Single-port-plus-one robot-assisted laparoscopic modified Lich-Gregoir direct nipple ureteral extravesical reimplantation in children with a primary obstructive megaureter
- 1Department of Pediatric Surgery, Children Medical Center, Fujian Provincial Hospital, Fuzhou, China
- 2Shengli Clinical Medical College of Fujian Medical University, Fuzhou, China
Purpose: To introduce a new technique of single-port-plus-one robotic laparoscopic-modified Lich-Gregoir direct nipple ureteral extravesical reimplantation and ascertain its validity in the treatment of pediatric primary obstructive megaureter.
Methods: Between January 2021 and November 2021, we retrospectively analyzed the clinical data of 12 children with primary obstructive megaureter who were admitted to the Department of Pediatric Surgery of Fujian Provincial Hospital. All 12 children were treated with single-port-plus-one robotic laparoscopic Lich-Gregoir direct nipple ureteral extravesical reimplantation. Five of them were female and seven were male, including nine cases were simple obstructive type, while the remaining three cases were obstructive with reflux type. The mean age of the children was 17.33 ± 6.99 (10–36) months and the mean follow-up time was 14.16 ± 1.75 (12–17) months. Changes in preoperative and first-year postoperative parameters were compared.
Results: The mean operative time for all 12 children was 123.58 ± 10.85 (110–145) min, with a mean internal operative time of 101.42 ± 0.85 (90–120) min, a mean operative bleeding time of 2.42 ± 0.67 (2–4) ml, and a mean hematuria duration of 16.08 ± 1.44 (14–19) h. The mean indwelling catheterization time was 2.25 ± 0.45 (2–3) days and the mean hospitalization time was 3.83 ± 0.39 (3–4) days. At the postoperative first year, the ureteral diameter, calyceal diameter, and anterior–posterior renal pelvic diameter were found to be significantly smaller than at the preoperative period (18.83 ± 3.21 mm vs. 6.83 ± 1.27 mm, 13.99 ± 3.58 mm vs. 3.5 ± 2.90 mm, and 34.92 ± 4.25 mm vs. 10.08 ± 1.88 mm, P < 0.001). There was a significant increase in renal cortical thickness and the percentage of differential renal function (3.63 ± 1.66 mm vs. 5.67 ± 1.88 mm, 33.75 ± 2.77 mm vs. 37.50 ± 1.31 mm, P < 0.001). The resolution rate of obstruction was 100% and no child developed DeNovo vesicoureteral reflux.
Conclusion: The technique of modified Lich-Gregoir direct nipple ureteral extravesical reimplantation can help maintain the physiological direction of the ureter and at the same time enhance the effectiveness of antirefluxing in robotic surgery. The design of a single-port-plus-one wound can produce a cosmetic appearance by concentrating and hiding the wound around the umbilicus. This modified reimplantation procedure has the potential to become a promising technique in the robot-assisted treatment of primary obstructive megaureter.
Introduction
The term Primary Obstructive Megaureter (POM), which was coined by Caulk in 1923 (1), results in hindered urine evacuation and dilates the ureter and renal collecting system (2). Most will resolve over time. However, in approximately 20% of patients with uncontrolled urinary tract infection (UTI) or high-grade or progressive obstruction, ureteral reimplantation is required (3). Traditionally, open ureteral reimplantation is the gold standard for primary obstructive megaureter. In the past decade, pediatric urologists have frequently implemented minimally invasive procedures by using laparoscopic, robotic-assisted, and other devices. The Da Vinci robot–assisted laparoscopic surgery platform, which is characterized by a 6° freedom articulation, tremor filtering, and stereoscopic vision, provides distinct advantages in intracorporeal reparation and suturing (4). These advantages have expanded the role of robot-assisted surgery in complex lower urinary tract reconstructive surgeries, which are possibly time-consuming due to the narrow pelvic space (5). To promote the use of robot-assisted surgery for the treatment of obstructed megaureter and to achieve optimal outcomes, herein, we implemented a novel technique called “single-port-plus-one robotic laparoscopic-modified direct nipple Lich-Gregoir extravesical reimplantation”, in an attempt to minimize technical difficulties. We reviewed our prospectively collected data and assessed the short-term safety and feasibility of the procedure.
