- 1Department of Breast Surgery, The Third Affiliated Hospital of Beijing University of Chinese Medicine, Beijing, China
- 2Department of Breast Surgery, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China
- 3Department of Breast Surgery, The Xiamen Hospital of Beijing University of Chinese Medicine, Xiamen, China
Background: Breast cancer persists as a major public health issue on a global scale. Lymphangiogenesis is an indispensable element in the promotion of breast cancer metastasis. Inhibiting the metastasis of breast cancer can be accomplished through targeting lymphangiogenesis. The purpose of this study was to examine research trends, major topics, and development directions of lymphangiogenesis in breast cancer through a bibliometric analysis, which may serve as a reference for future research and clinical practice.
Methods: English publications with article type article or review about lymphangiogenesis in breast cancer from inception to September 30, 2023, retrieved from the Web of Science Core Collection Database (WOSCC), and VOSviewer, CiteSpace, and Microsoft Excel were applied for bibliometric study.
Results: In this paper, a total of 369 articles and reviews were included. The 369 papers were written by 2120 authors from 553 organizations across 42 countries, published in 199 journals, and cited 12458 references from 1801 journals up to September 30, 2023. Moreover, the annual publications had a rising trajectory between 2004 to 2014 but declined from 2015. The US was the leading nation in publications and citations. Meanwhile, academics Mousumi Majumder and Peeyush Lala had the highest cumulative number of publications. Based on the number of publications/citations, Cancer Research was the most influential journal. The most cited paper was “Lymphangiogenesis: Molecular Mechanisms and Future Promise” by Tuomas Tammela, published in the Journal of Cell. Additionally, keywords frequency analysis demonstrated that “lymphangiogenesis,” “breast cancer,” “VEGF-C,” “angiogenesis,” and “metastasis” were the most frequent keywords, and the newly emergent topics could be represented by “tumor microenvironment,” “metastasis,” “stem-cell,” “triple-negative breast cancer,” and “blood vessels.”
Conclusions: Currently, there is a strong research basis for lymphangiogenesis in breast cancer. The core research team was primarily situated in the US. Investigating the mechanism of lymphangiogenesis in breast cancer will always remain a highly discussed topic. In particular, it was essential to emphasize the relationship between lymphangiogenesis and tumor microenvironment, stem cells, triple-negative breast cancer, and metastasis, which could be the frontiers.
1 Introduction
Breast cancer is the most frequently diagnosed cancer, the predominant cause of mortality in women, and has the highest incidence of malignant tumors. The percentage of breast cancer patients who may develop metastases post-diagnosis and primary tumor treatment is estimated to be between 20-30%, and around 90% of cancer-related deaths are attributed to metastasis (1). The 5-year overall survival (OS) rate of breast cancer patients without metastasis is greater than 80% (2). Nevertheless, distant metastasis can cause a remarkable decline in the rate, resulting in only about 25% (3). Therefore, systematic and deepened research on the molecular heterogeneity of metastatic breast cancer, which is a potential cause of a significant number of therapeutic failures, would afford the ability to explore more effective metastasis-targeting agents and improve patient prognosis. Blood vessels and lymphatic vessels are the main routes of tumor metastasis. However, in many solid tumors, the lymphatic system is the major escape route for cancer cells. Lymph nodes in the drainage area are usually the initial site of metastasis, especially for breast cancer.
Due to the rich mammary lymphatic network, cytokines such as chemokines, vascular endothelial growth factors (VEGF), and other immune factors, breast cancer cells tend to metastasize to the lymph nodes located in the drainage area. These factors not only cause tumor lymphangiogenesis but also facilitate the transfer of cancer cells to certain organs through the lymphatic system (4, 5). Lymphangiogenesis is deemed to be a vital step for cancer cells to metastasize through lymphatic vessels (6, 7). Research on cancer animal models and human tumor clinicopathological analysis also highlights that increased intratumoral and peritumoral lymphatic vessels are directly associated with cancer metastasis, and lymphangiogenesis is related to the poor clinical prognosis of breast cancer (5, 7). Recently, although anti-tumor angiogenesis drugs such as bevacizumab have had limited success in improving the OS of patients in clinical trials (8), this has prompted researchers to recognize that only targeting tumor angiogenesis cannot effectively impede breast cancer metastasis; rather, targeting lymphatic vessels and lymphangiogenesis may be a successful strategy to contain metastasis.
With the proliferation of literature, it is a challenge for researchers to accurately and promptly capture the research topics and trends in the academic realm without any prior knowledge. A quantitative and qualitative method of analysis of literature, bibliometrics utilizes statistical analysis methods to assess the structure, quantitative relationships, and changing patterns of relevant data in the literature. It can illustrate the current research progress, hotspots, and trends in a particular field from multiple perspectives, as well as indicate future development directions (9). Hence, it is imperative to acquire data and information from various pertinent publications on lymphangiogenesis in breast cancer in recent years in order to assist researchers in finding valuable and referential literature information on the topic. However, we have not found any bibliometric research in this field. Therefore, we executed this research to (a) help clinicians and researchers distinguish outstanding authors, countries, and journals with academic activity in this field, (b) summarize and evaluate the research achievements and trends, and (c) generate an effective reference for further research in the field of lymphangiogenesis in breast cancer.
2 Materials and methods
2.1 Data sources and collection
The Web of Science Core Collection (WOSCC) (http://apps.webofknowledge.com) database is the most commonly used and widely accepted database in scientific, among which the Science Citation Index Expanded (SCI-E) is regarded as the most suitable database for bibliometric analysis (10). In this study, literature on lymphangiogenesis in breast cancer was systematically retrieved from the SCI-E of WOSCC. “Breast cancer” and “lymphangiogenesis” were used as the search terms in the Medical Subject Headings (MeSH) database (https://www.ncbi.nlm.nih.gov/mesh). Ultimately, the full search strategy for obtaining data is as follows. [TS=(Breast Neoplasm OR Neoplasm, Breast OR Breast Tumors OR Breast Tumor OR Tumor, Breast OR Tumors, Breast OR Neoplasms, Breast OR Breast Cancer OR Cancer, Breast OR Mammary Cancer OR Cancer, Mammary OR Cancers, Mammary OR Mammary Cancers OR Malignant Neoplasm of Breast OR Breast Malignant Neoplasm OR Breast Malignant Neoplasms OR Malignant Tumor of Breast OR Breast Malignant Tumor OR Breast Malignant Tumors OR Cancer of Breast OR Cancer of the Breast OR Mammary Carcinoma, Human OR Carcinoma, Human Mammary OR Carcinomas, Human Mammary OR Human Mammary Carcinomas OR Mammary Carcinomas, Human OR Human Mammary Carcinoma OR Mammary Neoplasms, Human OR Human Mammary Neoplasm OR Human Mammary Neoplasms OR Neoplasm, Human Mammary OR Neoplasms, Human Mammary OR Mammary Neoplasm, Human OR Breast Carcinoma OR Breast Carcinomas OR Carcinoma, Breast OR Carcinomas, Breast)] AND TS=(Lymphangiogenesis OR Lymphangiogeneses), and set the time span from inception to September 30st 2023, the article type as “Article” or “Review”, and the language as “English”. The final retrieval execution time was October 10, 2023. Two researchers independently conducted the retrieval. During the retrieval process, disagreements were resolved by consulting a third colleague or the entire academic team. There were 1201 documents generated in this field. Following that, a manual review was conducted to examine the contents of each article to eliminate irrelevant publications that were not relevant to this study. Eventually, after excluding 823 articles that deviate significantly from the main topic, 369 articles were ultimately included, including 289 reviews and 70 articles. The 369 papers used in this study were from 2120 authors from 553 organizations in 42 countries, published in 199 journals, and cited 12458 references from 1801 journals up to September 30st 2023. All data used in this work were downloaded from a public database; therefore, no ethics approval or informed consent was required. The above processes were carried out under the guidance of breast cancer experts and bibliometrics experts.
2.2 Data analysis
We obtained a complete record of all the literature that satisfied these inclusion criteria from the WOSCC, including the titles, abstracts, countries, institutions, journals, authors, cited references, and keywords, all of which were downloaded as a txt file and imported into VOSviewer R1.6.19 and CiteSpace R6.2.2. After using VOSviewer and CiteSpace to analyze these documents, 369 articles were subjected to visualization analysis. VOSviewer was applied to analyze the targeted files and exported the top-cited or productive countries/regions, authors, journals, references, keywords. Microsoft Excel 2019 (https://www.microsoft.com/, accessed November 9, 2021). was applied to create line charts and tables. At the same time, the 2022 version of Impact factor (IF), Journal Citation Reports (JCR) quartile and H-index as important indicators to measure the scientific value of research, were also included in the analysis. Impact factor (IF) is a method of quantitatively evaluating the importance of absolute or total citation ratio. Journal Citation Reports (JCR) 2022 was used to obtain the impact factor (IF) and quartile of a journal category (11)(https://jcr.clarivate.com/jcr/browse-journals). The H-index, which indicates that an academic journal or scholar/country/region has published H papers, each of which was cited at least H times, was used to evaluate the scientific impact of an author or country (12)(https://www.scimagojr.com/journalrank.php).
Bradford’s law of concentrations was applied to the journals, fragmented into thirds of articles, avoiding the exponential decrease in decreasing performance by expanding the search of references in scientific journals peripheral to the topic under study (13). Bradford’s law proposed that each type of sci-tech periodical could be placed under a certain discipline classification. If the sci-tech journal is arranged in descending order based on the number of scholarly papers published in a particular specialty, then it can be categorized into core area, related area, and non-related area. The number of articles in every district is roughly equal, and the number of journals in the core area, related area, and non-related area becomes the relationship of 1: : during a certain period (14). Lotka’s law states that an author who publishes papers is one of an author who publishes 1 paper, and that about 60.79% of authors publish 1 paper in a field, unveils the relationship between the author and the quantity of papers published and outlines the frequency distribution pattern of scientific productivity (15). Price’s Law is a measure of the distribution pattern of authors in various research fields of literature, states that for a research field, half of the papers are written by a group of productive authors whose number is equal to the square root of the total number of authors (16). In the formula, signifies the number of authors who have written papers, is the number of papers published by the most productive authors in the field (calculated through VOSviewer generated a value of 10), is the total number of authors (calculated by VOSviewer was 2120), and is the minimum number of papers published by the core authors.
2.3 Bibliometric analysis and visualization software
VOSviewer (https://www.vosviewer.com/, Version 1.6.19, accessed May 10, 2023) and CitesSpace (https://citespace.podia.com/ download,Version 6.2.2, accessed May 10, 2023) are bibliometric softwares and both pay special attention to the graphical representation of bibliometric maps. VOSviewer is a widely-used software in bibliometric analysis created by the Science and Technology Research Center of Leiden University (17). VOSviewer applies probabilistic-based data normalization methods to produce bibliometric maps in fields such as keywords, countries, and authors, with prominent features of easy mapping and beautiful images. In this study, VOSviewer and Microsoft Excel were applied to analyze and map the distribution of publications, countries, authors, journals, keywords and citations of the retrieved 369 articles, and constructed overlay map of keywords. CiteSpace is a free Java application that can visualize and analyze scientific literature trends and patterns. It was developed by Professor Chaomei Chen in 2004 and has become a research tool for studying research trends in a certain field by presenting the structure, patterns, and distribution of scientific knowledge and providing a comprehensive overview of research status. In this study, CiteSpace was used for extraction of the annual distribution information of articles and burst word analysis (18).
3 Results
3.1 Analysis of global publishing trends
This study employed CiteSpace to extract the yearly publication data of the articles included in this study, and Microsoft Excel 2019 for depicting the trend of annual publication. The publication year related to lymphangiogenesis in breast cancer spanned from 2004 until 2023. Figure 1 illustrates the distribution of publication numbers by year. The annual number of publications in the field was 18. Since 2004, the number of publications in this field has remained fairly consistent, peaking in 2012 (35). The turning point occurred in 2015. Since then, the number of papers published has decreased, potentially signifying that this research field has reached a bottleneck. It is thus critical that prospective research, scientific analyses, and forward direction be addressed promptly.
