Interstitial cystitis—an imbalance of risk and protective factors?
- 1Department of Medicine and Epidemiology, UC Davis School of Veterinary Medicine, Davis, CA, United States
- 2Department of Comparative Pathobiology, Purdue University College of Veterinary Medicine, W. Lafayette, IN, United States
Interstitial cystitis (IC) presents as a chronic pain condition with variable combinations of symptoms depending on the species and individual patient. It is diagnosed by the presence of lower urinary tract signs and symptoms in combination with a variety of comorbid health problems, a history of life adversities, and the absence of other conditions that could cause the lower urinary tract signs. IC occurs naturally in humans and cats as a dimensional condition, with patients presenting with mild, moderate, and severe symptoms. Most patients appear to recover without specific treatment. A number of rodent models of IC have been used to study its causes and treatments. Unfortunately, current therapies generally fail to ameliorate IC symptoms long-term. The recent classification of IC as a chronic primary pain disorder calls for a rethinking of current clinical and research approaches to it. Beginning when a patient encounters a clinician, precipitating, perpetuating, and palliating risk factors can be addressed until a cause or reliably effective therapy is identified, and identifying predisposing and preventive factors can inform epidemiological studies and health promotion interventions. Predisposing, precipitating, and perpetuating risk factors, including environmental, psychological, and biological, increase the activity of the central threat response system (CTRS), which plays a clinically important role in IC symptoms. Studies in cats and rodent models have revealed that environmental enrichment (EE), in the absence of bladder-directed therapies, leads to amelioration of IC symptoms, implying a central role for the CTRS in symptom precipitation and perpetuation. Conceptually moving the source of IC pain to the brain as a motivational state rather than one resulting from peripheral nociceptive input offers both clinicians and researchers novel opportunities to improve care for patients with IC and for researchers to use more ecologically valid rodent models. It may even be that IC results from an excess of risk to protective factors, making this imbalance a targetable cause rather than a consequence of IC.
Introduction
Interstitial Cystitis (IC) presents as a chronic painful condition with variable combinations of social, psychological, and biological problems depending on the species and individual patient. In addition to afflicting humans, IC occurs commonly in pet cats (Felis catus), is studied in rodent models, and has been reported sporadically in other mammals, including cheetahs, dogs, llamas, and pot-bellied pigs (1–3). This article will focus mainly on humans, cats, a naturally occurring model for IC in humans, and induced rodent models of IC because there is a paucity of information available about other species.
Chronic pain is defined by the International Association for the Study of Pain (IASP) as an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage that persists or recurs for longer than three months1. The IASP has long recognized two classes of chronic pain: nociceptive pain, which results from tissue damage that doesn't heal, and neuropathic pain, which results from nerve injury. In 2019, the IASP classified IC as a chronic primary pain condition, which they define as one persisting for longer than three months, associated with significant emotional distress, functional disability, or a combination of the two, and not better accounted for by another condition (4). The IASP's 2019 revised chronic pain classification, although still controversial (5, 6), affords an opportunity to rethink current clinical and research approaches to IC.
Patient presentation
Humans and cats with IC present similarly (Table 1).
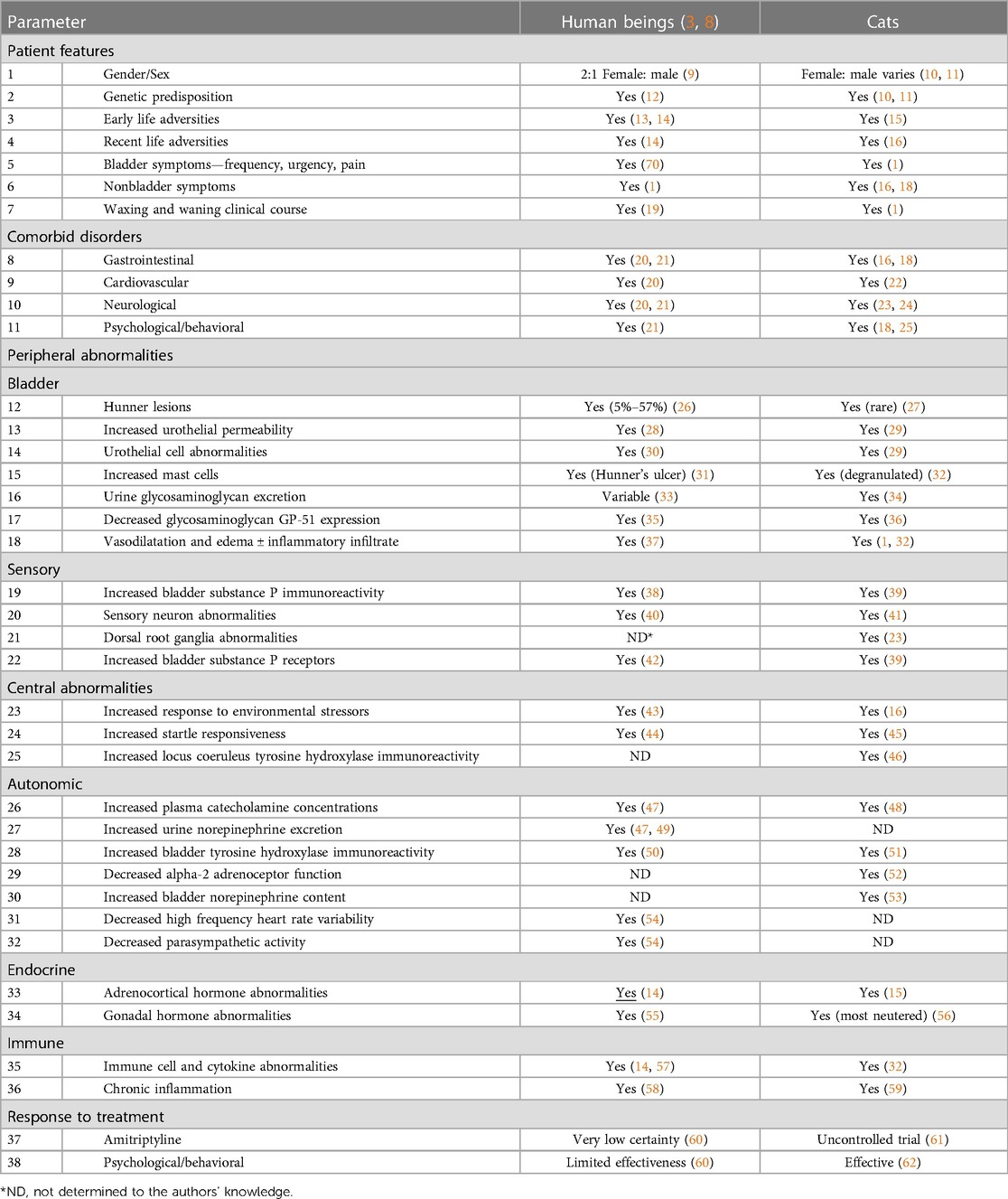
Table 1. Comparison of results of studies in humans and cats with IC [adapted from (7) and updated].
Commonalities between IC in humans and cats include variable severity, a waxing & waning course, sensitivity to threatening environmental contexts, various peripheral and central abnormalities, and a variety of comorbidities. In humans, these comorbidities are called chronic overlapping pain conditions (COPC), which include chronic low back pain, chronic prostatitis, painful endometriosis, fibromyalgia, irritable bowel syndrome, migraine/tension-type headache, myalgic encephalomyelitis/chronic fatigue syndrome, temporomandibular disorder, and vulvodynia (17). In cats, comorbidities are called sickness behaviors (SB), which include variable combinations of gastrointestinal, cardiovascular, and dermatologic signs, elimination abnormalities, decreased food intake, and behavioral problems (16). Furthermore, bladder-targeted therapies (e.g., glycosaminoglycans, intravesicular resiniferatoxin, bladder hydrodistension, surgery, and special diets) have minimal long-term beneficial effects for most humans and cats.
IC in humans and cats also appears to be a dimensional rather than a categorical condition; patients may have minimal, mild, moderate, or severe symptoms that can vary over time rather than simply being present or absent. This has been studied most commonly in women (63–65). IC is also dimensional in cats, and more commonly severe in males because they can develop urethral obstruction (66).
After centuries of investigation, research and clinical articles still commonly state that the cause of IC remains unknown. Cause has a disputed history in philosophy and medicine however (67, 68), and recent experience with the global SARS-CoV-2 pandemic underscores the importance of pathogen, person, and place (environmental) factors in the outcome of diseases, even those with known causes. Moreover, ignorance of a cause need not prevent effective action. For example, citrus fruit was known to prevent scurvy for more than 500 years before the cause, ascorbic acid deficiency, was identified (69). This review aims to expand and extend the risk and protective factor approach (15, 16, 70) to IC to suggest opportunities to improve care and research while the search for a cause continues.
Nosology
The classification and naming of IC, its nosology, has become complex and controversial in urology. Diseases may be classified by their presenting signs and symptoms, affected organ system(s), pathogenesis, and etiology (1). A significant challenge to accurate nosology exists because diseases may be named based on prominent signs and symptoms long before research identifies their pathogenesis or etiology. Feinstein (71) concluded that “An important principle in naming apparently new ailments is to avoid etiologic titles until the etiologic agent has been suitably demonstrated. A premature causal name can impair a patient's recovery from the syndrome, and impede research that might find the true cause.”
Presenting signs such as lower urinary tract signs (LUTS), which are often noted in patients with IC, have unfortunately affected the nosology of IC. The name given to any disease may reflect only a subset of the signs and problems associated with such a complex syndrome. By naming a disease for the organ associated with the primary presenting signs, we neglect the possibility that the disease may not originate from that organ, and many diseases can affect more than one organ. Different societies and organizations have created their own names and classifications for IC, leading to confusion and controversy among clinicians, patients, and researchers (72). Some of the names ascribed to IC are listed in Table 2 below. This review will use interstitial cystitis (IC) rather than one of the other names listed in Table 2 because of its heritage, familiarity, and common and Medical Subject Heading usage. Moreover, insurance systems in many countries or regions reimburse medical care for IC but not for its synonyms (80).
Risk and protective factors for IC
Beginning when a patient encounters a clinician, precipitating, perpetuating, palliating, predisposing, and preventive factors may be identified. These risk and protective factors can be addressed clinically until a cause or reliably effective therapy is identified, and identifying predisposing and preventive factors can inform epidemiological studies and health promotion interventions (Table 3).
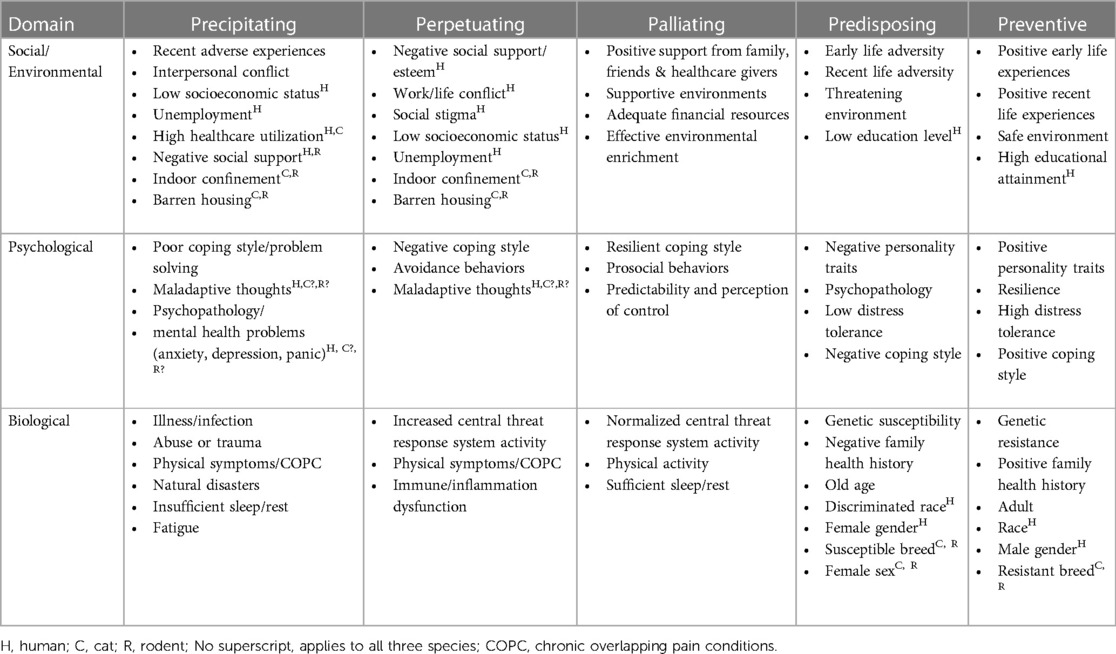
Table 3. Precipitating, perpetuating, palliating, predisposing, and protective factors related to IC across social, psychological, and biological domains [table structure adapted from (81–83)].