Patients and methods
Between January 2021 and November 2021, we retrospectively reviewed 12 patients with POM who were admitted to the Department of Pediatric Surgery of Fujian Provincial Hospital, of whom five were females and seven were males. Their mean age was 17.33 ± 6.99 (10–36) months and the mean follow-up time was 14.16 ± 1.75 (12–17) months. Out of 12 patients, five are on the right side and seven are on the left side. The patients on the right are all obstructive. Among the patients on the left, four are obstructive and three are obstructive with reflux. A prenatal diagnosis was carried out in all 12 patients. Two patients experienced abdominal pain and three presented with UTIs.
All patients underwent a preoperative magnetic resonance urography (MRU), urological ultrasound, diuretic renogram (99 m Tc-DTPA), renal static imaging (99 m Tc-DMSA), and voiding cystourethrography (VCUG). All patients presented with a worsening dilatation (6) of 5 UTDP3 grade and 7 UTDP2 grade, and all of them had a deteriorating differential renal function (DRF) and obstructive curves on serial scans. A preoperative VCUG was done in all patients, and three of them had a vesicoureteral reflux (VUR), with grade 1 in two and grade 3 in one.
All patients underwent single-port-plus-one robotic laparoscopic-modified direct nipple Lich-Gregoir extravesical reimplantation. Five of them underwent ureteral tapering repair, which was performed by using Hendren's technique. Informed consent was obtained from the parents of the children, and the ethical review of the study was performed in our institution (ethics approval number: K2020-12-033).
In the postoperative second month, the patients were readmitted to the hospital for cystoscopy, observation of the ureteral orifice pattern, and removal of the double J tube. On the first year after surgery, the patients again returned for a urological ultrasound, MRU, diuretic nephrography, renal static imaging (99m Tc-DMSA), and VCUG to assess their recovery. Also, on the first year after surgery, all patients underwent a cystoscopy for an observation of the ureteral orifice, and the F3 ureter catheter was passed through the ureteral orifice to confirm no kicking in the ureter.
Inclusion and exclusion criteria
Inclusion criteria: POM patients with symptoms such as febrile UTIs or pain were included in the study (7). Asymptomatic patients with a DRF below 40% and which was associated with massive or progressive hydronephrosis, or a drop of >5% in differential function, were included.
Exclusion criteria: Patients with secondary giant ureters caused by ectopic ureteral opening, neurogenic bladder, posterior urethral valve, or urethral stenosis were excluded from the study (7).
Surgical procedures
All robot-assisted laparoscopic surgeries were performed by the same surgeon and surgical team.
After administering a successful general anesthesia, the patients were kept in the supine position, with head low and foot high, the bilateral upper limbs were placed in a “surrender” position, and the bilateral upper limbs were slightly opened. All parts of the body under pressure were padded with a sponge and fixed with bandages. Disinfecting, draping, and urethral catheterization were performed before the starting the single-port-plus-one robotic laparoscopic-modified Lich-Gregoir direct nipple ureteral extravesical reimplantation.
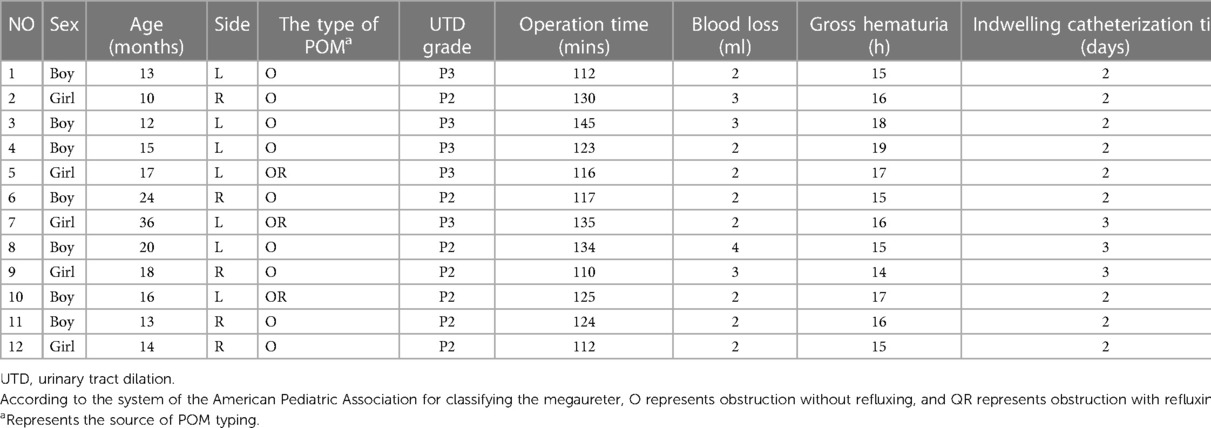
Although the da Vinci system Xi has four robotic arms, we used only three [three-dimensional (3D) camera arm III, operating arm II, and operating arm IV] and inserted a quadruple-channel puncture device to improve cosmetic appearance. We made a 2.5–3 cm curved incision along the edge of the umbilical cord to place a quadruple-channel puncture device, the four channels of which were used to insert an 8 mm 3D camera port III and an 8 mm operating port IV and were also used as assistant channels in a subsequent operation. Artificial pneumoperitoneum was established with a pressure rate of 10 mmH2O and a flow rate of 4 L/min. Another 8 mm incision was made 6 cm away from 3D camera port III on the right side of the abdomen for inserting robotic operating port II, as shown in Figures 1A,B.
