- 1Department of Hepatobiliary Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Shaanxi, China
- 2Department of Laboratory Medicine Center, Xi’an People’s Hospital (The Fourth Hospital of Xi’an), Shaanxi, China
- 3Health Management Department, The Second Affiliated Hospital of Xi’an Jiaotong University, Shaanxi, China
- 4Department of Surgical ICU (SICU), The First Affiliated Hospital of Xi’an Jiaotong University, Shaanxi, China
Background: NSAIDs are one of the most frequently used medications and a risk factor for AKI. However, the optimal time of NSAIDs in patients with AKI is unknown.
Methods: A secondary analysis of a multicenter, randomized clinical trial including adult inpatients with acute kidney injury was performed. Univariate, multivariate, and subgroup analyses were used to explore the impact of NSAIDs during the early onset of AKI on the outcome of patients with AKI.
Results: A total of 6,030 patients with AKI were enrolled in the study. Following are the findings of the multi-factor analysis: NSAID treatments within 72 and 24 h before the onset of AKI were not associated with AKI progression, dialysis, or discharge from dialysis; only NSAID treatment within the 24-h onset of AKI was associated with these outcomes, and their OR values were independently 1.50 (95% CI: 1.02–2.19, p = 0.037), 4.20 (95% CI: 1.47–11.97, p = 0.007), and 0.71 (95% CI: 0.54–0.92, p = 0.011); only NSAID treatment within the 24-h onset of AKI would decrease the 14-day mortality, and the OR value was 0.52 (95% CI: 0.33–0.82, p = 0.005). The subgroup analysis revealed that in patients with age ≥65 years, CKD (chronic kidney disease), congestive heart failure, hypertension, and liver disease, NSAID treatments within the 24-h onset of AKI would deteriorate the outcome of patients with AKI.
Conclusion: Before an early onset of AKI, NSAID treatment might be safe, but during the onset of AKI, even early NSAID treatment would deteriorate the outcome of patients with AKI.
Background
NSAIDs are one of the most often prescribed medications globally, accounting for around 5% of all prescribed medications (Bindu et al., 2020). NSAIDs are frequently used to treat rheumatic illnesses, rheumatoid arthritis, various acute and chronic pain, perioperative analgesia, and acute and chronic fever (Ghlichloo and Gerriets, 2021). Furthermore, the role of low-dose aspirin in the secondary prevention of ischemic stroke is well established (Frontera et al., 2016; Lip et al., 2018; Jia et al., 2021), and several studies have demonstrated that NSAIDs may be beneficial in preventing several cancers, including colorectal cancer, ovarian cancer, breast cancer, and prostate cancer (Ye et al., 2020; Kolawole and Kashfi, 2022).
The estimates of AKI prevalence range from <1% to 66% (Hoste et al., 2018), whereas AKI occurs at a rate of 10% in emergency patients, and its hospital mortality is also 10% (Challiner et al., 2014). The overall incidence of AKI is higher in ICUs (intensive care units), ranging from 20% to 50%, and the associated mortality is more than 50% (Selby et al., 2012). NSAIDs can result in renal damage, including AKI and CKD, depending on the dosage and duration of usage, particularly in older individuals and patients with comorbidities (Baker and Perazella, 2020). Due to the broad effects of NSAIDs, they are one of the most often prescribed medications (Bindu et al., 2020).
To summarize, NSAIDs increase the risk of AKI and CKD, and there is consensus on their use in CKD. However, there is a paucity of information about NSAID effects on the AKI progression and the prognosis of patients with AKI at different periods of AKI. Consequently, additional investigation into the impact of NSAIDs on AKI development and prognosis in individuals with AKI is required.
Objective
The study aimed to explore the effects of NSAIDs before and after an early onset of AKI on AKI progression and the prognosis of patients with AKI.
Methods
Study Design
A secondary analysis of a multicenter randomized clinical trial including adult inpatients with acute kidney injury was performed (Trial registration: ClinicalTrials.gov NCT02753751).
Setting
Six hospitals in the Yale New Haven Health System in Connecticut and Rhode Island, United States, were selected, including Bridgeport Hospital, Greenwich Hospital, The Hospital of St. Raphael, Lawrence and Memorial Hospital, Yale New Haven Hospital, and Westerly Hospital.
Ethics Approval and Consent to Participate
The original author had obtained the ethical approval prior to conducting this research, and our study involved retrospective data analysis. A new ethical approval was approved by the Medical Bioethics Committee, School of Medicine, Xi’an Jiaotong University, Xi’an, Shaanxi province, China (No. 2022-468).
Data Source
The data used in our study were shared by Wilson, Francis, Yale School of Medicine, which were stored in the Dryad database. The database is a publicly accessible data repository that authors have contributed to making their research data discoverable, freely reusable, and citable. For more details, this link can be referred: https://datadryad.org/stash/dataset/doi:10.5061/dryad.4f4qrfj95 (Su et al., 2021; Wilson, 2020).
Inclusion Criteria
1) Age ≥18 years old; 2) patients with AKI, which was diagnosed based on the KDIGO (Kidney Disease: Improving Global Outcomes) AKI criterion.
Exclusion Criteria
1) Previous dialysis patients; 2) end-stage renal disease; 3) initial serum creatinine ≥4.0 mg/L; 4) patients receiving hospice care; 5) patients undergoing kidney transplantation within 6 months.
Diagnosis of Acute Kidney Injury
The diagnostic algorithm for AKI (KDIGO AKI criteria) had been incorporated into the epic electronic health record system. A “pop-up” warning for AKI based on the electronic health record, along with an accompanying AKI order, was set when the clinician opens the patient’s medical record. When the system was turned on, the algorithm collected the patient’s associated indices for the purpose of determining if the patient was complicated with AKI. This procedure was part of a system that automatically collects essential indications and generates alerts without human interaction, and the researchers at all participating hospitals were trained to guarantee that the system was implemented correctly and reliably.
Participants
From 29 March 2018 to 14 December 2019, this research enrolled 6,030 individuals with AKI who satisfied the inclusion and exclusion criteria.
Outcome Indicators Involved in This Study
The primary outcomes were AKI progression within 14 days, 14-day dialysis, discharge to home within 14 days, and 14-day mortality.
AKI Progression
AKI progression was defined as a rise in the AKI stage within 14 days based on the KDIGO AKI criterion within 14 days.
