- Department of General Surgery, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Primary squamous cell carcinoma of the thyroid (PSCCT) is a rare malignant tumor. The incidence rate of PSCCT is less than 1%. However, the diagnosis and treatment of PSCCT are limited. Surgical resection is considered to be one of the few effective intervention methods. In this article, we reported a case of taking tyrosine kinase inhibitors (TKIs) combined with immune checkpoint inhibitors (ICIs) for PSCCT.
Case summary: An 80-year-old male was admitted to our hospital with dyspnea, cough, wheezing, and hoarseness for a giant thyroid mass. He underwent bronchoscopy and tracheal stent implantation to alleviate the respiratory obstruction. Then he accepted right partial thyroid and right lymph node biopsy. Postoperative pathology revealed squamous cell carcinoma. Subsequently, he underwent an endoscopy to exclude upper gastrointestinal squamous cell carcinoma. Finally, he was diagnosed with PSCCT. The patient was tentatively treated with a combination of Anlotinib and Sintilimab. After two courses, the tumor volume significantly reduced in MRI images and shrank further after five courses of combined treatment. Unfortunately, the patient died of fulminant liver failure and autoimmune liver disease after 5-month-treatment.
Conclusion: TKIs combined with ICIs may be an effective and novel way for PSCCT treatment, but immune-related complications, especially liver damage, should be cared.
1 Introduction
PSCCT is a rare disease, which is less than 1% of all thyroid neoplasms (1). Due to the rapid growth of the tumor, invasion of surrounding structures is usually observed, which could induce a poor prognosis. The median survival time is generally shorter than one year (2, 3). Diagnosis is difficult because squamous metaplasia is common in other primary thyroid cancers, and squamous cell carcinoma may have metastasized from elsewhere (4). Thus, bronchoscopy and gastrointestinal endoscopy are necessary for excluding other primary squamous cell carcinomas from the upper respiratory and digestive tract (1, 4). PSCCT has a poor response to radiotherapy and is resistant to chemotherapy. Some articles reported surgery appeared to be one of the few methods that can reduce tumor burden and local invasion, prolonging survival (5–7).
In this case, we treated PSCCT with Anlotinib plus Sintilimab. We reported the symptoms, histopathological findings, and diagnostic procedures. The combination of medicines rapidly reduced the tumor volume. We also reviewed the literature to evaluate the conventional treatment of PSCCT and the progression of TKIs in combination with ICIs in other solid tumors.
2 Case presentation
An 80-year-old male was admitted to our hospital for shortness of breath, cough, asthma, and hoarseness on November 19, 2020. Ultrasound showed a mass in the right lobe of the thyroid, about 38*51*78mm in size, partially involving the isthmus. Further MRI scans revealed a large right thyroid mass pressing on the airway (Figures 1A, D, F). Because of severe stenosis of the main trachea leading to chest tightness, palpitation, cough, and asthma, the patient was placed with a tracheal stent. Preoperative bronchoscopy showed that the vocal cords were fixed and compressed, and no new organisms were found in the airway wall. Owning to the failure of histopathological diagnosis by core needle biopsy, the patient underwent right partial thyroid and right neck lymph node surgical biopsy on November 25, 2020. Postoperative pathological examination revealed poorly differentiated squamous cell carcinoma. Scattered squamous cell islands and nuclear heterogeneous cells were seen. The right lymph node revealed a small round epithelial malignancy with low differentiation, and pathological mitotic figures could be seen (Figures 1H–K). Immunohistochemical staining was positive for CK5/6, CKH, P63, P40, CD5, CD117, and the Ki67 index was over 95%, while CK7, S100, CD56, SYN, NSE, and CHGA were negative. The results were consistent with squamous cell carcinoma (Figures 1L–S).
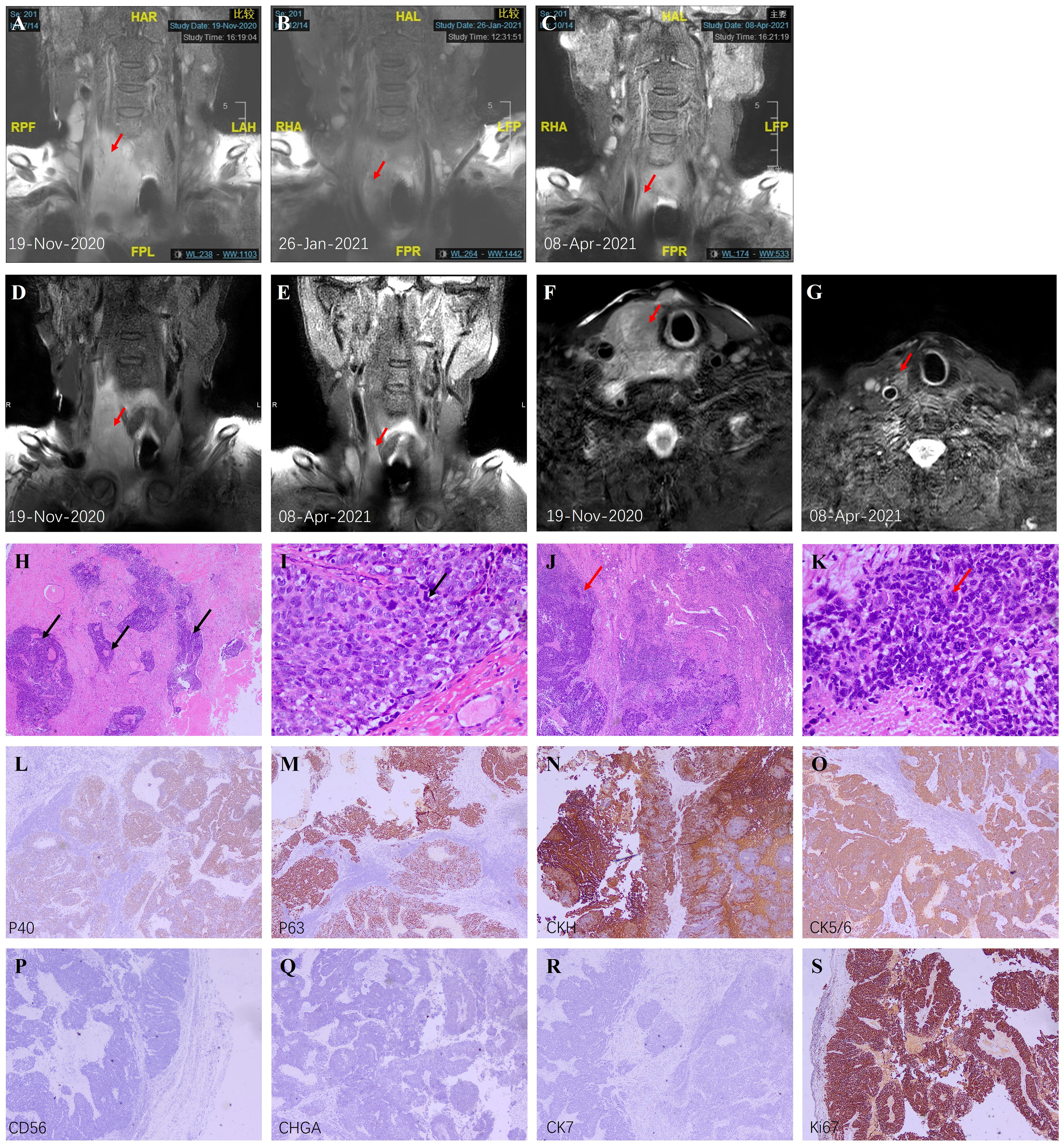
