- 1Department of Oncology, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Graduate School, Beijing University of Chinese Medicine, Beijing, China
Introduction: The incidence and mortality of gastric cancer ranks among the highest, and the 5-year survival rate of advanced gastric cancer (AGC) is less than 10%. Currently, chemotherapy is the main treatment for AGC, and oxaliplatin is an important part of the commonly used chemotherapy regimen for AGC. A large number of RCTs have shown that Chinese herbal medicine (CHM) combined with oxaliplatin-based chemotherapy can improve objective response rate (ORR) and disease control rate (DCR), reduce the toxic and side effects of chemotherapy. There is currently a lack of systematic evaluation of the evidence to account for the efficacy and safety of CHM combined with oxaliplatin-based chemotherapy in AGC. Therefore, we carried out this study and conducted the sensitivity analysis on the herbal composition to explore the potential anti-tumor efficacy.
Methods: Databases of PubMed, EMBASE, CENTRAL, Web of Science, the Chinese Biomedical Literature Database, the China National Knowledge Infrastructure, the Wanfang database, and the Chinese Scientific Journals Database were searched from their inception to April 2022. RCTs evaluating the efficacy of CHM combined with oxaliplatin-based chemotherapy on AGC were included. Stata 16 was used for data synthesis, RoB 2 for quality evaluation of included RCTs, and GRADE for quality of synthesized evidence. Additional sensitivity analysis was performed to explore the potential anti-tumor effects of single herbs and combination of herbs.
Results: Forty trials involving 3,029 participants were included. Most included RCTs were assessed as “Some concerns” of risk of bias. Meta-analyses showed that compare to oxaliplatin-based chemotherapy alone, that CHM combined with oxaliplatin-based chemotherapy could increase the objective response rate (ORR) by 35% [risk ratio (RR) = 1.35, 95% confidence intervals (CI) (1.25, 1.45)], and disease control rate (DCR) by 12% [RR = 1.12, 95% CI (1.08, 1.16)]. Subgroup analysis showed that compare to SOX, FOLFOX, and XELOX regimens alone, CHM plus SOX, CHM plus FOLFOX, and CHM plus XELOX could significantly increase the ORR and DCR. Sensitivity analysis identified seven herbs of Astragalus, Liquorice, Poria, Largehead Atractylodes, Chinese Angelica, Codonopsis, and Tangerine Peel with potentials to improve tumor response of oxaliplatin-based chemotherapy in AGC.
Conclusion: Synthesized evidence showed moderate certainty that CHM plus oxaliplatin-based chemotherapy may promote improvement in tumor response in AGC. CHM treatment is safe for AGC. Due to the poor quality of included RCTs and small samplesizes, the quality of synthesized evidence was not high. Specific combinations of herbs appeared to produce higher contributions to ORR than the herb individually. Each of this seven above mentioned herbs has been shown in experimental studies to potentially contribute to the improvement of tumor response. To support this conclusion, these seven herbs are worthy of further clinical research.
Systematic Review Registration: [http://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=262595], identifier [CRD42022262595].
1 Introduction
According to Global Cancer Statistics 2020, there were 1,089,000 new cases and 769,000 mortality cases of gastric cancer (GC) globally, ranked second of incidence and mortality rate of all malignant tumors of digestive system (Sung et al., 2021). The number of GC cases in China accounts for 43.9% of the global total. About 50% of GC patients were in advanced stage at the initial diagnosis. Advanced gastric cancer (AGC) has a poor prognosis, with a median overall survival (OS) of 10–12 months (Digklia et al., 2016), and the 5-year survival rate is no more than 10% (Song et al., 2017). AGC generally have distant metastasis and local infiltration, and 50% of recurrent patients were local lymph node positive (Peng et al., 2010). It is reported that the OS of patients with metastatic GC after palliative chemotherapy is only 7–15 months, and the 5-year survival rate is only 2% (Leong, 2005; Ajani et al., 2007). In recent years, the incidence and death of GC are on the rise. Therefore, AGC has become one of the main diseases endangering human life and health (Johnston et al., 2019).
Chemotherapy is the standard first-line treatment for AGC patients, and palliative chemotherapy has a statistically significant advantage over best supportive care in improving survival in AGC patients (Wagner et al., 2006). The NCCN guidelines recommend that combined chemotherapy containing platinum and fluorouracil is preferred for AGC patients (Ajani et al., 2022). Oxaliplatin is a third-generation platinum-type anticancer drug. Clinical studies have proved that it has a significant inhibitory effect on locally AGC or AGC, and its efficacy is no less than that of cisplatin (Ajani et al., 2016; Smyth et al., 2016; Yoshino et al., 2018). The median progression-free survical (mPFS) of first-line chemotherapy is 4–6 months and median overall survival (mOS) is 10–15 months (Van Cutsem et al., 2006; Cunningham et al., 2008; Koizumi et al., 2008; Kang et al., 2009). Moreover, oxaliplatin is better tolerated than cisplatin and has a better synergistic effect with fluorouracil (Al-Batran et al., 2008). Based on the above advantages, oxaliplatin has become the main platinum drug in AGC chemotherapy, which is used to form SOX, XELOX and FOLFOX regimens. However, peripheral neurotoxicity is the main side effect of oxaliplatin, which incidence of peripheral neurotoxicity is higher than that of cisplatin (63% vs. 22%), especially grade 3 or 4 neuropathy (Al-Batran et al., 2008; Cunningham et al., 2008). The targeted drugs approved for the treatment of AGC are mainly anti-angiogenic drugs, including trastuzumab and ramucirumab. The mOS of trastuzumab in the treatment of AGC patients with positive HER-2 is 7.9 months, but the incidence of anemia and visceral bleeding, two kinds of serious adverse events (AEs), is about 19% (Thuss-Patience et al., 2017). In addition, HER-2 overexpression only accounts for 15%–20% of AGC (Joshi et al., 2021), and the benefit in OS of other drugs is unclear, and the selection of targeted drugs is limited. There is no evidence supporting survival benefit of immunotherapy alone in patients with AGC (Shitara et al., 2018; Bang et al., 2019; Van Cutsem et al., 2021). Therefore, despite the variety of treatment options for AGC, chemotherapy is still the best choice for AGC, and chemotherapy drugs are constantly updated and iterated. However, obstacles such as serious AEs of chemotherapy, poor quality of life (QoL) and short survival of patients with AGC have not been well solved, and the treatment of AGC is still a major challenge.
CHM has been widely used in east Asia to fight against tumors for a long time. In particular, CHM combined with chemotherapy has advantages of synergistic efficacy and toxicity reduction, improving QoL and enhancing immune function (Cheng et al., 2021). A meta-analysis of 2,670 patients with AGC found that patients with astragalus-containing Chinese medicine combined with platinum-containing chemotherapy had better objective response rate (ORR) [risk ratio (RR) = 1.24, 95% confidence interval (CI): 1.15–1.34) ] and disease control rate (DCR) (RR = 1.10, 95% CI: 1.06–1.14), the AEs caused by chemotherapy were significantly reduced, and the QoL was significantly improved (Cheng et al., 2021). There was no previous meta-analysis of oxaliplatin-based chemotherapy regimen plus CHM, but some high quality clinical studies have demonstrated the important role of CHM combined with oxaliplatin. Yiqi Huoxue Jiedu formula combined with XELOX regimen could improve DCR (60.78% vs. 41.67%) (Hu, 2011). Shenlian capsules combined with SOX chemotherapy intervention in 157 patients with AGC, the ORR and DCR of the treatment group were 78.1% and 92.7%, respectively, while those of the control group were 66.4% and 79.9%, besides, which significantly reduced the incidence of neurotoxicity and other toxic and side effects (52.2% vs. 94.6%) and prolonged OS about 2.7 months (Diao et al., 2018). The ORR and DCR in the treatment group of Weifu formula combined with FOLFOX6 were significantly improved (ORR, 70.0% vs. 26.7%; DCR, 83.3% vs. 56.7%), and the KPS score was also improved (83.3% vs. 60.0%) (Fan et al., 2015). However, these individual studies are not enough to explain the clinical role of traditional Chinese medicine (TCM) in AGC. Evidence-based medicine should be used to comprehensively analyze existing clinical studies and to evaluate available evidence. Due to the complexity and diversity of Chinese medicine prescriptions, it is impossible to determine which CHM herbs play an important role to synergistic action with chemotherapy.
With the further development of basic research, the role and mechanism of TCM in inhibiting the division and proliferation of tumor cells, promoting the apoptosis of tumor cells, inhibiting the metastasis of tumor cells, and enhancing the curative effect with chemotherapy have been gradually revealed, laying a foundation for the effective application of anti-tumor (Zhou et al., 2016; Liu et al., 2020; Ren et al., 2020; Xiang et al., 2020; Zhang L. et al., 2021). Available experimental studies indicate that TCM compounds do have anti-tumor activity, but the more appropriate and accurate drug selection is still unknown. Therefore, future research will focus on finding individual herbs with special contributions to chemotherapy efficacy of AGC, and to improve survival benefit of AGC and precise selection of TCM.
There are several RCTs evaluated the efficacy and safety of CHM in AGC, but these clinical evidence were not systematically evaluated. Furthermore, whether CHM have a synergistic effect with oxaliplatin-based chemotherapy, the key component of the first-line treatment of AGC, also need further evaluation. The objective of this study was to systematically evaluate the available evidence of tumor response and safety of CHM combined with oxaliplatin-based chemotherapy in AGC. Furthermore, we performed sensitivity analysis of single herb and combination of herbs, to explore the potential anti-tumor effects of these herbs.
2 Methods
This study was performed by the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and checklist (Moher et al., 2009), PRISMA checklist is available in Supplementary Material S1. This study was registered on PROSPERO (No. CRD42022262595).
2.1 Eligibility criteria
2.1.1 Type of studies
This study included RCTs with or without the blinded method, observational studies and quasi-RCTs were excluded. Trials did not describe the randomization process in details were considered as non-RCTs, and were excluded. Animal studies were also excluded.
2.1.2 Types of participants
RCTs which participants diagnosed with AGC through cytological or pathological tests were included.
2.1.3 Types of intervention and control
The intervention of CHM combined with oxaliplatin-based chemotherapy, and control of oxaliplatin-based chemotherapy were included in this study. We only included CHM formulas or oral patented drugs as the treatment of intervention, Chinese medicine injections and plant extracts were excluded.
2.1.4 Types of outcomes
RCTs reporting outcomes of tumor response and safety of CHM in GC treatment were included in this study. Trials reported other efficacy outcomes were excluded. Given the strong correlation between the two anti-tumor treatment response evaluation criteria, WHO criteria, and RECIST criteria, the outcomes reported by these two criteria were considered homogeneous (Aras et al., 2016).
2.2 Search strategy
We searched PubMed, EMBASE, CENTRAL, Web of Science, the Chinese Biomedical Literature Database (CBM), the China National Knowledge Infrastructure (CNKI), the Wanfang database, and the Chinese Scientific Journals Database (VIP database). Searches were performed from the databases initiation to April 2022. The language restriction was English and Chinese. The search strategy was based on the combination of controlled vocabulary (MeSH terms and Emtree terms) and free-text terms. The terms of “Stomach Neoplasms,” “Oxaliplatin,” “Antineoplastic Combined Chemotherapy Protocols,” “Herbal Medicine,” “Medicine, Chinese Traditional,” and “Drugs, Chinese Herbal” were used to develop the search strategy for PubMed, which is shown in Supplementary Material S2. Modifications to the search strategy were used with other databases.
2.3 Screening and selection
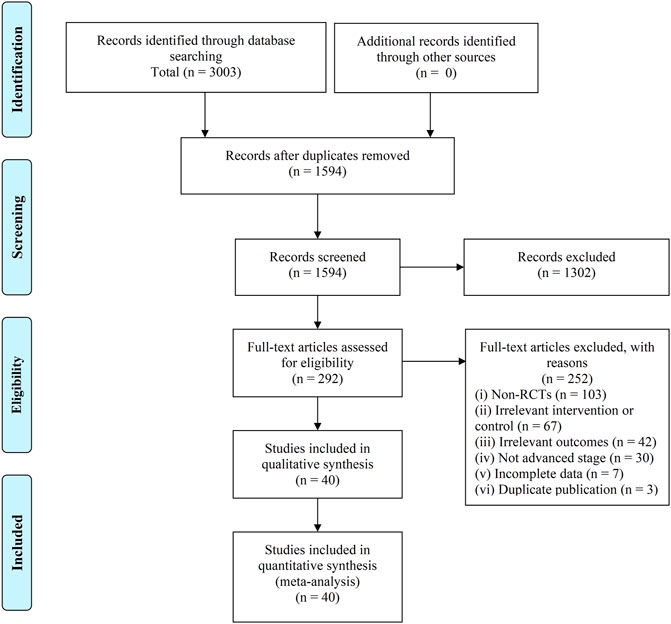
Search results were imported to EndNote 20. The titles and abstracts of retrievals were screened after duplicates removal, then full articles of potential trials were assessed for their eligibility. Screening and selection were independently and in duplicate performed by the review authors (YT and HW). RCTs that met the inclusion criteria were included. The process was summarized using a PRISMA flow diagram.
2.4 Data extraction
The following data were extracted from the included studies: 1) identification information (first author, year of publication); 2) general information (study setting, sample size, and duration of follow-up); 3) participants (clinical stage, age, and sex); 4) intervention details (name of CHM intervention, compositions, and duration); 5) comparison details (chemotherapy regimen, dose, frequency, and duration of treatment), and 6) outcomes details.
2.5 Quality assessment
The Risk of Bias 2 (RoB-2) tool was used to assess the methodological quality of included studies (Sterne et al., 2019). We evaluated included studies of quality of the randomization process, deviation from intended intervention, missing outcome data, outcome measurement, and selection of the reported result. The overall quality of RCTs were evaluated as low, some concerns or high RoB.
2.6 Evidence synthesis for RCTs
Stata 16 was used in data synthesis to perform a meta-analysis. The RR for dichotomous data with 95% CIs were evaluated. The random-effects model was used when synthesizing data for the meta-analysis. As for the outcomes reported with zero event, the Mantel-Haenszel methods were adopted. We quantified inconsistency by applying the I2 statistic; a value of I2 > 40% was considered important heterogeneity, and I2 > 75% was considerable heterogeneity (Higgins et al., 2019). Subgroup analysis were performed according to the different regimens of chemotherapy that patients received, and to explore the source of heterogeneity if substantial heterogeneity existed. Publication bias of the cumulative evidence among individual studies was evaluated using a graphical method of funnel plot (Egger et al., 1997).
2.7 Sensitivity analysis
We performed sensitivity analysis to investigate the potential contributions of specific herbs to tumor response. Previous studies proposed that if a particular herb possessed anti-tumor effects, they would be reflected in the pooled effect estimates of the studies which interventions containing this herb (Chen et al., 2016a; Chen et al., 2016b). Sensitivity analysis of ORR will be performed for studies on herbs used in AGC, herbs, or combinations of herbs presented in two or more studies, and the following principles will be applied:
1) Studies containing the same herb or combination of herbs will be treated as one, and the pooled RR (95% CI) and I2 will be calculated; 2) herbs or combinations of herbs will be excluded if there is no significant effect in the pooled results (95% CIs of RR overlap 1.0) and/or important heterogeneity exists between studies (I2 ≥ 40%); 3) the RR results will be listed in ascending order with 95% CI, the number of studies and I2 values; 4) the combination of herbs will be excluded when they have lower RRs than herbs alone; and 5) when herb combinations have higher RRs than herbs alone, they will be identified as potential examples of synergistic effects.
