- 1Molecular Chemistry, Materials and Catalysis Laboratory, Faculty of Sciences and Technologies, Sultan Moulay Slimane University, Beni-Mellal, Morocco
- 2Agro Bio Sciences, Mohammed VI Polytechnic University, Ben‐Guerir, Morocco
Tetraclinis articulata (Vahl) Masters, commonly known as Sandarac tree and Araâr, is the only species representing the genus Tetraclinis Masters. The plant has been extensively used for medicinal, artistic, and ritual purposes since its first recorded use in 1800 B.C. Recently, a full range of ethnobotanical investigations has been undertaken to document the plant’s empirical knowledge. They reported the use of different parts, such as leaves, stems, cones, bark, and roots, as part of folk healing practices to manage diabetes mellitus, hypertension, fever, stomach disorders, and diarrhea, among others. The phytochemical studies have identified at least 130 compounds from leaves, cones, resin, bark, and woods. These chemical constituents are categorized into phenolic acids, flavonoids and their derivatives, volatile compounds, phytosterols, and fatty acids, among others. Furthermore, they have strongly been correlated with the promising antimicrobial, antioxidant, neuroprotective, antiurolithiatic, anti-inflammatory, antidiabetic, and cytotoxic properties of the plant. Toxicological studies argued that the plant is quite safe and devoid of eventual toxicity; however, in-depth investigations are required to validate the safety of the plant. The remarkable antimicrobial and antioxidant potencies of various extracts from the plant against a wide range of foodborne pathogens support their possible use to increase the shelf life of foodstuffs in the food industry. Likewise, various plant-based extracts have been proven to exert substantial biocidal properties, making them potential alternatives to synthetic pesticides in agriculture. The present review provides an up-to-date comprehensive insight about the ethnobotanical uses of T. articulata, along with its phytochemistry and biological activities to furnish directions for further studies. We also discussed the biocidal potency of the plant and highlighted its usage to extend the shelf life of perishable foods.
Introduction
Since ancient times, medicinal and aromatic plants have been recognized as reliable sources of health-promoting bioactive agents and a valuable source for finding new drug leads (Kicel, 2020; Annaz et al., 2022). Recently, several serious concerns have been raised about the quality, efficacy, and safety of synthetic drugs (Sahoo et al., 2010). On the other hand, plant-based products are better for the environment and biologically sound as they are recognized by the cells of the body, allowing their metabolism to proceed (Van Wyk et Wink, 2015). As such, a plethora of medicinal and aromatic plants used in the traditional folk systems are progressively coming under the spotlight of scientific research to separate their active chemical ingredients for use in modern dispensing formulas (El-Hossary et al., 2020; Khatib et al., 2022).
The use of T. articulata dates back to Phoenician and Roman times. Due to its high flammability, the wood was used as a source of fuel (firewood and charcoal), incense sticks in religious ceremonies, and as an embalming material. It has been also used as planks in mining, while exquisite wood carvings have been made and exported to Mediterranean countries (Farjon, 2010; Morte and Honrubia, 1996). Nowadays, T. articulata covers an area of 566.000 ha in Morocco, categorized into six major distinct biogeographic zones, namely the Rif, eastern Middle Atlas and oriental, Western Middle Atlas, High Atlas, valleys of the central and the western plateau (Sadiki et al., 2018). However, T. articulata’s total area is constantly shrinking year by year due to the overbrowsing (especially by goats) (Kahouadji et al., 2022), making all attempts to preserve this plant and ensure its sustainability utterly critical (Bouhtoury-Charrier et al., 2009). As such, it is enlisted in the red list of IUCN of threatened conifer, and numerous countries have enacted legislation to ensure its protection (Sánchez-Gómez et al., 2013). Gum sandarac is the name given to the resin produced by this plant, which is released spontaneously in the form of nodules or retrieved by incisions made in the tree bark. The resin exudates are highly prized in the manufacture of varnishes, dental fillings, and pounce (Buhagiar et al., 2000). The tree resin also has a myriad of industrial applications, including replacing Canadian balsam in the preparation of microscope slides (www.conifers.org/cu/Tetraclinis.php, accessed on 18 November 2021). Essential oil derived from the tree resin is almost colorless or pale yellow with a slightly balsamic aroma applied as a fixative, relaxant, and treatment for stress relief and cold (El Moussaouiti et al., 2010). Wood derived from roots and logs is widely known as thuya wood or citron wood. The attractive burled root wood is potent and shines like glass when polished, making it a popular choice to fabricate one-of-a-kind and exquisitely handcrafted goods, such as tables, lamps, jewelry boxes, mirrors, trays, desktops items, and pens, among others (Buhagiar et al., 2000; El Moussaouiti et al., 2010). In North Africa, various parts of this tree, such as leaves, stems, roots, fruit, and seeds, have been used in the traditional folk medicine against multiple health conditions. Different parts of the plant have been reportedly prepared in the form of decoction, infusion, fumigation, and paste and applied topically or orally to treat diabetes mellitus, hypertension, diarrhea, rheumatism, intestinal, respiratory, and skin diseases (Kachmar et al., 2021; Idm’hand et al., 2020; Amel, 2013; Buhagiar et al., 2000). Recent studies have lent credence to several ethnomedicinal applications of T. articulata including, antioxidant (Djouahri et al., 2016), antimicrobial (Bahri et al., 2015), anti-inflammatory (Rached et al., 2018), neuroprotective (El Jemli et al., 2016; Sadiki et al., 2018b), vasorelaxant (Zidane et al., 2014), and anticancer properties (Calderón-Montaño et al., 2021). They also identified a wide range of bioactive compounds belonging to phenolic acids, flavonoids and their derivatives, fatty acids, terpenes, and phytosterols (Djouahri et al., 2014; Bahri et al., 2015; Rached et al., 2018).
Herein, we aimed at providing an up-to-date comprehensive overview of the ethnomedicinal uses of T. articulata (Vahl) Masters in the North Africa region, where this species thrives based on plenty of ethnobotanical surveys. The phytochemistry and biological activities of this tree are also critically summarized and reported in our review. We also discussed for the first time the ethnoveterinary applications and biopesticidal potential of T. articulata and the possibility of its application in agriculture to achieve pest management in an eco-friendly way. Moreover, we reported its-related patents published between 2001 and 2022 and highlighted the use of T. articulata extracts as a food preservative to extend the shelf life of perishable foodstuffs in the food industry.
Methods
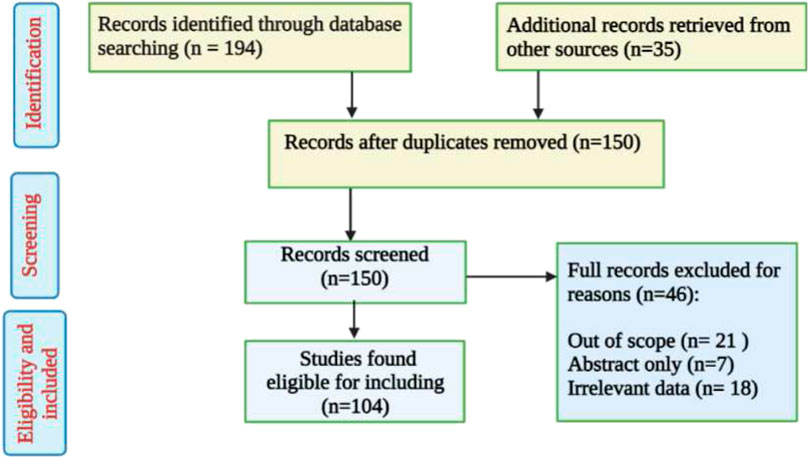
An extensive literature-based search was performed using different databases such as ScienceDirect, PubMed, Google Scholar, Scopus, Springer, and SciFinder. Only peer-reviewed publications were retrieved using the search key phrase ‘Tetraclinis articulata’ with no time limitation set. Duplicated and irrelevant works were excluded (Figure 1). Additionally, numerous books with botanical and ethnopharmacological material were also reviewed. Furthermore, the online web server ProTox-II (http://tox.charite.de/protox_II, accessed on 26 April 2022) was used to predict the organ toxicity and some toxicological endpoints of phenolic compounds, as well as the predominant volatile compounds, detected in T. articulata (Banerjee et al., 2018). The species name and its synonyms were checked based on the online database (http://www.worldfloraonline.org).
Botanical characteristics of T. articulata (Vahl) masters
Taxonomy
Tetraclinis articulata (Vahl) Masters, commonly known as Sandarac tree, and Araâr (Table 1), is a monoïc species that blooms in the spring with long-lasting foliage (Djouahri et al., 2014). Its taxonomy has long been a matter of debate; it was included in the Callitroideae Subfamily (genus Callitris Vent.), which has been around for a great many years. However, T. articulata is unique in this subfamily since all other species are found only in the southern hemisphere, whereas Tetraclinis spreads across the Mediterranean. It was also once classified as part of the genus Thuja L. and Widdringtonia Endl (Jagel and Stützel, 2003; Sánchez-Gómez et al., 2013). Recent morphological and molecular studies confirmed the occurrence of T. articulata within the Cupressaceae family as the only species of the genus Tetraclinis Masters (Jagel and Stützel, 2003; Sánchez-Gómez et al., 2013; Jlizi et al., 2018). The common names and synonyms of the tree have been collected and listed in Table 1.

TABLE 1. Synonyms and common names of T. articulata (Vahl) Masters according to the World Flora Online (http://www.worldfloraonline.org/taxon/wfo-0000456325. Accessed on: 03 August 2022).
Botanical description
T. articulata, widely known as Sandarac tree and Araâr (Table 1), is an evergreen, monoecious tree belonging to the family of Cupressaceae. It is a slow-growing medium-sized tree reaching 6–8 (rarely 15) m in height; the monopodial or multi-stemmed trunk is reddish-browns with vertical stripes and sweet-smelling, generally low branching, up to 50 cm diameter, converging from the base. The root system is profound and not very densely ramified. Young branches are bright green, flattened, flexible, and articulated. Many of these branches fall during the dry season, giving the species greater chances of surviving the long, and harsh summer. The leaves are grouped in opposite decussate pairs, with successive pairs closely and then gradually distanced, appearing in whorls of four on slender branchlets. The young leaves (appearing in the first year) are glaucous, needle-shaped, and about 5–10 cm long (the true leaves appear from the second year onwards). The flowers are monoecious. The male flowers are on the ends of the branches, and the female flowers are on the sides. The ovules are upright, bottle-shaped, and visible. The cone is ovoid, subglobose, with a diameter of approximately 8–12 mm. At first, it is glaucous, tetragonal, and then becomes light brown within 1 year from pollination with thick four woody scales placed in two opposite pairs. Two of the scales are truncated with deep, vertical depression, and the other two are sharp and wide. Seeds (34 × 11.5 mm) are elongated, with resinous peripheral sacs, and have two membranous wings up to 8 x 4-5 mm in size in the form of a samara. There are three–six cotyledons. The number of chromosomes is 2n = 22, (Figure 2) (Morte and Honrubia, 1996; Buhagiar et al., 2000; Farjon, 2010).
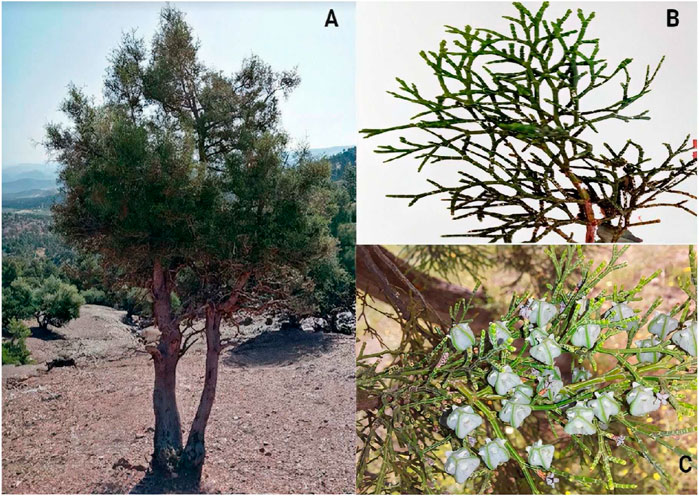
FIGURE 2. Different parts of T. articulata (Vahl) Masters (A) the whole plant; (B) Leaves; (C) Fruits (Pictures were taken in the region of Beni Mellal-khenifra, Morocco, 32⁰54′26.9″N 6⁰16′51.5″W) 2022©.
Geographical distribution
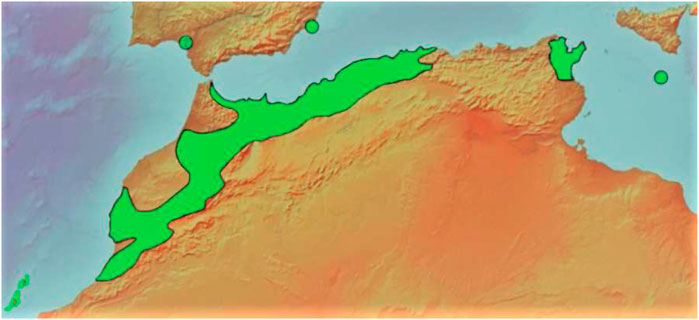
This evergreen coniferous tree is endemic to northwestern Africa in the Atlas Mountains of Morocco, Algeria, and Tunisia, with two small outlying populations in Malta and east Spain (nearby Cartagena and the province of Murcia) (Figure 3) (Bouhtoury-Charrier et al., 2009; Achmit et al., 2021). It is worth noting that the Sierra de Cartagena (Sierra de Cartagena) in east Spain is considered the only natural stronghold of the species in Europe (Morte and Honrubia, 1996). There are also scattered localities deemed naturalized or of unclear origin in the Canary Islands, Cyprus, and the southern Spanish provinces of Málaga (Monte San Antón), Granada (Barranco de Lanjarón), and Huelva (Doñana) (García-Castaño et al., 2021). Their occurrence might be accounted for by the abrupt climatic changes that have taken place in Mediterranean regions over the previous millennia, which may have permitted the import of a variety of plant species from North Africa (Morte and Honrubia, 1996).
Edaphic and pedoclimatic conditions
This thermophilous and xerophytic species thrives in a hot, dry subtropical Mediterranean climate at relatively low altitudes (El Moussaouiti et al., 2010). T. articulata grows from sea level up to 1,000-1,100 m on shady slopes and 1,500-1,600 m in sunny places (Morte and Honrubia, 1996; Sánchez-Gómez et al., 2013). Nonetheless, it has been spotted in Spain at altitudes beneath 370 m, where there is no threat of frost. T. articulata commonly emerges from tree stumps and can withstand wildfires and moderate levels of animals grazing due to its coppicing ability (El Moussaouiti et al., 2010). It grows mainly in calcareous soils, but it can acclimate to other soil types, such as limestone, dolomite, rhyolites, granite, and schist, except for sand-filled environments (Morte and Honrubia, 1996; Sánchez-Gómez et al., 2013). It also appears to stand up to drought (less than 250 mm of rainfall per year) and heavy metals like Zn and Pb in soils, rendering it a strong candidate for infertile and polluted land rehabilitation (Morte and Honrubia, 1996; Buhagiar et al., 2000; Sánchez-Gómez et al., 2013).
