- School of Pharmaceutical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
Actinidia eriantha Benth. (Called Maohuamihoutao in China) is a plant that has been utilized as a heat-clearing drug in She ethnic minority group for a long time in China. Specifically, it has been involved in the treatment of stomach cancer, colon cancer, cirrhosis with ascites, chronic hepatitis, leukemia, rectal prolapse, hernia and uterine prolapse. Pharmacological research provides partial evidence for the traditional use of A. eriantha and might have demonstrated the folk utilization of A. eriantha to combat many cancers. Crude extracts and relatively pure components of A. eriantha possess a variety of pharmacological activities, including anti-cancer, immunoregulatory, anti-angiogenic, neuroprotective, anti-inflammatory, and antioxidant activities. In addition, over 104 chemical substances have been determined from A. eriantha, involving terpenoids, alcohols, phenolics, aldehydes, organic acids, flavonoids glycosides, ketones, and glucoside. The existing literature reveals that a large proportion of the therapeutic effects of A. eriantha were rendered by the polysaccharides. However, the mechanisms of action and the structure-function correlations of these compounds, as well as the synergistic and antagonistic effects between them, need to be investigated further. Therefore, we propose that future studies on A. eriantha should focus on comprehensively assessing its medicinal quality, exploring its multi-target nature using network pharmacology approaches, and evaluating its long-term toxicity and efficacy in vivo.
Introduction
The genus Actinidia (Actinidiaceae) includes more than fifty-two perennial herbal species broadly distributed all over China (Flora of China Editorial Committee, 2015). Among them, Actinidia eriantha Benth. (AE) is a liana species commonly found in regions in the temperate zone (Xu et al., 2009a). The roots of AE (Actinidiaceae) have been utilized to treat various cancers, such as gastric cancer, mastocarcinoma, esophagus cancer, cervical carcinoma, nasopharyngeal cancer, and prostatic cancer, as well as cirrhosis with ascites, chronic hepatitis etc. in traditional Chinese medicine (Yu et al., 2017; Chen et al., 2019a). The AE roots are shown to have many biological activities, including anti-tumor (Xu et al., 2009b), immunoregulatory (Xu et al., 2009b), anti-angiogenic (Wu et al., 2017), neuroprotective (Cho et al., 2021), anti-inflammatory (Kim et al., 2018) and antioxidant (Huang et al., 2020) effects. Phytochemical investigations exhibited that AE contains a rich phytochemical like terpenoids, alcohols, phenolics, aldehydes, organic acids, flavonoids glycosides, ketones, glucoside. However, only a few of these compounds have been subjected to bioactivity studies, and their corresponding structures have not been adequately summarized and comprehensively presented in other publications. Although AE has showed its efficacy against cancers, its extracts contain chemical substances with so far undefined toxicity and biosafety. Moreover, both quality control research and rigorous pharmacological assessments of the associations between the extracts and the traditional utilization of AE are lacking.
We herein consistently organize the unsorted information on botanical characteristics, traditional uses, phytochemical properties, pharmacological activities, probable molecular mechanisms, quality control and biosafety of AE. Our paper displays phytochemical and pharmacological research on AE to testify its traditional application in the treatment of diseases. The information summarized in this paper will guide the design of future in vivo and clinical studies on AE and the development of new medicine containing AE or its active components.
Materials and methods
Research available on AE was retrieved from electronic databases of Baidu Scholar, Google Scholar, SciFinder, PubMed, ScienceDirect, Web of Science, and Springer using the keywords of “Actinidia eriantha,” “biological activity,” “phytochemistry,” “pharmacology,” “secondary metabolites,” “medicinal uses,” “safety,” “quality control,” “ethnobotanical survey,” “toxicology,” and related terms. Papers published on scientific journals, Chinese herbal medicine books and magazines, as well as thesises, were obtained. We utilized The Plant List (www.theplantlist.org) database to verify the nomenclature and acquire information on AE subspecies.
Botanical characteristics
Actinidia eriantha (Figure 1) is a traditional medicine of She minority in China (Yu et al., 2017). As per “The Plant List” (www.theplantlist.org) database, Actinidia eriantha Benth. is the plant’s most accepted name. Other five synonyms for the plant are Actinidia davidii Franch, Actinidia lanata Hemsl., Actinidia eriantha f. alba C. F. Gan, Actinidia eriantha var. brunnea C. F. Liang and Actinidia eriantha var. calvescens C. F. Liang (www.theplantlist.org). AE is distributed in at least 13 provinces in China, with the main distribution area being the Yangtze River basin and high plant abundances being observed in Fujian, Zhejiang, and Jiangxi Provinces.

FIGURE 1. The whole plant (A), flower portion (B), and commercial herbal root pieces (C) of A. eriantha.
AE grows primarily at streamside or in forests at low altitudes (i.e., 200–1,200 m above sea level) but is also found at relative high altitudes (i.e., 1800–2000 m above sea level) on the Yunnan-Guizhou Plateau. AE has no specific requirements for soil conditions and can tolerate acidic soils with a pH value of 4.5–5.5. An average annual temperature of 9.2°C–17.4°C supports the normal growth and development of AE plants. However, the species can also tolerate harsh temperature conditions, for instance, a high temperature of 42.6°C and a low temperature of −27.4°C. Wild AE plants generally climb tall trees. Current season shoots of AE are grayish green in color with dense white short hairs, which are even more conspicuous at the tip; older shoots of AE, with or without Sun light exposure, are reddish-brown or dark gray in color, respectively. Other characteristics of AE shoots include lamellar whitish pith, 6–10-cm-long internodes, and a 6–8-mm diameter. The leaves of AE are single, alternate and can be pointed or elliptical in shape; the upper surface of AE leaves is glabrous with a dark green color, while the lower surface is gray green in color with dense white fluff. AE plants possess fleshy fibrous roots, 85% of which are distributed in the 0–40-cm-deep soil zone, while some can extend to around 60 cm below the soil surface. Most of AE roots are distributed in the 20–80-cm zone around the tree trunk, while some can reach 150 cm away from the trunk (Liao et al., 2021). The roots for medicinal use were collected in the autumn. The roots soaked to a certain extent are taken out and then watered until the internal and external humidity of the medicinal materials is consistent, thick slices, dried, and used for medicinal purpose (Zhejiang Food and Drug Administration, 2015).