2.8 Quality of evidence
The quality of the cumulative evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system. Study limitations, inconsistency, indirectness, imprecision, and publication bias were evaluated. Quality of evidence was classified as high, moderate, low, or very low quality (Guyatt et al., 2008). We presented our findings in a Summary of Finding table.
3 Results
There were 3,003 retrievals exported from databases searches, and after the selection process, 40 trials involving 3,029 participants were included in this SR (Chen et al., 2007; Chi et al., 2010; Hu, 2011; Yuan, 2011; Zhao, 2011; Zhu et al., 2011; Qin, 2012; Guo, 2014; Huang, 2014; Liu et al., 2015; Li et al., 2016; Zhao et al., 2016; Chu et al., 2017; Feng, 2017; Huang et al., 2017; Wang N. et al., 2018; Yang et al., 2018; Yuan, 2018; Cai et al., 2019; Gu et al., 2019; Jiao, 2019; Liu et al., 2019; Xie, 2019; Yu et al., 2019; Zhai, 2019; Zhang, 2019; Zhong et al., 2019; Bao, 2020; Gong, 2020; Li D. H. et al., 2020; Sun et al., 2020; Zhang et al., 2020; Zhang, 2021a; Zhang, 2021b; Feng et al., 2021; Jiang et al., 2021; Long, 2021; Zhang H. O. et al., 2021; Zhao, 2021; Zhong et al., 2021). The selection process was summarized as a flowchart shown in Figure 1.
3.1 Details of included trials
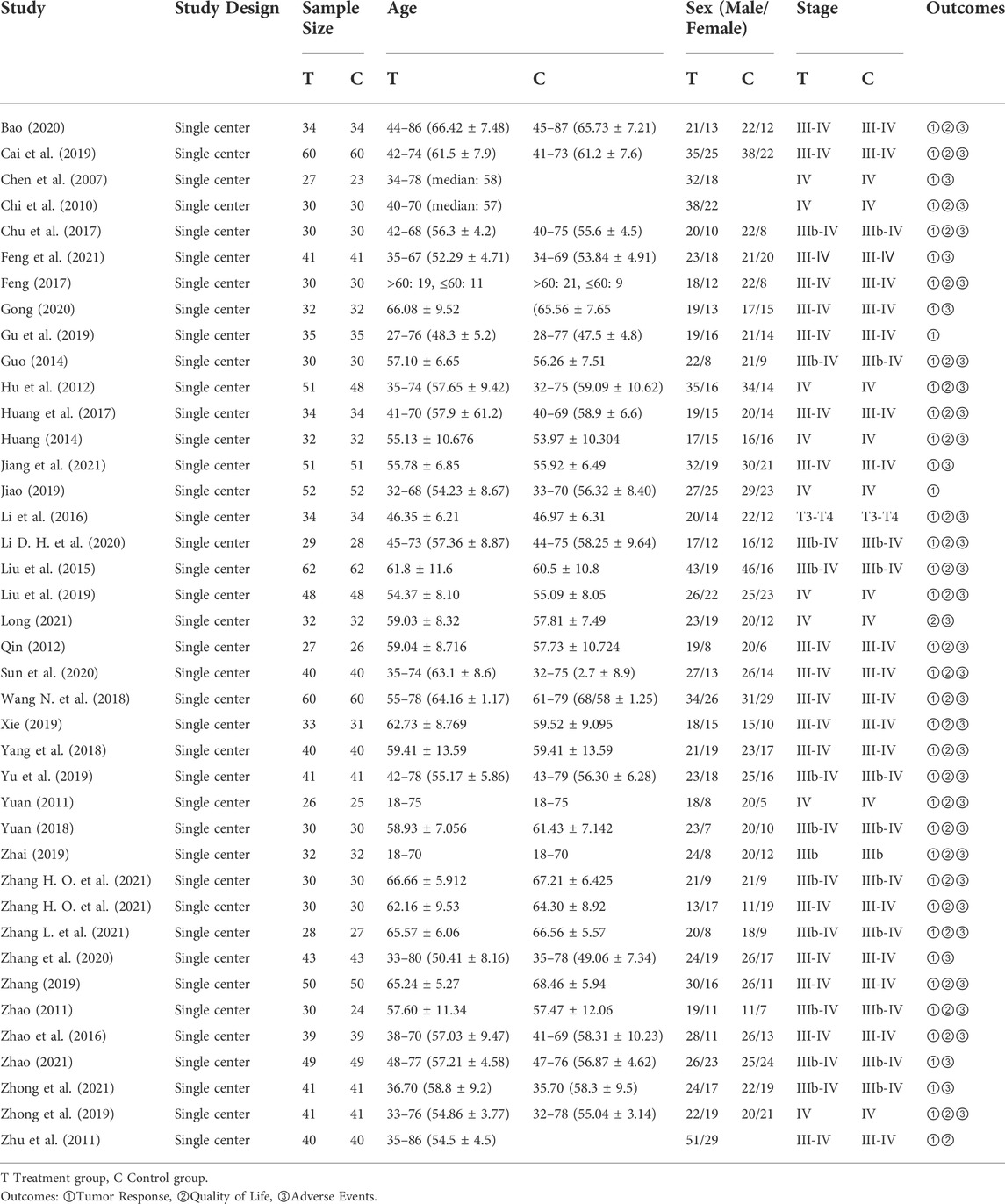
All these 40 trials are non-blinded RCTs that conducted in single-center. The sample sizes of included trials ranged from 50 to 124. Among these included trials, one published in an English journal (Li Y. et al., 2020), and other 39 trials were published in Chinese (Chen et al., 2007; Chi et al., 2010; Hu, 2011; Yuan, 2011; Zhao, 2011; Zhu et al., 2011; Qin, 2012; Guo, 2014; Huang, 2014; Liu et al., 2015; Li et al., 2016; Zhao et al., 2016; Chu et al., 2017; Feng, 2017; Huang et al., 2017; Wang Y. et al., 2018; Yang et al., 2018; Yuan, 2018; Cai et al., 2019; Gu et al., 2019; Jiao, 2019; Liu et al., 2019; Xie, 2019; Yu et al., 2019; Zhai, 2019; Zhang, 2019; Zhong et al., 2019; Bao, 2020; Gong, 2020; Sun et al., 2020; Zhang et al., 2020; Zhang, 2021a; Zhang, 2021b; Feng et al., 2021; Jiang et al., 2021; Long, 2021; Zhang L. et al., 2021; Zhao, 2021; Zhong et al., 2021). The characteristics of included RCTs were shown in Table 1.
3.1.1 Intervention details
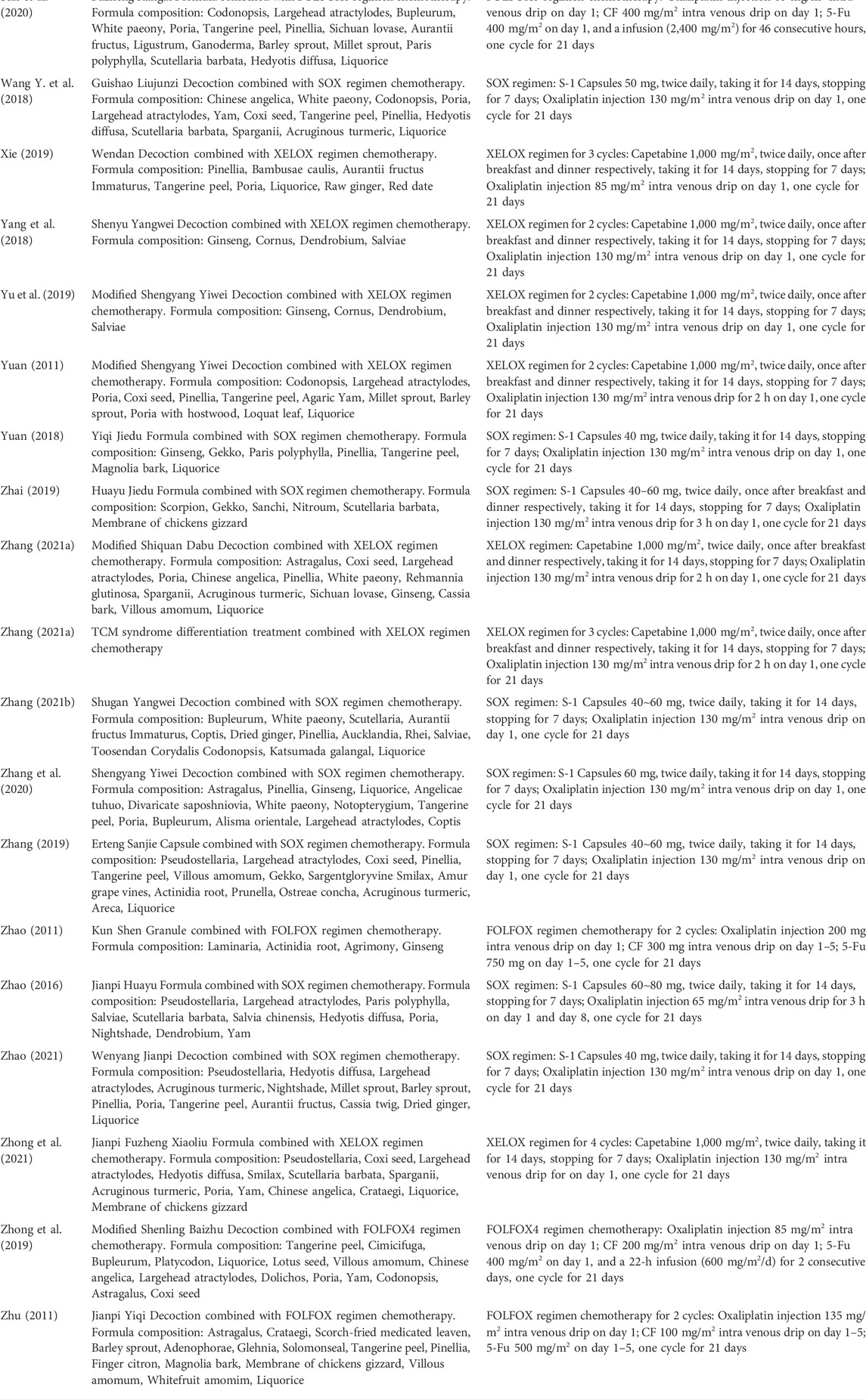
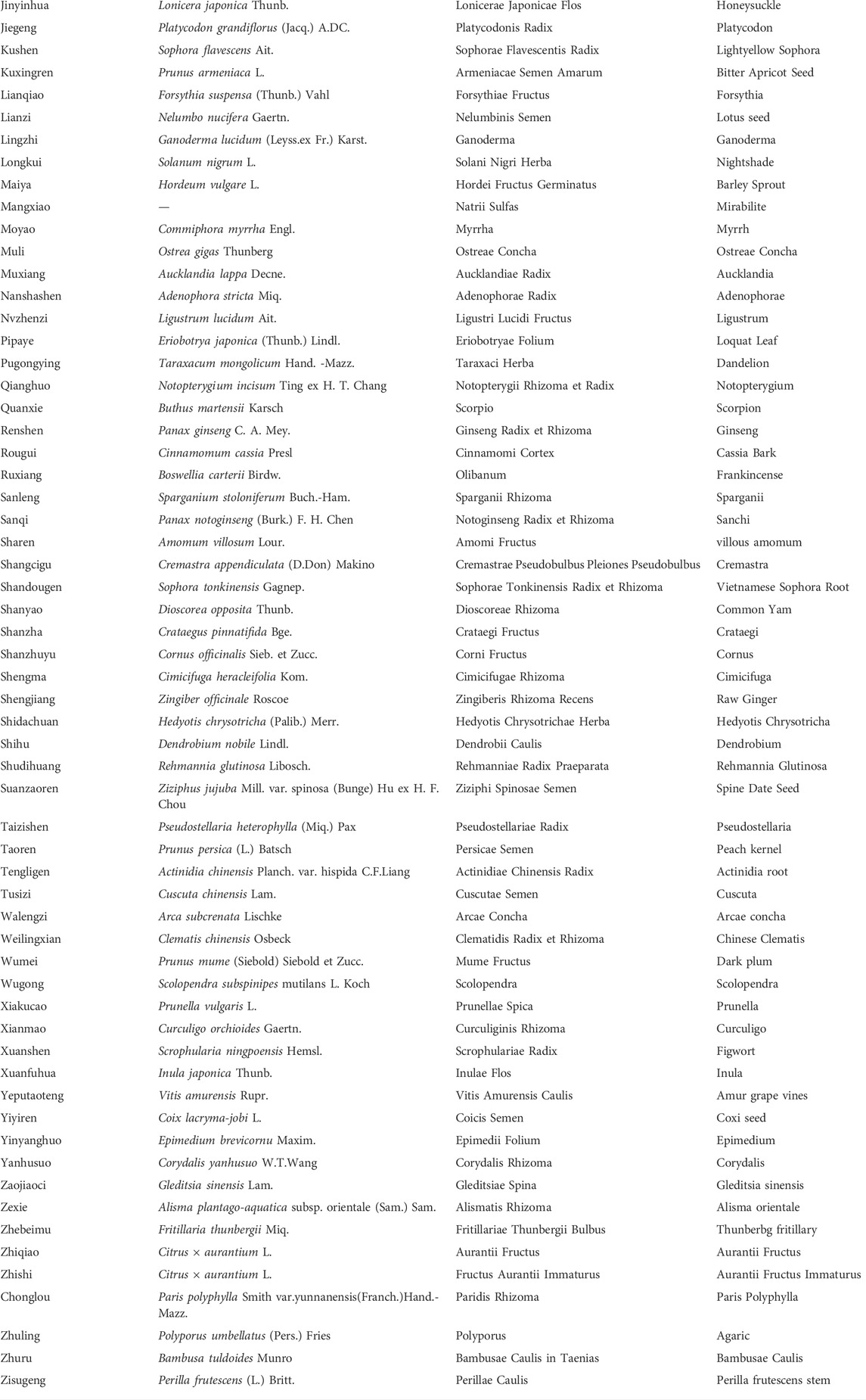
Among these 40 trials, three trials adopted TCM syndrome differentiation treatment, and patients in these three trials received more than one core prescription, other 37 trials used single formula as core prescription. As for the chemotherapy, SOX was the most frequent regimen that adopted by 16 trials (Qin, 2012; Guo, 2014; Liu et al., 2015; Zhao et al., 2016; Feng, 2017; Wang N. et al., 2018; Yuan, 2018; Zhai, 2019; Zhang, 2019; Bao, 2020; Gong, 2020; Li Y. et al., 2020; Zhang et al., 2020; Zhang, 2021a; Jiang et al., 2021; Zhao, 2021), FOLFOX regimen was adopted in 14 trials (Chen et al., 2007; Chi et al., 2010; Zhao, 2011; Zhu et al., 2011; Huang, 2014; Li et al., 2016; Chu et al., 2017; Huang et al., 2017; Gu et al., 2019; Jiao, 2019; Liu et al., 2019; Zhong et al., 2019; Sun et al., 2020; Long, 2021), and XELOX regimen in 10 trials (Hu, 2011; Yuan, 2011; Yang et al., 2018; Cai et al., 2019; Xie, 2019; Yu et al., 2019; Zhang, 2021b; Feng et al., 2021; Zhang H. O. et al., 2021; Zhong et al., 2021). Intervention details of included RCTs were shown in Table 2. The Chinese phonetic transcription, scientific name, Latin drug name, and English name of herbs adopted in the prescriptions of included trials were shown in Table 3.
