- 1Department of Thoracic Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2West China Biomedical Big Data Center, West China Hospital, Sichuan University, Chengdu, China
Many studies have confirmed that micro-RNA (mir) is related to the prognosis of esophageal carcinoma (EC), suggesting the mir could be used to guide the therapeutic strategy of EC. Some of mir molecules are considered as favorable prognostic factors for EC. The purpose of our study is to evaluate the prognostic potential of mir-375, 133, 143, 145 in primary EC, we summarized all the results from available studies, aiming delineating the prognostic role of mir in EC. Relevant studies were identified by searching databases including Medline, Embase, Web of science, Cochrane Library. The studies which explored the prognostic value of mir-375, 133, 143, 145 expressions on survival outcomes in patients with EC were included in this study. The hazard ratios (HR) and their responding 95% confidence interval (CI) were also extracted. A total of 25 studies were collected, including 1260 patients, and the prognostic values of four mirs in EC were analyzed. Survival outcomes including overall survival (OS), progression-free survival (PFS) and disease-free survival (DFS) were used as the primary endpoint to evaluate the prognostic value of mir. The pooled analysis results showed that up-regulation of mir-375 indicated favorable OS (HR=0.50; 95%CI: 0.37-0.69; P<0.001). In addition, the up-regulation of mir-133 (HR=0.40, 95%CI: 0.24-0.65, P<0.001), 143 (HR=0.40, 95%CI: 0.21-0.76, P < 0.001) and 145 (HR=0.55, 95%CI: 0.34-0.90, P<0.001) are also proved as protected factors in EC. Therefore, our study demonstrated that these mirs may have the potential to be used as prognostic biomarkers for EC in clinical practice.
Introduction
Esophageal carcinoma (EC) is a common and fatal gastrointestinal malignant tumor which is a group of heterogeneous malignancies including squamous cell carcinoma (ESCC) and adenocarcinoma (EAC). Its clinical characters are associated with races, geographical distribution and other risk factors. EC is currently the sixth most common cancer worldwide (1), and is also one of the most deadly malignant tumor (2). According to the latest research, in 2020, there were 604000 cases of EC and 544000 deaths worldwide (3). Since the symptoms of early stage in EC are easy to be neglected, patients with EC are often diagnosed at late stage, and the optimum opportunity for surgery is lost (4). As a result, the five-year survival rate of EC patients is low, only about 30% (5). Although the advancement in therapeutic technologies including surgery, chemotherapy, radiotherapy and other novel treatments for EC has been greatly upgraded (6), it is still essential to find new biological markers to assess the prognosis of EC patients after surgery (7).
Non-coding RNA plays a vital role in cancer biology and provides potential targets for cancer intervention (8, 9). Early studies had indicated that non-coding RNA had the potential clinical utility for diagnosing and prognosticating cancers (10). One type of non-coding RNA is called micro-RNA (mir), which ranges in size from 20-24 nucleotides (11). In recent years, many studies have confirmed that mirs regulate gene expression by targeting mRNA for translational inhibition or cleavage, thereby involving in various biological phenotypes such as cell proliferation, differentiation, and apoptosis (11–13). Some mirs are considered to be related to the occurrence and development of tumors. Meanwhile, some of them were used as biomarkers for cancer detection and prognosis (14, 15). Mir-375 has been confirmed by studies that it is widely present in various tissues, which has been characterized as an important cancer-related mir (16, 17). The expression of mir-375 has been proved significantly reduced in malignant tumor cells (18). Several studies had reported that mir-375 were related to the prognosis of EC with low-expression in cancer tissue (19, 20), but nowadays, some more new researches were conducted to detect the mechanism of mir-375 may involve in EC, especially in prognosis, we collected entire of these data to make an update. On the other side, no published meta-analysis had investigated the impact of mir-133, 143, 145 on EC prognosis. In order to comprehensively and systematically investigate these mirs in EC prognosis, we conducted this meta-analysis to investigate the prognostic value of mir-375, 133, 143 and 145 while discussing the therapeutic potentialities of these mirs.
Materials and methods
Literature retrieval strategy
In order to obtain potentially eligible documents, we carefully searched the Medline, Embase, Web of science, Cochrane Library databases using the following search strategies and terms: (((((((esophageal cancer [Title/Abstract]) OR esophageal carcinoma [Title/Abstract]) OR esophageal squamous cell carcinoma [Title/Abstract]) OR esophageal esophageal adenocarcinoma [Title/Abstract])) AND (((MicroRNA [Title/Abstract]) OR miRNAs[Title/Abstract]) OR RNA, Micro [Title/Abstract])) AND (((prognostic [Title/Abstract]) OR prognosis [Title/Abstract]) OR survival [Title/Abstract])) updated until July 1, 2022. The retrieved related reviews and meta-analysis lists are also manually checked to identify more relevant information.
Inclusion and exclusion criteria
The eligible studies included in this meta-analysis meet the following criteria (1): studies have reported specific methods of collecting mirs through surgical resection or blood collection and measuring their expression (2); the patients in studies had received treatment options such as surgery, radiotherapy or chemotherapy; (3) studies clearly illustrate the correlation between mir expression and survival outcomes of patients such as overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), et al; (4) the patients were grouped according to the level of mir expression; (5) sufficient data are reported, and the hazard ratio (HR) and 95% confidence interval (CI) of mir expression can be retrieved based on the data in the article. The exclusion criteria are: (1) non-esophageal carcinoma patients; (2) duplicated studies; (3) studies using only in vitro cell lines; (4) animal experiments; (5) reviews, letters, case reports and expert opinions; (6) data could not be extracted or original data was lost.
Data extraction and quality assessment
All eligible research data and information are independently extracted by two independent researchers (Fang and Zhou). The collected data information of various studies including: author, publication year, race, cancer type, patient number, sample type, mir detection method, survival outcomes, cut-off value and its 95% confidence interval. Based on the study population, intervention, comparison and outcome (PICO) format, the PICO of this study is defined bellow: P: patients with EC; I: patients with high mirs expression level; C: patients with low mirs expression level; O: survival outcomes. To reduce the bias of data extraction, the survival curve data was evaluated by other independent individual (Li), and the Engauge Digitizer version 4.1 software was used to analyze the survival curve. The original data and additional information needed for this study were obtained by contacting the authors of the included studies. The Newcastle-Ottawa Scale (NOS) was applied to assess the quality of all included studies (S1). The NOS scores including selection, comparability, and outcome, the score ≥6 was considered as study with high quality (21).
