Prevention of malaria in pregnancy: The threat of sulfadoxine-pyrimethamine resistance
- Department of Pediatrics, Children's Hospital of Philadelphia, The Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States
Malaria infection in pregnancy can lead to adverse outcomes for both the pregnant person and fetus. The administration of intermittent preventative therapy (IPTp) with sulfadoxine-pyrimethamine (SP) during pregnancy (IPTp-SP) improves outcomes, including severe maternal anemia, placental malaria infection, and low infant birth weight. The WHO recommends IPTp-SP for pregnant individuals living in areas of moderate or high malaria transmission in Africa. The current regimen consists of two or more doses of SP starting as early as possible in the second trimester, at least 1 month apart. Unfortunately, rising Plasmodium falciparum SP resistance throughout Africa threatens to erode the benefits of SP. Recent studies have shown a decrease in IPTp-SP efficacy in areas with high SP resistance. Thus, there is an urgent need to identify new drug regimens that can be used for intermittent preventative therapy in pregnancy. In this review, we discuss recent data on P. falciparum SP resistance in Africa, the effect of resistance on IPTp-SP, and studies of alternative IPTp regimens. Finally, we present a framework for the ideal pharmacokinetic and pharmacodynamic properties for future IPTp regimens.
Introduction
Malaria, caused by parasites of the genus Plasmodium, is a major cause of morbidity and mortality across the globe (1). The majority of malaria-related deaths occur in sub-Saharan Africa, and are caused by one parasite species, Plasmodium falciparum. Children, especially those under the age of five, are at highest risk of severe disease. Partial immunity, which develops through repeated exposure, provides some protection against both symptomatic and severe disease (2).
While the risks of malaria decrease with age and regular reinfections, they recur again in pregnancy, where both symptomatic and asymptomatic infection have significant consequences for the pregnant person and fetus. This burden is felt disproportionally by pregnant people in sub-Saharan Africa, where over 10 million pregnant individuals are likely exposed to malaria each year (1). Intermittent presumptive therapy for malaria in pregnancy (IPTp) with sulfadoxine pyrimethamine (SP) decreases the adverse effects of malaria in pregnancy, but the benefits of this intervention are threatened by increasing SP resistance throughout sub-Saharan Africa (3). New antimalarial therapies are, therefore, needed to ensure continued protection of pregnant people and fetuses.
Malaria in pregnancy and intermittent preventative therapy
The WHO estimates that over 10 million pregnant individuals (34% of all pregnancies worldwide) are exposed to malaria each year (1). The risks of malaria are increased in pregnancy (4, 5). Not only does pregnancy represent a state of transient immunosuppression, the malaria parasite Plasmodium falciparum, expresses a unique adhesion factor, var2csa, that binds chondroitin sulfate on the placenta (6). Parasite adhesion to the maternal surface of the placenta leads to increased inflammation and reduced placental blood flow (6). For these reasons, placental malaria contributes to poor outcomes for both the birthing parent and developing fetus. Malaria during pregnancy contributes to maternal anemia, low birthweight, intrauterine growth restriction, preterm delivery, stillbirth, and death in the neonatal period (7–9). In spite of these many potential complications, pregnant individuals may yet present with few or no symptoms, making prompt identification and treatment of infection difficult (5). Early studies showed that antimalarial prophylaxis in pregnancy reduced maternal anemia and increased infant birthweight (10, 11). This led the WHO to recommend that pregnant people in malaria endemic regions receive intermittent antimalarial prophylaxis.
Currently, intermittent preventative therapy for malaria in pregnancy (IPTp) consists of treatment doses of sulfadoxine pyrimethamine (SP), starting in the second trimester (12). IPTp-SP improves outcomes for both the pregnant person and fetus. Early clinical trials of SP during pregnancy showed a marked reduction in peripheral parasitemia, maternal anemia, placental malaria, preterm birth, and an increase in infant birth weight (13–16). Additional studies showed that three or more doses of SP, given at least four weeks apart, further decreases the overall prevalence of low birth weight and preterm birth (17, 18). The WHO estimates that current levels of IPTp coverage prevent over 400,000 cases of low birthweight each year.
