- 1Tobacco Research Institute of Technology Centre, China Tobacco Hunan Industrial Corporation, Changsha, China
- 2State Key Laboratory of Crop Genetics and Germplasm Enhancement, Ministry of Agriculture Key Laboratory of Plant Nutrition and Fertilization in Lower-Middle Reaches of the Yangtze River, Nanjing Agricultural University, Nanjing, China
- 3China Tobacco Gene Research Center, Zhengzhou Tobacco Research Institute of China National Tobacco Corporation, Zhengzhou, China
- 4State Key Laboratory of Hybrid Rice, College of Life Sciences, Wuhan University, Wuhan, China
Amino acids are vital nitrogen (N) sources for plant growth, development, and yield. The uptake and translocation of amino acids are mediated by amino acid transporters (AATs). The AATs family including lysine-histidine transporters (LHTs), amino acid permeases (AAPs), and proline transporters (ProTs) subfamilies have been identified in various plants. However, little is known about these genes in tobacco. In this study, we identified 23 LHT genes, the important members of AATs, in the tobacco genome. The gene structure, phylogenetic tree, transmembrane helices, chromosomal distribution, cis-regulatory elements, and expression profiles of NtLHT genes were systematically analyzed. Phylogenetic analysis divided the 23 NtLHT genes into two conserved subgroups. Expression profiles confirmed that the NtLHT genes were differentially expressed in various tissues, indicating their potential roles in tobacco growth and development. Cis-elements analysis of promoters and expression patterns after stress treatments suggested that NtLHT genes probable participate in abiotic stress responses of tobacco. In addition, Knock out and overexpression of NtLHT22 changed the amino acids homeostasis in the transgenic plants, the contents of amino acids were significantly decreased in NtLHT22 overexpression plants than wild-type. The results from this study provide important information for further studies on the molecular functions of the NtLHT genes.
Introduction
Nitrogen (N) is the major limiting factor among mineral nutrients for plant growth and development. In natural soil ecosystem, plant roots absorb both inorganic N and organic N including amino acids and peptides (Paungfoo-Lonhienne et al., 2008; Näsholm et al., 2009). Amino acids are the largest pool of dissolved organic N and can be acquired by plant roots. Soil amino acids are mainly derived from peptides of decaying organisms and decomposition of proteins (Kielland et al., 2007). In the soil solution, asparagine, aspartate, glutamine, glutamate, and glycine are the major forms of amino acids, and the concentrations of total free amino acids in the soil may be up to 150 μM (Senwo and Tabatabai, 1998; Weigelt et al., 2005).
In general, inorganic N is converted and transported in the form of amino acids in plants. Therefore, amino acids play crucial role in plants, including regulating root architecture, flowering time, and seed setting rate (Guo et al., 2021). Amino acid transporters (AATs) play vital roles in amino acid transport and translocation. Nowadays, plant AATs have been identified and are grouped into two superfamilies: amino acid-polyamine-choline transporters (APCs) and amino acid/auxin permeases (AAAPs) (Fischer et al., 1998). The APC superfamily includes L-type amino acid transporter (LAT) families and cationic amino acid transporter (CAT) (Tegeder, 2012; Li F. et al., 2021). The AAAP superfamily includes auxin transporters (AUXs), amino acid permeases (AAPs), γ-aminobutyric acid transporters (GATs), lysine and histidine transporters (LHTs), and proline transporters (ProTs) (Okumoto et al., 2002, 2004; Okumoto and Pilot, 2011).
Many AATs have been well researched in Arabidopsis. AtAAP1 is expressed in embryos, amino acid loaded into embryo via AtAAP1 is important for storage protein synthesis and seed yield (Sanders et al., 2009). AtAAP2 is expressed in the phloem, disruption of AtAAP2 increased amino acids allocation to leaves and resulted in higher seed yields (Zhang et al., 2010). AtAAP6 regulates phloem amino acids composition, in aap6 mutant, the concentrations of leucine, lysine, aspartate, and phenylalanine were significantly lower than wild-type plants (Hunt et al., 2010). AtAAP8 plays a crucial role in amino acids loading into the phloem. In aap8 mutants, amino acids loading into phloem and partitioning to sink tissues were decreased (Schmidt et al., 2007). AtLHT1, the first member identified in LHT family, was critical for amino acids uptake. AtLHT1 is expressed in various plant organs, including roots, flowers, and young leaves. Disruption of AtLHT1 decreased shoot biomass and seed yield, while overexpression of AtLHT1 enhanced amino acids uptake and improved the N use efficiency (Chen and Bush, 1997; Hirner et al., 2006). Pathogen infection activates the expression of AtLHT1, further studies suggested that AtLHT1 is a negative regulator of disease resistance, the lht1 mutants enhanced disease resistance to a broad spectrum of pathogens (Liu et al., 2010). AtLHT1 is also involved in the uptake of ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC), loss-of-function of AtLHT1 caused dose-dependent resistance to exogenous ACC (Shin et al., 2015). AtLHT2 is expressed preferentially in floral organs, transgenic expression of AtLHT2 could rescue the early senescence phenotypes of the lht1 mutant (Choi et al., 2019).
In rice, at least 85 putative AATs were identified (Zhao et al., 2012). OsAAP1 is mainly expressed in young panicles, leaves, andaxillary buds, and OsAAP1 protein is located in both the nuclear and plasma membranes. Overexpression of OsAAP1 increased tiller numbers and filled grain numbers, while OsAAP1 RNAi lines showed the opposite phenotype (Ji et al., 2020). OsAAP3 is highly expressed in lateral root, leaf sheath, leaf, panicle, and culm. Overexpression of OsAAP3 inhibited bud outgrowth and tillering, while downregulation of OsAAP3 expression promotes bud elongation and boosts grain yield (Lu et al., 2018). OsAAP5 is mainly expressed in panicles and leaves, OsAAP5 could transports anionic, neutral, and cationic amino acids. Loss-of-function of OsAAP5 could increase tiller number and grain yield (Svennerstam et al., 2008; Wang J. et al., 2019). OsLHT1 is expressed in root hairs and lateral roots and is a key transporter for root amino acids uptake (Guo et al., 2020a). Loss-of-function of OsLHT1 decreased amino acids allocation from root to shoot, thereby markedly reduced shoot growth and grain yields (Wang X. et al., 2019). The japonica subspecies take up aspartate 1.5-fold more efficiently than indica subspecies. Moreover, the expression levels of OsLHT1 in japonica is higher than that in indica. It has been shown that the expression of OsLHT1 and root aspartate uptake was positive correlation (Guo et al., 2020b).
Tobacco (Nicotiana tabacum L.) is one of the most important economic crops, it is cultivated worldwide and used as a model for plant biology research. AATs functions in long distance amino acids transport and are essential for plant growth and development. Lots of AAT genes, such as AAP, ProT, and LHT genes have been well studied and characterized in Arabidopsis, rice, tea, and other plants. However, the studies of AATs is limited in tobacco. In this study, we identified LHT gene family, the important members of AATs, in the tobacco genome with bioinformatics methods. The phylogenic relationships, gene structures, chromosomal distributions, syntenic regions, and cis-regulatory elements in these NtLHT genes were analyzed. Moreover, the expression profiles of NtLHT genes in different tissues and in response to drought and cold stresses were investigated. Our findings provide useful information for further functional research of NtLHT genes in tobacco.
Materials and methods
Plant materials and growth conditions
The N. tabacum L. K326 plants were cultured in a greenhouse with conditions described in previous report (Li Z. et al., 2021). For expression profiles of the NtLHT genes in different tissues, total RNA was isolated from the roots, axillary buds, stems, flowers, and leaves of tobacco at the flowering stage. For drought treatment, 3-week-old seedlings were not watered, the obviously wilted plants were termed as drought stress. For cold treatment, 3-week-old seedlings were exposed to 4°C for 5 h (Xie et al., 2021).
Annotation and identification of putative NtLHT genes
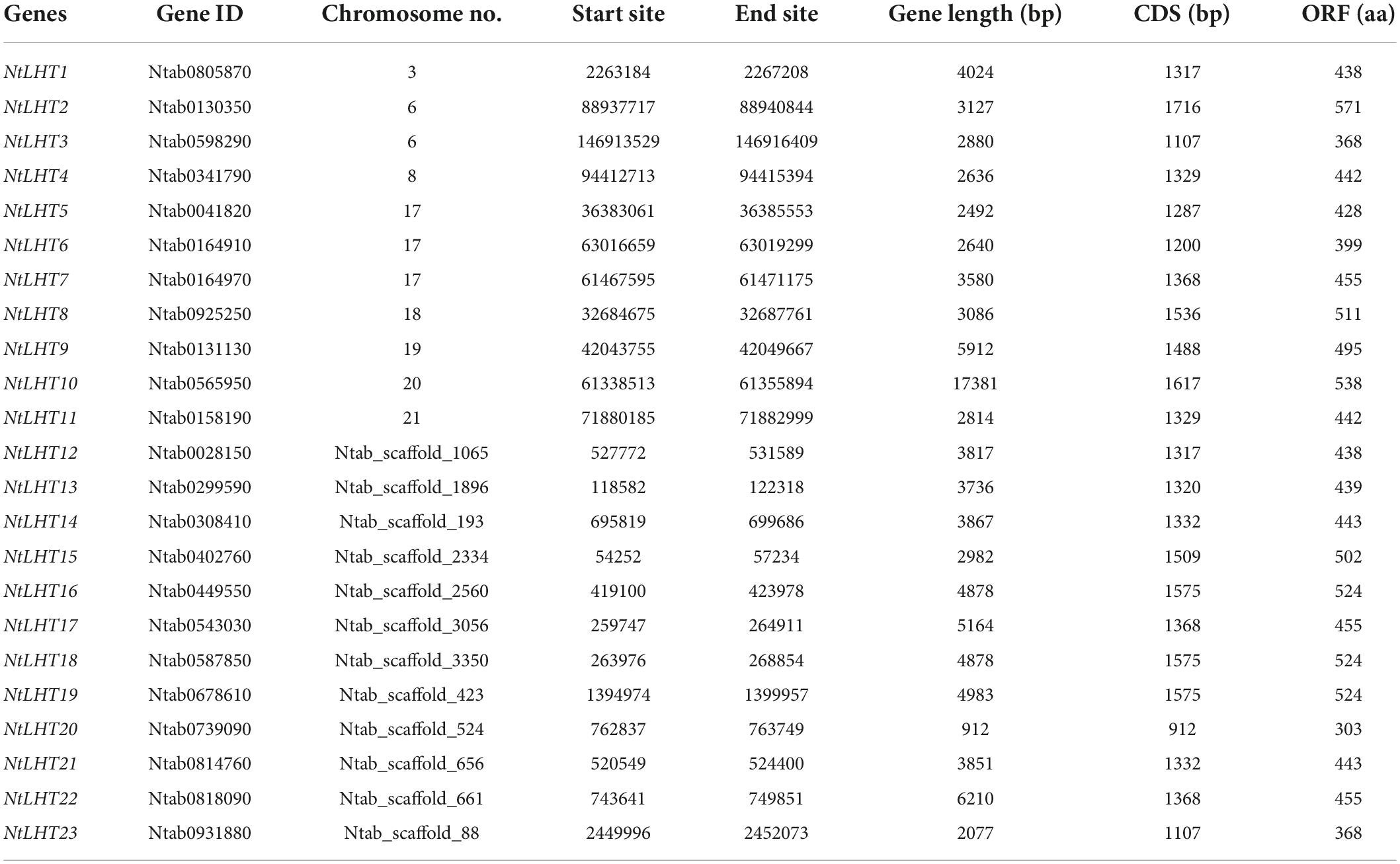
For NtLHT identification, the protein sequences of AtLHTs were retrieved from TAIR1 (Rhee et al., 2003), the obtained sequences were then used as a query to search putative NtLHT genes from the China tobacco genome database (data not shown). Top hits for putative NtLHT proteins were retained on the basis of high scores (score ≥ 0) and low E-values (E value ≤ 0.1), the NCBI conserved domain database (CDD)2 was used to analyze the conserved domain (PF01490) of candidate NtLHT proteins (Rabby et al., 2022). After manually removing the redundant sequences, 23 NtLHT genes were identified and used for further bioinformatics and expression analysis.