Statistical analysis
The high or low expression of mir is defined according to the cut-off value provided by the author. The pooled HR and the corresponding 95% confidence interval were used to assess the correlation between mir expression and prognosis. All statistical analyses of this Meta-analysis are performed using Stata12.0 software (StataCorp, College Station, TX, USA) in accordance with PRISMA (S2) guidelines. The heterogeneity among the included studies was determined by the chi-square-based Q test and the I2 statistics; the P value of the Q test was ≤0.05; the I2 value ≥50% considered that the study had significant heterogeneity, the random-effects model was used to pool the effect size (PQ ≤ 0.05, I2≥50%). If the P value of the Q test was >0.05; the I2 value <50%, we considered that the research heterogeneity is acceptable (PQ>0.05, I2<50%), and the fixed-effects model was used to analysis. To reduce the heterogeneity among studies and understand the prognostic value of mir-375, basing on multiple criteria we conducted a subgroup analysis of cancer type, sample type, race. To test the reliability of the main outcomes in our analysis, we conducted a sensitivity analysis of the included studies by removing one single study in turn and estimated whether there was publication bias among the studies using Begg’s test by assessing the asymmetry of an inverted funnel plot.
Results
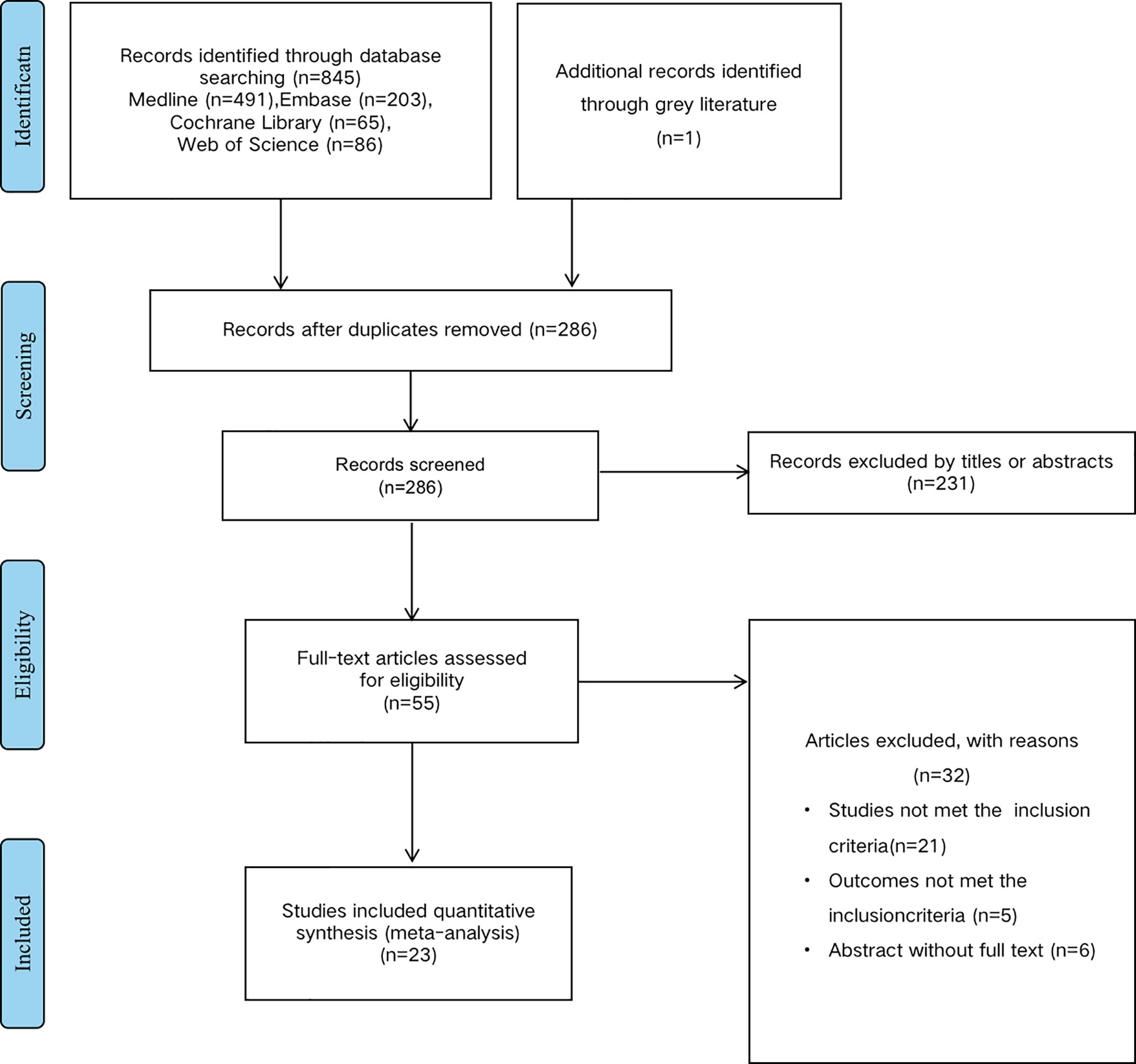
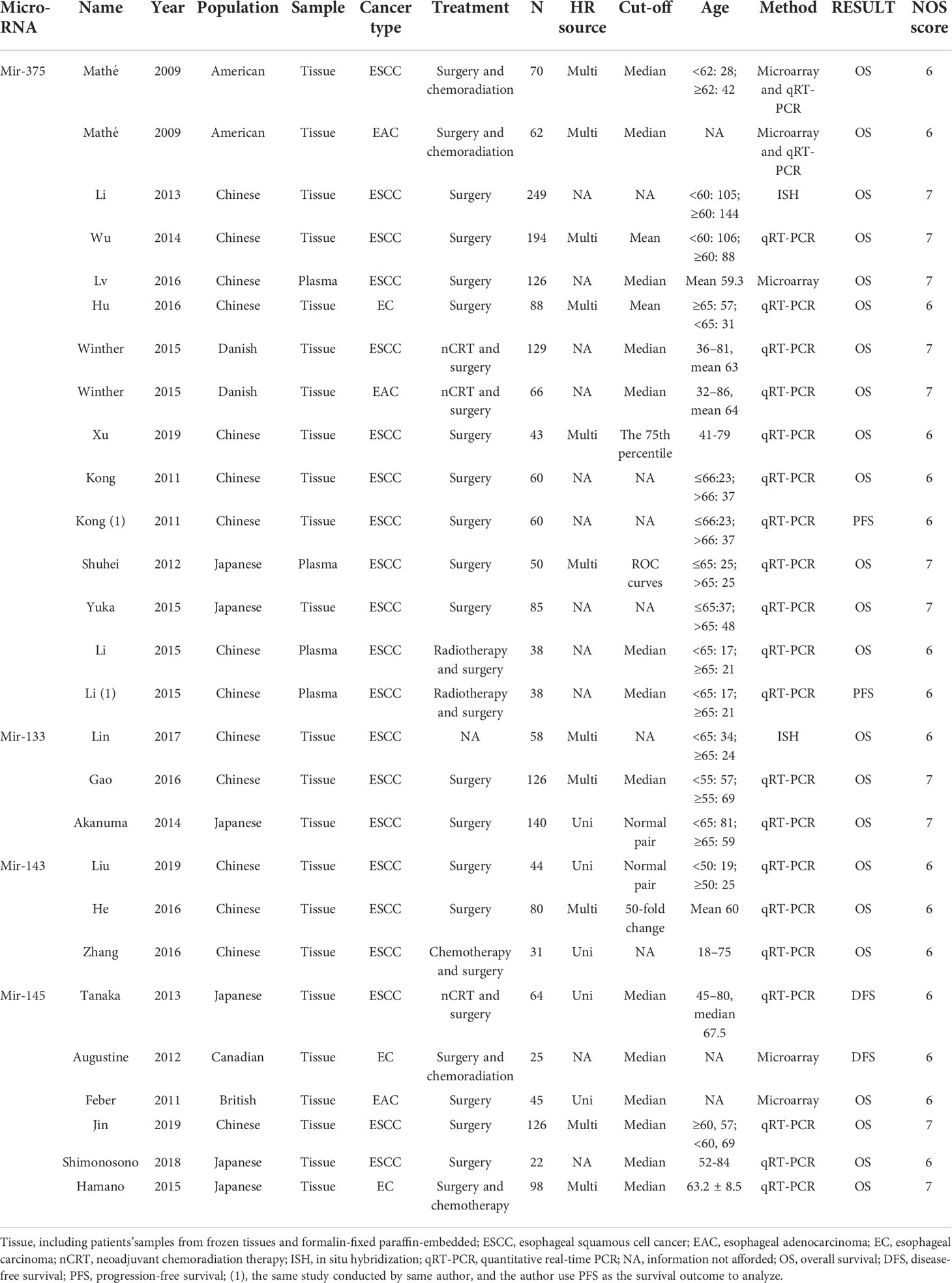
According to the mentioned criteria above, we have identified 25 studies from the preliminary literature search from databases shown in the flow diagram (Figure 1). Exclude duplicate documents 560 articles. After screening the research title and abstract, 231 articles that are not related to the research content are excluded. We read the full text of 55 articles, and further excluded 32 articles because relevant data could not be extracted or couldn’t meet the inclusion criteria. A total of 25 published studies met the inclusion criteria for our meta-analysis which had analyzed the correlation between mir and EC survival outcomes. There are a total of 13 studies reported the prognosis of mir-375 and EC (16, 22–31), 12 articles reported mir-133 (32–34), mir-143 (35–37) and mir-145 (38–43), and all of these studies discussed the prognosis of EC. The total number of patients included in the study was 1260, ranging from 22-249. The category of cancer is ESCC or EAC and quantitative real-time PCR (qRT-PCR), microarray et al, were used to detect the expression of mir in tissue or blood. The cut-off values of mir expression varied in different studies, 20 studies reported the cut-off value of mir, including the mean, median, percentile, and fixed value. For mir-375, 9 studies were conducted in Asian populations, and 4 were conducted in Western. The study populations of mir-143 and mir-133 were only Asian race, as for mir-145, both patients in Asia and Western countries were included. More detailed information is summarized in (Table 1).
To evaluate the OS for mir-375, a random-effects model was applied since there was heterogeneity among (I2 = 61.2%, P=0.002), pooled HR was 0.50 (95%CI: 0.37-0.69, P<0.001), in addition, the high expression of mir-375 showed no correlation with PFS the pooled HR was 0.88 (95%CI:0.37-2.06, P>0.05) (Figure 2), indicating that up-regulated mir-375 may be associated with better OS in EC. As for mir-143 (I2 = 0.0%, P=0.795) and mir-133 (I2 = 0.0%, P=0.823) studies evaluating OS were of no statistical heterogeneity, we use a fixed model to pool the HR. The results showed that up-regulated mir-143 and mir-133 was significantly associated with good OS outcome in ESCC with the pooled HR were 0.40 (95%CI: 0.21-0.76, P<0.001) and 0.40 (95%CI: 0.24-0.65, P<0.001) (Figure 3). On the other hand, to evaluate the prognosis of mir-145, we use fixed model to pool the HR, because there were no statistical heterogeneity found in the OS (I2 = 0.0%, P=0.592) and DFS (I2 = 0.0%, P=0.901) of mir-145, the results showed that high expression of mir-145 was associated with good OS in EC pooled HR was 0.55 (95%CI:0.34-0.90, P<0.001) but no correction was found in DFS because the pooled HR was 1.27 (95%CI: 0.41-3.98, P>0.05) (Figure 4). As shown in the figure, we have performed a sensitivity analysis picture of mir-375 to see if a single study could have significant impact on the pooled HR for survival, the results were not significant altered by removing anyone of the included studies, and Begg’s funnel chart (Figure 5) shows that no significant publication bias has been found (P=0.142). Based on the NOS assessment, the scores of these studies were from 6 to 7, indicating that the quality of the included studies is high, therefor all studies could be used in the subsequent analysis.
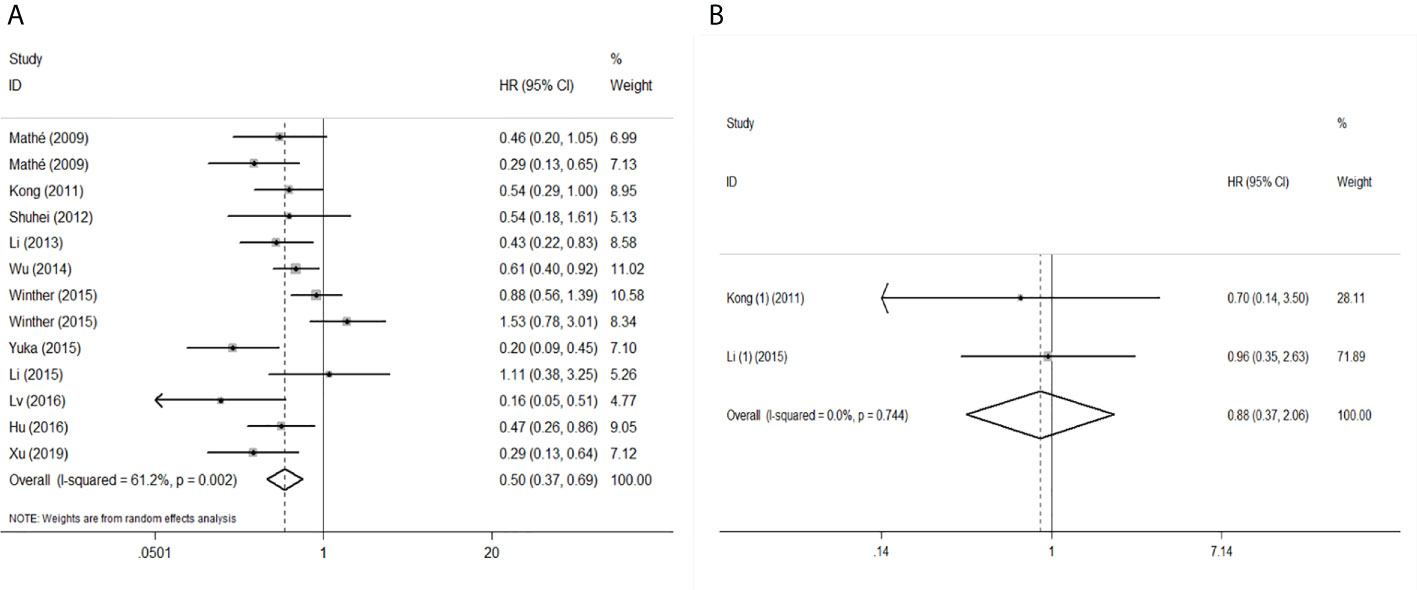
Figure 2 Forest plot of pooled HR of mir-375 in predicting survival outcomes in EC. (A) mir-375 and OS. (B) mir-375 and PFS.
