- 1Department of Nephrology, Xijing Hospital, Fourth Military Medical University of PLA, Xi’an, China
- 2Department of Nephrology, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, China
- 3M.S. in Biostatistics, Columbia University Mailman School of Public Health, New York, NY, United States
In this study, we aimed to evaluate the efficacy and safety of tacrolimus-based treatment for immunoglobulin A nephropathy (IgAN). We retrospectively reviewed 127 adult patients with primary IgAN with 24 h urine total protein quantity (24 h UTP) ≥ 1 g and serum creatinine ≤3 mg/dL. All patients were divided into tacrolimus (TAC) and control (non-TAC) groups according to the treatment strategy. Proteinuria remission, remission rate, and adverse events were compared between the two groups. Among the 127 patients, 61 received TAC-based treatment and 66 received non-TAC treatment. TAC group exhibited a more rapid decline in proteinuria than the non-TAC group at 3, 9, and 12 months (p = 0.049, 0.001, and 0.018, respectively). Remission rates at 1, 3, 6, 9, and 12 months were 41.0, 68.9, 80.3, 90.2, and 88.5%, respectively, in the TAC group. These rates were higher than those in the control group at 3, 9, and 12 months (p = 0.030, 0.008, and 0.026, respectively). Complete remission rates at 1, 3, 6, 9, and 12 months were 6.56, 19.7, 37.7, 54.1, and 62.3%, respectively, in the TAC group. These rates were higher than those in the control group at 9 and 12 months (p = 0.013 and 0.008, respectively). The estimated mean time to complete remission was significantly shorter in the TAC group than in the control group (p = 0.028). TAC did not increase the incidence of adverse events. In conclusion, TAC accelerated proteinuria remission in patients with non-rapidly progressive IgAN with no increased risk of adverse events. Further prospective randomized controlled trials are necessary to validate our findings.
Highlights
1. Tacrolimus increased proteinuria remission in patients with IgA nephropathy
2. Tacrolimus-based treatment resulted in a higher remission rate than non-tacrolimus treatment
3. Tacrolimus did not increase the incidence of adverse events
1 Introduction
Immunoglobulin A nephropathy (IgAN) is the most common form of glomerulonephritis that is characterized by prominent mesangial deposits of IgA (Rajasekaran et al., 2021). IgAN is the primary cause of end-stage renal disease worldwide, with up to 30%–50% of the affected patients developing end-stage renal disease within 20–30 years after diagnosis (Maixnerova & Tesar, 2020). Proteinuria, glomerular filtration rate, and hypertension have been reported as the independent indicators of poor outcomes in patients (Maixnerova et al., 2014; Tan et al., 2015). Increased neutrophil-to-lymphocyte ratio, serum uric acid levels, and deposition of complement C3 have also been identified as independent risk factors for the progression of IgAN (Caliskan et al., 2016; Wang et al., 2021). Patients with IgAN have various clinical presentations and histological lesions, resulting in highly variable outcomes. Clinical presentation of IgAN ranges from asymptomatic hematuria to rapidly progressing renal failure (Pattrapornpisut et al., 2021). Prevalence of IgAN varies according to age. IgAN is most prevalent in Asians, with high risk of disease progression, severe clinical manifestations, and commonly reported active lesions, than in Europeans (Zhang & Barratt, 2021), indicating the necessity of using different therapeutic strategies for different patient populations.
To date, there is no established standardized treatment for IgAN owing to variations in the prevalence and progression of this disease. Treatments for IgAN focus predominantly on supportive care, such as measures to reduce proteinuria, decrease blood pressure, and minimize lifestyle risk factors, and non-specific immunosuppression (Floege et al., 2021). Rapidly progressive IgAN is defined as a ≥50% decline in eGFR over ≤3 months. Patients with rapidly progressive IgAN have a poor outcome and should be offered treatment with cyclophosphamide and glucocorticoids (Kidney Disease: Improving Global Outcomes KDIGO, 2021). Nevertheless, the additive benefits of currently available immunosuppressive agents, such as glucocorticoids (GCs), acetazolamide, cyclophosphamide, and mycophenolate mofetil (MMF), remain controversial (Floege et al., 2021; Pattrapornpisut et al., 2021; Aron, 2022), especially in non-rapidly progressive diseases (Natale et al., 2020).
Tacrolimus (TAC) is a calcineurin inhibitor widely used to treat autoimmune diseases and an anti-rejection drug for organ transplants (Ong & Gaston, 2021; Schumacher et al., 2021). TAC binds to T lymphocyte-specific FK506-binding protein (FKBP) to form the TAC-FKBP12 complex, which then binds to calcineurin and inhibits the expression of cytokines related to T-lymphocyte activation, thereby inhibiting the immune response (Kandikattu & Mishra, 2018; Ong & Gaston, 2021). TAC is also used for the immunosuppressive treatment of primary and secondary glomerular diseases (Hannah et al., 2016; Zhu et al., 2017), including IgAN. Several studies have demonstrated the efficacy of TAC in the treatment of refractory IgAN (Zhang et al., 2012; Hu et al., 2018; Yan et al., 2022). However, its efficacy and safety in patients with non-rapidly progressive IgAN remain unknown. In this study, we retrospectively reviewed 127 cases of non-rapidly progressive IgAN to evaluate the safety and efficacy of TAC-based treatment for IgAN by comparing it with non-TAC treatment.
