- 1Department of General Surgery, the First Medical Centre, Chinese PLA General Hospital, Beijing, China
- 2Medical School of Chinese PLA, Beijing, China
Neutrophil extracellular traps (NETs), products of neutrophil death when exposed to certain stimuli, were first proposed as a type of response to bacterial infection in infectious diseases. Since then, extensive studies have discovered its involvement in other non-infectious inflammatory diseases including thromboembolism, autoimmune diseases, and cancer. Colorectal cancer (CRC) is one of the most common malignancies in the world. NET formation is closely associated with tumorigenesis, progression, and metastasis in CRC. Therefore, the application of NETs in clinical practice as diagnostic biomarkers, therapeutic targets, and prognostic predictors has a promising prospect. In addition, therapeutics targeting NETs are significantly efficient in halting tumor progression in preclinical cancer models, which further indicates its potential clinical utility in cancer treatment. This review focuses on the stimuli of NETosis, its pro-tumorigenic activity, and prospective clinical utility primarily in but not limited to CRC.
Introduction
Neutrophils, the most abundant white blood cells, play an important role in the immune system, especially for innate immunity (1). When exposed to exogenous pathogens, activated neutrophils can function in various ways, such as phagocytosis, cytokine release, and degranulation. Upon the activation of different signaling pathways, neutrophils may undergo different types of cell death, including apoptosis, necrosis, necroptosis, autophagy, pyroptosis, and NETosis (2). As a unique form of neutrophil programmed death, NETosis is characterized by the formation and release of NETs out of the cells. Neutrophil extracellular traps (NETs), mainly consisting of granule proteins and chromatin, were first described by Brinkmann et al. as a form of innate immune response to bacteria (3). Subsequently, several studies demonstrated the involvement of NETs in various non-infectious diseases, such as chronic inflammatory conditions, autoimmune disease, thrombosis, coronavirus disease 2019 (COVID-19) and malignancies (4–7). In addition, studies have reported that NETs mainly contribute to the progression of many types of tumors (7, 8). However, they can act as a double-edged sword, occasionally exerting anti-tumor effects (9).
Colorectal cancer (CRC) is the third most common cancer in the world, with approximately 1.3 million new cases and >600,000 deaths reported every year (10). CRC has a high probability of, and approximately half of the patients develop metastasis when diagnosed with CRC, which is also the main cause of death in patients (11). The currently available therapeutic strategies of CRC include surgical resection, chemotherapy, radiotherapy, and immunomodulatory therapy. However, approximately 40% of patients with CRC eventually have recurrence or metastasis, resulting in a 5-year survival rate of <15% (12, 13). Therefore, it is imperative to identify the exact mechanisms of CRC development and develop novel therapeutic strategies.
This review discusses stimuli that promote oncogenic NETosis, the structure of NETs and the involvement of NETs in non-neoplastic disease progression. Many studies have implicated that NETs can promote the progression of multiple tumors, including CRC, in different ways (14–16), indicating the importance of NETs in CRC and their potential value in clinical application. Therefore, in this review, we elucidated the impact of NETs mainly on CRC progression in terms of different stages and discussed the potential value of NETs in clinical application including diagnosis, therapeutic targeting, and prognostic prediction in cancer.
Basis of NETs
Stimuli of NETosis and Formation of NETs
NETosis is a process in which NETs are expelled out of neutrophils to the extracellular space. There are mainly two types of NETosis, namely, vital NETosis commonly occurring in infection and lytic NETosis often occurring in sterile injury (17, 18), which form NETs in two different ways, respectively. NETosis is also considered a form of cell death, different from apoptosis and necrosis, and is characterized by the extrusion of decondensed chromatin and protein contents to the extracellular space, forming NETs (19). A few important cellular events are involved in the process of NET formation, including the production of reactive oxygen species (ROS), migration of neutrophil elastase (NE) and myeloperoxidase (MPO) to the nucleus, histone citrullination, and chromatin decondensation (20). This series of events in neutrophils can be triggered under the influence of multiple cells and their paracrine components (Figure 1).
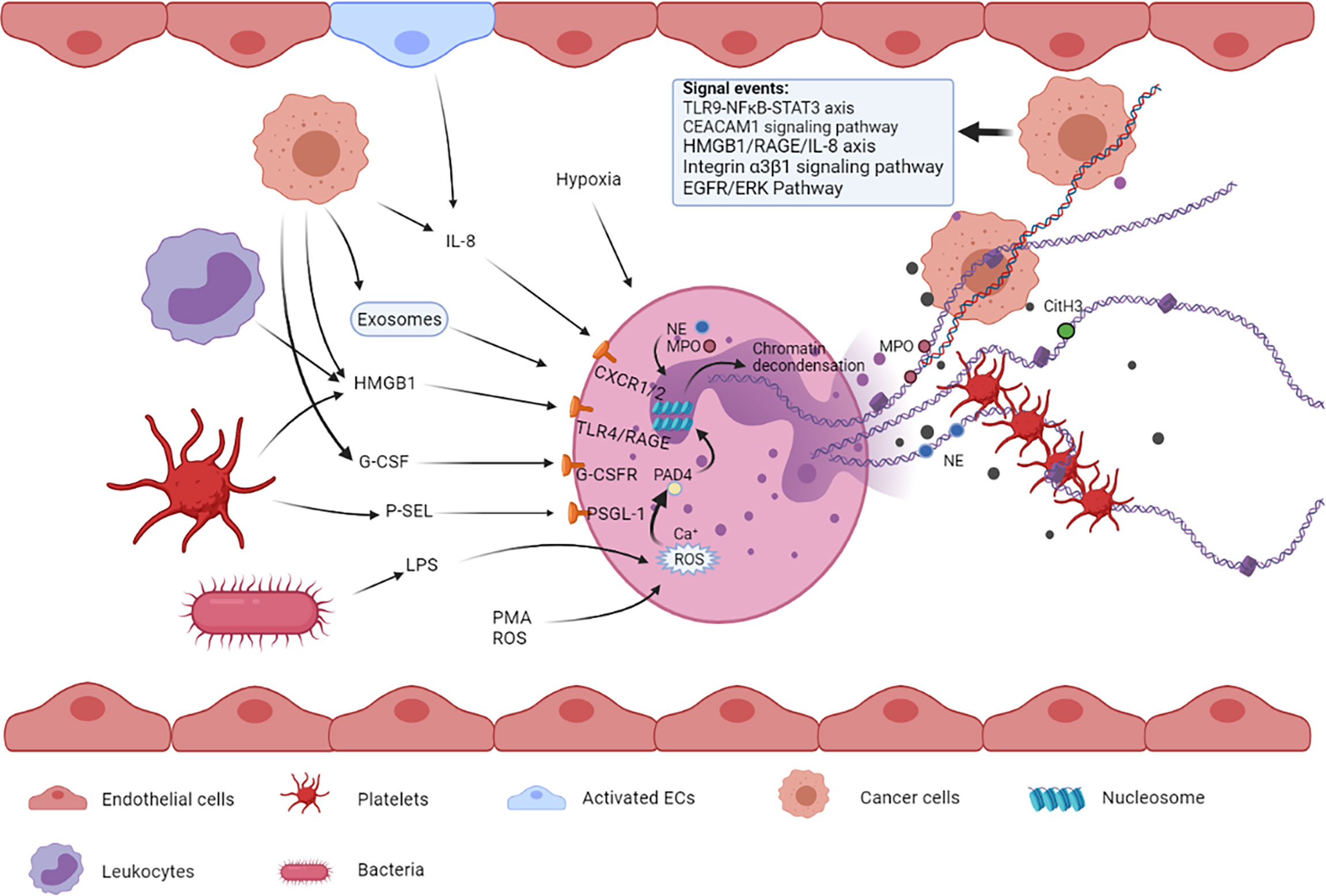
Figure 1 Multiple cells such as tumor cells, platelets, bacterial cells, and endothelial cells and their released factors are involved in NETosis. These factors from different types of cells can bind to their respective receptors on neutrophils, leading to NET formation. The released NETs are composed of a DNA skeleton and decorated granular and cytosolic proteins such as citH3, NE, and MPO. Thereafter, they can further activate and capture platelets, leading to venous thromboembolism. NETs can also entrap circulating tumor cells, promoting extravasation and metastasis.
Lipopolysaccharide (LPS), one of the dominant components of Gram-negative bacterial cell wall, is a common inducer of NETosis. Many studies have shown that the formation of NETs is increased in patients with infection and have verified the effect of LPS on the formation of NETs to some extent (3, 21). In addition, LPS promotes NETosis via a ROS-dependent mechanism, and ROS production is closely associated with inflammation, cancer, and neutrophil modulation (22, 23).
Endothelial cells (ECs) can promote NETosis upon activation via exposure to oxidative stress in injury, inflammation, or some compounds such as phorbol 12-myristate 13-acetate (PMA) and thapsigargin (24, 25). Activated ECs can release inflammatory cytokines including interleukin-8 (IL-8) (26), which has been validated to partially participate in EC-mediated NET formation when co-cultured with neutrophils in vitro (25).
Activated platelets are also one of the contributors to NET formation (27). Upon activation, platelets can directly bind to neutrophils through multiple molecular interactions and subsequently stimulate NETosis (28). On the one hand, activated platelets can translocate P-selectin to their surface (29), where it can bind to the neutrophil surface receptor P-selectin glycoprotein ligand-1 (PSGL-1) and further strengthen their adhesion (30), eventually leading to the release of NETs. On the other hand, platelets can express toll-like receptor-4 (TLR4) and high-mobility group box 1 (HMGB1), which are important contributors to platelet-stimulated NETosis (21, 27). Simultaneously, the newly generated NETs activate platelets to achieve a prothrombotic state, thus forming a positive feedback loop between NETosis and coagulation (31).