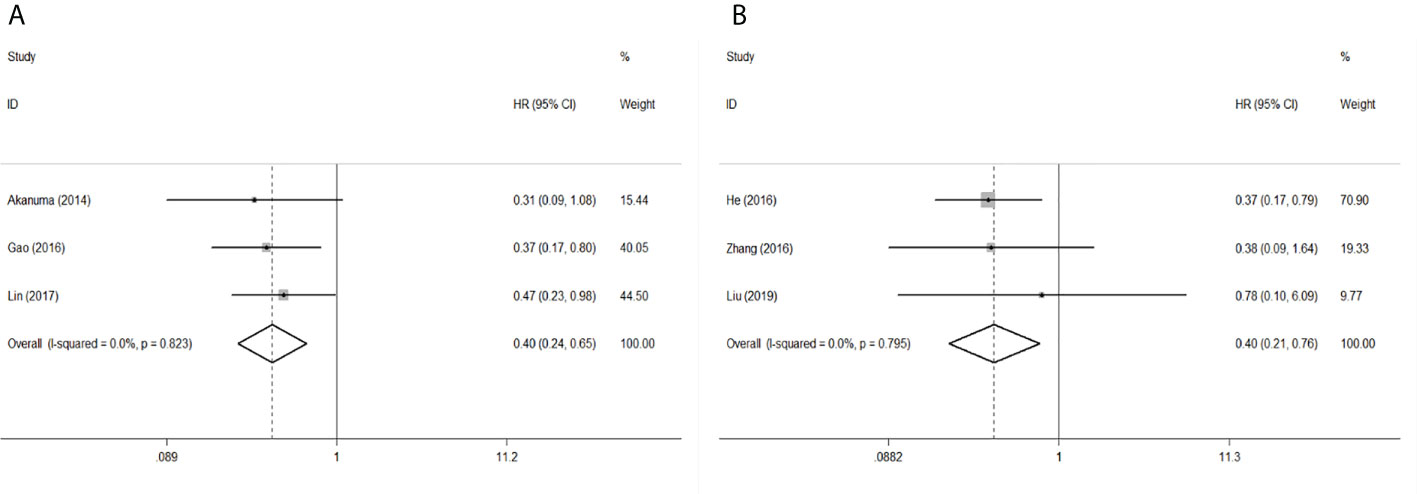
Figure 3 Forest plot of pooled HR of mir-133 and mir-143 in predicting survival outcomes OS in EC. (A) mir-133. (B) mir-143.
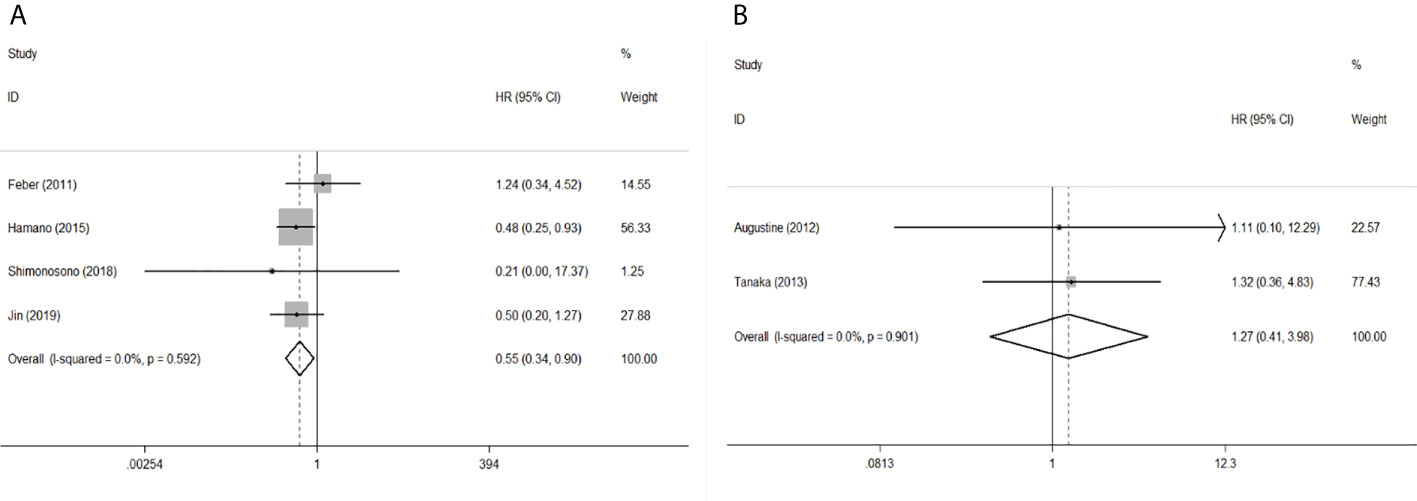
Figure 4 Forest plot of pooled HR of mir-145 in predicting survival outcomes in EC. (A) mir-145 and OS. (B) mir-145 and DFS.
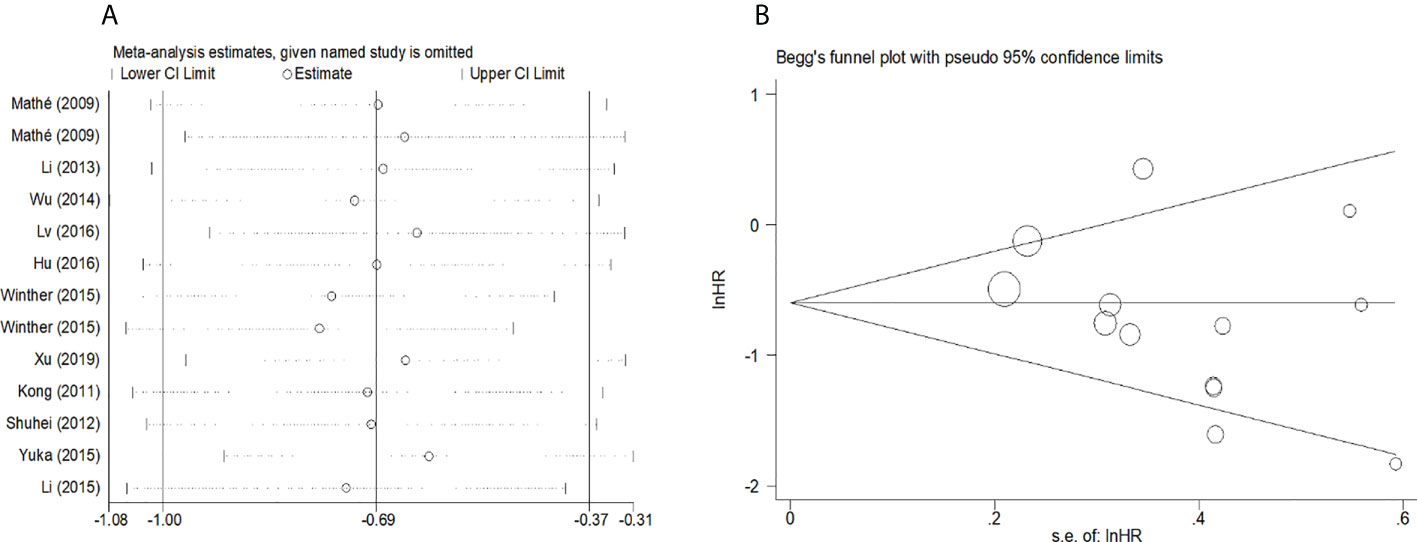
Figure 5 (A) Sensitivity analysis for meta-analysis of mir-375. (B)Funnel plots of publication bias for meta-analysis of mir-375.