Physicochemical characterization, transmembrane, and protein structure analysis
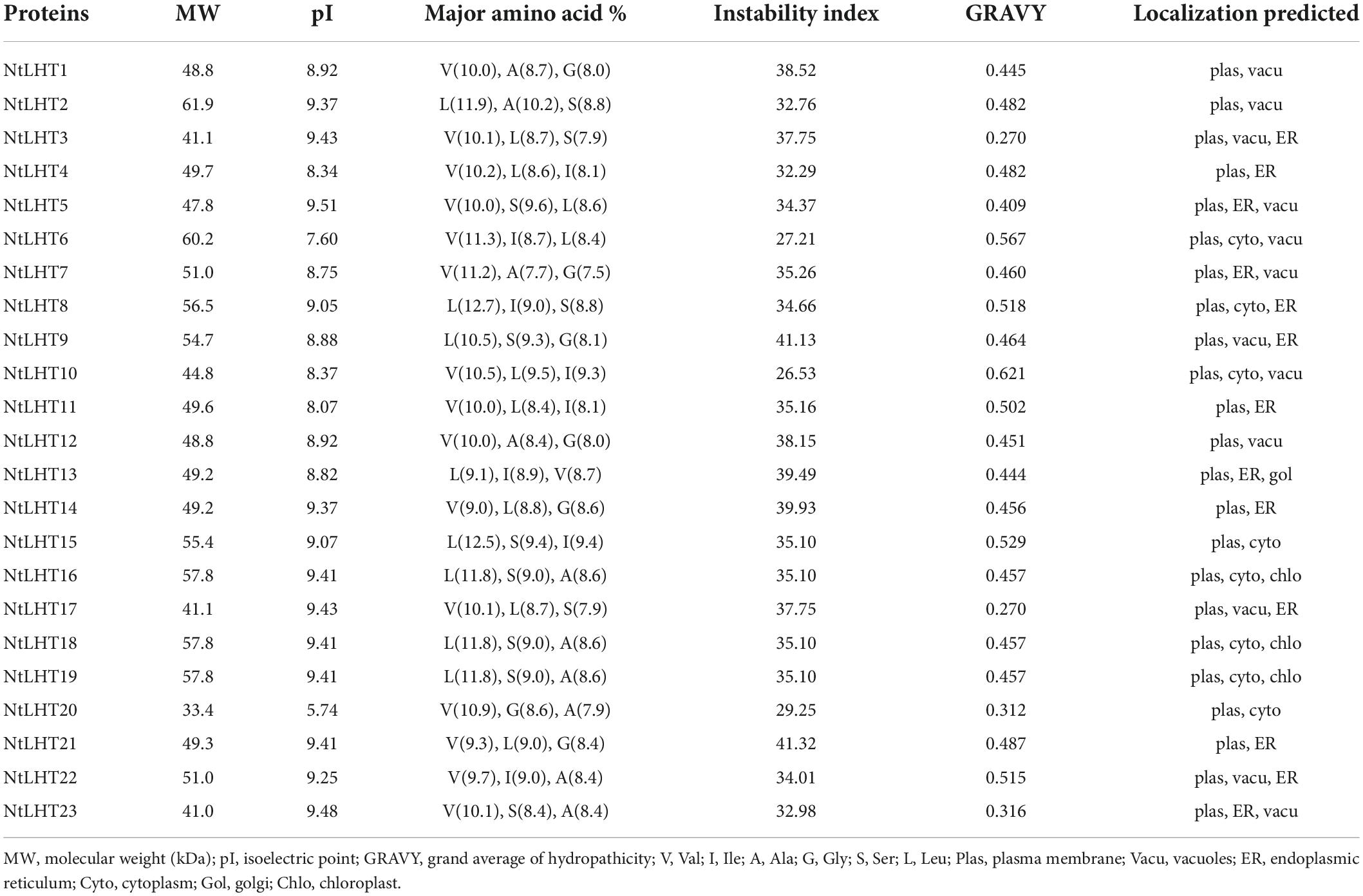
The physiochemical properties of NtLHT proteins were predicted using the ExPASy tool (Artimo et al., 2012). The subcellular location of NtLHT proteins were predicted using The WOLF PSORT II program.3 The PROTTER 1.0 was used to predict the presence of transmembrane helices,4 and the tertiary protein structures of NtLHT proteins were analyzed by PHYRE server v2.0.5
Phylogeny analysis, gene structure, and motifs analysis
The protein sequences of LHT from tobacco (NtLHT), tea (CsLHT), Arabidopsis (AtLHT), and rice (OsLHT) were used to construct a phylogenetic tree in MEGA X program via the neighbor-joining (NJ) method with 1,000 bootstrap replicates (Supplementary Table 1). The coding sequence of each NtLHT (Supplementary Table 2) was aligned with its corresponding genomic sequence to identify the exon–intron structure by GSDS tool.6 The conserved motifs of NtLHT proteins were analyzed using MEME tool with default parameter (Bailey et al., 2006).
Chromosomal mapping and gene duplication analysis
The chromosomal positions of NtLHT genes were mapped using MG2C 2.0.7 Segmental and tandem duplicated gene pairs among the tobacco, tea, Arabidopsis, and rice genomes were conducted using MCScanX. The collinearity map was drawn with Circos. The non-synonymous substitution (Ka) and synonymous substitution rate (Ks) of duplicated genes were determined using KaKs Calculator 2.0.
Cis-regulatory elements analysis
To assess the cis-regulatory elements of the NtLHT promoters, the 2 kb DNA sequence upstream from the transcription start site (start codon ATG) of NtLHT genes were extracted, the obtained sequences were analyzed using the PlantCARE program8 and classified according to their regulatory functions.
Expression analysis of NtLHT genes
Total RNA was isolated from plant tissue samples using the Plant RNA Kit (Transgen, Beijing, China). The first strand cDNA synthesis was performed according to the manufacturer’s directions (Thermo Fisher Scientific, Waltham, MA, United States). The quantitative real time PCR (qRT-PCR) was carried out using the CFX96 (Bio-Rad, Hercules, CA, United States) with 20 μL volume (containing 1 μL of 1:10 diluted cDNA, 200 nM of each gene-specific primer, and SYBR Green Mix from Bio-Rad). PCR cycling parameters were set as following: 95°C for 5 min, 40 cycles of 10 s at 95°C and 30 s at 60°C, then a final melting curve at 65°C for 5 s. The stably expressed gene NtL25 (ribosomal protein gene) was used as the internal control for data normalization. Expression data was carried out with three biological and technical replicates and calculated using the 2–ΔΔCt method (Schmittgen and Livak, 2008). The primers used in this study are listed in Supplementary Table 3.
Subcellular localization analysis
The coding sequence (CDS) of NtLHT22 without the stop codon was amplified and cloned into pEarlyGate101 vector (Invitrogen, Carlsbad, CA, United States) to produce 35S: NtLHT22-YFP construct, the empty pEarlyGate101 vector was used as a control. Tobacco protoplasts preparation and transformation for subcellular localization experiments were carried out as previously described (Schweiger and Schwenkert, 2014). After transformation, the fluorescence signals was observed via Leica SP8-X confocal laser scanning microscope (Leica, Wetzlar, Germany).
Plasmid construction and tobacco transformation
NtLHT22 was amplified and cloned into the modified overexpression vector pEarlyGate101 (Invitrogen, Carlsbad, CA, United States) to produce 35S: NtLHT22-Myc construct. The CRISPR/Cas9 vector was constructed as previously described (Gao et al., 2018). The CRISPR-P 2.0 software9 was used to design the target sites of NtLHT22. Two 20 bp DNA target sites were synthesized and inserted to the pORE-Cas9 binary vector, the resulting constructs was verified via PCR and sequencing. The overexpression and pORE-Cas9 vector was transformed into Agrobacterium tumefaciens (LBA4404), respectively. The Agrobacterium-mediated transformation and regeneration of transgenic tobacco plants were carried out as previously described (Wang et al., 2018; Supplementary Figure 1). Two independent knockout and overexpression lines of NtLHT22 were identified and used for subsequent experimental studies.
Free amino acid analysis
Free amino acid contents were measured according to the ninhydrin method with some modifications (Fang et al., 2013). 2.0 g sample was placed in 80% ethanol (10.0 mL) at 95°C for 20 min. The collected extracts were placed at 80°C in a drying oven to remove the ethanol, the residues were re-dissolved in 1 ml water. After centrifugation at 12000 g for 15 min, the supernatant was filtered through a 0.45 μm membrane. Amino acid contents were determined using an LA8080 automatic amino acid analyzer (Hitachi, Tokyo, Japan).
Results
Genome-wide identification and protein properties of NtLHT
To investigate the LHT genes in tobacco, we employed 10 AtLHT protein sequences as queries for BLAST search. As a result, 23 LHT genes were obtained from the tobacco genome. These genes were named in the order of their locations on the chromosomes and scaffolds. The gene and CDS lengths of NtLHTs ranged from 912 to 17381 bp and 912 to 1716 bp, respectively (Table 1). The length of NtLHT proteins ranged from 303 to 571 amino acids with a molecular weight of 33.4–61.9 kDa, and their pIs ranged from 5.74 to 9.51. The predominant amino acid residues were valine, alanine, and leucine. The instability index for most of the proteins (77.8%) were less than 40. Apart from these, the GRAVY values of all NtLHT proteins were positive, indicating that all NtLHT proteins were hydrophobic. The predicted subcellular locations suggested that most of the NtLHT proteins were localized in the plasma membrane, cytoplasm, and vacuoles (Table 2).
To comprehensive understand the information of NtLHT proteins, the transmembrane regions were predicted by PROTTER 1.0. The number of transmembrane regions in NtLHT proteins ranged from 5 to 11 (Supplementary Figure 2). The predicted protein structures for all NtLHT proteins revealed the presence of α helices, random coils, extended strands, and β turns. All NtLHT proteins have α helices, while β turns were the least common (Supplementary Figure 3).
Phylogenetic analysis of lysine-histidine transporter family in tobacco, tea, Arabidopsis, and rice
To explore the evolutionary relationships among LHT genes from Tobacco, tea, Arabidopsis, and rice, an unrooted NJ tree was constructed based on alignment of 23 NtLHT genes in tobacco, 7 CsLHT genes in tea, 10 AtLHT genes in Arabidopsis, 6 OsLHT genes in rice. According to the created phylogenetic tree, these LHT genes could be classified to two subgroups. Subgroup I contained AtLHT1/2/3/5/6/8/9/10, OsLHT1/2/3/4, CsLHT1/6, and NtLHT1/3/4/5/6/7/10/11/12/13/14/17/20/21/22/23. Subgroup II contained AtLHT4/7, OsLHT5/6, CsLHT2/3/4/5/7, and NtLHT2/8/9/15/16/18/19 (Figure 1). Therefore, subgroup I had the largest number of LHT members in Arabidopsis (8 genes), rice (4 genes), and tobacco (16 genes), whereas subgroup II had more LHT members from tea (5 genes).
