- 1Department of Food Science, Faculty of Life Sciences, Government College University, Faisalabad, Pakistan
- 2University Institute of Food Science and Technology, The University of Lahore, Lahore, Pakistan
- 3Department of Agriculture and Food Systems, The University of Melbourne, Melbourne, VIC, Australia
- 4Faculty of Food Engineering, “Ştefan cel Mare” University of Suceava, Suceava, Romania
- 5Faculty of Mechanical Engineering, Automotive and Robotics, “Ştefan cel Mare” University of Suceava, Suceava, Romania
- 6Integrated Center for Research, Development and Innovation in Advanced Materials, Nanotechnologies, and Distributed Systems for Fabrication and Control (MANSiD), “Ştefan cel Mare” University of Suceava, Suceava, Romania
This review aims to measure the different aspects of summer savory including biological activity, medicinal properties, nutritional value, food application, prospective health benefits, and its use as an additive in broiler feed. Furthermore, toxicity related to this is also overviewed. Summer savory leaves are abundant in total phenolic compounds (rosmarinic acid and flavonoids) that have a powerful antioxidant impact. Rosmarinic (α-O-caffeoyl-3,4-dihydroxy-phenyl lactic) acid has been identified in summer savory as a main component. According to phytochemical investigations, tannins, volatile oils, sterols, acids, gums, pyrocatechol, phenolic compounds, mucilage, and pyrocatechol are the primary compounds of Satureja species. Summer savory extract shows considerable biological potential in antioxidant, cytotoxic, and antibacterial assays. Regarding antioxidant activity, summer savory extract displays an inhibitory effect on lipid peroxidation. Summer savory also has Fe (III) reductive and free radical scavenging properties and contains minerals and vitamins. Summer savory has important biological properties, including antimicrobial activity and antioxidant activity, and protective effects against Jurkat T Cells, Alzheimer’s disease, cancer, infection, cardiovascular diseases, diabetes, and cholesterol. The leaves and stems of this plant are employed in the food, feed, and pharmacological industries due to their antioxidant properties and substantial nutritional content. Conclusively, summer savory is widely considered beneficial for human health due to its versatile properties and medicinal use.
1 Introduction
Summer savory (Satureja hortensis L.) is one of the most popular varieties of savory. It is a seasonal herb that displays similar function and flavor to the perennial winter savory. Autumn savory is used more regularly due to its bitter taste. From July to September in the Northern Hemisphere, this herb blossoms with violet tubular flowers. It has relatively thin brass foliage and rises to a height of 30–60 cm (1–2 ft). Satureja hortensis L. is a renowned herb in eastern Canada, and it can be used similarly to sage (Burlando et al., 2010). It is the predominant ingredient in many condiments for fowl, and it is used to produce cretonnade (cretonade). Summer savory is a very rich chicken vinaigrette that can be served with turkey, goose, and duck. It is also used in other food products, including fricot and mince pies. It is frequently accessible in dried form throughout the year in grocery stores, and it is used in varying amounts; for example, it is used in large quantities in cretonnade, whereas it is consumed in smaller quantities in other food products (Brown, 2009). It is popular for seasoning grilled meats and for barbecues, stews, and sauces. Summer savory is recommended for use in sausages over winter savory due to its richer aroma. It is used frequently in Bulgarian dishes, imparting a powerful fragrance in a wide range of meals. The traditional food of Sofia contains three ingredients for seasoning instead of just salt and pepper: salt, crimson chili, and summer savory. Sharena sol is the result of combining these ingredients. In Romanian cuisine, summer savory, also known as “cimbru,” is used in “sarmale” (stuffed cabbage and grape leaf rolls) and “mititei” (grilled ground meat rolls) (Cutler et al., 2010). Savory can grow from seeds propagated in a moderately fertile environment. It takes a long time to germinate. Spring season plants are frequently trimmed in June for new usage. The plants can be picked and dried for winter usage when they are in flower (Nybe, 2007). Apart from food preparation, this herb has been used as a traditional antibacterial medicine for gastrointestinal issues (Gelovani et al., 2012). Georgia cultivates native hybrids of summer savory (Akhalkatsi et al., 2012). For instance, Kondari is a variety containing one of the largest total flavonoid concentrations along with the strongest hydro antioxidant activity levels, as discovered in our earlier research on Georgian spices (Rodov et al., 2010). In principle, phenylpropanoid is a precursor of rosmarinic acid, which is the plant kingdom’s second leading ester of caffeic acid. Animal investigations have reported that S. hortensis powder and its polyphenolic fraction display anti-inflammatory characteristics (Hajhashemi et al., 2002; Uslu et al., 2003). To some extent, this activity has been credited to rosmarinic acid, which has been shown to have anti-inflammatory and anti-allergic effects in human and animal studies (Sanbongi et al., 2003). The antiallergic action of rosmarinic acid has been related to two distinct mechanisms, namely, reactive oxygen species filtration and modification of the inflammatory process (Osakabe et al., 2004). For instance, the nephroprotective impact of rosmarinic acid has been related to an increased antioxidant potency, particularly higher glutathione levels and the antioxidant impact of enzymes (Tavafi and Ahmadvand, 2011).
Summer savory (Satureja hortensis L.) also contains a variety of volatile oils (carvacrol and thymol) that have anti-inflammatory (Can Baser, 2008; Hashemipour et al., 2014), antioxidant (Güllüce et al., 2003), antimicrobial (Şahin et al., 2003), and antifungal (Boyraz and Özcan, 2006) properties. Summer savory extract may be valuable to the poultry industry. A previous study reported that dietary summer savory essence (SSE) may enhance and maintain broiler chicken productivity efficiency, blood components, immunological reaction, and ileal microbiota (Movahhedkhah et al., 2019). Furthermore, regarding the volatile oil composition, carvacrol and γ-terpinene are the primary components identified in a typical essential oil of this herb. Therefore, this review comprises different elements relating to summer savory, including an update on the chemical composition of summer savory and its known biological and medicinal properties associated with active substances. Interestingly, specific findings regarding the toxicity of its herb extracts are also provided.
2 Chemical composition of summer savory
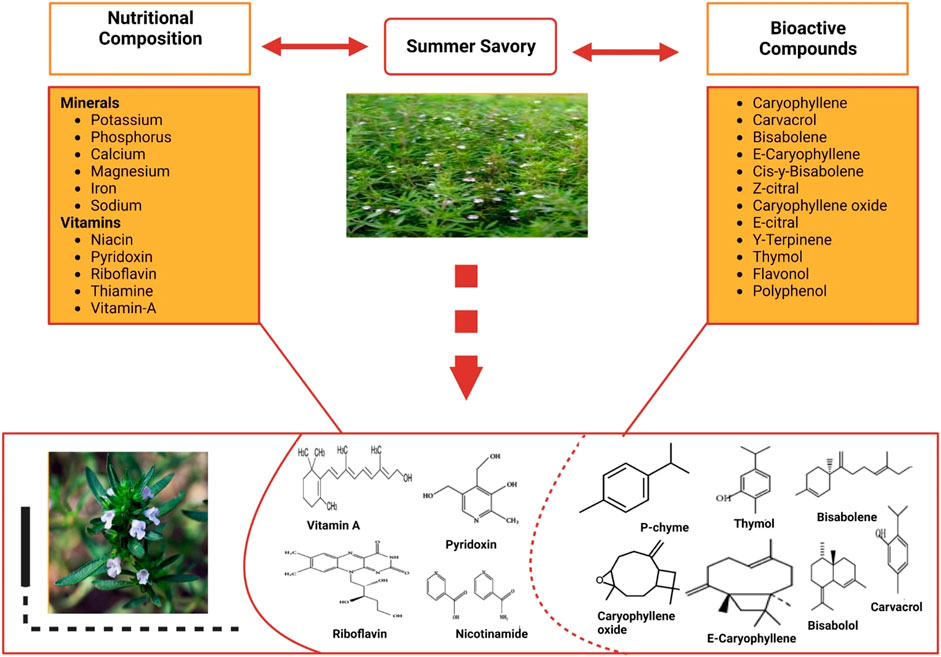
Many people in the food industry (herb, vegetable, and fruit growers) see improving human health as a principal goal for this century. Mineral elements are crucial for growth and can support and sustain the human ability to avoid ailments (Grusak, 2002). Herbs are valuable sources of quickly absorbable mineral elements among crops (Boyraz and Özcan, 2006). Summer savory (Satureja hortensis L.) is a common herbaceous plant from the Lamiaceae family that is grown in various regions across the world (Jafari et al., 2016) (Kudełka and Kosowska, 2008). Significant levels of minerals (potassium, phosphorus, calcium, magnesium, iron, and sodium) and vitamins (niacin, pyridoxine, riboflavin, thiamine, vitamin A, and vitamin C) were detected in the raw material of S. hortensis L. (Mumivand et al., 2010; Karimi et al., 2012; Soltani et al., 2014). Therefore, S. hortensis L. may be used as a nutritional basis for essential human minerals. The mineral rank of florae is critical not only for the nutritional content of food but also for the development, growth, and yield of crops. Crucial oils derived from several classes (Khan et al., 2020) in this family possess organic roles including physical function (photosynthesis) and environmental purpose (relationships between the flowers and their environment). Furthermore, the chemical structure of the oils from different Satureja strains has been discovered to vary greatly (Slavkovska et al., 2001). Various studies have shown that tannins, volatile oils, sterols, acids, gums, pyrocatechol, phenolic compounds, mucilage, and pyrocatechol are the main compounds of Satureja species.
Previous studies have shown the chemical composition of the oils from diverse Satureja species varies (Figure 1). The contents of the oils are determined by climatic, periodic, and terrestrial circumstances as well as yield period and purification practice (Baydar et al., 2004). Toward chemical profiling, water has been used to extract crucial oils from air-dried plants and strong spores through 4-h distillation in a Clevenger-type apparatus. Summer savory leaves and seeds had a total of 23 and 24 components, respectively (Farmanpour-Kalalagh et al., 2020). Furthermore, summer savory seeds contain chemicals including Carvacrol, Estragole (Methyl Chavicol), Caryophyllene, and E-Caryophyllene, whereas the leaves are a good source of Carvacrol, γ-Terpinene, and p-Cymene. Moreover, some chemical components are present in both (seeds and leaves), including Carvacrol, Caryophyllene, E-Caryophyllene, β-Bisabolene, cis-α-Bisabolene, Caryophyllene oxide, Z-Citral, E-Citral, γ-Terpinene, and δ-3-Carene (Farmanpour-Kalalagh et al., 2020). Dried summer savory is composed of volatile oil, which is a vital source of chemicals including carvacrol thymol and monoterpene hydrocarbons (beta-pinene, p-cymene, limonene, and camphene). Vitamins and minerals are present in the leaves of summer savory (Hamidpour et al., 2014). The results of many studies suggest that different parts of summer savory are chemically composed of Estragole, Carvacrol, E-Caryophyllene, Caryophyllene γ-Terpinene, Carvacrol, Thymol and p-Cymene, Caryophyllene, Carvacrol, β-Bisabolene, E-Caryophyllene, cis-α-Bisabolene, Z-Citral, Caryophyllene oxide, E-Citral, γ-Terpinene, and δ-3-Carene (Adiguzel et al., 2007; Kizil et al., 2009; Mahboubi and Kazempour, 2011; Katar et al., 2017; Mohtashami et al., 2018) (Figure 1).
