- 1Department of Internal Medicine, Chonnam National University Medical School and Hwasun Hospital, Hwasun, Republic of Korea
- 2Departments of Laboratory Medicine, Chonnam National University Medical School and Hwasun Hospital, Hwasun, Republic of Korea
- 3Dxome Co. Ltd., Seongnam-si, Gyeonggi-do, Republic of Korea
- 4Department of Ophthalmology, Chonnam National University Medical School and Hospital, Gwangju, Republic of Korea
- 5Department of Pathology, Chonnam National University Medical School and Hospital, Gwangju, Republic of Korea
Background: Cancer recurrence remains a significant problem, and most postoperative recurrences of non-small cell lung cancer (NSCLC) develop within 5 years after resection. We present a rare case of ultra-late recurrence of NSCLC accompanying choroidal metastasis with KIF13A-RET fusion 14 years after the definitive surgery.
Case description: A 48-year-old female patient who had never-smoked presented with decreased visual acuity. She had been treated with right upper lobe lobectomy followed by adjuvant chemotherapy 14 years prior. Fundus photographs revealed bilateral choroidal metastatic lesions. Positron emission tomography-computed tomography (PET-CT) scans showed extensive bone metastases and focal hypermetabolism in the left uterine cervix. An excision biopsy of the uterus showed primary lung adenocarcinoma with immunohistochemistry of TTF-1+. Plasma next-generation sequencing (NGS) identified the presence of KIF13A-RET fusion. After 6 months of selpercatinib therapy, PET-CT revealed a partial response for bone and uterine metastasis and stable disease for choroidal lesions.
Conclusion: In this case report, we are reporting a rare case of ultra-late recurrence of NSCLC in a patient with choroidal metastasis. Furthermore, the diagnosis of NSCLC with RET fusion was based on liquid-based NGS rather than tissue-based biopsy. The patient showed a good response to selpercatinib, which supports the efficacy of selpercatinib as a treatment for RET-fusion-positive NSCLC with choroidal metastasis.
1 Introduction
Lung cancer is the most common cause of cancer-related deaths worldwide. Complete surgical resection including adjuvant therapy is the most effective treatment for non-small cell lung cancer (NSCLC). Recurrence remains a major problem, and most postoperative recurrences of NSCLC develop within 5 years after resection. However, ultra-late recurrence developing more than 10 years after resection is extremely rare (1).
As many chemotherapeutic and targeted agents were developed, the survival of advanced NSCLC was extended. However, this improvement was not indicated for rare genetic alterations such as RET fusion, which is detected in 1% to 2% of NSCLC cases (2). Selpercatinib is a novel, adenosine triphosphate competitive, highly selective small-molecule inhibitor of RET kinase. As this medicine was approved for metastatic NSCLC with RET alteration, treatment response and survival have improved (3).
In this report, we present a rare case of ultra-late recurrence of NSCLC accompanying choroidal metastasis with KIF13A-RET fusion 14 years after the definitive surgery.