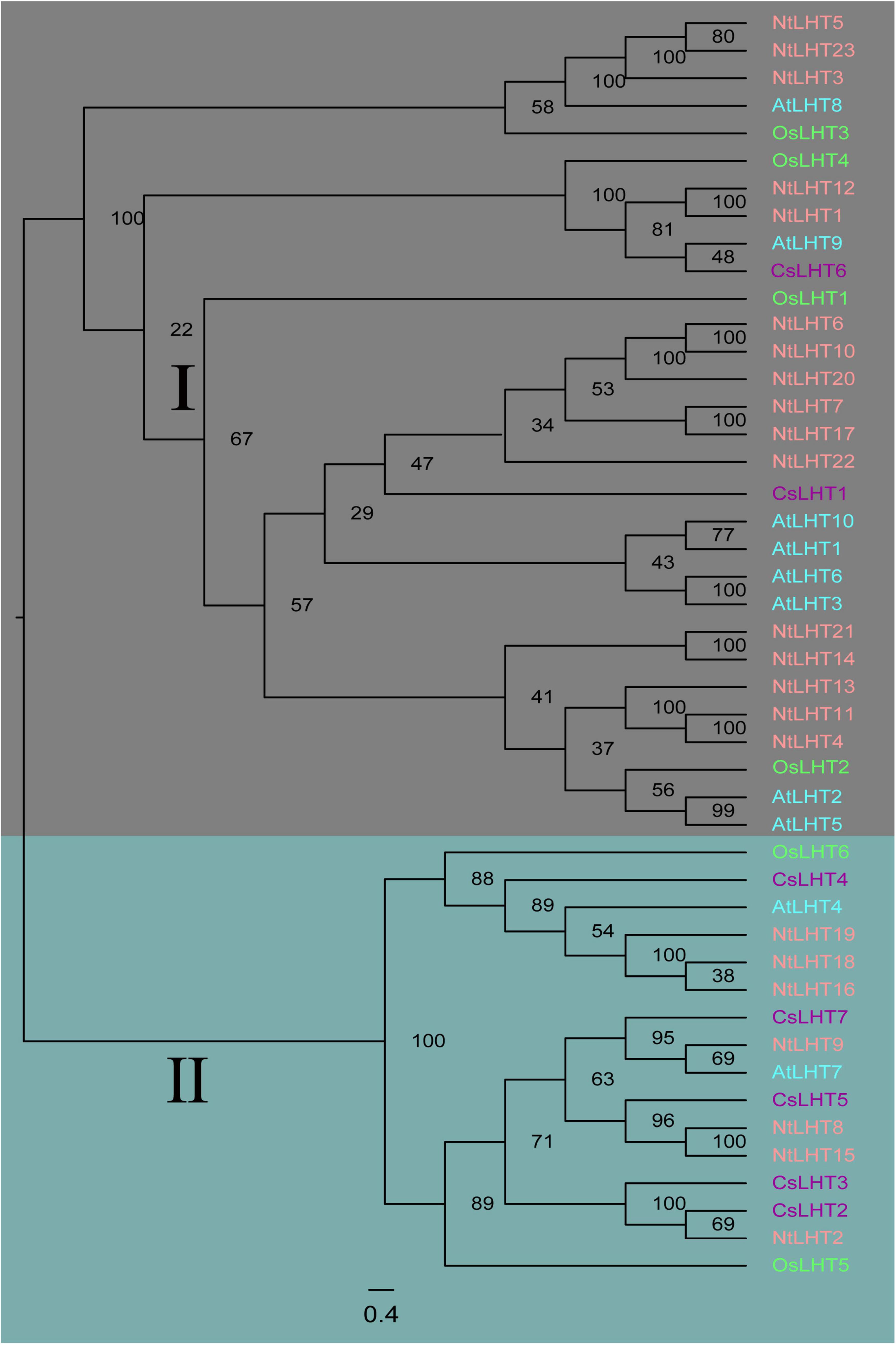
Figure 1. Phylogenetic relationship of LHT proteins from tobacco, tea, Arabidopsis, and rice. The LHT proteins were divided into two subgroups (marked as I and II), and distinguished by different colors: NtLHT labeled in pink, CsLHT labeled in purple, AtLHT labeled in cyan, and OsLHT labeled in green.
Gene structure and conserved motif analysis
To clarify the evolutionary relationships of NtLHT genes, a phylogenetic tree was generated between NtLHT genes. In concordance with the previous results, all the NtLHT genes were divided into two subgroups. Subgroup I contained 16 NtLHT genes, and subgroup II contained 7 NtLHT genes (Figure 2A). The gene structure analysis of NtLHT was performed by the GSDS program. The number of exons in subgroup I ranged from 0 to 8, while each member of subgroup II contained 5 exons (Figure 2B). The conserved motif analysis of NtLHT proteins was captured by MEME software. Several motifs were widespread among NtLHT proteins, such as motif 1, 2, 3, 4, 8, 9. The number of conserved motifs in subgroup I ranged from 6 to 11, while most of the members in subgroup II, except NtLHT2, contained 10 motifs (Figure 2C). These results indicating that subgroup II members is more conserved than subgroup I.
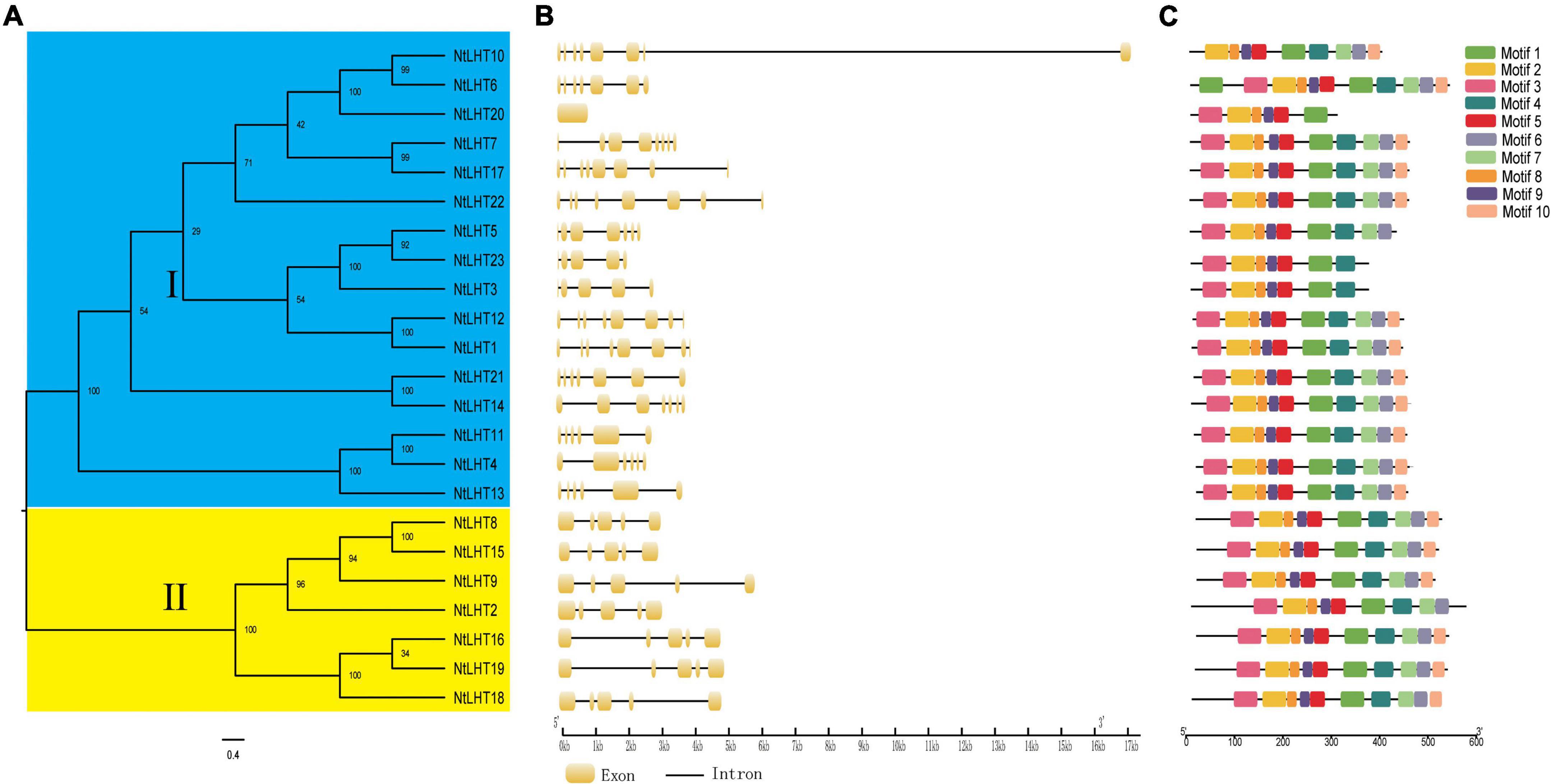
Figure 2. Phylogenetic relationship, exon–intron structures, and conserved motifs of NtLHT gene. (A) The phylogenetic tree was constructed based on NtLHT protein sequences, different subgroups were highlighted by different colors (subgroup I in gray, subgroup II in blue). (B) Exon–intron distribution of NtLHT genes. (C) Conserved motifs analysis. Different colors represent different motifs.
Chromosomal locations and evolutionary analysis of NtLHT genes
The chromosomal distribution of NtLHT genes in the tobacco genome were further determined. In the tobacco genome, 11 NtLHT genes were unevenly distributed in 8 chromosomes (Chr). There were three NtLHT genes on Chr 17, two NtLHT genes on Chr 6, Chr 3, 8, 18, 19, 20, and 21 contained one NtLHT gene each. The remaining 12 NtLHT genes could not be mapped on chromosomes but mapped on some scaffolds (Figure 3).