Phytochemistry
The phytochemical research on different AE organs, including root, leaf, and ripe fruit, has revealed the existence of various phytochemicals such as terpenoids, flavonoids glycoside, alcohols, aldehydes, ketones, organic acids, glucoside, and other compounds (Table 1). As far as the studies about the phytochemistry of AE are concerned, terpenoids that is the most deeply studied have been isolated and characterized from roots and aerial parts. What’s more, the phytochemicals extracted from root, the medicinal organ of AE, are even more worthy of further study. However, polysaccharides, the major type of bioactive compounds present in this herb (Guo et al., 2013), have not been isolated and still need further investigation. All in all, terpenoids and polysaccharides are the major biologically active constituents of AE.
Triterpenoids
Triterpenoids represent the main bioactive substances in AE. Totally, twenty-nine triterpenoids have been isolated from AE, including 2α, 3β, 24-trihydroxy-urs-12-en-28-oic acid 1 (Bai et al., 1997a), 2α,3α,24-trihydroxy-urs-12-en-28-oic acid 2 (Huang et al., 1986; Huang et al., 1988; Bai et al., 1997a; Zhang et al., 2017; Yu et al., 2020), 2β, 3β-dihydroxy-23-oxo-urs-12-en-28-oic acid 3 (Bai et al., 1997a), 2α,3α-dihydroxy-23-oxo-urs-12-en-28-oic acid 4 (Guo, 2013), 2α, 3β, 23-trihydroxy-urs-12-en-28-oic acid 5 (Bai et al., 1997b), 2α, 3β-dihydroxy-12-en-28-oic acid 6 (Bai et al., 1997b), 2α,3α-dihydroxy-23-methoxy-12-en-28-oic acid 7 (Guo, 2013), 3β,23,24-trihydroxyl-12-oleanen-28-oic acid 8 (Wu et al., 2017) β-sitosterol 9 (Huang et al., 1986; Huang et al., 1988; Bai et al., 1997a), daucosterol 10 (Huang et al., 1986; Huang et al., 1988; Bai et al., 1997a), etc. Triterpenoids are isolated from root and ripe fruits. However, the pharmacological effects of terpenoids are yet to be well defined. The chemical structures of the triterpenoids are displayed in Figure 2.
Alcohols
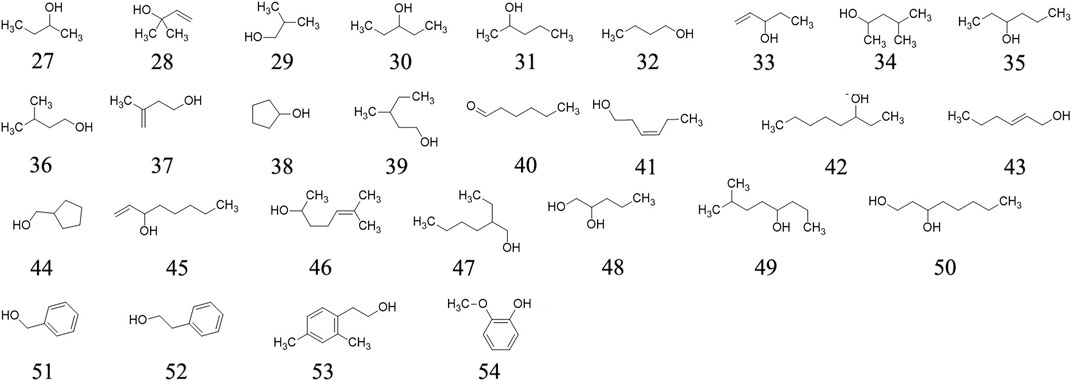
Twenty-four alcohols are determined in ripe fruits of AE plants through GC–MS, including 2-Butanol 27, 2-Methyl-3-buten-2-ol 28, Isobutanol 29, 3-Pentanol 30, 2-Pentanol 31, Butanol 32, 1-Penten-3-ol 33, 4-Methyl-2-pentanol 34, 3-Hexanol 35, Isoamyl alcohol 36, etc. (Garcia et al., 2012). These molecules’ chemical structures are presented in Figure 3.
Phenolics and aldehydes
Fifteen phenolics (methyl salicylate 55, phenol 56, p-Cresol 57, eugenol 58, 4-Vinylguaiacol 59, 4-Hydroxy-3-methylacetophenone 60, cis-Isoeugenol 61, (E)-Cinnamyl alcohol 62, trans-Isoeugenol 63, vanillin 64, homovanillic acid 65, p-Hydroxyphenethyl alcohol 66, 3,4,5-Trimethoxyphenol 67, coniferyl alcohol 68, Benzaldehyde 69) and two aldehydes (hexanal 70, (E)-2-Hexenal 71) are determined from AE ripe fruit by GC-MS (Garcia et al., 2012). The chemical structures of the phenolics and aldehydes are exhibited in Figure 4.
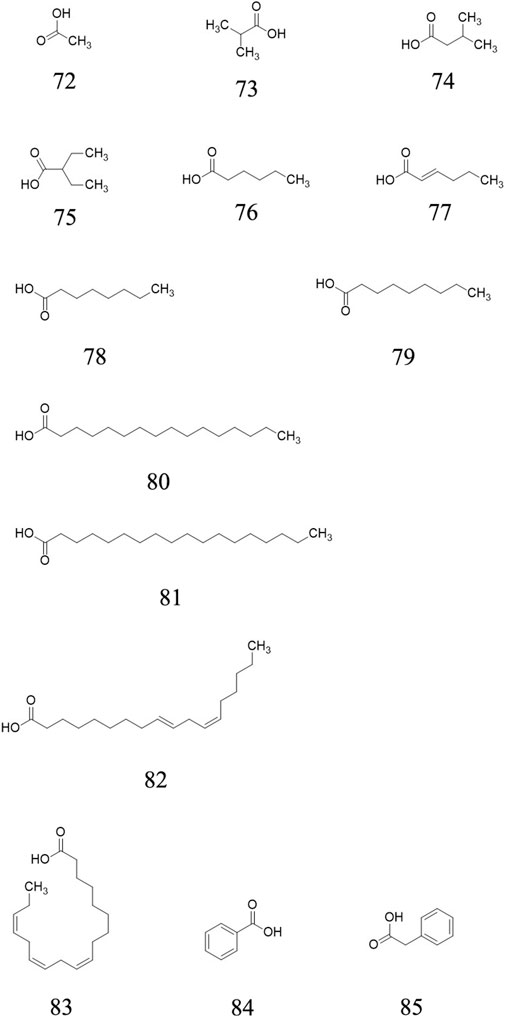
Organic acids
Twelve acid, including acetic acid 72, isobutyric acid 73, isovaleric acid 74, 2-Ethylbutanoic acid 75, hexanoic acid 76, trans-2-Hexenoic acid 77, octanoic acid 78, nonanoic acid 79, hexadecanoic acid 80, octadecanoic acid 81, linoletic acid 82, linolenic acid 83, benzoic acid 84 and phenylacetic acid 85 are confirmed from the ripe fruits of AE plants (Garcia et al., 2012). Their chemical structures are presented in Figure 5.