Precipitating factors
Any unexpected sensory stimulus producing a meaningful experience of the world and of oneself can result in seeking medical care depending on the individual and the context in which the perception arises (84). Patient appraisals of sensory perceptions include their long-term implications based on the person's unique genetic and epigenetic history, memories of past events, environmental context, imagination of future possibilities (expectations), and perception of control (agency). Complex interactions between facilitating and inhibitory neuronal networks in the central nervous system (CNS) influence these perceptions (85, 86). These complex CNS interactions appear to be present in humans with chronic primary pain conditions like IC (85, 87) and have been reported in rodent models of IC (3).
Perpetuating factors
Many risk factors can also perpetuate IC (88, 89). The IASP explicitly acknowledges that “Pain is always a subjective experience that is influenced to varying degrees by biological, psychological, and social factors.” (6). Relevant social factors are species-dependent. We humans are an ultra-social species, the most social mammal on earth. Our health and well-being depend on the quality of our social connections, which share the importance of safety, food, and sex for our existence (90, 91). We have survived evolution by connecting with other humans to forage, hunt, and fight off enemies together (92). Social factors influencing patients with IC include the quality of their social connections, healthcare, and collective support (93).
Chronic pain can result in social isolation, loneliness, conflict, exclusion, and invalidation, and depression, anxiety, and post-traumatic stress disorder afflict some patients with IC that may result from the presence of such negative social factors (94). In 2023, a report by the United States Surgeon General described what he saw as an epidemic of loneliness in America and explained the health effects of loneliness and isolation (95). In contrast, cats have a facultative matrilineal sociality (96), and sociality varies widely across rodents, which may differ in group size, composition (e.g., juveniles, adult peers, mates), and the roles of specific, selective relationships (97).
Biological factors can also perpetuate IC. The recently proposed “extended autonomic system” consists of four complexly interrelated components: (1) the central autonomic network, (2) the autonomic nervous system with its three sub-systems: the sympathetic, parasympathetic, and enteric nervous systems, (3) neuroendocrine systems including the hypothalamic-pituitary-adrenal (HPA), sympathetic adrenergic, renin-angiotensin-aldosterone, and arginine vasopressin systems, and (4) immune/inflammatory systems (98). In the extended autonomic system, perceptions of threat accompany experiences that motivate escape or avoidance. They activate sympathetic, adrenocortical, and adrenomedullary activation, homeostatic resetting, and a perceived inability to cope that produces instinctively communicated signs. These responses also have been described as resulting from activating a central threat response system (CTRS) (99). Activation of the CTRS plays a clinically important role in IC symptoms in humans, cats, and rodents (See below) (100).
Palliating factors
Palliating, making a disease or its symptoms less severe or unpleasant without removing or knowing its causes, applies directly to IC because its cause remains unknown. And despite the identification of a host of pathophysiological variables associated with IC, none has yet led to generally effective treatments for IC in humans (60). The goal of protective palliating factors is to normalize the activity of the CTRS. Palliating factors include creating surroundings that provide safety, predictability, choice, and positive social support. In humans, psychosocial factors include positive support from family, friends, coworkers, and healthcare providers, supportive physical and work environments, adequate financial resources, and compensatory coping behaviors. Palliative biological factors include voluntary physical activity, mindful presence, good sleep hygiene, and a healthy, nutritious diet. In cats and rodents, environmental modifications that increase perceptions of safety, predictability, and control reduce perceptions of threat and can alleviate their clinical signs.
Predisposing factors
Predisposing risk factors are those that can increase susceptibility to IC. These include genetic vulnerability and family medical and mental health history (12), personality traits (101), early (ELA) (102, 103) and recent (RLA) life adversities (14), age, (104), race (105), and gender (106). In cats and rodents, predisposing factors include genetic vulnerability, ELA, personality traits, breed, sex, housing, and RLA (e.g., social conflict) (1, 107).
Preventive factors
Preventive factors are protective factors that can mitigate or counterbalance predisposing risk factors (81–83). These include genetic resistance, resilience-building early-life experiences, positive psychological factors, and strong, positive social connections. Although generally less modifiable than precipitating, perpetuating, and palliative factors, identifying predisposing and preventive factors may facilitate early identification of susceptible individuals, regardless of species, to permit earlier preventive and therapeutic interventions (108) in addition to informing epidemiological studies and health promotion interventions.
Discussion
IC in human beings
Background
Although IC has been assumed to be a bladder disorder for centuries (109, 110), recent literature reviews of cystoscopy studies reported finding no convincing evidence that glomerulations (punctate petechial hemorrhages observed after hydrodistension) should be included in the diagnosis or phenotyping of IC since they did not correlate with symptoms and were found in patients without IC (111). Based on these results, the 2023 report of a committee of the International Continence Society concluded that “clinically at this time, the presence or absence of glomerulations on cystoscopy should not be used as a diagnostic or prognostic criterion for IC….” (112). Another recent study also cast doubt on the usefulness of bladder biopsy for the evaluation of severity in women with non-Hunner lesion IC (113). Moreover, a 2022 systemic review of uncontrolled studies of surgical interventions for IC found that fulgurating lesions in humans resulted in only a 31%–54% reduction in painful bladder symptoms, and no significant decrease in symptoms was noted in patients after a partial cystectomy with augmentation ileocystoplasty, suggesting the etiology of IC is more complex than one isolated to the bladder for many patients (114).
Precipitating factors
A variety of risk factors that can precipitate IC have been reported in women, including early symptom onset, symptoms increasing the week before menses, symptom flares after sex, and a family history of similar bladder symptoms (104). Additionally, ELA and RLA have been investigated as potential risk factors for IC. For example, Warren et al. (115), identified both prodromal (antecedent) and non-prodromal presentations of IC, and Lutgendorf et al., have reported inflammation and HPA abnormalities in women with IC (14). In their study, 154 women with IC and 32 healthy controls completed surveys, collected salivary cortisol, and provided a blood sample for analysis of seven lipopolysaccharide (LPS)-stimulated cytokines and chemokines. Patients with greater exposure to RLA or cumulative ELA and RLA of at least moderate severity had increased concentrations of a composite of all cytokines, but nocturnal cortisol and cortisol slope were not associated with RLA or inflammation. This altered stress response characterized by increased cytokines and lack of a cortisol response could contribute to the signs often noted in IC patients—urinary urgency, frequency, bladder or voiding pain, and painful intercourse, supporting the importance of adverse life events as risk factors for IC via a biological mechanism.
Perpetuating factors
As mentioned, the severity of IC is a perpetuating risk factor. Interstitial cystitis can vary in severity from mild to severe across three dimensions: social interference, psychological distress, and strength of intensity. Hay, et al. (116), recently proposed numerical rating scales for social interference—“How much did the pain interfere with your activities in the last week on average?”, psychological distress—“How much distress did you experience in the last week because of your pain on average?”, and biological strength of pain intensity—“How strong was your chronic pain in the last week on average?” on an 10-point scale ranging from 0 (no pain-related interference, distress, or strength) to 10 (unable to carry on activities, extreme pain-related distress, or worst pain imaginable). Considering all these dimensions can help differentiate between low and high-impact pain in IC patients, which may influence approaches to care and outcomes.
An additional perpetuating factor in humans is increased bladder permeability (28). Although commonly associated with IC, the cause of the increased permeability is unknown. Increased permeability led to attempts to use glycosaminoglycan compounds like pentosan polysulfate (Elmiron®, PPS) as a treatment for IC (117). Recent research showing cross-communication among the bladder, bowel and other organs, nerves, cytokine-responding cells, and the nervous system has led to the suggestion that IC may reflect the presence of a more complex systemic disorder (28).
Palliating factors
Although much IC research focuses on subjects with relatively severe symptoms, studies of IC patient outcomes in the years following diagnosis report that most patients have clinically relevant (≥50%) symptom improvement (63–65). Palliating factors can help patients live well with chronic pain, including IC. For example, a grounded theory study of 17 middle-aged women and men reported chronic pain severities similar to those found in primary care patients but with interference and emotional burden scores below the US national average (118). The subjects reported that their main problem was integrating their present experience with past experiences and motivations and finding meaning in their present experience to regain a sense of well-being and self-fulfillment to experience life meaningfully, even in difficult situations. Resolving this problem required them to make personal sense of their pain, to re-occupy their selves, and then to decide to turn their life from patient to person. This turn could be facilitated or hindered by interactions with clinicians and social others (family, friends, co-workers) and one's occupational drives, the things people need to, want to, and are expected to do in life. These steps were followed by a lifelong maintenance process of engaging in the everyday activities that other people do in families and communities to occupy time and bring meaning and purpose to their lives, which allowed them to live well despite their pain (118).
Predisposing factors
Predisposing risk factors that can increase susceptibility to IC include genetic vulnerability (12), family (104, 119) medical (120) and mental health history (121), personality traits (122), ELA and RLA (14), age (119). and gender (123). Associations between ELA and IC symptoms have been reported (13, 103, 124). A recent case-control study comparing women with IC (cases) with healthy controls found that cases had higher median numbers of ELA, with greater occurrence of abuse (emotional/physical/sexual) and household challenges. Cases had a seven-fold increased odds of having four or more ELA compared with controls (13).
Preventive factors
Preventive factors that can mitigate or counterbalance previously described risk factors include genetic resistance, resilience-building life experiences, positive psychological factors, and strong, positive social connections. Early identification of risk factors increases the time available for implementing effective protective measures (108). Assessing protective and ELA experiences is becoming more common in clinical practice in some regions; time will tell how effective such efforts are in identifying and implementing therapeutic strategies (125).
IC in cats
Background
Based on the many similarities between IC in humans and cats, replacing FIC with IC in cats as an initialism for either feline interstitial or idiopathic cystitis seems to be a reasonable nosological description of cats presented with cLUTS in the presence of compatible urinalysis and imaging findings that avoids an etiologic title. Cystoscopic or other advanced imaging evaluations could be considered for further investigation of cats that do not respond to treatment since many other causes of cLUTS have been reported in cats (126). However, similar to humans, cystoscopic findings (e.g., glomerulations) are not pathognomic for FIC, and lesion severity does not correlate with the severity of clinical signs (61).
Precipitating factors
Approximately 1.5% of cats presenting at veterinary practices exhibit one or more LUTS (127, 128). Poor outcomes for cats with elimination behaviors deemed inappropriate by owners are common and include euthanasia or relinquishment to shelters (129, 130). In cats with urethral obstruction due to IC, recurrence rates of 17.0%–58.0% have been reported (131). Rates of euthanasia have been reported to range from 8.5% and 26% in cats diagnosed with IC (132, 133).
Variable combinations of problems with the gastrointestinal, endocrine, and cardiovascular systems and fearful, anxious, or aggressive behaviors are commonly reported in cats with IC (134–136). Immune activation and pro-inflammatory cytokine release that can lead to SB have been reported in cats with IC (137, 138). Sickness behaviors are a well-documented group of non-specific clinical signs thought to reflect a change in motivation to conserve energy for recovery from infection (139) that have been reported in cats (16) and other species, including rodents (140). In cats, SB include anorexia or decreased appetite, vomiting of food, hair, or bile, eliminating outside of the litter pan, decreased social interactions and grooming behavior, and increased frequency and intensity of attempts to hide (16, 25, 141, 142).
Like humans with IC, recent adverse events are also a risk factor for cats with IC. Sickness behaviors in cats have been reported in response to environmental stressors, including changes in husbandry routine and caretakers and discontinuation of EE. These events resulted in a 3.2-fold increased relative risk for SB during weeks when unusual external events occurred compared to control weeks (16). Many cats also display SB before their first or recurrent episodes of LUTS, so identifying and monitoring SB may allow caretakers to intervene early to prevent the development of cLUTS.
Perpetuating factors
As with humans, the severity of clinical signs of IC in cats can vary from mild to severe and result in recurrent episodes of LUTS. Increased bladder permeability is also a perpetuating factor in cats (29), and the association also led to unsuccessful attempts to use PPS to treat IC in cats (143–145). Psychological distress can be affected by the quality of the housing environment, especially for cats strictly confined indoors. Cats likely experience the home environment very differently from humans' experience of it, so they may perceive many environmental features as aversive or stressful. Environmental factors that can influence cats' perception of threat include the quality of human-cat relationships and interactions with conspecifics and other animals (e.g., dogs) in the cat's social environment and their perception of predictability and control of their surroundings (142, 146–149). Cats with IC may respond with fearful, anxious, and/or aggressive behaviors toward their owners that can negatively impact the human-animal bond, feeding into a negative feedback loop that may exacerbate cLUTS and result in poor outcomes for the cat, including euthanasia.
Palliating factors
The number, frequency, and severity of episodes of IC may depend on several factors, arguably the most important being the dedication of the owner to reducing the cat's perception of threat by providing varied opportunities to engage in highly motivated, species-specific activities, cognitive challenges, and sensory experiences (62, 150, 151). Effective Multimodal Environmental MOdification (MEMO), designed to provide safety, predictability, and perception of control to confined cats, has been reported to reduce cLUTS and related signs of IC in both research and clinical studies of cats with IC (16, 62).