Ethnobotanical aspects of T. articulata (vahl) masters
Ethnomedicinal uses in humans
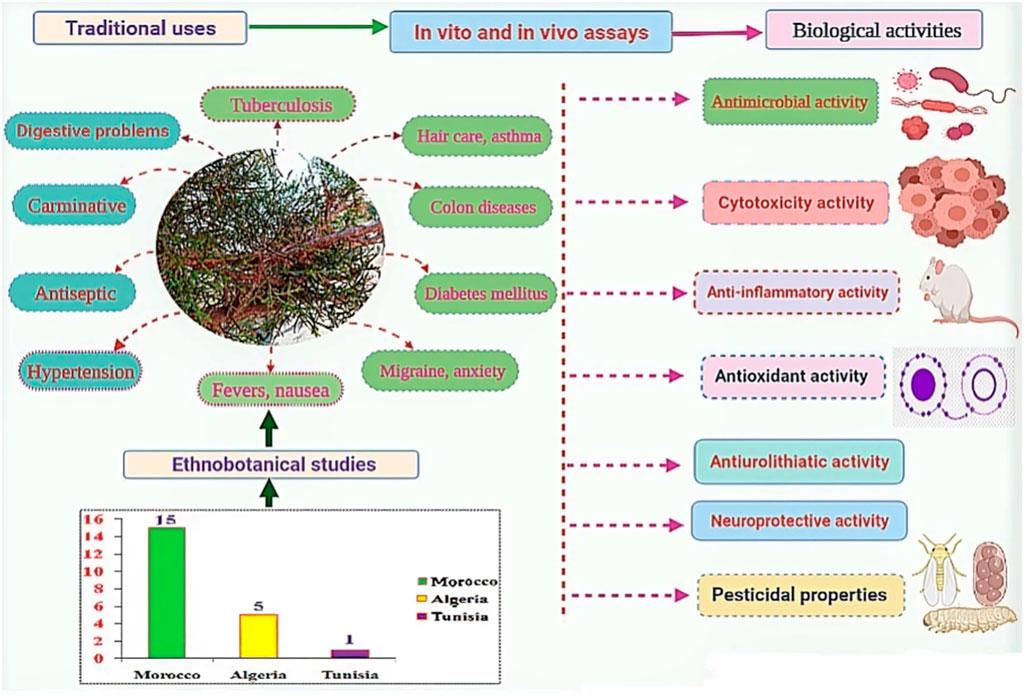
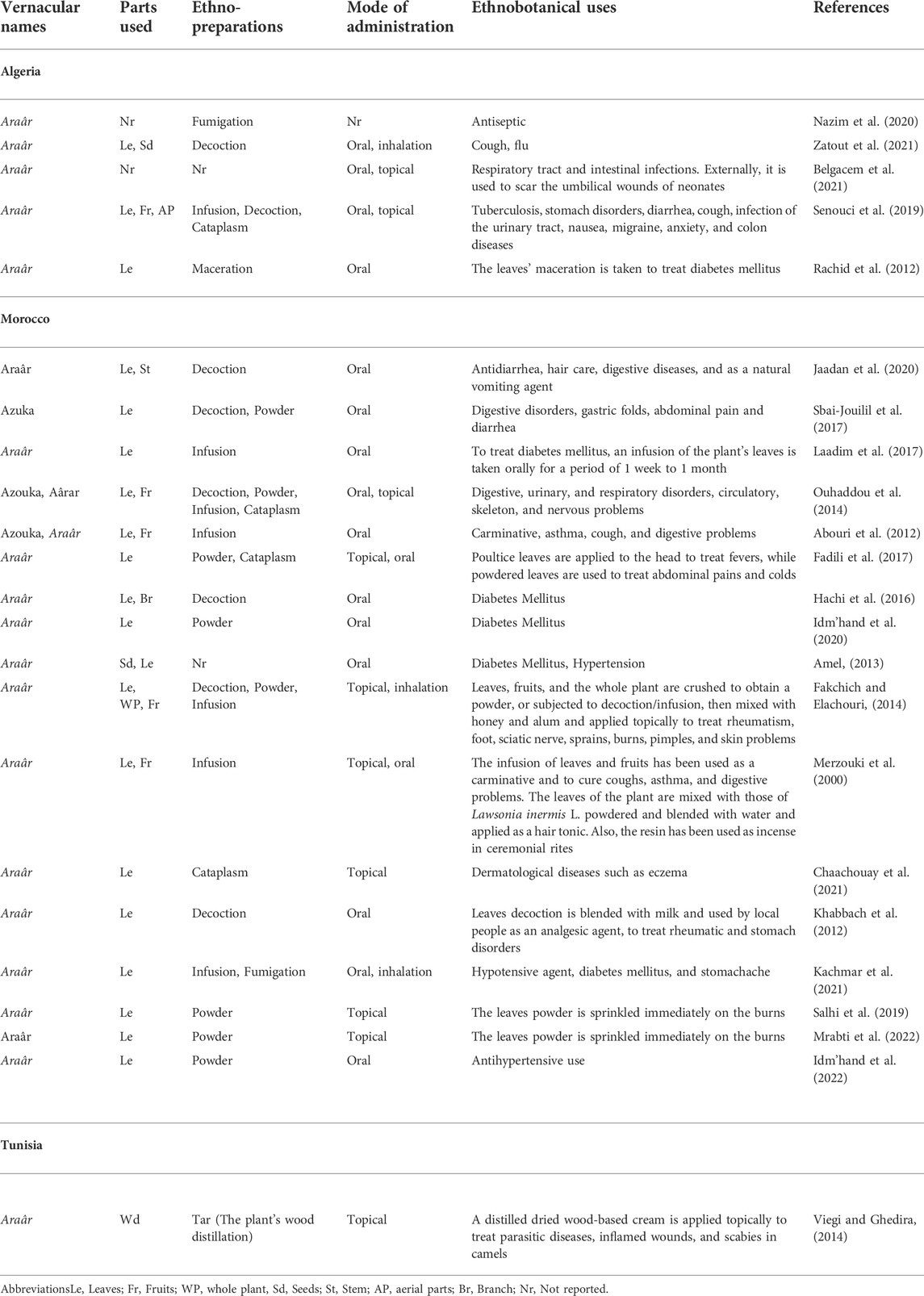
According to our thorough literature search, 21 ethnobotanical studies carried out in the North Africa region mentioned the ethnomedicinal and ethnoveterinary uses of T. articulata. These surveys were especially undertaken in Morocco with 15 ethnobotanical investigations (Figure 4). They reported that various parts of this tree, such as leaves, seeds, fruit, stems, have been used to prevent and treat multiple pathological health conditions. The plant’s aqueous extracts prepared as a decoction (29%), infusion (25%), maceration (4%), as well as cataplasm (14%), and fumigation (7%) were the predominantly reported mode of preparation; whereas, stomach pain, respiratory and intestinal infections, diabetes, and hypertension were the frequently treated diseases (Figure 4; Table 2). Merzouki et al. (2000) reported that aboriginal communities in the Ksar Lekbir district of Morocco used the leaf or fruit infusion orally as digestive, carminative, and for cough and asthma treatment. For hair care, they blended the powdered leaves of T. articulata with those of Lawsonia inermis L, added water, and applied the paste topically to their hair. To manage diabetes mellitus, Laadim et al. (2017) stated the local communities in northwestern Morocco drank leaf infusion over a period varying from 1 week to 1 month. This claim has been corroborated by several ethnobotanical investigations, such as the one conducted in the Central Middle Atlas region of Morocco by Hachi et al. (2016) and also by another one carried out by Rachid et al. (2012) in Algeria, wherein indigenous people took the leaves’ maceration to treat diabetes mellitus. In addition, the leaves or fruits of this plant are prepared as an infusion, decoction, or poultice and used topically or orally to treat digestive, urinary, circulatory, and respiratory disorders as well as skeleton and nervous problems (Ouhaddou et al., 2014). The skimmed milk combined with the leaves and cones decoction has been used as an expectorant and emetic to treat intoxication, diarrhea, and stomach pain (http://temperate.theferns.info/plant/Tetraclinis+articulata, 2021). Besides, the decoction of the leaf is believed to be beneficial against bruises and wounds when applied topically, whereas the macerated leaves serve to make tea (M’barek et al., 2018). Burnt resin is used as evening incense; its balsamic-like scent has been believed to have a relaxing and calming effect. It has also been said to be beneficial in cases of insomnia caused by stress (http://temperate.theferns.info/plant/Tetraclinis+articulata, 2021). In-depth details about the ethnobotanical uses of T. articulata, mode of preparations, routes of administration, and parts used were gathered and listed in Table 2. The figures below are based on 21 ethnobotanical studies conducted exclusively in the North Africa region.
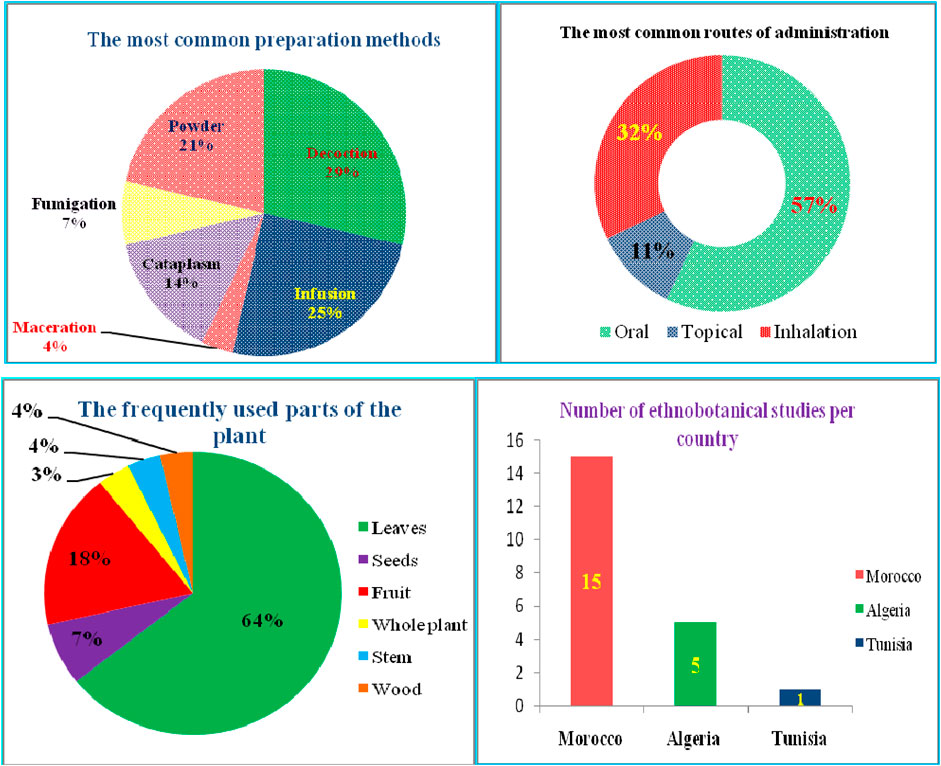
FIGURE 4. The most common preparation methods, routes of administration and used parts of T. articulata, as well as the number of ethnobotanical studies in each country.
Ethnoveterinary uses
Ethnoveterinary medicine is a traditional multifaceted system of concepts, methods, skills, and practices, which have been applied to prevent, cure, and maintain livestock health (McGaw and Eloff, 2008). It mainly relies on plant-based remedies for the treatment and prevention of microbial diseases and parasites in cattle. The ethnoveterinary practices are widespread in the areas where indigenous medical knowledge is deeply ingrained (Abdalla and McGaw, 2020). During the last decades, the overexploitation of standard veterinary drugs has led to severe hazardous effects on both humans and animals, including allergic reactions in hypersensitive subjects, as well as the emergence of resistant bacteria strains, rendering a plethora of these veterinary medications ineffective. Furthermore, the majority of livestock farmers in poor-resource countries neither have easy access to veterinary drugs nor can afford the costs (Lans et al., 2007). On the other hand, ethnoveterinary practices are inexpensive because they rely on readily available local sources. Hence, if appropriately harnessed, they may serve as a suitable alternative to modern veterinary medications for livestock care (Abdalla and McGaw, 2020). They also remain a choice even for rich livestock raisers, especially if animal-related veterinary charges are expensive, and the animal’s market value does not warrant the cost of modern veterinary care (Lans et al., 2007). Although these practices have been handed down orally over generations, recent ethnoveterinary surveys indicated that the know-how related to livestock healthcare is mainly held by elderly people, particularly men who are often in charge of herds (McGaw and Eloff, 2008; Ul Hassan et al., 2014; Piluzza et al., 2015; Miara et al., 2019). Thereby, these practices are likely to vanish along with their owners. In North Africa, many efforts have been invested into documenting and preserving ethnoveterinary knowledge in order to ensure its sustainability for the forthcoming generations. In Tunisia, Virgo and Ghedira, (2014) conducted an ethnoveterinary study to collect data about medicinal plants used locally to treat animals. They reported that indigenous people applied tar (a liquid obtained through destructive distillation or carbonization of dried wood)-based cream of T. articulata topically to treat parasitic diseases, scabies, and inflamed wounds in camels.
To manage several diseases in sheep, Miara et al. (2019) stated that indigenous nomadic people wildcrafted the aerial parts of this plant to prepare an infusion or decoction, which is then fed to livestock to cure stomach disorders, diarrhea, kidney problems, and tremor. In Morocco, herders and farmers hold advanced ethnoveterinary knowledge; they used their medical skills not only to treat animals but also to improve the quality of their herds’ milk, dairy products, and meat. They utilized dried wood tar of T. articulata to treat skin and dermal diseases, including inflamed wounds, scabies, and internal parasites (Abdalla and McGaw, 2020). Similarly, a recent study carried out in the Rif area, Northern Morocco, by Chaachouay et al. (2022) reported that the native people heavily rely on the crushed leaves from this species to heal goats’ Endo- and ectoparasite infections.