Collection of Clinical and Biochemical Data
1) Demographic data: age, sex, and ethnicity; 2) previous medical history: congestive heart failure, CKD, chronic obstructive pulmonary disease (COPD), diabetes, malignancy, and liver disease, as well as the complication index Elixhauser comorbidity score; 3) laboratory examination (when diagnosing AKI): anion gap, bicarbonate, BUN, chloridion, platelets, K+, and serum creatinine; 4) drug use: any diuretic within 24 h of AKI, NSAID treatment within 72 h before the onset of AKI, NSAIDs within 24 h before the onset of AKI, NSAIDs within the 24-h onset of AKI, PPI within 72 h before AKI, ACEi/ARB within 72 h before the onset of AKI, and fluid bolus within the 24-h onset of AKI; 5) other indicators: mean arterial pressure (MAP), SOFA score, nephrology consults, and contrast in prior 72 h; 6) related outcome indicators: 14-day mortality, AKI progression, and dialysis within 14 days. All of the aforementioned indicators were gathered prospectively, following the research protocol.
Statistical Analysis
1) Statistical description: continuous variables were described by mean ± SD, while calculated data were reported using the number of cases and percentage. When comparing two groups, the t-test was used if the continuous variables had a normal distribution, and the variance was homogeneous; otherwise, the non-parametric test was used, and the chi-squared test was used for counting data. 2) The association between NSAIDs and AKI progression, 14-day dialysis, discharge to home, and 14-day mortality was evaluated using univariate and multivariate logistic regression analyses. 3) We conducted a subgroup analysis of age ≥ 65 years, CKD, congestive heart failure, hypertension, and liver disease. 4) Adjustment variables were chosen if they impacted the effect estimate of NSAIDs by more than 10% and were recognized in the literature, and we also eliminated those that were collinear. 5) EmpowerStats 2.0 (Copyright 2009 X&Y Solutions, Inc.) and R software were used to conduct all statistical analyses (3.4.3). p < 0.05 was statistically significant.
Results
Baseline Characteristics of Included Patients