2 Materials and methods
2.1 Patients
This retrospective cohort study enrolled patients with primary IgAN who underwent treatmet at the Xijing Hospital, Fourth Military Medical University, between August 2017 and July 2020. Inclusion criteria were as follows: 1) patients diagnosed with primary IgAN via renal biopsy and 2) decrease in the estimated glomerular filtration rate (eGFR) to <25% within 3 months. Exclusion criteria were as follows: 1) patient age <16 or ≥75 years, 2) 24 hurine total protein quantity (24 hUTP) < 1 g, 3) serum creatinine >3 mg/dL, 4) follow-up period <1 year, 5) presence of comorbidities, such as diabetes, hepatitis B, cancer, and amyloidosis, and 6) incomplete data or self-discontinuation of treatment. A total of 127 patients were enrolled in the study and divided into TAC-based (n = 61) and non-TAC (control, n = 66) treatment groups based on their therapeutic strategy. This study was approved by the Ethics Committee of Xijing Hospital, and the requirement for informed consent was waived because of the retrospective study design.
2.2 Treatment protocol
TAC was administered at a dose of 0.05–0.1 mg/kg/d, orally divided twice a day, and which was adjusted according to the blood concentration as 5–10 ng/L (Kidney Disease: Improving Global Outcomes KDIGO, 2021). TAC dose was gradually decreased (25%–33% decrease every 2 months) 3 months later or at the time of complete remission of proteinuria and was not changed when the dose decreased to 1 mg/d, and this treatment plan was maintained for 12 months. The treatment regimen in the non-TAC group included GCs, GC plus cyclophosphamide (GC + CTX), GC plus MMF (GC + MMF), and MMF. GCs were initially administered at dose of 30–50 mg/d with oral administration once a day, which was gradually decreased to 5 mg/month after 8 weeks and then to 2.5 mg/month when the dose tapered to 20 mg. CTX was administered intravenously at a dose of 0.8 g (weight ≤70 kg) or 1.0 g (weight >70 kg) per month and stopped when the total dose reached 7–8 g. MMF was administered at a dose of 1.5 g/day with oral administration twice a day, which was gradually decreased to 0.5 g/day 6 months later or at the time of complete remission of proteinuria, and this treatment plan was maintained for 12 months. Renin angiotensin system (RAS) inhibitors were used in all patients who tolerated RAS inhibitors, and the dose was gradually increased to the maximum tolerable dose over 3 months and maintained until the end of the study.
2.3 Data collection
Two authors (L.Z. and Y.Y.) simultaneously collected and entered the data, and any disagreements were resolved via discussion. Data, including sex, age, complications, renal biopsy, biochemical indices (blood routine, alanine aminotransferase, aspartate transaminase, cholesterol, triglyceride, blood glucose, uric acid, electrolyte, creatinine, eGFR, and 24hUTP) before treatment, and medications, were retrieved from the medical records of the patients. All patients were followed-up at least once every one-to-three months, and the above-mentioned biochemical indices and adverse events were recorded.
2.4 Outcomes and definitions
Complete remission (CR) was defined as urinary protein excretion <0.3 g/day with a decrease in eGFR to <10% of baseline. Partial remission (PR) was defined as a decrease in proteinuria to >50% of baseline and a decrease in eGFR to <10% of baseline. Patients with decreased proteinuria (<50% of baseline) or eGFR (>10% of baseline) were considered to be resistant to TAC-based treatment. Remission time was defined as the time from the start of TAC-based treatment until a complete or partial response was achieved. CR, PR and total response rates were calculated and recorded separately at months 1, 3, 6, 9, and 12.
2.5 Statistical analysis
Continuous and categorical variables were expressed as the means with standard deviations and event numbers with percentages, respectively. Normally distributed continuous variables were compared using the t-test, while others were subjected to the Mann–Whitney rank test. Categorical variables were analyzed using the Chi-square or Fisher’s exact tests. Statistical analysis was conducted using the SPSS 16.0 and GraphPad Prism 9.0 software packages. Statistical significance was set at p < 0.05. Estimated mean complete remission time was calculated using Kaplan–Meier curves and compared using the log-rank test.