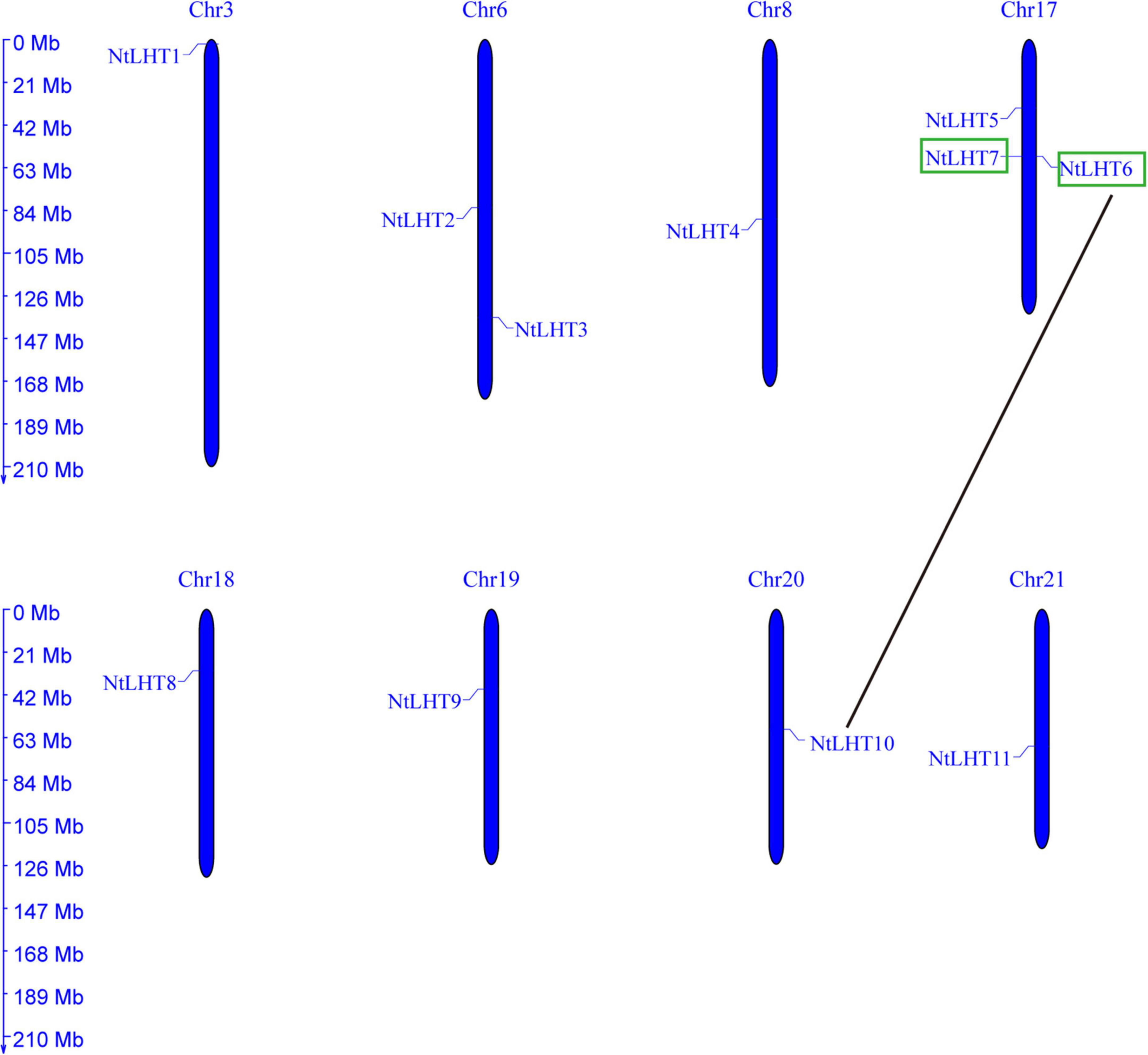
Figure 3. Chromosomal locations and duplication of NtLHT genes. Segmental duplication of NtLHT genes are connected by black line and green box shows tandem duplicated gene pairs.
Tandem and segmental duplicates play significant roles for the evolution of species (Xu et al., 2012). Two genes (NtLHT6 and NtLHT7) were tandemly duplicated, while six pairs (NtLHT1/NtLH12, NtLHT5/NtLHT23, NtLHT6/NtLHT10, NtLHT6/NtLHT22, NtLHT8/NtLHT15, and NtLHT14/NtLHT21) were segmental duplicated (Figure 4). The Ka/Ks values of all tandem and segmental duplicated gene pairs were less than 1 (Supplementary Table 4), indicating that these NtLHT genes were evolved under the influence of purifying selection.
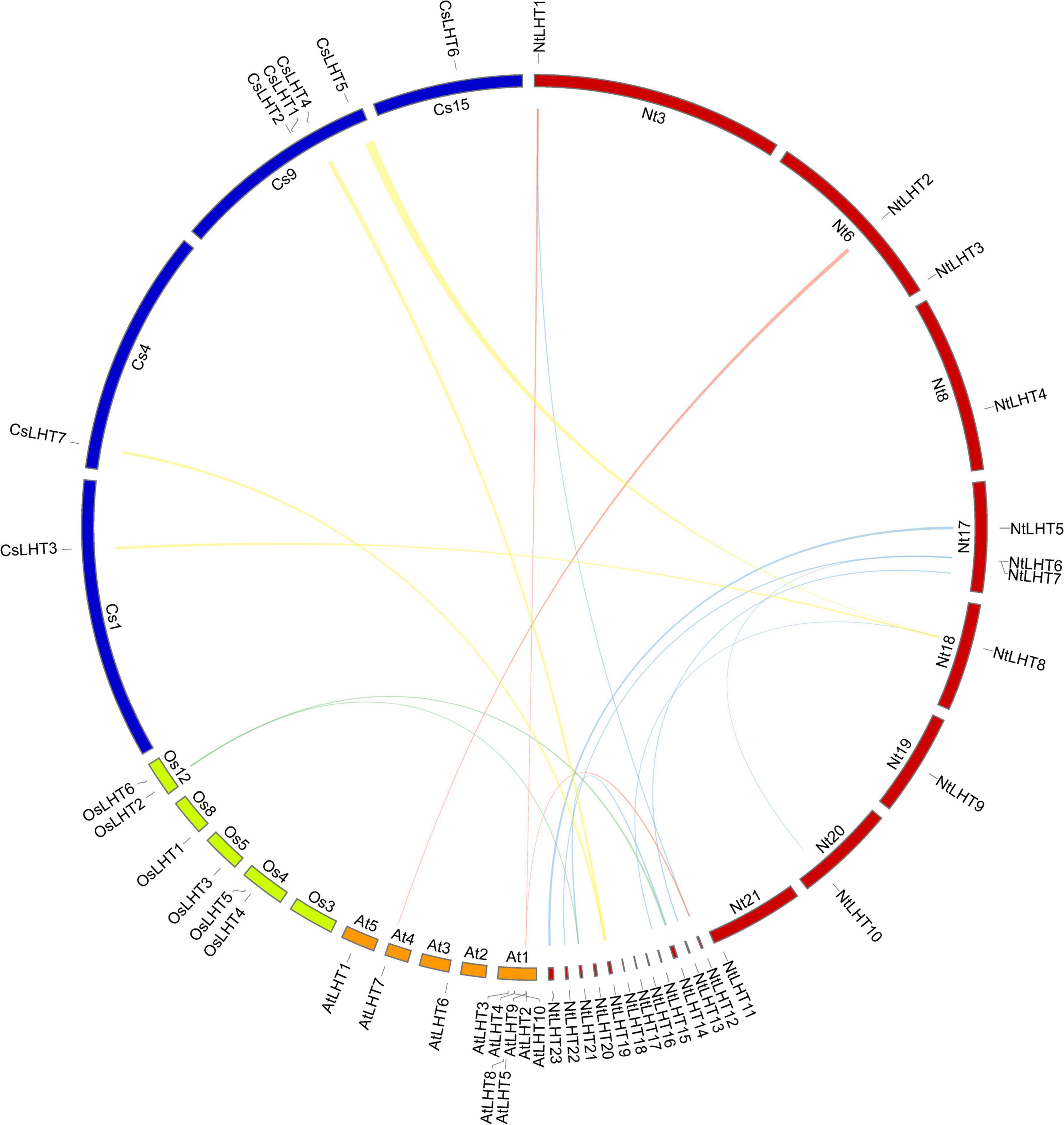
Figure 4. Collinear analysis of LHT genes between tobacco, tea, Arabidopsis, and rice. Blue lines highlight the segmental duplicated NtLHT genes. Yellow, cyan, and red lines highlight the collinear gene pairs between tobacco and tea, tobacco and rice, tobacco and Arabidopsis, respectively.
We constructed a collinearity plot to investigate the orthologous LHT genes in tobacco, tea, Arabidopsis, and rice (Figure 4). Two collinear gene pairs were identified between tobacco and rice, the collinear relationships between them were many-to-one matches. Three collinear gene pairs were identified between tobacco and Arabidopsis, the collinear relationships between them include many-to-one matches and one-to-one matches. Four collinear gene pairs were identified between tobacco and tea, the collinear relationships between them were one-to-many matches (Supplementary Table 5).
Tissue-specific expression profiles of NtLHT genes
The gene expression profiling in different tissues could provide preliminary clues for their function. To explore the tissue specific expression profiles of NtLHT genes, different tissues from tobacco were analyzed, including root, stem, leaf, flower, and axillary bud. The NtLHT genes showed diverse expression patterns among different tissues. NtLHT6/13/16/18/19 were expressed in all these tissues. Ten NtLHT genes, NtLHT2/3/4/7/10/11/14/20/21/23 showed high expression in flower, among which NtLHT2 also displayed high expression in root and NtLHT23 had high expression in leaf. NtLHT8/9/12/15 had the highest expression levels in root. NtLHT1 and NtLHT22 showed high expression in leaf. Notably, NtLHT5 was highly expressed in axillary bud (Figure 5). The results showed that the expression of NtLHT have a certain preference and most of these genes were highly expressed in flower, root, and leaf.
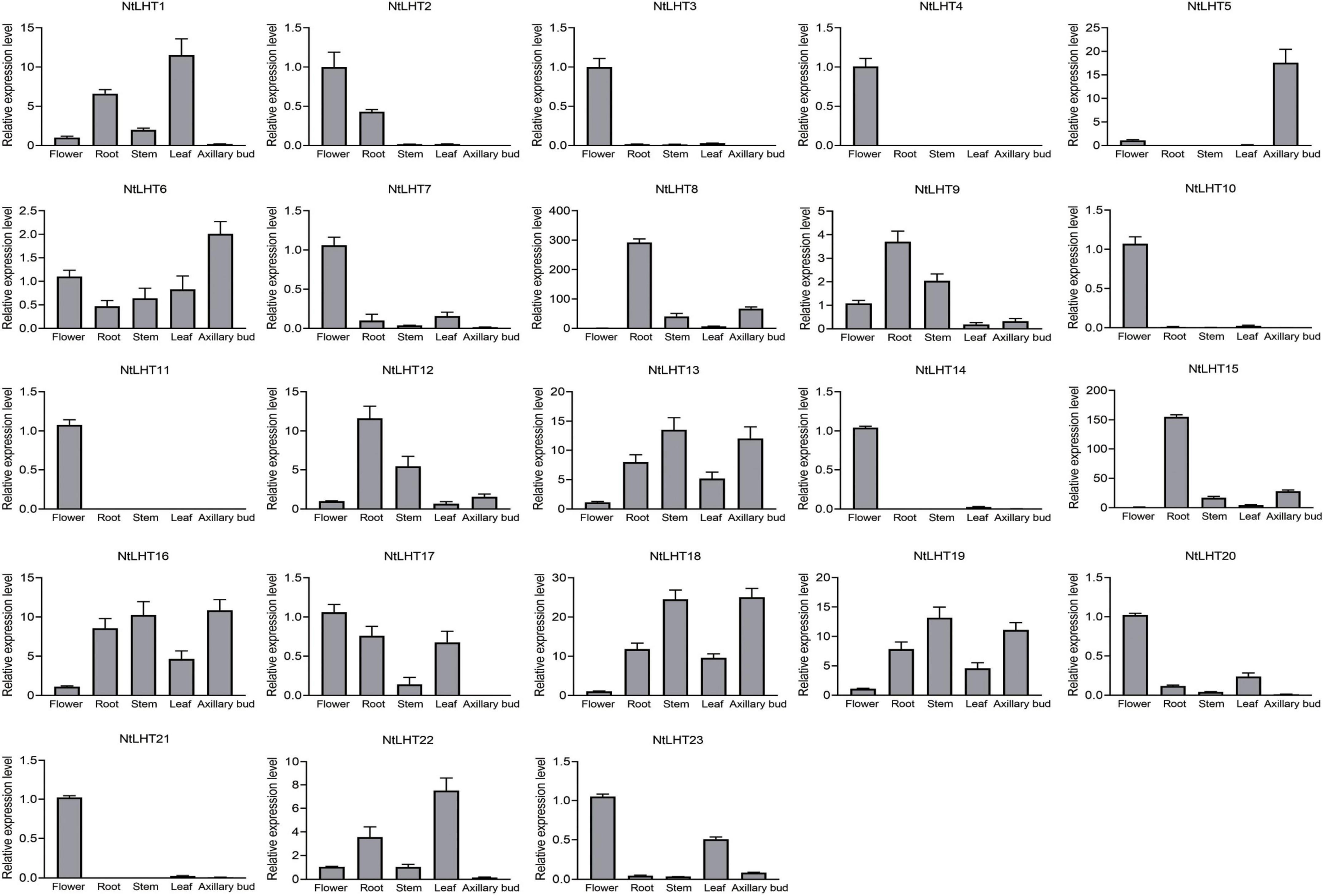
Figure 5. Analysis of NtLHT expression levels in various tissues. Expression levels in flowers were set to one. The data were presented as means ± SDs (n = 3).
