- Department of Dermatology, The Affiliated Hospital of Qingdao University, Qingdao, China
Objective: To investigate risk factors for advanced melanoma over 50 years of age and to develop and validate a new line chart and classification system.
Methods: The SEER database was screened for patients diagnosed with advanced melanoma from 2010 to 2019 and Cox regression analysis was applied to select variables affecting patient prognosis. The area under curve (AUC), relative operating characteristic curve (ROC), Consistency index (C-index), decision curve analysis (DCA), and survival calibration curves were used to verify the accuracy and utility of the model and to compare it with traditional AJCC tumor staging. The Kaplan-Meier curve was applied to compare the risk stratification between the model and traditional AJCC tumor staging.
Results: A total of 5166 patients were included in the study. Surgery, age, gender, tumor thickness, ulceration, the number of primary melanomas, M stage and N stage were the independent prognostic factors of CSS in patients with advanced melanoma (P<0.05). The predictive nomogram model was constructed and validated. The C-index values obtained from the training and validation cohorts were 0.732 (95%CI: 0.717-0.742) and 0.741 (95%CI: 0.732-0.751). Based on the observation and analysis results of the ROC curve, survival calibration curve, NRI, and IDI, the constructed prognosis model can accurately predict the prognosis of advanced melanoma and performs well in internal verification. The DCA curve verifies the practicability of the model. Compared with the traditional AJCC staging, the risk stratification in the model has a better identification ability for patients in different risk groups.
Conclusion: The nomogram of advanced melanoma and the new classification system were successfully established and verified, which can provide a practical tool for individualized clinical management of patients.
1 Background
The malignant transformation of melanocytes gives rise to the highly aggressive malignant tumor known as melanoma (1). Lymphocyte and hematogenous metastasis can occur in the early stage, with poor prognosis. Its incidence rate increases year by year all over the world, especially in western countries (2). The mean age of onset was 45 years old and increased with age after 50. Although melanoma accounts for only 5% of skin cancers, it has the highest mortality among skin cancers and is the most serious type (3–5). Skin melanoma is the primary subtype in the west, and most patients are in the early stage of diagnosis. Patients with early melanoma obtain a good prognosis by surgical resection of the primary lesion (6). However, for advanced melanoma, especially stage IV melanoma, studies have found that the average five-year survival rate is less than 10%, and the median progression-free survival period is only 1.7 months (7, 8). As a result, the focus and direction of research continue to be on the prognosis and therapy of advanced melanoma.
The prognosis of malignant melanoma is closely related to tumor staging. At present, the 8th edition of the American Joint Committee on Cancer (AJCC) staging system (9), jointly developed by the American Cancer Society and the union for international cancer control (UICC), is widely adopted. The system is based on assessing the primary tumor, regional lymph nodes and lymphatic drainage, and the presence or absence of distant metastasis without considering other clinically significant prognostic markers, including age, gender, ethnicity, and anatomic sites (10–15). Therefore, a more personalized and comprehensive prediction model is needed to predict the prognosis of patients with advanced melanoma.
Prognostic models of nomogram have been established for a variety of tumors (15–18). As a clinical prediction tool, the individual probability of clinical events can be generated by integrating different prognoses and determinant variables, which helps to make a personal prediction on the survival rate of patients (18, 19) and promotes personalized medical treatment (20, 21). Through a retrospective review of the SEER database, this study developed a prognostic nomogram and a new classification system for advanced melanoma in the current study, all while internally verifying their accuracy.
2 Information and methods
2.1 The choice of patients
Using SEER*STata, 5,166 eligible patients with advanced melanoma were selected from the SEER database. Inclusion criteria were as follows: (1) The diagnosis was made from 2010 to 2019; (2) The tissue type code of the International Classification of Tumor Diseases (ICD-O-3) III is 8720-8799, and the anatomical site code of the primary tumor is C 44.0-C 44.9; (3) The first primary tumor. Exclusion criteria: (1) Clinical information is incomplete, such as race, lymph node status, distant organ metastasis status, tumor stage (based on AJCC stage), and the operation type is unknown; (2) The sources of patient reports are limited to autopsy and death certificate; (3) The cause of death is unknown; (4) Follow-up survival time unknown; (5) age < 50 years old. See the flow chart for the specific screening process (Figure 1).
2.2 Building the nomograms
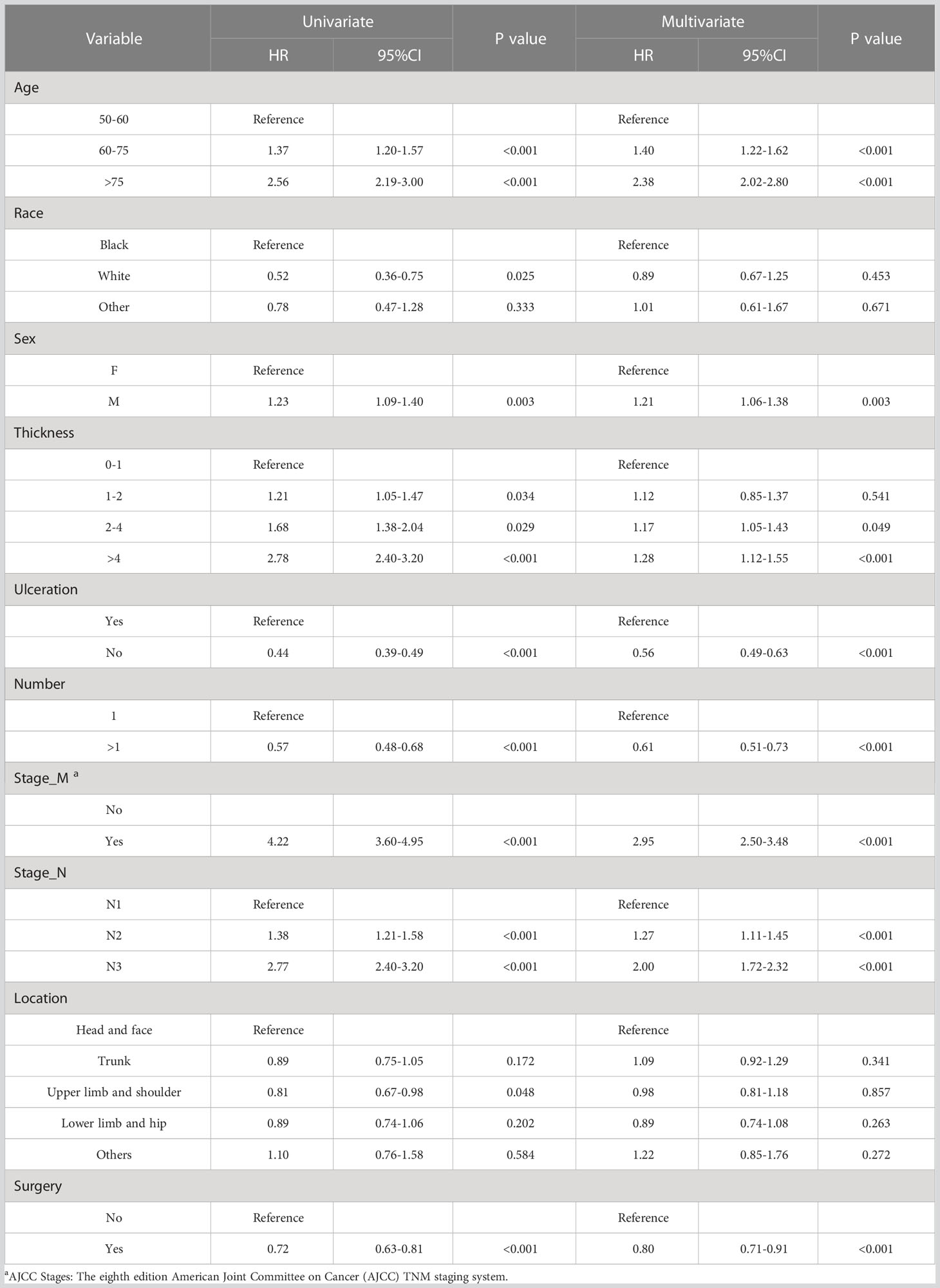
The 5166 samples were randomly allocated into training and validation cohorts in a 7: 3 ratio. The training cohort is used to filter variables and build the model. The validation cohort is used to verify the results obtained using the training cohort. Based on clinical experience and literature review, the following 10 variables were considered possible prognostic influencing factors: age, gender, race, tumor location, tumor thickness, ulceration, N stage, M stage, the number of primary melanomas, and surgery. Tumor staging is based on the Version 8 AJCC Staging System. Prognostic nomogram was constructed based on the variables identified from the univariate and multivariate Cox regression analyses (P<0.05).