2 Case presentation
A 48-year-old female patient who had never smoked presented with decreased visual acuity. On April 2008, she received right upper lobe lobectomy to treat lung adenocarcinoma with clinical stage IA (T1N0M0), according to the TNM Classification of Malignant Tumors, 7th edition. Her pathological stage was Stage IIIB (T4N1M0), and she received four cycles of adjuvant chemotherapy with paclitaxel and cisplatin. On August 2015, there was no evidence of disease, and regular cancer surveillance was terminated. In December 2021, she revisited our hospital with decreased visual acuity for about 3 months. Fundus photographs revealed bilateral choroidal metastatic lesions (Figures 1A–C, E), but computed tomography showed no definitive lung mass. Positron emission tomography-computed tomography (PET-CT) scans showed extensive bone metastatic lesions and focal hypermetabolism in the left uterine cervix (Figure 2). Magnetic resonance image (MRI) revealed two tiny metastases in the left middle frontal gyrus and lingual gyrus on brain. On pelvic MRI, there was a 6.0 × 4.3 × 2.1 cm heterogeneously enhancing mass in the endocervical canal. On January 2022, pathology from the excision biopsy of the uterus showed primary lung adenocarcinoma with an immunohistochemistry of TTF-1+, CK7+ and Ki-67+ without PD-L1 expression, which were similar findings as those from previously resected tissue in 2008 (Figures 3A, B). The real-time polymerase chain reactions to epidermal growth factor receptor and ROS proto-oncogene 1 receptor tyrosine kinase (ROS1) were