- School of Pharmacy, Key Laboratory of Molecular Pharmacology and Drug Evaluation, Ministry of Education, Collaborative Innovation Center of Advanced Drug Delivery System and Biotech Drugs in Universities of Shandong, Yantai University, Yantai, Shandong, China
The cortex of adrenal gland produces glucocorticoid, mineralocorticoid, and androgen. The medulla of adrenal gland secrets catecholamines. These hormones play an important role in regulating blood pressure, metabolism, and homeostasis of glucose or electrolytes. Hypersecretion or hyposecretion by the adrenal gland will cause a complex cascade of hormone effects and lead to diseases, including Addison’s disease, Cushing’s syndrome, and congenital adrenal cortical hyperplasia. Skin is the largest organ of body. It provides protection and acts as a barrier against external damage factors like infectious organisms, chemicals, and allergens. Endocrinologic disorders often induce cutaneous abnormalities. According to the previous evidences, natural products have the potential properties for attenuating skin disorders and improving dermatologic symptoms by inhibiting inflammation through MAPK or PI3K/AKT-dependent NF-κB pathways. The natural products may also promote skin wound healing by inhibiting the production of matrix metalloproteinase-9. We systematically searched the relevant articles from databases, including PubMed, Embase, and Cochrane library databases, to review the effects of natural products on skin disorders. This article summarized the effects of natural products on skin inflammation caused by abnormal hormone secreted by adrenal gland. And the published papers indicated that natural products might be a potential source for treating skin diseases.
1 Introduction
Skin is a diverse and extensive organ of the human body. The functions of the skin are important for the healthy and aesthetic conditions (Michalak, 2022). The skin is a well-known reflection of internal pathologic conditions. The unfavorable appearance resulting from cutaneous inflammation affects the mental condition of the patient. And both sides mentioned above play a key role in development and therapy of cutaneous diseases (Rashtak and Pittelkow, 2008). Immune dysregulations in allergic reactions, infections, and internal disorders usually cause cutaneous inflammation. Hormones secreted by adrenal gland play an important role in maintaining physiological function of body. Excessive or insufficient secretion of hormones will cause many diseases. These diseases may induce inflammation-mediated skin diseases. The inflammation of skin is also a main sign of endocrine disorder and chronic autoimmune inflammatory diseases, such as lupus erythematosus, atopic dermatitis, psoriasis, atopic dermatitis, and excessive pigmentation (Yang et al., 2022). Inflammation is an approach to protect cells and to regulate the stimulus including allergens, mechanical stress, irritants, toxins, and pathogens. However, excessive inflammation can lead to tissue lesion. Inflammation is a complex process which mediates skin healing and repairs after injury. Meanwhile, it is a main feature of skin disorders (Nicolaou, 2013). Inflammation is characterized by symptoms such as pain, heat, itching, redness, and swelling.
At present, the marketed drugs aim to reduce inflammation through regulating and/or suppressing immune responses and then improve function of skin, thus ameliorate clinical symptoms and signs with pruritus (Huang et al., 2022). It is well known that agents targeting IL-13, IL-4 (e.g., lebrikizumab, tralokinumab, and dupilumab) and JAK inhibitors (e.g., abrocitinib, upadacitinib, and baricitinib) are efficacious in treating atopic dermatitis. Novel topical agents to treat atopic dermatitis include phosphodiesterase 4 and JAK/STAT inhibitors (Suga and Sato, 2019; Munera-Campos and Carrascosa, 2020).
UP to date, there are no effective agents which can prevent the recurrence of skin disorder or block skin inflammation. Long-term treatment is difficult to be carried out because of the adverse reaction of therapeutic drugs and the inefficiency after application. There is a growing need to find valid therapeutic strategies to cure chronic inflammatory cutaneous diseases (Yang et al., 2022). Natural products or specifically botanicals are promising therapeutic agents in contemporary medicine. A suitable dosage of natural products or botanicals is effective to ameliorate the skin diseases and has less adverse effects when compared to most pharmaceutical medicines. It reported that curcumin can attenuate the skin disorders just causing gastrointestinal discomfort which is a mild adverse event (Zhang et al., 2022). Long-term application of escin does not induce drug resistance. And escin shows a more effective antioxidant activity, cell protection, and anti-aging properties (Dong et al., 2020). Hydroalcoholic extract of Sapium glandulatum is a potential source of anti-inflammatory compounds for the treatment of the inflammation-derived skin diseases. The mechanism of action may be partially related to activating the glucocorticoid receptor. Research showed multiple treatments with Hydroalcoholic extract of S.apium glandulatum did not cause major adverse effects when evaluated in the skin atrophy model (Mendes et al., 2016).
Escin (Figure 1A), an extract of Aesculus chinensis Bge., is a compound showing an anti-inflammatory effect. The compounds in A.esculus chinensis Bge. also have many beneficial effects on the skin and therefore they are used in cosmetic skin-care products (Sarikurkcu et al., 2020). Previous study showed that escin could attenuate the symptoms of atopic skin inflammation (Jeong et al., 2018). In diabetic rats, escin improved wound healing through its antioxidant activities and anti-inflammatory effects (Zhang et al., 2015). Furthermore, it reported that β-carotene (Figure 1B) which is abundant in fruits and vegetables has protective effects against skin injury induced by exposure of UV light (Balić and Mokos, 2019). β-carotene is also used to treat erythropoietic protoporphyria. It reports that β-carotene inhibits inflammation of skin by suppressing the expression of inflammatory factors, promoting expression offilaggrin in atopic dermatitis-like skin, as well as reducing the activity of matrix metalloproteinases (Kake et al., 2019; Takahashi et al., 2019). Ginsenosides extracted from Panax ginseng C. A. Mey (ginseng) are found to inhibit skin inflammation. It is reported that ginsenoside Rg1 (Figure 1C) could attenuate BB-UVB-induced resistance of glucocorticoids in keratinocytes trough Nrf2/HDAC2 signaling pathway (Li et al., 2016). Ginsenoside Rg1 abolishes imiquimod-induced psoriasiform dermatitis by lowering psoriasis severity index score and damage area, skin thickness, lipid peroxidation, and inflammation through downregulating NF-κB signaling pathway (Shi et al., 2019). Ginsenoside Rg3 (Figure 1D) ameliorates cutaneous disorders by blocking MDM2/HIF1α signal pathway (Han et al., 2021).
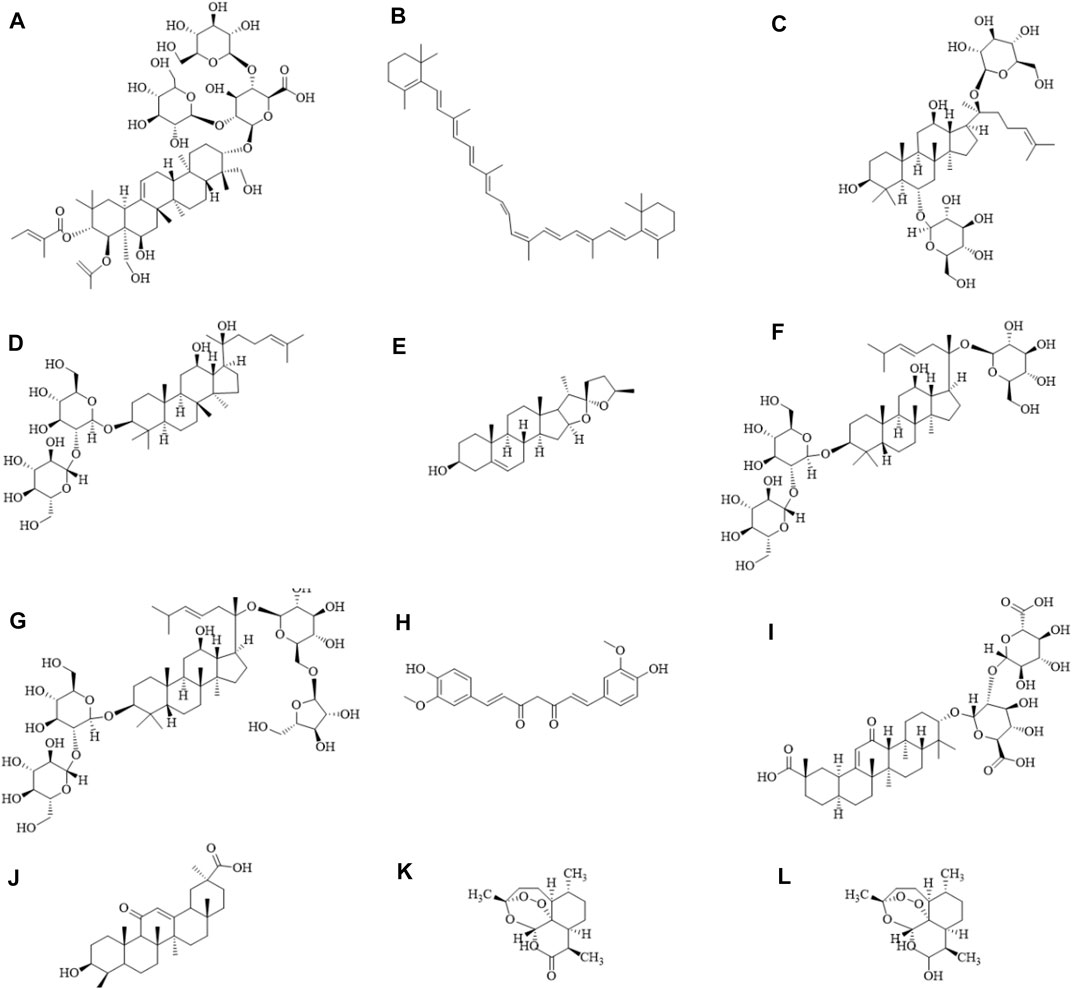
FIGURE 1. Diagram of the chemical formula structure of natural products. (A) Escin, (B) β-carotene, (C) Ginsenoside Rg1, (D) Ginsenoside Rg3, (E) Diosgenin, (F) Ginsenoside Rd, (G) Ginsenoside Rc, (H) Curcumin, (I) Glycyrrhizic acid, (J) Glycyrrhetinic acid, (K) Artemisinin, (L) Dihydroartemisinin. The schematic diagram was drawn using ChemDraw 20.0 software.