2.3 Validation the nomograms
The results of a multivariate Cox regression analysis were applied to establish the nomogram to predict the probability of tumor specific survival (CSS) at 3, 6 and 9 years for melanoma patients. The C-index, time-dependent ROC and calibration plots were employed to evaluate the performance of the model. C-index and AUC values with 0.50-0.70 being less accurate, Moderate accuracy between 0.71-0.90, and Above 0.90 being high accuracy. Values greater than 0.7 for C-index and AUC are generally considered good nomograms. The calibration curves were applied to evaluate the difference between the predicted and actual values of the model. The Net Reclassification Index (NRI), Integrated Discriminant Improvement (IDI) and Decision Curve Analysis (DCA) were utilized to demonstrate the strengths and weaknesses of the different performances of the nomogram compared to the AJCC staging system. NRI, IDI are applied to demonstrate the degree of predictive improvement of the nomogram relative to AJCC staging, and to measure the ability to apply them. DCA curves indicated the magnitude of the net clinical benefit of the nomogram compared to each model.
2.4 Establishment of a new risk classification system and comparison with AJCC staging
We calculated the total points for all patients based on the nomograms’ scores. Based on the X-tile software, the optimal cut-off point for patient risk score was selected to divide all patients into low-risk, middle-risk, and high-risk groups. The Kaplan-Meier survival curve was used to compare the difference between the newly established risk classification system and AJCC staging in predicting the prognosis and survival of patients.
2.5 Data analysis
The endpoint in this study was patient-specific survival for melanoma (CSS), which is the time from diagnosis to death due to melanoma in tumor patients. The data were extracted using SEER*Stat software (8.3.9.2 version), and the best cut-off value for the total points was selected using X-Tile (version 3.6.1). All data analyses were performed using the R software version 4.1.2 (http://www.r-project.org/). The nomograms were developed and validated with R packages “regplot,” “mstate,” “survival,” “cmprsk,” “Hmisc,” “timeROC,” “foreign,” “nricens,” “rmda,” and “DCA.” Chi-square test was used to assess the differences of distribution of the two cohorts. Bidirectional probability values P < 0.05 were considered statistically significant.
3 Results
3.1 Characteristics of patients and diseases