Cis-regulatory elements analysis of NtLHT genes
To explore the regulatory pathways of NtLHT genes, we isolated the 2 kb upstream sequences of the NtLHT genes for cis-element analysis. The largest number of cis-elements identified in the NtLHT genes promoter was involved in light-responsiveness. Moreover, cis-elements associated with anaerobic induction were detected in the promoters of most of the NtLHT genes. Apart from these, cis-elements associated with hormone response (e.g., abscisic acid, auxin, MeJA, salicylic acid, and gibberellins), stress response (e.g., low temperature, defense and stress, and drought), and meristem expression were also detected in the promoter sequences of NtLHT genes (Figure 6). The diversity of cis-elements suggest that NtLHT genes performed multiple functions in physiological and biological processes.
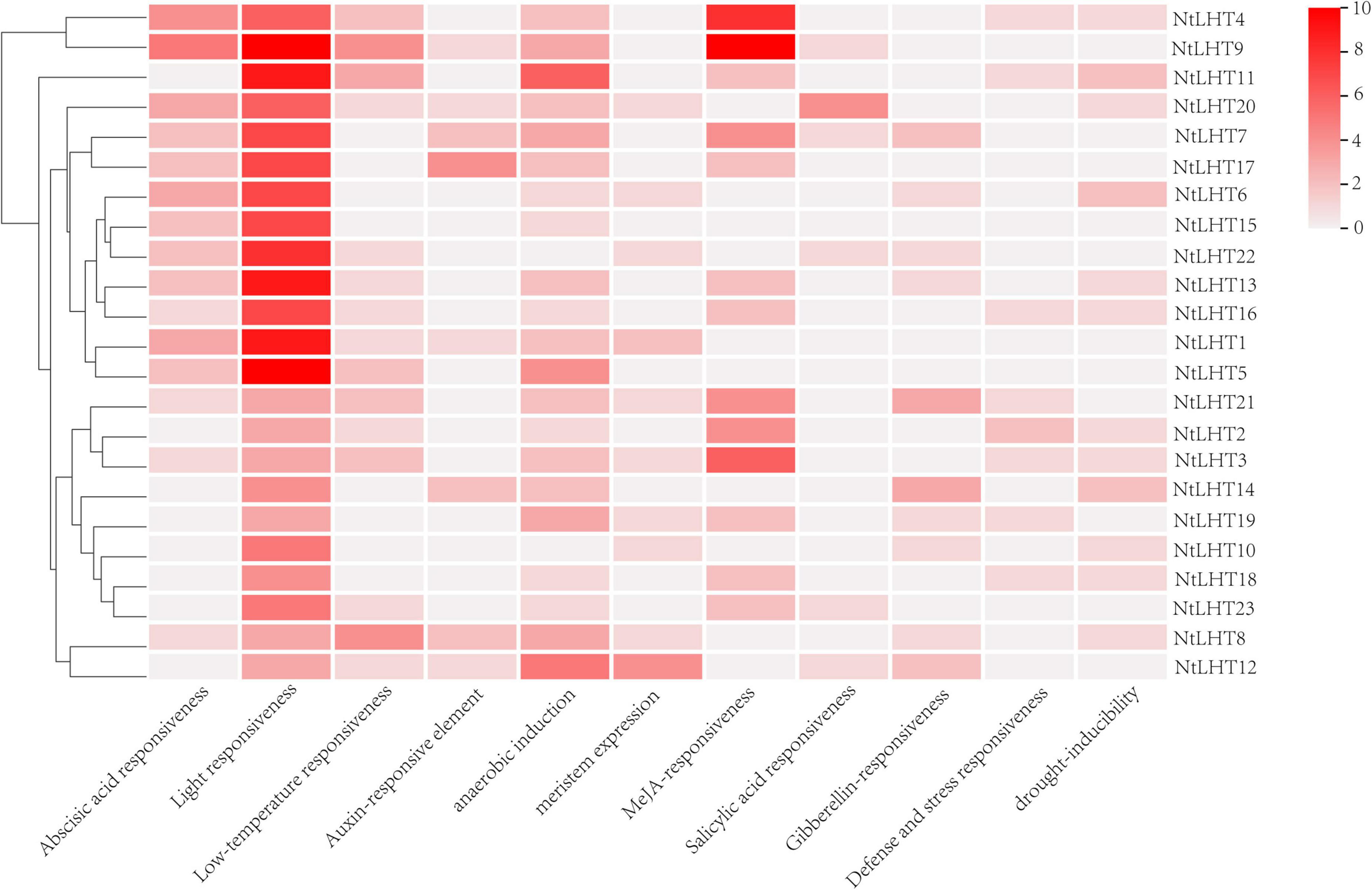
Figure 6. Cis-element analysis in the NtLHT genes promoter. The color represent the numbers of cis-element in NtLHT genes promoter.
Expression profiling of NtLHT genes under cold and drought stress
To analyze the roles of NtLHT genes to cold and drought stress, we analyze the expression profiling of NtLHT genes after cold and drought treatment. The expression levels of most NtLHT genes were differently changed under cold or drought stress (Figure 7). Under cold stress, the expression of NtLHT2/6/10/15/17 were significantly upregulated, the expression of NtLHT9/13/19/22 were significantly downregulated. After drought treatment, the expression of NtLHT1/2/3/5/6/8/12/15/23 were significantly upregulated, while the expression of NtLHT13/16/18/19/22 were significantly downregulated. In addition, the expression of NtLHT7 and NtLHT21 were not significantly changed after cold and drought treatment. Furthermore, four genes (NtLHT4/11/14/21) highly expressed in flower were not detected under drought and cold stresses. The results implied that NtLHT genes may play distinctive roles in plant response to environmental abiotic stresses.
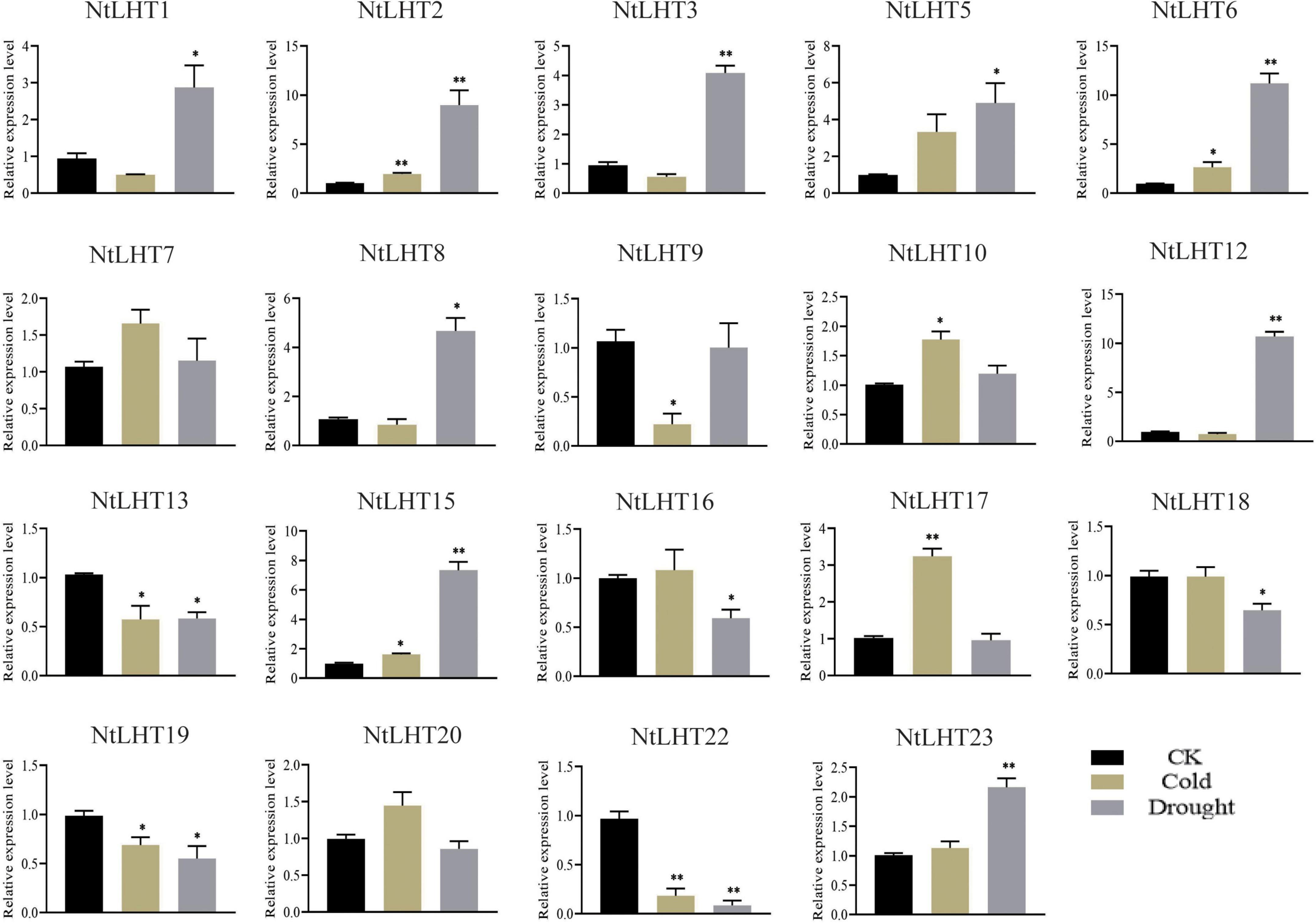
Figure 7. The expression profiles of NtLHT genes under cold and drought treatments. Seedlings grown under normal conditions used as controls. The data were presented as means ± SDs (n = 3). Significant differences was detected using Student’s t-test. *P < 0.05, **P < 0.01.
