Energy expenditure and weight-related behaviors in youth with Down syndrome: a protocol
- 1College of Nursing, University of Wisconsin - Milwaukee, Milwaukee, WI, United States
- 2Department of Nursing Research and Evidence-Based Practice, Children’s Wisconsin, Milwaukee, WI, United States
- 3Eunice Kennedy Shriver Center, University of Massachusetts Chan Medical School, Worcester, MA, United States
- 4Isotope Ratio Mass Spectrometry Laboratory, Biotechnology Center, University of Wisconsin, Madison, WI, United States
- 5Department of Rehabilitation Science and Technology, University of Pittsburgh, Pittsburgh, PA, United States
- 6Clinical and Translational Science Institute of Southeast Wisconsin, Medical College of Wisconsin, Milwaukee, WI, United States
- 7Zilber School of Public Health, University of Wisconsin – Milwaukee, Milwaukee, WI, United States
- 8Pediatric Translational Research Unit, Children’s Wisconsin, Milwaukee, WI, United States
Background: The consequences of obesity are ominous, yet healthcare professionals are not adequately preventing or treating obesity in youth with Down syndrome (DS). Total daily energy expenditure (TDEE) is the energy expended in 24 h through physical activity and life-sustaining physiologic processes. An individual's TDEE is essential for determining the daily caloric intake needed to maintain or change body weight. Successful prevention and treatment of obesity in youth with DS is severely compromised by the lack of data on TDEE and information on weight-related behaviors for this high-risk population. This manuscript describes the protocol for the federally funded study that is in process to determine daily energy expenditure in a large cohort of children with DS.
Methods: This observational cross-sectional study will include a national sample of 230 youth with DS, stratified by age (5–11 and 12–18 years of age) and sex. Doubly Labeled Water analysis will provide the criterion body fat%, fat-free mass, and TDEE. To increase accessibility and decrease the burden on participants, the entire study, including obtaining consent and data collection, is conducted virtually within the participant's home environment on weekdays and weekends. The study team supervises all data collection via a video conferencing platform, e.g., Zoom. This study will (1) examine and determine average TDEE based on age and sex, (2) develop a prediction equation based on measured TDEE to predict energy requirements with a best-fit model based on fat-free mass, sex, age, and height and/or weight, and (3) use 24-hour dietary recalls, a nutrition and physical activity screener, wearable devices, and sleep questionnaire to describe the patterns and quality of dietary intake, sleep, and physical activity status in youth with DS.
Discussion: The lack of accurate information on energy expenditure and weight-related behaviors in youth with DS significantly impedes the successful prevention and treatment of obesity for this vulnerable population. The findings of this study will provide a further understanding of weight-related behaviors as obesity risk factors, currently not well understood for this population. This study will advance the science of weight management in individuals with disabilities and shift clinical practice paradigms.
Introduction
The consequences of obesity are ominous, yet we are not adequately preventing or treating obesity in youth with Down syndrome (DS), who have a dramatically higher obesity prevalence (reported as high as 62.5%) (1) compared to their typically developing peers (18.5%) (2). Obesity is associated with life-long medical, economic, and psychological burdens which worsen with earlier age of onset (3–5). In children with disabilities, obesity limits independence, decreases the ability to self-manage health, increases the risk of social isolation, and is a barrier to caregivers' abilities to provide care (6, 7). In DS, obesity is linked to adverse health outcomes such as obstructive sleep apnea, dyslipidemia, hyperinsulinemia, impaired cardiorespiratory fitness, and orthopedic complications (1, 6–14). With dramatic increases in life expectancy for individuals with DS, it is imperative to ensure that they enter their adult years with optimal health (15). To address obesity in youth with disabilities, the National Institute of Child Health and Human Development (NICHD) expert panel's research agenda prioritized (1) addressing the need for accurate data on energy expenditure and (2) identifying and understanding weight-related behaviors as obesity determinants to inform potential interventions (16).
Obesity in youth often continues into adulthood impacting morbidity and mortality (17, 18). In the simplest terms, obesity is an outcome of an imbalance of excess energy intake as compared to energy expended (physical activity and physiologic processes) described for a 24-hour period as total daily energy expenditure (TDEE) (19). Characteristics inherent or related to DS (e.g., hypotonia, decreased fat-free mass, hypothyroidism, leptin resistance, less participation in physical activity) are associated with decreased TDEE (1, 12, 16, 20–24). These factors contribute to a lower level of energy expenditure resulting in a reduced caloric need and consequently increased risk of inadvertent overfeeding and subsequent weight gain (25). In addition, individuals and parents often overestimate the amount of energy expended through physical and sedentary activity further adding to unintentional excess intake (26–30). Growth retardation, decreased height velocity and muscle hypoplasia can further exacerbate the high percentage of body fat and can be accentuated with the youth's advancing age (1).
An individual's TDEE is essential for determining the energy intake required to maintain or change body weight (31) and is the foundation of anticipatory guidance provided by healthcare professionals to optimize growth and weight management. Specifically, the very limited data on the energy needs of youth with DS does not allow accurate recommendations for dietary intake (32). Successful prevention and treatment of obesity in youth with DS is severely compromised by the lack of data on TDEE and an understanding of weight-related behaviors.
Behaviors that contribute to obesity often begin in childhood or adolescent years (33). The onset of obesity in youth often continues into adulthood (17, 18, 34). Limitations in the current literature include a distinct void of inclusion of individuals with DS in weight-related research (23, 35). When individuals with disabilities are the focus of the study, limited studies have used “state-of-the-art measurement techniques” [i.e., doubly labeled water (DLW)] (24). While preliminary studies providing support for youth with developmental disabilities having poor quality diets, increased screen time, and needing less caloric intake per day are present, they are limited and recommend further study in larger samples (1, 16, 20, 23, 24, 36–38). Focusing on the prevention and treatment of obesity in youth with DS is a national priority (16, 39).
This study focuses on youth with DS and was a competitively reviewed supplement to our currently funded Body Composition and Energy Expenditure in Youth with Spina Bifida (R01HD096085). While the protocol is similar to the initial R01 aim addressing TDEE in children with spina bifida (SB), this protocol differs in the population of interest and specifics of the design, specifically the setting, recruitment, methods of data collection, and addition of DS-focused measures.
This protocol addresses gaps and weaknesses of prior research in this cohort as it pertains to establishing caloric need. This study will systematically investigate TDEE and develop an algorithm for use in youth with DS as stratified by age and sex to predict energy requirements. As a result, recommendations of daily caloric intake will be established. The second outcome will be information related to obesity determinants (i.e., dietary intake, sleep, and activity) in youth with DS.
The study aims are:
Aim 1. Using DLW, measure TDEE and develop a prediction equation for the energy requirements of youth with DS. We propose to: (a) Examine and describe average TDEE stratified by age and sex and (b) Develop a prediction equation based on actual TDEE to predict energy requirements with a best-fit model based on fat-free mass, sex, age, and height and/or weight.
Aim 2. Using 24-hour dietary recalls, a nutrition and physical activity screener, accelerometers, activity trackers, and a sleep questionnaire, describe the patterns and quality of dietary intake and sleep, and duration and frequency of activity (physical and sedentary) in youth with DS.
Methods
Design and participants
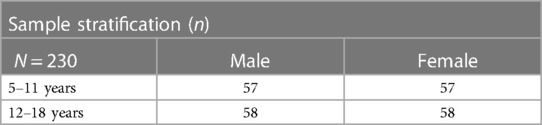
This observational, cross-sectional study will include a national sample of 230 youth with DS, stratified by age group (5–11 and 12–18 years of age) and biological sex (Table 1). The study protocol is approved by the Western Copernicus Group (WCG) Institutional Review Board (IRB) (#20214186) and acknowledged by the local IRB of the Principal Investigator (PI).