A total of 5,166 patients with advanced melanoma were enrolled and randomized 7:3 into a training group (3,614) and a validation group (1,552). What is the median follow-up time for the entire population 26 months [Quartile range (IQR): (11-66) months, Training cohort 27 months (IQR): 11-66 months, Validation cohort 26 months (IQR): (12-66.75) months. This study summarizes the demographic and clinical features of advanced melanoma patients > 50 years of age (Table 1). The demographic and clinical characteristics of the training and validation cohorts were comparable. (P>0.05).
3.2 Univariate and multivariate analysis
In the univariate regression analysis, age, race, gender, tumor thickness, ulceration, the number of primary melanomas, M stage, N stage, anatomic site, and whether to have surgery. were the prognostic factors for patients with advanced melanoma (P<0.05). After multivariate analysis of these factors, it was concluded that surgery, age, gender, tumor thickness, ulceration, the number of primary melanomas, M stage, and N stage were the independent prognostic factors for CSS in patients with advanced melanoma (P < 0.05). The above factors were included, and the nomogram was constructed (Table 2).
3.3 Construction and verification of nomograms
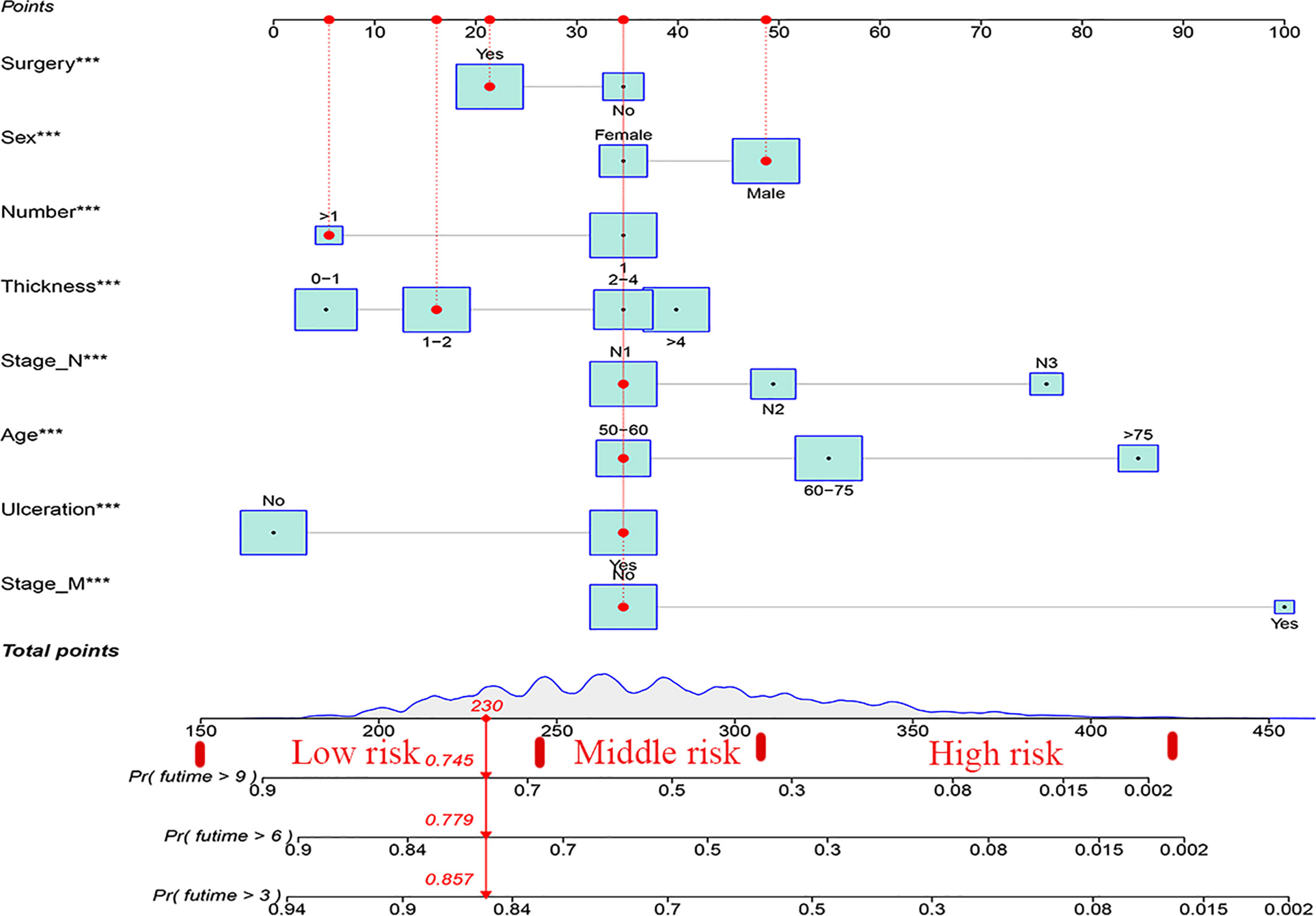
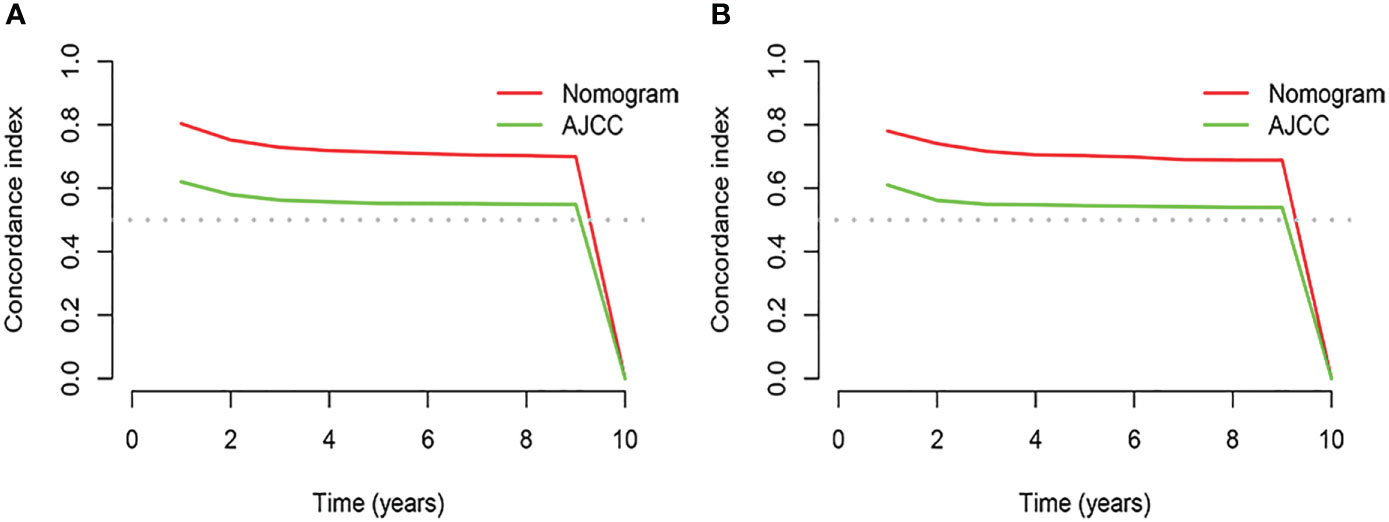
Based on the results of univariate and multivariate analyses, surgery, age, gender, tumor thickness, ulceration, number of primary melanoma, M-stage, and N-stage were included in the construction of the nomograms for the CSS probabilities of patients with advanced melanoma (Figure 2). The C-index, calibration curve, ROC curve, and DCA curve are shown in Figures 3–5. The C-index for the training and validation cohorts were 0.732 (95%CI: 0.717-0.742) and 0.741(95%CI: 0.732-0.751), respectively. The results of ROC curve analysis showed that the AUCs of the training cohort were 0.747,0.747, and 0.758 at 3, 6, and 9 years, respectively. The AUCs of the validation cohort were 0.742,0.737, and 0.789 at 3, 6, and 9 years, respectively, indicating that the model had good prediction performance. Additionally, calibration curves showed high agreement between predicted and observed CSS rates at 3, 6, and 9 years in both the training and validation cohorts.
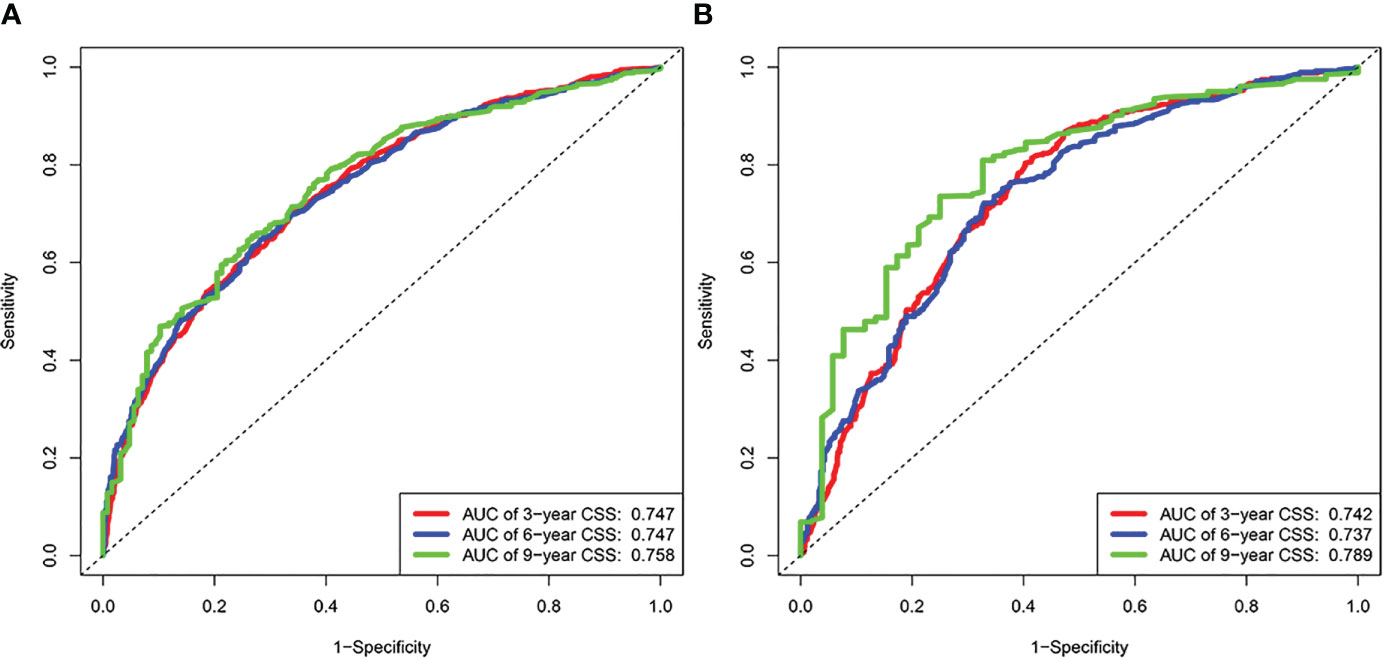
Figure 3 Results of time dependent ROC curve analysis based on the nomogram. (A) Based on the training cohorts; (B) Based on the validation cohorts.