Flavonoids glycoside
Eight flavonoids (isorhamnetin 3-O-rhamnose 1-6-glucose 86, kaempferol 3-O-rhamnose 1-6-glucose 87, quercetin 3,7-O-di, tri glycoside 88, quercetin 3-O-rhamnose rhamnose glucose 89, kaempferol 3-O-rhamnose 1-4 rhamnose 1-6 glucose 90, quercetin 3-O- glucose 91, kaempferol 3-O- glucose 92, quercetin 3-O-rhamnose 1-6 galactose 93) are isolated from the leaf of AE plants (Webby et al., 1994).
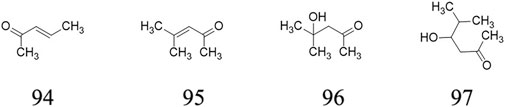
Ketones
Four ketones ((E)-3-Penten-2-one 94, 4-Methyl-3-penten-2-one 95, 4-Hydroxy-4-methyl-2- pentanone 96, 4-Hydroxy-5-methyl-2-hexanone 97) are determined from ripe fruits from AE plants (Garcia et al., 2012). These substances’ chemical structures are displayed in Figure 6.
Glucoside and others
Two Glucoside, (6R,7E,9S)-6,9-hydroxy-megastiman-4,7-dieu-3-one-9-O-β-D-glucopyranoside 98 (Wu et al., 2017), Oleanolic acid-23-O-β-D- glucopyranoside 99 (Wu et al., 2017) and two furans (Dihydro-3,5-dimethyl-2(3H)-furanone 100 (Garcia et al., 2012), Furfuryl alcohol 101 (Garcia et al., 2012)as well as other compounds, such as 3-Hydroxy-b-damascone 102 (Garcia et al., 2012), 2-(Methylthio)ethanol 103 (Garcia et al., 2012), 3-(Methylthio)-1-propanol 104 (Garcia et al., 2012), polysaccharide AEPS (Xu et al., 2009b; Chen et al., 2019b), are determined and isolated from roots and ripe fruits of AE plants. These compounds’ chemical structures are exhibited in Figure 7.
Pharmacological activities
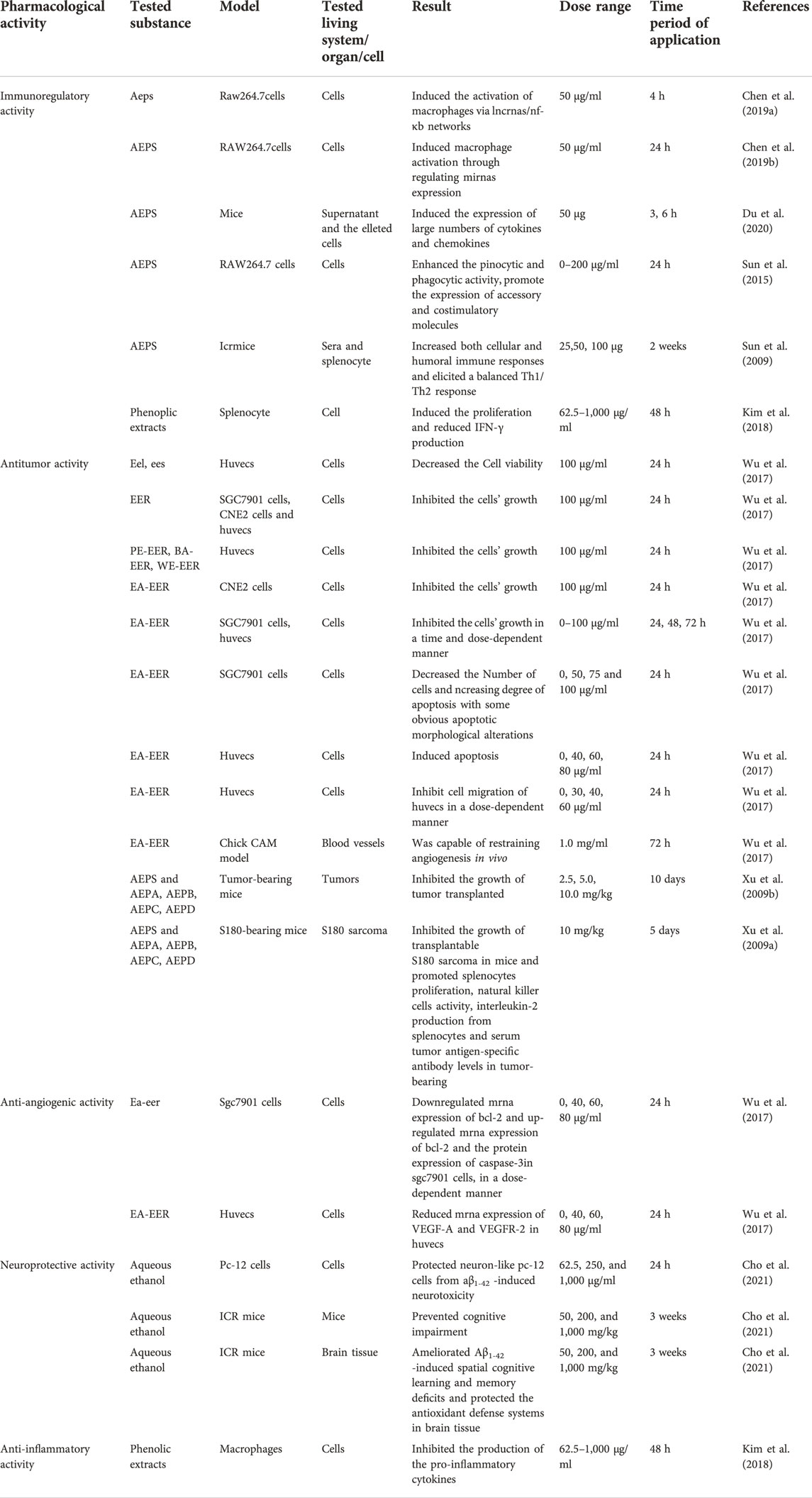
AE has been demonstrated to possess various pharmacological activities. The main pharmacological activities are anti-tumor activity, as well as other activities such as immunoregulatory, anti-inflammatory, anti-angiogenic, and neuroprotective and antioxidant activities. As a traditional medicine in She ethnic minority group, a minority who lived in Zhejiang and Fujian Provinces, AE is widely used to treat stomach cancer, colon cancer, cirrhosis with ascites, chronic hepatitis, leukemia, rectal prolapse, hernia and uterine prolapse. However, pharmacological studies only provide evidence for the traditional use of its anti-tumor effect, while other activities such as treating chronic hepatitis still need further research. The possible mechanism of anti-tumor, immunoregulatory, and anti-inflammatory activities are shown in Figure 8. And these activities have been displayed in Table 2 and will be discussed further in the following sections.