Recently, a large quantity of researches focused on using herbal natural products as potential agents to inhibit the skin inflammation. In this article, we review the anti-skin inflammatory effects of natural products, including diosgenin, ginsenoside, curcumin, glycyrrhizic acid, glycyrrhetinic acid, artemisinin, and dihydroartemisinin. The contents mainly focused on the skin inflammation caused by abnormal hormone secretion of adrenal gland.
2 Physiological function of hormones secreted by the adrenal gland
Adrenal gland is composed of adrenal medulla and adrenal cortex. Based on the physiological functions, adrenocortical hormones are divided into three categories: glucocorticoid, mineralocorticoid (aldosterone), and sex hormones. Adrenocorticotropin (ACTH) is secreted by the pituitary gland. ACTH binds to its receptor and promotes the conversion of cholesterol from cytoplasm to mitochondria into pregnenolone. Then, with the action of a series of enzymes, adrenocortical hormones are produced, which also become steroids hormone. Adrenal medulla secreted hormones including epinephrine, norepinephrine, and dopamine. The hormones secreted by adrenal glands are crucial for homeostasis of sodium, homeostasis of glucose, regulation of blood pressure and metabolism. Secretion of too much or not enough of the hormone will be life-threatening. Understanding the biological functions of adrenal hormones is a precondition to the treatment of adrenal gland disease (Kanczkowski et al., 2017).
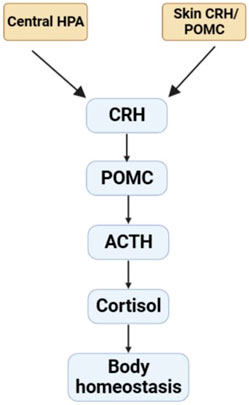
In addition, sebaceous gland of skin exhibits an independent peripheral endocrine function. Previous studies reported there was the presence of a corticotropin-releasing hormone (CRH) system in human sebaceous cell in vitro (Deacon et al., 2000; Krause et al., 2007). The mRNA and protein of CRH and the receptors are found at the skin of rodent or human. Skin also expresses pro-opiomelanocortin (POMC) and its products, including ACTH. It also demonstrates that POMC mRNA and protein are expressed in the skin of mouse during anagen, but not in telogen (the resting phase). And the immunolocalization of the POMC product is restricted to the sebaceous gland (Rassouli et al., 2018). Recent studies have shown that skin cell contain the apparatus which are necessary for production of glucocorticoid (Slominski et al., 2020), androgens (Ceruti et al., 2018; Bienenfeld et al., 2019), and estrogens (Zouboulis et al., 2022). Meanwhile, these hormones were transformed from precursors or cholesterol (Slominski et al., 2013). Furthermore, the CRH/POMC skin system fulfils analogous functions to the hypothalamic-pituitary-adrenal stress (HPA) that may act as a cutaneous defense system (Figure 2). Human skin expresses elements of the HPA axis including POMC, CRH, the CRH receptor-1 (CRH-R1), enzymes of corticosteroid synthesis and then synthesizes glucocorticoids. In melanocytes and fibroblasts, CRH-induced CRH-R1 stimulation upregulates the expression of POMC and the production of ACTH through activating cAMP dependent pathway (Slominski et al., 2013). It can act as a coordinator and executor of local responses to stress, preserving body homeostasis (Ziegler et al., 2007).
3 Effect of hormones secreted by the adrenal gland on skin inflammation
The endocrine system interacts with integumentary systems through a cohort of mechanisms. In the endocrinopathies, the dysregulation of endocrine hormones will lead to dermatologic disease (Lause et al., 2017). Abnormal hormones secreted by adrenal gland can also cause skin inflammation, and this is an important pathogenesis of skin diseases.
Glucocorticoids are cardinal regulators of epidermal growth, differentiation, and homeostasis (Bigas et al., 2018). Glucocorticoids are also widely used in pharmaceutical field. Biological activities were detected with topical glucocorticoids which make them meaningful in cutaneous medicine and diseases of the skin. Glucocorticoids have antiproliferative, anti-inflammatory, immune suppressive, and vasoconstrictive properties in treating cutaneous disorders (Niculet et al., 2020). However, the usage of glucocorticoids by a topical or inhaled route (local or systemic) is associated with lots of adverse side effects (Schoepe et al., 2006; Guillot, 2013). Metabolic side effects are characterized by skin atrophy (Jung et al., 2017), superficial erosions of skin, easy bleeding of skin, and thin skin. Other adverse side effects include the delayed wound healing, striae, hypertrichosis, and acne of papulopustular type (Guillot, 2013). Glucocorticoids can result in immunosuppressive side effect and lead to infection (McDonough et al., 2008), such as parasites diseases (Norwegian scabies), fungal dermopathy (dermatophytosis), viral infections, or bacterial infections. Aldosterone is recognized for its action on the kidney and the cardiovascular system. It modulates deposition of extracellular matrix in skin (Mitts et al., 2010). Human cutaneous tissues can express the local renin-angiotensin-aldosterone system (RAAS). The RAAS in skin exerts a moderating function in aging, cutaneous heating adaptation, wound healing, scarring, and epidermal proliferation. There are also signs demonstrating its role in the regulation of sebum secretion and hair growth. Impaired cutaneous wound healing relating with scleroderma, psoriasis, cancer development, and diabetes may be associated with changes in cutaneous RAAS (Aleksiejczuk et al., 2019). Adrenal gland produces a large quantity of androgens, such as dehydroepiandrosterone. Cutaneous tissue is a major target of androgens. Androgen receptors are expressed in the hair, sebaceous glands, epidermis, and dermis. Dysfunction of androgens in the adrenal gland and/or the skin is associated with acne (Horwitz et al., 2019), hirsutism (Fuchs et al., 2019), and androgenic alopecia (Nestor et al., 2021). The diseases of the adrenal cortex can be viewed from the dual perspectives of hyperfunction and hypofunction. Excessive corticosteroids may lead to several disorders including the adrenogenital syndrome, primary hyperaldosteronism, and Cushing’s syndrome. Addison’s disease and selective hypoaldosteronism are caused by adrenocortical hormone deficiency (Lause et al., 2017). It reported that Cushing’s syndrome can cause acanthosis nigricans, skin atrophy, bruising, hyperpigmentation, and steroid acne (Erden et al., 2019). While the cutaneous manifestations of Addison’s disease include oral mucosa, genital skin, recent scars, vermilion border, frictional surfaces, darkening of the skin in sun-exposed areas and hyperpigmentation of the palmar creases (Nieman and Chanco Turner, 2006). Dysregulation of androgens in the skin and/or the adrenals is associated with androgenic alopecia, hirsutism, and acne.
In patients with low blood pressure, norepinephrine is commonly used to improve hemodynamic stability. It is reported that noradrenaline may result in peripheral gangrene (Jung et al., 2018). Noradrenaline is regarded as a life-saving medicine. Noradrenaline administration may cause an insufficiency of circulation in limbs and therefore lead to necrosis or dry gangrene in skin (Wilson et al., 2021).
The hormones secreted by the adrenal gland play a key role in maintaining body homeostasis. If adrenal gland secreted hormones too much or too little, which will cause skin inflammation leading to skin diseases.
Recent evidence suggested that responses for endocrine stress not only are under control of the central nervous system but also occur in peripheral tissue (Lian et al., 2014; Zitkovsky et al., 2021; Churilov and Milton, 2022). Skin is a target of the POMC-derived neuropeptides alpha-melanocyte stimulating hormone, beta-endorphin, or ACTH. Skin expresses POMC or POMC/CRH peptides. The levels of expression of POMC or POMC/CRH peptides are not static. It is determined by physiological changes associated with hair cycle, ultraviolet radiation exposure, immune cytokine release, or the presence of cutaneous pathology (Bocheva et al., 2019; Ocampo-Garza et al., 2020). Previous work shows that the level of CRH is increased in acne-involved skin, especially in the sebaceous glands, possibly activating pathways which affect immune and inflammatory processes leading to the development and stress-induced exacerbation of acne (Ganceviciene et al., 2009; Khamdan et al., 2022).
