- Department of Radiotherapy, The First Affiliated Hospital of Zhejiang Chinese Medical University, Zhejiang Provincial Hospital of Traditional Chinese Medicine, Hangzhou, China
Immune checkpoint inhibitors (ICIs) are a revolutionary breakthrough in the field of cancer by modulating patient’s own immune system to exert anti-tumor effects. The clinical application of ICIs is still in its infancy, and their dosing regimens need to be continuously adjusted. Pharmacokinetic/pharmacodynamic studies showed a significant plateau in the exposure-response curve, with high receptor occupancy and plasma concentrations achieved at low dose levels. Coupled with concerns about drug toxicity and heavy economic costs, there has been an ongoing quest to reevaluate the current ICI dosing regimens while preserving maximum clinical efficacy. Many clinical data showed remarkable anticancer effects with ICIs at the doses far below the approved regimens, indicating the possibility of dose reduction. Our review attempts to summarize the clinical evidence for ICIs regimens with lower-dose, less-frequency, shorter-course, and provide clues for further ICIs regimen optimization.
1 Introduction
Immune checkpoint inhibitors (ICIs) are revolutionary breakthroughs in the field of cancer in recent years, which have changed the traditional treatment paradigm. At present, the relatively proven ICIs in clinical application include cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors and programmed cell death protein-1 (PD-1) receptor inhibitors/programmed cell death ligand 1 (PD-L1) inhibitors. CTLA-4 is a transmembrane receptor on T cells, which can compete with CD28 to prevent co-stimulation and induce T cell cycle arrest. CTLA-4 inhibitors block the above process and restore the function of T cells to eradicate tumor cells. The U.S. Food and Drug Administration (FDA) approved CTLA-4 inhibitors include: ipilimumab, tremelimumab. PD-1 is expressed on tumor-infiltrating lymphocytes (mainly CD4+ T cells), B cells, natural killer cells, monocytes and dendritic cells, while PD-L1 is highly expressed on tumor cells. The binding of PD-1 to PD-L1 mediates a co-inhibitory signal of T cell activation, thus leading to tumor immune escape. PD-1/PD-L1 inhibitors block the PD-1 signaling pathway, partially restoring T-cells recognition of tumors and inducing immune normalization. Currently FDA-approved PD-1 inhibitors include: nivolumab, pembrolizumab, cemiplimab, dostarlimab, and PD-L1 inhibitors include: atezolizumab, avelumab, durvalumab. In fact, most research on dose intensity reduction of ICI focused on nivolumab, pembrolizumab and ipilimumab, our review also mainly focused on these three ICIs.
ICIs belong to monoclonal antibody and their pharmacokinetic/pharmacodynamic properties are distinctly different from those of traditional cytotoxic and small molecule drugs; therefore, determining the optimal dose of ICIs using traditional drug models may face many difficulties. The recommended dosing regimens for ICIs have evolved as experience accumulates. ICIs were initially administered based on body weight, and as population pharmacokinetic data accumulated, fixed-dose regimen was found to improve convenience and reduce waste while preserving efficacy, thus FDA approved nivolumab 240 mg Q2W equivalent to 3 mg/kg Q2W and pembrolizumab 200 mg Q3W equivalent to 2 mg/kg Q3W. Subsequently, high-dose, extended-interval dosing regimens (e.g. nivolumab 480 mg Q4W and pembrolizumab 400 mg Q6W) were added to all approved adult indications based on silico simulations (1–4), and validated in prospective clinical trials (5–7). Notably, the choice of average body weight is not consistent across ICIs: 240mg fixed dose of nivolumab is numerically equivalent to 3mg/kg dose for 80kg patients, while 200mg pembrolizumab corresponds to 2mg/kg for 100kg patients and 750mg durvalumab corresponds to 10mg/kg for 75kg patients. Cancer patients are often combined with cachexia resulting in underweight. The average weight of patients using ICI is about 75 kg (8–10), and the Asian population tend to have lower weight. In clinical practice, clinicians may reduce the dose or delay the administration of ICIs concerns about the patient’s physical condition or adverse effects of drugs, as well as for economic reasons or patient requests, but significant survival benefits can still be seen, which may provide clues to optimize the dosing regimens. In terms of duration of therapy, the majority of ICIs are given for one year as adjuvant or consolidation therapy, which is entirely in reference to the duration of adjuvant chemotherapy, and there is no evidence to compare longer or shorter courses. For patients with advanced/metastatic tumors, treatment usually lasts two years or until disease progression or unacceptable toxicity occurs. For those patients with durable stability, there is no definitive answer as to when to discontinue ICIs therapy.
Considering the economic and convenience reasons, many scholars suggest the need to reevaluate the current ICI dosing regimens. They proposed the concept of interventional pharmacoeconomics, hoping to reduce health care costs and perhaps also adverse effects while maintaining treatment efficacy through the development of new dosing regimens (11, 12). The main strategies include lower doses, less frequent dosing, shorter duration of treatment and therapeutic substitution, which have successfully improved the clinical practice of many drugs (e.g., abiraterone, ibrutinib, trastuzumab) (11, 12), also provide opportunities for dose reduction of ICIs (13, 14). In this review, we mainly discuss the issues of dosing intensity reduction and treatment duration selection, and the administration strategy of ICIs in the context of the COVID-19 pandemic.
2 ICIs-Related Adverse Effects
The incidence of irAEs is 70-90% for any grade, 10-40% for grade 3/4, and 0.3-1.2% for fatal (grade 5) irAEs (15–22). An increased incidence and grade of irAEs as well as earlier onset could be observed in combination therapy (22). irAEs can occur in almost all organs throughout the body. Cutaneous toxicity is one of the most common irAEs, occurring in 1/3-1/2 of patients treated with ICIs, manifesting primarily as rash, pruritus, vitiligo (22, 23). The incidence of endocrine toxicity is 40% (16), mainly affecting thyroid, pituitary, and islet functions, requiring regular monitoring of hormone levels and timely hormone replacement therapy. Diarrhea is also a common side effect with an incidence of 15-45% (24). Colitis is the most common type of high-grade irAEs and one of the leading causes of discontinuation (22, 25). Immune pneumonitis is relatively rare but potentially fatal, with an incidence of 3-5% in clinical trials, and appears to be more common (9-19%) in real-world studies, among which grade 3-4 pneumonitis accounting for 30-50% of cases, and 10% patients may develop an infection that leads to death (22, 26–30). Other rare irAEs include hematologic toxicity, nephrotoxicity, immune hepatitis, immune myocarditis, neurological irAEs, etc. Distinguish from cytotoxic drugs, ICIs are generally administered continuously for a long time, chronic toxicity (even low-grade toxicity) is likely to be intolerable for patients. Approaches to reduce irAEs include more precise selection of targeted population, optimization of drug regimens, whole course management, and prophylactic application for high-risk patients.
3 Dosing Regimen Optimization
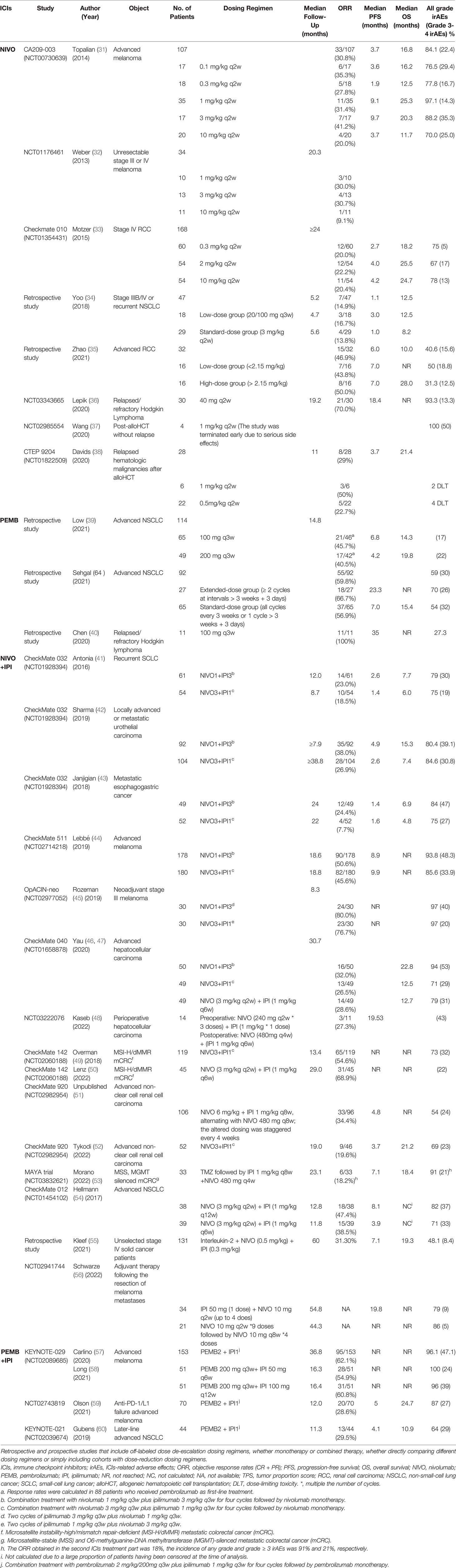
Dose optimization studies on ICIs are limited, focusing on nivolumab, pembrolizumab, and ipilimumab. An extensive literature search was conducted for the three most widely used ICIs to collect clinical data of each drug. Retrieval method: Firstly, in the PubMed database, the literature was searched by “Clinical Trial”, “Prospective Studies”, “Retrospective Studies” and “nivolumab”, “pembrolizumab”, “ipilimumab”. Secondly, search clinical trials of each drug in Clinicaltrials.gov. Inclusion criteria: retrospective and prospective studies of three ICIs that include off-label dosing regimens, whether monotherapy or combined therapy, whether directly comparing different dosing regimens or simply including cohorts with off-label dosing regimens. Although most attempts at off-labelled dosing regimens are pharmacokinetic/pharmacodynamic simulations, subgroup data from early clinical trials or retrospective studies with small samples, they can still provide clues to the optimization of ICIs (Table 1).
3.1 Pharmacokinetics/Pharmacodynamics Attempts
For most ICIs (except ipilimumab), there is no clear relationship between dose and efficacy or safety. The dose-response and exposure-response curves showed an obvious plateau, implying that increasing doses do not contribute to tumor control and that lower-dose ICIs may produce the same effect (61–63). Agrawal et al. found that the exposure-response relationship reached a plateau with nivolumab doses ≥1 mg/kg in melanoma and renal cell carcinoma, suggesting that low-dose regimen could be tried in high-immunogenic tumors (64). Receptor occupancy is maximized at low dose level, e.g., peripheral PD-1 receptor occupancy is saturated with 0.3 mg/kg nivolumab (64, 65), greater than 90% with 0.5 mg/kg pembrolizumab, 4 mg/kg atezolizumab, 3 mg/kg avelumab (66–68), and the soluble PD-L1 receptor was completely suppressed when durvalumab ≥ 0.3 mg/kg (69). The trough concentration (Cmin) at the recommended dose is also much higher than the target concentration. For example, the Cmin of 3 mg/kg atezolizumab exceeds the target concentration of 6 μg/mL (68, 70), while the labelled dose has a Cmin (>100 μg/mL) nearly 20 times higher than the target concentration (4, 71).
In terms of dosing intervals, prolonging the interval still maintains the pharmacodynamic parameters at effective levels. Simulated administration of nivolumab at 240mg Q4W/480mg Q8W regimen and pembrolizumab at 200mg Q6W regimen revealed that serum drug concentrations remained above the minimum effective concentration in more than 95% of patients (72). The Canadian Agency of Drugs and Technologies in Health simulated dosing regimens of pembrolizumab 4 mg/kg Q6W in patients weighing 70, 100, and 150 kg, all with trough target engagement above 97% (73). Comparison of the standard regimen of atezolizumab with several extended interval regimens showed that the predicted efficacy and safety of 1680 mg Q8W/1200 mg Q6W was not inferior to the standard 1200 mg Q3W (74).
Pharmacokinetic studies have shown that many variables can influence the clearance of ICIs, such as: gender, race, weight, performance status, tumor volume, drug response, and albumin levels (62, 75, 76). Over the treatment, drug clearance decreases as the responders’ performance status improves and tumor burden decreases (62). Therefore, it remains to be investigated whether less-frequent or lower-dose regimens could be administered in subsequent cycles for those patients who achieved good outcomes.