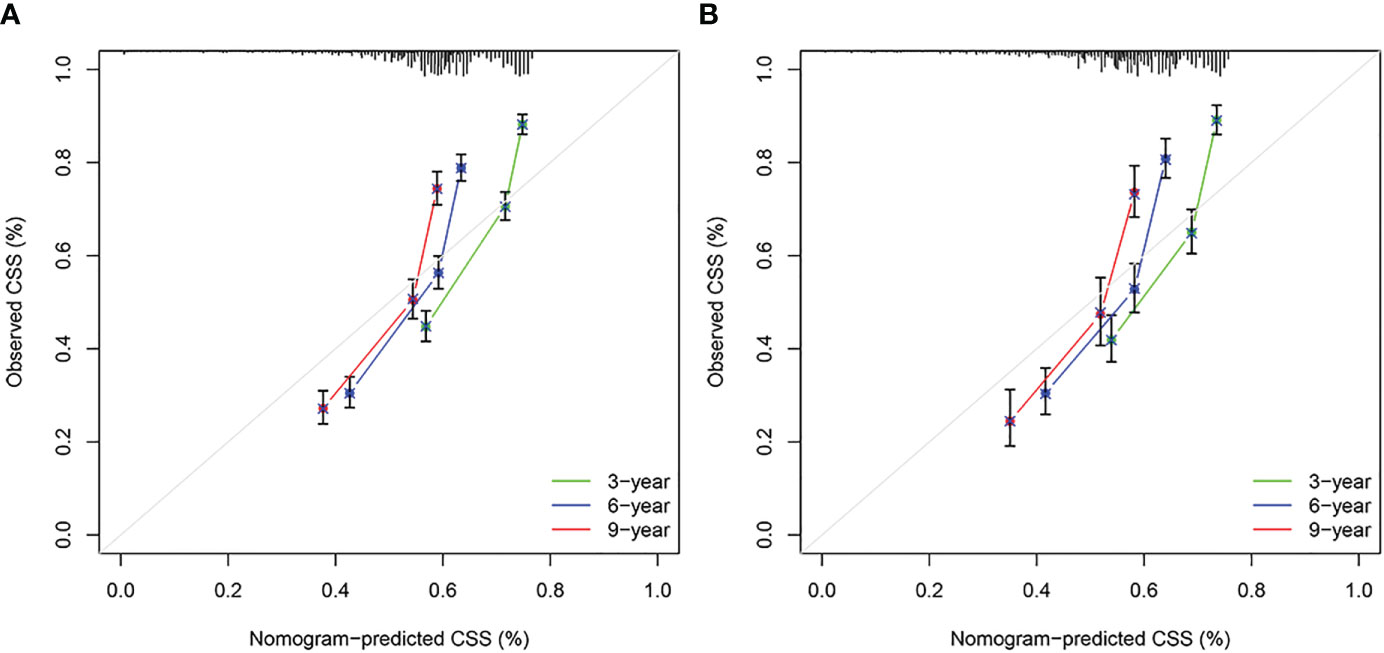
Figure 4 Calibration curves. (A) Based on the training cohorts; (B) Based on the validation cohorts.
3.4 Comparison of clinical values of nomograms and AJCC staging
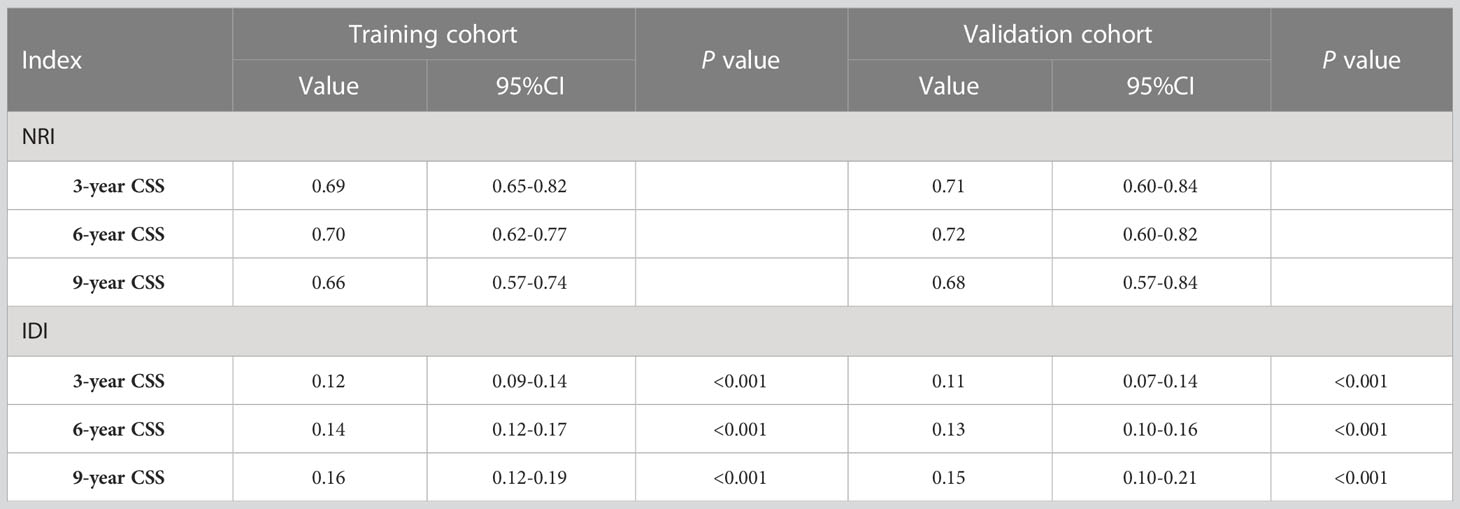
Changes in C-index, NRI, IDI, and DCA were used to compare the difference in nomograms with the ability to predict based on AJCC criteria. The nomogram-related C-index was higher in the training and validation cohorts than in the AJCC staging-related C-index. As can be seen from the DCA curve, the nomograms showed a much greater net gain compared to the AJCC standard tumor staging and the two extremes (Figure 6). The NRI of the 3-,6-, and 9-year CSS for the training cohort was 0.69 (95%CI=0.65-0.82), 0.70 (95%CI=0.62-0.77), and 0.66 (95%CI=0.57-0.74, and the IDI was 0.12 (95%CI=0.09-0.14), 0.14 (95%CI=0.12-0.17), and 0.16 (95%CI=0.12-0.19), respectively. These results were presented in the validation cohort (Table 3).