Subcellular localization analysis of NtLHT22
To explore the potential functions of the NtLHT genes, the subgroup I member NtLHT22 was selected for subcellular localization analysis. The 35S: NtLHT22-YFP vector and the 35S:YFP control vector were transiently expressed in tobacco protoplasts, subsequently, the subcellular localization of the YFP signal was detected by confocal laser scanning microscope. In control, the YFP signal was spread throughout the whole protoplasts. In contrast, the signal of the YFP protein fused to NtLHT22 was only observed in the plasma membrane, suggesting that NtLHT22 was targeted to the plasma membrane (Figure 8).
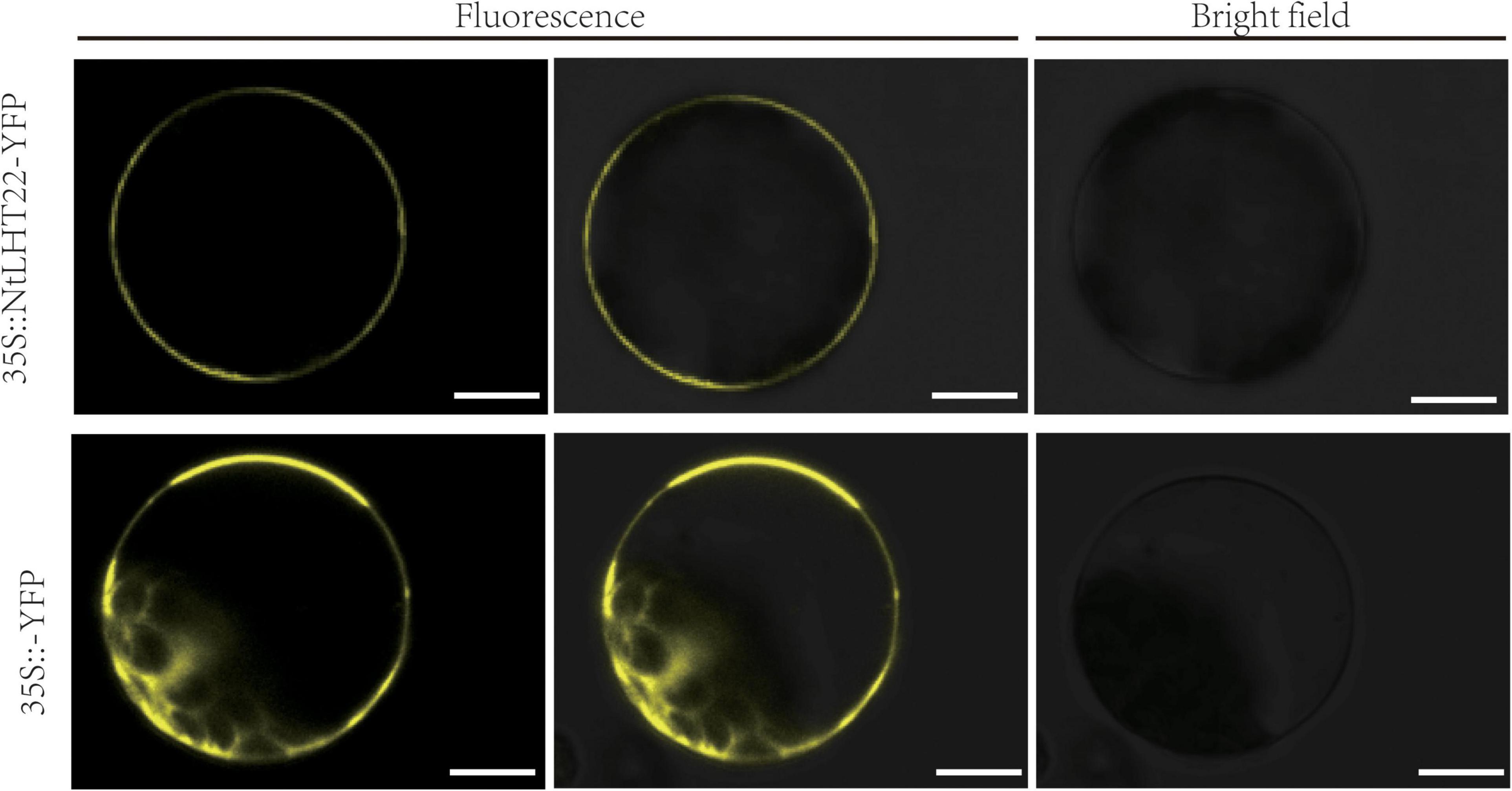
Figure 8. Subcellular localization of NtLHT22 proteins. The coding sequence (CDS) of NtLHT22 was inserted into pEarlyGate101 vector and transiently expressed in tobacco protoplasts. Bar = 10 μm.
NtLHT22 is involved in amino acids homeostasis in tobacco
To further explore the functions of NtLHT22 in amino acids homeostasis, transgenic plants with overexpressing and knock out of NtLHT22 were generated, respectively. Two overexpression (OE3 and OE6) and two mutant lines (ntlht22-1 and ntlht22-2)were chosen for further experiments (Supplementary Figures 4, 5). In leaf tissues, threonine, glycine, serine, aspartic acid, and alanine were the main amino acids. Compared with WT, the levels of six individual amino acids were significantly increased in ntlht22 mutants, including aspartic acid, serine, phenylalanine, lysine, arginine, and methionine, while the contents of threonine, aspartic acid and glycine were decreased in NtLHT22-OE plants (Figure 9A). In root tissues, the levels of threonine, alanine and serine were decreased in NtLHT22-OE plants than WT. In ntlht22 mutants roots, the levels of alanine was decreased but the levels of aspartic acid and lysine were increased than WT. In addition, the content of arginine in NtLHT22-OE plants and ntlht22 mutants roots were significantly higher than WT (Figure 9B). The amino acids content were significantly reduced in NtLHT22-OE plants than WT, however, total of amino acids were not changed between ntlht22 mutants and WT (Figures 9C,D). Taken together, the amino acids profile was significantly changed in NtLHT22-OE plants and ntlht22 mutants, suggesting that NtLHT22 is participate in amino acids homeostasis in tobacco.
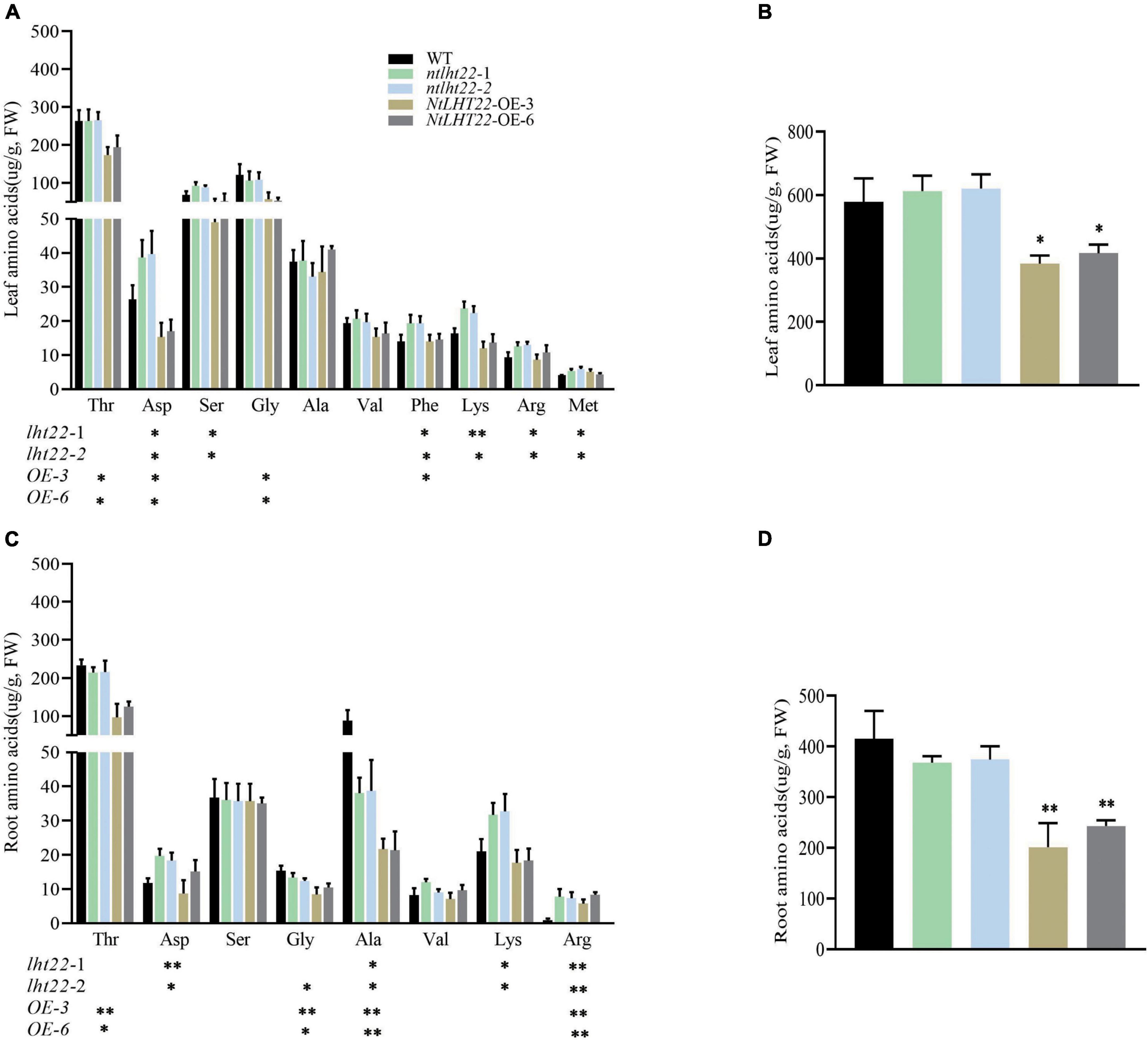
Figure 9. Analysis of amino acids levels of NtLHT22 overexpression, knock out, and wild-type plants. (A,B) Individual and total amino acids content in leaf. (C,D) Individual and total amino acids content in roots. FW, fresh weight. The data were presented as means ± SDs (n = 3). Significant differences was detected using Student’s t-test. *P < 0.05, **P < 0.01.
Discussion
Amino acids are important carriers of N exchange in plants, the absorption and translocation of amino acids are mainly mediated by AATs (Ortiz-Lopez et al., 2000). AATs play significant roles in plant growth and development, including long distance transport and translocate of amino acids, response to biotic and abiotic stresses (Tegeder and Ward, 2012; Ma et al., 2016). The functions of AATs in various plants have been characterized in detail. In potato, StAAP1 is induced during leaf maturation and the amino acid contents are decreased with reduced StAAP1 expression (Koch et al., 2003). In Vicia faba, VfAAP1 is highly expressed in the cotyledons and shows moderate expression in other sink tissues (Miranda et al., 2001). In Arabidopsis, AtProT is highly expressed in pollen and the epidermides of leaves, AtProT1 and AtProT2 were shown to facilitate the uptake of proline (Lehmann et al., 2011). In rice, OsProT1 is mainly expressed in roots, stems, and flowers and specifically mediated the transport of L-proline (Igarashi et al., 2000). OsLHT1 participate in root uptake and root-to-shoot allocation of acidic and neutral amino acids, loss-of-function of OsLHT1 significantly inhibited plants development and reduced seed yields (Wang X. et al., 2019). LHT gene family is an important part of AAT gene family, however, LHT gene family had not been well characterized in tobacco. In this study, we performed a comprehensive identification, classification, and expression profile to study LHT gene family in tobacco.