3.2 Clinical Evidence
3.2.1 Nivolumab
In the phase I CA209-003 trial (31), melanoma patients received 0.1, 0.3, 1 mg/kg nivolumab, and the objective response rates (ORR) were 35%, 28%, and 31%, respectively. Patients who did not respond to the lower dose remained unresponsive to the higher dose (31). In another phase I clinical trial of melanoma, the ORR of patients receiving 1, 3 mg/kg nivolumab was 30% and 31%, respectively (32). In the Checkmate 010 study in renal cancer, nivolumab was administered at doses of 0.3, 2, or 10 mg/kg with similar efficacy (33). All of the above early clinical trials suggested that nivolumab may be effective at low doses. A retrospective study from Korea compared the low-dose nivolumab group (20 mg/100 mg Q3W) with the standard-dose group (3 mg/kg Q2W) in non-small cell lung cancer (NSCLC) with no significant difference in ORR, progression-free survival (PFS), and overall survival (OS) (34). Results from another retrospective studies in Singapore also showed that low-dose (100 mg/140 mg) nivolumab did not reduce efficacy in renal cancers (35). In a single-arm, open-label phase II study conducted in Russia, the ORR for 40 mg Q2W nivolumab in relapsed/refractory Hodgkin lymphoma was 70%, with 13/30 (43.3%) achieving complete remission (CR) (36). The results of these clinical studies further confirmed the previous hypothesis that highly immunogenic tumors may be effective at low doses of ICIs.
Based on the results of the CheckMate 205 study (77), The FDA approved 3 mg/kg nivolumab for relapsed/refractory classic Hodgkin lymphoma after autologous hematopoietic cell transplantation (HCT), but did not recommend it for patients with allogeneic HCT (alloHCT), primarily due to the high risk of graft-versus-host disease (GVHD) (78–80). However, in retrospective studies and case reports, some physicians have also used low-dose nivolumab (0.3–1.5 mg/kg) in post-alloHCT patients with success (81–84). A clinical trial of 1 mg/kg Q2W nivolumab in the post-alloHCT population was terminated early due to serious side effects (37). In another study, nivolumab was started at 1 mg/kg and dose-limiting toxicity was observed in 2/6 patients, severe irAEs and fatal GVHD still occurred in 4/22 patients even after nivolumab was reduced to 0.5 mg/kg (38), indicating that the use of nivolumab in post-alloHCT patients requires more caution and further studies are needed.
3.2.2 Pembrolizumab
A retrospective study of advanced NSCLC found no significant differences in PFS, OS, or high grade irAEs between pembrolizumab 100mg and 200mg groups, either alone or in combination with chemotherapy (39). Similar survival outcomes were found between the extended-interval (>3 weeks + 3 days) and standard-interval groups of pembrolizumab in NSCLC patients, suggesting that extended dosing intervals may be available for patients with stable disease (85). Several cases have been reported in which complete remission was achieved with low-dose pembrolizumab in relapsed/refractory Hodgkin’s lymphoma, and even re-treatment with low-dose pembrolizumab remained effective (86, 87). In a series of studies in lymphoma, the ORR of 100 mg pembrolizumab was 100%, indicating the efficacy and safety of low-dose pembrolizumab, especially in the low-weight Asian population (40, 88). In addition, it is also recommended to administrate low dose pembrolizumab (50-100 mg) for post-alloHCT lymphoma (89).
3.2.3 Ipilimumab
Rationalized medication of ipilimumab is focused on combination therapy with PD-1 inhibitors. Various attempts have been made to reduce the dose in the combination, but the choice of which drug to reduce and by how much to preserve maximum efficacy while reducing toxicity is inconclusive. Given the dose-dependent toxicity of ipilimumab, we prefer to reduce the dose of ipilimumab in combination therapy.
3.2.3.1 Nivolumab+ Ipilimumab
3.2.3.1.1 N3I1 vs N1I3
Although the combination of 3mg/kg nivolumab with 3mg/kg ipilimumab provides a potential survival benefit, severe toxicity limits its use (90). Common modified combinations are N3I1 (3mg/kg nivolumab+1mg/kg ipilimumab) and N1I3 (1mg/kg nivolumab+3mg/kg ipilimumab). Currently, the FDA approved N1I3 for the treatment of melanoma and hepatocellular carcinoma (91), N3I1 for renal and colorectal cancer, and the nivolumab 360mg Q3W plus ipilimumab 1 mg/kg Q6W regimen is recommended for lung cancer and malignant pleural mesothelioma (92). However, there is much controversy over the FDA recommended dosing regimens. Some studies have concluded that the mode of N1I3 is more effective than N3I1, with an acceptable overall safety profile despite a slight increase in irAEs (41–43, 93). In melanoma, the CheckMate 511 and OpACIN-neo trials compared these two combinations and found that with similar efficacy, the grade 3-5 irAEs was significantly fewer in the N3I1 group, suggesting that the N3I1 combination maybe more appropriate for melanoma (44, 45). Although patients with recurrent/advanced hepatocellular carcinoma showed the greatest survival benefit with the N1I3 regimen (46, 47), nivolumab combined ipilimumab 1 mg/kg Q6W regimen also showed good results in perioperative treatment (48). Microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer patients treated with nivolumab every 2 weeks plus low-dose ipilimumab every 6 weeks achieved robust and durable benefit, seem fewer side effects than N3I1 regimen (49, 50). In conclusion, the optimal combination regimen of nivolumab plus ipilimumab is still inconclusive, which may be related to cancer type and disease stage.
3.2.3.1.2 Extended Interval Ipilimumab
Many studies have further extended the interval of ipilimumab to 6 weeks (49, 50, 94–97) or even longer. In the CheckMate 920 study of renal cancer, Cohort 1 patients received 6 mg/kg Q8W nivolumab plus 1 mg/kg Q8W ipilimumab, alternating with nivolumab 480 mg Q8W, with an ORR of 34.4%, median PFS 4.8 months, which was not significantly different from the results of several other cohorts using N3I1 regimen followed by nivolumab 480 mg Q4W (51, 52). The MAYA study evaluated the efficacy of temozolomide followed by nivolumab 480 mg Q4W plus ipilimumab 1 mg/kg Q8W in microsatellite-stable, O6-methylguanine–DNA methyltransferase–silenced metastatic colorectal cancer, with an ORR of 45% to the whole treatment strategy, median PFS of 7.1 months and median OS of 18.4 months (53). In CheckMate 012, nivolumab combined with ipilimumab 1 mg/kg Q6W or Q12W showed no significant differences in efficacy or safety in NSCLC patients (54).
A much lower-dose therapy of nivolumab (0.5 mg/kg) plus ipilimumab (0.3 mg/kg) combined with interleukin 2 and hyperthermia treating 131 cases of multiple advanced cancers showed an ORR of 31.3%, with median PFS reached 10 months, and the incidence of grade 3-4 irAEs was only 8.4% (55). In a Belgian single-center non-randomized phase II clinical trial, both nivolumab and ipilimumab were administrated as adjuvant therapy to melanoma at very low doses, with ipilimumab 50 mg (1 dose) plus nivolumab 10 mg Q2W (up to 4 does), and survival benefit was similar to the standard regimen (56).
3.2.3.2 Pembrolizumab + Ipilimumab
There are relatively few studies of the combination of pembrolizumab and ipilimumab. In the KEYNOTE-029 study, melanoma patients received pembrolizumab 2 mg/kg Q3W in combination with ipilimumab 1 mg/kg Q3W followed by pembrolizumab monotherapy had an ORR of 62% and a 3-year OS of 73% (57). Cohort C further compared pembrolizumab combined with two ipilimumab dosing regimens (50 mg Q6W vs. 100 mg Q12W) and found little difference in efficacy between the two groups, but the side effects were more severe in 100mg Q12W regimen (58). For melanoma patients after anti-PD-1/L1 failure, the ORR for pembrolizumab combined with low-dose ipilimumab (1mg/kg Q3W) was 29% (59).
In the KEYNOTE-021 study, receiving pembrolizumab plus low-dose ipilimumab in later-line treatment for NSCLC resulted in an ORR of 30% and grade 3-5 irAEs rate of 29% (60). However, in first-line treatment of NSCLC with PD-L1 tumor proportion score (TPS) ≥ 50%, the addition of ipilimumab to pembrolizumab did not improve the survival but did result in increased toxicity (98). This reminds us of the need to further enhance our patient selection and biomarker selection.
Given these published findings, there is immense potential to reduce the dose intensity of ICIs by applying available pharmacology or clinical data, aided by prospective interventional pharmacoeconomic trials. Given the fact that ICIs are packaged in fixed single vials and drug sharing is not agreed upon in most hospitals, single-dose reduction may not be cost effective. Alternatively, extend the frequency of administrations (with the minimum effective plasma concentration) could reduce costs, adverse events, and patient inconvenience, and is in line with the context of Covid-19 pandemic. In the opinion of Goldstein et.al., the simplest approach to reduce the dose intensity of Atezolizumab or Nivolumab was using a standard dose with an extended interval far greater than the labeled regimens (13, 14). The determination of the specific dosing frequency needs to be further investigated and can also be considered with the help of Therapeutic Drug Monitoring (TDM) to more accurately guide the individualized dosing frequency. This concept of reduced dosing frequency could be first applied to ICIs given at fixed dose as monotherapies rather than in combination ICIs therapy with doses based on body weight.
4 Optimal Durations of ICIs
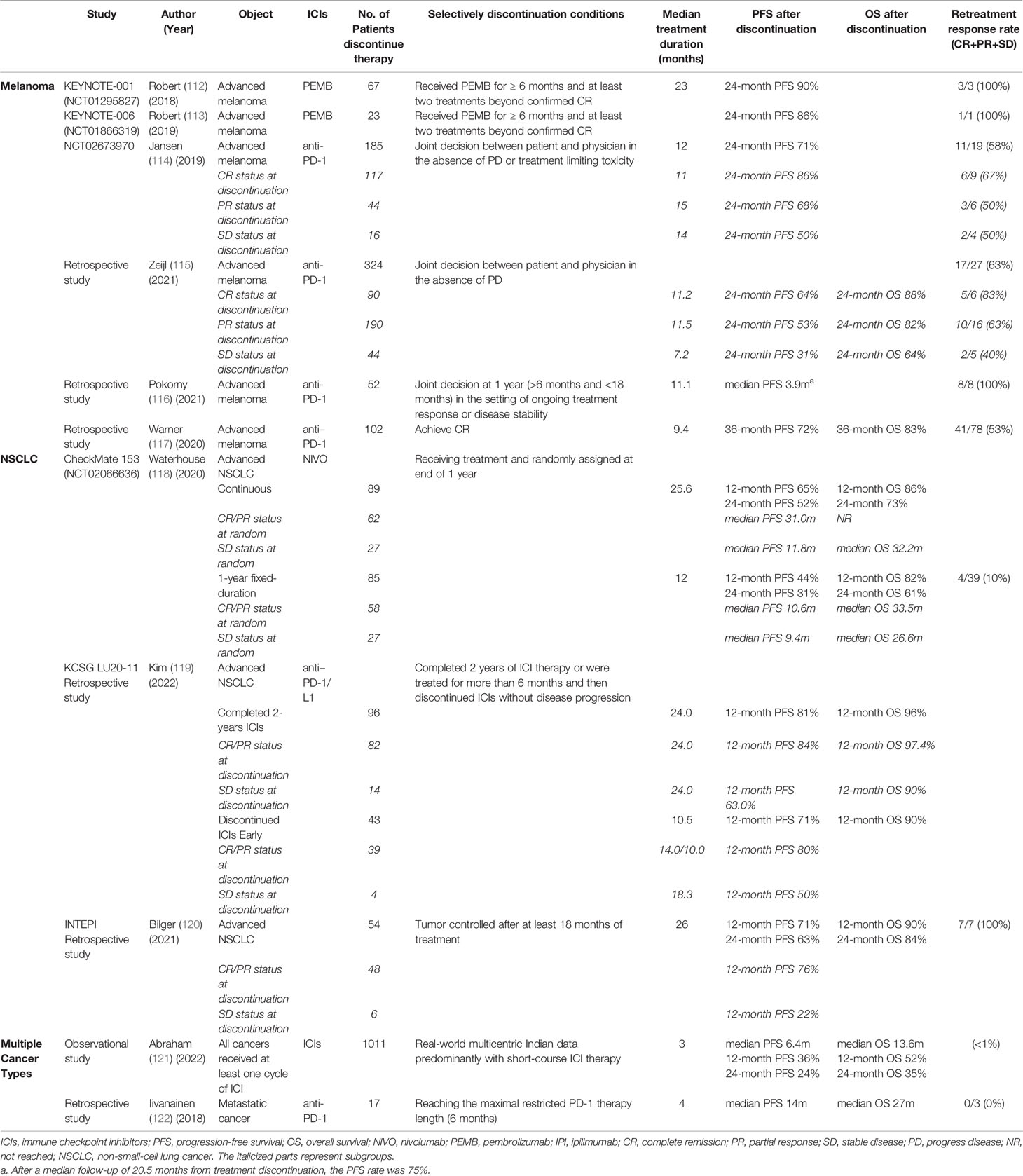
The treatment efficacy with different durations have been compared across several published clinical trials. Patients in the CA209-003 study were treated with nivolumab for up to 2 years with a 5-year OS of 16% (99), the CheckMate017/CheckMate057 study continued treatment until disease progression or intolerable toxicity with a 5-year OS of 13.4% (100); In the KEYNOTE-010 study in which treatment with pembrolizumab was administered for up to 2 years, 5-year OS was 15.6% (101), and the KEYNOTE-001 study required treatment until progression or intolerance, with a 5-year OS of 15.5% (102). Although inclusion criteria were not uniform across clinical trials, a long course of treatment does not necessarily mean long-term survival. Responders who discontinued due to irAEs had a similar survival benefit compared to those who completed the established course, implying that early discontinuation in these patients did not affect outcomes (103–105). Even more, it has been hypothesized that discontinuation of treatment due to severe irAE could itself serve as a biomarker of strong immune response and thus predict efficacy (103, 105, 106). While there is much evidence that short courses may provide durable benefits, this is not yet consistently standard practice worldwide currently. The National Comprehensive Cancer Network (NCCN)/the European Society for Medical Oncology (ESMO) guidelines generally recommend that, the duration of ICIs given as adjuvant or consolidation therapy was one year, and two years or until disease progression or unacceptable toxicity occurs for patients with later stage (107–111). Depth of response may play an important role in the determine of optimal treatment duration, and further exploration of prognostic biomarkers across different tumor types is needed (Table 2).