Tumor cells can activate neutrophils to release NETs by promoting the expression and release of multiple pro-NETotic factors such as granulocyte colony-stimulating factor (G-CSF) (32), IL-8, and extracellular vesicles (EVs). Some studies have indicated that G-CSF can be secreted abundantly by cancer cells in either murine or human tumors and can activate neutrophils by binding to the G-CSF receptor on the surface of these cells (33, 34). Mélanie et al. showed that overexpression of G-CSF in patients with cancer can lead to an overabundance of neutrophils in the blood and increased sensitivity toward NET generation in a ROS-dependent manner (35). IL-8 is a common cytokine to recruit neutrophils to the sites of inflammation, which can be released by multiple cancer cells including glioblastoma, diffuse large B-cell lymphoma (DLBCL), bladder cancer, and CRC, and the released IL-8 can stimulate NETosis in human neutrophils ex vivo (7, 14, 36–38). In addition, the cytokine IL-1β is associated with NETs in some tumors (39, 40). Protein arginine deiminase 4 (PAD4) is an essential enzyme of NETosis, and its expression increases either in the blood or tumor tissues of patients with malignancies (41). High PAD4 levels provide enough enzyme for histone citrullination, which may be considered another potential way for tumor cells to promote NET formation. As a type of membrane-enclosed particles formed by membranes of the parent cell (42), EVs are considered one of the most crucial components that affect NETosis in cancer (43). According to recent studies, EVs promote NETosis mainly in two ways: First, through the effect of bioactive contents present in EVs, including ILs and G-CSF that induce NETosis (44); second, by inducing proinflammatory activity of neutrophils, for example, IL-8 secretion (45).
In addition to the abovementioned factors, microRNAs (miRNAs), a type of small non-coding RNAs that are involved in almost all physiological processes in the human body, modulate the process of NETosis (46, 47). Arroyo et al. revealed that miR-146a, a negative regulator of the inflammatory response, inhibited NETosis in vivo, and miR-146a knockout in neutrophils resulted in a higher level of citH3 and NETs relative to the wild-type neutrophils upon PMA stimulation (47). In addition, miR-155 promotes the generation of NETs by positively regulating the neutrophil expression of PAD4 (48).
Because NETs are prone to be induced in the inflammatory environment, which, in turn, can promote inflammation, we speculate that NETs are closely related to inflammation-associated factors. Theoretically, factors that promote inflammation can also promote NETosis. This understanding expands the range of influencing factors associated with NETs and provides a direction for further exploration of the inducers of NETs.
Role of NETs in Disease Progression
In recent years, it has been discovered that NETs play an important role not only in the immune system but also in other pathologic processes such as thrombosis, autoimmune diseases, aseptic inflammation, metabolic disease, and the development of multiple tumors.
As a network in the circulatory system, NETs can capture and activate free platelets to promote thrombosis, especially during infection and inflammation (49). Many studies have validated the prothrombotic effect of NETs. For example, Fuchs et al. reported that NETs promoted fibrin deposition and thrombus formation, and blocking the production of NETs with DNA enzymes or PAD4 inhibitors reduced the incidence of deep vein thrombosis in animal models (50).
NETs released from neutrophils inevitably carry some intracellular proteins, that is, neutrophil-derived antigens. After these autoantigens are recognized by the immune system, they can drive the activation of autoreactive B cells to induce corresponding autoantibodies and result in autoimmune diseases (5). The association between NETs and autoimmune disease was firstly observed in patients with small-vessel vasculitis, whose serum was positive for anti-neutrophil cytoplasmic autoantibody (ANCA) against MPO and proteinase 3 in NETs (51). In some studies, NETs and autoantibodies have been detected in synovial fluid from patients with rheumatoid arthritis, and anti-citrulline protein antibody is the most significant of all autoantibodies (52). In addition, anti-ribonucleoprotein (anti-RNP) antibodies in patients with systemic lupus erythematosus (SLE) can induce NETosis, and SLE NETs activate plasmacytoid dendritic cells (PdCs) to produce high levels of IFN-α, which renders neutrophils more susceptible to NETosis upon stimulation of anti-RNP antibodies (53).
In addition, NETs are involved in some processes of aseptic inflammation. During the early inflammatory phase of atherosclerosis, cholesterol crystals can induce the generation of NETs, which, in turn, primes the transcriptional expression of IL-6 and IL-1β genes in macrophages and subsequently promotes the activation of T helper 17 cells, which enhance the recruitment of immune cells to atherosclerotic lesions (54). The pro-inflammatory effects of NETs have been demonstrated in the process of ischemia–reperfusion (IR) injury, during which neutrophils are recruited to the sinusoids of the ischemic liver lobes and release NETs in response to various stimuli, including the release of HMGB1 and histones from injured hepatocytes. In addition, inhibition of NET formation with PAD4 inhibitor or DNase I can protect hepatocytes and alleviate inflammation after liver IR injury, indicating the pathophysiological role of NETs in liver IR injury (55). Moreover, in mouse models of Alzheimer’s disease, NETs have been observed in areas with amyloid-β (Aβ) deposits. Depletion of neutrophils or blocking their trafficking process improves Alzheimer’s disease-like neuropathological features and cognitive performance in mice (56). As a nonspecific inflammatory bowel disease, ulcerative colitis (UC) has close relationship with NETs, which has been proven to be over-produced in inflamed colon of UC patients. The released NETs, in turn, significantly promoted IL-1β and TNF-α expression in mononuclear cells, leading to more NETs release (57). Meanwhile, the close relationship between UC and CRC has been acknowledged (58), which could be in part attributed to increased NETs in patients with UC.
Furthermore, NETs play a potential role in the pathogenesis of some metabolic diseases, such as obesity and diabetes mellitus (DM). Studies have shown that NET levels in patients with obesity are higher than those in the healthy population (59, 60), which is consistent with high levels of inflammatory cytokines in patients with obesity. Obesity is characterized by low-grade chronic inflammation, which underlies neutrophil activation and NET formation. Released NETs can, in turn, modulate inflammatory markers including IL-8, heat shock protein 90 (HSP90), and the E1 heat shock protein family (HSPE1), eventually contributing to metabolic profile alterations in obesity (60). Wong et al. reported that neutrophils derived from patients with DM were more susceptible to NETosis because of elevated PAD4 expression (61), which also means increased level of NETs in these patients compared to normal individuals. In addition, many studies have showed that obesity and DM were positively related to the incidence of CRC (14, 62, 63). Therefore, considering the role of NETs in CRC progression, high levels of NETs in patients with obesity and DM may be one of the reasons for obesity and DM being risk factors for CRC.
NETs Promote Colorectal Cancer Development and Metastasis
The initiation and development of tumors are complex pathophysiological processes involving a series of genetic, biochemical, and molecular biological changes. To date, many studies have shown that NETs are involved in the development of various tumors, such as breast cancer, pancreatic cancer, gastric cancer (GC), and CRC. Moreover, systemic neutrophils derived from patients with CRC and age-matched healthy volunteers possess different vulnerabilities to NETosis, and the serum levels of NETs between them are also distinct (64). Therefore, to some extent, NETs have potential effects on CRC development. Furthermore, the role of NETs in the growth and metastasis of CRC and beyond in terms of three important stages of tumor progression, namely, the local microenvironment, circulatory system, and pre-metastatic microenvironment, is described below (Figure 2).
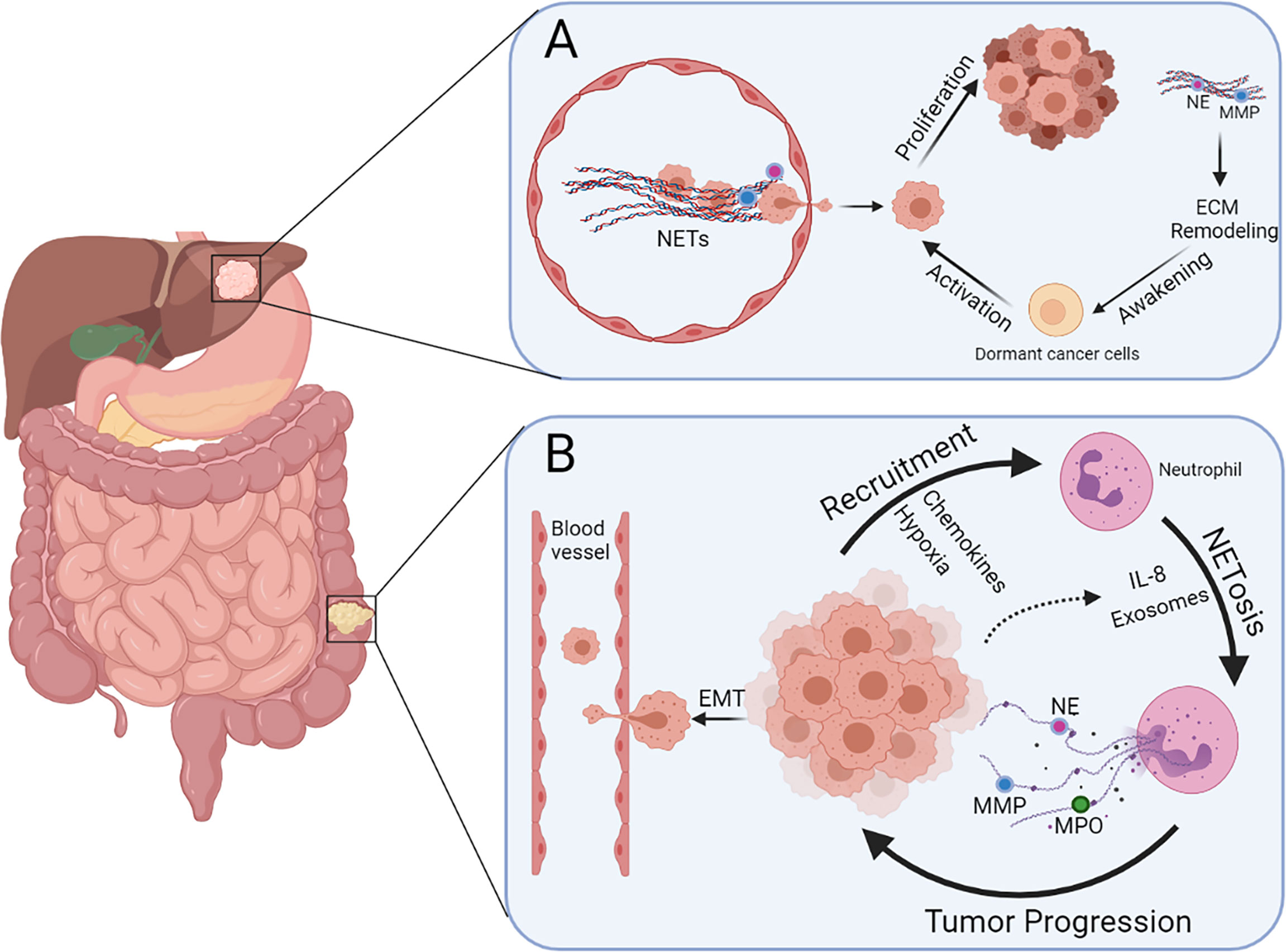
Figure 2 Multiple functions of NETs during colorectal cancer liver metastasis. (A) NETs present in blood vessels can entrap cancer cells and promote extravasation. Dormant cancer cells in the liver can be awakened via NET-induced ECM remodeling and hence promote metastasis and recurrence. (B) Neutrophils can be recruited by CRC cells because of the presence of chemokines and hypoxia in the tumor microenvironment, leading to more NETosis triggered by IL-8 and exosomes released by cancer cells. Cancer cells undergoing EMT are more prone to intravasate after stimulation of NETs.