Figure 1 Imaging and pathological findings. (A–C) Coronal T2-weighted MRI scans before treatment, after 2 courses, and after 5 courses; (D, E) Coronal high-resolution modified Dixon T1-weighted MRI scans of thyroid before and after 5 cycles; (F, G) Axial high-resolution modified Dixon T2-weighted MRI scans of thyroid before and after 5 cycles. The red arrows indicate the thyroid nodule. (H) Low magnification of PSCCT (H&E, ×40) showed scattered islands of squamous cells (black arrows) with few typical follicular structures. (I) High magnification of PSCCT (H&E, ×400) showed poorly differentiated tumor cells and typical nuclear heterogeneous cells (black arrows). (J) Low magnification of right lymph node metastasis (H&E, ×40) showed diffuse proliferation of tumor tissue (red arrow). (K) High magnification of right lymph node metastasis (H&E, ×400) showed epithelioid appearing neoplastic cells lacking differentiation, and abnormal mitotic figures (red arrow) could be seen. (L–S) Immunohistochemistry of the right lymph node metastasis (×40). P40 (L), P63 (M), CKH (N), and CK5/6 (O) were positive, indicating the source of squamous cells. CD56 (P) and CHGA (Q) are markers of neuroendocrine cells, while CK7 (R) is a marker of adenocarcinoma. Negative results suggested the low possibility of these two sources. (S) Ki67 index was over 95% suggesting vigorous proliferation excluding simple squamous metaplasia.
Subsequently, the patient underwent esophagoscopy, gastroscopy, and duodenoscope to rule out upper gastrointestinal tract origin tumors. Combined with previous bronchoscopy and pathological findings, the diagnosis of PSCCT was confirmed. Based on previous case reports (1, 8, 9) and retrospective studies (5, 6), the diagnosis was adequate. We also ran a genetic test to assess tumor mutation burden. Unfortunately, BRAF, HRAS, NRAS, TERT mutations and RET, PPARG, NTRK3 fusion were all negative. Due to the unresectable locally advanced tumor, the patient was treated with Anlotinib (Focus V®, Jiangsu Chia-Tai Tianqing Pharmaceutical and Advenchen Laboratories) 12 mg once daily taken orally continuously for 2 weeks of each 3-week cycle combined with Sintilimab (Tyvyt®, Innovent Biologics and Eli Lilly and Company) 200 mg once every 21 days. The patients were evaluated before every 3 treatment cycles. Surprisingly, after 2 courses, the volume of the tumor reduced significantly (maximum diameter from 11.17cm to 8.52cm, Figure 1B), and the symptoms of compression were relieved. Except for reversible leucopenia and skin pruritus, other laboratory tests were normal, and there were no other complications. MRI images showed almost no significant abnormalities in the original tumor site after 5 courses (Figures 1C, E, G).
On April 25, 2021, the patient was sent to the local hospital for malignant vomiting, anorexia, and disturbance of consciousness. He had an unexplained elevation of AST, ALT, GGT, and bilirubin with progressive aggravation (Supplement Table 1). He was diagnosed with fulminant liver failure and autoimmune liver disease in the local hospital. Three days later, the patient died there. The timeline of the major diagnosis and treatment is shown in Supplement Table 2.
3 Discussion
PSCCT usually appears in 50 to 60 years old women. Patients normally present with dyspnea or dysphagia for the airway and esophagus compressed by the tumor, and hoarseness for tumor invading recurrent laryngeal nerve (10). The origin of PSCCT is controversial because thyroid tissue itself does not have squamous cells. There are two common hypotheses, the “embryonic rest” theory (which holds that cancer cells develop from the remnants of the thyroglossal canal) and the “metaplasia” theory (4).
Pathologically, PSCCT showed islands of differentially differentiated squamous cells with distinct atypia and intercellular bridging (11). Immunohistochemistry is essential for the diagnosis of PSCCT. Typical positive stains include keratin, thyroglobulin, P40, P63, and Ki67 (1, 6, 12). P63 and P40 are sensitive markers of squamous cell differentiation, and P40 is more specific than P63 in recognizing squamous cell carcinoma (13). CK5/6 helps differentiate whether poorly differentiated squamous cell carcinoma is primary (14). Ki67 index is a marker of cell proliferation and about 80% PSCCT has a Ki67 index of 30% or above (6). Our patient Ki67 index was over 95%, demonstrating tumor proliferation and not just squamous metaplasia.