4.1 Melanoma
Melanoma is the most effective and well-studied tumor in immunotherapy, and a series of published studies on selectively discontinuation of ICIs have focused on melanoma. In the revised protocol of the KEYNOTE-001, 006 studies, patients who received pembrolizumab for more than 6 months, whose tumors reached CR and followed by at least two cycles of pembrolizumab could choose to discontinue treatment early. A total of 67 and 23 CR patients discontinued treatment, and their 2-year PFS were 89.9% and 86.4%, which were similar to the outcomes of other enrolled patients (112, 113). A Belgium real-world cohort study analyzed 185 melanoma patients who chose to discontinue anti-PD-1 therapy in the absence of disease progression or toxicity, with a median treatment duration of 12 months and 1- and 2-year PFS after discontinuation of 90% and 71% (114). The risk of recurrence was significantly lower in CR patients than in partial response (PR)/stable disease (SD) patients. In CR patients, the risk of relapse was significantly higher for treatment duration of less than 6 months than for treatment duration of more than 6 months, but did not differ between 6-12, 12-18, 18-24 or more than 24 months (114). Another Netherlands observational study reviewed 324 patients who discontinued first-line anti-PD-1 monotherapy without disease progression and found a better outcome in patients with CR/PR status compared to patients with SD. The 2-year PFS and OS were 64% and 88%, 53% and 82%, 31% and 64% for CR, PR, and SD patients, respectively (115). Pokorny reviewed 52 metastatic melanoma who responded well to 1-year treatment and selectively discontinue therapy, with a median follow-up of 20.5 months after discontinuation 39 (75%) patients had no disease progression (116). A study from Memorial Sloan Kettering Hospital showed that CR patients discontinued therapy after a median treatment duration of 9.4 months, with a 3-year PFS of 72.1% and a 3-year OS rate of 82.7% (117). To sum up, a large proportion of melanoma patients who achieved CR could get sustained efficacy after ICIs discontinuation, with a low recurrence rate and a 2-year PFS of 65-85%. Therefore, there is a premature suggestion for melanoma that early discontinuation of ICIs could be considered for CR patients with 6-months additional treatment after achieving CR.
4.2 NSCLC
The best treatment response for most NSCLC patients is PR/SD rather than CR, and the optimal treatment duration may vary from melanoma. The CheckMate 153 study is the first phase III randomized controlled study of ICIs duration to explore whether continuation treatment provides a survival benefit for advanced NSCLC who are progression-free after 1-year nivolumab treatment. The results showed that patients who discontinued nivolumab had an increased risk of relapse and that continued nivolumab could provide a survival benefit. Further stratified analysis showed that CR/PR patients is benefit from continuous treatment, while for SD patients, the median PFS of continuous treatment and 1-year fixed treatment was similar, suggesting that continued treatment was more meaningful for CR/PR patients (118). A multicenter retrospective study from Korea reported the long-term follow-up results in patients with advanced and/or metastatic NSCLC (119). For patients who completed 2-years ICIs therapy, the 1-year PFS and OS were 81.1% and 96.4%, respectively. And for patients who discontinued ICIs after more than 6 months of treatment without disease progression, the 1-year PFS and OS were 71.0% and 90.0%, respectively. Among them, the risk of relapse was significantly higher for treatment duration of 6-12 months but did not differ between 12-18 or 18-24 months (119). Bilger reported that NSCLC patients who chose to discontinue the drug after at least 18 months of ICIs therapy had 1-year PFS and OS of 71% and 90%, and 2-year PFS and OS of 63% and 84%, respectively (120). Comparing the results of the above studies, the duration of treatment for ICIs was set at >6 months, 1 year, >18 months, 2 years, and continued until progression or intolerability. The numerical trend of the longer the treatment duration the lower the risk of recurrence, independent of the patient’s best response status. Therefore, we believe that the duration of ICIs for NSCLC may be longer than for melanoma, with a more reasonable cut-off of 18-24 months.
4.3 Multiple Cancer Types
A multicenter observational study in India found that a short course of ICIs (0.5-13 months) was comparable in clinical benefit rate to standard ICIs therapy from literatures (121). Oulu University Hospital restricted maximal PD-(L)1 therapy length to 6 months, and reviewed 17 responders discontinued PD-1 therapy after 6 months therapy, 11 of whom remained stable after 1 year (122).
5 Ongoing Clinical Trials Related to ICIs Regimen Optimization
A randomized clinical trial initiated by the University of Chicago sought to compare the standard interval and extended interval dosing of nivolumab (240mg Q2W/480mg Q4W vs 240mg Q4W/480mg Q8W) or pembrolizumab (200mg Q3W/400mg Q6W vs 200mg Q6W) in locally advanced or metastatic cancers (123). Roswell Park Cancer Institute sponsored another multicenter randomized trial compared the regimen of pembrolizumab 200mg Q3W with 200mg Q12W in NSCLC patients benefit from pembrolizumab monotherapy, aiming to reduce the dose intensity of pembrolizumab in the maintenance phase (124). There is also a dose tapering and early discontinuation trial for NSCLC initiated by Radboud University Medical Center, where the labelled dose of pembrolizumab (200mg Q3W/400mg Q6W) will be reduced to 300mg Q6W (125). The ADAPT-IT study enrolled advanced melanoma underwent CT scan after 2 cycles of nivolumab combined with ipilimumab. According to CT results, patients with early favorable antitumor effect discontinued the combination and transferred to nivolumab monotherapy, otherwise received 4 cycles of combination therapy. Interim results were reported that 41/60 patients (68%) experienced only 2 cycles of combination, with 12-months PFS and OS of 68% and 85%, 18-months PFS and OS of 52% and 80%, respectively (126). Patients with early favorable antitumor effect who received additional combination therapy did not significantly improve their outcomes. There are also some small-sample studies focusing on reduced doses of ICIs combined with radiotherapy, chemotherapy, etc., and the use of artificial intelligence to guide medication (127–129).
A number of clinical trials focusing on ICIs discontinuation are carrying out. Similar to CheckMate 153, the Japanese phase III SAVE study (130)recruits NSCLCs with good response and no serious side effects after 1-year anti-PD-1/L1 therapy, the French DICIPLE study (131) recruits patients with stage IV NSCLC without progression after 6-months combination therapy of nivolumab plus ipilimumab, and the UK phase III DANTE study (132) recruits progression-free melanomas after 1-year anti-PD-1 therapy. The patients were randomized into the continuation and discontinuation groups to compare the survival differences. There are also some clinical trials exploring the modes of determining the treatment duration based on treatment response rather than setting a fixed duration, e.g., the Netherlands Safe Stop study (133) hopes to answer the question of whether ICIs can be discontinued after achieving CR/PR in melanoma. Moreover, many clinical studies exploring intermittent treatment patterns. For instance, Canadian STOP-GAP study (134) evaluates the clinical feasibility of stopping treatment after maximal tumor response and retreating when disease progresses. In another intermittent dosing mode, patients were evaluated periodically, and treatment was discontinued if tumors decreased by 10% or more, continued if tumors did not decrease, restarted in patients with a ≥ 10% tumor increase and again held with tumor reduction ≥10%. Although clinical trial was closed prior due to changes in standard of treatment, still providing experience for further investigation of intermittent immunotherapy dosing strategies (135). TITAN-RCC/TCC trial explored a response-based tailored immunotherapy approach, starting with nivolumab induction, followed by nivolumab monotherapy maintenance in responders, boost combination therapy of nivolumab plus ipilimumab in non-responders (136, 137). It is also important to find accurate markers for discontinuation and methods of monitoring after discontinuation. In a retrospective study at Georgetown Lombardi Cancer Center, inactive melanoma confirmed by PET/CT or tumor biopsy had a relapse rate of less than 10% at one year after anti-PD-1 withdrawal (138). Inspired by this, they initiated a clinical trial in which melanoma patients underwent PET-CT scan after 1-year ICIs treatment, and the negative PET-CT results guided drug discontinuation (138). We look forward to the results of all the above studies (Table 3), meanwhile, it is worth noting that most clinical studies are mainly limited to melanoma and NSCLC, and it is uncertain whether the results can be directly extrapolated to other tumor types; more prospective studies with predictive biomarkers are needed.