Predisposing factors
Many risk factors predispose cats to IC. Cat factors include male sex (107, 152–154), neutered (152, 155), overweight (107, 128, 155, 156), purebred (10, 107), middle-aged (11, 153), and fearful or anxious (128, 154). Environmental risk factors include indoor confinement (154, 156–158), low litter-box-to-cat ratio (152, 156, 157), and inadequate access to elevated resting areas or vantage points (128, 155). Other risk factors include decreased activity and opportunities to engage in species-typical predatory behaviors (156, 157), and social conflict within the home (107, 128, 155, 156). Indoor-housed cats have little or no control over who their social partners are, how much space they can put between themselves and others, the type, amount, or availability of food, or the quality or quantity of environmental stimuli, including lights, noise, odors, and temperature (159).
Preventive factors
The current recommendation for preventing recurrent signs of IC is to decrease the cat's perception of threat using MEMO (62). The provision of an enriched environment containing abundant resources optimally placed is the “gold standard” for all indoor-housed cats to ensure adequate welfare (160). This includes providing choice wherever possible, especially when introducing or changing essential resources such as resting areas, diet, litter, or litter boxes.
Rodent models of IC
Precipitating and perpetuating factors
Bladder-centric, physical, and psychological stressors are all used to study precipitating and/or perpetuating risk factors. Early bladder-centric rodent models have been reviewed (161). More recent models instill different noxious compounds, such as protamine sulfate, cyclophosphamide (intraperitoneally), acids, zymosan, and LPS, which can increase urothelial permeability, induce an inflammatory response, alter visceromotor responses, and inflict acute pain depending on the compound and dose. Additionally, treating neonatal rats with zymosan increased bladder plasma extravasation and urinary frequency in them as adults, demonstrating that neonatal bladder injury, an adverse early life experience, can increase urothelial permeability and bladder sensitivity in adults subjected to injury early in life (3).
Psychological and physical stressor models more accurately mimic naturally occurring IC. For example, an early psychological model applied to female mice found that moderate stress induced by prolonged illumination resulted in the detachment of urothelial tight junctions and the loss of the bladder permeability barrier, leading to expanded intercellular spaces among urothelial cells. Removal of the stressor led to rapid restoration of new tight junctions, preventing long-term malfunction of the blood-urine barrier (162). In an early model of physical stress (163), immobilizing adult male Sprague/Dawley rats for 30 min in a plexiglass immobilizing tube activated over 70% of bladder mast cells. Moreover, mast cell progenitors can be programmed toward a hyperactive phenotype and increased tissue activation by ELA, which may explain their presence in mast cell-related disorders like IC in adulthood (164, 165).
The psychological stressor water avoidance stress (WAS) model was developed to investigate the role of chronic stress as a perpetuating risk factor for visceral pain symptoms in patients with functional gastrointestinal disorders (166). In this model, a single rodent is placed on a small platform centered in the middle of a water-filled basin, usually for one hour each day for ten days. Rodents naturally avoid entering the water, which increases their perception of threat when no escape is possible. Exposure to WAS increases anxiety-like behaviors, urinary frequency, and bladder hyperalgesia. Histological changes in bladder tissue from WAS-exposed rats included ulcerated areas, edema, vascular congestion, inflammatory cell infiltration, increased angiogenesis and mucosal mast cell numbers (2), and increased bladder mucosal permeability in female Wistar rats (167). Additionally, Wang et al. (168), reported greater activation in brain regions of the central micturition circuit and increased engagement of portions of the micturition circuit responsive to urgency, viscerosensory perception, and its relay to motor regions coordinating imminent bladder contraction in adult female Wistar-Kyoto rats subjected to WAS to model IC.
Another psychological stressor is social defeat, which has been used to mimic the physiological and behavioral abnormalities resulting from the social conflict between individuals (169). For example, West, et al. (170), exposed adult male C57BL/6JArc mice to aggressive male ex-breeder Arc mice for one hour each day for 10 days. The social defeat mouse was placed into physical contact with the aggressor for a maximum of five minutes and then separated by a transparent perforated barrier for 55 min. This resulted in significant increases in plasma corticosterone concentrations and significant decreases in voiding frequency in the socially defeated mouse. Nerve-evoked bladder contractile responses were significantly increased in bladders from socially defeated mice at all frequencies, resembling changes after partial bladder outlet obstruction (2). This social defeat model has proven difficult to implement in female mice because, under most conditions, neither male nor female resident mice will attack intruder females (171), so alternatives have been proposed (172, 173).
Palliating factors
Studies in rodent models have also tested the effects of EE after threat induction. Like MEMO therapy for cats, EE aims to improve health by providing more complex and novel living conditions than standard housing offers by adding inherently enriching physical and/or social elements into animals' environments (174–177). Animal models of EE have been used for more than seventy years to promote healthy physiological responses (176). Physical EE includes providing home cages that are larger and more complex and objects that promote activity, play, nesting, and/or foraging to promote cognitive stimulation and physical activity, whereas social enrichment is implemented by housing animals together to provide opportunities for them to engage in species' typical social behaviors like social play, communal nesting, cooperative feeding, allogrooming, and nursing (175). Studies incorporating EE that more closely reflect the umwelt (178) of the animals studied seem to offer more ecologically valid models of IC in humans and cats (99).
Voluntary wheel running reportedly improves both physical and behavioral abnormalities in induced models of IC (179–182), as does EE in models of visceral pain after ELA (183–186). For example, maternal separation for six hours each day from postnatal day 1–21 resulted in visceral pain, anxiety, and depression in adult male C57BL/6J mice, which was effectively prevented by EE interventions in prepuberty and puberty (183). Environmental enrichment was more effective in male mice, whereas longer exposure to EE was required in female mice to demonstrate positive effects (184). In another study of EE, normal male and female Wistar rat pups received subcutaneous administration of LPS (50 µg/kg) 3 and 5 days after birth to induce an inflammatory response, after which they received EE from post-natal day 25–120. The EE increased motor and search activity and contextual conditioned fear in both sexes and reduced anxiety in males and depressive-like behavior in females. In contrast, LPS prevented the beneficial effects of EE on anxiety in females and on conditioned fear in both sexes (185). The systemic effects of EE on conditioned fear reactions have been reviewed (187).
In a 2024 study, adult Fischer-344 rats of both sexes were exposed to WAS daily for seven days. After confirming the rats had developed visceral hypersensitivity, they were housed in environmentally enriched cages with ample bedding, toys, food enrichment, and burrowing tunnels in groups of four rats/cage for two weeks, after which visceral and somatic sensitivity was assessed, followed by collection of colon tissue to measure permeability. In both sexes, EE reversed stress-induced visceral and somatic hypersensitivity and colonic hyperpermeability to control animal levels, suggesting that EE can ameliorate stress-induced pathophysiology (186).
Predisposing factors
Models of predisposing risk factors like ELA are relevant because ELA is common among humans and cats, and childhood maltreatment is a particularly potent risk factor for IC (102). In the United States, at least 1 in 7 children experienced child abuse or neglect in 20202, and about 1 in 20 boys and 1 in 4 girls experience sexual abuse during childhood3. This terrible prevalence is similar to the reported worldwide pooled overall rate of 21%–27% (188). Moreover, the prevalence of sexual abuse ranged between 1.3% and 50% in patients presenting with LUTS in a recent (2023) systematic review, which reported that urinary storage symptoms, voiding difficulties, voluntary holding of urine, and urinary tract infections were associated with psychosocial threats, depression, and anxiety (189). Among cats, orphaning and sheltering are common early adverse life events (190).
Animal models of ELA include limited bedding and maternal separation (191–193). In a study of limited bedding, female and male neonatal Wistar rats were exposed to limited bedding on postnatal days 2–9. Then, at 10–11 weeks, they underwent colorectal distension and abdominal electromyography, and cerebral blood flow was measured. Exposure to limited bedding increased abdominal electromyography responses to colorectal distension in rats of both sexes, whereas functional brain responses differed across circuits involved in the regulation of pain and emotion; responses were greater in female rats in the locus coeruleus/lateral parabrachial nucleus complex and brain noradrenergic system (the CTRS). In models of maternal separation, female (179) and male (182) C57/Bl6 mice were separated into clean glass containers with small amounts of their home cage bedding material and held for three hours on postnatal days 1–21. Maternal separation increased bladder sensitivity, void frequency, and mast cell degranulation and reduced corticotropin-releasing factor receptor 1 and glucocorticoid receptor mRNA levels when assessed four weeks later.
Early life adversity can trigger evolutionarily conserved survival responses that include epigenetic modification of gene expression in patients with IC and other primary chronic pain conditions (102, 194). Preclinical and clinical epigenetic findings include changes in DNA methylation, histone modification, noncoding RNA, RNA modification, and chromatin remodeling factors. The contribution of these mechanisms to trait variability after ELA and how such mechanisms might lead to identifying new therapeutic options has also been reviewed (195).
Recent life adversity and ongoing chronic threats have also been associated with neuroendocrine and inflammatory alterations in patients with chronic pain, including IC and related conditions (14). Animal models of RLA include WAS, social defeat, and various other threat-inducing events. Each has been tested in rodents of different ages, sexes, and strains, resulting in various LUTS, and each has advantages and disadvantages for understanding IC (2, 3).
Early and recent life adversity have also been combined in what are called “two-hit” models of IC, since patients with IC commonly have histories of both predisposing (ELA) and precipitating (RLA) risk factors (196). By itself, ELA is neither necessary nor sufficient to lead to IC but may potentiate susceptibility to adverse outcomes when a susceptible individual is exposed to threats later in life. These models also have limitations, as recently discussed (196).
Implications of the “5 Ps” risk and protective factor approach to IC for clinical care
Precipitating and Perpetuating factors
IC patients usually present with variable combinations of signs and symptoms resulting from underlying risk factors described previously, which are identified from the signalment and a careful history. Skepticism from medical providers can diminish patient satisfaction and outlook (197, 198), and a qualitative study reported that women with IC commonly experience disbelief and pain dismissal from healthcare providers, which can prevent effective communication with them and lead to suboptimal care (199).
Palliating factors
Providing trauma-informed care (200), identifying and mitigating identifiable risk factors to the extent possible, and providing positive, meaningful social connections and support (protective factors) can be therapeutic (201, 202). The authors of the qualitative study mentioned above proposed adopting a patient-centered approach by inviting patients to share their personal experiences, beliefs, and embodied knowledge during treatment discussions to elevate the interaction beyond typical urology considerations by affording patients the ability to share in decision-making when no clear “best” treatment option exists. They argued that appreciating the struggles, anxieties, and gendered inequities that women with IC experience during their diagnostic journey could enrich patient-centered treatment and improve the well-known delay in making the diagnosis of IC (199).
Although trauma-informed care is not specifically mentioned in all pelvic pain guidelines, clinicians are encouraged to listen attentively, convey interest and compassion, and validate patients’ symptom experiences (200). Screening for a family history of COPC beyond first-degree relatives may be useful during the initial evaluation of patients presenting with pain, anxiety, or depression because it could lead to earlier diagnosis of other relatives who could benefit from treatment (12).
Positive relational social support systems can also improve patient health (203), and IC patients may not receive enough support from their usual social support systems, given the general lack of understanding about their condition (204–206). The U. S. Surgeon General has identified various ways to improve social connections and health. When available, multidisciplinary pain clinics and positive support groups can provide social connections to help scaffold patients' recovery of positive social attachments.
Non-pharmacological psychological therapies have been recommended for IC, although their effectiveness is currently limited (60). Moreover, the most recent (2020) Cochrane review of psychological therapies for chronic pain in adults reported that, on average, people treated with cognitive behavior therapy probably experience slightly less pain and distress by the end of the treatment and six to 12 months later (moderate-quality evidence), may experience slightly less disability on average (low-quality evidence), and probably experience very slightly less pain and distress (moderate-quality evidence), but levels of disability may be similar to those of people who received a non-psychological treatment (low-quality evidence) (207).
As mentioned, biological treatments for IC have also met with little success (60). Schrepf, et al., recently reported that IC pain might have a treatable neuropathic component (208), but a recent, adequately powered randomized controlled trial of women with chronic pelvic pain without identified underlying disease reported gabapentin (used to treat some neuropathic pain conditions) to be equivalent to placebo in patients randomly assigned to receive gabapentin (titrated to a maximum dose of 2,700 mg daily) or matching placebo for 16 weeks (209). Gabapentin also resulted in significantly more self-reported serious adverse events (7%) than the placebo (2%).
Parsons' recommended approach to women presenting with symptoms of IC could be updated and combined with the compassionate assurance of ongoing vigilance and care to help prepare patients to begin evidence-based palliative approaches to care (104). In cats with IC, such a tailored case-specific approach (MEMO) has been successfully used to reduce clinical signs and related comorbidities for over three decades (16, 62) and is the current standard of care in veterinary medicine (210, 211). Cats with IC respond to effective environmental modification without any treatments directed toward the bladder, as do rodents subjected to physical and psychological stressors, emphasizing the role of the CTRS in IC. In contrast, people with IC are regularly given bladder treatments regardless of their demonstrated equivalence with a placebo (60). The placebo (regression to the mean and other statistical factors, and positive expectation) response rate among IC patients is reportedly 50% or more, suggesting that the CTRS plays a clinically important role in IC symptoms in humans as it does in cats and rodents (212). In fact, Parsons et al. (23), administered oral PPS, a treatment later demonstrated to be equivalent to a placebo4 (117), to 24 patients with IC. Within 4–8 weeks of initiation of therapy, 20 of the 24 patients experienced a decrease of at least 80 percent in pain, nocturia, and urinary urgency (excellent response), and 2 experienced a 50–80 percent reduction of symptoms (good response). The excellent overall success rate of 92 percent persisted during up to 24 months of follow-up despite the patient's history of longstanding, severely symptomatic disease. It is no surprise that Parsons et al., believed PPS to be an effective oral treatment for IC.