Pilot study
This application was supported by our pilot study (P20NR015339 and UL1TR000055) that confirmed the feasibility of measurement of energy expenditure with DLW in youth with DS, SB, and without disabilities. In this small sample, TDEE was significantly lower in youth with disabilities. When matched for fat-free mass, TDEE in youth with DS averaged 500 fewer calories per day to balance their caloric intake compared to youth without disabilities (32).
Setting
The proposed study is conducted virtually with consent, data collection, and testing occurring within the participant's home environment via a HIPAA-compliant video conferencing platform. The decision to conduct the study virtually was done to minimize the study burden and to increase accessibility for participants to join from anywhere in the lower 48 United States. Hawaii and Alaska were excluded due to shipping costs.
Coordinating sites
This study is coordinated through the two agencies where the PI holds a joint appointment as the Joint Research Chair in the Nursing of Children, Children's Wisconsin, a free-standing Children's Hospital, and the University of Wisconsin—Milwaukee College of Nursing, both located in Milwaukee Wisconsin. The coordination center for assembling study kits, shipping, receiving, and sterilizing supplies, and processing and storing samples is through the Pediatric Translational Research Unit (PTRU) located within Children's Wisconsin. Accelerometer and activity tracker analysis is completed at the Department of Rehabilitation Science and Technology at the University of Pittsburgh and DLW is supplied and analyzed through the Isotope Ratio Mass Spectrometry (IRMS) lab of the University of Wisconsin—Madison.
Recruitment, screening, and consent
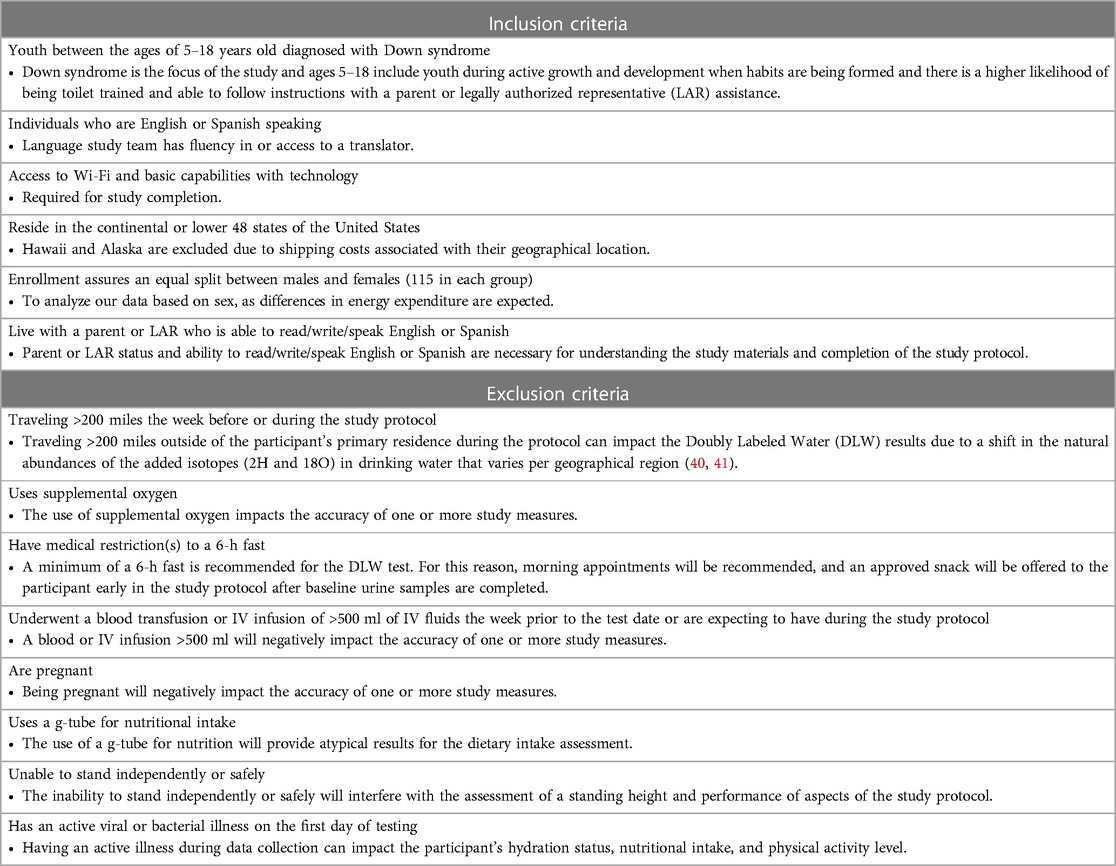
Primary recruitment strategies include the use of the National Institutes of Health (NIH) DS-Connect®, a national registry that connects individuals with DS and their families to research and healthcare providers. Once a study is reviewed and approved by the registry, the study description is shared with registry participants who meet inclusion and exclusion criteria (Table 2). In addition, the study is shared with family-focused DS organizations and their associated social media sites, specifically targeting organizations that have a diverse focus, and the use of snowball recruitment is incorporated. Through each of these recruitment methods, the interest in participating in the study is participant-driven to allow them to make an informed decision to participate.
Potential participants who are interested contact the study team through email or phone. A member of the study team meets with the potential participant via HIPAA-compliant video conferencing platform to review the study details, answer questions, screen for eligibility, and share additional study materials with them (e.g., a pictorial orientation booklet that provides a broad overview of the study protocol in an easy-to-read, child-friendly format). This allows the families to further consider and discuss as a family unit while making an informed decision to participate. If eligibility is confirmed and interest in the study remains, the team member performs the consenting/assenting (hereafter, consenting) process.
Consenting occurs via HIPAA-compliant video conferencing platform. This assists in ensuring the family's capabilities of using the virtual platform, and having sufficient Wi-Fi, and allows the consenting process to occur while seeing faces and/or body language to assist in confirmation of understanding and initiating the researcher-participant relationship that is helpful in the successful completion of the study. The study meets the requirements for a waiver of documentation of written consent under 45 CFR 46.117(c)(21)(ii). The consent forms are reviewed with the parent or legally authorized representative (LAR) by a Collaborative Institutional Training Initiative (CITI)-trained study team member. Due to the varying degrees of cognitive delays that may limit the ability to assent the IRB approved a waiver of assent. However, the study team member works with the parent/LAR to assess if the child is able to give verbal assent based on their maturity, psychological state, and cognitive ability on a case-by-case basis. While waiver of written documentation is present, all families are provided with a copy of the consent form for their records. All consents are professionally translated into Spanish. A data collector fluent in Spanish is employed for any families that primarily speak Spanish.
Upon informed consent completion, the study visit is scheduled. To accommodate families' schedules, weekday and weekend visits are available, preferably close to when the child wakes up due to early fasting requirements. Drinking up to 8 ounces of water and using a spoonful of yogurt or food for medication intake is acceptable if needed.
