Physical activity and physical fitness in children with heritable connective tissue disorders
- 1Center of Expertise Urban Vitality, Faculty of Health, University of Applied Sciences Amsterdam, Amsterdam, Netherlands
- 2Department of Rehabilitation Medicine, Amsterdam UMC Location University of Amsterdam, Amsterdam, Netherlands
- 3Amsterdam Movement Sciences, Rehabilitation and Development, Amsterdam, Netherlands
- 4Department of Pediatrics, Emma Children's Hospital, Academic Medical Center, Amsterdam, Netherlands
- 5Amsterdam Rheumatology and Immunology Center, Reade, Amsterdam, Netherlands
- 6Department of Physical and Rehabilitation Medicine, Child Rehabilitation, Ghent University Hospital, Ghent, Belgium
- 7Department of Pediatrics, Division of Pediatric Cardiology, Ghent University Hospital, Ghent, Belgium
- 8Center for Medical Genetics, Ghent University Hospital/Ghent University, Ghent, Belgium
- 9Department of Pediatric Cardiology, Amsterdam UMC, Location University of Amsterdam, Amsterdam, Netherlands
- 10Department of Pediatrics, Emma Children's Hospital Amsterdam UMC Follow-Me Program & Emma Neuroscience Group, Emma Children's Hospital, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands
- 11Amsterdam Reproduction and Development Research Institute, Amsterdam, Netherlands
Objectives: Health problems in patients with heritable connective tissue disorders (HCTD) are diverse and complex and might lead to lower physical activity (PA) and physical fitness (PF). This study aimed to investigate the PA and PF of children with heritable connective tissue disorders (HCTD).
Methods: PA was assessed using an accelerometer-based activity monitor (ActivPAL) and the mobility subscale of the Pediatric Evaluation of Disability Inventory Computer Adaptive Test (PEDI-CAT). PF was measured in terms of cardiovascular endurance using the Fitkids Treadmill Test (FTT); maximal hand grip strength, using hand grip dynamometry (HGD) as an indicator of muscle strength; and motor proficiency, using the Bruininks-Oseretsky Test of Motor Proficiency-2 (BOTMP-2).
Results: A total of 56 children, with a median age of 11.6 (interquartile range [IQR], 8.8–15.8) years, diagnosed with Marfan syndrome (MFS), n = 37, Loeys-Dietz syndrome (LDS), n = 6, and genetically confirmed Ehlers-Danlos (EDS) syndromes, n = 13 (including classical EDS n = 10, vascular EDS n = 1, dermatosparaxis EDS n = 1, arthrochalasia EDS n = 1), participated. Regarding PA, children with HCTD were active for 4.5 (IQR 3.5–5.2) hours/day, spent 9.2 (IQR 7.6–10.4) hours/day sedentary, slept 11.2 (IQR 9.5–11.5) hours/day, and performed 8,351.7 (IQR 6,456.9–1,0484.6) steps/day. They scored below average (mean (standard deviation [SD]) z-score −1.4 (1.6)) on the PEDI-CAT mobility subscale. Regarding PF, children with HCTD scored well below average on the FFT (mean (SD) z-score −3.3 (3.2)) and below average on the HGD (mean (SD) z-score −1.1 (1.2)) compared to normative data. Contradictory, the BOTMP-2 score was classified as average (mean (SD) z-score.02 (.98)). Moderate positive correlations were found between PA and PF (r(39) = .378, p < .001). Moderately sized negative correlations were found between pain intensity and fatigue and time spent actively (r(35) = .408, p < .001 and r(24) = .395 p < .001, respectively).
Conclusion: This study is the first to demonstrate reduced PA and PF in children with HCTD. PF was moderately positively correlated with PA and negatively correlated with pain intensity and fatigue. Reduced cardiovascular endurance, muscle strength, and deconditioning, combined with disorder-specific cardiovascular and musculoskeletal features, are hypothesized to be causal. Identifying the limitations in PA and PF provides a starting point for tailor-made interventions.
1. Introduction
Children with chronic disease often show physical inactivity leading to a reduction in PF level, thereby inducing a downward spiral of further physical inactivity (1). Physical activity (PA) and physical fitness (PF) have been described as important health-related outcomes in all age groups, especially for children with chronic conditions (2–5).
PF refers to the ability to perform physical activities and a full range of physiological and psychological qualities. PA can be defined as any bodily movement produced by muscle action that increases energy expenditure (5). PF is related to the components of fitness that benefit from a physically active lifestyle, including three main components: cardiorespiratory fitness, muscular fitness, and speed/agility (5–7).
Health problems in children with heritable connective tissue disorders (HCTD) are diverse and complex and characterized by multisystemic involvement (8–11). The phenotypes of the most common HCTD, Marfan syndrome (MFS) (9), Loeys-Dietz syndrome (LDS) (12) and Ehlers-Danlos syndrome (EDS) (11) show similarities in cardiovascular (aortic aneurism, mitral valve prolapse), musculoskeletal (e.g., scoliosis, foot deformities, joint hypermobility) and cutaneous features (e.g., skin hyperextensibility and tissue fragility). Children with MFS and EDS and their parents report problems with keeping up with peer activities and participation in school, sports, and other leisure activities due to fatigue, pain, and physical impairment (13–16).
To date, PA and PF have not been investigated in detail in children with HCTD. However, several studies have investigated PA and PF in children with other chronic conditions. These studies have reported significantly lower PA levels in children with juvenile idiopathic arthritis (JIA), type 1 diabetes, and obesity (17–19). More specifically, results showed that these children spent significantly less time in moderate to vigorous physical activities and more time in sedentary activities than healthy peers (17, 18, 20). In addition, children with JIA scored significantly lower on PF in terms of lower muscle strength, muscular endurance, and aerobic- and anaerobic- capacity than healthy peers (19). Children with generalized joint hypermobility (GJH) without a genetic diagnosis showed no differences in the level and duration of daily PA compared to healthy controls (21). However, another study reported a decrease in maximum exercise capacity in these children (22). It is unknown to what extent these findings translate to children with HCTD.