Resistance to sulfadoxine pyrimethamine
The benefits of IPTp-SP in pregnancy are threatened by rising SP resistance in P. falciparum. Sulfadoxine and pyrimethamine both inhibit folate synthesis in malaria parasites, acting on dihydropteroate synthase (dhps) and dihydrofolate reductase (dhfr), respectively. SP resistance develops through the accumulation of mutations in these enzymes (Figure 1). In East Africa, parasites containing a combination of three distinct dhfr mutations (N51I, N59R, and S108N) and two dhps mutations (A437G, and K540E) have become highly prevalent. The presence of these parasites, termed “quintuple mutants,” is highly predictive of treatment failure of clinical malaria in sub-Saharan Africa (19, 20). Acquired immunity likely also plays a role, as treatment failure is observed in young children infected with less resistant parasites (21).
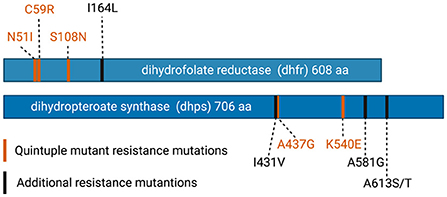
Figure 1. Mutations in dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) that confer resistance to sulfadoxine pyrimethamine. Mutations found in the highly resistant “quintuple mutant” are shown in orange. Additional mutations, that are increasing in prevalence in Africa, are shown in black. Figures generated in Biorender.
While the presence of quintuple mutants predicts poor treatment response in cases of acute uncomplicated malaria, P. falciparum continues to evolve higher levels of resistance. In East Africa, additional mutations, including dhps A581G, dhfr I164L, and dhps A613S/T have begun to emerge. These mutations, when present on the quintuple mutant background, further increase SP resistance and increase the risk of clinical treatment failure (3, 22). A581G and A613S/T mutations have also been detected in West Africa, in the absence of K540E (23). While the lack of K540E in these parasites increases their susceptibility to SP, the risk of highly resistant parasites emerging in West Africa, through the acquisition of K540E or other novel mutations, remains.
The effects of sulfadoxine pyrimethamine resistance on IPTp-SP
Early studies of IPTp-SP in areas of increasing SP resistance showed continued benefit. A 2007 meta-analysis by Kuile, et. al. found that IPTp-SP maintained effectiveness in preventing low birth weight, maternal anemia, maternal parasitemia at delivery, and placental malaria, even in geographic regions where SP treatment failure rates in children with acute malaria ranged from 9–39% (24). Importantly, treatment failure in children appears to occur at lower levels of SP resistance than in adults. Thus, pediatric malaria treatment failure rates are a surprisingly poor proxy for the effectiveness of ITPp-SP in pregnancy (21). More recent studies, using genetic markers of SP resistance, have found that IPTp-SP effectiveness is indeed reduced in areas where highly resistant parasites are prevalent. Van Eijk, et. al. found that the relative risk reduction of IPTp-SP on malaria-associated pregnancy outcomes decreased with increasing prevalence of the K540E mutation (25). While IPTp-SP confers some benefit in areas where the prevalence of K540E was >90%, the benefit was lost in areas where A581G prevalence was >10%. The loss of effectiveness, due to A581G, was also observed by Chico et al. (26). Given the increasing prevalence of A581G in East Africa (23), these data suggest that decreased IPTp-SP effectiveness will soon be widespread in this region.
Dihydroartemisinin-piperaquine as an alternative to IPTp-SP
As the efficacy of IPTp-SP wanes due to increasing resistance, there is an urgent need to identify alternative pharmaceutical strategies for preventing adverse pregnancy outcomes from malaria. While clinical studies have examined multiple alternatives, including amodiaquine, mefloquine, azithromycin, and chloroquine, most have failed due to adverse effects or lack of benefit (27–29). Recently, combination therapy with dihydroartemisinin plus piperaquine (DP) has been proposed as a replacement to IPTp-SP, given the tolerability and rapid, potent activity against asexual P. falciparum. IPTp-DP reduces the prevalence of both clinical and sub-patent malaria infection as compared to IPTp-SP (30–32). This has not, however, translated to a consistent improvement in birth outcomes. Thus, far, three randomized controlled trials have found IPTp-DP to be equivalent to IPTp-SP, and only one has found it to be superior in preventing adverse birth outcomes (30–33).