3 Results
3.1 Baseline characteristics of enrolled patients
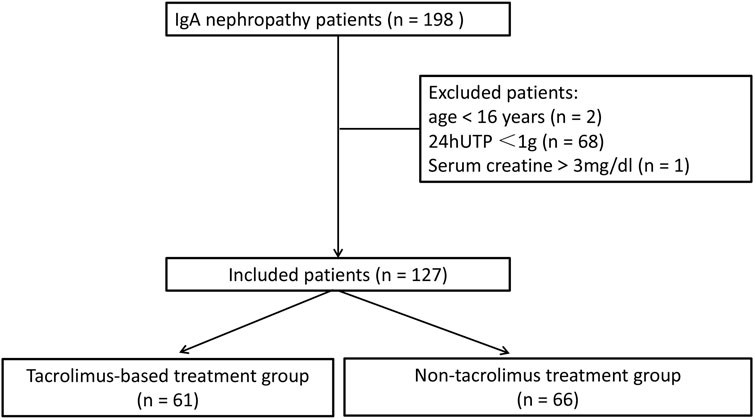
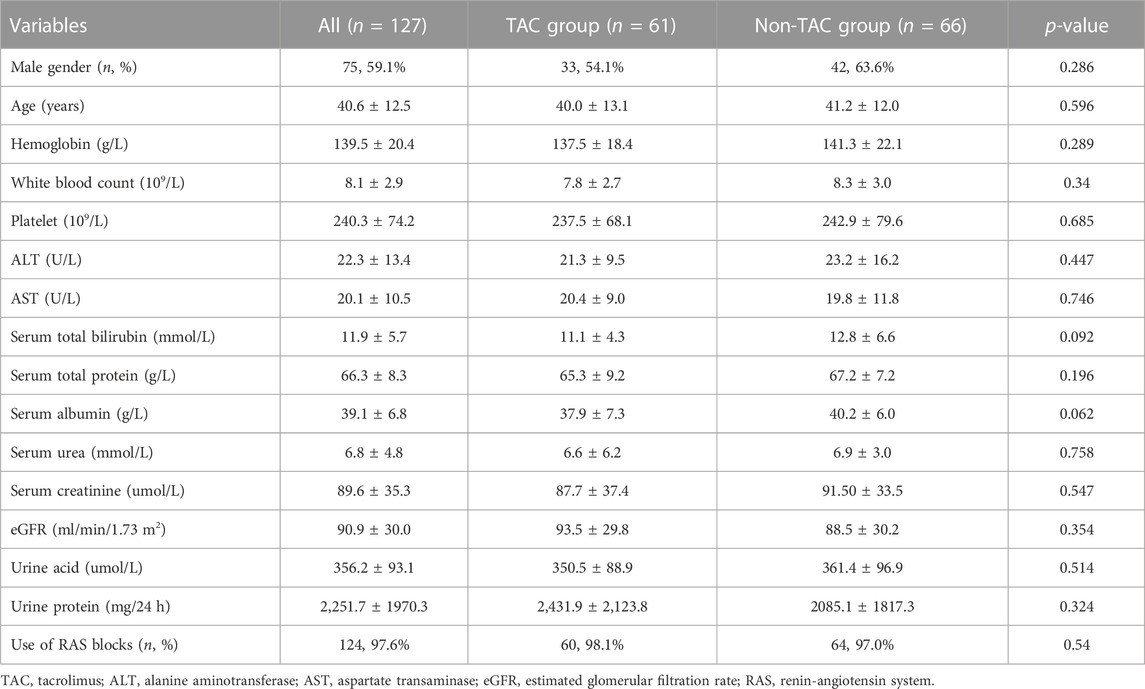
Between August 2017 and July 2020, 198 patients were diagnosed with primary IgAN. Of these, 71 patients who did not meet the inclusion criteria, including two patients aged <16 years, 68 patients with 24 hUTP <1 g, and one patient with serum creatinine >3 mg/dL, were excluded from the study. Finally, 127 patients were enrolled, of whom 61 received TAC-based treatment and 66 received non-TAC treatment (Figure 1). Baseline characteristics of the included patients are presented in Table 1. The mean age of 127 patients was 40.6 ± 12.5 years, and 59.1% patients (n = 75) were males. Baseline serum albumin was 39.1 ± 6.8 g/L. Baseline creatinine and eGFR were 89.6 ± 35.3 μmol/L and 90.9 ± 30.0 mL/min/1.73 m2, respectively. Baseline 24 hUTP was 2,251.7 ± 1970.3 mg. Of the 127 patients, approximately 97.6% (n = 124) were treated with RAS blockade. All baseline characteristics showed no statistically significant differences between the two groups, indicating the comparability of the clinical outcomes of the treatments.
3.2 Responses to treatment
3.2.1 Changes in urine protein and serum albumin levels
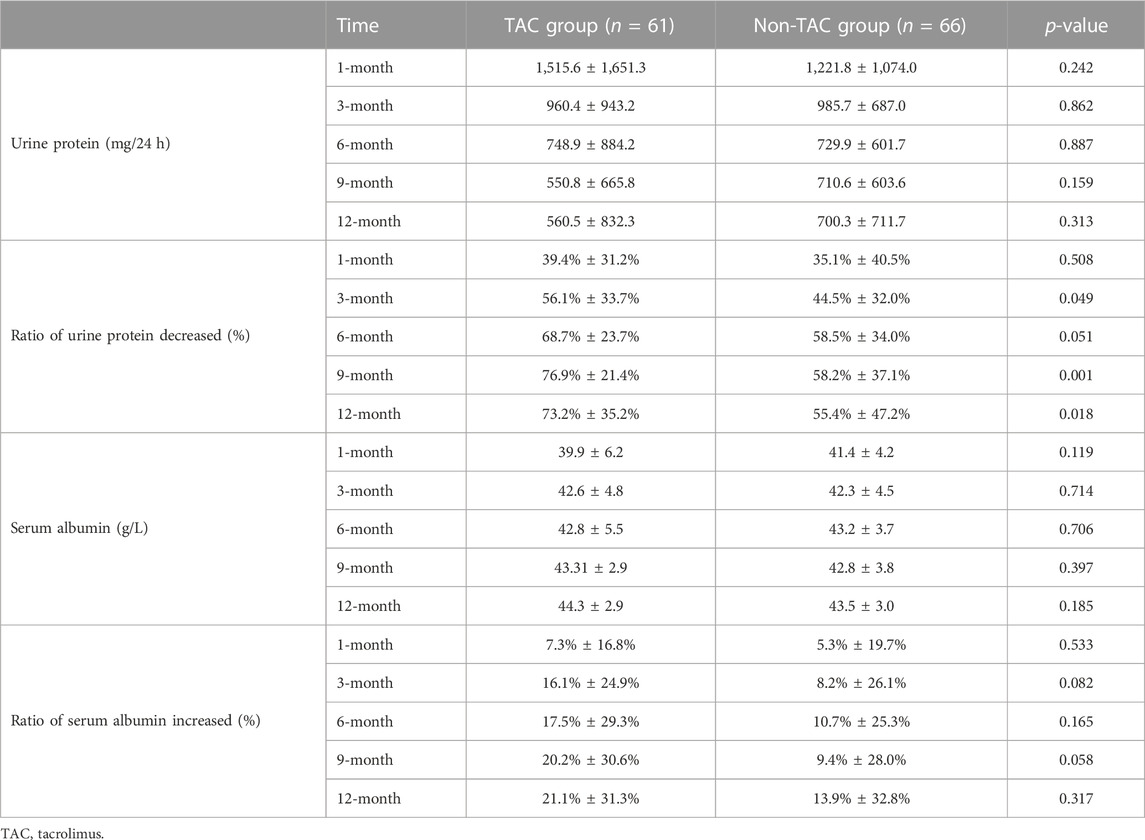
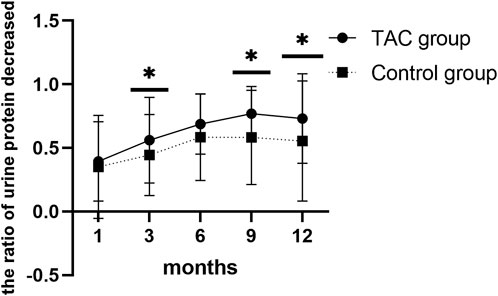
Urine protein levels decreased significantly 1 month after TAC-based treatment (2,431.9 ± 2,123.8 vs. 1,515.6 ± 1,651.3; p = 0.009). Serum albumin levels also increased 1 month after TAC-based treatment, with no statistical significance (37.9 ± 7.3 vs. 39.9 ± 6.2; p = 0.106), and 3 months after TAC-based treatment (37.9 ± 7.3 vs. 42.6 ± 4.8; p < 0.0001). Urine protein levels decreased (2085.1 ± 1817.3 vs. 