NETs in the Tumor Microenvironment
NETs promote tumor growth in different ways in the tumor microenvironment. First, NETs can affect the state and infiltration of mesenchymal cells in the microenvironment to mediate tumor progression. In addition, NETs can promote pancreatic tumor growth by activating pancreatic stellate cells (PSCs) by interacting with RAGE receptors; however, deficiency in RAGE receptors attenuates the carcinogenic effect. In a study, serum levels of NETs were strongly correlated with the tumor stage of patients with pancreatic duct adenocarcinoma, indicating the potential of NETs as biomarkers in human cancer (65). Dirk et al. discovered that neutrophil infiltration and NET formation were observed in a murine model of nonalcoholic steatohepatitis, followed by the presence of monocyte-derived macrophages, which produced inflammatory cytokines and promoted the progression of hepatocellular carcinoma (HCC) (66).
In addition, NETs can affect the malignant biological behavior of cancers by altering the metabolic characteristics of tumor cells. Yazdani et al. found that NETs controlled mitochondrial homeostasis in tumor cells (67), and inhibition of PAD4 remarkably reduced tumor mitochondrial density and mitochondrial DNA and ATP production and subsequently affected the growth of tumor cells.
NETs can affect the behavioral characteristics of tumor cells by directly activating some receptors and pathways associated with proliferation, migration, and metastasis. In a study, NETs increased cell proliferation and migration of DLBCL cells in vitro and tumor growth and lymph node dissemination in vivo. Mechanistically, NETs directly upregulated the TLR9 pathway in DLBCL and subsequently triggered the NF-κB, STAT3, and p38 pathways to promote tumor progression. Correspondingly, disruption of NETs or suppression of TLR9 inhibited tumor progression in preclinical models (37). Li et al. found that destruction of the integrity of NETs in vitro promoted apoptosis and inhibited invasion and metastasis of GC cells by regulating the Bcl-2, Bax, and NF-κB pathways, suggesting an anti-apoptotic effect of NETs on tumor cells (68).
The invasion and metastasis ability of tumor cells depend on various factors, such as tumor cell motor capacity, angiogenesis, remodeling of the extracellular matrix, and epithelial–mesenchymal transition (EMT). In a study, NETs were found to drive the transformation of typical epithelial morphology to the mesenchymal phenotype and increase the expression of tumor stem cell-related markers in human breast cancer MCF7 cells, which was accompanied by enhanced invasion and migration ability (8). In addition, NET-associated cathepsin G has been reported to facilitate the invasion and metastasis of HCC cells both in vitro and in vivo (69). The DNA component of NETs can directly act on the CCDC25 receptor on the surface of cancer cells and activates the ILK–β-parvin cascade to enhance the motor capacity of cells (70). Two NET-associated proteases, NE and matrix metalloproteinase 9 (MMP9), can cleave and remodel laminin to reveal an epitope that can subsequently activate FAK/ERK/MLCK/YAP signaling, eventually activating and awakening dormant tumor cells (71).
Furthermore, there is a positive feedback effect between the formation of NETs and CRC progression. For example, NETs can directly promote the secretion of IL-8 from CRC cells, although it can be secreted without NET stimulation (14, 36). As an inflammatory factor, IL-8 can recruit neutrophils and activate neutrophils to produce more NETs, thus aggravating CRC progression. Anquan et al. demonstrated the association between CRC cell-derived exosomes with NETosis, indicating that exosomes originating from KRAS-mutated CRC cells can activate neutrophils to promote NET generation (15), thereby accelerating the deterioration of CRC.
NETs have a close relationship with tumor immune evasion. As major executors of the killing of malignant cells in the tumor immune microenvironment, CD8+ T and natural killer (NK) cells determine the sensitivity of patients to immunotherapy to some extent. NETs may compromise the ability of these cells to kill cancer cells because NET aggregation can enwrap and shield tumor cells to avoid contact with CD8+ T and NK cells (72, 73). Therefore, inhibition of NET formation via PAD4 inhibitors may sensitize tumors to immunotherapy.
In addition to tumor progression, the role of NETs in tumor recurrence after treatment is also noteworthy. First, NETs can activate dormant cancer cells by cleaving laminin to remodel ECM in mouse models (71), leading to tumor recurrence. Second, cancer cells can acquire stem cell properties under the influence of NETs (8), and the generation of tumor stem cells is considered an important way through which tumors acquire drug resistance and relapse, which is another way for NETs to promote tumor recurrence.
Despite the carcinogenic effects of NETs revealed in extensive studies, controversial observations have also been reported, such as in vitro-produced NETs having anti-tumor effects on CRC. Arelaki et al. found that either PMA or sepsis serum-induced NETs could impede growth and induce apoptosis in CRC cells in vitro. However, these inhibitory effects could be abrogated through the destruction of NETs by DNase (74).
NETs in the Circulatory System
There are several pathways for tumor metastasis including lymphatic, hematogenous metastasis, and implantation metastases, of which hematogenous metastasis is the most common pathway for multiple tumors, such as colorectal liver metastasis. Circulating tumor cells (CTCs) are important for metastasis (75). After detachment from the primary tumor and intravasation, CTCs have to overcome many adverse factors to achieve successful colonization in target organs, a process in which NETs perform a series of functions.
NETs are widely distributed in the blood and physically sequester CTCs as a network structure in the microvessels of distant organs to develop metastasis (76). In addition, Rayes et al. reported that the levels of circulating NETs were higher in patients with multiple cancers, especially those at an advanced stage, than in healthy volunteers (77), indicating the potential role of NETs in distant metastases. However, in addition to physical capture, there may exist certain underlying molecular mechanisms through which NETs can trap CTCs to strengthen their adhesion.
The potential molecular nature of their adhesion was revealed by Najmeh et al. for the first time, and their data shed light on the important role of β1-integrins in mediating the adhesion of tumor cells to NETs (78). In their study, both tumor-derived and NET-derived β1-integrins mediated the adhesion of cancer cells to NETs in vitro and in vivo. Correspondingly, decreased cancer cell adhesion to liver sinusoids was observed in vivo through β1-integrin blockade by its antibody. Considering the multiple effects of NETs on cancer cells, the detailed molecular landscape of interaction between them may be complicated and warrants further investigation.
CTCs are present in the form of single cells and clusters, and the latter possesses greater metastatic capabilities than the former (79). In addition, single cells can form clusters through adhesive molecules in the circulatory system (80). Therefore, we speculated whether NETs affect the formation of clusters from single cells, which can be explained in terms of the following aspects. First, NETs provide scaffolds for the adhesion of CTCs and areas for their encounter, thus providing a spatial basis for the formation of tumor clusters from single cells. In addition, the adhesion of CTCs to NETs partially depends on integrins, which are reported to mediate cell–cell adhesion in tumors (81). CTCs that have successfully attached to NETs should have relatively high expression of integrins on the surface, which constitutes the molecular basis to form clusters. Therefore, we speculate that NETs drive the aggregation of single CTCs into cell clusters, although no direct evidence is available to validate this speculation.
One of the critical steps for the successful colonization of CTCs in target organs is extravasation; however, it is not easy for tumor cells to penetrate the intact blood vessel walls. NETs can facilitate vascular leakage and endothelial-to-mesenchymal transition through elastase-dependent proteolysis of the intercellular junction protein VE-cadherin and activation of β-catenin signaling (82). Therefore, NETs formation within the circulatory system can compromise endothelial integrity, which leaves tumor cells more prone to extravasation. In addition, the presence of NETs decreases the flow rate of CTCs in blood vessels and provides more time for their extravasation and colonization.
NETs in Liver Metastasis and Beyond
CRC, especially in the advanced stage, is prone to distant metastasis usually in the lung, bone, and liver, among which the most common site of metastasis is the liver (83). This phenomenon can be attributed to many factors. In terms of anatomy, blood from the vein draining the colorectum primarily flows toward the portal vein into the liver, which is also the first site CRC cells reach after detachment. Furthermore, the liver is rich in blood supply and hence provides sufficient nutrition for tumor cell proliferation. Pre-metastatic niche remodeling in the liver is also an important factor, and several studies have indicated the significance of NETs in this process.
Lee et al. reported that NETs could be detected in the omentum of ovarian tumor-bearing mice (TBM) and women with early-stage ovarian cancer without metastasis (84), indicating that NETs formation at the metastatic site occurs before metastasis. The existing NETs at the site capture cancer cells and promote metastasis. To further verify the crucial function of NETs in metastasis, decreased omental metastases were observed in TBM with neutrophil-specific deficiency of PAD4 or those treated with PAD4 pharmacological inhibitors (85). Rayes et al. also observed a significant increase in hepatic adhesion of intrasplenically injected colon cancer cells in TBM compared with non-TBM, DNase1- or NE inhibitor-treated TBM, and PAD4–/– TBM. The results demonstrated that colon primary tumor-induced NETs could promote adhesion of CTC to the liver (77).
