- 1Department of Pharmacy, College of Health Sciences, Debre Markos University, Debre Markos, Ethiopia
- 2Department of Pharmacognosy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 3Department of Pharmaceutics, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Euclea (Ebenaceae) is a genus of flowering shrubs and trees widely distributed in Africa, the Comoro Islands, and Arabia. This review aimed to evaluate the ethnobotanical uses, phytochemistry, and biological activities of the genus Euclea on available research reports. This was achieved through PubMed, Medline, Google Scholar, Science Direct, Taylor and Francis Online, Wiley Online Library which provides access to scientific and medical research. The extensive literature survey revealed that plants that belong to this genus are used as folkloric medicine for the treatment of diabetes mellitus, toothache, diarrhea, cancer, malaria, leprosy, and genital and oral diseases in the case of HIV/AIDS-related diseases. To date, more than 40 secondary metabolites have been isolated and identified from these plants, especially from E natalensis and E. divinorum. Among these, naphthoquinones, terpenes, and flavonoids are potential secondary metabolites with profound biological activities. Euclea plant extracts and their bioactive compounds possess outstanding pharmacological properties, especially antimalarial, antidiabetic, anticancer, antimicrobial, and antioxidant properties.
Introduction
The word “Euclea” comes from a Greek word “eukleia”, “eu” meaning “good”, and “kleios”meaning report (Maroyi, 2017). The genus Euclea belongs to the family Ebenaceae and is composed of 16 accepted species (Dhayalan et al., 2015; Botha, 2016).
The genus Euclea is distributed in the tropical and subtropical regions of the world. However, it is most abundant in Eastern and Southern Africa (Mebe et al., 1998) and South-East Asia (Botha, 2016). Euclea divinorum is distributed in Botswana, South Africa, Namibia, Swaziland, Zimbabwe, Tanzania, Uganda (Shumba, 2018), Sudan, Kenya, and Ethiopia (Woldemedhin et al., 2017). Euclea natalensis is widely found along the eastern coast of southern Africa (Johanna, 2007). Euclea latideus is well presented in the lowlands of the tropical and to a lesser extent, in subtropical regions of the world (Philip et al., 2018). A versatile medicinal plant in Ethiopia from this genus is Euclea divinorum. Traditionally it is used for the treatment of skin inflammation, scabies, cancer, hepatitis, urinary inconsistency, chest pain, pneumonia, gonorrhea, constipation, edema, abdominal and chest pain (Feyissa et al., 2013; Woldemedhin et al., 2017; Mekonnen et al., 2018).
Botanical profile and taxonomy of Euclea
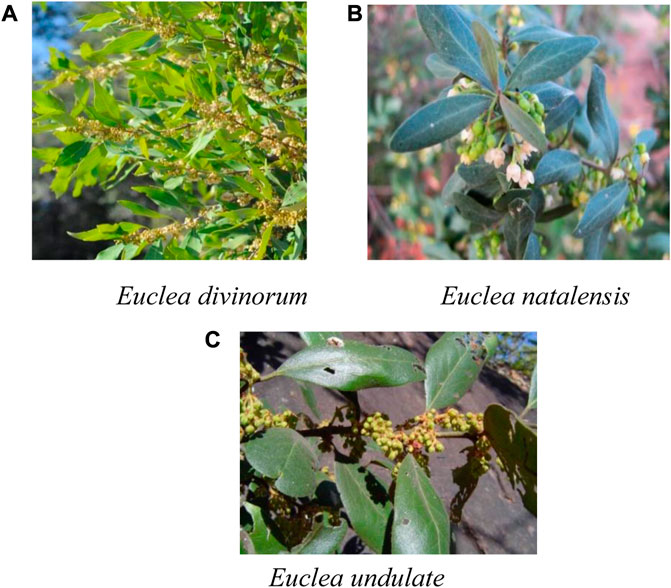
Most of the plants are trees, shrubs, and sub-shrubs, usually evergreen with alternate, opposite to sub-opposite, or in pseudo-whorls and diamond leaved (Figure 1A). Inflorescences: dioecious, axillary, or less frequently in branched pseudo-racemes, or flowers occasionally solitary (Figure 1B). Calyx: 4-5-lobed, usually polysepalous, not accrescent on fruits. Corolla: urceolate to subglobose, 5 - 8-lobed or campanulate and deeply 4-5-lobed. Stamens: 10-30; anthers dehiscing by large ellipsoidal apical pores, hairy or glabrous, oblong or lanceolate, 2-celled; filaments short, usually slender and glabrous. Staminodes: usually absent, glabrous; styles 2 (or 1, bifid), usually glabrous; stigmas bifid at apex. Ovary: ovoid or globular, hairy or glabrous, usually 4-celled; ovules 4, pendulous. Fruit: usually globose, 1-seeded berry (Halim et al., 2014), edible, spherical and one-seeded berries (Figure 1C) (Dhayalan et al., 2015). Many members of this genus are traditionally used to treat different diseases. Some are scientifically investigated for various biological activities and phytoconstituents. Previously, reviews that focus on single species, E undulata Thunb (Maroyi, 2017) and E. divinorum Hiern (Omara et al., 2020) have been conducted. To the authors’ knowledge, no study reviewed the ethnopharmacological use, phytochemistry, and biological activities of the whole genus.
Methodology
This review aims to critically evaluate available research reports on the genus and systematically organize and present the results. The review summarizes the existing knowledge on the ethnobotanical use, phytochemistry, and pharmacological activity of species belonging to the genus Euclea to bring the reader up to date with the current literature. Articles on the species of the genus Euclea that reported ethnobotanical uses, biological activities, and isolation and identification of compounds were included. It is attempted to include articles published from 1975–2023 while some articles published before 1975 were also included by considering their importance. In this review articles where the full text was not available in the database or even after contacting the author by email were excluded (Figure 2).
This review excluded unpublished results and publications unavailable online, articles written in languages other than English, and articles whose titles and abstracts did not contain the search terms. Chemical structures of only isolated and characterized compounds were provided, while structures of compounds identified from essential oils and other chemical analyses were not. Different databases, including PubMed, Google Scholar, Scopus, and Medline, were employed to search literature using “keywords such as “Euclea”, “ethnobotanical use”, “phytochemistry”, and “pharmacological activity” dated up to December 2023.”