Examples of tools to diagnose chronic pain type, assess physical functioning, emotional functioning, patients' beliefs and coping, multidimensional measures, and pharmacological treatments based on pain classification have recently become available in an open-access practical guide to Recognize, Assess, Treat, and Evaluate (RATE) patients with chronic pain seen in primary care (213). The guide also describes how to use this information to tailor treatment plans to meet individual patient's needs and to evaluate patients and the impact of treatment plans over time, with a particular focus on strategies to improve the patient's ability to self-manage their pain and related symptoms and to perform daily functions despite persistent pain. The article explicitly addresses chronic primary pain and could be adapted to IC and related conditions by acknowledging previously discussed pharmacological limitations.
Additional resources are also available; Darnall et al. reviewed several promising education and treatment technology innovations to improve access and scalability of evidence-based behavioral pain treatments (214). Identifying risk and protective factors early, in primary care if possible, might also shift the balance of perpetuating factors to palliating and protective factors to promote recovery.
Implications for research
Social and psychological palliating and protective factors
Research is just beginning to study the value of positive social connections for people with chronic pain (215). Despite the centrality of the effects of human sociality on our survival as a species, research into the effects of social factors on chronic pain in general and IC in particular remains in its infancy. Notwithstanding the remarkable work of some, much more remains to do. Social research that may be relevant to IC is starting to be published. For example, Groups 4 Health (G4H) is an emerging therapy program that targets social group disconnection (216). A recent study comparing G4H with treatment as usual for people with chronic low back pain (another COPC) found G4H to be significantly more effective in reducing pain intensity and disability post-intervention and at a one-month follow-up. More robust treatment group identification and cohesion early in the program among G4H recipients predicted better pain outcomes over time, which was fully mediated by perceptions of personal control (217).
The acceptability of G4H to clients and therapists and its feasibility for wider implementation have also been evaluated (218). Both client and therapist satisfaction was high, with all average ratings more than 70% positive, significantly exceeding the cognitive behavior therapy client comparison group. Retention was greater than 80%, and fewer than 10% of clients said that they had not attempted the homework. Therapists and clients both emphasized the contribution of the group context itself as a vehicle to achieve positive outcomes.
Other recently developed psychological therapies, such as emotional awareness and expression therapy (219), pain reprocessing therapy (220, 221), and cognitive functional therapy (222), have also shown promise for COPC, including IC (223). Although the studies need to be replicated in larger populations with longer follow-ups, effective therapies should also reduce the severity of associated comorbidities, as has been reported in humans with IBS (224) and cats with IC (62).
Biological factors
Evaluating the conserved transcriptional response to adversity (CTRA) or Toll-like Receptor-4-stimulated cytokines in the blood of patients with IC and other COPC might provide useful markers of CTRS activity and response to therapeutic interventions in both human and animal studies. The CTRA is a pattern of gene expression that reflects the activation of pro-inflammatory pathways and the suppression of antiviral and antibody-related pathways in response to stress, trauma, or social isolation (225). The CTRA has been linked to various adverse health outcomes, such as increased risk of infection, inflammation, and chronic disease. It also appears to be responsive to effective therapy, which may permit its use in clinical studies of palliative and other complementary approaches to care (91). Toll-like Receptor-4 (TLR-4) receptors can trigger an inflammatory response to bacterial components like LPS and immune stimulation by the CTRS in patients with IC and related COPC. Research using the blood of patients with IC and healthy controls has found that patients with IC have higher levels of TLR-4-stimulated cytokines and chemokines. TLR-4 may also interact with other factors, such as ELA or RLA, to increase the risk or severity of IC (14, 226).
Increased heart rate and changes in heart rate variability are also related to chronic primary pain conditions like IC (54). Dudarev et al. (227), recently reported a positive predictive relationship between increased sleep heart rate and next-day pain intensity in patients with two chronic primary pain conditions, fibromyalgia (228) and primary back pain (229), with no interaction between the types of chronic pain. These findings support the hypothesis that threat-driven increases in CTRS-mediated autonomic hyperactivation precede increases in next-day pain intensity in patients with chronic primary pain conditions.
Conclusions
The IASP's recent classification of IC and related conditions and the expansion of the dimensions of the effects of chronic pain on people's lives has thus afforded a unique opportunity to rethink these problems from a cause to a risk and protective factor perspective. The interconnected body-brain-mind creates the “organismic experience” of chronic pain. The standard urological explanation is that the experience of IC is initiated “bottom-up”, either by some abnormality of urothelial permeability that results from and/or leads to the absorption of toxins from the urine that results in increased nociceptive input to the central nervous system, or, more recently, by low bladder capacity (230). This input results in systemic and local bladder abnormalities and can result in pain, which activates the CTRS and its output to the peripheral adrenocortical, autonomic, and immune systems, creating a feedback loop that perpetuates the pain and results in IC's waxing and waning course.
More recent research has shown that the experience of IC can be initiated from the “top-down” by the persistent perception of environmental threat and/or a sensitized CTRS, leading to the activation of peripheral adrenocortical, autonomic, and immune systems, resulting in systemic and local bladder abnormalities and creating a feedback loop that perpetuates the pain and results in IC's waxing and waning course. To date, evidence from studies of both human and feline patients with IC, and rodent models of physical and psychological stressors lend more support to the “top-down” perspective. Moving the source of IC pain to the brain as a motivational state resulting from central sensitization rather than peripheral nociceptive input offers both clinicians and researchers novel opportunities to improve care for and study of IC for the better. It may even be that IC results from an excess of risk to protective factors, making this imbalance a cause rather than a consequence of IC.
Author contributions
JW: Writing – original draft, Writing – review & editing. JS: Writing – original draft, Writing – review & editing. CB: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1https://www.iasp-pain.org/advocacy/definitions-of-chronic-pain-syndromes accessed March 18, 2024.
2https://www.cdc.gov/violenceprevention/childabuseandneglect/fastfact.html Accessed March 18, 2024.
3https://www.cdc.gov/violenceprevention/childsexualabuse/fastfact.html Accessed March 18, 2024.
4https://beta.clinicaltrials.gov/study/NCT00086684?cond=Interstitial%20Cystitis&intr=elmiron&rank=2 Accessed March 18, 2024
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Buffington CA. Idiopathic cystitis in domestic cats-beyond the lower urinary tract. J Vet Intern Med. (2011) 25(4):784–96. doi: 10.1111/j.1939-1676.2011.0732.x
2. Gao Y, Rodríguez LV. The effect of chronic psychological stress on lower urinary tract function: an animal model perspective. Front Physiol (2022) 431:1–14. doi: 10.3389/fphys.2022.818993
3. Tay C, Grundy L. Animal models of interstitial cystitis/bladder pain syndrome. Front Physiol. (2023) 14:1–29. doi: 10.3389/fphys.2023.1232017
4. Nicholas M, Vlaeyen JW, Rief W, Barke A, Aziz Q, Benoliel R, et al. The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. (2019) 160(1):28–37. doi: 10.1097/j.pain.0000000000001390
5. Cohen M, Quintner J, Weisman A. “Nociplastic pain”: a challenge to nosology and to nociception. J Pain. (2023) 24(12):2131–9. doi: 10.1016/j.jpain.2023.07.019
6. Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. (2020) 161(9):1976–82. doi: 10.1097/j.pain.0000000000001939
7. Buffington CAT. Bladder pain syndrome/interstitial cystitis. In: Baranowski AP, Abrams P, Fall M, editors. Urogenital Pain in Clinical Practice. New York, USA: Informa Healthcare (2008). p. 169–83. doi: 10.3109/9781420021196
8. Lim Y, Leslie SW, O'Rourke S. Interstitial cystitis/bladder pain syndrome. [Updated 2023 Nov 12]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing (2024). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK570588/
9. Anger JT, Dallas KB, Bresee C, De Hoedt AM, Barbour KE, Hoggatt KJ, et al. National prevalence of IC/BPS in women and men utilizing veterans health administration data. Front Pain Res. (2022) 3:925834. doi: 10.3389/fpain.2022.925834
10. Willeberg P. Epidemiology of naturally-occurring feline urologic syndrome. Vet Clin North Am Small Anim Pract. (1984) 14(3):455–69. doi: 10.1016/s0195-5616(84)50053-9
11. Lekcharoensuk C, Osborne CA, Lulich JP. Epidemiologic study of risk factors for lower urinary tract diseases in cats. J Am Vet Med Assoc. (2001) 218(9):1429–35. doi: 10.2460/javma.2001.218.1429
12. Allen-Brady K, Fyer AJ, Weissman M. The multi-generational familial aggregation of interstitial cystitis, other chronic nociplastic pain disorders, depression, and panic disorder. Psychol Med. (2023) 53:7847–56. doi: 10.1017/S0033291723001885
13. Komesu YM, Petersen TR, Krantz TE, Ninivaggio CS, Jeppson PC, Meriwether KV, et al. Adverse childhood experiences in women with overactive bladder or interstitial cystitis/bladder pain syndrome. Female Pelvic Med Reconstr Surg. (2021) 27(1):e208–14. doi: 10.1097/SPV.0000000000000894
14. Lutgendorf SK, Zia S, Luo Y, O'Donnell M, van Bokhoven A, Bradley CS, et al. Early and recent exposure to adversity, TLR-4 stimulated inflammation, and diurnal cortisol in women with interstitial cystitis/bladder pain syndrome: a MAPP research network study. Brain Behav Immun (2023) 111:116–23. doi: 10.1016/j.bbi.2023.03.024
15. Westropp JL, Welk KA, Buffington CA. Small adrenal glands in cats with feline interstitial cystitis. J Urol. (2003) 170(6 Pt 1):2494–7. doi: 10.1097/01.ju.0000095566.63870.66
16. Stella JL, Lord LK, Buffington CA. Sickness behaviors in response to unusual external events in healthy cats and cats with feline interstitial cystitis. J Am Vet Med Assoc. (2011) 238(1):67–73. doi: 10.2460/javma.238.1.67
17. Schrepf A, Maixner W, Fillingim R, Veasley C, Ohrbach R, Smith S, et al. The chronic overlapping pain condition screener. J Pain. (2024) 25(1):265–72. doi: 10.1016/j.jpain.2023.08.009
18. Buffington C, Westropp J, Chew D, Bolus R. A case-control study of indoor-housed cats with lower urinary tract signs. J Am Vet Med Assoc. (2006) 228:722–5. doi: 10.2460/javma.228.5.722
19. Propert KJ, Schaeffer AJ, Brensinger CM, Kusek JW, Nyberg LM, Landis JR. A prospective study of interstitial, cystitis: results of longitudinal followup of the interstitial cystitis data base cohort. J Urol. (2000) 163(5):1434–9. doi: 10.1016/s0022-5347(05)67637-9
20. Keller JJ, Chen YK, Lin HC. Comorbidities of bladder pain syndrome/interstitial cystitis: a population-based study. BJU Int. (2012) 110(11 Pt C):E903–9. doi: 10.1111/j.1464-410X.2012.11539.x
21. Laden BF, Bresee C, De Hoedt A, Dallas KB, Scharfenberg A, Saxena R, et al. Comorbidities in a nationwide, heterogenous population of veterans with interstitial cystitis/bladder pain syndrome. Urology. (2021) 156:37–43. doi: 10.1016/j.urology.2021.04.015
22. Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990–1999). J Am Vet Med Assoc. (2002) 220(2):202–7. doi: 10.2460/javma.2002.220.202
23. Sculptoreanu A, deGroat WC, Buffington CAT, Birder LA. Protein kinase C contributes to abnormal capsaicin responses in DRG neurons from cats with feline interstitial cystitis. Neurosci Lett. (2005) 381(1-2):42–6. doi: 10.1016/j.neulet.2005.01.080
24. Sculptoreanu A, deGroat WC, Buffington CAT, Birder L. Abnormal excitability in capsaicin-responsive DRG neurons from cats with feline interstitial cystitis. Exp Neurol. (2005) 193(2):437–43. doi: 10.1016/j.expneurol.2005.01.011
25. Stella J, Croney C, Buffington T. Effects of stressors on the behavior and physiology of domestic cats. Appl Anim Behav Sci. (2013) 143(2):157–63. doi: 10.1016/j.applanim.2012.10.014
26. Whitmore KE, Fall M, Sengiku A, Tomoe H, Logadottir Y, Kim YH. Hunner lesion versus non-Hunner lesion interstitial cystitis/bladder pain syndrome. Int J Urol. (2019) 26:26–34. doi: 10.1111/iju.13971
27. Clasper M. A case of interstitial cystitis and Hunner’s ulcer in a domestic shorthaired cat. N Z Vet J. (1990) 38:158–60. doi: 10.1080/00480169.1990.35644
28. Hurst RE, Greenwood-Van Meerveld B, Wisniewski AB, VanGordon S, Lin H, Kropp BP, et al. Increased bladder permeability in interstitial cystitis/painful bladder syndrome. Transl Androl Urol. (2015) 4(5):563. doi: 10.3978/j.issn.2223-4683.2015.10.03
29. Lavelle JP, Meyers SA, Ruiz WG, Buffington CA, Zeidel ML, Apodaca G. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol Renal Physiol. (2000) 278(4):F540–53. doi: 10.1152/ajprenal.2000.278.4.F540
30. Jhang J-F, Ho H-C, Jiang Y-H, Lee C-L, Hsu Y-H, Kuo H-C. Electron microscopic characteristics of interstitial cystitis/bladder pain syndrome and their association with clinical condition. PLoS One. (2018) 13(6):e0198816. doi: 10.1371/journal.pone.0198816
31. Gamper M, Regauer S, Welter J, Eberhard J, Viereck V. Are mast cells still good biomarkers for bladder pain syndrome/interstitial cystitis? J Urol. (2015) 193(6):1994–2000. doi: 10.1016/j.juro.2015.01.036
32. Kullmann FA, McDonnell BM, Wolf-Johnston AS, Lynn AM, Getchell SE, Ruiz WG, et al. Inflammation and tissue remodeling in the bladder and urethra in feline interstitial cystitis. Front Syst Neurosci. (2018) 12:13. doi: 10.3389/fnsys.2018.00013
33. Ustundağ Y, Huysal K, Guzelsoy M, Genim CE, Yavuz A. Urine and serum glycosaminoglycan levels in the diagnosis of urological diseases and conditions: a narrative review of the literature. Urol J. (2021) 88(2):103–9. doi: 10.1177/0391560320960003
34. Buffington CA, Blaisdell JL, Binns SP Jr, Woodworth BE. Decreased urine glycosaminoglycan excretion in cats with interstitial cystitis. J Urol. (1996) 155(5):1801–4. doi: 10.1016/S0022-5347(01)66201-3
35. Shupp Byrne D, Sedor JF, Estojak J, Fitzpatrick KJ, Chiura AN, Mulholland SG. Mthe urinary glycoprotein GP51 as a clinical marker for interstitial cystitis. J Urol. (1999) 161(6):1786–90. doi: 10.1016/S0022-5347(05)68800-3
36. Press SM, Moldwin R, Kushner L, Buffington CAT, Schupp-Byrne D. Decreased expression of GP-51 glycosaminoglycan in cats afflicted with feline interstitial cystitis. J Urol (1995) 153:288A.