In general, it seems plausible that children with a higher level of PF spent more hours physically active during the day. However, the mutual relationship between PA and fitness has not been investigated recently (23). Insights into daily PA and PF in children with HCTD are mandatory to provide a starting point for tailored interventions (4). Therefore, the current study aimed to investigate PA and PF and their interrelationship in children with HCTD, specifically MFS, LDS, and genetically confirmed types of EDS.
2. Materials and methods
2.1. Study design and patient selection
This was a multicenter, observational, cross-sectional study. Participants were recruited from the Expert Center for MFS and related hereditary connective tissue disorders in Amsterdam, the Netherlands, and the Center for Medical Genetics of the Ghent University Hospital in Belgium. Eligible for inclusion were all children with HCTD, aged between 6 and 18 years, who were diagnosed with MFS (9), LDS (12) and genetically confirmed types of EDS, hereafter referred to as EDS (11). The exclusion criteria were comorbid prominent chronic diseases affecting physical functioning, PA, PF, and cognitive impairment (IQ < 80) and medical or psychiatric disorders that may affect the measurements in this study.
2.2. Procedure
The Medical Ethics Review Committee of the Amsterdam UMC (2019_121) and the Ethical Committee of Ghent University Hospital (EC2019/1958) approved the study protocol. The children and their parents were invited by letter. Informed consent was signed by the parents (for children aged <12 years), parents and children (for children aged 12 to 16 years), or adolescents (for participants aged ≥ 16 years). Approval from a pediatric cardiologist was required to participate in this study. All measurements were performed in a fixed order taking into account the intensity of the different tests and adequate rest pauses. Examiners were experienced with the specific tests and the standardized test protocol has been intensively trained. Tests were performed between March 2020 and March 2021.
2.3. Measures
2.3.1. Diagnostic and socio-demographic data
Disease-related data (diagnosis and use and type of cardiovascular medication) and socio-demographic data (age and sex) were collected using a custom-made questionnaire completed by the parents.
2.3.2. Clinical characteristics
The assessed clinical characteristics included body mass index (BMI) and the presence of GJH, as assessed using the Beighton scale (24).
Pain intensity over the last week was measured using a visual analog scale (VAS) (25) and scored on a 0–100 mm scale, with 0 mm referring to “no pain” and 100 mm to “very severe pain” (26). The validity and reliability of this method for assessing pain have been demonstrated in children with chronic diseases (25, 26).
Fatigue was measured using the Patient Reported Outcomes Measurement Information System (PROMIS) Fatigue 10a Pediatric v2.0 short form assessing self-reported fatigue in children 8–18 years of age or the Fatigue 10a Parent Proxy v2.0 short form assessing parent-reported fatigue in children <8 years of age. Both questionnaires demonstrated excellent psychometric properties (27, 28).
2.3.3. Physical activity
2.3.3.1. Accelerometry
PA was assessed using an ActivPAL™ accelerometer (Pal Technologies Ltd., Glasgow, United Kingdom). ActivPAL™ is a validated and reliable instrument for children (29, 30). The ActivPAL™ quantifies PA (standing, walking, and cycling), sedentary behavior (lying down, sitting, and passive traveling), and time spent asleep in daily life. Participants wore the ActivPAL™ for seven consecutive days during regular school weeks on the middle anterior line of the right thigh, sealed with a non-allergenic adhesive tape. The average time spent active, sedentary, sleeping, and daily steps were calculated.
2.3.3.2. Daily mobility
Daily mobility was assessed using the Pediatric Evaluation of Disability Inventory Computer Adaptive Test (PEDI-CAT). The PEDI-CAT is a validated and reliable assessment tool for parents and caregivers (31, 32) reporting on the daily mobility of their children and quantifying limitations in daily activity. Only the mobility subscale was used to assess five content areas: basic movement and transfers, standing and walking, steps and inclines, running and playing, and wheelchairs (33).
2.3.4. Physical fitness
2.3.4.1. Cardiovascular endurance
Cardiovascular endurance was assessed using the standardized Fitkids Treadmill Test Protocol (FTT) (34). The FTT is an incremental treadmill test consisting of 90 second stages with increments in speed and grade. After a warming-up period (3.5 km/h, 0% grade), the test starts at 3.5 km/h and a 1% gradient, followed by incremental increases in speed (0.5 km/h) and incline (2%) until exhaustion (34). The time to exhaustion (TTE) was defined as the point when the participant stopped the test despite verbal encouragement minus the 1.5 minute warming-up. The FTT has good validity and reproducibility in children aged 6–18 years of age (34).
2.3.4.2. Grip strength
Grip strength is a good indicator of overall muscle strength in children and adolescents (35). Grip strength was measured using the Biometrics E-Link Evaluation System (Biometrics Ltd., Gwent, UK) with a standard handgrip dynamometer (HGD). The standardized testing position recommended by the American Society of Hand Therapists (ASHT) to measure grip strength was used (36). The maximal grip strength was recorded in kilograms of force as the mean of three successive trials. The Biometrics E-Link Evaluation System is a validated and reliable instrument for assessing grip strength (37).
2.3.4.3. Motor proficiency
The Bruininks-Oseretsky Test of Motor Proficiency-2 (BOTMP-2) was used to assess motor performance (38). The BOTMP-2 generates scores on four composite measures: fine manual control, manual coordination, body coordination, and strength and agility. The BOTMP-2 has been validated and is reliable for assessing motor performance in children (39).
2.4. Statistical analysis
Data were exported from the Castor database (Electronic Data Capture, Ciwit BV, Amsterdam, The Netherlands, 2021) to the Statistical Package for Social Science (SPSS) version 26.0.
The percentage of missing values was <15% for the PF parameters (FTT 14.3%, HGD 5.4%, BOTMP-2 3.6%, respectively). Missingness was assessed as “missing at random”. Multivariate Imputation by Chained Equations (MICE) with predictive mean matching was used to impute the missing data (40, 41). The percentage of missing values on the PA parameters (including PROMIS V2.0 Shortform fatigue, PEDI-CAT, and ActivPAL) was >15% and therefore not suitable for imputation. Therefore, complete case analysis was used for analyzing PA parameters.
