Clinical and laboratory parameters associated with febrile seizure recurrence within the first 24 h: a ten-year cohort study
- 1Pediatric Emergency Department, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 2Department of Medicine and Surgery, University of Milan-Bicocca, Monza, Italy
- 3Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy
- 4Pediatric Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 5Department of Clinical Sciences and Community Health, Università Degli Studi di Milano, Milan, Italy
Introduction: We assessed clinical and laboratory parameters associated with early recurrence of febrile seizure in patients presenting at the Emergency Department with a first episode.
Methods: Case series of patients admitted to the emergency department with the first episode of febrile seizure for ten consecutive years. Exclusion criteria were focal features and prolonged duration (>15 min).
Results: We included 693 patients, 284 (41%) female. Median age of 20 (IQR 15–27) months. Fifty-two (8%) patients had a recurrence within 24 h. At univariate analysis, patients with recurrent seizures had higher use of antipyretics (88% vs. 74%, P = 0.03, OR 2.6, 95% CI: 1.1–7.7), higher median maximal body temperature (39.3 °C, IQR 38.9–39.9, vs. 38.9, IQR 38.4–39.3, P < 0.001, OR 2.3, 95% CI: 1.5–2.6) and presented with a lower proportion of respiratory tract infections (54% vs. 70%, P = 0.02) compared to patients without recurrence. A maximal body temperature equal to or higher than 39 °C was associated with a higher recurrence (11% vs. 4%, P < 0.001, OR 2.9, 95% CI: 1.6–5.6). Hyponatremia was not associated with a risk of recurrence. The multivariate analysis confirmed a direct association with body temperature (OR 2.3, 95% CI: 1.5–3.7, P < 0.001), and an inverse association with respiratory tract infections (OR 0.4, 95% CI: 0.2–0.9, P = 0.01), while antipyretic use was not correlated (OR 1.9, 95% CI: 0.8–5.2, P = 0.2).
Conclusions: High body temperature and respiratory tract infections were (directly and inversely) associated with recurrences. Consideration of these conditions might help for anticipating the probability of recurrence.
Introduction
Febrile seizures (FS) are events affecting children between 6 months and 5 years of age, with a peak incidence between 12 and 18 months, associated with fever not associated with any infection of the central nervous system (CNS) or well-defined clinical-related causes (1–3).
They are observed in 2%–5% of children between 6 months and 5 years of age in Western Europe and the United States, being the most common neurological diseases in childhood (1–4).
FS are divided into 2 categories, that is, simple febrile seizures (SFS), primary generalized and shorter than 15 min, without altered mental status following the episode, recurrence within 24 h, and pre-existing neurologic abnormalities. If seizures are prolonged (>15 min), focal or recurrent within 24 h, with pre-existing or post-critical neurologic abnormalities they are defined as complex febrile seizures (CFS) (5–7).
Approximately 30%–40% of children with FS have a recurrence during early childhood (2, 8, 9).
Although most children with FS have only one episode during the same febrile illness, 15%–25% present a recurrence of febrile seizures (RFS) within 24 h following the first episode of febrile seizure (10). Therefore, identifying predictors of RFS could be useful in recognizing these patients and optimizing their management. Few studies have evaluated so far this issue and results are still controversial. Accordingly, predictors of RFS identified in previous studies include a low body temperature at admission to the emergency department (ED), a family history of FS, hyponatremia, seizure type, duration of the seizure, and male sex (11). On the other hand, other studies revealed that these factors were either related or unrelated (12, 13). The aim of our study is, therefore, to identify the predictors of RFS based on a retrospective analysis of clinical and laboratory data collected in our pediatric ED.
Method
We performed a retrospective observational cohort study at a tertiary care Hospital (IRCCS Fondazione Cà Granda Ospedale Maggiore Policlinico) in Milan, Northern Italy, from, January 1st, 2012 to December, 31st, 2021. All patients aged 6 months to 5 years admitted to the ED with a first episode of FS were included. Exclusion criteria were focal features and prolonged duration (>15 min) of seizures, CNS infections, the use of anticonvulsant drugs to treat seizures, the presence of underlying diseases/conditions, such as epilepsy, chromosomal abnormalities, inborn errors of metabolism, perinatal abnormalities, delayed psychomotor development, brain tumors, intracranial hemorrhage, hydrocephalus, or a history of neurosurgery. We retrospectively collected data on demographic, clinical, and biochemical characteristics. The study was approved by The Milano Area 2 ethics committee.