1,221.8 ± 1,074.0; p = 0.091) and serum albumin levels (40.2 ± 6.0 vs. 41.4 ± 4.2; p = 0.105) also increased 1 month after non-TAC treatment with no statistical significance. Changes in urine protein and serum albumin levels during the 12-month treatment plan in both groups are presentedin Table 2. Urine protein levels persistently decreased and serum albumin levels persistently increased with increasing treatment time in both groups. However, TAC group showed higher decrease in urine protein levels than the non-TAC group at 3 months (56.1 ± 33.7 vs. 44.5 ± 32.0; p = 0.049), 9 months (76.9 ± 21.4 vs. 58.2 ± 37.1; p = 0.001) and 12 months (73.2% ± 35.2% vs. 55.4% ± 47.2%; p = 0.018) (Figure 2).
3.2.2 Remission rate
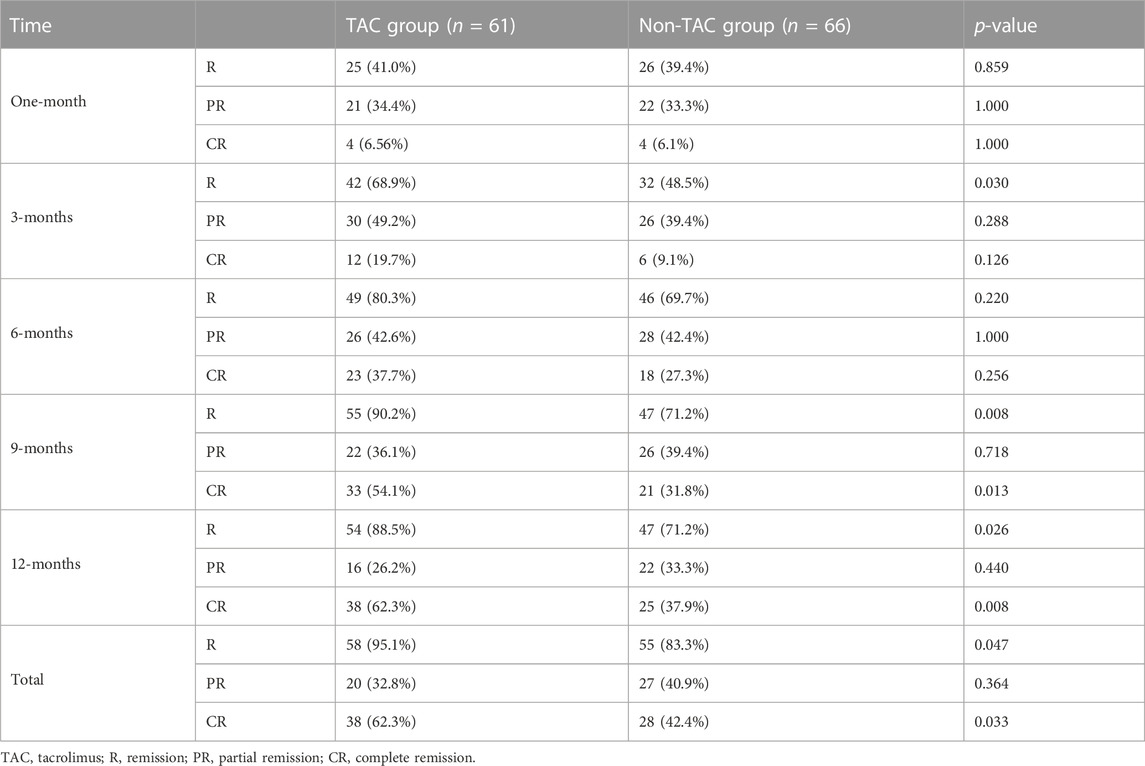
As shown in Table 3, the remission rates at 1, 3, 6, 9, and 12 months after TAC-based treatment were 41.0, 68.9, 80.3, 90.2, and 88.5%, respectively. In the non-TAC group, the remission rates at 1, 3, 6, 9, and 12 months were 39.4, 48.5, 69.7, 71.2, and 71.2%, respectively. Moreover, the remission rate was 68.9% 3 months after TAC-based treatment, which was significantly higher than that in the non-TAC group (68.9% vs. 48.5%; p = 0.030). Similarly, the remission rates 9 months (90.2% vs. 71.2%; p = 0.008) and 12 months (88.5% vs. 71.2%; p = 0.026) after TAC-based treatment were significantly higher than those in the non-TAC group. After TAC-based treatment, CR rates at 9 months (54.1% vs. 31.8%, p = 0.013) and 12 months (62.3% vs. 37.9%, p = 0.008) were significantly higher than those in the non-TAC group. Overall, the TAC group showed a markedly higher total remission (95.1% vs. 83.3%; p = 0.047) and CR (62.3% vs. 42.4%; p = 0.033) rates than the non-TAC group.