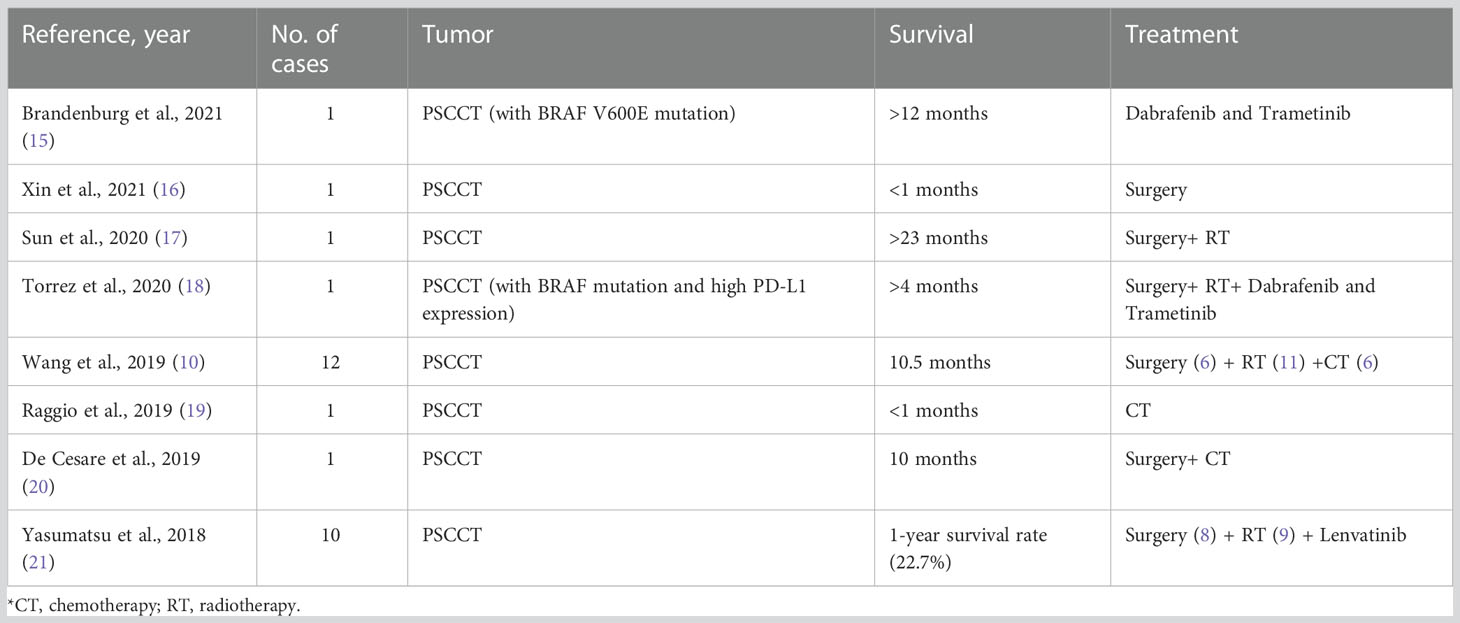
PSCCT has a poor response to radiotherapy, chemotherapy, and radioactive iodine ablation (5–7). Reviewing the literature on PSCCT in recent years (Table 1), surgery is a useful method for improving the survival rate. Moreover, it is worth noting that TKIs are increasingly being used as a trial treatment and are expected to prolong the survival of patients with PSCCT.
In the past decade, one significant advance in thyroid cancer is the molecular pathogenesis (22). The pathogenesis of thyroid cancer involves multiple steps. Changes in oncogenes and anti-oncogenes lead to abnormal cell proliferation, while changes in vascular growth factors lead to tumor invasion and spread (23). MAPK and PI3K/AKT are the most representative pathways which affect cell proliferation and differentiation (24). Common genetic changes in the MAPK pathway include proximal RET/PTC translocation and distal RAS and BRAF mutations, while common mutations in the PI3K pathway include RAS, PIK3CA, AKT1 and PTEN mutations (25). These two signaling pathways are coupled to the receptor tyrosine kinase (RTK), a transmembrane glycoprotein that conveys extracellular growth signals. RTK is the most frequently altered signaling pathway in all cancer types (26). Abnormal activation of RTK is related to the proliferation and metastasis of a variety of tumor cells (27). RTK inhibitors (TKIs) have shown significant antitumor activity in various tumors, including thyroid cancer (28, 29). Sorafenib, vandetanib and cabozantinib have been shown to be first-line therapies for advanced thyroid malignancies (30). Unfortunately, the classic genetic changes in MAPK and PI3K pathways were not detected in our patients, so there are no specific targeted drugs. Therefore, we chose to use Anlotinib, which has more targets and similar tolerances (31).
Anlotinib is a novel tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor (VEGFR), fibroblasts growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR) and C-Kit. The drug has a broad-spectrum inhibitory effect on cell growth and angiogenesis (32). Anlotinib is currently approved as a third-line drug in non-small cell lung cancer (NSCLC) (33), and has been studied in differentiated thyroid cancer and medullary thyroid cancer. In preclinical models of papillary thyroid carcinoma (PTC) and anaplastic thyroid carcinoma (ATC), Ruan et al. (34) found that Anlotinib can affect cell viability by interfering with spindle assembly, leading to G2/M phase stagnation and activation of TP53. In addition, Anlotinib inhibits the migration of tumor cells in vitro and affects the growth of xenograft thyroid tumors in vivo. In a randomized, placebo-controlled Phase IIB trial, 91 patients with histopathological proven and unresectable medullary thyroid carcinoma (MTC) were enrolled. Anlotinib significantly extended median progression-free survival (11.1 to 20.7 months) (35). These studies demonstrate the therapeutic potential of Anlotinib in thyroid tumors.