The comparability of the group of children with and without data of the PROMIS V2.0 Shortform fatigue, PEDI-CAT, and ActivPAL was assessed for age and sex using Chi-square tests and t-tests. The sample characteristics, age, and sex of the total HCTD group were comparable for the complete cases on the questionnaires and activity assessments (PROMIS V2.0 Shortform fatigue, PEDI-CAT, and ActivPAL, respectively) and proved to be representative.
Data of the total HCTD group and the MFS, LDS, and EDS subgroups are described in terms of mean and standard deviations (SD) or median and interquartile range (IQR) when indicated. Age- and sex-adjusted normative data were available for all measures (PEDI-CAT (42) FTT (34), HHD (43) and BOT-MP 2 (38)) except for ActivPAL. Data were converted to z-scores for the HCTD group and the MFS, LDS, and EDS subgroups. Z-scores were interpreted as well above average (z-score: ≥ 2), above average (z-score between 1 and 2), average (z-score between 1 and −1), below average (z-score between −1 and −2), and well below average (z-score ≤ −2). ActivPAL data were not converted to z-scores.
The exploratory analysis compared the PF data of children using and not using cardiovascular medication and children with and without GJH (Beighton score ≥6 yes/no). Z-scores between subgroups were compared using the t-test. Pearson's R or Spearman's rho was used to explore the relationships between PA, PF, and clinical characteristics of pain and fatigue in the HCTD group. The magnitude of correlations was interpreted according to Cohen, distinguishing between small-sized (0.10–0.30), medium-sized (0.30–0.50), and large-sized correlations (≥0.50) (44).
3. Results
3.1. Diagnostic and socio-demographic data
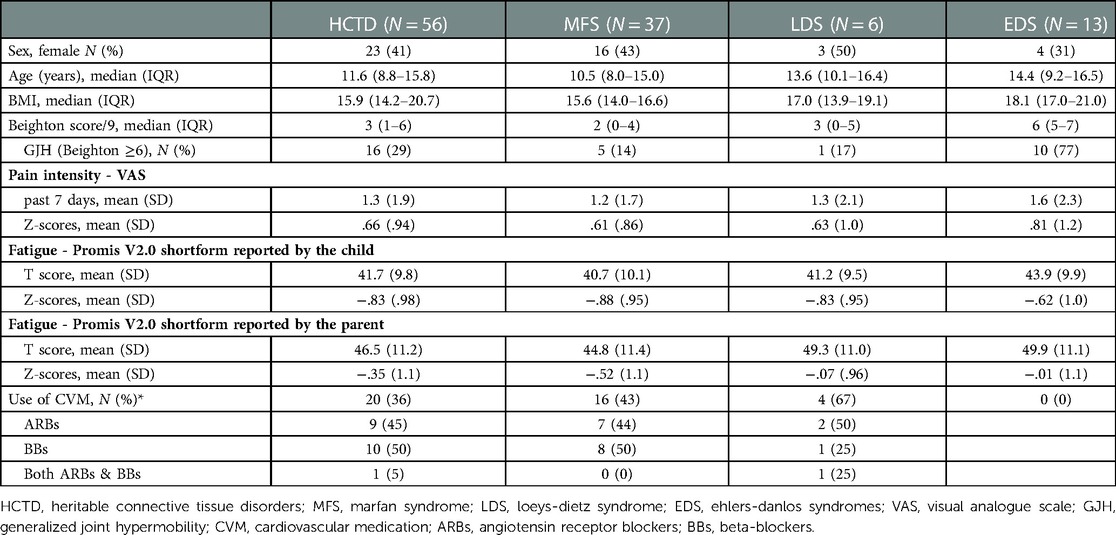
Table 1 shows the diagnostic and socio-demographic data and the clinical characteristics of the participants. Fifty-six children participated, of which 37 (66%) were diagnosed with MFS, 6 (11%) with LDS, and 13 (23%) with EDS (classical EDS n = 10, vascular EDS n = 1, dermatosparaxis EDS n = 1, arthrochalasia EDS n = 1), with a median age of 11.6 (IQR 8.8–15.8) years.
3.2. Clinical characteristics
Table 1 shows that the BMI was an average of 15.9 (IQR 14.2–20.7); 29% scored ≥6 on the Beighton scale, and 36% used cardiovascular medications in the HCTD group. The z-scores for pain intensity and fatigue for the HCTD group and the subgroups were in the average range compared to normative data.
3.3. Physical activity
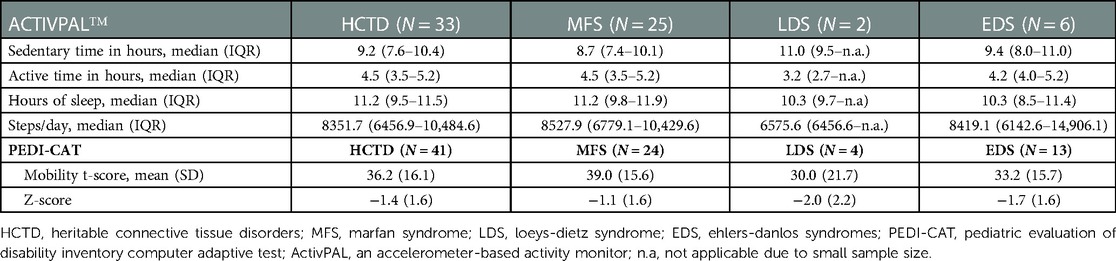
PA in children with HCTD is illustrated in Table 2.
3.3.1. Accelerometry
ActivPAL data were available for 33 of 56 (59%) children. Fifty-five children have worn the pre-set and working ActivPAL device. One child did not want to wear the devise (n = 1) and one child removed the device due to itching (n = 1). When loading the data afterwards, it turned out that a number of devices had not registered any data (n = 21). Concerning the complete cases, children with HCTD were active for 4.5 h/day (median = 4.5, IQR 3.5–5.2), spent 9.2 h/day sedentary (median = 9.2, IQR 7.6–10.4 h/day), slept 11.2 h/day (median = 11.2, IQR 9.5–11.5 h/day), and performed on average 8,351.7 steps a day (median = 8,351.7, IQR 6,456.9–10,484.6 steps a day).