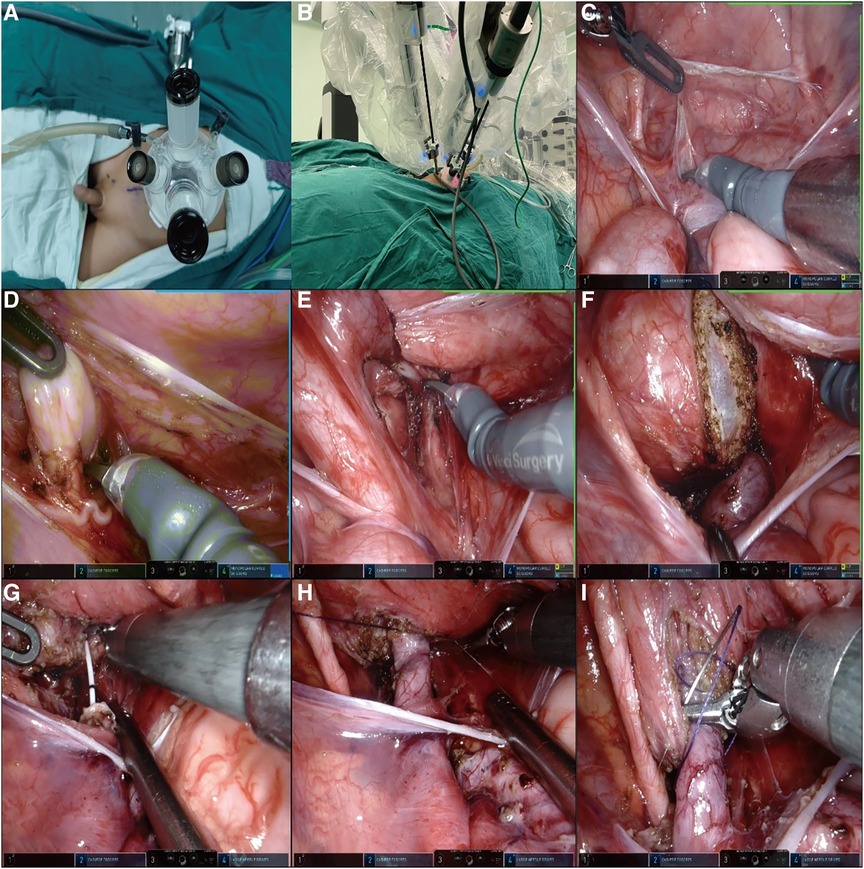
Figure 1. (A,B) A 2.5–3 cm curved incision was made along the edge of the umbilical cord to place a quadruple-channel puncture device. Another 8 mm incision was made 6 cm away from 3D camera port III on the right side of the abdomen for inserting the robotic operating port II. (C,D) We established a “peritoneal window,” in which the vas or uterine artery was directly identified from the ureter. (E,F) The direction of the detrusor tunnel was designed obliquely. The bladder detrusor was cut and disassembled until bulging of the bladder mucosa occurred, the length of the incision is five times the normal width of the ureter. (G,H) The overdilated ureter was cut and shaped and a double J tube was inserted into the ureter. The 1.5 cm end of the ureter was placed into the bladder. The seromuscular layer of the ureter and the bladder mucosa were sutured at 3, 6, 9, and 12 o' clock positions with 6-0 PDS. Finally, four stitches were made in each quadrant to complete the anastomosis. (I) A “down–top” method was applied to complete an intermittent incision of the cut detrusor suture with 4-0 Vicryl.
