- 1Department of Health Sciences, University “Magna Graecia” of Catanzaro, Catanzaro, Italy
- 2Department of Biotechnology, Dublin City University, Dublin, Ireland
- 3Pharmacotechnology Documentation and Transfer Unit, Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, Rende, Italy
- 4Department of Neurology, Headache Center, Regional Hospital “Pugliese-Ciaccio”, Catanzaro, Italy
- 5Pain Therapy Center, Provincial Health Authority (ASP), Cosenza, Italy
- 6Regional Center for Serious Brain Injuries, S. Anna Institute, Crotone, Italy
- 7Department of Brain and Behavioral Sciences, IRCCS C. Mondino Foundation Neurologic Institute, University of Pavia, Pavia, Italy
- 8German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany
Chronic migraine is a disabling neurovascular disorder that ranks amongst the top causes of years lived with disability worldwide. The duration and the frequency of migraine affect cognitive and affective domains, inducing worsening of memory, executive functions, orientation and causing anxiety. Population-based studies report a worrying level of resistance to treatments. Therefore, this study aims: 1) to assess efficacy of monoclonal antibodies (mAbs) directed towards the calcitonin gene-related peptide (CGRP) or its receptor (CGRP-R) for chronic migraine resistant to current preventatives; 2) to design a clinical trial protocol to evaluate the efficacy and safety of combination therapy utilizing anti-CGRP/CGRP-R together with onabotulinumtoxin A in patients suffering from resistant chronic migraine; 3) to provide a molecular rationale for combination therapy. A controlled trial is warranted as pooled analysis of real-world data from our group highlighted that combined treatment provides ≥50% reduction vs. baseline (onabotulinumtoxin A) of monthly headache days (MHDs) in up to 58.8% of patients, but there has been only sparse application of this combined therapy to date. The mAbs chosen are: erenumab, because its combination effect with onabotulinumtoxin A improved symptoms in 65% of patients; eptinezumab, due to its faster action. The results highlight that early diagnosis of migraine improves therapeutic outcomes with mAbs alone, confirming their effectiveness and the need for an adequately powered clinical trial evaluating the safety and potential superior effectiveness of eptinezumab/erenumab and onabotulinumtoxin A together.
1 Introduction
1.1 Migraine and refractoriness
Migraine is a disabling, primary headache endowed with a serious social impact, ranking as one of the top causes of years lived with disability worldwide, particularly in people under fifty (Steiner et al., 2018), is 3-to-4 times more frequent in females than in males (Rossi et al., 2022). Chronic migraine is a disease characterized by episodic manifestations (Haut et al., 2006), which the International Classification of Headache Disorders (ICHD, third revision) beta diagnostic criteria defines as at least 15 headache days per month, of which 8 days present the features of migraine, for 3 months consecutively (Headache Classification Committee of the International Headache Society, 2013). The social burden of chronic migraine is increased by its remarkable undertreatment and a high prevalence of resistance to current treatments such as the widely used triptans that stimulate serotonin receptors and the anti-epileptic topiramate which suppresses electrical overactivity in the central nervous system (Schulman et al., 2008). The mechanisms causing resistance to treatments have not yet been elucidated, but a role for genetic polymorphisms has been highlighted (Scuteri et al., 2021b). The duration and the frequency of migraine correlates with harm in both the cognitive and affective domains, damage to memory, executive functions and of orientation, as assessed through the Rey–Osterrieth complex figure test (ROCF) and the Montreal Cognitive Assessment (MoCA), and causing anxiety (Huang et al., 2017). Reduction of monthly headache days (MHDs) and monthly migraine days (MMDs) is a main goal of treatments, but the difficulty in treating chronic migraine in a large proportion of patients has prompted the development of novel therapies.
1.2 Game-changing novel therapies
The discovery of the involvement of the calcitonin gene-related peptide (CGRP) in the pathogenesis of migraine (Scuteri et al., 2019a) fostered the advance of novel specific small molecules (Scuteri et al., 2022b; Scuteri et al., 2022c) and biotechnological drugs (Scuteri et al., 2019b; Scuteri and Bagetta, 2021) targeting this peptide (Scuteri et al., 2020). Onabotulinumtoxin A has been approved since 2010 for the treatment of chronic migraine relying on data from the Phase III Research Evaluating Migraine Prophylaxis Therapy (PREEMPT) clinical program (Diener et al., 2010; Aurora et al., 2011; Aurora et al., 2014). This drug inhibits the exocytosis of CGRP from primary sensory neurons, as well as the release of several other neurotransmitters (Dolly, 2003; Aoki, 2005) by cleavage of the 25 kDa synaptosomal-associated protein (SNAP-25) (Welch et al., 2000). It is effective, well-tolerated (Herd et al., 2018) and despite a lack of homogeneous and long-term data, the available results indicate that it is safer than one of the most commonly used preventative drugs, topiramate. In fact, topiramate was reported to be associated to the highest rate of drop-out in comparison with onabotulinumtoxin A and the most novel antibodies (Frank et al., 2021) and with teratogenicity (Rothrock et al., 2019). Hence, more trials are needed to assess the effect of topiramate in chronic migraine with medication overuse (Giri et al., 2023). Side effects of onabotulinumtoxinA are rare, mild, self-limiting and usually resolve within a short time when used as directed by the label, but the potential for unwanted neuromuscular and/or autonomic side-effects precludes increasing the doses used above those specified in the label instructions. The most novel biotechnological drugs (authorized between 2018 and 2020) are the specific monoclonal antibodies (mAbs) directed towards CGRP (known as eptinezumab, galcanezumab and fremanezumab) or its receptor complex (erenumab). However, 38% of patients that failed all the available preventative drugs also did not respond to erenumab after 6 months of treatment (Lambru et al., 2020; Sacco et al., 2021). Such a large refractory group prompted the investigation of a treatment regime combining onabotulinumtoxin A and anti-CGRP mAbs.
1.3 Combined therapy with onabotulinumtoxin A and mAbs targeting the CGRP machinery
This afforded a pooled ≥50% reduction of MHDs with respect to baseline (onabotulinumtoxin A injections (Toni et al., 2021) of ≥ 2 consecutive cycles of (Blumenfeld et al., 2021; Mechtler et al., 2022)) in up to 58.8% of patients, with a decline of 35.5% after the 6th month (Scuteri et al., 2022d). Moreover, the combined therapy was more effective than erenumab, administered alone or with other preventative drugs, with the efficacy being prolonged by an average of 2 weeks, a fundamental improvement for refractory patients (Ailani and Blumenfeld, 2022). Nevertheless, head-to-head comparisons are still needed (Lu et al., 2021). The recent American Headache Society (AHS) consensus statement of 2021 (Ailani et al., 2021) reported the possible efficacy of the combination of CGRP-targeted mAbs and onabotulinumtoxin A for patients suffering from continued migraine and disability on a single preventive treatment, occurring when experiencing ≥4 MMDs with at least moderate disability, assessed as Migraine Disability Assessment ≥11, Headache Impact Test >50, or ≥8 MMDs (Ailani and Blumenfeld, 2022). Therefore, the purposes of the present study are: 1) to assess the resistance of chronic migraine to current preventative drugs and the consequent use and efficacy of the anti-CGRP/CGRP-R mAbs in a real-world setting; 2) to design a clinical trial protocol to evaluate the effectiveness and tolerability of combination therapy for resistant chronic migraine utilizing anti-CGRP/CGRP-R together with onabotulinumtoxin A; 3) to provide a molecular rationale for such combination therapy.
2 Methods
2.1 Objectives of the retrospective phase
This retrospective study was conducted in collaboration with the Pain Therapy Center of the Provincial Health Authority of Cosenza (Calabria, Italy). Anonymized data were collected concerning the following aspects: demographic characteristics of the patients; diagnosis of chronic migraine according to the ICHD 3 beta; failed preventative treatments; anti-CGRP mAbs administered; baseline MMDs; reduction of MMDs after 1, 3 and 6 months of treatment with mAbs; decrease of pain intensity measured by the numeric rating scale (NRS) after 6 months of treatment. The district consists of 298,000 inhabitants, 213,000 under 60 years of age, i.e., a sample typical for migraine occurrence. The patients enrolled in the study had no concomitant pathologies and were not undergoing concurrent treatments. The need for written informed consent and ethical approval was waived owing to the retrospective use of anonymized data only. The study was conducted in accordance with the Declaration of Helsinki.