Immune checkpoint proteins, including programmed cell death receptor 1 (PD-1), are involved in immune regulation of tumor (36). They may be utilized by tumor cells expressing PD-L1 to evade immune surveillance (37). Inhibiting immune checkpoints can promote antitumor immunity, thereby eliminating tumor cells (38). PD-1/PD-L1 inhibitors have been widely used in tumor immunotherapy, including thyroid tumors (39–41). Some subtypes, such as ATC, show a high level of PD-L1 expression, suggesting that such patients may be sensitive to PD-1/PD-L1 inhibitors (42). Currently, more than a dozen clinical studies have used PD-1/PD-L1 inhibitors in the treatment of unresectable, recurrent, and/or metastatic thyroid tumors (39), demonstrating that PD-1/PD-L1 inhibitors may be a “life-saving” option for advanced thyroid tumors.
Sintilimab is a monoclonal antibody of human origin IgG4. It binds to PD-1, blocking the interaction between PD-1 and its ligand, thereby restoring the function of endogenous T cell (43). According to a multicenter Phase II study, average PD-1 receptor occupancy ≥95% (44). Sintilimab was first used for relapsed or refractory Hodgkin’s lymphoma (43). Although it was widely used in various solid tumors such as NSCLC and squamous cell esophageal carcinoma, showing exciting antitumor activity (45), it has not been studied in thyroid tumors. We examined PD-L1 expression in accordance with previous clinical studies of Sintilimab (46). The 22C3 pharmDx (Dako) kit was used for staining, and positive and negative controls were set on the same slice. The whole experiment was carried out under the standard procedure (47). We used the combined positive score (CPS) for assessment because CPS may be more relevant to the benefit of anti-PD-1 therapy than the tumor proportion score (48). The PD-L1 CPS≥10 in thyroid tumor tissue sections indicated that the patient was likely to benefit from the treatment of Sintilimab (Supplement Figure 1).
A growing amount of evidence describes the interaction between tumor immune microenvironment and angiogenesis, which has also been mentioned in previous reviews (49–51). In short, VEGF not only promotes angiogenesis but also affects the immune regulation, helping tumor cells evade immune surveillance (52). It is currently believed that VEGF attenuates