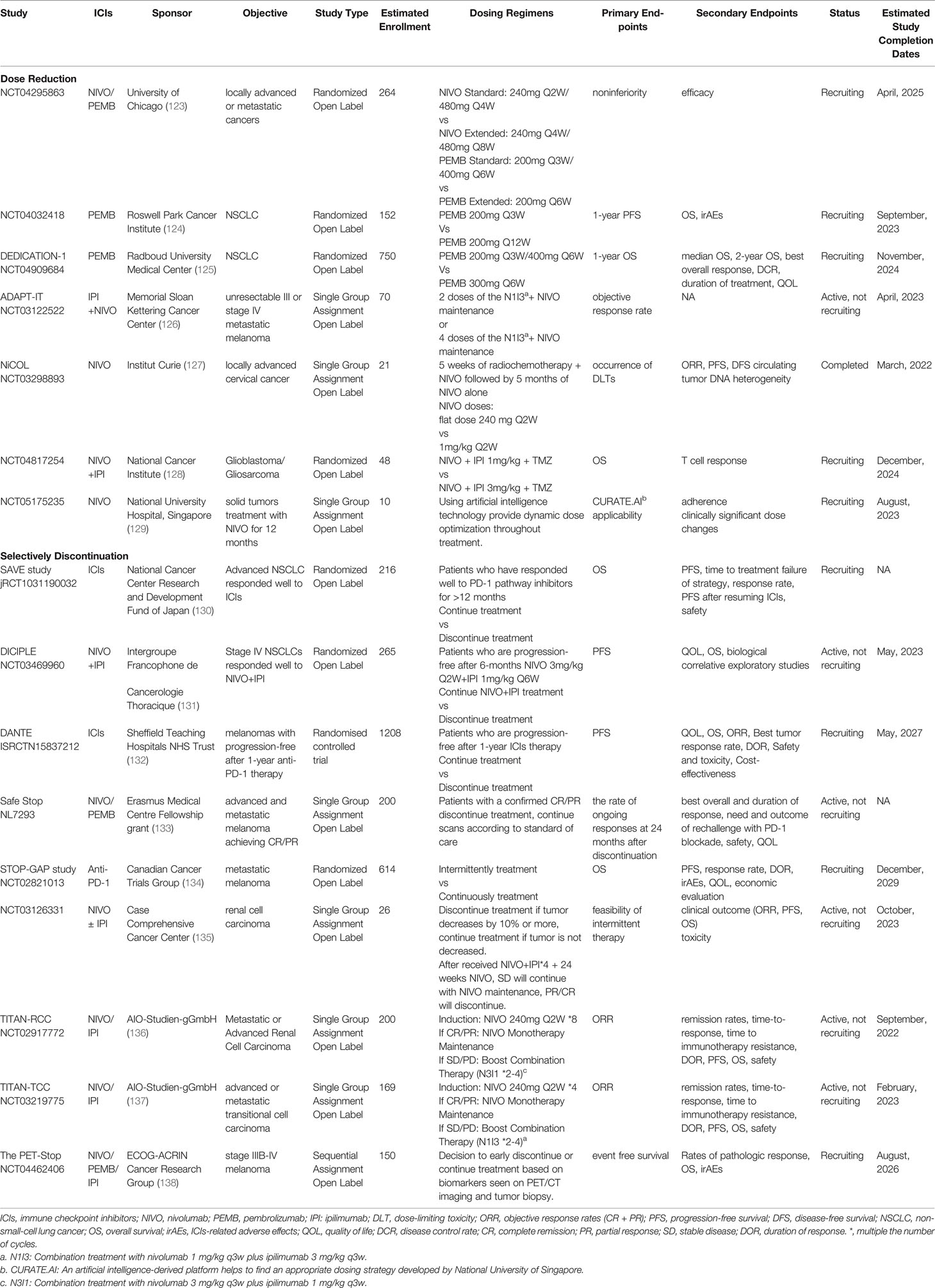
Table 3 Ongoing clinical trials related to ICIs regimen optimization. (Comparing the modified dosing regimens with the standard dosing regimens).
Previously established methodology of dose-finding in early-stage clinical trials has not progressed with the therapeutic improvements, and the concept of maximally tolerated doses (MTD) has much less instructive for ICIs recommended dose. If the MTD is not reached during dose escalation, the recommended phase 2 dose could be evaluated based on safety profile, pharmacokinetics/pharmacodynamics modelling simulations, early efficacy biomarkers, the variation of immunological composition reflecting immunomodulatory effect, target engagement receptor occupancy model and other new parameters (139–143). Besides, appropriate extension of the follow-up period may help to reduce the bias brought by unrecognized toxicity. It is a trend to design trials with reference to the minimum effective dose and supplementing it with in silico modelling and simulation, as well as incorporated TDM strategy to monitor trough concentrations within the therapeutic range, enable the provision of precision dosing of ICIs. Various novel phase I/II clinical trial designs have been proposed in the literature to select the optimal biological dose, such as Bayesian adaptive design (144–148). In terms of modified the current dosing schedule, the viewpoint published on JAMA ONCOLOGY proposed that Bayesian noninferiority studies could be more efficient to demonstrate the comparability between the modified regimen and the established standard one, especially when utilized some predictive biomarkers, pharmacokinetic, or pharmacodynamic end points (11). Besides, there is also support for noninferiority trials designed with relatively wide margins, considering the trade-off between statistical certainty, feasibility and population health (12).
6 Medication Strategies for ICIs Under the COVID-19 Pandemic
Cancer patients are in an immunosuppressed state and are at high risk for COVID-19 infection, with a high incidence of severe cases and mortality (149, 150). Due to the lack of evidence, experts are divided on the therapeutic management of ICIs in cancer patients during the COVID-19 pandemic. Some suggest that delayed or early discontinuation of therapy may be considered for elderly patients with comorbidities and low tumor burden, those who achieve (or near) CR, and those receiving adjuvant therapy (149, 151, 152). Others believe that the susceptibility and severity of cancer patients to COVID-19 may simply be an epidemiological coincidence caused by bias (153–155). Immunotherapy can restore the immune function, and patients receiving ICIs may be more resistant to the virus than those receiving chemotherapy and targeted therapy (156). The duration of the COVID-19 pandemic remains unpredictable and should not prevent the use of ICIs in patients with highly responsive tumors (157–159). The current consensus is that both physicians and patients preferred high-dose, extended-interval regimen to reduce the risk of exposure (73, 160–163). There was no difference in safety and efficacy between extended-interval dosing and standard dosing pembrolizumab or durvalumab in NSCLC patients during the COVID-19 pandemic, while PFS, OS were longer in patients treated with extended-interval nivolumab (163).
The safety and/or efficacy of the COVID-19 vaccine, as well as the interaction between the vaccine and the ICI, are inconclusive for patients treated with ICI whose immune systems are activated. The limited evidence available support COVID-19 vaccination in patients treated with ICIs. Chen YW (164) and Waissengrin (165) reported no serious vaccine-related adverse reactions observed in 81 and 137 patients receiving ICIs and COVID-19 mRNA vaccine, and no patients developed new irAEs or exacerbation of existing irAEs. The prospective VOICE study (vaccination against COVID in cancer), which included chemotherapy and immunotherapy patients, also confirmed the safety and efficacy of the mRNA-1273 vaccine (166). A recently published multicenter observational study included 2048 patients who had previously received anti-PD-1 therapy and were divided into vaccinated subgroup (receiving inactivated SARS-CoV-2 virus vaccine) and non-vaccinated subgroup for comparison. Both subgroups were similar in terms of ICIs efficacy, while in terms of safety, the vaccinated subgroup was more likely to have mild irAE, while the incidence of severe irAE was instead reduced (167). Although case of cytokine release syndrome occurring 5 days after BTN162b2 mRNA COVID-19 vaccination in patient with long-term anti-PD-1 therapy have been reported (168), the benefit-risk profile still strongly supports vaccination in cancer patients. The current recommendation is that patients with active cancer undergoing immunotherapy should receive COVID-19 vaccine at the earliest available opportunity but should avoid vaccination 48-72 hours within treatment to reduce confusion about the causality of adverse effects (169–172). The patients undergoing combined ICIs therapy should be more carefully evaluated and closely monitored at the time of vaccination (173, 174).
7 Conclusions
Rational dose selection and optimization of dosing regimens are of clinical importance and are prerequisites for enhancing patients’ medication compliance and obtaining maximum clinical benefits. The management of ICIs is still on research phase, and the approved dosing regimen may not be the best. The previously established approach of early-phase dose-finding clinical trials is not appropriate for current immunotherapy. Various phase I/II clinical trial designs have been proposed to select the optimal biological dose of ICIs, pending test of reasonableness in practice. There is an emergent need to explore the efficacy and safety of modified ICIs treatment strategies (e.g., lower dosage or shorter course) to promote the use of ICIs and reduce drug toxicity and economic wastage. Pharmacokinetic/pharmacodynamic studies, early clinical trials, and small sample attempts suggest that lower-dose and less-frequency administration of ICIs may have durable effects, similar to those of standard dosing regimens. Compared to reduce single-dose, the better way to reduce dose intensity is probably to extend the dosing frequency, which is more economical and convenient, especially in the context of COVID-19 pandemic. The search for the optimal duration of ICIs is also progressing, from a fixed course of treatment to determining the duration of treatment based on treatment response, and further searching for imaging and biological biomarkers to help determine the timing of drug discontinuation. It is important to note that the optimal dosing regimen of ICIs is related to the immunogenicity of the tumor, disease stage, and physical status of patients, and extrapolation of results requires caution. In the era of precision medicine, we pursue individualized treatment rather than using the same schedule for all patients. More pharmacokinetic/pharmacodynamic studies, interventional pharmacoeconomics clinical trials and real-world data, as well as in-depth studies on the mechanisms of ICIs are very essential. These off-labelled dose de-escalation of ICIs in clinical practice would be under the guidance and collaboration of pharmaceutical manufacturers. The correlated research potentially of interest to insurers, government payers, academic institutions, as well as professional/patient associations. Although the study funding and the dissemination of the concepts may be difficult, it is of great interest and urgently needed to reduce the medical stress on both individuals and society.
Author Contributions
MJ contributed to the study conception and design, data acquisition, data analysis and interpretation, manuscript writing and revision. MJ, YH and GL contributed to the study data acquisition, data analysis and interpretation, original draft preparation. MJ and CC contributed to review and revise the manuscript. All authors agree to be accountable for the content of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Long GV, Tykodi SS, Schneider JG, Garbe C, Gravis G, Rashford M, et al. Assessment of Nivolumab Exposure and Clinical Safety of 480 Mg Every 4 Weeks Flat-Dosing Schedule in Patients With Cancer. Ann Oncol (2018) 29(11):2208–13. doi: 10.1093/annonc/mdy408
2. Lala M, Li TR, de Alwis DP, Sinha V, Mayawala K, Yamamoto N, et al. A Six-Weekly Dosing Schedule for Pembrolizumab in Patients With Cancer Based on Evaluation Using Modelling and Simulation. Eur J Cancer (2020) 131:68–75. doi: 10.1016/j.ejca.2020.02.016
3. Zhao X, Shen J, Ivaturi V, Gopalakrishnan M, Feng Y, Schmidt BJ, et al. Model-Based Evaluation of the Efficacy and Safety of Nivolumab Once Every 4 Weeks Across Multiple Tumor Types. Ann Oncol (2020) 31(2):302–9. doi: 10.1016/j.annonc.2019.10.015
4. Morrissey KM, Marchand M, Patel H, Zhang R, Wu B, Phyllis Chan H, et al. Alternative Dosing Regimens for Atezolizumab: An Example of Model-Informed Drug Development in the Postmarketing Setting. Cancer Chemother Pharmacol (2019) 84(6):1257–67. doi: 10.1007/s00280-019-03954-8
5. Jacobs CR, Rapoport BL, Chan SW, Ruff P, Arance AM, Mujika K, et al. Keynote-555 Cohort B: Efficacy, Safety, and Pk of Pembrolizumab (Pembro) 400 Mg Every 6 Weeks (Q6w) as 1l Therapy for Advanced Melanoma. J Clin Oncol (2021) 39(15_suppl):9541. doi: 10.1200/JCO.2021.39.15_suppl.9541
6. Garon EB, Reinmuth N, Falchero L, Garcia YG, Hureaux J, Gore I, et al. Checkmate 384: Phase Iiib/Iv Trial of Nivolumab (Nivo) 480 Mg Q4w Versus 240 Mg Q2w After ≤ 12 Months of Nivo in Previously Treated Advanced Nsclc. J Clin Oncol (2019) 37(8_suppl):100. doi: 10.1200/JCO.2019.37.8_suppl.100