3 Different extraction methods of active compounds from summer savory
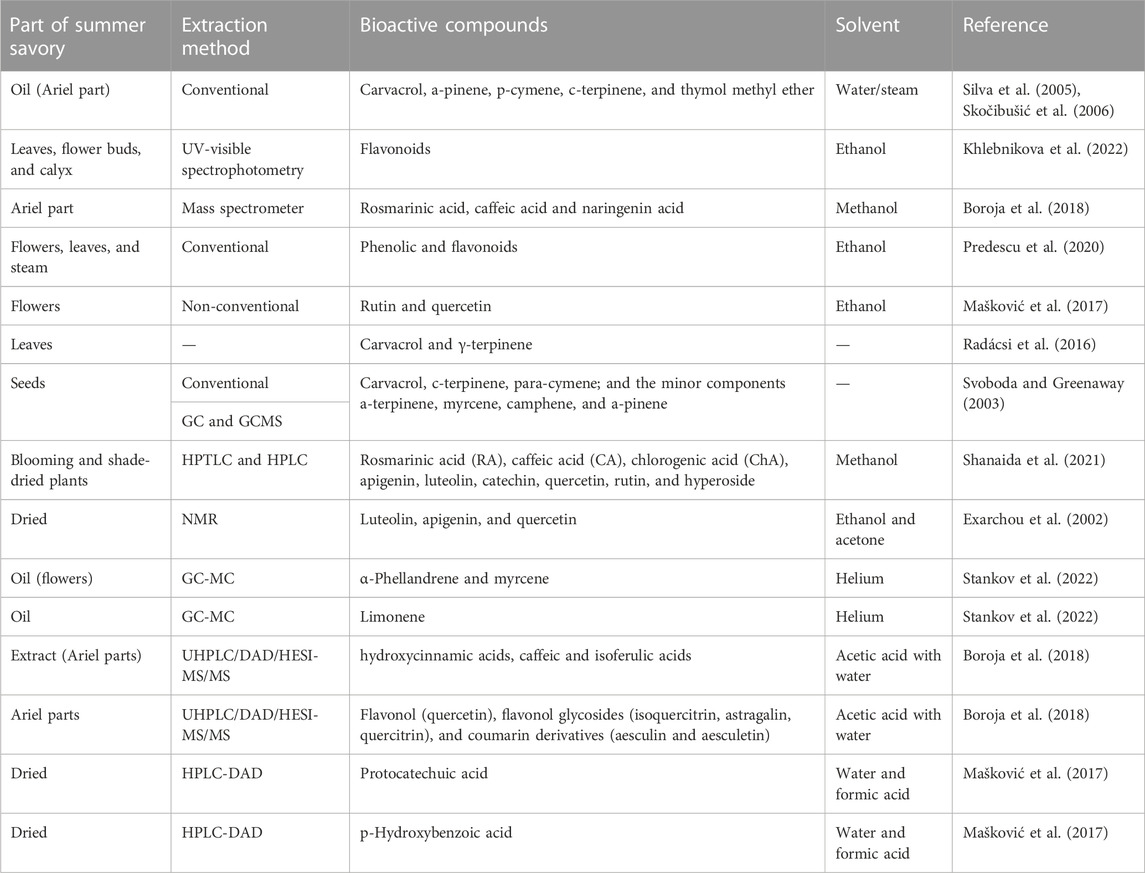
The cytotoxic, antioxidant, and bactericidal properties of several Satureja hortensis L. extracts have been investigated to perform genetic characterization (Cvetanović et al., 2017). Different separate tests relying on a distinct mechanism have been used in order to evaluate the antioxidant activity of the extracts, including total antioxidant activity, lipid peroxidation inhibition, and hydroxyl radical scavenging (Cvetanović et al., 2017). Phenolic content, including condensed tannins, anthocyanins, and gallotannins, is determined in extracts that are associated with biological activities. Flavonoids are extracted by traditional extraction methods, while rutin is predominantly extracted using unconventional methods (Brighente et al., 2007; Fatima et al., 2023). The previously mentioned bioactive molecules can be separated in different ways (Table 1), i.e., via conventional methods (maceration, solvent extraction, soxhlet extraction, and vapor or hydrodistillation) (Waliat et al., 2023) and innovative technologies (emulsion liquid membrane, ultrasound-assisted extraction, enzyme-associated extraction, pulsed electric field, microwave-assisted extraction, and supercritical fluid) (Aadil et al., 2015; Maqbool et al., 2022; Arshad et al., 2023). A previous study was conducted on the extraction of the chemical composition and biological potential of summer savory extracts using conventional and nonconventional methods. The results verified the domination of the subcritical water approach for the isolation of natural compounds, followed by microwave-assisted extraction (Mašković et al., 2017). Another study was conducted on the extraction of essential oils from summer savory extract, whereby Two extraction methods were compared, namely, microwave-assisted hydrodistillation (MAHD) and traditional hydrodistillation (HD) methods. The outcomes confirmed that the novel method is more suitable compared to the traditional method (Rezvanpanah et al., 2011). The current study of Šeregelj et al. (2022) evaluates the biological activities of ultrasound- and microwave-assisted extracts of S. kitaibelii. The findings confirm that microwave-assisted extraction with water solvent is a promising approach (Šeregelj et al., 2022).
4 An overview of the biological activities of summer savory extracts
Different extracts showed considerable biological potential in antioxidant, cytotoxic, and antibacterial assays (Exarchou et al., 2002). Specifically, the extracts obtained by subcritical water extraction extracts displayed the highest yield. In terms of antioxidant activity, the extract is found to have an inhibitory effect on lipid peroxidation. All manufactured extracts have biological properties, which opens up an extensive variety of potential claims in the nutrition and medicinal industries. Potentially, the extracts can be utilized as normal causes of antioxidants in place of synthetic substances for food preservation as well as the production of functional foods (Güllüce et al., 2003; Şahin et al., 2003).
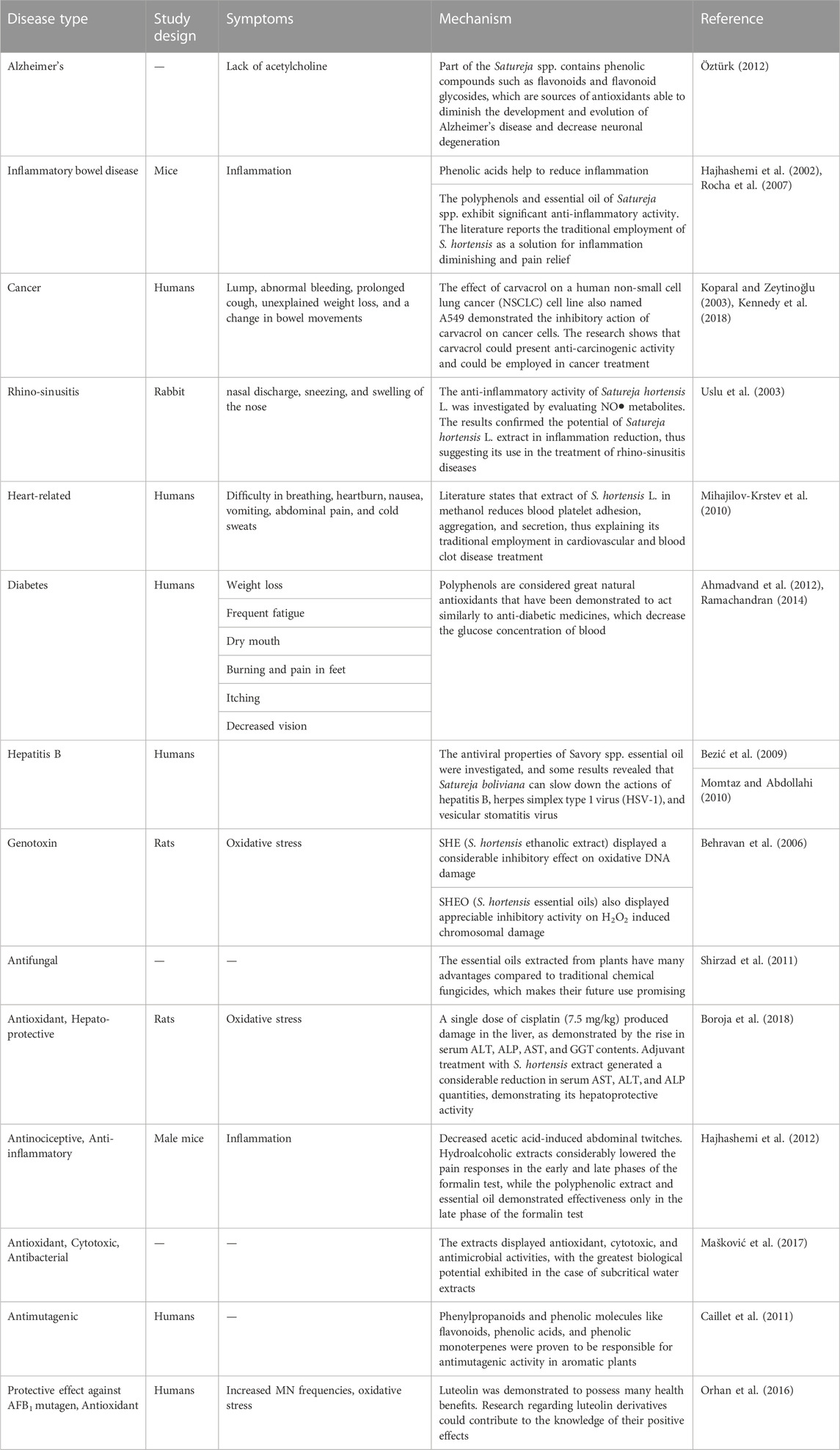
The medicinal properties of summer savory are shown in Table 2.
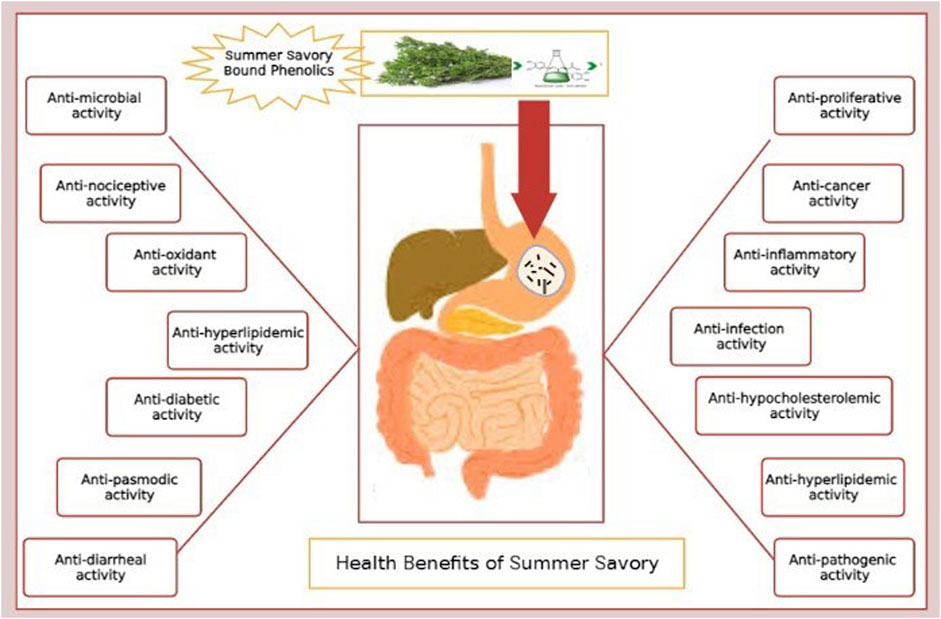
Since most of the compounds found in the herb display specific biological properties (Figure 2), these are presented in the following subsections.
4.1 Antimicrobial activity of summer savory
There has been a slew of research in recent years focusing on the antibacterial properties of fragrant florae essential oils and their possible relevance in nutrition protection (Ghalfi et al.). Many studies have been published suggesting a relationship between the chemical construction of essential oil mechanisms and antibacterial properties. In particular, essential oils affect the cell membrane by interacting with and disrupting the phospholipid bilayer and by affecting enzyme activity and genetic resources in bacteria (Rezvanpanah et al., 2011). Essential oil of S. hortensis is rich in carvacrol and thymol, which are isomeric composites with a phenylic acid group in their structure. Both thymol and carvacrol suppress the diversity of microbes including bacteria and fungus (Adiguzel et al., 2007; Razzaghi-Abyaneh et al., 2008). The Satureja family contains phenolic compounds and their metabolites, which alter the permeability of the cell crust while inhibiting cell respiration. In this way, the Satureja family performs antibacterial activity. (Gursoy et al., 2009; Mahboubi and Kazempour, 2011). The antiseptic properties of various herbs and spices have long been known, and they have been utilized in food preservation and healing. (Omidbeygi et al., 2007). Apart from deterioration in foods, fungus development leads to undesired metabolites such as aflatoxin, which may be derived from Aspergillus species (A. parasiticus and A. flavus). Additionally, mycotoxin (toxigenic fungus) can be found in food and grains that have been preserved for a long time. In both animals and humans, aflatoxins are known to be strong hepatocarcinogens. To some extent, antifungal properties have been discovered in several savory species (Dikbas et al., 2008). Summer savory ingredients can be used as an additional preservative in food items due to their antibacterial properties (Rezvanpanah et al., 2011). The essential oil from some savory species has been shown to be high in antiviral properties (Bezić et al., 2009). The seeds and leaves of summer savory are good sources of essential oil. The essential oil of summer savory is composed of chemical compounds (hydrophobic and hydrophilic molecules) that play an important role in antimicrobial activity. The hydrophilic and hydrophobic molecules play a favorable role in antimicrobial activity. The outer layer of Gram-positive bacteria is a peptidoglycan cell wall that allows hydrophobic molecules to penetrate and reach the internal material, whereas the outer layer of Gram-negative bacteria is lipopolysaccharide, which allows mainly small hydrophilic molecules to pass and is only partly permissive for hydrophobic molecules. The hydrophobicity of essential oils is responsible for the disruption of microbial structures. The essential oil has different mechanisms of action on the microbial population, including degradation of the cell wall and cytoplasmic membrane, cytoplasm coagulation, and diffusion through the double lipid layer of the membrane, together with alteration of its permeability and function (Nazzaro et al., 2013). In vitro investigations have shown that the Satureja boliviana could suppress the overall effects of vesicular stomatitis virus (VSV), hepatitis B, and herpes simplex type 1 virus (HSV-1). However, S. montana can protect against HIV-1 virus (Omidbeygi et al., 2007). It has been discovered that S. hortensis essential oil has excellent antifungal action compared to Aspergillus flavus. Moreover, it could be exploited as a cause of environmental plant antifungals to protect various food products from infection and saprophytic fungi. S. hortensis essential oil has a broad antibacterial spectrum that inhibits the progress of the social and phytopathogenic bacteria, fungi, and yeasts that cause food spoiling. Vital oils from S. hortensis were found to be effective against S. aureus, Listeria monocytogenes, S. typhimurium, and E. coli O157:H7 along with the Pseudomonas putida strain obtained from meat (Oussalah et al., 2006). Essential oils from summer savory reduce the mycelial development of plant-pathogenic fungi (Botrytis cinerea and Alternaria mali) due to their antifungal properties (Boyraz and Özcan, 2006). Furthermore, the oil was shown to reverse the progress of aflatoxin (AFG1 and AFB1) by Aspergillus parasiticus in vitro under storage conditions (Dikbas et al., 2008), in liquid standard and in tomato adhesive (Omidbeygi et al., 2007), and reverse the development and production of aflatoxin (AFG1 and AFB1) by A. parasiticus (Razzaghi-Abyaneh et al., 2008). The antiseptic effect of S. hortensis essential oil was tested and compared to selected strains using the broth micro-well dilution method. Vital oil was found to be effective against entirely medical insulates from injuries that were verified. The oil has the best antibacterial action against Acinetobacter spp. and S. aureus (Pintore et al., 2002). It also displays activity against Staphylococcus spp. and E. coli (Wilkinson et al., 2003). In identical concentrations, the oil remains efficacious against Enterobacter spp. and Enterococcus spp., S. pyogenes, P. mirabilis, (Wilkinson et al., 2003). The oil’s significant antibacterial activity is due to its high concentration of phenol component carvacrol, which has already been proven to have antimicrobial activity (Ben Arfa et al., 2006). Carvacrol has been found to have substantially strong antibacterial potential compared to other chemically similar compounds, such as eugenol (Ben Arfa et al., 2006).