2.2 Trial design
The trial assessing the efficacy and safety of the combined treatment with onabotulinumtoxin A and either erenumab or eptinezumab will be a double-blind, randomized single-center trial recruiting patients eligible for the intervention, i.e., those suffering from chronic migraine (according to the ICHD 3 beta) that are refractory to the most commonly used preventative treatments (see below for details). The patients allocated to the combination therapy arm of the study will be assigned randomly to erenumab or eptinezumab subgroups, whereas those allotted to the control arm will continue with their usual treatment. The study protocol included implements the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Checklist (Chan et al., 2013) and the Consolidated Standards of Reporting Trials (CONSORT) statement (Moher et al., 2010). It has been submitted for approval to the Calabria Region Ethics Committee and a request has been made for a ClinicalTrials.gov ID. According to the D.lgs 196/2003, the Helsinki agreements and subsequent amendments, the Good Clinical Practice and Guidelines for the treatment of personal data in clinical trials of 24 July 2008, and in accordance with European data protection legislation, each participant or his/her legal representative will be required to sign a consent form as acceptance of all aspects of the study contained in the patient information sheet and as a consequent expression of his/her willingness to participate in the study. The information sheet will be explained to the subjects or legal representatives by the study staff and the same staff will ensure that the consent form is properly signed and dated by all the parties involved before any procedure detailed in the protocol is carried out. Information on opt-out will be provided to the subjects or legal representatives by the study staff. The primary endpoint will be the mean change in MMDs from the baseline phase after 1, 3, 6, 9 and 12 months of treatment. The secondary endpoints are as follows: mean MHDs; numeric rating scale (NRS); six-item Headache Impact Test (HIT-6™); migraine disability assessment (MIDAS); MoCA; GAD-7; blood tests; anti-drug antibodies (ADA); safety end points that, according to usual safety assessment in clinical trials for chronic migraine with anti-CGRP mAbs, include patient incidences or exposure-adjusted patient incidences of adverse events (AEs) and serious AEs, identifying cardiovascular and cerebrovascular risk factors, such as diabetes, at baseline (Ashina et al., 2022). A complete CONSORT flow diagram for the study is reported in Figure 1.
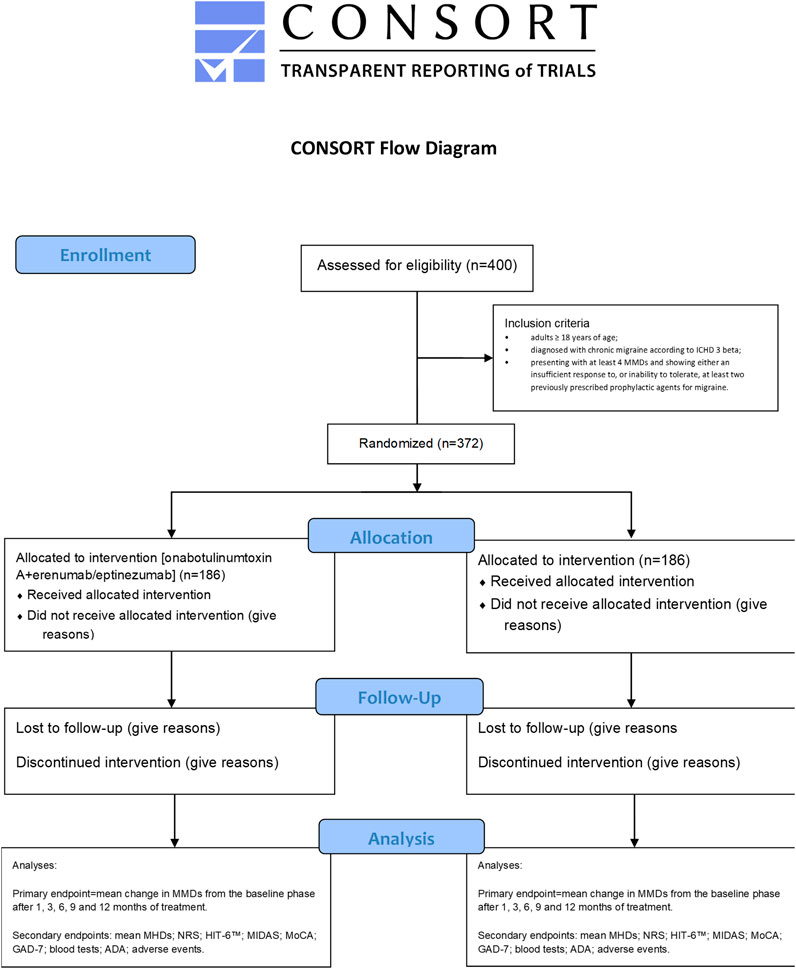
FIGURE 1. Consolidated Standards of Reporting Trials (CONSORT) flow diagram of the proposed clinical trial.
2.3 Inclusion criteria
Patients eligible for inclusion in the present clinical trial are:
• adults ≥ 18 years of age;
• diagnosed with chronic migraine according to ICHD 3 beta;
• presenting with at least 4 MMDs and showing either an insufficient response to, or inability to tolerate, at least two previously prescribed prophylactic agents for migraine.
2.4 Data analysis
Data for the retrospective observational study were extracted from anonymized migraine diaries of the patients and case report forms, then collated using Microsoft Office Excel 2010 (Microsoft, Milan, Italy). Statistical analyses on data expressed as percentage of reduction relative to baseline were performed using GraphPad Prism® 6.0 (GraphPad software Incorporated, San Diego, CA, United States). The results were statistically evaluated for differences using χ2 test for categorical variables considering p < 0.05 significant. The prespecified statistical analysis plan (SAP) for the combination therapy clinical trial consists of an assessment of differences for both the primary and the secondary endpoint measures, according to the calculation of the least-squares mean at each timepoint, evaluated through a linear mixed effects model including all the patient-level variables (Tepper et al., 2017).
3 Results
3.1 Effectiveness of anti-CGRP/R mAbs in a small sample in the real-world setting
The present retrospective, observational study identified n = 10 patients (9 females and 1 male) aged 38–66 years at the time of data collection (2022) that met the inclusion criteria after referral to the Pain Therapy Center of the Provincial Health Authority of Cosenza (Calabria, Italy) in the preceding decade (2010–2022). The gender distribution in this cohort is compatible with literature reporting that migraine is 3-to-4 times more frequent in females than in males (Rossi et al., 2022). The baseline MHDs and MMDs ranged from 15 to 25 and all the patients suffered from chronic migraine without aura (Table 1). Baseline migraine pain intensity was moderate to severe with NRS values from 7 to 10. The patients had all used two or three preventative drugs for an average of 7 years, and failed in the prophylaxis of chronic migraine (patient 017 even showed a worsening of MMDs), before then switching to anti-CGRP mAbs. Although patients 015 and 024 displayed a noteworthy reduction in MMDs with preventative drugs, they elected mAb therapy because their post-treatment MMDs remained high. The drugs used were a selection from the following: topiramate, amitriptyline, flunarizine, verapamil, propranolol, levosulpiride and gabapentin (Table 1). The baseline features of the patients recruited are illustrated in Table 1.
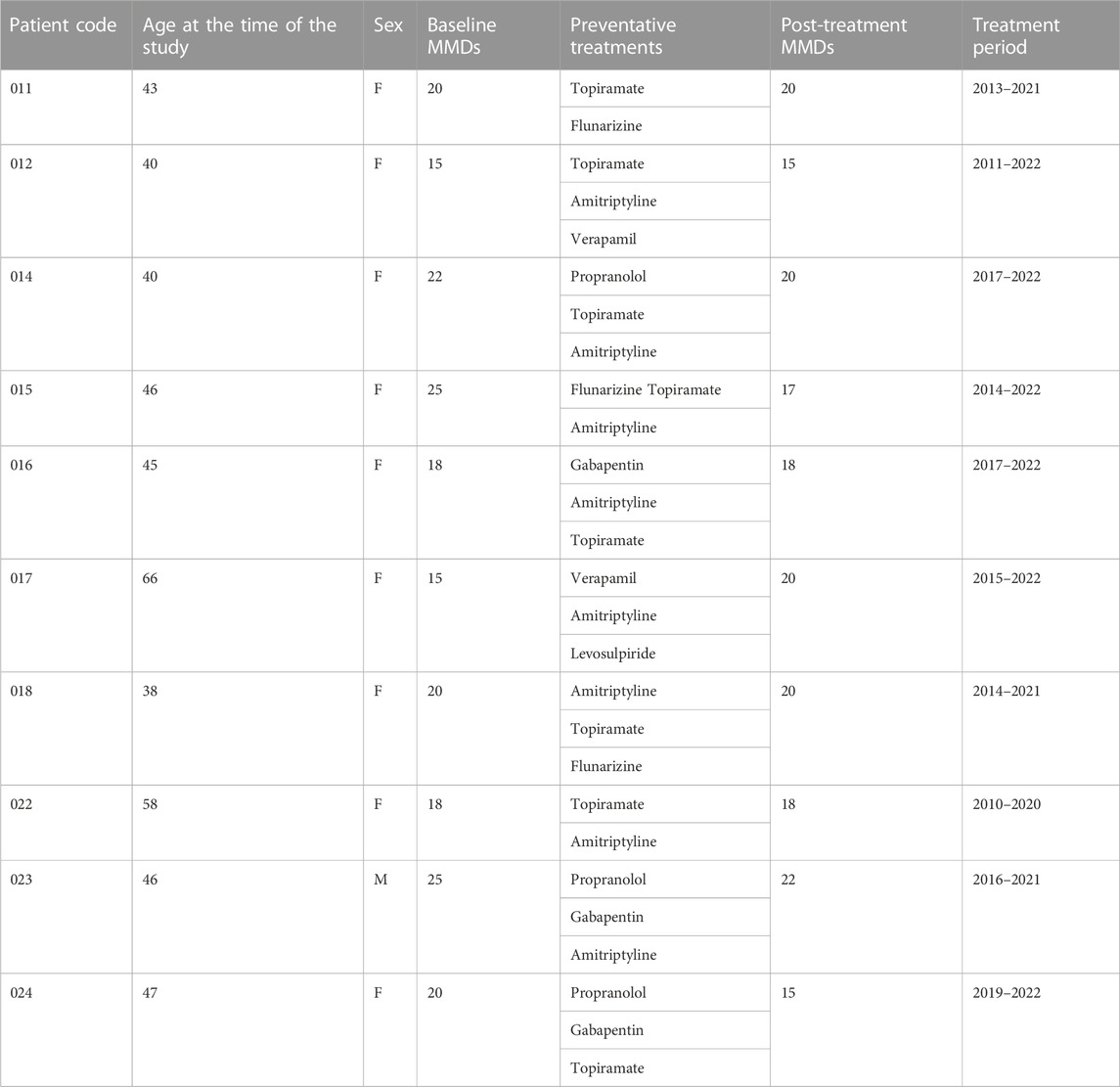
TABLE 1. Baseline characteristics of patients included in the study. The cohort were of predominantly females, with a late age of first chronic migraine observation that failed to achieve an adequate reduction of monthly migraine days (MMDs) with current preventative treatments.