negative, and anaplastic lymphoma kinase (ALK) fluorescence in situ hybridization was also negative. Subsequently, she received four cycles of chemotherapy with pemetrexed and cisplatin, and stable disease was observed. However, she suffered anaphylaxis during the 2nd cycle of cisplatin injection, so it was discontinued. We performed next-generation sequencing (NGS) of the uterine tissue and plasma, and then identified the presence of the KIF13A-RET fusion gene from the plasma sample (Figure 3C). Tissue NGS showed MDM2 copy gain (tier II) without RET fusion. The patient began treatment with selpercatinib 160 mg twice daily, based on her plasma result thanks to the Named Patient Program. She also received intravitreal injection of bevacizumab. After 6 months of RET-targeted therapy, PET-CT revealed a partial response for multiple bone metastases (Figure 2). Optical coherence tomography showed improvement of the subretinal fluid and choroidal folds after treatment (Figures 1D, F). She is continuing selpercatinib medication for more than 9 months without any safety issues.
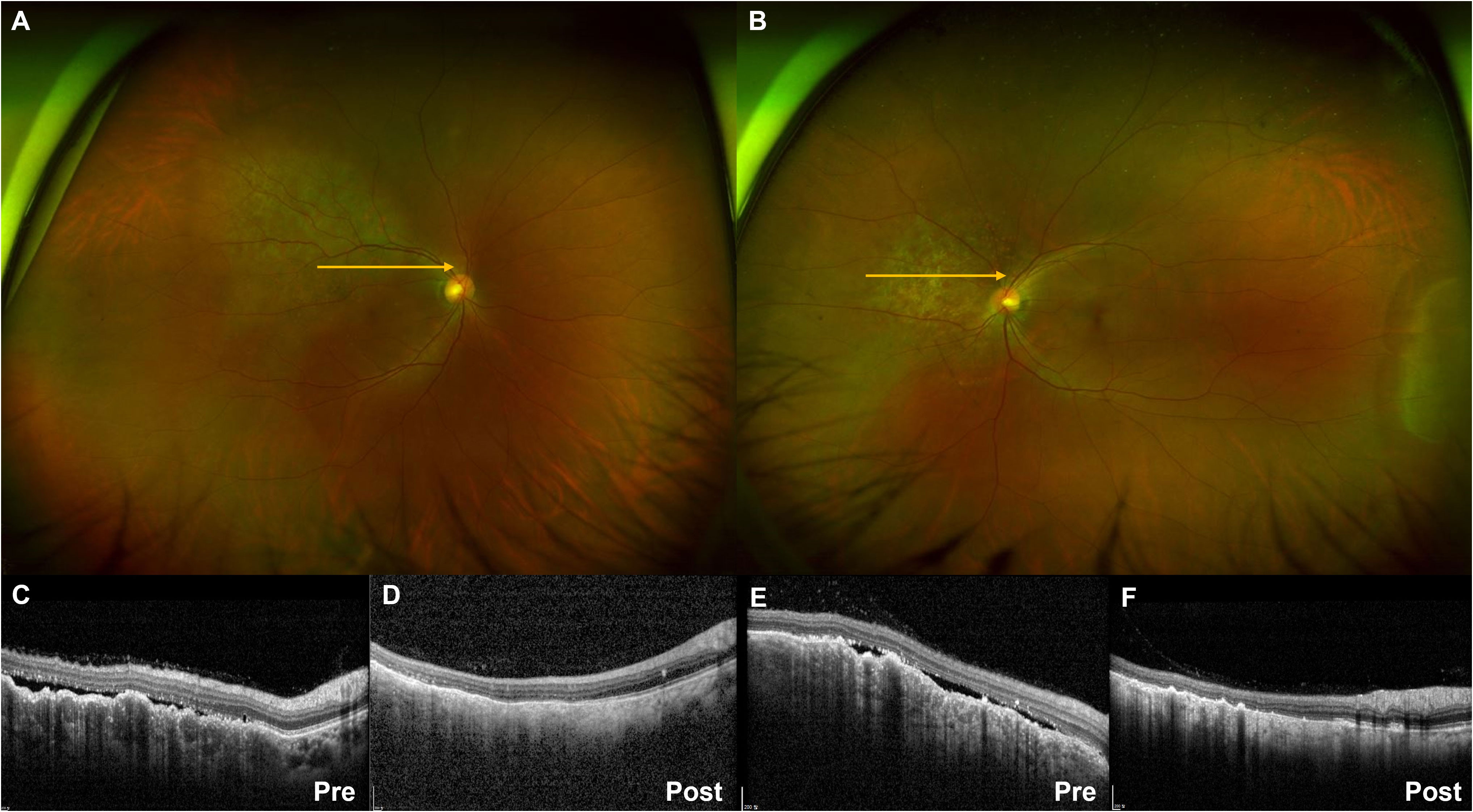
Figure 1 Ultra-widefield fundus photography showing pigmented choroidal elevated lesions in the right (A) and left (B) eye. Yellow arrows indicate the optical coherence tomography (OCT) scan direction. The subretinal fluid and choroidal folds on OCT images before treatment (C, E) showed improvement after selpercatinib treatment (D, F).
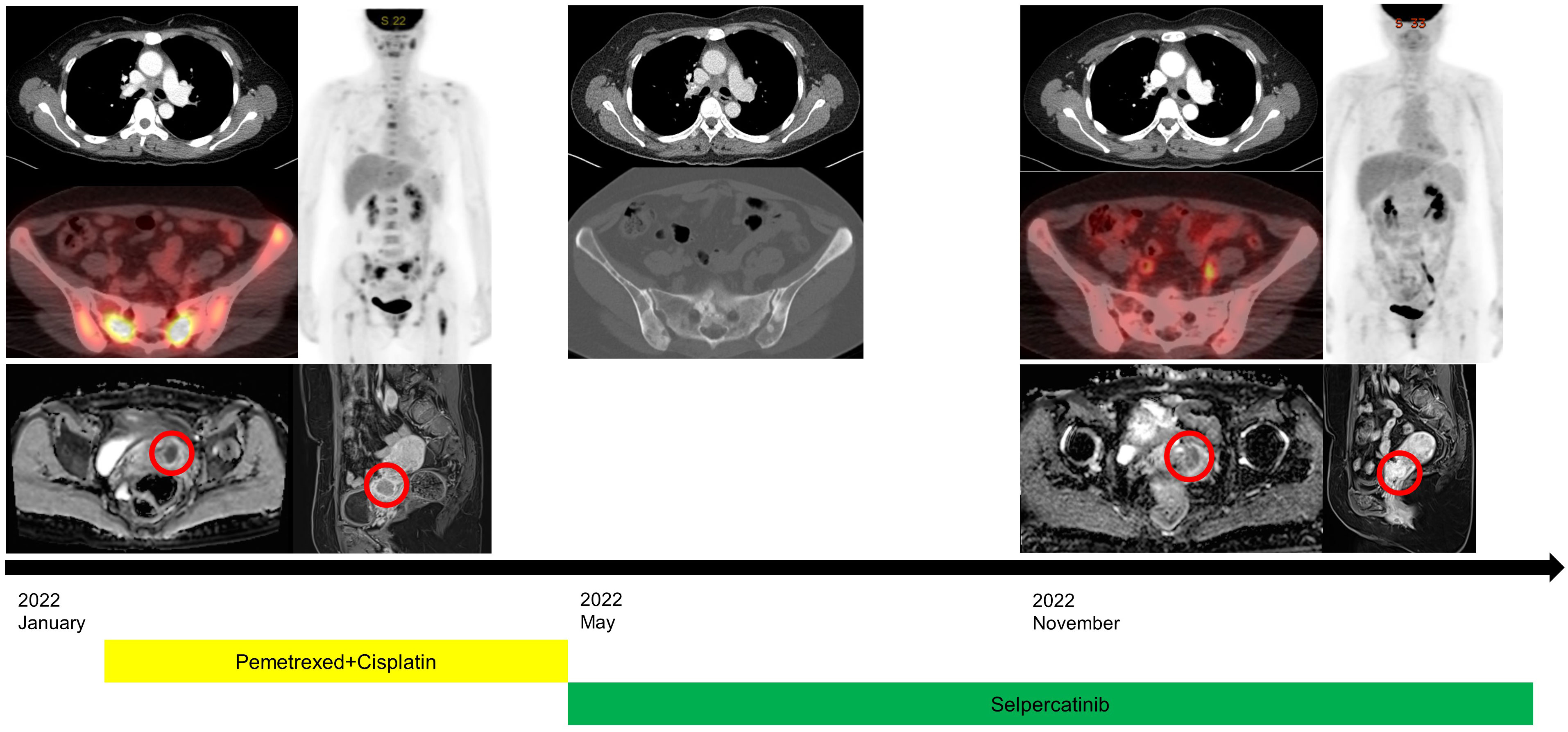