Regarding liver metastasis, a study reported that NETs generation in the liver preceded liver metastasis in a breast cancer-bearing mouse model, which occurred earlier than NET formation at the primary tumor site and was elevated in the plasma (70). Studies have also found that the NETs are evident in the liver but have very low levels in other organs, such as the skin and lungs (70, 86), suggesting that NETs favor the liver for metastasis over other sites. This may be partially attributed to the enhanced adhesive capacity of liver sinusoidal endothelial cells (LSECs) to neutrophils, especially during endotoxemia. LSECs can initiate neutrophil adhesion upon the activation of TLR4 receptors by LPS through a CD44/HA/SHAP-dependent mechanism, and Kupffer cells in the liver sinus also contribute to neutrophil recruitment (87). In addition, the liver can retain NETs through the expression of von Willebrand Factor (VWF) in liver sinusoids, which facilitate the adhesion of NETs to LSECs by binding to histones (86).
The formation of liver metastasis can be attributed to the activation of pre-existing dormant cancer cells in the liver, and NETs have been reported to participate in this process. In a murine model of inflammation, induced NETs remodeled ECM via NE and MMP9 (71), thus revealing an epitope that triggered dormant cancer cells through integrins and the FAK/ERK/MLCK/YAP signaling pathway. This is also one of the reasons for the postoperative recurrence of cancer.
Neutrophils and NETs can remodel the pre-metastatic niche by facilitating several pathological processes of hepatic diseases (88). Neutrophils are involved in the pathogenesis and progression of alcohol-associated liver disease (ALD); however, they also form an important defense line against infection, which is a leading cause of mortality (89–91). Terence et al. found that NET formation was decreased in a model of ALD, which simultaneously impaired the ability of macrophages to eliminate NETs in the liver, and this impaired ability of clearance aggravates hepatic injury and inflammation (92). Non-alcoholic fatty liver disease (NAFLD) is affected by peripheral metabolic conditions including hepatic fat and insulin resistance (93). Recent studies have implicated that NE participates in the pathogenesis of insulin resistance and obesity (94, 95) and hence influences the progression of NAFLD. Although there is no direct evidence to confirm the impact of NETs on metabolic syndrome and insulin resistance, these studies indicate that some components of NETs can affect the pathogenesis of NAFLD through peripheral mechanisms.
Some clinical studies have shown that neutrophils and NETs are closely related to the liver metastasis of tumors. In patients with HCC or colorectal liver metastasis undergoing hepatectomy (96, 97), a higher neutrophil-to-lymphocyte ratio often predicts a poorer clinical outcome. NETosis induced by postoperative infection promotes hepatic metastasis in a murine model of lung carcinoma (76). Similarly, in a study, a postoperative increase in the level of NETs promoted the progression of liver metastasis in a murine model of liver IR injury and was associated with reduced disease-free survival in a cohort of patients with colorectal liver metastasis undergoing curative liver resection (98).
Potential Value of NETs for Clinical Application
Diagnostic Biomarker
At present, liquid biopsy including CTCs, cell-free nucleic acids and extracellular vesicles, has become a promising method for oncology-related examination (99) owing to its characteristics of safety, convenience, and minimal invasion. NETs, which are widely present in the serum, are suitable for detection through liquid biopsy as well. In addition, cfDNA in liquid biopsy, whose clinical diagnostic value has been validated in various tumors (100–102), contains DNA from NETs. However, only a few studies are available to validate the value of solely NETs for tumor diagnosis.
Zhang et al. found that NETs had better diagnostic value than carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) in GC by comparing ROC curves (103). In addition, the serum levels of NETs were associated with some clinicopathological features such as lymph node metastasis; however, no relationship was found between the levels of NETs in the tumor tissue and clinicopathological factors. Another study reported that the levels of NETs increased in proportion to the stage of breast cancer, and the levels of NE–DNA complexes were higher in regional and metastatic disease than in localized disease, which was consistent with the speculation that the formation of NETs may result in cancer progression and metastasis (104). However, similar studies on CRC have not yet been reported.
We speculate that, as a new biomarker that can be detected in multiple tumors, NETs may lack specificity used for the diagnosis of a particular tumor type. However, NETs may have better sensitivity and specificity for tumor diagnosis when combined with other tumor-associated markers such as AFP, CEA, and CA199, and their clinical utility should be validated in future studies.
Therapeutic Target
The role of NETs in the pathogenesis of several tumors and inflammatory diseases has been validated in many studies (6, 105, 106). Therefore, targeting NETs may be of great potential for cancer therapy. NETs can be targeted by blocking the inducers of their formation, inhibiting their formation pathway, destroying their structure, and impeding the tumor–NET interaction (107). Recent studies have reported that therapeutics targeting NETs mainly focus on the following two aspects: inhibition of NETosis and destruction of NET integrity after formation (Table 1).
Extensive efforts have been made to inhibit the generation of NETs pharmacologically to suppress cancer progression. As a critical enzyme in NETosis, PAD4 has been considered the target to block the pro-tumoral effects of NETs. To date, several reports have demonstrated the efficacy of some compounds in inhibiting PAD activity including Cl-amidine, related compounds that are irreversible non-selective PAD inhibitors (108, 119), and the reversible selective PAD4 inhibitor GSK-484 (109). Another small-molecule inhibitor, BMS-P5, was demonstrated to abrogate NET formation induced by multiple myeloma cells and delay disease progression in a murine model (110). In addition, some clinical antitumor drugs affect NET formation. Zeng et al. found that kaempferol, whose inhibitory effects on primary tumor growth have been widely investigated, can suppress NETosis by inhibiting the ROS–PAD4 pathway (111). Anthracyclines, ranked among the most effective chemotherapeutics against cancer that act through DNA intercalation, oxidative stress, and topoisomerase II poisoning (120), suppress both NADPH oxidase-dependent and independent NETosis in human neutrophils (112), which further elucidates the antitumor mechanism of anthracyclines. Similarly, free 5FU, a chemotherapeutic agent, leads to a significant and rapid increase in the total amount of NETs in the blood. However, when 5FU-loaded amphiphilic poly-N-vinylpyrrolidone nanoparticles were studied under the same conditions, the level of NETs in the blood was not elevated, indicating the significant potential of nanoparticles as delivery agents for chemotherapeutic drugs in antitumor therapy (113).
Another way to suppress the pro-tumoral effects of NETs is to destroy their structural integrity by targeting DNA, comprising their backbone and affiliated proteins. DNase I has antimetastatic activity and can inhibit NETs (32); however, its short biological half-life limits its clinical utility. Xia et al. demonstrated that adeno-associated virus-mediated DNase I liver gene transfer inhibited neutrophil infiltration and NET formation, increases the proportion of CD8+ T cells at the tumor site, and eventually attenuates the progression of liver metastasis in a mouse model of colorectal liver metastasis (114), representing a novel and effective therapeutic strategy for CRC. A recent study reported that DNase-I-coated melanin-like nanospheres promoted dissolution of NET structure in sepsis-associated NETosis, thereby preventing further progression of the disease; however, its antitumor effects warrant further investigation (115). Some protein components of NETs play a significant role in the development of tumors, which can also be targeted to impair NET function. In a study, HMGB1 derived from NETs strengthened the malignancy of cancer cells in a mouse model of liver metastasis with inflammation. However, thrombomodulin (TM) potentiated HMGB1 proteolysis via thrombin and consequently inhibited the induction of NETs, thereby preventing pancreatic cancer metastasis to the liver (116). In addition, recombinant TM (rTM) can suppress histone-induced NET release both in vitro and in vivo by binding to histones (117). Rayes et al. discovered that NET-associated carcinoembryonic Ag cell adhesion molecule 1 (CEACAM1) was an important element for tumor progression and metastasis, and blocking CEACAM1 on NETs or its knockout in a mouse model significantly compromised cell adhesion, migration and metastasis in colon carcinoma (118). In a recent study, a novel method was introduced to use NETs as anti-tumor drug delivery vehicles by re-engineering neutrophils to express the apoptosis-inducing chimeric eGFP-TRAIL protein on NETs, which can simultaneously entrap and kill tumor cells while reserving their antibacterial capabilities (121), making NETs a promising candidate for the delivery of antitumoral agents.
Furthermore, NETs are associated with therapy resistance. Inhibition of neutrophils or PAD4-dependent NETosis can increase sensitivity to immune checkpoint blockade in pancreatic cancer (122), indicating that NETs are a potential candidate for improving immunotherapeutic efficacy.
Prognostic Prediction
The pro-tumoral effects of NETs are suggestive of their potential as a novel prognostic predictor of cancers (123), which has been reported in several studies. Elevated levels of NETs are strongly associated with a higher risk of metastasis. Decker et al. showed that increased NETosis in blood could be used as a biomarker to detect early head and neck cancer and predict the possibility of developing tumor metastasis (124).
Furthermore, increased levels of NETs have a strong correlation with unfavorable survival in many types of tumors. For example, higher preoperative serum NET levels were closely associated with shorter recurrence-free survival (RFS) and overall survival (OS) in patients with primary hepatic malignancies (125). In addition, preoperative moderate leucocytosis is correlated with increased levels of tumor-infiltrating NETs in esophageal cancer (EC), which is associated with worse OS and disease-free survival (DFS). The level of NETs is considered an independent prognostic factor for survival in EC after surgery (126). In patients with GC, higher baseline levels of NETs in the blood are significantly correlated with worse progression-free survival (PFS) (103). However, in a study on patients with high-grade ovarian cancer, a contradictory conclusion was proposed, indicating that NETs are related to favorable survival and better outcomes (127).