Continuous data are presented as median and interquartile range and categorical data as absolute and relative frequency. To compare patients with and without recurrence of febrile seizures, the chi-square test or Fisher's exact test were used for categorical variables, and the Student's t-test or Mann–Whitney U-test for continuous ones, depending on normal or abnormal distribution tested by Shapiro Wilk test. Variables that resulted significantly associated with recurrence were then included in a multiple logistic regression model. The maximal body temperature in ED was analyzed both as continuous and categorical variable (dichotomized for < or ≥39 °C). Statistical significance was considered as a p-value < 0.05. Statistical analysis was performed using R software (version 3.6.3 for Windows).
Results
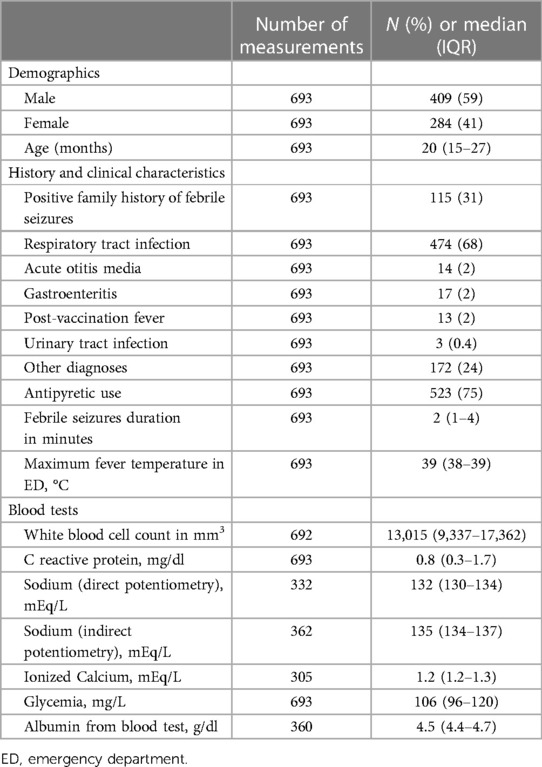
We included 693 patients, 284 (41%) female and 409 (59%) male, with a median age of 20 (IQR 15–27) months. Clinical and biochemical characteristics are summarized in Table 1. Overall, 52 (8%) patients had a recurrence within 24 h after the first event. Table 2 shows the demographic, clinical, and biochemical characteristics of patients with and without recurrence. Patients with RFS reported more frequent use of antipyretics compared with patients without recurrence (88% vs. 74%, P = 0.03, OR 2.6, 95% CI: 1.1–7.7) and higher median maximal body temperature (39.3 °C, IQR 38.9–39.9, vs. 38.9, IQR 38.4–39.3, P < 0.001, OR 2.3, 95% CI: 1.5–2.6). Patients with a maximal body temperature equal to or higher than 39 °C had a rate of recurrence more than twofold compared to other patients (11% vs. 4%, P < 0.001, OR 2.9, 95% CI: 1.6–5.6). Patients with RFS presented a lower rate of respiratory tract infection-related symptoms (54% vs. 70%, P = 0.02). We did not observe other significant differences in demographic, clinical, and biochemical characteristics between patients with or without recurrence of febrile seizures, including natremia, blood glucose, and calcium levels.
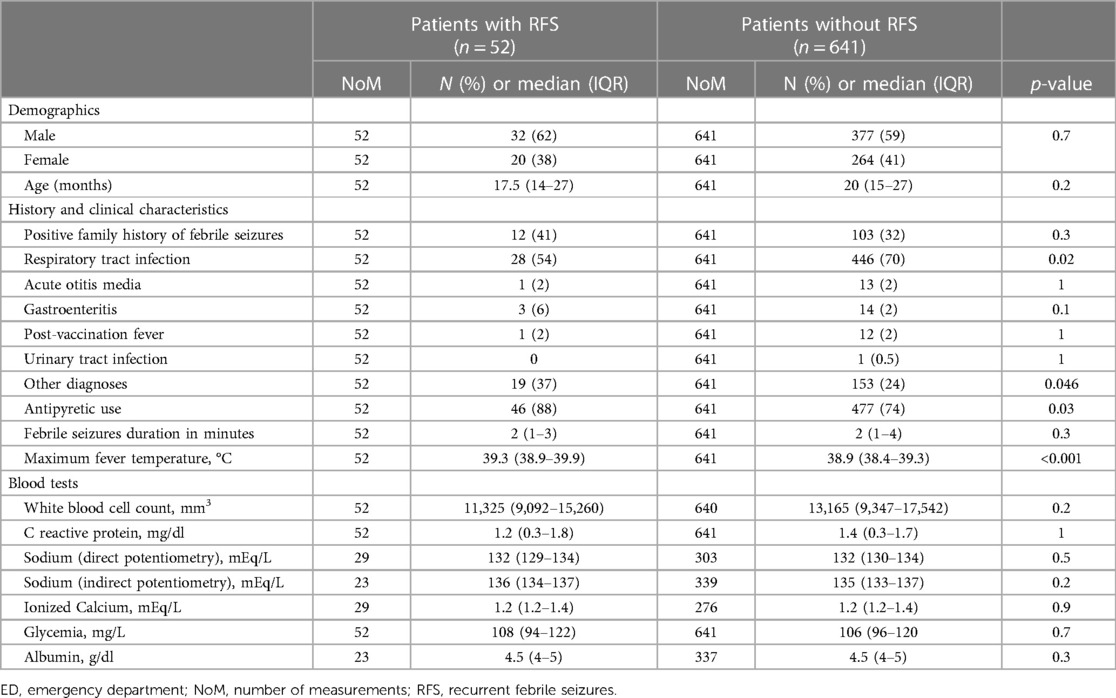
Table 2. Demographic, history, clinical and biochemical characteristics of patients by recurrence of febrile seizures within the first 24 h from the first event.