4.2 Antioxidant activity of summer savory
Antioxidants are substances that prevent the oxidation of other compounds by preventing or delaying the beginning or proliferation of oxidative chain reactions (Roobab et al., 2018; Mukhtar et al., 2023). Fears about the antagonistic properties of artificial antioxidants have recently prompted the consumption of natural antioxidants found in all plants and all their parts (Bakkali et al., 2008). Numerous studies have proven that Satureja strains display antioxidant action (Exarchou et al., 2002). Previous studies have stated that the oils of Satureja species are a rich source of isopropanoids and flavonoids, such as p-cymene, linalool, carvacrol, thymol, β-caryophyllene, and γ-terpinene. These compounds have powerful antioxidant properties (Ruberto and Baratta, 2000). It has been determined that the antioxidant consequence of fragrant plants may be related to the existence of hydroxyl groups in their phenolic chemicals. In S. montana, components with hydroxyl groups have relatively significant antioxidant activity (Radonic and Milos, 2003). During storage, the Satureja cilicica essential oil showed substantial antioxidant activity in butter. Moreover, with increased concentrations of oil, the antioxidant properties of rose oil also increased (Ozkan et al., 2007). The outcomes of the study of Ozkan et al. (2007) show that the essential oil of S. cilicica can be employed as a natural antioxidant and fragrance agent in fat. Oxidative damage is induced by the formation of sensitive oxygen species (SOS) throughout ordinary cell aerophilic respiration, and it plays a key part in the start and progression of several illnesses in the human body. Antioxidants play a significant role in defending cells from oxidative compensations and in the prevention of a variety of diseases (Szőllősi and Varga, 2002). This study suggests that the methanolic extract of the S. hortensis aerial part may be valuable against cisplatin-induced oxidative damage in the liver, kidney, and testes of rats (Boroja et al., 2018). Another study verified that S. hortensis extract is a good source of antioxidants. However, different methods were used to measure antioxidant properties. The outcomes of FRAP, ABTS, and DPPH measured the high activity of the S. hortensis extract. Moreover, the total phenolic and total flavonoid content was also determined as high in S. hortensis extract (Mašković et al., 2017). Previous studies have proven that leaves and essential oil are important sources of chemical compounds including isopropanoids, rosmarinic acid, and flavonoids. The outcomes confirm that both leaves and oils have a high antioxidant capacity (Momtaz and Abdollahi, 2010; Chkhikvishvili et al., 2013).
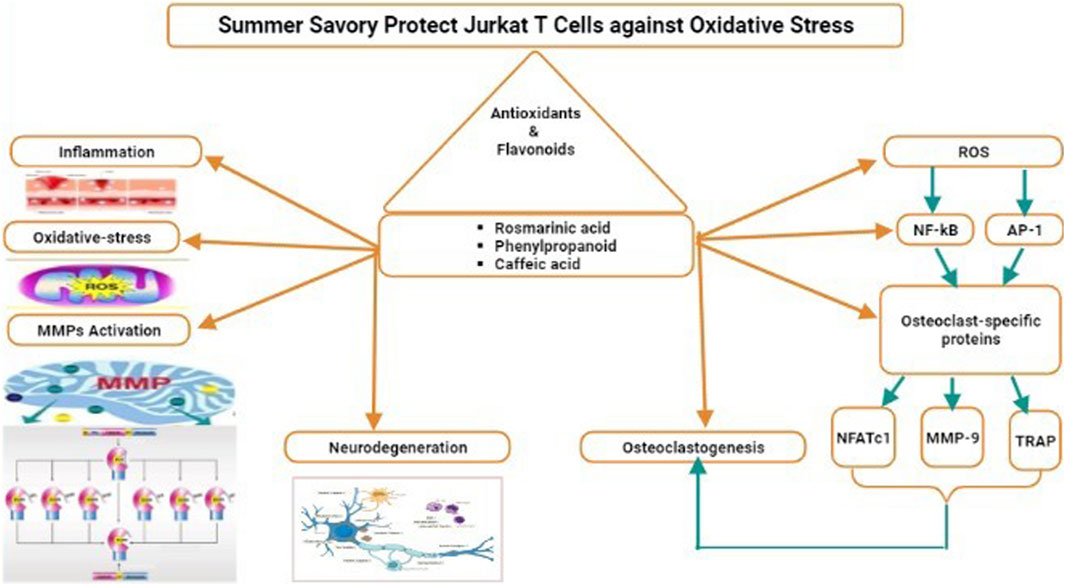
4.2.1 Summer savory protects Jurkat T Cells against oxidative stress
Rosmarinic acid is the main phenylpropanoid component in summer savory. Jurkat cells can be protected against oxidative stress generated by hydrogen peroxide by S. hortensis and its rich rosmarinic acid proportions. The results of Hajhashemi et al. (2002), Sanbongi et al. (2003), Osakabe et al. (2004) remain consistent through the cytoprotective, antiinflammatory, and antioxidant activities of S. hortensis (Hajhashemi et al., 2002) and rosmarinic acid (Sanbongi et al., 2003; Osakabe et al., 2004) in animals and humans. S. hortensis extracts exhibited significant protective antioxidant actions when administered to H2O2-stressed lymphocytes isolated from healthy rats’ blood (Behravan et al., 2006). Rosmarinic acid protected human neuronic cells from hydrogen peroxide-induced apoptosis in cell cultures (Lee et al., 2008) and inhibited the creation of reactive nitrogen and oxygen species in RAW264.7 macrophages encouraged with phorbol 12-myristate 13-acetate or lipopolysaccharides in a dose-dependent manner. However, maximum phenolic composites demonstrated pro-oxidant properties at small dosages in a metal catalyst system before switching to antioxidant activity at higher concentrations (Fukumoto and Mazza, 2000). Furthermore, high dosages (2–3-mM) of caffeic acid and phenylpropanoids have recently been demonstrated to protect Jurkat cells from H2O2-induced DNA impairment by chelating intracellular labile iron (Kitsati et al., 2012). In addition to rosmarinic acid, the existence of powerful antioxidants in the phenolic element may enhance its antioxidant potential. S. hortensis may also aid in the neutralization of hydrogen peroxide by increasing the activity of antioxidant enzymes. In Jurkat cells, SOD and Catalase play crucial roles in the regulation of apoptosis and oxidative stress (Kagan et al., 2002). The extract of Perilla frutescens rosmarinic acid in water from a Lamiaceae plant was demonstrated to have effects on the protein and mRNA appearance of the antioxidant enzymes in cultivated human vein endothelial cells (Saita et al., 2012). Anti-inflammatory features like IL-10 may be generated in stressful settings to counteract the rapid rise in pro-inflammatory cytokines and regulate the amount and interval of the inflammatory response. The accumulation of antioxidant-rich herbal ingredients in the diet of wildlife suffering from pro-inflammatory conditions has been demonstrated to enhance IL-10 levels (Kim et al., 2011) or both IL-2 and IL-10 (Zhang et al., 2012) in tandem, with a decrease in pro-inflammatory markers like IL-6, TNF-α, and IL-1β levels. Furthermore, nutritional interferences conserved standard antioxidant enzyme action, prevented fat peroxidation, and boosted HDL levels in the preserved animals, leading to improved immunity and the alleviation of diseases (Zhang et al., 2012). In a lipopolysaccharide-stimulated macrophage model, rosmarinic acid boosted 1L-10 productions (Mueller et al., 2010). Adding the S. hortensis extract or its phenolic component to H2O2-challenged Jurkat cells restored survival and proliferation, relieved the G0/G1 arrest, and regulated apoptosis. Overall, these findings are consistent with the overall reaction of cells to oxidative stress, whereby small quantities of reactive oxygen species encourage cell growth, in-between measures cause evolution arrest, and high oxidative stress causes cell death through necrotic or apoptotic mechanisms (Martindale and Holbrook, 2002). The accumulation of S. hortensis extracts appears to reduce the oxidative stress caused by hydrogen peroxide on the cells. These effects may be related to phenolic compounds and rosmarinic acid direct radical-scavenging activity but also to unintended processes like the increase in antioxidant enzymes and the production of anti-inflammatory gesturing particles like IL-10 (Figure 3).
4.2.2 Fe (III) reductive and free radical-scavenging properties of summer savory
The capacity of complexes and herb extracts to decrease Fe (III) is frequently employed as a measure of electron contributing procedure. This may be due to the phenolic antioxidant mechanism (Yildirim et al., 2000). The components of Summer Savory are resolvable in acidic aqueous methanol solvent and they have the ability to donate electrons to the unbalanced free radicals (Dorman et al., 2003). These are further constant non-reactive classes, with the EtOAc-soluble mechanisms acting as maximum active electron donors (Dorman et al., 2003). ABTS and DPPH artificial free radicals and hydroxyl radicals are used to examine the possible free radical-scavenging actions of the Satureja hortensis L. extract and subfractions (Jayasinghe et al., 2003). ABTS and DPPH are widely employed to estimate free radical-scavenging capabilities (Jayasinghe et al., 2003). There are often problems with solubility and interference with DPPH tests; thus, ABTS free radicals are frequently utilized (Arnao, 2000). However, it has been suggested that these approaches are not able to use a biologically or food-related reactive species and oxidizable substrate. Moreover, they can only indicate potential antioxidant activity and, therefore, provide no direct information on defensive presentation (Güllüce et al., 2003). Phospholipids are thought to play a key role in off-flavor development and oxidative deterioration in foodstuffs (Frankel and Meyer, 2000). The capability of the models to prevent ascorbate-Fe (III)-generated hydroxyl radical-mediated peroxidation of a heterogenous phospholipid-aqueous phosphate buffered system is resolute, and the hydroxyl radical is an extremely volatile radical that is used in in vivo study. In vitro studies have shown that the EtOAc-soluble mechanisms are activated at the maximum level, including crude extract and Fe (III) decrease assay. With hexane, EtOAc, and n-BuOH, an aliquot part of this extract was sub-fractioned by liquid-liquid breakdown against water (Kim et al., 2003). Fe (III) reduction assays as well as ABTS, DPPH, and hydroxyl free radical-scavenging assays were used to characterize these materials’ antioxidant capabilities (Hajhashemi et al., 2000). The EtOAc fraction and crude extract were the maximum active samples, showing much lower activity (Antolovich et al., 2002). The crude extract containing EtOAc-soluble components had predominantly significant action when used as preservative to prevent free radical-mediated destruction of vulnerable components. Moreover, free radicals play a key role in the deterioration of human health. The EtOAc subfraction may have favorable impacts on human biology when it is used in adequate amounts in foods (Niki et al.).