Because the heterogeneity among the effect size of mir-375, we conducted a subgroup analysis to find more information. The subgroups were stratified based on following criteria: cancer type, sample type, race. In the subgroup of patient race, we found that up-regulation of mir-375 significantly related to good prognosis in Asian patients (HR: 0.44, 95%CI: 0.32-0.60, P<0.01; random) but not in western patients (HR: 0.68, 95%CI: 0.35-1.31, P>0.05; random) and there was heterogeneity in the data, so we use random-effects model to analysis (Figure 6). In cancer type subgroup, higher mir-375 expression related to good prognosis in ESCC (HR:0.48, 95%CI: 0.34-0.67, P<0.01; random), no correlation was found in patients with EAC (HR: 0.68, 95%CI: 0.13-3.45, P>0.05; random) (Figure 7). The association between higher mir-375 expression and good OS outcomes was statistically significant in sample type from patients’ tissue (HR: 0.51, 95%CI: 0.36-0.71, P<0.01; random) but not from plasma (HR: 0.47, 95%CI: 0.16-1.39, P>0.05; random) (Figure 8). The pooled HR value and their 95%CI in each subgroup are demonstrated in figures above. By conducting such subgroup analysis could we find the impact of higher mir-375 expression on prognosis of EC patients with different clinical characteristics.
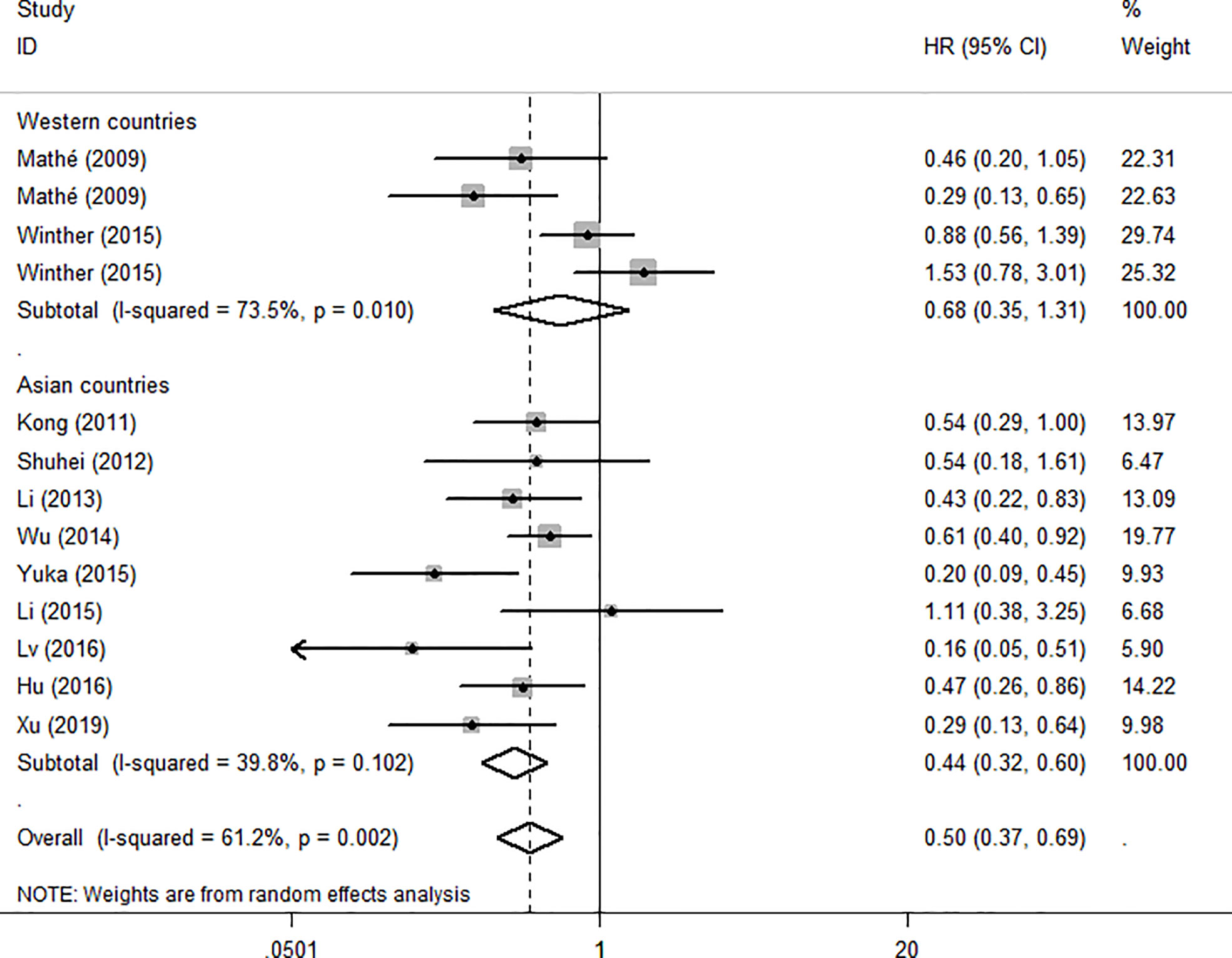
Figure 6 Forest plot showing subgroup analysis of the selected studies about the prognostic significance of mir-375 in patients with different races.