Ethno pharmacological uses
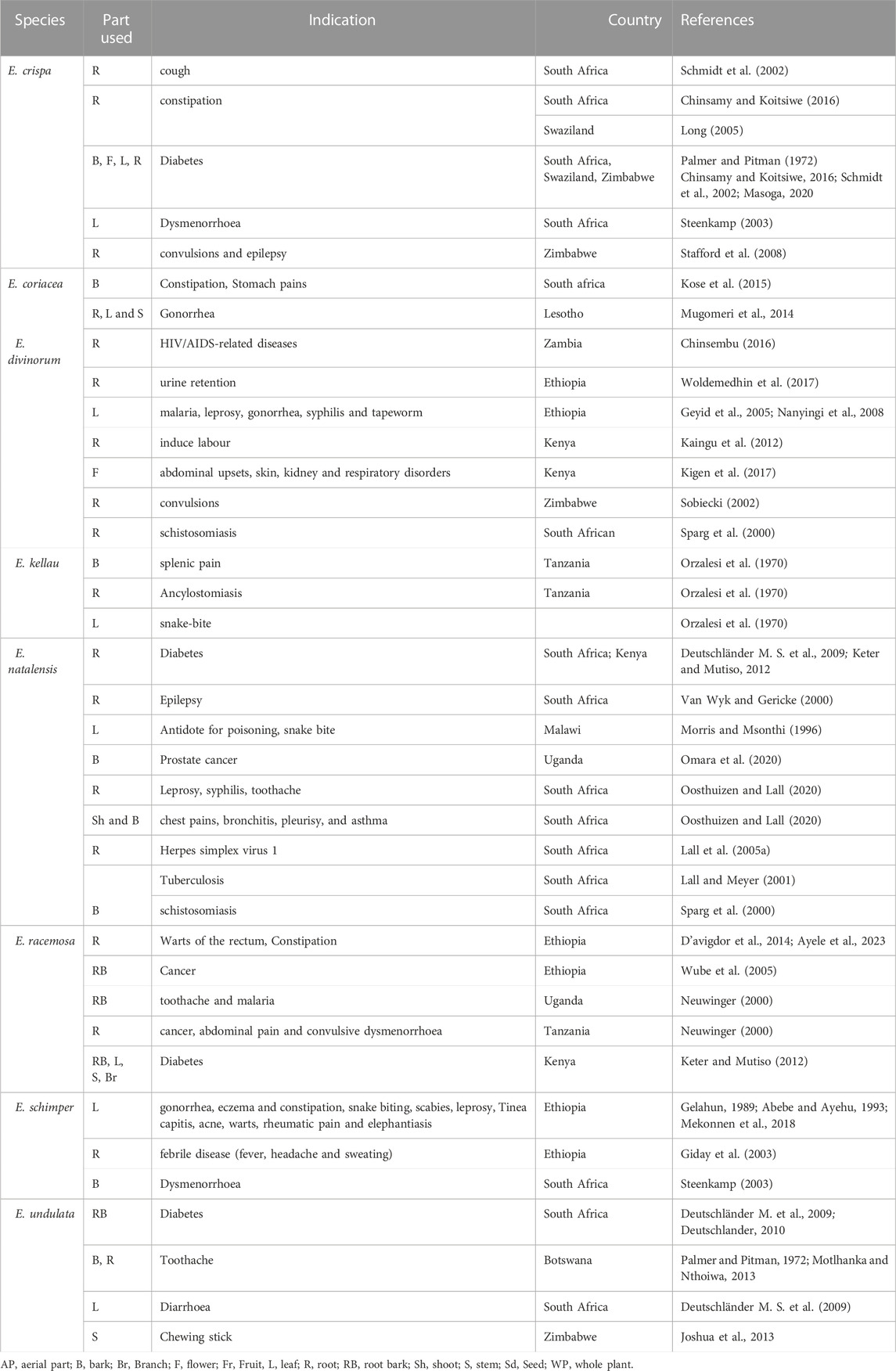
Ethnomedicinal claims on the genus Euclea to treat several ailments are illustrated in Table 1. The genus Euclea is used to treat hypnosis, toothache, headache (Bapela et al., 2008; Babula et al., 2009), chest complaints, bronchitis, pleurisy, chronic asthma, urinary tract infections, and venereal diseases (Lall & Meyer, 2000; Lall and Meyer, 2001; Weigenand et al., 2004; Kooy et al., 2006; Johanna, 2007; Bapela et al., 2008). An infusion of the roots of E. ceispa possesses antiepileptic activity (Dhayalan et al., 2015). The root bark of E. undulata is reported to be used for the management of body pains, diabetes, headache, and toothache while an infusion of its leaves is used for stomach problems or diarrhea, and leaf decoction for tonsillitis (Deutschländer M. et al., 2009; Dhayalan et al., 2015; Maroyi, 2017). This plant is a folk medicine for diabetes in the Venda area, Limpopo Province (Deutschländer M. S. et al., 2009; Babiaka et al., 2015; Maroyi, 2017). In the Western Cape, the root infusion of E. undulata is used as enemata or as an ingredient of inembe (herbal medication regularly taken during pregnancy to ensure trouble-free confinement). Emesis or purgation is induced with root preparations (Deutschländer M. et al., 2009).
The Zulu people use E. natalensis as a purgative (Lall and Meyer, 2001; Weigenand et al., 2004) and for abdominal complaints in the form of infusion (Deutschländer M. S. et al., 2009; Deutschländer, 2010). Its charred and powdered root is used treat leprosy, urinary tract infections, venereal diseases, dysmenorrhea, and ancylostomiasis among Shangaan people (Lall & Meyer, 2000; Lall and Meyer, 2001); Kooy et al., 2006; Deutschländer, 2010) while its root bark infusions for sores and wounds in South Africa (Lall and Meyer, 2001). Within the Tonga people, the same part of this plant exhibits toothache and headache relief (Deutschländer M. et al., 2009; Babiaka et al., 2015; Dhayalan et al., 2015).
In Swaziland, the stem bark decoction of E. divinorum is a folk medicine for constipation (Amusan et al., 2007). The root bark is used for diarrhea, convulsions, cancer, and skin diseases (Mebe et al., 1998; Babiaka et al., 2015). In Kenya, the root of this plant is a remedy for chest pain, pneumonia, and internal body swelling (Woldemedhin et al., 2017). In Ethiopia, the roots and leaves of this plant are used for treating urinary retention, malaria, leprosy, gonorrhea, syphilis, and tapeworm (Feyissa et al., 2013; Woldemedhin et al., 2017). E. schimperi is traditionally prescribed for managing wounds, teeth infection, eye disorder, headache, gonorrhea, eczema, skin disorder, snake biting, scabies, leprosy, and elephantiasis in Ethiopia (Mekonnen et al., 2018).
Phytochemistry
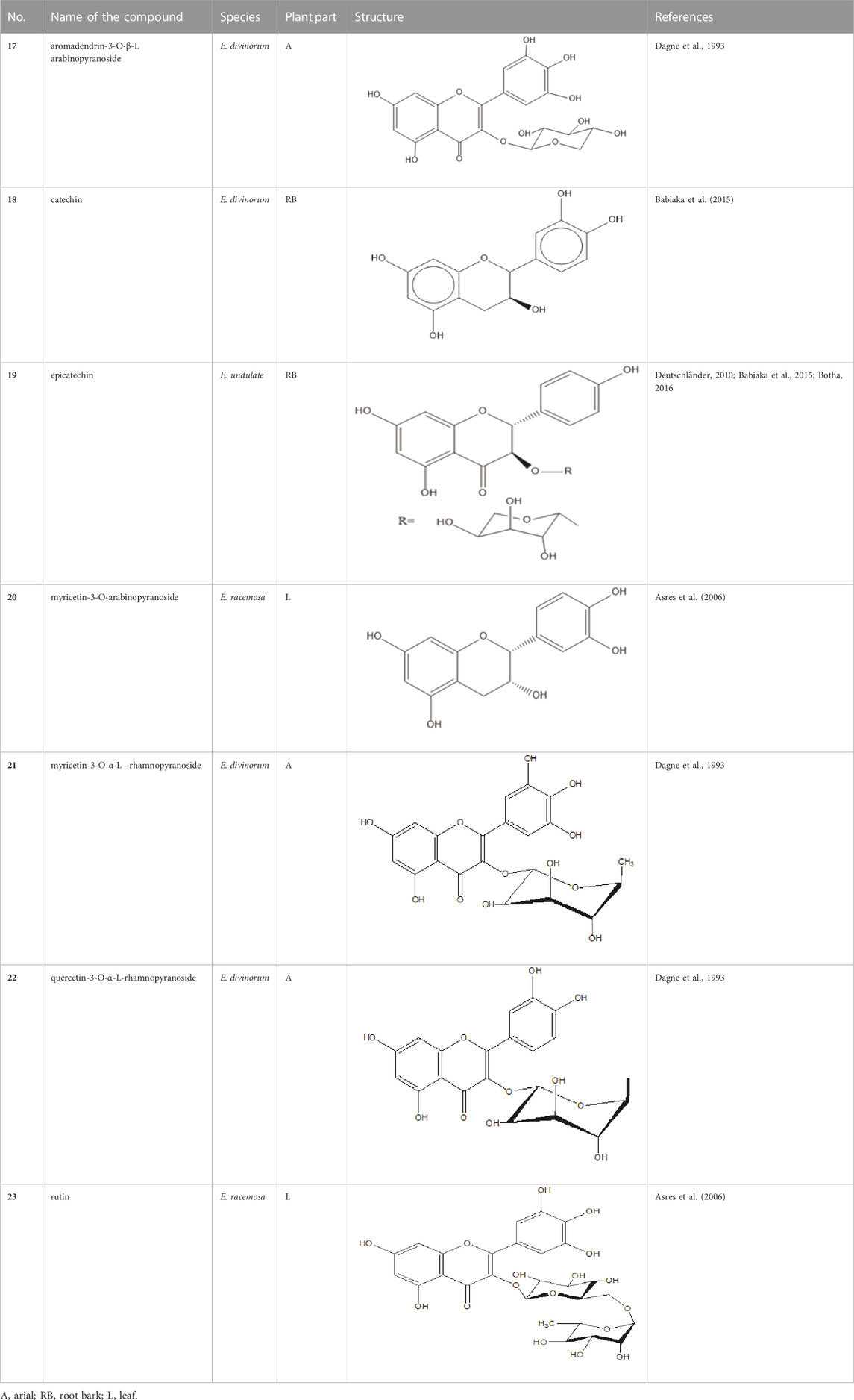