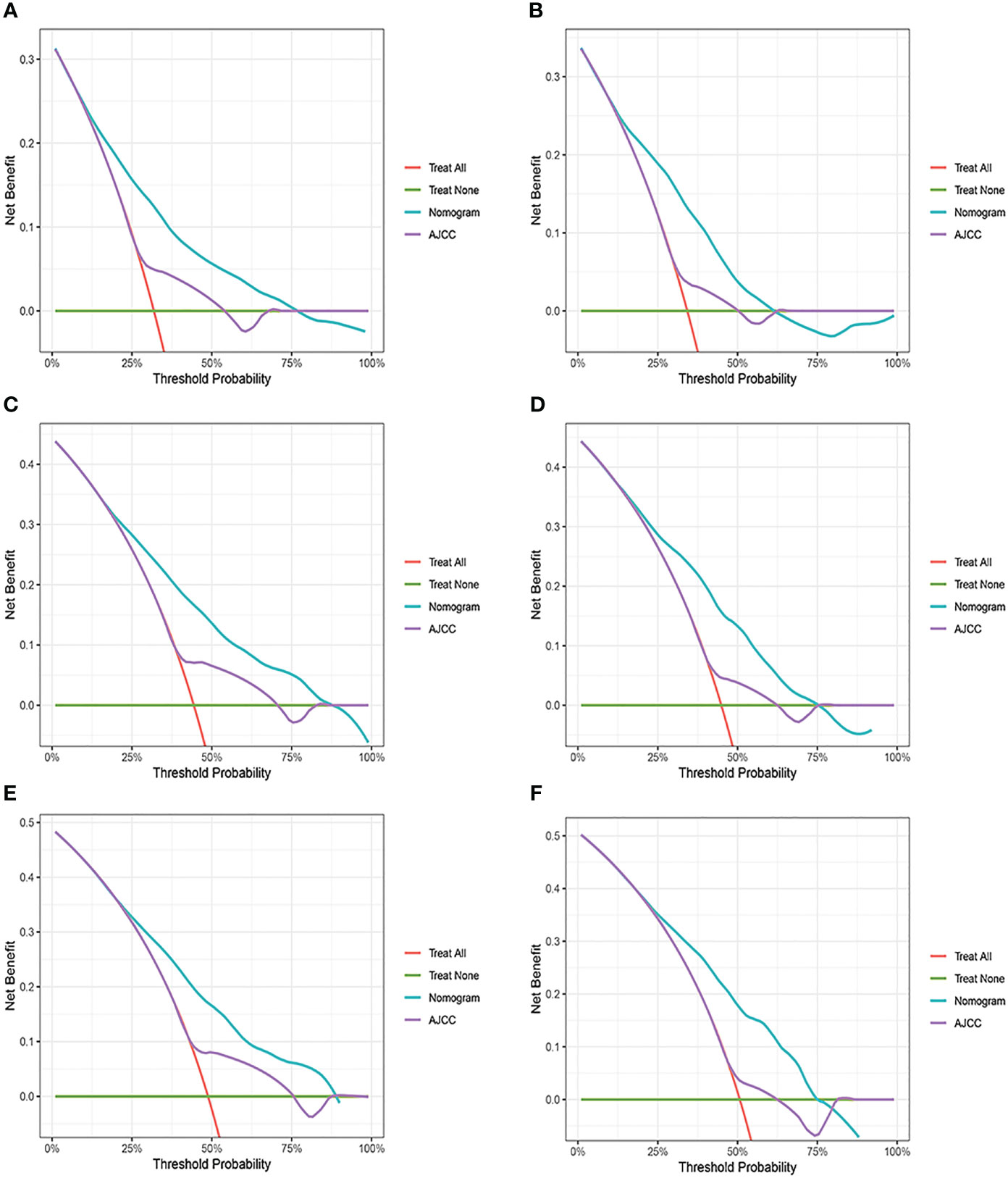
Figure 6 Decision curve analysis of the nomogram and AJCC tumor staging. (A) 3-year survival benefit in the training cohort. (B) 3-year survival benefit in the validation cohort. (C) 6-year survival benefit in the training cohort. (D) 6-year survival benefit in the validation cohort. (E) 9-year survival benefit in the training cohort. (F) 9-year survival benefit in the validation cohort.
3.5 Risk stratification of nomograms
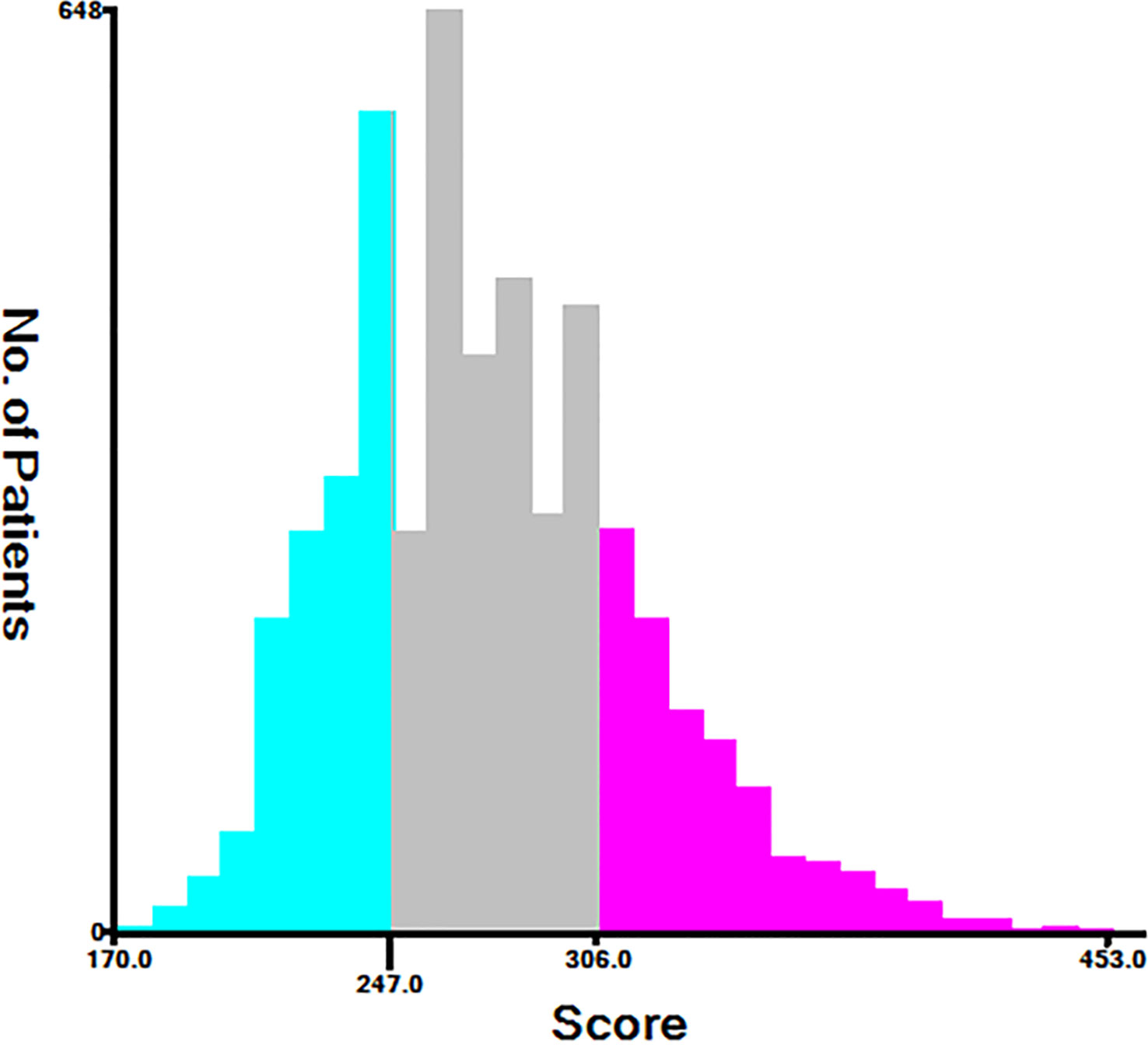
Using nomograms, total points are calculated and used to stratify risk. Three risk groups were formed for patients with advanced melanoma: low risk (total points < 247.0), middle risk (247.0≤ total points < 306.0) and high risk (total points ≥306.0) (Figure 7). The survival curve illustrated the ability of the new risk classification system to clearly distinguish between low and high-risk groups compared to AJCC staging, which will provide a practical tool for the clinical management of patients with advanced melanoma. (Figure 8).