4.3 Alzheimer’s disease
Alzheimer’s disease is generally caused by a deficiency of acetylcholine, which is a neurotransmitter. Alzheimer’s disease has been treated using acetylcholinesterase inhibitor tablets. Nevertheless, these treatments may involve adverse side effects. To prevent this sort of disease, the production of natural chemicals with antioxidant and anticholinesterase properties is preferable (Öztürk, 2012). Antioxidants can play a significant role as neuroprotective agents at the initial stage of Alzheimer’s disease (Silva et al., 2009). Some Satureja species are vital sources of phenolic compounds (flavonoids and flavonoid glycosides) that can play an important role as antioxidants. However, the antioxidant potential of Satureja species may reduce the chances of developing Alzheimer’s disease and neuronal degeneration (Öztürk, 2012). The activation of cell signaling pathways occurs due to oxidative stress. The results of Pritam et al. (2022) show reduced formations of toxic substances that foster the development of the disease. Antioxidants reduce free-radical-mediated damage in neuronal cells through detoxification. Moreover, the balance between antioxidants/oxidants is unfavorably unbalanced, which can have detrimental effects, such as Alzheimer’s disease (Sinyor et al., 2020). One study shows that the phenolic content of several Satureja species, specifically flavonoids and flavonoid glycosides, which delay the growth of Alzheimer’s disease and lower neural degeneration due to potent antioxidants (Öztürk, 2012). Thymol and carvacrol are abundant in Satureja species that can work as low cholinesterase inhibitors and protect people from oxidative stress and amnesia despite causing no adverse side effects (Öztürk, 2012). The study of Ross et al. (1999) shows that the progression of Alzheimer’s disease can be reduced by reducing neuronal damage. Previous studies have verified that different savory species can protect against various chronic diseases, including Alzheimer’s, diabetes, cancer, and cardiovascular diseases (Hamidpour et al., 2014).
4.4 Cancer
The plant materials are composed of phenolic chemicals that can help to protect or reduce oxidative destruction by both non-free-radical and free-radical species (Alizadeh et al., 2010; Ali et al., 2022). Regulation of oxidative chain reaction formation and growth helps to prevent various disorders, including oxidative stress dysfunctions, cancer, heart disease, and neural disorders (Szőllősi and Varga, 2002; Alizadeh et al., 2010). The anti-carcinogenic, vasoprotective, anti-allergic, anti-proliferative, anti-inflammatory, and antimicrobial properties of phenolic acids and flavonoids have been documented in several studies. Satureja montana L. has been used as medication in the treatment of several types of cancer (Cetojevic-Simin et al., 2008). The extracts suppressed the development of HT-29 (human colon adenocarcinoma) cells at levels over 0.7 mg/mL, and HeLa (human cervix epidermoid carcinoma) was shown to present the maximum sensitivity to the savory extract. Several Satureja spp. species are powerful sources of antioxidants that can prevent the growth of an extensive variety of human tumor cells (Cetojevic-Simin et al., 2008). Food products have been demonstrated to be effective and stable sources of innovative medicine. Carvacrol is a monoterpene present in the essential oils of a diversity of fragrant plants. The activity of carvacrol on the human non-small cell lung cancer (NSCLC) cell line named A549 indicates that it reduces cancer cells but has no impact on normal lung cells (HFL1). The findings of Koparal and Zeytinoğlu (2003) suggest that carvacrol has anti-carcinogenic properties and can be used as a cancer treatment medicine.
4.5 Anti-infection properties
Bacteria, fungi, and viruses are the most common sources of illnesses that affect both flora and fauna. Plant essential oils act as secondary metabolites to protect humans from natural enemies. It may be genetic or in reaction to pathogens. The antimicrobial activity of Satureja spp. was initially discovered in the 1950s, and it was discovered that the inhibitory action of savory is probably due to its high carvacrol and thymol content. These are the two most effective herbal antiseptic compounds (Oussalah et al., 2006). Essential oil concentration and composition are influenced by storage circumstances as well as the concentration and type of the mark microorganism (Baydar et al., 2004). Essential oil concentrations in Satureja parnassica and Satureja thymbra have been found to fluctuate. The oils extracted during the flowering time were have been determined as the most potent, with minimum inhibitory concentration (MIC) standards and significant antibacterial activities (Chorianopoulos et al., 2006). Essential oils have been shown to have inhibitory effects against a diverse variety of food-deteriorating bacteria due to their concentration in valuable components (Skočibušić et al., 2006). Furthermore, different varieties of Satureja have also been widely studied for their resistance to foodborne diseases. In Greece, the essential oils extracted from Satureja spp. contain monoterpene hydrocarbons and phenolic monoterpene. These oils have outstanding antibacterial properties against foodborne pathogens (Chorianopoulos et al., 2004). Summer savory (S. hortensis L.) extract was investigated in relation to the mycelial growth of food fungi due to the fungicidal activity of the hydrosol (Boyraz and Özcan, 2006). The extract was found to have dose-dependent fungicidal activity at all dosages. Carvacrol has been shown to be most effective in S. thymbra, followed by p-cymene and monoterpene hydrocarbons c-terpinene (Soković et al., 2002). The effect of S. boliviana against binary distinct VSV and viruses-HSV-1 has also been investigated. The active component in the aqueous extract of S. montana has been discovered to be non-polar water-soluble molecules. Non-polar chemicals like essential oils and extracts have substantial anti-HIV-1 action (Abad et al., 1999).
4.6 Cardiovascular diseases
Cardiovascular issues and thrombosis (blood accumulations in the vein or artery) occur due to platelet hyperactivity that contribute to their adsorption to the vascular wall (Yazdanparast and Shahriyary, 2008). Cardiovascular diseases (CVD) are usually illnesses that are directly related to blood arteries and the heart. Excessive oxidative stress produces reactive oxygen species (ROS) that are responsible for the pathophysiology of different CVDs such as ventricular remodeling, cardiomyopathy, myocardial infarction, heart failure, cardiac hypertrophy, and atherosclerosis. The body’s endogenous system fails to maintain normal physiology due to excessive oxidative stress. However, different sources of antioxidants are necessary to scavenge free radicals (Jain and K Mehra N, 2015). Different investigations have shown that S. hortensis has anticoagulant blood properties. Flavonoid, monoterpene hydrocarbons, and carvacrol, including phenolic acids and apigenin (labiatic acid), may all play a role in S. hortensis anti-platelet activity (Yazdanparast and Shahriyary, 2008). The methanol extract of S. hortensis has been shown to reduce secretion, aggregation, and blood platelet adhesion, which may explain its conventional usage in the treatment of blood clots and cardiovascular issues (Mihajilov-Krstev et al., 2010). The current study of Khalid et al. (2023) suggests that essential oil, antioxidants, and phenolic compounds protect CVDs by reducing cholesterol, preventing oxidation, and reducing platelet aggregation, respectively. Previous studies have suggested that phenolic compounds reduce the risk of CVDs by preventing atherothrombosis and platelet activity (Torres-Urrutia et al., 2011; Fuentes et al., 2012).
4.7 Antidiabetic and anticholesterol effects
Hypertension and hyperlipidemic raise the threat of cardiac ailment. It has been recognized that hypercholesterolemia has been linked to a variety of clinical conditions including atherosclerosis, diabetes mellitus, thromboembolic, and cardiovascular diseases. Antioxidants play a vital role in the handling of illnesses involving oxidative stress damage (diabetes mellitus) (Vosough-Ghanbari et al., 2010). Antioxidant medication is the best way to prevent and decrease the development of diabetic consequences, including hyperlipidemia and liver issues. Natural antioxidants derived from medicinal herbs have recently captivated researchers’ curiosity as a possible replacement for artificial antioxidants (Ahmadvand et al., 2012). Polyphenols are well-recognized natural antioxidants that have been shown to reduce blood glucose levels in a manner comparable to antidiabetic medications. Satureja khuzestanica (SKE) is an Iranian Satureja plant with antioxidant activities and anti-diabetic actions (Momtaz and Abdollahi, 2010). Malondialdehyde levels and serum glucose were controlled in diabetic patients using SKE (Momtaz and Abdollahi, 2010). SKE essential oil has been investigated for its hepatoprotective, hypolipidemic, and antiatherogenic properties. In diabetic patients, it can reduce the risk of cardiovascular mortality and liver injury (Ahmadvand et al., 2012). Thymol and carvacrol are the essential components of Satureja species. Essential oils have been revealed to reduce serum cholesterol levels. Flavonoids are abundant in Satureja species that have been shown to have anti-hyperlipidemic and antioxidant effects. SKE has been shown to significantly reduce the triglyceride levels and fasting blood glucose in hyperlipidemic and diabetic rats along with ATP levels and lipid peroxidation in numerous trials (Momtaz and Abdollahi, 2010). The study of Momtaz and Abdollahi (2010) indicates that some Satureja species, including SKE, can be utilized as an enhancement in hyperlipidemic diabetic patients due to their lipid-lowering and antioxidant characteristics (Vosough-Ghanbari et al., 2010). The flavonoids extracted from summer savory reduce cholesterol in rabbits and lead to a considerable decrease in serum cholesterol (Mchedlishvili et al., 2005). Rats ingesting S. khuzestanica essential oils showed a considerable increase in total antioxidant capacity and a reduction in normal blood lipid peroxidation levels S. khuzestanica oil’s antioxidant properties may explain its triglyceride-lowering properties (Abdollahi et al., 2003). Isopropanoids such as carvacrol, thymol, and flavonoids are also important components of S. khuzestanica. Thymol and carvacrol have been demonstrated to lower serum cholesterol stages considerably. Flavonoids have also been revealed to have anti-hyperlipidemic effects (Momtaz and Abdollahi, 2008).
4.8 Anti-inflammatory and analgesic effects
Inflammation is the body’s natural defense process in relation to pathophysiological conditions. Several examinations have been conducted in order to discover extra influential anti-inflammatory treatments with less harmful properties (Amanlou et al., 2005). Plants of the Lamiaceae family are recognized for their pain-relieving and antispasmodic effects. In animal experiments, many components of Satureja species (flavonoids) have been found to be essential for analgesic, relaxing, and vasodilatory actions (Momtaz and Abdollahi, 2010). In addition to analgesic effects, various Satureja species have been identified to exhibit anti-inflammatory properties (Momtaz and Abdollahi, 2010). S. hortensis L. has been used as a bone pain and muscular reliever in traditional remedies Previous studies have shown that polyphenolic and essential oil from Satureja spp. have strong anti-inflammatory characteristics. The research confirms the traditional use of S. hortensis as a pain killer and an anti-inflammatory (Hajhashemi et al., 2002). According to several investigations, Satureja species, including S. hortensis and SKE, act as anti-inflammatory medications and are equivalent to morphine, indomethacin, and prednisolone (Momtaz and Abdollahi, 2010). Animal and human trials have shown the anti-inflammatory and anti-allergic actions of savory linked to the polyphenolic fraction (rosmarinic acid) (Chkhikvishvili et al., 2013).
4.9 Summer savory and reproduction stimulatory effects
A study was conducted on male rat fertility in which S. khuzestanica essential oil significant gains in fertility index, fecundity, litter index, and potency Additionally, it reduced the post-implant loss (Haeri et al., 2006). Moreover, the seminal vesicles, ventral prostate, and weights of the testes were greatly elevated, and the weight of the testes, testosterone, and FSH were concentrated. These alterations could be linked to the antioxidant potential of the essential oils. The principal antioxidants in Satureja spp. are pcymene, carvacrol, and flavonoids. The results indicate the stimulatory effects of this genus on reproduction (Radonic and Milos, 2003).