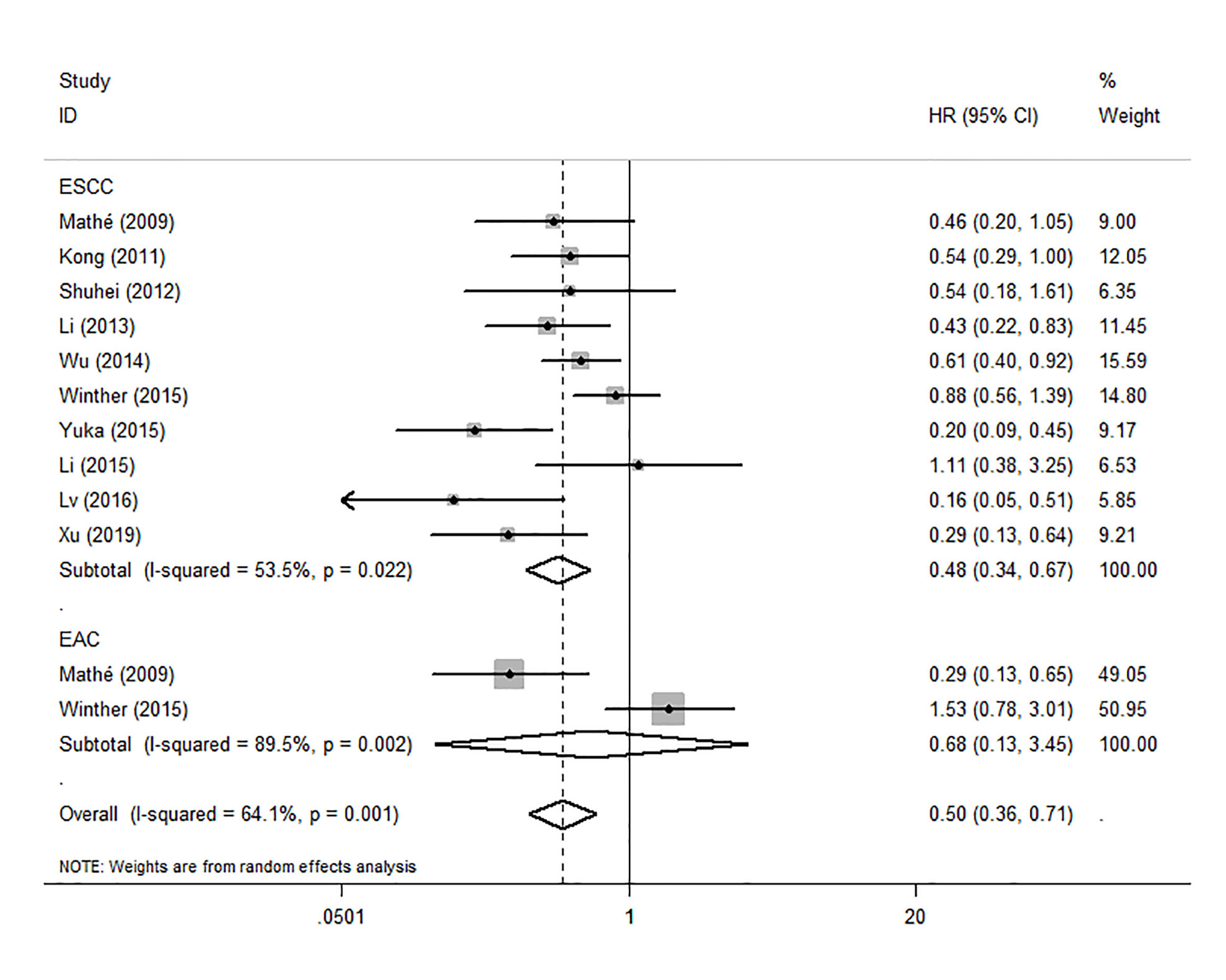
Figure 7 Forest plot showing subgroup analysis of the selected studies about the prognostic significance of mir-375 in patients with different cancer types.
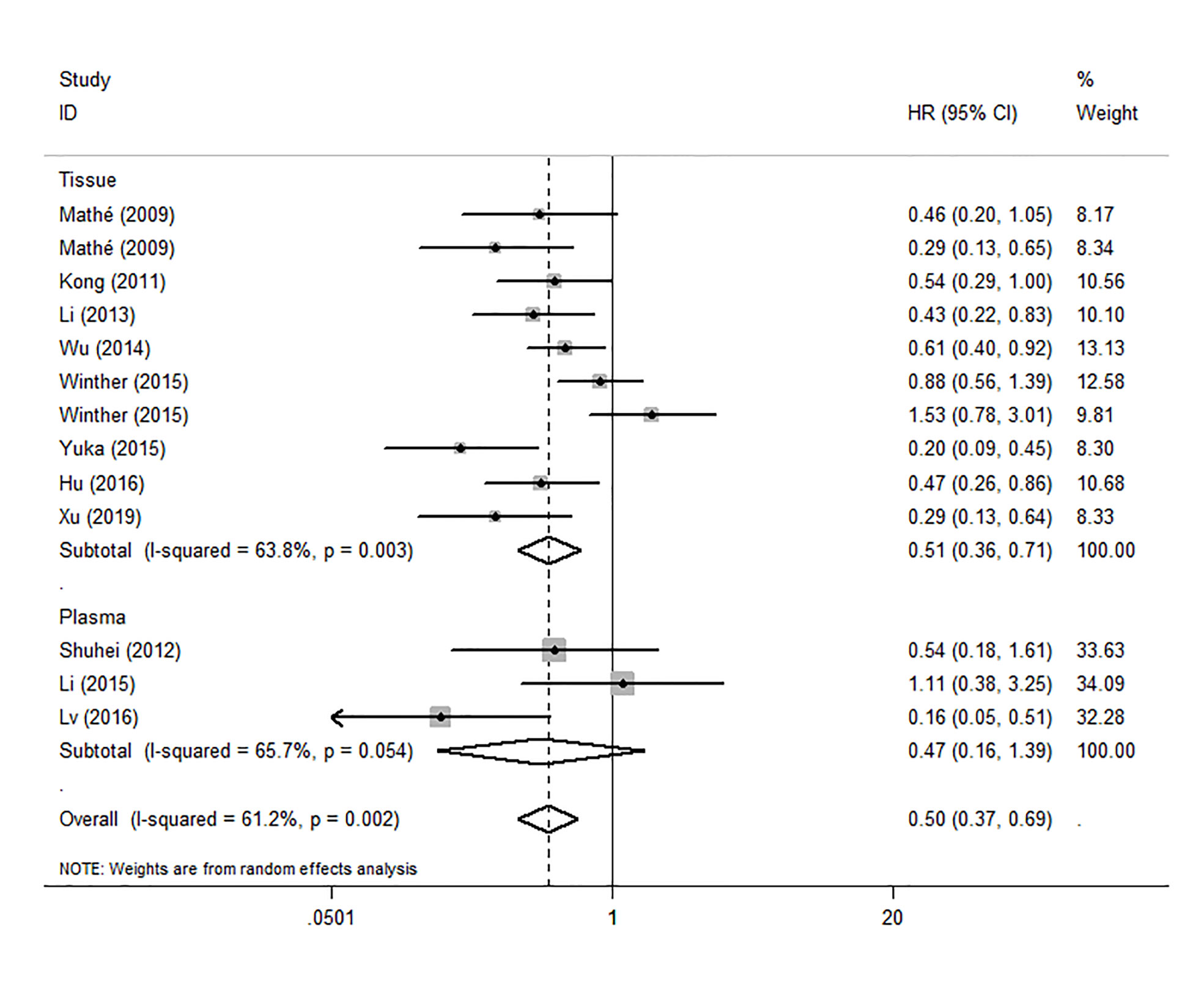
Figure 8 Forest plot showing subgroup analysis of the selected studies about the prognostic significance of mir-375 in patients with different sample types.