the antitumor response through two modes of action. Firstly, VEGF can directly affect the immune cells, for example, inhibiting T cell differentiation and dendritic cell maturation (53, 54). Secondly, by affecting the adhesion molecules of endothelial cells, VEGF affects the transport of lymphocytes and T cells to tumor cells (55). As for Anlotinib, a previous study found that it can down-regulate the expression of PD-L1 on vascular endothelial cells, thereby increasing CD8+T cell infiltration, decreasing FoxP3+T cell aggregation, and relieving immunosuppression (56). Conversely, the enhanced immune microenvironment also affects angiogenesis. IFN-γ is thought to play an essential role in this process. Previous studies have shown that IFN-γ secreted by activated CD8+T cells can mediate anti-angiogenesis (57). IFN-γ has also been shown to directly down-regulate delta-like protein 4 and VEGF mRNA expression on endothelial cells (58, 59). Therefore, we believed that there was a positive feedback regulation before immune regulation and angiogenesis. Accordingly, we speculated the mechanism by which the coordination of Anlotinib and Sintilimab plays an antitumor role (Figure 2).
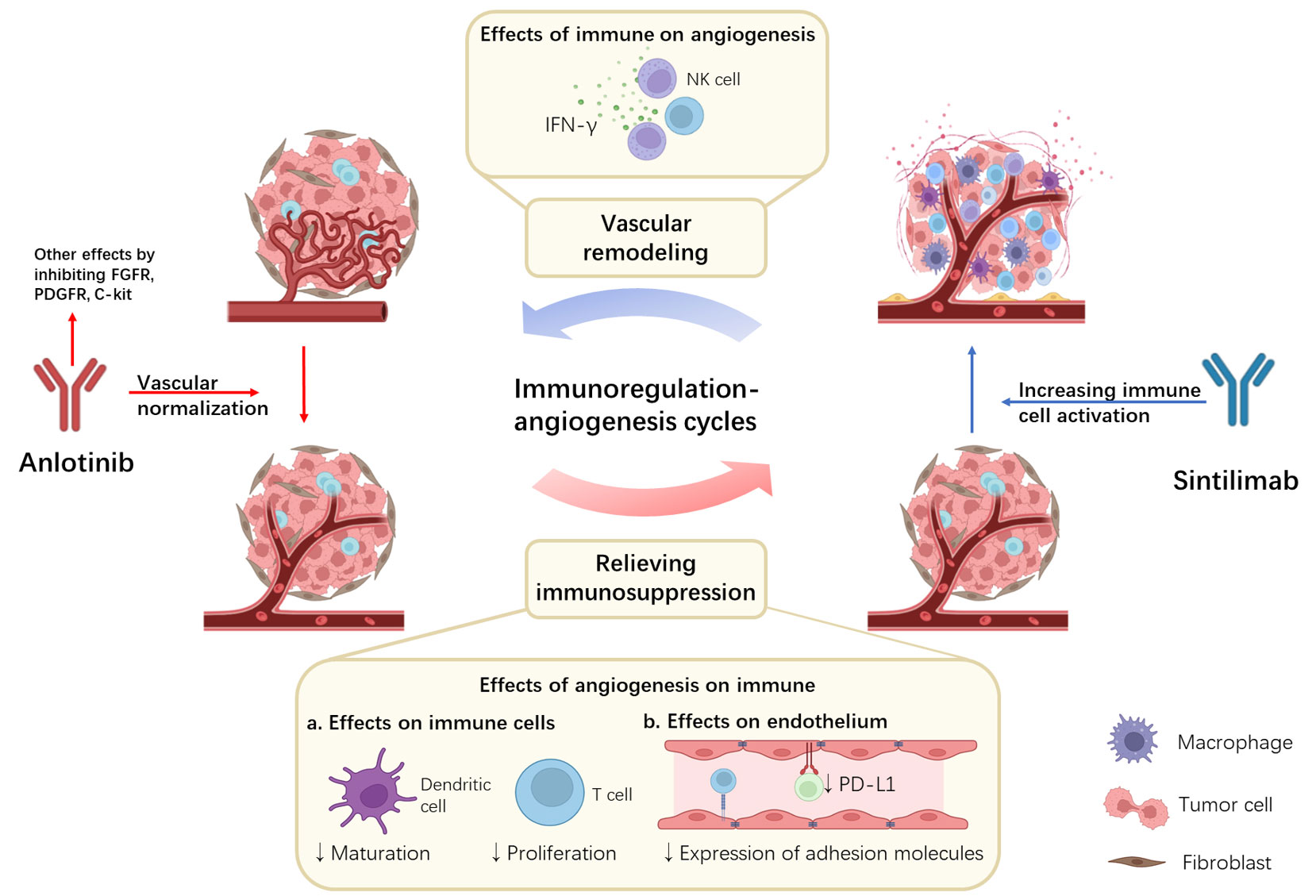
Figure 2 Potential synergistic mechanisms of Anlotinib combined with Sintilimab and the immunoregulation-angiogenesis cycles. Anlotinib inhibits angiogenesis by targeting VEGF. At the same time, the inhibition of VEGF can also relieve immunosuppression in the tumor microenvironment by directly affecting immune cells and indirectly affecting endothelium. Sintilimab binds to PD-1 and blocks its binding to PD-L1 and PD-L2, activating immune cells. At the same time, activated immune cells release large amounts of IFN-γ, further leading to vascular remodeling. Positive feedback is formed between immune regulation and angiogenesis.
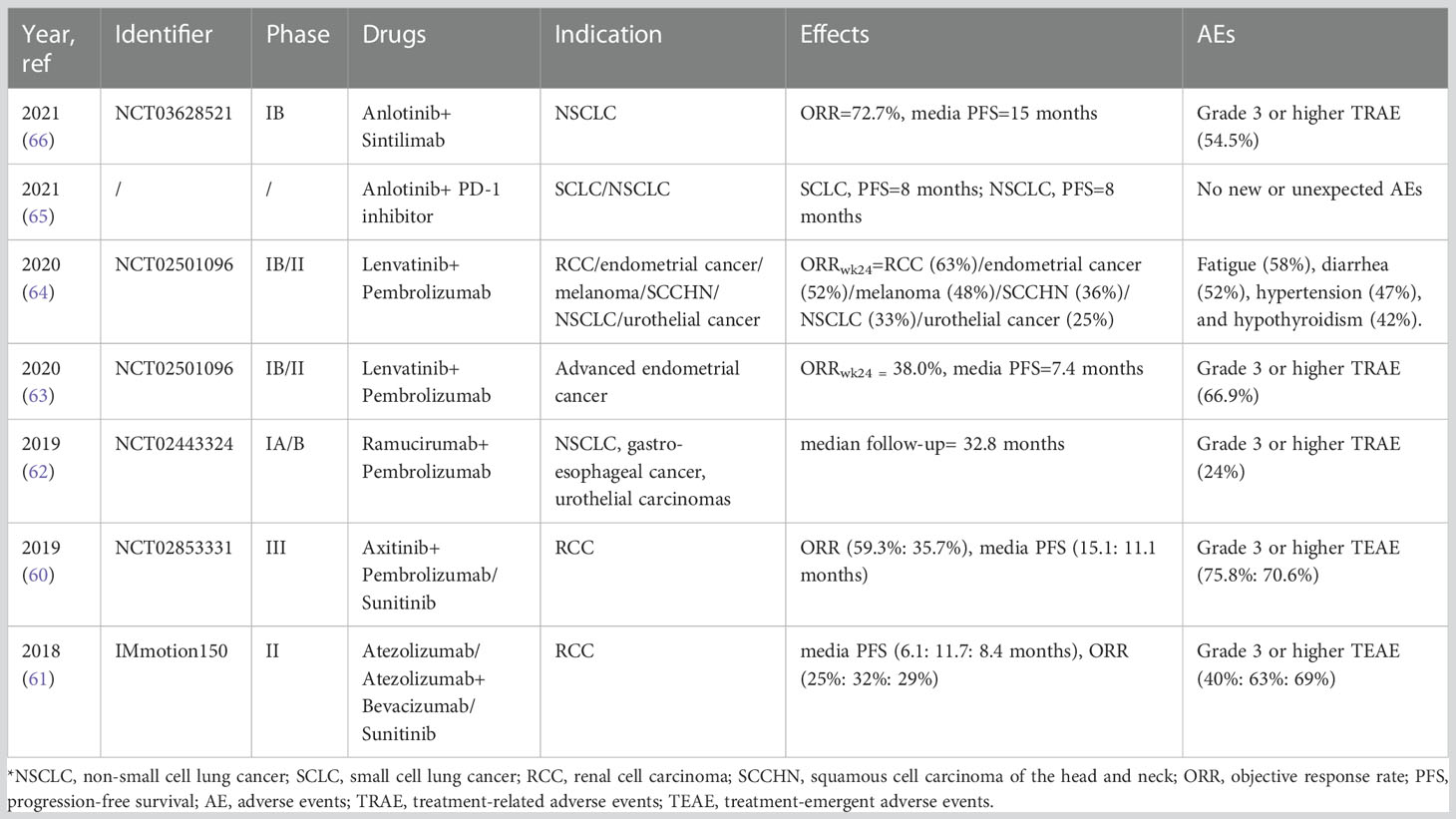
Clinical studies of multiple solid tumors have also reported clinical benefits from the combination of ICIs and antiangiogenic agents (60–64). Among them, the combination of Anlotinib and PD-1 inhibitors (including Sintilimab) has also received widespread attention (65, 66). (Table 2) Moreover, because Anlotinib has a broader range of targets than VEGF inhibitors, it is also thought to have better efficacy (66).