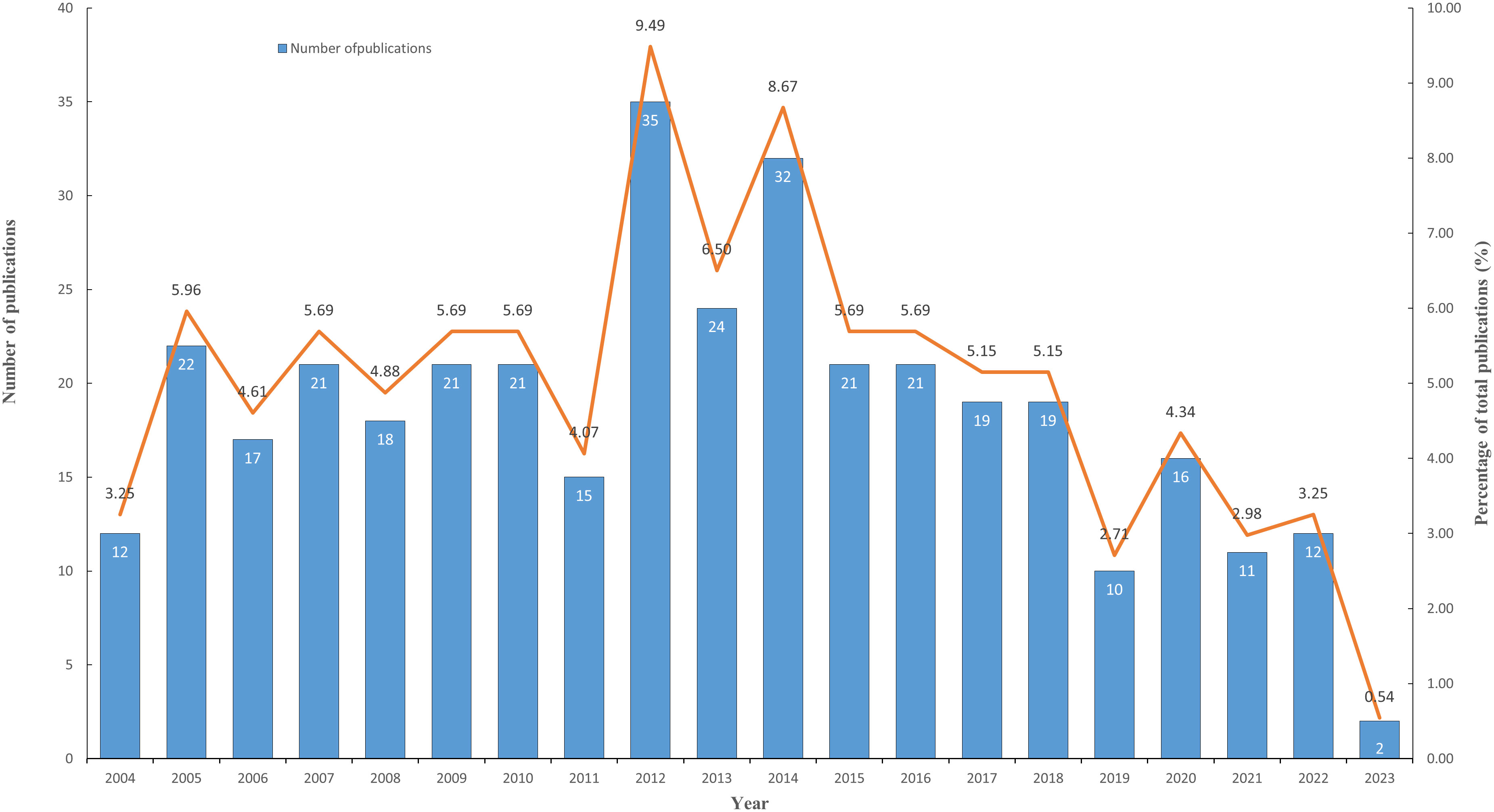
Figure 1 The changing trend of annual publications on breast cancer lymphangiogenesis from 2004 to 2023. (The blue column represents the annual publication number, while the orange line shows the percentage of publications’ development trend.).
3.2 Analysis of distribution and cooperation of leading countries/regions
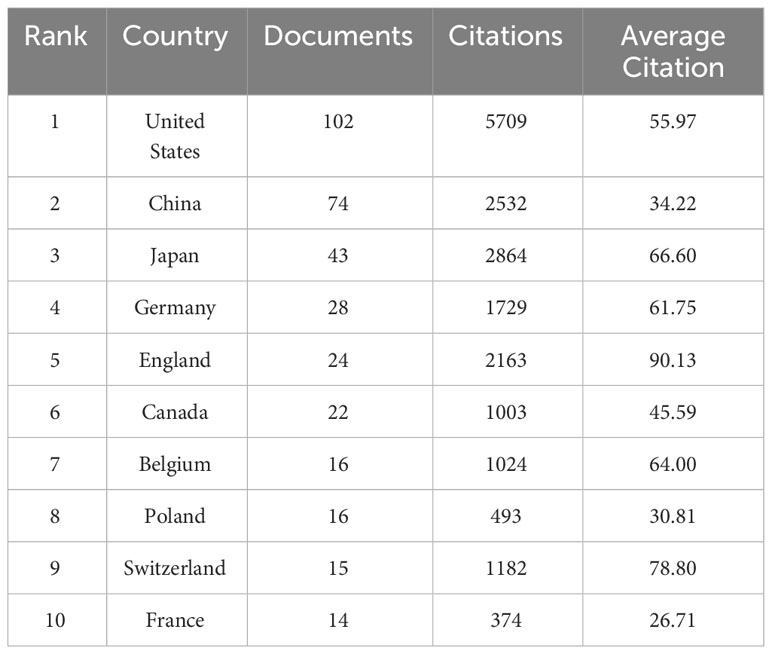
Countries/regions are platforms for researchers to conduct scientific research, providing them with financial, equipment, and human support as well as the rights to the findings. In order to elucidate the extent of involvement of several nations in research regarding lymphangiogenesis in breast cancer, this study carried out a bibliometric analysis of the 42 countries involved. Setting the minimum number of national publications at 14 through VOSviewer, we obtained the top 10 countries in terms of the number of publications (Table 1) and visualized the cooperation between them (Figure 2). This demonstrates an extremely unbalanced spread of publishing countries in this area and has a significant top effect. The vast majority of the studies have been authored by researchers from a few countries. The US had the largest number of publications (102, 27.64%), followed by China (74, 20.05%) and Japan (43, 11.65%). The top three cited countries were the US, Japan, and China. Additionally, the three countries with the highest average citation rate were the England, Switzerland, and Japan (Table 1). The results revealed that the stated countries were more predisposed to researching lymphangiogenesis in breast cancer.
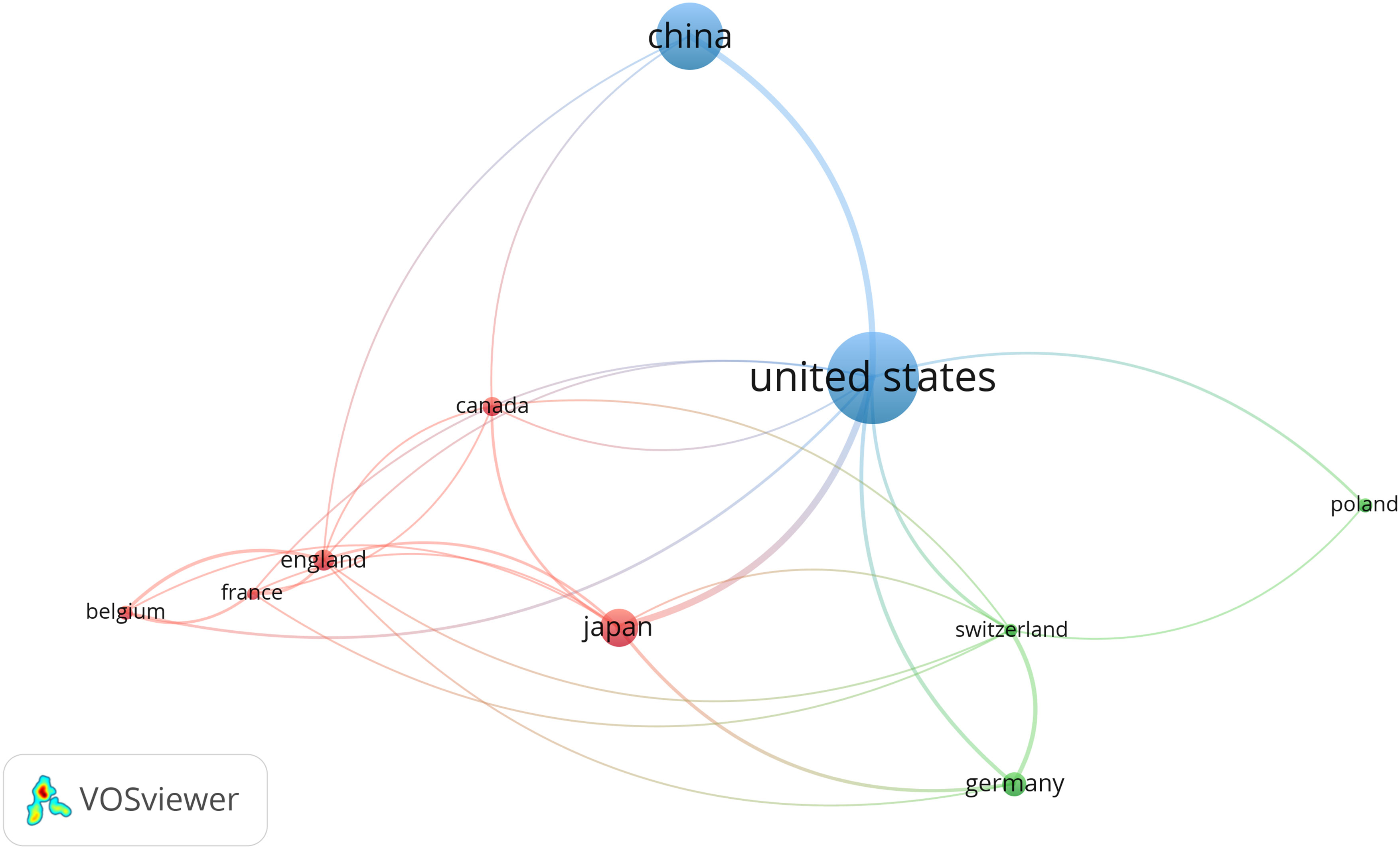
Figure 2 The co-occurrence of the top ten productive countries. (The node size represents the number of articles; the curve thickness indicates the strength of collaboration between countries; and the colors denote various collaboration groups.). From: VOSviewer.
This study used VOSviewer to analyze the cooperation of different countries. The line between nodes indicates the co-authorship between countries, the larger nodes suggested more postings, and the thicker lines between two nations suggested a stronger degree of cooperation. The findings underlined that the US had more cooperation with China and Japan. The US, with the largest amount of publications, has collaborated with all nations (Figure 2). The abovementioned results indicate that the US remains the leader in the research area of lymphangiogenesis in breast cancer while engaging in more interchange and collaboration in the field of lymphangiogenesis in breast cancer.
3.3 Analysis of the authors and co-cited authors
The authors’ scientific research activities in a certain subject area and the quantity and quality of their publications are instrumental in advancing the development of the discipline, to some extent. Looking at the works of the most influential authors in the present research field and examining their articles allows us to ascertain the current research trends. According to Price’s Law, the minimum number of articles published by the core authors in a specific field is =0.749× ≈2.36 is the number of the most productive author’s publication.) Core authors, with 3 or more publications, are hereby perceived as the most prolific authors in this field, resulting in a total of 255 papers written by 65 core authors, amounting to 69.1% (255/369) of the total number of papers. This falls in line with Price’s criterion that half of the papers should be created by a productive set of authors in a certain area. Substituting the above values into the formula computation is also largely consistent with Price’s Law.
It thus appears that a cohesive cohort of researchers has emerged in the field of lymphangiogenesis in breast cancer. Utilizing VOSviewer, we conducted an analysis of 2120 authors and determined the top five authors by setting the minimum number of publications at 7. Table 2 itemizes the top five with the highest number of publications and citations.

Table 2 Top 5 productive authors and co-cited authors in the field of lymphangiogenesis in breast cancer.