3.3.2. Daily mobility
The PEDI-CAT mobility subscale data were available for 41 of the 56 children (73%). Missingness was due to absent of the parent during the measurement (n = 2) or technical issues with the software or computer (n = 13). Regarding the complete cases, the reported score on the mobility subscale of the PEDI-CAT was below average for the total HCTD group and subgroups compared to normative data, indicating reduced daily physical mobility in children with HCTD.
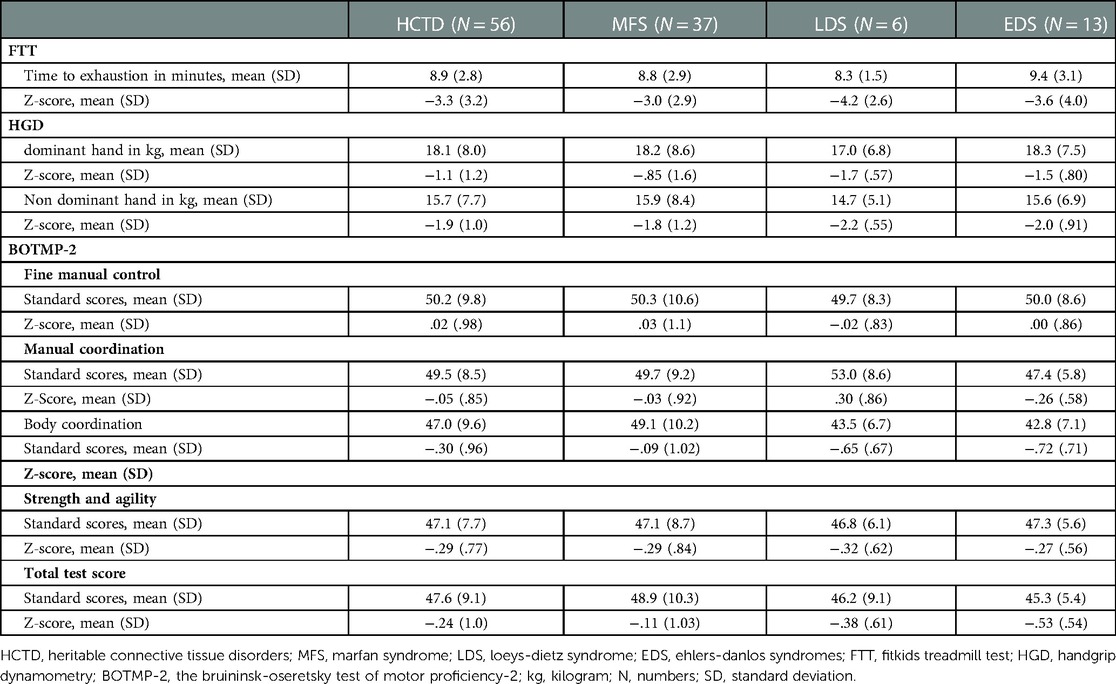
3.4. Physical fitness
PF parameters in children with HCTD are shown in Table 3.
3.4.1. Cardiovascular endurance
Compared to normative data, the TTE on the treadmill test was well below the average for the HCTD group and the HCTD subgroups, indicating considerably reduced cardiovascular endurance in children with HCTD.
3.4.2. Grip strength
The GHD of the dominant- and non-dominant hand was below average for the HCTD group and the EDS and LDS subgroups, indicating reduced muscle strength in children with HCTD.
3.4.3. Motor proficiency
BOTMP-2 scores were interpreted as the average of the total score and all subscale scores for the HCTD and HCTD subgroups, indicating normal motor performance.
3.5. Exploratory subgroup analysis
There were no significant differences in PF between children who did or did not use cardiovascular medication (FFT p = 0.739, HGD p = 0.563, BOTMP-2 p = 0.750) or between children classified with and without GJH (FFT p = 0.825, HGD p = 0.439, BOTMP-2 p = 0.912).
3.6. Relationship between physical activity and physical fitness
PA (daily mobility) and PF and cardiovascular endurance were moderately positively correlated (r(39) = 0.378, p ≤ .001). The clinical characteristics of pain intensity and fatigue were moderately sized and negatively correlated with the time spent actively (r(35) = 0.408, p ≤ .001, r(24) = 0.395, p ≤ .001, respectively).
4. Discussion
This is the first study to investigate PA and PF in children with HCTD. We found that children with HCTD have less than average cardiovascular endurance, below-average grip strength as an indicator of total muscle strength, normal motor proficiency, and below-average mobility in daily PA compared to normative data. PA and PF were moderately correlated.
Our findings regarding PF agree with previous research on children with various chronic conditions, such as JIA and diabetes (17, 18, 20). However, several disorder-specific clinical characteristics, including cardiovascular and musculoskeletal systems, may lead to decreased daily PA and PF in children with HCTD.
For PA, we measured daily activity and mobility. Our results on daily activity were comparable with those of studies in children with other chronic conditions, such as JIA, measured using questionnaires and diaries. They concluded that children with JIA had lower PA, spent less time in moderate to vigorous PA, and spent more time sedentary than healthy controls (20, 45). Other studies have indicated that children with or without a chronic condition have lower PA levels than those recommended in exercise guidelines (46).
In addition to measuring the quantity of activities, it is also important to provide information about the ability to perform activities that are important in daily life (daily mobility). A previously conducted qualitative study of adolescents with HCTD and their parents reported that they experienced difficulties performing everyday activities related to leisure activities and sports (13, 14, 16). These observations are consistent with the below-average mobility on the PEDI-CAT mobility subscale in our study group.