After adjusting for antipyretic use and clinical presentation, body temperature remained positively correlated with a febrile seizure recurrence in the first 24 h (OR 2.3, 95% CI: 1.5–3.7, P < 0.001), upper respiratory tract infections, and inversely associated with RFS (OR 0.4, 95% CI: 0.2–0.9, P = 0.01). The use of antipyretics was not associated with recurrence (OR 1.9, 95% CI: 0.8–5.2, P = 0.2). Table 3 figures out the variables included in the logistic regression model.
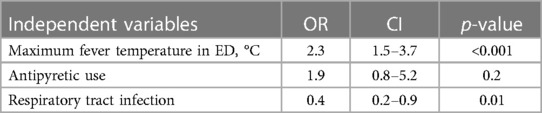
Table 3. Independent variables included in the multiple logistic regression model and their odds ratios, 95% confidence intervals and p-values. The dependent variable was the recurrence of febrile seizure within 24 h from the first event.
Discussion
To our knowledge, this is the largest study evaluating the risk factors for a recurrence of febrile seizures within the first 24 h in a European cohort. Our findings show that patients with a maximum body temperature equal to or higher than 39 °C had higher risk of RFS than other patients. The role of fever as predictor of RFS is still a matter of debate, and different results are found in the literature. In a recent prospective study by Kubota et al. with a total of 109 children, a body temperature <39.2 °C was associated with RFS (P = 0.02) (10). Similar results were observed in a previous retrospective pilot study in 2020 conducted by the same group on 132 children (11). Indeed, those with a body temperature below 39 °C were more likely to experience RFS than those with higher body temperature (14). On the other hand, Jeong et al. did not find an association between body temperature and RFS (13). Moreover, there is a well-known association between a low temperature at the onset of the febrile seizure with late recurrence (after 24 h) (1). While patients with late recurrence are likely to have a low seizure threshold, our findings suggest that physicians should account for high temperature as an early recurrence trigger. We found an association between the use of antipyretics with early recurrence, that was not confirmed by the multivariate analysis. This association is likely secondary to the correlation between fever and recurrence, as it becomes not significant using a multivariate model including temperature. The relationship between antipyretic use and febrile seizure recurrence during the same febrile illness remains controversial. A recent large randomized controlled study suggested the efficacy of antipyretics compared to placebo for preventing RFS within a single event of febrile illness (15). However, a recent systematic review concluded that further studies are required to evaluate the effectiveness of antipyretics in the prevention of RFS (16).