Euclea is a good source of naphthoquinones, pentacyclic triterpenes (Dagne et al., 1993; Joubert et al., 2006; Kwon et al., 2011; Dhayalan et al., 2015), flavonoids, naphthols (Dagne et al., 1993) and diosindigo (Dhayalan et al., 2015). Members of the genus Euclea contain primarily naphtoquinones and the root/root bark of the plant is the main source of the naphtoquinones. Phytochemical screening revealed that the leaf of E. schimperi contains saponins, terpenoids, tannins, steroids, polyphenols, and flavonoids after extraction with methanol and chloroform (Mekonnen et al., 2018). Aqueous and 80% methanol root extract of E. divinorum had shown to contain saponins, flavonoids, glycosides, steroids, tannins, and terpenoids (Woldemedhin et al., 2017; Al-fatimi, 2019) but alkaloids and anthraquinones were absent (Woldemedhin et al., 2017). On the other hand, the root bark of this plant produces alkaloids, terpenoids, flavonoids, tannins, and saponins (Shumba, 2018). Methanol leaf and stem extracts of E. undulata contained alkaloids, diterpenes, glycosides, phytosterols, reducing sugars, saponins, and tannins (Maroyi, 2017). Essential oils, saponins, terpenoid derivatives, alkaloids, and flavonoids are the constituents of E. crispa subsp. crispa (Kwon et al., 2011).
Naphthoquinone
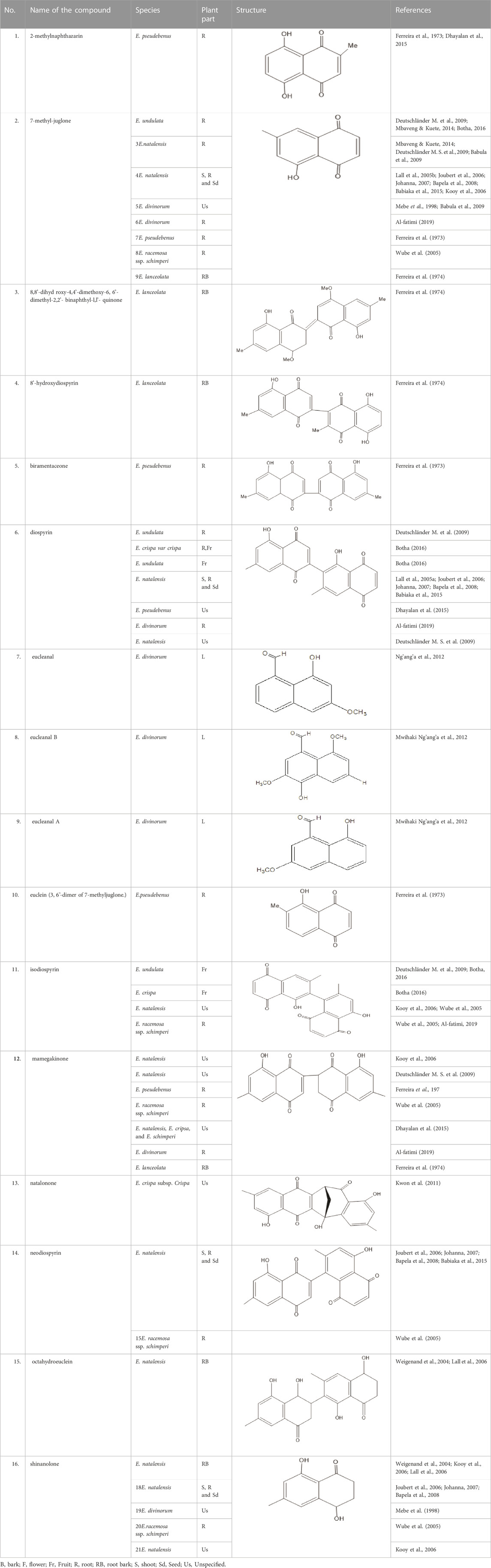
Quinones are one of the plant-derived secondary metabolites. Based on the number of benzene rings in the structural fused and skeleton, they are mainly classified as naphthoquinone, phenanthrenequinone, anthraquinone, and benzoquinone (Demir, 2020). Naphthoquinones are phenolic compounds derived from naphthalene occurring in plants (common) and fungi (Mbaveng & Kuete, 2014; Botha, 2016). They were mainly detected from the root barks of the genus Euclea (Khan, 1985). Naphthoquinone isolated from the genus Euclea is presented in Table 2.
Flavonoids
Flavonoids are phenolic compounds having two benzene rings linked through a heterocyclic pyrane ring (Shumba, 2018). Quercetin, kaempferol (Al-fatimi, 2019), new aromadendrin-3-O-β-L-arabinopyranoside (17), and known flavonoids such as catechin (Dagne et al., 1993; Mebe et al., 1998), myricetin-3-O-α-L–rhamnopyranoside (21) and quercetin-3-O-α-L-rhamnopyranoside (22) were isolated from the extract of ethanol aerial part of E. divinorum (Dagne et al., 1993), (Table 3). Acetone leaves extract of E. racemosa ssp. Schimperi yields quercetrin, myricitrin, myricetin-3-O-arabinopyranoside (20) and rutin (23), (Asres et al., 2006).
HPLC detects large amounts of myricitrin and small amounts of isoquercitrin and quercitrin in E. schimperi (Mueller-harvey et al., 1987). Root bark extracts of E. undulata (acetone) (Deutschländer, 2010; Babiaka et al., 2015; Botha, 2016), E. divinorum (chloroform) and E. undulata (acetone) resulted in the isolation of epicatechin (19) and catechin (18) respectively (Babiaka et al., 2015). Hyperoside, quercitrin, epicatechin, catechins and gallocatechin were isolated from the leaves of E. crispa subsp. Crispa (Rademana et al., 2019).