Here, 23 NtLHT genes were identified from the tobacco genome. Physicochemical analysis showed that NtLHT proteins exhibited similar physical properties. For example, all NtLHT proteins were alkaline (pI > 7) and hydrophobic (Table 2). The 3D protein structure analysis indicated that NtLHT proteins have highly conservative structure characterized by several α helices, random coils, extended strands, and β turns (Supplementary Figure 3). NtLHT genes were divided into two subgroups (subgroup I and subgroup II) and subgroup I contained more LHT genes than subgroup II (Figure 1).
Gene structure is closely related with gene evolution and can provide useful information for the study of gene function (Guo et al., 2013). The number of introns in subgroup I ranged from 0 to 8, in subgroup II, all members of NtLHT genes contained 5 exons, indicating that the gene structure in subgroup I is more conserved than subgroup II (Figure 2B). We identified ten conserved motifs in NtLHT proteins. The number of conserved motifs in subgroup I ranged from 6 to 11, whereas all NtLHT proteins in subgroup II, except NtLHT2, contained 10 motifs (Figure 2C). Together, these results show that the number of exons/introns and motifs in NtLHT gene family were closely related with function conservation or diversification.
Gene duplication, such as whole genome duplication, tandem duplication, and segmental duplication, is the most important pathway for the expansion and evolution of gene families. Moreover, segmental duplications were more important than tandem duplications for the expansion of gene family (Vision Todd et al., 2000; Paterson et al., 2010; Zhang et al., 2020). We obtained one pair of tandem duplicated NtLHT genes and six pairs of segmental duplicated NtLHT genes in tobacco genome (Figure 4), these results corroborates the previous studies. Additionally, the Ka/Ks values of these duplicated gene pairs were less than 1 (Supplementary Table 4), indicating that duplicated genes have evolved under the influence of purifying selection.
To better understand the functions of NtLHT genes, we analyzed the expression profiles of these genes in different tissues (Figure 5). Several NtLHT genes (i.e., NtLHT6 and NtLHT16) were highly expressed in most tissues analyzed. Some NtLHT genes were highly expressed in specific tissues (i.e., NtLHT5 in axillary bud, NtLHT3/4/10/11/14 in flower, NtLHT8 and NtLHT15 in root), suggesting that these genes may play different physiological functions in different tissues. Cis-element contribute to the regulation of gene expression, therefore, the analysis of cis-elements is helpful for gene functional characterization. Light-responsiveness elements were ubiquitously identified in the promoter region of NtLHT genes, indicating that light might regulate the expression of NtLHT genes. In addition, cis-elements related to hormone response and stress response were identified in most of the NtLHT genes (Figure 6), suggesting that NtLHT genes play essential roles in plant growth and development. According to cis-elements analysis of NtLHT genes, we explored the expression profiling of these genes under cold and drought treatment. The results indicated that 9 and 14 NtLHT genes responded to cold and drought stress, respectively. Notably, the expression levels of NtLHT4/11/14/21 were not detected after cold and drought treatment (Figure 7). These results indicate that the expression of NtLHT genes might be regulated by several cis-elements, and there also exist unidentified cis-elements to regulating the expression of these genes under cold and drought stresses.
Various membrane-localized transporters involved in amino acids uptake, accumulation and translocation, in addition, efficient use of the N inside the crops for quality and yield relies on the proper amino acid metabolism. Previous work suggests that amino acid distribution processes in leaves affect N uptake, probably via changes in amino acid metabolism and shoot-to-root signaling processes (Tegeder, 2014). Genetic manipulation of AATs is potentially able to alter plant performance with coordinating amino acid assimilation and metabolism, and ultimately influence quality and yield. A large amount of N is needed in the process of tobacco growing, and the content of amino acids in leaves is also closely related to the quality of tobacco. Our results indicated that overexpression and mutant of NtLHT22 changed the amino acids profile in leaf and root, and the accumulation of amino acids were significantly decreased in NtLHT22-OE plants than WT (Figures 9A–D). Therefore, NtLHT22 might be involved in the homeostasis of amino acids in different tissues, the alterations of amino acids accumulaion may trigger the changes in N metabolism and quality. Furthermore, NtLHT22 could be used as a potential gene for quality and yield breeding, and future studies will explore the essential roles of NtLHT22 in quality as well as N use efficiency.
Conclusion
In summary, we discovered 23 NtLHT genes and analyzed their physicochemical characteristics, phylogenetic relationships, chromosomal locations, and cis-elements. We also explored the expression profiles of NtLHT genes in different tissues and under abiotic stresses. Furthermore, overexpression and mutant of NtLHT22 in tobacco changed the amino acids profile, suggesting that NtLHT22 might be involved in amino acids homeostasis. Overall, our studies provided useful information for the role of NtLHT and laid a solid foundation for further investigations of the biological mechanisms of NtLHT genes.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
ZL and JG conceived and designed the study. SW, XX, and ZW conducted the bioinformatics analysis. YP, XY, WP, and YW assisted in data collection. ZL and XF wrote the manuscript. All authors read and approved the manuscript.
Funding
This work was supported by the key funding of CNTC [No. 110202101003(JY-03)], the Science and Technology Research Foundation of HNTI (No. KY2022YC0005), the Policy Guidance Program of Jiangsu Province in 2020 (International Science and Technology Cooperation, BZ2020064), and the sub-project 4 of Jiangsu middle and late mature garlic industrial cluster construction: Garlic mechanical intelligent operation technology and demonstration and promotion of green production technology. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this manuscript, or the decision to submit it for publication.
Conflict of interest
ZL, JG, SW, YP, XY, WP, and YW were employed by China Tobacco Hunan Industrial Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.927844/full#supplementary-material
Supplementary Figure 1 | The process of tobacco genetic transformation.
Supplementary Figure 2 | The transmembrane helices analysis of NtLHT proteins.
Supplementary Figure 3 | Predicted 3D structures of the NtLHT proteins.
Supplementary Figure 4 | Expression levels of NtLHT22 in wild-type plants and two overexpression plants.
Supplementary Figure 5 | Construction and verification of NtLHT22 knock out transgeneic plants. (A) Two separated target sites designed for knock out the NtLHT22 by CRISPR/Cas9. (B) Verification of the knockout lines of NtLHT22 by PCR-based sequencing.
Supplementary Table 1 | The LHT protein sequences of tobacco, tea, Arabidopsis, and rice.
Supplementary Table 2 | The LHT coding sequences of tobacco.
Supplementary Table 3 | Primers used in this study.
Supplementary Table 4 | Ka and Ks ratios for seven paralogous NtLHT genes.
Supplementary Table 5 | Collinear LHT genes between tobacco, tea, Arabidopsis, and rice.
Footnotes
- ^ http://www.arabidopsis.org
- ^ https://www.ncbi.nlm.nih.gov/cdd/
- ^ http://www.genscript.com/wolf-psort.html
- ^ http://wlab.ethz.ch/protter/start/
- ^ http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index
- ^ http://gsds.cbi.pku.edu.cn
- ^ http://mg2c.iask.in/mg2c_v2.0/
- ^ http://bioinformatics.psb.ugent.be/webtools/plantcare/html/search_CARE.html
- ^ http://crispr.hzau.edu.cn/CRISPR2/
References
Artimo, P., Jonnalagedda, M., Arnold, K., Baratin, D., Csardi, G., de Castro, E., et al. (2012). ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 40:W597–W603. doi: 10.1093/nar/gks400
Bailey, T. L., Williams, N., Misleh, C., and Li, W. W. (2006). MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 34:W369–W373. doi: 10.1093/nar/gkl198
Chen, L., and Bush, D. R. (1997). LHT1, a lysine- and histidine-specific amino acid transporter in Arabidopsis. Plant Physiol. 115, 1127–1134. doi: 10.1104/pp.115.3.1127
Choi, J., Eom, S., Shin, K., Lee, R. A., Choi, S., Lee, J. H., et al. (2019). Identification of Lysine Histidine Transporter 2 as an 1-Aminocyclopropane Carboxylic Acid Transporter in Arabidopsis thaliana by Transgenic Complementation Approach. Front. Plant Sci. 10:1092. doi: 10.3389/fpls.2019.01092
Fang, Z., Xia, K., Yang, X., Grotemeyer, M. S., Meier, S., Rentsch, D., et al. (2013). Altered expression of the PTR/NRT1 homologue OsPTR9 affects nitrogen utilization efficiency, growth and grain yield in rice. Plant Biotechnol. J. 11, 446–458. doi: 10.1111/pbi.12031
Fischer, W.-N., André, B., Rentsch, D., Krolkiewicz, S., Tegeder, M., Breitkreuz, K., et al. (1998). Amino acid transport in plants. Trends Plant Sci. 3, 188–195. doi: 10.1016/S1360-1385(98)01231-X
Gao, J., Zhang, T., Xu, B., Jia, L., Xiao, B., Liu, H., et al. (2018). CRISPR/Cas9-Mediated Mutagenesis of Carotenoid Cleavage Dioxygenase 8 (CCD8) in Tobacco Affects Shoot and Root Architecture. Int. J. Mol. Sci. 19:1062. doi: 10.3390/ijms19041062
Guo, N., Gu, M., Hu, J., Qu, H., and Xu, G. (2020a). Rice OsLHT1 Functions in Leaf-to-Panicle Nitrogen Allocation for Grain Yield and Quality. Front. Plant Sci. 11:1150. doi: 10.3389/fpls.2020.01150
Guo, N., Hu, J., Yan, M., Qu, H., Luo, L., Tegeder, M., et al. (2020b). Oryza sativa Lysine-Histidine-type Transporter 1 functions in root uptake and root-to-shoot allocation of amino acids in rice. Plant J. 103, 395–411. doi: 10.1111/tpj.14742
Guo, N., Zhang, S., Gu, M., and Xu, G. (2021). Function, transport, and regulation of amino acids: What is missing in rice? Crop J. 9, 530–542. doi: 10.1016/j.cj.2021.04.002