Mousumi Majumder and Peeyush Lala, the two most productive authors, collaborated on nine articles related to lymphangiogenesis in breast cancer (19–27). Following an assessment of the published studies, the two scientists persisted in their research on this topic from 2012 to 2021, with a focus on prostaglandins (PGs) and their role in this condition. They found that PG, particularly Prostaglandin E2 (PGE2), significantly influences breast cancer-associated lymphangiogenesis. Moreover, Cyclooxygenase (COX)-2 expression by breast cancer cells or tumor stroma leads to high PGE2 levels in the tumor milieu. PGE2 and prostaglandin E2 receptor EP4 (EP4) subtype combine on multiple cell types: tumor cells, tumor-infiltrating immune cells, and lymphatic endothelial cells (LECs), EP4 activation on cancer cells, and macrophages upregulated vascular endothelial growth factor c/d (VEGF-C/D) production. Their purpose is to stimulate the sprouting of LECs and promote lymphangiogenesis and lymphatic metastases. The ligation of EP4 with PGE2 on cancer or host cells can initiate a new cascade of molecular events resulting in the cross-talk between cancer cells and LECs, facilitating lymphangiogenesis and the lympho-vascular transport of cancer cells. Hence, EP4 can be considered a promising therapeutic target for lymphangiogenesis in breast cancer (19, 21, 22, 24, 26).
3.4 Analysis of leading journals
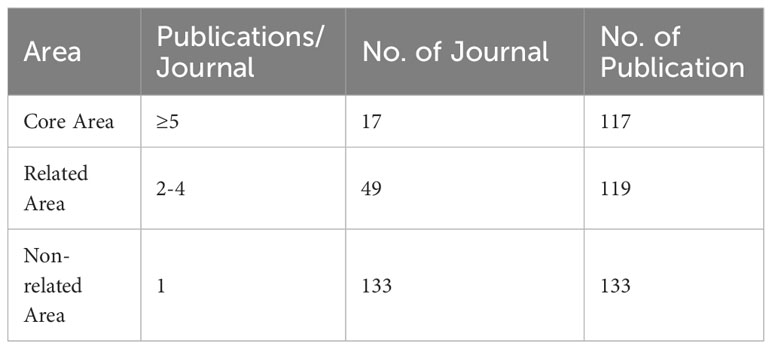
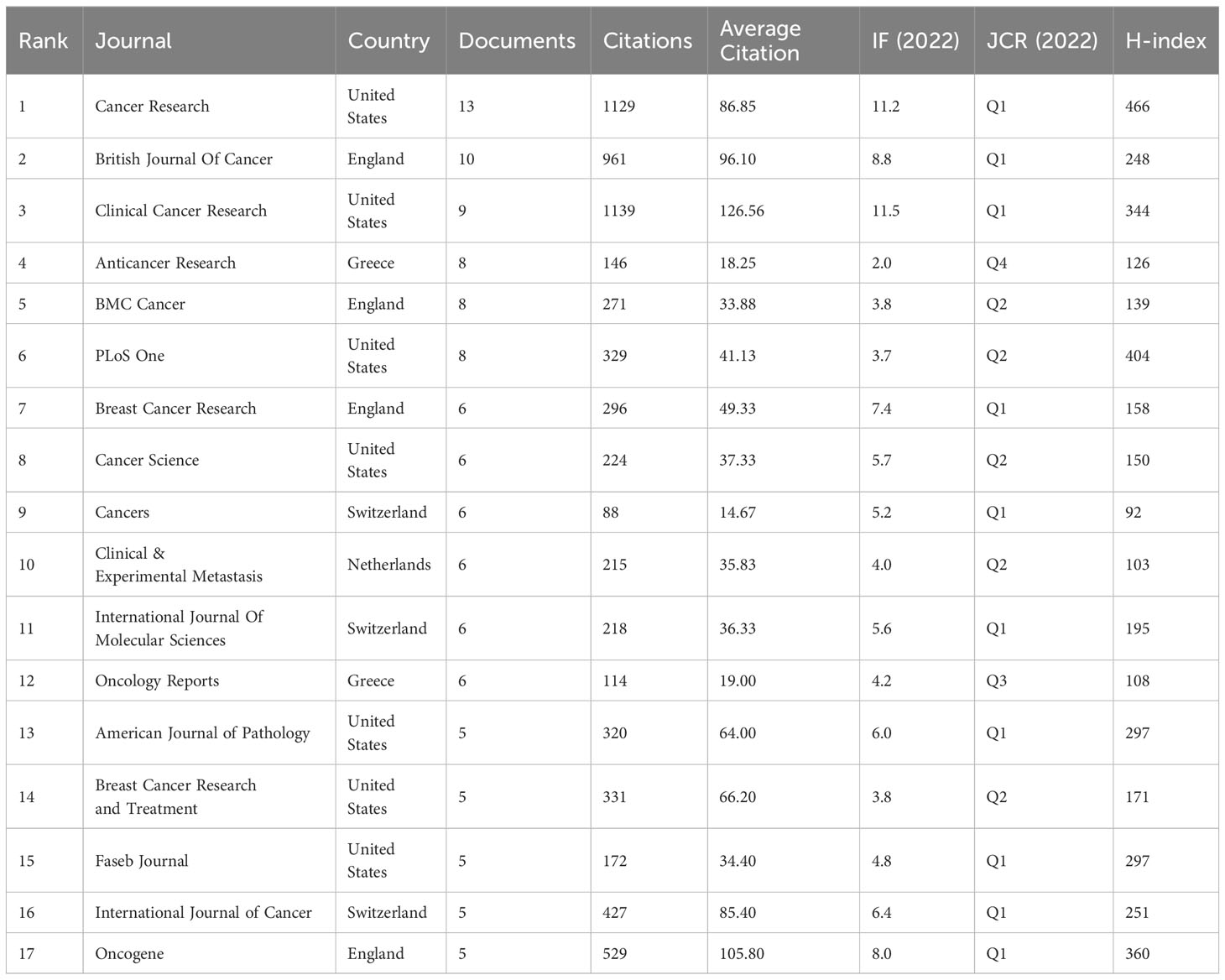
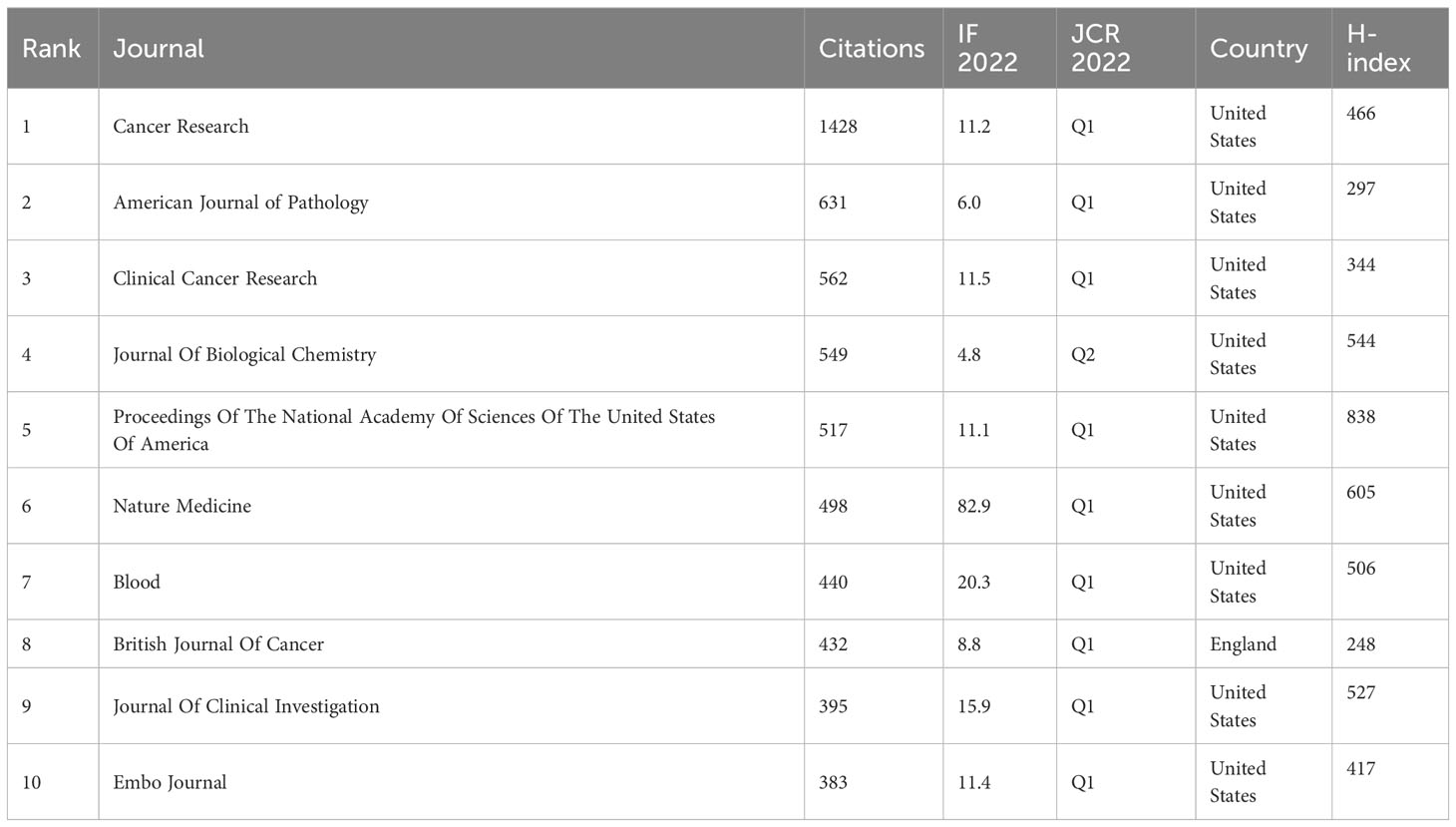
According to Bradford’s law, this investigation comprised a total of 199 journals, segmented into three sections based on the number of publications. Moreover, the number of publications in each area was generally the same, and the ratio of journal numbers was close to 1:3:9 (1:3:32). The journal distribution of studies in the realm of lymphangiogenesis conforms to Bradford’s law. Table 3 lists the three areas of 199 journals based on the number of publications. Finally, 17 journals were considered as core journals in this field. Table 4 displays the 17 core journals and their IF, JCR, and H-index.
Cancer Research, which had an IF of 11.2 in 2022, produced the greatest volume of research in the cancer field, with the British Journal of Cancer (BJC) and Clinical Cancer Research (CCR) in second and third place, respectively. Equally important, CCR had the highest impact factors. From an annual IF standpoint, CCR, Cancer Research, and BJC are viewed as authoritative journals in this field. The JCR standard of 2022 demonstrates that 10 out of the 17 most productive journals fall into the Q1 category, with the remaining 5 in Q2, testifying to the high quality of research in this field.
3.5 Analysis of keywords
Utilizing keywords as signifiers of an article, an examination of said keywords can enable us to rapidly comprehend the theme of the article and grasp the research hotspots and research frontiers in this domain. A rise in the prevalence of keyword citations or a greater frequency of keyword occurrence over a certain interval serves as an indicator for evaluating the most cutting-edge topics or emerging trends. Therefore, the keyword analysis of references in a certain field can reveal current research hotspots and predict the direction of future research hotspots through the frequency of keyword occurrences. There are a total of 1407 keywords appearing in 369 articles pertaining to lymphangiogenesis in breast cancer. VOSviewer was used to draw a keywords co-occurrence diagram in this field, and keywords with a frequency greater than 5 were selected for visualization (Figure 3). When the threshold was selected at the value of “4,” 137 keywords met the conditions, and the visualization map was somewhat mixed. Meanwhile, when the threshold was set to “6,” 90 keywords conformed to the criterion, and the map was somewhat straightforward. Against this backdrop, we used the threshold value of 5. At this time, there were 103 keywords to meet, the conclusion was evident, and the distinct branches of the field could be observed. Figure 4 shows the top 10 keywords in terms of their frequencies.