Terpenoids
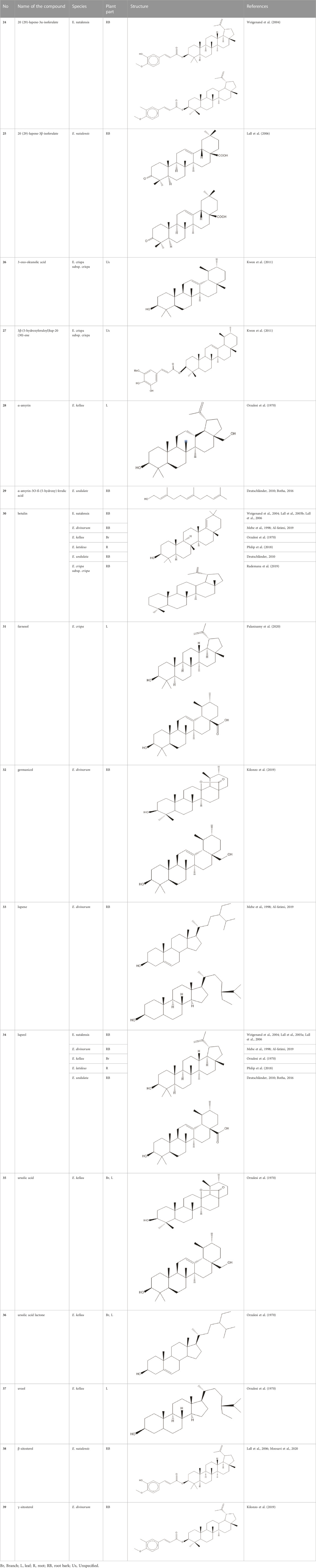
Triterpenes are a group of natural products, derived from isoprene units. In nature, triterpenoids are often existed as tetra- or penta-cyclic structures but some acyclic, mono-, bi-, tri- and hexa acyclic. As described in Table 4, Lupeol, lupine, botulin and oleanolic acid are some examples of pentacyclic triterpenoids (Furtado et al., 2017). Triterpenoids were detected from root and stem barks of E. natalensis (Khan, 1985). Phytol (0.66%) and squalene (5.85%) were detected from hexane extract of E. crispa using GC-MS (Palanisamy & Ashafa, 2018).
Miscellaneous
The following bioactive compounds with their composition were identified from hexane extract of E. crispa using GC-MS: tetracosane (14.98%), dodecane (10.76%), 2-ethyl-1-decanol (8.00%), tridecane (7.53%), diphenyl vinyl phosphine (6.38%), triacontane (5.27%), 2,6-dimethylheptadec-ane (5.02%), docosane (3.68%), tetradecane (3.59%), 1-hepten-3-ol (2.63%), orthotolidine (2.31%), Phenyl glucuronide (2.25%), 5-tridecy-lbenzene-1,3-diol (1.90%), and Pentadec-ane (1.68%) (Palanisamy & Ashafa, 2018). Vitamin E, fatty acid methyl esters such as saturated (C14, C20) and unsaturated (C16, C18:1, C18:2, and C18:3 were isolated from twigs and leaves of E. undulate (Maroyi, 2017). VTLC identified gallic and ellagic acid esters in E. schimperi (Mueller-harvey et al., 1987).
Biological activities
Antimicrobial activity
The acetone and aqueous extract of E. natalensis inhibited the growth of Bacillus cereus, B. pumilus, B. subtilis, Micrococcus kristinae, and Staphylococcus aureus at concentrations ranging between 0.1 and 6.0 mg/mL (Lall and Meyer, 2000). Isolated compounds from the root extract also demonstrated a significant antimicrobial effect. Diospyrin and 7-methyljuglone were more effective against Gram-positive bacteria than Gram-negative bacteria.
Shinanolone, 7-methyljuglone, diospyrin, isodiospyrin and neodiospyrin in the genus Euclea especially E. natalensis are potent for the treatment of both drug-sensitive and resistant tuberculosis (Joubert et al., 2006; Johanna, 2007; Bapela et al., 2008; Babula et al., 2009; Babiaka et al., 2015). On the other hand, diospyrin, lupeol, betulin and 7-methyl juglone presented in E. natalensis has inhibitory activity against drug-sensitive M. tuberculosis at MIC of 8.0 and 0.5 mg/mL respectively (Maroyi, 2017). The intracellular and extracellular inhibition of the latter compound is greater than that of the anti-tuberculosis drugs streptomycin and ethambutol (Lall et al., 2005b; Mcgaw et al., 2008).
7-methyl juglone and mamegakinone are effective against M. tuberculosis (Kooy et al., 2006), Neisseria gonorrhoeae, Shigella dysenteriae and Shigella flexneri. Aqueous and acetone extracts of the roots of E. natalensis inhibited the growth of Mycobacterium tuberculosis at MIC value of 0.5 mg/mL while MIC values for B. cereus, B. pumilus, B. subtilis, M. kristinae and S. aureus ranged from 0.1–6.0 mg/mL (Lall & Meyer, 2000; Lall and Meyer, 2001). 7-methyl juglone is also effective against Saccharomyces cerevisiae, M. bovis, M. smegmatis and M. fortuitum (Mbaveng & Kuete, 2014). Due to Shinanolone, E. natalensis inhibits the growth of Gram-positive bacterial strains and a drug-sensitive strain of M. tuberculosis at a concentration of 0.1 mg/mL (Weigenand et al., 2004).
Ethanolic extract of E. crispa leaves elicit antimicrobial activity with maximum inhibition zone against Staphylococcus aureus, Streptococcus aureus, Escherichia coli, Klebsiella pneumonia, Aspergillus niger and Aspergillus terreus (Palanisamy et al., 2019). Previous literatures demonstrated that E. lanceolata, E. undulata and E. multiflora possess antifungal activity due to the presence of lawsone, juglone and 7-methyljuglone (Lall & Meyer, 2000; Lall and Meyer, 2001). Euclea natalensis comprises β-sitosterol (Lall et al., 2006; Moosavi et al., 2020), 20 (29)-lupine-3β-isoferulic and shinanolone that have inhibitory activity against Aspergillus niger at 0.01 mg/mL. The former compound and octahydro euclein significantly show fungistatic activity against C. cladosporioides at 0.01 mg/mL. Besides this, octahydro euclein present in this plant is very effective for Phytophthora sp. at 0.1 mg/mL (Lall et al., 2006).
Ethyl acetate root extract of E. divinorum has inhibitory activity against Gram-negative bacteria like E. coli but is ineffective for S. aureus. Alkaloids and terpenoids in this plant contribute to this kind of antibacterial activity (Shumba, 2018). The MIC values of the extracts of E. divinorum against bacterial activity for root bark ethyl acetate and leaf aqueous ranges from 0.048-0.871 mg/mL and 0.781-1.562 mg/mL respectively. The first extract is very effective against S. typhi followed by stem bark aqueous and root bark petroleum ether extract against S. aureus (Kilonzo et al., 2019).
The non-polar dichloromethane root extract of E. divinorum root bark has better antifungal activity than the nystatin for Absidia corymbifera, Aspergillus fumigatus, Candida krusei, Microsporum gypseum, Mucor sp. and Trichophyton mentagrophytes. This activity is maintained with lupeol, lupine, botulin, 7-methyl juglone, diospyrin, iso diospyrin and shinalone (Al-fatimi, 2019).
Antiviral activity
The acetone extract of E. natalensis demonstrated moderate antiviral activity against HSV-1, at concentrations of 0.1–0.02 mg ml−1 (Lall et al., 2005a). In a study conducted by Tshikalange et al. (2007) 7-methyljuglone (potent), diospyrin, neodiospyrin, isodiospyrin, and 6-methyljuglone isolated from that E. natalensis exhibited HIV-1 reverse transcriptase activity at the concentrations ranging from 25 to 50 μg/mL. The leaf extract of E. schimperi showed good antiviral activity against Influenza A virus and herpes simplex virus (HSV-1) with IC50 values of 6.22 6 μg/mL and 67.5 μg/mL, respectively (Gebre-Mariam et al., 2006).
Antimalarial activity
Aqueous, dichloromethane, and methanol leaf and twig extracts of E. undulata have shown antimalarial activity against Plasmodium falciparum using the parasite lactate dehydrogenase assay (Maroyi, 2017). E. latideus is also effective against P. falciparum especially for the chloroquine resistant strain of P. falciparum due to the presence of lupeol, betulin, and 3β-(5-hydroxy feruloyl) lup-20 (30)-ene (Philip et al., 2018). The dichloromethane and methanol (1:1) root and leaf extracts of E. natalensis demonstrated promising activity in a research by Clarkson et al. (2004) employing the parasite lactate dehydrogenase assay, with (IC50) values of 5.1 and 5.3 mg/mL, respectively, against P. falciparum. A study done by Philip et al. (2018) indicated that the extracts and isolated compounds from E. latideus demonstrated antiplasmodial activity against chloroquine sensitive and chloroquine resistant strains of P. falciparum. The leaves of E. natalensis also showed antiplasmodial activity with an IC50 of 25.6 μg/mL (Tajuddeen et al., 2022). The in vivo antimalarial assay of the aqueous root extract of E. divinorum possessed significant parasitemia suppression (Girmaw and Engidawork, 2022).