5 Applications of summer savory
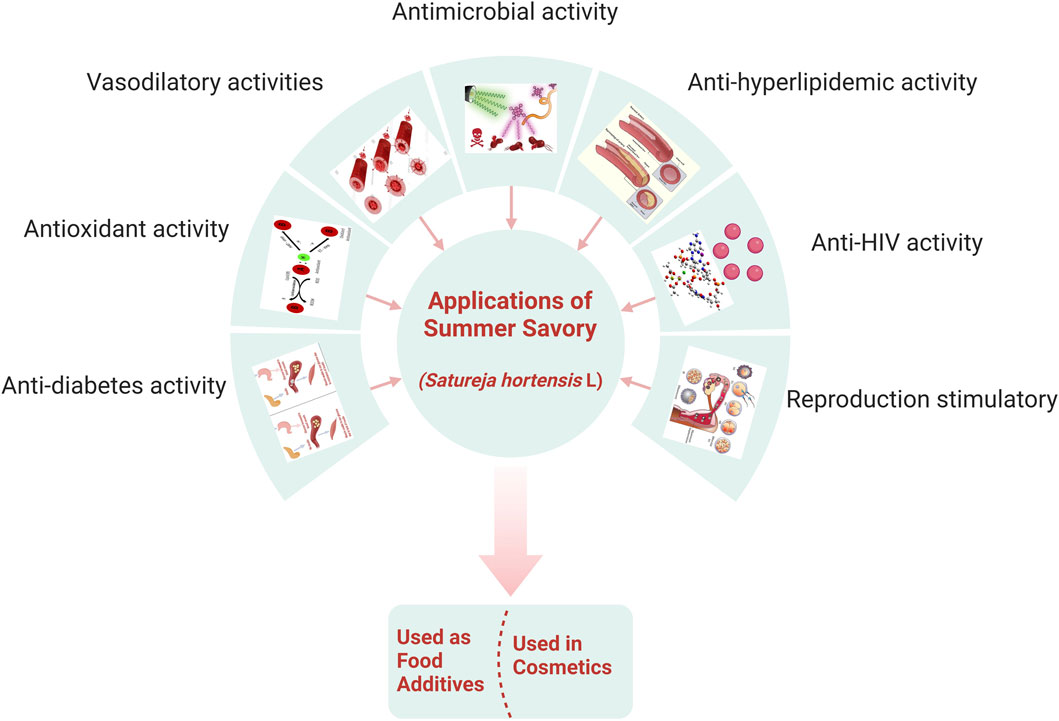
S. hortensis is used worldwide as a food additive, flavoring, and spice and in herbal beverages due to its pre-eminent ethnomedical activity and pharmaceutical and food applications (Figure 4). Furthermore, summer savory oil has been used in the cosmetic industry and in perfumes, both alone and in combination with essential oils (Sefidkon et al., 2004).
Primarily, savory species are used to treat muscle pain, flatus, and intestinal disorders such as indigestion, cramps, nausea, and diarrhea (Abdollahi et al., 2003). Other properties of S. hortensis include antifungal, antibacterial, antioxidant, anti-hyperlipidemic, anti-HIV, anti-diabetes, expectorant, reproduction stimulatory, and vasodilatory activities (Şahin et al., 2003; Amanlou et al., 2005; Basiri et al., 2007). In ancient medical books, it is demonstrated that Satureja spp. has a medicinal impact on respiratory diseases, such as coughs and asthma (Vagionas et al., 2007).
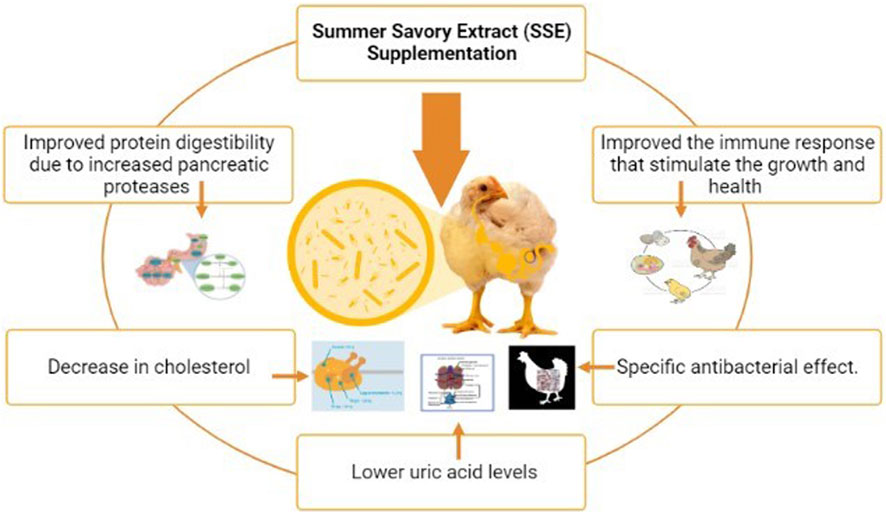
5.1 Summer savory used as a native additive in broiler feed
Essential oils have been revealed to have various favorable properties in broiler feed, including increased feed intake (Jang et al., 2007), improved digestibility, higher digestive enzyme secretion (Jamroz et al., 2005), and microbial ecosystem balancing (Liolios et al., 2009). The beneficial qualities of the essential oils of this plant may account for the increased performance reported, with the addition of summer savory essence (SSE). The plasma content of feed could be linked to the effects of the essential oils in summer savory foods on digestion. In broilers, feed supplementation with thymol was found to greatly improve pancreatic action (Lee et al., 2003). Protein digestibility improved due to increased pancreatic proteases, which could explain why the SSE-supplemented sets had lower uric acid levels. The cholesterolemic action of essential oils (Nobakht et al., 2012) could explain the considerable decrease in cholesterol SSE addition in the diet at 200 and 300 ppm, along with LDL at 300 and 400 mg/kg SSE. SSE-supplemented diets improved the immune response of broilers as they grew older; the immune response, being dependent on age, may be attributable to the essential oil content and antioxidants (Attia and Al-Harthi, 2015). The considerable decrease in E. coli, correspondence to Lactobacillus spp., and a greater ratio of Lactobacillus to coliform suggest that SSE-treated birds have better gut health. This array of IB and IBD virus titers, Lactobacilli counts, and E. coli may indicate that savory extract has a specific antibacterial effect (Attia et al., 2017). Furthermore, better gut biology could explain the rise in antibody titers due to the nutrition sparing-effect (Attia et al., 2017). Although there was no substantial impact of SSE supplementation in broiler feed, conversion ratio and body weight gain were considerably enhanced when 400 mg/kg SSE was used (Bombik et al., 2012). SSE supplementation enhanced the majority of the blood indicators and immunological response criteria evaluated (Bombik et al., 2012). Lactobacilli count was unaffected by diet. However, SSE lowered the Escherichia coli count and improved the Lactobacillus to coliform ratio (Bombik et al., 2012). Supplementing the broiler feed up to 400 mg/kg with SSE maintained growth features and increased health and feed efficiency (Pourhossein et al., 2015). There was no impact on broilers’ weight gain or feed intake during the initial growth period (Hajhashemi et al., 2002; Sanbongi et al., 2003; Uslu et al., 2003; Osakabe et al., 2004; Nybe, 2007; Can Baser, 2008; Brown, 2009; Burlando et al., 2010; Cutler et al., 2010; Rodov et al., 2010; Tavafi and Ahmadvand, 2011; Akhalkatsi et al., 2012; Gelovani et al., 2012; Hashemipour et al., 2014) (Ghazvinian et al., 2018). During the 15–28-day growth phase, broilers were fed up to 300 mg/kg SSE, and the feed intake was gradually lowered (Ghazvinian et al., 2018). During the finisher phase (Adiguzel et al., 2007; Brighente et al., 2007; Kizil et al., 2009; Mahboubi and Kazempour, 2011; Hamidpour et al., 2014; Aadil et al., 2015; Cvetanović et al., 2017; Katar et al., 2017; Mašković et al., 2017; Mohtashami et al., 2018; Maqbool et al., 2022; Arshad et al., 2023; Fatima et al., 2023; Waliat et al., 2023), dietary interventions had no effect on any of the growth indices examined. Finally, dietary supplementation with summer savory extract at 400 mg/kg as a natural feed addition helped broiler chickens maintain their growth and enhance their health (Daferera et al., 2000). (Figure 5).
6 Summer savory and toxicity
Toxicity is a condition in which chemical compounds or parts of a chemical mixture can damage an organ. Antioxidants are consumed on a large scale as nutraceuticals and food supplements that can preserve optimal health. It is well known that “excess of everything is bad”, yet it is not generally recognized that a high intake of antioxidants may also have adverse effects. On the other hand, some antioxidants are used to illustrate general considerations on the toxicity of antioxidants. The toxicity of antioxidants can be evaluated with some recommendations, including classical safety factors, the knowledge of the efficacity mechanism, bio-kinetic/bio-efficacy modeling, and antioxidant supplementation changes into therapy being also of interest (Bast and Haenen, 2002). The study of Boroja et al. (2018) suggests that the methanolic extract of the summer savory aerial part protects against cisplatin-induced oxidative damage. Cisplatin was induced to produce toxicity using intraperitoneal injection (Boroja et al., 2018). The results confirm that summer savory could be a valuable source of dietary and therapeutic phenolic compounds. However, summer savory can maintain normal health conditions or may be a remedy for different oxidative damage diseases (Boroja et al., 2018). Furthermore, there are fewer known side effects of S. hortensis, but people who are taking medication for chronic diseases are cautioned about its use. The advice of a healthcare provider is mandatory before starting any new therapy or consumption of medicinal plants. S. hortensis is not recommended for children, pregnant women, or breastfeeding women due to a lack of sufficient evidence of its safe use in these populations (Hamidpour et al., 2014). In general, essential oils are comprised of a large variety of elements; hence, they do not appear to have any unique cellular targets. The presence of phenols, aldehydes, and alcohols in essential oils contributes to their cytotoxic effect (Bakkali et al., 2008). It appears that a distinction can be made between Satureja spp. and poisonous effects in eukaryotic cells and cytotoxic effects on microorganisms (yeast, viruses, fungi, and bacteria). The anti-pathogenic action of Satureja spp. has been well documented. Many people throughout the world consume Satureja spp. in the form of spice, herbal tea, and extracts. S. hortensis is consumed as a vegetable on a daily basis and has no known negative effects (Hajhashemi et al., 2000). Furthermore, an in vitro study showed that both the ethanolic extract and the essential oil of summer savory protected rat lymphocytes from hydrogen peroxide-induced damage (Behravan et al., 2006). In rats, the essential oil of S. khuzestanica was found to protect against the toxicity of Malathion (a commonly used organophosphorus) (Basiri et al., 2007). The leaves of S. gilliesii contain two isomeric monoterpene peroxides and they were found to be poisonous to Artemia salina in other investigations (brine shrimp bioassay) (Labbe et al., 1993).
7 Conclusion
It is concluded that the leaves and seeds of summer savory (Satureja hortensis L.) contain different chemical components. Summer savory leaves are abundant in total phenolic components, especially flavonoids and rosmarinic acid. It has strong antioxidant, antimicrobial, and antifungal effects that play a preventative role in human health. Furthermore, their oxidant activity suppresses the growth of many large tumor cells and the growth of HT-29 (human colon adenocarcinoma) cells. Carvacrol and Thymol are suppressing a rich diversity of microbes in S. hortensis, which have medicinal properties such as anti-diabetic, antispasmodic, anti-hyperlipidemic, anti-inflammatory, anti-proliferative, and anti-nociceptive properties.
Author contributions
Conceptualization, MA and AE; methodology, WK; software, WK; validation, SW, WK, and MK; formal analysis, HR; investigation, HR; resources, M-IL; data curation, WK; writing—original draft preparation, AE; writing—review and editing, SW, MU-I, and IC; visualization, M-IL, AB, and CM; supervision, MA., SM; project administration, SM; funding acquisition, SM. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Ministry of Research, Innovation, and Digitalization within Program 1—Development of national research and development system, Subprogram 1.2—Institutional Performance—RDI excellence funding projects, under contract No. 10PFE/2021.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aadil, R. M., Zeng, X., Ali, A., Zeng, F., Farooq, M. A., Han, Z., et al. (2015). Influence of different pulsed electric field strengths on the quality of the grapefruit juice. Int. J. food Sci. Technol. 50, 2290–2296. doi:10.1111/ijfs.12891
Abad, M. J., Bermejo, P., Gonzales, E., Iglesias, I., Irurzun, A., and Carrasco, L. (1999). Antiviral activity of Bolivian plant extracts. General Pharmacol. Vasc. Syst. 32, 499–503. doi:10.1016/s0306-3623(98)00214-6
Abdollahi, M., Salehnia, A., Mortazavi, S. H., Ebrahimi, M., Shafiee, A., Fouladian, F., et al. (2003). Antioxidant, antidiabetic, antihyperlipidemic, reproduction stimulatory properties and safety of essential oil of Satureja khuzestanica in rat in vivo: A oxicopharmacological study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 9, BR331–5.
Adiguzel, A., Ozer, H., KiliC, H., and CetiN, B. (2007). Screening of antimicrobial activity of essential oil and methanol extract of Satureja hortensis on foodborne bacteria and fungi. Czech J. food Sci. 25, 81–89. doi:10.17221/753-cjfs
Ahmadvand, H., Tavafi, M., and Khalatbary, A. R. (2012). Hepatoprotective and hypolipidemic effects of Satureja khuzestanica essential oil in alloxan-induced type 1 diabetic rats. Iran. J. Pharm. Res. IJPR 11, 1219–1226. doi:10.22037/ijpr.2012.1213
Akhalkatsi, M., Ekhvaia, J., and Asanidze, Z. (2012). Diversity and genetic erosion of ancient crops and wild relatives of agricultural cultivars for food: Implications for nature conservation in Georgia (caucasus). Perspectives on nature conservation-patterns, pressures and prospects rijeka. Croatia: InTech, 51–92.