Chemical composition
Essential oil
T. articulata has been proven to be a rich repository of essential oil that might obtained from various parts, including leaves, stems, cones, leafy twigs, using advanced techniques such as microwave-assisted hydrodistillation, supercritical fluid extraction, or conventional ones such as hydrodistillation using a Clevenger-type apparatus. The chemical composition of T. articulata’s essential oil extracted from woody branches, cones, resin, woods, wood sawdust, and roots has been the subject of numerous studies in North Africa countries and Malta (Buhagiar et al., 2000; Djouahri et al., 2016; Ben Ghnaya et al., 2016; Harmouzi et al., 2016). They reported that the chemical composition showed slight variations depending on the origin and organs from which they were originated, with bornyl acetate and camphor being the dominant encountered chemical components (Table 3). So far, the EOs contained mainly monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, and oxygenated sesquiterpene (Figure 5) (Abi-Ayad et al., 2013). Bornyl acetate, camphor, and α-pinene are essential oil markers in leaf identification at various phenological stages (Bahri et al., 2015). In this sense, Sadiki et al. (2018) reported that the chemical composition of the essential oil from leaves of T. articulata collected in the eastern region of Morocco was dominated by monoterpene hydrocarbons (47.08%), with α-pinene (22.68%), bornyl acetate (16.87%), camphor (14.52%), and limonene (7.34%). These findings are in agreement with those obtained by Bahri et al. (2015), who examined the EO of T. articulata leaves (fresh and dried) collected in Algeria. They indicated that α-pinene (36.1%; 44.1%), bornyl acetate (18.3%; 3.1%), camphor (1.7%; 20.1%), and limonene (2.9%; 5.0%) were the most representative constituents. Similar results were also mentioned by Achak et al. (2009), Bourkhiss et al. (2007), and Sadiki et al. (2018). These outcomes slightly disagree with those of Ben Ghnaya et al. (2016), who showed that the major constituents of the EO of T. articulata leaves collected in Nabeul, Tunisia, were α-pinene (56.21%), followed by 1,8-cineole (9.91%), isobornyl acetate (7.46%), and β-myrcene (3.08%). On the other hand, a large body of phytochemical studies revealed that the chemotype of plant leaves differed from wood sawdust, root, resin, and cones. For instance, the oxygenated monoterpenes α-campholenal, trans-pinocarveol, verbenone, and cis-verbenol were identified as the preponderant components of the EO of T. articulata cones taken from the area of Ain-Defla, Algeria (Djouahri et al., 2013), whereas trans-pinocarveol, fenchyl acetate, p-cymene-8-ol, and β-phellandrene were the main constituents of the EO of T. articulata cones collected from the area of Zaghouan, Tunisia (Tékaya-Karoui and Mighri, 2007). Indeed, differences in chemical composition can be linked to several factors, including the time of harvest and drying techniques, which may result in the destruction of small molecules with low vaporization points. The variability in soil nutrient concentrations and their storage in leaves can also lead to plenty of metabolic processes and the synthesis of various bio-products and volatile components (Djouahri et al., 2013).
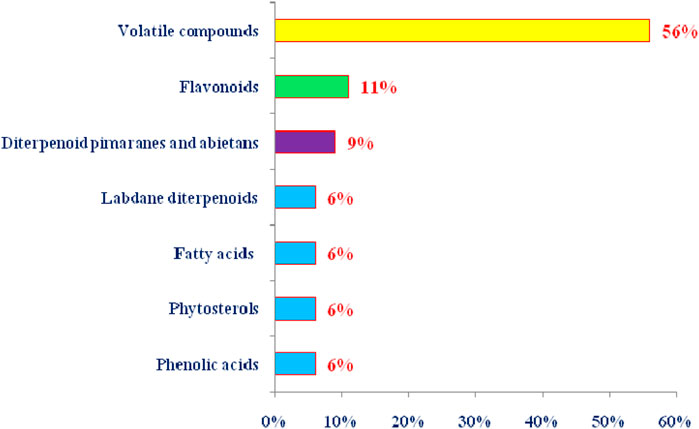
FIGURE 5. Summary of the phytoconstituents from T. articulata by class of natural products (The percentage of each class was calculated based on the total number of chemical components).
Phenolic compounds
There are relatively few studies that have been devoted to investigating the plant’s phenolic compounds. These studies identified 21 phenolic compounds from leaf and cones crude extracts (Djouahri et al., 2014; Zidane et al., 2014; Dane et al., 2016; Rached et al., 2018). These compounds are represented by phenolic acids (gallic and caffeic acids), flavonoid aglycones, and flavonoid glycosides and they were identified from 100% MeOH, 100% EtOH, and 70% EtOH extracts of air-dried cones of T. articulata (Figure 5; Table 3) (Djouahri et al., 2014). On the other hand, the aqueous extract of T. articulata air-dried cones was shown to contain exclusively tannic and gallic acids, while caffeic acid was lacking (Djouahri et al., 2014). Moreover, nine phenolic compounds have been identified in crude aqueous extract, ethyl acetate fraction, and butanol fraction of T. articulata leaves, including three flavan-3-ols and six flavanols (Rached et al., 2018). Accordingly, flavan-3-ols were the most common phenolic chemicals in the three samples, constituting up to 71% of the total phenolic content. B-type epicatechin dimer was the primary constituent in the aqueous crude extract and the butanol fraction, whereas catechin was by far the most abundant component in the ethyl acetate fraction (Rached et al., 2018). Flavonols made up an average of 29% of the total phenolic content, with the ethyl acetate fraction having the highest composition and the glycoside myricetin-3-O-rhamnoside as the major constituent (Rached et al., 2018). Furthermore, cupressuflavone and amentoflavone, two naturally occurring biflavonoids with hydroxyl substituent, were identified in the methanol extract and ethyl acetate extract (decoction) of T. articulata leaves, whereas they were absent in the aqueous extract (Zidane et al., 2014).
Diterpenoid pimaranes, labdanes and abietans of T. articulata sandarac resin
T. articulata resin is a highly balsamic and viscous substance with a light yellow color and a melting point of about 145°C (Kononenko, 2017). It is secreted from specialized internal and external structures and released for different purposes including, defense, protection against predators, and interaction with its surrounding environment (Zona, 2004; Kononenko, 2017). The use of sandarac resin is traced back to 1800 BC, and since then, it has been frequently used for artistic, therapeutic, and ritual purposes (Romero-Noguera et al., 2014; Kononenko, 2017). Previous research on the chemical composition of sandarac resin revealed that communic acid, a natural bicyclic diterpenoid with a labdane skeleton makes up over 70% of the resin. The communic acid was shown to be responsible for the polar properties and poor solubility of the resin after aging (Romero-Noguera et al., 2014). So far, a total of 42 compounds were identified during the chemical analysis of sandarac resin (Sugimoto et al., 2006; Romero-Noguera et al., 2014; Jlizi et al., 2018). These compounds consist primarily of labdane diterpenes such as cis-communic acid, trans-communic acid, and agathalic acid. Sandarac resin also contains six pimaranes and abietans diterpenoids, including sandaracopimarinol, sandaracopimaric acid, and laevopimaric acid, as well as small amounts of phenolic components such as totarol and manool (Figure 5; Table 3) (Sugimoto et al., 2006; Romero-Noguera et al., 2014).
In silico toxicity prediction of phenolic compounds as well as the major volatile compounds found in T. articulata
The study of the toxicity of a candidate compound is a crucial step in the drug development process before proceeding into clinical trials. Thus, the in silico toxicity tools emerged as a time-saving and inexpensive alternative to animal experiments (Bai and Abernethy, 2013). On the other hand, given the scarcity of toxicological research on the plant species, we have relied on the online web Tool ProTox-II (http://tox.charite.de/protox_II, accessed on 26 April 2022) to predict the organ toxicity (hepatotoxicity) (since the liver is the organ where these compounds are metabolized) as well as four toxicological endpoints, namely cytotoxicity, mutagenicity, carcinogenicity, and immunotoxicity of the phenolic compounds alongside the major volatile compounds found in different T. articulata extracts. The predicted descriptors are listed in Table 4.
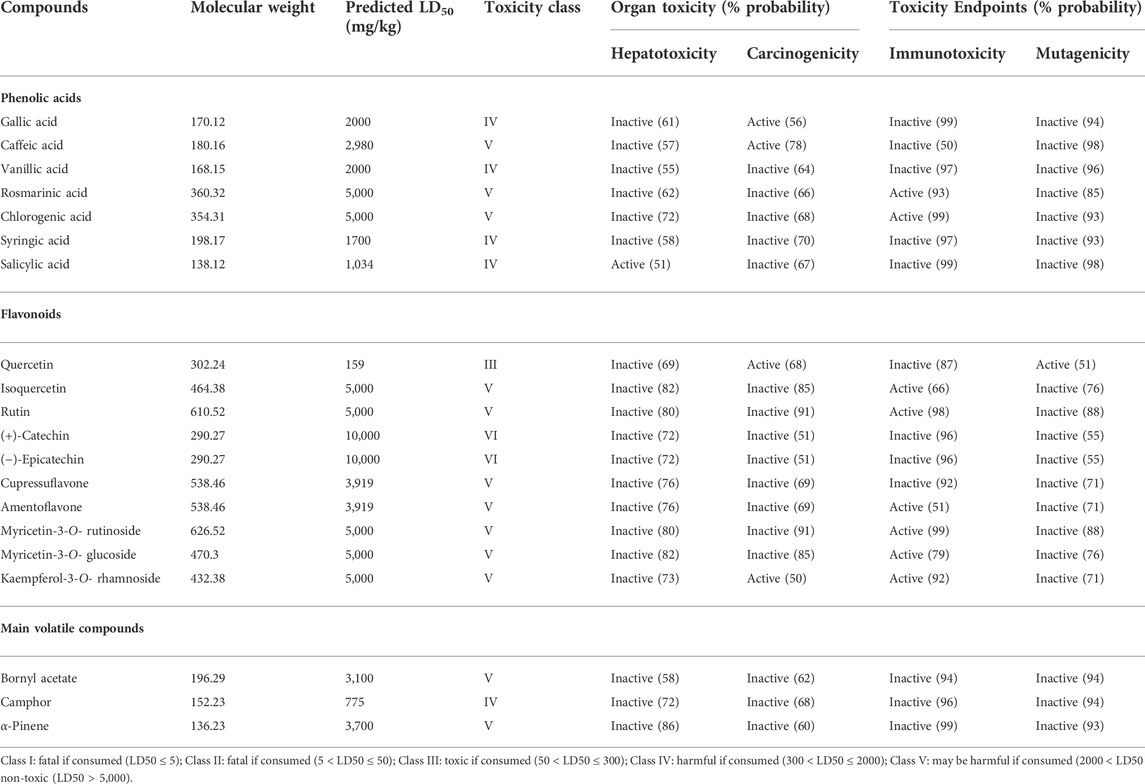
TABLE 4. The predicted organ toxicity and toxicological endpoints of phenolic compounds and the main volatile compounds using the ProTox-II server.
Regarding organ toxicity, results revealed that salicylic acid was assumed to be hepatotoxic, while the other compounds were expected to be hepatotoxic-free. Proceeding in vivo and in vitro studies have corroborated the previous in silico findings showing that salicylic acid may occasionally induce lipid peroxidation and severe liver damage, especially under oxidative stress (Doi and Horie, 2010). When it came to toxicological endpoints, all the chemical constituents were predicted to be devoid of any cytotoxicity. Quercetin, a naturally occurring flavonol, showed two toxicological endpoints, namely mutagenicity and carcinogenicity, while gallic acid, caffeic acid, and kaempferol-3-O-rhamnoside were predicted as both immunotoxic and carcinogenic. Previous in vivo and in vitro investigations revealed that quercetin displayed positive mutagenicity and carcinogenicity, which is consistent with in silico prediction (Ukoroije and Otayor, et al., 2020 Utesch et al., 2008). The median lethal dose (LD50) values were estimated to be between 159 and 5,000 mg/kg (Table 4). According to the globally harmonized system of classification of labeling of chemicals (GHS) (Drwal et al., 2014), the majority of phenolic acids fall in the category of ‘harmful if consumed’ (Toxicity class IV), while caffeic acid, rosmarinic acid, and chlorogenic acid were categorized as ‘may be harmful if swallowed’ (Toxicity class V) with LD50 values of 2,980, 5,000, and 5,000 mg/kg, respectively. Similarly, most flavonoids were assigned to toxicity classes IV or V, except for quercetin, which was allocated in category III (Toxic if swallowed). However, in depth studies are needed to evaluate the toxicity of T. articulata due to the synergetic effects and possible interactions its multiple components with several proteins and body organs.
Biological activities of T. articulata
Cytotoxicity properties
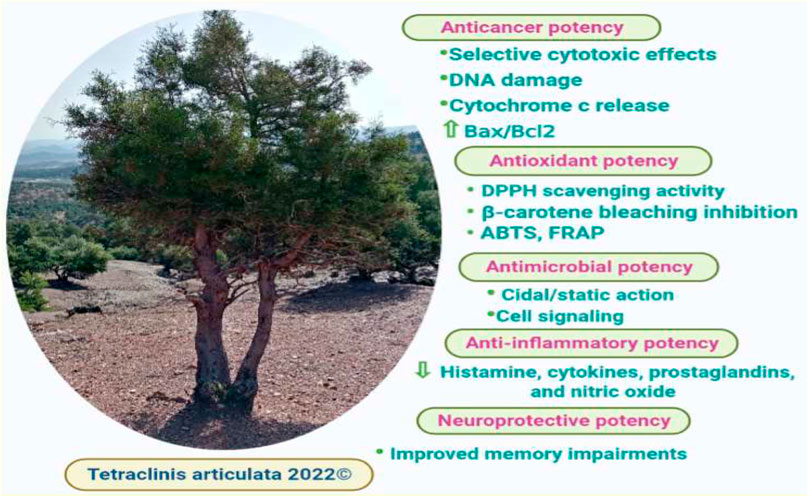
The in vitro anticancer activity of T. articulata extracts was examined against various cancer cell lines, offering data on the bioactivity of both the extract and the individual compounds (Figure 6). To overcome multidrug resistance in MDA-MB-231 breast cancer cells and investigate whether T. articulata essential oil possesses substantial in vitro cytotoxic properties (Table 5). Jlizi et al. (2021) tested the in vitro cytotoxic activity of T. articulata trunk bark essential oil against MDA-MB-231 breast cancer and SW620 colon cancer using the cell viability assay (MTT). They reported that the EO exhibited moderate dose-dependent cytotoxic potency against both cell lines. They also recorded IC50 values of 25.7 and 83.0 μg/ml against SW620 and MDA-MB-231cells, respectively, after 48 h of exposure. To improve the extract’s cytotoxicity, the authors fractionated the EO on a silica gel column using a step gradient of hexane/ethyl acetate and reassessed each fraction against both cell types. Accordingly, the fraction hexane/ethyl acetate (90:10) exhibited better activity against SW620 cells, while the fraction (80:20) was effective against MDA cells. They correlated the cytotoxic effect with the presence of particular sesquiterpene compounds, such as caryophyllene and caryophyllene oxide. Using the same method, Rached et al. (2018) reported that the crude aqueous extract of T. articulata and its fractions disclosed good cytotoxic effects toward four human cancer cells, namely CI-H460 (non-small cell lung cancer), HeLa (cervical carcinoma), MCF-7 (breast carcinoma), and HepG2 (hepatocellular carcinoma), with ethyl acetate fraction being the most prominent against all the tested cell lines. They reported that the ethyl acetate fraction was the richest in total flavonoids 93.1 mg/g, compared to 21.2 mg/g in the crude aqueous extract, and ascribed the effects to certain flavonoids, such as flavonols (catechin, and epicatechin). To seek cytotoxic agents with high selectivity towards cancer cells to counteract metastatic cancers, Calderón-Montaño et al. (2021) screened 65 extracts prepared from 45 plants growing in Spain for their cytotoxic capacity against normal lung cells (MRC-5) and lung cancer cells (A549). They reported that T. articulata leaves ethanol/ethyl acetate/water (1:1:1) extract exhibited the highest activity against A549 cells (IC50 = 0.37 ± 0.03 μg/ml); Meanwhile, displaying less toxicity towards lung normal cells IC50 = 129.5 ± 64.0 μg/ml, (high selectivity). Moreover, the diterpene trans-communic acid and cis-trans mixture were isolated from the resin of T. articulata and screened for their cytotoxic potential against HeLa and A549 cell lines, following the MTT assay. Both trans-communic acid and cis-trans mixture exhibited potent cytotoxic effects on the HeLa cell line, with IC50 values of 12.8 ± 0.5 and 09.5 ± 1.0, respectively (Jlizi et al., 2018). Moreover, the essential oil of this tree contained thymoquinone, a monoterpene with promising anti-cancer properties; It has been reported to induce apoptosis in many solid and liquid tumors through increasing Bax/Bcl2 ratio and the expression of pro-apoptotic molecules, including caspase-3 and caspase-9, triggering cytochrome c release, DNA damage, and through P53 dependent pathway (Ahmad et al., 2019). These studies disclosed the anticancer potential of T. articulata; however, in-depth in vivo, toxicological, and clinical trials are mandatory to ensure its efficacy and safety.