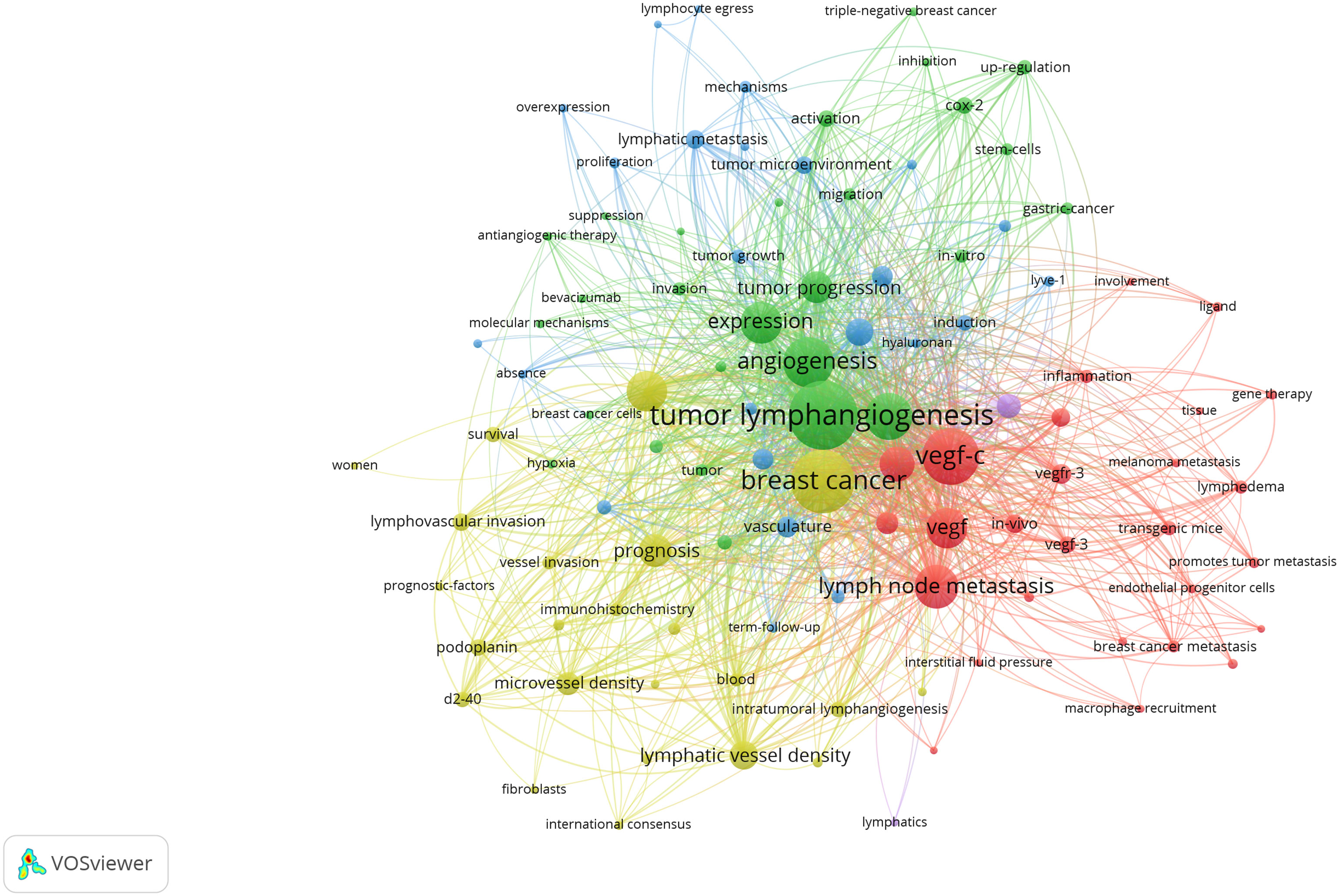
Figure 3 Network visualization of keywords drawn by VOSviewer. [The size of the nodes represents the number of occurrences; the thickness of the curve represents the strength of connection; the different colors represent the different clusters. The key words were classified into 4 clusters, presented by four colors (red, green, blue, and yellow)]. From: VOSviewer.
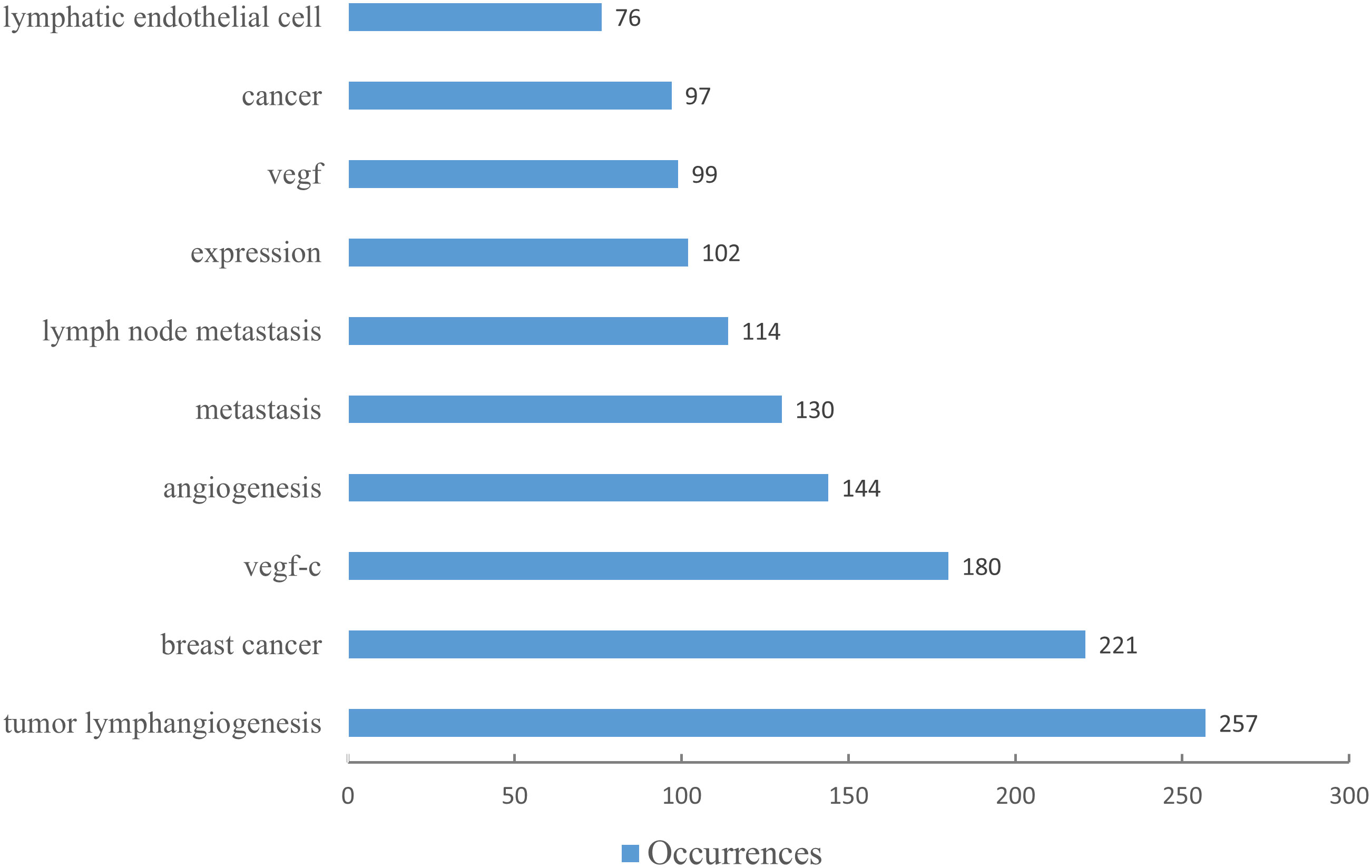
Figure 4 The top ten keywords by number of occurrences. (The blue column indicates the number of occurrences.).
Figure 3 illustrates that all keywords revolve around the topic of “tumor lymphangiogenesis” and are divided into four main clusters: I. keywords associated with the prognosis and related detection indicators of tumor lymphangiogenesis (yellow clusters), II. keywords centered around the vascular endothelial growth factor (VEGF) (red and purple clusters), III. keywords linked to the influence of tumor lymphangiogenesis and angiogenesis on tumor progression (green clusters), and IV. keywords related to the metastasis mechanism of the lymphatic system and the role of tumor microenvironment (blue clusters). Figure 5 displays the overlay visualization map of the 103 keywords. The evolution of high-frequency keywords over time can provide an intuitive insight into the research focus. The yellow nodes were indicative of the keywords that were emerging around 2016. Among these, tumor microenvironment (TME), epithelial-mesenchymal transition (EMT), stem cells, triple-negative breast cancer, and bevacizumab may become the future research hotspots in the field of lymphangiogenesis in breast cancer.
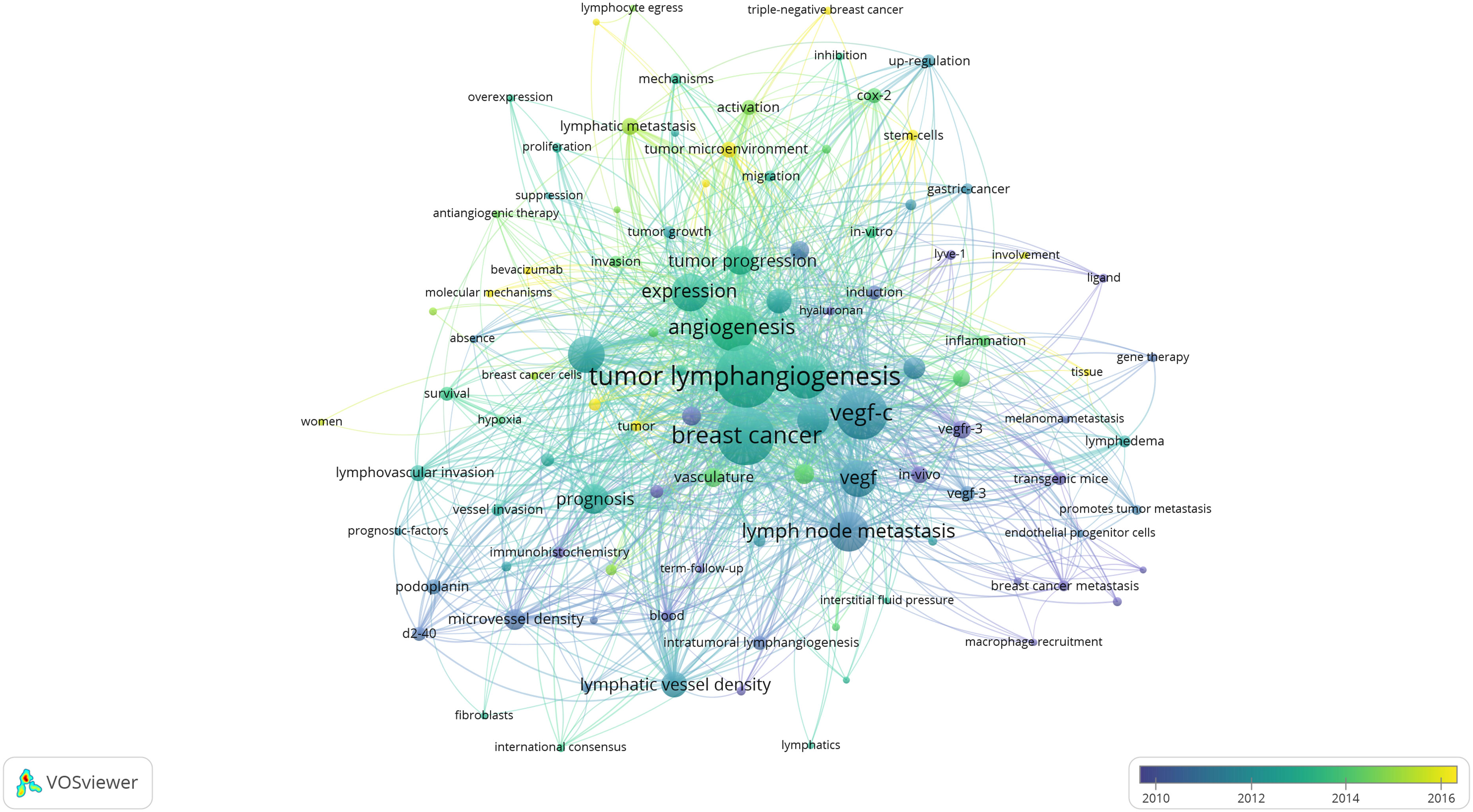
Figure 5 An overlay visualization of keywords drawn using VOSviewer. (The node size represents the number of occurrences; the curve thickness indicates the connection strength; and the colors denote the average publication years, with keywords in blue appearing before those in yellow.). From: VOSviewer.
