- 1Department of Breast Surgery, The 1st Affiliated Hospital, China Medical University, Shenyang, China
- 2Department of Pediatrics, The First Hospital of China Medical University, Shenyang, China
- 3Department of Cell Biology, Key Laboratory of Cell Biology, Ministry of Public Health, Key Laboratory of Medical Cell Biology, Ministry of Education, China Medical University, Shenyang, China
- 4Research Unit of General Surgery, Department of Breast Surgery and Surgical Oncology, The First Hospital of China Medical University, Shenyang, China
Background: The implementation of sentinel lymph node biopsy (SLNB) and further completion axillary lymph node dissection (cALND) after positive sentinel lymph nodes (SLNs) on early invasive breast cancer patients should be cautiously tailored. Identifying predictors for SLN and non-sentinel lymph node (nSLN) metastases can help surgeons make better surgical decisions.
Methods: A retrospective case-control study was designed and a total of 560 eligible patients were enrolled consecutively. They were all diagnosed in our center and received appropriate medical care. According to the metastasis of SLN and nSLN, they were divided into metastatic and non-metastatic groups on two successive occasions to investigate the relationship between clinical factors, pathological factors, hematological factors and lymph node metastasis.
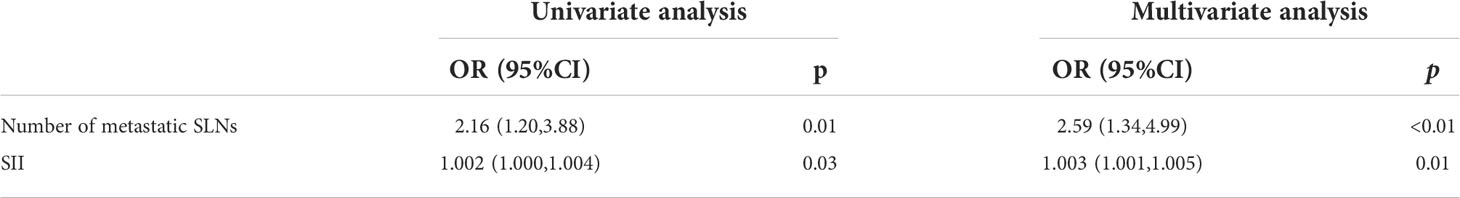
Results: In total, 101 (18.04%) patients developed SLN metastases, including 98 patients with macro-metastases and 3 patients with micro-metastases. Out of 97 patients receiving further cALND, 20 patients (20.62%) developed nSLN metastases. Multivariate analysis revealed that “high expression of Ki-67” and “lymphatic invasion” predicted a higher risk of SLN metastasis; and “increased number of positive SLNs” and “increased systemic inflammation index (SII)” predicted a higher risk of nSLN metastasis.
Conclusion: Surgery for early invasive breast cancer patients should be more customized and precise. Appropriate axillary management is necessary for patients with the associated predictors.
Introduction
Axillary management of early invasive breast cancer patients contributes to a favorable prognosis by attaining local control and obtaining information on postoperative systemic therapy decisions (1). SLNB has been authorized a safe and reliable alternative to one-stage ALND for assessing axillary lymph node status in clinically node-negative breast cancer patients (2). For patients with negative SLNs, SLNB alone can attain satisfactory local control and a non-inferior prognosis compared with cALND while avoiding serious side-effects, thus improving the patient’s quality of life (3). On the other hand, in patients with positive SLNs, SLNB provides surgeons with information to decide the extent of further surgical resection (4). And for such patients, cALND has long been considered the gold standard (5).
However, this notion has now been challenged. Do we really need cALND in all patients with positive SLNs? Existing studies revealed that less than one-third of SLN-positive patients had nSLN metastases, implying that for most patients, cALND was not able to contribute to a better local control at a cost of heavier financial pressure and more serious side-effects (6). Meanwhile, as the understanding of tumor biology deepens, the postoperative systemic treatment decision is based more on molecular typing and genetic patterns, resulting in a decrease in the impact of information obtained from cALND (7). According to the ACOSOG Z0011 trial and the IBCSG 23-01 trial, cALND could be conditionally omitted in patients with limited disease in SLNs, and the American Society of Clinical Oncology (ASCO) Expert Panel have made pertinent recommendations on this top (8–11). However, this strategy has been criticized due to the high rates of micro-metastasis and rigorous enrollment requirements of the cornerstone studies, which were believed to reduce the persuasiveness (12).
Given the trend towards minimizing intrusive surgical procedures, SLNB also loses much of its importance. Since SLN-negative patients have a satisfactory prognosis; further cALND makes a limited contribution to local control; and the impact of lymph node status on systemic treatment decisions is declining, do we really need to perform SLNB in all invasive breast cancer patients? (9, 13, 14)
Axillary management of breast cancer patients should be more customized and precise. If we can screen out patients with only SLN metastasis, unnecessary surgeries could be avoided. In this study, we retrospectively reviewed 560 patients’ electronic medical records. Clinical, pathological and hematological constants were analyzed to explore potential predictors for SLN and nSLN metastasis.
Methods
Study design
We designed a retrospective case-control study to investigate which factors were independently associated with SLN metastasis and further nSLN metastasis in SLN-positive patients. All enrolled patients were divided into SLN metastasis group and SLN non-metastasis group for the first analysis. Then, within the SLN metastasis group, eligible patients were further divided into nSLN metastasis group and nSLN non-metastasis group for the second analysis. All enrolled patients received standard surgical treatment as recommended by the national comprehensive cancer network (NCCN) Clinical Practice Guidelines (15).
Inclusion and exclusion criteria
Inclusion criteria: Female patients diagnosed with invasive breast cancer and underwent SLNB from December 2019 to December 2021 at the Department of Breast Surgery, The First Affiliated Hospital of China Medical University. Exclusion criteria: (1) Male patients (2) Patients with pathologically diagnosed pure ductal carcinoma in situ, lobular carcinoma in situ, encapsulated papillary carcinoma or Paget’s disease. (3) Patients who have received neoadjuvant chemotherapy (NAC) or are proposed to receive neoadjuvant chemotherapy. (4) Patients with inflammatory or blood disorders. (5) Patients who are taking drugs able to affect the results of hematology tests, including antibiotics, anti-inflammatory drugs and anticoagulants.