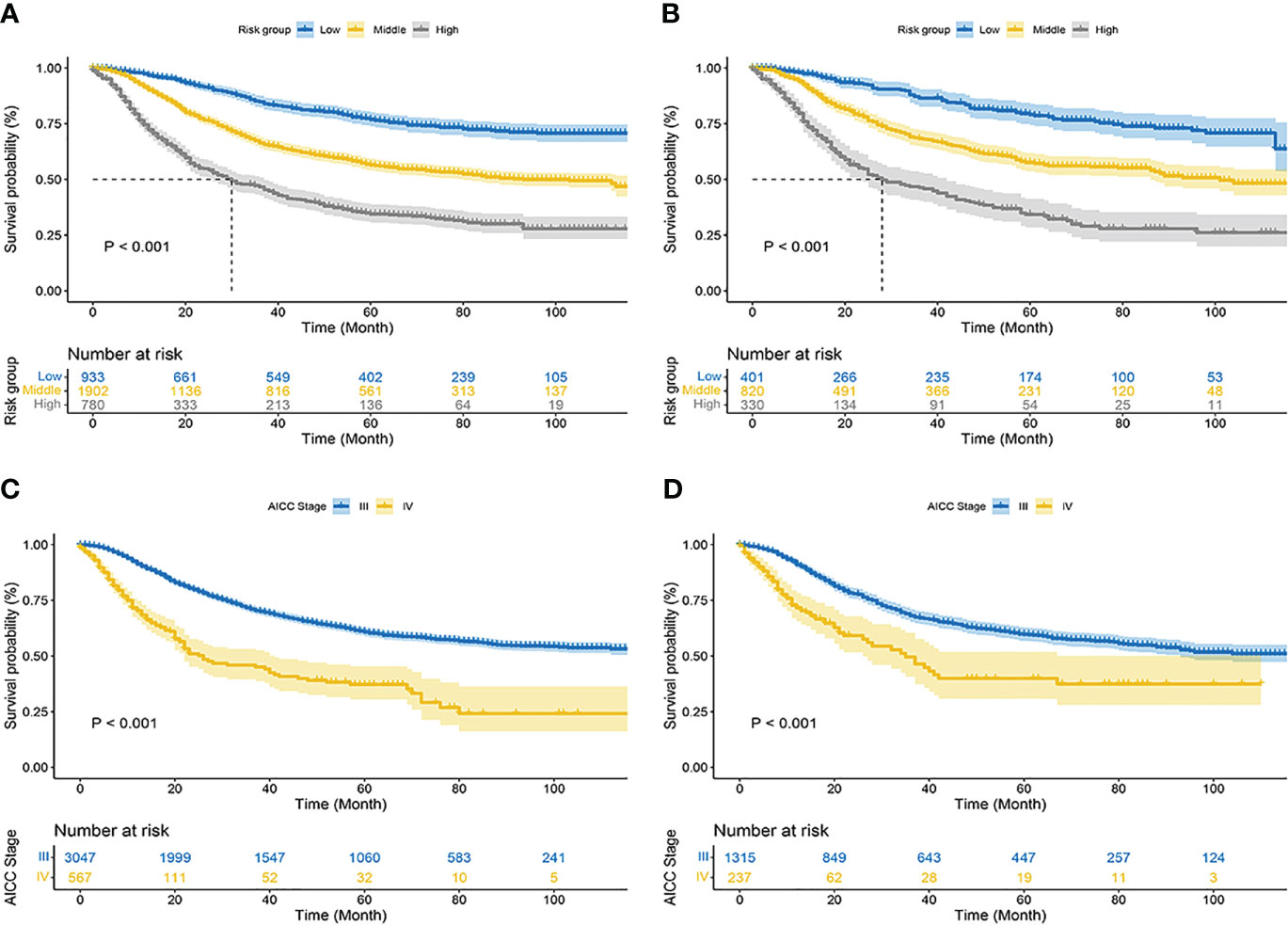
Figure 8 Kaplan–Meier curves of cancer-specific survival for new risk classification and the AJCC tumor staging. (A) The new risk classification in the training cohort; (B) The new risk classification in the validation cohort; (C) The AJCC tumor staging in the training cohort; (D) The AJCC tumor staging in the validation cohort.
4 Discussion
Melanoma is a highly malignant neoplasm with a much lower incidence of skin cancer than other skin cancers, such as basal cell carcinoma (70%) and squamous cell carcinoma (25%). Still, melanoma accounts for the majority of skin cancer deaths (22). Like most malignant tumors, the stage of melanoma determines its prognosis and directly affects the choice of treatment plan for patients. Although breakthrough has been made in chemotherapy (23), immunotherapy (24, 25), targeted therapy (26), and other therapies in recent years, surgery and pathological examination are still the first choice for diagnosis and treatment (27). Now that the survival rate of elderly patients with advanced melanoma is still low, which is highly harmful to human beings, accurate prognosis prediction is crucial for better management of elderly patients with advanced melanoma. The AJCC staging system is currently widely used for melanoma staging. The new version of the revision focuses more on the evidence-based revision of stage I to stage III melanoma without establishing a stage IV database or analysis for patients with stage IV melanoma (9). Second, biological factors such as age, gender, and ethnicity, independent risk factors for melanoma, were not included in the AJCC system due to their limitations (28, 29). Only using TNM staging to guide the treatment and prognosis of patients cannot rule out individual differences of patients, which will cause difficulties for personalized medicine.