3.6 Analysis of burst keywords
Burst words represent new discoveries or turning points in the development of a research field. They have been subject to extensive research in a short time, represent the frontier of the field, and can highlight the hotspots of future research and the evolution of the discipline. Applying CiteSpace, the research hotspots and emerging trends of lymphangiogenesis in breast cancer over a period of time were identified and analyzed by recognizing and studying keywords with citation bursts. The map presents the top 10 keywords with the strongest citation bursts from 2004 to 2023 (Figure 6). The red bar shows that the keyword was frequently cited, and the green bar indicates that the keyword was rarely cited. In the early days (2004-2011), “transgenic mice,” “vessel density,” “microvessel density,” and “gene expression” appeared. Meanwhile, in the mid-term (2012–2017), “vascular endothelial growth factor-c,” “lymphangiogenesis,” and “vegf-d promotes” emerged, and in the later stage (from 2018 to the present), “tumor microenvironment,” “metastasis,” and “blood vessels” surfaced. Among them, “transgenic mice” had a burst strength of 3.24, implying that it garnered the most attention and research from 2004 to 2011. Then, the keyword “tumor microenvironment” received the strongest citation burst (strength = 5.42) from 2018 to 2023, followed by “metastasis” (strength = 5.41), and “blood vessels” (strength = 4.96), which highlights that these three could represent important content and frontiers in the research domain of lymphangiogenesis in breast cancer and could remain a hot research topic today and in the coming years.
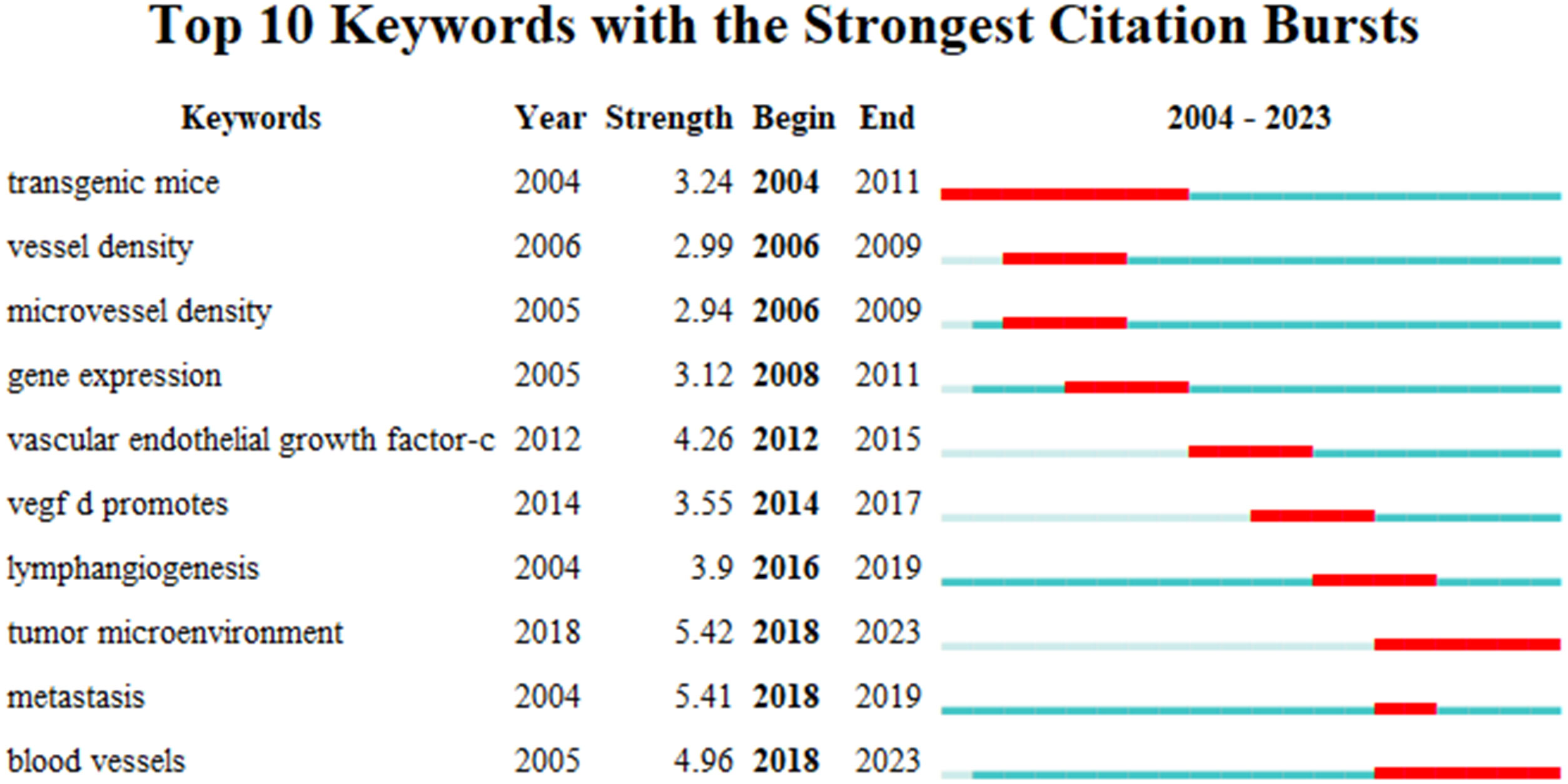
Figure 6 The top 10 burst words. (The strongest citation burst indicates a significant change in a variable over a short period, with red bars signifying the burst duration.).
3.7 Analysis of references co-citation
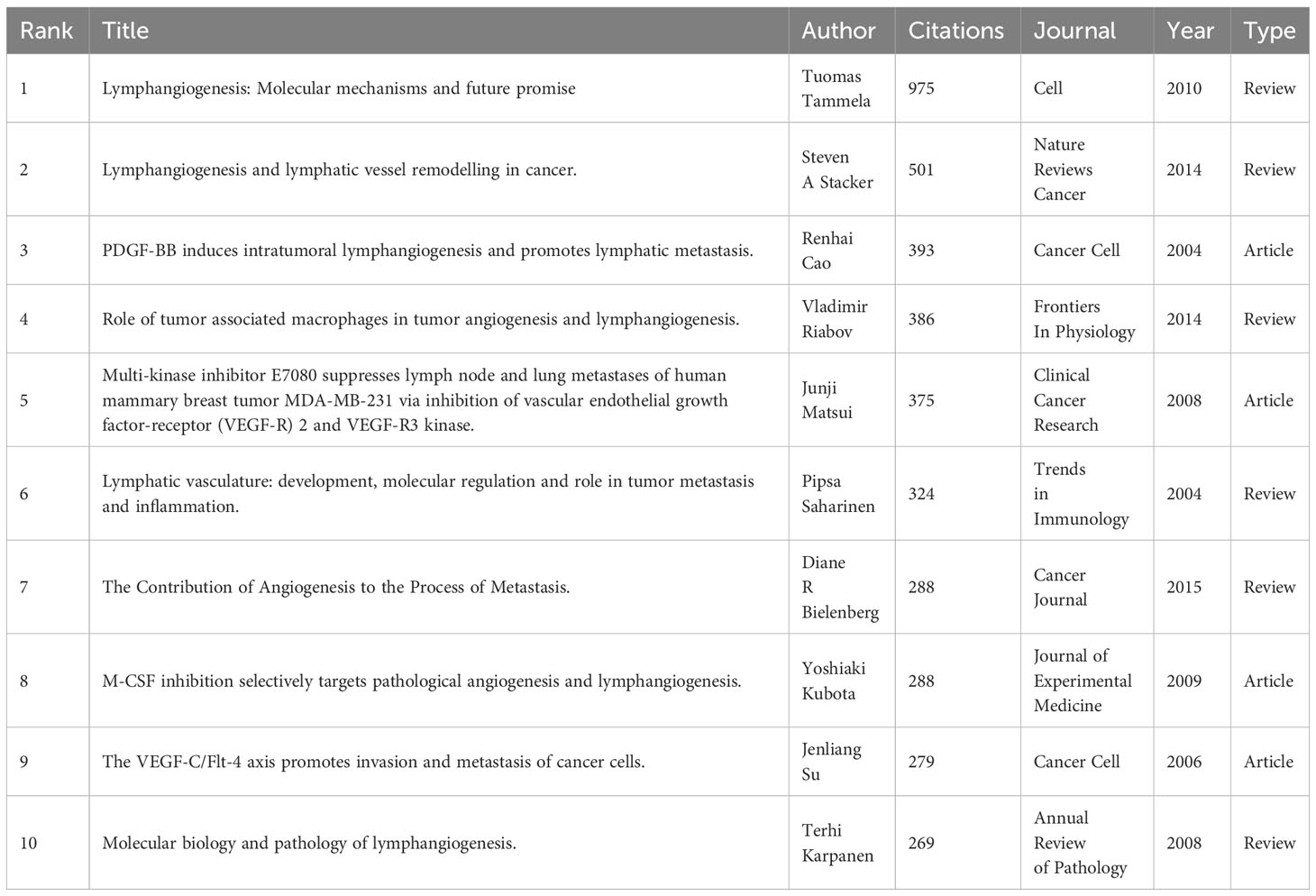
VOSviewer was employed to filter the top 10 cited articles among 369 articles, and the minimum number of citations was set to 261 to generate Table 5. Among the 10 cited articles, 6 were review articles, primarily focusing on the role and molecular mechanisms of lymphangiogenesis in cancer progression. Applying VOSviewer to filter 12458 citations from 1801 journals and establish the minimum number of citations to 85, we identified the top 50 cited journals. Figure 7 shows their co-citation relationships. A profound nexus between journals concerning lymphangiogenesis in breast cancer was unveiled, the three highest-cited journals being Cancer Research, American Journal of Pathology, and Clinical Cancer Research. Table 6 details the top 10 co-cited journals ranked by the number of citations. The co-citation network of the journal comprised three clusters, including journals related to medical oncology, biochemistry, and molecular biology (red cluster), tumor-pathology-related (green cluster), and cancer direction (blue clusters).
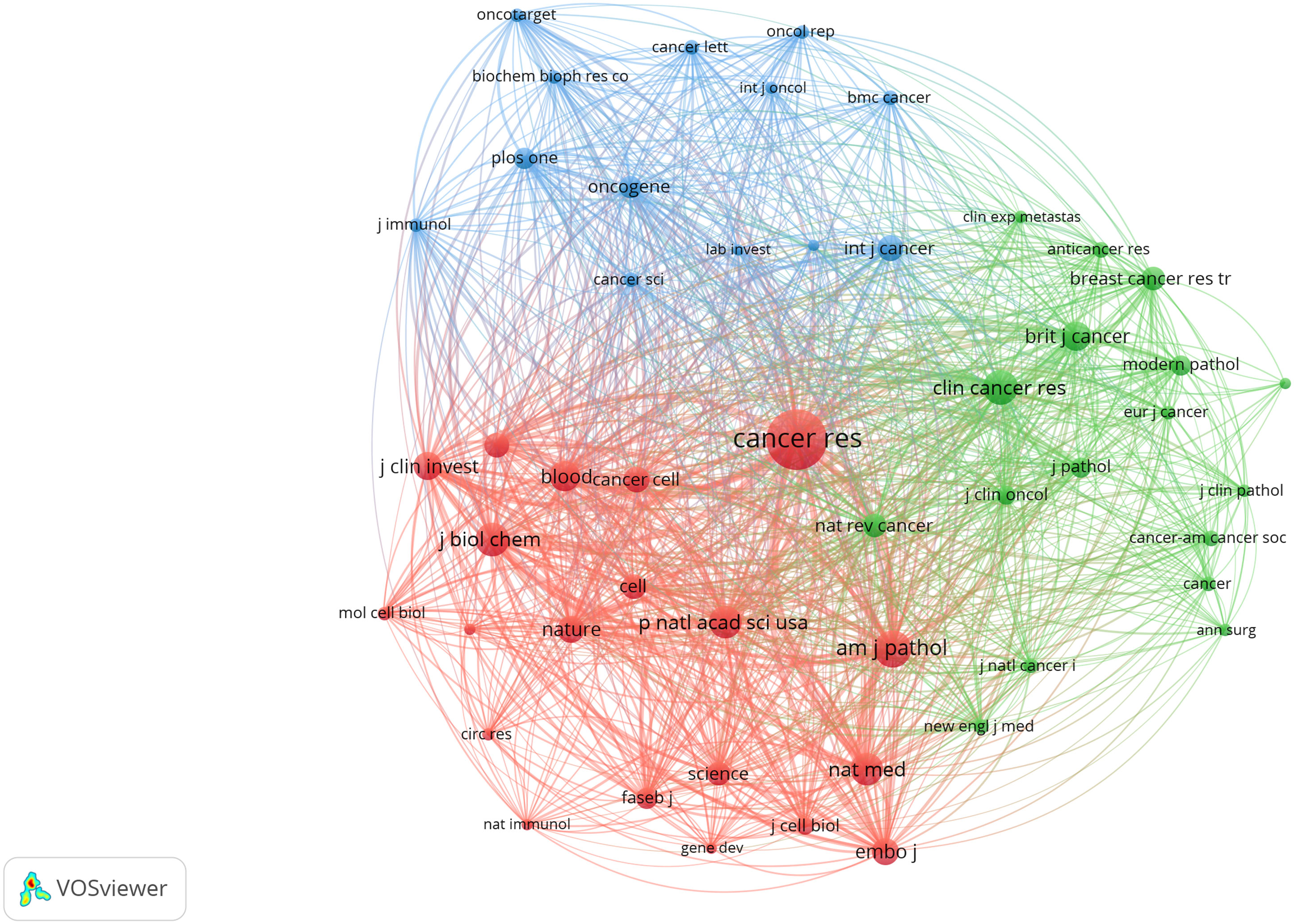
Figure 7 The co-occurrence of the top 50 cited journals. (The node size represents the number of articles; the curve thickness indicates the connection strength between journals; and the colors denote varying journal types.).