Figure 2 Clinical course with radiological images at initial diagnosis, the beginning of selpercatinib treatment, and after treatment. Positron emission tomography-computed tomography revealed a partial response for multiple bone metastases and slightly decreased size of uterine cervical metastasis (red circles) on pelvic magnetic resonance image.
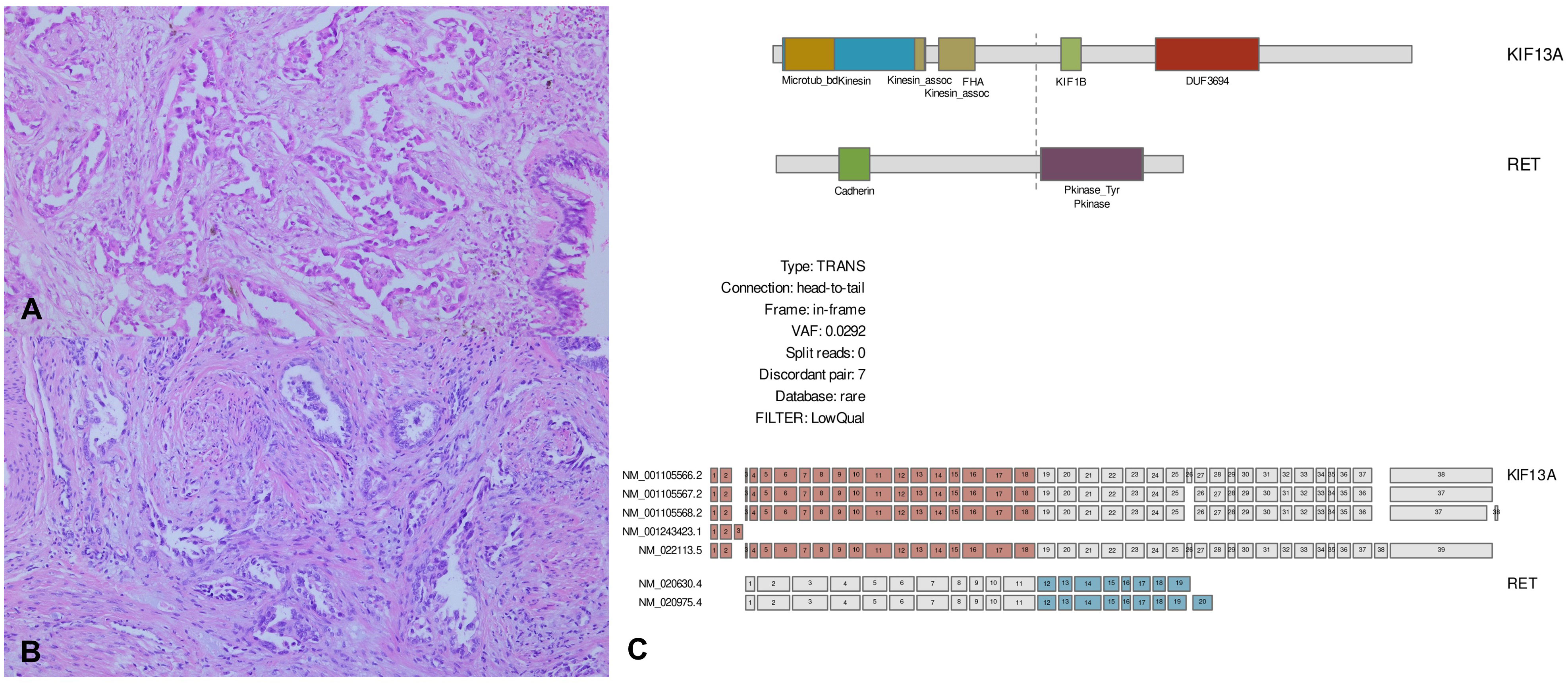
Figure 3 Pathological images and next-generation sequencing (NGS). (A, B) Microscopic findings of the lung surgical specimen and uterine biopsy. Lung adenocarcinoma exhibits an acinar growth pattern in invasive component, which is similar to the histology seen in uterine biopsy (H&E, 100×). (C) Plasma NGS shows KIF13A-RET rearrangement with a variant allele fraction of 0.0292%. Library preparation and target capture were performed using a commercial kit and custom target gene panel (Dxome). Massive parallel sequencing was performed using a high-throughput sequencing machine (Illumina).
3 Discussion
This case exhibits several rare clinical features. First, choroidal metastasis is a relatively rare complication in lung cancer, but lung cancer is the second most common tumor that causes metastasis development in the choroid (4). In choroidal metastasis, adenocarcinoma is the most common histology, and left eye involvement is more common than right eye involvement. Patients who received systemic chemotherapy for choroidal metastasis tend to be younger than median age (4). Choroidal metastasis typically presents only when another organ system is affected by metastasis, and is usually diagnosed before or early after diagnosis of primary lung cancer (5).