4 Effect of natural products on hormones secreted by the adrenal gland
The hormones secreted by adrenal gland play an important role in the metabolism (Sevilla et al., 2013), regulation of blood pressure (Reincke et al., 2021), and homeostasis of sodium or glucose (Kuo et al., 2015; Christa et al., 2022). Natural products may influence secretion of hormones from adrenal gland.
4.1 Diosgenin
Diosgenin (Figure 1E) is a natural steroidal sapogenin (Schwarz et al., 2022). Diosgenin is found in Dioscorea alata L., Smilax china L., and Trigonella foenum-graecum L. (Jesus et al., 2016). Diosgenin can also be extracted from roots of dioscoreae rhizoma. The extraction business is originated in Mexico. The extraction method of the steroids field was introduced to Chinese companies (Herráiz, 2017). Diosgenin is a precursor of steroid hormones. And it shares a similar steroidal structure with glucocorticoids (Junchao et al., 2017). Diosgenin has a variety of biological activities including anti-inflammatory effect (Ran et al., 2020). Diosgenin in Dioscorea nipponica exerts an anti-trachea inflammatory effect through suppressing the secretion of TNF-α, IL-1β, and IL-6 by the interactions with glucocorticoid receptor α (Junchao et al., 2017). Long-term usage of glucocorticoids may cause side effects and thereby limits their use. In the process of searching for compounds that are as effective as glucocorticoids but with less side effects, it is proved that diosgenin can interact with glucocorticoid receptor (GR) (Morsy et al., 2019) using computer molecular docking. The structure of diosgenin is similar as that of glucocorticoid. And diosgenin can interact with GR and then exerts its therapeutic role. It is speculated that diosgenin may replace glucocorticoid and combine with GR to regulate the secretion of glucocorticoid in adrenal gland.
4.2 Ginsenoside
Panax ginseng C. A. Mey (ginseng) is a traditional Chinese medicine (Liu et al., 2020). In China, it has been used to treat a series of diseases for more than 1,000 years. Ginseng is now used in the therapy of inflammation as alternative medicines. Ginsenosides, the main active ingredients of ginseng, are triterpenoid saponins that have a rigid steroidal skeleton with sugar moieties. Ginsenosides have multifarious pharmacological effects (Mancuso and Santangelo, 2017). Based on the chemical structure, ginsenosides are divided into two groups: protopanaxatriols and protopanaxadiols (Hou et al., 2021). The protopanaxadiols include ginsenoside Rb1, Rb2, Rb3, Rc, Rd, Rg3, Rh2. While the sugar moieties in the protopanaxatriols group are attached at the 6-position of ginsenosides including Rf, Re, Rg1, and so on. Ginsenosides show different pharmacological effects and the mechanisms of actions are due to their different chemical structures. Ginsenosides have similar structures to steroids (Yue et al., 2007) and they can prevent stress-associated diseases through reducing glucocorticoid secretion (Zhang et al., 2016). Studies suggested that ginsenoside Rd (Figure 1F) attenuated corticosterone secretion induced by ACTH via inhibiting the MC2R-cAMP/PKA/CREB signal pathway in adrenocortical cells of mouse (Jin et al., 2020). It has also been reported that ginsenoside Rg1 displayed an antidepressant activity via regulating hypothalamic-pituitary-adrenal axis to decrease serum corticosterone level (Mou et al., 2017). The effects of preparations of saponin mixture and ginsenosides on plasma corticosterone and corticotropin in rats were detected via the competitive protein binding methods and radioimmunoassay. Ginseng saponin was found to act on the hypophysis primarily and/or hypothalamus. And it stimulated ACTH secretion, resulting in increased biological synthesis of corticosterone in the adrenal cortex. (Li et al., 2014). In addition, the total saponin of ginseng and ginsenoside Rc (Figure 1G) significantly attenuated the ACTH-induced increase of plasma corticosterone (Kim et al., 2003). These results demonstrated that ginsenosides which has different structures and act as analogues of steroids can regulate the hormone secretion of adrenal gland.
5 Treatment of natural products on skin inflammation caused by abnormal hormones secreted by the adrenal gland
5.1 Curcumin
Curcumin (Figure 1H) is a compound which is extracted from Curcuma longa L. (turmeric) (Farzaei et al., 2018). Turmeric has various pharmacological activities and curcumin is the major active component in turmeric (Kocaadam and Şanlier, 2017; Chopra et al., 2021). Previous studies indicated that curcumin could inhibit vascular remodeling. Curcumin shows antitumor, antioxidant, and anti-inflammatory properties (Aggarwal and Harikumar, 2009; Walker and Mittal, 2020; Mahjoob and Stochaj, 2021). Hormone abnormalities of adrenal gland are the major cause leading to Cushing’s syndrome (Staack et al., 2020; Uehara et al., 2020), Addison’s disease (Jabbour, 2003; Nieman and Chanco Turner, 2006). Hormone abnormalities of adrenal gland can also induce skin inflammation including acne (Nikolakis et al., 2016), skin atrophy (Schoepe et al., 2006), bruising (Niculet et al., 2020), hyperpigmentation (Benner et al., 2019), hirsutism (Bienenfeld et al., 2019), androgenetic alopecia (Alesci and Bornstein, 2000). Up to date, there are a growing number of evidences suggesting curcumin can be used to improve chronic pain, inflammatory dermatoses, skin infections, psoriasis (Panahi et al., 2019), acne, skin cancer, as well as dyspigmentation (Nguyen and Friedman, 2013). It is reported that hairless were topically treated with 500 µL of solutions containing 10% curcumin, 3% ginger extract or the combination of the two, daily for 21 days, ameliorated abrasion wound healing in the corticosteroid-impaired skin in rats. In the healed skin, the production of collagen was increased while matrix metalloproteinase-9 was decreased (Bhagavathula et al., 2009). Curcumin can improve psoriatic cutaneous lesions alone or in combination with other medicines via inhibiting the release of inflammatory factors and keratinocyte proliferation (Zhang et al., 2022). Curcumin oral administration (40 mg/kg, for 20 days) resulted in significant reduction of the serum levels of IL-2, IL-12, IL-22, IL-23, INF-γ, and TNF-α in psoriatic mice, reducing psoriasis-associated inflammation as well as hyper-proliferation of keratinocytes. Clinically, a phase II clinical trial confirmed the efficacy of oral curcumin on cutaneous symptoms of plaque psoriasis, reporting an excellent safety profile (Vollono et al., 2019). A clinical study investigated the use of an herbal combination gel containing turmeric, rosemary, and gotu kola (Tricutan®) for improving signs of photoaging. The treatment showed a significant improvement in skin firmness and self-evaluations after 4 weeks usage (Sommerfeld, 2007). Clinical applications of curcumin are difficult because of its insufficient solubility, chemical instability, and poor absorption. Recent reports demonstrated that these limitations can be overcome by using a nanotechnology-based delivery system.