FIGURE 8. Possible mechanism of anti-tumor, immunoregulatory, and anti-inflammatory activities of A. eriantha. S, Synthesis phase, G, Gap phase, M, mitotic period. AE inhibited tumor development through arresting cell cycle, inducing apoptosis and inhibiting tumor angiogenesis and migration. AE regulated immunity through inducing the responses of M1 and M2 macrophages. AE exhibited anti-inflammatory activity through inhibiting cytokine production.
Anti-tumor activity
Cancer is the second most common disease worldwide and is difficult to treat (Zhu et al., 2018). Investigations utilizing in vivo and in vitro systems have demonstrated that AE extracts, including ethanol extracts, n-BuOH extracts, ethyl acetate extracts, petroleum ether extract, aqueous extract, chloroform extract and methanol extract, possessed anti-tumor activity. The aqueous extracts of AE roots can not only inhibit the proliferation, migration and invasion of H1299 cells in dose-dependently manner via upregulated the PCDH10 gene expression by downregulating mir-182-5p gene expression (Zhao et al., 2020), but also inhibited M21 cell proliferation, invasion and migration by downregulating the expression of pD-L1 and PD-L2 molecular genes and proteins closely related to immune escape of tumor cells (Wang et al., 2013).
A 24 h administration of ethyl acetate fraction from the root of AE viz. EA-EER (100 μg/ml) significantly suppressed the proliferation of HUVECs by blocking G1 to S cell cycle progression and downregulating VEGF-A and VEGFR-2 expression (Wu et al., 2017). In addition, EA-EER inhibited Bcl-2 expression and enhanced Bax and caspase-3 expression in SGC7901 cells (Wu et al., 2017). These results suggested that EA-EER has the potential to serve as a source of anti-cancer drugs. However, it was necessary to explore the bioactivity of the compounds extracted and the trial lacked a positive control. Tang et al. confirmed the main active ingredient of EA-EER to be 2α,3α,24-trihydroxyurs-12-en-28-oic acid by HPLC, and found that its nano-micelles could inhibit the survival rate of U87MG cells in an administration of 0–80 μg/ml dose-dependently. After being loaded with nano-micelles, the compound exhibited better inhibiting effect on U87MG cells than monomeric compound, while the enhanced effect can be attributed to nano-micelles’ improving water solubility of compound (Tang et al., 2019). Nevertheless, the administration of chloroform extract from the AE roots (4.5–450 μg/ml) dose-dependently suppressed SMMC-7721 cell proliferation (Wang et al., 2011). In another study, administration of chloroform extract from the AE roots (87.5–200 μg/ml) also dose-dependently suppressed BEL-7404 cell proliferation and it was considered that the active components are alkaloids (Guo et al., 2013).
Xu et al. (2009b) has found that the administration of total polysaccharides (AEPS) and four polysaccharides (namely AEPA-AEPD) extracted from AE roots (10 mg/kg) could suppress the growth of S180 sarcoma or H22 hepatoma xenografts in mice. However, the research lacked a positive control for the tumor-suppressing effects of the polysaccharides from AE.
Immunoregulatory activity
Spleen, as an essential peripheral lymphatic organ, it key for innate immunological responses because it can participate in phagocytosis and immune memory (Zhu et al., 2018). In the models of OVA-immunized mice and OVA-induced splenocytes, the administration of AEPS (62.5–1,000 μg/ml) significantly promoted the proliferation of splenocytes treated with Con A and LPS and reduced the production of INF-c relative to the splenocytes stimulated by an anti-CD3 mAb (Kim et al., 2018). However, there was no positive control for that study, and an analysis of dose-effect relationship was also lacking, which had negatively affected the reliability of the study. In another study, administration of AEPA-AEPD could significantly enhance the proliferation of splenocytes, increase the activity of natural killer (NK) cells, stimulate the secretion of interleukin-2 by splenocytes and upregulate the level of tumor antigen-specific antibody in the serum of tumor-bearing mice, which suggested that the anti-tumor activity of AEPA-AEPD was realized by enhancing immune response (Xu et al., 2009a). Furthermore, administration of AEPS (25, 50, or 100 μg) and OVA for 2 weeks significantly increased the serum level of OVA-specific antibody, the cytotoxic activity of NK cells, and the expression and secretion of Th1/Th2 cytokines by activating related transcription factors in the splenocytes of mice treated with OVA (Sun et al., 2009). In OVA induced BALB/c mice, the administration of AEPS triggered an immune effector process manifesting as monocyte, dendritic cell, and neutrophil recruitment and higher expression levels of CXCL2, CXCL3, CXCL5, CXCL10, CCL2, CCL3, CCL4, CCL7, IL-12β, and IL-23α mRNA (Du et al., 2020). Nevertheless, a 24-h administration of AEPS (10 mg/ml) resulted in the differential expression of 82 miRNAs in RAW264.7 cells, among which 43 and 39 were up- and downregulated, respectively (Chen et al., 2019a). In in vitro studies on RAW264.7 cells, AEPS evoked the responses of M1 and M2 macrophages via the NF-κB pathway (Sun et al., 2015; Chen et al., 2019a). These findings reflected that AEPS might have induced macrophage activation by regulating the expression of miRNAs and the activity of the NF-κB pathway. However, it is necessary to carry out further in vivo studies to investigate the physicochemical characteristics of these extracts and their mechanisms of action from the perspective of intracellular signaling pathways. In addition, there existed robust patterns of lncRNAs expression corresponding to the specific adjuvants used during the biological processes mentioned above, implying that lncRNAs are involved in the immune responses stimulated by AEPS(Du et al., 2020). This research shed more light on the adjuvants’ molecular mechanisms and provided guidance for the reasonable design of vaccines with high efficacy. However, other factors should also be considered when making conclusive decisions on the predictor organism.