The lack of benefit of IPTp-DP, relative to IPTp-SP, for birth outcomes, in spite of a decrease in detectable peripheral parasitemia, suggests that the latter may not adequately reflect pathology at the placenta (33, 34). Indeed, randomized controlled trials comparing IPTp-SP to intermittent screening and treatment (IST), where pregnant individuals are screened by rapid diagnostic test or peripheral smear and treated only if positive, have found IST to be inferior (33, 35). Moreover, an analysis of over 1,500 patients found that placental malaria infection was associated with lower birth weight regardless of whether parasites were detected in the peripheral blood, and that the presence of peripheral parasitemia, without placental infection, was not associated with lower birth weight (36).
There are multiple possible explanations for the lack of consistent benefit of IPTp-DP over IPTp-SP. First, levels of SP resistance may not have reached thresholds that compromise IPTp-SP. To date, trials comparing IPTp-DP to IPTp-SP have been conducted in areas where the prevalence of A581G and other highly resistance genotypes is low (0–5.8%) (31, 33, 37, 38). In the absence of these highly SP resistant parasites, it is possible that IPTp-SP and IPTp-DP are equally effective in preventing adverse birth outcomes. Second, the frequency of DP dosing may be insufficient to maintain protection. Pharmacokinetic analyses of pregnant individuals given IPTp-DP found that higher exposure to piperaquine was associated with reduced odds of placental malaria, preterm birth, and low birth weight (39). Future studies of IPTp-DP may need to test alternative DP dosing regimens, such as weekly administration, and should be focused in areas of high SP resistance (39).
Future directions
The decreasing effectiveness of SP in East Africa, and lack of a clear alternative IPTp regimen, highlight the importance of continued research into IPTp options. Important areas of focus will include the development of novel therapeutics, establishment of drug resistance markers that correlate with loss of IPTp effectiveness, and discovery of non-invasive methods to detect the presence of placental malaria infection prior to delivery.
Development of novel therapeutics
If DP proves superior to SP for IPTp, its usefulness may unfortunately be short-lived due to evolving resistance patterns in P. falciparum. There is evidence for emerging artemisinin resistance in Africa, and piperaquine resistance in South East Asia (40, 41). The rise of both SP and DP resistance highlights the need for continued development of new antimalarials for the treatment of clinical malaria and intermittent preventative treatment in pregnancy. To address this ongoing need, the Medicines for Malaria Venture (MMV) has proposed two Target Product Profiles (TPPs) for antimalarial drug development (42). TPP-1 applies to medications for acute malaria treatment, with essential parameters that include activity against resistant parasites, rapid onset of action, and a large (>12 log10) reduction in asexual parasite load. TPP-2 applies to medications for chemoprotection, with essential parameters that include a long dosing interval (weekly or longer) and efficacy against the pre-erythrocytic liver stages. The clinical benefit of IPTp-SP is likely due to both eradication of any ongoing malaria infection and temporary prophylaxis against new infection (43); both sulfadoxine and pyrimethamine remain detectable in serum for more 40 days after dosing (44). An ideal IPTp regimens should thus aim to meet both TPPs for optimum benefit (Figure 2). However, antigametocyte and antihypnozoite activities, to eradicate the sexual transmission stages or the latent liver stages of P. vivax or, respectively, are not necessary for IPTp.
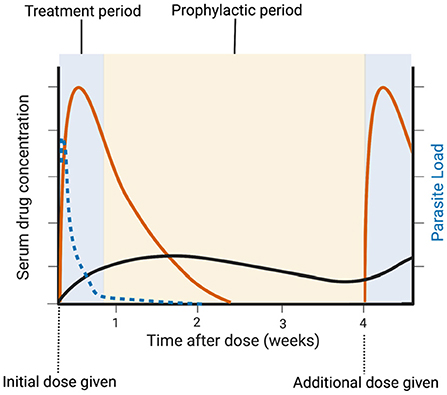
Figure 2. Idealized IPTp regimen. A treatment dose of a drug that meets MMV TPP-1 (management, orange line) eradicates existing asexual and placental infection (dotted blue line). A drug that meets TPP-2 (chemoprevention, black line) provides long-term protection against re-infection until the next prenatal visit.