Our study enrolled a total of 6,030 patients with AKI, the median of age was 69.32 67.00 ± 15.38 years, 3,148 (52.21%) patients were male, and the median of mean arterial pressure (MAP) was 84.82 ± 14.65 mmHg. The prevalence estimates of congestive heart failure, CKD, chronic obstructive pulmonary disease (COPD), diabetes, malignancy, and liver disease were 2,658 (44.08%), 2,290 (37.98%), 2,064 (34.23%), 2,290 (37.98%), 931 (15.44%), and 855 (14.18%), respectively. The NSAID treatments within 72 h before the onset of AKI, within 24 h before the onset of AKI, and within the 24-h onset of AKI were , respectively, 791 (13.12%), 600 (9.95%), and 310 (5.14%). The progression estimates of AKI within 14 days, 14-day dialysis, discharge to home, and 14-day mortality in patients with AKI, were, respectively, 948 (15.72%), 199(3.30%), 2,997 (49.70%), and 537 (8.91%) (Table 1).
The Relationship Between NSAIDs and AKI Progression Within 14 Days in Patients With AKI
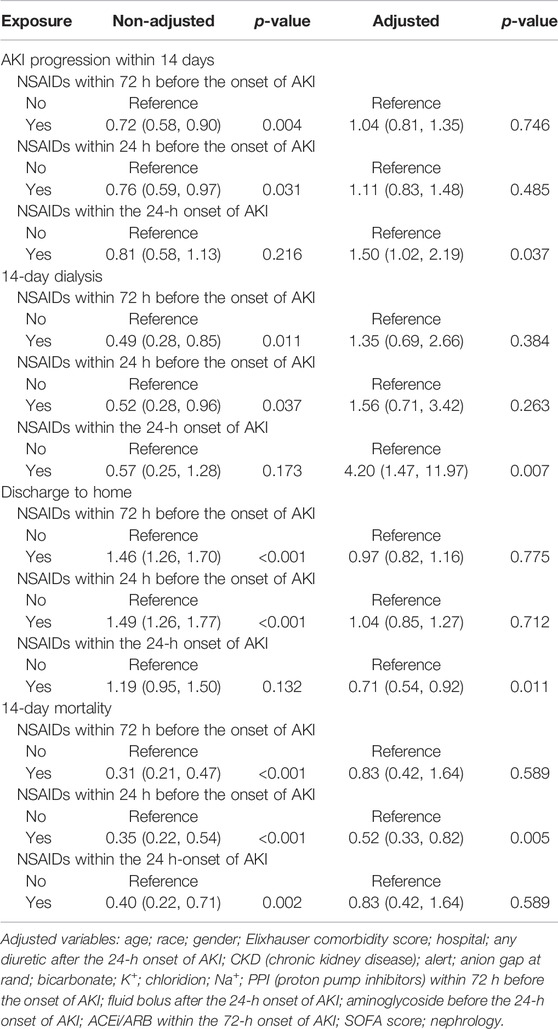
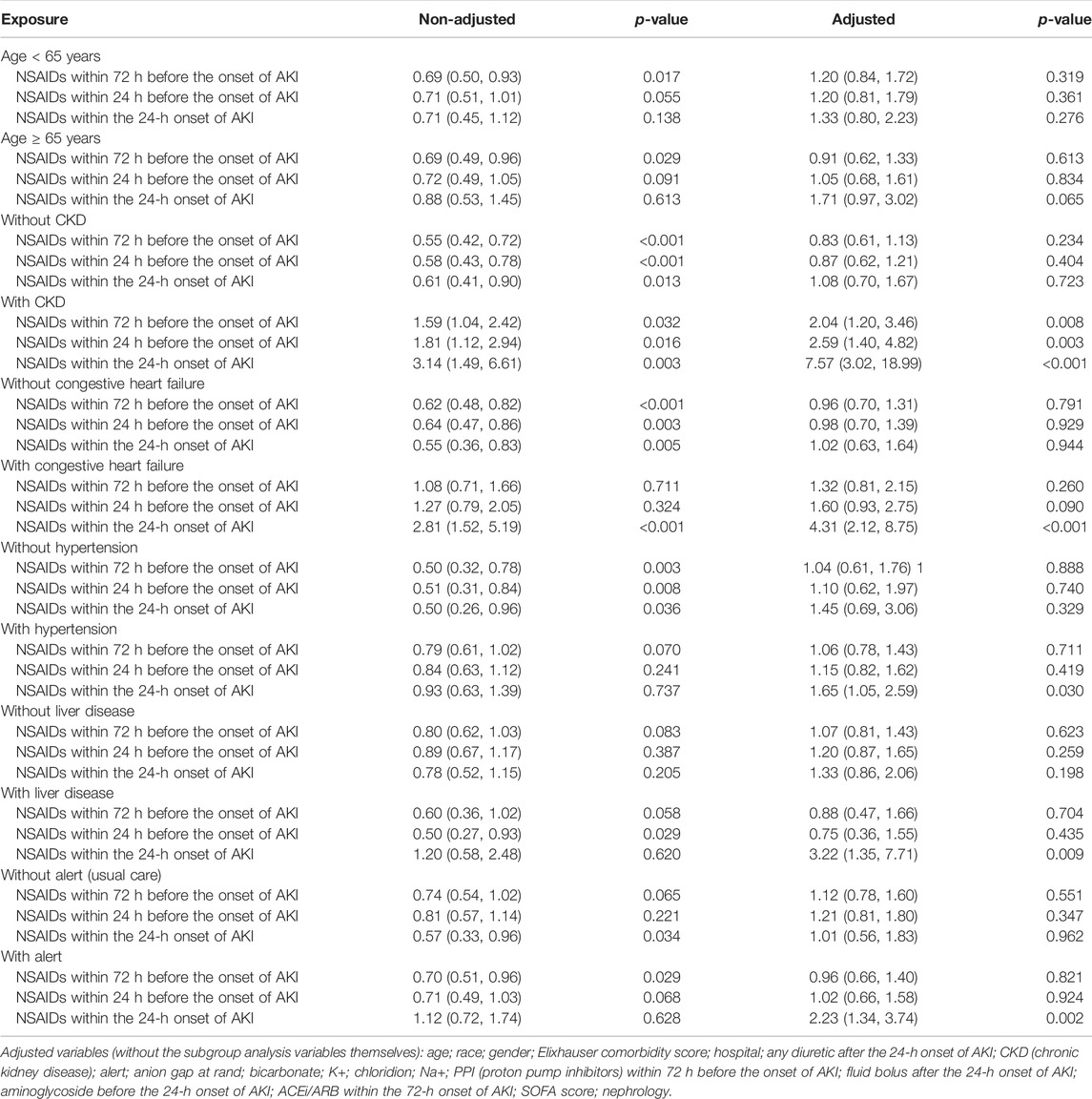
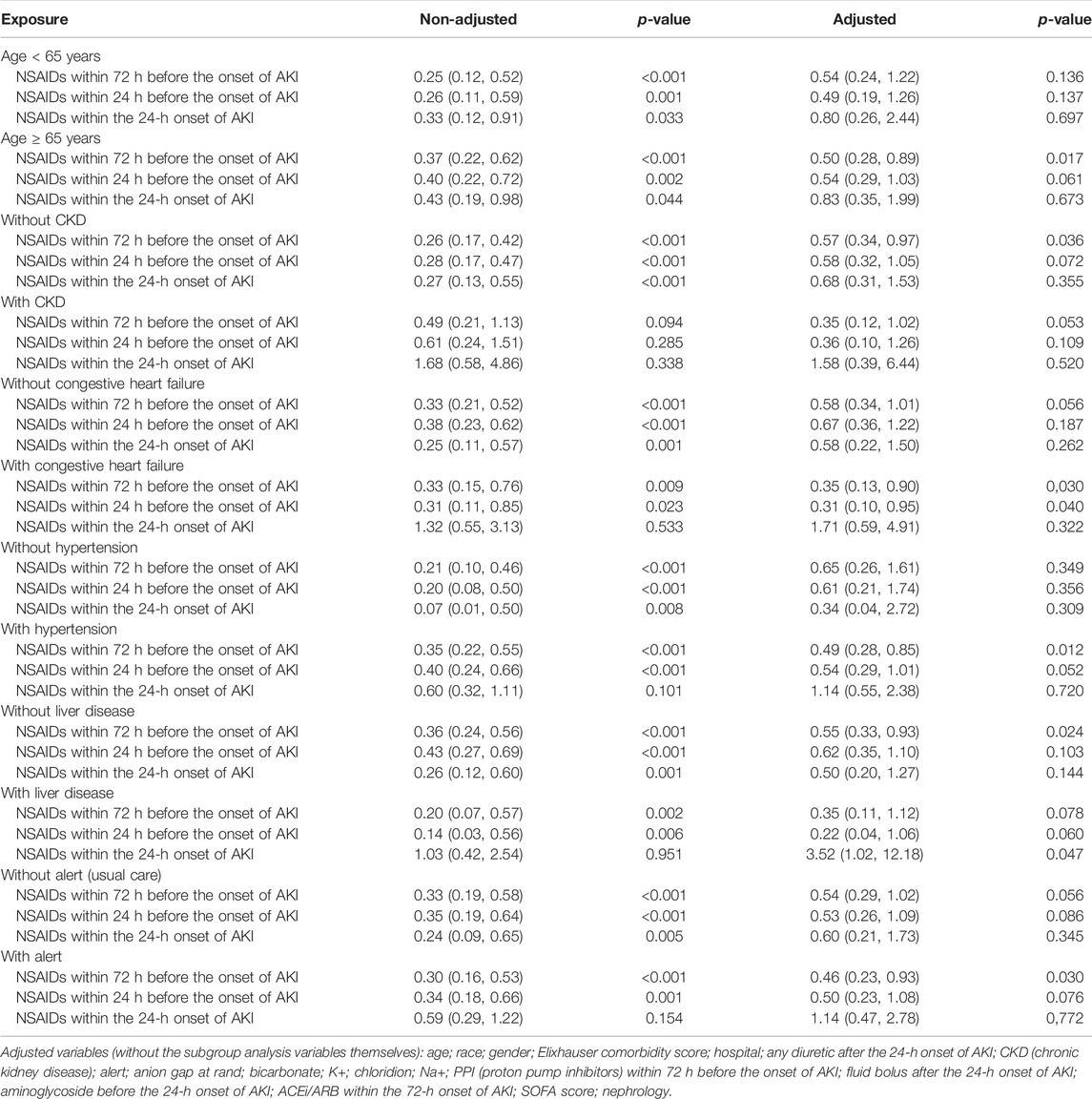
Univariate analysis revealed that NSAIDs within 72 h before the onset of AKI and NSAIDs within 24 h before the onset of AKI might significantly lower the AKI progression, and the OR values were 0.72 (95% CI: 0.58–0.90, p = 0.004) and 0.76 (95% CI: 0.59–0.97, p = 0.031). After adjusting the related confounding variables, it was determined that NSAID treatment 72 h before the onset of AKI and 24 h before the onset of AKI was not associated with the AKI progression. However, NSAID treatments within the 24-h onset of AKI could develop AKI progression; their adjusted OR values were, respectively, 1.04 (95% CI: 0.81–1.35, p = 0.746), 1.11 (95% CI: 0.83–1.48, p = 0.485), and 1.50 (95% CI: 1.02–2.19, p = 0.037). The subgroup analysis showed that in addition to the presence of CKD, NSAID treatments within 72 h before the onset of AKI and NSAID treatments within 24 h before the onset of AKI promoted AKI progression, while other conditions did not promote renal disease progressions, such as whether the age ≥ 65 years or age < 65 years, and whether it was accompanied by congestive heart failure, hypertension, and liver disease. However, NSAID treatment within the 24-h onset of AKI could significantly promote AKI progression in patients with CKD, congestive heart failure, hypertension, liver disease, and alert; their OR values were, respectively, 7.57 (95% CI: 3.02–18.99, p < 0.001), 4.31 (95% CI: 2.12–8.75, p < 0.001), 1.65 (95% CI: 1.05–2.59, p = 0.03) 3.22 (95% CI: 1.35–7.71, p = 0.009), and 2.23 (95% CI: 1.34–3.74, p = 0.002) (Tables 2, 3).