Study procedures
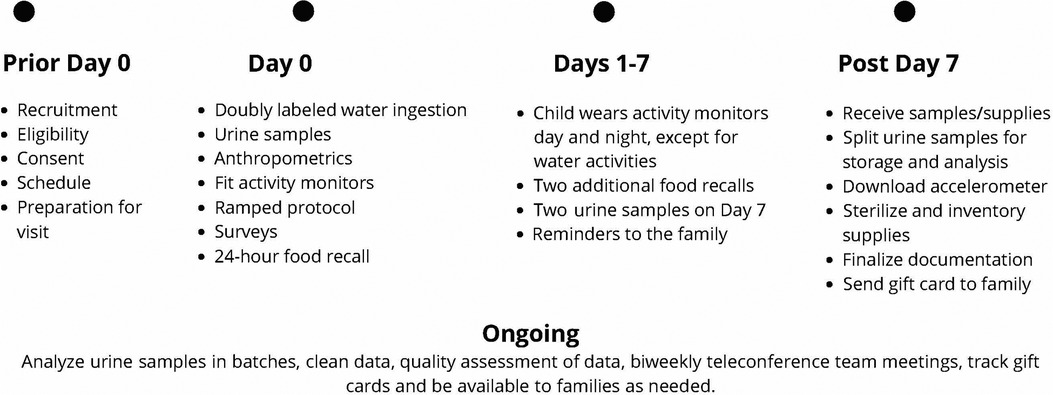
The study includes an 8-day protocol that is conducted virtually within the family's home with a trained study team member working with the family via a HIPAA-compliant video conferencing platform, phone (call and text), and email communication. See Table 3 for a detailed study protocol and Figure 1 for a timeline of the study protocol. Once consenting has been completed, the parent/LAR is asked to provide an estimate of the child's weight (to dose DLW), share their home address to send supplies, and schedule their visit (Day 0). This initial data collection visit is estimated to last 4.5 h but it is recommended that 5 h be scheduled in case of unforeseen issues. Prior to the scheduled visit date, two boxes are shipped to the family home that includes the supplies needed to execute the study protocol. All materials are clearly marked, color-coded, and have an easy-to-read family-friendly procedure manual with them.
Box 1 includes supplies for measurement and assessment (portable scale for body weight, portable stadiometer, flexible tape measure, accelerometer, activity tracker, iPad for questionnaires, urine collection supplies (e.g., urine hats, urine cups, double bag, absorbent materials, preprinted labels, and parafilm), measuring cups, calorie-controlled snack, and paperwork. Box 2 is insulated and includes the DLW dose, shipping materials for returning urine samples and items used past Day 0 (accelerometer, activity tracker, ice packs). The week before the study visit, the family meets with a study team member to review fasting requirements, study kit materials, and the plan for the study visit including the collection of a baseline urine sample using the child's first-morning urine and refrigeration of DLW for taste purposes. In addition, written instructions, and materials for collecting urine samples as well as gloves and storage materials are provided. This allows the family to collect the baseline urine sample upon the child's waking but before the start of the virtual meeting depending on the start time of the visit.
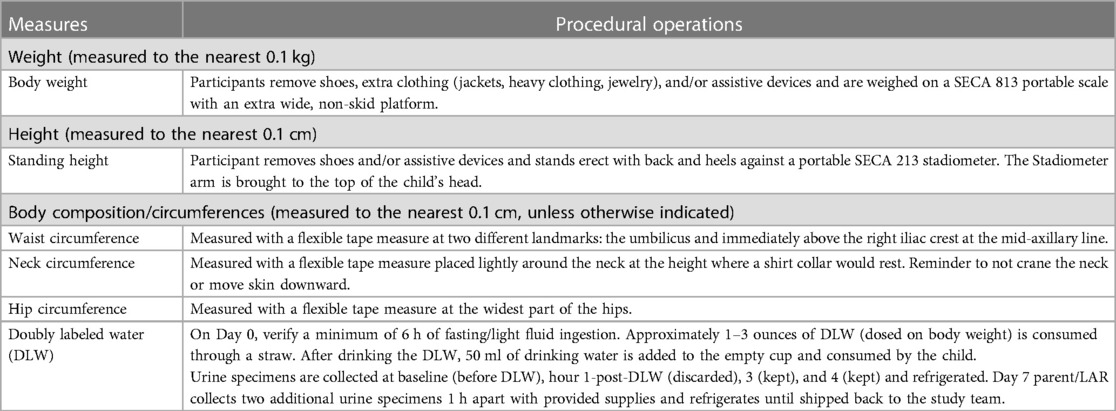
On Day 0, a study team member connects via a video conferencing platform with the participant and a minimum of one parent/LAR. The child's fasting status and lack of any active illness are confirmed. If the family has not already collected a baseline urine sample, they are asked to collect this sample with the team member's guidance at the start of the visit. If they did collect the sample, confirmation of proper collection and timing of the sample is obtained. The parent/LAR is asked to obtain the child's body weight with excess clothing removed on the SECA 813 portable scale provided. The research assistant uses this weight to confirm that the dose of DLW is appropriate for the child's weight. If the DLW is spilled, the protocol would be stopped, the visit would be rescheduled with a new dose of DLW shipped and the baseline urine will be stored and analyzed. If the wrong dose was given and it is smaller than planned, the center staff calls the IRMS lab to discuss options.
The child drinks the DLW from the provided bottle and straw as spillage reduces accuracy. After the child drinks the DLW from the bottle that it was sent in, the parent/LAR is asked to add 50 ml of tap or bottled water to the now empty bottle with a provided measuring cup and a marked line identifying 50 ml on the DLW bottle. The child drinks the added water with the same straw used for the DLW. This ensures that all DLW is consumed. Three additional urine samples beyond the baseline sample are obtained during the Day 0 visit. At 1 h after the child drinks the DLW, a second urine sample is obtained and discarded to flush the bladder. Two urine samples are collected and saved at 3- and 4 h post DLW consumption. These two samples are labeled with the child's coded identification number, date, and time and stored in the refrigerator. An example would be if the DLW was consumed at 08:15 a.m., the Hour 1 urine sample is obtained at 09:15 a.m. (discarded), the Hour 3 urine at 11:15 a.m., and the Hour 4 urine at 12:15 p.m. (both labeled and refrigerated). If the child is unable to void, the child is asked to try again in 15 min. If still unable to void, 60 ml of drinking water is provided, and the child is asked to try again in 15 min. This continues until the child is able to void. If a urine sample is delayed, the timing of the next urine collection is adjusted based on the actual time of the urine sample. In between the urine sample collection, ample time is available to complete survey instruments and other study measures and to provide the family with downtime to be off camera as needed.
Between the timed urine sample collection, families are guided on the completion of additional measurements including a standing height with the SECA 213 portable stadiometer. A flexible tape measure is used to obtain waist, hip, and neck circumferences. These measures are collected under the guidance of study staff to confirm landmarks and fidelity of the measures. All circumferences are measured three times and the average of the measures is calculated. If an extreme outlier (>3 cm) is obtained, the parent/LAR is asked to obtain an additional measure. The study team member documents all measures in the REDCap database. A study-provided calorie-controlled snack is included in the study box to be available after Hour 1 urine but before Hour 3 urine per DLW protocol. The snack is discussed during consenting to confirm no allergies and preferences of the child. If a family prefers their own snack, confirmation from the study team is needed to ensure that the snack is 250 kcal or less. During this snack time, the child is also offered 8 ounces of water. This supports the collection of the Hour 3 and Hour 4 urine samples. The snack (kcal) and drink (ounces) are documented in the amount and time consumed.