Drug resistance markers to predict IPTp effectiveness
While combined dhps K540E and A581G mutations are an important genetic marker of decreasing IPTp-SP effectiveness in East Africa, the generalizability of these markers to other parts of Africa will likely be limited. There is significant variability in the prevalence of dhfr and dhps resistance mutations across the continent. In West Africa, the A581G mutation is found in the absence of K540E and is associated with increased susceptibility to SP, relative to parasites containing both mutations (3, 25, 45, 46). However, other mutations, such as dhps I431V, appear to be emerging. Additional studies are necessary to determine the effects of this I431V on the effectiveness of IPTp-SP. Phenotypic drug resistance studies of field isolates to SP, will also be useful. While traditional in vitro assays are limited by the substantial technical challenges of culture-adapting large numbers of field isolates, short-term ex vivo drug sensitivity assays can be used to phenotypically screen fresh clinical isolates (47–49). The identification of ex vivo phenotypic markers, such as MIC or IC50, that correlate with clinical IPTp-SP failure could allow for generalizability to areas with distinct dhfr and dhps genetic backgrounds.
Detection of placental malaria infection
Current studies of IPTp are hindered by the inability to assess the presence and degree of placental malaria infection. While the goal of IPTp is to improve outcomes for both the pregnant person and fetus, confounders make it difficult to monitor the clinical effectiveness of IPTp and to compare it across populations or studies. Accurate identification of placental malaria requires labor-intensive histopathology or studies of placental blood, and can only be performed after delivery. Data from studies of IPTp-DP suggest that peripheral parasitemia, even when identified by molecular methods, may overestimate effects on placental infection (33, 36). Future studies would benefit from the identification of new biomarkers of placental infection. These could be derived either from infecting parasites (i.e., levels of var2csa antigen), or the host (i.e., profiles of inflammatory cytokines) (50). Such biomarkers would facilitate longitudinal monitoring of the effectiveness of current IPTp regimens, identification of phenotypic or genotypic resistance markers that predict IPTp treatment failure, and comparisons of new IPTp regimens to current standard of care. They would also help differentiate the benefits conferred by IPTp from other pregnancy interventions, improving our understanding of the effects of IPTp in the evolving field of maternal-fetal health.
Author contributions
AO and SS conceived of this work, and both contributed to drafting and editing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
AO was supported by NIH/NIAID R01AI103280, R21AI154370, and R21AI144472. AO is an Investigator in the Pathogenesis of Infectious Diseases of the Burroughs Wellcome Fund. Funders had no role in conception or preparation of this manuscript. Institutional funds will be used for open access publication. SS is supported by the PIDS-St. Jude Children's Research Hospital Fellowship Award in Basic and Translational Science.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization (WHO). World Malaria Report 2021. Geneva: World Health Organization (2021).
2. Langhorne J, Ndungu FM, Sponaas A-M, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. (2008) 9:725–32. doi: 10.1038/ni.f.205
3. Naidoo I, Roper C. Mapping “partially resistant,” “fully resistant”, and “super resistant” malaria. Trends Parasitol. (2013) 29:505–15. doi: 10.1016/j.pt.2013.08.002
4. Bray RS, Anderson MJ. Falciparum malaria and pregnancy. T Roy Soc Trop Med H. (1979) 73:427–31. doi: 10.1016/0035-9203(79)90170-6
5. Desai M. ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. (2007) 7:93–104. doi: 10.1016/s1473-3099(07)70021-x
6. Fried M, Duffy PE. Malaria during pregnancy. CSH Perspect Med. (2017) 7:a025551. doi: 10.1101/cshperspect.a025551
7. Snow RW, Guyatt HL. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Am J Trop Med Hyg. (2001) 64:36–44. doi: 10.4269/ajtmh.2001.64.36
8. Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg. (1996) 55:33–41. doi: 10.4269/ajtmh.1996.55.33
9. Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. Am J Trop Med Hyg. (2001) 64:57–67. doi: 10.4269/ajtmh.2001.64.57
10. Morley DC, Woodland M, Cuthbertson WFJ. Controlled trial of pyrimethamine in pregnant women in an African village. Brit Med J. (1964) 1:667. doi: 10.1136/bmj.1.5384.667
11. Greenwood BM, Greenwood AM, Snow RW, Byass P, Bennett S. Hatib-N'Jie AB. The effects of malaria chemoprophylaxis given by traditional birth attendants on the course and outcome of pregnancy. T Roy Soc Trop Med H. (1989) 83:589–94. doi: 10.1016/0035-9203(89)90362-3
12. World Health Organization (WHO). WHO policy brief for the implementation of intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP). Geneva: World Health Organization (2014).