5.2 Glycyrrhizic acid and glycyrrhetinic acid
Glycyrrhizae Radix et Rhizoma is a native natural products to Asia and Mediterranean area (Sabbadin et al., 2019). Glycyrrhizae Radix et Rhizoma contains a variety of active constituents. It has been used as medicinal agent since ancient times. Glycyrrhizae Radix et Rhizoma shows many biological activities including anti-inflammatory effect (Yang et al., 2017), neuro-protective effect (Lim et al., 2018), anti-cancer action (Wen et al., 2021), and hepatoprotective activities (Kuang et al., 2017). The fresh leaves of Glycyrrhizae Radix et Rhizoma are traditionally used for wounds (Liu et al., 2018). The roots of licorice have been used as a traditional drug to treat cough and stomachache (Liu et al., 2018). Moreover, the stem of Glycyrrhizae Radix et Rhizoma was used to control the symptom of diabetes (Gaur et al., 2014). Glycyrrhizic acid (Figure 1I) is one of the main components of Glycyrrhizae Radix et Rhizoma (Liu et al., 2018). It has been confirmed that glycyrrhizic acid is responsible for the main bioactivities of Glycyrrhizae Radix et Rhizoma’s, such as anti-inflammatory activity. Both ears of the mice were topically treated with a total of 30 μL of Glycyrrhizic acid solution at doses of 50 mM, 100 mM, 150 mM, and 200 mM at 15 min before topical application of a total of 30 μL of TPA solution (25 μg/mL). It demonstrated that glycyrrhizic acid inhibited inflammatory responses via blocking PI3K/Akt signaling pathway, thereby attenuating NF-κB activation in skin inflammation. Glycyrrhizic acid can downregulate the expression of inducible nitric oxide synthase and cyclooxygenase-2 (Liu et al., 2018). Previous data suggested that the deficiency of glucocorticoid in psoriasis skin induced a localized and sustained inflammatory response (Sarkar et al., 2017). An intraperitoneal injection with glycyrrhizic acid at dose of 50 mg/kg/d for 7 days can attenuate dermatitis and improve psoriasis-like cutaneous inflammation in mice through reducing intercellular adhesion molecule-1 expression. Glycyrrhizic acid regulates the ERK/p38 MAPK and NF-κB signaling pathways in keratinocytes (Xiong et al., 2015). Patients of erythrodermic psoriasis with bullous pemphigoid were treated with methotrexate at a dose of 15 mg weekly and compound glycyrrhizin (150 mg/d). After 2 weeks, the patient’s condition had been improved. And the diffuse flushing and the infiltrative swelling were ameliorated (Si et al., 2014). Clinical study showed that, glycyrrhizin and UVB combination therapy led to improvement active-stage generalized vitiligo in disease stage from active to stable (Mou et al., 2016). Glycyrrhizic acid being hydrolyzed and then transformed into glycyrrhetinic acid (Figure 1J) in stomach and duodenum (Armanini et al., 2004), which is responsible for its pharmacological properties. 18β-glycyrrhetinic acid is a major active component of Glycyrrhizae Radix et Rhizoma root and has a wide range of dermatological applications. From the viewpoint of dermatology and cosmetology, inflammation process is the basis of many skin disorders, such as ageing skin, atopic skin, or acne. 18β-glycyrrhetinic acid exerts an anti-inflammatory activity through inhibiting the expression of pro-inflammatory genes, suppressing the production of inflammatory cytokines, and blocking the transformation of arachidonic acid into pro-inflammatory leukotrienes (Kowalska and Kalinowska-Lis, 2019). 18β-glycyrrhetinic acid, a 18β-glycyrrhetinic acid-3-O-β-D-glucuronid metabolite in human intestine, also reduced skin inflammation and the passive skin anaphylaxis (Park et al., 2004). It is reported that a dose of 50 mg/cm2 18β-glycyrrhetinic acid cream or vehicle cream (without 18β-glycyrrhetinic acid) was applied twice daily for 7 consecutive days alleviates psoriasis-like diseases in mice and induces apoptosis of keratinocytes via PI3K/Akt signaling pathway (Gao et al., 2020). All patients of scalp seborrheic dermatitis were instructed to rinse and massage their scalps with 6% glycyrrhetinic acid. After 10–20 s, rinsing off with water, followed by a second massage for 1–2 min with the same product, every day for 5 weeks. The results found that this new formulation can attenuate seborrheic dermatitis of the scalp (Wang et al., 2022). In clinic, glycyrrhizic acid or glycyrrhetinic acid alone were not effective. Therefore, they are often combined with other drugs to elevate efficacy.
5.3 Artemisinin and dihydroartemisinin
Artemisinin (Figure 1K) is a sesquiterpene lactone isolated from artemisiae annuae herba which is a Chinese plant. It has been used for treating different forms of malarial parasites (Uehara et al., 2020). The potential application of artemisinin and its derivatives in coronavirus disease 2019 (COVID-19) treatment has also been recently proposed (Shi et al., 2022). Studies also demonstrate that artemisinin possesses effects of anti-inflammation (Wang et al., 2017) and anti-angiogenesis (Wei and Liu, 2017). Rosacea is a skin disorder showing vascular and immune system dysfunction. Disturbance of corticosteroid homeostasis (Nikolakis et al., 2016; Saric-Bosanac et al., 2020) and topical glucocorticoids treatment (Hengge et al., 2006; Nikolakis and Zouboulis, 2014) play a role in rosacea. In a mouse model, artemisinin was diluted in filtered DMSO and 200 mg/kg feed by means of gavage daily for consecutive 7 days. It found that artemisinin ameliorated rosacea-like dermatitis via inhibiting IL-1β, IL-6, and TNF-α. Artemisinin also reduces the productions of chemokines of immune cells, such as CXCL2, CCL2, CCL20 and CXCL10 (Yuan et al., 2019). Dihydroartemisinin (Figure 1L) is an active metabolite of artemisinin (Dai et al., 2021). It has been used for the treating malaria and show a beneficial efficacy. Dihydroartemisinin showed good bioavailability and anti-malarial effects (Morris et al., 2011; Dai et al., 2021). Recent studies confirmed that dihydroartemisinin inhibited melanoma through regulating immunity and cell apoptosis (Yu et al., 2020). Dihydroartemisinin also alleviates atopic dermatitis by inhibiting mast cell infiltration (Xue et al., 2020). Studies demonstrate that the mice in DHA-treated groups were intraperitoneally administered with dihydroartemisininat at dose of 25 or 50 mg/kg/day for 7 days, which can attenuate psoriasis through inhibiting memory CD8+ T-cells (Chen et al., 2020), targeting fibroblast growth factor receptor 1 to IL-17A (Chen et al., 2022), and regulating the IL-23/Th17 axis (Liu et al., 2021). Further randomized, comparative clinical trials are needed in order to clarify the potential role of dihydroartemisinin in the treatment of skin inflammation. Nevertheless, the clinical application of artemisinin is limited due to its poor solubility and short plasma half-life. However, the problems mentioned above can be solved by preparing controlled release formulations.
Other natural products also alleviate skin inflammation induced by abnormal hormone secretion of adrenal gland through a range of mechanisms (Table 1). For example, quercetin improved skin wound healing by increasing the expression of growth factors via Wnt/β-catenin signaling pathway, by inhibiting inflammation, or by enhancing the migration and proliferation of fibroblasts (Mi et al., 2022). Aloesin ameliorates inflammation through regulating Smad and MAPK/Rho signaling pathways. These findings indicate that aloesin has a therapeutic potential for treating cutaneous wounds (Wahedi et al., 2017).
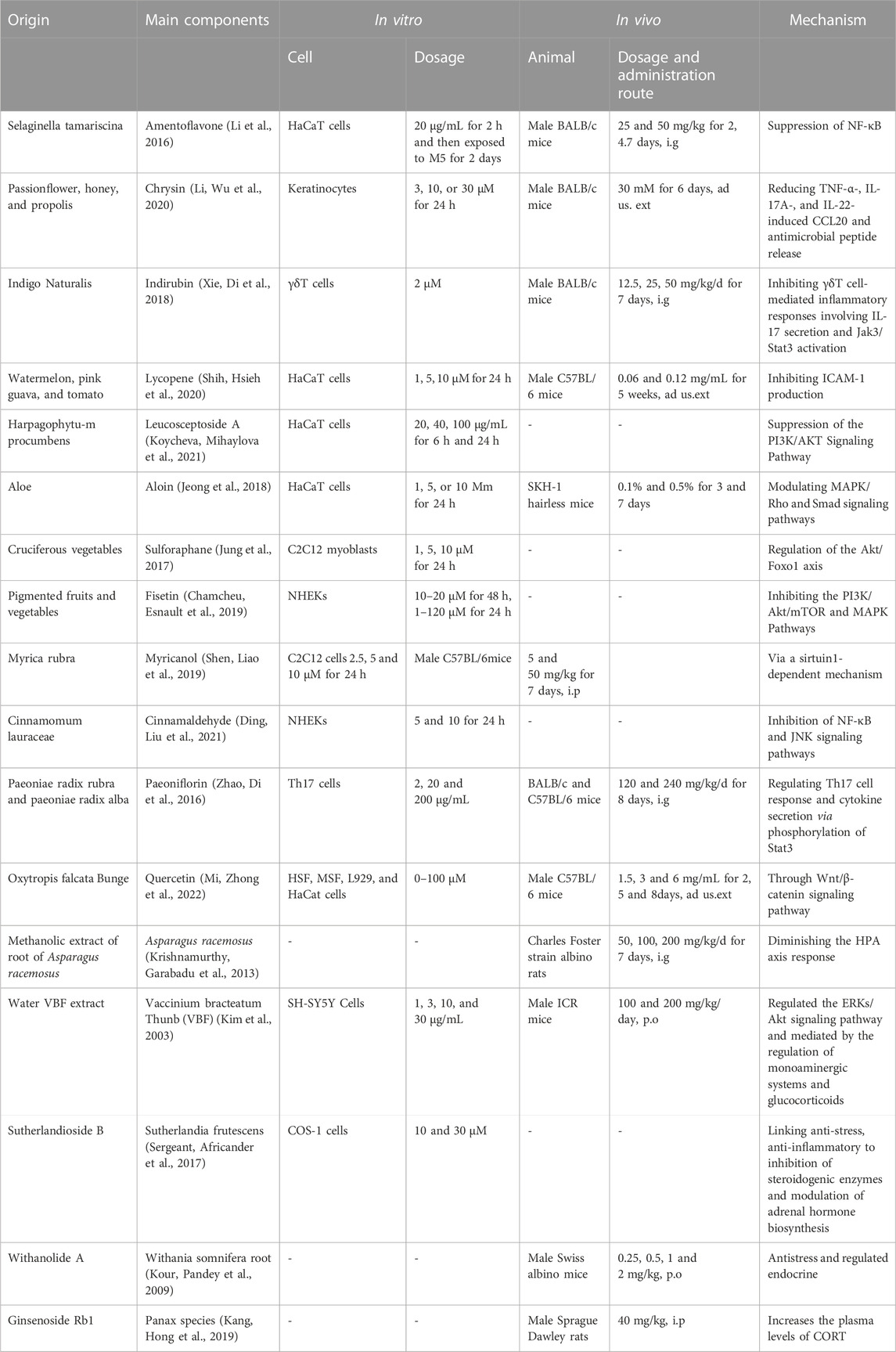
TABLE 1. Natural products for the treatment skin inflammation caused abnormal hormone secreted by adrenal gland.