Therefore, our patient received Anlotinib and Sintilimab combination therapy. The result was outstanding. After 2 courses, the tumor significantly decreased, and the compression symptoms were relieved. The tumor was almost invisible after five months of treatment, indicating the ability of ICIs and TKIs combination therapy.
However, the increasing adverse events (AEs) associated with combination therapy should be considered. Although the combination therapy in the current study was generally well tolerated (60–64), the incidence of treatment-related hepatic AE was slightly higher than in the whole study population (although elevated liver enzymes were generally Grade 1/2) (65). Immune-related adverse events (IRAE) caused by immunotherapy have also been widely concerned (67). This patient died of fulminant liver failure and autoimmune liver disease after 5 months of treatment. Typically, most IRAEs occur 3-6 months after ICIs administration (68) and are sensitive to steroids, which resolve within 6-12 weeks (69). About 5% of patients developed immune-associated hepatitis, presenting with an unexplained elevation of AST or ALT (70). Most patients lack symptomatic but abnormal in laboratory tests (71). In addition, Anlotinib by itself causes hypertriglyceridemia and hypercholesterolemia (32), which in combination may increase the risk of immune liver disease. Therefore, the AEs of combination therapy, especially immune liver disease, need special attention.
4 Conclusion
In summary, PSCCT is a rare disease characterized by locally advanced symptoms and poor prognosis. The treatment of PSCCT is limited, and surgical resection is currently the dominant treatment. TKIs combined with ICIs maybe is an effective way for PSCCT treatment. Since this study is a single case report, more randomized controlled clinical studies are expected to be carried out in the future to determine the efficacy. In addition, immune-related adverse reactions should be careful. When TKIs plus ICIs are used together, we need to pay attention to the occurrence of severe liver failure.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine (SH9H-2020-T346-1). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the patients/participants for the publication of this case report.
Author contributions
ZL: literature research and manuscript preparation. MY: manuscript preparation. FZ: treatment of the patient and clinical data collection. CZ: treatment of the patient and guarantee of the integrity of the whole research process. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Clinical Research Program of 9th People’s Hospital, Shanghai Jiao Tong University School of Medicine (JYLJ202016).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.976415/full#supplementary-material
References
1. Booya F, Sebo TJ, Kasperbauer JL, Fatourechi V. Primary squamous cell carcinoma of the thyroid: report of ten cases. Thyroid (2006) 16(1):89–93. doi: 10.1089/thy.2006.16.89
2. Cook AM, Vini L, Harmer C. Squamous cell carcinoma of the thyroid: outcome of treatment in 16 patients. Eur J Surg Oncol (1999) 25(6):606–9. doi: 10.1053/ejso.1999.0715
3. Struller F, Senne M, Falch C, Kirschniak A, Konigsrainer A, Muller S. Primary squamous cell carcinoma of the thyroid: Case report and systematic review of the literature. Int J Surg Case Rep (2017) 37:36–40. doi: 10.1016/j.ijscr.2017.06.011
4. Syed MI, Stewart M, Syed S, Dahill S, Adams C, McLellan DR, et al. Squamous cell carcinoma of the thyroid gland: primary or secondary disease? J Laryngol Otol (2011) 125(1):3–9. doi: 10.1017/S0022215110002070
5. Au JK, Alonso J, Kuan EC, Arshi A, St John MA. Primary squamous cell carcinoma of the thyroid: A population-based analysis. Otolaryngol Head Neck Surg (2017) 157(1):25–9. doi: 10.1177/0194599817698436
6. Lam AK-Y. Squamous cell carcinoma of thyroid: a unique type of cancer in world health organization classification. Endocr Relat Cancer (2020) 27(6):R177–R92. doi: 10.1530/ERC-20-0045
7. Yang S, Li C, Shi X, Ma B, Xu W, Jiang H, et al. Primary squamous cell carcinoma in the thyroid gland: A population-based analysis using the SEER database. World J Surg (2019) 43(5):1249–55. doi: 10.1007/s00268-019-04906-2
8. Shrestha M, Sridhara SK, Leo LJ, Coppit GL, Ehrhardt NM. Primary squamous cell carcinoma of the thyroid gland: a case report and review. Head Neck (2013) 35(10):E299–303. doi: 10.1002/hed.23152
9. Liu G, Xu X, Chen G, Liu Z. Analysis of primary and secondary squamous cell carcinoma of the thyroid gland: a retrospective study. Gland Surg (2021) 10(2):559–66. doi: 10.21037/gs-20-628
10. Wang W, Ouyang Q, Meng C, Jing L, Li X. Treatment optimization and prognostic considerations for primary squamous cell carcinoma of the thyroid. Gland Surg (2019) 8(6):683–90. doi: 10.21037/gs.2019.11.07
11. Sahoo M, Bal CS, Bhatnagar D. Primary squamous-cell carcinoma of the thyroid gland: new evidence in support of follicular epithelial cell origin. Diagn Cytopathol (2002) 27(4):227–31. doi: 10.1002/dc.10178
12. Lam KY, Lo CY, Liu MC. Primary squamous cell carcinoma of the thyroid gland: an entity with aggressive clinical behaviour and distinctive cytokeratin expression profiles. Histopathology (2001) 39(3):279–86. doi: 10.1046/j.1365-2559.2001.01207.x