Operating methods
The single tracer method was adopted for all patients, with nanocarbon or methylene blue as the tracer. Methylene blue was injected intracutaneously or subcutaneously around the ipsilateral areola 5-15 minutes prior to surgery. Nanocarbon was injected in the same way, 6-8 hours before surgery (16, 17). SLN refers to one or a few lymph nodes to which breast cancer cells metastasize at first (5). Frozen sections of the SLNs were used for intraoperative pathology examination, and remaining tissues from the section were paraffin-embedded for further pathological examination. Macro-metastases were defined as tumor deposits with a maximum diameter >2 mm. Micro-metastases were defined as tumor deposits with a maximum diameter >0.2 mm and ≤2 mm or over 200 tumor cells being seen in one frozen section. Both macro- and micro-metastases of sentinel lymph nodes were considered positive. The presence of isolated tumor cells or absence of tumor cells in frozen sections was considered negative (18).
Diagnosis of lesions
The pathological types of tumors were classified as invasive ductal carcinoma, invasive lobular carcinoma and others, including septate carcinoma, mucinous carcinoma, invasive papillary carcinoma, invasive micropapillary carcinoma, septate carcinoma and carcinoma with neuroendocrine differentiation. Immunohistochemical markers estrogen receptors (ER), progesterone receptors (PR), human epidermal growth factor receptor 2 (HER2) and Ki-67 were detected and interpreted as specified (19, 20). For ER and PR, 10% was regarded as the threshold to distinguish between high expression and low/no expression groups. For HER2, 3+ was considered the label of high expression group, and 0+, 1+ and 2+ were considered the labels of low/no expression group. For Ki-67, 30% was regarded as the threshold to distinguish between high expression and low/no expression groups (21, 22). The unifocal and multifocal nature of lesions was evaluated preoperatively and confirmed intraoperatively. The location of lesions was divided into upper outside quadrant and other quadrants, including upper inside quadrant, lower inside quadrant, lower outside quadrant and central area, due to the character of upper outside quadrant as the most frequent location of breast cancers, also seeming to imply that tumors in this area are more likely to metastasize to the axillary region (23, 24).
Collection of blood samples
All blood samples were obtained within one week prior to surgery. If two sets of hematological data were available, the one closer to the date of surgery would be used for analysis to ensure the representativeness. We recorded platelet count, neutrophil count, lymphocyte count and calculated three parameters including platelet lymphocyte ratio (PLR), neutrophil lymphocyte ratio (NLR) and systemic inflammation index (SII) on this basis. PLR was calculated as platelet/lymphocyte. NLR was calculated as neutrophil/lymphocyte. And SII was calculated as platelet × neutrophil/lymphocyte.
Statistical analysis
Age was the only continuous variable conforming to a normal distribution tested by the Shapiro-Wilk method and was presented as the form of mean ± standard deviation. Other continuous variables were presented as median joint quartiles. The intergroup difference in average of age was comprised by Student’s t-test. Intergroup differences in non-normally distributed continuous variables and hierarchical variables were compared by Mann Whitney-U test. Intergroup differences in counting variables were comprised by chi-square test. Independent predictors for metastasis were determined by the logistic regression model. Variables with p<0.1 in univariate analysis were enrolled in multivariate analysis. All analyses were two-tailed with 0.05 as the statistical threshold. The SPSS version 26.0 was used for all statistical analysis.
Results
Participants and lymph node metastasis
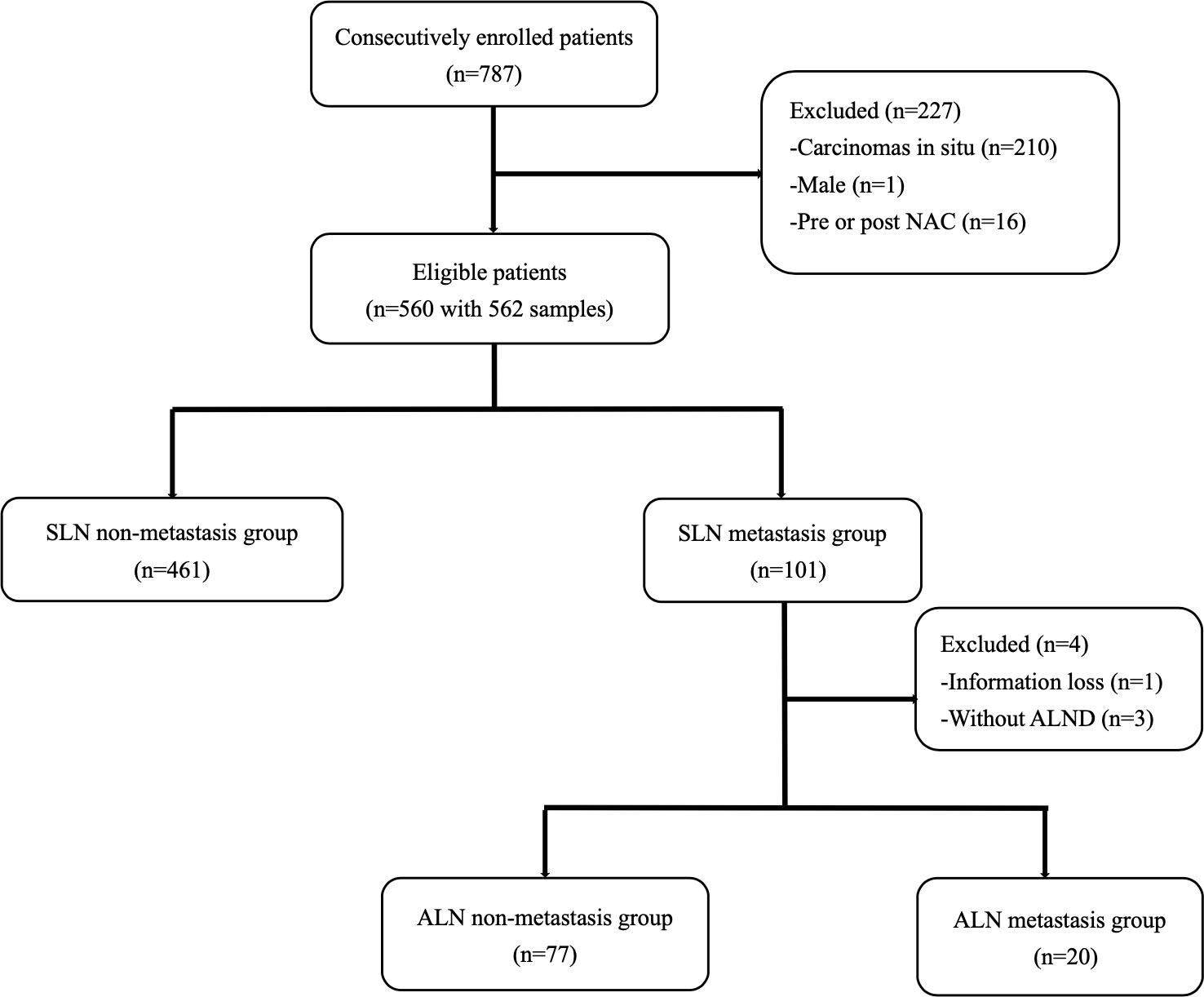
A total of 787 breast cancer patients received SLNB in our center from December 2019 to December 2021. After screening, 562 samples from 560 patients who met the inclusion criteria were consecutively included (Figure 1). The mean age of all eligible patients was 51.79±10.54. Two patients received bilateral SLNB, and they were both divided into the non-metastasis group. All patients were staged cT1-2N0M0 according to the Eighth Edition of AJCC Cancer Staging Manual (25).