6 Conclusion
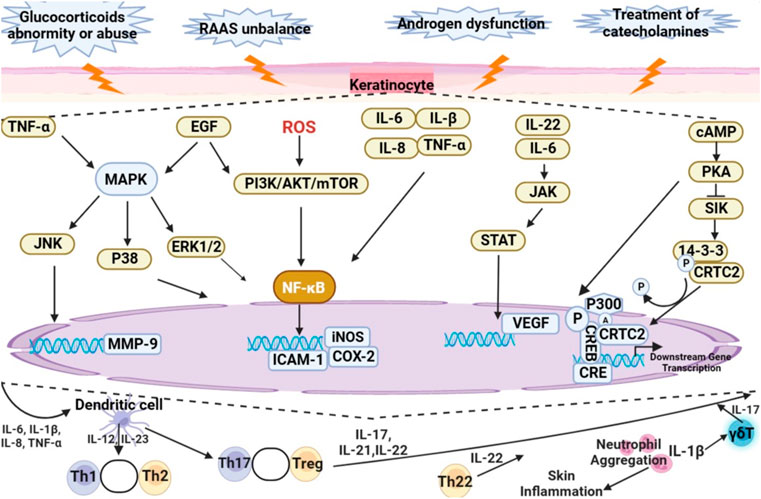
Disorder of hormone secretion by adrenal gland are closely associated with cutaneous lesions. Clinical presentation of skin atrophy, alopecia, psoriasis, skin pigmentation, rosacea, acne, atopic dermatitis, and hirsutism are frequently characterized by skin inflammation. The mechanism of skin inflammation is shown in Figure 3. Abnormal secretion or improper use of glucocorticoids, unbalance of RAAS, androgen secretion dysfunction and treatment of catecholamines will regulate the expression of intracellular inflammatory factors through regulating MAPK, PI3K/AKT/mTOR, JAK/STAT, and cAMP/PKA signaling pathways, resulting in skin inflammation. In conclusion, this review summarizes natural products can attenuate skin inflammation by improving the abnormality of hormones of adrenal gland, including inhibiting the proliferation of keratinocytes, increasing the production of collagen, and reducing the activity of matrix metalloproteinase-9. Based on the published-papers, it is showed that curcumin, glycyrrhizic acid, glycyrrhetinic acid, artemisinin, dihydroartemisinin are the main natural products to be evaluated during trials as a source of drug discovery for skin inflammation caused by abnormal secretion of hormones of adrenal gland.
Author contributions
WX and FF conceived the idea. WX and CZ drafted the manuscript. TW, JW and FF supervised the process and contributed to editing. All authors contributed to the article and approved the submitted version.
Funding
The present study was supported by grants from the Taishan Scholar Project of Shandong Province.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aggarwal, B. B., and Harikumar, K. B. (2009). Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 41, 40–59. doi:10.1016/j.biocel.2008.06.010
Aleksiejczuk, M., Gromotowicz-Poplawska, A., Marcinczyk, N., Przylipiak, A., and Chabielska, E. (2019). The expression of the renin-angiotensin-aldosterone system in the skin and its effects on skin physiology and pathophysiology. J. Physiol. Pharmacol. 70. doi:10.26402/jpp.2019.3.01
Alesci, S., and Bornstein, S. R. (2000). Neuroimmunoregulation of androgens in the adrenal gland and the skin. Horm. Res. 54, 281–286. doi:10.1159/000053272
Armanini, D., Mattarello, M. J., Fiore, C., Bonanni, G., Scaroni, C., Sartorato, P., et al. (2004). Licorice reduces serum testosterone in healthy women. Steroids 69, 763–766. doi:10.1016/j.steroids.2004.09.005
Balić, A., and Mokos, M. (2019). Do We Utilize Our Knowledge of the skin protective effects of Carotenoids enough? Antioxidants (Basel) 8, 259. doi:10.3390/antiox8080259
Benner, B. J. M., Alsma, J., and Feelders, R. A. (2019). Hyponatraemia and hyperpigmentation in primary adrenal insufficiency. BMJ Case Rep. 12, e227200. doi:10.1136/bcr-2018-227200
Bhagavathula, N., Warner, R. L., DaSilva, M., McClintock, S. D., Barron, A., Aslam, M. N., et al. (2009). A combination of curcumin and ginger extract improves abrasion wound healing in corticosteroid-impaired hairless rat skin. Wound Repair Regen. 17, 360–366. doi:10.1111/j.1524-475X.2009.00483.x
Bienenfeld, A., Azarchi, S., Lo Sicco, K., Marchbein, S., Shapiro, J., and Nagler, A. R. (2019). Androgens in women: Androgen-mediated skin disease and patient evaluation. J. Am. Acad. Dermatol 80, 1497–1506. doi:10.1016/j.jaad.2018.08.062
Bigas, J., Sevilla, L. M., Carceller, E., Boix, J., and Pérez, P. (2018). Epidermal glucocorticoid and mineralocorticoid receptors act cooperatively to regulate epidermal development and counteract skin inflammation. Cell Death Dis. 9, 588. doi:10.1038/s41419-018-0673-z
Bocheva, G., Slominski, R. M., and Slominski, A. T. (2019). Neuroendocrine Aspects of skin aging. Int. J. Mol. Sci. 20, 2798. doi:10.3390/ijms20112798
Ceruti, J. M., Leirós, G. J., and Balañá, M. E. (2018). Androgens and androgen receptor action in skin and hair follicles. Mol. Cell Endocrinol. 465, 122–133. doi:10.1016/j.mce.2017.09.009
Chen, B., Li, C., Chang, G., and Wang, H. (2022). Dihydroartemisinin targets fibroblast growth factor receptor 1 (FGFR1) to inhibit interleukin 17A (IL-17A)-induced hyperproliferation and inflammation of keratinocytes. Bioengineered 13, 1530–1540. doi:10.1080/21655979.2021.2021701
Chen, Y., Yan, Y., Liu, H., Qiu, F., Liang, C. L., Zhang, Q., et al. (2020). Dihydroartemisinin ameliorates psoriatic skin inflammation and its relapse by diminishing CD8(+) T-cell memory in wild-type and humanized mice. Theranostics 10, 10466–10482. doi:10.7150/thno.45211
Chopra, H., Dey, P. S., Das, D., Bhattacharya, T., Shah, M., Mubin, S., et al. (2021). Curcumin Nanoparticles as promising therapeutic agents for drug targets. Molecules 26, 4998. doi:10.3390/molecules26164998
Christa, M., Hahner, S., Köstler, H., Bauer, W. R., Störk, S., and Weng, A. M. (2022). Primary hyperaldosteronism induces congruent alterations of sodium homeostasis in different skeletal muscles: A 23Na-MRI study. Eur. J. Endocrinol. 186, K33–k38. doi:10.1530/eje-22-0074
Churilov, A. N., and Milton, J. G. (2022). Modeling pulsativity in the hypothalamic-pituitary-adrenal hormonal axis. Sci. Rep. 12, 8480. doi:10.1038/s41598-022-12513-w
Dai, X., Zhang, X., Chen, W., Chen, Y., Zhang, Q., Mo, S., et al. (2021). Dihydroartemisinin: A potential natural Anticancer drug. Int. J. Biol. Sci. 17, 603–622. doi:10.7150/ijbs.50364
Deacon, C. F., Nauck, M. A., Meier, J., Hücking, K., and Holst, J. J. (2000). Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J. Clin. Endocrinol. Metab. 85, 3575–3581. doi:10.1210/jcem.85.10.6855
Dong, Y., Hou, Q., Sun, M., Sun, J., and Zhang, B. (2020). Targeted isolation of antioxidant Constituents from Plantago asiatica L. And in vitro activity assay. Molecules 25, 1825. doi:10.3390/molecules25081825
Erden, F., Borlu, M., Simsek, Y., and Kelestemur, H. F. (2019). Differences in skin lesions of endogenous and exogenous Cushing's patients. Postepy Dermatol Alergol. 36, 272–275. doi:10.5114/ada.2018.74639