Second, our case developed recurrence 14 years after surgical resection. Few cancers tend to recur more than 10 years after resection, which is called ‘ultra-late recurrence.’ There are only a few reported cases of ultra-late recurrence, and those is not well known. Dai et al. studied cases of ultra-late recurrence that developed in 2.5% of total patients who had surgical resection for NSCLC. Their histologic type was adenocarcinoma, and lymphatic invasion significantly influenced ultra-late recurrence (1). Matsuo et al. reported a very rare case of recurrent EML4-ALK fusion-positive lung adenocarcinoma with an interval of 16 years after curative surgery (6). Therefore, patients with ALK and RET gene rearrangement may need long-term follow-up of more than 15 years after surgery.
Third, NGS was performed on both tissue and plasma specimens, and KIF13A-RET fusion was found only in plasma in our case. Liquid biopsy using NGS is a rapidly evolving application because it is convenient and less invasive. Compared to tissue biopsies, liquid biopsies can relieve patient’s discomfort and overcome tumor heterogeneity (7). In our case, thirty nanograms of DNA were used for the experiments, and library preparation was performed using the DxSeq ctDNA Pan100 for the Illumina Platform Kit (Dxome, Seoul, Republic of Korea) according to the manufacturer’s instructions. Paired-end sequencing was performed on the NovaSeq 6000 System (Illumina) with a 300-cycle protocol targeting at least 150 million sequencing reads and >20,000× mean depth.