3.1.2 Risk of bias of included trials
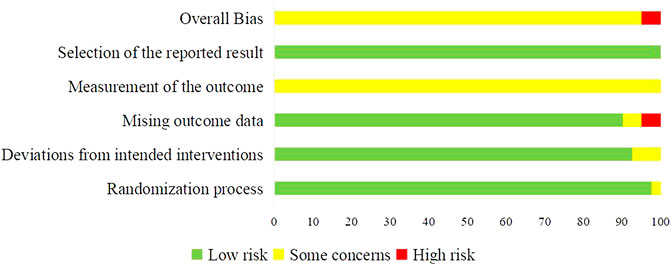
RoB 2 was used to assess the quality of 40 included trials. Two trials were assessed as “High” risk of bias (Zhao, 2011; Zhang, 2019), and 38 trials were assessed as “Some concerns” (Chen et al., 2007; Chi et al., 2010; Hu, 2011; Yuan, 2011; Zhu et al., 2011; Qin, 2012; Guo, 2014; Huang, 2014; Liu et al., 2015; Li et al., 2016; Zhao et al., 2016; Chu et al., 2017; Feng, 2017; Huang et al., 2017; Wang Y. et al., 2018; Yang et al., 2018; Yuan, 2018; Cai et al., 2019; Gu et al., 2019; Jiao, 2019; Liu et al., 2019; Xie, 2019; Yu et al., 2019; Zhai, 2019; Zhong et al., 2019; Bao, 2020; Gong, 2020; Li D. H. et al., 2020; Sun et al., 2020; Zhang et al., 2020; Zhang, 2021a; Zhang, 2021b; Feng et al., 2021; Jiang et al., 2021; Long, 2021; Zhang L. et al., 2021; Zhao, 2021; Zhong et al., 2021). Most concerns were caused by the measurement of the outcomes, since the assessment of outcomes could be influenced by knowledge of interventions patients received. The imbalanced missing data in two trials lead to the “High” risk in domain of missing outcome data, and in overall bias. The summary of RoB was shown in Figure 2.
3.2 The tumor response of CHM combined with oxaliplatin-based chemotherapy in AGC
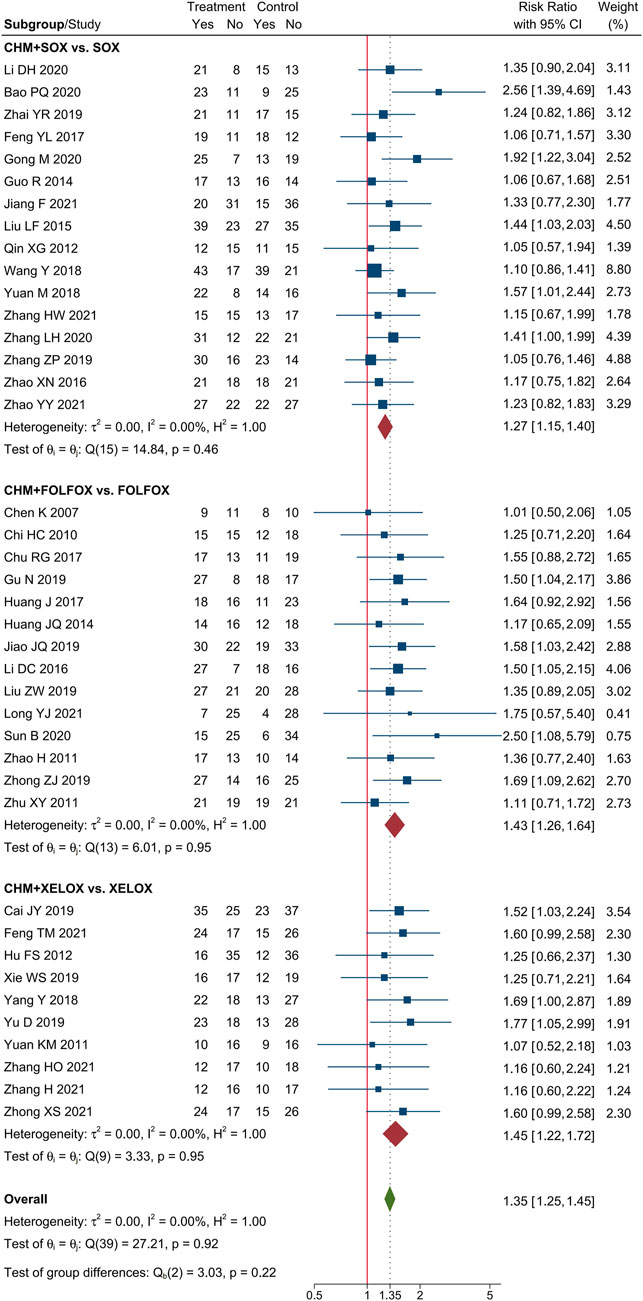
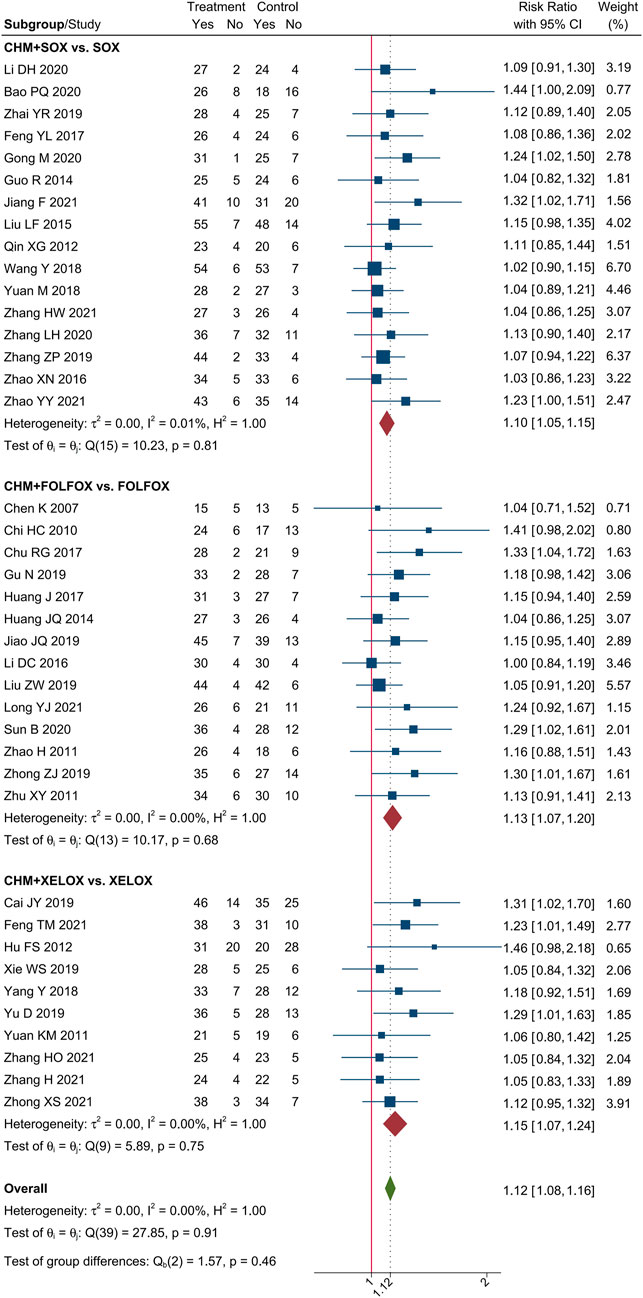
Meta-analysis of 40 trials showed that CHM combined with oxaliplatin-based chemotherapy could increase the ORR by 35% [RR = 1.35, 95% CI (1.25, 1.45)]. The value of I2 = 0 indicates that there was no statistical heterogeneity among these trials. The forest plot of ORR was shown in Figure 3. Meta-analysis of 40 trials showed that CHM combined with oxaliplatin-based chemotherapy could increase the DCR by 12% [RR = 1.12, 95% CI (1.08, 1.16)]. I2 = 0 indicates that there was no statistical heterogeneity among these trials. The forest plot of DCR was shown in Figure 4.
3.3 The safety of CHM combined with oxaliplatin-based chemotherapy in AGC
Safety outcomes were reported in 37 trials. We evaluated the incidence of AEs of blood system, gastrointestinal reaction, hepatorenal toxicity, and peripheral neurotoxicity.
3.3.1 AEs of blood system
Incidence of AEs of blood system were reported in 35 trials. Meta-analysis showed that compare oxaliplatin-based chemotherapy alone, CHM combined with oxaliplatin-based chemotherapy could decrease the incidence of myelosuppression by 50% [RR = 0.50, 95% CI (0.41, 0.61)], leucopenia by 46% [RR = 0.54, 95% CI (0.48, 0.61)], anemia by 23% [RR = 0.77, 95% CI (0.64, 0.92)], and thrombocytopenia by 43% [RR = 0.57, 95% CI (0.47, 0.70)]. Forest plots of incidence of AEs of blood system were shown in Figure 5.
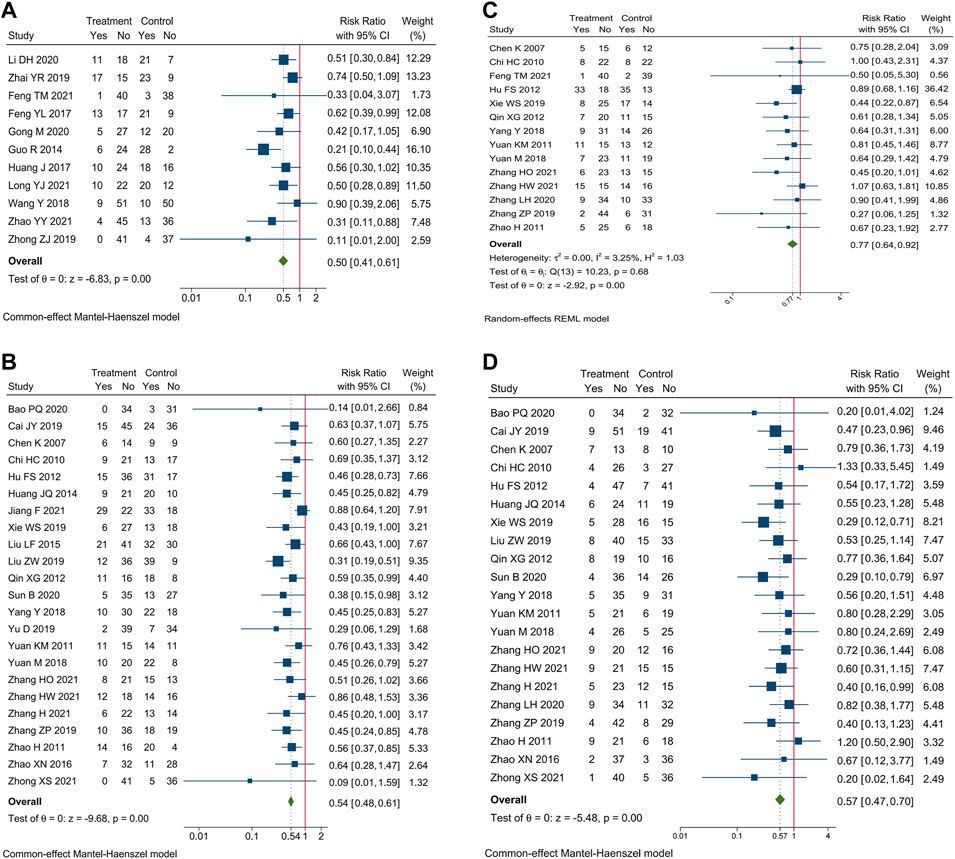
FIGURE 5. Forest plot of incidence of AEs of blood system. Incidence of (A) myelosuppression, (B) leucopenia, (C) anemia, and (D) thrombocytopenia.
3.3.2 Gastrointestinal reaction
Incidence of gastrointestinal reaction were reported in 34 trials. Meta-analysis showed that compare oxaliplatin-based chemotherapy alone, CHM combined with oxaliplatin-based chemotherapy could decrease the incidence of gastrointestinal reaction by 45% [RR = 0.55, 95% CI (0.47, 0.64)], nausea and vomiting by 39% [RR = 0.61, 95% CI (0.51, 0.73)], and diarrhea by 46% [RR = 0.54, 95% CI (0.42, 0.69)]. Forest plots of incidence of gastrointestinal reaction were shown in Figure 6.
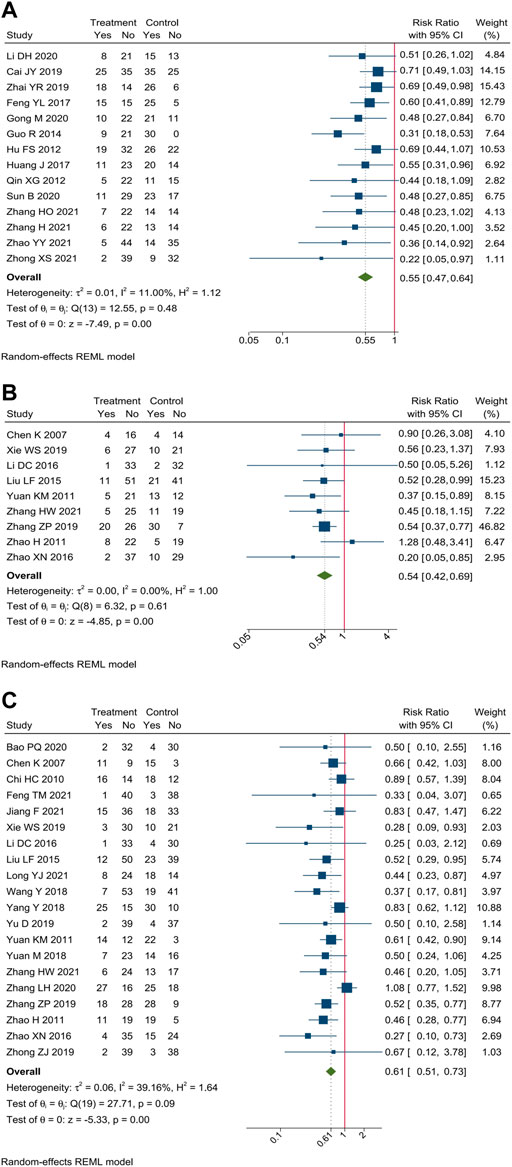
FIGURE 6. Forest plot of incidence of gastrointestinal reaction. Incidence of (A) gastrointestinal reaction, (B) diarrhea, and (C) nausea and vomiting.