13. Bishop JA, Teruya-Feldstein J, Westra WH, Pelosi G, Travis WD, Rekhtman N. p40 (ΔNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol (2012) 25(3):405–15. doi: 10.1038/modpathol.2011.173
14. Kaufmann O, Fietze E, Mengs J, Dietel M. Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am J Clin Pathol (2001) 116(6):823–30. doi: 10.1309/21TW-2NDG-JRK4-PFJX
15. Brandenburg T, Muchalla P, Theurer S, Schmid KW, Führer D. Therapeutic effect of combined dabrafenib and trametinib treatment of BRAF V600E-mutated primary squamous cell carcinoma of the thyroid: A case report. Eur Thyroid J (2021) 10(6):511–6. doi: 10.1159/000518055
16. Xin S, Li W, Yuan N, Shen C, Zhang D, Chai S. Primary squamous cell carcinoma of the thyroid: a case report. J Int Med Res (2021) 49(4):3000605211004702. doi: 10.1177/03000605211004702
17. Sun B-H, Yu S-T, Ge J-N, Lei S-T. Primary squamous cell carcinoma (PSCC) of the thyroid: a case report and review of the literature. Gland Surg (2020) 9(2):474–7. doi: 10.21037/gs.2020.02.18
18. Torrez M, Braunberger RC, Yilmaz E, Agarwal S. Primary squamous cell carcinoma of thyroid with a novel BRAF mutation and high PDL-1 expression: A case report with treatment implications and review of literature. Pathol Res Pract (2020) 216(10):153146. doi: 10.1016/j.prp.2020.153146
19. Raggio B, Barr J, Ghandour Z, Friedlander P. Primary squamous cell carcinoma of the thyroid. Ochsner J (2019) 19(3):290–2. doi: 10.31486/toj.18.0002
20. De Cesare A, Di Cristofano C, Di Filippo AR, Salesi N, Spaziani M, Picchio M, et al. Total thyroidectomy associated to chemotherapy in primary squamous cell carcinoma of the thyroid. Clin Ter (2019) 170(4):e231–e4. doi: 10.7417/CT.2019.2138
21. Yasumatsu R, Sato M, Uchi R, Nakano T, Hashimoto K, Kogo R, et al. The treatment and outcome analysis of primary squamous cell carcinoma of the thyroid. Auris Nasus Larynx (2018) 45(3):553–7. doi: 10.1016/j.anl.2017.07.009
22. Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA (2013) 309(14):1493–501. doi: 10.1001/jama.2013.3190
23. Fagin JA, Mitsiades N. Molecular pathology of thyroid cancer: diagnostic and clinical implications. Best Pract Res Clin Endocrinol Metab (2008) 22(6):955–69. doi: 10.1016/j.beem.2008.09.017
24. Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet (2013) 381(9871):1058–69. doi: 10.1016/S0140-6736(13)60109-9
25. Gild ML, Bullock M, Robinson BG, Clifton-Bligh R. Multikinase inhibitors: a new option for the treatment of thyroid cancer. Nat Rev Endocrinol (2011) 7(10):617–24. doi: 10.1038/nrendo.2011.141
26. Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell (2018) 173(2):321–37.e10. doi: 10.1016/j.cell.2018.03.035
27. Regad T. Targeting RTK signaling pathways in cancer. Cancers (Basel) (2015) 7(3):1758–84. doi: 10.3390/cancers7030860
28. Ferguson FM, Gray NS. Kinase inhibitors: the road ahead. Nat Rev Drug Discovery (2018) 17(5):353–77. doi: 10.1038/nrd.2018.21
29. Gild ML, Tsang VHM, Clifton-Bligh RJ, Robinson BG. Multikinase inhibitors in thyroid cancer: timing of targeted therapy. Nat Rev Endocrinol (2021) 17(4):225–34. doi: 10.1038/s41574-020-00465-y
30. Pacini F, Castagna MG, Brilli L, Pentheroudakis G. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2012) 23 Suppl 7:vii110–vii9. doi: 10.1093/annonc/mds230
31. Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol (2018) 11(1):120. doi: 10.1186/s13045-018-0664-7
32. Sun Y, Niu W, Du F, Du C, Li S, Wang J, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol (2016) 9(1):105. doi: 10.1186/s13045-016-0332-8
33. Zhou M, Chen X, Zhang H, Xia L, Tong X, Zou L, et al. China National medical products administration approval summary: anlotinib for the treatment of advanced non-small cell lung cancer after two lines of chemotherapy. Cancer Commun (Lond) (2019) 39(1):36. doi: 10.1186/s40880-019-0383-7
34. Ruan X, Shi X, Dong Q, Yu Y, Hou X, Song X, et al. Antitumor effects of anlotinib in thyroid cancer. Endocr Relat Cancer (2019) 26(1):153–64. doi: 10.1530/ERC-17-0558
35. Li D, Chi Y, Chen X, Ge M, Zhang Y, Guo Z, et al. Anlotinib in locally advanced or metastatic medullary thyroid carcinoma: A randomized, double-blind phase IIB trial. Clin Cancer Res (2021) 27(13):3567–75. doi: 10.1158/1078-0432.CCR-20-2950
36. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin (2020) 70(2):86–104. doi: 10.3322/caac.21596
37. Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother (2005) 54(4):307–14. doi: 10.1007/s00262-004-0593-x
38. Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A (2002) 99(19):12293–7. doi: 10.1073/pnas.192461099
39. D'Andréa G, Lassalle S, Guevara N, Mograbi B, Hofman P. From biomarkers to therapeutic targets: the promise of PD-L1 in thyroid autoimmunity and cancer. Theranostics (2021) 11(3):1310–25. doi: 10.7150/thno.50333
40. Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer (2022) 21(1):28. doi: 10.1186/s12943-021-01489-2
41. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu Rev Pathol (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741
42. Mehnert JM, Varga A, Brose MS, Aggarwal RR, Lin C-C, Prawira A, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer (2019) 19(1):196. doi: 10.1186/s12885-019-5380-3
43. Hoy SM. Sintilimab: First global approval. Drugs (2019) 79(3):341–6. doi: 10.1007/s40265-019-1066-z