Antidiabetic activity
E. undulata containing α-amyrin-3-O-β-(5-hydroxy) ferulic acid inhibits α-glucosidase and epicatechin lowers glucose levels in the blood (Botha, 2016). Phenolic acids and flavonoids of E. crispa inhibit alpha amylase with IC50 values of 1.001 mg/mL and 1.65 mg/mL (Tinevimbo, 2017). Lowering of blood glucose can be achieved with acetone root bark extracts of E. undulata by displaying a glucose uptake of 162.2% by changing liver cells at 50 mg/mL (Maroyi, 2017). E. coriacea contains phytosterols that possess antidiabetic activity (Mugomeri et al., 2014). Acetone root bark extracts of E. undulata effectively reduced fasting blood glucose levels, raised cholesterol, and triglyceride levels to close to normal without causing weight gain in an in vivo model of streptozotocin-nicotinamide-induced type-2 diabetes (Deutschländer et al., 2012).
Antioxidant activity
Ethanolic root bark and leaf extracts of E. crispa have radical scavenging activity because of flavonoids, phenolics (Tinevimbo, 2017) and (6E, 10E)-2, 6, 24-trimethylpentane cosa-2, 6, 10-triene isolated from the leaves of E. crispa exhibited potent antioxidant activity (Palanisamy et al., 2019). The leaves of E. crispa were tested for antioxidant activity and showed IC50 values of 113.79, 109.59, and 116.65 μg/mL for DPPH, hydroxyl and nitric oxide radical scavenging assays. Farnesol contributes to such activity (Palanisamy et al., 2020). At a 2000 mg/mL concentration, E. divinorum inhibits DPPH by 82.5%, 74.5% and 62.5% for the methanol fraction, aqueous fraction and crude extract, respectively (Feyissa et al., 2013). Fatty acids, flavonoids, and phenolics of E. undulata showed antioxidant activity using the DPPH, ABTS and FRAP assays (Maroyi, 2017). The free radical scavenging effect of methanol and chloroform leaf extracts of E. schimperi was demonstrated. The methanol and chloroform extracts were able to scavenge the DPPH radical with a percentage scavenging activity of 85.4% and 58.5% at the concentration of 40 μg/mL, respectively (Mekonnen et al., 2018).
Anticancer activity
The leaves of E. crispa subsp. crispa extract exhibited anti-proliferative activity on human breast adenocarcinoma (MCF-7) and human epidermoid carcinoma (A431) cell lines with IC50 values of 45.7 μg/mL and 41.8 μg/mL, respectively (Rademana et al., 2019). 7-methyl juglone and 3β-(5-hydroxy feruloyl) lup-20 (30)-ene, which are the main constituents of E. divinorum, showed anticancer effects against human breast cancer, colon cancer, fibrosarcoma, nasopharyngeal carcinoma, lung cancer, and human melanoma (Mebe et al., 1998). Diterpenes isolated from E. coriacea has been reported to possess an anticancer effect in human cells (Mugomeri et al., 2014). 7-Methyl juglone isolated from E. racemosa ssp. schimperi has been described to possess significant cytotoxic properties against human colon carcinoma cells (Wube et al., 2005). Euclea natalensis also contains this compound that has anticancer activity on several cancer cell lines, such as KB, Lu1, and LNCaP (Mbaveng & Kuete, 2014).
Other activities
E. coriacea contains phytosterols that possess anti-inflammatory and anti-pain activity (Mugomeri et al., 2014). A study showed that E. natalensis shoot extract has in vivo hepatoprotective activity by reducing the level of alanine transaminase liver enzyme by 15% (50 mg/kg) and 40% (100 mg/kg). This plant also provides an immunomodulatory activity by increasing T-helper 1 cell cytokines such as Interleukin 2, Interleukin 12, and Interferon α by 12 fold and decreasing the T-helper 2 cell cytokine, interleukin 10 by 4 fold when compared to baseline cytokine production (Lall et al., 2016). The in vivo evaluation of the antidiuretic activity of E. divinorum revealed that the aqueous and methanol root extract of the plant possessed a significant diuretic activity by increasing urine volume and electrolyte excretion (Woldemedhin et al., 2017). Feyissa et al. (2013) demonstrated that the crude extract and solvent fractions of E. divinorum leaves restored gentamicin-induced nephrotoxicity by decreasing tubular necrosis, serum and oxidant markers and by increasing in antioxidant molecules. The methanol fraction provided the most renoprotection, implying that semi-polar antioxidant principles may be involved.
Acute toxicity, gentotoxicity and cytotoxiciy
Acute toxicity studies of the crude and methanolic extract of E. divinorum leaves indicated that it was safe when administered orally at 2000 mg/kg (Feyissa et al., 2013; Woldemedhin et al., 2017). After a period of 72 h, the animals tolerated the administered dose, and there were no appreciable changes in behavior such as motor activity, diarrhoea, breathing, alertness, restlessness, convulsions, coma and appearance. Since no mortality was recorded within 14 days, the lethal dose (LD50) was indicated to be more than 2000 mg/kg. Shauli, (2023) evaluated the acute and sub-acute oral toxicity of E. natalensis and the results demonstrate that no treatment related deaths or toxic signs were observed. Another study done by Ayele et al. (2023) revealed that E. racemosa was safe after oral toxicity study with LD50 greater than 2000 mg/kg. E. latideus is considered as a non-toxic plant since acute toxicity studies showed that crude extracts had LD50 > 5,000 mg/kg (Kodi et al., 2018).
Taylor et al. (2003) investigated genotoxicity in human peripheral blood lymphocytes of South African medicinal plants. The results reported that the dichloromethane root extract of E. divinorum induced DNA damage (more cells with high tail DNA content), which was however lower than that of the positive control (1 mM potassium bichromate). However, the bark extract of E. natalensis showed positive results for genotoxicity in the micronucleus test.
Conclusion
The genus Euclea is well known for its use in the treatment of diabetic and body pain manifestations. The traditional claims were justified by different biological evaluations. The genus Euclea is known to be a source of biologically active compounds. More than 40 compounds were isolated from the genus and naphthoquinones, pentacyclic triterpenes and flavonoids are the most abundant bioactive secondary metabolites which are responsible for the observed biological activity. Most of these secondary metabolites are found in the roots and root bark while some in fruit, seeds, leaves and shoots. According to the present review, it has been noted that the potential uses of the species in the treatment of viral infections and nerve-related diseases have not been scientifically explored. We believe the scientific community researching on the genus will benefit from the material compiled in this review.
Author contributions
AT designed the study, conducted the literature review, extracted relevant information to the study, write the manuscript. AK and GB contributed in writing, editing and revising the manuscript. All authors read and approved the manuscript.
Conflict of interest
The authors declare that they no conflict of financial interests or personal relationships that could have appeared to influence the work reported in this review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abebe, D., and Ayehu, A. (1993). “Medicinal plants and enigmatic health practices of Northern Ethiopia,” in Addis ababa, Ethiopia: B. S. P. E, 511.
Ahvazi, M., Charkhchiyan, M., Khalighi-Sigaroodi, F., Mojab, F., Mozaffarian, A., and Zakeri, H. (2012). Introduction of medicinal plants species with the most traditional usage in Alamut Region. Iran. J. Pharm. Res. 11 (1), 185–194.