4 Discussion
Through the application of VOSviewer and CiteSpace software, this study conducted a bibliometric analysis of the literature surrounding lymphangiogenesis in breast cancer. This enabled researchers to swiftly and methodically acquire the development trajectory, current research status, and focal points in the area of lymphangiogenesis in breast cancer from 2004 to 2023, thereby predicting the global trends in this field. From 2004 to 2023, a total of 369 articles on lymphangiogenesis in breast cancer were published. Between 2004 and 2012, there was a steady rise in the number of publications, and from 2015 to 2023, the figure decreased, likely signifying a plateau in this area of research. This bottleneck could be due to the following reasons. The 2013 St. Gallen International Breast Cancer Consensus Conference redefined breast cancer molecular typing, dividing breast cancer into four subtypes: luminal A, luminal B, HER2-positive, and basal-like or triple-negative (TNBC) phenotype (28, 29). It marks the beginning of the precision treatment era for breast cancer based on molecular typing, as well as the emergence of numerous low-toxic and efficient molecularly targeted drugs for various molecular types. For example, the Food and Drug Administration (FDA) approved patozumab and TDM-1 in HER2-positive advanced breast cancer patients in 2012 and 2013, respectively (30, 31), Pfizer’s Palbiclib as an initial endocrine therapy scheme in 2015, and the PARP inhibitor Olaparib for treating BRCA1 or BRCA2 gene mutation breast cancer patients (32). Marketing these drugs has significantly benefited breast cancer patients. Apart from the research and development of new drugs and current treatment schemes with varying action mechanisms, including combining chemotherapy with targeted therapy, immunotherapy, and biological therapy, it offers a novel approach to breast cancer. Therefore, researchers shifted their focus away from traditional anti-tumor angiogenesis and anti-tumor lymphangiogenesis and toward investigating the biological behavior and therapeutic targets of various breast cancer molecular subtypes, thereby reducing the research literature on breast cancer lymphangiogenesis. In this context, prospective research, scientific analysis, and forward-looking guidance are imperative.
The research development in various countries or regions in the field of lymphangiogenesis in breast cancer is unbalanced. The majority of these articles originate from US, China, and Japan-based institutions and researchers, with the US having the most consistent collaborations with China and Japan. It is worth noting that the country with the highest citations is the US, while the UK has the highest average citation. However, the average citation rate in China stands at only 34.22, and there are no authors from Chinese institutions represented in the set of exceptionally productive authors who have published more than 7 articles. This indicates that the Chinese research community benefits from a larger population and the ability to produce more research and literature, though the quality of the studies still needs to be improved. Ultimately, Western researchers have a greater bearing on the topic of lymphangiogenesis in breast cancer. From the perspective of journals, it is found that journals in the core area are consistent with Bradford’s Law in terms of journal distribution. Among the 17 core journals, 10 belong to Q1, and 9 of the top 10 co-cited journals belong to Q1, emphasizing that articles associated with lymphangiogenesis in breast cancer are of high quality and have high scientific reference value.
In the keyword analysis, the keywords are divided into four clusters. The yellow clusters stand for the detection indicators and prognosis connected to lymphangiogenesis in breast cancer. Among them, the high frequency of “lymphatic vessel density,” “microvessel density,” “d2-40,” “diagnosis,” “lymphatic invasion,” and “survival” suggests that these keywords have garnered considerable interest. Lymphatic vessel density (LVD) or microvessel density (MVD) is a surrogate marker of tumor lymphangiogenesis. In view of this, quantitative assessment of lymphangiogenesis or angiogenesis in breast cancer can more precisely determine those with high recurrence risk, particularly those with negative lymph nodes, which is of value in the prognosis and treatment of breast cancer at its early stages. High LVD has adverse effects on disease-free survival (DFS) (HR 2.02, 95% CI 1.69 to 2.40) and OS (HR 2.88, 95% CI 2.07 to 4.01). LVD has a greater prognostic significance than vascular density in breast cancer. High LVD and the presence of lymphovascular invasion both predict poor prognosis in breast cancer (33).
Immunohistochemistry was put forward by Van der Auwera et al. as the method to detect LVD (34), however, there is a lack of clinical consensus on the quantitative method for LVD. Weidner’s initial strategy from 1991 is regularly utilized to assess LVD (35), a widely adopted method, to quantify intratumoral angiogenesis in breast cancer. Weidner’s approach commences with the identification by light microscopy of the area of highest neovessel density (the “hotspot”), by scanning the entire tumoral section at low power. Then, individual microvessels are counted at a higher power (×200 field) in a suitable area. However, there are several issues. Firstly, the amplification factor chosen for representative regions in different studies can vary, leading to discrepancies in the number of lymphatic vessels present in the region and resulting in distinct results concerning the number of lymphatic vessels tallied. Secondly, since the asset value of lymphatic vessel density is not a normal distribution, its data representation varies, yielding a different cutoff value. Finally, there are no established standards for the classification of high, medium, and low lymphatic vessel densities. Therefore, studies with more standardized and stringent designs are required in the assessment of LVD, especially in the methods of counting the number of lymphatic vessels, the selection of appropriate critical values, and the gradient classification of lymphatic vessel density, which are crucial points to consider.
The lymphatic endothelial-specific markers used for detecting lymphangiogenesis primarily include vascular endothelial growth factor receptor 3 (VEGFR-3), prospero homeobox protein 1 (Prox-1), lymphatic endothelial receptor-1 (LYVE-1), platelet endothelial cell adhesion molecule-1 (CD31), podoplanin, and D2-40 (36). Our research findings underscore that podoplanin and D2-40 had a higher frequency in the keyword clusters network, suggesting that these two markers hold a significant role in lymphangiogenesis. Podoplanin, a transmembrane protein, is highly expressed on the surface of LECs but not on vascular endothelial cells, thus rendering it a distinctive marker for LECs (37). The widely used commercial monoclonal antibody D2-40 binds to a fixation-resistant epitope of podoplanin (38). Additionally, in comparison to Prox-1 and LYVE-1, it is more sensitive in recognizing lymphangiogenesis in breast cancer and displays the strongest immunoreactivity in both intratumoral and peritumoral LECs (39–41). The detection of these distinct lymphatic endothelial markers renders us able to differentiate between lymphatic and blood vessels with efficacy (42). With the development of immunohistochemistry technology, we can utilize specific markers like podoplanin/D2-40/LYVE-1/Proxl to evaluate lymphangiogenesis in our research (43, 44).
VEGF-C and VEGF-D, two components of the vascular endothelial growth factors (VEGFs) family, are the first and sole representatives of vascular endothelial growth factors that possess specificity for lymphatic endothelial cells (45, 46). Simultaneously, burst words showed that from 2012 to 2017, the strength of VEGF-C and VEGF-D were 4.26 and 3.55, respectively, demonstrating that VEGFs linked to lymphangiogenesis received more attention. During this period, investigations into the molecular mechanisms of lymphangiogenesis continued. The overexpression of VEGF-C and VEGF-D can stimulate lymphangiogenesis and is associated with lymphovascular invasion and lymph node metastasis (47–49). VEGFR-3, as a receptor for VEGF-C and VEGF-D, is overexpressed and solely expressed in LECs. Meanwhile, VEGF-C and VEGF-D can activate VEGFR-3, enabling LECs to bud from embryonic veins and initiating the formation of primitive lymphatic sacs, thereby playing a vital role in the earliest stages of lymphatic vessel development (45). On the other hand, the interaction between VEGF-C/VEGF-D and VEGFR-3 triggers receptor phosphorylation and activates the Akt signaling pathway, which ultimately promotes the survival, proliferation, and migration of LECs, subsequently leading to the lymphangiogenesis in breast cancer (50). The intra- and peri-tumoral LVD of invasive breast cancer was quantified through the D2-40 immunohistochemical method. Studies showed that the high expression of VEGF-C/D in breast cancer may induce lymphangiogenesis in the peri-tumoral region, leading to elevated levels of peri-tumoral LVD, increased tumor invasiveness, and a higher risk of lymph node invasion, distant metastasis, and a poor prognosis (49, 51).
The green clusters demonstrate the effect of tumor lymphatic vessels and angiogenesis on tumor progression. The lymph nodes in the drainage area of the breast are the most common sites for metastasis of breast cancer cells (52). Cancer cells must undergo lymphangiogenesis in order for them to metastasize through the lymphatic vessels. The newly formed lymphatic vessels provide pathways for cancer cells to reach regional lymph nodes, thus priming cancer cells for distant metastasis (51). Meanwhile, lymphangiogenesis also expands the contact area between invasive cancer cells and LECs, contributing to metastasis (53). Therefore, lymphangiogenesis is the pivotal element to stimulate the invasion and metastasis of breast cancer.
The network visualization of keywords has revealed that in recent times, there has been an increased focus on the TME, epithelial-mesenchymal transformation (EMT), stem cells, triple-negative breast cancer, and bevacizumab in the field of lymphangiogenesis. Simultaneously, the analysis of burst words has unveiled that “tumor microenvironment” has been gaining traction in the research field since 2018. Therefore, we anticipate TME to become a focus of research into lymphangiogenesis with regard to breast cancer going forward. Growing evidence shows that the progression of a tumor to a malignant phenotype is heavily dependent on the interplay between tumor cells and the TME. Different TME components may stimulate or inhibit tumor progression by regulating gene expression in tumor cells and stromal cells. Lymphangiogenesis is generated by the dynamic interaction of breast cancer cells, lymphatic endothelial cells, and TME (54, 55).
VEGF-C/D, secreted by tumor cells, functions upon VEGFR-3, providing a basic signal for the proliferation, migration, and tubular structure formation of LECs (56). Along with passively receiving signals from tumor cells, LECs can also engage with multiple cytokines, such as chemokines, extracellular matrix (ECM), immune cells, tumor-associated macrophages (TAM), inflammatory cytokines, and other cytokines, thereby fostering lymphangiogenesis and tumor progression. These cytokines are important TME components. Chemokines, a class of small cytokines secreted by tumor cells, are essential in stimulating the migration of LECs and prompting tumor-related lymphangiogenesis when combined with their receptors. Among all known chemokine receptors, chemokine receptor 4 (CXCR4) and chemokine receptor 7 (CCR7) display a relatively high expression in breast malignant tumors and metastatic tissues (57). Moreover, CXCR4 and CCR7 interact with their respective ligands chemokine (C-X-C motif) ligand 12 (CXCL12) and chemokine (C-X-C motif) ligand 21 (CCL21), enabling tumor cells to have “chemotaxis” and promoting their metastasis to the intended organs (23, 58).