Farzaei, M. H., Zobeiri, M., Parvizi, F., El-Senduny, F. F., Marmouzi, I., Coy-Barrera, E., et al. (2018). Curcumin in Liver diseases: A systematic review of the Cellular mechanisms of oxidative stress and clinical perspective. Nutrients 10, 855. doi:10.3390/nu10070855
Fuchs, A., Matonóg, A., Sieradzka, P., Pilarska, J., Hauzer, A., Czech, I., et al. (2019). Anti-androgenic therapy in young patients and its impact on intensity of hirsutism, acne, menstrual pain intensity and sexuality - a preliminary study. Ginekol. Pol. 90, 520–526. doi:10.5603/gp.2019.0091
Ganceviciene, R., Graziene, V., Fimmel, S., and Zouboulis, C. C. (2009). Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br. J. Dermatol 160, 345–352. doi:10.1111/j.1365-2133.2008.08959.x
Gao, J., Guo, J., Nong, Y., Mo, W., Fang, H., Mi, J., et al. (2020). 18β-Glycyrrhetinic acid induces human HaCaT keratinocytes apoptosis through ROS-mediated PI3K-Akt signaling pathway and ameliorates IMQ-induced psoriasis-like skin lesions in mice. BMC Pharmacol. Toxicol. 21, 41. doi:10.1186/s40360-020-00419-0
Gaur, R., Yadav, K. S., Verma, R. K., Yadav, N. P., and Bhakuni, R. S. (2014). In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine 21, 415–422. doi:10.1016/j.phymed.2013.10.015
Guillot, B. (2013). Glucocorticoid-induced cutaneous adverse events. Rev. Med. Interne 34, 310–314. doi:10.1016/j.revmed.2012.12.004
Han, N. R., Ko, S. G., Moon, P. D., and Park, H. J. (2021). Ginsenoside Rg3 attenuates skin disorders via down-regulation of MDM2/HIF1α signaling pathway. J. Ginseng Res. 45, 610–616. doi:10.1016/j.jgr.2021.06.008
Hengge, U. R., Ruzicka, T., Schwartz, R. A., and Cork, M. J. (2006). Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol 54, 1–15. quiz 16-18. doi:10.1016/j.jaad.2005.01.010
Herráiz, I. (2017). Chemical pathways of corticosteroids, Industrial synthesis from sapogenins. Methods Mol. Biol. 1645, 15–27. doi:10.1007/978-1-4939-7183-1_2
Horwitz, H., Andersen, J. T., and Dalhoff, K. P. (2019). Health consequences of androgenic anabolic steroid use. J. Intern Med. 285, 333–340. doi:10.1111/joim.12850
Hou, M., Wang, R., Zhao, S., and Wang, Z. (2021). Ginsenosides in Panax genus and their biosynthesis. Acta Pharm. Sin. B 11, 1813–1834. doi:10.1016/j.apsb.2020.12.017
Huang, I. H., Chung, W. H., Wu, P. C., and Chen, C. B. (2022). JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Front. Immunol. 13, 1068260. doi:10.3389/fimmu.2022.1068260
Jabbour, S. A. (2003). Cutaneous manifestations of endocrine disorders: A guide for dermatologists. Am. J. Clin. Dermatol 4, 315–331. doi:10.2165/00128071-200304050-00003
Jeong, N. H., Yang, E. J., Jin, M., Lee, J. Y., Choi, Y. A., Park, P. H., et al. (2018). Esculetin from Fraxinus rhynchophylla attenuates atopic skin inflammation by inhibiting the expression of inflammatory cytokines. Int. Immunopharmacol. 59, 209–216. doi:10.1016/j.intimp.2018.04.005
Jesus, M., Martins, A. P., Gallardo, E., and Silvestre, S. (2016). Diosgenin: Recent Highlights on pharmacology and Analytical methodology. J. Anal. Methods Chem. 2016, 4156293. doi:10.1155/2016/4156293
Jin, W., Ma, R., Zhai, L., Xu, X., Lou, T., Huang, Q., et al. (2020). Ginsenoside Rd attenuates ACTH-induced corticosterone secretion by blocking the MC2R-cAMP/PKA/CREB pathway in Y1 mouse adrenocortical cells. Life Sci. 245, 117337. doi:10.1016/j.lfs.2020.117337
Junchao, Y., Zhen, W., Yuan, W., Liying, X., Libin, J., Yuanhong, Z., et al. (2017). Anti-trachea inflammatory effects of diosgenin from Dioscorea nipponica through interactions with glucocorticoid receptor α. J. Int. Med. Res. 45, 101–113. doi:10.1177/0300060516676724
Jung, K. J., Nho, J. H., Cho, H. K., Hong, S., Won, S. H., Chun, D. I., et al. (2018). Amputation of multiple limbs caused by use of inotropics: Case report, a report of 4 cases. Med. Baltim. 97, e9800. doi:10.1097/md.0000000000009800
Jung, S., Lademann, J., Darvin, M. E., Richter, C., Pedersen, C. B., Richter, H., et al. (2017). In vivo characterization of structural changes after topical application of glucocorticoids in healthy human skin. J. Biomed. Opt. 22, 76018. doi:10.1117/1.Jbo.22.7.076018
Kake, T., Imai, M., and Takahashi, N. (2019). Effects of β-carotene on oxazolone-induced atopic dermatitis in hairless mice. Exp. Dermatol 28, 1044–1050. doi:10.1111/exd.14003
Kanczkowski, W., Sue, M., and Bornstein, S. R. (2017). The adrenal gland microenvironment in health, disease and during regeneration. Horm. (Athens) 16, 251–265. doi:10.14310/horm.2002.1744
Khamdan, F. A., Shah, M. A., Khamdan, M. A., and Albasri, E. (2022). Acromegaly presenting with resistant acne vulgaris. Case Rep. Dermatol 14, 151–156. doi:10.1159/000525069
Kim, D. H., Moon, Y. S., Jung, J. S., Min, S. K., Son, B. K., Suh, H. W., et al. (2003). Effects of ginseng saponin administered intraperitoneally on the hypothalamo-pituitary-adrenal axis in mice. Neurosci. Lett. 343, 62–66. doi:10.1016/s0304-3940(03)00300-8
Kocaadam, B., and Şanlier, N. (2017). Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 57, 2889–2895. doi:10.1080/10408398.2015.1077195
Kowalska, A., and Kalinowska-Lis, U. (2019). 18β-Glycyrrhetinic acid: Its core biological properties and dermatological applications. Int. J. Cosmet. Sci. 41, 325–331. doi:10.1111/ics.12548
Krause, K., Schnitger, A., Fimmel, S., Glass, E., and Zouboulis, C. C. (2007). Corticotropin-releasing hormone skin signaling is receptor-mediated and is predominant in the sebaceous glands. Horm. Metab. Res. 39, 166–170. doi:10.1055/s-2007-961811
Kuang, Y., Lin, Y., Li, K., Song, W., Ji, S., Qiao, X., et al. (2017). Screening of hepatoprotective compounds from licorice against carbon tetrachloride and acetaminophen induced HepG2 cells injury. Phytomedicine 34, 59–66. doi:10.1016/j.phymed.2017.08.005
Kuo, T., McQueen, A., Chen, T. C., and Wang, J. C. (2015). Regulation of glucose homeostasis by glucocorticoids. Adv. Exp. Med. Biol. 872, 99–126. doi:10.1007/978-1-4939-2895-8_5
Lause, M., Kamboj, A., and Fernandez Faith, E. (2017). Dermatologic manifestations of endocrine disorders. Transl. Pediatr. 6, 300–312. doi:10.21037/tp.2017.09.08
Li, H., Liu, S. Y., and Wang, B. (2014). Progress of the regulation effect of ginsenosides on HPA axis. Yao Xue Xue Bao 49, 569–575.