7. Leighl N, Alexandru A, Ohe Y, Ruff P, Hida T, Nishio M, et al. Fp04. 01 Nivolumab 480 Mg Every 4 Weeks as De Novo Second-Line Treatment for Advanced Non-Small Cell Lung Cancer: Checkmate 907. J Thorac Oncol (2021) 16(10):S949–S50. doi: 10.1016/j.jtho.2021.08.215
8. Hall E, Zhang J, Kim EJ, Hwang G, Chu G, Bhatia S, et al. Economics of Alternative Dosing Strategies for Pembrolizumab and Nivolumab at a Single Academic Cancer Center. Cancer Med (2020) 9(6):2106–12. doi: 10.1002/cam4.2888
9. Ogungbenro K, Patel A, Duncombe R, Nuttall R, Clark J, Lorigan P. Dose Rationalization of Pembrolizumab and Nivolumab Using Pharmacokinetic Modeling and Simulation and Cost Analysis. Clin Pharmacol Ther (2018) 103(4):582–90. doi: 10.1002/cpt.875
10. Goldstein DA, Gordon N, Davidescu M, Leshno M, Steuer CE, Patel N, et al. A Phamacoeconomic Analysis of Personalized Dosing Vs Fixed Dosing of Pembrolizumab in Firstline Pd-L1-Positive Non-Small Cell Lung Cancer. J Natl Cancer Inst (2017) 109(11):djx063. doi: 10.1093/jnci/djx063
11. Ratain MJ, Goldstein DA, Lichter AS. Interventional Pharmacoeconomics-A New Discipline for a Cost-Constrained Environment. JAMA Oncol (2019) 5(8):1097–8. doi: 10.1001/jamaoncol.2019.1341
12. Goldstein DA, Strohbehn GW, Serritella AV, Hyman DA, Lichter AS, Ratain MJ. Interventional Pharmacoeconomics. Cancer J (2020) 26(4):330–4. doi: 10.1097/PPO.0000000000000461
13. Goldstein DA, Ratain MJ. Alternative Dosing Regimens for Atezolizumab: Right Dose, Wrong Frequency. Cancer Chemother Pharmacol (2019) 84(6):1153–5. doi: 10.1007/s00280-019-03971-7
14. Ratain MJ, Goldstein DA. Time Is Money: Optimizing the Scheduling of Nivolumab. J Clin Oncol (2018) 36(31):JCO1800045. doi: 10.1200/JCO.18.00045
15. Bajwa R, Cheema A, Khan T, Amirpour A, Paul A, Chaughtai S, et al. Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J Clin Med Res (2019) 11(4):225–36. doi: 10.14740/jocmr3750
16. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse Effects of Immune-Checkpoint Inhibitors: Epidemiology, Management and Surveillance. Nat Rev Clin Oncol (2019) 16(9):563–80. doi: 10.1038/s41571-019-0218-0
17. Nebhan CA, Cortellini A, Ma W, Ganta T, Song H, Ye F, et al. Clinical Outcomes and Toxic Effects of Single-Agent Immune Checkpoint Inhibitors Among Patients Aged 80 Years or Older With Cancer: A Multicenter International Cohort Study. JAMA Oncol (2021) 7(12):1856–61. doi: 10.1001/jamaoncol.2021.4960
18. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol (2018) 4(12):1721–8. doi: 10.1001/jamaoncol.2018.3923
19. Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Prolonged Survival in Stage Iii Melanoma With Ipilimumab Adjuvant Therapy. N Engl J Med (2016) 375(19):1845–55. doi: 10.1056/NEJMoa1611299
20. Xu C, Chen YP, Du XJ, Liu JQ, Huang CL, Chen L, et al. Comparative Safety of Immune Checkpoint Inhibitors in Cancer: Systematic Review and Network Meta-Analysis. BMJ (2018) 363:k4226. doi: 10.1136/bmj.k4226
21. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-Related Adverse Events of Pd-1 and Pd-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. JAMA Oncol (2019) 5(7):1008–19. doi: 10.1001/jamaoncol.2019.0393
22. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of Toxicities From Immunotherapy: Esmo Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2017) 28(suppl_4):iv119–iv42. doi: 10.1093/annonc/mdx225
23. Villadolid J, Amin A. Immune Checkpoint Inhibitors in Clinical Practice: Update on Management of Immune-Related Toxicities. Transl Lung Cancer Res (2015) 4(5):560–75. doi: 10.3978/j.issn.2218-6751.2015.06.06
24. Dougan M. Checkpoint Blockade Toxicity and Immune Homeostasis in the Gastrointestinal Tract. Front Immunol (2017) 8:1547. doi: 10.3389/fimmu.2017.01547
25. Assoun S, Lemiale V, Azoulay E. Molecular Targeted Therapy-Related Life-Threatening Toxicity in Patients With Malignancies. A Systematic Review of Published Cases. Intensive Care Med (2019) 45(7):988–97. doi: 10.1007/s00134-019-05650-w
26. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol (2017) 35(7):709–17. doi: 10.1200/JCO.2016.68.2005
27. Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. J Thorac Oncol (2018) 13(12):1930–9. doi: 10.1016/j.jtho.2018.08.2035
28. Cho JY, Kim J, Lee JS, Kim YJ, Kim SH, Lee YJ, et al. Characteristics, Incidence, and Risk Factors of Immune Checkpoint Inhibitor-Related Pneumonitis in Patients With Non-Small Cell Lung Cancer. Lung Cancer (2018) 125:150–6. doi: 10.1016/j.lungcan.2018.09.015
29. Naidoo J, Cottrell TR, Lipson EJ, Forde PM, Illei PB, Yarmus LB, et al. Chronic Immune Checkpoint Inhibitor Pneumonitis. J Immunother Cancer (2020) 8(1):e000840. doi: 10.1136/jitc-2020-000840
30. Atchley WT, Alvarez C, Saxena-Beem S, Schwartz TA, Ishizawar RC, Patel KP, et al. Immune Checkpoint Inhibitor-Related Pneumonitis in Lung Cancer: Real-World Incidence, Risk Factors, and Management Practices Across Six Health Care Centers in North Carolina. Chest (2021) 160(2):731–42. doi: 10.1016/j.chest.2021.02.032
31. Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients With Advanced Melanoma Receiving Nivolumab. J Clin Oncol (2014) 32(10):1020–30. doi: 10.1200/JCO.2013.53.0105
32. Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, et al. Safety, Efficacy, and Biomarkers of Nivolumab With Vaccine in Ipilimumab-Refractory or -Naive Melanoma. J Clin Oncol (2013) 31(34):4311–8. doi: 10.1200/JCO.2013.51.4802
33. Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase Ii Trial. J Clin Oncol (2015) 33(13):1430–7. doi: 10.1200/JCO.2014.59.0703
34. Yoo SH, Keam B, Kim M, Kim SH, Kim YJ, Kim TM, et al. Low-Dose Nivolumab Can Be Effective in Non-Small Cell Lung Cancer: Alternative Option for Financial Toxicity. ESMO Open (2018) 3(5):e000332. doi: 10.1136/esmoopen-2018-000332
35. Zhao JJ, Kumarakulasinghe NB, Muthu V, Lee M, Walsh R, Low JL, et al. Low-Dose Nivolumab in Renal Cell Carcinoma: A Real-World Experience. Oncology (2021) 99(3):192–202. doi: 10.1159/000512000
36. Lepik KV, Fedorova LV, Kondakova EV, Zalyalov YR, Babenko EV, Lepik EE, et al. A Phase 2 Study of Nivolumab Using a Fixed Dose of 40 Mg (Nivo40) in Patients With Relapsed/Refractory Hodgkin Lymphoma. Hemasphere (2020) 4(5):e480. doi: 10.1097/HS9.0000000000000480
37. Wang AY, Kline J, Stock W, Kosuri S, Artz A, Larson RA, et al. Unexpected Toxicities When Nivolumab Was Given as Maintenance Therapy Following Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant (2020) 26(5):1025–7. doi: 10.1016/j.bbmt.2020.01.021
38. Davids MS, Kim HT, Costello C, Herrera AF, Locke FL, Maegawa RO, et al. A Multicenter Phase 1 Study of Nivolumab for Relapsed Hematologic Malignancies After Allogeneic Transplantation. Blood (2020) 135(24):2182–91. doi: 10.1182/blood.2019004710
39. Low JL, Huang Y, Sooi K, Ang Y, Chan ZY, Spencer K, et al. Low-Dose Pembrolizumab in the Treatment of Advanced Non-Small Cell Lung Cancer. Int J Cancer (2021) 149(1):169–76. doi: 10.1002/ijc.33534
40. Chan TSY, Hwang YY, Khong PL, Leung AYH, Chim CS, Tse EWC, et al. Low-Dose Pembrolizumab and Nivolumab Were Efficacious and Safe in Relapsed and Refractory Classical Hodgkin Lymphoma: Experience in a Resource-Constrained Setting. Hematol Oncol (2020) 38(5):726–36. doi: 10.1002/hon.2787
41. Antonia SJ, Lopez-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab Alone and Nivolumab Plus Ipilimumab in Recurrent Small-Cell Lung Cancer (Checkmate 032): A Multicentre, Open-Label, Phase 1/2 Trial. Lancet Oncol (2016) 17(7):883–95. doi: 10.1016/S1470-2045(16)30098-5
42. Sharma P, Siefker-Radtke A, de Braud F, Basso U, Calvo E, Bono P, et al. Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: Checkmate 032 Nivolumab 1 Mg/Kg Plus Ipilimumab 3 Mg/Kg Expansion Cohort Results. J Clin Oncol (2019) 37(19):1608–16. doi: 10.1200/JCO.19.00538
43. Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. Checkmate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol (2018) 36(28):2836–44. doi: 10.1200/JCO.2017.76.6212
44. Lebbe C, Meyer N, Mortier L, Marquez-Rodas I, Robert C, Rutkowski P, et al. Evaluation of Two Dosing Regimens for Nivolumab in Combination With Ipilimumab in Patients With Advanced Melanoma: Results From the Phase Iiib/Iv Checkmate 511 Trial. J Clin Oncol (2019) 37(11):867–75. doi: 10.1200/JCO.18.01998
45. Rozeman EA, Menzies AM, van Akkooi ACJ, Adhikari C, Bierman C, van de Wiel BA, et al. Identification of the Optimal Combination Dosing Schedule of Neoadjuvant Ipilimumab Plus Nivolumab in Macroscopic Stage Iii Melanoma (Opacin-Neo): A Multicentre, Phase 2, Randomised, Controlled Trial. Lancet Oncol (2019) 20(7):948–60. doi: 10.1016/S1470-2045(19)30151-2
46. El-Khoueiry AB, Yau T, Kang Y-K, Kim T-Y, Santoro A, Sangro B, et al. Nivolumab (Nivo) Plus Ipilimumab (Ipi) Combination Therapy in Patients (Pts) With Advanced Hepatocellular Carcinoma (Ahcc): Long-Term Results From Checkmate 040. J Clin Oncol (2021) 39(3_suppl):269. doi: 10.1200/JCO.2021.39.3_suppl.269
47. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The Checkmate 040 Randomized Clinical Trial. JAMA Oncol (2020) 6(11):e204564. doi: 10.1001/jamaoncol.2020.4564
48. Kaseb AO, Hasanov E, Cao HST, Xiao L, Vauthey JN, Lee SS, et al. Perioperative Nivolumab Monotherapy Versus Nivolumab Plus Ipilimumab in Resectable Hepatocellular Carcinoma: A Randomised, Open-Label, Phase 2 Trial. Lancet Gastroenterol Hepatol (2022) 7(3):208–18. doi: 10.1016/S2468-1253(21)00427-1
49. Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol (2018) 36(8):773–9. doi: 10.1200/JCO.2017.76.9901
50. Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase Ii Checkmate 142 Study. J Clin Oncol (2021) 40(2):161–70. doi: 10.1200/JCO.21.01015
51. A Study to Evaluate the Safety of Nivolumab and Ipilimumab in Subjects With Previously Untreated Advanced or Metastatic Renal Cell Cancer. Available at: https://ClinicalTrials.gov/show/NCT02982954.