The relationship between LECs and ECM is fundamental to tumor lymphangiogenesis (59). On the one hand, ECM proteins, consisting of collagen, laminin, and fibronectin, can facilitate the proliferation of LECs and the development of tumor lymphangiogenesis by providing essential structural backing (60). On the other hand, ECM remodeling, catalyzed by matrix metalloproteinases (MMPs), may generate a more conducive environment for LECs, thereby promoting lymphangiogenesis (61). MMP-2, as an efficient collagenase, can effectively drive LECs to migrate to distant areas through the collagen matrix. Invasive cancer cells actively recruit stromal cells and interact with them to create a TME suitable for tumor growth and potential metastasis. Meanwhile, TME remodeling results in excessive amounts of low molecular weight hyaluronic acid (HA) in the TME, further activating macrophages to demonstrate lymphatic endothelial characteristics and encourage tumor lymphangiogenesis (62). This finding reveals a new mechanism of lymphangiogenesis induced by TME remodeling and provides a potential target for inhibiting the lymphatic metastasis of breast cancer.
Recent evidence shed light on the bidirectional crosstalk between LECs and immune cells. Through the action of immune cells, white blood cells are mobilized to produce VEGF-C and secrete some lymphangiogenic factors, such as lipocalin 2 (LCN2) from macrophages. This activates VEGF-C through a multicellular signaling pathway, thus promoting lymphangiogenesis in the TME (62). Additionally, cytokines produced by infiltrating immune cells can directly affect the proliferation, migration, and tubular structure formation of LECs. For instance, IL-17 secreted from Th17 cells can induce LEC proliferation, hence promoting lymphangiogenesis (63, 64).
Concerning the occurrence and development of lymphangiogenesis, tumor-associated macrophages (TAM) are also involved. TAM expresses a considerable amount of (a) VEGF-C/D, which promotes lymphangiogenesis, and (b) lymphatic endothelial progenitor cells that can be transformed into LECs under pathological conditions, serving as a direct structural contributor to new lymphatic vessels (65). Weichand et al. observed that the sphingolipid sphingosine-1-phosphate (S1PR1) signaling circuit in TAM facilitated lymphangiogenesis via the inflammasome component Nlrp3-dependent IL-1β secretion (66). In addition to the aforementioned TME factors, inflammation-induced lymphangiogenesis should not be overlooked, encompassing IL-1 β recruiting M2 type macrophages that can promote tumor lymphangiogenesis and lymph node metastasis (67). The anti-cytokine IL-10 with anti-inflammatory properties can upregulate the expression of VEGF-C in macrophages, thereby augmenting lymphangiogenesis (68). Among them, Prox1 and NF-kappaB co-regulate LECs, causing an increased expression of VEGFR-3 on the surface of LECs, thus disclosing the mechanism of inflammation-induced lymphangiogenesis (69).
Among the top 10 cited articles pertaining to the lymphangiogenesis of breast cancer, 6 were focused on the review of the essential concepts. All six articles furnish a review of the molecular mechanisms of lymphangiogenesis. Collectively, through these articles, we could grasp the structure of lymphatic vessels, the development and remodeling of tumor lymphangiogenesis, the function of lymphatic vessel growth factors in lymphangiogenesis, and the influence of lymphangiogenesis on tumor metastasis. The other four articles are specialized. Among them, the platelet-derived growth factor BBs (PDGF-BB) are novel potent and direct lymphangiogenic factors that can induce tumor lymphangiogenesis and promote lymphatic metastasis. Additionally, the blockage of the VEGF-C/-D-VEGFR-3 axle does not block PDGF-BB-induced lymphangiogenesis in vitro and in vivo. It is imperative in stimulating tumor lymphangiogenesis and lymphatic metastasis. Therefore, the formulation of antagonists for PDGF-BB may be a beneficial method for the control of tumor growth and metastasis (70).
Investigations into the VEGF-C/Flt-4 axis have revealed its capacity to take part in tumor progression by promoting the movement and invasiveness of epithelial tumor cells, as well as LEC proliferation (e.g., lymphangiogenesis) and possible vascular endothelial cell proliferation and migration (e.g., angiogenesis). With regard to controlling tumor growth or metastasis, targeting the VEGF-C/Flt-4 axis may be an effective method (71). Concerning the inhibition of tumor lymphangiogenesis and metastasis, multi-kinase inhibitor E7080 and macrophage colony-stimulating factor (M-CSF) have produced newly identified results. E7080 functions as a dual tyrosine kinase inhibitor for VEGFR-2 and VEGFR-3. It has been demonstrated to effectively inhibit both MVD and LVD in the lymph node metastasis of a transplanted tumor in a nude mouse model of breast cancer, showing greater efficacy in anti-lymphangiogenesis and anti-angiogenesis than bevacizumab, and can effectively impede regional lymph nodes and distant lung metastasis (72). M-CSF, a cytokine required for monocyte lineage cell differentiation, can selectively inhibit tumor angiogenesis and lymphangiogenesis while reducing tumor recurrence after treatment interruption (73). It is evident from these results that E7080 and M-CSF are ideal strategies for inhibiting lymphangiogenesis and progression in breast cancer. These extensively referenced texts offer insight into the clinical and basic research of lymphangiogenesis in breast cancer, aiding in the comprehension of the pathological mechanism of tumor lymphangiogenesis and forming a platform from which to explore new mechanisms.
Although lymphangiogenesis has been proposed to be a potential target for preventing cancer metastasis, there are few antilymphangiogenic drugs approved for clinical trials. The main obstacles are the heterogeneity involved in tumor-induced lymphangiogenesis in gene signature, structure, cellular origins and functional plasticity, which is governed by complex factors (74). Elucidating the mechanism that causes lymphangiogenesis heterogeneity in solid tumors may provide better insight into the role of lymphangiogenesis in tumor progression and potential implications for tumor treatment. In addition, similar to tumor angiogenesis, multiple lymphangiogenic pathways are active in some tumor microenvironments, meaning that single pathway targeting drugs might not be efficient in all cases.
5 Strengths and limitations
To the best of our knowledge, this is the first scientometrics-based bibliometric examination of the development and trends of lymphangiogenesis in breast cancer. However, several potential limitations exist in our study. First, this research was restricted to certain studies (the documents in SCI-E of WoSCC, English language, publication type of article, and review) in order to meet the format requirements of CiteSpace and VOSviewer. Inevitably, this led to the partial inclusion of articles, thus this study may not fully represent the available information in this domain. Given that a bibliometric analysis does not address a specific research query, narrow areas of the topic were not identified. Secondly, due to the regular updating of the database, there was a certain lag in the data obtained, such as the number of articles, citations, and H-index. Finally, evaluating prior literature, the dearth of keywords or abstracts may increase the chances of being excluded due to poor discoverability.
Notwithstanding the limitations, bibliometric analysis is advantageous for gaining keener insight into the essential components and a far-reaching quantity of studies on lymphangiogenesis in the context of breast cancer. Our study clearly displayed the countries/regions, institutions, authors, journals, references, keywords, and other elements of literature related to lymphangiogenesis in breast cancer in the past 20 years. We collated data sets and reviewed the publications of experts in relevant fields, which enabled us to understand the development process, research status, and knowledge hotspots of lymphangiogenesis in breast cancer. Through keyword analysis, the future research directions for lymphangiogenesis in breast cancer were delineated and outlined for reference. We are confident that this bibliometric research could offer a few inspirations and viable ideas for researchers in related fields.
6 Conclusion
Based on the published literature, this study provided a comprehensive overview of the research trend involving lymphangiogenesis in breast cancer. Since 2015, the number of relevant literature being published has declined, possibly suggesting that this research topic has reached a bottleneck. Consequently, further research, scientific investigation, and future direction are critically necessary. The US and China have the highest number of publications in this field, but the latter needs to conduct higher-quality research. Peeyush Lala and Mousumi Majumder, as highly productive authors in this field, have extensive collaborations. Journals with the most publications and citations are mostly in Q1 and Q2, demonstrating excellence in research in this area. Moreover, a keyword analysis reveals that the primary focus of research in this area is the molecular mechanism of lymphangiogenesis, with the connection between TME and lymphangiogenesis being a popular research topic in recent years and is expected to remain so in the future. To sum up, this study will afford scholars an extensive comprehension of the current state and trends in this domain, in addition to yielding ideas for future investigations.
Author contributions
LX: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. XW: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. BW: Investigation, Software, Validation, Writing – review & editing. BM: Investigation, Validation, Writing – review & editing. XP: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Beijing (7232275), the National Natural Science Foundation of China (82374463).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1337124/full#supplementary-material
References
1. Network CGA. Comprehensive molecular portraits of human breast tumors. Nature. (2012) 490:61–70. doi: 10.1038/nature11412
2. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
3. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. (2011) 147:275–92. doi: 10.1016/j.cell.2011.09.024
4. Zwaans BMM, Bielenberg DR. Potential therapeutic strategies for lymphatic metastasis. Microvasc Res. (2007) 74:145–58. doi: 10.1016/j.mvr.2007.08.006
5. Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. (2004) 25:387–95. doi: 10.1016/j.it.2004.05.003
6. Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodeling in cancer. Nat Rev Cancer. (2014) 14:159–72. doi: 10.1038/nrc3677
7. Achen MG, Mann GB, Stacker SA. Targeting lymphangiogenesis to prevent tumor metastasis. Brit J Cancer. (2006) 94:1355–60. doi: 10.1038/sj.bjc.6603120
8. Rossari JR, Metzger-Filho O, Fau - Paesmans M, Paesmans M Fau - Saini KS, Saini Ks Fau - Gennari A, Gennari a Fau - De Azambuja E, et al. Bevacizumab and breast cancer: A meta-analysis of first-line phase III studies and a critical reappraisal of available evidence. J Oncol. (2012) 2012:1687–8469. doi: 10.1155/2012/417673
9. Roldan-Valadez E, Salazar-Ruiz SY, Ibarra-Contreras R, Rios C. Current concepts on bibliometrics: a brief review about impact factor, Eigenfactor score, CiteScore, SCImago Journal Rank, Source-Normalised Impact per Paper, H-index, and alternative metrics. Irish J Med Sci. (2019) 188:939–51. doi: 10.1007/s11845-018-1936-5
10. Shen Z, Wu H, Chen Z, Hu J, Pan J, Kong J, et al. The global research of artificial intelligence on prostate cancer: A 22-year bibliometric analysis. Front Oncol. (2022) 12:843735. doi: 10.3389/fonc.2022.843735
11. Garfield E. Citation analysis as a tool in journal evaluation. Science. (1972) 178:471–9. doi: 10.1126/science.178.4060.471
12. Hirsch JE. An index to quantify an individual's scientific research output. P Natl Acad Sci USA. (2005) 102:16569–72. doi: 10.1073/pnas.0507655102
13. Bulick S. Book use as a bradford-zipf phenomenon. Coll Res Libraries. (1978) 39:215–9. doi: 10.5860/crl-39-03-215
14. Bradford SC. Sources of information on specific subjects. J Inf Science. (1985) 10:173–80. doi: 10.1177/016555158501000406
15. Coile RC. Lotka's frequency distribution of scientific productivity. J Am Soc Inf Science. (1977) 28:366–70. doi: 10.1002/asi.4630280610
16. Price D, Merton R, Garfield E. Little science, big science. and beyond. New York: Columbia University Press (1986). p. 301.