The Relationship Between NSAIDs and 14-Day Dialysis in Patients With AKI
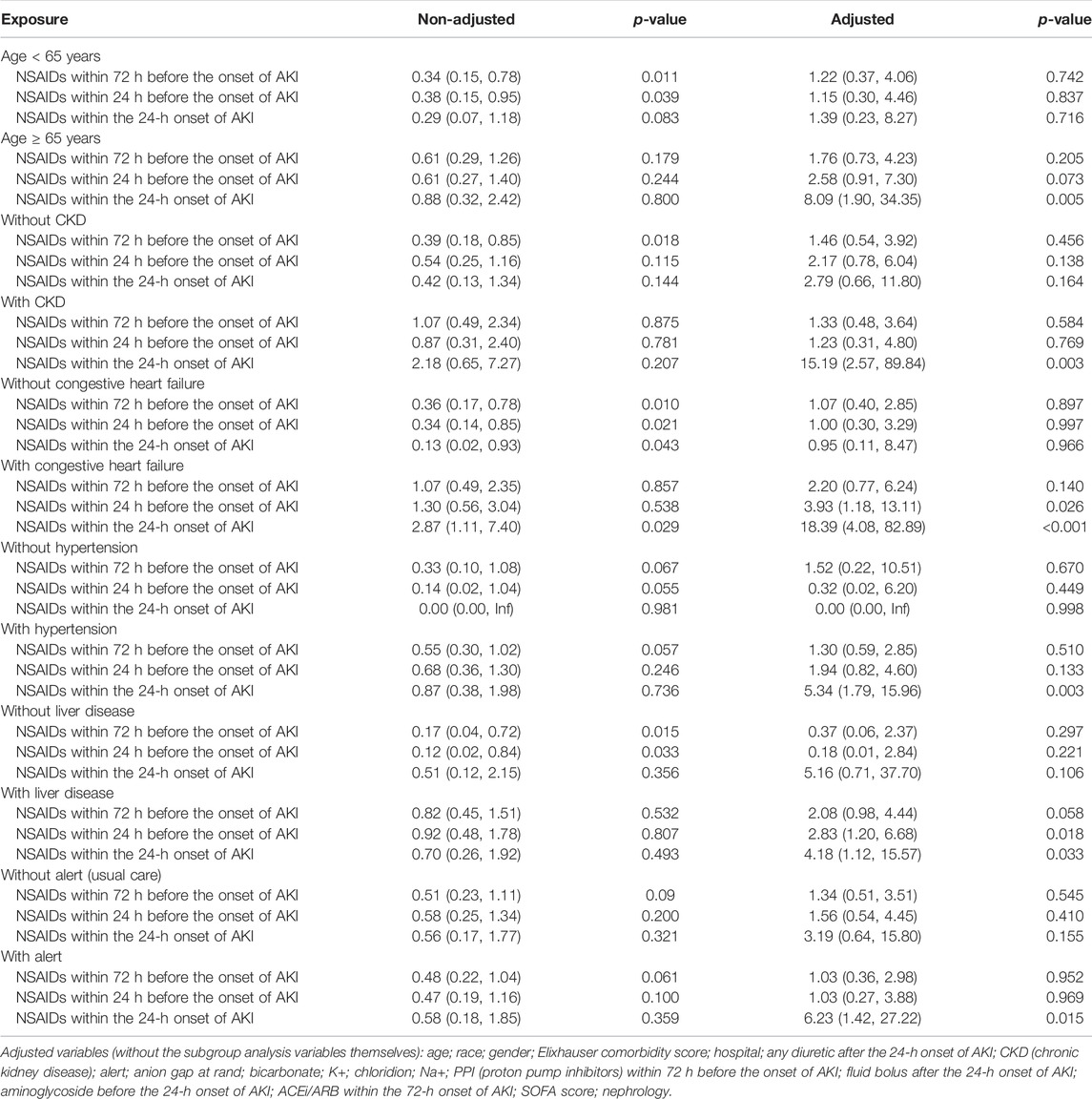
Univariate analysis showed that NSAID treatments within 72 h before the onset of AKI and NSAID treatments within 24 h before the onset of AKI could reduce the 14-day dialysis, their OR values were, respectively, 0.49 (95% CI: 0.28–0.85, p = 0.011) and 0.52 (95% CI: 0.28–0.96, p = 0.037) and that NSAIDs within the 24-h onset of AKI were not associated with the 14-day dialysis; the OR value was 0.57 (95% CI: 0.25–1.28, p = 0.173). After adjusting the related confounding variables, only NSAIDs within the 24-h onset of AKI were associated with the 14-day dialysis, and the adjusted OR was 4.20 (95% CI: 1.47–11.97, p = 0.007). The subgroup analysis revealed that when AKI patients were co-occurring with the following conditions, age ≥ 65 years, CKD, congestive heart failure, hypertension, liver disease, and alert, NSAIDs within the 24-h onset of AKI significantly improved the 14-day dialysis and that in patients with liver disease, NSAIDs within 24 h before the onset of AKI also significantly improved the 14-day dialysis (p < 0.05) (Tables 2, 4).