Instead of using the traditional two peritoneal incisions to identify the ureter, we devised a new method of creating a “peritoneal window.” We used a transverse incision to open the peritoneal layer along the surface of the posterior bladder wall, dissected the peritoneal layer from the posterior bladder wall on both sides, and then used an undamaged vessel clip to fix the peritoneal layer with the surrounding tissue. In these ways, we established a “peritoneal window,” in which the vas or uterine artery could be directly identified from the ureter, as shown in Figures 1C,D.
The direction of the detrusor tunnel was designed obliquely and a local electrocoagulation marker was generated. Traction sutures of the posterior bladder wall were inserted through the abdominal wall above the middle of the pubic symphysis and pulled out from the inserted position. In this way, the posterior bladder wall was pulled obliquely to the abdominal wall, and the detrusor tunnel was kept straight, which was convenient for cutting and dissecting. Inject saline into the bladder through a catheter to fill it. The bladder detrusor was cut and disassembled until bulging of the bladder mucosa occurred, the length of the incision is five times the normal width of the ureter. The outward and upward detrusor tunnel was more in line with the physiological direction of the ureter, as shown in Figures 1E,F.
The stricture segment of the ureter was resected, the overdilated ureter was cut and shaped as needed, and a double J tube was inserted into the ureter. Then, the 1.5 cm end of the ureter was placed into the bladder. Next, we sutured the seromuscular layer of the ureter and the bladder mucosa at 3, 6, 9, and 12 o’ clock positions with 6-0 PDS, and finally, four stitches were made in each quadrant to complete the anastomosis, as shown in Figures 1G,H.
The “down–top” method was applied to complete an intermittent incision of the cut detrusor suture with 4-0 Vicryl, as shown in Figure 1I. Finally, the peritoneal layer of the posterior bladder wall was closed and the wound was sutured.
Results
The mean operative time for all 12 children was 123.58 ± 10.85 (110–145) min, with a mean internal operative time of 101.42 ± 0.85 (90–120) min, a mean operative bleeding of 2.42 ± 0.67 (2–4) ml, and a mean hematuria duration of 16.08 ± 1.44 (14–19) h. The mean indwelling catheterization time was 2.25 ± 0.45 (2–3) days, and the mean hospitalization time was 3.83 ± 0.39 (3–4) days, as shown in Table 1.
At the postoperative first year, the ureteral diameter, calyceal diameter, and anterior–posterior renal pelvic diameter (APRPD) were found to be significantly smaller than at the preoperative period (18.83 ± 3.21 mm vs. 6.83 ± 1.27 mm, 13.99 ± 3.58 mm vs. 3.5 ± 2.90 mm, and 34.92 ± 4.25 mm vs. 10.08 ± 1.88 mm, P < 0.001) (Figures 2A,B: the yellow arrow). There was a significant increase in renal cortical thickness and the DRF percentage (3.63 ± 1.66 mm vs. 5.67 ± 1.88 mm, 33.75 ± 2.77 mm vs. 37.50 ± 1.31 mm, P < 0.001), as shown in Table 2.