3.3.3 Hepatorenal toxicity
Incidence of hepatorenal toxicity were reported in 29 trials. Meta-analysis showed that compare oxaliplatin-based chemotherapy alone, CHM combined with oxaliplatin-based chemotherapy could decrease the incidence of hepatorenal toxicity by 29% [RR = 0.71, 95% CI (0.56, 0.89)], hepatotoxicity by 35% [RR = 0.65, 95% CI (0.52, 0.81)], and renal toxicity by 45% [RR = 0.55, 95% CI (0.40, 0.77)]. Forest plots of incidence of hepatorenal toxicity were shown in Figure 7.
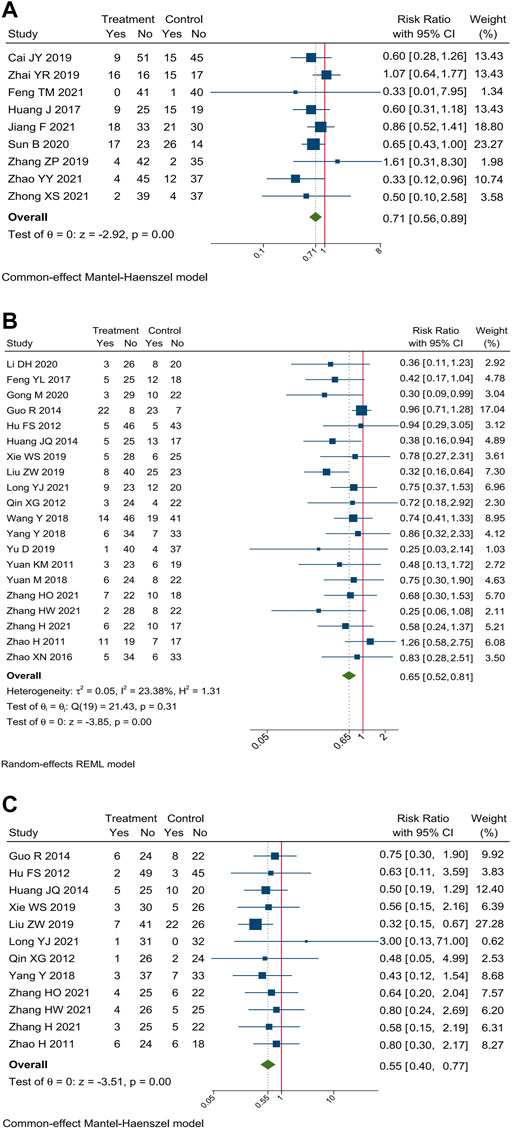
FIGURE 7. Forest plot of incidence of hepatorenal toxicity. Incidence of (A) hepatorenal toxicity, (B) hepatotoxicity, and (C) renal toxicity.
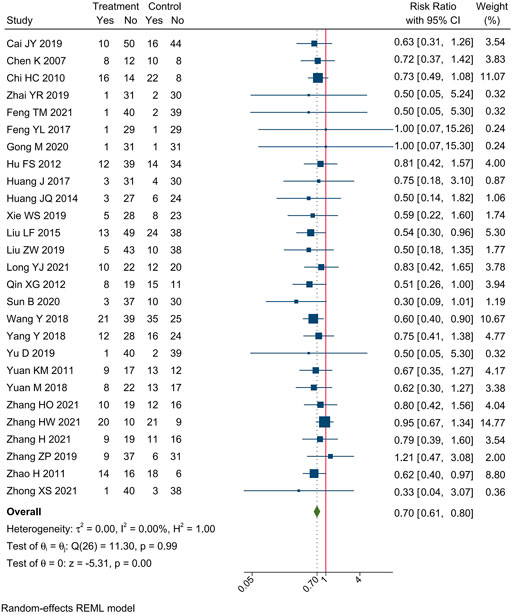
3.3.4 Peripheral neurotoxicity
Incidences of peripheral neurotoxicity were reported in 27 trials. Meta-analysis showed that compare oxaliplatin-based chemotherapy alone, CHM combined with oxaliplatin-based chemotherapy could decrease the incidence of peripheral neurotoxicity by 30% [RR = 0.70, 95% CI (0.61, 0.80)]. Forest plots of incidence of hepatorenal toxicity were shown in Figure 8.
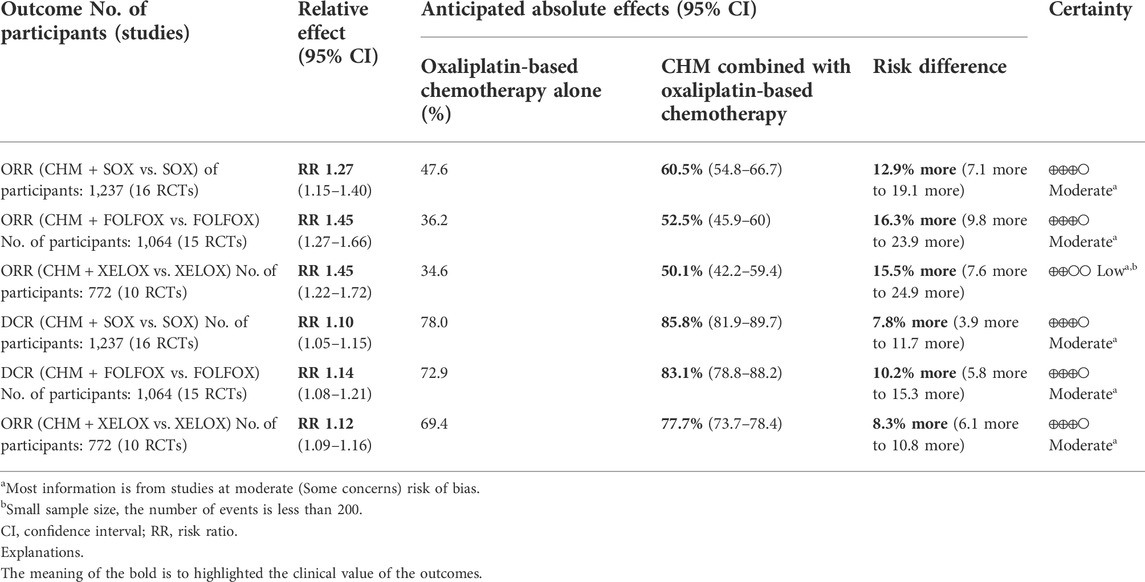
3.4 Subgroup analysis
We performed subgroup analysis of the outcomes of tumor-response, according to the different regimen of chemotherapy that patients received. Compare to SOX regimen alone, CHM combined with SOX regimen chemotherapy could increase the ORR by 27% [RR = 1.27, 95% CI (1.15, 1.40)], and DCR by 10% [RR = 1.10, 95% CI (1.05, 1.15)]. Compare to FOLFOX regimen alone, CHM combined with FOLFOX regimen chemotherapy could increase the ORR by 43% [RR = 1.43, 95% CI (1.26, 1.64)], and DCR by 14% [RR = 1.13, 95% CI (1.07, 1.20)]. Compare to XELOX regimen alone, CHM combined with XELOX regimen chemotherapy could increase the ORR by 27% [RR = 1.35, 95% CI (1.26, 1.45)], and DCR by 12% [RR = 1.12, 95% CI (1.09, 1.16)]. The details were shown in Figures 3, 4.
3.5 Sensitivity analysis
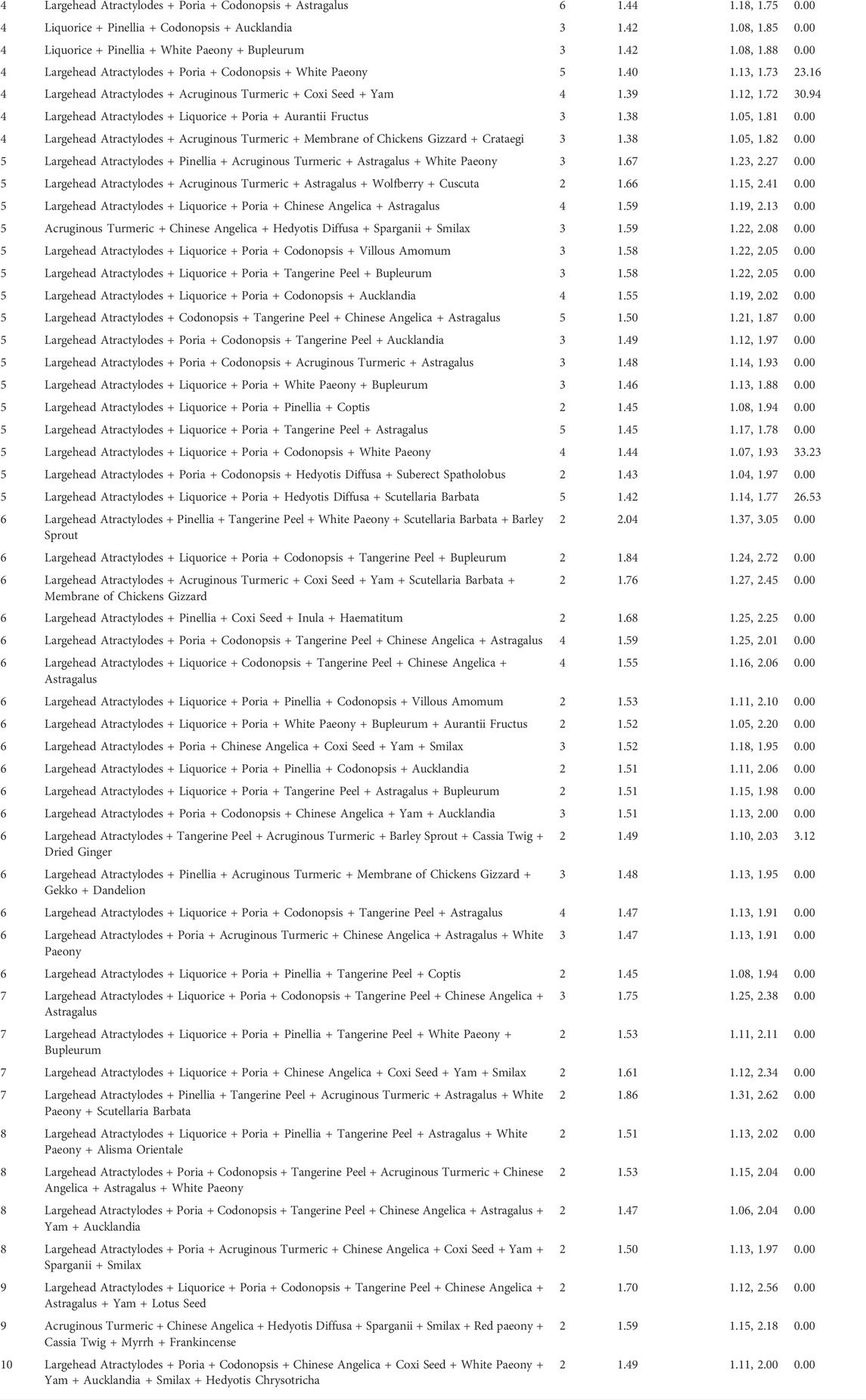
We performed sensitivity analysis to investigate the potential contributions of specific herbs to tumor response. Three trials which adopted multiple core prescriptions were excluded from sensitivity analysis, and we analyzed the composition of core prescriptions in other 37 trials that adopted single core prescription. There are 114 herbs in the formulas of included trials, 47 of these herbs were used only in one trial and were excluded from the sensitivity analysis. Among 67 herbs analyzed, 43 herbs were only used with other herbs as a combination, which means that when herb was used in several formulas, there was always an herb in the same formulas. RRs of the group of trials that included each specific herb or herb combination were calculated. Among 380 sensitivity analyses performed, 21 single herbs and 129 herb combinations were located, and 3 single herbs along with 227 herb combinations were excluded according to the predetermined principle.
3.5.1 Tier 1: Single herbs
The five most frequently used single herbs were Largehead Atractylodes (n = 26), Liquorice (n = 23), Poria (n = 21), Pinellia (n = 20), and Codonopsis Pilosula (n = 18). The single herbs with the five highest RR were Dandelion [RR = 1.47, 95% CI (1.17, 1.83), n = 5], Paris Polyphylla [RR = 1.47, 95% CI (1.07, 2.02), n = 3], Red Paeony [RR = 1.46, 95% CI (1.13, 1.88) n = 4], Chinese Angelica [RR = 1.44, 95% CI (1.27, 1.64), n = 14], and Astragalus [RR = 1.43, 95% CI (1.27, 1.64), n = 13]. Other details of the contributions of single herbs were shown in Table 4.
3.5.2 Tier 2: Combination of 2 herbs
The three most frequently used combination of 2 herbs were: Liquorice + Poria (n = 17), Largehead Atractylodes + White Paeony (n = 9), and Largehead Atractylodes + Yam (n = 9). The combination of 2 herbs with the five highest RR were Codonopsis Pilosula + Villous Amomum [RR = 1.70, 95% CI (1.34, 2.16), n = 4], Cornus + Ginseng [RR = 1.56, 95% CI (1.16,2.10), n = 2], Acruginous Turmeric + Astragalus [RR = 1.55, 95% CI (1.28, 1.88), n = 7], Chinese Angelica + Astragalus [RR = 1.55, 95% CI (1.28, 1.89), n = 7], and Chinese Angelica + Smilax [RR = 1.55, 95% CI (1.27, 1.88), n = 5]. Other sensitivity analysis of combination of 2 herbs were shown in Table 4.
3.5.3 Tier 3: Combination of 3 herbs
The combination of 3 herbs with the five highest RR were Chinese Angelica + Astragalus + Villous Amomum [RR = 1.73, 95% CI (1.26, 2.36), n = 3], Largehead Atractylodes + Wolfberry + Psoralea [RR = 1.65, 95% CI (1.24, 2.19), n = 2], Codonopsis + Chinese Angelica + Astragalus [RR = 1.60, 95% CI (1.30, 1.97), n = 6], Liquorice + Codonopsis + Bupleurum [RR = 1.57, 95% CI (1.14, 2.15), n = 3], and Pinellia + White Paeony + Dried Ginger [RR = 1.56, 95% CI (1.10, 2.21), n = 2]. Other sensitivity analysis of combination of 3 herbs were shown in Table 4.
3.5.4 Tier 4: Combination of 4 herbs
Two combination of 4 herbs were used more than five times: Largehead Atractylodes + Poria + Tangerine Peel + Astragalus (n = 6), and Largehead Atractylodes + Poria + Codonopsis + Astragalus (n = 6). The combination of 3 herbs with the five highest RR were Codonopsis + Chinese Angelica + Astragalus + Villous Amomum [RR = 1.95, 95% CI (1.36, 2.78), n = 2], Largehead Atractylodes + Pinellia + Tangerine Peel + Dandelion [RR = 1.76, 95% CI (1.24, 2.52), n = 2], Acruginous Turmeric + Chinese Angelica + Astragalus + Villous Amomum [RR = 1.75, 95% CI (1.01, 3.03), n = 2], Liquorice + Pinellia + Tangerine Peel + Paris Polyphylla [RR = 1.74, 95% CI (1.18, 2.56), n = 2], and Largehead Atractylodes + Tangerine Peel + Astragalus + Yam [RR = 1.63, 95% CI (1.30, 2.05), n = 4]. Other sensitivity analyses were shown in Table 4.