3.2.3 Remission time
Based on the Kaplan–Meier curve of remission time, the estimated mean time to achieve remission was 4.20 months (95% confidence interval [CI]: 2.978–5.415) in the TAC group, which was shorter than the mean time of 8.26 months (95% CI: 5.515–11.000) in the non-TAC group; however, the difference was not statistically significant (p = 0.077; Figure 3A). Meanwhile, the estimated mean time to achieve PR in the TAC group was similar to that in the non-TAC group (p = 0.907; Figure 3B). Notably, the estimated mean time to achieve CR was 18.44 months (95% CI: 14.157–22.723) in the TAC group, which was significantly shorter than the mean time of 27.07 months (95% CI: 22.484–31.660) in non-TAC group (p = 0.028; Figure 3C).
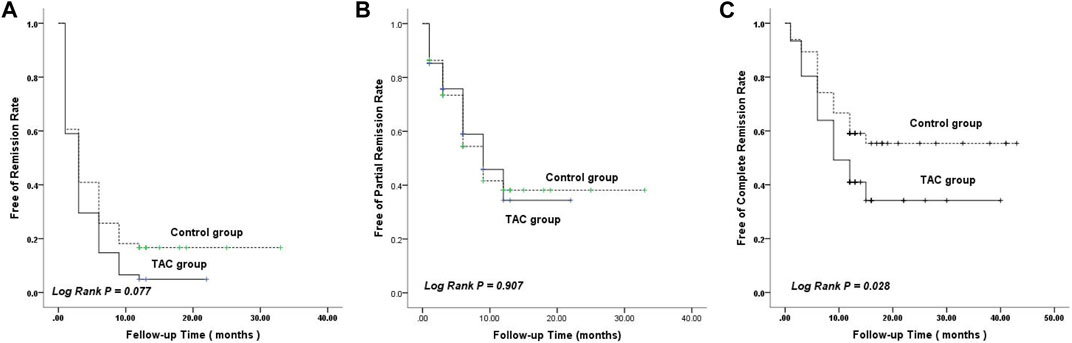
FIGURE 3. Kaplan–Meier curves ofremission rate. (A) Remission. (B) Partial remission. (C) Complete remission. TAC, tacrolimus.
3.3 Adverse events
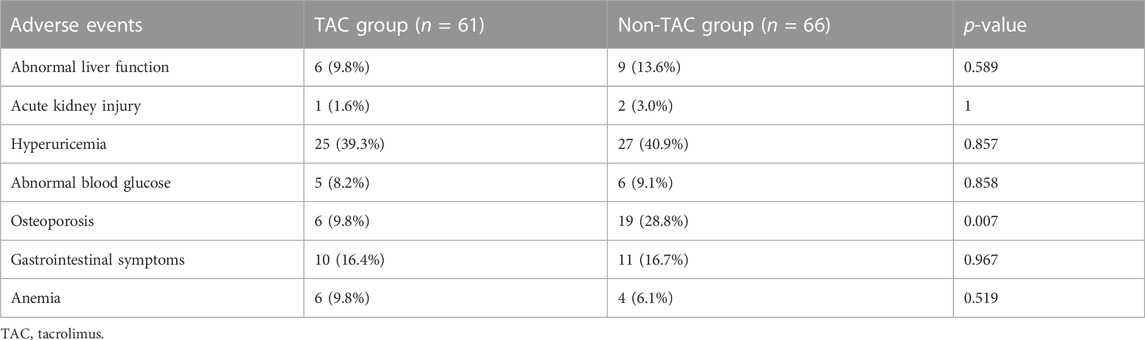
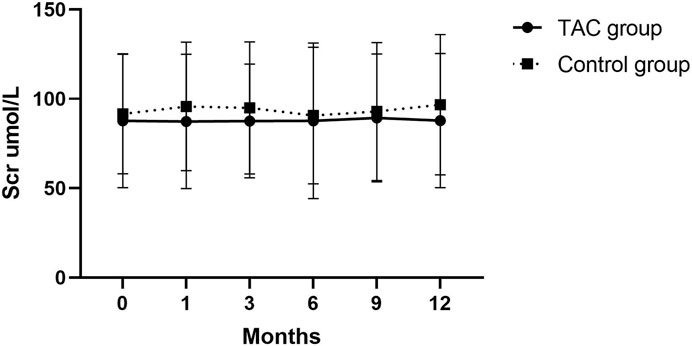
No severe infectious events occurred during the follow-up period. Main adverse events were abnormal liver function, acute kidney injury, hyperuricemia, elevated blood glucose levels, GC-related osteoporosis, gastrointestinal symptoms, and anemia (Table 4). In addition to GC-related osteoarthritis, the incidence rates of other adverse events were not significantly different between the two groups. Incidence rate of GC-related osteoarthritis (defined as prednisone ≥7.5 mg for >3 months and bone pain) in the TAC group was significantly lower than that in the non-TAC group (9.8% vs. 28.8%; p = 0.007). Total prednisone dosage was 269, 895 mg administered to 28 (45.9%) patients in the TAC group and 473,167 mg administered to 52 (78.8%) patients in the non-TAC group. Changes in serum creatinine levels were also analyzed during the follow-up period and no significant differences were observed between the two groups (Figure 4).