Al-fatimi, M. (2019). Antifungal activity of Euclea divinorum root and study of its ethnobotany and phytopharmacology. Processes 7, 680. doi:10.3390/pr7100680
Amusan, O., Sukati, N., Dlamini, P., and Sibandze, F. (2007). Some Swazi phytomedicines and their constituents. Afr. J. Biotechnol. 6 (3), 267–272.
Asres, K., Gibbons, S., and Bucar, F. (2006). Radical scavenging compounds from Ethiopian medicinal plants. Ethiop. Pharm.J. 24, 23–30. doi:10.4314/epj.v24i1.35095
Ayele, A. G., Mulugeta, B., and Wondmkun, Y. T. (2023). Evaluations of the in vivo laxative effects of aqueous root extracts of Euclea racemosa L. in mice. Metab. Open 17, 100222. doi:10.1016/j.metop.2022.100222
Babiaka, S., Ntie-kang, F., Ndingkokhar, B., Mbah, J., Sippl, W., and Yong, J. (2015). The chemistry and bioactivity of southern african flora II: Flavonoids, quinones and minor compound classes. RSC Adv. 5, 57704–57720. doi:10.1039/c5ra05524e
Babula, P., Adam, V., Havel, L., and Kizek, R. (2009). Noteworthy secondary metabolites naphthoquinones – their occurrence, pharmacological properties and analysis. Curr. Pharm. Anal. 5, 47–68. doi:10.2174/157341209787314936
Bapela, J., Kuete, V., Toit, E., Meyer, M., and Lall, N. (2008). Fertilization-induced changes in growth parameters and antimycobacterial activity of Euclea natalensis (Ebenaceae). S. A. J. Bot. 74, 244–250. doi:10.1016/j.sajb.2007.11.011
Bapela, M. J. (2008). Variation of active constituents in Euclea natalensis based on seedling stages, seasons, and fertilizers(Doctoral dissertation, University of Pretoria).
Botha, L. E. (2016). Investigating the production of secondary metabolites effective in lowering blood glucose levels in Euclea Undulata Thunb. Var Myrtina (Ebenaceae)(Doctoral dissertation).
Chinsamy, M., and Koitsiwe, M. (2016). Traditional knowledge of medicinal and food plant uses for sustainable community livelihoods: A case of batswana communities in South Africa. J. Soc. Sci. 46 (2), 146–154. doi:10.1080/09718923.2016.11893522
Chinsembu, K. C. (2016). Ethnobotanical study of plants used in the management of HIV/AIDS-related diseases in Livingstone, Southern Province, Zambia. Evid. Based Complement. Altern. Med. 2016, 4238625. Article ID 4238625. doi:10.1155/2016/4238625
Clarkson, C., Maharaj, V. J., Crouch, N. R., Grace, O. M., Pillay, P., Matsabisa, M. G., et al. (2004). In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 92 (2-3), 177–191. doi:10.1016/j.jep.2004.02.011
Dagne, E., Alemu, M., and Sterner, O. (1993). Flavonoids from Euclea divinorum. Bull. Chem. Soc. Ethiop. 7 (2), 87–92.
D’avigdor, E., Wohlmuth, H., Asfaw, Z., and Awas, T. (2014). The current status of knowledge of herbal medicine and medicinal plants in Fiche, Ethiopia. J. Ethnobiol. Ethnomedicine 10 (1), 38–33. doi:10.1186/1746-4269-10-38
Demir, Y. (2020). Naphthoquinones, benzoquinones and anthraquinones: Molecular docking, ADME and inhibition studies on human serum paraoxonase-1 associated with cardiovascular diseases. Drug Dev. Res. 81 (5), 628–636. doi:10.1002/ddr.21667
Deutschländer, M., Lall, N., and Venter, M. (2009). Plant species used in the treatment of diabetes by South African traditional healers: An inventory. Pharm. Biol. 47 (4), 348–365. doi:10.1080/13880200902752959
Deutschländer, M. S., Van de Venter, M., Roux, S., Louw, J., and Lall, N. (2009). Hypoglycaemic activity of four plant extracts traditionally used in South Africa for diabetes. J. Ethnopharmacol. 124 (3), 619–624. doi:10.1016/j.jep.2009.04.052
Dhayalan, M., Jegadeeshwari, A., and Gandhi, N. (2015). Biological activity sources from traditionally used Tribe and herbal plants material. Asian J. Pharm. Clin. Res. 8 (6), 11–23.
Ferreira, A., Lopes, M., Costa, M., and Alves, C. (1974). Eucleolatin: A dimeric methyl naphthazarin from Euclea lanceolata. Phytochemistry 13, 499–501. doi:10.1016/s0031-9422(00)91243-3
Ferreira, M., Costa, M., Alves, A., and Lopes, M. (1973). Euclein: A new naphthaquinone from Euclea pseudebenus. Phytochemistry 12, 433–435. doi:10.1016/0031-9422(73)80035-4
Feyissa, T., Asres, K., and Engidawork, E. (2013). Renoprotective effects of the crude extract and solvent fractions of the leaves of Euclea divinorum Hierns against gentamicin-induced nephrotoxicity in rats. J. Ethnopharmacol. 145 (3), 758–766. doi:10.1016/j.jep.2012.12.006
Furtado, N., Pirson, L., Edelberg, H., Miranda, L., Loira-Pastoriza, C., Preat, V., et al. (2017). Pentacyclic triterpene bioavailability: An overview of in vitro and in vivo studies. Molecules 22 (3), 1–24.
Gebre-Mariam, T., Neubert, R., Schmidt, P. C., Wutzler, P., and Schmidtke, M. (2006). Antiviral activities of some Ethiopian medicinal plants used for the treatment of dermatological disorders. J. Ethnopharmacol. 104 (1-2), 182–187. doi:10.1016/j.jep.2005.08.071
Gelahun, A. (1989). “Etse debdabe, Ethiopian traditional medicine,” in Biology department, science faculty, addis ababa university. Editor D. Sebsebe, 64–123.
Geyid, A., Abebe, D., Debella, A., Makonnen, Z., Aberra, F., Teka, F., et al. (2005). Screening of some medicinal plants of Ethiopia for their anti-microbial properties and chemical profiles. J. Ethnopharmacol. 97 (3), 421–427. doi:10.1016/j.jep.2004.08.021
Giday, M., Asfaw, Z., Elmqvist, T., and Woldu, Z. (2003). An ethnobotanical study of medicinal plants used by the Zay people in Ethiopia. J. Ethnopharmacol. 85 (1), 43–52. doi:10.1016/s0378-8741(02)00359-8
Girmaw, F., and Engidawork, E. (2022). In vivo anti-malarial activity of the aqueous root extract of Euclea divinorum hiern (Ebenaceae) against Plasmodium berghei ANKA. Evid. Based Complement. Altern. Med. 2022, 2640648. Article ID 2640648. doi:10.1155/2022/2640648
Gowdhami, T., Rajalakshmi, A. K., and Sugumar, N. (2015). Pharmacognostical and preliminary phytochemical screening of the leaf extract of Jasminum auriculatum Vahl. Int. Lett. Nat. Sci. 43, 69–75. doi:10.56431/p-23h92l
Halim, A., Mohamed, A., Habeeb, R., Azer, A., Safwat, R., and Hafeez, A. (2014). Taxonomic revision of Ebenaceae in Egypt. Curr. Sci. Int. 3 (4), 414–425.