Ali, A., Manzoor, M. F., Ahmad, N., Aadil, R. M., Qin, H., Siddique, R., et al. (2022). The burden of cancer, government strategic policies, and challenges in Pakistan: A comprehensive review. Front. Nutr. 9, 940514. doi:10.3389/fnut.2022.940514
Alizadeh, A., Khoshkhui, M., Javidnia, K., Firuzi, O., Tafazoli, E., and Khalighi, A. (2010). Effects of fertilizer on yield, essential oil composition, total phenolic content and antioxidant activity in Satureja hortensis L. (Lamiaceae) cultivated in Iran. J. Med. Plants Res. 4, 33–40. doi:10.5897/JMPR09.361
Amanlou, M., Dadkhah, F., Salehnia, A., Farsam, H., and Dehpour, A. R. (2005). An anti-inflammatory and anti-nociceptive effects of hydroalcoholic extract of Satureja khuzistanica Jamzad extract. J. Pharm. Pharm. Sci. 8, 102–106.
Antolovich, M., Prenzler, P. D., Patsalides, E., McDonald, S., and Robards, K. (2002). Methods for testing antioxidant activity. Analyst 127, 183–198. doi:10.1039/b009171p
Arnao, M. B. (2000). Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 11, 419–421. doi:10.1016/s0924-2244(01)00027-9
Arshad, M. S., Khalid, W., Khalid, M. Z., Maqbool, Z., Ali, A., Kousar, S., et al. (2023). Sonication microwave synergistic extraction of bioactive compounds from plants, animals and others agro-industrial waste sources.,” Ultrasound and Microwave for Food Processing. China: Elsevier, 345–361.
Attia, Y. A., and Al-Harthi, M. A. (2015). Nigella seed oil as an alternative to antibiotic growth promoters for broiler chickens. Eur. Poult. Sci. 79, 10–1399. doi:10.1399/eps.2015.80
Attia, Y. A., Bakhashwain, A. A., and Bertu, N. K. (2017). Thyme oil (Thyme vulgaris L.) as a natural growth promoter for broiler chickens reared under hot climate. Italian J. Animal Sci. 16, 275–282. doi:10.1080/1828051x.2016.1245594
Bakkali, F., Averbeck, S., Averbeck, D., and Idaomar, M. (2008). Biological effects of essential oils–a review. Food Chem. Toxicol. 46, 446–475. doi:10.1016/j.fct.2007.09.106
Basiri, S., Esmaily, H., Vosough-Ghanbari, S., Mohammadirad, A., Yasa, N., and Abdollahi, M. (2007). Improvement by Satureja khuzestanica essential oil of malathion-induced red blood cells acetylcholinesterase inhibition and altered hepatic mitochondrial glycogen phosphorylase and phosphoenolpyruvate carboxykinase activities. Pesticide Biochem. physiology 89, 124–129. doi:10.1016/j.pestbp.2007.04.006
Bast, A., and Haenen, G. R. M. M. (2002). The toxicity of antioxidants and their metabolites. Environ. Toxicol. Pharmacol. 11, 251–258. doi:10.1016/s1382-6689(01)00118-1
Baydar, H., Sağdiç, O., Özkan, G., and Karadoğan, T. (2004). Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food control. 15, 169–172. doi:10.1016/s0956-7135(03)00028-8
Behravan, J., Mosaffaa, F., Karimi, G., and Iranshahi, M. (2006). Antigenotoxic effects of Satureja hortensis L. on rat lymphocytes exposed to oxidative stress. Planta Medica 72, S_003. doi:10.1055/s-2006-949736
Ben Arfa, A., Combes, S., Preziosi-Belloy, L., Gontard, N., and Chalier, P. (2006). Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 43, 149–154. doi:10.1111/j.1472-765X.2006.01938.x
Bezić, N., Šamanić, I., Dunkić, V., Besendorfer, V., and Puizina, J. (2009). Essential oil composition and internal transcribed spacer (ITS) sequence variability of four South-Croatian Satureja species (Lamiaceae). Molecules 14, 925–938. doi:10.3390/molecules14030925
Bombik, T., Bombik, E., Frankowska, A., Trawińska, B., and Saba, L. (2012). Effect of herbal extracts on some haematological parameters of calves during rearing. J. Veterinary Res. 56, 655–658. doi:10.2478/v10213-012-0115-0
Boroja, T., Katanić, J., Rosić, G., Selaković, D., Joksimović, J., Mišić, D., et al. (2018). Summer savory (Satureja hortensis L.) extract: Phytochemical profile and modulation of cisplatin-induced liver, renal and testicular toxicity. Food Chem. Toxicol. 118, 252–263. doi:10.1016/j.fct.2018.05.001
Boyraz, N., and Özcan, M. (2006). Inhibition of phytopathogenic fungi by essential oil, hydrosol, ground material and extract of summer savory (Satureja hortensis L.) growing wild in Turkey. Int. J. food Microbiol. 107, 238–242. doi:10.1016/j.ijfoodmicro.2005.10.002
Brighente, I. M. C., Dias, M., Verdi, L. G., and Pizzolatti, M. G. (2007). Antioxidant activity and total phenolic content of some Brazilian species. Pharm. Biol. 45, 156–161. doi:10.1080/13880200601113131
Burlando, B., Verotta, L., Cornara, L., and Bottini-Massa, E. (2010). Herbal principles in cosmetics: Properties and mechanisms of action. Florida: CRC Press.
Caillet, S., Lessard, S., Lamoureux, G., and Lacroix, M. (2011). Umu test applied for screening natural antimutagenic agents. Food Chem. 124, 1699–1707. doi:10.1016/j.foodchem.2010.07.082
Can Baser, K. H. (2008). Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 14, 3106–3119. doi:10.2174/138161208786404227
Cetojevic-Simin, D. D., Bogdanovic, G. M., Cvetkovic, D. D., and Velicanski, A. S. (2008). Antiproliferative and antimicrobial activity of traditional Kombucha and Satureja Montana L. Kombucha. J. BUON 13, 395–401.
Chkhikvishvili, I., Sanikidze, T., Gogia, N., Mchedlishvili, T., Enukidze, M., Machavariani, M., et al. (2013). Rosmarinic acid-rich extracts of summer savory (Satureja hortensis L.) protect Jurkat T cells against oxidative stress. Oxid. Med. Cell. Longev. 2013. doi:10.1155/2013/456253
Chorianopoulos, N., Evergetis, E., Mallouchos, A., Kalpoutzakis, E., Nychas, G-J., and Haroutounian, S. A. (2006). Characterization of the essential oil volatiles of Satureja thymbra and Satureja parnassica: Influence of harvesting time and antimicrobial activity. J. Agric. food Chem. 54, 3139–3145. doi:10.1021/jf053183n
Chorianopoulos, N., Kalpoutzakis, E., Aligiannis, N., Mitaku, S., Nychas, G-J., and Haroutounian, S. A. (2004). Essential oils of Satureja, origanum, and thymus species: Chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food Chem. 52, 8261–8267. doi:10.1021/jf049113i
Cutler, K. D., Fisher, K., DeJohn, S., and Association, N. G. (2010). Herb gardening for dummies. USA: John Wiley & Sons.
Cvetanović, A., Švarc-Gajić, J., Gašić, U., Ž, Tešić, Zengin, G., Zeković, Z., et al. (2017). Isolation of apigenin from subcritical water extracts: Optimization of the process. J. Supercrit. Fluids 120, 32–42. doi:10.1016/j.supflu.2016.10.012
Daferera, D. J., Ziogas, B. N., and Polissiou, M. G. (2000). GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J. Agric. food Chem. 48, 2576–2581. doi:10.1021/jf990835x
Dikbas, N., Kotan, R., Dadasoglu, F., and Sahin, F. (2008). Control of Aspergillus flavus with essential oil and methanol extract of Satureja hortensis. Int. J. food Microbiol. 124, 179–182. doi:10.1016/j.ijfoodmicro.2008.03.034
Dorman, H. J. D., Peltoketo, A., Hiltunen, R., and Tikkanen, M. J. (2003). Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 83, 255–262. doi:10.1016/s0308-8146(03)00088-8
Exarchou, V., Nenadis, N., Tsimidou, M., Gerothanassis, I. P., Troganis, A., and Boskou, D. (2002). Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J. Agric. food Chem. 50, 5294–5299. doi:10.1021/jf020408a
Farmanpour-Kalalagh, K., Mohebodini, M., and Sabaghnia, N. (2020). Comparison and correlation of the compositions in volatile constituents from different parts of summer Savory (Satureja hortensis L.). Int. J. Hortic. Sci. Technol. 7, 295–304. doi:10.22059/ijhst.2020.292759.325
Fatima, K., Imran, M., Ahmad, M. H., Khan, M. K., Khalid, W., Al-Farga, A., et al. (2023). Ultrasound-assisted extraction of protein from Moringa oleifera seeds and its impact on techno-functional properties. Molecules 28, 2554. doi:10.3390/molecules28062554
Frankel, E. N., and Meyer, A. S. (2000). The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 80, 1925–1941. –1941. doi:10.1002/1097-0010(200010)80:13<1925::aid-jsfa714>3.0.co;2-4
Fuentes, E. J., Astudillo, L. A., Gutiérrez, M. I., Contreras, S. O., Bustamante, L. O., Rubio, P. I., et al. (2012). Fractions of aqueous and methanolic extracts from tomato (Solanum lycopersicum L.) present platelet antiaggregant activity. Blood coagulation fibrinolysis 23, 109–117. doi:10.1097/MBC.0b013e32834d78dd
Fukumoto, L. R., and Mazza, G. (2000). Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. food Chem. 48, 3597–3604. doi:10.1021/jf000220w
Gelovani, N., Tsintsadze, T., Tzikarishvili, Kh., and Targamadze, L. L. (2012). Savory (Saturea hortensis L.) in the Georgian historical chronicles. Trans. Georgian Tech. Univ. 483, 59–63.
Ghalfi, H., Benkerroum, N., Doguiet, D. D. K., Bensaid, M., and Thonart, P. (2007). Effectiveness of cell-adsorbed bacteriocin produced by Lactobacillus curvatus CWBI-B28 and selected essential oils to control Listeria monocytogenes in pork meat during cold storage. Lett. Appl. Microbiol. 44, 268–273. doi:10.1111/j.1472-765X.2006.02077.x
Ghazvinian, K., Seidavi, A., Laudadio, V., Ragni, M., and Tufarelli, V. (2018). Effects of various levels of organic acids and of virginiamycin on performance, blood parameters, immunoglobulins and microbial population of broiler chicks. South Afr. J. Animal Sci. 48, 961–967. doi:10.4314/sajas.v48i5.16
Grusak, M. A. (2002). Enhancing mineral content in plant food products. J. Am. Coll. Nutr. 21, 178S–183S. doi:10.1080/07315724.2002.10719263
Güllüce, M., Sökmen, M., Daferera, D., Aǧar, G., Özkan, H., Kartal, N., et al. (2003). In vitro antibacterial, antifungal, and antioxidant activities of the essential oil and methanol extracts of herbal parts and callus cultures of Satureja hortensis L. J. Agric. food Chem. 51, 3958–3965. doi:10.1021/jf0340308
Gursoy, U. K., Gursoy, M., Gursoy, O. V., Cakmakci, L., Könönen, E., and Uitto, V-J. (2009). Anti-biofilm properties of Satureja hortensis L. essential oil against periodontal pathogens. Anaerobe 15, 164–167. doi:10.1016/j.anaerobe.2009.02.004
Haeri, S., Minaie, B., Amin, G., Nikfar, S., Khorasani, R., Esmaily, H., et al. (2006). Effect of Satureja khuzestanica essential oil on male rat fertility. Fitoterapia 77, 495–499. doi:10.1016/j.fitote.2006.05.025
Hajhashemi, V., Ghannadi, A., and Pezeshkian, S. K. (2002). Antinociceptive and anti-inflammatory effects of Satureja hortensis L. extracts and essential oil. J. Ethnopharmacol. 82, 83–87. doi:10.1016/s0378-8741(02)00137-x
Hajhashemi, V., Sadraei, H., Ghannadi, A. R., and Mohseni, M. (2000). Antispasmodic and anti-diarrhoeal effect of Satureja hortensis L. essential oil. J. Ethnopharmacol. 71, 187–192. doi:10.1016/s0378-8741(99)00209-3
Hajhashemi, V., Zolfaghari, B., and Yousefi, A. (2012). Antinociceptive and anti-inflammatory activities of Satureja hortensis seed essential oil, hydroalcoholic and polyphenolic extracts in animal models. Med. Princ. Pract. 21, 178–182. doi:10.1159/000333555
Hamidpour, R., Hamidpour, S., Hamidpour, M., Shahlari, M., and Sohraby, M. (2014). Summer savory: From the selection of traditional applications to the novel effect in relief, prevention, and treatment of a number of serious illnesses such as diabetes, cardiovascular disease, Alzheimer’s disease, and cancer. J. Traditional Complementary Med. 4, 140–144. doi:10.4103/2225-4110.136540
Hashemipour, H., Kermanshahi, H., Golian, A., and Khaksar, V. (2014). Effects of carboxy methyl cellulose and thymol+ carvacrol on performance, digesta viscosity and some blood metabolites of broilers. J. animal physiology animal Nutr. 98, 672–679. doi:10.1111/jpn.12121
Jafari, F., Ghavidel, F., and Zarshenas, M. M. (2016). A critical overview on the pharmacological and clinical aspects of popular Satureja species. J. Acupunct. meridian Stud. 9, 118–127. doi:10.1016/j.jams.2016.04.003
Jain, K. A., and K Mehra N, K. Swarnakar N. (2015). Role of antioxidants for the treatment of cardiovascular diseases: Challenges and opportunities. Curr. Pharm. Des. 21, 4441–4455. doi:10.2174/1381612821666150803151758
Jamroz, D., Wiliczkiewicz, A., Wertelecki, T., Orda, J., and Skorupińska, J. (2005). Use of active substances of plant origin in chicken diets based on maize and locally grown cereals. Br. Poult. Sci. 46, 485–493. doi:10.1080/00071660500191056
Jang, I. S., Ko, Y. H., Kang, S. Y., and Lee, C. Y. (2007). Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Animal Feed Sci. Technol. 134, 304–315. doi:10.1016/j.anifeedsci.2006.06.009
Jayasinghe, C., Gotoh, N., Aoki, T., and Wada, S. (2003). Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 51, 4442–4449. doi:10.1021/jf034269o
Kagan, V. E., Gleiss, B., Tyurina, Y. Y., Tyurin, V. A., Elenstrom Magnusson, C., Liu, S-X., et al. (2002). A role for oxidative stress in apoptosis: Oxidation and externalization of phosphatidylserine is required for macrophage clearance of cells undergoing fas-mediated apoptosis. J. Immunol. 169, 487–499. doi:10.4049/jimmunol.169.1.487
Karimi, N., Yari, M., and Ghasmpour, H. R. (2012). Identification and comparison of essential oil composition and mineral changes in different phenological stages of Satureja hortensis L. Iran. J. Plant Physiol. 3 (1), 577–582.