52. Tykodi SS, Gordan LN, Alter RS, Arrowsmith E, Harrison MR, Percent I, et al. Safety and Efficacy of Nivolumab Plus Ipilimumab in Patients With Advanced Non-Clear Cell Renal Cell Carcinoma: Results From the Phase 3b/4 Checkmate 920 Trial. J Immunother Cancer (2022) 10(2):e003844. doi: 10.1136/jitc-2021-003844
53. Morano F, Raimondi A, Pagani F, Lonardi S, Salvatore L, Cremolini C, et al. Temozolomide Followed by Combination With Low-Dose Ipilimumab and Nivolumab in Patients With Microsatellite-Stable, O(6)-Methylguanine-DNA Methyltransferase-Silenced Metastatic Colorectal Cancer: The Maya Trial. J Clin Oncol (2022) 40(14):1562–73. doi: 10.1200/JCO.21.02583
54. Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab Plus Ipilimumab as First-Line Treatment for Advanced Non-Small-Cell Lung Cancer (Checkmate 012): Results of an Open-Label, Phase 1, Multicohort Study. Lancet Oncol (2017) 18(1):31–41. doi: 10.1016/S1470-2045(16)30624-6
55. Kleef R, Nagy R, Baierl A, Bacher V, Bojar H, McKee DL, et al. Low-Dose Ipilimumab Plus Nivolumab Combined With Il-2 and Hyperthermia in Cancer Patients With Advanced Disease: Exploratory Findings of a Case Series of 131 Stage Iv Cancers - a Retrospective Study of a Single Institution. Cancer Immunol Immunother (2021) 70(5):1393–403. doi: 10.1007/s00262-020-02751-0
56. Schwarze JK, Garaud S, Jansen YJL, Awada G, Vandersleyen V, Tijtgat J, et al. Low-Dose Nivolumab With or Without Ipilimumab as Adjuvant Therapy Following the Resection of Melanoma Metastases: A Sequential Dual Cohort Phase Ii Clinical Trial. Cancers (2022) 14(3):682. doi: 10.3390/cancers14030682
57. Carlino MS, Menzies AM, Atkinson V, Cebon JS, Jameson MB, Fitzharris BM, et al. Long-Term Follow-Up of Standard-Dose Pembrolizumab Plus Reduced-Dose Ipilimumab in Patients With Advanced Melanoma: Keynote-029 Part 1b. Clin Cancer Res (2020) 26(19):5086–91. doi: 10.1158/1078-0432.CCR-20-0177
58. Long GV, Robert C, Butler MO, Couture F, Carlino MS, O'Day S, et al. Standard-Dose Pembrolizumab Plus Alternate-Dose Ipilimumab in Advanced Melanoma: Keynote-029 Cohort 1c, A Phase 2 Randomized Study of Two Dosing Schedules. Clin Cancer Res (2021) 27(19):5280–88. doi: 10.1158/1078-0432.CCR-21-0793
59. Olson DJ, Eroglu Z, Brockstein B, Poklepovic AS, Bajaj M, Babu S, et al. Pembrolizumab Plus Ipilimumab Following Anti-Pd-1/L1 Failure in Melanoma. J Clin Oncol (2021) 39(24):2647–55. doi: 10.1200/JCO.21.00079
60. Gubens MA, Sequist LV, Stevenson JP, Powell SF, Villaruz LC, Gadgeel SM, et al. Pembrolizumab in Combination With Ipilimumab as Second-Line or Later Therapy for Advanced Non-Small-Cell Lung Cancer: Keynote-021 Cohorts D and H. Lung Cancer (2019) 130:59–66. doi: 10.1016/j.lungcan.2018.12.015
61. Wang X, Feng Y, Bajaj G, Gupta M, Agrawal S, Yang A, et al. Quantitative Characterization of the Exposure-Response Relationship for Cancer Immunotherapy: A Case Study of Nivolumab in Patients With Advanced Melanoma. CPT Pharmacometr Syst Pharmacol (2017) 6(1):40–8. doi: 10.1002/psp4.12133
62. Liu C, Yu J, Li H, Liu J, Xu Y, Song P, et al. Association of Time-Varying Clearance of Nivolumab With Disease Dynamics and Its Implications on Exposure Response Analysis. Clin Pharmacol Ther (2017) 101(5):657–66. doi: 10.1002/cpt.656
63. Feng Y, Wang X, Bajaj G, Agrawal S, Bello A, Lestini B, et al. Nivolumab Exposure-Response Analyses of Efficacy and Safety in Previously Treated Squamous or Nonsquamous Non-Small Cell Lung Cancer. Clin Cancer Res (2017) 23(18):5394–405. doi: 10.1158/1078-0432.CCR-16-2842
64. Agrawal S, Feng Y, Roy A, Kollia G, Lestini B. Nivolumab Dose Selection: Challenges, Opportunities, and Lessons Learned for Cancer Immunotherapy. J Immunother Cancer (2016) 4:72. doi: 10.1186/s40425-016-0177-2
65. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and Activity of Anti-Pd-L1 Antibody in Patients With Advanced Cancer. N Engl J Med (2012) 366(26):2455–65. doi: 10.1056/NEJMoa1200694
66. Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, et al. Phase I Study of Pembrolizumab (Mk-3475; Anti-Pd-1 Monoclonal Antibody) in Patients With Advanced Solid Tumors. Clin Cancer Res (2015) 21(19):4286–93. doi: 10.1158/1078-0432.CCR-14-2607
67. Elassaiss-Schaap J, Rossenu S, Lindauer A, Kang SP, de Greef R, Sachs JR, et al. Using Model-Based “Learn and Confirm” to Reveal the Pharmacokinetics-Pharmacodynamics Relationship of Pembrolizumab in the Keynote-001 Trial. CPT Pharmacometr Syst Pharmacol (2017) 6(1):21–8. doi: 10.1002/psp4.12132
68. Deng R, Bumbaca D, Pastuskovas CV, Boswell CA, West D, Cowan KJ, et al. Preclinical Pharmacokinetics, Pharmacodynamics, Tissue Distribution, and Tumor Penetration of Anti-Pd-L1 Monoclonal Antibody, an Immune Checkpoint Inhibitor. MAbs (2016) 8(3):593–603. doi: 10.1080/19420862.2015.1136043
69. Song X, Pak M, Chavez C, Liang M, Lu H, Schwickart M, et al. Pharmacokinetics and Pharmacodynamics of Medi4736, a Fully Human Anti-Programmed Death Ligand 1 (Pd-L1) Monoclonal Antibody, in Patients With Advanced Solid Tumors. J Clin Oncol (2015) 33(15_suppl):e14009–e. doi: 10.1200/jco.2015.33.15_suppl.e14009
70. Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. Mpdl3280a (Anti-Pd-L1) Treatment Leads to Clinical Activity in Metastatic Bladder Cancer. Nature (2014) 515(7528):558–62. doi: 10.1038/nature13904
71. Peer CJ, Goldstein DA, Goodell JC, Nguyen R, Figg WD, Ratain MJ. Opportunities for Using in Silico-Based Extended Dosing Regimens for Monoclonal Antibody Immune Checkpoint Inhibitors. Br J Clin Pharmacol (2020) 86(9):1769–77. doi: 10.1111/bcp.14369
72. Peer CJ, Heiss BL, Goldstein DA, Goodell JC, Figg WD, Ratain MJ. Pharmacokinetic Simulation Analysis of Less Frequent Nivolumab and Pembrolizumab Dosing: Pharmacoeconomic Rationale for Dose Deescalation. J Clin Pharmacol (2022) 62(4):532–40. doi: 10.1002/jcph.1984
73. Goldstein DA, Ratain MJ, Saltz LB. Weight-Based Dosing of Pembrolizumab Every 6 Weeks in the Time of Covid-19. JAMA Oncol (2020) 6(11):1694–5. doi: 10.1001/jamaoncol.2020.2493
74. Chou CH, Hsu LF. Model-Based Simulation to Support the Extended Dosing Regimens of Atezolizumab. Eur J Clin Pharmacol (2021) 77(1):87–93. doi: 10.1007/s00228-020-02980-3
75. Li H, Yu J, Liu C, Liu J, Subramaniam S, Zhao H, et al. Time Dependent Pharmacokinetics of Pembrolizumab in Patients With Solid Tumor and Its Correlation With Best Overall Response. J Pharmacokinet Pharmacodyn (2017) 44(5):403–14. doi: 10.1007/s10928-017-9528-y
76. Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y. Model-Based Population Pharmacokinetic Analysis of Nivolumab in Patients With Solid Tumors. CPT Pharmacometr Syst Pharmacol (2017) 6(1):58–66. doi: 10.1002/psp4.12143
77. Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase Ii Checkmate 205 Trial. J Clin Oncol (2018) 36(14):1428–39. doi: 10.1200/JCO.2017.76.0793
78. Haverkos BM, Abbott D, Hamadani M, Armand P, Flowers ME, Merryman R, et al. Pd-1 Blockade for Relapsed Lymphoma Post-Allogeneic Hematopoietic Cell Transplant: High Response Rate But Frequent Gvhd. Blood (2017) 130(2):221–8. doi: 10.1182/blood-2017-01-761346
79. Herbaux C, Gauthier J, Brice P, Drumez E, Ysebaert L, Doyen H, et al. Efficacy and Tolerability of Nivolumab After Allogeneic Transplantation for Relapsed Hodgkin Lymphoma. Blood (2017) 129(18):2471–8. doi: 10.1182/blood-2016-11-749556
80. Nguyen LS, Raia L, Lebrun-Vignes B, Salem JE. Graft Versus Host Disease Associated With Immune Checkpoint Inhibitors: A Pharmacovigilance Study and Systematic Literature Review. Front Pharmacol (2020) 11:619649. doi: 10.3389/fphar.2020.619649
81. Albring JC, Inselmann S, Sauer T, Schliemann C, Altvater B, Kailayangiri S, et al. Pd-1 Checkpoint Blockade in Patients With Relapsed Aml After Allogeneic Stem Cell Transplantation. Bone Marrow Transplant (2017) 52(2):317–20. doi: 10.1038/bmt.2016.274
82. Davids MS, Kim HT, Costello C, Herrera AF, Locke FL, McSweeney P, et al. Optimizing Checkpoint Blockade as a Treatment for Relapsed Hematologic Malignancies After Allogeneic Hematopoietic Cell Transplantation. Blood (2017) 130:275. doi: 10.1182/blood.V130.Suppl_1.275.275
83. Yared JA, Hardy N, Singh Z, Hajj S, Badros AZ, Kocoglu M, et al. Major Clinical Response to Nivolumab in Relapsed/Refractory Hodgkin Lymphoma After Allogeneic Stem Cell Transplantation. Bone Marrow Transplant (2016) 51(6):850–2. doi: 10.1038/bmt.2015.346
84. Onizuka M, Kojima M, Matsui K, Machida S, Toyosaki M, Aoyama Y, et al. Successful Treatment With Low-Dose Nivolumab in Refractory Hodgkin Lymphoma After Allogeneic Stem Cell Transplantation. Int J Hematol (2017) 106(1):141–5. doi: 10.1007/s12185-017-2181-9
85. Sehgal K, Bulumulle A, Brody H, Gill RR, Macherla S, Qilleri A, et al. Association of Extended Dosing Intervals or Delays in Pembrolizumab-Based Regimens With Survival Outcomes in Advanced Non-Small-Cell Lung Cancer. Clin Lung Cancer (2021) 22(3):e379–e89. doi: 10.1016/j.cllc.2020.05.028
86. Kwong YL, Lopes D, Khong PL. Low-Dose Pembrolizumab Induced Remission in Patients With Refractory Classical Hodgkin Lymphoma. Br J Haematol (2017) 176(1):131–2. doi: 10.1111/bjh.13920
87. Kwong YL, Loong F, Khong PL. Low-Dose Pembrolizumab Re-Treatment Induced Complete Radiologic and Molecular Remission in Hodgkin Lymphoma Recurring From a Previous Relapse Successfully Treated by Pembrolizumab. Ann Hematol (2019) 98(10):2451–5. doi: 10.1007/s00277-019-03762-3
88. Chan TS, Luk TH, Lau JS, Khong PL, Kwong YL. Low-Dose Pembrolizumab for Relapsed/Refractory Hodgkin Lymphoma: High Efficacy With Minimal Toxicity. Ann Hematol (2017) 96(4):647–51. doi: 10.1007/s00277-017-2931-z
89. Minson A, Douglas G, Bilmon I, Grigg A. Low Dose Pd-1 Inhibition in Relapsed Refractory Hodgkin Lymphoma After Allogeneic Stem Cell Transplant With Concomitant Active Gvhd. Br J Haematol (2019) 184(5):840–4. doi: 10.1111/bjh.15186
90. Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, et al. Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The Checkmate 016 Study. J Clin Oncol (2017) 35(34):3851–8. doi: 10.1200/JCO.2016.72.1985
91. Saung MT, Pelosof L, Casak S, Donoghue M, Lemery S, Yuan M, et al. Fda Approval Summary: Nivolumab Plus Ipilimumab for the Treatment of Patients With Hepatocellular Carcinoma Previously Treated With Sorafenib. Oncologist (2021) 26(9):797–806. doi: 10.1002/onco.13819
92. U.S. Food and Drug Administration. Yervoy- Ipilimumab Injection (2021). Available at: https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/2265ef30-253e-11df-8a39-0800200c9a66/spl-doc?hl=ipilimumab.