Anti-inflammatory activity
The overproduction of pro-inflammatory factors, such as TNF-α and IL-6, has been associated with several inflammatory disorders, including rheumatoid arthritis, Alzheimer’s disease, and cancer7. It was found that the phenolic extracts from AE kiwifruit could decrease the concentrations of TNF-α, IL-6, and IL-12 in the cell culture medium for primary macrophages obtained from male BALB/c mice (Kim et al., 2018). Moreover, the phenolic extracts significantly attenuated INF-γ production in splenocytes. However, there is a need to investigate the chronic toxicity of AE and identified the isolated activity compounds (Kim et al., 2018).
Other activities
In classic chick CAM model, the treatment of EA-EER profoundly decreased the number of blood vessels relative to the untreated control, indicating that EA-EER could restrain angiogenesis in vivo (Wu et al., 2017). In addition, the Aβ 1–42 -treated ICR mice experiment showed that AEE (A. eriantha cv. Bidan extract) at doses of 50, 200, and 1,000 mg/kg body weight per day could alleviate learning and spatial memory deficiencies and activate intracellular antioxidant systems (CAT, SOD, and GSH/GSSG) in brain tissues of mice. These results indicated that the mechanisms underlying the cell protection effects of AEE include the inhibition of apoptosis-related signaling pathways and the protection of mitochondra (Cho et al., 2021). Furthermore, AE also exhibited strong antioxidant capacity with high ferric reducing ability of plasma (FRAP) value and ·O2− clearance rate (Huang et al., 2020). The antioxidant capacities of AE kiwifruit were determined to be 608.9, 620.9 and 1,016.8 mg VCE/100 g fresh weight, respectively, by the ABTS, DPPH and ORAC experiments (Kim et al., 2018). However, many traditional uses of AE have not been the focus of recent research. For example, although many traditional documents have revealed that AE can benefit cirrhosis with ascites, chronic hepatitis, leukemia, rectal prolapse, hernia and uterine prolapse, these beneficial effects themselves and the mechanisms underlying them have not been widely investigated. Therefore, these traditional uses of AE warrant further investigations.
Traditional uses
The genus Actinidia was first recorded in Book of Songs (《诗经》) which dates to the Xizhou and Chunqiu period (B. C. 1,000–600). Actinidia has been widely used in China as both functional foods (Fruit) and medicines (Root). Root of Actinidia is recorded in many ancient herbal texts, such as Supplement to Herbology (《本草拾遗》) (Tang Dynasty, A. D. 739), Kaibao Herbology (《开宝本草》) (Song Dynasty, A. D. 973–974), and Compendium of Materia Medica (《本草纲目》) (Ming Dynasty, A. D. 1,578), and is widely used to treat arthrosis pain, traumatic injury, haemorrhoids, filariasis, dysentery, and pyogenic infections (Di et al., 2014). However, it is still not sure when the root of Actinidia was distinguished into Actinidia Chinensis (one sibling species of AE) root and AE root.
AE has many folk names, including Baishanmaotaogen, Baimaotao, Maohuayangtao, Baitengli, Tengligen (Lei and Li 2007; Zhu et al., 2015). In TCM, AE is used to treat stomach cancer, colon cancer, cirrhosis with ascites, chronic hepatitis, leukemia, rectal prolapse, hernia and uterine prolapse (Zhu et al., 2015). AE roots has been used in many She ethnic minority group traditional preparations. AE roots is often used in combination with Scutellaria barbata, Hedyotis diffusa and Rabdosia amethystoides to treat tumors, colon cancer, cirrhosis of the liver, and leukemia. In addition to this, it also used in combination with Aralia chinensis roots and Ampelopsis sinica to treat fracture and sprain. Examples of traditional Chinese medicine prescriptions containing AE are listed in the Table 3. However, the potential interactions and synergistic effects between the active components of AE and those of other herbal medicines, as well as the underlying mechanism of action, have not been clarified and warrant further investigation.
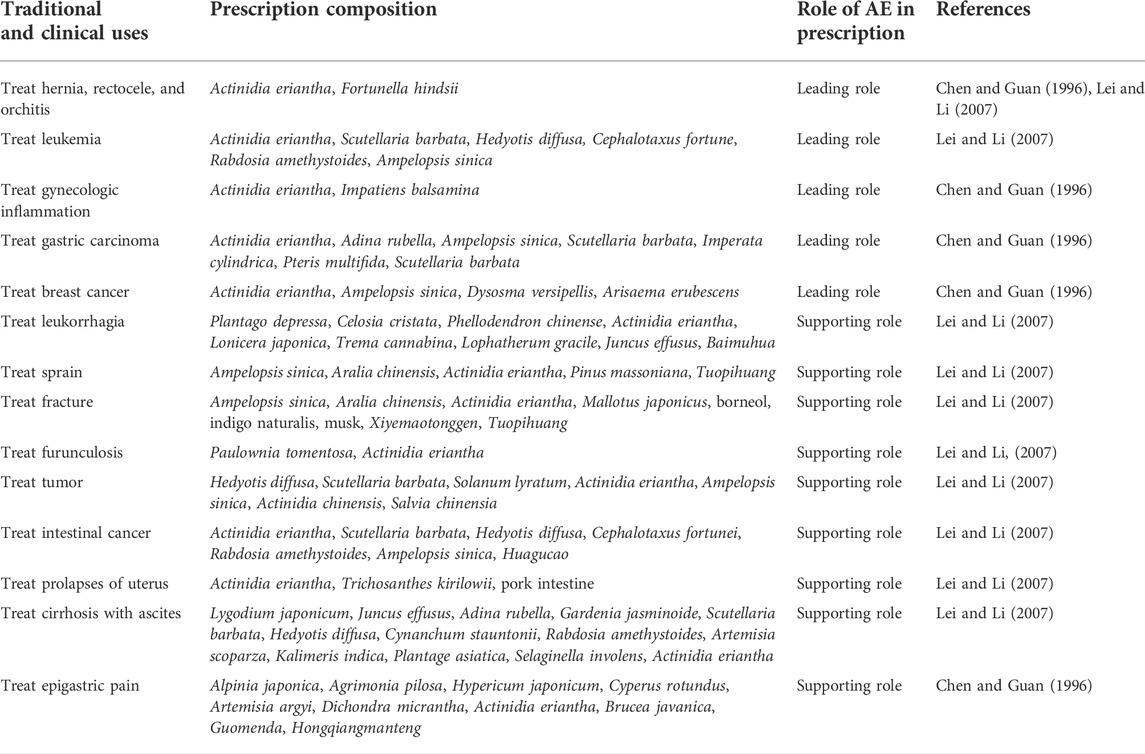
TABLE 3. Examples of traditional Chinese medicine prescriptions containing Actinidia eriantha Benth.