In addition to anthropometric measurements, the family is asked to complete questionnaires and is guided to set up data collection devices and place them on their child. All questionnaires, except the Block Nutrition Screener and Block Physical Activity Screener are provided via REDCap on the study iPad that is provided. The study team member, who is trained in conducting 24-hour dietary recalls, works with the parent/LAR and child if able to assist, to document the child's previous day's intake using a multiple-pass approach. Measuring cups, spoons, and visual aids are included in the supplies for the family to assist in quantification and portion sizes. The final item is conducting a ramped protocol to assess physical activity. This includes the child wearing the study-provided preprogrammed accelerometer (ActiGraph GT3X-BT, ActiGraph LLC, Pensacola, FL, USA) on their waist and the activity tracker (Fitbit Inspire 2, Fitbit, Inc, San Francisco, CA, USA) on their non-dominant wrist. With guidance from the study team, the parent fits each device to their child, activation is confirmed, and the device is time synced. The activity tracker is configured to minimize or remove notifications for the child/family to decrease the risk of altering the child's health habits for the week. The team members guide the child with the parent/LAR's assistance through a ramped protocol divided into five intervals that include the child moving at light, moderate and vigorous activity levels, with perceived exertion reported at each level. The five intervals include (1) obtaining a resting heart rate (3 min), (2) standing, and wiping surfaces for arm movement (3 min), (3) walking at a normal pace (3 min), (4) walking at a fast pace (6 min), and (5) dancing in place (2 min). Between intervals, the child's heart rate must return to baseline before doing the next activity. The child and parent/LAR discuss and provide the child's perceived exertion rate for that interval based on a pictorial perceived exertion chart (42), This allows the study team to individualize the analysis of the activity tracker and accelerometer raw data for the study protocol.
After the Hour 4 urine collection, it is expected that Day 0 study requirements are completed. The study team uses the end of the visit to review the remainder of the protocol including the continued wearing of the accelerometer on the child's waist and the activity tracker on their non-dominant wrist during wake and sleep times but removed during water activities (e.g., bathing, swimming). The benefit of the activity tracker worn with the accelerometer is the continuous recording of heart rate and physical activity information from both research-grade and commercially available wearable devices. The family is provided with a daily wear/sleep log sheet and asked to document when the child goes to bed, wakes up, and is not wearing the accelerometer or activity tracker over the following week. Guidance is also provided to assist the family in repackaging equipment and supplies. Appointment times for two additional 24-hour dietary recalls are set up based on the family's availability to include a total of 2 weekdays and 1 weekend day. The family is asked to continue with their normal daily routine during the protocol. The final action item of the study is the collection of two remaining urine samples a minimum of 1 h apart on Day 7, preferably close to the time of day that Day 0 samples are collected. The study team reviews this during the final food recall and is available to assist the family if needed.
After the study protocol is complete and the final two urine collections are obtained, the parent/LAR is guided to ship the boxes back using a prepaid shipping label. Specific instructions to schedule optional home pick up of the boxes are included to add convenience for the family. The refrigerated urine samples are shipped back using the insulated storage box and frozen gel bags that were provided. The prepaid shipping label for the insulated box that includes urine samples is for overnight shipment and the family is instructed to only ship this box on Monday, Tuesday, or Wednesday of each week to ensure that personnel will be available to accept the shipment. The other supplies in the second box use a prepaid shipping label for ground transportation. Upon receipt of the urine samples and supplies, the family is provided an e-gift card for $250.00 through email. If the family is uncomfortable with the electronic gift card, a physical gift card can be mailed via the United States Postal Service, but a signature will be needed to confirm receipt.
The two boxes are shipped back to the coordination center (i.e., PTRU Children's Wisconsin) where the urine samples are processed, documented, and split into two. One sample is frozen and remains at the PTRU for quality control purposes. The second sample is mailed in batches to the UW-Madison IRMS Lab for analysis. All samples are de-identified and labeled with the time and date of collection and participant study identification number. The remaining equipment and supplies are inventoried, sterilized, and confirmed to be in good working condition.
Measures
Anthropometric measures
To provide contextual support for body composition and risk factors for obesity-related comorbidities the participant's weight, height, waist, hip, and neck circumference are obtained. Circumference measures are repeated three times with the average used for analysis. All measures are obtained by the parent/LAR with direction and guidance provided by the study team during the virtual session. Pictorial directions for measurements are also provided in a family-friendly procedure manual. See Table 4 for detailed measures and procedures.
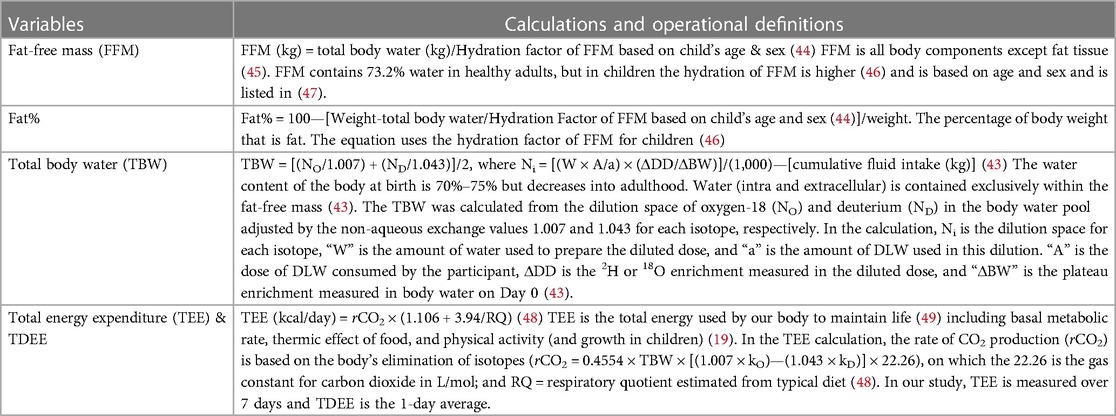
DLW variables and calculations
DLW is a valid and reliable tool that provides a measure of body fat and TDEE (43). DLW uses drinking water mixed with two stable isotopes, deuterium (2H) and oxygen-18 (18O) which act as tracers when ingested. DLW is dosed based on the individual's body weight. The analysis is performed on the individual's body fluids (urine) by isotope ratio mass spectrometry to measure the elimination of the tracers over a specific time frame (43). The difference in the rate at which the tracers are eliminated from body water allows for the calculation of carbon dioxide production, a product of energy metabolism that is used to compute TDEE. The added tracers also can be used to calculate the individual's total body water. Based on total body water, fat-free mass and fat mass can be calculated. These equations are typically applied to DLW-derived total body water to determine body fat%, an age-related adjustment for chemical maturation of fat-free mass.
While DLW is an objective reference measure of TDEE and valid for body fat%, it is not practical for clinical use due to its high cost, specialized equipment and expertise that is required (43). See Table 5 for DLW variables and standard calculations.
Weight-related behaviors
Sleep and activity data are collected by an accelerometer and activity tracker that are worn day and evening but removed during water activities. See Table 6 for physical activity assessment devices.