Six out of the 10 patients eligible for anti-CGRP/R mAbs treatment received monthly administrations of erenumab with titration from 70 mg to 140 mg in two cases, while two patients in treatment with 225 mg of fremanezumab monthly and the other 2 patients with monthly galcanezumab titrated from 240 mg loading dose to 120 mg. Data are reported in Table 2. Nine of the ten patients reported a reduction of MMDs after 1 month of treatment and all recorded a reduction upon assessment after 3 and 6 months of treatment, confirming efficacy. Moreover, 8 of the 10 patients scored a reduced pain intensity at 6 months. Interestingly, older patients (017 and 022) showed more resistance to anti-CGRP mAbs in terms of a less pronounced reduction of MMDs with respect to the whole sample, although the possible age-dependency for treatment outcomes will require further investigation in a larger sample because 2 of the younger patients (012 and 023) also exhibited similarly small reductions in MMDs. Eptinezumab and onabotulinumtoxin A were not used in this sample taken from real-world clinical practice. A mean 42% reduction of MMDs was observed with erenumab producing a 41.83% decline, fremanezumab 41.5% and galcanezumab 42.5%. Data are summarized in Table 2.
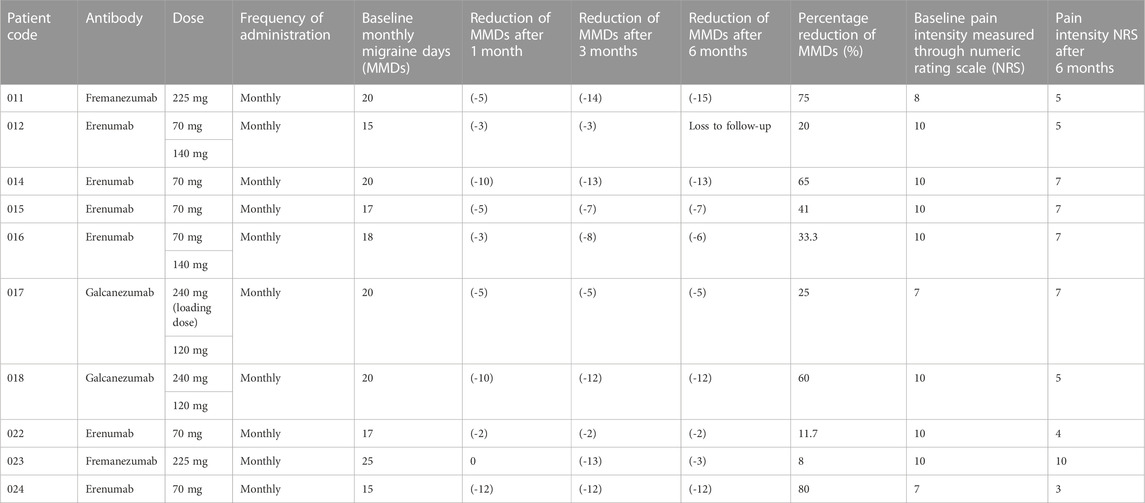
TABLE 2. Reduction of monthly migraine days (MMDs) and of migraine pain intensity (NRS) after 1, 3 and 6 months of treatment with mAbs targeting either the CGRP (fremanezumab or galcanezumab) or the receptor (erenumab).
3.2 Clinical trial protocol for combination therapy of onabotulinumtoxin A and anti-CGRP mAbs
A double-blind, randomized clinical trial will be conducted at the Headache Center of the Regional Hospital “Pugliese-Ciaccio” directed by Dr. Rosario Iannacchero to assess the effectiveness of combination therapy utilizing onabotulinumtoxin A together with an anti-CGRP mAb compared to patients’ continuation with their current treatment with regard to the mAbs to be used in the combination therapy, both erenumab and eptinezumab will be assessed separately in individual subgroups of the study. Applying the structure of the exploratory analysis of patient-reported outcomes (PROs) for superiority of the intervention in the primary endpoint, that consists in the decrease of mean MMDs (from baseline), NCT02066415, a sample size of n = 186 patients for the intervention group is required assuming a treatment effect of −1.9 days with a standard deviation of 6.1, providing 85% power using a two-sample t-test with a two-sided significance level of 0.04 (Tepper et al., 2017). Eligible patients will be randomly assigned in ratio 1:1 to the combination therapy or usual treatment group. Patients in the combined therapy group will receive per label administration of onabotulinumtoxin A and one of either erenumab or eptinezumab. In this way it will be possible to cover all the possible mechanisms, responding either to the inhibition of the signaling of the CGRP ligand (eptinezumab) or its receptor (erenumab) and with the fastest onset of action. Patients, administrators, raters and data analysts will be blinded to the assignments to the intervention or usual care groups, although the administration route can differ. In order to obtain long-term efficacy and safety data the trial will last 52 weeks. Demographic characteristics and baseline information of the patients will be collected through a migraine diary to be completed each day with details including incidence of headache; incidence of migraine with or without aura; time of onset of headache; time to resolution of headache; headache intensity assessed as NRS; pain features; migraine symptoms and most bothersome symptom; use of acute drug treatment during aura or headaches. Baseline assessments will be carried out during the first month immediately before allocation to either of the groups detailed in 2.2. The primary endpoint will be the mean change in MMDs from the baseline phase after 1, 3, 6, 9 and 12 months of treatment. MMDs will be identified based on headache duration, symptoms, pain features and use of migraine-specific drugs use. Migraine is defined as follows: headache (with or without aura) lasting for at least 4 h continuously, with two or more pain features (unilateral, throbbing, moderate to severe intensity, or aggravation by exercise or physical activity) or one or more associated non pain features (nausea, vomiting, or photophobia accompanied by phonophobia) (Tepper et al., 2017). Secondary endpoints include: reduction of mean MHDs; decrease of pain severity measured as NRS; improvement in impact of headache and disability evaluated by the HIT-6™ (Ware et al., 2000; Kosinski et al., 2003; Yang et al., 2011) and the MIDAS (Lipton et al., 2001) scores, respectively; decrease of need for rescue medications; assessment of tolerability. Moreover, MoCA and the Generalized Anxiety Disorder Scale 7-item (GAD-7), validated for migraineurs, will be performed to assess the efficacy of treatment on the cognitive and affective domains (Seo and Park, 2015). Blood tests and searches for neutralizing antibodies against the mAbs will be performed at the beginning and at the end of the study. Any adverse events will be recorded on the case report form during the trial. No sponsor will participate in the trial.