3.5.5 Tier 5: Combination of 5 herbs
The three most frequently used combination of 5 herbs were: Largehead Atractylodes + Codonopsis + Tangerine Peel + Chinese Angelica + Astragalus (n = 5), Largehead Atractylodes + Liquorice + Poria + Tangerine Peel + Astragalus (n = 5), and Largehead Atractylodes + Liquorice + Poria + Hedyotis Diffusa + Scutellaria Barbata (n = 5). The combination of 5 herbs with the two highest RR were Largehead Atractylodes + Pinellia + Acruginous Turmeric + Astragalus + White Paeony [RR = 1.67, 95% CI (1.23, 2.27), n = 3] and Largehead Atractylodes + Acruginous Turmeric + Astragalus + Wolfberry + Cuscuta [RR = 1.66, 95% CI (1.15, 2.41), n = 2]. Other sensitivity analyses were shown in Table 4.
3.5.6 Tier 6: Combination of 6 herbs or above
The three most frequently used combination of 6 or more herbs were Largehead Atractylodes + Poria + Codonopsis + Tangerine Peel + Chinese Angelica + Astragalus (n = 4), Largehead Atractylodes + Liquorice + Codonopsis + Tangerine Peel + Chinese Angelica + Astragalus (n = 4), and Largehead Atractylodes + Liquorice + Poria + Codonopsis + Tangerine Peel + Astragalus (n = 4). Other combination of 6 or more herbs only used 2 or 3 times in the formulas. The combination of 6 or more herbs with the three highest RR were Largehead Atractylodes + Pinellia + Tangerine Peel + White Paeony + Scutellaria Barbata + Barley Sprout [RR = 2.04, 95% CI (1.37, 3.05), n = 2], Largehead Atractylodes + Pinellia + Tangerine Peel + Acruginous Turmeric + Astragalus + White Paeony + Scutellaria Barbata [RR = 1.86, 95% CI (1.31, 2.62), n = 2], and Largehead Atractylodes + Liquorice + Poria + Codonopsis + Tangerine Peel + Bupleurum [RR = 1.84, 95% CI (1.24, 2.72), n = 2]. Other sensitivity analyses were shown in Table 4.
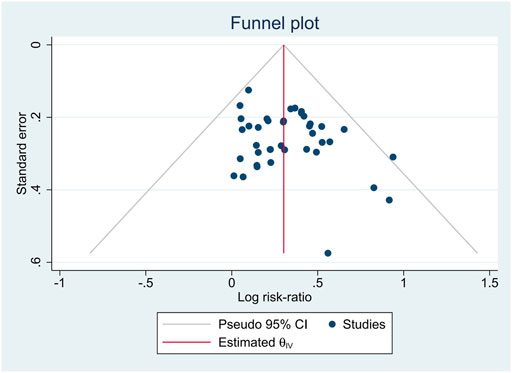
3.6 Publication bias
We assessed the publication bias with a funnel plot and Egger test. The funnel plot was shown in Figure 9A p-value = 0.1197 in Egger test indicated that no serious publication bias was observed.
3.7 Quality of evidence
We assessed the quality of synthesized evidence of tumor response with GRADE approach. The results in subgroup analysis were adopted, since the subgroup of different chemotherapy regimen minimize the clinical heterogeneity. We got moderate to low quality of evidence, and the reasons to downgrade were that the majority of the evidence was from the trials with moderate methodological quality, and small sample size. The summary of findings was shown in Table 5.
4 Discussion
4.1 Meta-analysis of trial results
Our study showed with a moderate certainty that compare with SOX, FOLFOX or XELOX regimen alone, CHM combined with SOX, FOLFOX or XELOX could significantly improve the ORR without heterogeneity, which has a practical guiding value for the clinical application of CHM synergistic chemotherapy regimen. In addition, we also found that although the relative effect of SOX regimen was lower than that of FOLFOX and XELOX, which was due to the higher anticipated absolute effects of SOX regimen alone than that of FOLFOX and XELOX, so the improvement was not particularly significant. When SOX combined with CHM, the anticipated absolute effects had increased to 60.5%, which was higher than about 50% of other regimens. The results of our study were consistent with another previously published meta-analysis of CHM combined with paclitaxel-based chemotherapy in the treatment of AGC (Li Y. et al., 2020). Compared with paclitaxel-based chemotherapy, TCM combined with paclitaxel-based chemotherapy could also significantly improve the ORR [RR = 1.39, 95% CI (1.24–1.57), I2 = 12%]. But the prescriptions of CHM included in the above studies are not consistent, which brings more uncertain factors to the comparison.
In reducing the incidence of AEs, CHM combined with oxaliplatin-based chemotherapy showed consistent efficacy in leukopenia, thrombocytopenia, nausea and vomiting with paclitaxel-based chemotherapy, but had more improvement advantages in anemia, gastrointestinal reactions, diarrhea, hepatorenal toxicity and peripheral neurotoxicity. It is worth noting that both paclitaxel-based and oxaliplatin-based chemotherapy are clinically useful chemotherapy regimens that easily lead to peripheral neurotoxicities (Yamashita et al., 2021). However, inconsistent clinical outcomes occurred between these regimens. CHM prescriptions in our study were effective in reducing the incidence of peripheral neurotoxicity, indicating that different herbal combinations may have different effects in reducing the incidence of AEs. Therefore, it is important to excavate the effects of herbs and their combinations which may affect the efficacy to guide clinical application. In addition, this study also found that the interventions of CHM was safe in AGC patients, and the participation of CHM did not increase the occurrence of AEs.
Furthermore, in order to explore the effect of CHM on the survival time of AGC, we reviewed the following studies and hope that will provide a useful reference, which is also the focus for future studies. A cohort study that 1:1 matched in Taiwan found that CHM could improve the OS rate of GC and reduce the risk of death by 45% (Hung et al., 2017). Afterward, using propensity score matching according to 1:3 also found that regardless of short-term or long-term application of TCM, compared with non-users, it could effectively reduce the risk of death by 41% or 59%, and pointed out that the most frequently used single herbal medicine was Astragalus (11.8%), and the most frequently used formula was Xiangsha Liujunzi Decoction (15.5%) [Codonopsis pilosula, Largehead Atractylodes, Poria, Liquorice, Muxiang, Amomum villosum] (Shih et al., 2021). Interestingly, these single herbs or formulas were generally consistent with the listed herbs in our study. In addition, a meta-analysis evaluating the efficacy of Astragalus-containing TCM combined with platinum-based chemotherapy for AGC suggested that compare with platinum-based chemotherapy alone, chemotherapy combined with Astragalus-containing TCM could improve ORR, DCR, 1 or 2-year survival rate, QoL and lower incidence of AEs (Cheng et al., 2021). These above studies strongly corroborated the credibility of the herbal compositions obtained in this study.
4.2 Analysis of TCM contribution
It is well-known that CHM formulas were prescriptions composed of varieties of herbs, which plays a complex and multiple effect role in clinical efficacy. Based on the characteristics of syndrome differentiation treatment, the CHM prescriptions issued by different physicians for the same disease may be different from others. Similarly, this unique treatment characteristic is significantly highlighted in this study: the specific herbs of interventions is inconsistent in clinical studies of different CHM treatments for AGC. It should be emphasized that the same herbs may be used in different studies, which provides an important basis for findings the special contribution of different herbs.
However, these herbs are not only focused on improving ORR, and perhaps some herbs pay more attention to the reduction of the occurrence of AEs to chemotherapy, or the improvement of QoL. Therefore, to eliminate the interference of other herbs, we applied sensitivity analysis to explore the effect of individual herbs on ORR in AGC one by one firstly. Then analyze the multi-herb combination containing the same herbs to further evaluate which specific herbs and their combinations are most likely to improve the ORR of chemotherapy for AGC.
Inferred to previous studies (Chen et al., 2016a; Chen et al., 2016b; Chen MH. et al., 2016), to analyze the results more cautiously, our selected herbs and compositions must have more significant differences, no heterogeneity, and equal or higher RRs within each level. Based on the above criteria, from the results of multiple sensitivity analyses, we identified seven herbs as potential effect of synergistic action with chemotherapy for AGC: Astragalus (n = 13), Liquorice (n = 23), Poria (n = 21), Largehead Atractylodes (n = 26), Chinese Angelica (n = 14), Codonopsis (n = 18), and Tangerine Peel (n = 17). Among them, Dandelion and Paris Polyphylla possessed the highest effect estimate (RR = 1.47) in individual herb analysis. In addition, this study also analyzed the combination of herbs. It is reported that the composition of Codonopsis and Liquorice, and the composition of Codonopsis, Liquorice, Largehead Atractylodes and Poria both significantly improved the ORR of TCM combined with paclitaxel-based chemotherapy, which also provided supporting evidence for our study (Li Y. et al., 2020).
Surprisingly, some relatively high-frequency herbs such as Pinellia (n = 20), Acruginous Turmeric (n = 15), or some herbs with relatively high RR such as Dandelion [1.47 (1.17, 1.83)], Paris Polyphylla [1.47 (1.07, 2.02)], and Red Paeony [1.46 (1.13, 1.88)] in the individual herb analysis were not included in the final combination. We found that compared to the ORR under multi-herb combinations, the above potential herbs may not show a consistent effect with other herbs under multi-herb combinations. Nevertheless, these herbs also have the potential to contribute to ORR, and we found that these individual herbs with relatively high RR were mostly combined with the above 7 herbs in multi-herb combinations showing higher ORR value. Therefore, these herbs can also be considered for subsequent development.
In addition, we also excluded some highly heterogeneous herbs, such as Panax notoginseng (I2 = 73.56%), although commonly used in AGC, which seems to significantly reduce ORR [1.71, (0.84, 3.48)], but only two studies were evaluated. Notably, no negative RRs emerged for any of the herbs or combinations, suggesting that these herbs did not impair the effects of chemotherapy.
4.3 Strength and limitations
To our knowledge, this is the first meta-analysis evaluating the efficacy and safety of CHM combined with oxaliplatin-based chemotherapy in AGC. Our study suggests that the synergistic treatment of CHM may be more TRR-improving on FOLFOX and XELOX regimens, and has high safety for clinical application. In addition, this study complied strict inclusion and exclusion criteria to exclude studies without a clear randomization method, which reduces the RoB in studies and improves the reliability of results. Therefore, quality of only one outcome was classified as low, and other qualities of evidence were moderate. There was also no serious publication bias.
At the same time, our study also has several limitations. Firstly, lacks multi-center, large-sample RCTs and some of the original study sample sizes are small, which causes bias in the results, which requires the publication of more high-quality clinical studies. Secondly, the included studies had an insufficient assessment of long-term survival indicators (OS, PFS), and our study showed that CHM could improve TRR in AGC, but whether it could translate into a benefit in long-term outcomes, also requires reassessment. In addition, CHMs are a part of TCM preparations, including Chinese patent medicines and TCM injections, and these preparation types should also be widely evaluated. Even if there have been studies on the anti-tumor efficacy of TCM preparations such as cinobufotalin (Sun et al., 2019) and brucea javanica oil injection (Wang et al., 2021) for AGC. A more comprehensive evaluation of CHM is needed to obtain more real, effective and safe evidence.
In addition, an obvious innovation point lies in exploring special herbs that have a synergistic action with chemotherapy on the ORR for AGC. The advantage of this methodology is based on the contribution of each herb to the contribution of ORR and is not simply based on the total frequency of herb emergence. A sensitivity analysis was chosen to identify the contribution of each herb in the study to ORR without missing it at a lower frequency. In summary, one cannot simply choose according to the overall frequency or the order of effect sizes of herbs in the dataset, but rather needs to consider the overall effect sizes at multiple levels simultaneously. For the selection process of final results, these herbs do not show improvement in ORR in only one clinical intervention study. In contrast, these herbs have shown consistent or higher effects in multiple studies and multiple combinations. Another limitation is that all possible combinations cannot be evaluated, and as the number of multi-herb combinations increases, the number of combinations with a significant difference and no heterogeneity is smaller, the number of corresponding clinical studies and their sample size is also reduced, and the interpretation of the results should be more cautious. And there is no clear clinical trial to evaluate the clinical efficacy of this formula, and the corresponding RCT should be carried out for evaluation in the future.
4.4 Review of anti-gastric cancer mechanisms of seven selected herbs
Similarly, we cannot clarify that the listed herbs are all for improving the efficacy of chemotherapy, however, seven herbs have been used for AGC treatment in clinical in China (Cao et al., 2009). Among them, four herbs constitute a typical formula in China, Sijunzi decoction, which has been shown to reduce the nuclear accumulation and DNA-binding activity of β-catenin, thereby repressing cell growth and inducing apoptosis in human GC MKN74 and MKN45 cells (Li et al., 2021). Network pharmacology and experimental validation suggested that Sijunzi decoction could inhibit tumor proliferation and angiogenesis by down-regulating the expression of VEGFA, iNOS, COX-2, and Bax/Bcl2 proteins in NCG-bearing mice with human gastric adenocarcinoma cell NUGC-4, regulate the PI3K/AKT pathway, and induce apoptosis (Ding et al., 2022). To further explore how seven herbs improve the short-term efficacy of platinum-based chemotherapy for AGC, we will review each herb to assess the mechanistic evidence of the specific anti-tumor activity. We found that the number of published mechanistic studies of these herbs was uneven, with Astragalus and Liquorice being more intensively studied.
4.4.1 Astragalus
Previous study has shown that Astragalus dose-dependently stimulates dendritic cells (DCs) to express Toll-like receptor 4 (TLR4), thereby enhancing cellular immune function, and inhibiting IκB-α protein expression and regulating NF-kB signaling pathways (Tian et al., 2014). Secondly, after co-culture with MKN45 in vitro, MTT showed that Astragalus could reduce cell viability and induce apoptosis, and significantly reduce the number of cells. In vitro experiments also confirmed that Astragalus could significantly inhibit tumor diameter and weight, with a tumor inhibition rate of 57.1%. Another study also found that the aqueous extract of Astragalus could mediate antigen-presenting cells (APCs) to stimulate CXCR5 + Tfh-like cells to highly express IL-21, enhance humoral immunity and regulate CD8 + T cell activity (Dong et al., 2021).
In addition, the pharmacologically active components of Astragalus include polysaccharides, saponins and flavonoids, of which Astragalus polysaccharide (APS) is the most widely studied (Ma et al., 2002). In one study evaluating the anti-tumor activity of APS combined with adriamycin in human GC cell SGC-7901 and SGC-7901/ADR, the results showed that APS reduced cell viability and enhanced the rate of apoptosis in a time-dosage dependent manner, up-regulated p-AMPK and caspase-3 expression levels, induced apoptosis through the AMPK pathway, and improved the sensitivity of GC cells to adriamycin, suggesting that APS can be used as a chemosensitizer (Song et al., 2020). This paper speculated that the effect may be related to the decreased expression of MDR1, and the overexpression of tumor suppressor genes such as SEMA3F, P21WAF1/CIP1 and FBXW7. Similarly, APS can also inhibit the expression of phosphorylated AKT (p-AKT) and MMP-9, and then inhibit the proliferation, migration and invasion of GC AGS cells through the AKT pathway, and also induce autophagy (Wu et al., 2018). In addition, APS also has a variety of immunoregulatory activities, such as regulating B lymphocytes and cytokines, especially inducing the activation and differentiation of splenic DCs, followed by the completion of Th2 to Th1 transition and enhancing the immune function of T lymphocytes (Liu et al., 2011).