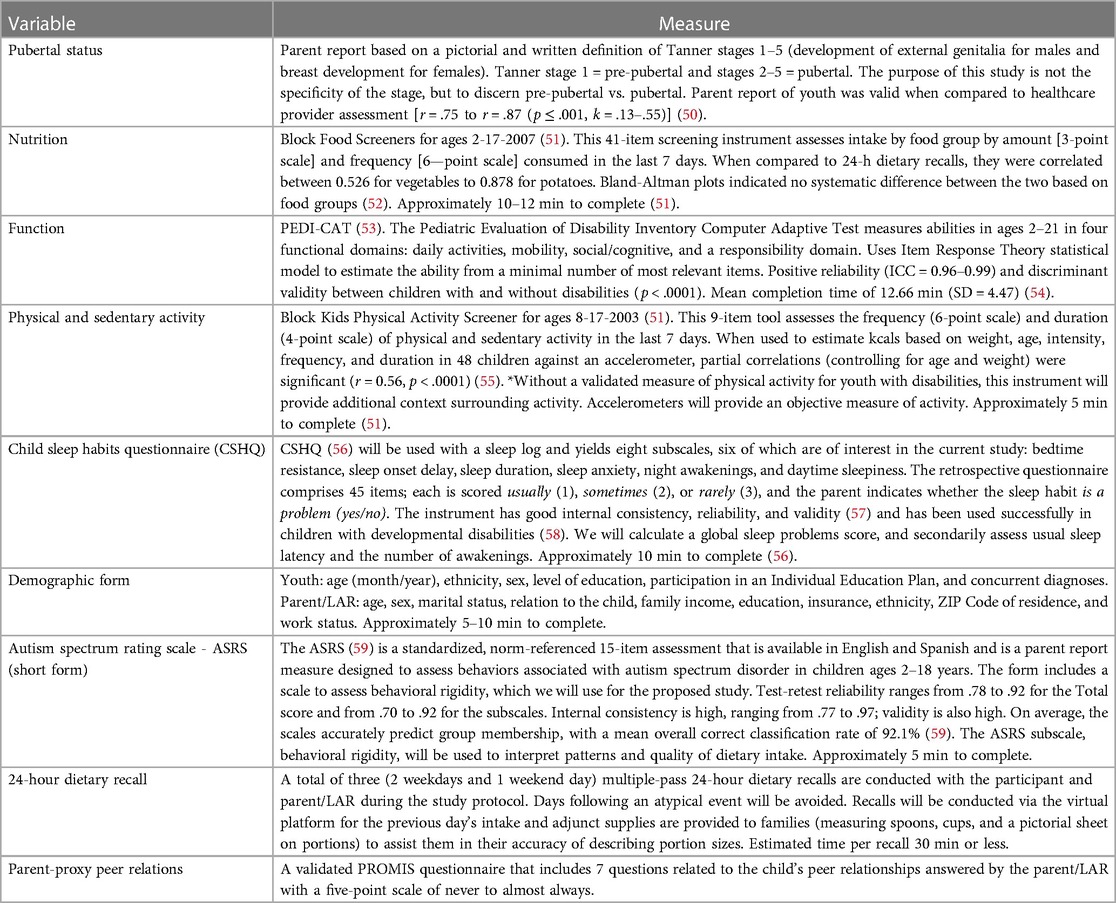
Instruments
Parent/LAR complete all instruments with child assistance as able. All instruments are available in Spanish and English. See Table 7 for a detailed description of the instruments.
Retention
To ensure retention, we employ multiple strategies. Our initial strategy is to be clear and transparent as to what participation in the study involves. This allows the family to make an informed decision and be less likely to be surprised by the protocol which could lead to them to withdraw from the study. We also try to remain consistent with the study team member who is interacting with the family to support the building of a relationship and comfort with the study.
The next strategy is to support the family through the study. We provide information to guide the family verbally and in print with easy-to-follow instructions. We are flexible and available for their questions. Examples of being flexible include offering data collection visits on weekday and weekend mornings through early evening hours and accommodating the different time zones. We proactively consider potential concerns the family may have and provide reminders throughout the study. Our team is committed to reducing the study burden and supporting the completion of the protocol.
To accommodate unforeseen scheduling difficulties, we expect and accommodate appointment changes to the best of our ability. During the initial visit, we set up the additional two 24-hour dietary recalls (2 weekdays and 1 weekend day) per the family's schedule.
To support the families' participation in the study, each family is offered a $250 gift card for completing the study protocol. The gift card is not sent to the family until the Day 7 sample collections and supplies are returned and received by the study's coordinating center. The process and requirements for receipt of the gift card are discussed during the consenting process and reiterated during the Day 0 visit, so the family is aware of what to expect.
In summary, retaining study participants to complete the study protocol is enhanced by replicating strategies successfully used in our pilot study and current R01, including (1) the provision for a comprehensive explanation of what the study entails (aided by the pictorial study manual) so the family can make an informed choice to participate, (2) the provision of flexible scheduling options, (3) confirmation of understanding of the study protocol during the consenting process, (4) clear instructions provided verbally and in print, and (5) provision of all materials, clearly marked, that are needed for the collection and the return of the Day 7 urine samples, activity tracker and accelerometer with mailing supplies provided, and (6) touchpoints or interactions with the family during the study protocol to increase opportunities for questions, the reiteration of directions, and building of relationships with the family. To assist the family with the Day 7 urine collection, we call and/or text them on Day 5 and/or 6 to remind them of the final urine collection, review instructions, and answer questions.
While we do not anticipate challenges with retention based on the above strategies and our previous success, if we begin to have problems with retention, we will ask the families if they were willing to share what the challenges are and what led to their decision to not complete the study. Dealing with challenges early in the process allows us to make changes if able with our protocol and IRB amendments.
Statistical data analysis
Descriptive statistics, including mean, standard deviation, median, and range will be calculated for each variable/measurement. Descriptive analysis will be conducted to assess for missingness, describe sample characteristics, and estimate the reliability of scores for all instruments. For body mass index (kg/m2), the average standing height measured will be used.
Missingness analysis
Univariate or regression analysis in both Aim 1 and Aim 2 will be performed using multiple imputation under the missing-at-random (MAR) assumption. Sensitivity analysis will be performed to check for potential violation of MAR assumption (60). Simulation under the MAR assumption will be performed to quantify the efficiency loss defined as 1−E [Ŷ (X*)−Y]2/E[Ŷ (X)−Y]2 where X* represent the observed data and the X is the data with missingness.
Analysis Aim 1, Bland-Altman plot analysis and concordance correlation will be used to evaluate the agreement between DLW body fat% and body mass index. We will predict TDEE by estimating basal metabolic rate and physical activity level and using these as our primary predictors. Several basal metabolic rate equations from cross-sectional studies with good model performance (i.e., R2 0.7–0.8) have been reported85 which mainly used age, fat-free mass, and fat mass weight as key predictors. Thus, we will use those as our base model predictors and further consider height, race, sex, and pubertal status as potential predictors. Stepwise linear regression procedures in conjunction with Akaike information criterion (AIC) will be used for model selection and to develop prediction equations. Potential interactions between age/race and other variables will be examined. The resulting predicted TDEE is a weighted sum of a subset of potential predictors, where the weights are the parameter estimates associated with each predictor in the regression model. Predictive accuracy will be further evaluated by mean square error through a 10-fold cross-validation to test the predictive accuracy between the predicted and DLW TDEE.
Analysis Aim 2, The Block Food and Physical Activity Screeners, will be analyzed through Nutrition Quest, the child's function is scored through Pedi-Cat and the Autism Rating Scale is hand scored. The heart rate and sleep data obtained with the Fitbit activity tracker will be extracted and exported by Fitabase (Small Steps Labs LLC, San Diego, CA, USA.), a comprehensive data management platform. Descriptive analysis and univariate test (i.e., ANOVA or Chi-square test) on frequency and duration of physical activities, sleep, autism rating, sedentary activity, and youth's dietary intake by food groups and servings will be presented. Overall pattern by sex will be analyzed by principal component analysis or multiple correspondence analysis (61). Raw accelerometer signals at a sampling frequency of 30 Hz will be collected. Each data file will be manually cleaned to extract data collected and assessed for non-wear time using validated algorithms with ActiLife software (62). We plan to use the physical activity intensity thresholds based on counts per minute (CPM) that were validated in typically developing youth (63, 64); sedentary (<100 CPM), light (101–2,295 CPM), moderate (2,296–4,011 CPM), and vigorous intensity (>4,011 CPM). The CPM will be obtained based on a 10-second epoch to capture intermittent movement. We will also perform a 20-minute ramped protocol and collect participant perceived exertion and heart rate during Day 0 when parents can provide assistance as instructed. The ramped protocol will assist us in further validating the CPM thresholds with our participants with DS at individual levels and allow us to make any adjustments if needed based on raw data collected. The Physical Activity Screener will complement the objective accelerometer data. The 24-hour dietary recalls will be analyzed using Nutrition Data Systems for Research (65) (a nutrient analyses software) to identify energy intakes at the macronutrient levels and general Health Eating Index scores to compare with other youth groups and the Dietary Guidelines for Americans 2020–2025 (66).