In total, 101 patients developed SLN metastases, accounting for 18.04%. Macro-metastases were found in 98 patients, accounting for 97.03%. Micro-metastases were found in only three patients, accounting for 2.97%, and none of them had nSLN metastasis. A total of 97 patients in the metastasis group received cALND, and 20 of them had nSLN metastases, accounting for 20.62%. Nine patients had ≥3 positive SLNs, of whom 4 patients had nSLN metastases, accounting for 44.44%. While in the remaining 88 patients with 1-2 positive SLNs, nSLN metastases were found in 16 patients, accounting for 18.18%.
Baseline characteristics
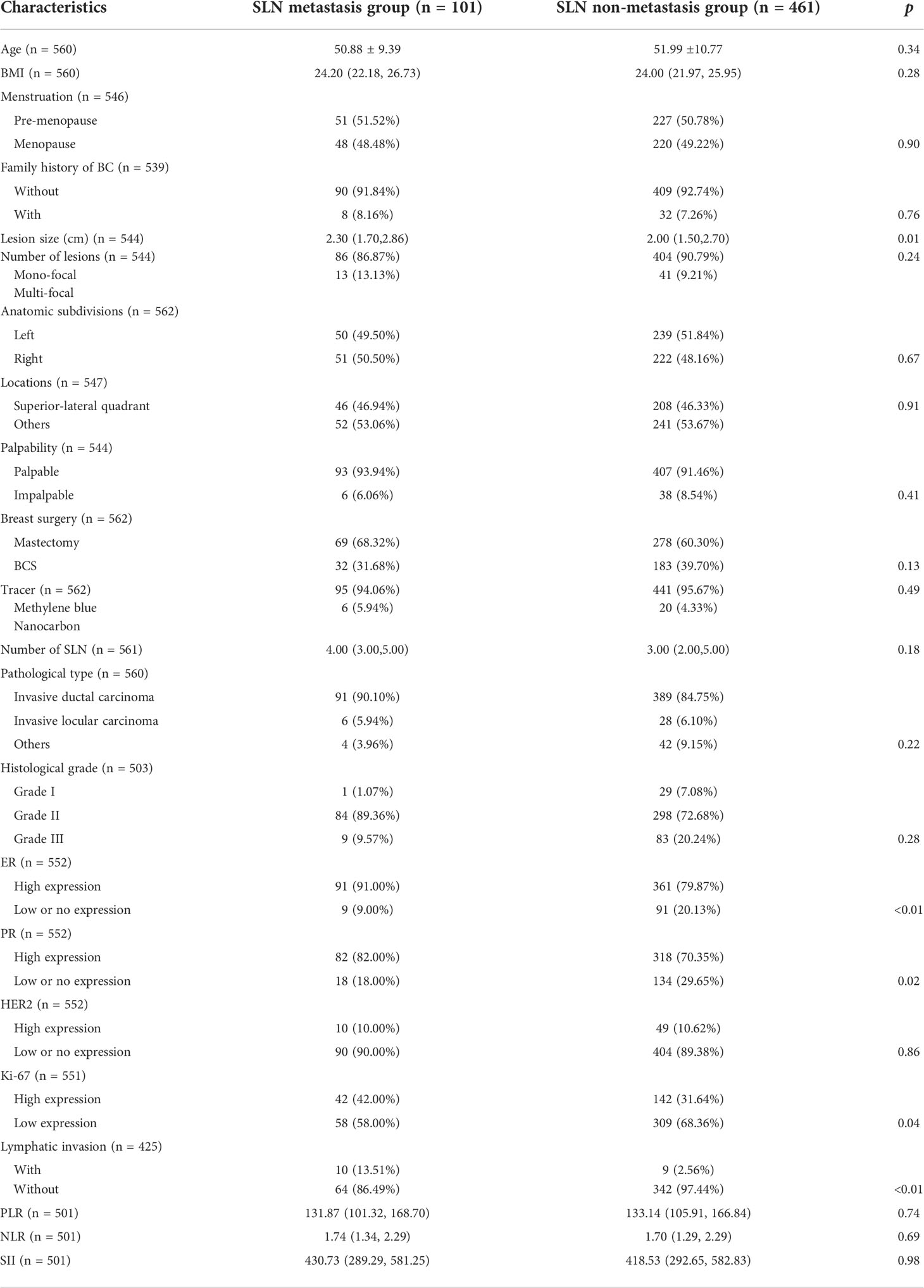
The mean age of patients in SLN metastasis and non-metastasis groups was comparable (50.88 ± 9.39 vs 51.99 ± 10.77, p=0.34), accompanied by a similar distribution of menstrual status (p=0.90). The difference in body mass index (BMI) was not statistically significant (24.20 vs 24.00, p=0.28). A minority of patients in both groups have family history of breast cancer (8.16% in metastasis group and 7.26% in non-metastasis group, p=0.76).
Patients in the metastasis group had statistically significant larger lesions than those in non-metastasis group (2.30 cm vs 2.00 cm, p=0.01). In both groups, mono-focal lesions were in the majority and the tiny intergroup differences were not statistically significant (86.87% vs 90.79%, p=0.24). For other properties of the lesion, including anatomic subdivisions (p=0.67), locations (p=0.91) and palpability (p=0.41), intergroup differences were minor and without statistical significance.
In the metastatic group, 69(68.32%) patients received mastectomy and 32(31.68%) patients received breast conserving surgery (BCS). Methylene blue was used as the tracer in 95 patients (94.06%) and nanocarbon in 6 patients (5.94%). An average of 4 SLNs were resected intraoperatively. In the non-metastatic group, 278(60.30%) patients received mastectomy and 183(39.70%) patients received BCS. Methylene blue was used as the tracer in 441 patients (95.67%) and nanocarbon in 6 patients (4.33%). An average of 3 SLNs were resected intraoperatively. None of the intergroup differences were statistically significant (p=0.13 for breast surgery, p=0.49 for tracer and p=0.18 for SLN number).