Quality control
For quality control of AE-derived medicines, the Zhejiang Processing Standard of Traditional Chinese Medicine (ZPSTCM) suggested morphological, microscopic and chemical identification (Zhejiang Food and Drug Administration, 2015). According to the requirements of ZPSTCM, moisture shall be not exceed 12% (“Chinese Pharmacopoeia “moisture determination drying method), while the total ash shall be not exceed 7% (“Chinese Pharmacopoeia” ash determination method) (Zhejiang Food and Drug Administration, 2015). What’s more, the bioactive components of AE, including general flavone and total triterpenes, have been identified by ultraviolet spectroscopy. A good linearity of rutin was shown in ranges of 0.00942–0.05650 mg mL−1 (r = 0.9991). The average recovery rate was 97.33% (RSD = 1.98%). A good linearity of ursolic acid was shown in ranges of 0.004 89–0.01712 mg/ml (r = 0.9987) and the average recovery rate was 96.58% (RSD = 1.79%) (Yu et al., 2017). In terms of dried products, the total flavonoids in the form of anhydrous rutin (C27H30O16) and total three pieces in the form of ursolic acid (C30H48O3) should not be less than 1.0% (Yu et al., 2017). What’s more, via optimizing the conditions of ultrasonic extraction, the average total flavonoids content in the root of AE could up to 1.058% (Zheng et al., 2012). By optimizing ultrasonic extraction conditions, the total triterpenoid content in the root of AE could reach to 1.19% ± 0.08% (Li et al., 2015). In further study, using the combination of ultrasonic extraction and percolation extraction, the content of triterpenoids in the ethyl acetate extract of the root of AE can be as high as 45% (Zhang et al., 2019). However, adopting only one crude, quantitative marker to ascertain the quality of AE extract may not be convincing enough.
AE root is a traditional medicine used in folk cancer treatment of she people, and is widely used in the treatment of gastric cancer, esophageal cancer, breast cancer, nasopharyngeal cancer, and liver cancer, etc. Triterpenoid acid is one of the main active substance (Gong et al., 2016). Guo et al. used HPLC-PDA to compare the contents of 2α,3α,24-trihydroxy-12-ene-28-ursolic acid in the roots of AE from different origins, finding it existed in the roots of AE from producing areas, but the content was different (Li et al., 2015). These results provided reference for the quality evaluation of AE roots from different origins. What’s more, total flavonoids were used as the main index to evaluate the quality of Actinidia chinensis Planch, which is indicated that can be used as a candidate index component for evaluation of AE (Shaanxi Food and Drug Administration, 2015).
Safety
Based on available animal trails, AE administration seems to cause no or little toxicity. According to the results of body weight measurement and microscopic examination of organs (intestinal tract, liver and kidney), there have been no observed toxic effects of AEPS (the total polysaccharide from the root of AE) and its polysaccharides on mice at the maximum dose of 10 mg/kg (Xu et al., 2009a; Xu et al., 2009b). In addition, when the mice were subjected to subcutaneous administration of AEPS at the doses of 0.5–5.0 mg/kg twice a week, no local swelling or hair loss was observed. These results implied that the upper safe dosage of AEPS for humans and animals may be higher than 200 mg/kg (Sun et al., 2009). What’s more, AEPS did not elicit cytotoxicity in RAW264.7 cells at a high concentration of 200 μg/ml; in fact, AEPS at the concentrations of 25–100 μg/ml could even promote the proliferation of RAW264.7 cells (Sun et al., 2015). However, further investigations specifically focusing on the chronic toxicity of AE should be carried out.
Discussion
A. eriantha is a liana species that has been extensively utilized in TCM to treat various diseases in China. Our paper summarizes the existing knowledge on the botanical characteristics, traditional uses, phytochemical properties, pharmacological activities, and toxicity of AE. In classical TCM documents and ZPSTCM, AE is commonly used to treat stomach cancer, colon cancer, cirrhosis with ascites, chronic hepatitis, leukemia, rectal prolapse, hernia and uterine prolapse. Pharmacological studies have demonstrated many bioactivities of AE, including anti-cancer, immunoregulatory, anti-inflammatory, anti-angiogenic, neuroprotective and antioxidant activities. However, many traditional uses of AE have not been supported by pharmacological research. To date, over 104 chemical components have been extracted from AE, among which triterpenoids and polysaccharides are the main bioactive substances.
Our current understanding of the phytochemical properties and pharmacological activities of AE is insufficient. Firstly, although AE administration exhibited almost no toxicity in most animal studies, its long-term toxicity should be further assessed. The possible adverse effects and biotoxicity of AE extracts and their active components should be evaluated when they are used for in vitro, in vivo, or clinical studies. Secondly, most pharmacological investigations on AE have been performed on its crude extracts and fractions, of which few have been analyzed for phytochemical properties. For example, AE polysaccharides possess antitumor, immunomodulatory, and anti-inflammatory activities, but how these activities of AE polysaccharides correlate with their structural characteristics remains clear. Thus, these bioactive components of AE should be isolated and studied for their molecular mechanisms, bioavailability, and pharmacokinetic characteristics in future research. Thirdly, although AE-derived polysaccharides have been shown to possess similar anti-tumor activity as ethyl acetate fraction (representative compounds: triterpenoids), the mechanisms underlying absorption, distribution, metabolism, and excretion, as well as the synergistic or antagonistic effects between the two constituents are unknown and should be further studied. Moreover, the synergistic anti-tumor and therapeutic action, as well as other pharmacological activities, such as anti-diabetic, renal protective, and neuro-protective effects, should be taken into consideration in future study. Last but not the least, the idea that AE is responsible for cirrhosis with ascites, chronic hepatitis, rectal prolapse, hernia and uterine prolapse should be validated by modern pharmacological research; once validated, the mechanisms underlying these bioactivities of AE should be further explored. Given that most of the existing research was conducted at the cellular level, more in vivo studies adopting animal models and clinical samples are needed to testify the efficacy of AE in the treatment of eat stomach cancer, colon cancer, cirrhosis with ascites, chronic hepatitis, leukemia, rectal prolapse, hernia and uterine prolapse. Nevertheless, a lot of the traditional uses of AE have not been the focus of recent research, which warrant further investigations.
Author contributions
SW collated documents and wrote manuscript; XG and QS helped perform the arrangement of tables; YZ helped to check chemical structure formula. LQ and BZ contributed significantly to analysis and manuscript preparation. All authors have read and approved the final version of the manuscript.