As for natremia, we did not find any difference in sodium levels in patients with RFS and patients without recurrence. The median sodium value at venous gas analysis was 132 mEq/Lboth in patients with and without recurrence. The median value of natremia measured at laboratory analysis was 135 mEq/L in those patients without recurrence and 136 mEq/L in RFS patients. None of these results resulted statistically significant (P = 0.5 for venous gas analysis natremia, P = 0.2 for laboratory analysis). There is no consensus on the potential effect of hyponatremia on RFS. Different studies demonstrated that hyponatremia could be a predictor for recurrence. In a prospective study of 69 children, natremia was significantly lower in children with RFS than in patients without recurrence (17). Similar results were obtained in a recent retrospective study conducted by Alp et al. in which they observed that serum sodium levels were lower in children with RFS than in those without recurrence (134.20 ± 3.55 vs. 138.50 ± 2.38, P < 0.001) (18). A recent meta-analysis conducted by Miyagi et al. concluded that a serum sodium level lower than 134.72 mmol/L was significantly associated with RFS and it could be used as a predictor for recurrent febrile seizure (19). Nevertheless, in a large retrospective study on 315 children by Maksikharin et al. serum sodium levels were not different in children with RFS and in patients without recurrence (134.5 ± 3.2 vs. 134.9 ± 3.1, P = 0.41) (12). Similar results were obtained in the retrospective study of Navaeifar et al. (20). Differently from previous studies, we considered both blood gas analysis and laboratory analysis sodium measurement. According to the literature, sodium measured by blood gas analysis should be preferred because of its higher accuracy compared to laboratory analysis because less interfering with the hemoglobin levels and the circulating non-water fractions (albumin, immunoglobulins, clotting and non-clotting factors, lipids) (21–23).
The association between blood glucose levels and other laboratory parameters with the risk of RFS is controversial. In the retrospective study of Kubota et al., a lower blood glucose level was associated with RFS at univariate analysis (P = 0.047) (11). On the other hand, in another study by Kubota et al. serum glucose, C-reactive protein, and calcium levels were not different between the two groups (10). Similar results were obtained in our study.
Finally, we have found that children with RFS had a lower rate of respiratory tract infections compared to those without recurrence (P = 0.02). On the contrary, this result has not been observed in a retrospective study of Kubota et al. (10). Further studies are needed to better understand the correlation between clinical presentation and the risk of RFS.
Although the large sample size, our study had some limitations. At first, it is a retrospective monocentric study. Moreover, serum sodium was measured by two techniques, but data on this parameter were missing in some cases.
In a large sample of children presenting through 10 years at an Emergency Room, a high body temperature and respiratory tract infections are (directly and inversely) associated with RFS within the first 24 h. On the other hand, serum sodium, calcium, and glucose levels were not associated with a higher risk of recurrence. These findings might help for anticipating the probability of febrile seizure recurrence in childhood.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Milano Area 2 ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
MC: Conceptualization, Writing – original draft, Writing – review & editing. AV: Conceptualization, Formal Analysis, Writing – original draft, Writing – review & editing. MS: Data curation, Writing – original draft. CA: Supervision, Writing – original draft, Writing – review & editing. GD: Writing – original draft. GM: Conceptualization, Formal Analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was (partially) funded by Italian Ministry of Health—Current research IRCCS.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Waruiru C, Appleton R. Febrile seizures: an update. Arch Dis Child. (2004) 89(8):751–6. doi: 10.1136/adc.2003.028449
2. Eilbert W, Chan C. Febrile seizures: a review. J Am Coll Emerg Physicians Open. (2022) 3(4):e12769. doi: 10.1002/emp2.12769
3. Leung AK, Hon KL, Leung TN. Febrile seizures: an overview. Drugs Context. (2018) 7:212536. doi: 10.7573/dic.212536
4. Tiwari A, Meshram RJ, Kumar Singh R. Febrile seizures in children: a review. Cureus. (2022) 14(11):e31509. doi: 10.7759/cureus.31509
5. Febrile seizures: guideline for the neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics. (2011) 127(2):389–94. doi: 10.1542/peds.2010-3318