When it comes to pathological type and histological grade, “Invasive ductal carcinoma” and “Grade II” were in the majority in both groups (90.1% of “Invasive ductal carcinoma” and 89.36% of “Grade II” in the metastatic group; 84.75% of “Invasive ductal carcinoma” and 72.68% of “Grade II” in the non-metastatic group) and no statistically significant intergroup differences were noticed (p=0.22 for pathological type and p=0.28 for histological grade). There were more patients with high expression of ER and PR in the metastatic group compared to those in the non-metastatic group (91.00% vs 79.87% for ER, p<0.01 and 82.00% vs 70.35% for PR, p=0.02). As for the expression of Ki-67, although low-expressing patients occupied the majority in both groups (58.00% in the metastasis group and 68.36% in the non-metastasis group), the proportion of high-expressing patients in the metastatic group was significantly higher than those in the non-metastatic group (42.00% vs 31.64%, p=0.04). The expression of HER2 was comparable (p=0.04). In addition, a higher percentage of “Lymphatic invasion” and “Nerve invasion” was noticed in the metastasis group (13.51% vs 2.56% for Lymphatic invasion, p<0.01 and 8.11% vs 2.28% for Nerve invasion, p=0.02). No statistically significant intergroup differences in hematological constants were noticed (p=0.74 for PLR, p=0.69 for NLR and p=0.68 for SII) (Table 1).
Independent predictors for SLN metastasis
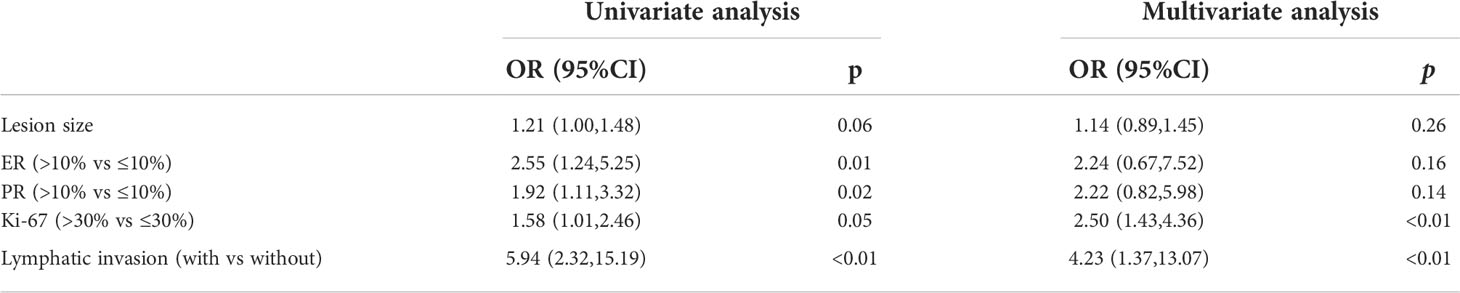
The receiver operating characteristic (ROC) analysis was adopted to determine the cut-off value of lesion size and assess its discriminative power. The cut-off value was 3.25 and the area under the curve (AUC) was 0.583 (p=0.01) (Figure 2). The expression of Ki-67 (odds ratio, 2.52; confidence interval, 0.67–7.58; p<0.01) and lymphatic invasion (odds ratio, 4.12; confidence interval, 1.35–12.97; p=0.01) were established as independent predictors for SLN metastasis after univariate and multivariate logistic regression analysis (Table 2).
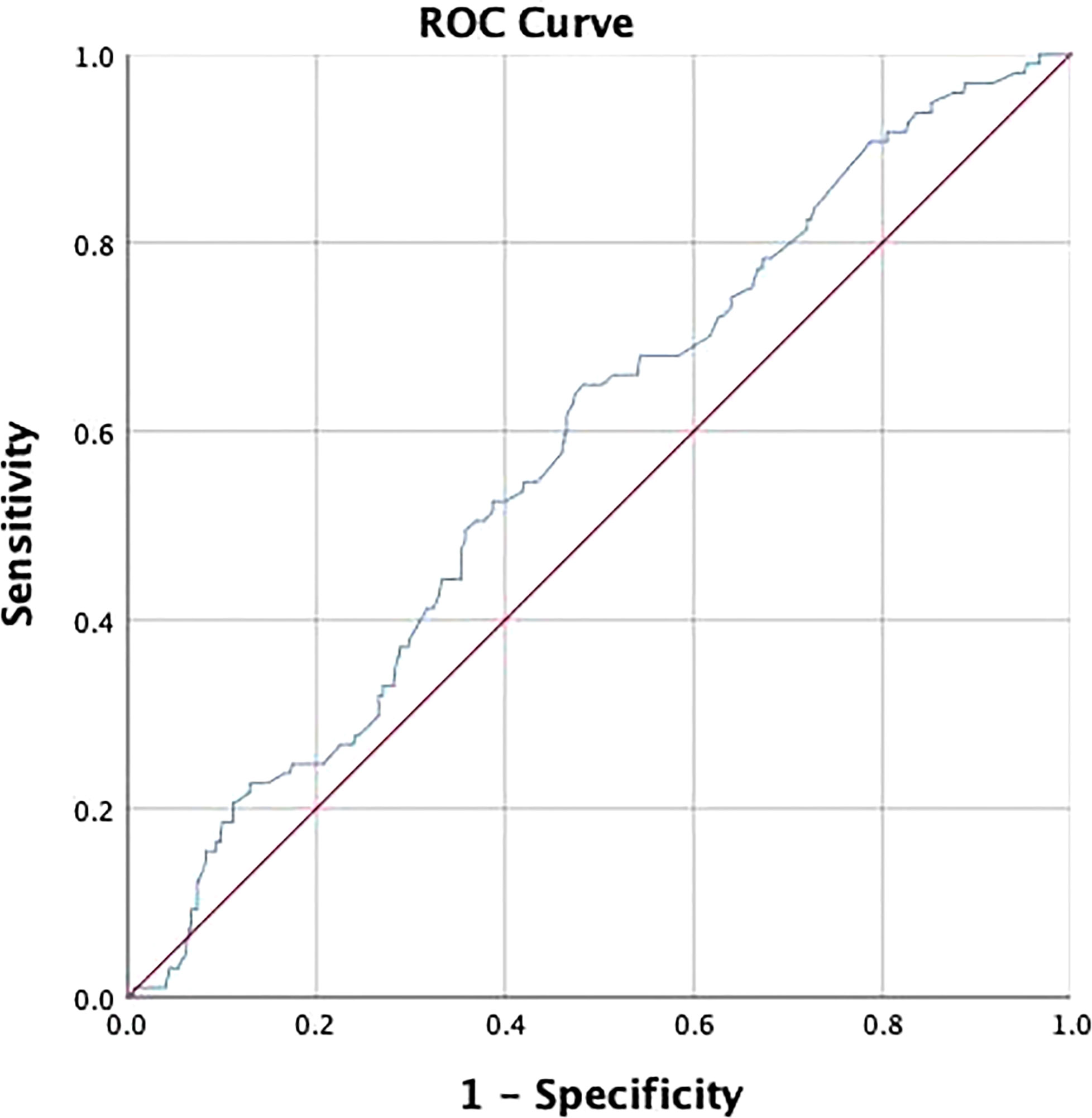
Figure 2 Receiver operating characteristic (ROC) curve was used to determine the cut-off value of lesion size and assess its discriminative power. The cut-off value was 3.25 and the area under the curve (AUC) was 0.583 (P = 0.01).