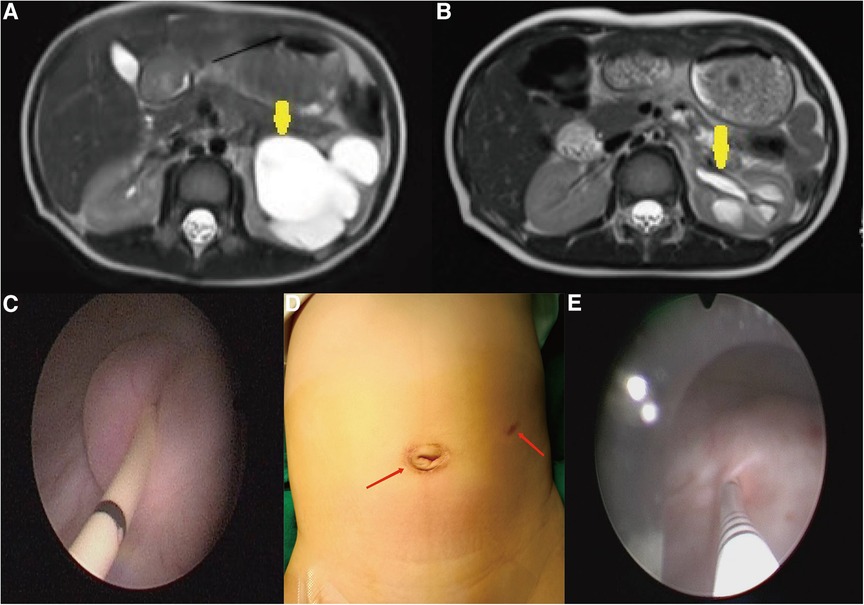
Figure 2. (A,B) At the postoperative first year, there was a significant reduction in APRPD and ureteral diameter compared with those before the operation (yellow arrow). (C) The end of the ureter that was placed into the bladder was found to turn into nipples automatically in cystoscopy at the postoperative second month. (D) Cosmetic appearance of the wound at the postoperative second month (the scar is marked by a red arrow). (E) In the first year after surgery, the F3 ureter catheter was passed through the ureteral orifice smoothly into the ureter, confirming no kicking in the ureter.
In the postoperative period, no patient developed urinary retention, urinary extravasation, and wound infection. Two patients developed postoperative UTI treated conservatively and no one demonstrated reflux on VCUG. The resolution rate of obstruction was 100% and no patient developed DeNovo VUR.
At the postoperative second month, the patients were readmitted to the hospital for a cystoscopy for removal of the double J catheter, and the end of the ureter that was placed into the bladder was found to turn into nipples automatically, as shown in Figure 2C, and the wounds were cosmetic, as shown in Figure 2D. On the first year after surgery, all patients underwent cystoscopy for observation of the ureteral orifice again, and the F3 ureter catheter was passed through the ureteral orifice smoothly into the ureter, which confirmed no kicking in the ureter, as shown in Figure 2E. The trends of variations in the ureteral diameter, APRPD, and calyceal diameter at the preoperative and 1-year postoperative time points are shown in Figure 3. The trends of renal cortex thickness and DRF variation at the preoperative and 1-year postoperative time points are shown in Figure 4.
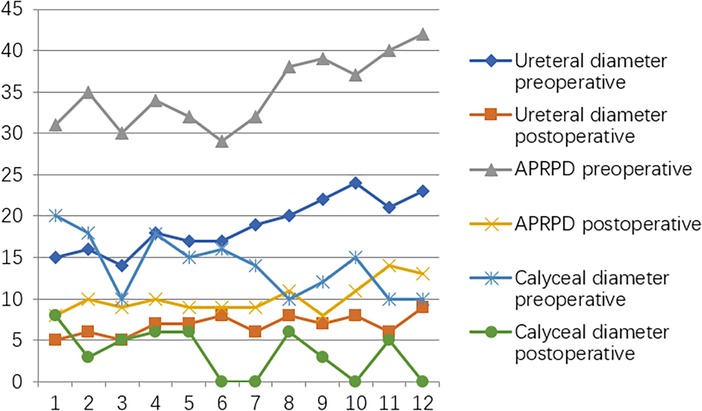
Figure 3. Trends in variations in ureteral diameter, APRPD, and calyceal diameter at the preoperative and one-year postoperative time points.
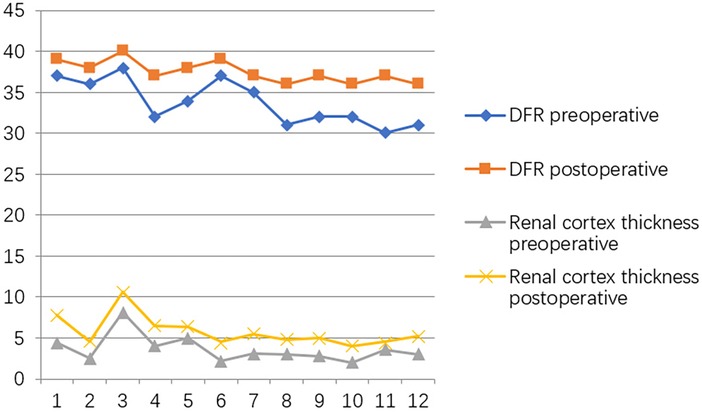
Figure 4. Trends in variations in renal cortex thickness and DRF variation at the preoperative and one-year postoperative time points.