4 Discussion
4.1 Real-world data and delay in diagnosis
According to age-standardized data from the Global Burden of Disease Study 2016, Italy exhibits the highest calculated prevalence of migraine in the world at 20,000 to 21,000 patients per 100,000 inhabitants population (Stovner et al., 2018). Chronic migraine is a debilitating condition consequent to the process of transformation and progression of episodic migraine (Bigal et al., 2006). Therefore, the prevention of migraine chronification and of medication-overuse headache (MOH) is fundamental. For a long time triptans have represented the sole specific treatment for migraine attacks, which offer 2-h sustained freedom from pain to some 18%–50% patients and sustained headache relief at 24 h to some 29%–50% of patients (Cameron et al., 2015). However, the discovery of CGRP as a mediator of chronic migraine pathophysiology (Edvinsson et al., 2018) has caused a revolution in the treatment and prophylaxis of this condition. Data presented herein, gathered from current clinical practice in Calabria in Italy, reports the efficacy of antibody therapies targeting CGRP or its receptor for reducing MHDs, MMDs and NRS pain score. Interestingly, our sample included patients arriving to clinical observation at the age of over 50 years. This highlights a serious delay in diagnosis, in accordance with an underestimation of the problem of migraine (Gupta and Gaurkar, 2022). Notably nine out of the ten patients analyzed showed MMDs decrease after 1 month of treatment and a reduction after 3 and 6 months. Older patients (017 and 022) showed more resistance to anti-CGRP mAbs. Moreover, the eldest patient (at 66 years old) not only proved completely refractory to current prophylactic therapies, the treatments failed to prevent the further deterioration in symptoms, so further investigations into a possible relationship between age and refractory migraine is warranted. Pain processing alters during aging (Hamm and Knisely, 1985; Jourdan et al., 2000; Jourdan et al., 2002) and this could foster new studies investigating the role of natural products with analgesic activity as potentially useful and safe add-on therapies (Scuteri et al., 2021a; Scuteri et al., 2022a). The development of migraine in over 50 year old patients is unusual (Herrero et al., 2013) and, consequently, this population is often neglected from clinical trials for painkillers in general (Bayer and Tadd, 2000). Another issue for refractory migraine treatment, also in the population of over 50-year-old, is MOH induced by the overuse of drugs. By contrast to the experience of the older patients in this study, a recent real-life multicentre analysis of 162 over 65 year-old patients, showing that anti-CGRP mAbs provided a reduction of MMDs ≥ 50% and ≥ 75% to some 57% and 33% patients, respectively (Muñoz-Vendrell et al., 2023). The present clinical practice data analyzed herein highlight a preference to switch patients from non-specific, preventative, small molecules to mAbs rather than onabotulinumtoxin A. Interestingly, no patients were transferred to eptinezumab, in spite of its rapid action. The use of mAbs proved to be effective, but to different extents in individual recipients, a finding that corroborates international data, possibly due to differences in hypothalamic modulation (Torres-Ferrús et al., 2021; Basedau et al., 2022; Iannone et al., 2022). The real-world data on anti-CGRP-mAbs suffer from the following limitations: retrospective collection of data, small sample size, short follow-up periods (Pavelic et al., 2023). This is confirmed by a recent systematic search that included randomized controlled trials reporting the outcomes of change in MHDs, MMDs, ≥50% response rates and change in MOH status (Giri et al., 2023).
4.2 Neuropharmacology of resistant, chronic migraine
As an alternative to mAbs targeting CGRP signalling, onabotulinumtoxin A has been approved for use in chronic migraine since 2010 (Simpson et al., 2016). It proteolyses SNAP-25, one of the proteins required for the membrane fusion reaction that mediates the exocytosis of CGRP, as well as other neuropeptides and various neurotransmitters (Meng et al., 2009; Cernuda-Morollón et al., 2015; Belinskaia et al., 2023). Among these neurotransmitters, it is possible to find acetylcholine, glutamate, pituitary adenylate cyclase activating peptide 38 (PACAP 38) and substance P, inhibition of the trafficking of transient receptor potential cation channel subfamily V member 1 [TRPV1], transient receptor potential cation channel subfamily A member 1 [TRPA1] and purinergic receptor P2X ligand-gated ion channel 3 [P2X3] (Devesa et al., 2014; Meng et al., 2016) is also implicated in the mechanism of action of onabotulinumtoxin A (Burstein et al., 2020). The effect is long-lasting, as supported by the evidence that SNAP-25 is cleaved over 80 days in cultured spinal cord cells (Keller et al., 1999) and a minimum of 3 months is recommended between clinical injections for chronic migraine (BOTOX® label information). Botulinum toxin can be useful in several other types of pain, as including neuropathic. In fact, a double-blind, crossover, pilot trial investigating the effectiveness of botulinum toxin type A for diabetic neuropathy, demonstrated a significant reduction of pain intensity assessed through visual analog scale (VAS) at 1-4-8 and 12 weeks after administration (Yuan et al., 2009). In relation to its mechanism of action, onabotulinumtoxinA reduced interictal plasma levels of CGRP, determined in samples obtained from the right antecubital vein using ELISA, one month after treatment, in chronic migraineurs who are responders to treatment, but not in nonresponders (Cernuda-Morollón et al., 2015). By contrast, the levels of vasoactive intestinal peptide (VIP), investigated in samples obtained from the right antecubital vein by ELISA, were significantly increased in responders (Cernuda-Morollón et al., 2014). The gathered evidence suggests that measurements of the interictal levels of CGRP may be helpful to predict the response to onbaotulinumtoxin A (Cernuda-Morollón et al., 2015), supporting the importance of an additive/synergic mechanism with antibody therapy. The synergic mechanism of the combination in migraine may be related to the inhibition of CGRP release from thin unmyelinated C fiber dural nociceptors produced by onabotulinumtoxin A, while anti-CGRP mAbs prevent the binding of the ligand to its receptor (Figure 2; reproduced with permission from (Pellesi et al., 2020)). This is corroborated by the finding that fremanezumab can prevent the activation of Aδ- but not C-fibers, whilst onabotulinumtoxin A might selectivley interact with C- but not Aδ-fibers, in agreement with distribution of CGRP in C-fibers and CGRP receptors in Aδ-fibers (Strassman et al., 2004; Eftekhari et al., 2013; Pellesi et al., 2020). It has also been proposed that onabotulinumtoxin A reverses mechanical hypersensitivity of sensitized C-units by interference with the expression of high-threshold mechanosensitive ion channels on the surface of nerve cells (Burstein et al., 2014). Thus, onabotulinumtoxin A can be exploited as a multipurpose drug offering long-lasting relief from several forms of pain including migraine (Sandrini et al., 2017; De Icco et al., 2019) and neuropathic pain in experimental conditions (Wang et al., 2017). Accordingly, clinical use of onabotulinumtoxin A has been shown to decrease the need for rescue medications (Sandrini et al., 2011). Chronic migraine is a form of complex, neurological disorder characterized by sensory, cognitive and affective comorbidities, likely due to network disruption (DeSouza et al., 2020), that needs to be prevented to avoid patients’ disability. So to prevent patients’ disability, it is fundamental to provide relief from all these associated modalities. Indeed, a prospective cross-sectional study on 165 patients highlighted that some 89.7% of them experienced cognitive symptoms with consequent dysfunction involved in the attack-related disability (Gil-Gouveia et al., 2016), thus representing a pathological outcome of the utmost importance. This can be, at least in part, due to the perturbations of brain areas important for cognition, i.e., amygdala, hypothalamus, periaqueductal gray (PAG), ventral pontine tegmentum, ventral and dorsal medulla and also spinal cord (Kozlowska et al., 2015; Gil-Gouveia and Martins, 2019). Executive functions, working memory, visual-spatial processing and attention are among the most affected cognitive skills (Gil-Gouveia and Martins, 2019). Cognitive symptoms rank second in the symptoms related to the attack of migraine (Vuralli et al., 2018; Gil-Gouveia and Martins, 2019) and these are accompanied by low-rate depression, anxiety and apathy (Santangelo et al., 2016), supporting the need for long-term assessment of cognitive impairment in chronic migraineurs. CGRP is thought to be a trigger factor for migraine because injections of levels of this neuropeptide can induce migraine-like headache symptoms. Also, in some migraineurs, its levels are elevated (compared to healthy controls) in the inter-ictal phase and have been seen to increase even further during the pre-ictal to headache onset phase (Kamm, 2022, Front. Neurol.). Anti-CGRP mAb therapies, or botulinum toxin injections, are thought to reduce migraine incidence and severity by suppressing interictal and ictal levels of this neuropeptide, thereby, halting migraine progression and alleviating the development of cognitive and affective comorbidity. Hence, the inclusion in the clinical study herein of the MoCA and GAD-7 analyses to investigate the impact of combination therapy on these facets.