Regarding PF, children with MFS and LDS are commonly diagnosed with aortic dilatation, regardless of whether it is combined with mitral valve regurgitation (8, 47). This may affect PF. We investigated whether cardiac involvement contributed to cardiovascular endurance. Almost 40% of the children in our population used cardiovascular medications (angiotensin receptor blockers [ARBs] or beta-blockers [BB]), which are only prescribed in cases of evident aortic root dilatation (48). However, we did not find significant differences in cardiovascular endurance between children with and without cardiovascular medication. The effect of medication and its relation to cardiovascular endurance have been studied in various adult populations, but the findings are contradictory (49, 50). Additionally, children with EDS did not use cardiovascular medication, and their cardiovascular endurance was in line with that of the other subgroups.
In addition to the cardiovascular system, some musculoskeletal features may also affect cardiovascular endurance and muscle strength. GJH is present in approximately 30% of our population and has been previously linked to lower PF (51). In contrast, in line with more recent studies (52, 53), we did not find significant differences between children with and without GJH. Contributing factors, such as pain, fatigue, multi-systemic dysfunction, loss of postural control, and pain-related fear, may cause decreased muscle strength, motor performance, and lower PA levels in adolescents and adults with chronic musculoskeletal pain, with or without GJH (52, 54–56). Features related to decreased exercise capacity and muscle weakness, such as scoliosis and anterior chest deformation, are frequently reported in children with HCTD (8) and other populations (51, 57, 58).
There is an international trend to promote PA in children with and without chronic conditions. The general advice is to stimulate all children to be more physically active, resulting in beneficial health effects (59, 60). There is a need for interventions to address the limitations in PF and create optimal conditions for increasing daily activity and beneficial health effects in children with HCTD. However, specific advice regarding aortic status should be considered (61).
Our results should be viewed within the limitations of the present study. First, due to mainly technical problems, there were missing data on ActivPAL and PEDI-CAT. The reasons for missing data were beyond the influence of the assessors or researchers and labelled as missing at random. However, the samples without ActivPAL and PEDI-CAT data were still comparable to the HCTD group in terms of sex and age distribution and were, therefore, representative. Second, ActivPAL data were collected during the COVID-19 pandemic. There are indications that Dutch children had significantly lower PA and higher sedentary screen time during the pandemic (62, 63). Nevertheless, our results agree with those of studies conducted on children with JIA before the COVID-19 pandemic (20, 45). Third, we could not perform statistical tests to compare the MFS, LDS, and EDS subgroups because of the small sample size.
In conclusion, this study is the first to demonstrate reduced PA and PF in children with HCTD. PA and PF were moderately positively correlated in children with HCTD. Reduced cardiovascular endurance, muscle strength, and deconditioning combined with disorder-specific cardiovascular and musculoskeletal features are hypothesized to be causal. Identifying the limitations in PA and PF provides a starting point for tailor-made interventions.
Data availability statement
The datasets generated for this study are available on request to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The Medical Ethics Review Committee of the Amsterdam UMC (2019_121) and the Ethical Committee of Ghent University Hospital (EC2019/1958). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
The Pediatric Heritable Connective Tissue Disorders Study Group
Marieke J. H. Baars (University of Amsterdam, Amsterdam); Eelco Dulfer (University Medical Center Groningen, Groningen); Yvonne Hilhorst-Hofstee (Leiden University Medical Center, Leiden); Marlies J. E. Kempers (Radboud University Medical Center, Nijmegen); Ingrid P. C. Krapels (Maastricht University Medical Center, Maastricht); Bart L. Loeys (Radboud University Medical Center, Nijmegen; University of Antwerp and Antwerp University Hospital, Antwerp); and Femke Stoelinga (University of Amsterdam, Amsterdam; Rehabilitation and Development, Amsterdam).
Author contributions
LK: Study design, data acquisition, data analysis and interpretation, and writing of the original draft. JW: Research idea, study design, data acquisition, data analysis and interpretation, and writing—review and editing. MR, SL, RL, LM, AH: Writing—review and editing. JO: Research idea, writing—review and editing. LR: Research idea, study design, writing—review and editing, supervision. RE: Research idea, study design, writing—review and editing, supervision. All authors contributed important intellectual content during manuscript drafting or revision, accepted personal accountability for their contributions, and agreed to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Funding
SIA RAAK-PRO, part of the Dutch Organization for Scientific Research (NWO; SVB.RAAK > PRO02.007), a 5-year research grant allocated to the project “Follow You – a follow-up program on physical, psychosocial functioning and participation in children and adolescents with (Heritable) connective tissue disorders”.
Acknowledgments
We thank the parents and children who participated in this study. We are grateful to SIA RAAK-PRO, part of the Dutch Organization for Scientific Research, for funding this project (NWO, SVB. RAAK > PRO02.007), which is part of a 5-year research grant for the project “Follow You—a follow-up program on physical and psychosocial functioning and participation in children and adolescents with (heritable) connective tissue disorders. We also acknowledge the members of the Pediatric Heritable Connective Tissue Disorders study group, the European Reference Network Skin—Mendelian Connective Tissue Disorders, the Dutch Network Marfan and related disorders, and both the Marfan and Ehlers-Danlos patient associations for productive discussions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ABRs, angiotensin receptor blockers; ASHT, american society of hand therapists; BB, beta-blockers; BMI, body mass index; BOTMP-2, bruininks-oseretsky test of motor proficiency-; EDS, ehlers-danlos; FTT, fitkids treadmill test; GJH, generalized joint hypermobility; HCTD, heritable connective tissue disorders; HGD, hand grip dynamometry; IQR, interquartile range; JIA, juvenile idiopathic arthritis; LDS, loeys-dietz syndrome; MFS, marfan syndrome; PA, physical activity; PEDI-CAT, pediatric evaluation of disability inventory computer adaptive test; PF, physical fitness; PROMIS, patient reported outcomes measurement information system; SD, standard deviation; SPSS, statistical package for social science; TTE, time to exhaustion; VAS, visual analogue scale.
References
1. Bar-Or O, Rowland TW. Pediatric exercise medicine: From physiologic principles to health care application. Champaign, IL: Human Kinetics (2004).