The predictive nomogram model has been shown to be superior to traditional staging systems in various cancers (30–33). We constructed a nomogram to predict the prognosis of patients with advanced melanoma. Eight variables were selected and incorporated into the nomogram. The 3-, 6- and 9-year survival rates of the patients can be calculated by the scores of the corresponding factors in the nomogram to guide clinical practice. Our nomogram indicates that a poorer prognosis in patients is associated with male gender, absence of surgery, advanced age, increased tumor thickness, concurrent tumor ulceration, and later N and M stages. The above conclusions are consistent with the results in the existing studies (34–36). Currently, the effect of the number of primary melanomas on the prognosis of melanoma is still controversial (37–43).. Based on samples from SEER database 5166, this study found that patients with a single primary melanoma had a worse prognosis according to both univariate and multivariate Cox regression analyses, which is consistent with some existing studies. In addition, the nomogram was constructed utilizing a substantial sample size, resulting in a relatively comprehensive prognostic evaluation for melanoma that is not limited to a single factor. The predictive and calibration abilities of the nomogram have also been confirmed through multi-angle validation.
In this study, several limitations were identified. First, it is important to note that the SEER database has certain limitations of its own. Specifically, the database lacks comprehensive data on newer treatment modalities, such as immunotherapy and targeted therapy. Furthermore, the database does not categorize primary types of melanomas with great specificity, which may limit the current clinical relevance of diagnosis and treatment plan. Secondly, retrospective data collection may inherently introduce certain biases, and the exclusion of patients with missing data could result in a selection bias. Thirdly, we randomly divided the enrolled patients into a training cohort and a validation cohort according to the ratio of 7: 3 and developed a nomogram for internal validation. While the C-index and AUC values obtained were relatively high, and the risk stratification of the nomogram was superior to the AJCC stage prediction model, it is important to note that the data used for modeling and calibration are from the same database, which limits the model’s generalizability. Finally, while the nomograms developed in this study have not been tested in clinical trials, their accuracy and utility remain controversial. Therefore, further research is required to confirm the findings of this study, particularly through randomized clinical trials that can serve as a gold standard for evaluating the performance of the nomograms.
5 Conclusion
The nomogram and risk classification system for patients over 50 years of age with advanced cutaneous melanoma based on data from the SEER database, which can help develop personalized treatment options for these patients. However, its clinical utility needs to be evaluated, which is also where the nomogram needs further improvement.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Data analysis were performed by QX. The manuscript was written by QX and revised by HC. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Science and Technology Bureau of Qingdao West Coast New Area (No. 2020-61).
Acknowledgments
We would like to thank our esteemed supervisor, HC. HC always encourages us to overcome a sea of barriers in our prospective life.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1166877/full#supplementary-material
References
1. Guo W, Wang H, Li C. Signal pathways of melanoma and targeted therapy. Signal Transduct Target Ther (2021) 6(1):424. doi: 10.1038/s41392-021-00827-6
2. Dzwierzynski WW. Melanoma risk factors and prevention. Clin Plast Surg (2021) 48(4):543–50. doi: 10.1016/j.cps.2021.05.001
3. Turner N, Ware O, Bosenberg M. Genetics of metastasis: melanoma and other cancers. Clin Exp Metastasis (2018) 35(5-6):379–91. doi: 10.1007/s10585-018-9893-y
4. Guy GP Jr., Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC. Vital signs: melanoma incidence and mortality trends and projections - united states, 1982-2030. MMWR Morb Mortal Wkly Rep (2015) 64(21):591–6.
5. Arnold M, Singh D, Laversanne M, Vignat J, Vaccarella S, Meheus F, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol (2022) 158(5):495–503. doi: 10.1001/jamadermatol.2022.0160
6. Carlson JA, Slominski A, Linette GP, Mihm MC Jr., Ross JS. Biomarkers in melanoma: staging, prognosis and detection of early metastases. Expert Rev Mol Diagn (2003) 3(3):303–30. doi: 10.1586/14737159.3.3.303
7. Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist (2011) 16(1):5–24. doi: 10.1634/theoncologist.2010-0190
8. McDermott D, Lebbé C, Hodi FS, Maio M, Weber JS, Wolchok JD, et al. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev (2014) 40(9):1056–64. doi: 10.1016/j.ctrv.2014.06.012
9. Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. Melanoma staging: evidence-based changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin (2017) 67(6):472–92. doi: 10.3322/caac.21409
10. Lasithiotakis K, Leiter U, Meier F, Eigentler T, Metzler G, Moehrle M, et al. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer (2008) 112(8):1795–804. doi: 10.1002/cncr.23359
11. Joosse A, Collette S, Suciu S, Nijsten T, Patel PM, Keilholz U, et al. Sex is an independent prognostic indicator for survival and relapse/progression-free survival in metastasized stage III to IV melanoma: a pooled analysis of five European organisation for research and treatment of cancer randomized controlled trials. J Clin Oncol (2013) 31(18):2337–46. doi: 10.1200/JCO.2012.44.5031
12. Laskar R, Ferreiro-Iglesias A, Bishop DT, Iles MM, Kanetsky PA, Armstrong BK, et al. Risk factors for melanoma by anatomical site: an evaluation of aetiological heterogeneity. Br J Dermatol (2021) 184(6):1085–93. doi: 10.1111/bjd.19705
13. Ettl T, Irga S, Müller S, Rohrmeier C, Reichert TE, Schreml S, et al. Value of anatomic site, histology and clinicopathological parameters for prediction of lymph node metastasis and overall survival in head and neck melanomas. J Craniomaxillofac Surg (2014) 42(5):e252–8. doi: 10.1016/j.jcms.2013.09.007
14. Lam M, Zhu JW, Hu A, Beecker J. Racial differences in the prognosis and survival of cutaneous melanoma from 1990 to 2020 in north America: a systematic review and meta-analysis. J Cutan Med Surg (2022) 26(2):181–8. doi: 10.1177/12034754211052866