Discussion
In recent years, various mirs have been confirmed to exhibit prognostic value in a variety of human cancers, and a great many of mirs have also been proved acting as a co-effector with other elements in tumor progression such as: cellular proliferation, survival, migration, and immune evasion. Thus, the mirs play an important role in the field of malignant tumor (10). The study by Zhao et al (44), showed that mir-375 not only reduced the stemness, but also decreased adriamycin resistance of breast cancer cells, indicating that mir-375 may inhibit the stemness breast cancer cells by targeting JAK2. Xie et al (45), have proved that low expression of mir-375 are significantly correlated with the occurrence and development of liver cancer. Furthermore, Zhang et al (46), found that mir-133 could inhibit the growth of triple-negative breast cancer by targeting YES1. In general, mir-143 and 145 were also clarified that they played a crucial role in suppressing tumor developing in other human cancer (47, 48). Beyond that, Yi et al (49), proved in their study that mir-375 could suppresses ESCC cells invasion and metastasis by direct targeting of SHOX2 and the interaction of mir-375 and SHOX2 could still affect EMT phenotypes. In addition, the down-regulated level of mir-375 was detected in primary ESCC, and was significantly associated with advanced stage and distant metastasis. The results of the study conducted by Osako et al (50), showed that mir-375 markedly inhibits cancer cell migration and invasion by regulation of MMP13 in ESCC, demonstrating that mir-375 may involve in MMP13 axis and affect the occurrence and development of EC.
Mirs played a key role in tumor pathology, that might partly clarify the association between their expression and EC prognosis and made mirs become a hotspot in oncology researching field. Considering the high mortality rates of EC may partly due to the lack of accurate prognostic biomarkers, in recent years, growing number of researchers had investigated the prognostic value of mirs in EC. Gao et al (51), conducted a meta-analysis focusing on mirs affecting EC prognosis. In their study, the number of literatures investigating these mirs was only 12 which was dated up to 2018. In our study, we made an upgradation of data focusing on mir-375,133,143 and 145 (the specific number of included studies is double to Gao’s) published up to November 20, 2021. Importantly, we included 15 studies which investigated the correlation between mir-375 expression and clinical survival outcomes of EC patients. With sufficient studies amount, we could achieve subgroup analysis to comprehensively and systematically investigate these mirs in EC prognosis and to make the conclusion more convincing. Up-regulations of mir-375,133,143 and 145 are associated with favorable prognosis. In addition, according to recent reports, mir was also used as a potential therapeutic targeted molecule in tumor. Many researches had tested the mechanism mirs involving in therapy to anti various human cancers. A study conducted by Campayo showed that up-regulation of mir-375 can increase the sensitivity of rectal cancer cells to chemoradiotherapy (52). On the other hand, Xu et al (53), showed mir-375 could enhance chemosensitivity to 5-fluorouracil in colorectal cancer. Fu et al (54), demonstrated that mir-375 could degrade HOXB3 and inhibit tamoxifen resistance in human breast cancer. Furthermore, Weidle et al (55), showed in their study that mir-133 had a negative impact on regulating cell-cycle and apotosis in gastric carcinoma. Záveský et al (56), illustrated that mir-143 were capable to inhibit the proliferation of ovarian cancer cells by in vitro experiment. Liu et al (57), found that up-regulation of mir-145 could perform as a tumor suppressor inhibiting the growth of non-small-cell lung cancer by targeting RIOK2 and NOB1. Nowadays, molecular-targeted therapy is becoming a vital treatment for malignant tumors and has attracted comprehensive attention worldwide. Therefore, these mirs may also have the potential to be used as targeted molecules for EC treatment in clinic practice.
Although the prognostic value of mir-375 was proved by our study statistically, it should be prudently comprehended because of the following reasons. First, according to the results of subgroups analysis, the pooled HR value showed no significant correlation in EAC which indicated that in different EC types mir-375 may involve in different mechanism which are still unclear to us. Similarly, Mathe et al,[14] also found down-regulation of mir-375 significantly associated with poor EC prognosis only in EAC patients with Barrett’ s esophagus. Considering of such discrepancy, more well-designed clinical researches with EAC samples should be conducted in the future to elucidate the relationship from different cancer types. Second, subgroups analysis also showed that sample types also affected a lot, when sample from plasma, the pooled HR showed no association in mir-375 expression and EC prognosis, contradicting to the sample from tissue which indicated the blood mir-375 may not be used as biomarker to detect the tumorigenesis and monitor tumor recurrence. Currently, mir-375 from tissues were more to be used to study. However, circulating biomarkers were more likely to be applied in clinic because they could be easily got and could be monitored through the whole progression of disease. Therefore, deeper clinical studies should be completed to verify whether there is a correlation between mir-375 expression both in tissue and blood. Third, in different races subgroups, up-regulation of mir-375 wasn’t related to good prognosis in western patients, this may because of the sample sizes from western countries were small and the conclusion might be biased. Fourth, previous studies had investigated the mirs overexpressed in EC patients. Therefore, in this study, we mainly focused on those mirs down-regulated in EC. According to the pooled HRs results, the included four mirs all act as protective factors, and the result might be different from the reality to a certain extent.
Though our meta-analysis proved that mir-375 has an association on the prognosis of patients with EC, there are still some limitations in this study. First, EC is a series of malignant tumor, ESCC and EAC consisted of the most common histological subtypes, but some of studies included in our research did not strictly distinguish the subtypes of EC, leading to a bias in the classification of cancer types. Second, most researchers have used the median or average as the cut-off value in their studies, lacking uniform standards for the cut-off value of mir expression in different studies, the pooled survival outcomes may deviate from the true value. Third, some HR values in this study were calculated by using software Engauge Digitizer to extract data from the survival curve which may bring minor deviations to the outcome. Fourth, there is significant methodological heterogeneity in clinical and baseline demographic characteristics, especially tumor stage, treatment methods which are most likely to affect prognosis in patents. Detailed staging statistics are not provided on patients in the included studies, and many studies have not described the treatment of patients with EC in detail, so subgroup analysis cannot fully reflect the sources of heterogeneity between studies and may lead to bias in patients’ homogeneity. Finally, our meta-analysis only collected perspective mirs evidence from databases but have no specific basic research to verify the exact mechanism mirs involving in EC, so the conclusion was less convincing. Hence, more necessary basic research based on EC patients’ tissues and cells should be completed to obtain accurate results and conclusions.