Discussion
According to the International Classification of Diseases, congenital megaureter can be classified either as obstructed and refluxing or as unobstructed and unrefluxing (7). The treatment goal of POM is to relieve obstruction while establishing a new and effective antireflux mechanism. The primary minimally invasive surgical approaches for the correction of an obstructed megaureter that were used so far and are still used today are laparoscopic extravesical reimplantation and the pneumovesical laparoscopic approach (8, 9). Pneumovesical laparoscopic approaches such as Cohen and Leadbetter are often technically challenging and require a long curve, even for the most experienced laparoscopic surgeons. In infants and fat adolescents, they are often more challenging due to the small bladder volume and thickness of the abdominal wall (10, 11). In recent years, the robot-assisted laparoscopic platform with a stereoscopic vision and flexible arms has provided a new opportunity for the laparoscopic extravesical approach for POM.
Two robotic-assisted laparoscopic ureteral extravesical reimplantation methods for the treatment of POM have been described in recent studies. One is robotic-assisted extravesical cross-trigonal ureteral reimplantation (12, 13). In 2020 (13), Neheman et al. described a novel surgical approach in the pediatric population and found that anastomosis was more ergonomic when performing a horizontal detrusorrhaphy, as opposed to a vertical one, and the submucosal tunnel was easier to extend. But the technique also had disadvantages in the form of challenges with endoscopic ureteral intubation during follow-up, if needed, postoperative cytoplasm, and urinary retention.
The other method is robotic-assisted extravesical Lich-Gregoir ureteral reimplantation, which is mainly used in high-grade VUR surgeries (14, 15). We reviewed the currently published literature on robot-assisted laparoscopic ureteral reimplantation (RALUR) and found that the VUR resolution rates widely ranged from 66.7% to 100%, which may be attributed to a submucosal tunnel of insufficient length (15).
In this investigate, we implemented a new method by maintaining the vertical submucosal tunnel in the Lich-Gregoir technique and adding ureteral direct nipple implantation to produce an antireflux effect. As indicated by previous reports, traditional ureteral nipple implantation is not convenient to perform in adults (16, 17). Al-Shukri and Alwan (18) first reported direct nipple ureteroneocystostomy in adults with a ureteral stricture (19). In 2014, Fu et al. also used this method in robot surgeries in adult megaureters. In our new technique, we used this method in the pediatric population. In all 12 children in our study, the new ureteral orifice automatically acquired a papillary shape upon cystoscopy 2 months after surgery. At the postoperative first year, the ureteral diameter, calyceal diameter, and APRPD were found to be significantly smaller than at the preoperative period. There was a significant increase in renal cortical thickness and DRF percentage. The resolution rate of obstruction was 100% and no child developed DeNovo VUR on VCUG. The results of the postoperative tests showed that this strategy was simple, safe, and feasible. In particular, the ureteral direct nipple implantation also shortened the anastomosis time, and the vertical submucosal tunnel remained in the physiological direction of the ureter, which was confirmed by the smooth passage of the 3F ureteral catheter upon cystoscopy at the postoperative 1-year period.
According to research by Leissner et al. of human cadaver dissection, the main portion of the pelvic plexus is located at about 1.5 cm dorsal and medial to the ureterovesical junction. The bundles of the pelvic plexus end at the distal ureter, trigone, and rectum (20). The oblique detrusor tunnel designed in the Lich-Gregoir technique may help preserve the neurovascular bundles and avoid potential voiding issues such as urinary retention during mobilization of the ureter.
Traumas experienced by children will get magnified as they grow up. That is why minimally invasive surgery has gradually become the mainstream treatment in the pediatric population in recent years. Unlike the four scattered wounds (three robotic port wounds and one assisted laparoscopic port wound) in the abdomen wall (21, 22), the wounds created by the single-site-plus-one technique are concentrated and hidden around the umbilical cord, which are more concealed when children grow up. When 5 mm robotic arms or the Da Vinci SP system are used by Chinese surgeons in the future, there is a possibility that all robotic arms are concentrated in a single-site port to realize a successful single-site robot-assisted surgery.