Sex as a biological variable analysis
The proposed sample of 230 youth with DS will be stratified by sex with 115 males and 115 females being recruited. For Aim 1, sex as a biological variable will be used to describe the average TDEE and tested as a potential predictor in the equation described above (Statistical Data Analysis, Aim 1) to predict energy requirements for youth with DS. This is important as TDEE is expected to be different for males as compared to females. For Aim 2, sex as a biological variable will be used to describe and examine differences between the weight-related behaviors (dietary intake, sleep, and activity) by univariate analysis (i.e., ANOVA or Chi-Square test as appropriate) for the sample based on sex. This will inform if obesity risk factors are different based on sex and used in future interventions.
Power analysis
The power calculation is based on the increased coefficient of determination (R2) from a baseline linear model to a multivariate model that regresses TDEE on potential predictors (i.e., height, race, age, sex, puberty status). Pilot studies from the literature suggest that the R2 for a regression model of TDEE measured by DLW on basal metabolic rate and physical activity level is in the range of 0.7–0.8. As we will use 10-fold cross-validation to construct our predictive models for two sex groups and assume that 10% of subjects will not complete all measures based on Pilot One data, a sample size of 93 (230/2*0.9*0.9) achieves 90% power to detect an increase of R2 ≥ 0.045 attributed to additional four independent variables using an F-test with a significance level of 0.05. The variables tested are adjusted for two independent variables (basal metabolic rate and physical activity level) in the baseline linear model with an R2 of 0.7.
Discussion
Individuals with DS are recognized to be at higher risk for obesity, yet there is insufficient evidence related to energy expenditure and weight-related behaviors that are integral to the prevention and treatment of obesity. This is critical as individuals with DS are living longer and the added burden of obesity can affect the individual's ability to self-manage their health and transition to independence. Furthermore, obesity is linked to multiple medical conditions. This lack of evidence-based information places added stress on the family and limits the ability of the healthcare provider to provide anticipatory guidance.
The study protocol was designed with the goal of obtaining clinically relevant information on TDEE and weight-related behaviors in youth with DS while reducing the burden on the child and their family. Specific burden-reducing attributes of this study include flexibility in scheduling and collecting data within the family's residence via a video conferencing platform along with the provision of family-centric, easy-to-understand study-related materials. This supports the family's ability to make an informed decision to participate, increases accessibility, supports recruitment efforts, aids in the collection of accurate data, and decreases the study burden on the participant. Post Covid-19, families have increased awareness and comfort with using virtual platforms such as Zoom which can be a benefit to conducting research studies and reduce the need to enter medical research facilities if not needed.
An added benefit of conducting the visit within the family's residence includes the ability to have the family be in a comfortable environment during data collection. This assists the child with comfort during the study and with obtaining data. A specific example of this is during the 24-hour dietary food recall, the family can go to their kitchen and show the study team actual serving sizes and brand names of food. The extended study visit allows the building of a relationship with the family and provides the ability to answer questions as they come up. The study is also designed to ensure that the family does not need to leave their house for participation by having all study-related equipment and supplies shipped to the family and picked up from the family's residence. The successful completion of this study with the use of virtual platforms, family-centric study materials, and a focus on reducing family burden during study participation may provide evidence for alternative study designs in the future.
The study outcomes will shift current research and clinical practice paradigms by providing an evidence-based prediction equation to support healthcare providers to provide accurate anticipatory guidance for youth with DS and their caregivers/families. Our protocol can serve as a template for research into other developmental disabilities and the virtual methodology can provide a model that supports study participation and potentially can increase the diversity of the sample. The Aim 1 evidence-based prediction equation for TDEE will be immediately useful and translatable into clinical practice. Our findings will also provide a requisite foundation for future intervention research, and when compared to our ongoing R01 findings, will generate knowledge about whether intervention components need to be tailored to specific disabilities (e.g., different TDEE in DS vs. SB) or if intervention components can be more broadly tested across populations. The findings from Aim 2 are foundational as the origin of obesity is multifactorial and can be influenced by behavioral modifications. The Aim 2 outcomes will inform both providers and future research on current weight-related behaviors as obesity risk factors in youth with DS. Evidence-based information on weight-related behaviors (nutrition, activity, sleep) can be used independently [e.g., recommendations for dietary modifications or opportunities to modify activity once we have a better understanding of the daily activities (physical and sedentary) of youth with DS] and with the TDEE information obtained in Aim 1 to developing interventions both from a clinical and research perspective.
The lack of accurate information on energy expenditure and weight-related behaviors (nutrition, activity and sleep) in youth with DS and the inability to provide daily caloric recommendations significantly impedes the successful prevention and treatment of obesity for this vulnerable population. The findings from this study will provide a foundational understanding of weight-related behaviors (energy expenditure, activity, nutrition, and sleep) as obesity risk factors, currently not well understood for this vulnerable population. This information will optimize clinical appointments and support and enhance the anticipatory guidance provided by healthcare providers. This innovative study will advance the science of weight management in individuals with disabilities, address national research priorities and shift clinical practice paradigms.
Ethics statement
The study protocol is approved by the Western Copernicus Group (WCG) Institutional Review Board (IRB) (#20214186).
Author contributions
Contributions to the study design include MP, LB, ZH, AM, DS, C-CH, DD, CB, and KS. Preparation of the database was completed by MR, ZH, AM, DS, and TS. The manuscript was drafted by MP and KS. Critical review and final approval were completed by all authors. All authors contributed to the article and approved the submitted version.
Funding
The research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health INCLUDE (INvestigation of Co-occurring conditions across the Lifespan to Understand Down syndromE) Project under Award Number R01HD096085. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. In addition, this work was supported by the Clinical and Translational Science Institute of Southeastern Wisconsin through the Advancing a Healthier Wisconsin Endowment of the Medical College of Wisconsin under the award 2UL1TR001436.
Acknowledgments
We extend our sincere gratitude to the families who have or will participate in our study, DS Connect®: The Down Syndrome Registry, and the many Down syndrome organizations that assist us with recruitment. A special note of appreciation to the team members of the Children's Wisconsin Pediatric Translational Research for their work on the study.
Conflict of interest
LB is a guest associate editor for the special topic and a member of the editorial team.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bertapelli F, Pitetti K, Agiovlasitis S, Guerra-Junior G. Overweight and obesity in children and adolescents with down syndrome-prevalence, determinants, consequences, and interventions: a literature review. Res Dev Disabil. (2016) 57:181–92. doi: 10.1016/j.ridd.2016.06.018
2. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. no 288. Hyattsville, MD: National Center for Health Statistics (2017).