17. Van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
18. Chen CM. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Tec. (2006) 57:359–77. doi: 10.1002/asi.20317
19. De Paz Linares GA, Opperman RM, Majumder M, Lala PK. Prostaglandin E2 receptor 4 (EP4) as a therapeutic target to impede breast cancer-associated angiogenesis and lymphangiogenesis. Cancers. (2021) 13:942. doi: 10.3390/cancers13050942
20. Lala PK, Nandi P, Majumder M. Roles of prostaglandins in tumor-associated lymphangiogenesis with special reference to breast cancer. Cancer Metast Rev. (2018) 37:369–84. doi: 10.1007/s10555-018-9734-0
21. Majumder M, Nandi P, Omar A, Ugwuagbo KC, Lala PK. EP4 as a therapeutic target for aggressive human breast cancer. Int J Mol Sci. (2018) 19:1019. doi: 10.3390/ijms19041019
22. Nandi P, Girish GV, Majumder M, Xin XP, Tutunea-Fatan E, Lala PK. PGE2 promotes breast cancer-associated lymphangiogenesis by activation of EP4 receptor on lymphatic endothelial cells. BMC Cancer. (2017) 17:11. doi: 10.1186/s12885-016-3018-2
23. Tutunea-Fatan E, Majumder M, Xin XP, Lala PK. The role of CCL21/CCR7 chemokine axis in breast cancer-induced lymphangiogenesis. Mol Cancer. (2015) 10:35. doi: 10.1186/s12943-015-0306-4
24. Majumder M, Xin XP, Liu L, Girish GV, Lala PK. Prostaglandin E2 receptor EP4 as the common target on cancer cells and macrophages to abolish angiogenesis, lymphangiogenesis, metastasis, and stem-like cell functions. Cancer Sci. (2014) 105:1142–51. doi: 10.1111/cas.12475
25. Majumder M, Xin XP, Lala PK. A practical and sensitive method of quantitating lymphangiogenesis. Lab Invest. (2013) 93:779–91. doi: 10.1038/labinvest.2013.72
26. Xin XP, Majumder M, Girish GV, Mohindra V, Maruyama T, Lala PK. Targeting COX-2 and EP4 to control tumor growth, angiogenesis, lymphangiogenesis and metastasis to the lungs and lymph nodes in a breast cancer model. Lab Invest. (2012) 92:1115–28. doi: 10.1038/labinvest.2012.90
27. Majumder M, Tutunea-Fatan E, Xin X, Rodriguez-Torres M, Torres-Garcia J, Wiebe R, et al. Co-expression of alpha9beta1 integrin and VEGF-D confers lymphatic metastatic ability to a human breast cancer cell line MDA-MB-468LN. PloS One. (2012) 7:e35094. doi: 10.1371/journal.pone.0035094
28. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. (2013) 24:2206–23. doi: 10.1093/annonc/mdt303
29. Untch M, Gerber B, Harbeck N, Jackisch C, Marschner N, Möbus V, et al. 13th st. Gallen international breast cancer conference 2013: primary therapy of early breast cancer evidence, controversies, consensus - opinion of a german team of experts (Zurich 2013). Breast Care. (2013) 8:221–9. doi: 10.1159/000351692
30. FDA grants regular approval to pertuzumab for adjuvant treatment of HER2-positive breast cancer (2012). Available online at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-pertuzumab-adjuvant-treatment-her2-positive-breast-cancer.
31. Tanzi MG. Ado-trastuzumab emtansine: Therapy for metastatic breast cancer. Pharmacy Today (2013). doi: 10.1016/S1042-0991(15)31372-4
32. Novel Drug Approvals for (2015). Available online at: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2015.
33. Zhang S, Zhang D, Gong MF, Wen L, Liao CW, Zou LG. High lymphatic vessel density and presence of lymphovascular invasion both predict poor prognosis in breast cancer. BMC Cancer. (2017) 17:335. doi: 10.1186/s12885-017-3338-x
34. Van Der Auwera I, Cao Y, Tille JC, Pepper MS, Jackson DG, Fox SB, et al. First international consensus on the methodology of lymphangiogenesis quantification in solid human tumors. Brit J Cancer. (2006) 95:1611–25. doi: 10.1038/sj.bjc.6603445
35. Weidner N SJ, Welch WR, Folkman J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. (1991) 324:1–8. doi: 10.1056/NEJM199101033240101
36. Chen JM, Luo B, Ma R, Luo XX, Chen YS, Li Y. Lymphatic endothelial markers and tumor lymphangiogenesis assessment in human breast cancer. Diagnostics. (2022) 12:4. doi: 10.3390/diagnostics12010004
37. Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. (1999) 154:385–94. doi: 10.1016/S0002-9440(10)65285-6
38. Sonne SB, Herlihy AS, Hoei-Hansen CE, Nielsen JE, Almstrup K, Skakkebaek NE, et al. Identity of M2A (D2-40) antigen and gp36 (Aggrus, T1A-2, podoplanin) in human developing testis, testicular carcinoma in situ and germ-cell tumors. Virchows Arch. (2006) 449:200–6. doi: 10.1007/s00428-006-0223-4
39. Evangelou E, Kyzas PA, Trikalinos TA. Comparison of the diagnostic accuracy of lymphatic endothelium markers: Bayesian approach. Modern Pathol. (2005) 18:1490–7. doi: 10.1038/modpathol.3800457
40. Wahal SP, Goel MM, Mehrotra R. Lymphatic vessel assessment by podoplanin (D2-40) immunohistochemistry in breast cancer. J Cancer Res Ther. (2015) 11:798–804. doi: 10.4103/0973-1482.146123
41. Gujam FJA, Going JJ, Mohammed ZMA, Orange C, Edwards J, Mcmillan DC. Immunohistochemical detection improves the prognostic value of lymphatic and blood vessel invasion in primary ductal breast cancer. BMC Cancer. (2014) 14:674. doi: 10.1186/1471-2407-14-676
42. Gujam FJA, Going JJ, Edwards J, Mohammed ZMA, Mcmillan DC. The role of lymphatic and blood vessel invasion in predicting survival and methods of detection in patients with primary operable breast cancer. Crit Rev Oncol Hemat. (2014) 89:231–41. doi: 10.1016/j.critrevonc.2013.08.014
43. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
44. Bono P, Wasenius VM, Heikkilä P, Lundin J, Jackson DG, Joensuu H. High LYVE-1-positive lymphatic vessel numbers are associated with poor outcome in breast cancer. Clin Cancer Res. (2004) 10:7144–9. doi: 10.1158/1078-0432.Ccr-03-0826
45. Kukk E, Lymboussaki A, Kaipainen A, Taira S, Jeltsch M, Joukov V, et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. (1996) 122:3829–37. doi: 10.1242/dev.122.12.3829
46. Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci U S A. (1998) 95:548–53. doi: 10.1073/pnas.95.2.548
47. Wang J, Guo Y, Wang B, Bi J, Li K, Liang X, et al. Lymphatic microvessel density and vascular endothelial growth factor-C and -D as prognostic factors in breast cancer: a systematic review and meta-analysis of the literature. Mol Biol Rep. (2012) 39:11153–65. doi: 10.1007/s11033-012-2024-y
48. Varney ML, Singh RK. VEGF-C-VEGFR3/Flt4 axis regulates mammary tumor growth and metastasis in an autocrine manner. Am J Cancer Res. (2015) 5:616–28.
49. Eroglu A, Ersoz C, Karasoy D, Sak S. Vascular endothelial growth factor (VEGF)-C, VEGF-D, VEGFR-3 and D2-40 expressions in primary breast cancer: Association with lymph node metastasis. Adv Clin Exp Med. (2017) 26:245–9. doi: 10.17219/acem/58784
50. Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. (2001) 20:4762–73. doi: 10.1093/emboj/20.17.4762
51. Zhao YC, Ni XJ, Li Y, Dai M, Yuan ZX, Zhu YY, et al. Peritumoral lymphangiogenesis induced by vascular endothelial growth factor C and D promotes lymph node metastasis in breast cancer patients. World J Surg Oncol. (2012) 10:165. doi: 10.1186/1477-7819-10-165
52. Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. (2010) 140:460–76. doi: 10.1016/j.cell.2010.01.045
53. El-Gendi S, Abdel-Hadi M. Lymphatic vessel density as prognostic factor in breast carcinoma: relation to clinicopathologic parameters. J Egypt Natl Canc Inst. (2009) 21:139–49.
54. Arneth B. Tumor microenvironment. Medicina (Kaunas). (2019) 56:15. doi: 10.3390/medicina56010015
55. Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. (2019) 79:4557–66. doi: 10.1158/0008-5472.Can-18-3962
56. Schorr A. Lotka s law and map librarianship. J Am Soc Inf Sci. (1975) 26:189–90. doi: 10.1002/asi.4630260308
57. Korbecki J, Grochans S, Gutowska I, Barczak K, Baranowska-Bosiacka I. CC chemokines in a tumor: A review of pro-cancer and anti-cancer properties of receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 ligands. Int J Mol Sci. (2020) 21:7619. doi: 10.3390/ijms21207619
58. Zhuo W, Jia L, Song N, Lu XA, Ding Y, Wang X, et al. The CXCL12-CXCR4 chemokine pathway: a novel axis regulates lymphangiogenesis. Clin Cancer Res. (2012) 18:5387–98. doi: 10.1158/1078-0432.CCR-12-0708
59. Wiig H, Keskin D, Kalluri R. Interaction between the extracellular matrix and lymphatics: Consequences for lymphangiogenesis and lymphatic function. Matrix Biol. (2010) 29:645–56. doi: 10.1016/j.matbio.2010.08.001
60. Garmy-Susini B, Avraamides CJ, Schmid MC, Foubert P, Ellies LG, Barnes L, et al. Integrin α4β1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res. (2010) 70:3042–51. doi: 10.1158/0008-5472.Can-09-3761
61. Detry B, Erpicum C, Paupert J, Blacher S, Maillard C, Bruyère F, et al. Matrix metalloproteinase-2 governs lymphatic vessel formation as an interstitial collagenase. Blood. (2012) 119:5048–56. doi: 10.1182/blood-2011-12-400267
62. Jung M, Ören B, Mora J, Mertens C, Dziumbla S, Popp R, et al. Lipocalin 2 from macrophages stimulated by tumor cell-derived sphingosine-1-phosphate promotes lymphangiogenesis and tumor metastasis. Sci Signal. (2016) 9:ra64. doi: 10.1126/scisignal.aaf3241
63. Mazzone M, Bergers G. Regulation of blood and lymphatic vessels by immune cells in tumors and metastasis. Annu Rev Physiol. (2019) 81:535–60. doi: 10.1146/annurev-physiol-020518-114721
64. Chauhan SK, Jin YP, Goyal S, Lee HS, Fuchsluger TA, Lee HK, et al. A novel pro-lymphangiogenic function for Th17/IL-17. Blood. (2011) 118:4630–4. doi: 10.1182/blood-2011-01-332049
65. Ran S, Volk-Draper L. Lymphatic endothelial cell progenitors in the tumor microenvironment. Adv Exp Med Biol. (2020) 1234:87–105. doi: 10.1007/978-3-030-37184-5_7
66. Weichand B, Popp R, Dziumbla S, Mora J, Strack E, Elwakeel E, et al. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1beta. J Exp Med. (2017) 214:2695–713. doi: 10.1084/jem.20160392
67. Watari K, Shibata T, Kawahara A, Murakami Y, Nabeshima H, Shinoda A, et al. Tumor-derived interleukin-1 promotes lymphangiogenesis and lymph node metastasis through activation of M2-type macrophages by lung cancer cells. Cancer Res. (2014) 74:e99568. doi: 10.1158/1538-7445.Am2014-3662
68. Hos D, Bucher F, Regenfuss B, Dreisow ML, Bock F, Heindl LM, et al. IL-10 indirectly regulates corneal lymphangiogenesis and resolution of inflammation via macrophages. Am J Pathol. (2016) 186:159–71. doi: 10.1016/j.ajpath.2015.09.012
69. Flister MJ, Wilber A, Hall KL, Iwata C, Miyazono K, Nisato RE, et al. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-κB and Prox1. Blood. (2010) 115:418–29. doi: 10.1182/blood-2008-12-196840
70. Cao RH, Björndahl MA, Religa P, Clasper S, Garvin S, Galter D, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. (2004) 6:333–45. doi: 10.1016/j.ccr.2004.08.034
71. Su JL, Yang PC, Shih JY, Yang CY, Wei LH, Hsieh CY, et al. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell. (2006) 9:209–23. doi: 10.1016/j.ccr.2006.02.018
72. Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. (2008) 14:5459–65. doi: 10.1158/1078-0432.Ccr-07-5270
73. Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, et al. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. (2009) 206:1089–102. doi: 10.1084/jem.20081605
Keywords: bibliometric analysis, breast cancer, lymphangiogenesis, visualization, Citespace, VOSviewer
Citation: Xu L, Wang X, Wang B, Meng B and Pei X (2024) The global landscape and research trend of lymphangiogenesis in breast cancer: a bibliometric analysis and visualization. Front. Oncol. 14:1337124. doi: 10.3389/fonc.2024.1337124
Received: 12 November 2023; Accepted: 27 February 2024;
Published: 14 March 2024.
Edited by:
Cristian Scatena, University of Pisa, ItalyReviewed by:
Aadya Nagpal, Peter MacCallum Cancer Center, AustraliaMichele Zanoni, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), Italy
Copyright © 2024 Xu, Wang, Wang, Meng and Pei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohua Pei, pxh_127@163.com
†These authors have contributed equally to this work and share first authorship
 Liuyan Xu
Liuyan Xu Xuan Wang
Xuan Wang Beibei Wang2
Beibei Wang2 Xiaohua Pei
Xiaohua Pei