The Relationship Between NSAIDs and the Discharge to Home in Patients With AKI
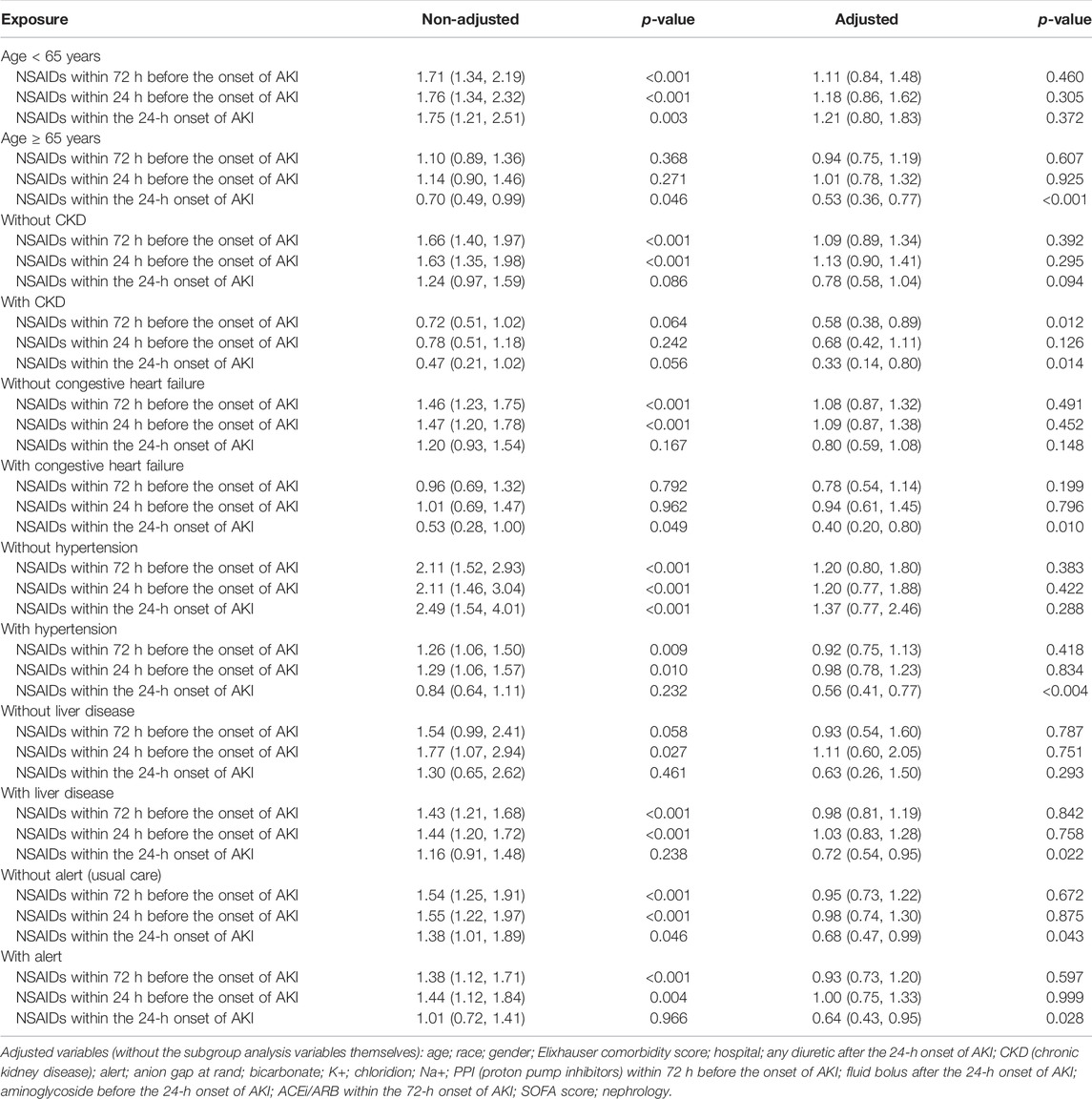
Univariate analysis showed that NSAID treatments within 72 h before the onset of AKI and NSAID treatments within 24 h before the onset of AKI could increase the discharge to home, and their ORs were, respectively, 1.46 (95% CI: 1.26–1.70, p < 0.001) and 1.49 (95% CI: 1.26–1.77, p < 0.001) and that NSAIDs within the 24-h onset of AKI were not associated with the discharge to home, and the OR value was 1.19 (95% CI: 0.95–1.50, p = 0.132). After adjusting the related confounding variables, only NSAIDs within the 24-h onset of AKI decreased the discharge to home, and the adjusted OR was 0.71 (95% CI: 0.54–0.92, p = 0.011). The subgroup analysis revealed that when AKI patients were co-occurring with the following conditions, age ≥ 65 years, CKD, congestive heart failure, hypertension, liver disease, and whether with or without, alert, NSAIDs within the 24-h onset of AKI significantly reduced the discharge to home (p < 0.05) (Tables 2, 5).
The Relationship Between NSAIDs and Death Within 14 Days in Patients With AKI
Univariate analysis showed that NSAID treatments within 72 h before the onset of AKI, NSAID treatments within 24 h before the onset of AKI, and NSAID treatments within the 24-h onset of AKI could decrease the 14-day mortality; their ORs were, respectively, 0.31 (95% CI: 0.21–0.47, p < 0.001), 0.35 (95% CI: 0.22–0.54, p < 0.001), and 0.40 (95% CI: 0.22–0.71, p = 0.002). After adjusting the related confounding variables, only NSAIDs within 24 h before the onset of AKI could reduce the death within 14 days, and the OR value was 0.52 (95% CI: 0.33–0.82, p = 0.005). The subgroup analysis revealed that when AKI patients were complicated with the following conditions, age ≥ 65 years, congestive heart failure, hypertension, and alert, NSAIDs within 72 h before the onset of AKI significantly reduced the 14-day mortality and that in patients with congestive heart failure, NSAIDs within 24 h before the onset of AKI would also significantly reduce the 14-day mortality and that in patients with the liver disease, NSAID treatments within the 24-h onset of AKI could increase the 14-day mortality (Tables 2, 6).