3. Rankin J, Matthews L, Cobley S, Han A, Sanders R, Wiltshire HD, et al. Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolesc Health Med Ther. (2016) 7:125–46. doi: 10.2147/AHMT.S101631
4. Spieker EA, Pyzocha N. Economic impact of obesity. Prim Care. (2016) 43(1):83–95, viii-ix. doi: 10.1016/j.pop.2015.08.013
5. Tremmel M, Gerdtham UG, Nilsson PM, Saha S. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. (2017) 14(4):435. doi: 10.3390/ijerph14040435
6. Neter JE, Schokker DF, de Jong E, Renders CM, Seidell JC, Visscher TL. The prevalence of overweight and obesity and its determinants in children with and without disabilities. J Pediatr. (2011) 158(5):735–9. doi: 10.1016/j.jpeds.2010.10.039
7. Rimmer JH, Yamaki K, Lowry BM, Wang E, Vogel LC. Obesity and obesity-related secondary conditions in adolescents with intellectual/developmental disabilities. J Intellect Disabil Res. (2010) 54(9):787–94. doi: 10.1111/j.1365-2788.2010.01305.x
8. Basil JS, Santoro SL, Martin LJ, Healy KW, Chini BA, Saal HM. Retrospective study of obesity in children with down syndrome. J Pediatr. (2016) 173:143–8. doi: 10.1016/j.jpeds.2016.02.046
9. Chen CJJ, Ringenbach SDR. Walking performance in adolescents and young adults with down syndrome: the role of obesity and sleep problems. J Intellect Disabil Res. (2018) 62(4):339–48. doi: 10.1111/jir.12474
10. Dierssen M, Fructuoso M, Martínez de Lagrán M, Perluigi M, Barone E. Down syndrome is a metabolic disease: altered insulin signaling mediates peripheral and brain dysfunctions. Front Neurosci. (2020) 14:670. doi: 10.3389/fnins.2020.00670
11. Fonseca CT, Amaral DM, Ribeiro MG, Beserra IC, Guimarães MM. Insulin resistance in adolescents with down syndrome: a cross-sectional study. BMC Endocr Disord. (2005) 5:6. doi: 10.1186/1472-6823-5-6
12. Murray J, Ryan-Krause P. Obesity in children with down syndrome: background and recommendations for management. Pediatr Nurs. (2010) 36(6):314–9.21291048
13. Ordóiez-Munoz FJ, Rosety-Rodríguez M, Rosety-Rodríguez JM, Rosety-Plaza M. Anthropometrical measurements as predictor of serum lipid profile in adolescents with down syndrome. Rev Invest Clin. (2005) 57(5):691–4.
14. Shires CB, Anold SL, Schoumacher RA, Dehoff GW, Donepudi SK, Stocks RM. Body mass index as an indicator of obstructive sleep apnea in pediatric down syndrome. Int J Pediatr Otorhinolaryngol. (2010) 74(7):768–72. doi: 10.1016/j.ijporl.2010.03.050
15. Tsou AY, Bulova P, Capone G, Chicoine B, Gelaro B, Harville TO, et al. Medical care of adults with down syndrome: a clinical guideline. J Am Med Assoc. (2020) 324(15):1543–56. doi: 10.1001/jama.2020.17024
16. Bandini L, Danielson M, Esposito LE, Foley JT, Fox MH, Frey GC, et al. Obesity in children with developmental and/or physical disabilities. Disabil Health J. (2015) 8(3):309–16. doi: 10.1016/j.dhjo.2015.04.005
17. Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. (2015) 33(7):673–89. doi: 10.1007/s40273-014-0243-x
18. Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med. (2017) 377(22):2145–53. doi: 10.1056/NEJMoa1703860
19. Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation. (2012) 126(1):126–32. doi: 10.1161/CIRCULATIONAHA.111.087213
20. González-Agüero A, Ara I, Moreno LA, Vicente-Rodríguez G, Casajús JA. Fat and lean masses in youths with down syndrome: gender differences. Res Dev Disabil. (2011) 32(5):1685–93. doi: 10.1016/j.ridd.2011.02.023
21. Hill DL, Parks EP, Zemel BS, Shults J, Stallings VA, Stettler N. Resting energy expenditure and adiposity accretion among children with down syndrome: a 3-year prospective study. Eur J Clin Nutr. (2013) 67(10):1087–91. doi: 10.1038/ejcn.2013.137
22. Luke A, Roizen NJ, Sutton M, Schoeller DA. Energy expenditure in children with down syndrome: correcting metabolic rate for movement. J Pediatr. (1994) 125(5, Part 1):829–38. doi: 10.1016/S0022-3476(06)80193-9
23. Martínez-Espinosa RM, Molina Vila MD, Reig García-Galbis M. Evidences from clinical trials in down syndrome: diet, exercise and body composition. Int J Environ Res Public Health. (2020) 17(12):4294. doi: 10.3390/ijerph17124294
24. Must A, Curtin C, Hubbard K, Sikich L, Bedford J, Bandini L. Obesity prevention for children with developmental disabilities. Curr Obes Rep. (2014) 3(2):156–70. doi: 10.1007/s13679-014-0098-7
25. Dulloo AG, Jacquet J, Miles-Chan JL, Schutz Y. Passive and active roles of fat-free mass in the control of energy intake and body composition regulation. Eur J Clin Nutr. (2017) 71(3):353–7. doi: 10.1038/ejcn.2016.256
26. Almiron-Roig E, Solis-Trapala I, Dodd J, Jebb SA.. estimating food portions. Influence of unit number, meal type and energy density. Appetite. (2013) 71:95–103. doi: 10.1016/j.appet.2013.07.012
27. Corder K, Crespo NC, Van Sluijs EM, Lopez NV, Elder JP. Parent awareness of young children’s physical activity. Prev Med. (2012) 55:201–5. doi: 10.1016/j.ypmed.2012.06.021
28. Corder K, van Sluijs EM, McMinn AM, Ekelund U, Cassidy A, Griffin SJ. Perception versus reality awareness of physical activity levels of British children. Am J Prev Med. (2010) 38:1–8. doi: 10.1016/j.amepre.2009.08.025
29. Hesketh KR, McMinn AM, Griffin SJ, Harvey NC, Godfrey KM, Inskip HM, et al. Maternal awareness of young children’s physical activity: levels and cross-sectional correlates of overestimation. BMC Public Health. (2013) 13(1):924. doi: 10.1186/1471-2458-13-924
30. Polfuss M, Moosreiner A, Boushey CJ, Delp EJ, Zhu F. Technology-based dietary assessment in youth with and without developmental disabilities. Nutrients. (2018) 10(10):1482. doi: 10.3390/nu10101482
31. Müller MJ, Enderle J, Bosy-Westphal A. Changes in energy expenditure with weight gain and weight loss in humans. Curr Obes Rep. (2016) 5(4):413–23. doi: 10.1007/s13679-016-0237-4
32. Polfuss M, Sawin KJ, Papanek PE, Bandini L, Forseth B, Moosreiner A, et al. Total energy expenditure and body composition of children with developmental disabilities. Disabil Health J. (2018) 11(3):442–6. doi: 10.1016/j.dhjo.2017.12.009
33. Enright G, Allman-Farinelli M, Redfern J. Effectiveness of family-based behavior change interventions on obesity-related behavior change in children: a realist synthesis. Int J Environ Res Public Health. (2020) 17(11):4099. doi: 10.3390/ijerph17114099
34. Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. (2010) 91(5):1499s–505s. doi: 10.3945/ajcn.2010.28701B
35. U.S. Department of Health & Human Services. The INCLUDE project research plan (2022). Available at: https://www.nih.gov/include-project/include-project-research-plan (Accessed July 9, 2022).