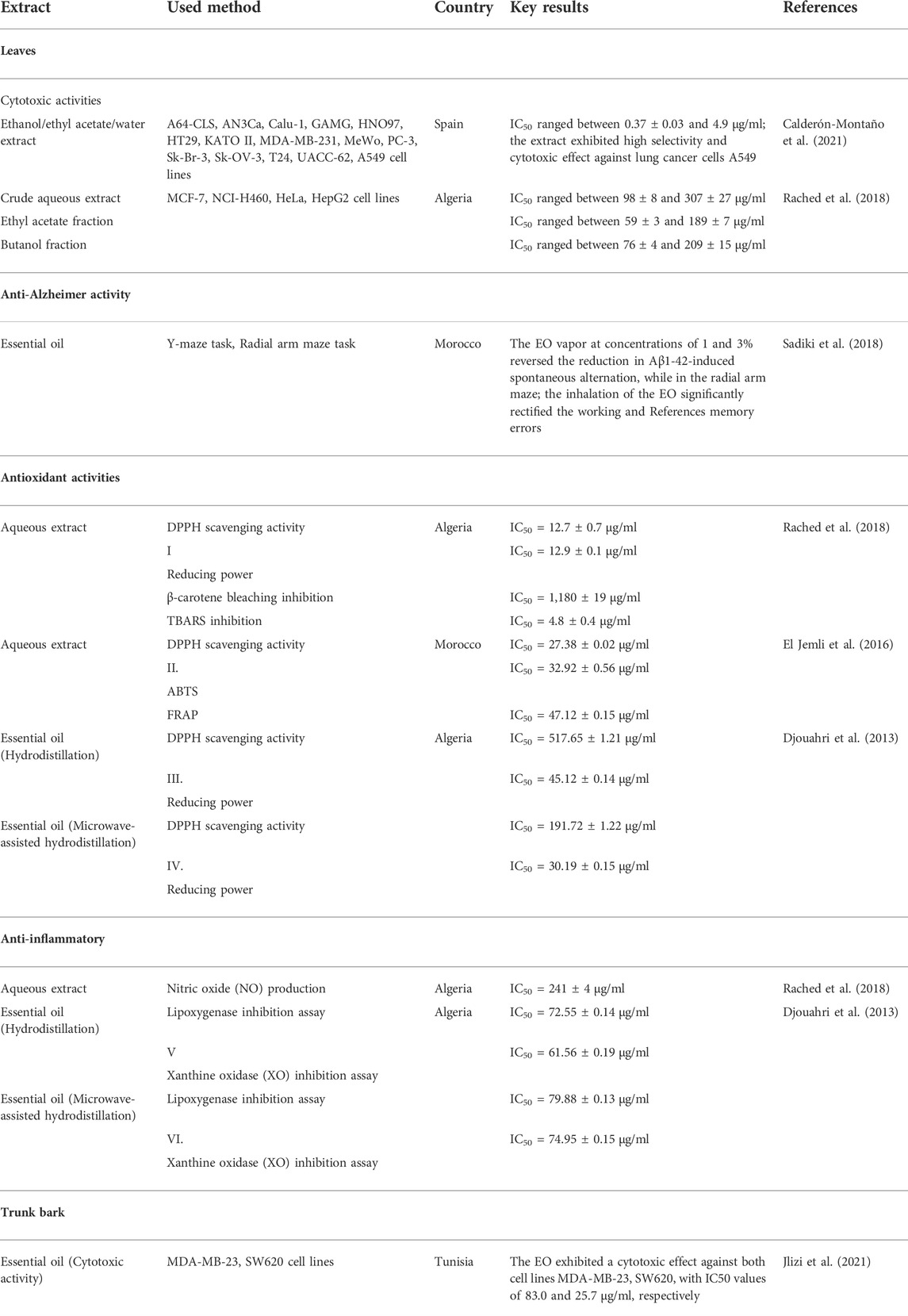
TABLE 5. Summary of cytotoxic, neuroprotective, anti-inflammatory, and antioxidant potencies of various T. articulata extracts.
Antioxidant activity
Recently, there has been a keen interest in identifying new plant-based antioxidants to protect the human body from free radical-related disorders such as diabetes, cancer, cardiovascular and neurodegenerative diseases, atherosclerosis, arthritis, asthma, anemia, inflammation, and so forth (Pham-Huy et al., 2008; Yassir et al., 2022). Many research studies showed that various plant species contained significant amounts of polyphenolic compounds and flavonoids, which could also be applied as promising antioxidant agents in dietary supplements and as food additives to postpone or prevent rancidity. Therefore, the antioxidant properties of T. articulata have been the subject of a great deal of research in North Africa countries. However, they all reported low-to-moderate antiradical activity of the essential oil. Meanwhile, they noticed a potent antioxidant capacity of the aqueous and acetone extracts, as mentioned in Table 5. Djouahri et al. (2016) evaluated the antioxidant potency of leaves essential oil of T. articulata from Algeria using scavenging reducing power activities and DPPH free radicals. According to the authors, the extract exhibited poor antiradical activity with an IC50 value of 517.6 ± 1.75 μg/ml, which was very weak compared with both standards BHA and BHT (IC50 = 21.28 ± 0.12 μg/ml and 12.76 ± 0.08 μg/ml, respectively). Another investigation that corroborated the previous findings was carried out by Rabib et al. (2020). They reported that the essential oil of T. articulata leaves from Morocco has moderate antioxidant power compared with conventional antioxidants at a dose of 2 mg/ml. It is widely known that the antiradical activity of a plant extract is strongly correlated with the total amounts of polyphenolic compounds and flavonoids, which are highly stable molecules capable of scavenging free radicals by H-atom donating. However, essential oils, particularly those obtained by hydrodistillation, are utterly bereft of phenolic compounds, which may explain why T. articulata essential oil exhibited poor antioxidant efficacy (Sliti et al., 2016). On the other hand, El Jemli et al. (2016) tested the in vitro antioxidant power of leaf aqueous extract of T. articulata using DPPH, ABTS, and FRAP. The authors reported that the extract showed strong scavenging activity displaying IC50 values of 27.38 ± 0.02 μg/ml (DPPH), 32.92 ± 0.56 μg/ml (ABTS) and 47.12 ± 0.15 μg/ml (FRAP). They also indicated a strong link between the antioxidant capacity and phenolic content of the extract (11.78 ± 0.30 µg (QE)/mg edw), demonstrating that phenolic compounds are major contributors to antioxidant properties of T. articulata aqueous extract. The previous findings were backed up by the investigation conducted by Ben Jemia et al. (2012). The authors used four different assay systems, namely DPPH, reducing power, β-carotene/linoleic acid, and metal chelating activity test to assess the in vitro antioxidant activities of T. articulata leaves 80% aqueous acetone extract. They indicated that the extract exhibited potent free radical scavenging activity in the DPPH system displaying an IC50 value of 5.5 mg/ml, which was two times higher than the standard positive control (BHT). They associated the powerful antioxidant effect with the high amount of the total phenolics, flavonoids, and condensed tannins in the extract. The previous findings supported the possible application of T. articulata extracts in the fields of food industry and cosmetics as well as to manage several free radical-related diseases. However, further in vivo studies and clinical trials are mandatory to validate the species safety and define appropriate doses.
Anti-inflammatory activity
To lend credence to the ethnomedicinal uses of T. articulata as an anti-inflammatory agent, El Jemli et al. (2016) tested the in vivo anti-inflammatory activity of T. articulata leaves essential oils on carrageenan and trauma-induced rats paw edema. They reported that the EO exhibited dose-dependent anti-inflammatory effects toward carrageenan-induced rat paw edema with a maximal effect (68.42% inhibition) achieved at 200 mg/kg after 3h, which was almost identical to the standard anti-inflammatory agent indomethacin (72.63% inhibition). They also mentioned dose-dependent anti-inflammatory effects on trauma-induced rat paw edema displaying substantial inhibition (84.51%) at the concentration of 200 mg/kg, which was also the same as indomethacin (84.08% inhibition). They hypothesized that the anti-inflammatory effects could be associated with the synthesis inhibition or release of cyclooxygenase products, such as histamine, cytokines, prostaglandins, and nitric oxide. They also indicated that the inflammatory mediators’ inhibition is likely produced by specific components of the essential oils like monoterpene hydrocarbons and oxygenated monoterpenes. Furthermore, T. articulata leaves have been widely used in North Africa’s folk medicine system to cure inflammatory infections. To validate the ethnomedicinal uses, Rached et al. (2018) evaluated the in vitro anti-inflammatory potential of T. articulata leaves aqueous extract on LPS-induced NO production by Murine macrophage. They found that the extract and its organic fractions ethyl acetate fraction and butanol fraction considerably reduced NO production. They linked the significant anti-inflammatory effect with the richness of the plant extract with flavonoids, especially catechin, epicatechin, and myricetin-3-O-rhamnoside.
Antiurolithiatic activity
The antiurolithiatic activity of T. articulata aqueous extract (infusion), collected in Algeria, was tested in vitro on the formation and inhibition of calcium oxalate monohydrate using polarized light microscopy. According to the results, the extract prevented urinary stones growth, with the highest inhibitory effects (87.94 and 84.12%) noticed at the concentration of 100 and 50%, respectively. However, in-depth in vivo and in vitro studies are needed to elucidate the curative and prophylactic potential of T. articulata extracts in preventing urolithiasis (Beghalia et al., 2007).
Neuroprotective activity
There are relatively few studies on the neuroprotective effect of T. articulata to back up the traditional applications and provide scientific evidence for further studies. Sadiki et al. (2018) investigated the potency of T. articulata leaves essential oil in amyloid-β peptide 1-42 (Aβ1-42)-induced spontaneous alternation, working, and reference memory errors in rats, using the Y-maze and radial arm maze tests. Results indicated that in the Y-maze test, the EO vapor at concentrations of 1 and 3% reversed the reduction in Aβ1-42-induced spontaneous alternation, which was almost similar to the donepezil effect (Sadiki et al., 2018). Results also showed that in the radial arm maze, the inhalation of the EO significantly rectified the working and reference memory errors. Moreover, the EO attenuated A1-42-induced cholinergic deficits and lowered acetylcholinesterase activity in the rat hippocampus. Similarly, Postu et al. (2022) assessed the in vivo neuroprotective properties of T. articulata EO (3%) in the rat model of amyloid-beta 1-42 (A1-42)-induced Alzheimer’s disease (AD) after administering the EO by inhalation once daily for 21 days. Results showed that the EO substantially attenuated memory impairments, mainly through improving the expressions of Brain-derived neurotrophic factor (BDNF) and down-regulating the expression of interleukin-1β (IL-1β) gene expressions associated with Aβ1-42-induced toxicity in the rat model. These findings proved that the plant EO might be a natural neuroprotective agent against Aβ1-42-induced neurotoxicity.
Antimicrobial activity
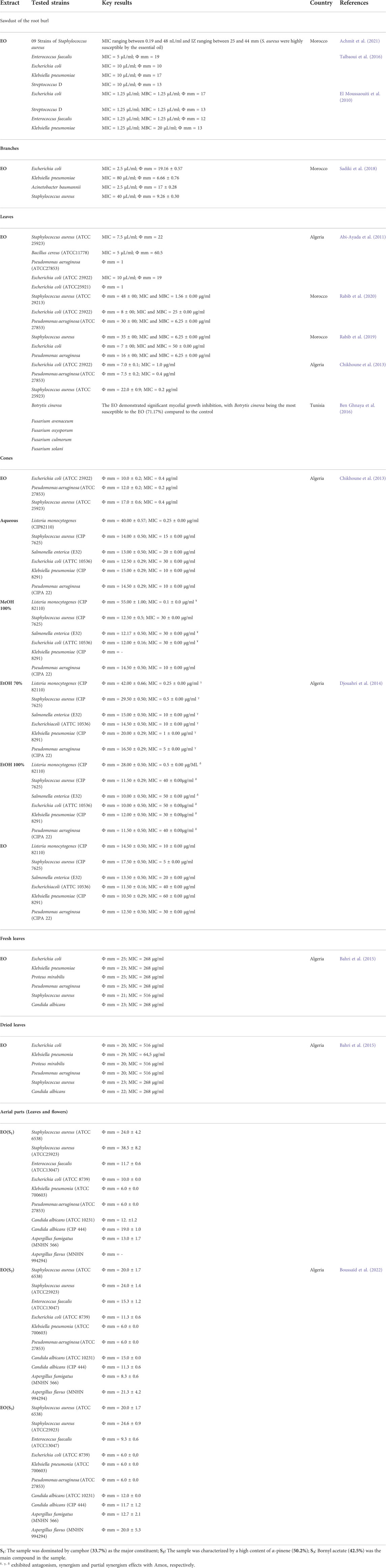
T. articulata has been widely used in folk medicine as a natural antibiotic agent to manage intestinal and urinary tract infections (Belgacem et al., 2021). These empirical practices were the springboard to seek better alternatives to standard antimicrobial drugs, especially with the harmful effects attributed to these antibiotics and the growth of multidrug resistance in a full range of Gram-positive and Gram-negative bacteria. Previous studies have described the antimicrobial activity of crude extracts from T. articulata. The antibacterial activity of the plant extracts was evaluated using disc diffusion and the disc volatilization assays, agar well diffusion, and broth microdilution procedures. As indicated in Table 6, microbial growth inhibition zones and percentages, as well as minimum inhibitory concentrations (MICs), showed that T. articulata exhibited a potent bacteriostatic effect.