In addition, the level of NETs in the blood can be used to predict the effectiveness of treatment regimens in patients with cancer. Zhang et al. found that the level of NETs was inversely correlated with short-term therapeutic efficacy in patients with GC who had received advanced first-line treatment (103). This study indicated the possibility of enhanced chemotherapeutic efficacy through NETosis inhibition.
Venous thromboembolism (VTE), a common complication in patients with cancer, can be induced and aggravated by NET formation (128). Extensive studies have confirmed the relationship between increased NET levels and a higher risk of thrombosis in many diseases including cancer (50, 129), which indicates the involvement of NETs in cancer-associated thrombosis and the significance of NETs as prognostic biomarkers to predict the risk of thromboembolism.
Conclusion and Perspectives
Several studies have validated the role of NETs in tumorigenesis, metastasis spread, and associated complications, indicating the significant potential of targeting NETs for cancer therapy. On the one hand, further investigation is required to study the detailed molecular mechanisms of NETs formation and pro-tumoral pathways affected by NETs to identify more therapeutic targets and develop corresponding agents. On the other hand, solely inhibiting overall NET formation may compromise immunity because NETosis is a part of the immune system. Therefore, it is necessary to develop therapeutics precisely targeting NETs in tumor tissues but without adverse effects on immune function. At present, a gene therapy vector has been reported to specifically express DNase in the liver and effectively inhibit colorectal liver metastasis, which indicates the possibility of achieving tumor precision therapy targeting NETs.
NETs can be used for tumor diagnosis and prognosis combined with some classical tumor markers; however, the results may not necessarily be reliable, limited by sample number, species specificity, and other unknown factors. Therefore, large-scale, multicenter studies should be performed to further verify the potential of NETs as diagnostic and prognostic biomarkers. In addition, fecal testing is currently one of the key methods for early CRC screening. Therefore, could NETs be present in the stool of patients with CRC and considered an early screening indicator?
NETs are involved in inflammation in chronic liver diseases. In patients with CRC, increased levels of NETs in the liver often indicate a high metastatic rate. Therefore, the management of chronic liver disease in patients with CRC is very important. Controlling the levels of NETs in the liver as early as possible may prevent or decrease metastasis. In addition, developing a method for rapid, minimally invasive, inexpensive, and stable detection of NETs is the basis for further clinical utility; however, the currently available detection technology cannot meet these requirements, which should be further improved.
CRC refers to colon cancer, including left and right colon cancer, and rectal cancer, which have different molecular landscapes in tumorigenesis despite a close relationship. Therefore, the role of NETs in tumorigenesis is also different and should be investigated in future studies.
Author Contributions
SJ and GD: conception of the work. DL, JS and BC: literature search and manuscript drafting. PC, LJ and LC: design of the table and figures. RZ, HL, and WG: critical revision of the work and final version approval. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lehman HK, Segal BH. The Role of Neutrophils in Host Defense and Disease. J Allergy Clin Immunol (2020) 145(6):1535–44. doi: 10.1016/j.jaci.2020.02.038
2. Dąbrowska D, Jabłońska E, Iwaniuk A, Garley M. Many Ways-One Destination: Different Types of Neutrophils Death. Int Rev Immunol (2019) 38(1):18–32. doi: 10.1080/08830185.2018.1540616
3. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil Extracellular Traps Kill Bacteria. Science (2004) 303(5663):1532–5. doi: 10.1126/science.1092385
4. Delgado-Rizo V, Martínez-Guzmán MA, Iñiguez-Gutierrez L, García-Orozco A, Alvarado-Navarro A, Fafutis-Morris M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front Immunol (2017) 8:81. doi: 10.3389/fimmu.2017.00081
5. Lee KH, Kronbichler A, Park DD, Park Y, Moon H, Kim H, et al. Neutrophil Extracellular Traps (Nets) in Autoimmune Diseases: A Comprehensive Review. Autoimmun Rev (2017) 16(11):1160–73. doi: 10.1016/j.autrev.2017.09.012
6. Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting Potential Drivers of Covid-19: Neutrophil Extracellular Traps. J Exp Med (2020) 217(6):e20200652. doi: 10.1084/jem.20200652
7. Zha C, Meng X, Li L, Mi S, Qian D, Li Z, et al. Neutrophil Extracellular Traps Mediate the Crosstalk Between Glioma Progression and the Tumor Microenvironment Via the Hmgb1/Rage/Il-8 Axis. Cancer Biol Med (2020) 17(1):154–68. doi: 10.20892/j.issn.2095-3941.2019.0353
8. Martins-Cardoso K, Almeida VH, Bagri KM, Rossi MID, Mermelstein CS, Konig S, et al. Neutrophil Extracellular Traps (Nets) Promote Pro-Metastatic Phenotype in Human Breast Cancer Cells Through Epithelial-Mesenchymal Transition. Cancers (Basel) (2020) 12(6):1542. doi: 10.3390/cancers12061542
9. Schedel F, Mayer-Hain S, Pappelbaum KI, Metze D, Stock M, Goerge T, et al. Evidence and Impact of Neutrophil Extracellular Traps in Malignant Mel Anoma. Pigment Cell Melanoma Res (2020) 33(1):63–73. doi: 10.1111/pcmr.12818
10. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551
11. Jin M, Frankel WL. Lymph Node Metastasis in Colorectal Cancer. Surg Oncol Clin N Am (2018) 27(2):401–12. doi: 10.1016/j.soc.2017.11.011
12. Tsikitis VL, Larson DW, Huebner M, Lohse CM, Thompson PA. Predictors of Recurrence Free Survival for Patients With Stage Ii and Iii Colon Cancer. BMC Cancer (2014) 14:336. doi: 10.1186/1471-2407-14-336
13. Miyamoto Y, Hayashi N, Sakamoto Y, Ohuchi M, Tokunagam R, Kurashige J, et al. Predictors of Long-Term Survival in Patients With Stage Iv Colorectal Cancer With Multi-Organ Metastases: A Single-Center Retrospective Analysis. Int J Clin Oncol (2015) 20(6):1140–6. doi: 10.1007/s10147-015-0835-2
14. Yang L, Liu L, Zhang R, Hong J, Wang Y, Wang J, et al. Il-8 Mediates a Positive Loop Connecting Increased Neutrophil Extracellular Traps (Nets) and Colorectal Cancer Liver Metastasis. J Cancer (2020) 11(15):4384–96. doi: 10.7150/jca.44215
15. Shang A, Gu C, Zhou C, Yang Y, Chen C, Zeng B, et al. Exosomal Kras Mutation Promotes the Formation of Tumor-Associated Neutrophil Extracellular Traps and Causes Deterioration of Colorectal Cancer by Inducing Il-8 Expression. Cell Commun Signal (2020) 18(1):52. doi: 10.1186/s12964-020-0517-1
16. Khan U, Chowdhury S, Billah MM, Islam KMD, Thorlacius H, Rahman M. Neutrophil Extracellular Traps in Colorectal Cancer Progression and Metastasis. Int J Mol Sci (2021) 22(14):7260. doi: 10.3390/ijms22147260
17. Jorch SK, Kubes P. An Emerging Role for Neutrophil Extracellular Traps in Noninfectious Disease. Nat Med (2017) 23(3):279–87. doi: 10.1038/nm.4294
18. Rochael NC, Guimaraes-Costa AB, Nascimento MT, DeSouza-Vieira TS, Oliveira MP, Garcia e Souza LF, et al. Classical Ros-Dependent and Early/Rapid Ros-Independent Release of Neutrophil Extracellular Traps Triggered by Leishmania Parasites. Sci Rep (2015) 5:18302. doi: 10.1038/srep18302
19. Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast Cells and Neutrophils Release Il-17 Through Extracellular Trap Formation in Psoriasis. J Immunol (2011) 187(1):490–500. doi: 10.4049/jimmunol.1100123
20. Grayson PC, Kaplan MJ. At the Bench: Neutrophil Extracellular Traps (Nets) Highlight Novel Aspects of Innate Immune System Involvement in Autoimmune Diseases. J Leukoc Biol (2016) 99(2):253–64. doi: 10.1189/jlb.5BT0615-247R
21. Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet Tlr4 Activates Neutrophil Extracellular Traps to Ensnare Bacteria in Septic Blood. Nat Med (2007) 13(4):463–9. doi: 10.1038/nm1565
22. Kirchner T, Moller S, Klinger M, Solbach W, Laskay T, Behnen M. The Impact of Various Reactive Oxygen Species on the Formation of Neutrophil Extracellular Traps. Mediators Inflamm (2012) 2012:849136. doi: 10.1155/2012/849136
23. Warnatsch A, Tsourouktsoglou TD, Branzk N, Wang Q, Reincke S, Herbst S, et al. Reactive Oxygen Species Localization Programs Inflammation to Clear Microbes of Different Size. Immunity (2017) 46(3):421–32. doi: 10.1016/j.immuni.2017.02.013