37. Westropp JL, Buffington CAT. In vivo models of interstitial cystitis. J Urol. (2002) 167(2):694–702. doi: 10.1016/S0022-5347(01)69129-8
38. Pang X, Marchand J, Sant GR, Kream RM, Theoharides TC. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol. (1995) 75:744–50. doi: 10.1111/j.1464-410x.1995.tb07384.x
39. Buffington CAT, Wolfe SA. High affinity binding sites for [3H]substance P in urinary bladders of cats with interstitial cystitis. J Urol. (1998) 160:605–11. doi: 10.1016/S0022-5347(01)62967-7
40. Sanses T, McCabe P, Zhong L, Taylor A, Chelimsky G, Mahajan S, et al. Sensory mapping of pelvic dermatomes in women with interstitial cystitis/bladder pain syndrome. Neurourol Urodyn. (2018) 37(1):458–65. doi: 10.1002/nau.23330
41. Roppolo JR, Tai C, Booth AM, Buffington CA, de Groat WC, Birder LA. Bladder A-delta afferent nerve activity in normal cats and cats with feline interstitial cystitis. J Urol. (2005) 173(3):1011–5. doi: 10.1097/01.ju.0000145591.35569.9e
42. Marchand JE, Sant GR, Kream RM. Increased expression of substance P receptor-encoding mRNA in bladder biopsies from patients with interstitial cystitis. Br J Urol. (1998) 81(2):224–8. doi: 10.1046/j.1464-410x.1998.00507.x
43. Barker ES, Chiu K, Brown VL, Morsy H, Yaeger LH, Catna A, et al. Urologic chronic pelvic pain syndrome flares: a comprehensive, systematic review and meta-analysis of the peer-reviewed flare literature. J Urol (2024) 211:341–53. doi: 10.1097/JU.0000000000003820
44. Twiss C, Kilpatrick L, Craske M, Buffington CA, Ornitz E, Rodriguez LV, et al. Increased startle responses in interstitial cystitis: evidence for central hyperresponsiveness to visceral related threat. J Urol. (2009) 181(5):2127–33. doi: 10.1016/j.juro.2009.01.025
45. Hague DW, Stella JL, Buffington CA. Effects of interstitial cystitis on the acoustic startle reflex in cats. Am J Vet Res. (2013) 74(1):144–7. doi: 10.2460/ajvr.74.1.144
46. Reche AJ, Buffington CAT. Increased tyrosine hydroxylase immunoreactivity in the locus coeruleus of cats with interstitial cystitis. J Urol. (1998) 159:1045–8. doi: 10.1016/s0022-5347(01)63833-3
47. Charrua A, Pinto R, Taylor A, Canelas A, Ribeiro-da-Silva A, Cruz CD, et al. Can the adrenergic system be implicated in the pathophysiology of bladder pain syndrome/interstitial cystitis? A clinical and experimental study. Neurourol Urodyn. (2015) 34(5):489–96. doi: 10.1002/nau.22542
48. Buffington C, Pacak K. Increased plasma norepinephrine concentration in cats with interstitial cystitis. J Urol. (2001) 165(6 Part 1):2051–4. doi: 10.1016/S0022-5347(05)66292-1
49. Stein PC, Torri A, Parsons L. Elevated urinary norepinephrine in interstitial cystitis. Urology. (1999) 53(6):1140–3. doi: 10.1016/s0090-4295(98)00663-3
50. Peeker R, Aldenborg F, Dahlstrom A, Johansson SL, Li JY, Fall M. Increased tyrosine hydroxylase immunoreactivity in bladder tissue from patients with classic and nonulcer interstitial cystitis. J Urol. (2000) 163(4):1112–5. doi: 10.1016/S0022-5347(05)67704-X
51. Reche A, Buffington T, Hagiwara MK, Daniel AG. Increased tyrosine hydroxylase immunoreactivity in the urinary bladder of cats with interstitial cystitis. Online J Vet Res. (2004) 8:42–7.
52. Westropp JL, Kass PH, Buffington CA. In vivo evaluation of alpha(2)-adrenoceptors in cats with idiopathic cystitis. Am J Vet Res. (2007) 68(2):203–7. doi: 10.2460/ajvr.68.2.203
53. Buffington CAT, Teng BY, Somogyi GT. Norepinephrine content and adrenoceptor function in the bladder of cats with feline interstitial cystitis. J Urol. (2002) 167(4):1876–80. doi: 10.1016/S0022-5347(05)65253-6
54. Williams DP, Chelimsky G, McCabe NP, Koenig J, Singh P, Janata J, et al. Effects of chronic pelvic pain on heart rate variability in women. J Urol. (2015) 194(5):1289–94. doi: 10.1016/j.juro.2015.04.101
55. Hellman KM, Oladosu FA, Garrison EF, Roth GE, Dillane KE, Tu FF. Circulating sex steroids and bladder pain sensitivity in dysmenorrhea. Mol Pain. (2021) 17:17448069211035217. doi: 10.1177/17448069211035217
56. Kovarikova S, Simerdova V, Bilek M, Honzak D, Palus V, Marsalek P. Clinicopathological characteristics of cats with signs of feline lower urinary tract disease in the Czech Republic. Vet Med. (2020) 65(3):123–33. doi: 10.17221/146/2019-VETMED
57. Moldwin RM, Nursey V, Yaskiv O, Dalvi S, Macdonald EJ, Funaro M, et al. Immune cell profiles of patients with interstitial cystitis/bladder pain syndrome. J Transl Med. (2022) 20(1):97. doi: 10.1186/s12967-022-03236-7
58. Jiang Y-H, Peng C-H, Liu H-T, Kuo H-C. Increased pro-inflammatory cytokines, C-reactive protein and nerve growth factor expressions in serum of patients with interstitial cystitis/bladder pain syndrome. PLoS One. (2013) 8(10):e76779. doi: 10.1371/journal.pone.0076779
59. Mohamaden WI, Hamad R, Bahr HI. Alterations of pro-inflammatory cytokines and tissue protein expressions in cats with interstitial cystitis. Pak Vet J. (2019) 39(2):151–6. doi: 10.29261/pakvetj/2019.026
60. Imamura M, Scott NW, Wallace SA, Ogah JA, Ford AA, Dubos YA, et al. Interventions for treating people with symptoms of bladder pain syndrome: a network meta-analysis. Cochrane Database Syst Rev. (2020) 7:1–7. doi: 10.1002/14651858.CD013325.pub2
61. Chew DJ, Buffington CT, Kendall MS, DiBartola SP, Woodworth BE. Amitriptyline treatment for severe recurrent idiopathic cystitis in cats. J Am Vet Med Assoc. (1998) 213(9):1282–6. doi: 10.2460/javma.1998.213.09.1282
62. Buffington CAT, Westropp JL, Chew DJ, Bolus RR. Clinical evaluation of multimodal environmental modification in the management of cats with lower urinary tract signs. J Feline Med Surg. (2006) 8:261–8. doi: 10.1016/j.jfms.2006.02.002
63. Yeh HL, Jhang JF, Kuo YC, Kuo HC. Long-term outcome and symptom improvement in patients with interstitial cystitis/bladder pain syndrome with or without regular follow-up and treatment. Neurourol Urodyn. (2019) 38(7):1985–93. doi: 10.1002/nau.24104
64. Mishra NN. Interstitial cystitis/bladder pain syndrome (IC/BPS): single-center 20 year experience and treatment results in India. Neurourol Urodyn (2022) 41(6):1390–8. doi: 10.1002/nau.24959
65. De Cian M, Tricard T, Saussine C. Syndrome de la douleur vésicale: résultats à long terme (15 ans) d’une expérience monocentrique. Prog Urol. (2022) 32(10):681–90. doi: 10.1016/j.purol.2022.03.003
66. He C, Fan K, Hao Z, Tang N, Li G, Wang S. Prevalence, risk factors, pathophysiology, potential biomarkers and management of feline idiopathic cystitis: an update review. Front Vet Sci. (2022) 758:1–18. doi: 10.3389/fvets.2022.900847
67. Klement RJ, Bandyopadhyay PS. Emergence and evidence: a close look at bunge’s philosophy of medicine. Philosophies. (2019) 4(3):50. doi: 10.3390/philosophies4030050
68. Anjum RL, Copeland S, Rocca E. Rethinking causality, complexity and evidence for the unique patient: a causehealth resource for healthcare professionals and the clinical encounter. Cham, CH: Springer Nature (2020). doi: 10.1007/978-3-030-41239-5
69. Martini E. How did Vasco da Gama sail for 16 weeks without developing scurvy? Lancet. (2003) 361(9367):1480. doi: 10.1016/S0140-6736(03)13131-5
70. Clemens JQ, Erickson DR, Varela NP, Lai HH. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. (2022) 208(1):34–42. doi: 10.1097/JU.0000000000002756
71. Feinstein AR. The blame-X syndrome: problems and lessons in nosology, spectrum, and etiology. J Clin Epidemiol (2001) 54(5):433–9. doi: 10.1016/S0895-4356(00)00374-7
72. Meijlink JM. Interstitial cystitis and the painful bladder: a brief history of nomenclature, definitions and criteria. Int J Urol. (2014) 21:4–12. doi: 10.1111/iju.12307
73. Berry SH, Bogart LM, Pham C, Liu K, Nyberg L, Stoto M, et al. Development, validation and testing of an epidemiological case definition of interstitial cystitis/painful bladder syndrome. JUrol. (2010) 183(5):1848–52. doi: 10.1016/j.juro.2009.12.103
74. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the international continence society. Neurourol Urodyn. (2002) 21(2):167–78. doi: 10.1002/nau.10052
75. Hanno P, Lin A, Nordling J, Nyberg L, van Ophoven A, Ueda T, et al. Bladder pain syndrome committee of the international consultation on incontinence. Neurourol Urodyn. (2010) 29(1):191–8. doi: 10.1002/nau.20847
76. Landis JR, Williams DA, Lucia MS, Clauw DJ, Naliboff BD, Robinson NA, et al. The MAPP research network: design, patient characterization and operations. BMC Urol. (2014) 14(1):1–17. doi: 10.1186/1471-2490-14-58
77. Homma Y. Hypersensitive bladder: a solution to confused terminology and ignorance concerning interstitial cystitis. Int J Urol. (2014) 21:43–7. doi: 10.1111/iju.12314
78. Gori M, Onesti E, Ceccanti M, Cambieri C, Nasta L, Cervigni M, et al. Central sensitization in the bladder pain syndrome. JSM Pain Manag. (2016) 1(1):1–6. https://hdl.handle.net/11573/989068
79. Yunus MB. Editorial review: an update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr Rheumatol Rev (2015) 11(2):70–85. doi: 10.2174/157339711102150702112236
80. Homma Y, Akiyama Y, Niimi A, Nomiya A, Igawa Y. Classification, characterization, and sub-grouping of interstitial cystitis. Curr Bladder Dysfunct Rep. (2019) 14:294–300. doi: 10.1007/s11884-019-00542-7
81. Bolton JW. Case formulation after engel—the 4P model: a philosophical case conference. Philos Psychiatr Psychol. (2014) 21(3):179–89. doi: 10.1353/ppp.2014.0027
82. Wright CD, Tiani AG, Billingsley AL, Steinman SA, Larkin KT, McNeil DW. A framework for understanding the role of psychological processes in disease development, maintenance, and treatment: the 3P-disease model. Front Psychol. (2019) 10:2498. doi: 10.3389/fpsyg.2019.02498
83. Kitselaar WM, Van Der Vaart R, Perschl J, Numans ME, Evers AW. Predictors of persistent somatic symptoms in the general population: a systematic review of cohort studies. Psychosom Med (2023) 85(1):71–8. doi: 10.1097/PSY.0000000000001145
84. De Ridder D, Elgoyhen AB, Romo R, Langguth B. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad SciC. (2011) 108(20):8075–80. doi: 10.1073/pnas.1018466108
85. Treede R-D, Hoheisel U, Wang D, Magerl W. Central sensitization: clinical utility of a physiological concept for the international statistical classification of diseases and related health problems and for nociplastic pain. Pain. (2022) 163(11):S99–107. doi: 10.1097/j.pain.0000000000002740
86. De Ridder D, Adhia D, Vanneste S. The anatomy of pain and suffering in the brain and its clinical implications. Neurosci Biobehav Rev. (2021) 130:125–46. doi: 10.1016/j.neubiorev.2021.08.013
87. Fitzcharles M-A, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. (2021) 397(10289):2098–110. doi: 10.1016/S0140-6736(21)00392-5
88. Kirkham A, Swainston K. Women’s experiences of interstitial cystitis/painful bladder syndrome. West J Nurs Res. (2022) 44(2):125–32. doi: 10.1177/0193945921990730
89. Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain. (2016) 17(9):T70–92. doi: 10.1016/j.jpain.2016.01.001
90. Holt-Lunstad J, Perissinotto C. Social isolation and loneliness as medical issues. N Engl J Med. (2023) 288(3):193–5. doi: 10.1056/NEJMp2208029