4.4.2 Liquorice
A large number of bioactive components can be isolated from Liquorice, including triterpenoid saponins, flavonoids, isoflavones and chalcone (Asl et al., 2008). Glycyrrhizic acid (GA) is usually considered to be the main active component. The study has shown that GA can induce G1/S phase arrest of cell cycle and apoptosis by down-regulating the expression of cyclin D1, D2, D3, E1, E2, Bcl-2, survivin and p65, up-regulating the protein levels of Bax, PARP and pro-caspase-3, -8, -9. The proliferation of human GC cells (MGC-803, BGC-823, SGC-7901) has a time- and dose-dependent inhibitory effect by down-regulating PI3K/AKT signaling pathway (Wang et al., 2020).
Isoliquiritigenin (ISL) is a flavonoid extracted from Liquorice. Studies have shown that ISL can down-regulate Bcl-2 protein and p62, up-regulate Bax protein, caspase-3, Beclin 1 and LC3II/LC3I ratio, mediate apoptosis and autophagy of human GC MKN28 cells by inhibiting PI3K/AKT/mTOR signaling pathway, thereby inhibiting cell proliferation, and reducing invasion and migration (Zhang et al., 2018). ISL can also induce apoptosis in human GC MGC-803 cells by increasing free calcium concentration and decreasing mitochondrial transmembrane potential (Ma et al., 2001). However, licochalcone A (LCA), as a cytotoxic flavonoid, alone or in combination with 5-FU, increased the expression levels of Bax, PARP, tumor proteins 21, 27, 53 and caspase 3, and decreased the expression levels of Bcl-2, cyclin A, cyclin B and MDM2, which in turn blocked G 2/M cell cycle progression and induced apoptosis, inhibiting SGC7901 and MKN-45 cell proliferation in a time-dependent manner (Xiao et al., 2011; Lin et al., 2017). In addition, LCA can also increase the level of reactive oxygen species (ROS) and induce oxidative stress and apoptosis of BGC-823 cells through PI3K/AKT/mTOR and MAPK signaling cascades (Hao et al., 2015).
In addition, glycyrrhetinic acid (GA) can inhibit the viability of GC cells in a dose- and time-dependent manner, but its toxic and side effects limit its wide application, thus making many beneficial explorations on related derivatives (Xu et al., 2017). For example, 11-deoxy glycyrrhetinic acid can effectively inhibit GC formation by up-regulating p21 and down-regulating cdc2 and cyclin B1, mediating BID translocation from the nucleus to mitochondria, thereby inducing apoptosis and G2 phase arrest in GC cell (Lin et al., 2014). For another example, 18β-glycyrrhetinic acid (18β-GA) can inhibit MMP-2 and MMP-9 activities in a dose-dependent manner, up-regulate E-cadherin expression and down-regulate vimentin expression, and reduce the metastatic potential of human GC cell line SGC-7901 cells through ROS/PKC-α/ERK signaling pathway and inhibition of EMT (Cai et al., 2018). In addition, new isoliquiritigenin (ISL) analogues (Huang et al., 2020), licochalcone A derivatives (LCA) (Shibata, 1994) can also provide more options for anti-GC drug research and development.
4.4.3 Poria
In vitro studies confirmed that Poria combined with oxaliplatin significantly decreased Snail, Twist, vimentin, and N-cadherin mRNA and protein expression, significantly increased E-cadherin mRNA and protein expression, inhibited the EMT process, and decreased the invasion and migration of SGC7901 (Wang N. et al., 2018). This study also found that Poria could reduce the tumor volume, and improve the morphological parameters of GC cells. In addition, both dehydroebriconic acid and dehydrotrametenonic acid found from Poria sclerotia inhibited the growth of GC cells by arresting the G1 phase of the cell cycle, with LD (50) values of 63.6 and 38.4 microM, respectively (Mizushina et al., 2004).
4.4.4 Largehead Atractylodes
Atractylenolide (AT) is the main anticancer active ingredient of Largehead Atractylodes. Atractylenolide I (AT-I) has been found to inactivate the Notch signaling pathway by down-regulating the protein and mRNA expression of Notch1, Jagged1, Hes1, Hey1 and CD44, inhibiting the sphere formation ability and cell viability of HGC-27, MGC-803 and MKN-45, thereby inhibiting the self-renewal ability and cell proliferation of GC stem-like cells (GCSC) and inducing apoptosis (Ma et al., 2014). A randomized controlled trial also verified the therapeutic effect of AT-1 on GC cachexia (Liu et al., 2008). Similarly, for Atractylenolide II (AT-II), Bax expression could also be up-regulated, and B-cell lymphoma 2 (Bcl-2), phosphorylated protein kinase B (p-Akt), and phosphorylated ERK (p-ERK) expression could be down-regulated, which induced apoptosis and inhibited proliferation and migration of HGC-27 and AGS in a concentration- and time-dependent manner by inactivating Ras/ERK and PI3K/Akt signaling pathways (Tian et al., 2017). AT-1 and AT-2 also inhibit Akt/IκBα/NF-κB signaling pathway to play a role, thereby inhibiting gastritis to GC transformation (Amin et al., 2022). In addition, the aqueous extract of Largehead Atractylodes could inhibit the proliferation of BGC-823 and SGC-7901, decrease the mitochondrial transmembrane potential, and induce apoptosis and cell cycle arrest in a dose- and time-dependent manner (Zhao et al., 2014).
4.4.5 Chinese Angelica
Decursin is one of the active components of Chinese Angelica, which has been confirmed to down-regulate CXC chemokine receptor 7 (CXCR7) and Bcl-2 expression in a dose-dependent manner, mediate STAT3/c-Myc signaling pathway, induce apoptosis, and inhibit the proliferation, migration, and invasion of SNU484 and SNU216 (Kim et al., 2019). Another study by the same team also confirmed that Decursin could reduce cell viability, inhibited cell growth and induce G0/G1 arrest in vitro in a dose- and time-dependent manner (Kim et al., 2021). And by promoting the accumulation of LC3 and SQSTM1, inhibiting CTSC and E2F3 expression, and reducing the activity of lysosomal protein cathepsin C (CTSC), thereby inducing autophagic flux disorders. While in vivo studies, Decursin decreased the growth of tumor spheroids and patient-derived gastric organoids, and regulated the expression of CTSC and autophagy-related proteins, which in turn validated the in vivo experimental results.
In addition, a clinical data from Taiwan verified that Chinese Angelica could prolong the survival rate of GC patients [adjusted hazard ratio (HR) 0.72 [95% CI, 0.57–0.92] (p = 0.009)], and experimental studies also found that N-butylidenephthalide (BP), the active component of Chinese Angelica, could induce increased REDD1 expression, inhibit mTOR signaling, activate apoptosis through the mitochondrial pathway, and inhibit the proliferation and EMT of AGS, NCI-N87, and TSGH-9201 cells (Liao et al., 2018).
4.4.6 Codonopsis
Lobetyolin (LBT) and Lobetyol are the essential components of Codonopsis. The anti-GC activity of LBT mainly depends on the regulation of glutamine metabolism, the decrease of mRNA and protein expression of amino acid transporter alanine-serine-cysteine transporter 2 (ASCT2), the induction of apoptosis and the inhibition of GC cell proliferation (Bailly, 2021). Lobetyol induced apoptosis and G1/S phase cell cycle arrest in MKN45 cells by mediating MAPK signaling pathway in a time- and dose-dependent manner (Shen et al., 2016). Both can be further considered as potential anti-GC active ingredients. In addition, the Chinese medicine formula “Weikang Keli”, containing Codonopsis and Largehead Atractylodes, can induce autophagic cell death in SGC-7901 cels. In vitro experiments showed that compared with the positive control of 5-FU, “Weikang Keli” aqueous extract could reduce the tumor volume in the GC model. Although the difference was not statistically significant, the motility, response sensitivity and food intake were increased (Huo et al., 2013).
4.4.7 Tangerine Peel
Nobiletin (Nob), a poly methoxy flavonoid extracted from Tangerine Peel, has been shown to inhibit MMP-9 activity in a concentration-dependent manner and to significantly reduce the total weight and number of disseminated nodules in a SCID mouse model (0.07g vs. 0.78g, p = 0.0059; 7.5 vs. 69.3/body, p = 0.0001), thereby inhibiting the proliferation of TMK-1 cell with peritoneal disseminated nodule formation, which is beneficial in preventing GC metastasis (Minagawa et al., 2001). In addition, it could also up-regulate E-cadherin protein expression and down-regulate vimentin, fibronectin, MMP-9 protein and p-STAT3 expression in SGC-7901 cell, by inhibiting the STAT3 pathway, followed by inhibiting EMT (Yang et al., 2020).
In addition, Nob-induced apoptosis is mediated by activating ER stress, decreasing phosphorylated Akt and mTOR, and mediating protective autophagy, thereby inhibiting GC proliferation in SNU-16 cells (Moon et al., 2016). And it increased Bax/Bcl-2 ratio, caspase-3/9 proteolytic activation with degradation of poly (ADP-ribose) polymerase (PARP) protein, induced apoptosis and had a synergistic anticancer effect with 5-fluorouracil in p53-mutant SNU-16 cell (Moon et al., 2013).
5 Conclusion
Overall, CHM has a positive effect on improving oxaliplatin-based chemotherapy for AGC, which can improve short-term efficacy and reduce the incidence of AEs. In the sensitivity analysis of herbal medicines, it was found that herbal medicines such as Astragalus, Liquorice, Poria, Largehead Atractylodes, Chinese Angelica, Codonopsis, and Tangerine Peel had more advantages in increasing ORR. Previous mechanistic studies also confirmed their anti-GC activity, and the results of our study will provide beneficial evidence support for the combination therapy of CHM. Considering factors such as low quality of literature and insufficient sample size for clinical trials corresponding to shortlisted herbal medicines, quality of evidence was not high, definite conclusions cannot be drawn. More rigorously designed, large-sample, multi-center RCTs of herbal synergistic chemotherapy are needed in the future to better validate the action characteristics of CHM and its potential herbal medicines.
Author contributions
YT conceived this study. YT and HW registered the protocol and performed the search, screen, inclusion, and quality assessment of the included trials. HW and BX performed the evidence synthesis. YT, HW, and BX drafted the first version of this manuscript. XZ, GZ, YG, and TL provided critical revisions and edited the manuscript. JL and RG revised the manuscript. All authors read and approved the final manuscript for submission.
Funding
This work was supported by Scientific and technological innovation project of China Academy of Chinese Medical Sciences (grant number CI2021A01802).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SC declared a shared parent affifiliation with the authors BX, GZ, YG, and TL at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.977708/full#supplementary-material
References
Ajani, J. A., D'Amico, T. A., Almhanna, K., Bentrem, D. J., Chao, J., Das, P., et al. (2016). Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 10, 1286–1312. doi:10.6004/jnccn.2016.0137
Ajani, J. A., D'Amico, T. A., Bentrem, D. J., Chao, J., Cooke, D., Corvera, C., et al. (2022). Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 20, 167–192. doi:10.6004/jnccn.2022.0008
Ajani, J. A., Moiseyenko, V. M., Tjulandin, S., Majlis, A., Constenla, M., Boni, C., et al. (2007). Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: The V-325 study group. J. Clin. Oncol. 22, 3205–3209. doi:10.1200/JCO.2006.10.4968
Al-Batran, S. E., Hartmann, J. T., Probst, S., Schmalenberg, H., Hollerbach, S., Hofheinz, R., et al. (2008). Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the arbeitsgemeinschaft internistische onkologie. J. Clin. Oncol. 9, 1435–1442. doi:10.1200/JCO.2007.13.9378
Amin, A., Hossen, M. J., Fu, X. Q., Chou, J. Y., Wu, J. Y., Wang, X. Q., et al. (2022). Inhibition of the Akt/NF-κB pathway is involved in the anti-gastritis effects of an ethanolic extract of the rhizome of Atractylodes macrocephala. J. Ethnopharmacol. 293, 115251. doi:10.1016/j.jep.2022.115251
Asl, M. N., and Hosseinzadeh, H. (2008). Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 6, 709–724. doi:10.1002/ptr.2362
Bailly, C. (2021). Anticancer properties of lobetyolin, an essential component of radix Codonopsis (dangshen). Nat. Prod. Bioprospect. 2, 143–153. doi:10.1007/s13659-020-00283-9
Bang, Y. J., Kang, Y. K., Catenacci, D. V., Muro, K., Fuchs, C. S., Geva, R., et al. (2019). Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: Results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer 4, 828–837. doi:10.1007/s10120-018-00909-5
Bao, P. Q. (2020). Effect of Yiqi Huoxue Recipe combined with SOX chemotherapy on serum tumor markers, T lymphocyte subsets and quality of life in patients with stage III-IV gastric cancer. Int. Med. Health Guid. 2, 241–243+248. doi:10.3760/cma.j.issn.1007-1245.2020.02.028
Cai, H., Chen, X., Zhang, J., and Wang, J. (2018). 18β-glycyrrhetinic acid inhibits migration and invasion of human gastric cancer cells via the ROS/PKC-α/ERK pathway. J. Nat. Med. 1, 252–259. doi:10.1007/s11418-017-1145-y
Cai, J. Y., Jin, Y., Lin, H., Gan, J. W., Yue, S. B., and Chen, Q. T. (2019). Efficacy of tumor response and quality of life of traditional Chinese medicine of strengthening spleen and reinforcing qi in the treatment of advanced gastric cancer. Mod. J. Integr. Traditional Chin. West. Med. 33, 3711–3714. doi:10.3969/j.issn.1008-8849.2019.33.016
Cao, W., and Zhao, A. G. (2009). Prescription rules of Chinese herbal medicines in treatment of gastric cancer. Zhong Xi Yi Jie He Xue Bao 1, 1–8. doi:10.3736/jcim20090101
Chen, K., Wang, Q. C., Yin, H., Duan, W. M., and Gao, Y. (2007). Clinical observation of chemotherapy combined with TCM syndrome differentiation treatment in advanced gastric cancer. Chin. J. Clin. Healthc. 3, 275–277. doi:10.3969/j.issn.1672-6790.2007.03.021
Chen, M., May, B. H., Zhou, I. W., Sze, D. M., Xue, C. C., and Zhang, A. L. (2016a). Oxaliplatin-based chemotherapy combined with traditional medicines for neutropenia in colorectal cancer: A meta-analysis of the contributions of specific plants. Crit. Rev. Oncol. Hematol. 105, 18–34. doi:10.1016/j.critrevonc.2016.07.002
Chen, M., May, B. H., Zhou, I. W., Xue, C. C., and Zhang, A. L. (2016b). Meta-analysis of oxaliplatin-based chemotherapy combined with traditional medicines for colorectal cancer: Contributions of specific plants to tumor response. Integr. Cancer Ther. 1, 40–59. doi:10.1177/1534735415596424
Chen, M. H., May, B. H., Zhou, I. W., Zhang, A. L., and Xue, C. C. (2016c). Integrative medicine for relief of nausea and vomiting in the treatment of colorectal cancer using oxaliplatin-based chemotherapy: A systematic review and meta-analysis. Phytother. Res. 5, 741–753. doi:10.1002/ptr.5586
Cheng, M. Q., Hu, J. Q., Zhao, Y., Jiang, W. J. L., Qi, R. Z., Chen, S. T., et al. (2021). Efficacy and safety of astragalus-containing traditional Chinese medicine combined with platinum-based chemotherapy in advanced gastric cancer: A systematic review and meta-analysis. Front. Oncol. 11, 632168. doi:10.3389/fonc.2021.632168
Chi, H. C., Zhao, W. S., Yang, Z., Hu, F. S., and Zhang, Q. (2010). Effective observation on treating advanced gastric cancer by Yiqi Huoxue plus FOLFOX4. Clin. J. Chin. Med. 11, 77–79. doi:10.3969/j.issn.1674-7860.2010.11.047
Chu, R. G., Guo, H. F., Xie, H., Wu, L., Wu, H. Y., Shi, M., et al. (2017). Effect of zhangshi yiwei decoction on T-lymphocyte subsets of advanced gastric cancer. Guangming J. Chin. Med. 4, 499–502. doi:10.3969/j.issn.1003-8914.2017.04.018
Cunningham, D., Starling, N., Rao, S., Iveson, T., Nicolson, M., Coxon, F., et al. (2008). Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 1, 36–46. doi:10.1056/NEJMoa073149
Diao, Z., Wang, C., Liu, X. C., and Tang, Y. H. (2018). The clinic effects of Shenlian capsule, trastuzumab combined with SOX regimen in the treatment for HER-2 positive advanced gastric carcinoma. Int. J. Trad. Chin. Med. 11, 1020–1024. doi:10.13288/j.11-2166/r.2015.02.009
Digklia, A., and Wagner, A. D. (2016). Advanced gastric cancer: Current treatment landscape and future perspectives. World J. Gastroenterol. 8, 2403–2414. doi:10.3748/wjg.v22.i8.2403
Ding, P., Guo, Y., Wang, C., Chen, J., Guo, C., Liu, H., et al. (2022). A network pharmacology approach for uncovering the antitumor effects and potential mechanisms of the Sijunzi decoction for the treatment of gastric cancer. Evid. Based. Complement. Altern. Med. 2022, 9364313. doi:10.1155/2022/9364313
Dong, Q., Pu, J., Du, T., Xu, S., Liu, W., Liu, L., et al. (2021). Astragalus-mediated stimulation on antigen-presenting cells could result in higher IL-21 production from CXCR5+ Tfh-like cells and better IL-21-mediated effector functions. Hum. Immunol. 6, 429–437. doi:10.1016/j.humimm.2021.03.012
Egger, M., Smith, G. D., Schneider, M., and Minder, C. (1997). Bias in meta analysis detected by a simple, graphical test. BMJ 315, 629–634. doi:10.1136/bmj.315.7109.629
Fan, J. L., Li, D. F., Jiao, J., Tang, J. Y., Hou, F. F., and Hu, Y. (2015). Effect of combined treatment of Weifu formula and chemotherapy on the quality of life in advanced gastric carcinoma patients with spleen deficiency and toxin accumulation syndrome. J. Tradit. Chin. Med. 2, 120–123. doi:10.13288/j.11-2166/r.2015.02.009
Feng, T. M., Wang, E. Q., and Wu, D. P. (2021). Effects of modified xuezhen decoction combined with western medicine on serum CEA and CA199 in patients with advanced gastric cancer. West. J. Traditional Chin. Med. 8, 126–129. doi:10.12174/j.issn.2096-9600.2021.08.29
Feng, Y. L. (2017). Clinical observation of gancao xiexin decoction combined with SOX chemotherapy in treating gastric adenocarcinoma (syndrome of dampness-heat due to spleen deficiency). Chendu: Chendu University of Traditional Chinese Medicine.