2. Thornton JS, Frémont P, Khan K, Poirier P, Fowles J, Wells GD, et al. Physical activity prescription : a critical opportunity to address a modi fi able risk factor for the prevention and management of chronic disease : a position statement by the Canadian academy of sport and exercise medicine. Br J Sports Med. (2016) 50(18):1109–14. doi: 10.1136/bjsports-2016-096291
3. Dimitri P, Joshi K, Jones N, Medicine M. Moving more: physical activity and Its positive effects on long term conditions in children and young people. Arch Dis Child. (2020) 105(11):1035–40. doi: 10.1136/archdischild-2019-318017
4. van Brussel M, van der Net J, Hulzebos E, Helders PJM, Takken T. The Utrecht approach to exercise in chronic childhood conditions: the decade in review. Pediatr Phys Ther. (2011) 23(1):2–14. doi: 10.1097/PEP.0b013e318208cb22
5. Bouchard C, Blair SN, Haskell WL. Physical activity and health. 2nd edi. Bouchard C, Blair SN, Haskell WL, editors. Champaign, IL: Human Kinetics; (2012). p. 1–426.
6. Baranowski T, Bouchard C, Bar-Or O, Bricker T, Heath G, Kimm SY, et al. Assessment, prevalence, and cardiovascular benefits of physical activity and fitness in youth. Med Sci Sports Exerc. (1992) 24(6 Suppl):S237–47. PMID: 1625549.1625549
7. Ortega FB, Ruiz JR, Castillo MJ, Sjöström M. PEDIATRIC REVIEW physical fitness in childhood and adolescence : a powerful marker of health. Int J Obes (Lond). (2008) 32(1):1–11. doi: 10.1038/sj.ijo.0803774
8. Meester JAN, Verstraeten A, Schepers D, Alaerts M, van Laer L, Loeys BL. Differences in manifestations of marfan syndrome, ehlers-danlos syndrome, and loeys-dietz syndrome. Ann Cardiothorac Surg. (2017) 6(6):582–94. doi: 10.21037/acs.2017.11.03
9. Loeys BL, Dietz HC, Braverman AC, Callewaert BL, de Backer J, Devereux RB, et al. The revised Ghent nosology for the marfan syndrome. J Med Genet. (2010) 47(7):476–85. doi: 10.1136/jmg.2009.072785
10. Velchev JD, van Laer L, Luyckx I, Dietz H, Loeys B. Loeys-Dietz syndrome. Adv Exp Med Biol. (2021) 1348:251–64. doi: 10.1007/978-3-030-80614-9_11
11. Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 international classification of the ehlers-danlos syndromes. Am J Med Genet C Semin Med Genet. (2017) 175(1):8–26. doi: 10.1002/ajmg.c.31552
12. Van Laer L, Dietz H, Loeys B. Loeys-Dietz syndrome. In: Halper J, editors. Progress in heritable soft connective tissue diseases. Dordrecht: Springer Netherlands (2014). p. 95–105.
13. Warnink-Kavelaars J, Beelen A, Dekker S, Nollet F, Menke LA, Engelbert RHH. Marfan syndrome in childhood: parents’ perspectives of the impact on daily functioning of children, parents and family; A qualitative study. BMC Pediatr. (2019) 19(1):1–10. doi: 10.1186/s12887-019-1612-6
14. Warnink-Kavelaars J, Beelen A, Goedhart TMHJ, de Koning LE, Nollet F, Alsem MW, et al. Marfan syndrome in adolescence: adolescents’ perspectives on (physical) functioning, disability, contextual factors and support needs. Eur J Pediatr. (2019) 178(12):1883–92. doi: 10.1007/s00431-019-03469-7
15. Wesley A, Bray P, Munns CF, Pacey V. Impact of heritable disorders of connective tissue on daily life of children: parent perspectives. J Paediatr Child Health. (2020) 57:626–30. doi: 10.1111/jpc.15284
16. Warnink-Kavelaars J, de Koning LE, Rombaut L, Alsem MW, Menke LA, Oosterlaan J, et al. Heritable connective tissue disorders in childhood: increased fatigue, pain, disability and decreased general health. Genes (Basel). (2021) 12(6):1–12. doi: 10.3390/genes12060831
17. Gueddari S, Amine B, Rostom S, Badri D, Mawani N, Ezzahri M, et al. Physical activity, functional ability, and disease activity in children and adolescents with juvenile idiopathic arthritis. Clin Rheumatol. (2014) 33(9):1289–94. doi: 10.1007/s10067-014-2576-4
18. Maggio ABR, Hofer MF, Martin XE, Marchand LM, Beghetti M, Farpour-Lambert NJ. Reduced physical activity level and cardiorespiratory fitness in children with chronic diseases. Eur J Pediatr. (2010) 169(10):1187–93. doi: 10.1007/s00431-010-1199-2
19. van Brussel M, Lelieveld OTHM, van der Net J, Engelbert RHH, Helders PJM, Takken T. Aerobic and anaerobic exercise capacity in children with juvenile idiopathic arthritis. Arthritis Rheum. (2007) 57(6):891–7. doi: 10.1002/art.22893
20. Bos GJFJ, Lelieveld OTHM, Armbrust W, Sauer PJJ, Geertzen JHB, Dijkstra PU. Physical activity in children with juvenile idiopathic arthritis compared to controls. Pediatr Rheumatol Online J. (2016) 14(1):42. doi: 10.1186/s12969-016-0102-8
21. Juul-Kristensen B, Kristensen JH, Frausing B, Jensen DV, Røgind H, Remvig L. Motor competence and physical activity in 8-year-old school children with generalized joint hypermobility. Pediatrics. (2009) 124(5):1380–7. doi: 10.1542/peds.2009-0294
22. Engelbert RHH, van Bergen M, Henneken T, Helders PJM, Takken T. Exercise tolerance in children and adolescents with musculoskeletal pain in joint hypermobility and joint hypomobility syndrome. Pediatrics. (2006) 118(3):e690–6. doi: 10.1542/peds.2005-2219
23. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. (2020) 54(24):1451–62. doi: 10.1136/bjsports-2020-102955