24. Joshi MB, Philippova M, Ivanov D, Allenspach R, Erne P, Resink TJ. T-Cadherin Protects Endothelial Cells From Oxidative Stress-Induced Apoptosis. FASEB J (2005) 19(12):1737–9. doi: 10.1096/fj.05-3834fje
25. Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, et al. Activated Endothelial Cells Induce Neutrophil Extracellular Traps and Are Susceptible to Netosis-Mediated Cell Death. FEBS Lett (2010) 584(14):3193–7. doi: 10.1016/j.febslet.2010.06.006
26. Jeannin P, Delneste Y, Gosset P, Molet S, Lassalle P, Hamid Q, et al. Histamine Induces Interleukin-8 Secretion by Endothelial Cells. Blood (1994) 84(7):2229–33. doi: 10.1182/blood.V84.7.2229.2229
27. Maugeri N, Campana L, Gavina M, Covino C, De Metrio M, Panciroli C, et al. Activated Platelets Present High Mobility Group Box 1 to Neutrophils, Inducing Autophagy and Promoting the Extrusion of Neutrophil Extracellular Traps. J Thromb Haemost (2014) 12(12):2074–88. doi: 10.1111/jth.12710
28. Etulain J, Martinod K, Wong SL, Cifuni SM, Schattner M, Wagner DD. P-Selectin Promotes Neutrophil Extracellular Trap Formation in Mice. Blood (2015) 126(2):242–6. doi: 10.1182/blood-2015-01-624023
29. Li J, Kim K, Hahm E, Molokie R, Hay N, Gordeuk VR, et al. Neutrophil Akt2 Regulates Heterotypic Cell-Cell Interactions During Vascular Inflammation. J Clin Invest (2014) 124(4):1483–96. doi: 10.1172/JCI72305
30. Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, et al. Neutrophils Scan for Activated Platelets to Initiate Inflammation. Science (2014) 346(6214):1234–8. doi: 10.1126/science.1256478
31. Andrews RK, Arthur JF, Gardiner EE. Neutrophil Extracellular Traps (Nets) and the Role of Platelets in Infection. Thromb Haemost (2014) 112(4):659–65. doi: 10.1160/TH14-05-0455
32. Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. Cancer Cells Induce Metastasis-Supporting Neutrophil Extracellular DNA Traps. Sci Transl Med (2016) 8(361):361ra138. doi: 10.1126/scitranslmed.aag1711
33. DuPre SA, Hunter KW Jr. Murine Mammary Carcinoma 4t1 Induces a Leukemoid Reaction With Splenomegaly: Association With Tumor-Derived Growth Factors. Exp Mol Pathol (2007) 82(1):12–24. doi: 10.1016/j.yexmp.2006.06.007
34. Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, et al. Granulocyte-Colony Stimulating Factor Promotes Lung Metastasis Through Mobilization of Ly6g+Ly6c+ Granulocytes. Proc Natl Acad Sci U.S.A. (2010) 107(50):21248–55. doi: 10.1073/pnas.1015855107
35. Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, et al. Cancers Predispose Neutrophils to Release Extracellular DNA Traps That Contribute to Cancer-Associated Thrombosis. Proc Natl Acad Sci USA (2012) 109(32):13076–81. doi: 10.1073/pnas.1200419109
36. Alfaro C, Teijeira A, Oñate C, Pérez G, Sanmamed MF, Andueza MP, et al. Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (Nets). Clin Cancer Res (2016) 22(15):3924–36. doi: 10.1158/1078-0432.ccr-15-2463
37. Nie M, Yang L, Bi X, Wang Y, Sun P, Yang H, et al. Neutrophil Extracellular Traps Induced by Il8 Promote Diffuse Large B-Cell Lymphoma Progression Via the Tlr9 Signaling. Clin Cancer Res (2019) 25(6):1867–79. doi: 10.1158/1078-0432.ccr-18-1226
38. Liu K, Sun E, Lei M, Li L, Gao J, Nian X, et al. Bcg-Induced Formation of Neutrophil Extracellular Traps Play an Important Role in Bladder Cancer Treatment. Clin Immunol (2019) 201:4–14. doi: 10.1016/j.clim.2019.02.005
39. Gomes T, Várady CBS, Lourenço AL, Mizurini DM, Rondon AMR, Leal AC, et al. Il-1β Blockade Attenuates Thrombosis in a Neutrophil Extracellular Trap-Dependent Breast Cancer Model. Front Immunol (2019) 10:2088. doi: 10.3389/fimmu.2019.02088
40. Jin W, Yin H, Li H, Yu XJ, Xu HX, Liu L. Neutrophil Extracellular DNA Traps Promote Pancreatic Cancer Cells Migration and Invasion by Activating Egfr/Erk Pathway. J Cell Mol Med (2021) 25(12):5443–56. doi: 10.1111/jcmm.16555
41. Chang X, Han J, Pang L, Zhao Y, Yang Y, Shen Z. Increased Padi4 Expression in Blood and Tissues of Patients With Malignant Tumors. BMC Cancer (2009) 9:40. doi: 10.1186/1471-2407-9-40
42. Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of Extracellular Vesicles (Ev): Exosomes, Microvesicles, Retrovirus-Like Vesicles, and Apoptotic Bodies. J Neurooncol (2013) 113(1):1–11. doi: 10.1007/s11060-013-1084-8
43. Leal AC, Mizurini DM, Gomes T, Rochael NC, Saraiva EM, Dias MS, et al. Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications for the Establishment of Cancer-Associated Thrombosis. Sci Rep (2017) 7(1):6438. doi: 10.1038/s41598-017-06893-7
44. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular Vesicles in Cancer: Cell-To-Cell Mediators of Metastasis. Cancer Cell (2016) 30(6):836–48. doi: 10.1016/j.ccell.2016.10.009
45. Chennakrishnaiah S, Meehan B, D'Asti E, Montermini L, Lee TH, Karatzas N, et al. Leukocytes as a Reservoir of Circulating Oncogenic DNA and Regulatory Targets of Tumor-Derived Extracellular Vesicles. J Thromb Haemost (2018) 16(9):1800–13. doi: 10.1111/jth.14222
46. Aguila S, de Los Reyes-Garcia AM, Fernandez-Perez MP, Reguilon-Gallego L, Zapata-Martinez L, Ruiz-Lorente I, et al. Micrornas as New Regulators of Neutrophil Extracellular Trap Formation. Int J Mol Sci (2021) 22(4):2116. doi: 10.3390/ijms22042116
47. Arroyo AB, de Los Reyes-García AM, Rivera-Caravaca JM, Valledor P, García-Barberá N, Roldán V, et al. Mir-146a Regulates Neutrophil Extracellular Trap Formation That Predicts Adverse Cardiovascular Events in Patients With Atrial Fibrillation. Arterioscler Thromb Vasc Biol (2018) 38(4):892–902. doi: 10.1161/atvbaha.117.310597
48. Hawez A, Al-Haidari A, Madhi R, Rahman M, Thorlacius H. Mir-155 Regulates Pad4-Dependent Formation of Neutrophil Extracellular Traps. Front Immunol (2019) 10:2462. doi: 10.3389/fimmu.2019.02462
49. Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Kollnberger M, et al. Von Willebrand Factor-Mediated Platelet Adhesion Is Critical for Deep Vein Thrombosis in Mouse Models. Blood (2011) 117(4):1400–7. doi: 10.1182/blood-2010-05-287623
50. Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr, et al. Extracellular DNA Traps Promote Thrombosis. Proc Natl Acad Sci USA (2010) 107(36):15880–5. doi: 10.1073/pnas.1005743107
51. Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, et al. Netting Neutrophils in Autoimmune Small-Vessel Vasculitis. Nat Med (2009) 15(6):623–5. doi: 10.1038/nm.1959
52. Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. Nets Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci Transl Med (2013) 5(178):178ra40. doi: 10.1126/scitranslmed.3005580
53. Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting Neutrophils Are Major Inducers of Type I Ifn Production in Pediatric Systemic Lupus Erythematosus. Sci Transl Med (2011) 3(73):73ra20. doi: 10.1126/scitranslmed.3001201
54. Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil Extracellular Traps License Macrophages for Cytokine Production in Atherosclerosis. Science (2015) 349(6245):316–20. doi: 10.1126/science.aaa8064
55. Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, et al. Damage-Associated Molecular Pattern-Activated Neutrophil Extracellular Trap Exacerbates Sterile Inflammatory Liver Injury. Hepatology (2015) 62(2):600–14. doi: 10.1002/hep.27841
56. Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, et al. Neutrophils Promote Alzheimer's Disease-Like Pathology and Cognitive Decline Via Lfa-1 Integrin. Nat Med (2015) 21(8):880–6. doi: 10.1038/nm.3913
57. Dinallo V, Marafini I, Di Fusco D, Laudisi F, Franzè E, Di Grazia A, et al. Neutrophil Extracellular Traps Sustain Inflammatory Signals in Ulcerative Colitis. J Crohns Colitis (2019) 13(6):772–84. doi: 10.1093/ecco-jcc/jjy215
58. Yashiro M. Ulcerative Colitis-Associated Colorectal Cancer. World J Gastroenterol (2014) 20(44):16389–97. doi: 10.3748/wjg.v20.i44.16389
59. D'Abbondanza M, Martorelli EE, Ricci MA, De Vuono S, Migliola EN, Godino C, et al. Increased Plasmatic Nets by-Products in Patients in Severe Obesity. Sci Rep (2019) 9(1):14678. doi: 10.1038/s41598-019-51220-x
60. Freitas DF, Colón DF, Silva RL, Santos EM, Guimarães VHD, Ribeiro GHM, et al. Neutrophil Extracellular Traps (Nets) Modulate Inflammatory Profile in Obese Humans and Mice: Adipose Tissue Role on Nets Levels. Mol Biol Rep (2022) 49(4):3225–36. doi: 10.1007/s11033-022-07157-y
61. Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, et al. Diabetes Primes Neutrophils to Undergo Netosis, Which Impairs Wound Healing. Nat Med (2015) 21(7):815–9. doi: 10.1038/nm.3887
62. Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging Cancer Trends Among Young Adults in the USA: Analysis of a Population-Based Cancer Registry. Lancet Public Health (2019) 4(3):e137–47. doi: 10.1016/s2468-2667(18)30267-6
63. De Bruijn KM, Arends LR, Hansen BE, Leeflang S, Ruiter R, van Eijck CH. Systematic Review and Meta-Analysis of the Association Between Diabetes Mellitus and Incidence and Mortality in Breast and Colorectal Cancer. Br J Surg (2013) 100(11):1421–9. doi: 10.1002/bjs.9229
64. Richardson JJR, Hendrickse C, Gao-Smith F, Thickett DR. Neutrophil Extracellular Trap Production in Patients With Colorectal Cancer in Vitro. Int J Inflam (2017) 2017:4915062. doi: 10.1155/2017/4915062
65. Miller-Ocuin JL, Liang X, Boone BA, Doerfler WR, Singhi AD, Tang D, et al. DNA Released From Neutrophil Extracellular Traps (Nets) Activates Pancreatic Stellate Cells and Enhances Pancreatic Tumor Growth. Oncoimmunology (2019) 8(9):e1605822. doi: 10.1080/2162402X.2019.1605822
66. van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, et al. Neutrophil Extracellular Traps Promote Inflammation and Development of Hepatocellular Carcinoma in Nonalcoholic Steatohepatitis. Hepatology (2018) 68(4):1347–60. doi: 10.1002/hep.29914
67. Yazdani HO, Roy E, Comerci AJ, van der Windt DJ, Zhang H, Huang H, et al. Neutrophil Extracellular Traps Drive Mitochondrial Homeostasis in Tumors to Augment Growth. Cancer Res (2019) 79(21):5626–39. doi: 10.1158/0008-5472.CAN-19-0800
68. Li R, Zou X, Zhu T, Xu H, Li X, Zhu L. Destruction of Neutrophil Extracellular Traps Promotes the Apoptosis and Inhibits the Invasion of Gastric Cancer Cells by Regulating the Expression of Bcl-2, Bax and Nf-Kappab. Onco Targets Ther (2020) 13:5271–81. doi: 10.2147/OTT.S227331
69. Guan X, Lu Y, Zhu H, Yu S, Zhao W, Chi X, et al. The Crosstalk Between Cancer Cells and Neutrophils Enhances Hepatocellular Carcinoma Metastasis Via Neutrophil Extracellular Traps-Associated Cathepsin G Component: A Potential Therapeutic Target. J Hepatocell Carcinoma (2021) 8:451–65. doi: 10.2147/jhc.s303588
70. Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, et al. DNA of Neutrophil Extracellular Traps Promotes Cancer Metastasis Via Ccdc25. Nature (2020) 583(7814):133–8. doi: 10.1038/s41586-020-2394-6
71. Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil Extracellular Traps Produced During Inflammation Awaken Dormant Cancer Cells in Mice. Science (2018) 361(6409):eaao4227. doi: 10.1126/science.aao4227
72. Teijeira Á, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, et al. Cxcr1 and Cxcr2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps That Interfere With Immune Cytotoxicity. Immunity (2020) 52(5):856–71.e8. doi: 10.1016/j.immuni.2020.03.001
73. Ireland AS, Oliver TG. Neutrophils Create an Impenetrable Shield Between Tumor and Cytotoxic Immune Cells. Immunity (2020) 52(5):729–31. doi: 10.1016/j.immuni.2020.04.009
74. Arelaki S, Arampatzioglou A, Kambas K, Papagoras C, Miltiades P, Angelidou I, et al. Gradient Infiltration of Neutrophil Extracellular Traps in Colon Cancer and Evidence for Their Involvement in Tumour Growth. PloS One (2016) 11(5):e0154484. doi: 10.1371/journal.pone.0154484
75. Rodrigues P, Vanharanta S. Circulating Tumor Cells: Come Together, Right Now, Over Metastasis. Cancer Discov (2019) 9(1):22–4. doi: 10.1158/2159-8290.cd-18-1285
76. Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil Extracellular Traps Sequester Circulating Tumor Cells and Promote Metastasis. J Clin Invest (2013) 123(8):3446–58. doi: 10.1172/JCI67484
77. Rayes RF, Mouhanna JG, Nicolau I, Bourdeau F, Giannias B, Rousseau S, et al. Primary Tumors Induce Neutrophil Extracellular Traps With Targetable Metastasis Promoting Effects. JCI Insight (2019) 5(16):e128008. doi: 10.1172/jci.insight.128008
78. Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, et al. Neutrophil Extracellular Traps Sequester Circulating Tumor Cells Via B1-Integrin Mediated Interactions. Int J Cancer (2017) 140(10):2321–30. doi: 10.1002/ijc.30635
79. Amintas S, Bedel A, Moreau-Gaudry F, Boutin J, Buscail L, Merlio JP, et al. Circulating Tumor Cell Clusters: United We Stand Divided We Fall. Int J Mol Sci (2020) 21(7):2653. doi: 10.3390/ijms21072653
80. Liu X, Taftaf R, Kawaguchi M, Chang YF, Chen W, Entenberg D, et al. Homophilic Cd44 Interactions Mediate Tumor Cell Aggregation and Polyclonal Metastasis in Patient-Derived Breast Cancer Models. Cancer Discovery (2019) 9(1):96–113. doi: 10.1158/2159-8290.cd-18-0065
81. An T, Zhang Z, Li Y, Yi J, Zhang W, Chen D, et al. Integrin B1-Mediated Cell-Cell Adhesion Augments Metformin-Induced Anoikis. Int J Mol Sci (2019) 20(5):1161. doi: 10.3390/ijms20051161
82. Pieterse E, Rother N, Garsen M, Hofstra JM, Satchell SC, Hoffmann M, et al. Neutrophil Extracellular Traps Drive Endothelial-To-Mesenchymal Transition. Arterioscler Thromb Vasc Biol (2017) 37(7):1371–9. doi: 10.1161/atvbaha.117.309002
83. Teng S, Li YE, Yang M, Qi R, Huang Y, Wang Q, et al. Tissue-Specific Transcription Reprogramming Promotes Liver Metastasis of Colorectal Cancer. Cell Res (2020) 30(1):34–49. doi: 10.1038/s41422-019-0259-z
84. Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H. Neutrophils Facilitate Ovarian Cancer Premetastatic Niche Formation in the Omentum. J Exp Med (2019) 216(1):176–94. doi: 10.1084/jem.20181170
85. Lee W, Naora H. Neutrophils Fertilize the Pre-Metastatic Niche. Aging (Albany NY) (2019) 11(17):6624–5. doi: 10.18632/aging.102258
86. Kolaczkowska E, Jenne CN, Surewaard BG, Thanabalasuriar A, Lee WY, Sanz MJ, et al. Molecular Mechanisms of Net Formation and Degradation Revealed by Intravital Imaging in the Liver Vasculature. Nat Commun (2015) 6:6673. doi: 10.1038/ncomms7673
87. McDonald B, Jenne CN, Zhuo L, Kimata K, Kubes P. Kupffer Cells and Activation of Endothelial Tlr4 Coordinate Neutrophil Adhesion Within Liver Sinusoids During Endotoxemia. Am J Physiol Gastrointest Liver Physiol (2013) 305(11):G797–806. doi: 10.1152/ajpgi.00058.2013
88. Hilscher MB, Shah VH. Neutrophil Extracellular Traps and Liver Disease. Semin Liver Dis (2020) 40(2):171–9. doi: 10.1055/s-0039-3399562
89. Louvet A, Wartel F, Castel H, Dharancy S, Hollebecque A, Canva-Delcambre V, et al. Infection in Patients With Severe Alcoholic Hepatitis Treated With Steroids: Early Response to Therapy Is the Key Factor. Gastroenterology (2009) 137(2):541–8. doi: 10.1053/j.gastro.2009.04.062
90. Michelena J, Altamirano J, Abraldes JG, Affò S, Morales-Ibanez O, Sancho-Bru P, et al. Systemic Inflammatory Response and Serum Lipopolysaccharide Levels Predict Multiple Organ Failure and Death in Alcoholic Hepatitis. Hepatology (2015) 62(3):762–72. doi: 10.1002/hep.27779
91. Taylor NJ, Nishtala A, Manakkat Vijay GK, Abeles RD, Auzinger G, Bernal W, et al. Circulating Neutrophil Dysfunction in Acute Liver Failure. Hepatology (2013) 57(3):1142–52. doi: 10.1002/hep.26102
92. Bukong TN, Cho Y, Iracheta-Vellve A, Saha B, Lowe P, Adejumo A, et al. Abnormal Neutrophil Traps and Impaired Efferocytosis Contribute to Liver Injury and Sepsis Severity After Binge Alcohol Use. J Hepatol (2018) 69(5):1145–54. doi: 10.1016/j.jhep.2018.07.005
93. van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, et al. Visceral Fat: A Key Mediator of Steatohepatitis in Metabolic Liver Disease. Hepatology (2008) 48(2):449–57. doi: 10.1002/hep.22350
94. Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, et al. Neutrophils Mediate Insulin Resistance in Mice Fed a High-Fat Diet Through Secreted Elastase. Nat Med (2012) 18(9):1407–12. doi: 10.1038/nm.2885
95. Mansuy-Aubert V, Zhou QL, Xie X, Gong Z, Huang JY, Khan AR, et al. Imbalance Between Neutrophil Elastase and Its Inhibitor A1-Antitrypsin in Obesity Alters Insulin Sensitivity, Inflammation, and Energy Expenditure. Cell Metab (2013) 17(4):534–48. doi: 10.1016/j.cmet.2013.03.005
96. Mano Y, Shirabe K, Yamashita Y, Harimoto N, Tsujita E, Takeishi K, et al. Preoperative Neutrophil-To-Lymphocyte Ratio Is a Predictor of Survival After Hepatectomy for Hepatocellular Carcinoma: A Retrospective Analysis. Ann Surg (2013) 258(2):301–5. doi: 10.1097/SLA.0b013e318297ad6b
97. Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, et al. Preoperative Prognostic Score for Predicting Survival After Hepatic Resection for Colorectal Liver Metastases. Ann Surg (2007) 246(5):806–14. doi: 10.1097/SLA.0b013e318142d964
98. Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, et al. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases After Surgical Stress. Cancer Res (2016) 76(6):1367–80. doi: 10.1158/0008-5472.can-15-1591
99. Mader S, Pantel K. Liquid Biopsy: Current Status and Future Perspectives. Oncol Res Treat (2017) 40(7-8):404–8. doi: 10.1159/000478018
100. Choudhury AD, Werner L, Francini E, Wei XX, Ha G, Freeman SS, et al. Tumor Fraction in Cell-Free DNA as a Biomarker in Prostate Cancer. JCI Insight (2018) 3(21):e122109. doi: 10.1172/jci.insight.122109
101. Phallen J, Sausen M, Adleff V, Leal A, Hruban C, White J, et al. Direct Detection of Early-Stage Cancers Using Circulating Tumor DNA. Sci Transl Med (2017) 9(403):eaan2415. doi: 10.1126/scitranslmed.aan2415
102. Alimirzaie S, Bagherzadeh M, Akbari MR. Liquid Biopsy in Breast Cancer: A Comprehensive Review. Clin Genet (2019) 95(6):643–60. doi: 10.1111/cge.13514
103. Zhang Y, Hu Y, Ma C, Sun H, Wei X, Li M, et al. Diagnostic, Therapeutic Predictive, and Prognostic Value of Neutrophil Extracellular Traps in Patients With Gastric Adenocarcinoma. Front Oncol (2020) 10:1036. doi: 10.3389/fonc.2020.01036
104. Rivera-Franco MM, Leon-Rodriguez E, Torres-Ruiz JJ, Gomez-Martin D, Angles-Cano E, de la Luz Sevilla-Gonzalez M. Neutrophil Extracellular Traps Associate With Clinical Stages in Breast Cancer. Pathol Oncol Res (2020) 26(3):1781–5. doi: 10.1007/s12253-019-00763-5
105. Blasco A, Coronado MJ, Hernandez-Terciado F, Martin P, Royuela A, Ramil E, et al. Assessment of Neutrophil Extracellular Traps in Coronary Thrombus of a Case Series of Patients With Covid-19 and Myocardial Infarction. JAMA Cardiol (2020) 6(4):1–6. doi: 10.1001/jamacardio.2020.7308
106. Cahilog Z, Zhao H, Wu L, Alam A, Eguchi S, Weng H, et al. The Role of Neutrophil Netosis in Organ Injury: Novel Inflammatory Cell Death Mechanisms. Inflammation (2020) 43(6):2021–32. doi: 10.1007/s10753-020-01294-x
107. Yang D, Liu J. Neutrophil Extracellular Traps: A New Player in Cancer Metastasis and Therapeutic Target. J Exp Clin Cancer Res (2021) 40(1):233. doi: 10.1186/s13046-021-02013-6
108. Causey CP, Jones JE, Slack JL, Kamei D, Jones LE, Subramanian V, et al. The Development of N-A-(2-Carboxyl)Benzoyl-N(5)-(2-Fluoro-1-Iminoethyl)-L-Ornithine Amide (O-F-Amidine) and N-A;-(2-Carboxyl)Benzoyl-N(5)-(2-Chloro-1-Iminoethyl)-L-Ornithine Amide (O-Cl-Amidine) as Second Generation Protein Arginine Deiminase (Pad) Inhibitors. J Med Chem (2011) 54(19):6919–35. doi: 10.1021/jm2008985
109. Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, et al. Inhibition of Pad4 Activity Is Sufficient to Disrupt Mouse and Human Net Formation. Nat Chem Biol (2015) 11(3):189–91. doi: 10.1038/nchembio.1735
110. Li M, Lin C, Deng H, Strnad J, Bernabei L, Vogl DT, et al. A Novel Peptidylarginine Deiminase 4 (Pad4) Inhibitor Bms-P5 Blocks Formation of Neutrophil Extracellular Traps and Delays Progression of Multiple Myeloma. Mol Cancer Ther (2020) 19(7):1530–8. doi: 10.1158/1535-7163.MCT-19-1020
111. Zeng J, Xu H, Fan PZ, Xie J, He J, Yu J, et al. Kaempferol Blocks Neutrophil Extracellular Traps Formation and Reduces Tumour Metastasis by Inhibiting Ros-Pad4 Pathway. J Cell Mol Med (2020) 24(13):7590–9. doi: 10.1111/jcmm.15394
112. Khan MA, D'Ovidio A, Tran H, Palaniyar N. Anthracyclines Suppress Both Nadph Oxidase- Dependent and -Independent Netosis in Human Neutrophils. Cancers (Basel) (2019) 11(9):1328. doi: 10.3390/cancers11091328
113. Basyreva LY, Voinova EV, Gusev AA, Mikhalchik EV, Kuskov AN, Goryachaya AV, et al. Fluorouracil Neutrophil Extracellular Traps Formation Inhibited by Polymer Nanoparticle Shielding. Mater Sci Eng C Mater Biol Appl (2020) 108:110382. doi: 10.1016/j.msec.2019.110382
114. Xia Y, He J, Zhang H, Wang H, Tetz G, Maguire CA, et al. Aav-Mediated Gene Transfer of Dnase I in the Liver of Mice With Colorectal Cancer Reduces Liver Metastasis and Restores Local Innate and Adaptive Immune Response. Mol Oncol (2020) 14(11):2920–35. doi: 10.1002/1878-0261.12787
115. Park HH, Park W, Lee YY, Kim H, Seo HS, Choi DW, et al. Bioinspired Dnase-I-Coated Melanin-Like Nanospheres for Modulation of Infection-Associated Netosis Dysregulation. Adv Sci (Weinh) (2020) 7(23–:2001940. doi: 10.1002/advs.202001940
116. Kajioka H, Kagawa S, Ito A, Yoshimoto M, Sakamoto S, Kikuchi S, et al. Targeting Neutrophil Extracellular Traps With Thrombomodulin Prevents Pancreatic Cancer Metastasis. Cancer Lett (2021) 497:1–13. doi: 10.1016/j.canlet.2020.10.015
117. Shrestha B, Ito T, Kakuuchi M, Totoki T, Nagasato T, Yamamoto M, et al. Recombinant Thrombomodulin Suppresses Histone-Induced Neutrophil Extracellular Trap Formation. Front Immunol (2019) 10:2535. doi: 10.3389/fimmu.2019.02535
118. Rayes RF, Vourtzoumis P, Bou Rjeily M, Seth R, Bourdeau F, Giannias B, et al. Neutrophil Extracellular Trap-Associated Ceacam1 as a Putative Therapeutic Target to Prevent Metastatic Progression of Colon Carcinoma. J Immunol (2020) 204(8):2285–94. doi: 10.4049/jimmunol.1900240
119. Knuckley B, Causey CP, Jones JE, Bhatia M, Dreyton CJ, Osborne TC, et al. Substrate Specificity and Kinetic Studies of Pads 1, 3, and 4 Identify Potent and Selective Inhibitors of Protein Arginine Deiminase 3. Biochemistry (2010) 49(23):4852–63. doi: 10.1021/bi100363t
120. Martins-Teixeira MB, Carvalho I. Antitumour Anthracyclines: Progress and Perspectives. ChemMedChem (2020) 15(11):933–48. doi: 10.1002/cmdc.202000131
121. Cao TM, King MR. Supercharged Egfp-Trail Decorated Nets to Ensnare and Kill Disseminated Tumor Cells. Cell Mol Bioeng (2020) 13(4):359–67. doi: 10.1007/s12195-020-00639-8
122. Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada PR, Rakoski A, et al. Interleukin-17-Induced Neutrophil Extracellular Traps Mediate Resistance to Checkpoint Blockade in Pancreatic Cancer. J Exp Med (2020) 217(12):e20190354. doi: 10.1084/jem.20190354
123. Rivera-Franco MM, Leon-Rodriguez E, Sevilla-Gonzalez ML. Could Neutrophil Extracellular Traps Be the New Prognostic Markers of Cancer? Rev Invest Clin (2019) 71(6):365–8. doi: 10.24875/RIC.19003155
124. Decker AS, Pylaeva E, Brenzel A, Spyra I, Droege F, Hussain T, et al. Prognostic Role of Blood Netosis in the Progression of Head and Neck Cancer. Cells (2019) 8(9):946. doi: 10.3390/cells8090946
125. Kaltenmeier CT, Yazdani H, van der Windt D, Molinari M, Geller D, Tsung A, et al. Neutrophil Extracellular Traps as a Novel Biomarker to Predict Recurrence-Free and Overall Survival in Patients With Primary Hepatic Malignancies. HPB (Oxford) (2021) 23(2):309–20. doi: 10.1016/j.hpb.2020.06.012
126. Zhang H, Lv H, Weng M, Wang H, Cata JP, Chen W, et al. Preoperative Leukocytosis Is Associated With Increased Tumor-Infiltrating Neutrophil Extracellular Traps and Worse Outcomes in Esophageal Cancer. Ann Transl Med (2020) 8(7):441. doi: 10.21037/atm.2020.03.190
127. Muqaku B, Pils D, Mader JC, Aust S, Mangold A, Muqaku L, et al. Neutrophil Extracellular Trap Formation Correlates With Favorable Overall Survival in High Grade Ovarian Cancer. Cancers (Basel) (2020) 12(2):505. doi: 10.3390/cancers12020505
128. Hamza MS, Mousa SA. Cancer-Associated Thrombosis: Risk Factors, Molecular Mechanisms, Future Management. Clin Appl Thromb Hemost (2020) 26:1076029620954282. doi: 10.1177/1076029620954282
Keywords: neutrophil extracellular traps, colorectal cancer, metastasis, clinical application, tumor microenvironment
Citation: Li D, Shao J, Cao B, Zhao R, Li H, Gao W, Chen P, Jin L, Cao L, Ji S and Dong G (2022) The Significance of Neutrophil Extracellular Traps in Colorectal Cancer and Beyond: From Bench to Bedside. Front. Oncol. 12:848594. doi: 10.3389/fonc.2022.848594
Received: 04 January 2022; Accepted: 09 May 2022;
Published: 07 June 2022.
Edited by:
Nadia M. Hamdy, Ain Shams University, EgyptReviewed by:
Robson Q. Monteiro, Federal University of Rio de Janeiro, BrazilTiziana Schioppa, University of Brescia, Italy
Copyright © 2022 Li, Shao, Cao, Zhao, Li, Gao, Chen, Jin, Cao, Ji and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuaifei Ji, jiflyfly301@163.com; Guanglong Dong, gldong301@163.com
†These authors have contributed equally to this work
 Dingchang Li
Dingchang Li Jiakang Shao
Jiakang Shao Bo Cao
Bo Cao Ruiyang Zhao
Ruiyang Zhao Hanghang Li
Hanghang Li Wenxing Gao1,2
Wenxing Gao1,2 Li Cao
Li Cao Shuaifei Ji
Shuaifei Ji Guanglong Dong
Guanglong Dong