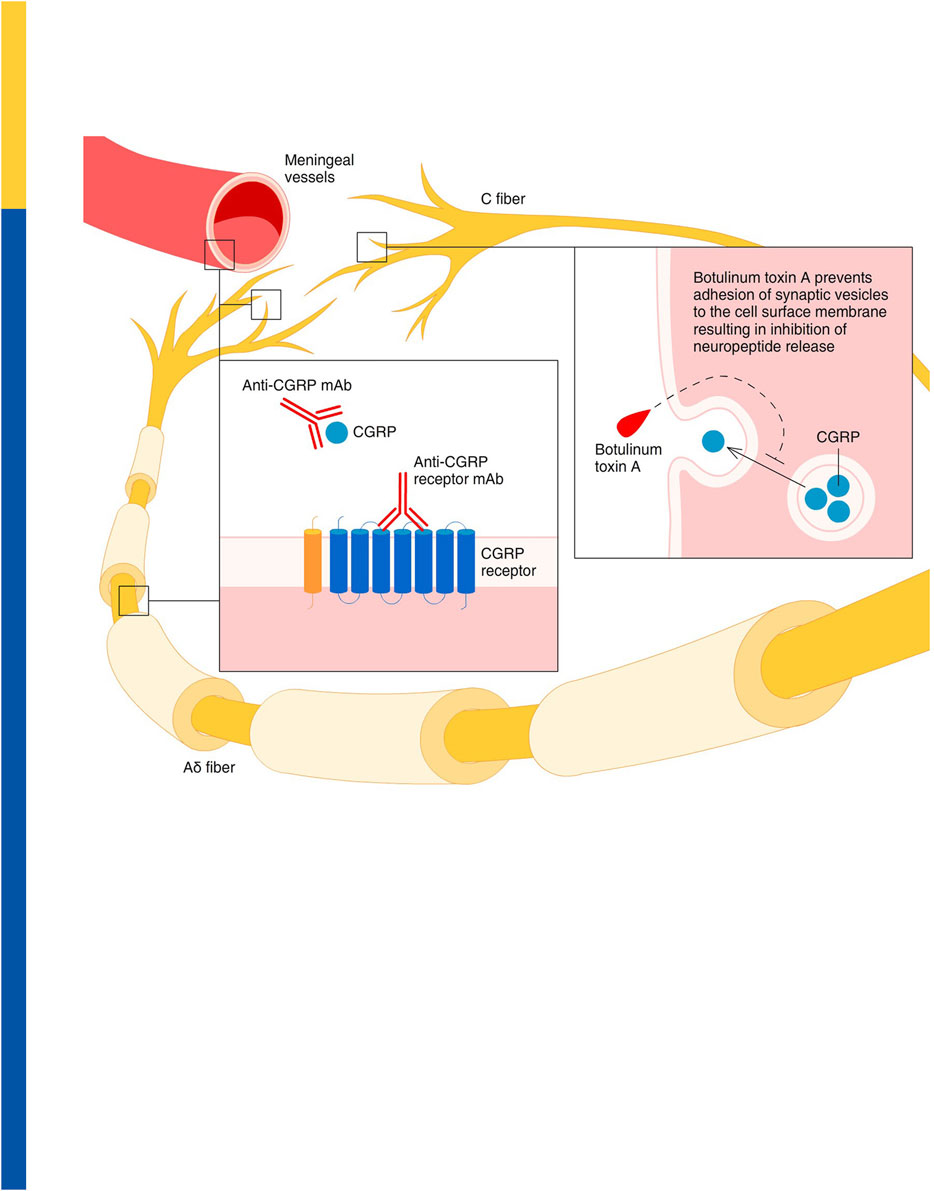
FIGURE 2. Proposed synergic activity of onabotulinumtoxin A and anti-CGRP mAbs in migraine. Onabotulinumtoxin A inhibits the release of CGRP from thin unmyelinated C fiber meningeal nociceptors in the dura, thus preventing a CGRP-dependent activation of meningeal vessels and thick myelinated Aδ nociceptors. At the same time, anti-CGRP mAbs prevent the interaction between the CGRP and its receptor within the meningeal vessel walls, as well as in the extremities and along the fibers in at the nodes of Ranvier of the Aδ nociceptors. Reproduced with permission from (Pellesi et al., 2020).
4.3 Rational basis for co-administration of onabotulinumtoxin A and mAbs directed towards the CGRP ligand or receptor
Notably, retrospective studies indicate that co-administering onabotulinumtoxin A with anti-CGRP/R mAbs can more successfully reduce MMDs and MHDs and prolong the suppression of migraine impact with induced disability than either individual intervention alone. In fact, the combination therapy was associated with statistically significant reductions of 8.1 MHDs (p < 0.001) and of 7.4 MMDs [30% (p < 0.001)] at 90 days (Armanious et al., 2021). In this regard, an interesting study by Blumenfeld and coworkers of 2021 (Blumenfeld et al., 2021) reported a wide primary analysis cohort (n = 257) and sensitivity analysis cohort (n = 172), including only patients suffering from moderate disability defined by MIDAS score>11 or HIT-6TM score >50. This study demonstrated that, after 6–12 months of combined therapy, one-third (31.5%–36.7%) of patients presented a reduction of MHDs ≥50% and a reduction in migraine-related disability ≥30%. Furthermore, the mean MIDAS score for 27.1%–29.6% of the cohort was significantly reduced from baseline by between 6.1 and 11.1 points. Another retrospective study likewise highlighted the effectiveness of onabotulinumtoxin A as an add-on therapy to mAbs in patients suffering from refractory chronic migraine, who failed two oral migraine preventative drugs, three onabotulinumtoxin A cycles and three sessions with either fremanezumab or erenumab delivered sequentially as monotherapies (Argyriou et al., 2022). Furthermore, addition of an anti-CGRP mAb to the treatment for MOH in chronic migraineurs has been recently suggested to reduce headache frequency and symptomatic medication use (Krymchantowski et al., 2023). Although patient persistence with onabotulinumtoxinA is better than that seen with anti-CGRP mAbs (Schwedt et al., 2023), the doses of botulinum toxin A that can be delivered are strictly limited to restrict the unwanted spread of the toxin beyond the treatment area and to preclude the development of potentially debilitating motor and autonomic side-effects. Consideration of all these data together prompted the design of a prospective clinical trial to evaluate the effectiveness and tolerability of combination treatment using mAbs together with onabotulinumtoxin A (Guerzoni et al., 2022). Therefore, here we propose a study protocol for a 52-week, randomized, adequately powered, clinical trial to provide long-term evidence for effectiveness and tolerability of the combined treatment of onabotulinumtoxin A and anti-CGRP mAbs. The mAbs chosen are erenumab, because its combination beneficial effect was demonstrated in some 65% patients (Boudreau, 2020) and eptinezumab due to its faster action. The main goal of this prospective randomized clinical trial is to fill the gap between clinical practice and research due to the still unmet need for wide prospective clinical trials assessing long-term follow-up of combined therapies and to offer a new therapeutic approach for refractory patients. This trial falls in the novel field considering chronic migraine management of patients with frequent and disabling attacks as a multimodal strategy including the control of the following milestones: 1) comorbidities; 2) modifiable risk factors involved in the process of progression as the overuse of medications and of caffeine; 3) secondary headaches; 4) tailored acute and preventative therapies with the aim of reducing pain, allodynia, cognitive and affective impairment and consequent disability (Blumenfeld et al., 2023). Moreover, statistical modeling deserves further investigation since it can represent an important aid in the prediction of synergistic or additive effects of treatments combinations also in acute management of the attacks (Blumenfeld et al., 2012).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The requirement of ethical approval was waived by the need for written informed consent and ethical approval was waived owing to the retrospective use of anonymized data only for the studies involving humans because the studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the need for written informed consent and ethical approval was waived owing to the retrospective use of anonymized data only.