Conclusion
The technique of modified Lich-Gregoir ureteral direct nipple extravesical reimplantation can help maintain the physiological direction of the ureter and at the same time enhance the effectiveness of antirefluxing in robotic surgery. The design of a single-port-plus-one wound can produce a cosmetic appearance by concentrating and hiding the wound around the umbilicus. This modified reimplantation procedure has the potential to become a promising surgical technique in the robot-assisted treatment of POM.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the institutional review board (IRB) of the Fujian Provincial Hospital (Ethics No: K2020-12-033). The studies were conducted in accordance with local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YH wrote the manuscript. SL and XX collected data, performed the statistical analysis, and revised the manuscript. SH, HX, and GY collected data and performed the statistical analysis. SL, XX, and DX conceptualized the study. JC and DX revised the manuscript and funded the work. DX was the primary surgeon. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Talent Induction Projection of Fujian Province (Grant Number: 2021YJ03), the Natural Science Foundation of Fujian Province (Grant Number: 2021J01386), the Medical Innovation Projection of Fujian Provincial Health Department Foundation (Grant Number: 2020CXB004), the Science and Technology Innovation Joint of Fujian Province (Grant Number: 2021Y9019), and the Natural Science Foundation of Fujian Province (Grant Number: 2022J05208).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
UR, ureteral reimplantation; VUR, vesicoureteral reflux; POM, primary obstructive megaureter; DRF, differential renal function; APRPD, anterior–posterior renal pelvic diameter; MIS, minimally invasive surgery; UTIs, urinary tract infections; UTDS, urinary tract dilation risk stratification; VCUG, voiding cystourethrogram; MRU, magnetic resonance urography; DMSA, dimercaptosuccinic acid.
References
2. Pfister RC, Hendren WH. Primary megaureter in children and adults. Clinical and pathophysiologic features of 150 ureters. Urology. (1978) 12(2):160–76. doi: 10.1016/0090-4295(78)90327-8
3. Zhu W, Zhou H, Cao H, Li P, Tao Y, Ma L, et al. Modified robot-assisted laparoscopic infantile ureteral reimplantation technique for obstructive megaureter. J Pediatr Surg. (2022) 57(12):1011–7. doi: 10.1016/j.jpedsurg.2022.05.015
4. Bindi E, Todesco C, Nino F, Torino G, Gentilucci G, Cobellis G. Robotic surgery: is there a possibility of increasing its application in pediatric settings? A single-center experience. Children (Basel). (2022) 9(7):1021. doi: 10.3390/children9071021
5. Arlen AM, Broderick KM, Travers C, Smith EA, Elmore JM, Kirsch AJ. Outcomes of complex robot-assisted extravesical ureteral reimplantation in the pediatric population. J Pediatr Urol. (2016) 12(3):169.e1–6. doi: 10.1016/j.jpurol.2015.11.007
6. Hwang J, Kim PH, Yoon HM, Song SH, Jung AY, Lee JS, et al. Application of the postnatal urinary tract dilation classification system to predict the need for surgical intervention among neonates and young infants. Ultrasonography. (2023) 42(1):136–46. doi: 10.14366/usg.22035
7. Wein AJ, Kavoussi LR, Partin AW, Peters CA. Campbell-Walsh urology: 4-volume set. 11th ed. Philadelphia, PA: Elsevier Science Health Science (2016).