93. Chen J, Li S, Yao Q, Du N, Fu X, Lou Y, et al. The Efficacy and Safety of Combined Immune Checkpoint Inhibitors (Nivolumab Plus Ipilimumab): A Systematic Review and Meta-Analysis. World J Surg Oncol (2020) 18(1):150. doi: 10.1186/s12957-020-01933-5
94. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med (2022) 386(5):449–62. doi: 10.1056/NEJMoa2111380
95. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med (2019) 381(21):2020–31. doi: 10.1056/NEJMoa1910231
96. Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, et al. First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (Checkmate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J Clin Oncol (2019) 37(12):992–1000. doi: 10.1200/JCO.18.01042
97. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-Line Nivolumab Plus Ipilimumab Combined With Two Cycles of Chemotherapy in Patients With Non-Small-Cell Lung Cancer (Checkmate 9la): An International, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2021) 22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0
98. Boyer M, Sendur MAN, Rodriguez-Abreu D, Park K, Lee DH, Cicin I, et al. Pembrolizumab Plus Ipilimumab or Placebo for Metastatic Non-Small-Cell Lung Cancer With Pd-L1 Tumor Proportion Score >/= 50%: Randomized, Double-Blind Phase Iii Keynote-598 Study. J Clin Oncol (2021) 39(21):2327–38. doi: 10.1200/JCO.20.03579
99. Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the Ca209-003 Study. J Clin Oncol (2018) 36(17):1675–84. doi: 10.1200/JCO.2017.77.0412
100. Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro Carpeno J, et al. Five-Year Outcomes From the Randomized, Phase Iii Trials Checkmate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J Clin Oncol (2021) 39(7):723–33. doi: 10.1200/JCO.20.01605
101. Herbst RS, Garon EB, Kim DW, Cho BC, Gervais R, Perez-Gracia JL, et al. Five Year Survival Update From Keynote-010: Pembrolizumab Versus Docetaxel for Previously Treated, Programmed Death-Ligand 1-Positive Advanced Nsclc. J Thorac Oncol (2021) 16(10):1718–32. doi: 10.1016/j.jtho.2021.05.001
102. Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-Year Overall Survival for Patients With Advanced Nonsmall-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I Keynote-001 Study. J Clin Oncol (2019) 37(28):2518–27. doi: 10.1200/JCO.19.00934
103. Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P, et al. Efficacy and Safety Outcomes in Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase Ii and Iii Trials. J Clin Oncol (2017) 35(34):3807–14. doi: 10.1200/JCO.2017.73.2289
104. Horiguchi M, Uno H, Wei LJ. Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab as a Result of Adverse Events Lived Significantly Longer Than Patients Who Continued Treatment. J Clin Oncol (2018) 36(7):720–1. doi: 10.1200/JCO.2017.76.0983
105. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-Year Survival With Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2019) 381(16):1535–46. doi: 10.1056/NEJMoa1910836
106. Feng S, Coward J, McCaffrey E, Coucher J, Kalokerinos P, O'Byrne K. Pembrolizumab-Induced Encephalopathy: A Review of Neurological Toxicities With Immune Checkpoint Inhibitors. J Thorac Oncol (2017) 12(11):1626–35. doi: 10.1016/j.jtho.2017.08.007
107. National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 3.2022) (2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
108. National Comprehensive Cancer Network. Melanoma: Cutaneous (Version 3.2022) (2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf.
109. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic Non-Small Cell Lung Cancer: Esmo Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2018) 29(Suppl 4):iv192–237. doi: 10.1093/annonc/mdy275
110. Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, et al. Pan-Asian Adapted Clinical Practice Guidelines for the Management of Patients With Metastatic Non-Small-Cell Lung Cancer: A Csco-Esmo Initiative Endorsed by Jsmo, Ksmo, Mos, Sso and Tos. Ann Oncol (2019) 30(2):171–210. doi: 10.1093/annonc/mdy554
111. Park K, Vansteenkiste J, Lee KH, Pentheroudakis G, Zhou C, Prabhash K, et al. Pan-Asian Adapted Esmo Clinical Practice Guidelines for the Management of Patients With Locally-Advanced Unresectable Non-Small-Cell Lung Cancer: A Ksmo-Esmo Initiative Endorsed by Csco, Ismpo, Jsmo, Mos, Sso and Tos. Ann Oncol (2020) 31(2):191–201. doi: 10.1016/j.annonc.2019.10.026
112. Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Durable Complete Response After Discontinuation of Pembrolizumab in Patients With Metastatic Melanoma. J Clin Oncol (2018) 36(17):1668–74. doi: 10.1200/JCO.2017.75.6270
113. Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab Versus Ipilimumab in Advanced Melanoma (Keynote-006): Post-Hoc 5-Year Results From an Open-Label, Multicentre, Randomised, Controlled, Phase 3 Study. Lancet Oncol (2019) 20(9):1239–51. doi: 10.1016/S1470-2045(19)30388-2
114. Jansen YJL, Rozeman EA, Mason R, Goldinger SM, Geukes Foppen MH, Hoejberg L, et al. Discontinuation of Anti-Pd-1 Antibody Therapy in the Absence of Disease Progression or Treatment Limiting Toxicity: Clinical Outcomes in Advanced Melanoma. Ann Oncol (2019) 30(7):1154–61. doi: 10.1093/annonc/mdz110
115. van Zeijl MCT, van den Eertwegh AJM, Wouters M, de Wreede LC, Aarts MJB, van den Berkmortel F, et al. Discontinuation of Anti-Pd-1 Monotherapy in Advanced Melanoma-Outcomes of Daily Clinical Practice. Int J Cancer (2022) 150(2):317–26. doi: 10.1002/ijc.33800
116. Pokorny R, McPherson JP, Haaland B, Grossmann KF, Luckett C, Voorhies BN, et al. Real-World Experience With Elective Discontinuation of Pd-1 Inhibitors at 1 Year in Patients With Metastatic Melanoma. J Immunother Cancer (2021) 9(1):e001781. doi: 10.1136/jitc-2020-001781
117. Betof Warner A, Palmer JS, Shoushtari AN, Goldman DA, Panageas KS, Hayes SA, et al. Long-Term Outcomes and Responses to Retreatment in Patients With Melanoma Treated With Pd-1 Blockade. J Clin Oncol (2020) 38(15):1655–63. doi: 10.1200/JCO.19.01464
118. Waterhouse DM, Garon EB, Chandler J, McCleod M, Hussein M, Jotte R, et al. Continuous Versus 1-Year Fixed-Duration Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Checkmate 153. J Clin Oncol (2020) 38(33):3863–73. doi: 10.1200/JCO.20.00131
119. Kim H, Kim DW, Kim M, Lee Y, Ahn HK, Cho JH, et al. Long-Term Outcomes in Patients With Advanced and/or Metastatic Non-Small Cell Lung Cancer Who Completed 2 Years of Immune Checkpoint Inhibitors or Achieved a Durable Response After Discontinuation Without Disease Progression: Multicenter, Real-World Data (Kcsg Lu20-11). Cancer (2021) 128(4):778–87. doi: 10.1002/cncr.33984
120. Bilger G, Girard N, Doubre H, Levra MG, Giroux-Leprieur E, Giraud F, et al. Discontinuation of Immune Checkpoint Inhibitor (Ici) Above 18 Months of Treatment in Real-Life Patients With Advanced Non-Small Cell Lung Cancer (Nsclc): Intepi, a Multicentric Retrospective Study. Cancer Immunol Immunother (2021). doi: 10.1007/s00262-021-03114-z
121. Abraham G, Noronha V, Rajappa S, Agarwal A, Batra U, Somani N, et al. The Clinical Utility and Safety of Short-Course Immune Checkpoint Inhibitors in Multiple Tumours-A Real-World Multicentric Study From India. Int J Cancer (2021) 150(6):1045–52. doi: 10.1002/ijc.33868
122. Iivanainen S, Koivunen JP. Early Pd-1 Therapy Discontinuation in Responding Metastatic Cancer Patients. Oncology (2019) 96(3):125–31. doi: 10.1159/000493193
123. Chicago Uo. Comparing Dosing Intervals of Nivolumab or Pembrolizumab in Locally Advanced or Metastatic Cancers (2020). Available at: https://ClinicalTrials.gov/show/NCT04295863.
124. Institute RPC. Pembrolizumab Every 12 Weeks Versus Every 3 Weeks in Treating Patients With Non-Small Cell Lung Cancer (2020). Available at: https://ClinicalTrials.gov/show/NCT04032418.
125. Center RUM. Pulmonology DSoPf, Tuberculosis. Dose Tapering and Early Discontinuation to Increase Cost-Effectiveness of Immunotherapy for Nsclc (2020). Available at: https://ClinicalTrials.gov/show/NCT04909684.
126. Postow MA, Goldman DA, Shoushtari AN, Betof Warner A, Callahan MK, Momtaz P, et al. Adaptive Dosing of Nivolumab + Ipilimumab Immunotherapy Based Upon Early, Interim Radiographic Assessment in Advanced Melanoma (the Adapt-It Study). J Clin Oncol (2022) 40(10):1059–67. doi: 10.1200/JCO.21.01570
127. Curie I, Squibb B-M. Nivolumab in Association With Radiotherapy and Cisplatin in Locally Advanced Cervical Cancers Followed by Adjuvant Nivolumab for Up to 6 Months (2017). Available at: https://ClinicalTrials.gov/show/NCT03298893.
128. Institute NC, Center NIoHC. Association of Peripheral Blood Immunologic Response to Therapeutic Response to Adjuvant Treatment With Immune Checkpoint Inhibition (Ici) in Patients With Newly Diagnosed Glioblastoma or Gliosarcoma (2021). Available at: https://ClinicalTrials.gov/show/NCT04817254.
129. National University Hospital S, Health TNIf. Nivolumab Dose Optimisation in Solid Tumours With Curate.Ai Platform and Sequential Ctdna Measurements (2021). Available at: https://ClinicalTrials.gov/show/NCT05175235.
130. Nomura S, Goto Y, Mizutani T, Kataoka T, Kawai S, Okuma Y, et al. A Randomized Phase Iii Study Comparing Continuation and Discontinuation of Pd-1 Pathway Inhibitors for Patients With Advanced Non-Small-Cell Lung Cancer (Jcog1701, Save Study). Jpn J Clin Oncol (2020) 50(7):821–5. doi: 10.1093/jjco/hyaa054
131. Zalcman G, Toffart A, Flandin A-CM, Molinier O, Dayen C, Egenod T, et al. Ifct-1701 Diciple: A Randomized Phase Iii Trial Comparing Continuation Nivolumab-Ipilimumab Doublet Immunotherapy Until Progression Versus Observation in Patients With Pdl1-Positive Stage Iv Non-Small Cell Lung Cancer (Nsclc) After Nivolumab-Ipilimumab Induction Treatment. Ann Oncol (2019) 30:v658. doi: 10.1093/annonc/mdz260.113
132. Coen O, Corrie P, Marshall H, Plummer R, Ottensmeier C, Hook J, et al. The Dante Trial Protocol: A Randomised Phase Iii Trial to Evaluate the Duration of Anti-Pd-1 Monoclonal Antibody Treatment in Patients With Metastatic Melanoma. BMC Cancer (2021) 21(1):761. doi: 10.1186/s12885-021-08509-w
133. Mulder E, de Joode K, Litiere S, Ten Tije AJ, Suijkerbuijk KPM, Boers-Sonderen MJ, et al. Early Discontinuation of Pd-1 Blockade Upon Achieving a Complete or Partial Response in Patients With Advanced Melanoma: The Multicentre Prospective Safe Stop Trial. BMC Cancer (2021) 21(1):323. doi: 10.1186/s12885-021-08018-w
134. Baetz TD, Song X, Ernst DS, McWhirter E, Petrella TM, Savage KJ, et al. A Randomized Phase Iii Study of Duration of Anti-Pd-1 Therapy in Metastatic Melanoma (Stop-Gap): Canadian Clinical Trials Group Study (Cctg) Me.13. J Clin Oncol (2018) 36(15_suppl):TPS9600–TPS. doi: 10.1200/JCO.2018.36.15_suppl.TPS9600
135. Ornstein MC, Wood LS, Hobbs BP, Allman KD, Martin A, Bevan M, et al. A Phase Ii Trial of Intermittent Nivolumab in Patients With Metastatic Renal Cell Carcinoma (Mrcc) Who Have Received Prior Anti-Angiogenic Therapy. J Immunother Cancer (2019) 7(1):127. doi: 10.1186/s40425-019-0615-z
136. AIO-Studien-gGmbH, Squibb B-M. Tailored Immunotherapy Approach With Nivolumab in Subjects With Metastatic or Advanced Renal Cell Carcinoma (2016). Available at: https://ClinicalTrials.gov/show/NCT02917772.