Author contributions
MC: Conceptualization. GL: Conceptualization, Language review. GB: Conceptualization. RI: Methodology, Data curation. AT: Methodology, Data curation. AM: Methodology, Data curation. MP: Methodology, Data curation. PT: Methodology, Data curation. GS: Conceptualization. PN: Conceptualization. DS: Conceptualization, Methodology, Data curation. All authors have read and agreed to the published version of the manuscript.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research is coordinated by DS and received partial financial support from: 1) Phase 2 RIABEO Funding (Executive Decree n.6790 of 22 June 2022) Progetto Ingegno POR Calabria FESR 2014/2020—Azione 1 1 5—Sostegno all’Avanzamento tecnologico delle Imprese Attraverso il Finanziamento di Linee Pilota e Azioni di Validazione Precoce di Prodotti e di Dimostrazione su Larga Scala (DDG N. 12814 DEL 17 October 2019); 2) by the Italian Ministry of Health: NET-2016-02361805 (WP 5).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ailani, J., and Blumenfeld, A. M. (2022). Combination CGRP monoclonal antibody and onabotulinumtoxinA treatment for preventive treatment in chronic migraine. Headache J. Head Face Pain 62 (1), 106–108. doi:10.1111/head.14244
Ailani, J., Burch, R. C., and Robbins, M. S.Board of Directors of the American Headache Society (2021). The American Headache Society Consensus Statement: update on integrating new migraine treatments into clinical practice. Headache 61 (7), 1021–1039. doi:10.1111/head.14153
Aoki, K. R. (2005). Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. NeuroToxicology 26 (5), 785–793. doi:10.1016/j.neuro.2005.01.017
Argyriou, A. A., Dermitzakis, E. V., Xiromerisiou, G., and Vikelis, M. (2022). OnabotulinumtoxinA add-on to monoclonal anti-CGRP antibodies in treatment-refractory chronic migraine. Toxins 14 (12), 847. doi:10.3390/toxins14120847
Armanious, M., Khalil, N., Lu, Y., and Jimenez-Sanders, R. (2021). Erenumab and OnabotulinumtoxinA combination therapy for the prevention of intractable chronic migraine without aura: a retrospective analysis. J. Pain and Palliat. Care Pharmacother. 35 (1), 1–6. doi:10.1080/15360288.2020.1829249
Ashina, M., Goadsby, P. J., Dodick, D. W., Tepper, S. J., Xue, F., Zhang, F., et al. (2022). Assessment of erenumab safety and efficacy in patients with migraine with and without aura: a secondary analysis of randomized clinical trials. JAMA Neurol. 79 (2), 159–168. doi:10.1001/jamaneurol.2021.4678
Aurora, S. K., Dodick, D. W., Diener, H. C., DeGryse, R. E., Turkel, C. C., Lipton, R. B., et al. (2014). OnabotulinumtoxinA for chronic migraine: efficacy, safety, and tolerability in patients who received all five treatment cycles in the PREEMPT clinical program. Acta Neurol. Scand. 129 (1), 61–70. doi:10.1111/ane.12171
Aurora, S. K., Winner, P., Freeman, M. C., Spierings, E. L., Heiring, J. O., DeGryse, R. E., et al. (2011). OnabotulinumtoxinA for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache 51 (9), 1358–1373. doi:10.1111/j.1526-4610.2011.01990.x
Basedau, H., Sturm, L.-M., Mehnert, J., Peng, K.-P., Schellong, M., and May, A. (2022). Migraine monoclonal antibodies against CGRP change brain activity depending on ligand or receptor target – an fMRI study. eLife 11, e77146. doi:10.7554/eLife.77146
Bayer, A., and Tadd, W. (2000). Unjustified exclusion of elderly people from studies submitted to research ethics committee for approval: descriptive study. BMJ 321 (7267), 992–993. doi:10.1136/bmj.321.7267.992
Belinskaia, M., Wang, J., Kaza, S. K., Antoniazzi, C., Zurawski, T., Dolly, J. O., et al. (2023). Bipartite activation of sensory neurons by a TRPA1 agonist allyl isothiocyanate is reflected by complex Ca(2+) influx and CGRP release patterns: enhancement by NGF and inhibition with VAMP and SNAP-25 cleaving botulinum neurotoxins. Int. J. Mol. Sci. 24 (2), 1338. doi:10.3390/ijms24021338
Bigal, M. E., Liberman, J. N., and Lipton, R. B. (2006). Age-dependent prevalence and clinical features of migraine. Neurology 67 (2), 246–251. doi:10.1212/01.wnl.0000225186.76323.69
Blumenfeld, A., Gennings, C., and Cady, R. (2012). Pharmacological synergy: the next frontier on therapeutic advancement for migraine. Headache 52 (4), 636–647. doi:10.1111/j.1526-4610.2011.02058.x
Blumenfeld, A. M., Frishberg, B. M., Schim, J. D., Iannone, A., Schneider, G., Yedigarova, L., et al. (2021). Real-world evidence for control of chronic migraine patients receiving CGRP monoclonal antibody therapy added to OnabotulinumtoxinA: a retrospective chart review. Pain Ther. 10 (2), 809–826. doi:10.1007/s40122-021-00264-x
Blumenfeld, A. M., Lipton, R. B., Silberstein, S., Tepper, S. J., Charleston, L. t., Landy, S., et al. (2023). Multimodal migraine management and the pursuit of migraine freedom: a narrative review. Neurol. Ther. 12, 1533–1551. doi:10.1007/s40120-023-00529-x
Boudreau, G. (2020). Treatment of chronic migraine with erenumab alone or as an add on therapy: a real-world observational study. Anesth. Pain Res. 4, 4. doi:10.33425/2639-846x.1037
Burstein, R., Blumenfeld, A. M., Silberstein, S. D., Manack Adams, A., and Brin, M. F. (2020). Mechanism of action of OnabotulinumtoxinA in chronic migraine: a narrative review. Headache J. Head Face Pain 60 (7), 1259–1272. doi:10.1111/head.13849
Burstein, R., Zhang, X., Levy, D., Aoki, K. R., and Brin, M. F. (2014). Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: therapeutic implications for migraine and other pains. Cephalalgia 34 (11), 853–869. doi:10.1177/0333102414527648
Cameron, C., Kelly, S., Hsieh, S. C., Murphy, M., Chen, L., Kotb, A., et al. (2015). Triptans in the acute treatment of migraine: a systematic review and network meta-analysis. Headache 55 (Suppl. 4), 221–235. doi:10.1111/head.12601
Cernuda-Morollón, E., Martínez-Camblor, P., Ramón, C., Larrosa, D., Serrano-Pertierra, E., and Pascual, J. (2014). CGRP and VIP levels as predictors of efficacy of Onabotulinumtoxin type A in chronic migraine. Headache 54 (6), 987–995. doi:10.1111/head.12372
Cernuda-Morollón, E., Ramón, C., Martínez-Camblor, P., Serrano-Pertierra, E., Larrosa, D., and Pascual, J. (2015). OnabotulinumtoxinA decreases interictal CGRP plasma levels in patients with chronic migraine. Pain 156 (5), 820–824. doi:10.1097/j.pain.0000000000000119
Chan, A. W., Tetzlaff, J. M., Altman, D. G., Laupacis, A., Gøtzsche, P. C., Krleža-Jerić, K., et al. (2013). SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann. Intern. Med. 158 (3), 200–207. doi:10.7326/0003-4819-158-3-201302050-00583
De Icco, R., Perrotta, A., Berra, E., Allena, M., Alfonsi, E., Tamburin, S., et al. (2019). OnabotulinumtoxinA reduces temporal pain processing at spinal level in patients with lower limb spasticity. Toxins 11 (6), 359. doi:10.3390/toxins11060359
DeSouza, D. D., Woldeamanuel, Y. W., Sanjanwala, B. M., Bissell, D. A., Bishop, J. H., Peretz, A., et al. (2020). Altered structural brain network topology in chronic migraine. Brain Struct. Funct. 225 (1), 161–172. doi:10.1007/s00429-019-01994-7
Devesa, I., Ferrándiz-Huertas, C., Mathivanan, S., Wolf, C., Luján, R., Changeux, J. P., et al. (2014). αCGRP is essential for algesic exocytotic mobilization of TRPV1 channels in peptidergic nociceptors. Proc. Natl. Acad. Sci. U. S. A. 111 (51), 18345–18350. doi:10.1073/pnas.1420252111
Diener, H. C., Dodick, D. W., Aurora, S. K., Turkel, C. C., DeGryse, R. E., Lipton, R. B., et al. (2010). OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 30 (7), 804–814. doi:10.1177/0333102410364677
Dolly, O. (2003). Synaptic transmission: inhibition of neurotransmitter release by botulinum toxins. Headache J. Head Face Pain 43 (s1), 16–24. doi:10.1046/j.1526-4610.43.7s.4.x
Edvinsson, L., Haanes, K. A., Warfvinge, K., and Krause, D. N. (2018). CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat. Rev. Neurol. 14 (6), 338–350. doi:10.1038/s41582-018-0003-1
Eftekhari, S., Warfvinge, K., Blixt, F. W., and Edvinsson, L. (2013). Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J. Pain 14 (11), 1289–1303. doi:10.1016/j.jpain.2013.03.010