Discussion
This study discovered that taking NSAIDs within 72 and 24 h before the onset of AKI had no association with AKI progression, 14-day dialysis, and discharge to home in patients with AKI. In comparison, only NSAID treatment within the 24-h onset of AKI would promote AKI progression and worsen patient prognosis, especially in patients with age ≥ 65 years, CKD, congestive heart failure, hypertension, liver disease, and alert.
Previous research has established that NSAIDs increase the AKI risk and that patients with AKI have a considerably poorer prognosis. NSAIDs were usually considered a risk factor for triggering AKI, and healthcare workers have been advised to use NSAID medications with caution. However, the reality is that NSAIDs are one of the most commonly used drugs in the clinic and lack effective alternatives, and the standard use of NSAIDs cautiously lacks operability. This study found that the use of NSAIDs before the disease progressed to AKI did not promote the deterioration of renal function, increase dialysis rates, affect discharge to home, and increase mortality in AKI patients. In contrast, NSAIDs in AKI, even if used at the early stage of AKI, would lead to AKI progression and worsen the prognosis of patients with AKI. In a retrospective study of 2,340 people admitted to the ICU due to fractures, who had taken one or more doses of NSAIDs before 48 h of hospitalization, NSAID exposure was not associated with increased AKI progression, decreased AKI improvement, prolonged duration, or increased mortality (Hatton et al., 2020). Another systematic review and meta-analysis showed that perioperative NSAID treatment might be associated with increased disease-free and overall survival after cancer surgery (Shaji et al., 2021). The aforementioned two studies showed that the treatment of NSAIDs in either the early stage of AKI or perioperative periods did not confer adverse outcomes, which was consistent with the conclusions of our study.
Previous studies had shown that the use of NSAIDs in patients with AKI could significantly worsen the disease progression and prognosis (Su et al., 2021). However, our study found that even within the 24-h onset of AKI, 5.14% of patients continued to use NSAIDs and that NSAID treatments within the 24-h onset of AKI promoted AKI progression and 14-day dialysis and reduced the discharge to home. However, our study showed that NSAID treatments within the 24-h onset of AKI did not increase the 14-day mortality. The possible reasons are as follows: 1) first of all, the population involved in this study was different from the previous study population; the previous study population was the population without AKI, to explore whether the use of NSAIDs could induce AKI and worsen the prognosis of patients, while our study explored the effect of the use of NSAIDs on AKI progression and the prognosis of patients based on the fact that the patient’s disease had progressed to AKI. 2) The death outcome of our study was 14-day mortality. Because AKI due to NSAIDs was time- and dose-dependent (Zhang et al., 2017; Lucas et al., 2019), it might be that the dose and temporal effects of NSAIDs had not yet been fully realized, which resulted in a negative result.
Bruce Guthrie discovered that the incidence of AKI caused by NSAIDs was substantially greater in older patients than that in non-elderly individuals in their meta-analysis and systematic evaluation research, and the OR value was 2.51 (95% CI: 1.52–2.68) (Zhang et al., 2017). Kurina’s retrospective study also discovered that the older patients taking NSAIDs have greater risk of AKI (Nelson et al., 2019). Randomized controlled research on the metabolism of NSAIDs discovered that the metabolic rate of NSAIDs dropped considerably with age and that the degree of metabolic rate reduction was strongly related to the age-related loss in the renal function (McKeand et al., 2018). Pharmacologically, NSAIDs have a dose-dependent influence on the severity and risk of AKI. With increasing age, NSAID metabolism slowed, and NSAIDs accumulated in the body; this might be the reason why the elderly’s AKI progression and dialysis with 14 days rose obviously.
In addition, this study discovered that in patients with CKD, NSAIDs promoted AKI progression and decreased discharge to home in patients with AKI. Patients with CKD were more prone to accumulate NSAIDs and further impair kidney function due to their kidney dysfunction (Wu et al., 2015; Wolfe et al., 2021).
Congestive heart failure exacerbates cardiac ejection dysfunction, and hypertension can result in glomerular focal sphericity and local segmental sclerosis, which contribute to renal hypoperfusion (Dalal et al., 2022; Joslin et al., 2022). NSAIDs might reduce prostaglandin production, resulting in renal blood vessel constriction and decreased renal blood flow (Bindu et al., 2020). As a result, using NSAIDs in individuals with congestive heart failure and hypertension might exacerbate renal ischemia and damage. In addition, related studies demonstrated that patients with pre-renal parenchymal damage such as dehydration and hypovolemia and that even short-term NSAID usage might result in renal failure (Makris and Spanou, 2016), which corroborates the preceding assertion.
The use of NSAIDs in patients with liver disorders was observed to promote AKI progression and dialysis within 14 days and decrease the discharge to home in patients with AKI. NSAIDs might disrupt the intestinal barrier, resulting in bacterial translocation and toxic properties entering the liver and causing liver injury or inflammation (Licata et al., 2017; Utzeri and Usai, 2017). The liver is the primary site of NSAID metabolism (Ghlichloo and Gerriets, 2021). When a liver injury occurs, the metabolic rate of NSAIDs in the liver will inevitably decrease, resulting in NSAID buildup in the body and subsequent induction or aggravation of kidney damage. This might be the reason why NSAIDs promote the deterioration of renal function in patients with liver diseases.
This study found that taking NSAIDs in the early stages of AKI had a significant impact on the prognosis of patients with alertness of AKI but had no effect on the prognosis of patients receiving standard therapy. In our opinion, the reason why a patient with AKI was still receiving NSAID medications was that the patient might have additional conditions that continually needed the use of NSAIDs, making it difficult to avoid the ongoing use of NSAIDs.