36. Buttimer J, Tierney E. Patterns of leisure participation among adolescents with a mild intellectual disability. J Intellect Disabil. (2005) 9(1):25–42. doi: 10.1177/1744629505049728
37. Fox MH, Witten MH, Lullo C. Reducing obesity among people with disabilities. J Disabil Policy Stud. (2014) 25(3):175–85. doi: 10.1177/1044207313494236
38. Shields N, Dodd KJ, Abblitt C. Do children with down syndrome perform sufficient physical activity to maintain good health? A pilot study. Adapt Phys Activ Q. (2009) 26(4):307–20. doi: 10.1123/apaq.26.4.307
39. McPherson AC, Ball GD, Maltais DB, Swift JA, Cairney J, Knibbe TJ, et al. A call to action: setting the research agenda for addressing obesity and weight-related topics in children with physical disabilities. Childhood Obesity. (2016) 12(1):59–69. doi: 10.1089/chi.2015.0119
40. Bowen GJ, Ehleringer JR, Chesson LA, Stange E, Cerling TE. Stable isotope ratios of tap water in the contiguous United States. Water Resour Res. (2007) 43(3). doi: 10.1029/2006WR005186
41. Valenzuela LO, O’Grady SP, Ehleringer JR. Variations in human body water isotope composition across the United States. Forensic Sci Int. (2021) 327:110990. doi: 10.1016/j.forsciint.2021.110990
42. Yelling M, Lamb K, Swaine I. Validity of a pictorial perceived exertion scale for effort estimation and effort production during stepping exercise in adolescent children. Eur Phy Educ Rev. (2002) 8(2):157–75. doi: 10.1177/1356336X020082007
43. International Atomic Energy Agency. Assessment of body composition and total energy expenditure in humans using stable isotope techniques. Vienna, Austria (2009).
44. Schoeller DA. Hydrometry. In: Heyesfield S, Lohman T, Wang Z, Going S, editors. Human body composition. Champaign, IL: Human Kinetics Press (2005). p. 35–50.
45. Toomey CM, Cremona A, Hughes K, Norton C, Jakeman P. A review of body composition measurement in the assessment of health. Top Clin Nutr. (2015) 30(1):16–32. doi: 10.1097/TIN.0000000000000017
46. Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. (1982) 35(5 Suppl):1169–75. doi: 10.1093/ajcn/35.5.1169
47. Wells JC, Fuller NJ, Dewit O, Fewtrell MS, Elia M, Cole TJ. Four-component model of body composition in children: density and hydration of fat-free mass and comparison with simpler models. Am J Clin Nutr. (1999) 69(5):904–12. doi: 10.1093/ajcn/69.5.904
48. Speakman JR, Yamada Y, Sagayama H, Berman ESF, Ainslie PN, Andersen LF, et al. A standard calculation methodology for human doubly labeled water studies. Cell Rep Med. (2021) 2(2):100203. doi: 10.1016/j.xcrm.2021.100203
49. Heydenreich J, Kayser B, Schutz Y, Melzer K. Total energy expenditure, energy intake, and body composition in endurance athletes across the training season: a systematic review. Sports Med Open. (2017) 3(1):8. doi: 10.1186/s40798-017-0076-1
50. Dorn LD, Biro FM. Puberty and its measurement: a decade in review. J Adolesc Res. (2011) 21(1):180–95. doi: 10.1111/j.1532-7795.2010.00722.x
51. NutritionQuest. Questionnaires and screeners 2014. Available at: https://www.nutritionquest.com/assessment/.
52. Hunsberger M, O'Malley J, Block T, Norris JC. Relative validation of block kids food screener for dietary assessment in children and adolescents. Matern Child Nutr. (2015) 11(2):260–70. doi: 10.1111/j.1740-8709.2012.00446.x
53. Haley S, Coster W., Dumas H., Fragala-Pinkham M., Moed R. Pediatric evaluatioin of disability inventory computer adaptive test (PEDI-CAT) (2016). Available at: www.pedicat.com.
54. Dumas HM, Fragala-Pinkham MA, Haley SM, Ni P, Coster W, Kramer JM, et al. Computer adaptive test performance in children with and without disabilities: prospective field study of the PEDI-CAT. Disabil Rehabil. (2012) 34(5):393–401. doi: 10.3109/09638288.2011.607217
55. Drahovzal DN, Bennett TM, Campagne PD, Vallis TM, Block TJ. Comparison of the block child activity screener with an objective measure of physical activity. In: Poster session at annual meeting of the International Society of Behavioral Nutrition and Physical Activity (ISBNPA). (2003) Available at: https://www.nutritionquest.com/company/our-research-questionnaires/.
56. Owens JA, Spirito A, McGuinn M. The children’s sleep habits questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. (2000) 23(8):1043–51. doi: 10.1093/sleep/23.8.1d
57. Markovich AN, Gendron MA, Corkum PV. Validating the children’s sleep habits questionnaire against polysomnography and actigraphy in school-aged children. Front Psychiatry. (2014) 5:188. doi: 10.3389/fpsyt.2014.00188
58. Honomichl RD, Goodlin-Jones BL, Burnham M, Gaylor E, Anders TF. Sleep patterns of children with pervasive developmental disorders. J Autism Dev Disord. (2002) 32(6):553–61. doi: 10.1023/A:1021254914276
59. Goldstein S, Naglieri J. Autism spectrum rating scales (ASRS): product overview (2009). Available at: https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Behavior/Autism-Spectrum-Rating-Scales/p/100000354.html.
60. Resseguier N, Giorgi R, Paoletti X. Sensitivity analysis when data are missing not-at-random. Epidemiology. (2011) 22(2):282. doi: 10.1097/EDE.0b013e318209dec7
61. Greenacre M, Blasius J. Multiple correspondence analysis and related methods. Boca Raton, FL: Chapman & Hall/CRC (2006).
62. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. (2011) 43(2):357–64. doi: 10.1249/MSS.0b013e3181ed61a3
63. Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. (2008) 26(14):1557–65. doi: 10.1080/02640410802334196
64. Trost SG, Loprinzi PD, Moore R, Pfeiffer KA. Comparison of accelerometer cut points for predicting activity intensity in youth. Med Sci Sports Exerc. (2011) 43(7):1360–8. doi: 10.1249/MSS.0b013e318206476e
65. Nutrition data system for research (NDSR) (2020). Available at: http://www.ncc.umn.edu/products/information-for-grant-writers/ (Accessed January 17, 2023).
66. U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary guidelines for Americans 2020-2025, 9th Edition2020. Available at: https://dietaryguidelines.gov.
Keywords: Down syndrome, trisomy 21 (Down syndrome), nutrition, physical activity, energy expenditure, obesity, doubly labeled water (DLW), wearable devices
Citation: Polfuss M, Bandini LG, Ravelli MN, Huang Z, Moosreiner A, Schoeller DA, Huang C-C, Ding D, Berry C, Marston E, Hussain A, Shriver TC and Sawin KJ (2023) Energy expenditure and weight-related behaviors in youth with Down syndrome: a protocol. Front. Pediatr. 11:1151797. doi: 10.3389/fped.2023.1151797
Received: 26 January 2023; Accepted: 5 July 2023;
Published: 20 July 2023.
Edited by:
Aviva Must, Tufts University, United StatesReviewed by:
Monica Guglielmetti, University of Pavia, ItalyRosaura Leis, University of Santiago de Compostela, Spain
© 2023 Polfuss, Bandini, Ravelli, Huang, Moosreiner, Schoeller, Huang, Ding, Berry, Marston, Hussain, Shriver and Sawin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michele Polfuss mpolfuss@uwm.edu
 Michele Polfuss
Michele Polfuss Linda G. Bandini
Linda G. Bandini Michele N. Ravelli
Michele N. Ravelli Zijian Huang5
Zijian Huang5  Dale A. Schoeller
Dale A. Schoeller Dan Ding
Dan Ding