44. Shi Y, Su H, Song Y, Jiang W, Sun X, Qian W, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, phase 2 trial. Lancet Haematol (2019) 6(1):e12–e9. doi: 10.1016/S2352-3026(18)30192-3
45. Liu X, Yi Y. Recent updates on sintilimab in solid tumor immunotherapy. biomark Res (2020) 8(1):69. doi: 10.1186/s40364-020-00250-z
46. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ (2022) 377:e068714. doi: 10.1136/bmj-2021-068714
47. Lin D, Liu D, Chen G, Li Y, Shen W, Yang W, et al[Consensus on the immunohistochemical tests of PD-L1 in solid tumors (2021 version)]. Zhonghua Bing Li Xue Za Zhi (2021) 50(7):710–8. doi: 10.3760/cma.j.cn112151-20210228-00172
48. Sun J-M, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet (2021) 398(10302):759–71. doi: 10.1016/S0140-6736(21)01234-4
49. Rahma OE, Hodi FS. The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res (2019) 25(18):5449–57. doi: 10.1158/1078-0432.CCR-18-1543
50. Manegold C, Dingemans A-MC, Gray JE, Nakagawa K, Nicolson M, Peters S, et al. The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol (2017) 12(2):194–207. doi: 10.1016/j.jtho.2016.10.003
51. Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol (2018) 15(5):310–24. doi: 10.1038/nrclinonc.2018.9
52. Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res (2001) 23(2-3):263–72. doi: 10.1385/IR:23:2-3:263
53. Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med (1996) 2(10):1096–103. doi: 10.1038/nm1096-1096
54. Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood (1998) 92(11):4150–66. doi: 10.1182/blood.V92.11.4150
55. Bouzin C, Brouet A, De Vriese J, Dewever J, Feron O. Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J Immunol (2007) 178(3):1505–11. doi: 10.4049/jimmunol.178.3.1505
56. Liu S, Qin T, Liu Z, Wang J, Jia Y, Feng Y, et al. Anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis (2020) 11(5):309. doi: 10.1038/s41419-020-2511-3
57. Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H, et al. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res (2003) 63(14):4095–100.
58. Deng J, Liu X, Rong L, Ni C, Li X, Yang W, et al. IFNγ-responsiveness of endothelial cells leads to efficient angiostasis in tumours involving down-regulation of Dll4. J Pathol (2014) 233(2):170–82. doi: 10.1002/path.4340
59. Lu Y, Yang W, Qin C, Zhang L, Deng J, Liu S, et al. Responsiveness of stromal fibroblasts to IFN-gamma blocks tumor growth via angiostasis. J Immunol (2009) 183(10):6413–21. doi: 10.4049/jimmunol.0901073
60. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med (2019) 380(12):1116–27. doi: 10.1056/NEJMoa1816714
61. McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med (2018) 24(6):749–57. doi: 10.1038/s41591-018-0053-3
62. Herbst RS, Arkenau H-T, Santana-Davila R, Calvo E, Paz-Ares L, Cassier PA, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol (2019) 20(8):1109–23. doi: 10.1016/S1470-2045(19)30458-9
63. Makker V, Taylor MH, Aghajanian C, Oaknin A, Mier J, Cohn AL, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol (2020) 38(26):2981–92. doi: 10.1200/JCO.19.02627
64. Taylor MH, Lee C-H, Makker V, Rasco D, Dutcus CE, Wu J, et al. Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J Clin Oncol (2020) 38(11):1154–63. doi: 10.1200/JCO.19.01598
65. Zhang X, Zeng L, Li Y, Xu Q, Yang H, Lizaso A, et al. Anlotinib combined with PD-1 blockade for the treatment of lung cancer: a real-world retrospective study in China. Cancer Immunol Immunother (2021) 70(9):2517–28. doi: 10.1007/s00262-021-02869-9
66. Chu T, Zhong R, Zhong H, Zhang B, Zhang W, Shi C, et al. Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J Thorac Oncol (2021) 16(4):643–52. doi: 10.1016/j.jtho.2020.11.026
67. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016
68. Weber JS, Antonia SJ, Topalian SL, Schadendorf D, Larkin JMG, Sznol M, et al. Safety profile of nivolumab (NIVO) in patients (pts) with advanced melanoma (MEL): A pooled analysis. J Clin Oncol (2015) 33(15). doi: 10.1200/jco.2015.33.15_suppl.9018
69. Weber JS, Dummer R, de Pril V, Lebbé C, Hodi FS. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer (2013) 119(9):1675–82. doi: 10.1002/cncr.27969
70. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med (2015) 372(26):2521–32. doi: 10.1056/NEJMoa1503093
Keywords: primary squamous cell carcinoma of the thyroid, tyrosine kinase inhibitors, immune checkpoint inhibitors, Anlotinib, Sintilimab, immune-related adverse reactions, autoimmune liver disease
Citation: Liu Z, Yu M, Zhao F and Zhu C (2023) Anlotinib combined with Sintilimab is win-win cooperation for primary squamous cell carcinoma of the thyroid: A case report and literature review. Front. Oncol. 13:976415. doi: 10.3389/fonc.2023.976415
Received: 23 June 2022; Accepted: 06 March 2023;
Published: 16 March 2023.
Edited by:
Wu Jing Bo, The Affiliated Hospital of Southwest Medical University, ChinaReviewed by:
Lei Sheng, Qilu Hospital, Shandong University, ChinaPeng Huang, Xiangya Hospital, Central South University, China
Copyright © 2023 Liu, Yu, Zhao and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenfang Zhu, sammizz1977@126.com; Feng Zhao, phillip_zhao@126.com
†These authors have contributed equally to this work
 Zichang Liu
Zichang Liu Maosheng Yu
Maosheng Yu Feng Zhao
Feng Zhao Chenfang Zhu*
Chenfang Zhu*