Achmit et al. (2021) tested the antibacterial activity of the essential oil of T. articulata sawdust from the root burl against nine strains of Staphylococcus aureus using the disc diffusion assay. The nine strains contained eight clinical isolates and one standard strain (ATCC 25923). They showed that the EO exhibited pronounced antibacterial properties against all Staphylococcus aureus tested strains, including two methicillin-resistant and one multidrug-resistant. The broth microdilution assay confirmed the high susceptibility of these strains to the EO, displaying MIC values ranging from 0.19 to 48 nL/ml and inhibition Zone Diameter (IZD) values ranging from 25 to 44 mm. The high inhibitory effects were ascribed to the significant amounts of monoterpenic phenols in the EO, such as carvacrol (37.8%). The antibacterial activity of T. articulata sawdust essential oil was corroborated by previous studies conducted by Talbaoui et al. (2016) and El Moussaouiti et al. (2010). Using disc diffusion assay, Talbaoui et al. (2016) demonstrated that the EO exhibited a strain-dependent inhibitory effect. Escherichia coli was the most sensitive to the EO, with MIC values of 5 μL/ml and inhibition zone diameter value of 19 mm, whereas Enterococcus faecalis, Klebsiella pneumonia, and Streptococcus D (Group D Streptococcus) were less susceptible to the EO. The authors identified thymol (22.83%) and 3-tert-butyl-4-methoxy phenol (35.02%) as the main compounds in the EO and correlated the effect with these key compounds or potential synergistic/antagonistic effects of all the mixture (Talbaoui et al., 2016). Likewise, T. articulata leaves essential oil demonstrated strong antibacterial effects towards Escherichia coli, Bacillus subtilis, Staphylococcus aureus, and Micrococcus luteus, with Staphylococcus aureus being the most vulnerable to the EO (Bourkhiss et al., 2007). In the same line, the antibacterial activity of T. articulata leaves essential oil was also assessed against five bacteria strains by Abi-Ayad et al. (2011) using the disc diffusion and the disc volatilization method. The EO inhibited the growth of Bacillus cereus (ATCC11778), Staphylococcus aureus (ATCC 25923), and Escherichia coli (ATCC 25922), with MIC values of 5, 7, and 10 μL/ml, and IZ values of 60.5, 22, and 19 mm, respectively. However, Escherichia coli (ATCC 25921) and Pseudomonas aeruginosa (ATCC 27853) have manifested resistance towards the EO, with an IZ value of 1 mm. In a recent study, Boussaïd et al. (2022) used the disc diffusion method to investigate the antimicrobial capacity of three samples obtained from T. articulata aerial parts (flowers and leaves) against three Gram-positive bacteria, three Gram-negative bacteria, two yeasts, and two filamentous fungi. Camphor (33.7%), α-pinene (50.2%), and bornyl acetate (42.5%) were the predominant compounds in sample 1, sample 2, and sample 3, respectively. Results suggested that all three samples were ineffective against the bacterial strains Pseudomonas aeruginosa (ATCC 27853) and Klebsiella pneumonia (ATCC 700603), while Staphylococcus aureus (ATCC 25923), Candida albicans (ATCC 10231), and Aspergillus fumigatus (MNHN 566) were the most susceptible to the EOs. On the other hand, the sample containing camphor as the dominant component was proven to be most prominent with MICs ranging between 1 and 3 μL/ml, whereas the essential oil enclosing α-pinene as the main ingredient was the least effective with MICs ranging between 2 and 6 μL/ml. Moreover, Djouahri et al. (2014) reported the potency of T. articulata extracts to increase the efficacy of the antibiotic Amox towards bacteria strains exhibiting the MDR phenotype. When they associated the antibiotic Amox with ETOH 100%, ETOH 70%, or water extracts of T. articulata fresh cones, they witnessed a synergism or partially synergism effect against Staphylococcus aureus (CIP 7625), Salmonella enterica (E32), Klebsiella pneumoniae (CIP 8291), Escherichia coli (ATTC 10536), Listeria monocytogenes (CIP 82110), and Pseudomonas aeruginosa (CIPA 22). However, when the antibiotic and MeOH 100% extract were combined, an antagonistic effect was noticed for all tested strains.
Previous studies revealed that T. articulata has strong in vitro antifungal effects. Ben Ghnaya et al. (2016) tested the antifungal potency of T. articulata leaves essential oil against phytopathogenic fungi, namely Botrytis cinerea, Fusarium avenaceum, Fusarium oxysporum, Fusarium culmorum, and Fusarium solani using in vitro contact tests. Results demonstrated that the essential oil exhibited significant mycelial growth inhibition against all the tested fungi. Botrytis cinerea was the most sensitive to the EO, with an inhibition percentage of 71.17%, whereas Fusarium solani was less susceptible to the EO (25.36%). In the context of investigating new eco-effective and environmentally friendly fungicides to neutralize the hazardous impacts of mycotoxins on the stored grains, Abi-Ayad et al. (2013) examined the antifungal properties of T. articulata leaves EO towards three fungal strains, namely Aspergillus niger, Aspergillus flavus, and Fusarium spp., through the contact method. The EO, at the following concentrations 5, 10, 15, and 20 μL/ml, exhibited significant inhibitory effects on the growth of the three fungi. After 6 days of incubation, the highest activity was shown against Fusarium spp., which was fully suppressed at a concentration of 20 µL/ml. In the same line, Bourkhiss et al. (2007) reported strong antifungal effects of T. articulata leaves essential oil against Penicillium parasiticus and Aspergillus niger at the concentration of 1/500 (V/V). The authors credited the significant antifungal capacity to the chemical profile of EO, which was dominated by bornyl acetate. Nevertheless, they did not rule out the possibility of a whole-compound synergism, which has yet to be validated. In spite of being endowed with strong antimicrobial properties, the majority of the assays were conducted using the disk diffusion test, which, while useful for evaluating the antimicrobial activity, may not always compare the potential of various extracts due to differences in physical features such a solubility, volatility, and diffusion in the in agar medium (Scorzoni et al., 2007). In contrast, the broth microdilution method is more accurate and gives better visualization of the inhibitory concentrations. Therefore, it is a feasible alternative for confirming the antibacterial efficacy of plant extracts. Furthermore, antibacterial research has largely been focused on plant crude extracts and essential oil, with no studies looking into the antimicrobial potential of isolated chemicals, particularly those recognized for their antimicrobial properties. Thus, in-depth studies incorporating in vivo and in vitro are recommended to offer a valid basis for investigating potential and low harmful antimicrobial compounds from the plant.
Other activities
The in vitro antidiabetic activity of extracts from T. articulata was tested against α-amylase. Results revealed a dose-dependent inhibitory effect against α-amylase (IC50 of 57.74 μg/ml) following a competitive inhibition way (Toumi et al., 2022). However, further in vitro and in vivo studies are highly required to validate the ethnomedicinal use of the plant as a hypoglycemic agent. Moreover, the essential oil from T. articulata displayed moderate leishmanicidal potency against L. infantum, while no effect was recorded against L. major up to 8 μg/ml (Ahmed et al., 2011).
Potential application of T. articulata essential oils as food preservatives
During the last decades, commercial food preservatives have extensively been applied to prevent spoilage and biodeterioration of processed foodstuffs, while retaining their natural flavors, taste, color, nutritional value, and appearance (Carocho et al., 2014). The severe defects linked to the employment of synthetic additives have fueled more and more controversies regarding their injurious effects on the behavior and health of consumers, including genotoxicity, carcinogenicity, asthma attacks, eczema, skin rashes, and nervousness, among others (Carocho et al., 2014; Amchova et al., 2015; Pandey et al., 2021). On the other hand, widespread distrust of synthetic additives, especially in developed countries, has driven scientists toward seeking new natural-based and health-beneficial preservatives with pronounced antimicrobial and antioxidant properties (Pandey et al., 2021). Indeed, a myriad of plant species from the Cupressaceae family are distinguished by high amounts of volatiles components, which could serve as a renewable supply of food additive agents due to their substantial cidal/static action (Benjemaa et al., 2022; Sadiki et al., 2022). T. articulata essential oil is almost colorless or pale yellow, featured by its sweet balsamic scent with a broad spectrum of antimicrobial and antioxidant properties (Djouahri et al., 2016; Achmit et al., 2021; Boussaïd et al., 2022; Sadiki et al., 2022). In a recent study, the effect of T. articulata EO on the shelf life of refrigerated storage chicken fillets was explored by Salem et al. (2022). Results disclosed the capacity of T. articulata EO at the concentration of 200 ppm/100 g of product to drastically drop lipid oxidation (p < 0.05) over 12 days of refrigerated storage. Interestingly, T. articulata EO showed significant antioxidant activity (IC50 = 1,000 μg/ml) with no toxicity on murine macrophage cells and the ability to reduce the acidity of the treated fillets (1.3 g/kg) from the third day of storage compared with the control. Results also revealed the capacity of T. articulata EO to significantly decrease the flora charges and inhibit the growth of food-borne pathogen bacteria strain Enterococcus faecalis (ATCC 29212) with MIC <0.031 mg/ml (Salem et al., 2022). Thereby, T. articulata EO could be a potential source of chemical compounds that might be used to prevent biodeterioration and spoiling of perishable foods such as meat and poultry during storage time.
Toxicity studies
It is compulsory to evaluate the safety of T. articulata as one of the most frequently used medicinal plants in North Africa countries. El Jemli et al. (2016) examined the acute toxicity of the leaves EO at doses of 2 and 5 g/kg after oral administration to Swiss mice. For the first 6 h and within the next 2 weeks following the administration of the EO, animals were examined for bodyweight fluctuations, mortality, neurotoxicity, and convulsions signs. As a result, the administration of T. articulata EO at both doses (2 and 5 g/kg) showed relatively low acute toxicity and did not cause mortality. Interestingly, at the dose of 2 g/kg, there was no change in animals’ weight, growth, or functions as compared to the control group. However, at 5 g/kg, a substantial drop in body weight and activities were recorded, which is likely related to a neurotoxic effect. Moreover, Rachid et al. (2018) have also demonstrated that the crude aqueous extract of the leaves of T. articulata did not exhibit cytotoxicity towards PLP 2 (porcine liver cells), with an IC50 higher than 400 μg/ml, compared to Ellipticine, which has an IC50 of 3.2 μg/ml. It is worth noting that the crude extract in this study was highly selective for cancer cell lines and had no effect on non-tumor cells PLP2. Nevertheless, for plant applications and novel drugs development, toxicology safety evaluation is mandatory. On the other hand, toxicological investigations of extracts and components obtained from T. articulata have yet to be completely addressed. As a result, more toxicity studies are required to establish the appropriateness of the plant extracts and related bioactive constituents.
T. articulata extracts as a botanical pesticide for a sustainable agriculture
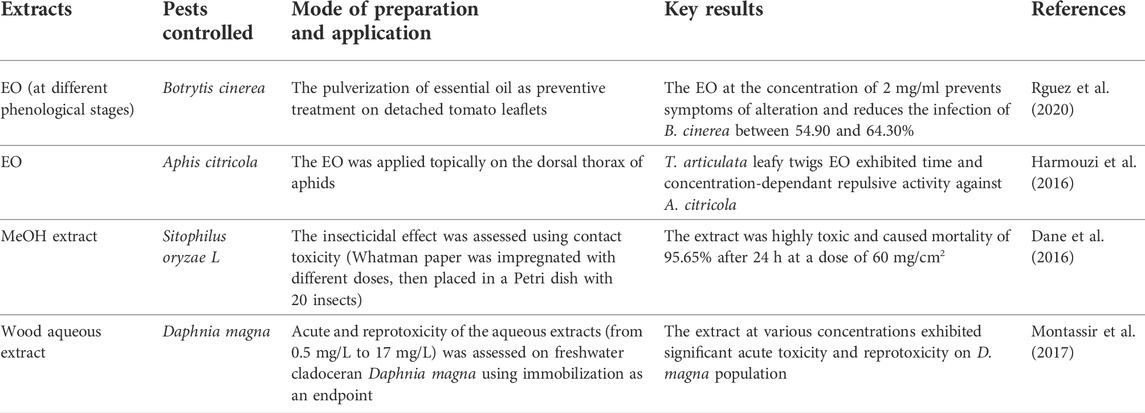
Botanical pesticides are naturally occurring agents derived from plants of different families, which are applied similarly to chemical pesticides as plant extracts, essential oils, or both to achieve pest management in an eco-friendly way (Ukoroije and Otayor, 2020; Bitchagno et al., 2022). Biopesticides would account for nearly 20% of the worldwide pesticide market by 2025 compared to 4–5% in 2014 (Isman, 2015). Due to the harmful effects associated with the use of synthetic pesticides on human health and biodiversity, the recent trend is being shifted towards plant-based pesticides. Indeed, several plant families, including Apiaceae, Asteraceae, Cupressaceae, Lamiaceae, and Rutaceae, have been proven to exhibit promising pesticidal properties due to the presence of a broad spectrum of secondary metabolites, such as alkaloids, phenols, flavonoids, steroids, and tannins, among others (Lengai et al., 2020). To make plant-based pesticide, various parts from these plants, such as leaves, stems, bark, roots, rhizome, are dried, powdered then subjected to extraction with the suitable solvent to maximize the yield of the target bioactive compounds, which are then concentrated, formulated, and evaluated for their safety (Duke et al., 2010; Lengai et al., 2020; Ukoroije and Otayor, 2020). In fact, several plant-based pesticides have been successfully formulated and commercialized as safe pesticides for crop pest management, such as pyrethroids derived from the dried flowers of Tanacetum cinerariaefolium and azadiractin from Azadirachta indica. Botanical pesticides have a variety of mechanisms of action on pests, including repellence, molluscicides, attractants, nematicides, and phytotoxins (Ramdani et al., 2021; Souto et al., 2021). Botanical pesticides, unlike conventional pesticides, are made up of several bioactive components rather than a single active agent. Thus, they may affect both physiological and behavioral processes owing to their internal synergism or antagonism (Miresmailli and Isman, 2014; Niroumand et al., 2016). Consequently, it is unlikely for pests to develop resistance to such a mixture of natural pesticidal agents (Niroumand et al., 2016). For example, Pyrethrums disrupt the normal transmission of nerve impulses by altering the sodium and potassium ion exchange process in insect nerve fibers, which result in paralysis and death (neurotoxicity) (Laxmishree and Singh, 2018). Botanical pesticides may also inhibit digestive enzymes, such as amylase, invertase, lipase, and protease. They can affect the rate of enzyme-substrate complex breakdown by decreasing enzyme affinity to the substrate and increasing Km (Zibaee, 2011; Sengottayan, 2013).