Frank, F., Ulmer, H., Sidoroff, V., and Broessner, G. (2021). CGRP-antibodies, topiramate and botulinum toxin type A in episodic and chronic migraine: a systematic review and meta-analysis. Cephalalgia 41 (11-12), 1222–1239. doi:10.1177/03331024211018137
Gil-Gouveia, R., and Martins, I. P. (2019). Cognition and cognitive impairment in migraine. Curr. Pain Headache Rep. 23 (11), 84. doi:10.1007/s11916-019-0824-7
Gil-Gouveia, R., Oliveira, A. G., and Martins, I. P. (2016). Subjective cognitive symptoms during a migraine attack: a prospective study of a clinic-based sample. Pain Physician 19 (1), E137–E150. doi:10.36076/ppj/2016.19.e137
Giri, S., Tronvik, E., Linde, M., Pedersen, S. A., and Hagen, K. (2023). Randomized controlled studies evaluating Topiramate, Botulinum toxin type A, and mABs targeting CGRP in patients with chronic migraine and medication overuse headache: a systematic review and meta-analysis. Cephalalgia 43 (4), 3331024231156922. doi:10.1177/03331024231156922
Guerzoni, S., Baraldi, C., and Pani, L. (2022). The association between onabotulinumtoxinA and anti-CGRP monoclonal antibodies: a reliable option for the optimal treatment of chronic migraine. Neurol. Sci. 43 (9), 5687–5695. doi:10.1007/s10072-022-06195-5
Gupta, J., and Gaurkar, S. S. (2022). Migraine: an underestimated neurological condition affecting billions. Cureus 14 (8), e28347. doi:10.7759/cureus.28347
Hamm, R. J., and Knisely, J. S. (1985). Environmentally induced analgesia: an age-related decline in an endogenous opioid system. J. Gerontol. 40 (3), 268–274. doi:10.1093/geronj/40.3.268
Haut, S. R., Bigal, M. E., and Lipton, R. B. (2006). Chronic disorders with episodic manifestations: focus on epilepsy and migraine. Lancet Neurol. 5 (2), 148–157. doi:10.1016/s1474-4422(06)70348-9
Headache Classification Committee of the International Headache Society (IHS) (2013). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33 (9), 629–808. doi:10.1177/0333102413485658
Herd, C. P., Tomlinson, C. L., Rick, C., Scotton, W. J., Edwards, J., Ives, N., et al. (2018). Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst. Rev. 6 (6), Cd011616. doi:10.1002/14651858.CD011616.pub2
Herrero, S., Guerrero, A. L., Ruiz, M., Pedraza, M. I., Mulero, P., Barón, J., et al. (2013). Migraine in the elderly: clinical characteristics in a series of 71 cases. J. Headache Pain 14 (1), P152. doi:10.1186/1129-2377-14-S1-P152
Huang, L., Juan Dong, H., Wang, X., Wang, Y., and Xiao, Z. (2017). Duration and frequency of migraines affect cognitive function: evidence from neuropsychological tests and event-related potentials. J. Headache Pain 18 (1), 54. doi:10.1186/s10194-017-0758-6
Iannone, L. F., Fattori, D., Benemei, S., Chiarugi, A., Geppetti, P., and De Cesaris, F. (2022). Long-term effectiveness of three anti-CGRP monoclonal antibodies in resistant chronic migraine patients based on the MIDAS score. CNS Drugs 36 (2), 191–202. doi:10.1007/s40263-021-00893-y
Jourdan, D., Boghossian, S., Alloui, A., Veyrat-Durebex, C., Coudore, M. A., Eschalier, A., et al. (2000). Age-related changes in nociception and effect of morphine in the Lou rat. Eur. J. Pain 4 (3), 291–300. doi:10.1053/eujp.2000.0188
Jourdan, D., Pickering, G., Marchand, F., Gaulier, J. M., Alliot, J., and Eschalier, A. (2002). Impact of ageing on the antinociceptive effect of reference analgesics in the Lou/c rat. Br. J. Pharmacol. 137 (6), 813–820. doi:10.1038/sj.bjp.0704944
Keller, J. E., Neale, E. A., Oyler, G., and Adler, M. (1999). Persistence of botulinum neurotoxin action in cultured spinal cord cells. FEBS Lett. 456 (1), 137–142. doi:10.1016/s0014-5793(99)00948-5
Kosinski, M., Bayliss, M. S., Bjorner, J. B., Ware, J. E., Garber, W. H., Batenhorst, A., et al. (2003). A six-item short-form survey for measuring headache impact: the HIT-6. Qual. Life Res. 12 (8), 963–974. doi:10.1023/a:1026119331193
Kozlowska, K., Walker, P., McLean, L., and Carrive, P. (2015). Fear and the defense cascade: clinical implications and management. Harv Rev. Psychiatry 23 (4), 263–287. doi:10.1097/hrp.0000000000000065
Krymchantowski, A. V., Jevoux, C., Krymchantowski, A. G., and Silva-Néto, R. P. (2023). Monoclonal antibodies for chronic migraine and medication overuse headache: a real-world study. Front. Neurology 14, 1129439. doi:10.3389/fneur.2023.1129439
Lambru, G., Hill, B., Murphy, M., Tylova, I., and Andreou, A. P. (2020). A prospective real-world analysis of erenumab in refractory chronic migraine. J. Headache Pain 21 (1), 61. doi:10.1186/s10194-020-01127-0
Lipton, R. B., Stewart, W. F., Sawyer, J., and Edmeads, J. G. (2001). Clinical utility of an instrument assessing migraine disability: the migraine disability assessment (MIDAS) questionnaire. Headache J. Head Face Pain 41 (9), 854–861. doi:10.1111/j.1526-4610.2001.01156.x
Lu, J., Zhang, Q., Guo, X., Liu, W., Xu, C., Hu, X., et al. (2021). Calcitonin gene–related peptide monoclonal antibody versus botulinum toxin for the preventive treatment of chronic migraine: evidence from indirect treatment comparison. Front. Pharmacol. 12, 631204. doi:10.3389/fphar.2021.631204
Mechtler, L., Saikali, N., McVige, J., Hughes, O., Traut, A., and Adams, A. M. (2022). Real-world evidence for the safety and efficacy of CGRP monoclonal antibody therapy added to OnabotulinumtoxinA treatment for migraine prevention in adult patients with chronic migraine. Front. Neurology 12, 788159. doi:10.3389/fneur.2021.788159
Meng, J., Ovsepian, S. V., Wang, J., Pickering, M., Sasse, A., Aoki, K. R., et al. (2009). Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J. Neurosci. 29 (15), 4981–4992. doi:10.1523/jneurosci.5490-08.2009
Meng, J., Wang, J., Steinhoff, M., and Dolly, J. O. (2016). TNFα induces co-trafficking of TRPV1/TRPA1 in VAMP1-containing vesicles to the plasmalemma via Munc18-1/syntaxin1/SNAP-25 mediated fusion. Sci. Rep. 6, 21226. doi:10.1038/srep21226
Moher, D., Hopewell, S., Schulz, K. F., Montori, V., Gøtzsche, P. C., Devereaux, P. J., et al. (2010). CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 340, c869. doi:10.1136/bmj.c869
Muñoz-Vendrell, A., Campoy, S., Caronna, E., Alpuente, A., Torres-Ferrus, M., Nieves Castellanos, C., et al. (2023). Effectiveness and safety of anti-CGRP monoclonal antibodies in patients over 65 years: a real-life multicentre analysis of 162 patients. J. Headache Pain 24 (1), 63. doi:10.1186/s10194-023-01585-2
Pavelic, A. R., Wöber, C., Riederer, F., and Zebenholzer, K. (2023). Monoclonal antibodies against calcitonin gene-related peptide for migraine prophylaxis: a systematic review of real-world data. Cells 12 (1), 143. doi:10.3390/cells12010143
Pellesi, L., Do, T. P., Ashina, H., Ashina, M., and Burstein, R. (2020). Dual therapy with anti-CGRP monoclonal antibodies and botulinum toxin for migraine prevention: is there a rationale? Headache J. Head Face Pain 60 (6), 1056–1065. doi:10.1111/head.13843
Rossi, M. F., Tumminello, A., Marconi, M., Gualano, M. R., Santoro, P. E., Malorni, W., et al. (2022). Sex and gender differences in migraines: a narrative review. Neurol. Sci. 43 (9), 5729–5734. doi:10.1007/s10072-022-06178-6
Rothrock, J. F., Adams, A. M., Lipton, R. B., Silberstein, S. D., Jo, E., Zhao, X., et al. (2019). FORWARD study: evaluating the comparative effectiveness of OnabotulinumtoxinA and topiramate for headache prevention in adults with chronic migraine. Headache 59 (10), 1700–1713. doi:10.1111/head.13653
Sacco, S., Lampl, C., Maassen van den Brink, A., Caponnetto, V., Braschinsky, M., Ducros, A., et al. (2021). Burden and attitude to resistant and refractory migraine: a survey from the European Headache Federation with the endorsement of the European Migraine and Headache Alliance. J. Headache Pain 22 (1), 39. doi:10.1186/s10194-021-01252-4
Sandrini, G., De Icco, R., Tassorelli, C., Smania, N., and Tamburin, S. (2017). Botulinum neurotoxin type A for the treatment of pain: not just in migraine and trigeminal neuralgia. J. Headache Pain 18 (1), 38. doi:10.1186/s10194-017-0744-z