Subgroup comparisons and the independent predictors for nSLN metastasis
A total of 4 patients were excluded from the second analysis in the SLN metastasis group, including one with missing information and three without further cALND. Based on whether nSLN metastases were detected, 20 of all eligible patients were divided into the metastatic group and the remaining 77 patients were divided into the non-metastatic group.
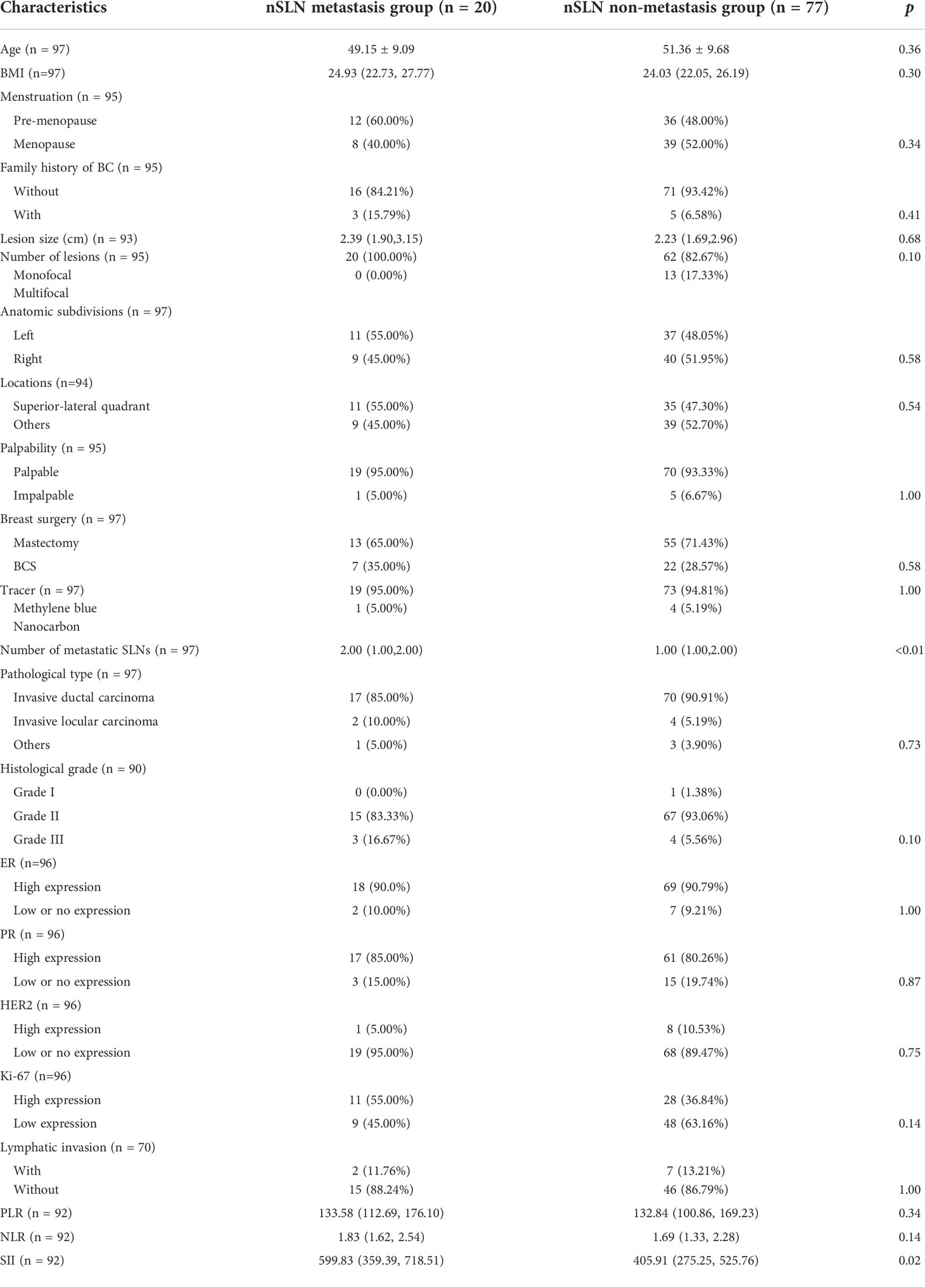
Patients in the nSLN metastasis group had more SLN metastases than those in the non-metastasis group (2.00 vs1.00, p<0.01). Besides, the analysis of hematological parameters revealed statistically significant intergroup difference in the expression of SII (599.83 vs 405.91, p=0.02) (Table 3). No statistically significant intergroup differences were noticed in other patient characteristics, lesion characteristics, surgical approaches and nature of the pathology. However, it was worth mentioning that patients in the metastatic group were younger than those in the non-metastatic group (49.15 vs 51.36, p=0.10). Patients in the metastatic group had larger lesion size compared with those in the non-metastatic group (2.39 cm vs 2.23 cm, p=0.68).
After univariate and multivariate logistic analysis, both ‘number of metastatic SLN’ (odds ratio, 2.59; confidence interval, 1.34–4.99; P<0.01) and ‘SII’ (odds ratio, 1.003; confidence interval, 1.001–1.005; P=0.01) were determined independent predictors for nSLN metastasis (Table 4).
Discussion
Axillary management has been considered indispensable for breast cancer patients although the scope of surgery is still up for debate (1). Patients tend to have smaller tumors and lower axillary burdens as a result of early diagnosis and advances in imaging techniques (26). With this comes the constant de-escalating of surgical procedure, and SLNB is a significant milestone.