13. Shulman CE, Dorman EK, Cutts F, Kawuondo K, Bulmer JN, Peshu N, et al. Intermittent sulphadoxine-pyrimethamine to prevent severe anaemia secondary to malaria in pregnancy: a randomised placebo-controlled trial. Lancet. (1999) 353:632–6. doi: 10.1016/s0140-6736(98)07318-8
14. Challis K, Osman NB, Cotiro M, Nordahl G, Dgedge M, Bergström S. Impact of a double dose of sulphadoxine–pyrimethamine to reduce prevalence of pregnancy malaria in southern Mozambique. Trop Med Int Health. (2004) 9:1066–73. doi: 10.1111/j.1365-3156.2004.01307.x
15. Parise ME, Ayisi JG, Nahlen BL, Schultz LJ, Roberts JM, Misore A, et al. Efficacy of sulfadoxine-pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. Am J Trop Med Hyg. (1998) 59:813–22. doi: 10.4269/ajtmh.1998.59.813
16. Kayentao K, Kodio M, Newman RD, Maiga H, Doumtabe D, Ongoiba A, et al. Comparison of intermittent preventive treatment with chemoprophylaxis for the prevention of malaria during pregnancy in Mali. J Infect Dis. (2005) 191:109–16. doi: 10.1086/426400
17. Diakite OSM, Maiga OM, Kayentao K, Traoré BT, Djimde A, Traoré B, et al. Superiority of 3 over 2 doses of intermittent preventive treatment with sulfadoxine-pyrimethamine for the prevention of malaria during pregnancy in Mali: a randomized controlled trial. Clin Infect Dis. (2011) 53:215–23. doi: 10.1093/cid/cir374
18. Kayentao K, Garner P, van Eijk AM, Naidoo I, Roper C, Mulokozi A, et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: systematic review and meta-analysis. JAMA. (2013) 309:594–604. doi: 10.1001/jama.2012.216231
19. Kublin JG, Dzinjalamala FK, Kamwendo DD, Malkin EM, Cortese JF, Martino LM, et al. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis. (2002) 185:380–8. doi: 10.1086/338566
20. Picot S, Olliaro P, Monbrison F de, Bienvenu A-L, Price RN, Ringwald P, et al. systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malaria J. (2009) 8:89. doi: 10.1186/1475-2875-8-89
21. Staedke SG, Sendagire H, Lamola S, Kamya MR, Dorsey G, Rosenthal PJ. Relationship between age, molecular markers, and response to sulphadoxine–pyrimethamine treatment in Kampala, Uganda. Trop Med Int Health. (2004) 9:624–9. doi: 10.1111/j.1365-3156.2004.01239.x
22. Gesase S, Gosling RD, Hashim R, Ord R, Naidoo I, Madebe R, et al. High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in Northern Tanzania and the emergence of dhps resistance mutation at codon 581. PLoS ONE. (2009) 4:e4569. doi: 10.1371/journal.pone.0004569
23. Okell LC, Griffin JT, Roper C. Mapping sulphadoxine-pyrimethamine-resistant Plasmodium falciparum malaria in infected humans and in parasite populations in Africa. Sci Rep-uk. (2017) 7:7389. doi: 10.1038/s41598-017-06708-9
24. Kuile FO, van Eijk AM. Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. (2007) 297:2603–16. doi: 10.1001/jama.297.23.2603
25. van Eijk AM, Larsen DA, Kayentao K, Koshy G, Slaughter DEC, Roper C, et al. Effect of Plasmodium falciparum sulfadoxine-pyrimethamine resistance on the effectiveness of intermittent preventive therapy for malaria in pregnancy in Africa: a systematic review and meta-analysis. Lancet Infect Dis. (2019) 19:546–56. doi: 10.1016/s1473-3099(18)30732-1
26. Chico RM, Cano J, Ariti C, Collier TJ, Chandramohan D, Roper C, et al. Influence of malaria transmission intensity and the 581G mutation on the efficacy of intermittent preventive treatment in pregnancy: systematic review and meta-analysis. Trop Med Int Health. (2015) 20:1621–33. doi: 10.1111/tmi.12595