15. Kadakia S, Chan D, Mourad M, Ducic Y. The prognostic value of age, sex, and subsite in cutaneous head and neck melanoma: a clinical review of recent literature. Iran J Cancer Prev (2016) 9(3):e5079. doi: 10.17795/ijcp-5079
16. Morlacco A, Modonutti D, Motterle G, Martino F, Dal Moro F, Novara G. Nomograms in urologic oncology: lights and shadows. J Clin Med (2021) 10(5):980. doi: 10.3390/jcm10050980
17. Kotecha R, Tonse R, Rubens M, Appel H, Albrecht F, Kaywin P, et al. Meta-analysis of survival and development of a prognostic nomogram for malignant pleural mesothelioma treated with systemic chemotherapy. Cancers (Basel) (2021) 13(9):2186. doi: 10.3390/cancers13092186
18. Yang Y, Shen C, Shao J, Wang Y, Wang G, Shen A. Based on the development and verification of a risk stratification nomogram: predicting the risk of lung cancer-specific mortality in stage IIIA-N2 unresectable Large cell lung neuroendocrine cancer compared with lung squamous cell cancer and lung adenocarcinoma. Front Oncol (2022) 12:825598. doi: 10.3389/fonc.2022.825598
19. Song K, Song J, Shi X, Wang H, Ma X, Xia X, et al. Development and validation of nomograms predicting overall and cancer-specific survival of spinal chondrosarcoma patients. Spine (Phila Pa 1976) (2018) 43(21):E1281–e9. doi: 10.1097/BRS.0000000000002688
20. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol (2008) 26(8):1364–70. doi: 10.1200/JCO.2007.12.9791
21. Naoum GE, Ho AY, Shui A, Salama L, Goldberg S, Arafat W, et al. Risk of developing breast reconstruction complications: a machine-learning nomogram for individualized risk estimation with and without postmastectomy radiation therapy. Plast Reconstr Surg (2022) 149(1):1e–12e. doi: 10.1097/PRS.0000000000008635
22. Fijałkowska M, Koziej M, Antoszewski B. Detailed head localization and incidence of skin cancers. Sci Rep (2021) 11(1):12391. doi: 10.1038/s41598-021-91942-5
23. Scatena C, Murtas D, Tomei S. Cutaneous melanoma classification: the importance of high-throughput genomic technologies. Front Oncol (2021) 11:635488. doi: 10.3389/fonc.2021.635488
24. Bai R, Huang H, Li M, Chu M. Temporal trends in the incidence and mortality of skin malignant melanoma in China from 1990 to 2019. J Oncol (2021) 2021:9989824. doi: 10.1155/2021/9989824
25. Knackstedt R, Smile T, Yu J, Gastman BR. Non-operative options for loco-regional melanoma. Clin Plast Surg (2021) 48(4):631–42. doi: 10.1016/j.cps.2021.05.007
26. Tarhini AA, Pahuja S, Kirkwood JM. Neoadjuvant therapy for high-risk bulky regional melanoma. J Surg Oncol (2011) 104(4):386–90. doi: 10.1002/jso.21882
27. Howard MD, Wee E, Wolfe R, McLean CA, Kelly JW, Pan Y. Anatomic location of primary melanoma: survival differences and sun exposure. J Am Acad Dermatol (2019) 81(2):500–9. doi: 10.1016/j.jaad.2019.04.034
28. Tracey EH, Vij A. Updates in melanoma. Dermatol Clin (2019) 37(1):73–82. doi: 10.1016/j.det.2018.08.003
29. Isaksson K, Katsarelias D, Mikiver R, Carneiro A, Ny L, Olofsson Bagge R. A population-based comparison of the AJCC 7th and AJCC 8th editions for patients diagnosed with stage III cutaneous malignant melanoma in Sweden. Ann Surg Oncol (2019) 26(9):2839–45. doi: 10.1245/s10434-019-07448-y
30. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol (2013) 31(9):1188–95. doi: 10.1200/JCO.2012.41.5984
31. Wang S, Yang L, Ci B, Maclean M, Gerber DE, Xiao G, et al. Development and validation of a nomogram prognostic model for SCLC patients. J Thorac Oncol (2018) 13(9):1338–48. doi: 10.1016/j.jtho.2018.05.037
32. Zeng Q, Yao Y, Zhao M. Development and validation of a nomogram to predict cancer-specific survival of uveal melanoma. BMC Ophthalmol (2021) 21(1):230. doi: 10.1186/s12886-021-01968-6
33. Tang J, Jiang S, Gao L, Xi X, Zhao R, Lai X, et al. Construction and validation of a nomogram based on the log odds of positive lymph nodes to predict the prognosis of medullary thyroid carcinoma after surgery. Ann Surg Oncol (2021) 28(8):4360–70. doi: 10.1245/s10434-020-09567-3
34. Xiao Y, Peng S, Hu Y, Zhang J, Cao X. Development and validation of prognostic nomogram in patients with nonmetastatic malignant melanoma: a SEER population-based study. Cancer Med (2020) 9(22):8562–70. doi: 10.1002/cam4.3318
35. Li W, Xiao Y, Xu X, Zhang Y. A novel nomogram and risk classification system predicting the cancer-specific mortality of patients with initially diagnosed metastatic cutaneous melanoma. Ann Surg Oncol (2021) 28(7):3490–500. doi: 10.1245/s10434-020-09341-5
36. Muinonen-Martin AJ, O'Shea SJ, Newton-Bishop J. Amelanotic melanoma. Bmj (2018) 360:k826. doi: 10.1136/bmj.k826
37. Absil G, Collins P, Seidel L, Damsin T, Nikkels AF. Clinical features and survival of multiple primary melanoma: a Belgian single center cohort. Dermatol Ther (Heidelb) (2023) 13(2):641–9. doi: 10.1007/s13555-022-00884-x
38. Aviles-Izquierdo JA, Lazaro-Ochaita P, Suarez-Fernandez R, Marquez-Rodas I, Parra-Blanco V, Escat-Cortes JL. Epidemiological changes in cutaneous melanoma: retrospective study of 969 cases (1996-2010). Rev Clin Esp (Barc) (2013) 213(2):81–7. doi: 10.1016/j.rce.2012.06.006
39. Kricker A, Armstrong BK, Goumas C, Thomas NE, From L, Busam K, et al. Survival for patients with single and multiple primary melanomas: the genes, environment, and melanoma study. JAMA Dermatol (2013) 149(8):921–7. doi: 10.1001/jamadermatol.2013.4581
40. Menzies S, Barry R, Ormond P. Multiple primary melanoma: a single centre retrospective review. Melanoma Res (2017) 27(6):638–40. doi: 10.1097/CMR.0000000000000395
41. Nosrati A, Yu WY, McGuire J, Griffin A, de Souza JR, Singh R, et al. Outcomes and risk factors in patients with multiple primary melanomas. J Invest Dermatol (2019) 139(1):195–201. doi: 10.1016/j.jid.2018.07.009
42. Youlden DR, Baade PD, Soyer HP, Youl PH, Kimlin MG, Aitken JF, et al. Ten-year survival after multiple invasive melanomas is worse than after a single melanoma: a population-based study. J Invest Dermatol (2016) 136(11):2270–6. doi: 10.1016/j.jid.2016.03.014
Keywords: advanced melanoma, SEER, nomogram, classification system, AJCC staging
Citation: Xi Q, Lu X, Zhang J, Wang D, Sun Y and Chen H (2023) A practical nomogram and risk stratification system predicting the cancer-specific survival for patients aged >50 with advanced melanoma. Front. Oncol. 13:1166877. doi: 10.3389/fonc.2023.1166877
Received: 15 February 2023; Accepted: 23 June 2023;
Published: 13 July 2023.
Edited by:
Vladimir Spiegelman, Penn State Milton S. Hershey Medical Center, United StatesReviewed by:
Eszter Anna Janka, University of Debrecen, HungaryHan Yu, Roswell Park Comprehensive Cancer Center, United States
Copyright © 2023 Xi, Lu, Zhang, Wang, Sun and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongquan Chen, chenhongquan0811@163.com
 Qiufen Xi
Qiufen Xi Xiaoou Lu
Xiaoou Lu