Li, J., Liu, D., Wu, J., Zhang, D., Cheng, B., Zhang, Y., et al. (2016). Ginsenoside Rg1 attenuates ultraviolet B-induced glucocortisides resistance in keratinocytes via Nrf2/HDAC2 signalling. Sci. Rep. 6, 39336. doi:10.1038/srep39336
Lian, Y., Xiao, J., Wang, Q., Ning, L., Guan, S., Ge, H., et al. (2014). The relationship between glucocorticoid receptor polymorphisms, stressful life events, social support, and post-traumatic stress disorder. BMC Psychiatry 14, 232. doi:10.1186/s12888-014-0232-9
Lim, C., Lim, S., Lee, B., Kim, B., and Cho, S. (2018). Licorice Pretreatment protects against Brain damage induced by Middle Cerebral Artery Occlusion in mice. J. Med. Food 21, 474–480. doi:10.1089/jmf.2017.4044
Liu, H., Lv, C., and Lu, J. (2020). Panax ginseng C. A. Meyer as a potential therapeutic agent for organ fibrosis disease. Chin. Med. 15, 124. doi:10.1186/s13020-020-00400-3
Liu, J. M., Jin, Q. X., Fujimoto, M., Li, F. F., Jin, L. B., Yu, R., et al. (2021). Dihydroartemisinin alleviates imiquimod-induced psoriasis-like skin lesion in mice involving modulation of IL-23/Th17 Axis. Front. Pharmacol. 12, 704481. doi:10.3389/fphar.2021.704481
Liu, W., Huang, S., Li, Y., Li, Y., Li, D., Wu, P., et al. (2018). Glycyrrhizic acid from licorice down-regulates inflammatory responses via blocking MAPK and PI3K/Akt-dependent NF-κB signalling pathways in TPA-induced skin inflammation. Medchemcomm 9, 1502–1510. doi:10.1039/c8md00288f
Mahjoob, M., and Stochaj, U. (2021). Curcumin nanoformulations to combat aging-related diseases. Ageing Res. Rev. 69, 101364. doi:10.1016/j.arr.2021.101364
Mancuso, C., and Santangelo, R. (2017). Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem. Toxicol. 107, 362–372. doi:10.1016/j.fct.2017.07.019
McDonough, A. K., Curtis, J. R., and Saag, K. G. (2008). The epidemiology of glucocorticoid-associated adverse events. Curr. Opin. Rheumatol. 20, 131–137. doi:10.1097/BOR.0b013e3282f51031
Mendes, D. A., Soley, B. D., Prudente, A. D., Sponchiado, G., Ferreira, B. G., Dos Santos, M. C., et al. (2016). Hydroalcoholic extract of Sapium glandulatum (Vell) Pax displays potent anti-inflammatory activities through a glucocorticoid receptor-dependent pathway. Phytomedicine 23, 1610–1620. doi:10.1016/j.phymed.2016.10.003
Mi, Y., Zhong, L., Lu, S., Hu, P., Pan, Y., Ma, X., et al. (2022). Quercetin promotes cutaneous wound healing in mice through Wnt/β-catenin signaling pathway. J. Ethnopharmacol. 290, 115066. doi:10.1016/j.jep.2022.115066
Michalak, M. (2022). Plant-derived antioxidants: Significance in skin health and the ageing process. Int. J. Mol. Sci. 23, 585. doi:10.3390/ijms23020585
Mitts, T. F., Bunda, S., Wang, Y., and Hinek, A. (2010). Aldosterone and mineralocorticoid receptor antagonists modulate elastin and collagen deposition in human skin. J. Invest Dermatol 130, 2396–2406. doi:10.1038/jid.2010.155
Morris, C. A., Duparc, S., Borghini-Fuhrer, I., Jung, D., Shin, C. S., and Fleckenstein, L. (2011). Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration. Malar. J. 10, 263. doi:10.1186/1475-2875-10-263
Morsy, M. A., Patel, S. S., El-Sheikh, A. A. K., Savjani, J. K., Nair, A. B., Shah, J. N., et al. (2019). Computational and biological Comparisons of plant steroids as Modulators of inflammation through interacting with glucocorticoid receptor. Mediat. Inflamm. 2019, 3041438. doi:10.1155/2019/3041438
Mou, K. H., Han, D., Liu, W. L., and Li, P. (2016). Combination therapy of orally administered glycyrrhizin and UVB improved active-stage generalized vitiligo. Braz J. Med. Biol. Res. 49, e5354. doi:10.1590/1414-431x20165354
Mou, Z., Huang, Q., Chu, S. F., Zhang, M. J., Hu, J. F., Chen, N. H., et al. (2017). Antidepressive effects of ginsenoside Rg1 via regulation of HPA and HPG axis. Biomed. Pharmacother. 92, 962–971. doi:10.1016/j.biopha.2017.05.119
Munera-Campos, M., and Carrascosa, J. M. (2020). Innovation in atopic dermatitis: From pathogenesis to treatment. Actas Dermosifiliogr. Engl. Ed. 111, 205–221. doi:10.1016/j.ad.2019.11.002
Nestor, M. S., Ablon, G., Gade, A., Han, H., and Fischer, D. L. (2021). Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol 20, 3759–3781. doi:10.1111/jocd.14537
Nguyen, T. A., and Friedman, A. J. (2013). Curcumin: A novel treatment for skin-related disorders. J. Drugs Dermatol 12, 1131–1137.
Nicolaou, A. (2013). Eicosanoids in skin inflammation. Prostagl. Leukot. Essent. Fat. Acids 88, 131–138. doi:10.1016/j.plefa.2012.03.009
Niculet, E., Bobeica, C., and Tatu, A. L. (2020). Glucocorticoid-induced skin atrophy: The Old and the new. Clin. Cosmet. Investig. Dermatol 13, 1041–1050. doi:10.2147/ccid.S224211
Nieman, L. K., and Chanco Turner, M. L. (2006). Addison's disease. Clin. Dermatol 24, 276–280. doi:10.1016/j.clindermatol.2006.04.006
Nikolakis, G., Stratakis, C. A., Kanaki, T., Slominski, A., and Zouboulis, C. C. (2016). Skin steroidogenesis in health and disease. Rev. Endocr. Metab. Disord. 17, 247–258. doi:10.1007/s11154-016-9390-z
Nikolakis, G., and Zouboulis, C. C. (2014). Skin and glucocorticoids: Effects of local skin glucocorticoid impairment on skin homeostasis. Exp. Dermatol 23, 807–808. doi:10.1111/exd.12519
Ocampo-Garza, J., Salinas-Santander, M., Welsh, O., Herz-Ruelas, M., and Ocampo-Candiani, J. (2020). Expression of melanocortin 1 receptor before and after narrowband UVB phototherapy treatment in patients with stable vitiligo: A prospective study. Exp. Ther. Med. 19, 1649–1654. doi:10.3892/etm.2020.8435
Panahi, Y., Fazlolahzadeh, O., Atkin, S. L., Majeed, M., Butler, A. E., Johnston, T. P., et al. (2019). Evidence of curcumin and curcumin analogue effects in skin diseases: A narrative review. J. Cell Physiol. 234, 1165–1178. doi:10.1002/jcp.27096
Park, H. Y., Park, S. H., Yoon, H. K., Han, M. J., and Kim, D. H. (2004). Anti-allergic activity of 18beta-glycyrrhetinic acid-3-O-beta-D-glucuronide. Arch. Pharm. Res. 27, 57–60. doi:10.1007/bf02980047
Ran, X., Yan, Z., Yang, Y., Hu, G., Liu, J., Hou, S., et al. (2020). Dioscin improves Pyroptosis in LPS-induced mice mastitis by activating AMPK/Nrf2 and inhibiting the NF-κB signaling pathway. Oxid. Med. Cell Longev. 2020, 8845521. doi:10.1155/2020/8845521
Rashtak, S., and Pittelkow, M. R. (2008). Skin involvement in systemic autoimmune diseases. Curr. Dir. Autoimmun. 10, 344–358. doi:10.1159/000131754
Rassouli, O., Liapakis, G., and Venihaki, M. (2018). Role of central and peripheral CRH in skin. Curr. Mol. Pharmacol. 11, 72–80. doi:10.2174/1874467209666161026144219
Reincke, M., Bancos, I., Mulatero, P., Scholl, U. I., Stowasser, M., and Williams, T. A. (2021). Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. 9, 876–892. doi:10.1016/s2213-8587(21)00210-2
Sabbadin, C., Bordin, L., Donà, G., Manso, J., Avruscio, G., and Armanini, D. (2019). Licorice: From Pseudohyperaldosteronism to therapeutic Uses. Front. Endocrinol. (Lausanne) 10, 484. doi:10.3389/fendo.2019.00484
Saric-Bosanac, S., Clark, A. K., Sivamani, R. K., and Shi, V. Y. (2020). The role of hypothalamus-pituitary-adrenal (HPA)-like axis in inflammatory pilosebaceous disorders. Dermatol Online J. 26. doi:10.5070/d3262047430
Sarikurkcu, C., Locatelli, M., Tartaglia, A., Ferrone, V., Juszczak, A. M., Ozer, M. S., et al. (2020). Enzyme and biological activities of the water extracts from the Plants Aesculus hippocastanum, Olea europaea and Hypericum perforatum that are used as Folk Remedies in Turkey. Molecules 25, 1202. doi:10.3390/molecules25051202
Sarkar, M. K., Kaplan, N., Tsoi, L. C., Xing, X., Liang, Y., Swindell, W. R., et al. (2017). Endogenous glucocorticoid deficiency in psoriasis promotes inflammation and abnormal differentiation. J. Invest Dermatol 137, 1474–1483. doi:10.1016/j.jid.2017.02.972
Schoepe, S., Schäcke, H., May, E., and Asadullah, K. (2006). Glucocorticoid therapy-induced skin atrophy. Exp. Dermatol 15, 406–420. doi:10.1111/j.0906-6705.2006.00435.x