To efficiently and sustainably control Aphis citricola, a citrus crop pest, while minimizing the reliance on hazardous synthetic pesticides. Harmouzi et al. (2016) investigated T. articulata EO chemical composition and tested its toxicity on A. citricola. They showed that EO exhibited significant acute toxicity against A. citricola by contact in a dose and time-dependent fashion (Table 7). The authors recorded an LC50 of 54.03 μL/L after 24 h of exposure. They attributed the significant toxicity to the significant amounts of oxygenated monoterpenes like isobornyl acetate, borneol, and camphor, which can interfere with several neurotransmitters such as Octopamine and causes neurotoxicity. To come up with new effective eco-friendly methods to control mosquitoes responsible for Vector-borne diseases, Aouinty et al. (2006) assessed the larvicidal potency of T. articulata wood aqueous extract against four mosquito species. Results showed that the extract exhibited a significant larvicidal effect against all mosquitoes’ larvae at a concentration of 4%, with a mortality rate of 100% after 24 h of exposure. Botrytis cinerea is a necrotrophic fungus that causes gray mold in tomatoes and other crops such as peppers, cabbage, beans, etc. To seek safe, efficacious, and natural fungicides, Rguez et al. (2020) assessed the in vivo and in vitro antifungal properties of T. articulata essential oil at different phenological stages against B. cinerea. The authors reported that T. articulata EO exhibited the highest antifungal activity at the blooming phase. They stated that T. articulata EO applied as a means of prevention on tomato plants under greenhouse conditions improved plant growth and decreased B. cinerea infection to 17.72% compared to 52.1% documented in non-treated plants. When they applied 100 μg/ml of EO on tomato fruit, they observed that the infection rate recedes by 64.01%. They associated the antifungal effect with the presence of oxygenated monoterpenes and their hydrocarbons and supported the possible application of T. articulata EO as a natural fungicide.
Patents related to T. articulata published between 2001 and 2022
Recently, there has been a global cumulative increase in the number of T. articulata-related patents, demonstrating the intensive ongoing research on this plant tree to provide high-value-added products and support its usage to treat various health conditions. As reported in (Table 8) various parts from the plant, especially resin, have been formulated as patents and found applications mainly in the pharmaceutical field to prevent and treat neurodegenerative diseases such as Alzheimer’s disease, diabetes mellitus, fibrotic conditions, cancers, among others. These patents have the specificity to combine cutting-edge extraction methods with tried-and-true formulas to ensure both efficacy and safety. For instance, a cis- and trans-communic acid rich-fraction obtained from T. articulata resin using a two-step or three-step extraction procedure (with a carrier) was designed to prevent and treat fibrotic conditions, gliosis, surgical adhesions, and impaired neurological function such as vascular dementia and Alzheimer’s disease (Hazan and Lucassen, 2015). Moreover, an herbal formulation consisting of several plant genera, including T. articulata, displayed the capacity to prevent and treat diabetes mellitus and its related health complications and dyslipidemia (Fogel, 2010). The usefulness of T. articulata in cancer prevention and treatment has been alarmed by a couple of inventions (Brooks and Norris, 2007). The first patent was invented by Brooks and Norris, (2007), claiming the use of Pheophorbide derivative compounds from plants as potential agents (high oral bioavailability) to inhibit cell proliferation and angiogenesis. Another patent invented by Benoit, (2006) furnished a method for the extraction and purification of potential compounds from various plants, including T. articulata, having the ability to inhibit, slow down, or prevent cell migration of abnormal cells. In the cosmetic field, Mitsuharu and Michimasa (2001) developed a cosmetic formulation with the steam distillate of T. articulata, which aimed at providing dry skin gloss and tension. Several formulations from plant extracts, including T. articulata, claimed the capacity to prevent and treat various dermatological conditions, including skin sagging, irradiation-induced skin damage, skin lines, and elastotic changes in the skin. The dermatological action of the plant extracts seemed to be related to the inhibition of one or more extracellular proteases such as Matrix metalloproteinase-1 (MMP-1), Matrix metalloproteinase-2 (MMP-2), and human leukocyte elastase (HLE). Other patents included the one issued in 2019 by Toni, which claimed the use of T. articulata sandarac and beeswax to manufacture a self-healing cutting board or a storage container characterized by their water-resistant, non-skid, and antimicrobial properties (Toni, 2019). Further details about patents granted for therapeutic and cosmetic applications of T. articulata are reported in Table 8.
Conclusion and perspectives
T. articulata has long been exploited for therapeutic, artistic, and ritual purposes. Recent ethnobotanical surveys reported the use of different parts, such as leaves, resin, bark, and cones, to cure several pathological conditions, including chronic ones such as diabetes mellitus and hypertension. According to several phytochemical studies, the plant extracts contain significant amounts of phenolic compounds, which are primarily made up of phenolic acids, flavonoids and their derivatives. Phenolic compounds, especially, flavonoids are excellent free radical scavengers. They may play a crucial role in mitigating and protecting the human body from free radicals-related diseases such as diabetes mellitus, cancers, and neurodegenerative diseases. As such, they have strongly been associated with the significant cytotoxic, antioxidant, anti-Alzheimer, and anti-inflammatory properties of T. articulata extracts.
Despites rich literature about the plant, the chemistry and biology of T. articulata have yet to be thoroughly examined. For instance, the phytochemical screening of T. articulata cones methanolic extract has positively identified other classes of compounds such as alkaloids and saponosides (Saber et al., 2021). Likewise, the individual compounds and their pharmacological effects have yet to be determined.
T. articulata has long been used by indigenous people in Morocco, Algeria, and Tunisia to treat digestive and respiratory disorders, cough, and diarrhea. These empirical practices were the springboard that has incentivized further antimicrobial studies over the last 2 decades. Recent phytochemical investigations of the essential oil revealed the presence of monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, and oxygenated sesquiterpene. These chemical compounds have strongly been linked to the significant antimicrobial potency of the essential oil towards Gram-negative and Gram-positive bacteria exhibiting multidrug resistance phenotype (MDR). Interestingly, a synergistic effect has been recorded between the plant extracts (EtOH 70%, EtOH 100%), and the antibiotic Amoxicillin, improving the effectiveness of the antimicrobial agent.
Although this plant has long been used to manage a variety of chronic diseases such as diabetes mellitus and hypertension, limited pharmacological research has been conducted to corroborate and support these ethnomedicinal claims with scientific evidence. Therefore, further in vitro studies are highly required to evaluate the antidiabetic properties of the T. articulata-based extracts against α-amylase, α-glucosidase, and β-glucosidase. In vivo studies of the plant extracts in normal and streptozotocin (or alloxan)-induced diabetic rats are also mandatory to assess the extracts’ ability to lower blood glucose levels, restore the body cells’ sensitivity, and protect pancreatic cells. T. articulata extracts have also demonstrated potent in vitro cytotoxic effects with high selectivity towards various cancer cell lines. However, in-depth in vivo studies are also needed to explore the ability of the extracts to induce apoptosis in cancer cells through the intrinsic and extrinsic pathways. Further in vivo toxicological and clinical studies are required to ensure the plant’s efficacy and safety. In line with the traditional uses of the plant, in vitro and in vivo studies about the claimed anti-asthmatic, anti-tuberculosis, and wound healing properties are also mandatory. Likewise, locals in North Africa have also utilized T. articulata tar to cure parasitic illnesses, scabies, and inflamed wounds in livestock. Nevertheless, no anthelmintic studies have been conducted to back up these claims and provide scientific evidence for ethnoveterinary applications. The EO from the plant disclosed also the capacity to extend the shelf life of refrigerated storage chicken fillets, which indicates that the EO might be a source of chemical compounds that can be utilized as natural food additives in the food industry and as a platform for biodegradable active packaging.
Author contributions
LB and SK designed the study, drafted the manuscript, collected and arranged the references; LB and MS analyzed the data, reviewed and edited the manuscript, and supervised the final version of the paper.
Acknowledgments
We would like to thank Sultan Moulay Slimane University, Beni-Mellal, and Mohammed VI Polytechnic University, Benguerir, Morocco for the partial support of this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
A549, Human lung cancer cell line; A64-CLS, Cellosaurus cell line; ABTS, 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); DPPH, 2,2-Diphenyl-1-picrylhydrazyl; EO:, Essential oil; FRAP, Ferric reducing antioxidant power; HeLa, Cervical epithelial carcinom; HepG2, Liver hepatocellular carcinoma; HT29, Colon cancer cell line; IUCN, International Union for Conservation of Nature; IZD, Inhibition Zone Diameter; MBC, Minimum Bactericidal Concentration; MCF-7, Human breast cancer cell line; MDR, Multidrug resistance; MeOH, Methanolic extract; MeWo, Human melanoma cell line; MIC, Minimum inhibitory concentration; NCI-H460, Human Lung Carcinoma; PC-3, Human Prostate Adenocarcinoma; Sk-Br-3, Human breast cancer; Sk-OV-3, Human ovarian cancer; T24, Urinary bladder carcinoma; UACC-62, Melanoma cell line; edw, equivalent dry weight.
References
Abdalla, M. A., and McGaw, L. J. (2020). Ethno-veterinary medicine: Present and future concepts. Switzerland: Springer NatureSpringer International Publishing, 391–426.
Abi-Ayad, F. Z., Abi-Ayad, M., Lazzouni, H. A., Rebiahi, S. A., and Bessiere, J. M. (2011). Antibacterial activity of essential oil extracted from leaves ofTetraclinis articulata (Vahl) Masters from Algeria flora. J. Microbiol. Biotech. Res. 1 (1), 1–6.
Abi-Ayad, F. Z., Abi-Ayad, M., Lazouni, H. A., and Rebiahi, S. A. (2013). Evaluation of Tetraclinis Articulata essential oil from Algeria flora as a potential source of antifungal activity and study of its chemical composition. J. Indian Acad. Wood Sci. 10, 9–15. doi:10.1007/s13196-013-0086-7
Abouri, M., ElMousadik, A., Msanda, F., Boubaker, H., Saadi, B., and Cherifi, K. (2012). An ethnobotanical survey of medicinal plants used in the Tata Province, Morocco. Int. J. Med. Plants Res. 1, 99–123.
Achak, N., Romane, A., Alifriqui, M., and Markouk, M. (2009). Chemical composition, organic and mineral contents of leaves of Tetraclinis articulata (Vahl) Masters. From the Tensift-Al Haouz, Marrakech region (Morocco). J. Essent. Oil Bear. Plants 12, 198–204. . doi:10.1080/0972060X.2009.10643711
Achmit, M., Aoussar, N., Mellouki, F., Mhand, R. A., Ibáñez, M. D., Blázquez, M. A., et al. (2021). In vitro antibacterial and biofilm inhibitory activity of the sawdust essential oil of Tetraclinis articulata (vahl) against catheter-associated Staphylococcus aureus clinical isolates. Curr. Res. Biotechnol. 3, 1–5. doi:10.1016/j.crbiot.2020.12.001
Ahmad, A., Mishra, R. K., Vyawahare, A., Kumar, A., Rehman, M. U., Qamar, W., et al. (2019). Thymoquinone (2-Isoprpyl-5-methyl-1, 4-benzoquinone) as a chemopreventive/anticancer agent: Chemistry and biological effects. Saudi Pharm. J. 27, 1113–1126. doi:10.1016/j.jsps.2019.09.008
Ahmed, S. B. H., Sghaier, R. M., Guesmi, F., Kaabi, B., Mejri, M., Attia, H., et al. (2011). Evaluation of antileishmanial, cytotoxic and antioxidant activities of essential oils extracted from plants issued from the leishmaniasis-endemic region of Sned (Tunisia). Nat. Prod. Res. 25 (12), 1195–1201. doi:10.1080/14786419.2010.534097
Amchova, P., Kotolova, H., and Ruda-Kucerova, J. (2015). Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 73, 914–922. doi:10.1016/j.yrtph.2015.09.026
Amel, B. (2013). Traditional treatment of high blood pressure and diabetes in Souk Ahras District. J. Pharmacogn. Phytother. 5, 12–20. doi:10.5897/JPP11.065
Annaz, H., Sane, Y., Bitchagno, G. T., Ben Bakrim, W., Drissi, B., Mahdi, I., et al. (2022). Caper (Capparis spinosa L.): An updated review on its phytochemistry, nutritional value, traditional uses, and therapeutic potential. Front. Pharmacol. 13, 878749. doi:10.3389/fphar.2022.878749
Bahri, F., Romane, A., Höferl, M., Wanner, J., Schmidt, E., and Jirovetz, L. (2015). Chemical composition and antimicrobial activity of essential oil of Algerian Tetraclinis articulata (Vahl) Masters. J. Essent. Oil Res. 28, 42–48. . doi:10.1080/10412905.2015.1076739
Bai, J. P. F., and Abernethy, D. R. (2013). Systems pharmacology to predict drug toxicity: Integration across levels of biological organization. Annu. Rev. Pharmacol. Toxicol. 53, 451–473. doi:10.1146/annurev-pharmtox-011112-140248
Banerjee, P., Eckert, A. O., Schrey, A. K., and Preissner, R. (2018). ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 46 (W1), W257–W263. doi:10.1093/nar/gky318
Beghalia, M., Ghalem, S., Allali, H., Belouatek, A., and Marouf, A. (2007). Effect of herbal extracts of Tetraclinis Articulata and Chamaerops humilis on calcium oxalate crystals in vitro. Gomal J. Med. Sci. 5 (2), 55–58.
Behr, S., Duret, P., and Gendron, N. (2011). Plant extracts and dermatological uses thereof. Patents No US20110311661. Quebec: Lucas Meyer Cosmetics Canada Inc.
Belgacem, N., Okkacha, H., Benchohra, M., and Elhadj, T. (2021). Inventory, diversity and therapeutic uses of medicinal plants in the Tiaret mountains (Western Algeria). Biodivers. J. 12, 129–138. doi:10.31396/biodiv.jour.2021.12.1.129.138
Ben Ghnaya, A., Amri, I., Hanana, M., Gargouri, S., Jamoussi, B., Romane, A., et al. (2016). Tetraclinis articulata (Vahl.) Masters essential oil from Tunisia: Chemical characterization and herbicidal and antifungal activities assessment. Ind. Crops Prod. 83, 113–117. doi:10.1016/j.indcrop.2015.12.026
Ben Jemia, M., Chaabane, S., Senatore, F., Bruno, M., and Kchouk, M. E. (2012). Studies on the antioxidant activity of the essential oil and extract of Tunisian Tetraclinis articulata (vahl) mast.(cupressaceae). Nat. Prod. Res. 27, 1419–1430. doi:10.1080/14786419.2012.717289
Benjemaa, M., Snoussi, M., Falleh, H., Hessini, K., Msaada, K., Flamini, G., et al. (2022). Chemical composition, antibacterial and antifungal activities of four essential oils collected in the North-East of Tunisia. J. Essent. Oil Bear. Plants 25, 338–355. doi:10.1080/0972060X.2022.2068971
Benoit, C. (2006). Plant extracts for treatment of angiogenesis and metastasis. Patent No US20060228426. Menlo Park: Heller Ehrman LLP.