27. Kimani J, Phiri K, Kamiza S, Duparc S, Ayoub A, Rojo R, et al. Efficacy and safety of azithromycin-chloroquine versus sulfadoxine-pyrimethamine for intermittent preventive treatment of Plasmodium falciparum malaria infection in pregnant women in Africa: an open-label, randomized trial. PLoS ONE. (2016) 11:e0157045. doi: 10.1371/journal.pone.0157045
28. González R, Mombo-Ngoma G, Ouédraogo S, Kakolwa MA, Abdulla S, Accrombessi M, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-negative women: a multicentre randomized controlled trial. PLoS Med. (2014) 11:e1001733. doi: 10.1371/journal.pmed.1001733
29. Clerk CA, Bruce J, Affipunguh PK, Mensah N, Hodgson A, Greenwood B, et al. randomized, controlled trial of intermittent preventive treatment with sulfadoxine-pyrimethamine, amodiaquine, or the combination in pregnant women in Ghana. J Infect Dis. (2008) 198:1202–11. doi: 10.1086/591944
30. Kakuru A, Jagannathan P, Muhindo MK, Natureeba P, Awori P, Nakalembe M, et al. Dihydroartemisinin–piperaquine for the prevention of malaria in pregnancy. New Engl J Med. (2016) 374:928–39. doi: 10.1056/nejmoa1509150
31. Mlugu EM, Minzi O, Kamuhabwa AAR, Aklillu E. Effectiveness of intermittent preventive treatment with dihydroartemisinin-piperaqunine against malaria in pregnancy in Tanzania: a randomized controlled trial. Clin Pharmacol Ther. (2021) 10:1478–89. doi: 10.1002/cpt.2273
32. Kajubi R, Ochieng T, Kakuru A, Jagannathan P, Nakalembe M, Ruel T, et al. Monthly sulfadoxine–pyrimethamine versus dihydroartemisinin–piperaquine for intermittent preventive treatment of malaria in pregnancy: a double-blind, randomised, controlled, superiority trial. Lancet. (2019) 393:1428–39. doi: 10.1016/s0140-6736(18)32224-4
33. Desai M, Gutman J., L'lanziva A, Otieno K, Juma E, Kariuki S, Ouma P, Were V, Laserson K, Katana A, et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin–piperaquine versus intermittent preventive treatment with sulfadoxine–pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial Lancet. (2015) 386:2507–19. doi: 10.1016/s0140-6736(15)00310-4
34. Kakuru A, Jagannathan P, Kajubi R, Ochieng T, Ochokoru H, Nakalembe M, et al. Impact of intermittent preventive treatment of malaria in pregnancy with dihydroartemisinin-piperaquine versus sulfadoxine-pyrimethamine on the incidence of malaria in infancy: a randomized controlled trial. BMC Med. (2020) 18:207. doi: 10.1186/s12916-020-01675-x
35. Madanitsa M, Kalilani L, Mwapasa V, van Eijk AM, Khairallah C, Ali D, et al. Scheduled intermittent screening with rapid diagnostic tests and treatment with dihydroartemisinin-piperaquine versus intermittent preventive therapy with sulfadoxine-pyrimethamine for malaria in pregnancy in Malawi: an open-label randomized controlled trial. PLoS Med. (2016) 13:e1002124. doi: 10.1371/journal.pmed.1002124
36. Roh ME., ter Kuile FO, Rerolle F, Glymour MM, Shiboski S, Gosling R, et al. Overall, anti-malarial, and non-malarial effect of intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine on birthweight: a mediation analysis. Lancet Glob Health. (2020) 8:e942–53. doi: 10.1016/s2214-109x(20)30119-4
37. Nayebare P, Asua V, Conrad MD, Kajubi R, Kakuru A, Nankabirwa JI, et al. Associations between malaria-preventive regimens and Plasmodium falciparum drug resistance-mediating polymorphisms in Ugandan pregnant women. Antimicrob Agents Chemother. (2020) 64:20. doi: 10.1128/aac.01047-20
