et al. (2021). Recent advances in the roles of HSFs and HSPs in heat stress response in woody plants. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.704905
Usman, M. G., Rafii, M. Y., Ismail, M. R., Abdul Malek, M., Abdul Latif, M. (2015). Expression of target gene Hsp70 and membrane stability determine heat tolerance in chili pepper. J. Am. Soc Hortic. Sci. 140, 144–150. doi: 10.21273/JASHS.140.2.144
Vetter, J. L., Steinberg, M. P., Nelson, A. I. (1958). Enzyme assay, quantitative determination of peroxidase in sweet corn. J. Agric. Food Chem. 6, 39–41. doi: 10.1021/jf60083a006
Vidya, M. K., Kumar, V. G., Sejian, V., Bagath, M., Krishnan, G., Bhatta, R. (2018). Toll-like receptors: significance, ligands, signaling pathways, and functions in mammals. Int. Rev. Immunol. 37, 20–36. doi: 10.1080/08830185.2017.1380200
Zhang, M., Xu, G., Teng, Z., Liu, G., Zhang, X. (2021). Effects of simulated acid rain on growth and photosynthetic physiological characteristics of Populus simonii × p. nigra. J. Nanjing For. Univ. Nat. Sci. Ed. 45, 57–64. doi: 10.12302/j.issn.1000-2006.202003068
Zhang, Y., Yang, J., Zhu, L., Xue, J., Hu, H., Cui, J., et al. (2021). Identification of microRNAs and their target genes related to needle discoloration of evergreen tree Chinese cedar (Cryptomeria fortunei) in cold winters. Planta. 254, 31. doi: 10.1007/s00425-021-03685-2
Zhang, Y., Zhu, L., Xue, J., Yang, J., Hu, H., Cui, J., et al. (2021). Selection and verification of appropriate reference genes for expression normalization in Cryptomeria fortunei under abiotic stress and hormone treatments. Genes. 12, 791. doi: 10.3390/genes12060791
Zhang, Y., Zhu, Q., Zhang, M., Guo, Z., Yang, J., Mo, J., et al. (2020). Individual Cryptomeria fortunei hooibrenk families show varying degrees of chilling stress resistance. Forests. 11, 189. doi: 10.3390/f11020189
Zhou, Y. W., Chen, J. H., Lu, L., Cheng, J., Yang, L., Shi, J. (2018). Changes on leaf chloroplast ultrastructure and photosynthetic characteristics of Liriodendron sino-americanum somatic embryo regeneration seedlings under waterlogging stress. Scientia Silvae Sinicae. 54, 19–28. doi: 10.11707/j.1001-7488.20180303
Keywords: Cryptomeria fortunei Hooibrenk, heat stress, physiological analysis, plant cell ultrastructure, gene expression
Citation: Xue J, Zeng P, Cui J, Zhang Y, Yang J, Zhu L, Hu H and Xu J (2023) Physiological and gene expression changes of Cryptomeria fortunei Hooibrenk families under heat stress. Front. Plant Sci. 14:1083847. doi: 10.3389/fpls.2023.1083847
Received: 29 October 2022; Accepted: 16 January 2023;
Published: 30 January 2023.
Edited by:
Biao Jin, Yangzhou University, ChinaReviewed by:
Jemaa Essemine, Partner Institute for Computational Biology, ChinaMartina Spundova, Palacký University, Olomouc, Czechia
Copyright © 2023 Xue, Zeng, Cui, Zhang, Yang, Zhu, Hu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Xu, xjinhsh@njfu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Jinyu Xue
Jinyu Xue Pingsheng Zeng2†
Pingsheng Zeng2† Yingting Zhang
Yingting Zhang Jin Xu
Jin Xu