SLNB entails preoperative injection of a tracer and intraoperative excision of SLNs for pathological examination; if the result is negative, further cALND is eliminated. However, in case of a positive result, the need for further cALND is debated.
The role of cALND was challenged because of the low rate of nSLN metastasis in SLN-positive patients and the serious accompanied side-effects. In the Z0011, IBCSG 23-01 and AMAROS trials, the rate of nSLN metastasis was 27.3%, 13% and 33% respectively (6, 10, 27). On the other hand, the long-term follow-up results of NSABP B-32 trial revealed that incidence of upper limb lymphedema in patients receiving cALND was 4 times higher than those receiving SLNB alone (28). In our study, a total of 97 SLN-positive patients received cALND, and only 20 of them were with nSLN metastases, accounting for 20.6%.
Furthermore, the safety of eliminating cALND seemed to be proven. The Z0011 trial reveled that for cT1-2N0M0 breast cancer patients with 1-2 positive SLNs, cALND contributed neither to a better local control nor to a longer disease-free survival (DFS) or overall survival (OS) if patients received BCS, systemic therapy, or radiotherapy. The IBCSG 23-01 trial reached similar results after 10-years follow-up. Besides, the AMAROS trial confirmed the safety of radiotherapy in place of cALND.
Noteworthily, in the 3 clinical trials mentioned, the percentage of patients with SLN micro-metastases was 41.2%, 28.8% and 98% respectively, which was also the reason why they were criticized. It was well known that patients with micro-metastasis had a better outcome than those with macro-metastasis (29). However, there are still a large number of patients with SLN macro-metastases in the clinic. In our study, there were 98 patients with macro-metastases out of 101 SLN-positive patients, accounting for 97%. It would be irresponsible to omit cALND in such patients who are ineligible for present clinical trials.
Concerns regarding the need for cALND have led to questions about the role of SLNB. Is it really necessary to carry out SLNB in all clinically node-negative patients? First, the rate of SLN metastasis is extremely low, ranging from 15% to 35% (30–32). Second, although being reduced, side-effects of SLNB were still present. Hanne Verbelen et al. followed 126 SLN-negative patients for 7 years and discovered that one-quarter of them suffered from arm and shoulder complaints (33). Gebruers et al. reviewed 28 articles and found that lymphedema was still a problem with 0%-63% incidence for SLN-negative patients (34). In our study, only 101 patients discovered SLN metastases, accounting for 18.04%.
These considerations suggested that the implementation of SLNB and further cALND should be tailored more cautiously. In our study, 4 independent predictors for SLN and nSLN metastases were identified.
Ki-67, a proliferation marker, has been commonly used as a prognosis and treatment selection signal despite substantial inter-laboratory variability (35). Breast cancer patients with high Ki-67 expression have been proven to have a higher incidence of distant metastasis and recurrence, as well as a worse overall survival (21). Surprisingly, Ki-67 isn’t included in any of the common nomograms for predicting non-sentinel metastasis after a positive SLN (36). Similarly, our study suggested that high expression of Ki-67 was an independent predictor for SLN metastasis. However, it did not show statistical relevance for nSLN metastases after a positive SLN, coinciding with existing studies.
Lymphatic invasion is one of the most essential steps in cancer cell metastasis, and it has been linked to a poorer DFS and OS for breast cancer patients (37). SLN-positive patients with lymphatic invasion have a higher risk of further metastasis, according to a study by Kimberly J. Van Zee et al. (38) Similarly, patients with lymphatic invasion had a higher chance of SLN metastases in our study.
The number of positive SLNs has been identified as a determinant in surgical decision-making and prognosis evaluation. The cALND can be skipped in breast cancer patients with 1-2 positive SLNs, according to ASCO guidelines (11). In our study, patients with ≥3 positive SLNs had a nSLN metastasis rate of 40%, which was considerably higher than the 20% rate for patients with 1-2 positive SLNs. Further analysis revealed that the number of positive SLNs was an independent predictor for nSLN metastasis, with patients having a 2.6-fold greater risk of nSLN metastases for each increase in the number of positive SLNs.
Hematological constants are thought to be a simple way to assess a patient’s systemic immunological and inflammatory condition (39). SII has been proposed as a factor with abilities to reflect distant metastasis, local recurrence and prognosis by outlining changes in platelets, neutrophils and lymphocytes in the circulatory system (40). In our study, elevated SII served as an independent predictor for nSLN metastasis following positive SLNs.
Furthermore, certain other factors, while not yielding favorable findings in the multivariate analysis, are nonetheless instructive to us.
Tumor size is one of the most important factors in determining the surgical approach and assessing patient prognosis. In the study by Seung Ki Min et al, increased tumor size predicted increased number of lymph node metastases (41). Also, tumor size served as a significant predictor for nSLN metastases in SLN-positive patients in the study by A. M. Moorman et al. (31) The present study showed that patients with larger lesion size were more likely to develop SLN metastasis. And the cut-off value was 3.2 cm on ROC analysis. However, in further logistic regression and analysis for nSLN metastasis, lesion size did not show positive results. The reason for this phenomenon, we speculate, in addition to the limitations of the single-center sample, may also be the bias caused by the single-tracer method and the subjective choice of surgeons.
The presence of a functional estrogen-signaling pathway and a better prognosis are assumed to be linked to high expression of ER and PR. Our findings appeared to imply a trend that patients with higher ER and PR expression got a higher risk of lymph node metastasis, even though it was not statistically significant in the multivariate logistic analysis. This is counterintuitive. Other trials, not coincidentally, came to similar results (38, 42, 43). The link between ER, PR, and lymph node metastases needs to be further investigated.
Age has been shown to be closely related to the incidence of breast cancer and the biological behavior of tumor cells (44). Breast cancers in younger patients tend to have a worse immunophenotype, such as a higher histological staging and a lower expression of hormone receptors (45). In our study, the mean age of all patients was 51.8. And in both comparisons, patients in the metastatic group were younger than those in the non-metastatic group, which was consistent with the existing theory.
BMI was included in this study for analysis because obesity was thought to be linked to a worse prognosis for breast cancer patients (46). In both comparisons, patients in the metastatic group had a higher BMI than those in the non-metastasis group, according to the findings.
In this study, we investigated SLN and nSLN metastasis together, looking longitudinally at factors related to lymph node metastasis in breast cancer. However, there are some limitations. First, the size of the intraoperatively removed SLNs was thought to be an important factor linked to nSLN metastasis, but due to lacking of data, we were unable to incorporate it in our analysis (47). Second, we are unable to investigate prognosis and surgical side-effects of patients due to a paucity of follow-up data. Finally, this is a single-center retrospective study with a limited sample size, more large-scale and multi-center studies are needed.