Katar, D., Kacar, O., Kara, N., Aytaç, Z., Göksu, E., Kara, S., et al. (2017). Ecological variation of yield and aroma components of summer savory (Satureja hortensis L.). J. Appl. Res. Med. aromatic plants 7, 131–135. doi:10.1016/j.jarmap.2017.07.005
Kennedy, M. P. T., Cheyne, L., Darby, M., Plant, P., Milton, R., Robson, J. M., et al. (2018). Lung cancer stage-shift following a symptom awareness campaign. Thorax 73, 1128–1136. doi:10.1136/thoraxjnl-2018-211842
Khalid, W., Arshad, M. S., Aziz, A., Rahim, M. A., Qaisrani, T. B., Afzal, F., et al. (2023). Chia seeds (Salvia hispanica L.): A therapeutic weapon in metabolic disorders. Food Sci. Nutr. 11, 3–16. doi:10.1002/fsn3.3035
Khan, M. R., Batool, M., Amir, R. M., Shabbir, A., Siddique, F., Adil, R. M., et al. (2020). Ameliorating effects of okra (Abelmoschus esculentus) seed oil on hypercholesterolemia. Food Sci. Technol. 41, 113–119. doi:10.1590/fst.38919
Khlebnikova, D. A., Efanova, E. M., Danilova, N. A., Shcherbakova, Y. V., and Rivera Sidorova, I. (2022). Flavonoid accumulation in an aseptic culture of summer savory (Satureja hortensis L.). Plants 11, 533. doi:10.3390/plants11040533
Kim, J. E., Kim, A. R., Chung, H. Y., Han, S. Y., Kim, B. S., and Choi, J. S. (2003). In vitro peroxynitrite scavenging activity of diarylheptanoids from Curcuma longa. Phytotherapy Res. 17, 481–484. doi:10.1002/ptr.1179
Kim, M. J., Ohn, J., Kim, J. H., and Kwak, H-K. (2011). Effects of freeze-dried cranberry powder on serum lipids and inflammatory markers in lipopolysaccharide treated rats fed an atherogenic diet. Nutr. Res. Pract. 5, 404–411. doi:10.4162/nrp.2011.5.5.404
Kitsati, N., Fokas, D., Ouzouni, M-D., Mantzaris, M. D., Barbouti, A., and Galaris, D. (2012). Lipophilic caffeic acid derivatives protect cells against H2O2-Induced DNA damage by chelating intracellular labile iron. J. Agric. food Chem. 60, 7873–7879. doi:10.1021/jf301237y
Kizil, S., Turk, M., Özguven, M., and Khawar, K. M. (2009). Full blooming stage is suitable for herbage yield and essential oil content of summer savory (Satureja hortensis L.). J. Essent. Oil Bear. Plants 12, 620–629. doi:10.1080/0972060x.2009.10643765
Koparal, A. T., and Zeytinoğlu, M. (2003). Effects of carvacrol on a human non-small cell lung cancer (NSCLC) cell line, A549. Animal Cell. Technol. Basic & Appl. Aspects –211, 207. doi:10.1023/b:cyto.0000039917.60348.45
Kudełka, W., and Kosowska, A. (2008). Components of spices and herbs determining their functional properties and their role in human nutrition and prevention of diseases. Crac. Rev. Econ. Manag. 78, 83–111.
Labbe, C., Castillo, M., and Connolly, J. D. (1993). Mono and sesquiterpenoids from Satureja gilliesii. Phytochemistry 34, 441–444. doi:10.1016/0031-9422(93)80026-o
Lee, H. J., Cho, H-S., Park, E., Kim, S., Lee, S-Y., Kim, C-S., et al. (2008). Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology 250, 109–115. doi:10.1016/j.tox.2008.06.010
Lee, K-W., Everts, H., Kapperst, H. J., Yeom, K-H., and Beynen, A. C. (2003). Dietary carvacrol lowers body weight gain but improves feed conversion in female broiler chickens. J. Appl. Poult. Res. 12, 394–399. doi:10.1093/japr/12.4.394
Liolios, C. C., Gortzi, O., Lalas, S., Tsaknis, J., and Chinou, I. (2009). Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 112, 77–83. doi:10.1016/j.foodchem.2008.05.060
Mahboubi, M., and Kazempour, N. (2011). Chemical composition and antimicrobial activity of Satureja hortensis and Trachyspermum copticum essential oil. Iran. J. Microbiol. 3, 194–200.
Maqbool, Z., Arshad, M. S., Ali, A., Aziz, A., Khalid, W., Afzal, M. F., et al. (2022). Potential role of phytochemical extract from saffron in development of functional foods and protection of brain-related disorders. Oxidative Med. Cell. Longev. 2022, 6480590. doi:10.1155/2022/6480590
Martindale, J. L., and Holbrook, N. J. (2002). Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. physiology 192, 1–15. doi:10.1002/jcp.10119
Mašković, P., Veličković, V., Mitić, M., Đurović, S., Zeković, Z., Radojković, M., et al. (2017). Summer savory extracts prepared by novel extraction methods resulted in enhanced biological activity. Industrial Crops Prod. 109, 875–881. doi:10.1016/j.indcrop.2017.09.063
Mchedlishvili, D., Kuchukashvili, Z., Tabatadze, T., and Davitaia, G. (2005). Influence of flavonoids isolated from Satureja hortensis L. on hypercholesterolemic rabbits. Indian J. Pharmacol. 37, 259. doi:10.4103/0253-7613.16577
Mihajilov-Krstev, T., Radnović, D., Kitić, D., Stojanović-Radić, Z., and Zlatković, B. (2010). Antimicrobial activity of Satureja hortensis L. essential oil against pathogenic microbial strains. Archives Biol. Sci. 62, 159–166. doi:10.2298/abs1001159m
Mohtashami, S., Rowshan, V., Tabrizi, L., Babalar, M., and Ghani, A. (2018). Summer savory (Satureja hortensis L.) essential oil constituent oscillation at different storage conditions. Industrial crops Prod. 111, 226–231. doi:10.1016/j.indcrop.2017.09.055
Momtaz, S., and Abdollahi, M. (2008). A systematic review of the biological activities of Satureja L. species. Pharmacologyonline 2, 34–54.
Momtaz, S., and Abdollahi, M. (2010). An update on pharmacology of Satureja species; from antioxidant, antimicrobial, antidiabetes and anti-hyperlipidemic to reproductive stimulation. Int. J. Pharmacol. 6 (4), 346–353.
Movahhedkhah, S., Rasouli, B., Seidavi, A., Mazzei, D., Laudadio, V., and Tufarelli, V. (2019). Summer savory (Satureja hortensis L.) extract as natural feed additive in broilers: Effects on growth, plasma constituents, immune response, and ileal microflora. Animals 9, 87. doi:10.3390/ani9030087
Mueller, M., Hobiger, S., and Jungbauer, A. (2010). Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 122, 987–996. doi:10.1016/j.foodchem.2010.03.041
Mukhtar, S., Xiaoxiong, Z., Khalid, W., Moreno, A., and Lorenzo, J. M. (2023). Vitro antioxidant capacity of purified bioactive compounds in milk thistle seed (Silybum marianum) along with phenolic profile. Food Anal. Methods, 1–13. doi:10.1007/s12161-023-02449-w
Mumivand, H., Babalar, M., Hadian, J., and Tabatabaei, S. M. F. (2010). Influence of nitrogen and calcium carbonate application rates on the minerals content of summer savory (Satureja hortensis L.) leaves. Hortic. Environ. Biotechnol. 51, 173–177.
Nazzaro, F., Fratianni, F., De Martino, L., Coppola, R., and De Feo, V. (2013). Effect of essential oils on pathogenic bacteria. Pharmaceuticals 6, 1451–1474. doi:10.3390/ph6121451
Niki, E., and Noguchi, N. (2000). Evaluation of antioxidant capacity. What capacity is being measured by which method? IUBMB life 50, 323–329. doi:10.1080/713803736
Nobakht, A., Nobakht, M., and Safamehr, A. R. (2012). The effect of different levels of savory medicinal plant (Satureja hortensis L.) on growth performance, carcass traits, immune cells and blood biochemical parameters of broilers. Afr. J. Agric. Res. 7, 1456–1461. doi:10.5897/AJAR11.2120
Omidbeygi, M., Barzegar, M., Hamidi, Z., and Naghdibadi, H. (2007). Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food control. 18, 1518–1523. doi:10.1016/j.foodcont.2006.12.003
Orhan, F., Ceker, S., Anar, M., Agar, G., Arasoglu, T., and Gulluce, M. (2016). Protective effects of three luteolin derivatives on aflatoxin B 1-induced genotoxicity on human blood cells. Med. Chem. Res. 25, 2567–2577. doi:10.1007/s00044-016-1681-0
Osakabe, N., Takano, H., Sanbongi, C., Yasuda, A., Yanagisawa, R., Inoue, K., et al. (2004). Anti-inflammatory and anti-allergic effect of rosmarinic acid (RA); inhibition of seasonal allergic rhinoconjunctivitis (SAR) and its mechanism. Biofactors 21, 127–131. doi:10.1002/biof.552210125
Oussalah, M., Caillet, S., Saucier, L., and Lacroix, M. (2006). Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat. Meat Sci. 73, 236–244. doi:10.1016/j.meatsci.2005.11.019
Ozkan, G., Simsek, B., and Kuleasan, H. (2007). Antioxidant activities of Satureja cilicica essential oil in butter and in vitro. J. food Eng. 79, 1391–1396. doi:10.1016/j.jfoodeng.2006.04.020
Öztürk, M. (2012). Anticholinesterase and antioxidant activities of Savoury (Satureja thymbra L.) with identified major terpenes of the essential oil. Food Chem. 134, 48–54. doi:10.1016/j.foodchem.2012.02.054
Pintore, G., Usai, M., Bradesi, P., Juliano, C., Boatto, G., Tomi, F., et al. (2002). Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils from Sardinia and Corsica. Flavour Fragr. J. 17, 15–19. doi:10.1002/ffj.1022
Pourhossein, Z., Qotbi, A. A. A., Seidavi, A., Laudadio, V., Centoducati, G., and Tufarelli, V. (2015). Effect of different levels of dietary sweet orange (Citrus sinensis) peel extract on humoral immune system responses in broiler chickens. Animal Sci. J. 86, 105–110. doi:10.1111/asj.12250
Predescu, C., Papuc, C., Ștefan, G., and Petcu, C. (2020). Phenolics content, antioxidant and antimicrobial activities of some extracts obtained from Romanian summer savory and Lebanon wild thyme. Sci. Work. Ser. C. Vet. Med., 17–22.