A retrospective study published in the American Journal of Medicine in 2021, including a total of 31,340 acute pancreatitis patients, found that prior exposure to non-steroidal anti-inflammatory drugs reduces the rate of organ failure and in-hospital mortality in acute pancreatitis (Ladd et al., 2022). Therefore, treatment with NSAIDs before and during the early stages of AKI decreased the chance of developing AKI, which might be related to NSAIDs preventing organ failure. At this time, the precise mechanism by which NSAIDs exert this beneficial effect was unknown. NSAIDs were best known for inhibiting cyclooxygenases, which produce prostaglandins and thromboxanes. Its anti-inflammatory effects extend far beyond cyclooxygenase inhibition, including the inhibition of the pivotal nuclear factor kappa-light-chain-enhancer of activated B cells (NF-B) and the induction of certain types of eicosanoids, which reduced pro-inflammatory mediators such as TNF-, IL-1, and IL-6 (Deligiannidou et al., 2021; Pekacar et al., 2021; Ladd et al., 2022).
Strength of the Study
1) AKI could be diagnosed intermittently in this study using a “pop-up” alert system based on the electronic health record, thereby avoiding missed or delayed diagnosis of AKI; 2) the data for this study were derived from prospective RCT research, and the collected indicators were all objective, ensuring the data’s authenticity and reliability; 3) prior research on NSAIDs had primarily focused on determining if they enhance the risk of AKI. However, little data exist on the optimal usage of NSAIDs in individuals with AKI. This study had practical and educational implications for the appropriate usage of NSAIDs in patients with AKI.
Limitations to the Study
1) The study’s applicability was restricted since the original data did not include the particular medication property name, dosage, and duration of usage for NSAIDs and co-administration of other nephrotoxic medications, including beta lactams and glycopeptides. As a result, further studies were needed to determine the relationship between the different types, dosages, and duration of usage of NSAIDs, and co-administration of other nephrotoxic medications and the outcome of individuals with AKI. 2) The follow-up period was relatively short. It might result in an underestimate of the risk of mortality associated with NSAIDs in patients with AKI. It was required to perform a longer follow-up to elucidate the NSAID mortality risk better. 3) In addition, the reason for admission was not provided, which might also present a potential risk of bias.
Conclusion
Before the onset of AKI, NSAID treatment might be safe, but during the early onset of AKI, it might hasten the progression of AKI and worsen the patient’s prognosis, especially in patients with age ≥ 65 years, CKD, congestive heart failure, hypertension, and liver disease. For the rational use of NSAIDs, the vigilance of AKI should be strengthened; the timely detection of AKI patients and the critical monitoring of special populations were important means to avoid NSAID-related side effects.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://datadryad.org/stash/dataset/doi:10.5061/dryad.4f4qrfj9.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. A new ethical approval was approved by the Medical Bioethics Committee, School of Medicine, Xian Jiaotong University, Xian, Shaanxi province, China (No.2022-468).
Author Contributions
HW participated in the research design, the writing of the manuscript, and data analysis; TL and QL participated in data analysis; RC, XF, YT, and SM participated in the improving and revising of the manuscript; CL and JZ provided substantial advice in designing the study and assisting in the division of labor, writing, and revising the manuscript.
Funding
The study was funded by the Basic Research Program of Natural Science of Shaanxi Province Youth programs, funding number: 2020JQ-548. National Natural Science Foundation of China, fund number: 8207214.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NSAIDs, non-steroidal anti-inflammatory drugs; MAP, mean arterial pressure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; BUN, blood urea nitrogen; SOFA, sequential organ failure assessment; AKI, acute kidney injury; PPI, proton pump inhibitor; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; KDIGO, Kidney Disease: Improving Global Outcomes; ICUs, intensive care units.
References
Baker, M., and Perazella, M. A. (2020). NSAIDs in CKD: Are They Safe? Am. J. Kidney Dis. 76, 546–557. doi:10.1053/j.ajkd.2020.03.023
Bindu, S., Mazumder, S., and Bandyopadhyay, U. (2020). Non-steroidal Anti-inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 180, 114147. doi:10.1016/j.bcp.2020.114147
Challiner, R., Ritchie, J. P., Fullwood, C., Loughnan, P., and Hutchison, A. J. (2014). Incidence and Consequence of Acute Kidney Injury in Unselected Emergency Admissions to a Large Acute UK Hospital Trust. BMC Nephrol. 15, 84. doi:10.1186/1471-2369-15-84
Dalal, R., Bruss, Z. S., and Sehdev, J. S. (2022). Physiology, Renal Blood Flow and Filtration. Treasure Island (FL): StatPearls.
Deligiannidou, G.-E., Gougoula, V., Bezirtzoglou, E., Kontogiorgis, C., and Constantinides, T. K. (2021). The Role of Natural Products in Rheumatoid Arthritis: Current Knowledge of Basic In Vitro and In Vivo Research. Antioxidants 10, 599. doi:10.3390/antiox10040599
Frontera, J. A., Lewin, J. J., Rabinstein, A. A., Aisiku, I. P., Alexandrov, A. W., Cook, A. M., et al. (2016). Guideline for Reversal of Antithrombotics in Intracranial Hemorrhage: A Statement for Healthcare Professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit. Care 24, 6–46. doi:10.1007/s12028-015-0222-x
Ghlichloo, I., and Gerriets, V. (2021). Nonsteroidal Anti-inflammatory Drugs (NSAIDs). Treasure Island (FL): StatPearls.