RET is a transmembrane glycoprotein receptor-tyrosine kinase that is encoded by the RET proto-oncogene on chromosome 10. This kinase helps kidneys and the enteric nervous system during embryogenesis (8). RET fusion is an oncogenic driver that occurs in 1% to 2% of NSCLC patients and has a high occurrence rate in young women, non-smokers, and lung adenocarcinoma (9). KIF5B-RET (68%) is the most frequently observed RET fusion in NSCLC, followed by CCDC6-RET (12%), and KIF13A-RET fusion was classified as one of the rare noncanonical fusion (10). There were two available kinase inhibitors for RET fusions; selpercatinib and pralsetinib. Selpercatinib is the first-developed RET inhibitor. In a phase 1/2 study (LIBRETTO-001, ClinicalTrials.gov Identifier: NCT03157128), the objective response rate (ORR) was 64%, and median progression-free survival was 16.5 months in patients who had previously received platinum-based chemotherapy (3). Selpercatinib treatment showed relatively high intracranial activity: ORR was 82% and the intracranial disease control rate was 100%, and at 6 months, 79% of patients were intracranial progression/death-free (11). Besides, a phase III study (LIBRETTO-432, ClinicalTrials.gov Identifier: NCT04819100) about selpercatinib as an adjuvant therapy after surgery or radiation is enrolling study subject (12). Pralsetinib showed overall responses was 61% of patients who had previous platinum-based chemotherapy in a phase 1/2 study (13).
4 Conclusion
In this report, we treated a rare case of ultra-late recurrence of NSCLC in a patient with choroidal metastasis. Furthermore, the patient was diagnosed with NSCLC with RET-fusion according to liquid-based NGS, rather than tissue-based biopsy. The patient showed a good response to selpercatinib, which supports the efficacy of selpercatinib for treating RET fusion-positive NSCLC with choroidal metastasis.
Data availability statement
The datasets presented in this article are not readily available because of ethical/privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review board of the Chonnam National University Hwasun Hospital. Written informed consent was obtained from the patient for the publication of this case report, including any potentially-identifying images or data.
Author contributions
Conceptualization: H-YP and I-JO. Data curation: H-YP, J-HP, SH, TL and I-JO. Analysis and interpretation of data: H-YP, J-HP, TL and I-JO. Funding acquisition: I-JO. Writing-original draft: H-YP, Y-SJ and, TL. Writing-review & editing: Y-DC and I-JO. Manuscript approval: all authors. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. NRF-2020M3A9G3080330).
Acknowledgments
The biospecimens and data used for this study were provided by the Biobank of Chonnam National University Hwasun Hospital, a member of the Korea Biobank Network. Dxome Co. Ltd performed liquid-based NGS using plasma specimen. This company had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
Author SJH is employed by Dxome Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sonoda D, Matsuura Y, Ichinose J, Nakao M, Ninomiya H, Mun M, et al. Ultra-late recurrence of non-small cell lung cancer over 10 years after curative resection. Cancer Manag Res (2019) 11:6765. doi: 10.2147/CMAR.S213553
2. Wang R, Hu H, Pan Y, Li Y, Ye T, Li C, et al. Ret fusions define a unique molecular and clinicopathologic subtype of non–small-cell lung cancer. J Clin Oncol (2012) 30(35):4352–9. doi: 10.1200/JCO.2012.44.1477