Bitchagno, G. T. M., El Bouhssini, M., Mahdi, I., Ward, J. L., and Sobeh, M. (2022). Toward the allelopathy of Peganum sp. and related chemical constituents in agriculture. Front. Plant Sci. 21 (12), 796103. doi:10.3389/fpls.2021.796103
Bouhtoury-Charrier, F. E., Hakam, A., Famiri, A., Ziani, M., and Charrier, B. (2009). Wood characterization of Tetraclinis Articulata and evaluation of its resistance against lignilolytic fungi. IRG Wood Protection, Doc No.IRG/WPþ, 09–10697.
Bourkhiss, M., Hnach, M., Bourkhiss, B., Ouhssine, M., and Chaouch, A. (2007). Composition chimique et propriétés antimicrobiennes de l’huile essentielle extraite des feuilles de Tetraclinis articulata (Vahl) du Maroc. Afr. Sci. Rev. Int. Sci. Technol. 3, 232–242. doi:10.4314/afsci.v3i2.61267
Boussaïd, M., Bekhechi, C., Tomi, P., and Tomi, F. (2022). Antimicrobial activity of Tetraclinis Articulata aerial parts essential oil from Tlemcen, Northwestern of Algeria. jnpra. 1, 29–40. doi:10.46325/jnpra.v1i03.26
Brooks, M. N., and Norris, A. J. (2006). Orally available light-independent antineoplastic compounds. Patent No US20070299046. United States: BCN Biosciences L.L.C.
Buhagiar, J., Podestà, M. C., Cioni, P. L., Flamini, G., and Morelli, I. (2000). Essential oil composition of different parts of Tetraclinis Articulata. J. Essent. Oil Res. 12, 29–32. doi:10.1080/10412905.2000.9712034
Calderón-Montaño, J. M., Martínez-Sánchez, S. M., Jiménez-González, V., Burgos-Morón, E., Guillén-Mancina, E., and Jiménez-Alonso, J. J. (2021). Screening for selective anticancer activity of 65 extracts of plants collected in Western Andalusia, Spain. Plants 10. doi:10.3390/plants10102193
Carocho, M., Barreiro, M. F., Morales, P., and Ferreira, I. C. F. R. (2014). Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 13, 377–399. doi:10.1111/1541-4337.12065
Chaachouay, N., El Mostafa Douiri, N. F., Zidane, L., and Douira, A. (2021). Use of medicinal plants in dermato-cosmetology: An ethnobotanical study among the population of Izarène. Jordan J. Pharm. Sci. 14, 323–340.
Chaachouay, N., Azeroual, A., Bencharki, B., Douira, A., and Zidane, L. (2022). Ethnoveterinary medicinal plants for animal therapy in the Rif, North of Morocco. S. Afr. J. Bot. 147, 176–191. doi:10.1016/j.sajb.2021.12.037
Chikhoune, A., Hazzit, M., Kerbouche, L., Baaliouamer, A., and Aissat, K. (2013). Tetraclinis articulata (Vahl) Masters essential oils: Chemical composition and biological activities. J. Essent. Oil Res. 25 (4), 300–307. doi:10.1080/10412905.2013.774625
Dane, Y., Mouhouche, F., Canela-Garayoa, R., and Delpino-Rius, A. (2016). Phytochemical analysis of methanolic extracts of Artemisia Absinthium L. 1753 (Asteraceae), Juniperus phoenicea L., and Tetraclinis Articulata (Vahl) Mast, 1892 (Cupressaceae) and evaluation of their biological activity for stored grain protection. Arab. J. Sci. Eng. 41, 2147–2158. doi:10.1007/s13369-015-1977-2
Djouahri, A., Boudarene, L., and Meklati, B. Y. (2013). Effect of extraction method on chemical composition, antioxidant and anti-inflammatory activities of essential oil from the leaves of Algerian Tetraclinis articulata (Vahl) Masters. Ind. Crops Prod. 44, 32–36. doi:10.1016/j.indcrop.2012.10.021
Djouahri, A., Saka, B., Boudarene, L., Benseradj, F., Aberrane, S., Aitmoussa, S., et al. (2014). In vitro synergistic/antagonistic antibacterial and anti-inflammatory effect of various extracts/essential oil from cones of Tetraclinis articulata (Vahl) Masters with antibiotic and anti-inflammatory agents. Ind. Crops Prod. 56, 60–66. doi:10.1016/J.INDCROP.2014.02.035
Djouahri, A., Saka, B., Boudarene, L., Lamari, L., Sabaou, N., and Baaliouamer, A. (2016). Essential oil variability of Tetraclinis Articulata (Vahl) Mast.parts during its phenological cycle and incidence on the antioxidant and antimicrobial activities. Chem. Biodivers. 14, e1600216. doi:10.1002/cbdv.201600216
Doi, H., and Horie, T. (2010). Salicylic acid-induced hepatotoxicity triggered by oxidative stress. Chem. Biol. Interact. 183, 363–368. doi:10.1016/j.cbi.2009.11.024
Drwal, M. N., Banerjee, P., Dunkel, M., Wettig, M. R., and Preissner, R. (2014). ProTox: A web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res. 42, 53–58. doi:10.1093/nar/gku401
Duke, S. O., Cantrell, C. L., Meepagala, K. M., Wedge, D. E., Tabanca, N., and Schrader, K. K.(2010). Natural toxins for use in pest management. Toxins. 2, 1943–1962. doi:10.3390/toxins2081943
El Jemli, M., Kamal, R., Marmouzi, I., Zerrouki, A., Cherrah, Y., and Alaoui, K. (2016). Radical-scavenging activity and ferric reducing ability of Juniperus Thurifera (L.), J. oxycedrus (L.), J. phoenicea (L.) and Tetraclinis articulata (L.). Adv. Pharmacol. Sci. 2016, 6392656. doi:10.1155/2016/6392656
El Jemli, M. (2019). Contribution à l’étude ethnobotanique, toxicologique, pharmacologique et phytochimique de quatre Cupressacées marocaines : Juniperus Thurifera L., Juniperus Oxycedrus L., Juniperus Phoenicea L. et Tetraclinis Articulata L. Doctoral dissertation. Rabat, Morocco: Mohammed V University.
El Moussaouiti, M., Talbaoui, A., Gmouh, S., Aberchane, M., Benjouad, A., Bakri, Y., et al. (2010). Chemical composition and bactericidal evaluation of essential oil of Tetraclinis Articulata burl wood from Morocco. J. Indian Acad. Wood Sci. 7, 14–18. doi:10.1007/s13196-010-0003-2
El-Hossary, E. M., Abdel-Halim, M., Ibrahim, E. S., Pimentel-Elardo, S. M., Nodwell, J. R., Handoussa, H., et al. (2020). Natural products repertoire of the red sea. Mar. Drugs 18 (9), 457. doi:10.3390/md18090457
Fadili, K., Sekkate, C., Alistiqsa, F., Haloui, Z., Chakir, S., and Zair, T. (2017). Ethnobotanical study of medicinal plants from Er-Rich region (Moroccan High Atlas). Adv. Environ. Biol. 11, 27–41.
Fakchich, J., and Elachouri, M. (2014). Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. J. Ethnopharmacol. 154 (1), 76–87. doi:10.1016/j.jep.2014.03.016
Farjon, A. (2010). A handbook of the world’s conifers (two vol. set), 2. Leiden: Brill. Brill, Leiden ; Boston.
García-Castaño, J. L., Balao, F., Lorenzo, M. T., Véla, E., Hadjadj-Aoul, S., Mifsud, S., et al. (2021). A complex genetic structure of Tetraclinis articulata (Cupressaceae) in the western mediterranean. Bot. J. Linn. Soc. 197, 420–438. doi:10.1093/botlinnean/boab030
Hachi, M., Ouafae, B., Hachi, T., Mohamed, E. B., Imane, B., Atmane, R., et al. (2016). Contribution to the ethnobotanical study of antidiabetic medicinal plants of the central Middle Atlas region (Morocco). Lazaroa 37, 135–144. doi:10.5209/LAZAROA.51854
Harmouzi, A., Boughdad, A., El Ammari, Y., and Chaouch, A. (2016). Chemical composition and toxicity of Moroccan Tetraclinis Articulata and Juniperus phoenicea essential oils against Aphis citricola Goot, 1912 (Homoptera, Aphididae). Res. Chem. Intermed. 42, 7185–7197. doi:10.1007/s11164-016-2528-5
Hazan, Z., and Lucassen, A. C. B. (2015). Extracts and therapeutic uses thereof. Patent No US20150246087. Rehovot: Regenera pharma LTD.
Idm'hand, E., Msanda, F., and Cherifi, K. (2020). Ethnobotanical study and biodiversity of medicinal plants used in the Tarfaya Province, Morocco. Acta Ecol. Sin. 40, 134–144. doi:10.1016/j.chnaes.2020.01.002
Idm'hand, E., El-Ouazzani, Y., and Cherifi, K. (2022). Ethnopharmacological review of medicinal plants used to manage hypertension in Morocco. Med. Plants - Int. J. Phytomed.Relat. Ind. 14, 202–220. doi:10.5958/0975-6892.2022.00024.7
Isman, M. B. (2015). A renaissance for botanical insecticides? Pest Manag. Sci. 71, 1587–1590. doi:10.1002/ps.4088
Jaadan, H., Akodad, M., Moumen, A., Baghour, M., Skalli, A., Ezrari, S., et al. (2020). Ethnobotanical survey of medicinal plants growing in the region of" oulad daoud zkhanine" (nador province), in northeastern Morocco. Eth. Res. Appl. 19, 1–12. doi:10.32859/era.19.39.1-12
Jagel, A., and Stützel, T. (2003). On the occurrence of non‐axillary ovules in Tetraclinis Articulata (Vahl) Mast. (Cupressaceae s. str.). Feddes Repert. 114, 497–507. doi:10.1002/fedr.200311012
Jamila, F., and Mostafa, E. (2014). Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. J. Ethnopharmacol. 154, 76–87. doi:10.1016/j.jep.2014.03.016
Jlizi, S., Zardi-Bergaoui, A., Znati, M., Flamini, G., Ascrizzi, R., and Jannet, H. B. (2018). Chemical composition and biological evaluation of the resin from Tetraclinis articulata (vahl.) masters: A promising source of bioactive secondary metabolites. Ind. Crops Prod. 124, 74–83. doi:10.1016/J.INDCROP.2018.07.055
Jlizi, S., Lahmar, A., Zardi-Bergaoui, A., Ascrizzi, R., Flamini, G., Harrath, A. H., et al. (2021). Chemical composition and cytotoxic activity of the fractionated trunk bark essential oil from Tetraclinis Articulata (Vahl) Mast. growing in Tunisia. Molecules 26, 1110. doi:10.3390/molecules26041110
Kachmar, M. R., Naceiri Mrabti, H., Bellahmar, M., Ouahbi, A., Haloui, Z., El Badaoui, K., et al. (2021). Traditional knowledge of medicinal plants used in the Northeastern part of Morocco. Evid. Based. Complement. Altern. Med. 2021. 6002949, doi:10.1155/2021/6002949
Kahouadji, M. S., Hseini, S., Jaafar, B., Zaid, Y., Kahouadji, A., and Zidane, L. (2022). Floristic diversity and evaluation of the potential of spontaneous medicinal plants in the Bigoudine Watershed (Moroccan Western High Atlas). Int. J. For. Res. 2022, 1. doi:10.1155/2022/2485502
Khabbach, A., Libiad, M., Ennabili, A., and Bousta, D. (2012). Medicinal and cosmetic use of plants from the province of Taza, Northern Morocco. Bol. Latinoam. Caribe Plant Med. Arom. 11, 46–60.
Khatib, S., Faraloni, C., and Bouissane, L. (2022). Exploring the use of iris species: Antioxidant properties, phytochemistry, medicinal and industrial applications. Antioxidants 11, 526. doi:10.3390/antiox11030526
Kicel, A. (2020). An overview of the genus Cotoneaster (Rosaceae): Phytochemistry, biological activity, and toxicology. Antioxidants 9, 1002. doi:10.3390/antiox9101002
Kononenko, I. (2017). Molecular characterization and aging of the sandarac resin and its principal component communic acid, Doctoral dissertation. France: Paris 6 University.
Laadim, M., Ouahidi, M. L., Zidane, L., El Hessni, A., Ouichou, A., and Mesfioui, A. (2017). Ethnopharmacological survey of plants used for the treatment of diabetes in the town of Sidi Slimane (Morocco). J. Pharmacogn. Phytother. 9, 101–110. doi:10.5897/JPP2016.0437
Lans, C., Khan, T. E., Curran, M. M., and McCorkle, C. M. (2007). Ethnoveterinary medicine: Potential solutions for large-scale problems? United States: Veterinary herbal medicine, 17–32.
Laxmishree, C., and Singh, N. (2018). Review of mode of action of some major botanical pesticides. Int. Res. J. Sci. Eng. 6, 129–132.
Lengai, G. M., Muthomi, J. W., and Mbega, E. R. (2020). Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 7, e00239. doi:10.1016/j.sciaf.2019.e00239
M'barek, K., Zribi, I., and Haouala, R. (2018). Allelopathic effects of Tetraclinis Articulata on barley, lettuce, radish and tomato. Allelopathy J. 43, 187–202. doi:10.26651/allelo.j./2018-43-2-1140
McBride, W., Kim, K., and Damoiseaux, R. (2010). Radioprotective drugs. Patent No US20100292193. Oakland: Weaver Austin Villeneuve and Sampson LLP.
McGaw, L. J., and Eloff, J. N. (2008). Ethnoveterinary use of Southern African plants and scientific evaluation of their medicinal properties. J. Ethnopharmacol. 119, 559–574. doi:10.1016/j.jep.2008.06.013
Merzouki, A., Ed-Derfoufi, F., and Mesa, J. M. (2000). Contribution to the knowledge of Rifian traditional medicine. II: Folk medicine in Ksar Lakbir district (NW Morocco). Fitoterapia 71, 278–307. doi:10.1016/S0367-326X(00)00139-8
Miara, M. D., Bendif, H., Ouabed, A., Rebbas, K., Hammou, M. A., Amirat, M., et al. (2019). Ethnoveterinary remedies used in the Algerian steppe: Exploring the relationship with traditional human herbal medicine. J. Ethnopharmacol. 244, 112164. doi:10.1016/j.jep.2019.112164
Miresmailli, S., and Isman, M. B. (2014). Botanical insecticides inspired by plant–herbivore chemical interactions. Trends Plant Sci. 19, 29–35. doi:10.1016/j.tplants.2013.10.002
Mitsuharu, O., and Michimasa, H. (2001). Cosmetic composition containing steam distillate of plant. Patent No JP2001220312. Japan: Ichimaru Pharcos Co Ltd.
Montassir, L., Berrebaan, I., Mellouki, F., Zkhiri, F., Boughribil, S., and Bessi, H. (2017). Acute toxicity and reprotoxicity of aqueous extract of a Moroccan plant (Tetraclinis Articulata) on freshwater cladoceran Daphnia magna. J. Mat. Environ. Sci. 8, 770–776.