4 Discussion
In this study, we found that TAC effectively reduces proteinuria in patients with non-rapidly progressive IgAN, with a more rapid decline in proteinuria than that observed in the non-TAC group at 3, 9, and 12 months after the onset of treatment. Total remission (95.1%) and CR (62.3%) rates were significantly higher in the TAC group than in the non-TAC group. These results were also observed 9 and 12 months after the onset of treatment. Estimated mean time to achieve CR was 18.44 months in the TAC group, which was significantly shorter than the mean time of 27.07 months in the non-TAC group. In addition, the TAC group did not show an increase in the occurrence of adverse events. These results demonstrate the safety and efficacy of TAC treatment in patients with non-rapidly progressive IgAN. To the best of our knowledge, our study has the largest sample size to date for TAC treatment of IgAN.
Anti-proteinuric effects of TAC in IgAN have been reported in various studies (Hu et al., 2018; Yan et al., 2022), and their findings are consistent with those of our study. For example, TAC was reported to effectively decrease proteinuria in a prospective cohort study of 50 patients with IgAN (24 hUTP ≥ 2.0 g, estimated GFR ≥50 mL/min/1.73 m2) (Yan et al., 2022) and a retrospective observational study of 34 patients with refractory IgAN (Hu et al., 2018). A network meta-analysis revealed that TAC improved the remission of proteinuria (RR = 3.67; 95% CI = 1.06–12.63) in patients with IgAN better than that in patients receiving supportive care alone (Han et al., 2020), and the proteinuria remission rate of TAC treatment showed no significant differences (p = 0.7) with full-dose GCs (Yan et al., 2022). In our study, TAC treatment led to a significantly higher decrease in proteinuria at 3, 9 and 12 months and significantly higher total remission and CR rates than non-TAC treatment (including immunosuppressive agents CTX and MMF), which has not been reported in previous studies. In our study, the estimated mean time to achieve remission was 4.20 months (95% CI = 2.978–5.415) in the TAC group, which was longer than the mean remission time of 7.0 ± 4.7 weeks in the study of Hu et al. (Hu et al., 2018).
Yan et al. reported an increase in serum creatinine levels after TAC treatment, which later decreased to normal levels when the use of TAC was stopped, indicating a high incidence of renal insufficiency with TAC treatment (Yan et al., 2022). This finding is inconsistent with our results. We found no significant changes in the serum creatinine levels of patients during the follow-up period (12 months) (Figure 4), and acute kidney injury was observed in only one patient (1.6%), which was not significantly different from that in the non-TAC group (3.0%). Moreover, the anti-proteinuric effect and safety of TAC were evaluated based on a smaller sample size in these previous studies, whereas a larger sample size was included in our study. Therefore, the results of this study are more reliable. However, the anti-proteinuric effect of TAC can be reversed after stopping TAC use for 3 months (Yu et al., 2017; Han et al., 2020), indicating the necessity of investigating the long-term effects of TAC in future studies.
We also found that TAC significantly reduced the need for GCs. Only 45.9% of the patients in the TAC group received GCs compared to 78.8% in the non-TAC group. In TESTING studies (Effect of Oral Methylprednisolone on Clinical Outcomesin Patients with IgA Nephropathy: The TESTING Randomized Clinical Trial), GC-related side effects cannot be ignored, although GCs can restore the IgAN uroprotein levels. Our retrospective study found that TAC not only significantly reduced urinary protein levels but also significantly reduced GC consumption, thereby reducing GC-related adverse effects, such as osteoporosis.