91. Slavich GM, Mengelkoch S, Cole SW. Human social genomics: concepts, mechanisms, and implications for health. Lifestyle Med. (2023) 4(2):1–15. doi: 10.1002/lim2.75
92. Tomasello M. The adaptive origins of uniquely human sociality. Philos Trans R Soc B. (2020) 375(1803):20190493. doi: 10.1098/rstb.2019.0493
93. Sanford MT, Rodriguez LV. The role of environmental stress on lower urinary tract symptoms. Curr Opin Urol. (2017) 27(3):268–73. doi: 10.1097/MOU.0000000000000379
94. Syme KL, Hagen EH. Mental health is biological health: why tackling “diseases of the mind” is an imperative for biological anthropology in the 21st century. Am J Phys Anthropol (2020) 171:87–117. doi: 10.1002/ajpa.23965
95. Jaffe S. US surgeon general: loneliness is a public health crisis. Lancet. (2023) 401(10388):1560. doi: 10.1016/S0140-6736(23)00957-1
96. Bradshaw JW. Sociality in cats: a comparative review. J Vet Behav. (2016) 11:113–24. doi: 10.1016/j.jveb.2015.09.004
97. Lee NS, Beery AK. Neural circuits underlying rodent sociality: a comparative approach. In: Coolen LM, Grattan DR, editors. Neuroendocrine Regulation of Behavior. Cham, CH: Springer Nature (2019). p. 211–38. doi: 10.1007/7854_2018_77
98. Goldstein DS. Stress and the “extended” autonomic system. Auton Neurosci. (2021) 236:102889. doi: 10.1016/j.autneu.2021.102889
99. Buffington C, Bain M. Stress and feline health. Vet Clin North Am Small Anim Pract (2020) 50(4):653–62. doi: 10.1016/j.cvsm.2020.03.001
100. Quaghebeur J, Petros P, Wyndaele J-J, De Wachter S. The innervation of the bladder, the pelvic floor, and emotion: a review. Auton Neurosci (2021) 235:102868. doi: 10.1016/j.autneu.2021.102868
101. Magariños López M, Lobato Rodríguez MJ, Menéndez García Á, García-Cid S, Royuela A, Pereira A. Psychological profile in women with chronic pelvic pain. J Clin Med. (2022) 11(21):6345. doi: 10.3390/jcm11216345
102. Teicher MH, Gordon JB, Nemeroff CB. Recognizing the importance of childhood maltreatment as a critical factor in psychiatric diagnoses, treatment, research, prevention, and education. Mol Psychiatry. (2022) 27(3):1331–8. doi: 10.1038/s41380-021-01367-9
103. Chiu C-D, Lee M-H, Chen W-C, Ho HL, Wu H-C. Childhood trauma perpetrated by close others, psychiatric dysfunction, and urological symptoms in patients with interstitial cystitis/bladder pain syndrome. J Psychosom Res. (2017) 93:90–5. doi: 10.1016/j.jpsychores.2016.12.014
104. Parsons CL. How does interstitial cystitis begin? Transl Androl Urol. (2015) 4(6):605–10. doi: 10.3978/j.issn.2223-4683.2015.11.02
105. Gonzalez DC, Khorsandi S, Mathew M, Enemchukwu E, Syan R. A systematic review of racial/ethnic disparities in female pelvic floor disorders. Urology. (2022) 163:8–15. doi: 10.1016/j.urology.2021.09.018
106. Windgassen SS, Sutherland S, Finn MT, Bonnet KR, Schlundt DG, Reynolds WS, et al. Gender differences in the experience of interstitial cystitis/bladder pain syndrome. Front Pain Res. (2022) 126:1–15. doi: 10.3389/fpain.2022.954967
107. Cameron M, Casey R, Bradshaw J, Waran N, Gunn-Moore D. A study of environmental and behavioural factors that may be associated with feline idiopathic cystitis. J Small Anim Pract. (2004) 45(3):144–7. doi: 10.1111/j.1748-5827.2004.tb00216.x
108. Bryan R, Beitz JM. Critical connections among embedding of childhood adversity and adult chronic gastrointestinal and genitourinary disorders: a review of the literature. Wound Manag Prev. (2021) 67(11):33–47. doi: 10.25270/wmp.2021.11.3347
109. Parsons JK, Parsons CL. The historical origins of interstitial cystitis. J Urol. (2004) 171(1):20–2. doi: 10.1097/01.ju.0000099890.35040.8d
110. Meijlink J. Historical perspectives. In: Hanno PM, Nordling J, Staskin DR, Wein AJ, and Wyndaele JJ, editors. Bladder Pain Syndrome–An Evolution. Cham, CH: Springer (2018). p. 3–10. doi: 10.1007/978-3-319-61449-6_2
111. Wennevik GE, Meijlink JM, Hanno P, Nordling J. The role of glomerulations in bladder pain syndrome: a review. J Urol. (2016) 195(1):19–25. doi: 10.1016/j.juro.2015.06.112
112. Hanno P, Cervigni M, Choo MS, Clemens JQ, Lee M-H, Malde S, et al. Summary of the 2023 report of the international consultation on incontinence interstitial cystitis/bladder pain syndrome (IC/BPS) committee. Continence. (2023) 8:101056. doi: 10.1016/j.cont.2023.101056
113. Natale F, Campagna G, Marturano M, Caramazza D, Panico G, Vacca L, et al. Is there a role for bladder biopsy in the diagnosis of non-Hunner lesions interstitial cystitis? Urol Int. (2023) 107(3):257–62. doi: 10.1159/000525849
114. Abelleyra Lastoria DA, Raison N, Aydin A, Khan S, Dasgupta P, Ahmed K. Comparing surgical interventions for interstitial cystitis: a systematic review. Low Urin Tract Symptoms. (2022) 14(4):218–41. doi: 10.1111/luts.12441
115. Warren JW, Jian N, Gallicchio L, Wu D, Clauw DJ. Prodrome and non-prodrome phenotypes of bladder pain syndrome/interstitial cystitis. Urology. (2018) 118:52–8. doi: 10.1016/j.urology.2018.05.004
116. Hay G, Korwisi B, Rief W, Smith BH, Treede R-D, Barke A. Pain severity ratings in the 11th revision of the international classification of diseases: a versatile tool for rapid assessment. Pain. (2022) 163(12):2421–9. doi: 10.1097/j.pain.0000000000002640
117. Nickel JC, Herschorn S, Whitmore KE, Forrest JB, Hu P, Friedman AJ, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: insights from a randomized, double-blind, placebo controlled study. J Urol. (2015) 193(3):857–62. doi: 10.1016/j.juro.2014.09.036
118. Lennox Thompson B, Gage J, Kirk R. Living well with chronic pain: a classical grounded theory. Disabil Rehabil. (2020) 42(8):1141–52. doi: 10.1080/09638288.2018.1517195
119. Koziol JA, Clark DC, Gittes RF, Tan EM. The natural history of interstitial cystitis: a survey of 374 patients. J Urol (1993) 149:465–9. doi: 10.1016/s0022-5347(17)36120-7
120. Warren JW, Morozov V, Howard FM, Wesselmann U, Gallicchio L, Langenberg P, et al. Before the onset of interstitial cystitis/bladder pain syndrome, the presence of multiple non-bladder syndromes is strongly associated with a history of multiple surgeries. J Psychosom Res. (2014) 76(1):75–9. doi: 10.1016/j.jpsychores.2013.10.013
121. Bendrick TR, Sitenga GL, Booth C, Sacco MP, Erie C, Anderson DJ, et al. The implications of mental health and trauma in interstitial cystitis. Health Psychol Res. (2022) 10(4):1–5. doi: 10.52965/001c.40321
122. Fazio RL, Wunderlich T, Wilson N, Akeson S. MMPI-2-RF characteristics of individuals with interstitial cystitis. J Psychosom Res. (2014) 77(5):359–62. doi: 10.1016/j.jpsychores.2014.09.010
123. Suskind AM, Berry SH, Ewing BA, Elliott MN, Suttorp MJ, Clemens JQ. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND interstitial cystitis epidemiology male study. J Urol. (2013) 189(1):141–5. doi: 10.1016/j.juro.2012.08.088
124. Schrepf A, Naliboff B, Williams DA, Stephens-Shields AJ, Landis JR, Gupta A, et al. Adverse childhood experiences and symptoms of urologic chronic pelvic pain syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Ann Behav Med. (2018) 52(10):865–77. doi: 10.1093/abm/kax060
125. Narayan AJ, Merrick JS, Lane AS, Larson MD. A multisystem, dimensional interplay of assets versus adversities: revised benevolent childhood experiences (BCEs) in the context of childhood maltreatment, threat, and deprivation. Dev Psychopathol. (2023) 35(5):2444–63. doi: 10.1017/S0954579423000536
126. Osborne CA, John MK, Jody PL. Feline lower urinary tract disorders: definition of terms and concepts. Vet Clin North Am Small Anim Pract. (1996) 26(2):169–79. doi: 10.1016/S0195-5616(96)50200-7
127. Lund EM, Armstrong PJ, Kirk CA, Kolar LM, Klausner JS. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc. (1999) 214(9):1336–41. doi: 10.2460/javma.1999.214.09.1336
128. Lund HS, Sævik BK, Finstad ØW, Grøntvedt ET, Vatne T, Eggertsdóttir AV. Risk factors for idiopathic cystitis in Norwegian cats: a matched case-control study. J Feline Med Surg. (2016) 18(6):483–91. doi: 10.1177/1098612X15587955
129. Patronek GJ, Glickman LT, Beck AM, McCabe GP, Ecker C. Risk factors for relinquishment of cats to an animal shelter. J Am Vet Med Assoc. (1996) 209(3):582–8. doi: 10.2460/javma.1996.209.03.582
130. Casey RA, Vandenbussche S, Bradshaw JW, Roberts MA. Reasons for relinquishment and return of domestic cats (Felis silvestris catus) to rescue shelters in the UK. Anthrozoös. (2009) 22(4):347–58. doi: 10.2752/089279309X12538695316185
131. Kaul E, Hartmann K, Reese S, Dorsch R. Recurrence rate and long-term course of cats with feline lower urinary tract disease. J Feline Med Surg. (2020) 22(6):544–56. doi: 10.1177/1098612X19862887
132. Eggertsdóttir AV, Blankvandsbråten S, Gretarsson P, Olofsson AE, Lund HS. Retrospective interview-based long-term follow-up study of cats diagnosed with idiopathic cystitis in 2003–2009. J Feline Med Surg. (2021) 23(10):945–51. doi: 10.1177/1098612X21990302
133. Jones E, Palmieri C, Thompson M, Jackson K, Allavena R. Feline idiopathic cystitis: pathogenesis, histopathology and comparative potential. J Comp Pathol. (2021) 185:18–29. doi: 10.1016/j.jcpa.2021.03.006
134. Buffington CAT. Comorbidity of interstitial cystitis with other unexplained clinical conditions. J Urol. (2004) 172:1242–8. doi: 10.1097/01.ju.0000137953.49304.6c
135. Buffington CA, Westropp JL, Chew DJ, Bolus RR. Risk factors associated with clinical signs of lower urinary tract disease in indoor-housed cats. J Am Vet Med Assoc. (2006) 228(5):722–5. doi: 10.2460/javma.228.5.722
136. Westropp JL, Delgado M, Buffington C. Chronic lower urinary tract signs in cats: current understanding of pathophysiology and management. Vet Clin North Am Small Anim Pract (2019) 49(2):187–209. doi: 10.1016/j.cvsm.2018.11.001
137. Parys M, Yuzbasiyan-Gurkan V, Kruger J. Serum cytokine profiling in cats with acute idiopathic cystitis. J Vet Intern Med. (2018) 32(1):274–9. doi: 10.1111/jvim.15032
138. Gülersoy E, Maden M, Parlak TM, Sayin Z. Comparative evaluation of selected serum and urine biomarkers in cats with interstitial and bacterial cystitis. Vet Clin Pathol. (2023) 52(1):79–87. doi: 10.1111/vcp.13174
139. Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. (2009) 29(2):247–64. doi: 10.1016/j.iac.2009.02.002
140. Broom DM. Behaviour and welfare in relation to pathology. Appl Anim Behav Sci. (2006) 97(1):73–83. doi: 10.1016/j.applanim.2005.11.019
141. Stella JL, Croney CC, Buffington CT. Behavior and welfare of domestic cats housed in cages larger than US norm. J Appl Anim Welf Sci. (2017) 20:296–312. doi: 10.1080/10888705.2017.1317252
142. Stella J, Croney C, Buffington T. Environmental factors that affect the behavior and welfare of domestic cats (Felis silvestris catus) housed in cages. Appl Anim Behav Sci. (2014) 160:94–105. doi: 10.1016/j.applanim.2014.08.006
143. Chew DJ, Bartges JW, Adams LG, Kruger JM, Buffington CAT. Randomized, placebo-controlled clinical trial of pentosan polysulfate sodium for treatment of feline interstitial (idiopathic) cystitis. In: 2009 ACVIM Forum. Montreal, Quebec: JVIM (2009). p. 690.