Pritam, P., Deka, R., Bhardwaj, A., Srivastava, R., Kumar, D., Jha, A. K., et al. (2022). Antioxidants in Alzheimer’s disease: Current therapeutic significance and future prospects. Biology 11, 212. doi:10.3390/biology11020212
Radácsi, P., Inotai, K., Sárosi, S., and Németh, É. (2016). Effect of soil water content on the physiological parameters, production and active substances of summer savory (Satureja hortensis L.). Acta Sci. Pol. Hortorum Cultus 15, 3–12.
Radonic, A., and Milos, M. (2003). Chemical composition and in vitro evaluation of antioxidant effect of free volatile compounds from Satureja Montana L. Free Radic. Res. 37, 673–679. doi:10.1080/1071576031000105643
Razzaghi-Abyaneh, M., Shams-Ghahfarokhi, M., Yoshinari, T., Rezaee, M-B., Jaimand, K., Nagasawa, H., et al. (2008). Inhibitory effects of Satureja hortensis L. essential oil on growth and aflatoxin production by Aspergillus parasiticus. Int. J. food Microbiol. 123, 228–233. doi:10.1016/j.ijfoodmicro.2008.02.003
Rezvanpanah, S., Rezaei, K., Golmakani, M-T., and Razavi, S. H. (2011). Antibacterial properties and chemical characterization of the essential oils from summer savory extracted by microwave-assisted hydrodistillation. Braz. J. Microbiol. 42, 1453–1462. doi:10.1590/S1517-838220110004000031
Rocha, M., Searcy, C., and Karapetrovic, S. (2007). Integrating sustainable development into existing management systems. Total Qual. Manag. Bus. Excell. 18, 83–92. doi:10.1080/14783360601051594
Rodov, V., Vinokur, Y., Gogia, N., and Chkhikvishvili, I. (2010). Hydrophilic and lipophilic antioxidant capacities of Georgian spices for meat and their possible health implications. Georgian Med. News 179, 61–66.
Roobab, U., Aadil, R. M., Madni, G. M., and Bekhit, A. E. (2018). The impact of nonthermal technologies on the microbiological quality of juices: A review. Compr. Rev. Food Sci. Food Saf. 17, 437–457. doi:10.1111/1541-4337.12336
Ross, D., Mendiratta, S., Qu, Z., Cobb, C. E., and May, J. M. (1999). Ascorbate 6-palmitate protects human erythrocytes from oxidative damage. Free Radic. Biol. Med. 26, 81–89. doi:10.1016/s0891-5849(98)00198-1
Ruberto, G., and Baratta, M. T. (2000). Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 69, 167–174. doi:10.1016/s0308-8146(99)00247-2
Şahin, F., Karaman, I., Güllüce, M., Öğütçü, H., Şengül, M., Adıgüzel, A., et al. (2003). Evaluation of antimicrobial activities of Satureja hortensis L. J. Ethnopharmacol. 87, 61–65. doi:10.1016/s0378-8741(03)00110-7
Saita, E., Kishimoto, Y., Tani, M., Iizuka, M., Toyozaki, M., Sugihara, N., et al. (2012). Antioxidant activities of Perilla frutescens against low-density lipoprotein oxidation in vitro and in human subjects. J. oleo Sci. 61, 113–120. doi:10.5650/jos.61.113
Sanbongi, C., Takano, H., Osakabe, N., Sasa, N., Natsume, M., Yanagisawa, R., et al. (2003). Rosmarinic acid inhibits lung injury induced by diesel exhaust particles. Free Radic. Biol. Med. 34, 1060–1069. doi:10.1016/s0891-5849(03)00040-6
Sefidkon, F., Jamzad, Z., and Mirza, M. (2004). Chemical variation in the essential oil of Satureja sahendica from Iran. Food Chem. 88, 325–328. doi:10.1016/j.foodchem.2003.12.044
Šeregelj, V., Šovljanski, O., Švarc-Gajić, J., Cvanić, T., Ranitović, A., Vulić, J., et al. (2022). Modern green approaches for obtaining Satureja kitaibelii Wierzb. ex Heuff extracts with enhanced biological activity. J. Serbian Chem. Soc. 87, 1359–1365. doi:10.2298/jsc220314043s
Shanaida, M., Jasicka-Misiak, I., and Wieczorek, P. P. (2021). Chromatographic analysis of polyphenols in the Satureja hortensis L. (Lamiaceae Martinov) herb. Pharmacol. OnLine 2, 1337–1345. doi:10.11603/2312-0967.2022.4.13748
Shirzad, H., Hassani, A., Abdollahi, A., Ghosta, Y., and Finidokht, S. R. (2011). Assessment of the preservative activity of some essential oils to reduce postharvest fungal rot on kiwifruits (Actinidia deliciosa). J. Essent. Oil Bear. Plants 14, 175–184. doi:10.1080/0972060x.2011.10643919
Silva, F. V. M., Martins, A., Salta, J., Neng, N. R., Nogueira, J. M. F., Mira, D., et al. (2009). Phytochemical profile and anticholinesterase and antimicrobial activities of supercritical versus conventional extracts of Satureja montana. J. Agric. Food Chem. 57, 11557–11563. doi:10.1021/jf901786p
Silva, L. V., Nelson, D. L., Drummond, M. F. B., Dufossé, L., and Glória, M. B. A. (2005). Comparison of hydrodistillation methods for the deodorization of turmeric. Food Res. Int. 38, 1087–1096. doi:10.1016/j.foodres.2005.02.025
Sinyor, B., Mineo, J., and Ochner, C. (2020). Alzheimer’s disease, inflammation, and the role of antioxidants. J. Alzheimer’s Dis. Rep. 4, 175–183. doi:10.3233/ADR-200171
Skočibušić, M., Bezić, N., and Dunkić, V. (2006). Phytochemical composition and antimicrobial activities of the essential oils from Satureja subspicata Vis. growing in Croatia. Food Chem. 96, 20–28. doi:10.1016/j.foodchem.2005.01.051
Slavkovska, V., Jancic, R., Bojovic, S., Milosavljevic, S., and Djokovic, D. (2001). Variability of essential oils of Satureja Montana L. and Satureja kitaibelii Wierzb. ex Heuff. from the central part of the Balkan peninsula. Phytochemistry 57, 71–76. doi:10.1016/s0031-9422(00)00458-1
Soković, M., Tzakou, O., Pitarokili, D., and Couladis, M. (2002). Antifungal activities of selected aromatic plants growing wild in Greece. Food/Nahrung 46, 317–320. doi:10.1002/1521-3803(20020901)46:5<317::AID-FOOD317>3.0.CO;2-B
Soltani, F., Heidari, S., Azizi, M., and Hadian, J. (2014). Influence of Ca(NO3)2 and KNO3 application on biomass, yield, oil and mineral contents of Tarragon in “Ray” region. J. Agric. Sci. 7, 19–29. doi:10.5539/jas.v7n1p19
Stankov, S., Fidan, H., Lazarov, A., Gandova, V., Stoyanova, A., and Dincheva, I. Evaluation of the chemical composition and antimicrobial activity of summer savory (Satureja hortensis L.) essential oil. 2022International Conference on Energy Efficiency and Agricultural Engineering (EE&AE). 29-june-2023, Bulgaria, IEEE.
Svoboda, K. P., and Greenaway, R. I. (2003). Investigation of volatile oil glands of Satureja hortensis L. (summer savory) and phytochemical comparison of different varieties. Int. J. Aromather. 13, 196–202. doi:10.1016/s0962-4562(03)00038-9
Szőllősi, R., and Varga, I. S. I. (2002). Total antioxidant power in some species of Labiatae: Adaptation of FRAP method. Acta Biol. Szeged. 46, 125–127.
Tavafi, M., and Ahmadvand, H. (2011). Effect of rosmarinic acid on inhibition of gentamicin induced nephrotoxicity in rats. Tissue Cell. 43, 392–397. doi:10.1016/j.tice.2011.09.001
Torres-Urrutia, C., Guzman, L., Schmeda-Hirschmann, G., Moore-Carrasco, R., Alarcon, M., Astudillo, L., et al. (2011). Antiplatelet, anticoagulant, and fibrinolytic activity in vitro of extracts from selected fruits and vegetables. Blood coagulation fibrinolysis 22, 197–205. doi:10.1097/MBC.0b013e328343f7da
Uslu, C., Karasen, R. M., Sahin, F., Taysi, S., and Akcay, F. (2003). Effects of aqueous extracts of Satureja hortensis L. on rhinosinusitis treatment in rabbit. J. Ethnopharmacol. 88, 225–228. doi:10.1016/s0378-8741(03)00236-8
Vagionas, K., Graikou, K., Ngassapa, O., Runyoro, D., and Chinou, I. (2007). Composition and antimicrobial activity of the essential oils of three Satureja species growing in Tanzania. Food Chem. 103, 319–324. doi:10.1016/j.foodchem.2006.07.051
Vosough-Ghanbari, S., Rahimi, R., Kharabaf, S., Zeinali, S., Mohammadirad, A., Amini, S., et al. (2010). Effects of Satureja khuzestanica on serum glucose, lipids and markers of oxidative stress in patients with type 2 diabetes mellitus: A double-blind randomized controlled trial. Evidence-Based Complementary Altern. Med. 7, 465–470. doi:10.1093/ecam/nen018
Waliat, S., Arshad, M. S., Hanif, H., Ejaz, A., Khalid, W., Kauser, S., et al. (2023). A review on bioactive compounds in sprouts: Extraction techniques, food application and health functionality. Int. J. Food Prop. 26, 647–665. doi:10.1080/10942912.2023.2176001
Wilkinson, J. M., Hipwell, M., Ryan, T., and Cavanagh, H. M. A. (2003). Bioactivity of Backhousia citriodora: Antibacterial and antifungal activity. J. Agric. Food Chem. 51, 76–81. doi:10.1021/jf0258003
Yazdanparast, R., and Shahriyary, L. (2008). Comparative effects of Artemisia dracunculus, Satureja hortensis and Origanum majorana on inhibition of blood platelet adhesion, aggregation and secretion. Vasc. Pharmacol. 48, 32–37. doi:10.1016/j.vph.2007.11.003
Yildirim, A., Mavi, A., Oktay, M., Kara, A. A., Algur, O. F., and Bilaloglu, V. (2000). Comparison of antioxidant and antimicrobial activities of tilia (Tilia argentea Desf ex DC), sage (Salvia triloba L.), and black tea (Camellia sinensis) extracts. J. Agric. food Chem. 48, 5030–5034. doi:10.1021/jf000590k
Keywords: antimicrobial activity, chemical composition, antioxidant activity, health benefits, nutraceutical, additive, health benefits γ-terpinene, food applications
Citation: Ejaz A, Waliat S, Arshad MS, Khalid W, Khalid MZ, Rasul Suleria HA, Luca M-I, Mironeasa C, Batariuc A, Ungureanu-Iuga M, Coţovanu I and Mironeasa S (2023) A comprehensive review of summer savory (Satureja hortensis L.): promising ingredient for production of functional foods. Front. Pharmacol. 14:1198970. doi: 10.3389/fphar.2023.1198970
Received: 02 April 2023; Accepted: 03 July 2023;
Published: 24 July 2023.
Edited by:
Monica Trif, Centre for innovative process engineering, GermanyReviewed by:
Sidra Jabeen, University of Agriculture, PakistanKhalid Ali Khan, King Khalid University, Saudi Arabia
Copyright © 2023 Ejaz, Waliat, Arshad, Khalid, Khalid, Rasul Suleria, Luca, Mironeasa, Batariuc, Ungureanu-Iuga, Coţovanu and Mironeasa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Sajid Arshad, msajidarshad@gcuf.edu.pk; Waseem Khalid, waseem.khalid@uifst.uol.edu.pk; Mădălina Ungureanu-Iuga, madalina.iuga@usm.ro; Silvia Mironeasa, silviam@fia.usv.ro
 Afaf Ejaz
Afaf Ejaz Sadaf Waliat1
Sadaf Waliat1 Waseem Khalid
Waseem Khalid Muhammad Zubair Khalid
Muhammad Zubair Khalid Hafiz Ansar Rasul Suleria
Hafiz Ansar Rasul Suleria Ana Batariuc
Ana Batariuc Mădălina Ungureanu-Iuga
Mădălina Ungureanu-Iuga Ionica Coţovanu
Ionica Coţovanu Silvia Mironeasa
Silvia Mironeasa