24. Scheper MC, Engelbert RHH, Rameckers EAA, Verbunt J, Remvig L, Juul-Kristensen B. Children with generalised joint hypermobility and musculoskeletal complaints: state of the art on diagnostics, clinical characteristics, and treatment. Biomed Res Int. (2013) 2013:121054. doi: 10.1155/2013/121054
25. Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. (1983) 16(1):87–101. doi: 10.1016/0304-3959(83)90088-X
26. Michaleff ZA, Kamper SJ, Stinson JN, Hestbaek L, Williams CM, Campbell P, et al. Measuring musculoskeletal pain in infants, children, and adolescents. J Orthop Sports Phys Ther. (2017) 47(10):712–30. doi: 10.2519/jospt.2017.7469
27. Haverman L, Grootenhuis MA, Raat H, van Rossum MAJ, van Dulmen-den Broeder E, Hoppenbrouwers K, et al. Dutch-Flemish translation of nine pediatric item banks from the patient-reported outcomes measurement information system (PROMIS)®. Qual Life Res. (2016) 25(3):761–5. doi: 10.1007/s11136-015-0966-y
28. Luijten MAJ, van Litsenburg RRL, Terwee CB, Grootenhuis MA, Haverman L. Psychometric properties of the patient-reported outcomes measurement information system (PROMIS®) pediatric item bank peer relationships in the Dutch general population. Qual Life Res. (2021) 30(7):2061–70. doi: 10.1007/s11136-021-02781-w
29. Adib N, Davies K, Grahame R, Woo P, Murray KJ. Joint hypermobility syndrome in childhood. A Not So benign multisystem disorder? Rheumatology (Oxford). (2005) 44(6):744–50. doi: 10.1093/rheumatology/keh557
30. De Decker E, De Craemer M, Santos-Lozano A, Van Cauwenberghe E, De Bourdeaudhuij I, Cardon G. Validity of the ActivPALTM and the ActiGraph monitors in preschoolers. Med Sci Sports Exerc. (2013) 45(10):2002–11. doi: 10.1249/MSS.0b013e318292c575
31. Dumas HM, Fragala-Pinkham MA, Haley SM, Ni P, Coster W, Kramer JM, et al. Computer adaptive test performance in children with and without disabilities: prospective field study of the PEDI-CAT. Disabil Rehabil. (2012) 34(5):393–401. doi: 10.3109/09638288.2011.607217
32. Bos N, Engel MF, van Rijswijk NJ, Verheijden JMA, Coster W, Moed R, et al. Translation and cross-cultural adaptation of the PEDI-CAT: dutch version. J Pediatr Rehabil Med. (2019) 12(1):57–64. doi: 10.3233/PRM-180544
33. Haley SM, Coster WJ, Dumas HM, Fragala-Pinkham MA, Moed R. PEDI-CAT: Development, Standardisation and Administration Manual. Boston University; Boston, MA, USA: (2012). p. 1–109.
34. Kotte EMW, de Groot JF, Bongers BC, Winkler AMF, Takken T. Fitkids treadmill test: age- and sex-related normative values in Dutch children and adolescents. Phys Ther. (2016) 96(11):1764–72. doi: 10.2522/ptj.20150399
35. Wind AE, Takken T, Helders PJM, Engelbert RHH. Is Grip strength a predictor for total muscle strength in healthy children, adolescents, and young adults? Eur J Pediatr. (2010) 169(3):281–7. doi: 10.1007/s00431-009-1010-4
36. Fess Elaine & Moran, Christine. (1981). American Society of Hand Therapists Clinical Assessment Recommendations.
37. Allen D, Barnett F. Reliability and validity of an electronic dynamometer for measuring grip strength. Int J Ther Rehabil. (2011) 18:258–64. doi: 10.12968/ijtr.2011.18.5.258
38. Bruininks R, Bruininks B. Bruininks-Oseretsky test of motor proficiency. 2nd ed., MN: NCS Pearson. editor. Minneapolis, 2005.
39. Griffiths A, Toovey R, Morgan PE, Spittle AJ. Psychometric properties of gross motor assessment tools for children: a systematic review. BMJ Open. (2018) 8(10):e021734. doi: 10.1136/bmjopen-2018-021734
40. Eekhout I, de Vet HCW, Twisk JWR, Brand JPL, de Boer MR, Heymans MW. Missing data in a multi-item instrument Were best handled by multiple imputation at the item score level. J Clin Epidemiol. (2014) 67(3):335–42. doi: 10.1016/j.jclinepi.2013.09.009
41. Heymans MW, Twisk JWR. Handling missing data in clinical research. J Clin Epidemiol. (2022) 151:185–88. doi: 10.1016/j.jclinepi.2022.08.016
42. Haley SM, Coster WJ, Dumas HM, Fragala-Pinkham MA, Kramer J, Ni P, et al. Accuracy and precision of the pediatric evaluation of disability inventory computer-adaptive tests (PEDI-CAT). Dev Med Child Neurol. (2011) 53(12):1100–6. doi: 10.1111/j.1469-8749.2011.04107.x
43. Mathiowetz V, Wiemer DM, Federman SM. Grip and pinch strength: norms for 6- to 19-year-olds. Am J Occup Ther. (1986) 40(10):705–11. doi: 10.5014/ajot.40.10.705
45. Fazaa A, Sellami M, Ouenniche K, Miladi S, Kassab S, Chekili S, et al. Physical activity assessment in children and adolescents with juvenile idiopathic arthritis compared with controls. Arch Pediatr. (2021) 28(1):47–52. doi: 10.1016/j.arcped.2020.10.008
46. Elmesmari R, Reilly JJ, Martin A, Paton JY. Accelerometer measured levels of moderate-to-vigorous intensity physical activity and sedentary time in children and adolescents with chronic disease: a systematic review and meta-analysis. PLoS One. (2017) 12(6):e0179429. doi: 10.1371/journal.pone.0179429
47. Attias D, Stheneur C, Roy C, Collod-Béroud G, Detaint D, Faivre L, et al. Comparison of clinical presentations and outcomes between patients with TGFBR2 and FBN1 mutations in marfan syndrome and related disorders. Circulation. (2009) 120(25):2541–9. doi: 10.1161/CIRCULATIONAHA.109.887042