Jackson, P. W., and Miller, J. (2015). Developing a world flora online - a 2020 challenge to the world’s botanists from the international community. Rodriguésia 66 (4), 939–946. doi:10.1590/2175-7860201566402
Joubert, A., Kooy, F., Meyer, J. M., and Lall, N. (2006). HPLC in the comparative study of the content of naphthoquinones (quinonoid constituents) in Euclea species of South Africa. Chromatographia 64 (7/8), 399–403. doi:10.1365/s10337-006-0055-z
Kaingu, C. K., Oduma, J. A., and Kanui, T. (2012). Preliminary investigation of contractile activity of Ricinus communis and Euclea divinorum extracts on isolated rabbit uterine strips. J. Ethnopharmacol. 142 (2), 496–502. doi:10.1016/j.jep.2012.05.026
Karunamoorthi, K., Jagajeevanram, K., Vijayalakshmi, J., and Mengistie, E. (2013). Traditional medicinal plants: A source of phytotherapeutic modality in resource-constrained health care settings. J. Evid. Based Complement. Altern. Med. 18 (1), 67–74. doi:10.1177/2156587212460241
Keskin, C. (2018). Medicinal plants and their traditional uses. J. Adv. plant Biol. 1 (2), 8–12. doi:10.14302/issn.2638-4469.japb-18-2423
Keter, L. K., and Mutiso, P. C. (2012). Ethnobotanical studies of medicinal plants used by traditional health practitioners in the management of diabetes in lower eastern Province, Kenya. J. Ethnopharmacol. 139 (1), 74–80. doi:10.1016/j.jep.2011.10.014
Kigen, G., Kipkore, W., Wanjohi, B., Haruki, B., and Kemboi, J. (2017). Medicinal plants used by traditional healers in sangurur, elgeyo marakwet county, Kenya. Pharmacogn. Res. 9 (4), 333–347. doi:10.4103/pr.pr_42_17
Kilonzo, M., Rubanza, C., Richard, U., and Sangiwa, G. (2019). Antimicrobial activities and phytochemical analysis of extracts from Ormocarpum trichocarpum and Euclea divinorum used as traditional medicines in Tanzania. Tanzan J. Health Res. 21 (2), 1–12. doi:10.4314/thrb.v21i2.6
Kodi, P., Mwangi, M. E., Cheplogoi, P. K., Langat, M., and Hoseah, M. A. (2018). In vitro antiplasmodial and toxicity activities of crude extracts and compounds from Euclea latideus (Ebenaceae). Int. J. Biochem. Res. Rev. 21 (1), 1–22. doi:10.9734/ijbcrr/2018/39603
Kose, L. S., Moteetee, A., and Van Vuuren, S. (2015). Ethnobotanical survey of medicinal plants used in the Maseru district of Lesotho. J. Ethnopharmacol. 170, 184–200. doi:10.1016/j.jep.2015.04.047
Kwon, H., Cha, J., Park, J., Chun, Y., Moodley, N., Maharaj, V., et al. (2011). Rapid identification of bioactive compounds reducing the production of amyloid β -Peptide (A β) from South African plants using an automated HPLC/SPE/HPLC coupling system. Biomol. Ther. 19 (1), 90–96. doi:10.4062/biomolther.2011.19.1.090
Lall, N., Kumar, V., Meyer, D., Gasa, N., Hamilton, C., Matsabisa, M., et al. (2016). In vitro and in vivo antimycobacterial, hepatoprotective and immunomodulatory activity of Euclea natalensis and its mode of action. J. Ethnopharmacol. 194, 740–748. doi:10.1016/j.jep.2016.10.060
Lall, N., and Meyer, J. J. M. (2001). Inhibition of drug-sensitive and drug-resistant strains of Mycobacterium tuberculosis by diospyrin, isolated from Euclea natalensis. J. Ethnopharmacol. 78 (2-3), 213–216. doi:10.1016/s0378-8741(01)00356-7
Lall, N., Meyer, J. J. M., Taylor, M. B., and van Staden, J. (2005b). Anti-HSV-1 activity of Euclea natalensis. S. Afr. J. Bot. 71 (3-4), 444–446. doi:10.1016/s0254-6299(15)30118-6
Lall, N., and Meyer, J. M. (2000). Antibacterial activity of water and acetone extracts of the roots of Euclea natalensis. J. Ethnopharmacol. 72, 313–316. doi:10.1016/s0378-8741(00)00231-2
Lall, N., Meyer, J., Wang, Y., Bapela, N., Rensburg, C., Fourie, B., et al. (2005a). Characterization of intracellular activity of antitubercular constituents from the roots of Euclea natalensis. Pharm. Biol. 43 (4), 353–357. doi:10.1080/13880200590951829
Lall, N., Weiganand, O., Hussein, A., and Meyer, J. (2006). Antifungal activity of naphthoquinones and triterpenes isolated from the root bark of Euclea natalensis. Euclea natalensis S. Afr. J. Bot. 72, 579–583. doi:10.1016/j.sajb.2006.03.005
Long, C. (2005). Swaziland’s flora: SiSwati names and uses. Swaziland National Trust Commission. [Last accessed on 2021 June 15] Available at: http://www.sntc.org.sz/index.asp.
Maroyi, A. (2017). Euclea undulata thumb: Review of its botany, ethnomedicinal uses, phytochemistry and biological activities. Asian pac. J. Trop. Med., 1–7.
Masoga, M. A. (2020). “Critical reflections on selected local narratives of contextual South African indigenous knowledge,” in African studies: Breakthroughs in Research and practice (IGI Global), 295–316.
Mbanga, J., Ncube, M., and Magumura, A. (2013). Antimicrobial activity of Euclea undulata, Euclea divinorum and Diospyros lycioides extracts on multi-drug resistant Streptococcus mutans. J. Med. Plant Res. 7 (37), 2741–2746.
Mbaveng, A., and Kuete, V. (2014). Review of the chemistry and pharmacology of 7-Methyl jugulone. Afr. Health Sci. 14 (1), 201–205. doi:10.4314/ahs.v14i1.31
Mcgaw, L., Lall, N., Hlokwe, T., Michel, A., Meyer, J. M., and Eloff, J. (2008). Purified compounds and extracts from Euclea species with antimycobacterial activity against Mycobacterium bovis and fast-growing Mycobacteria. Biol. Pharm. Bull. 31 (7), 1429–1433. doi:10.1248/bpb.31.1429
Mebe, P., Cordell, G., and Pezzuto, J. (1998). Pentacyclic triterpenes and naphthoquinones from Euclea divinorum. Phytochemistry 47 (2), 311–313. doi:10.1016/s0031-9422(97)00398-1
Mekonnen, A., Atlabachew, M., Kassie, B., and Brien, J. A. E. (2018). Investigation of antioxidant and antimicrobial activities of Euclea schimperi leaf extracts. Chem. Biol. Technol. Agric. 5 (1), 1–24. doi:10.1007/s40801-017-0125-6
Moosavi, B., Liu, S., Wang, N., Zhu, X., and Yang, G. (2020). The anti-fungal β-sitosterol targets the yeast oxysterol-binding protein Osh4. Pest Manag. Sci. 76 (2), 704–711. doi:10.1002/ps.5568
Morris, B., and Msonthi, J. D. (1996). Chewa medical botany: A study of herbalism in southern Malawi, 2. LIT Verlag Münster.
Motlhanka, D. M. T., and Nthoiwa, G. P. (2013). Ethnobotanical survey of medicinal plants of tswapong north, in eastern Botswana: A case of plants from mosweu and seolwane villages. Eur. J. Med. Plants. 3 (1), 10–24. doi:10.9734/ejmp/2013/1871
Mueller-harvey, I., Reed, J., and Hartley, R. (1987). Characterisation of phenolic compounds, including flavonoids and tannins, of ten ethiopian browse species by high performance liquid chromatography. J. Sci. Food Agri. 39, 1–14. doi:10.1002/jsfa.2740390102
Mugomeri, E., Chatanga, P., Hlapisi, S., and RahaL, (2014). Phytochemical characterisation of selected herbal products in Lesotho. Lesotho Med. Assoc. J. 12 (1), 38–47.
Nanyingi, M. O., Mbaria, J. M., Lanyasunya, A. L., Wagate, C. G., Koros, K. B., Kaburia, H. F., et al. (2008). Ethnopharmacological survey of Samburu district, Kenya. J. Ethnobiol. Ethnomed. 4 (1), 14–12. doi:10.1186/1746-4269-4-14
Neuwinger, H. D. (2000). African traditional medicine: A dictionary of plant use and applications. With supplement: Search system for diseases. Stuttgart, Germany: Medpharm. Scientific Publ., 213.