137. AIO-Studien-gGmbH, Squibb B-M. Tailored Immunotherapy Approach With Nivolumab in Subjects With Metastatic or Advanced Transitional Cell Carcinoma(2017). Available at: https://ClinicalTrials.gov/show/NCT03219775.
138. Gibney GT, Zaemes J, Shand S, Shah NJ, Swoboda D, Gardner K, et al. Pet/Ct Scan and Biopsy-Driven Approach for Safe Anti-Pd-1 Therapy Discontinuation in Patients With Advanced Melanoma. J Immunother Cancer (2021) 9(10):e002955. doi: 10.1136/jitc-2021-002955
139. Creemers JHA, Pawlitzky I, Grosios K, Gileadi U, Middleton MR, Gerritsen WR, et al. Assessing the Safety, Tolerability and Efficacy of Plga-Based Immunomodulatory Nanoparticles in Patients With Advanced Ny-Eso-1-Positive Cancers: A First-In-Human Phase I Open-Label Dose-Escalation Study Protocol. BMJ Open (2021) 11(11):e050725. doi: 10.1136/bmjopen-2021-050725
140. Papadopoulos KP, Lakhani N, Falchook GS, Riley G, Baeck J, Brown KS, et al. First-In-Human Trial of Programmed Cell Death Receptor-1 (Pd-1) Inhibitor, Jtx-4014, in Adult Patients With Advanced, Refractory, Solid Tumors. Cancer Immunol Immunother (2021) 70(3):763–72. doi: 10.1007/s00262-020-02730-5
141. Schoffski P, Tan DSW, Martin M, Ochoa-de-Olza M, Sarantopoulos J, Carvajal RD, et al. Phase I/Ii Study of the Lag-3 Inhibitor Ieramilimab (Lag525) +/- Anti-Pd-1 Spartalizumab (Pdr001) in Patients With Advanced Malignancies. J Immunother Cancer (2022) 10(2):e003776. doi: 10.1136/jitc-2021-003776
142. Curigliano G, Gelderblom H, Mach N, Doi T, Tai D, Forde PM, et al. Phase I/Ib Clinical Trial of Sabatolimab, an Anti-Tim-3 Antibody, Alone and in Combination With Spartalizumab, an Anti-Pd-1 Antibody, in Advanced Solid Tumors. Clin Cancer Res (2021) 27(13):3620–9. doi: 10.1158/1078-0432.CCR-20-4746
143. Stein AM, Ramakrishna R. Afir: A Dimensionless Potency Metric for Characterizing the Activity of Monoclonal Antibodies. CPT Pharmacometr Syst Pharmacol (2017) 6(4):258–66. doi: 10.1002/psp4.12169
144. Zhang Y, Cao S, Zhang C, Jin IH, Zang Y. A Bayesian Adaptive Phase I/Ii Clinical Trial Design With Late-Onset Competing Risk Outcomes. Biometrics (2021) 77(3):796–808. doi: 10.1111/biom.13347
145. Zhou Y, Lin R, Lee JJ, Li D, Wang L, Li R, et al. Tite-Boin12: A Bayesian Phase I/Ii Trial Design to Find the Optimal Biological Dose With Late-Onset Toxicity and Efficacy. Stat Med (2022) 41(11):1918–31. doi: 10.1002/sim.9337
146. Lin R, Yin G, Shi H. Bayesian Adaptive Model Selection Design for Optimal Biological Dose Finding in Phase I/Ii Clinical Trials. Biostatistics (2021) kxab028. doi: 10.1093/biostatistics/kxab028
147. Zang Y, Lee JJ. A Robust Two-Stage Design Identifying the Optimal Biological Dose for Phase I/Ii Clinical Trials. Stat Med (2017) 36(1):27–42. doi: 10.1002/sim.7082
148. Zhou Y, Lee JJ, Yuan Y. A Utility-Based Bayesian Optimal Interval (U-Boin) Phase I/Ii Design to Identify the Optimal Biological Dose for Targeted and Immune Therapies. Stat Med (2019) 38(28):5299–316. doi: 10.1002/sim.8361
149. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer Patients in Sars-Cov-2 Infection: A Nationwide Analysis in China. Lancet Oncol (2020) 21(3):335–7. doi: 10.1016/S1470-2045(20)30096-6
150. Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients With Cancer Appear More Vulnerable to Sars-Cov-2: A Multicenter Study During the Covid-19 Outbreak. Cancer Discovery (2020) 10(6):783–91. doi: 10.1158/2159-8290.CD-20-0422
151. Quaglino P, Fava P, Brizio M, Marra E, Rubatto M, Agostini A, et al. Metastatic Melanoma Treatment With Checkpoint Inhibitors in the Covid-19 Era: Experience From an Italian Skin Cancer Unit. J Eur Acad Dermatol Venereol (2020) 34(7):1395–6. doi: 10.1111/jdv.16586
152. Sullivan RJ, Johnson DB, Rini BI, Neilan TG, Lovly CM, Moslehi JJ, et al. Covid-19 and Immune Checkpoint Inhibitors: Initial Considerations. J Immunother Cancer (2020) 8(1):e000933. doi: 10.1136/jitc-2020-000933
153. Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of Covid-19 for Patients With Cancer. Lancet Oncol (2020) 21(4):e180. doi: 10.1016/S1470-2045(20)30150-9
154. Lazarus G, Budiman RA, Rinaldi I. Does Immune Checkpoint Inhibitor Increase the Risks of Poor Outcomes in Covid-19-Infected Cancer Patients? A Systematic Review and Meta-Analysis. Cancer Immunol Immunother CII (2022) 71(2):373–86. doi: 10.1007/s00262-021-02990-9
155. Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of Pd-1 Blockade on Severity of Covid-19 in Patients With Lung Cancers. Cancer Discovery (2020) 10(8):1121–8. doi: 10.1158/2159-8290.CD-20-0596
156. Di Cosimo S, Malfettone A, Perez-Garcia JM, Llombart-Cussac A, Miceli R, Curigliano G, et al. Immune Checkpoint Inhibitors: A Physiology-Driven Approach to the Treatment of Coronavirus Disease 2019. Eur J Cancer (2020) 135:62–5. doi: 10.1016/j.ejca.2020.05.026
157. Bersanelli M. Controversies About Covid-19 and Anticancer Treatment With Immune Checkpoint Inhibitors. Immunotherapy (2020) 12(5):269–73. doi: 10.2217/imt-2020-0067
158. Maio M, Hamid O, Larkin J, Covre A, Altomonte M, Calabro L, et al. Immune Checkpoint Inhibitors for Cancer Therapy in the Covid-19 Era. Clin Cancer Res (2020) 26(16):4201–5. doi: 10.1158/1078-0432.CCR-20-1657
159. Moritz RKC, Gutzmer R, Zimmer L, Meier F, Ahmed MS, Sell S, et al. Sars-Cov-2 Infections in Melanoma Patients Treated With Pd-1 Inhibitors: A Survey of the German Adoreg Melanoma Registry. Eur J Cancer (2021) 144:382–5. doi: 10.1016/j.ejca.2020.11.015
160. Ottaviano M, Curvietto M, Rescigno P, Tortora M, Palmieri G, Giannarelli D, et al. Impact of Covid-19 Outbreak on Cancer Immunotherapy in Italy: A Survey of Young Oncologists. J Immunother Cancer (2020) 8(2):e001154. doi: 10.1136/jitc-2020-001154
161. Tagliamento M, Spagnolo F, Poggio F, Soldato D, Conte B, Ruelle T, et al. Italian Survey on Managing Immune Checkpoint Inhibitors in Oncology During Covid-19 Outbreak. Eur J Clin Invest (2020) 50(9):e13315. doi: 10.1111/eci.13315
162. Sehgal K, Costa DB, Rangachari D. Extended-Interval Dosing Strategy of Immune Checkpoint Inhibitors in Lung Cancer: Will It Outlast the Covid-19 Pandemic? Front Oncol (2020) 10:1193. doi: 10.3389/fonc.2020.01193
163. Hijmering-Kappelle LBM, Hiltermann TJN, Bensch F. Safety and Efficacy of Extended Interval Dosing for Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer During the Covid-19 Pandemic. Clin Lung Cancer (2021) 23(2):143–50. doi: 10.1016/j.cllc.2021.12.005
164. Chen YW, Tucker MD, Beckermann KE, Iams WT, Rini BI, Johnson DB. Covid-19 Mrna Vaccines and Immune-Related Adverse Events in Cancer Patients Treated With Immune Checkpoint Inhibitors. Eur J Cancer (2021) 155:291–3. doi: 10.1016/j.ejca.2021.07.017
165. Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short-Term Safety of the Bnt162b2 Mrna Covid-19 Vaccine in Patients With Cancer Treated With Immune Checkpoint Inhibitors. Lancet Oncol (2021) 22(5):581–3. doi: 10.1016/S1470-2045(21)00155-8
166. Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, Fehrmann RSN, van Binnendijk RS, Dingemans AC, et al. Mrna-1273 Covid-19 Vaccination in Patients Receiving Chemotherapy, Immunotherapy, or Chemoimmunotherapy for Solid Tumours: A Prospective, Multicentre, Non-Inferiority Trial. Lancet Oncol (2021) 22(12):1681–91. doi: 10.1016/S1470-2045(21)00574-X
167. Mei Q, Hu G, Yang Y, Liu B, Yin J, Li M, et al. Impact of Covid-19 Vaccination on the Use of Pd-1 Inhibitor in Treating Patients With Cancer: A Real-World Study. J Immunother Cancer (2022) 10(3):e004157. doi: 10.1136/jitc-2021-004157
168. Au L, Fendler A, Shepherd STC, Rzeniewicz K, Cerrone M, Byrne F, et al. Cytokine Release Syndrome in a Patient With Colorectal Cancer After Vaccination With Bnt162b2. Nat Med (2021) 27(8):1362–6. doi: 10.1038/s41591-021-01387-6
169. The Society for Immunotherapy of Cancer (SITC). Sitc Statement on Sars-Cov-2 Vaccination and Cancer Immunotherapy (2020). Available at: https://www.sitcancer.org/aboutsitc/press-releases/2020/sitc-statement-sars-cov-2-vaccination-cancer-immunotherapy.
170. National Comprehensive Cancer Network. Cancer and Covid-19 Vaccination (Version 6, 2022) (2022). Available at: https://www.nccn.org/docs/default-source/covid-19/2021_covid-19_vaccination_guidance_v5-0.pdf?sfvrsn=b483da2b_114.
171. Desai A, Gainor JF, Hegde A, Schram AM, Curigliano G, Pal S, et al. Covid-19 Vaccine Guidance for Patients With Cancer Participating in Oncology Clinical Trials. Nat Rev Clin Oncol (2021) 18(5):313–9. doi: 10.1038/s41571-021-00487-z
172. Switzer B, Haanen J, Lorigan PC, Puzanov I, Turajlic S. Clinical and Immunologic Implications of Covid-19 in Patients With Melanoma and Renal Cell Carcinoma Receiving Immune Checkpoint Inhibitors. J Immunother Cancer (2021) 9(7):e002835. doi: 10.1136/jitc-2021-002835
173. Luo B, Li J, Hou X, Yang Q, Zhou Y, Ye J, et al. Indications for and Contraindications of Immune Checkpoint Inhibitors in Cancer Patients With Covid-19 Vaccination. Future Oncol (2021) 17(26):3477–84. doi: 10.2217/fon-2021-0288
Keywords: immune checkpoint inhibitors, adverse effects, optimization dosing regimens, lower dosage, selectively discontinuation
Citation: Jiang M, Hu Y, Lin G and Chen C (2022) Dosing Regimens of Immune Checkpoint Inhibitors: Attempts at Lower Dose, Less Frequency, Shorter Course. Front. Oncol. 12:906251. doi: 10.3389/fonc.2022.906251
Received: 28 March 2022; Accepted: 24 May 2022;
Published: 20 June 2022.
Edited by:
Alessandro Poggi, San Martino Hospital (IRCCS), ItalyReviewed by:
Dominique Leveque, Hôpital d’Hautepierre, FranceLawrence Kasherman, The University of Sydney, Australia
Copyright © 2022 Jiang, Hu, Lin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Chen, 20053006@zcmu.edu.cn
 Mengjie Jiang
Mengjie Jiang Yujie Hu
Yujie Hu Gang Lin
Gang Lin Chao Chen
Chao Chen