Gong, M. (2020). Efficacy and safety of wenyang sanjie decoction combined with chemotherapy in the treatment of advanced gastric adenocarcinoma. Chongqing Yixue S02, 248–250.
Gu, N., Wang, Z. X., and Li, Z. G. (2019). Clinical efficacy of chemotherapy combined with traditional Chinese medicine in advanced gastric cancer. Henan Med. Res. 18, 3383–3385. doi:10.3969/j.issn.1004-437X.2019.18.066
Guo, R. (2014). The clinical research of jianpi xiaoji decoction combined with S-1 combined oxaliplatin chemotherapy in the treatment of advanced middle-late gastric cancer. Zhengzhou: Zhengzhou University.
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). Grade: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 7650, 924–926. doi:10.1136/bmj.39489.470347.AD
Hao, W., Yuan, X., Yu, L., Gao, C., Sun, X., Wang, D., et al. (2015). Licochalcone A-induced human gastric cancer BGC-823 cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT signaling pathways. Sci. Rep. 5, 10336. doi:10.1038/srep10336
Hu, F. S. (2011). Summary of professor Yu rencun’s academic thought and clinical experience and clinical observation of yiqi huoxue Jiedu recipe combined with chemotherapy in the treatment of advanced gastric cancer. Beijing: Beijing University of Chinese Medicine.
Hu, F., Li, C., Yang, G., Yang, Z., Zhao, W., and Zhang, Q. (2012). Clinical observation of yiqi huoxue jiedu formula combined with chemotherapy on advanced gastric cancer. Int. J. Trad. Chin. Med. 34, 1024–1026. doi:10.3760/cma.j.issn.1673-4246.2012.11.023
Huang, F., Wang, J., Xu, Y., Zhang, Y., Xu, N., and Yin, L. (2020). Discovery of novel isoliquiritigenin analogue ISL-17 as a potential anti-gastric cancer agent. Biosci. Rep. 6, BSR20201199. doi:10.1042/BSR20201199
Huang, J., and Hu, K. (2017). Clinical observation of jianpi yangwei prescription combined with FOLFOX4 regimen for advanced gastric cancer. J. New Chin. Med. 4, 121–124. doi:10.13457/j.cnki.jncm.2017.04.043
Huang, J. Q. (2014). Clinical observation of jianpi huayu decoction combined with chemotherapy for advanced gastric cancer of spleen deficiency and phlegm stasis syndrome. Changsha: Hunan University of Chinese Medicine.
Hung, K. F., Hsu, C. P., Chiang, J. H., Lin, H. J., Kuo, Y. T., Sun, M. F., et al. (2017). Complementary Chinese herbal medicine therapy improves survival of patients with gastric cancer in taiwan: A nationwide retrospective matched-cohort study. J. Ethnopharmacol. 199, 168–174. doi:10.1016/j.jep.2017.02.004
Huo, J., Qin, F., Cai, X., Ju, J., Hu, C., Wang, Z., et al. (2013). Chinese medicine formula "Weikang Keli" induces autophagic cell death on human gastric cancer cell line SGC-7901. Phytomedicine. 2, 159–165. doi:10.1016/j.phymed.2012.10.001
J. P. T. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M. J. Pageet al. Editors (2019). Cochrane handbook for systematic reviews of interventions version 6.0 (updated july 2019) (London, United Kingdom: Cochrane). Available at: http://www.training.cochrane.org/handbook.
Jiang, F., Liu, X. F., Gao, Y. W., Sun, X. H., and Jiang, S. Q. (2021). Effect of Jiedu sanjie recipe combined with chemotherapy on patients with advanced gastric cancer and the effect of serum VEGF and VEGFR-1. Shaanxi J. Traditional Chin. Med. 5, 582–585. doi:10.3969/j.issn.1000-7369.2021.05.009
Jiao, J. Q. (2019). Application of dialectical addition and subtraction of xuezheng decoction combined with chemotherapy in patients with advanced gastric cancer and its effects on serum tumor markers and immune status. J. Sichuan Traditional Chin. Med. 12, 99–101.
Johnston, F. M., and Beckman, M. (2019). Updates on management of gastric cancer. Curr. Oncol. Rep. 21, 67. doi:10.1007/s11912-019-0820-4
Joshi, S. S., and Badgwell, B. D. (2021). Current treatment and recent progress in gastric cancer. Ca. Cancer J. Clin. 3, 264–279. doi:10.3322/caac.21657
Kang, Y. K., Kang, W. K., Shin, D. B., Chen, J., Xiong, J., Wang, J., et al. (2009). Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: A randomised phase III noninferiority trial. Ann. Oncol. 4, 666–673. doi:10.1093/annonc/mdn717
Kim, S., Kim, J. E., Kim, N., Joo, M., Lee, M. W., Jeon, H. J., et al. (2019). Decursin inhibits tumor growth, migration, and invasion in gastric cancer by down-regulating CXCR7 expression. Am. J. Cancer Res. 9, 2007–2018.
Kim, S., Lee, S. I., Kim, N., Joo, M., Lee, K. H., Lee, M. W., et al. (2021). Decursin inhibits cell growth and autophagic flux in gastric cancer via suppression of cathepsin C. Am. J. Cancer Res. 4, 1304–1320.
Koizumi, W., Narahara, H., Hara, T., Takagane, A., Akiya, T., Takagi, M., et al. (2008). S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet. Oncol. 3, 215–221. doi:10.1016/S1470-2045(08)70035-4
Leong, T. (2005). Chemotherapy and radiotherapy in the management of gastric cancer. Curr. Opin. Gastroenterol. 6, 673–678. doi:10.1097/01.mog.0000179833.28158.b7
Li, D. C., and Xiong, Y. J. (2016). Fuzheng kang'ai prescription combined with mFOLFOX4 chemotherapy in treatment of advanced gastric cancer. Acta Chin. Med. 9, 1253–1257. doi:10.16368/j.issn.1674-8999.2016.09.352
Li, D. H., Su, Y. F., Sun, C. X., and Fan, H. F. (2020). Effect of shunqi yiwei decoction combined with sox chemotherapy on advanced gastric cancer. Acta Medica Mediterr. 36, 2313. doi:10.19193/0393-6384_2020_4_360
Li, Y., Sui, X., Su, Z., Yu, C., Shi, X., Johnson, N. L., et al. (2020). Meta-analysis of paclitaxel-based chemotherapy combined with traditional Chinese medicines for gastric cancer treatment. Front. Pharmacol. 11, 132. doi:10.3389/fphar.2020.00132
Li, Y. J., Liao, L. L., Liu, P., Tang, P., Wang, H., and Peng, Q. H. (2021). Sijunzi decoction inhibits stemness by suppressing β-catenin transcriptional activity in gastric cancer cells. Chin. J. Integr. Med. 28, 702–710. doi:10.1007/s11655-021-3314-9
Liao, K. F., Chiu, T. L., Huang, S. Y., Hsieh, T. F., Chang, S. F., Ruan, J. W., et al. (2018). Anti-cancer effects of radix Angelica sinensis (danggui) and N-butylidenephthalide on gastric cancer: Implications for REDD1 activation and mTOR inhibition. Cell. Physiol. biochem. 6, 2231–2246. doi:10.1159/000492641
Lin, D., Zhong, W., Li, J., Zhang, B., Song, G., and Hu, T. (2014). Involvement of BID translocation in glycyrrhetinic acid and 11-deoxy glycyrrhetinic acid-induced attenuation of gastric cancer growth. Nutr. Cancer 3, 463–473. doi:10.1080/01635581.2013.877498
Lin, X., Tian, L., Wang, L., Li, W., Xu, Q., and Xiao, X. (2017). Antitumor effects and the underlying mechanism of licochalcone A combined with 5-fluorouracil in gastric cancer cells. Oncol. Lett. 3, 1695–1701. doi:10.3892/ol.2017.5614
Liu, L. F., Liu, Z. R., Cheng, Z. R., and Wang, C. X. (2015). Jianpi-Xiaozheng recipe combined with chemotherapy for late gastric cancer. Int. J. Traditional Chin. Med. 2, 126–129. doi:10.3760/cma.j.issn.1673-4246.2015.02.008
Liu, Q. Y., Yao, Y. M., Zhang, S. W., and Sheng, Z. Y. (2011). Astragalus polysaccharides regulate T cell-mediated immunity via CD11c(high)CD45RB(low) DCs in vitro. J. Ethnopharmacol. 3, 457–464. doi:10.1016/j.jep.2010.06.041
Liu, Y., Jia, Z., Dong, L., Wang, R., and Qiu, G. (2008). A randomized pilot study of atractylenolide I on gastric cancer cachexia patients. Evid. Based. Complement. Altern. Med. 3, 337–344. doi:10.1093/ecam/nem031
Liu, Z. M., Yang, X. L., Jiang, F., Pan, Y. C., and Zhang, L. (2020). Matrine involves in the progression of gastric cancer through inhibiting miR-93-5p and upregulating the expression of target gene AHNAK. J. Cell. Biochem. 3, 2467–2477. doi:10.1002/jcb.29469
Liu, Z. W., Wang, D. L., Zhao, X. F., and Zhao, P. (2019). Effect of jianpi huayu decoction on immune function and toxicity for patients with gastric cancer before and after chemotherapy. J. Sichuan Traditional Chin. Med. 10, 92–96.
Long, Y. J. (2021). Clinical observation of yiqi fuyuan paste combined with chemotherapy in the treatment of advanced gastric cancer of spleen qi deficiency syndrome. Changsha: Hunan University of Chinese Medicine.