Morte, M. A., and Honrubia, M. (1996). “Tetraclinis articulata (Cartagena cypress),”. Trees IV. Biotechnology in agriculture and forestry. Editor Y. P. S Bajaj (Berlin, Heidelberg: Springer), 35.
Mrabti, H. N., Doudach, L., Mekkaoui, M., Khalil, Z., Harraqui, K., Fozia, F., et al. (2022). Profile of medicinal plants traditionally used for the treatment of skin burns. Evid. Based. Complement. Altern. Med. 2022, 3436665. doi:10.1155/2022/3436665
Nazim, B., Houari, T., and Benhaddou, I. (2020). Ethnobotanical Survey of Some Plants Used in Tessala Region. Algeria. Curr. Pers. MAPs 3 (1), 25–30. doi:10.38093/cupmap.652708
Niroumand, M. C., Farzaei, M. H., Razkenari, E. K., Amin, G., Khanavi, M., Akbarzadeh, T., et al. (2016). An evidence-based review on medicinal plants used as insecticide and insect repellent in traditional Iranian medicine. Iran. Red. Crescent Med. J. 18, e22361. doi:10.5812/ircmj.22361
Ouhaddou, H., Boubaker, H., Msanda, F., and El Mousadik, A. (2014). An ethnobotanical study of medicinal plants of the Agadir Ida Ou Tanane province (Southwest Morocco). J. Appl. Biosci. 84, 7707–7722. doi:10.4314/jab.v84i1.5
Pandey, A. K., Chávez-González, M. L., Silva, A. S., and Singh, P. (2021). Essential oils from the genus Thymus as antimicrobial food preservatives: Progress in their use as nanoemulsions - a new paradigm. Trends Food Sci. Technol. 111, 426–441. doi:10.1016/j.tifs.2021.02.076
Patron, A., Noncovich, A., and Ung, J. (2017). Compounds useful as modulators of TRPM8. Patent No US20170096418. San Diego: Senomyx, Inc.
Patron, A., Manam, R., Colquitt, J., Servant, N., Noriega, C. E., Hammaker, J. R., et al. (2022). High intensity sweeteners. Patent No US11357246. San Diego: Firmenich Incorporated.
Pham-Huy, L. A., He, H., and Pham-Huy, C. (2008). Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 4, 89–96.
Piluzza, G., Virdis, S., Serralutzu, F., and Bullitta, S. (2015). Uses of plants, animal and mineral substances in Mediterranean ethno-veterinary practices for the care of small ruminants. J. Ethnopharmacol. 168, 87–99. doi:10.1016/j.jep.2015.03.056
Postu, P. A., Tiron, A., Tiron, C. E., Gorgan, D., Mihasan, M., and Hritcu, L. (2022). Conifer essential oils reversed amyloid beta1-42 action by modulating BDNF and ARC expression in the rat hippocampus. CNS Neurol. Disord. Drug Targets 21 (1), 85–94. doi:10.2174/1871527320666210303111537
Rabib, H., Zougagh, S., Hsain, M., Badri, W., and Koussa, T. (2019). GC/MS analysis and antibacterial activity of the essential oil of Moroccan Tetraclinis articulata (vahl) Masters. Mediterr. J. Chem. 8 (4), 302–307. doi:10.13171/mjc84190706041316hr
Rabib, H., Elagdi, C., Hsaine, M., Fougrach, H., Koussa, T., and Badri, W. (2020). Antioxidant and antibacterial activities of the essential oil of Moroccan Tetraclinis Articulata (Vahl) masters. Biochem. Res. Int. 2020, 9638548. doi:10.1155/2020/9638548
Rached, W., Zeghada, F. Z., Bennaceur, M., Barros, L., Calhelha, R. C., Heleno, S., et al. (2018). Phytochemical analysis and assessment of antioxidant, antimicrobial, anti-inflammatory and cytotoxic properties of Tetraclinis Articulata (Vahl) Masters leaves. Ind. Crops Prod. 112, 460–466. doi:10.1016/j.indcrop.2017.12.037
Rachid, A., Rabah, D., Farid, L., Zohra, S. F., Houcine, B., and Nacéra, B. (2012). Ethnopharmacological survey of medicinal plants used in the traditional treatment of diabetes mellitus in the North Western and South Western Algeria. J. Med. Plant Res. 6, 2041–2050. doi:10.5897/JMPR11.1796
Ramdani, C., El Fakhouri, K., Sbaghi, M., Bouharroud, R., Boulamtat, R., Aasfar, A., et al. (2021). Chemical composition and insecticidal potential of six essential oils from Morocco against Dactylopius opuntiae (Cockerell) under field and laboratory conditions. Insects 12 (11), 1007. doi:10.3390/insects12111007
Rguez, S., Ben Slimene, I., Abid, G., Hammemi, M., Kefi, A., Elkahoui, S., et al. (2020). Tetraclinis Articulata essential oil reduces Botrytis cinerea infections on tomato. Sci. Hortic. 266, 109291. doi:10.1016/j.scienta.2020.109291
Romero-Noguera, J., Martín-Sánchez, I., Doménech-Carbó, M. T., Osete-Cortina, L., López-Miras, M. M., and Bolívar-Galiano, F. (2014). Analytical characterization of the biodeterioration of diterpenoid labdanic varnishes used in pictorial techniques: Sandarac and Manila copal. Int. Biodeterior. Biodegrad. 90, 99–105. doi:10.1016/j.ibiod.2014.01.017
Saber, M., Harhar, H., El Hattabi, L., Zengin, G., Bouyahya, A., and Tabyaoui, M. (2021). Chemical composition and antioxidant activities of essential oils and extracts from cones of Tetraclinis Articulata (Vahl) Masters. Int. J. Second. Metab. 8, 352–363. doi:10.21448/ijsm.989436
Sadiki, F. Z., El Idrissi, M., Cioanca, O., Trifan, A., Hancianu, M., Hritcu, L., et al. (2018). Tetraclinis Articulata essential oil mitigates cognitive deficits and brain oxidative stress in an Alzheimer's disease amyloidosis model. Phytomedicine. 15, 57–63. doi:10.1016/j.phymed.2018.10.032
Sadiki, F. Z., El Idrissi, M., Sbiti, M., Lemrhari, A., Trifan, A., Cioanca, O., et al. (2018). Chemical composition and antibacterial activity of essential oil of Tetraclinis Articulata (Vahl) Masters branches of eastern Morocco. Chem. Biol. Technol. Agric. 5, 24–26. doi:10.1186/s40538-018-0137-9
Sadiki, F. Z., Bouymajane, A., Sbiti, M., Channaoui, S., Micalizzi, G., Cacciola, F., et al. (2022). Chemical profile, antibacterial, antioxidant and insecticidal properties of the essential oil from Tetraclinis Articulata (Vahl) masters cones. J. Essent. Oil Res. 1-11. doi:10.1080/10412905.2022.2072962
Sahoo, N., Manchikanti, P., and Dey, S. (2010). Herbal drugs: Standards and regulation. Fitoterapia 81, 462–471. doi:10.1016/j.fitote.2010.02.001
Salem, N., Boulares, M., Zarrouk, Y., Kammoun el euch, S., Essid, R., Jemai, M., et al. (2022). Preservation of poultry meat using Tetraclinis Articulata essential oil during refrigerated storage. Food Sci. Technol. Int., 1177. doi:10.1177/10820132221108710
Salhi, N., Bouyahya, A., Fettach, S., Zellou, A., and Cherrah, Y. (2019). Ethnopharmacological study of medicinal plants used in the treatment of skin burns in Occidental Morocco (area of Rabat). S. Afr. J. Bot. 121, 128–142. doi:10.1016/j.sajb.2018.10.038
Sánchez-Gómez, P., Jiménez, J. F., Vera, J. B., Sánchez-Saorín, F. J., Martínez, J. F., and Buhagiar, J. (2013). Genetic structure of Tetraclinis articulata, an endangered conifer of the western mediterranean basin. Silva Fenn. Hels. 45, 14. doi:10.14214/sf.1073
Sbai-Jouilil, H., Fadli, A., and Zidane, L. (2017). Survey of ethnomedicinal plants used for the treatment of gastrointestinal disorders in Seksaoua region (Western High Moroccan Atlas). Annu. Res. Rev. Biol. 16, 1–9. doi:10.9734/ARRB/2017/36112
Scorzoni, L., Benaducci, T., Almeida, A. M. F., Silva, D. H. S., Bolzani, V. D. S., and Gianinni, M. J. S. M. (2007). The use of standard methodology for determination of antifungal activity of natural products against medical yeasts Candida sp and Cryptococcus sp. Braz. J. Microbiol. 38, 391–397. doi:10.1590/S1517-83822007000300001
Sengottayan, S. N. (2013). Physiological and biochemical effect of Neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front. Physiol. 4, 359. doi:10.3389/fphys.2013.00359
Senouci, F., Ababou, A., and Chouieb, M. (2019). Ethnobotanical survey of the medicinal plants used in the southern mediterranean. Case study: The region of bissa (northeastern dahra mountains, Algeria). Pharmacogn. J. 11, 647–659. doi:10.5530/pj.2019.11.103
Sliti, S., Ayadi, S., Dumarcay, S., Khouja, M., Gerardin, P., André, E., et al. (2016). Evaluation of essential oil composition and antioxidant capacity of hydromethanolic extracts of Tetraclinis Articulata, depending on location and seasonal variations. J. Mat. Environ. Sci. 7 (3), 968–980.
Souto, A. L., Sylvestre, M., Tölke, E. D., Tavares, J. F., Barbosa-Filho, J. M., and Cebrián-Torrejón, G. (2021). Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules 26, 4835. doi:10.3390/molecules26164835
Sugimoto, N., Kuroyanagi, M., Kato, T., Sato, K., Tada, A., Yamazaki, T., et al. (2006). Identification of the main constituents in Sandarac Resin, a natural gum base. Shokuhin Eiseigaku Zasshi. 47, 76–79. doi:10.3358/shokueishi.47.76
Takashi, S. (2021). Space adjustment device and space adjustment method. Patent No JP2021104190. Japan: Toyota Central Research and Development Laboratories.
Talbaoui, A., El Hamdaoui, L., El Moussaouiti, M., Aneb, M., Amzazi, S., and Bakri, Y. (2016). GC–MS analysis and antibacterial activity of hydro-distillation oil from Tetraclinis articulata wood grown in Khemisset (Morocco). J. Indian Acad. Wood Sci. 13 (2), 114–117. doi:10.1007/s13196-016-0173-7
Tékaya-Karoui, A., and Mighri, H, Z. (2007). Essential oil composition of terminal branches, cones and roots of Tetraclinis Articulata from Tunisia. Pak. J. Biol. Sci. 10, 2495–2499. doi:10.3923/pjbs.2007.2495.2499
Toni, M. (2019). Combination of an organic substrate and organic formulation for use as a cutting board and storage container. Patent No US10337139. Victoria: Abeego Designs.
Toumi, M. N., Bouzidi, M. A., Benyamina, A., Tilmatine, A., Megharbi, A., and Toutmi, F. (2022). Plants acting on alpha-amylase: Trigonella foenum-graecum and Tetraclinis articulata. Phytothérapie 18, 17–29. doi:10.3166/phyto-2022-0310
Ukoroije, R. B., and Otayor, R. A. (2020). Review on the bio-insecticidal properties of some plant secondary metabolites: Types, formulations, modes of action, advantages and limitations. AJRiZ. 3, 27–60. doi:10.9734/ajriz/2020/v3i430099
Ul Hassan, H., Murad, W., Tariq, A., and Ahmad, A. (2014). Ethnoveterinary study of medicinal plants in malakand valley, district dir (lower), khyber pakhtunkhwa, Pakistan. Ir. Vet. J. 67, 6. doi:10.1186/2046-0481-67-6
Utesch, D., Feige, K., Dasenbrock, J., Broschard, T. H., Harwood, M., Danielewska-Nikiel, B., et al. (2008). Evaluation of the potential in vivo genotoxicity of quercetin. Mutat.Res. - Genet.Toxicol. Environ. Mutagen. 654, 38–44. doi:10.1016/j.mrgentox.2008.04.008
Van Wyk, B-E., and Wink, M. (2015). Phytomedicines, herbal drugs, and poisons. Chicago: RoyalBotanic Gardens, Kew: The University of Chicago Press; Kew Publishing.
Viegi, L., and Ghedira, K. (2014). Preliminary study of plants used in ethnoveterinary medicine in Tunisia and in Italy. Afr. J. Tradit. Complement. Altern. Med. 11, 189–199. doi:10.4314/ajtcam.v11i3.27
Wicker, S., and Ditschun, T. (2017). Compositions incorporating an Umami flavor agent. Patent No US20170143022. San Diego: Senomyx, Inc.
Yassir, M., Bakrim, W. B., Mahmoud, M. F., Drissi, B., Kouisni, L., and Sobeh, M. (2022). Watery rose apple: A comprehensive review of its traditional uses, nutritional value, phytochemistry, and therapeutic merits against inflammation-related disorders. Oxid. Med. Cell. Longev. 2022, 7502185. doi:10.1155/2022/7502185
Zatout, F., Benarba, B., Bouazza, A., Babali, B., Nacer Bey, N., and Morsli, A. (2021). Ethnobotanical investigation on medicinal plants used by local populations in Tlemcen National Park (extreme North West Algeria). Mediterr. Bot. 42, e69396. doi:10.5209/mbot.69396
Zibaee, A. (2011). Botanical insecticides and their effects on insect biochemistry and immunity. London, United Kingdom: pesticides in the modern world - pests control and pesticides exposure and toxicity assessment.
Zidane, A., Tits, M., Angenot, L., Wauters, J. N., Frederich, M., Dib, I., et al. (2014). Phytochemical analysis of Tetraclinis Articula in relation to its vasorelaxant property. J. Mat. Environ. Sci. 5, 1368–1375.
Keywords: Tetraclinis articulata (Vahl) Masters, ethnopharmacology, biocide, phytochemistry, toxicity
Citation: Khatib S, Sobeh M and Bouissane L (2022) Tetraclinis articulata (vahl) masters: An insight into its ethnobotany, phytochemistry, toxicity, biocide and therapeutic merits. Front. Pharmacol. 13:977726. doi: 10.3389/fphar.2022.977726
Received: 24 June 2022; Accepted: 08 August 2022;
Published: 05 September 2022.
Edited by:
Alessandro Venditti, Sapienza University of Rome, ItalyReviewed by:
Abdellah Aghraz, University College London, United KingdomAbdelhakim Bouyahya, Mohammed V University, Morocco
Motlalepula Gilbert Matsabisa, University of the Free State, South Africa
Muhammad Riaz, Shaheed Benazir Bhutto University, Pakistan
Copyright © 2022 Khatib, Sobeh and Bouissane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mansour Sobeh, mansour.sobeh@um6p.ma; Latifa Bouissane, l.bouissane@usms.ma
 Sohaib Khatib
Sohaib Khatib Mansour Sobeh
Mansour Sobeh Latifa Bouissane
Latifa Bouissane