Funding
This study is supported by Young Innovative Talents Project of Zhejiang Medical Health Science and Technology 2022RC052.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bai, S., Huang, C., and Chen, X. (1997a). Studies on the chemical constituents of the aerial parts of Maohuamihoutao (Actinidia eriantha). Chin. Herb. Med. 9, 69–72.
Bai, S., Huang, C., and Chen, X. (1997b). Studies on triterpen compounds from Actinidia eriantha Benth. Nat. Prod. Res. Dev. 28, 15–17.
Chen, X., Du, J., Zhu, Y., Zhang, C., and Sun, H. (2019a). Comprehensive analysis of lncRNA and mRNA expression profiles in macrophages activated by Actinidia eriantha polysaccharide. Int. J. Biol. Macromol. 136, 980–993. doi:10.1016/j.ijbiomac.2019.06.091
Chen, X., Yuan, L., Du, J., Zhang, C., and Sun, H. (2019b). The polysaccharide from the roots of Actinidia eriantha activates RAW264.7 macrophages via regulating microRNA expression. Int. J. Biol. Macromol. 132, 203–212. doi:10.1016/j.ijbiomac.2019.03.158
Cho, C. H., Jung, Y. S., Kim, J. M., Nam, T. G., Lee, S.-H., Cho, H. S., et al. (2021). Neuroprotective effects of Actinidia eriantha cv. Bidan kiwifruit on amyloid beta-induced neuronal damages in PC-12 cells and ICR mice. J. Funct. Foods 79, 104398. doi:10.1016/j.jff.2021.104398
Di, X., Wang, H., Zhang, J., Zai, Y., and Wang, Y. (2014). Radix actinidiae chinensis' herbal textual. J. Liaoning Univ. TCM 16, 132–134. doi:10.13194/j.issn.1673-842x.2014.05.047
Du, J., Chen, X., Ye, Y., and Sun, H. (2020). A comparative study on the mechanisms of innate immune responses in mice induced by Alum and Actinidia eriantha polysaccharide. Int. J. Biol. Macromol. 156, 1202–1216. doi:10.1016/j.ijbiomac.2019.11.158
Garcia, C. V., Quek, S.-Y., Stevenson, R. J., and Winz, R. A. (2012). Characterisation of bound volatile compounds of a low flavour kiwifruit species: Actinidia eriantha. Food Chem. 134, 655–661. doi:10.1016/j.foodchem.2012.02.148
Gong, M., Yaohan, L., Ling;, X., Qing, P., and Xing, L. H. (2016). Content determination of 2α, 3α, 24-trihydroxy-12-alkene-28-ursolic acid in the roots of actinidia erian- tha Benth as an active component by HPLC. Chin. J. Pharm. 51, 1219–1223. doi:10.11669/cpj.2016.14.015
Guo, H. (2013). Study on the chemical constituents and antitumor mechanism of the chloroform fraction of Actinidia eriantha Benth. Hangzhou: Master, Zhejiang Sci-Tech University. doi:10.3969/j.issn.1673-3851.2013.05.024
Guo, H., Ying, S., Wang, X., Jin, X., Ma, C., and Xu, C. (2013). Study on antineoplastic activity of chloroform extraction of actindia eriantha Benth. J. Zhejiang Sci-Tech Univ. 30, 758–761.
Huang, C., Li, G., Fan, H., Zhang, Z., and Zhou, J. (1986). A new triterpen from roots of Actinidia eriantha. Acta Bot. Yunnanica 8, 489–491.
Huang, C., Tao, J., Liao, G., Xie, M., Qu, X., Lu, C., et al. (2020). Dynamic changes of phenol and antioxidant capacity during fruit development of three Actinidia species (kiwifruit). Sci. Hortic. 273, 109571. doi:10.1016/j.scienta.2020.109571
Huang, C., Zhang, Z., Li, G., and Zhou, J. (1988). Isolation and identification of two new trierpenes from Actinidia eriantha. Acta Bot. Yunnanica 8, 93–100.
Kim, Y.-E., Cho, C.-H., Kang, H., Heo, H. J., Cho, Y.-S., and Kim, D.-O. (2018). Kiwifruit of Actinidia eriantha cv. Bidan has in vitro antioxidative, anti-inflammatory and immunomodulatory effects on macrophages and splenocytes isolated from male BALB/c mice. Food Sci. Biotechnol. 27, 1503–1511. doi:10.1007/s10068-018-0321-5
Lei, H., and Li, S. (2007). Chinese she medicine. Beijing: China Traditional Chinese Medicine Publishing House.
Li, H., Zhang, L., and Tian, J. (2015). The establishment of fingerprint on the root of Actinida eriantha Benth and the content determination of 2α, 3α, 24-three hydroxy-12-alkene-28-ursolic acid as an active component. Chin. Sci. Technol. Pap. 1, 1–5.
Liao, G., Xu, X., Huang, C., Zhong, M., and Jia, D. (2021). Resource evaluation and novel germplasm mining of Actinidia eriantha. Sci. Hortic. 282, 110037. doi:10.1016/j.scienta.2021.110037
Shaanxi Food and Drug Administration (2015). Standard for medicinal materials of shaanxi province. Xi 'an: Shaanxi Science and Technology Press.
Sun, H., Wang, H., Xu, H., and Ni, Y. (2009). Novel polysaccharide adjuvant from the roots of Actinidia eriantha with dual Th1 and Th2 potentiating activity. Vaccine 27, 3984–3991. doi:10.1016/j.vaccine.2009.04.037
Sun, H., Zhang, J., Chen, F., Chen, X., Zhou, Z., and Wang, H. (2015). Activation of RAW264.7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr. Polym. 121, 388–402. doi:10.1016/j.carbpol.2014.12.023
Tang, L., Jiang, Z., Li, J., and Wu, A. (2019). Anti-glioma active compounds and their nanomicells preparation of the root of actinidiaeriantha Benth. Chem. J. Chin. Univ. 40, 468–472. doi:10.7503/cjcu20180507
Wang, S., Sun, Y., Jin, H., and Cheng, X. (2013). Research on effect and potenial mechanism of extractive of radix actindiate erianthae on proliferation inhibition in human melanoma M21 cells. Liaoning J. Traditional Chin. Med. 10, 2101–2104. doi:10.13192/j.issn.1000-1719.2013.10.059
Wang, X., Yang, Z., Shi, Y., and Xu, C. (2011). The inhibiting effect of actindia eriantha Benth in liver cance line SMMC-7721. J. Zhejiang Sci-Tech Univ. 4, 606–610.