Hatton, G. E., Bell, C., Wei, S., Wade, C. E., Kao, L. S., and Harvin, J. A. (2020). Do early Non-steroidal Anti-inflammatory Drugs for Analgesia Worsen Acute Kidney Injury in Critically Ill Trauma Patients? an Inverse Probability of Treatment Weighted Analysis. J. Trauma Acute Care Surg. 89, 673–678. doi:10.1097/TA.0000000000002875
Hoste, E. A. J., Kellum, J. A., Selby, N. M., Zarbock, A., Palevsky, P. M., Bagshaw, S. M., et al. (2018). Global Epidemiology and Outcomes of Acute Kidney Injury. Nat. Rev. Nephrol. 14, 607–625. doi:10.1038/s41581-018-0052-0
Jia, W., Jia, Q., Zhang, Y., Zhao, X., and Wang, Y. (2021). Effect of Prediabetes on Asprin or Clopidogrel Resistance in Patients with Recent Ischemic Stroke/TIA. Neurol. Sci. 42, 2829–2835. doi:10.1007/s10072-020-04881-w
Joslin, J. R., Lioudaki, E., and Androulakis, E. (2022). Interrelation between Heart Failure with Preserved Ejection Fraction and Renal Impairment. Rev. Cardiovasc. Med. 23, 69. doi:10.31083/j.rcm2302069
Kolawole, O. R., and Kashfi, K. (2022). NSAIDs and Cancer Resolution: New Paradigms beyond Cyclooxygenase. Ijms 23, 1432. doi:10.3390/ijms23031432
Ladd, A. M., Conwell, D., Burroughs, T. E., and Satish, M. (2022). Prior Exposure to Nonsteroidal Anti-inflammatory Drugs Reduces the Rate of Organ Failure and In-Hospital Mortality in Acute Pancreatitis. Am. J. Med. 135, 471–477. doi:10.1016/j.amjmed.2021.10.020
Licata, A., Minissale, M. G., Calvaruso, V., and Craxì, A. (2017). A Focus on Epidemiology of Drug-Induced Liver Injury: Analysis of a Prospective Cohort. Eur. Rev. Med. Pharmacol. Sci. 21, 112–121.
Lip, G. Y. H., Banerjee, A., Boriani, G., Chiang, C. E., Fargo, R., Freedman, B., et al. (2018). Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest 154, 1121–1201. doi:10.1016/j.chest.2018.07.040
Lucas, G. N. C., Leitão, A. C. C., Alencar, R. L., Xavier, R. M. F., Daher, E. F., Silva Junior, G. B. D., et al. (2019). Pathophysiological Aspects of Nephropathy Caused by Non-steroidal Anti-inflammatory Drugs. J. Bras Nefrol 41, 124–130. doi:10.1590/2175-8239-JBN-2018-0107
Makris, K., and Spanou, L. (2016). Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 37, 85–98.
McKeand, W., Ermer, J., and Korth-Bradley, J. (2018). Assessment of the Effects of Age and Renal Function on Pharmacokinetics of Bazedoxifene in Postmenopausal Women. Clin. Pharmacol. Drug Dev. 7, 920–926. doi:10.1002/cpdd.607
Nelson, D. A., Marks, E. S., Deuster, P. A., O'Connor, F. G., and Kurina, L. M. (2019). Association of Nonsteroidal Anti-inflammatory Drug Prescriptions with Kidney Disease Among Active Young and Middle-Aged Adults. JAMA Netw. Open 2, e187896. doi:10.1001/jamanetworkopen.2018.7896
Pekacar, S., Bulut, S., Özüpek, B., and Orhan, D. D. (2021). Anti-Inflammatory and Analgesic Effects of Rosehip in Inflammatory Musculoskeletal Disorders and its Active Molecules. Curr. Mol. Pharmacol. 14, 731–745. doi:10.2174/1874467214666210804154604
Selby, N. M., Kolhe, N. V., McIntyre, C. W., Monaghan, J., Lawson, N., Elliott, D., et al. (2012). Defining the Cause of Death in Hospitalised Patients with Acute Kidney Injury. PLoS One 7, e48580. doi:10.1371/journal.pone.0048580
Shaji, S., Smith, C., and Forget, P. (2021). Perioperative NSAIDs and Long-Term Outcomes after Cancer Surgery: a Systematic Review and Meta-Analysis. Curr. Oncol. Rep. 23, 146. doi:10.1007/s11912-021-01133-8
Su, L., Li, Y., Xu, R., Luo, F., Gao, Q., Chen, R., et al. (2021). Association of Ibuprofen Prescription with Acute Kidney Injury Among Hospitalized Children in China. JAMA Netw. Open 4, e210775. doi:10.1001/jamanetworkopen.2021.0775
Utzeri, E., and Usai, P. (2017). Role of Non-steroidal Anti-inflammatory Drugs on Intestinal Permeability and Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 23, 3954–3963. doi:10.3748/wjg.v23.i22.3954
Wilson, F. (2020). Randomized Trial of AKI Alerts in Hospitalized Patients. Dryad, Dataset. doi:10.5061/dryad.4f4qrfj95
Wolfe, R., Wetmore, J. B., Woods, R. L., McNeil, J. J., Gallagher, H., Roderick, P., et al. (2021). Subgroup Analysis of the ASPirin in Reducing Events in the Elderly Randomized Clinical Trial Suggests Aspirin Did Not Improve Outcomes in Older Adults with Chronic Kidney Disease. Kidney Int. 99, 466–474. doi:10.1016/j.kint.2020.08.011
Wu, J., Ginsberg, J. S., Zhan, M., Diamantidis, C. J., Chen, J., Woods, C., et al. (2015). Chronic Pain and Analgesic Use in CKD: Implications for Patient Safety. Clin. J. Am. Soc. Nephrol. 10, 435–442. doi:10.2215/CJN.06520714
Ye, Y., Wang, X., Jeschke, U., and von Schönfeldt, V. (2020). COX-2-PGE2-EPs in Gynecological Cancers. Arch. Gynecol. Obstet. 301, 1365–1375. doi:10.1007/s00404-020-05559-6
Keywords: non-steroidal anti-inflammatory drugs, acute kidney injury, AKI progression, dialysis, discharge to home, 14-day mortality
Citation: Wang H, Liu T, Li Q, Cui R, Fan X, Tong Y, Ma S, Liu C and Zhang J (2022) NSAID Treatment Before and on the Early Onset of Acute Kidney Injury Had an Opposite Effect on the Outcome of Patients With AKI. Front. Pharmacol. 13:843210. doi: 10.3389/fphar.2022.843210
Received: 25 December 2021; Accepted: 05 April 2022;
Published: 17 May 2022.
Edited by:
Norberto Perico, Mario Negri Pharmacological Research Institute (IRCCS), ItalyReviewed by:
Rizaldy Taslim Pinzon, Duta Wacana Christian University, IndonesiaBrittany Bissell, University of Kentucky, United States
Copyright © 2022 Wang, Liu, Li, Cui, Fan, Tong, Ma, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chang Liu, liuchangdoctor@163.com; Jingyao Zhang, you12ouy@163.com
†These authors have contributed equally to this work
 Hai Wang
Hai Wang Tong Liu1†
Tong Liu1† Chang Liu
Chang Liu Jingyao Zhang
Jingyao Zhang