Schwarz, P. F., Perhal, A. F., Schöberl, L. N., Kraus, M. M., Kirchmair, J., and Dirsch, V. M. (2022). Identification of the natural steroid sapogenin diosgenin as a Direct dual-Specific RORα/γ Inverse Agonist. Biomedicines 10, 2076. doi:10.3390/biomedicines10092076
Sevilla, L. M., Latorre, V., Sanchis, A., and Pérez, P. (2013). Epidermal inactivation of the glucocorticoid receptor triggers skin barrier defects and cutaneous inflammation. J. Invest Dermatol 133, 361–370. doi:10.1038/jid.2012.281
Shi, Q., He, Q., Chen, W., Long, J., and Zhang, B. (2019). Ginsenoside Rg1 abolish imiquimod-induced psoriasis-like dermatitis in BALB/c mice via downregulating NF-κB signaling pathway. J. Food Biochem. 43, e13032. doi:10.1111/jfbc.13032
Shi, Q., Xia, F., Wang, Q., Liao, F., Guo, Q., Xu, C., et al. (2022). Discovery and repurposing of artemisinin. Front. Med. 16, 1–9. doi:10.1007/s11684-021-0898-6
Si, X., Ge, L., Xin, H., Cao, W., Sun, X., and Li, W. (2014). Erythrodermic psoriasis with bullous pemphigoid: Combination treatment with methotrexate and compound glycyrrhizin. Diagn Pathol. 9, 102. doi:10.1186/1746-1596-9-102
Slominski, A., Zbytek, B., Nikolakis, G., Manna, P. R., Skobowiat, C., Zmijewski, M., et al. (2013). Steroidogenesis in the skin: Implications for local immune functions. J. Steroid Biochem. Mol. Biol. 137, 107–123. doi:10.1016/j.jsbmb.2013.02.006
Slominski, R. M., Tuckey, R. C., Manna, P. R., Jetten, A. M., Postlethwaite, A., Raman, C., et al. (2020). Extra-adrenal glucocorticoid biosynthesis: Implications for autoimmune and inflammatory disorders. Genes Immun. 21, 150–168. doi:10.1038/s41435-020-0096-6
Sommerfeld, B. (2007). Randomised, placebo-controlled, double-blind, split-face study on the clinical efficacy of Tricutan on skin firmness. Phytomedicine 14, 711–715. doi:10.1016/j.phymed.2007.09.015
Staack, S. O., Rosenthal, A. C., Cook, C. B., and Yang, M. (2020). Glucocorticoid-induced Hypermetabolism in white Adipose tissue in Cushing syndrome. J. Nucl. Med. Technol. 48, 285–286. doi:10.2967/jnmt.119.237545
Suga, H., and Sato, S. (2019). Novel topical and systemic therapies in atopic dermatitis. Immunol. Med. 42, 84–93. doi:10.1080/25785826.2019.1642727
Takahashi, N., Kake, T., Hasegawa, S., and Imai, M. (2019). Effects of post-administration of β-carotene on Diet-induced atopic dermatitis in hairless mice. J. Oleo Sci. 68, 793–802. doi:10.5650/jos.ess19092
Uehara, M., Yamazaki, H., Yoshikawa, N., Kuribara-Souta, A., and Tanaka, H. (2020). Correlation among body composition and metabolic regulation in a male mouse model of Cushing's syndrome. Endocr. J. 67, 21–30. doi:10.1507/endocrj.EJ19-0205
Vollono, L., Falconi, M., Gaziano, R., Iacovelli, F., Dika, E., Terracciano, C., et al. (2019). Potential of curcumin in skin disorders. Nutrients 11, 2169. doi:10.3390/nu11092169
Wahedi, H. M., Jeong, M., Chae, J. K., Do, S. G., Yoon, H., and Kim, S. Y. (2017). Aloesin from Aloe vera accelerates skin wound healing by modulating MAPK/Rho and Smad signaling pathways in vitro and in vivo. Phytomedicine 28, 19–26. doi:10.1016/j.phymed.2017.02.005
Walker, B. C., and Mittal, S. (2020). Antitumor activity of curcumin in Glioblastoma. Int. J. Mol. Sci. 21, 9435. doi:10.3390/ijms21249435
Wang, H. C., Wang, C. S., Hsieh, S. C., Hung, Y. T., and Chen, H. H. (2022). Evaluation of a new-formula shampoo containing 6% glycyrrhetinic acid complex for scalp seborrheic dermatitis: A pilot study. J. Cosmet. Dermatol 21, 3423–3430. doi:10.1111/jocd.14623
Wang, K. S., Li, J., Wang, Z., Mi, C., Ma, J., Piao, L. X., et al. (2017). Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharmacol. Immunotoxicol. 39, 28–36. doi:10.1080/08923973.2016.1267744
Wei, T., and Liu, J. (2017). Anti-angiogenic properties of artemisinin derivatives (Review). Int. J. Mol. Med. 40, 972–978. doi:10.3892/ijmm.2017.3085
Wen, Y., Chen, H., Zhang, L., Wu, M., Zhang, F., Yang, D., et al. (2021). Glycyrrhetinic acid induces oxidative/nitrative stress and drives ferroptosis through activating NADPH oxidases and iNOS, and depriving glutathione in triple-negative breast cancer cells. Free Radic. Biol. Med. 173, 41–51. doi:10.1016/j.freeradbiomed.2021.07.019
Wilson, M., Schafer, K., Goldschmidt, E., Wu, B., and Simman, R. (2021). Norepinephrine-induced peripheral Ischemia leading to gangrene: A Case series. Adv. Skin. Wound Care 34, 273–277. doi:10.1097/01.ASW.0000741528.49437.2c
Xiong, H., Xu, Y., Tan, G., Han, Y., Tang, Z., Xu, W., et al. (2015). Glycyrrhizin ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice and inhibits TNF-α-induced ICAM-1 expression via NF-κB/MAPK in HaCaT cells. Cell Physiol. Biochem. 35, 1335–1346. doi:10.1159/000373955
Xue, X., Dong, Z., Deng, Y., Yin, S., Wang, P., Liao, Y., et al. (2020). Dihydroartemisinin alleviates atopic dermatitis in mice by inhibiting mast cell infiltration. Nan Fang. Yi Ke Da Xue Xue Bao 40, 1480–1487. doi:10.12122/j.issn.1673-4254.2020.10.14
Yang, R., Yuan, B. C., Ma, Y. S., Zhou, S., and Liu, Y. (2017). The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 55, 5–18. doi:10.1080/13880209.2016.1225775
Yang, S. C., Alalaiwe, A., Lin, Z. C., Lin, Y. C., Aljuffali, I. A., and Fang, J. Y. (2022). Anti-inflammatory microRNAs for treating inflammatory skin diseases. Biomolecules 12, 1072. doi:10.3390/biom12081072
Yu, R., Jin, L., Li, F., Fujimoto, M., Wei, Q., Lin, Z., et al. (2020). Dihydroartemisinin inhibits melanoma by regulating CTL/Treg anti-tumor immunity and STAT3-mediated apoptosis via IL-10 dependent manner. J. Dermatol Sci. 99, 193–202. doi:10.1016/j.jdermsci.2020.08.001
Yuan, X., Li, J., Li, Y., Deng, Z., Zhou, L., Long, J., et al. (2019). Artemisinin, a potential option to inhibit inflammation and angiogenesis in rosacea. Biomed. Pharmacother. 117, 109181. doi:10.1016/j.biopha.2019.109181
Yue, P. Y., Mak, N. K., Cheng, Y. K., Leung, K. W., Ng, T. B., Fan, D. T., et al. (2007). Pharmacogenomics and the Yin/Yang actions of ginseng: Anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chin. Med. 2, 6. doi:10.1186/1749-8546-2-6
Zhang, H., Li, Z., Zhou, Z., Yang, H., Zhong, Z., and Lou, C. (2016). Antidepressant-like effects of ginsenosides: A comparison of ginsenoside Rb3 and its four deglycosylated derivatives, Rg3, Rh2, compound K, and 20(S)-protopanaxadiol in mice models of despair. Pharmacol. Biochem. Behav. 140, 17–26. doi:10.1016/j.pbb.2015.10.018
Zhang, S., Wang, J., Liu, L., Sun, X., Zhou, Y., Chen, S., et al. (2022). Efficacy and safety of curcumin in psoriasis: Preclinical and clinical evidence and possible mechanisms. Front. Pharmacol. 13, 903160. doi:10.3389/fphar.2022.903160
Zhang, Z., Cao, G., Sha, L., Wang, D., and Liu, M. (2015). The efficacy of sodium Aescinate on cutaneous wound healing in diabetic rats. Inflammation 38, 1942–1948. doi:10.1007/s10753-015-0174-5
Ziegler, C. G., Krug, A. W., Zouboulis, C. C., and Bornstein, S. R. (2007). Corticotropin releasing hormone and its function in the skin. Horm. Metab. Res. 39, 106–109. doi:10.1055/s-2007-961809
Zitkovsky, E. K., Daniels, T. E., and Tyrka, A. R. (2021). Mitochondria and early-life adversity. Mitochondrion 57, 213–221. doi:10.1016/j.mito.2021.01.005
Keywords: natural products, skin inflammation, abnormality of hormone, hormone of adrenal gland, adrenal gland
Citation: Xie W, Zhang C, Wang T, Wang J and Fu F (2023) Effects of natural products on skin inflammation caused by abnormal hormones secreted by the adrenal gland. Front. Pharmacol. 14:1156271. doi: 10.3389/fphar.2023.1156271
Received: 01 February 2023; Accepted: 02 March 2023;
Published: 03 May 2023.
Edited by:
Tiantai Zhang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Xin Wenyu, Binzhou Medical University, ChinaAndrzej T. Slominski, University of Alabama at Birmingham, United States
Copyright © 2023 Xie, Zhang, Wang, Wang and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fenghua Fu, fenghua@luye.com
†These authors have contributed equally to this work
 Wei Xie†
Wei Xie† Ce Zhang
Ce Zhang Tian Wang
Tian Wang Fenghua Fu
Fenghua Fu