Conclusion
For clinically node-negative breast cancer patients, “high expression of Ki-67” and “lymphatic invasion” imply a higher risk of SLN metastasis; for SLN-positive patients, “increased number of positive SLN” and “increased SII” imply a higher risk of nSLN metastasis. For such patients, appropriate axillary lymph node management is necessary.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by Ethics Committee of China Medical University (Approval number: AF-SOP-07-1.1-01). All methods were performed in accordance with the relevant guidelines and regulations. All enrolled patients supported this study and signed an informed consent form.
Author contributions
XW conceived this study. FJ was the director for the fund. YL and YF collected medical records and drafted manuscript. ZJ, MC, and XY assisted in revising the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China (No. 82073282), China Postdoctoral Science Foundation (No. 2020M681018) and Natural Science Foundation of Liaoning Province-Doctoral Research Program (No. 2021-BS-115).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SLNB, sentinel lymph node biopsy; cALND, completion axillary lymph node dissection; SLN, sentinel lymph nodes; nSLN, non-sentinel lymph node; SII, systemic inflammation index; ASCO, American Society of Clinical Oncology; ER, estrogen receptors; PR, progesterone receptors; HER2, human epidermal growth factor receptor 2; PLR, platelet lymphocyte ratio; NLR, neutrophil lymphocyte ratio; AUC, area under the curve; ROC, receiver operating characteristic; DFS, disease-free survival; OS, overall survival; NAC, neoadjuvant chemotherapy; BCS, breast conserving surgery; NCCN, national comprehensive cancer network.
References
1. Park KU, Caudle A. Management of the axilla in the patient with breast cancer. Surg Clin North Am (2018) 98(4):747–60. doi: 10.1016/j.suc.2018.04.001
2. Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med (2003) 349(6):546–53. doi: 10.1056/NEJMoa012782
3. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP b-32 randomised phase 3 trial. Lancet Oncol (2010) 11(10):927–33. doi: 10.1016/S1470-2045(10)70207-2
4. Esposito E, Di Micco R, Gentilini OD. Sentinel node biopsy in early breast cancer. a review on recent and ongoing randomized trials. Breast (2017) 36:14–9. doi: 10.1016/j.breast.2017.08.006
5. Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al. American Society of clinical oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol (2005) 23(30):7703–20. doi: 10.1200/JCO.2005.08.001
6. Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol (2014) 15(12):1303–10. doi: 10.1016/S1470-2045(14)70460-7
7. Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ. Thresholds for therapies: highlights of the St gallen international expert consensus on the primary therapy of early breast cancer 2009. Ann Oncol (2009) 20(8):1319–29. doi: 10.1093/annonc/mdp322
8. Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. Jama (2011) 305(6):569–75. doi: 10.1001/jama.2011.90
9. Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American college of surgeons oncology group Z0011 randomized trial. Ann Surg (2010) 252(3):426–32. doi: 10.1097/SLA.0b013e3181f08f32
10. Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol (2013) 14(4):297–305. doi: 10.1016/S1470-2045(13)70035-4
11. Voutsadakis IA, Spadafora S. Recommendation for omitting axillary lymph node dissection should be individualized in patients with breast cancer with one or two positive sentinel lymph nodes. J Clin Oncol (2014) 32(34):3901–2. doi: 10.1200/JCO.2014.57.1190
12. Tinterri C, Canavese G, Bruzzi P, Dozin B. SINODAR ONE, an ongoing randomized clinical trial to assess the role of axillary surgery in breast cancer patients with one or two macrometastatic sentinel nodes. Breast (2016) 30:197–200. doi: 10.1016/j.breast.2016.06.016
13. Veronesi U, Galimberti V, Paganelli G, Maisonneuve P, Viale G, Orecchia R, et al. Axillary metastases in breast cancer patients with negative sentinel nodes: a follow-up of 3548 cases. Eur J Cancer (2009) 45(8):1381–8. doi: 10.1016/j.ejca.2008.11.041
14. Straver ME, Meijnen P, van Tienhoven G, van de Velde CJ, Mansel RE, Bogaerts J, et al. Role of axillary clearance after a tumor-positive sentinel node in the administration of adjuvant therapy in early breast cancer. J Clin Oncol (2010) 28(5):731–7. doi: 10.1200/JCO.2008.21.7554
15. Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20(6):691–722. doi: 10.6004/jnccn.2022.0030
16. Ahmed M, Purushotham AD, Horgan K, Klaase JM, Douek M. Meta-analysis of superficial versus deep injection of radioactive tracer and blue dye for lymphatic mapping and detection of sentinel lymph nodes in breast cancer. Br J Surg (2015) 102(3):169–81. doi: 10.1002/bjs.9673
17. Kern KA. Concordance and validation study of sentinel lymph node biopsy for breast cancer using subareolar injection of blue dye and technetium 99m sulfur colloid. J Am Coll Surg (2002) 195(4):467–75. doi: 10.1016/S1072-7515(02)01312-1
18. Ye JM, Guo BL, Liu Q, Ma F, Liu HJ, Wu Q, et al. Clinical practice guidelines for sentinel lymph node biopsy in patients with early-stage breast cancer: Chinese society of breast surgery (CSBrS) practice guidelines 2021. Chin Med J (Engl) (2021) 134(8):886–94. doi: 10.1097/CM9.0000000000001410
19. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of clinical Oncology/College of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med (2010) 134(7):e48–72. doi: 10.5858/134.7.e48
20. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of clinical Oncology/College of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med (2007) 131(1):18–43. doi: 10.5858/2007-131-18-ASOCCO
21. Bonacho T, Rodrigues F, Liberal J. Immunohistochemistry for diagnosis and prognosis of breast cancer: a review. Biotech Histochem (2020) 95(2):71–91. doi: 10.1080/10520295.2019.1651901
22. Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol (2020) 38(12):1346–66. doi: 10.1200/JCO.19.02309