Webby, R. F., Wllsont, R. D., and Ferguson, A. R. (1994). Leaf flavonoids of actinidia. Biochem. Syst. Ecol. 22, 277–286. doi:10.1016/0305-1978(94)90101-5
Wu, J., Ma, L., Lin, S., Wu, Y., Yi, J., Yang, B., et al. (2017). Anticancer and anti-angiogenic activities of extract from Actinidia eriantha Benth root. J. Ethnopharmacol. 203, 1–10. doi:10.1016/j.jep.2017.03.013
Xu, H., Li, Y., Sun, H., and Wu, Y. (2009a). Chemical composition and antitumor activity of different polysaccharides from the roots of Actinidia eriantha. Carbohydr. Polym. 78, 316–322. doi:10.1016/j.carbpol.2009.04.007
Xu, H., Wu, Y., Xu, S., Sun, H., Chen, F., and Yao, L. (2009b). Antitumor and immunomodulatory activity of polysaccharides from the roots of Actinidia eriantha. J. Ethnopharmacol. 125, 310–317. doi:10.1016/j.jep.2009.06.015
Yu, L., Chen, Z., Wang, W., Ye, B., Fan, L., and Li, S. (2017). Study on quality standard of traditional she medicine white peach radix. Chin. Archives Traditional Chin. Med. 35, 654–655. doi:10.13193/j.issn.1673-7717.2017.03.039
Yu, X., Wang, X., Wang, X., Zhou, Y., Li, Y., Wang, A., et al. (2020). TEOA, inhibits proliferation and induces DNA damage of diffuse large B-cell lymphoma cells through activation of the ROS-dependent p38 MAPK signaling pathway. Front. Pharmacol. 11, 554736. doi:10.3389/fphar.2020.554736
Zhang, D., Gao, C., Li, R., Zhang, L., and Tian, J. (2017). TEOA, a triterpenoid from Actinidia eriantha, induces autophagy in SW620 cells via endoplasmic reticulum stress and ROS-dependent mitophagy. Arch. Pharm. Res. 40, 579–591. doi:10.1007/s12272-017-0899-9
Zhang, J., Zhang, C., Wang, P., Wang, J., Zhu, W., and Tong, Y. (2019). Screening of antitumor active fraction extracted from roots of Actinidia erianthain vitro and determination of total triterpenoids. Chin. J. Mod. Appl. Pharm. 38, 1232–1235. doi:10.13748/j.cnki.issn1007-693.2019.10.012
Zhao, Z., Chen, J., and Wang, M. (2020). Inhibitory effect of extracts of radix actinidiae erianthae on non-small cell lung cancer cella by regulating miR-182-5p/PCDH10 expression. J. Guangxi Med. Univ. 37, 2166–2172. doi:10.3969/j.issn.1005-9202.2021.23.052
Zhejiang Food and Drug Administration (2015). Zhejiang province standard of TCM decoction. Beijing: China Medical And Technology Press.
Zheng, Y., Li, R., Zhou, X., and Wu, J. (2012). Ultrasonic extraction technology of total flavonoids from Actinidia eriantha Benth. J. Fujian Univ. TCM, 50–51. doi:10.13261/j.cnki.jfutcm.002640
Zhu, B., Hua, J., Chen, W., Ji, Q., Wu, J., and Qi, C. (2015). High quality cultivation techniques of Actinidia eriantha, a medicinal and edible plant. Inn. Mong. Agric. Sci. Technol. 43, 84–87. doi:10.3969/j.issn.1007-0907.2015.04.030
Zhu, B., Zhang, Q., Hua, J., Cheng, W., and Qin, L. (2018). The traditional uses, phytochemistry, and pharmacology of atractylodes macrocephala koidz: A review. J. Ethnopharmacol. 226, 143–167. doi:10.1016/j.jep.2018.08.023
Glossary
ABTS 2, 2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
AE Actinidia eriantha
Bax BCL2-associated X
Bcl-2 B-cell lymphoma-2
CAM Chick embryo chorioallantoic membrane
CAT Catalase
CD3 Cluster of differentiation 3
ConA Concanavalin A
CXCL Chemokines
DPPH 1,1-diphenyl-2-picrylhydrazyl
EA-EER Ethyl acetate fraction of AE root
FRAP High ferric reducing ability of plasma
GSH/GSSG L-Glutathione
HPLC High purity liquid chromatography
HPLC-PDA High purity liquid chromatography-photo-diode array
IL Interleukin
INF Interferon
lncRNA Long noncoding RNA
LPS Lipopolysaccharide
mAb Monoclonal antibody
miRNA Micro RNA
mRNA MessengerRNA
n-BuOH Normal butanol
NK cell Natural killer
ORAC Oxygen-radical absorbance capacity
OVA Ovalbumin
PCDH10 Protocadherin 10
PD-L1 Programmed death 1 ligand 1
PD-L2 Programmed death 1 ligand 2
RSD Rlative sandard deviation
SOD Superoxide dismutase
TCM Traditional chinese medicine
TNF-α Tumor necrosis factor α
VEGF-A Vascular endothelial growth factor A
VEGFR-2 Vascular endothelial growth factor receptor 2
ZPSTCM Zhejiang processing standard of traditional chinese medicine
Keywords: Actinidia eriantha, traditional use, phytochemistry, pharmacology, quality control
Citation: Wang S, Gao X, Sun Q, Zhu Y, Qin L and Zhu B (2022) The phytochemical properties, pharmacological effects and traditional uses of Actinidia eriantha Benth.: A review. Front. Pharmacol. 13:959900. doi: 10.3389/fphar.2022.959900
Received: 02 June 2022; Accepted: 21 July 2022;
Published: 19 August 2022.
Edited by:
Shuai Ji, Xuzhou Medical University, ChinaReviewed by:
Kamran Ashraf, Universiti Teknologi MARA Puncak Alam, MalaysiaSadia Sultan, Universiti Teknologi MARA Puncak Alam, Malaysia
Hardeep Singh Tuli, Maharishi Markandeshwar University, Mullana, India
Copyright © 2022 Wang, Gao, Sun, Zhu, Qin and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luping Qin, lpqin@zcmu.edu.cn; Bo Zhu, zhubo@zcmu.edu.cn
 Shiyu Wang
Shiyu Wang Xiaoqi Gao
Xiaoqi Gao Qingmei Sun
Qingmei Sun Yichun Zhu
Yichun Zhu Luping Qin
Luping Qin Bo Zhu
Bo Zhu