Increasing evidence has demonstrated that the mechanism by which TAC reduces proteinuria in patients with IgAN is multifactorial. The first is an immunosuppressive mechanism, in which TAC inhibits the immune response by suppressing the transcription factors involved in activated T cell activity. However, other immunosuppressive agents, including CTX and MMF, were administered to the control group. TAC group showed a more rapid decline in proteinuria than non-TAC group at 9 months (76.9% ± 21.4% vs. 58.2% ± 37.1%; p = 0.001) and 12 months (73.2% ± 35.2% vs. 55.4% ± 47.2%; p = 0.018). Therefore, other non-immunosuppressive mechanisms may be involved in this process. TAC can induce renal vasoconstriction and increase renal vascular resistance (Textor et al., 1993; Textor et al., 1995); such changes in hemodynamics can decrease the permeability of proteins. This can be confirmed by the findings of a previous study, in which TAC was found to inhibit the production of vascular permeability factor (VPF) derived from T lymphocytes, which is considered the leading cause of massive proteinuria in minimal change nephrotic syndrome (Maruyama et al., 1994). Additionally, an effect of TAC on podocyte cytoskeletal stabilization has been proposed (Zhang et al., 2012). Qi et al. revealed that TAC protects podocytes from injury by upregulating the expression of podocin and nephrin and inhibiting the activation of macrophages (Qi et al., 2016). TAC can inhibit the redistribution of calcineurin and nephrin at the slit diaphragm, which is a unique cell–cell junction of podocytes that functions as an important barrier preventing the plasma proteins from leaking into urine (Wakamatsu et al., 2016). In addition, TAC can decrease proteinuria and restore podocyte injury by inhibiting the expression of angiopoietin-like-4 (a marker for predicting early podocyte injury) (Peng et al., 2014; Li et al., 2015) and calcineurin-binding protein 1 (Wen et al., 2016). Another study revealed that decreased expression of transient receptor potential canonical channels and calcineurin may be involved in the therapeutic effect of TAC on IgAN (Wei et al., 2017).
This study has several limitations: 1) The inherent limitations of the retrospective study design and small sample size, 2) the treatment protocol and doses were not standardized across patients in each group, which maybe confounding factors affecting the results, and 3) renal function and long-term effects of TAC were not investigated. Therefore, a well-designed, randomized controlled study with a larger sample size and long follow-up period is necessary to validate our findings.
In conclusion, TAC accelerated proteinuria remission in patients with non-rapidly progressive IgAN with no increased risk of adverse events. Moreover, TAC decreased the levels of GCs, thereby reducing GC-related side effects.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Xijing Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
GX conceived and designed the study and wrote the first draft of the manuscript. YY conducted the patient follow-up and collected the data. LZ sorted the data and wrote the manuscript. HX conducted data analysis and statistical processing. WL collected the data. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by the Key Project of Shaanxi Province (2022ZDLSF03-12), Project of Xijing Hospital (2022XJZT-YH08), and National Natural Science Foundation of China (Nos 81470993 and 81272621).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1189608/full#supplementary-material
References
Aron, A.W (2022). Is there a role for more intense immunosuppression in IgA nephropathy? Kidney360 3, 410–412. doi:10.34067/KID.0000512022
Caliskan, Y., Ozluk, Y., Celik, D., Oztop, N., Aksoy, A., Ucar, A S., et al. 2016.The clinical significance of uric acid and complement activation in the progression of IgA nephropathy. Kidney Blood Press. Res.41:148–157. doi:10.1159/000443415
Floege, J., Rauen, T., and Tang, S C W. (2021). Current treatment of IgA nephropathy. Semin. Immunopathol. 43, 717–728. doi:10.1007/s00281-021-00888-3
Han, S., Yao, T., Lu, Y., Chen, M., Xu, Y., and Wang, Y. (2020). Efficacy and safety of immunosuppressive monotherapy agents for IgA nephropathy: A network meta-analysis. Front. Pharmacol. 11, 539545. doi:10.3389/fphar.2020.539545
Hannah, J., Casian, A., and D'cruz, D. (2016). Tacrolimus use in lupus nephritis: A systematic review and meta-analysis. Autoimmun. Rev. 15, 93–101. doi:10.1016/j.autrev.2015.09.006
Hu, T., Liu, Q., Xu, Q., Liu, H., Qiu, W., Huang, F., et al. (2018). Tacrolimus decreases proteinuria in patients with refractory IgA nephropathy. Med. Baltim. 97, e0610. doi:10.1097/MD.0000000000010610
Kandikattu, H. K., and Mishra, A. (2018). Immunomodulatory effects of tacrolimus (FK506) for the treatment of allergic diseases. Int. J. Cell Biol. physiology 1, 5–13. doi:10.5281/zenodo.2530969
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group (2021). KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 100, S1–S276. doi:10.1016/j.kint.2021.05.021
Li, J.S., Chen, X., Peng, L., Wei, S Y., Zhao, S L., Diao, T T., et al. (2015). Angiopoietin-Like-4, a potential target of tacrolimus, predicts earlier podocyte injury in minimal change disease. PLoS One 10, e0137049. doi:10.1371/journal.pone.0137049