38. Conrad MD, Mota D, Foster M, Tukwasibwe S, Legac J, Tumwebaze P, et al. Impact of intermittent preventive treatment during pregnancy on Plasmodium falciparum drug resistance–mediating polymorphisms in Uganda. J Infect Dis. (2017) 216:1008–17. doi: 10.1093/infdis/jix421
39. Savic RM, Jagannathan P, Kajubi R, Huang L, Zhang N, Were M, et al. Intermittent preventive treatment for malaria in pregnancy: optimization of target concentrations of dihydroartemisinin-piperaquine. Clin Infect Dis. (2018) 67:1079–88. doi: 10.1093/cid/ciy218
40. Balikagala B, Fukuda N, Ikeda M, Katuro OT, Tachibana S-I, Yamauchi M, et al. Evidence of artemisinin-resistant malaria in Africa. New Engl J Med. (2021) 385:1163–71. doi: 10.1056/nejmoa2101746
41. Pluijm RW., van der, Imwong M, Chau NH, Hoa NT, Thuy-Nhien NT, et al. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis. (2019) 19:952–61. doi: 10.1016/s1473-3099(19)30391-3
42. Burrows JN, Duparc S, Gutteridge WE, van Huijsduijnen RH, Kaszubska W, Macintyre F, et al. New developments in anti-malarial target candidate and product profiles. Malaria J. (2017) 16:26. doi: 10.1186/s12936-016-1675-x
43. White NJ. Intermittent presumptive treatment for malaria. PLoS Med. (2005) 2:e3. doi: 10.1371/journal.pmed.0020003
44. Karunajeewa HA, Salman S, Mueller I, Baiwog F, Gomorrai S, Law I, et al. Pharmacokinetic properties of sulfadoxine-pyrimethamine in pregnant women. Antimicrob Agents Chemother. (2009) 53:4368–76. doi: 10.1128/aac.00335-09
45. Oguike MC, Falade CO, Shu E, Enato IG, Watila I, Baba ES, et al. Molecular determinants of sulfadoxine-pyrimethamine resistance in Plasmodium falciparum in Nigeria and the regional emergence of dhps 431V. Int J Parasitol-Drug. (2016) 6:220–9. doi: 10.1016/j.ijpddr.2016.08.004
46. Chauvin P, Menard S, Iriart X, Nsango SE, Tchioffo MT, Abate L, et al. Prevalence of Plasmodium falciparum parasites resistant to sulfadoxine/pyrimethamine in pregnant women in Yaoundé, Cameroon: emergence of highly resistant pfdhfr/pfdhps alleles. J Antimicrob Chemother. (2015) 70:2566–71. doi: 10.1093/jac/dkv160
47. Marfurt J, Chalfein F, Prayoga P, Wabiser F, Wirjanata G, Sebayang B, et al. Comparative ex vivo activity of novel endoperoxides in multidrug-resistant Plasmodium falciparum and P. vivax Antimicrob Agents Chemother. (2012) 56:5258–63. doi: 10.1128/aac.00283-12
48. Kosaisavee V, Suwanarusk R, Nosten F, Kyle DE, Barrends M, Jones J, et al. Plasmodium vivax: Isotopic, PicoGreen, and microscopic assays for measuring chloroquine sensitivity in fresh and cryopreserved isolates. Exp Parasitol. (2006) 114:34–9. doi: 10.1016/j.exppara.2006.02.006
49. Wirjanata G, Handayuni I, Prayoga P, Apriyanti D, Chalfein F, Sebayang BF, et al. Quantification of Plasmodium ex vivo drug susceptibility by flow cytometry. Malaria J. (2015) 14:417. doi: 10.1186/s12936-015-0940-8
Keywords: malaria, drug resistance, low birth weight, antimalarial, IPTp
Citation: Sundararaman SA and Odom John AR (2022) Prevention of malaria in pregnancy: The threat of sulfadoxine-pyrimethamine resistance. Front. Pediatr. 10:966402. doi: 10.3389/fped.2022.966402
Received: 10 June 2022; Accepted: 26 July 2022;
Published: 18 August 2022.
Edited by:
Britt Nakstad, University of Botswana, BotswanaReviewed by:
Olugbenga A. Mokuolu, University of Ilorin, NigeriaCopyright © 2022 Sundararaman and Odom John. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Audrey R. Odom John, johna3@chop.edu
 Sesh A. Sundararaman
Sesh A. Sundararaman Audrey R. Odom John
Audrey R. Odom John