Guo, R., Xu, X., Carole, B., Li, X., Gao, M., Zheng, Y., et al. (2013). Genome-wide identification, evolutionary and expression analysis of the aspartic protease gene superfamily in grape. BMC Genom. 14:554. doi: 10.1186/1471-2164-14-554
Hirner, A., Ladwig, F., Stransky, H., Okumoto, S., Keinath, M., Harms, A., et al. (2006). Arabidopsis LHT1 is a high-affinity transporter for cellular amino acid uptake in both root epidermis and leaf mesophyll. Plant Cell 18, 1931–1946. doi: 10.1105/tpc.106.041012
Hunt, E., Gattolin, S., Newbury, H. J., Bale, J. S., Tseng, H.-M., Barrett, D. A., et al. (2010). A mutation in amino acid permease AAP6 reduces the amino acid content of the Arabidopsis sieve elements but leaves aphid herbivores unaffected. J. Exp. Bot. 61, 55–64. doi: 10.1093/jxb/erp274
Igarashi, Y., Yoshiba, Y., Takeshita, T., Nomura, S., Otomo, J., Yamaguchi-Shinozaki, K., et al. (2000). Molecular cloning and characterization of a cDNA encoding proline transporter in rice. Plant Cell Physiol. 41, 750–756. doi: 10.1093/pcp/41.6.750
Ji, Y., Huang, W., Wu, B., Fang, Z., and Wang, X. (2020). The amino acid transporter AAP1 mediates growth and grain yield by regulating neutral amino acid uptake and reallocation in Oryza sativa. J. Exp. Bot. 71, 4763–4777. doi: 10.1093/jxb/eraa256
Kielland, K., McFarland, J. W., Ruess, R. W., and Olson, K. (2007). Rapid Cycling of Organic Nitrogen in Taiga Forest Ecosystems. Ecosystems 10, 360–368. doi: 10.1007/s10021-007-9037-8
Koch, W., Kwart, M., Laubner, M., Heineke, D., Stransky, H., Frommer, W. B., et al. (2003). Reduced amino acid content in transgenic potato tubers due to antisense inhibition of the leaf H+/amino acid symporter StAAP1. Plant J. 33, 211–220. doi: 10.1046/j.1365-313X.2003.01618.x
Lehmann, S., Gumy, C., Blatter, E., Boeffel, S., Fricke, W., and Rentsch, D. (2011). In planta function of compatible solute transporters of the AtProT family. J. Exp. Bot. 62, 787–796. doi: 10.1093/jxb/erq320
Li, F., Dong, C., Yang, T., Bao, S., Fang, W., Lucas, W. J., et al. (2021). The tea plant CsLHT1 and CsLHT6 transporters take up amino acids, as a nitrogen source, from the soil of organic tea plantations. Hortic. Res. 8:178. doi: 10.1038/s41438-021-00615-x
Li, Z., Gao, J., Wang, G., Wang, S., Chen, K., Pu, W., et al. (2021). Genome-Wide Identification and Characterization of GASA Gene Family in Nicotiana tabacum. Front. Genet. 12:768942. doi: 10.3389/fgene.2021.768942
Liu, G., Ji, Y., Bhuiyan, N. H., Pilot, G., Selvaraj, G., Zou, J., et al. (2010). Amino acid homeostasis modulates salicylic acid-associated redox status and defense responses in Arabidopsis. Plant Cell 22, 3845–3863. doi: 10.1105/tpc.110.079392
Lu, K., Wu, B., Wang, J., Zhu, W., Nie, H., Qian, J., et al. (2018). Blocking amino acid transporter OsAAP3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol. J. 16, 1710–1722. doi: 10.1111/pbi.12907
Ma, H., Cao, X., Shi, S., Li, S., Gao, J., Ma, Y., et al. (2016). Genome-wide survey and expression analysis of the amino acid transporter superfamily in potato (Solanum tuberosum L.). Plant Physiol. Biochem. 107, 164–177. doi: 10.1016/j.plaphy.2016.06.007
Miranda, M., Borisjuk, L., Tewes, A., Heim, U., Sauer, N., Wobus, U., et al. (2001). Amino acid permeases in developing seeds of Vicia faba L.: Expression precedes storage protein synthesis and is regulated by amino acid supply. Plant J. 28, 61–71. doi: 10.1046/j.1365-313X.2001.01129.x
Näsholm, T., Kielland, K., and Ganeteg, U. (2009). Uptake of organic nitrogen by plants. New Phytol. 182, 31–48. doi: 10.1111/j.1469-8137.2008.02751.x
Okumoto, S., Koch, W., Tegeder, M., Fischer, W. N., Biehl, A., Leister, D., et al. (2004). Root phloem-specific expression of the plasma membrane amino acid proton co-transporter AAP3. J. Exp. Bot. 55, 2155–2168. doi: 10.1093/jxb/erh233
Okumoto, S., and Pilot, G. (2011). Amino Acid Export in Plants: A Missing Link in Nitrogen Cycling. Mol. Plant 4, 453–463. doi: 10.1093/mp/ssr003
Okumoto, S., Schmidt, R., Tegeder, M., Fischer, W. N., Rentsch, D., Frommer, W. B., et al. (2002). High Affinity Amino Acid Transporters Specifically Expressed in Xylem Parenchyma and Developing Seeds of Arabidopsis. J. Biol. Chem. 277, 45338–45346. doi: 10.1074/jbc.M207730200
Ortiz-Lopez, A., Chang, H. C., and Bush, D. R. (2000). Amino acid transporters in plants. Biochim. Biophys. Acta 1465, 275–280. doi: 10.1016/S0005-2736(00)00144-9
Paterson, A. H., Freeling, M., Tang, H., and Wang, X. (2010). Insights from the Comparison of Plant Genome Sequences. Annu. Rev. Plant Biol. 61, 349–372. doi: 10.1146/annurev-arplant-042809-112235
Paungfoo-Lonhienne, C., Lonhienne, T. G. A., Rentsch, D., Robinson, N., Christie, M., Webb, R. I., et al. (2008). Plants can use protein as a nitrogen source without assistance from other organisms. Proc. Natl. Acad. Sci. U.S.A. 105:4524. doi: 10.1073/pnas.0712078105
Rabby, M. G., Hossen, M. M., Kamal, M. M., and Islam, M. N. (2022). Genome-Wide Identification and Functional Analysis of Lysine Histidine Transporter (LHT) Gene Families in Maize. Genet. Res. 2022:2673748. doi: 10.1155/2022/2673748
Rhee, S. Y., Beavis, W., Berardini, T. Z., Chen, G., Dixon, D., Doyle, A., et al. (2003). The Arabidopsis Information Resource (TAIR): A model organism database providing a centralized, curated gateway to Arabidopsis biology, research materials and community. Nucleic Acids Res. 31, 224–228. doi: 10.1093/nar/gkg076
Sanders, A., Collier, R., Trethewy, A., Gould, G., Sieker, R., and Tegeder, M. (2009). AAP1 regulates import of amino acids into developing Arabidopsis embryos. Plant J. 59, 540–552. doi: 10.1111/j.1365-313X.2009.03890.x
Schmidt, R., Stransky, H., and Koch, W. (2007). The amino acid permease AAP8 is important for early seed development in Arabidopsis thaliana. Planta 226, 805–813. doi: 10.1007/s00425-007-0527-x
Schmittgen, T. D., and Livak, K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. doi: 10.1038/nprot.2008.73
Schweiger, R., and Schwenkert, S. (2014). Protein-protein interactions visualized by bimolecular fluorescence complementation in tobacco protoplasts and leaves. J. Vis. Exp. 85:51327. doi: 10.3791/51327
Senwo, Z. N., and Tabatabai, M. A. (1998). Amino acid composition of soil organic matter. Biol. Fertil. Soils. 26, 235–242. doi: 10.1007/s003740050373
Shin, K., Lee, S., Song, W. Y., Lee, R. A., Lee, I., Ha, K., et al. (2015). Genetic identification of ACC-RESISTANT2 reveals involvement of LYSINE HISTIDINE TRANSPORTER1 in the uptake of 1-aminocyclopropane-1-carboxylic acid in Arabidopsis thaliana. Plant Cell Physiol. 56, 572–582. doi: 10.1093/pcp/pcu201
Svennerstam, H., Ganeteg, U., and Näsholm, T. (2008). Root uptake of cationic amino acids by Arabidopsis depends on functional expression of amino acid permease 5. New Phytol. 180, 620–630. doi: 10.1111/j.1469-8137.2008.02589.x
Tegeder, M. (2012). Transporters for amino acids in plant cells: Some functions and many unknowns. Curr. Opin. Plant Biol. 15, 315–321. doi: 10.1016/j.pbi.2012.02.001
Tegeder, M. (2014). Transporters involved in source to sink partitioning of amino acids and ureides: Opportunities for crop improvement. J. Exp. Bot. 65, 1865–1878. doi: 10.1093/jxb/eru012
Tegeder, M., and Ward, J. M. (2012). Molecular Evolution of Plant AAP and LHT Amino Acid Transporters. Front. Plant Sci. 3:21. doi: 10.3389/fpls.2012.00021
Vision Todd, J., Brown Daniel, G., and Tanksley Steven, D. (2000). The Origins of Genomic Duplications in Arabidopsis. Science 290, 2114–2117. doi: 10.1126/science.290.5499.2114
Wang, G., Wang, P., Gao, Y., Li, Y., Wu, L., Gao, J., et al. (2018). Isolation and functional characterization of a novel FLOWERING LOCUS T homolog (NtFT5) in Nicotiana tabacum. J. Plant Physiol. 231, 393–401. doi: 10.1016/j.jplph.2018.10.021
Wang, J., Wu, B., Lu, K., Wei, Q., Qian, J., Chen, Y., et al. (2019). The Amino Acid Permease 5 (OsAAP5) Regulates Tiller Number and Grain Yield in Rice. Plant Physiol. 180, 1031–1045. doi: 10.1104/pp.19.00034
Wang, X., Yang, G., Shi, M., Hao, D., Wei, Q., Wang, Z., et al. (2019). Disruption of an amino acid transporter LHT1 leads to growth inhibition and low yields in rice. BMC Plant Biol. 19:268. doi: 10.1186/s12870-019-1885-9
Weigelt, A., Bol, R., and Bardgett, R. D. (2005). Preferential uptake of soil nitrogen forms by grassland plant species. Oecologia 142, 627–635. doi: 10.1007/s00442-004-1765-2
Xie, X., Cao, P., Wang, Z., Gao, J., Wu, M., Li, X., et al. (2021). Genome-wide characterization and expression profiling of the PDR gene family in tobacco (Nicotiana tabacum). Gene 788:145637. doi: 10.1016/j.gene.2021.145637
Xu, G., Guo, C., Shan, H., and Kong, H. (2012). Divergence of duplicate genes in exon-intron structure. Proc. Natl. Acad. Sci. U.S.A. 109, 1187–1192. doi: 10.1073/pnas.1109047109
Zhang, L., Tan, Q., Lee, R., Trethewy, A., Lee, Y.-H., and Tegeder, M. (2010). Altered xylem-phloem transfer of amino acids affects metabolism and leads to increased seed yield and oil content in Arabidopsis. Plant Cell 22, 3603–3620. doi: 10.1105/tpc.110.073833
Zhang, Z., Chen, J., Liang, C., Liu, F., Hou, X., and Zou, X. (2020). Genome-Wide Identification and Characterization of the bHLH Transcription Factor Family in Pepper (Capsicum annuum L.). Front. Genet. 11:570156. doi: 10.3389/fgene.2020.570156
Keywords: Nicotiana tabacum, LHT, bioinformatics, expression profile, amino acids
Citation: Li Z, Gao J, Wang S, Xie X, Wang Z, Peng Y, Yang X, Pu W, Wang Y and Fan X (2022) Comprehensive analysis of the LHT gene family in tobacco and functional characterization of NtLHT22 involvement in amino acids homeostasis. Front. Plant Sci. 13:927844. doi: 10.3389/fpls.2022.927844
Received: 11 May 2022; Accepted: 15 August 2022;
Published: 13 September 2022.
Edited by:
Weiqiang Li, RIKEN, JapanReviewed by:
Chengjian Xie, Chongqing Normal University, ChinaMuhammad Waseem, Riphah International University, Pakistan
Copyright © 2022 Li, Gao, Wang, Xie, Wang, Peng, Yang, Pu, Wang and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaofu Wang, yaofuwang@163.com; Xiaorong Fan, xiaorongfan@njau.edu.cn
†These authors have contributed equally to this work
 Zhaowu Li
Zhaowu Li Junping Gao
Junping Gao Shuaibin Wang
Shuaibin Wang Xiaodong Xie
Xiaodong Xie Zhangying Wang4
Zhangying Wang4 Yaofu Wang
Yaofu Wang Xiaorong Fan
Xiaorong Fan