Nordeng, H., and Havnen, G. C. (2005). Impact of socio-demographic factors, knowledge and attitude on the use of herbal drugs in pregnancy. Acta Obstetricia Gynecol. Scand. 84 (1), 26–33. doi:10.1111/j.0001-6349.2005.00648.x
Omara, T., Kiprop, A. K., Ramkat, R. C., Cherutoi, J., Kagoya, S., Moraa Nyangena, D., et al. (2020). Medicinal plants used in traditional management of cancer in Uganda: A review of ethnobotanical surveys, phytochemistry, and anticancer studies. Evid. Based Complement. Altern. Med. 2020, 1–26. Article ID 3529081. doi:10.1155/2020/3529081
Oosthuizen, C. B., and Lall, N. (2020). “Euclea natalensis,” in Underexplored medicinal plants from sub-saharan Africa (Academic Press), 111–116.
Orzalesi, G., Mezzetti, T., Rossi, C., and Bellavita, V. (1970). Pentacyclic triterpenoids from Euclea kellau. Planta Medica 19 (1), 30–36. doi:10.1055/s-0028-1099801
Palanisamy, C., and Ashafa, T. (2018). Analysis of novel C-X-C chemokine receptor type 4 (CXCR4) inhibitors from hexane extract of Euclea crispa (Thunb.) leaves by chemical fingerprint identification and molecular docking analysis. J. Young Pharm. 10 (2), 173–177. doi:10.5530/jyp.2018.10.39
Palanisamy, C., Cui, B., Zhang, H., Trung, N., Tran, H., Khanh, T., et al. (2020). Characterization of (2E,6E)-3,7,11trimethyldodecane-2,6,10-trien-1-ol with antioxidant and antimicrobial potentials from Euclea crispa (Thunb.) leaves. Int. Lett. Nat. Sci. 80, 51–63. doi:10.56431/p-v34u92
Palanisamy, C., Selvarajan, R., Balogun, F., Kanakasabapathy, D., and Ashafa, A. (2019). Antioxidant and antimicrobial activities of (6E, 10E)-2, 6, 24-trimethyl pentacosa-2, 6, 10-triene from Euclea crispa leaves. S. Afr. J. Bot. 124, 311–319. doi:10.1016/j.sajb.2019.03.019
Palmer, E., and Pitman, N. (1972). Trees of southern Africa: Covering all known indigenous species in the republic of South Africa, south-west Africa, Botswana, Lesotho & Swaziland.volumes 1 & 2.Trees of southern Africa: Covering all known indigenous species in the Republic of South Africa, south-west Africa, Botswana, Lesotho & Swaziland. Volumes 1 & 2.
Philip, K., Elizabeth, M., Cheaplogoi, P., Langat, M., and Hoseah, M. (2018). In vitro antiplasmodial and toxicity activities of crude extracts and compounds from Euclea latideus (Ebenaceae). Int. J. Biochem. Res. Rev. 21 (1), 1–22. doi:10.9734/ijbcrr/2018/39603
Rademana, S., Anantharaju, P., Madhunapantulab, S., and Lalla, N. (2019). The antiproliferative and antioxidant activity of four indigenous South African plants. Afr. J. Tradit. Complement. Altern. Med. 16 (1), 13–23. doi:10.21010/ajtcam.v16i1.2
Schmidt, E., Lotter, M., and McCleland, W. (2002). Trees and shrubs of Mpumalanga and Kruger national park. Jacana Media.
Shauli, M. (2023). Acute toxicity and 28-day oral administration of Euclea natalesis extract in Swiss albino mice. J. Med. Lab. Sci. Technol. S. Afr. 5 (1), 1–10.
Sobiecki, J. F. (2002). A preliminary inventory of plants used for psychoactive purposes in southern African healing traditions. Trans. R. Soc. S. Afr. 57 (1-2), 1–24. doi:10.1080/00359190209520523
Sparg, S. G., Van Staden, J., and Jäger, A. K. (2000). Efficiency of traditionally used South African plants against schistosomiasis. J. Ethnopharmacol. 73 (1-2), 209–214. doi:10.1016/s0378-8741(00)00310-x
Stafford, G. I., Pedersen, M. E., van Staden, J., and Jäger, A. K. (2008). Review on plants with CNS-effects used in traditional South African medicine against mental diseases. J. Ethnopharmacol. 119 (3), 513–537. doi:10.1016/j.jep.2008.08.010
Steenkamp, V. (2003). Traditional herbal remedies used by South African women for gynaecological complaints. J. Ethnopharmacol. 86 (1), 97–108. doi:10.1016/s0378-8741(03)00053-9
Taylor, J. L., Elgorashi, E. E., Maes, A., Van Gorp, U., De Kimpe, N., Van Staden, J., et al. (2003). Investigating the safety of plants used in South African traditional medicine: Testing for genotoxicity in the micronucleus and alkaline comet assays. Environ. Mol. Mutagen 42 (3), 144–154. doi:10.1002/em.10184
Tinevimbo, C. (2017). Inhibition of α-amylase by Euclea crispa flavonoids and phenolics: Implication for herbal management of Diabetes Mellitus.
Tshikalange, T., Lall, N., Meyer, J., and Mahapatra, A. (2007). In vitro HIV-1 reverse transcriptase inhibitory activity of naphthoquinones and derivatives from Euclea natalensis. S.Afr. J. Bot. 73 (2), 339. doi:10.1016/j.sajb.2007.02.200
Van Wyk, B. E., and Gericke, N. (2000). People's plants: A guide to useful plants of southern Africa. Briza publications.
Weigenand, O., Hussein, A., Lall, N., and Meyer, J. (2004). Antibacterial activity of naphthoquinones and triterpenoids from Euclea natalensis root bark. J. Nat. Prod. 67 (11), 1936–1938. doi:10.1021/np030465d
WHO (2019). WHO Global report on traditional and complementary medicine 2019. Available at: https://www.who.int/news/item-the-who-global-report-on-traditional-and-complementary-medicine (accessed September, 2022).
Woldemedhin, B., Nedi, T., Shibeshi, W., and Shiferaw, M. (2017). Evaluation of the diuretic activity of the aqueous and 80% methanol extracts of the root of Euclea divinorum Hiern (Ebenaceae) in sprague dawley rats. J. Ethnopharmacol. 1, 114–121. doi:10.1016/j.jep.2017.01.015
Wube, A. A., Streit, B., Gibbons, S., Asres, K., and Bucar, F. (2005). In vitro 12 (S)-HETE inhibitory activities of naphthoquinones isolated from the root bark of Euclea racemosa ssp. schimperi. J. Ethnopharmacol. 102 (2), 191–196. doi:10.1016/j.jep.2005.06.009
Keywords: Euclea, naphtoquinones, phytochemistry, ethnobotanical use, pharmacological activity
Citation: Taye AD, Bizuneh GK and Kasahun AE (2023) Ethnobotanical uses, phytochemistry and biological activity of the genus Euclea: A review. Front. Pharmacol. 14:1170145. doi: 10.3389/fphar.2023.1170145
Received: 20 February 2023; Accepted: 05 April 2023;
Published: 19 April 2023.
Edited by:
François Chassagne, IRD UMR152 Pharmacochimie et Biologie Pour le Développement (PHARMADEV), FranceReviewed by:
Smith B. Babiaka, University of Buea, CameroonLatifa Bouissane, Université Sultan Moulay Slimane, Morocco
Copyright © 2023 Taye, Bizuneh and Kasahun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gizachew Kassahun Bizuneh, gizachewkassahun4@gmail.com
 Abebe Dagne Taye1
Abebe Dagne Taye1 Gizachew Kassahun Bizuneh
Gizachew Kassahun Bizuneh Asmamaw Emagn Kasahun
Asmamaw Emagn Kasahun