Ma, J., Fu, N. Y., Pang, D. B., Wu, W. Y., and Xu, A. L. (2001). Apoptosis induced by isoliquiritigenin in human gastric cancer MGC-803 cells. Planta Med. 8, 754–757. doi:10.1055/s-2001-18361
Ma, L., Mao, R., Shen, K., Zheng, Y., Li, Y., Liu, J., et al. (2014). Atractylenolide I-mediated Notch pathway inhibition attenuates gastric cancer stem cell traits. Biochem. Biophys. Res. Commun. 1, 353–359. doi:10.1016/j.bbrc.2014.05.110
Ma, X. Q., Shi, Q., Duan, J. A., Dong, T. T., and Tsim, K. W. (2002). Chemical analysis of radix astragali (huangqi) in China: A comparison with its adulterants and seasonal variations. J. Agric. Food Chem. 17, 4861–4866. doi:10.1021/jf0202279
Minagawa, A., Otani, Y., Kubota, T., Wada, N., Furukawa, T., Kumai, K., et al. (2001). The citrus flavonoid, nobiletin, inhibits peritoneal dissemination of human gastric carcinoma in SCID mice. Jpn. J. Cancer Res. 12, 1322–1328. doi:10.1111/j.1349-7006.2001.tb02156.x
Mizushina, Y., Akihisa, T., Ukiya, M., Murakami, C., Kuriyama, I., Xu, X., et al. (2004). A novel DNA topoisomerase inhibitor: Dehydroebriconic acid, one of the lanostane-type triterpene acids from Poria cocos. Cancer Sci. 4, 354–360. doi:10.1111/j.1349-7006.2004.tb03215.x
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G.Prisma Group (2009). Preferred reporting Items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 7, e1000097. doi:10.1371/journal.pmed.1000097
Moon, J. Y., Cho, M., Ahn, K. S., and Cho, S. K. (2013). Nobiletin induces apoptosis and potentiates the effects of the anticancer drug 5-fluorouracil in p53-mutated SNU-16 human gastric cancer cells. Nutr. Cancer 2, 286–295. doi:10.1080/01635581.2013.756529
Moon, J. Y., and Cho, S. K. (2016). Nobiletin induces protective autophagy accompanied by ER-stress mediated apoptosis in human gastric cancer SNU-16 cells. Molecules 7, 914. doi:10.3390/molecules21070914
Peng, W. H., Tien, Y. C., Huang, C. Y., Huang, T. H., Liao, J. C., Kuo, C. L., et al. (2010). Fraxinus rhynchophylla ethanol extract attenuates carbon tetrachloride-induced liver fibrosis in rats via down-regulating the expressions of uPA, MMP-2, MMP-9 and TIMP-1. J. Ethnopharmacol. 3, 606–613. doi:10.1016/j.jep.2009.12.016
Qin, X. G. (2012). Clinical observation of buzhong yiqi decoction combined with SOX therapy on the patient with advanced gastric cancer. J. Pract. Traditional Chin. Intern. Med. 10, 39–41. doi:10.3969/j.issn.1671-7813.2012.10.24
Ren, L. Q., Li, Q., and Zhang, Y. (2020). Luteolin suppresses the proliferation of gastric cancer cells and acts in synergy with oxaliplatin. Biomed. Res. Int. 2020, 9396512. doi:10.1155/2020/9396512
Shen, J., Lu, X., Du, W., Zhou, J., Qiu, H., Chen, J., et al. (2016). Lobetyol activate MAPK pathways associated with G1/S cell cycle arrest and apoptosis in MKN45 cells in vitro and in vivo. Biomed. Pharmacother. 81, 120–127. doi:10.1016/j.biopha.2016.03.046
Shih, W. T., Yang, P. R., Shen, Y. C., Yang, Y. H., and Wu, C. Y. (2021). Traditional Chinese medicine enhances survival in patients with gastric cancer after surgery and adjuvant chemotherapy in taiwan: A nationwide matched cohort study. Evid. Based. Complement. Altern. Med. 2021, 7584631. doi:10.1155/2021/7584631
Shitara, K., Özgüroğlu, M., Bang, Y. J., Di Bartolomeo, M., Mandalà, M., Ryu, M. H., et al. (2018). Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 10142, 123–133. doi:10.1016/S0140-6736(18)31257-1
Smyth, E. C., Verheij, M., Allum, W., Cunningham, D., Cervantes, A., Arnold, D., et al. (2016). Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27 (5), v38–v49. doi:10.1093/annonc/mdw350
Song, J., Chen, Y., He, D., Tan, W., Lv, F., Liang, B., et al. (2020). Astragalus polysaccharide promotes adriamycin-induced apoptosis in gastric cancer cells. Cancer Manag. Res. 12, 2405–2414. doi:10.2147/CMAR.S237146
Song, Z., Wu, Y., Yang, J., Yang, D., and Fang, X. (2017). Progress in the treatment of advanced gastric cancer. Tumour Biol. 7, 1010428317714626. doi:10.1177/1010428317714626
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in RandomisedTrials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Sun, B., Zhou, L., Wang, X. L., Zhou, D. Y., and Li, Z. Q. (2020). Clinical study of self-made fuzheng kang'ai decoction combined with FOLFOX6 chemotherapy on symptoms, signs and quality of life for patients with stage III-IV gastric cancer. J. Sichuan Traditional Chin. Med. 12, 101–104.
Sun, H., Wang, W., Bai, M., and Liu, D. (2019). Cinobufotalin as an effective adjuvant therapy for advanced gastric cancer: A meta-analysis of randomized controlled trials. Onco. Targets. Ther. 12, 3139–3160. doi:10.2147/OTT.S196684
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 3, 209–249. doi:10.3322/caac.21660
Thuss-Patience, P. C., Shah, M. A., Ohtsu, A., Van Cutsem, E., Ajani, J. A., Castro, H., et al. (2017). Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet. Oncol. 5, 640–653. doi:10.1016/S1470-2045(17)30111-0
Tian, S., and Yu, H. (2017). Atractylenolide II inhibits proliferation, motility and induces apoptosis in human gastric carcinoma cell lines HGC-27 and AGS. Molecules 11, 1886. doi:10.3390/molecules22111886
Tian, Y., Li, X., Li, H., Lu, Q., Sun, G., and Chen, H. (2014). Astragalus mongholicus regulate the Toll-like-receptor 4 meditated signal transduction of dendritic cells to restrain stomach cancer cells. Afr. J. Tradit. Complement. Altern. Med. 3, 92–96. doi:10.4314/ajtcam.v11i3.13
Van Cutsem, E., Moiseyenko, V. M., Tjulandin, S., Majlis, A., Constenla, M., Boni, C., et al. (2006). Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 study group. J. Clin. Oncol. 31, 4991–4997. doi:10.1200/JCO.2006.06.8429
Van Cutsem, E., Valderrama, A., Bang, Y. J., Fuchs, C. S., Shitara, K., Janjigian, Y. Y., et al. (2021). Quality of life with first-line pembrolizumab for PD-L1-positive advanced gastric/gastroesophageal junction adenocarcinoma: Results from the randomised phase III KEYNOTE-062 study. ESMO Open 4, 100189. doi:10.1016/j.esmoop.2021.100189
Wagner, A. D., Grothe, W., Haerting, J., Kleber, G., Grothey, A., and Fleig, W. E. (2006). Chemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate data. J. Clin. Oncol. 18, 2903–2909. doi:10.1200/JCO.2005.05.0245
Wang, H., Ge, X., Qu, H., Wang, N., Zhou, J., Xu, W., et al. (2020). Glycyrrhizic acid inhibits proliferation of gastric cancer cells by inducing cell cycle arrest and apoptosis. Cancer Manag. Res. 12, 2853–2861. doi:10.2147/CMAR.S244481
Wang, N., Liu, D., Guo, J., Sun, Y., Guo, T., and Zhu, X. (2018). Molecular mechanism of Poria cocos combined with oxaliplatin on the inhibition of epithelial-mesenchymal transition in gastric cancer cells. Biomed. Pharmacother. 102, 865–873. doi:10.1016/j.biopha.2018.03.134
Wang, X., Wang, H., Cao, L., Wu, J., Lu, T., Li, S., et al. (2021). Efficacy and safety of brucea javanica oil emulsion injection in the treatment of gastric cancer: A systematic review and meta-analysis. Front. Nutr. 8, 784164. doi:10.3389/fnut.2021.784164
Wang, Y., Li, Z. P., Qian, W. T., Jia, Z. J., Li, W. B., He, Y. M., et al. (2018). Treated HER2 negative advanced metastatic gastric cancer by combining guishao Liujunzi decoction with SOX regimen. J. Anhui Sci. Technol. Univ. 5, 48–52.
Wu, J., Yu, J., Wang, J., Zhang, C., Shang, K., Yao, X., et al. (2018). Astragalus polysaccharide enhanced antitumor effects of Apatinib in gastric cancer AGS cells by inhibiting AKT signalling pathway. Biomed. Pharmacother. 100, 176–183. doi:10.1016/j.biopha.2018.01.140
Xiang, S., Zhao, Z., Zhang, T., Zhang, B., Meng, M., Cao, Z., et al. (2020). Triptonide effectively suppresses gastric tumor growth and metastasis through inhibition of the oncogenic Notch1 and NF-κB signaling pathways. Toxicol. Appl. Pharmacol. 388, 114870. doi:10.1016/j.taap.2019.114870
Xiao, X. Y., Hao, M., Yang, X. Y., Ba, Q., Li, M., Ni, S. J., et al. (2011). Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett. 1, 69–75. doi:10.1016/j.canlet.2010.12.016
Xie, W. S. (2019). The clinical observation of wendan decoction combined with chemotherapy in the treatment of advanced gastric cancer with the type of phlegm-damp coagulation. Taiyuan: Shanxi University of Chinese Medicine.
Xu, B., Wu, G. R., Zhang, X. Y., Yan, M. M., Zhao, R., Xue, N. N., et al. (2017). An overview of structurally modified glycyrrhetinic acid derivatives as antitumor agents. Molecules 6, 924. doi:10.3390/molecules22060924
Yamashita, K., Hosoda, K., Niihara, M., and Hiki, N. (2021). History and emerging trends in chemotherapy for gastric cancer. Ann. Gastroenterol. Surg. 4, 446–456. doi:10.1002/ags3.12439
Yang, X. Z., Zhang, H., Cui, X. Y., Su, P. Z., and Li, Z. T. (2020). Study on the mechanism of nobiletin inhibiting the invasiveness of SGC-7901 gastric cancer cells. Mod. Oncol. 18, 3099–3104. doi:10.3969/j.issn.1672-4992.2020.18.001
Yang, Y., Ma, J., and Zhang, H. Y. (2018). Clinical study on 40 cases of advanced gastric cancer treated with shenyu yangwei decoction combined with capeox chemotherapy. Jiangsu J. Traditional Chin. Med. 4, 40–43. doi:10.3969/j.issn.1672-397X.2018.04.017
Yoshino, T., Arnold, D., Taniguchi, H., Pentheroudakis, G., Yamazaki, K., Xu, R. H., et al. (2018). Pan-asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 1, 44–70. doi:10.1093/annonc/mdx738
Yu, D., Huang, L. P., Shao, L. L., and Tian, Y. Z. (2019). Effect of shengyang yiwei decoction combined with chemotherapy on advanced gastric cancer and its effect on immune function. Chin. J. Traditional Med. Sci. Technol. 4, 556–557.
Yuan, K. M. (2011). The clinical research of effect of WD-3 combined with XELOX scheme on the quality of life in treating patients with Ⅳ period gastric carcinoma. Nanjing: Nanjing University of Chinese Medicine.
Yuan, M. (2018). Clinical observation of YiQi Jiedu decoction combined with chemotherapy for gastric cancer in stage ⅢB-Ⅳ. Zhengzhou: Henan University of Chinese Medicine.
Zhai, Y. R. (2019). Clinical observation on treatment of advanced GastricCancer (syndrome of intermin-gled phlegm and bloodstasis) by huayu Jiedu method. Zhengzhou: Henan University of Chinese Medicine.
Zhang, H. O., Chen, W. J., Yang, S. W., Huang, H. Q., Huang, M. H., Tang, S. H., et al. (2021). Evaluation of curative effect of traditional Chinese medicine treatment for advanced gastric cancer. Fujian J. Traditional Chin. Med. 11, 15–17. doi:10.3969/j.issn.1000-338X.2021.11.004
Zhang, H. (2021b2021). The clinical observation of all nourishing decoction combined with chemotherapy in the treatment of advanced gastric cancer with qi blood deficiency and stasis syndrome. Fuzhou: Fujian University of Traditional Chinese Medicine.
Zhang, H. W. (2021a2021). Pei's shugan yangwei decoction combined with SOX regimen chemotherapy under treatment clinical observation of advanced gastric cancer. Lanzhou: Gansu University of Chinese Medicine.
Zhang, L., Xiao, Y., Yang, R., Wang, S., Ma, S., Liu, J., et al. (2021). Systems pharmacology to reveal multi-scale mechanisms of traditional Chinese medicine for gastric cancer. Sci. Rep. 1, 22149. doi:10.1038/s41598-021-01535-5
Zhang, L. H., Zhao, C. J., Wu, H., Dang, Y. Y., and Zheng, J. (2020). Exploration on the effect of shengyang yiwei decoction plus reduction in chemotherapy for advanced gastric cancer patients with wet resistance syndrome of Yang deficiency. World Chin. Med. 24, 3792–3796. doi:10.3969/j.issn.1673-7202.2020.24.012
Zhang, X. R., Wang, S. Y., Sun, W., and Wei, C. (2018). Isoliquiritigenin inhibits proliferation and metastasis of MKN28 gastric cancer cells by suppressing the PI3K/AKT/mTOR signaling pathway. Mol. Med. Rep. 3, 3429–3436. doi:10.3892/mmr.2018.9318
Zhang, Z. P. (2019). Clinical observation of erteng sanjie capsule combined with SOX regimen in the treatment of advanced gastric cancer with spleen deficiency and phlegm stasis syndrome. Taiyuan: Shanxi Provincial Institute of Tradtional Chinese Medicine.
Zhao, H. (2011). Experimental research on effect of kunshen granule to antiangiogenesis factors and clinical research on it treating advanced gastric cancer. Jinan: Shandong University of Traditional Chinese Medicine.
Zhao, M., Wang, Q., Ouyang, Z., Han, B., Wang, W., Wei, Y., et al. (2014). Selective fraction of Atractylodes lancea (Thunb.) DC. and its growth inhibitory effect on human gastric cancer cells. Cytotechnology 2, 201–208. doi:10.1007/s10616-013-9559-1
Zhao, X. N., and Xue, W. H. (2016). Effect of jianpi huayu decoction combined with chemotherapy on advanced gastric cancer and its impact on quality of life. Acta Chin. Med. Pharmacol. 3, 105–108. doi:10.19664/j.cnki.1002-2392.2016.03.034
Zhao, Y. Y. (2021). Clinical efficacy of wenyang jianpi decoction supplemented with SOX regimen in the treatment of patients with advanced gastric cancer. Acta Med. Sin. 4, 63–67. doi:10.19296/j.cnki.1008-2409.2021-04-016
Zhong, X. S., Shen, G. Z., Qin, H. H., Liang, W. J., and Zhou, Z. L. (2021). Clinical effect of weijixiao combined with chemotherapy in advanced gastric cancer and its effect on MDSCs, treg cells and immune factors. Mod. J. Integr. Traditional Chin. West. Med. 9, 974–978. doi:10.3969/j.issn.1008-8849.2021.09.015
Zhong, Z. J., Hu, C., and Liu, J. Y. (2019). Efficacy of shenling baizhu san plus FOLFOX4 on advanced gastric cancer. Clin. J. Chin. Med. 31, 84–86. doi:10.3969/j.issn.1674-7860.2019.31.029
Zhou, X., Wang, W., Li, P., Zheng, Z., Tu, Y., Zhang, Y., et al. (2016). Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in inducing gastric cancer cell apoptosis both in vitro and in vivo. Oncol. Res. 1-2, 29–34. doi:10.3727/096504015X14452563486011
Keywords: advanced gastric cancer, Chinese herbal medicine, oxaliplatin, meta-analysis, efficacy, tumor response, synergistic action
Citation: Tan Y, Wang H, Xu B, Zhang X, Zhu G, Ge Y, Lu T, Gao R and Li J (2022) Chinese herbal medicine combined with oxaliplatin-based chemotherapy for advanced gastric cancer: A systematic review and meta-analysis of contributions of specific medicinal materials to tumor response. Front. Pharmacol. 13:977708. doi: 10.3389/fphar.2022.977708
Received: 24 June 2022; Accepted: 22 July 2022;
Published: 25 August 2022.
Edited by:
Aftab Ullah, Jiangsu University, ChinaReviewed by:
Shuntai Chen, Beijing University of Chinese Medicine, ChinaHuanli Xu, Capital Medical University, China
Copyright © 2022 Tan, Wang, Xu, Zhang, Zhu, Ge, Lu, Gao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Li, qfm2020jieli@yeah.net
†These authors have contributed equally to this work
 Ying Tan
Ying Tan Heping Wang
Heping Wang Bowen Xu
Bowen Xu Xiaoxiao Zhang
Xiaoxiao Zhang Guanghui Zhu
Guanghui Zhu Yuansha Ge
Yuansha Ge Taicheng Lu
Taicheng Lu Ruike Gao
Ruike Gao Jie Li
Jie Li