144. Delille M, Fröhlich L, Müller RS, Hartmann K, Dorsch R. Efficacy of intravesical pentosan polysulfate sodium in cats with obstructive feline idiopathic cystitis. J Feline Med Surg. (2016) 18(6):492–500. doi: 10.1177/1098612X15588934
145. Bradley AM, Lappin MR. Intravesical glycosaminoglycans for obstructive feline idiopathic cystitis: a pilot study. J Feline Med Surg. (2014) 16(6):504–6. doi: 10.1177/1098612X13510918
146. Stella JL, Buffington CAT. Individual and environmental effects on health and welfare. In: Turner DC, Bateson P, editors. The Domestic Cat-the Biology of Its Behavior. Cambridge: Cambridge University Press (2014). p. 186–200.
147. Amat M, Camps T, Manteca X. Stress in owned cats: behavioural changes and welfare implications. J Feline Med Surg. (2016) 18(8):577–86. doi: 10.1177/1098612X15590867
148. Vinke C, Godijn L, Van der Leij W. Will a hiding box provide stress reduction for shelter cats? Appl Anim Behav Sci. (2014) 160:86–93. doi: 10.1016/j.applanim.2014.09.002
149. Finka LR, Ward J, Farnworth MJ, Mills DS. Owner personality and the wellbeing of their cats share parallels with the parent-child relationship. PLoS One. (2019) 14(2):e0211862. doi: 10.1371/journal.pone.0211862
150. Buffington CAT. Multimodal environmental modification (MEMO) for prevention and treatment of disease in cats: part 1. Feline Focus. (2015) 1(8):275–80.
151. Buffington CAT. Multimodal environmental modification (MEMO) for prevention and treatment of disease in cats: part 2. Feline Focus. (2015) 1(9):311–7.
152. Piyarungsri K, Tangtrongsup S, Thitaram N, Lekklar P, Kittinuntasilp A. Prevalence and risk factors of feline lower urinary tract disease in Chiang Mai, Thailand. Sci Rep. (2020) 10(1):196. doi: 10.1038/s41598-019-56968-w
153. Lew-Kojrys S, Mikulska-Skupien E, Snarska A, Krystkiewicz W. Evaluation of clinical signs and causes of lower urinary tract disease in Polish cats. Vet Med. (2017) 62(7):386–93. doi: 10.17221/170/2016-VETMED
154. Saevik BK, Trangerud C, Ottesen N, Sorum H, Eggertsdottir AV. Causes of lower urinary tract disease in Norwegian cats. J Feline Med Surg. (2011) 13(6):410–7. doi: 10.1016/j.jfms.2010.12.012
155. Kim Y, Kim H, Pfeiffer D, Brodbelt D. Epidemiological study of feline idiopathic cystitis in Seoul, South Korea. J Feline Med Surg. (2018) 20(10):913–21. doi: 10.1177/1098612X17734067
156. Defauw PA, Van de Maele I, Duchateau L, Polis IE, Saunders JH, Daminet S. Risk factors and clinical presentation of cats with feline idiopathic cystitis. J Feline Med Surg. (2011) 13(12):967–75. doi: 10.1016/j.jfms.2011.08.001
157. Jones BR, Sanson RL, Morris RS. Elucidating the risk factors of feline urologic syndrome. N Z Vet J. (1997) 45:100–8. doi: 10.1080/00480169.1997.36003
158. Longstaff L, Gruffydd-Jones TJ, Buffington CT, Casey RA, Murray JK. Owner-reported lower urinary tract signs in a cohort of young cats. J Feline Med Surg. (2017) 19(6):609–18. doi: 10.1177/1098612X16643123
159. Morgan KN, Tromborg CT. Sources of stress in captivity. Appl Anim Behav Sci. (2007) 102(3-4):262–302. doi: 10.1016/j.applanim.2006.05.032
160. Ellis SL, Rodan I, Carney HC, Heath S, Rochlitz I, Shearburn LD, et al. AAFP and ISFM feline environmental needs guidelines. J Feline Med Surg. (2013) 15(3):219–30. doi: 10.1177/1098612X13477537
161. Ruggieri M, Monson F, Levin R, Wein A, Hanno P. Interstitial cystitis: animal models. In: Hanno PM, Staskin DR, Krane RJ, Wein AJ, editors. Interstitial Cystitis. London: Springer (1990). p. 49–62. doi: 10.1007/978-1-4471-3293-6_5
162. Veranic P, Jezernik K. Succession of events in desquamation of superficial urothelial cells as a response to stress induced by prolonged constant illumination. Tissue Cell. (2001) 33(3):280–5. doi: 10.1054/tice.2001.0175
163. Spanos C, Pang XZ, Ligris K, Letourneau R, Alferes L, Alexacos N, et al. Stress-induced bladder mast cell activation: implications for interstitial cystitis. J Urol. (1997) 157(2):669–72. doi: 10.1016/S0022-5347(01)65247-9
164. Duque-Wilckens N, Teis R, Sarno E, Stoelting F, Khalid S, Dairi Z, et al. Early life adversity drives sex-specific anhedonia and meningeal immune gene expression through mast cell activation. Brain Behav Immun. (2022) 103:73–84. doi: 10.1016/j.bbi.2022.03.009
165. Mackey E, Moeser AJ. Sex differences in mast cell–associated disorders: a life span perspective. Cold Spring Harb Perspect Biol (2022) 14(10):a039172. doi: 10.1101/cshperspect.a039172
166. Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. (2005) 289(1):G42–53. doi: 10.1152/ajpgi.00500.2004
167. Saito T, Hitchens TK, Foley LM, Singh N, Mizoguchi S, Kurobe M, et al. Functional and histologic imaging of urinary bladder wall after exposure to psychological stress and protamine sulfate. Sci Rep. (2021) 11(1):1–9. doi: 10.1038/s41598-021-98504-9
168. Wang Z, Chang HH, Gao Y, Zhang R, Guo Y, Holschneider DP, et al. Effects of water avoidance stress on peripheral and central responses during bladder filling in the rat: a multidisciplinary approach to the study of urologic chronic pelvic pain syndrome (MAPP) research network study. PLoS One. (2017) 12(9):e0182976. doi: 10.1371/journal.pone.0182976
169. Toyoda A. Social defeat models in animal science: what we have learned from rodent models. An Sci J. (2017) 88(7):944–52. doi: 10.1111/asj.12809
170. West EG, Sellers DJ, Chess-Williams R, McDermott C. Voiding behavior and efferent bladder function altered in mice following social defeat but not witness trauma. Front Physiol. (2020) 11:247. doi: 10.3389/fphys.2020.00247
171. Radley JJ, Herman JP. Preclinical models of chronic stress: adaptation or pathology? Biol Psychiatry. (2023) 94(3):194–202. doi: 10.1016/j.biopsych.2022.11.004
172. Harris AZ, Atsak P, Bretton ZH, Holt ES, Alam R, Morton MP, et al. A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology. (2018) 43(6):1276–83. doi: 10.1038/npp.2017.259
173. Zhai X, Ai L, Chen D, Zhou D, Han Y, Ji R, et al. Multiple integrated social stress induces depressive-like behavioral and neural adaptations in female C57BL/6J mice. Neurobiol Dis. (2024) 190:106374. doi: 10.1016/j.nbd.2023.106374
174. van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. (2000) 1(3):191–8. doi: 10.1038/35044558
175. Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. (2006) 7(9):697–709. doi: 10.1038/nrn1970
176. Sparling JE, Barbeau K, Boileau K, Konkle AT. Environmental enrichment and its influence on rodent offspring and maternal behaviours, a scoping style review of indices of depression and anxiety. Pharmacol Biochem Behav. (2020) 197:172997. doi: 10.1016/j.pbb.2020.172997
177. Ranaldi R, Hannan AJ, Grimm J. Influence of exercise and other forms of environmental enrichment on drug effects, brain function and behavioral change (2022). Available online at: https://www.sciencedirect.com/journal/pharmacology-biochemistry-and-behavior/special-issue/10090ZZKPVL (Accessed January 4, 2024).