3. Drilon A, Oxnard GR, Tan DS, Loong HH, Johnson M, Gainor J, et al. Efficacy of selpercatinib in ret fusion–positive non–Small-Cell lung cancer. New Engl J Med (2020) 383(9):813–24. doi: 10.1056/NEJMoa2005653
4. Singh N, Kulkarni P, Aggarwal AN, Mittal BR, Gupta N, Behera D, et al. Choroidal metastasis as a presenting manifestation of lung cancer: a report of 3 cases and systematic review of the literature. Medicine (2012) 91(4):179–94. doi: 10.1097/MD.0b013e3182574a0b
5. Kreusel K-M, Wiegel T, Stange M, Bornfeld N, Hinkelbein W, Foerster MH. Choroidal metastasis in disseminated lung cancer: Frequency and risk factors. Am J Ophthalmol (2002) 134(3):445–7. doi: 10.1016/s0002-9394(02)01580-5
6. Matsuo S, Tsukamoto Y, Mabuchi E, Negoro S, Hirota S. A case of late distant Recurrence/Metastasis (≧ 10 years after curative surgery) of anaplastic lymphoma kinase-rearranged lung cancer and the review of similar cases in the literature. Hum Pathology: Case Rep (2020) 22:200421. doi: 10.1016/j.ehpc.2020.200421
7. Pisapia P, Costa JL, Pepe F, Russo G, Gragnano G, Russo A, et al. Next generation sequencing for liquid biopsy based testing in non-small cell lung cancer in 2021. Crit Rev Oncol Hematol (2021) 161:103311. doi: 10.1016/j.critrevonc.2021.103311
8. Drilon A, Hu ZI, Lai GG, Tan DS. Targeting ret-driven cancers: Lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol (2018) 15(3):151–67. doi: 10.1038/nrclinonc.2017.175
9. Chung EK, Yong SH, Lee EH, Kim EY, Chang YS, Lee SH. New targeted therapy for non-small cell lung cancer. Tuberc Respir Dis (2023) 86(1):1–13. doi: 10.4046/trd.2022.0066
10. Xiang C, Guo L, Zhao R, Teng H, Wang Y, Xiong L, et al. Identification and validation of noncanonical RET fusions in non-small-cell lung cancer through DNA and RNA sequencing. J Mol Diagn (2022) 24(4):374–85. doi: 10.1016/j.jmoldx.2021.12.004
11. Subbiah V, Gainor JF, Oxnard GR, Tan DS, Owen DH, Cho BC, et al. Intracranial efficacy of selpercatinib in ret fusion-positive non–small cell lung cancers on the libretto-001 trial. Clin Cancer Res (2021) 27(15):4160–7. doi: 10.1158/1078-0432.CCR-21-0800
12. Tsuboi M, Goldman JW, Wu YL, Johnson ML, Paz-Ares L, Yang JC, et al. LIBRETTO-432, a phase III study of adjuvant selpercatinib or placebo in stage IB-IIIA RET fusion-positive non-small-cell lung cancer. Future Oncol (2022) 18(28):3133–41. doi: 10.2217/fon-2022-0656
Keywords: non-small cell lung cancer, selpercatinib, RET fusion, choroidal metastasis, recurrence
Citation: Park H-Y, Park J-H, Shin M-G, Han SJ, Ji Y-S, Oh H-J, Kim Y-C, Lee T, Choi Y-D and Oh I-J (2023) Case Report: A case of ultra-late recurrence of KIF13A-RET fusion non-small cell lung cancer response to selpercatinib. Front. Oncol. 13:1178762. doi: 10.3389/fonc.2023.1178762
Received: 03 March 2023; Accepted: 12 April 2023;
Published: 25 April 2023.
Edited by:
Sang-Won Um, Sungkyunkwan University, Republic of KoreaReviewed by:
Jung Seop Eom, Pusan National University Hospital, Republic of KoreaGuozhu Hou, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2023 Park, Park, Shin, Han, Ji, Oh, Kim, Lee, Choi and Oh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: In-Jae Oh, droij@chonnam.ac.kr
 Ha-Young Park1
Ha-Young Park1 Seung Jung Han
Seung Jung Han Yong-Sok Ji
Yong-Sok Ji In-Jae Oh
In-Jae Oh