6. Laino D, Mencaroni E, Esposito S. Management of pediatric febrile seizures. Int J Environ Res Public Health. (2018) 15(10):2232. doi: 10.3390/ijerph15102232
7. Smith DK, Sadler KP, Benedum M. Febrile seizures: risks, evaluation, and prognosis. Am Fam Physician. (2019) 99(7):445–50. 30932454
8. Hampers LC, Spina LA. Evaluation and management of pediatric febrile seizures in the emergency department. Emerg Med Clin North Am. (2011) 29(1):83–93. doi: 10.1016/j.emc.2010.08.008
9. Offringa M, Bossuyt PM, Lubsen J, Ellenberg JH, Nelson KB, Knudsen FU, et al. Risk factors for seizure recurrence in children with febrile seizures: a pooled analysis of individual patient data from five studies. J Pediatr. (1994) 124(4):574–84. doi: 10.1016/s0022-3476(05)83136-1
10. Kubota J, Higurashi N, Hirano D, Okabe S, Yamauchi K, Kimura R, et al. Body temperature predicts recurrent febrile seizures in the same febrile illness. Brain Dev. (2021) 43(7):768–74. doi: 10.1016/j.braindev.2021.03.002
11. Kubota J, Higurashi N, Hirano D, Isono H, Numata H, Suzuki T, et al. Predictors of recurrent febrile seizures during the same febrile illness in children with febrile seizures. J Neurol Sci. (2020) 411:116682. doi: 10.1016/j.jns.2020.116682
12. Maksikharin A, Prommalikit O. Serum sodium levels do not predict recurrence of febrile seizures within 24 h. Paediatr Int Child Health. (2015) 35(1):44–6. doi: 10.1179/2046905514Y.0000000159
13. Jeong JH, Lee JH, Kim K, Jo YH, Rhee JE, Kwak YH, et al. Rate of and risk factors for early recurrence in patients with febrile seizures. Pediatr Emerg Care. (2014) 30(8):540–5. doi: 10.1097/PEC.0000000000000191
14. El-Radhi AS. Lower degree of fever at the initial febrile convulsion is associated with increased risk of subsequent convulsions. Eur J Paediatr Neurol. (1998) 2(2):91–6. doi: 10.1016/s1090-3798(98)80047-0
15. Murata S, Okasora K, Tanabe T, Ogino M, Yamazaki S, Oba C, et al. Acetaminophen and febrile seizure recurrences during the same fever episode. Pediatrics. (2018) 142(5):e20181009. doi: 10.1542/peds.2018-1009
16. Hashimoto R, Suto M, Tsuji M, Sasaki H, Takehara K, Ishiguro A, et al. Use of antipyretics for preventing febrile seizure recurrence in children: a systematic review and meta-analysis. Eur J Pediatr. (2021) 180(4):987–97. doi: 10.1007/s00431-020-03845-8
17. Hugen CA, Oudesluys-Murphy AM, Hop WC. Serum sodium levels and probability of recurrent febrile convulsions. Eur J Pediatr. (1995) 154(5):403–5. doi: 10.1007/BF02072115
18. Alp EK, Elmacı AM. The association between serum sodium levels and febrile seizures recurrence: is the degree of hyponatremia a risk factor? J Pediatr Neurol. (2022) 20:024–7. doi: 10.1055/s-0041-1722851
19. Miyagi Y, Sasano T, Kato H, Kin K. Hyponatremia and recurrent febrile seizures during febrile episodes: a meta-analysis. Cureus. (2022) 14(4):e24398. doi: 10.7759/cureus.24398
20. Navaeifar MR, Abbaskhanian A, Farmanbarborji A. Relation between febrile seizure recurrence and hyponatremia in children: a single-center trial. J Pediatr Neurosci. (2020) 15(1):5–8. doi: 10.4103/JPN.JPN_4_19
21. Milani GP, Edefonti V, De Santis R, Agostoni C, Spolidoro GCI, Pelucchi C, et al. Disagreement between direct and indirect potentiometric na+ determination in infancy and childhood. Clin Chem Lab Med. (2020) 58(4):e117–9. doi: 10.1515/cclm-2019-0931
22. Lavagno C, Milani GP, Uestuener P, Simonetti GD, Casaulta C, Bianchetti MG, et al. Hyponatremia in children with acute respiratory infections: a reappraisal. Pediatr Pulmonol. (2017) 52(7):962–7. doi: 10.1002/ppul.23671
Keywords: febrile seizure, recurrence, children, risk factors, fever
Citation: Castellazzi ML, La Vecchia A, Scali M, Agostoni C, Di Pietro G and Milani GP (2024) Clinical and laboratory parameters associated with febrile seizure recurrence within the first 24 h: a ten-year cohort study. Front. Pediatr. 12:1373848. doi: 10.3389/fped.2024.1373848
Received: 20 January 2024; Accepted: 21 February 2024;
Published: 4 March 2024.
Edited by:
Piero Pavone, University of Catania, ItalyReviewed by:
Doina Anca Plesca, Carol Davila University of Medicine and Pharmacy, RomaniaMohammad Bagher Rahmati, Hormozgan University of Medical Sciences, Iran
© 2024 Castellazzi, La Vecchia, Scali, Agostoni, Di Pietro and Milani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Luca Castellazzi luca.castellazzi@policlinico.mi.it
Abbreviations FS, febrile seizures; CNS, central nervous system; SFS, simple febrile seizures; CFS, complex febrile seizures; RFS, recurrence of febrile seizures; ED, emergency department.
 Massimo Luca Castellazzi
Massimo Luca Castellazzi Adriano La Vecchia
Adriano La Vecchia Martina Scali3
Martina Scali3  Carlo Agostoni
Carlo Agostoni Gregorio Paolo Milani
Gregorio Paolo Milani