178. Kull K. On the logic of animal umwelten: the animal subjective present and zoosemiotics of choice and learning. In: Marrone G, Mangano D, editors. Semiotics of Animals in Culture: Zoosemiotics 2.0. Cham, CH: Springer (2018). p. 135–48. doi: 10.1007/978-3-319-72992-3_10
179. Pierce AN, Eller-Smith OC, Christianson JA. Voluntary wheel running attenuates urinary bladder hypersensitivity and dysfunction following neonatal maternal separation in female mice. Neurourol Urodyn. (2018) 37(5):1623–32. doi: 10.1002/nau.23530
180. Sanford MT, Yeh JC, Mao JJ, Guo Y, Wang Z, Zhang R, et al. Voluntary exercise improves voiding function and bladder hyperalgesia in an animal model of stress-induced visceral hypersensitivity: a multidisciplinary approach to the study of urologic chronic pelvic pain syndrome research network study. Neurourol Urodyn. (2020) 39(2):603–12. doi: 10.1002/nau.24270
181. Holschneider DP, Wang Z, Guo Y, Sanford MT, Yeh J, Mao JJ, et al. Exercise modulates neuronal activation in the micturition circuit of chronically stressed rats: a multidisciplinary approach to the study of urologic chronic pelvic pain syndrome (MAPP) research network study. Physiol Behav. (2020) 215:112796. doi: 10.1016/j.physbeh.2019.112796
182. Fuentes IM, Jones BM, Brake AD, Pierce AN, Eller OC, Supple RM, et al. Voluntary wheel running improves outcomes in an early life stress–induced model of urologic chronic pelvic pain syndrome in male mice. Pain. (2021) 162(6):1681–91. doi: 10.1097/j.pain.0000000000002178
183. Ji N-N, Jiang H, Xia M. The influence of the enriched environment in different periods on neonatal maternal separation-induced visceral pain, anxiousness, and depressive behaviors. Transl Pediatr. (2022) 11(9):1562–9. doi: 10.21037/tp-22-475
184. Ji N-N, Jiang H, Xia M. Sex-dependent effects of postweaning exposure to an enriched environment on visceral pain and anxiety-and depression-like behaviors induced by neonatal maternal separation. Transl Pediatr. (2022) 11(9):1570–6. doi: 10.21037/tp-22-476
185. Pavlova IV, Broshevitskaya ND, Zaichenko MI, Grigoryan GA. The influence of long-term housing in enriched environment on behavior of normal rats and subjected to neonatal pro-inflammatory challenge. Brain Behav Immun Health. (2023) 30:100639. doi: 10.1016/j.bbih.2023.100639
186. Orock A, Johnson A, Mohammadi E, Greenwood-Van Meerveld B. Environmental enrichment reverses stress-induced changes in the brain-gut axis to ameliorate chronic visceral and somatic hypersensitivity. Neurobiol Stress. (2024) 28:100590. doi: 10.1016/j.ynstr.2023.100590
187. Grigoryan GA. The systemic effects of the enriched environment on the conditioned fear reaction. Front Behav Neurosci. (2023) 17:1–13. doi: 10.3389/fnbeh.2023.1227575
188. Pan Y, Lin X, Liu J, Zhang S, Zeng X, Chen F, et al. Prevalence of childhood sexual abuse among women using the childhood trauma questionnaire: a worldwide meta-analysis. Trauma Violence Abuse. (2021) 22(5):1181–91. doi: 10.1177/1524838020912867
189. Selai C, Elmalem MS, Chartier-Kastler E, Sassoon N, Hewitt S, Rocha MF, et al. Systematic review exploring the relationship between sexual abuse and lower urinary tract symptoms. Int Urogynecol J. (2023) 34(3):635–53. doi: 10.1007/s00192-022-05277-4
190. Nutter FB, Levine JF, Stoskopf MK. Reproductive capacity of free-roaming domestic cats and kitten survival rate. J Am Vet Med Assoc. (2004) 225(9):1399–402. doi: 10.2460/javma.2004.225.1399
191. Greenwood-Van Meerveld B, Prusator DK, Johnson AC. Animal models of gastrointestinal and liver diseases. Animal models of visceral pain: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. (2015) 308(11):G885–903. doi: 10.1152/ajpgi.00463.2014
192. Louwies T. Epigenetic regulation of stress-induced visceral pain. In: Brierley SM, Spencer NJ, editors. Visceral Pain. Cham. CH: Springer (2023). p. 71-80. doi: 10.1007/978-3-031-25702-5_6
193. Kamijo TC, Miyazato M. The influence of maternal separation on the development of voiding and behavior in rat pups. Continence. (2023) 5:100570. doi: 10.1016/j.cont.2022.100570
194. Levasseur BM, Young EE, Christianson JA. Evidence of early life stress exposure and epigenetic modifications in functional chronic pain disorders. In: Brierley SM, Spencer NJ, editors. Visceral Pain. Cham, CH: Springer (2023). p. 55–69. doi: 10.1007/978-3-031-25702-5_5
195. Yuan M, Yang B, Rothschild G, Mann JJ, Sanford LD, Tang X, et al. Epigenetic regulation in major depression and other stress-related disorders: molecular mechanisms, clinical relevance and therapeutic potential. Signal Transduct Target Ther. (2023) 8(1):1–30. doi: 10.1038/s41392-023-01519-z
196. Kuhlman KR. Pitfalls and potential: translating the two-hit model of early life stress from pre-clinical non-human experiments to human samples. Brain Behav Immun Health. (2024) 35:100711. doi: 10.1016/j.bbih.2023.100711
197. Wygant JN, McGuire LJ, Bush NM, Burnett TL, Green IC, Breitkopf DM. What makes a chronic pelvic pain patient satisfied? J Psychosom Obstet Gynecol. (2019) 40(3):239–42. doi: 10.1080/0167482X.2018.1476486
198. Comen E. All in Her Head—the Truth and Lies Early Medicine Taught us About Women’s Bodies and Why it Matters Today. New York, NY: Harper Wave (2024).
199. Brown VL, James A, Hunleth J, Bradley CS, Farrar JT, Gupta P, et al. Believing women: a qualitative exploration of provider disbelief and pain dismissal among women with interstitial cystitis/bladder pain syndrome from the MAPP research network. Int Urogynecol J. (2024) 35(1):139–48. doi: 10.1007/s00192-023-05677-0
200. Lamvu G, Carrillo J, Ouyang C, Rapkin A. Chronic pelvic pain in women: a review. JAMA. (2021) 325(23):2381–91. doi: 10.1001/jama.2021.2631
201. Ashton-James CE, Anderson SR, Mackey SC, Darnall BD. Beyond pain, distress, and disability: the importance of social outcomes in pain management research and practice. Pain. (2022) 163(3):e426–31. doi: 10.1097/j.pain.0000000000002404
202. Ross WT, Snyder B, Stuckey H, Ross IR, McCall-Hosenfeld J, Harkins GJ, et al. Gynaecological care of women with chronic pelvic pain: patient perspectives and care preferences. BJOG. (2023) 130(5):476–84. doi: 10.1111/1471-0528.17355
203. Fernández-Peña R, Molina JL, Valero O. Satisfaction with social support received from social relationships in cases of chronic pain: the influence of personal network characteristics in terms of structure, composition and functional content. Int J Environ Res Public Health. (2020) 17(8):2706. doi: 10.3390/ijerph17082706
204. McKernan LC, Bonnet KR, Finn MT, Williams DA, Bruehl S, Reynolds WS, et al. Qualitative analysis of treatment needs in interstitial cystitis/bladder pain syndrome: implications for intervention. Can J Pain. (2020) 4(1):181–98. doi: 10.1080/24740527.2020.1785854
205. Kanter G, Volpe KA, Dunivan GC, Cichowski SB, Jeppson PC, Rogers RG, et al. Important role of physicians in addressing psychological aspects of interstitial cystitis/bladder pain syndrome (IC/BPS): a qualitative analysis. Int Urogynecol J. (2017) 28(2):249–56. doi: 10.1007/s00192-016-3109-2
206. Zulman DM, Haverfield MC, Shaw JG, Brown-Johnson CG, Schwartz R, Tierney AA, et al. Practices to foster physician presence and connection with patients in the clinical encounter. JAMA. (2020) 323(1):70–81. doi: 10.1001/jama.2019.19003
207. de C Williams AC, Fisher E, Hearn L, Eccleston C. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. (2020) 8:1–6. doi: 10.1002/14651858.CD007407.pub4
208. Schrepf A, Gallop R, Naliboff B, Harte SE, Afari N, Lai HH, et al. Clinical phenotyping for pain mechanisms in urologic chronic pelvic pain syndromes: a MAPP research network study. J Pain. (2022) 23:1594–603. doi: 10.1016/j.jpain.2022.03.240
209. Horne AW, Vincent K, Hewitt CA, Middleton LJ, Koscielniak M, Szubert W, et al. Gabapentin for chronic pelvic pain in women (GaPP2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. (2020) 396(10255):909–17. doi: 10.1016/S0140-6736(20)31693-7
210. Hostutler RA, Chew DJ, DiBartola SP. Recent concepts in feline lower urinary tract disease. Vet Clin North Am Small Anim Pract. (2005) 35(1):147–1170. doi: 10.1016/j.cvsm.2004.08.006
211. Buffington T, Delgado MM. Pandora syndrome (feline interstitia cystitis). In: Ettinger SJ, Feldman EC, Cote E, editors. Ettinger’s Textbook of Veterinary Internal Medicine. Philadelphia: Elsevier (2024). p. 2171–8.
212. Mostafaei H, Jilch S, Carlin GL, Mori K, Quhal F, Pradere B, et al. The placebo and nocebo effects in functional urology. Nat Rev Urol. (2021) 19(3):171–89. doi: 10.1038/s41585-021-00545-2
213. Gebke KB, McCarberg B, Shaw E, Turk DC, Wright WL, Semel D. A practical guide to recognize, assess, treat and evaluate (RATE) primary care patients with chronic pain. Postgrad Med. (2023) 135(3):244–53. doi: 10.1080/00325481.2021.2017201
214. Darnall BD, Edwards KA, Courtney RE, Ziadni MS, Simons LE, Harrison LE. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res. (2023) 4:1–11. doi: 10.3389/fpain.2023.1223172
215. Baumgartner JN, Haupt MR, Case LK. Chronic pain patients low in social connectedness report higher pain and need deeper pressure for pain relief. Emotion. (2023) 23(8):2156–68. doi: 10.1037/emo0001228
216. Haslam C, Jetten J, Cruwys T, Dingle GA, Haslam SA. The New Psychology of Health: Unlocking the Social Cure. London: Routledge (2018).
217. Haslam C, Bertschy K, Cruwys T, Griffin J, Johnson D. The group mechanism in treatment: group identification and cohesion contributes to reducing chronic lower back pain by increasing personal control. Disabil Rehabil. (2023) 45(8):1332–42. doi: 10.1080/09638288.2022.2057602
218. Cruwys T, Haslam C, Haslam SA, Rathbone JA, Donaldson JL. Acceptability and feasibility of an intervention to enhance social group belonging: evidence from three trials of groups 4 health. Behav Ther. (2022) 53(6):1233–49. doi: 10.1016/j.beth.2022.06.011
219. Lumley MA, Schubiner H. Emotional awareness and expression therapy for chronic pain: rationale, principles and techniques, evidence, and critical review. Curr Rheumatol Rep (2019) 21:1–8. doi: 10.1007/s11926-019-0829-6
220. Ashar YK, Lumley MA, Perlis RH, Liston C, Gunning FM, Wager TD. Reattribution to mind-brain processes and recovery from chronic back pain: a secondary analysis of a randomized clinical trial. JAMA Network Open. (2023) 6(9):e2333846. doi: 10.1001/jamanetworkopen.2023.33846
221. Ashar YK, Gordon A, Schubiner H, Uipi C, Knight K, Anderson Z, et al. Effect of pain reprocessing therapy vs placebo and usual care for patients with chronic back pain: a randomized clinical trial. JAMA psychiatry. (2022) 79(1):13–23. doi: 10.1001/jamapsychiatry.2021.2669
222. Trevlaki E, Trevlakis E, Iakovidis P, Notopoulos P, Papadopoulou O, Hristara-Papadopoulou A. The effects of cognitive functional therapy in chronic pain management: a systematic review. Int Res J Pub Environ Health. (2023) 3(10):88–97. doi: 10.15739/irjpeh.23.010
223. Carty JN, Ziadni MS, Holmes HJ, Tomakowsky J, Peters K, Schubiner H, et al. The effects of a life stress emotional awareness and expression interview for women with chronic urogenital pain: a randomized controlled trial. Pain Med. (2019) 20(7):1321–9. doi: 10.1093/pm/pny182
224. Quigley BM, Naliboff B, Gudleski GG, Braun A, Jaccard J, Mayer EA, et al. Effects of patient education and cognitive behavior therapy for irritable bowel syndrome on overlapping pain conditions of non-gastrointestinal origin. IASP World Congress on Pain; Boston, MA (2018).
225. Cole SW. The conserved transcriptional response to adversity. Curr Opin Behav Sci. (2019) 28:31–7. doi: 10.1016/j.cobeha.2019.01.008
226. Schrepf A, Kaplan C, Harris RE, Williams DA, Clauw DJ, As-Sanie S, et al. Stimulated whole blood cytokine/chemokine responses are associated with interstitial cystitis/bladder pain syndrome phenotypes and features of nociplastic pain: a MAPP research network study. Pain. (2023) 164(5):1148–57. doi: 10.1097/j.pain.0000000000002813
227. Dudarev V, Barral O, Radaeva M, Davis G, Enns JT. Night time heart rate predicts next-day pain in fibromyalgia and primary back pain. PAIN Rep. (2024) 9(2):e1119. doi: 10.1097/PR9.0000000000001119
228. Tracy LM, Ioannou L, Baker KS, Gibson SJ, Georgiou-Karistianis N, Giummarra MJ. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain. (2016) 157(1):7–29. doi: 10.1097/j.pain.0000000000000360
229. Bandeira PM, Reis FJ, Sequeira VC, Chaves AC, Fernandes O, Arruda-Sanchez T. Heart rate variability in patients with low back pain: a systematic review. Scand J Pain. (2021) 21(3):426–33. doi: 10.1515/sjpain-2021-0006
Keywords: urinary bladder, chronic primary pain, chronic pelvic pain, bladder pain syndrome, biopsychosocial model, one health, cats, animal models
Citation: Westropp JL, Stella JL and Buffington CAT (2024) Interstitial cystitis—an imbalance of risk and protective factors?. Front. Pain Res. 5:1405488. doi: 10.3389/fpain.2024.1405488
Received: 22 March 2024; Accepted: 15 April 2024;
Published: 9 May 2024.
Edited by:
Kevin M. Hellman, NorthShore University HealthSystem, United StatesReviewed by:
Michel Pontari, Temple University, United StatesMargaret A. Vizzard, University of Vermont, United States
© 2024 Westropp, Stella and Buffington. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: C. A. Tony Buffington, drbuffington@ucdavis.edu
†These authors have contributed equally to this work and share first authorship
 Jodi L. Westropp1,†
Jodi L. Westropp1,†  Judith L. Stella
Judith L. Stella C. A. Tony Buffington
C. A. Tony Buffington