Maixnerova, D., Neprasova, M., Skibova, J., Mokrisova, J., Rysava, R., Reiterova, J., et al. (2014). IgA nephropathy in Czech patients--are we able reliably predict the outcome? Kidney blood press. Res 39, 555–562. doi:10.1159/000368467
Maixnerova, D., and Tesar, V. (2020). Emerging modes of treatment of IgA nephropathy. Int. J. Mol. Sci. 21, 9064. doi:10.3390/ijms21239064
Maruyama, K., Tomizawa, S., Seki, Y., Arai, H., Ogawa, T., and Kuroume, T. (1994). FK 506 for vascular permeability factor production in minimal change nephrotic syndrome. Nephron 66, 486–487. doi:10.1159/000187876
Natale, P., Palmer, S C., Ruospo, M., Saglimbene, V M., Craig, J C., and Vecchio, M., (2020). Immunosuppressive agents for treating IgA nephropathy. Cochrane database Syst. Rev. 3, Cd003965. doi:10.1002/14651858.CD003965
Ong, S C., and Gaston, R S. (2021). Thirty years of tacrolimus in clinical practice. Transplantation 105, 484–495. doi:10.1097/TP.0000000000003350
Pattrapornpisut, P., Avila-Casado, C., and Reich, H N. (2021). IgA nephropathy: Core curriculum 2021. Am. J. Kidney Dis. 78, 429–441. doi:10.1053/j.ajkd.2021.01.024
Peng, L., Ma, J., Cui, R., Chen, X., Wei, S Y., Wei, Q J., et al. (2014). The calcineurin inhibitor tacrolimus reduces proteinuria in membranous nephropathy accompanied by a decrease in angiopoietin-like-4. PLoS One 9, e106164. doi:10.1371/journal.pone.0106164
Qi, X M., Wang, J., Xu, X X., Li, Y Y., and Wu, Y G. (2016). FK506 reduces albuminuria through improving podocyte nephrin and podocin expression in diabetic rats. Inflamm. Res. 65, 103–114. doi:10.1007/s00011-015-0893-y
Rajasekaran, A., Julian, B A., and Rizk, D V. (2021). IgA nephropathy: An interesting autoimmune kidney disease. Am. J. Med. Sci. 361, 176–194. doi:10.1016/j.amjms.2020.10.003
Schumacher, L., Leino, A D., and Park, J M. (2021). Tacrolimus intrapatient variability in solid organ transplantation: A multiorgan perspective. Pharmacotherapy 41, 103–118. doi:10.1002/phar.2480
Tan, M., Li, W., Zou, G., Zhang, C., and Fang, J. (2015). Clinicopathological features and outcomes of IgA nephropathy with hematuria and/or minimal proteinuria. Kidney Blood Press Res. 40, 200–206. doi:10.1159/000368495
Textor, S C., Burnett, J C., Romero, J C., Canzanello, V J., Taler, S J., and Wiesner, R., (1995). Urinary endothelin and renal vasoconstriction with cyclosporine or FK506 after liver transplantation. Kidney Int. 47, 1426–1433. doi:10.1038/ki.1995.200
Textor, S C., Wiesner, R., Wilson, D J., Porayko, M., Romero, J C., Burnett, J C., et al. (1993). Systemic and renal hemodynamic differences between FK506 and cyclosporine in liver transplant recipients. Transplantation 55, 1332–1339. doi:10.1097/00007890-199306000-00023
Wakamatsu, A., Fukusumi, Y., Hasegawa, E., Tomita, M., Watanabe, T., and Narita, I., (2016). Role of calcineurin (CN) in kidney glomerular podocyte: CN inhibitor ameliorated proteinuria by inhibiting the redistribution of CN at the slit diaphragm. Physiol. Rep. 4, e12679. doi:10.14814/phy2.12679
Wang, S., Dong, L., Pei, G., Jiang, Z., Qin, A., Tan, J., et al. (2021). High neutrophil-to-lymphocyte ratio is an independent risk factor for end stage renal diseases in IgA nephropathy. Front. Immunol. 12, 700224. doi:10.3389/fimmu.2021.700224
Wei, L., Du, Y., Jia, L., Ma, X., Chen, Z., Lu, J., et al. (2017). Therapeutic effects of FK506 on IgA nephropathy rat. Kidney Blood Press Res. 42, 983–998. doi:10.1159/000485346
Wen, Y., Liu, L., Zhou, P., Li, H., Wang, Z., and Zhang, Y., (2016). Tacrolimus restores podocyte injury and stabilizes the expression of Cabin1 in 5/6 nephrectomized rats. Ren. Fail. 38, 564–570. doi:10.3109/0886022X.2016.1148936
Yan, Z., Wang, J., Huang, T., Liu, X., Wang, L., and Xu, G. (2022). Effectiveness and safety of tacrolimus treatment for IgA nephropathy: A prospective cohort study. Med. Clin. (Barc.). 158, 596–602. doi:10.1016/j.medcli.2021.07.030
Yu, M Y., Kim, Y C., Koo, H S., and Chin, H J. (2017). Short-term anti-proteinuric effect of tacrolimus is not related to preservation of the glomerular filtration rate in IgA nephropathy: A 5-year follow-up study. PLoS One 12, e0188375. doi:10.1371/journal.pone.0188375
Zhang, H., and Barratt, J. (2021). Is IgA nephropathy the same disease in different parts of the world? Semin. Immunopathol. 43, 707–715. doi:10.1007/s00281-021-00884-7
Zhang, Q., Shi, S F., Zhu, L., Lv, J C., Liu, L J., Chen, Y Q., et al. (2012). Tacrolimus improves the proteinuria remission in patients with refractory IgA nephropathy. Am. J. Nephrol. 35, 312–320. doi:10.1159/000337175
Keywords: IgA nephropathy, tacrolimus, proteinuria, immunosuppressive agents, adverse events
Citation: Zhao L, Yang Y, Xu H, Leng W and Xu G (2023) Efficacy and safety of tacrolimus-based treatment for non-rapidly progressive IgA nephropathy. Front. Pharmacol. 14:1189608. doi: 10.3389/fphar.2023.1189608
Received: 19 March 2023; Accepted: 10 May 2023;
Published: 18 May 2023.
Edited by:
Anis Ahmad, University of Miami Health System, United StatesReviewed by:
Whidul Hasan, Harvard Medical School, United StatesAshraf Hussain, University of Miami, United States
Copyright © 2023 Zhao, Yang, Xu, Leng and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoshuang Xu, xugsh882003@163.com
†These authors have contributed equally to this work
 Lijuan Zhao
Lijuan Zhao Yanyan Yang1,2†
Yanyan Yang1,2† Hao Xu
Hao Xu Guoshuang Xu
Guoshuang Xu