23. Siotos C, McColl M, Psoter K, Gilmore RC, Sebai ME, Broderick KP, et al. Tumor site and breast cancer prognosis. Clin Breast Cancer (2018) 18(5):e1045–52. doi: 10.1016/j.clbc.2018.05.007
24. Kroman N, Wohlfahrt J, Mouridsen HT, Melbye M. Influence of tumor location on breast cancer prognosis. Int J Cancer (2003) 105(4):542–5. doi: 10.1002/ijc.11116
25. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin (2017) 67(2):93–9. doi: 10.3322/caac.21388
26. Goyal A, Dodwell D. POSNOC: A randomised trial looking at axillary treatment in women with one or two sentinel nodes with macrometastases. Clin Oncol (R Coll Radiol) (2015) 27(12):692–5. doi: 10.1016/j.clon.2015.07.005
27. Galimberti V, Cole BF, Viale G, Veronesi P, Vicini E, Intra M, et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol (2018) 19(10):1385–93. doi: 10.1016/S1470-2045(18)30380-2
28. Kopec JA, Colangelo LH, Land SR, Julian TB, Brown AM, Anderson SJ, et al. Relationship between arm morbidity and patient-reported outcomes following surgery in women with node-negative breast cancer: NSABP protocol B-32. J Support Oncol (2013) 11(1):22–30. doi: 10.1016/j.suponc.2012.05.003
29. Hoda SA, Chiu A, Prasad ML, Giri D, Hoda RS. Are microinvasion and micrometastasis in breast cancer mountains or molehills? Am J Surg (2000) 180(4):305–8. doi: 10.1016/S0002-9610(00)00464-5
30. Reimer T, Stachs A, Nekljudova V, Loibl S, Hartmann S, Wolter K, et al. Restricted axillary staging in clinically and sonographically node-negative early invasive breast cancer (c/iT1-2) in the context of breast conserving therapy: First results following commencement of the intergroup-Sentinel-Mamma (INSEMA) trial. Geburtshilfe Frauenheilkd (2017) 77(2):149–57. doi: 10.1055/s-0042-122853
31. Moorman AM, Rutgers EJT, Kouwenhoven EA. Omitting SLNB in Breast Cancer: Is aNomogram the Answer? Ann Surg Oncol (2022) 29(4):2210–8. doi: 10.1245/s10434-021-11007-9
32. Hwang RF, Gonzalez-Angulo AM, Yi M, Buchholz TA, Meric-Bernstam F, Kuerer HM, et al. Low locoregional failure rates in selected breast cancer patients with tumor-positive sentinel lymph nodes who do not undergo completion axillary dissection. Cancer (2007) 110(4):723–30. doi: 10.1002/cncr.22847
33. Verbelen H, Tjalma W, Meirte J, Gebruers N. Long-term morbidity after a negative sentinel node in breast cancer patients. Eur J Cancer Care (Engl) (2019) 28(5):e13077. doi: 10.1111/ecc.13077
34. Gebruers N, Verbelen H, De Vrieze T, Coeck D, Tjalma W. Incidence and time path of lymphedema in sentinel node negative breast cancer patients: a systematic review. Arch Phys Med Rehabil (2015) 96(6):1131–9. doi: 10.1016/j.apmr.2015.01.014
35. Soliman NA, Yussif SM. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol Med (2016) 13(4):496–504. doi: 10.20892/j.issn.2095-3941.2016.0066
36. Coutant C, Olivier C, Lambaudie E, Fondrinier E, Marchal F, Guillemin F, et al. Comparison of models to predict nonsentinel lymph node status in breast cancer patients with metastatic sentinel lymph nodes: a prospective multicenter study. J Clin Oncol (2009) 27(17):2800–8. doi: 10.1200/JCO.2008.19.7418
37. Sahoo PK, Jana D, Mandal PK, Basak S. Effect of lymphangiogenesis and lymphovascular invasion on the survival pattern of breast cancer patients. Asian Pac J Cancer Prev (2014) 15(15):6287–93. doi: 10.7314/APJCP.2014.15.15.6287
38. Van Zee KJ, Manasseh DM, Bevilacqua JL, Boolbol SK, Fey JV, Tan LK, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol (2003) 10(10):1140–51. doi: 10.1245/aso.2003.03.015
39. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
40. Hua X, Long ZQ, Zhang YL, Wen W, Guo L, Xia W, et al. Prognostic value of preoperative systemic immune-inflammation index in breast cancer: A propensity score-matching study. Front Oncol (2020) 10:580. doi: 10.3389/fonc.2020.00580
41. Min SK, Lee SK, Woo J, Jung SM, Ryu JM, Yu J, et al. Relation between tumor size and lymph node metastasis according to subtypes of breast cancer. J Breast Cancer (2021) 24(1):75–84. doi: 10.4048/jbc.2021.24.e4
42. Gann PH, Colilla SA, Gapstur SM, Winchester DJ, Winchester DP. Factors associated with axillary lymph node metastasis from breast carcinoma: descriptive and predictive analyses. Cancer (1999) 86(8):1511–9. doi: 10.1002/(SICI)1097-0142(19991015)86:8<1511::AID-CNCR18>3.0.CO;2-D
43. Ahlgren J, Stål O, Westman G, Arnesson LG. Prediction of axillary lymph node metastases in a screened breast cancer population. south-East Sweden breast cancer group. Acta Oncol (1994) 33(6):603–8. doi: 10.3109/02841869409121769
44. Radecka B, Litwiniuk M. Breast cancer in young women. Ginekol Pol (2016) 87(9):659–63. doi: 10.5603/GP.2016.0062
45. Rossi L, Mazzara C, Pagani O. Diagnosis and treatment of breast cancer in young women. Curr Treat Options Oncol (2019) 20(12):86. doi: 10.1007/s11864-019-0685-7
46. Gondo N, Sawaki M, Hattori M, Yoshimura A, Kotani H, Adachi Y, et al. Impact of BMI for clinical outcomes in Japanese breast cancer patients. Jpn J Clin Oncol (2020) 50(3):230–40. doi: 10.1093/jjco/hyz175
Keywords: surgery, invasive breast cancer, sentinel lymph node biopsy, completion axillary lymph node dissection, metastasis
Citation: Liu Y, Fan Y, Jin Z, Cui M, Yu X, Jin F and Wang X (2022) Axillary management for early invasive breast cancer patients: Who will truly benefit? Front. Oncol. 12:989975. doi: 10.3389/fonc.2022.989975
Received: 09 July 2022; Accepted: 19 July 2022;
Published: 15 August 2022.
Edited by:
Umer Farooq Awan, Government College University, Lahore, PakistanReviewed by:
Jia Wang, Second Affiliated Hospital of Dalian Medical University, ChinaYan Zhang, Jilin University, China
Copyright © 2022 Liu, Fan, Jin, Cui, Yu, Jin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Wang, soloman2003@163.com
†These authors have contributed equally to this work
 Yanbiao Liu
Yanbiao Liu Yan Fan2,3,4†
Yan Fan2,3,4† Xinmiao Yu
Xinmiao Yu Feng Jin
Feng Jin