Sandrini, G., Perrotta, A., Tassorelli, C., Torelli, P., Brighina, F., Sances, G., et al. (2011). Botulinum toxin type-A in the prophylactic treatment of medication-overuse headache: a multicenter, double-blind, randomized, placebo-controlled, parallel group study. J. Headache Pain 12 (4), 427–433. doi:10.1007/s10194-011-0339-z
Santangelo, G., Russo, A., Trojano, L., Falco, F., Marcuccio, L., Siciliano, M., et al. (2016). Cognitive dysfunctions and psychological symptoms in migraine without aura: a cross-sectional study. J. Headache Pain 17 (1), 76. doi:10.1186/s10194-016-0667-0
Schulman, E. A., Lake, A. E., Goadsby, P. J., Peterlin, B. L., Siegel, S. E., Markley, H. G., et al. (2008). Defining refractory migraine and refractory chronic migraine: proposed criteria from the refractory headache special interest section of the American headache society. Headache J. Head Face Pain 48 (6), 778–782. doi:10.1111/j.1526-4610.2008.01132.x
Schwedt, T. J., Lee, J., Knievel, K., McVige, J., Wang, W., Wu, Z., et al. (2023). Real-world persistence and costs among patients with chronic migraine treated with onabotulinumtoxinA or calcitonin gene-related peptide monoclonal antibodies. J. Manag. Care Spec. Pharm. 29 (10), 1119–1128. doi:10.18553/jmcp.2023.29.10.1119
Scuteri, D., Adornetto, A., Rombolà, L., Naturale, M. D., De Francesco, A. E., Esposito, S., et al. (2020). Pattern of triptans use: a retrospective prescription study in Calabria, Italy. Neural Regen. Res. 15 (7), 1340–1343. doi:10.4103/1673-5374.272630
Scuteri, D., Adornetto, A., Rombolà, L., Naturale, M. D., Morrone, L. A., Bagetta, G., et al. (2019a). New trends in migraine Pharmacology: targeting calcitonin gene-related peptide (CGRP) with monoclonal antibodies. Front. Pharmacol. 10, 363. doi:10.3389/fphar.2019.00363
Scuteri, D., and Bagetta, G. (2021). Progress in the treatment of migraine attacks: from traditional approaches to eptinezumab. Pharm. (Basel) 14 (9), 924. doi:10.3390/ph14090924
Scuteri, D., Cassano, R., Trombino, S., Russo, R., Mizoguchi, H., Watanabe, C., et al. (2021a). Development and translation of NanoBEO, a nanotechnology-based delivery system of bergamot essential oil deprived of furocumarins, in the control of agitation in severe dementia. Pharmaceutics 13 (3), 379. doi:10.3390/pharmaceutics13030379
Scuteri, D., Corasaniti, M. T., Tonin, P., and Bagetta, G. (2019b). Eptinezumab for the treatment of migraine. Drugs Today (Barc) 55 (11), 695–703. doi:10.1358/dot.2019.55.11.3069864
Scuteri, D., Corasaniti, M. T., Tonin, P., Nicotera, P., and Bagetta, G. (2021b). Role of CGRP pathway polymorphisms in migraine: a systematic review and impact on CGRP mAbs migraine therapy. J. Headache Pain 22 (1), 87. doi:10.1186/s10194-021-01295-7
Scuteri, D., Rombolà, L., Crudo, M., Watanabe, C., Mizoguchi, H., Sakurada, S., et al. (2022a). Preclinical characterization of antinociceptive effect of bergamot essential oil and of its fractions for rational translation in complementary therapy. Pharmaceutics 14 (2), 312. doi:10.3390/pharmaceutics14020312
Scuteri, D., Tarsitano, A., Tonin, P., Bagetta, G., and Corasaniti, M. T. (2022b). Focus on zavegepant: the first intranasal third-generation gepant. Pain Manag. 12 (8), 879–885. doi:10.2217/pmt-2022-0054
Scuteri, D., Tonin, P., Nicotera, P., Bagetta, G., and Corasaniti, M. T. (2022c). Real world considerations for newly approved CGRP receptor antagonists in migraine care. Expert Rev. Neurother. 22 (3), 221–230. doi:10.1080/14737175.2022.2049758
Scuteri, D., Tonin, P., Nicotera, P., Vulnera, M., Altieri, G. C., Tarsitano, A., et al. (2022d). Pooled analysis of real-world evidence supports anti-CGRP mAbs and OnabotulinumtoxinA combined trial in chronic migraine. Toxins (Basel) 14 (8), 529. doi:10.3390/toxins14080529
Seo, J. G., and Park, S. P. (2015). Validation of the generalized anxiety disorder-7 (GAD-7) and GAD-2 in patients with migraine. J. Headache Pain 16, 97. doi:10.1186/s10194-015-0583-8
Simpson, D. M., Hallett, M., Ashman, E. J., Comella, C. L., Green, M. W., Gronseth, G. S., et al. (2016). Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 86 (19), 1818–1826. doi:10.1212/wnl.0000000000002560
Steiner, T. J., Stovner, L. J., Vos, T., Jensen, R., and Katsarava, Z. (2018). Migraine is first cause of disability in under 50s: will health politicians now take notice? J. Headache Pain 19 (1), 17. doi:10.1186/s10194-018-0846-2
Stovner, L. J., Nichols, E., Steiner, T. J., Abd-Allah, F., Abdelalim, A., Al-Raddadi, R. M., et al. (2018). Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology 17 (11), 954–976. doi:10.1016/S1474-4422(18)30322-3
Strassman, A. M., Weissner, W., Williams, M., Ali, S., and Levy, D. (2004). Axon diameters and intradural trajectories of the dural innervation in the rat. J. Comp. Neurol. 473 (3), 364–376. doi:10.1002/cne.20106
Tepper, S., Ashina, M., Reuter, U., Brandes, J. L., Doležil, D., Silberstein, S., et al. (2017). Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 16 (6), 425–434. doi:10.1016/s1474-4422(17)30083-2
Toni, T., Tamanaha, R., Newman, B., Liang, Y., Lee, J., Carrazana, E., et al. (2021). Effectiveness of dual migraine therapy with CGRP inhibitors and onabotulinumtoxinA injections: case series. Neurol. Sci. 42 (12), 5373–5376. doi:10.1007/s10072-021-05547-x
Torres-Ferrús, M., Gallardo, V. J., Alpuente, A., Caronna, E., Gine-Cipres, E., and Pozo-Rosich, P. (2021). The impact of anti-CGRP monoclonal antibodies in resistant migraine patients: a real-world evidence observational study. J. Neurology 268 (10), 3789–3798. doi:10.1007/s00415-021-10523-8
Vuralli, D., Ayata, C., and Bolay, H. (2018). Cognitive dysfunction and migraine. J. Headache Pain 19 (1), 109. doi:10.1186/s10194-018-0933-4
Wang, J., Casals-Diaz, L., Zurawski, T., Meng, J., Moriarty, O., Nealon, J., et al. (2017). A novel therapeutic with two SNAP-25 inactivating proteases shows long-lasting anti-hyperalgesic activity in a rat model of neuropathic pain. Neuropharmacology 118, 223–232. doi:10.1016/j.neuropharm.2017.03.026
Ware, J. E., Bjorner, J. B., and Kosinski, M. (2000). Practical implications of item response theory and computerized adaptive testing: a brief summary of ongoing studies of widely used headache impact scales. Med. Care 38 (9 Suppl. l), Ii73–82. http://www.jstor.org/stable/3768065.
Welch, M. J., Purkiss, J. R., and Foster, K. A. (2000). Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon 38 (2), 245–258. doi:10.1016/S0041-0101(99)00153-1
Yang, M., Rendas-Baum, R., Varon, S. F., and Kosinski, M. (2011). Validation of the headache impact test (HIT-6™) across episodic and chronic migraine. Cephalalgia 31 (3), 357–367. doi:10.1177/0333102410379890
Keywords: onabotulinumtoxin A, chronic migraine, anti-CGRP monoclonal antibodies, anti-CGRP-R monoclonal antibodies, erenumab, eptinezumab
Citation: Corasaniti MT, Lawrence GW, Bagetta G, Iannacchero R, Tarsitano A, Monteleone A, Pagliaro M, Tonin P, Sandrini G, Nicotera P and Scuteri D (2023) Combination of anti-CGRP/CGRP-R mAbs with onabotulinumtoxin A as a novel therapeutic approach for refractory chronic migraine: a retrospective study of real-world clinical evidence and a protocol for a double-blind, randomized clinical trial to establish the efficacy and safety. Front. Pharmacol. 14:1296577. doi: 10.3389/fphar.2023.1296577
Received: 18 September 2023; Accepted: 22 November 2023;
Published: 13 December 2023.
Edited by:
Danilo De Gregorio, Vita-Salute San Raffaele University, ItalyReviewed by:
Stefania Ceruti, University of Milan, ItalyNico Antenucci, Texas Tech University Health Sciences Center, United States
Antonella Scorziello, University of Naples Federico II, Italy
Copyright © 2023 Corasaniti, Lawrence, Bagetta, Iannacchero, Tarsitano, Monteleone, Pagliaro, Tonin, Sandrini, Nicotera and Scuteri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: D. Scuteri, damiana.scuteri@unical.it
†These authors share first authorship
 M. T. Corasaniti
M. T. Corasaniti G. W. Lawrence
G. W. Lawrence G. Bagetta
G. Bagetta R. Iannacchero4
R. Iannacchero4 A. Monteleone
A. Monteleone M. Pagliaro
M. Pagliaro P. Tonin
P. Tonin G. Sandrini
G. Sandrini D. Scuteri
D. Scuteri