48. Hilhorst-Hofstee Y. Multidisciplinaire richtlijn ‘Marfan syndroom’ [Multidisciplinary practice guideline ‘Marfan syndrome’]. Ned Tijdschr Geneeskd. (2013) 157(50):A6658. Dutch. PMID: 24326138.24326138
49. Nielen JTH, de Vries F, van der Velde JHPM, Savelberg HHCM, Schaper NC, Dagnelie PC, et al. The association between β-blocker use and cardiorespiratory fitness: the Maastricht study. J Cardiovasc Pharmacol Ther. (2019) 24(1):37–45. doi: 10.1177/1074248418778551
50. Dore A, Houde C, Chan KL, Ducharme A, Khairy P, Juneau M, et al. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation. (2005) 112(16):2411–6. doi: 10.1161/CIRCULATIONAHA.105.543470
51. Scheper MC, de Vries JE, Juul-Kristensen B, Nollet F, Engelbert RHH. The functional consequences of generalized joint hypermobility: a cross-sectional study. BMC Musculoskelet Disord. (2014) 15:243. doi: 10.1186/1471-2474-15-243
52. van Meulenbroek T, Huijnen IPJ, Simons LE, Conijn AEA, Engelbert RHH, Verbunt JA. Exploring the underlying mechanism of pain-related disability in hypermobile adolescents with chronic musculoskeletal pain. Scand J Pain. (2021) 21(1):22–31. doi: 10.1515/sjpain-2020-0023
53. van Meulenbroek T, Huijnen IP, Engelbert RH, Verbunt JA. Are Chronic musculoskeletal pain and generalized joint hypermobility disabling contributors to physical functioning? Eur J Phys Rehabil Med. (2021) 57(5):747–57. doi: 10.23736/S1973-9087.21.06455-8
54. Scheper MC, Nicholson LL, Adams RD, Tofts L, Pacey V. The natural history of children with joint hypermobility syndrome and ehlers-danlos hypermobility type: a longitudinal cohort study. Rheumatology (United Kingdom). (2017) 56(12):2073–83. doi: 10.1093/rheumatology/kex148
55. Vlaeyen JWS, Linton SJ. Fear-avoidance and Its consequences in chronic musculoskeletal pain: a state of the art. Pain. (2000) 85(3):317–32. doi: 10.1016/S0304-3959(99)00242-0
56. Leeuw M, Goossens MEJB, Linton SJ, Crombez G, Boersma K, Vlaeyen JWS. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. (2007) 30(1):77–94. doi: 10.1007/s10865-006-9085-0
57. Shamberger RC. Cardiopulmonary effects of anterior chest wall deformities. Chest Surg Clin N Am. (2000) 10(2):245–52, v–vi. PMID: 10803331.10803331
58. de Loos ER, Höppener PF, Busari JO, Lenderink T, Hulsewé KWE, Vissers YLJ. Trechterborst: niet alleen een cosmetisch probleem [Pectus excavatum: not just a cosmetic problem]. Ned Tijdschr Geneeskd. (2020) 164:D4509. Dutch. PMID: 32749790. 32749790
59. Health TLCA. Enabling participation in physical activity. Lancet Child Adolesc Health. (2022) 6(2):71. doi: 10.1016/S2352-4642(22)00003-7
60. Carbone PS, Smith PJ, Lewis C, LeBlanc C. Promoting the participation of children and adolescents with disabilities in sports, recreation, and physical activity. Pediatrics. (2021) 148(6):e2021054664. doi: 10.1542/peds.2021-054664
61. Iung B. New ESC guidelines: aortic disease. Heart (British Cardiac Society). (2015) 101:421–3. doi: 10.1136/heartjnl-2014-306777
62. Ten Velde G, Lubrecht J, Arayess L, van Loo C, Hesselink M, Reijnders D, et al. Physical activity behaviour and screen time in Dutch children during the COVID-19 pandemic: pre-, during- and post-school closures. Pediatr Obes. (2021) 16(9):e12779. doi: 10.1111/ijpo.12779
63. Krijger A, Dulfer K, van Oers H, Teela L, de Jong-van Kempen B, van Els A, et al. Perceived stress, family impact, and changes in physical and social daily life activities of children with chronic somatic conditions during the COVID-19 pandemic. BMC Public Health. (2022) 22(1):1106. doi: 10.1186/s12889-022-13544-8
Keywords: Heritable Connective Tissue Disorders, Marfan Syndrome, Ehlers Danlos Syndromes, Loeys Dietz Syndrome, physical activity, physical fitness
Citation: de Koning L, Warnink-Kavelaars J, van Rossum M, Limmen S, Van der Looven R, Muiño-Mosquera L, van der Hulst A, Oosterlaan J, Rombaut L and Engelbert R (2023) Physical activity and physical fitness in children with heritable connective tissue disorders. Front. Pediatr. 11:1057070. doi: 10.3389/fped.2023.1057070
Received: 17 October 2022; Accepted: 21 February 2023;
Published: 17 March 2023.
Edited by:
Abdulsamet Erden, Yıldırım Beyazıt University, TürkiyeReviewed by:
Shea Palmer, Centre for Care Excellence, United KingdomSarah Bennett, The University of Bristol, United Kingdom
© 2023 de Koning, Warnink-Kavelaars, van Rossum, Limmen, Van der Looven, Muiño-Mosquera, van der Hulst, Oosterlaan, Rombaut and Engelbert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisanne de Koning l.e.de.koning@hva.nl
†These authors have contributed equally to this work and share last authorship
Specialty Section: This article was submitted to Pediatric Rheumatology, a section of the journal Frontiers in Pediatrics
 Lisanne de Koning
Lisanne de Koning Jessica Warnink-Kavelaars2,3
Jessica Warnink-Kavelaars2,3  Laura Muiño-Mosquera
Laura Muiño-Mosquera Annelies van der Hulst
Annelies van der Hulst