8. Soulier V, Scalabre AL, Lopez M, Li C-Y, Thach S, Vermersch S, et al. Laparoscopic vesicoureteral reimplantation with Lich-Gregoir approach in children: medium-term results of 159 renal units in 117 children. World J Urol. (2017) 35(11):1791–8. doi: 10.1007/s00345-017-2064-y
9. Teklali Y, Robert Y, Boillot B, Overs C, Piolat C, Rabattu PY. Endoscopic management of primary obstructive megaureter in pediatrics. J Pediatr Urol. (2018) 14(5):382–7. doi: 10.1016/j.jpurol.2018.05.027
10. Baek M, Han DH. Transvesicoscopic Politano-Leadbetter ureteral reimplantation in children with vesicoureteral reflux: a novel surgical technique. Investig Clin Urol. (2019) 60(5):405–11. doi: 10.4111/icu.2019.60.5.405
11. Ansari MS, Yadav P, Arora S, Singh P, Sekhon V. Bilateral transvesicoscopic cross-trigonal ureteric reimplantation in children: surgical subtleties and a prospective summary. Urology. (2017) 101:67–72. doi: 10.1016/j.urology.2016.11.034
12. Rappaport YH, Kord E, Noh PH, Koucherov S, Gaber J, Shumaker A, et al. Minimally invasive dismembered extravesical cross-trigonal ureteral reimplantation for obstructed megaureter: a multi-institutional study comparing robotic and laparoscopic approaches. Urology. (2021) 149:211–5. doi: 10.1016/j.urology.2020.10.018
13. Neheman A, Kord E, Koucherov S, Kafka I, Gaber J, Noh PH, et al. A novel surgical technique for obstructed megaureter: robot-assisted laparoscopic dismembered extravesical cross-trigonal ureteral reimplantation-short-term assessment. J Endourol. (2020) 34(3):249–54. doi: 10.1089/end.2019.0192
14. Akhavan A, Avery D, Lendvay TS. Robot-assisted extravesical ureteral reimplantation: outcomes and conclusions from 78 ureters. J Pediatr Urol. (2014) 10(5):864–8. doi: 10.1016/j.jpurol.2014.01.028
15. Grimsby GM, Dwyer ME, Jacobs MA, Ost MC, Schneck FX, Cannon GM, et al. Multi-institutional review of outcomes of robot-assisted laparoscopic extravesical ureteral reimplantation. J Urol. (2015) 193(5 Suppl):1791–5. doi: 10.1016/j.juro.2014.07.128
16. Tatlişen A, Ekmekçioğlu O. Direct nipple ureteroneocystostomy in adults with primary obstructed megaureter. J Urol. (2005) 173(3):877–80. doi: 10.1097/01.ju.0000152533.93716.3c
17. Chung H, Jeong BC, Kim HH. Laparoscopic ureteroneocystostomy with vesicopsoas hitch: non-refluxing ureteral reimplantation using cystoscopy-assisted submucosal tunneling. J Endourol. (2006) 20(9):632–8. doi: 10.1089/end.2006.20.632
18. Al-Shukri S, Alwan MH. Bilharzial strictures of the lower third of the ureter: a critical review of 560 strictures. Br J Urol. (1983) 55(5):477–82. doi: 10.1111/j.1464-410x.1983.tb03352.x
19. Fu W, Zhang X, Zhang X, Zhang P, Gao J, Dong J, et al. Pure laparoscopic and robot-assisted laparoscopic reconstructive surgery in congenital megaureter: a single institution experience. PLoS One. (2014) 9(6):e99777. doi: 10.1371/journal.pone.0099777
20. Leissner J, Allhoff EP, Wolff W, Feja C, Höckel M, Black P, et al. The pelvic plexus and antireflux surgery: topographical findings and clinical consequences. J Urol. (2001) 165(5):1652–5. doi: 10.1016/S0022-5347(05)66384-7
21. Alizai NK, Dawrant MJ, Najmaldin AS. Robot-assisted resection of choledochal cysts and hepaticojejunostomy in children. Pediatr Surg Int. (2014) 30(3):291–4. doi: 10.1007/s00383-013-3459-5
Keywords: single-port-plus-one, robotic laparoscopic surgery, primary obstructive megaureter, direct nipple, ureteral extravesical reimplantation
Citation: He Y, Lin S, Xu X, He S, Xu H, You G, Chen J and Xu D (2023) Single-port-plus-one robot-assisted laparoscopic modified Lich-Gregoir direct nipple ureteral extravesical reimplantation in children with a primary obstructive megaureter. Front. Pediatr. 11:1238918. doi: 10.3389/fped.2023.1238918
Received: 12 June 2023; Accepted: 21 September 2023;
Published: 2 November 2023.
Edited by:
Giuseppe Lauriti, G. d’Annunzio University of Chieti and Pescara, ItalyReviewed by:
Giovanni Cobellis, Marche Polytechnic University, ItalyMiguel Alfedo Castellan, University of Miami, United States
© 2023 He, Lin, Xu, He, Xu, You, Chen and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Di Xu fjsleyk@163.com
†These authors have contributed equally to this work
 Yufeng He2,1,†
Yufeng He2,1,†  Shan Lin
Shan Lin Shaohua He
Shaohua He Di Xu
Di Xu