Conclusion
The results of this meta-analysis show that high expression of mir-375, mir-143, mir-133 and mir-145 is significantly associated with a better prognosis in EC, indicating that these mirs could be used as prognostic factors for EC. Meanwhile, the recurrence of EC whether could be early detected by monitoring these mirs are still unknown. Furthermore, these novel mirs may also be utilized as potential therapeutic targets for EC treatment. For ESCC in Asian population, high expression of mir-375 is a significantly favorable factor, implying a potential therapeutic target for ESCC. Therefore, more high quality clinical researches are needed to investigate the prognostic and therapeutic value of mir-375.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YYu conceptualized the study, revise and proof the manuscript. PF, JZ and XL conceptualized the study and drafted the manuscript. SL, XX, QS, HZ, YYa and XZ collected the literature. All authors contributed to the manuscript revision and read and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81970481)
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.828339/full#supplementary-material
References
1. Enzinger PC, Mayer RJ. Esophageal cancer. New Engl J Med (2003) 349(23):2241–52. doi: 10.1056/NEJMra035010
2. Karamanou M, Markatos K, Papaioannou TG, Zografos G, Androutsos G. Hallmarks in history of esophageal carcinoma. J BUON: Off J Balkan Union Oncol (2017) 22(4):1088–91.
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
4. Malhotra GK, Yanala U, Ravipati A, Follet M, Vijayakumar M, Are C. Global trends in esophageal cancer. J Surg Oncol (2017) 115(5):564–79. doi: 10.1002/jso.24592
5. McCann J. Esophageal cancers: Changing character, increasing incidence. J Natl Cancer Inst (1999) 91(6):497–8. doi: 10.1093/jnci/91.6.497
6. Li X, Chen L, Luan S, Zhou J, Xiao X, Yang Y, et al. The development and progress of nanomedicine for esophageal cancer diagnosis and treatment. Semin Cancer Biol (2022) S1044-579X(22)00013-X. doi: 10.1016/j.semcancer.2022.01.007
7. Orringer MB. Multimodality therapy for esophageal carcinoma–update. Chest (1993) 103(4 Suppl):406s–9s. doi: 10.1378/chest.103.4_supplement.406s
8. Kent OA, Mendell JT. A small piece in the cancer puzzle: Micrornas as tumor suppressors and oncogenes. Oncogene (2006) 25(46):6188–96. doi: 10.1038/sj.onc.1209913
9. Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, et al. Human microrna genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA (2004) 101(9):2999–3004. doi: 10.1073/pnas.0307323101
10. Notarte KI, Senanayake S, Macaranas I, Albano PM, Mundo L, Fennell E, et al. Microrna and other non-coding rnas in Epstein-Barr virus-associated cancers. Cancers (Basel) (2021) 13(15):3909. doi: 10.3390/cancers13153909
11. Bartel DP. Micrornas: Genomics, biogenesis, mechanism, and function. Cell (2004) 116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5
12. Gao W, Zhang C, Li W, Li H, Sang J, Zhao Q, et al. Promoter methylation-regulated mir-145-5p inhibits laryngeal squamous cell carcinoma progression by targeting Fscn1. Mol Therapy: J Am Soc Gene Ther (2019) 27(2):365–79. doi: 10.1016/j.ymthe.2018.09.018
13. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: Microrna biogenesis pathways and their regulation. Nat Cell Biol (2009) 11(3):228–34. doi: 10.1038/ncb0309-228
14. Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microrna expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA (2006) 103(7):2257–61. doi: 10.1073/pnas.0510565103
15. Trang P, Medina PP, Wiggins JF, Ruffino L, Kelnar K, Omotola M, et al. Regression of murine lung tumors by the let-7 microrna. Oncogene (2010) 29(11):1580–7. doi: 10.1038/onc.2009.445
16. Mathé EA, Nguyen GH, Bowman ED, Zhao Y, Budhu A, Schetter AJ, et al. Microrna expression in squamous cell carcinoma and adenocarcinoma of the esophagus: Associations with survival. Clin Cancer Research: An Off J Am Assoc Cancer Res (2009) 15(19):6192–200. doi: 10.1158/1078-0432.Ccr-09-1467
17. Meng XR, Lu P, Mei JZ, Liu GJ, Fan QX. Expression analysis of mirna and target mrnas in esophageal cancer. Braz J Med Biol Res = Rev Bras pesquisas medicas e biolog (2014) 47(9):811–7. doi: 10.1590/1414-431x20143906
18. He XX, Chang Y, Meng FY, Wang MY, Xie QH, Tang F, et al. Microrna-375 targets aeg-1 in hepatocellular carcinoma and suppresses liver cancer cell growth in vitro and in vivo. Oncogene (2012) 31(28):3357–69. doi: 10.1038/onc.2011.500
19. Fu C, Dong W, Wang Z, Li H, Qin Q, Li B. The expression of mir-21 and mir-375 predict prognosis of esophageal cancer. Biochem Biophys Res Commun (2014) 446(4):1197–203. doi: 10.1016/j.bbrc.2014.03.087
20. Wang P, Xu L, Li L, Ren S, Tang J, Zhang M, et al. The microrna-375 as a potentially promising biomarker to predict the prognosis of patients with head and neck or esophageal squamous cell carcinoma: A meta-analysis. Eur Arch oto-rhino-laryngol: Off J Eur Fed Oto-Rhino-Laryngol Societies (EUFOS): affiliated German Soc Oto-Rhino-Laryngol - Head Neck Surgery (2019) 276(4):957–68. doi: 10.1007/s00405-019-05325-8
21. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
22. Li J, Li X, Li Y, Yang H, Wang L, Qin Y, et al. Cell-specific detection of mir-375 downregulation for predicting the prognosis of esophageal squamous cell carcinoma by mirna in situ hybridization. PLoS One (2013) 8(1):e53582. doi: 10.1371/journal.pone.0053582
23. Wu C, Li M, Hu C, Duan H. Clinical significance of serum mir-223, mir-25 and mir-375 in patients with esophageal squamous cell carcinoma. Mol Biol Rep (2014) 41(3):1257–66. doi: 10.1007/s11033-013-2970-z
24. Lv H, He Z, Wang H, Du T, Pang Z. Differential expression of mir-21 and mir-75 in esophageal carcinoma patients and its clinical implication. Am J Trans Res (2016) 8(7):3288–98.
25. He Y, Jin J, Wang L, Hu Y, Liang D, Yang H, et al. Evaluation of mir-21 and mir-375 as prognostic biomarkers in oesophageal cancer in high-risk areas in China. Clin Exp Metastasis (2017) 34(1):73–84. doi: 10.1007/s10585-016-9828-4
26. Winther M, Alsner J, Tramm T, Baeksgaard L, Holtved E, Nordsmark M. Evaluation of mir-21 and mir-375 as prognostic biomarkers in esophageal cancer. Acta Oncol (Stockholm Sweden) (2015) 54(9):1582–91. doi: 10.3109/0284186x.2015.1064161
27. Xu H, Jiang J, Zhang J, Cheng L, Pan S, Li Y. Microrna-375 inhibits esophageal squamous cell carcinoma proliferation through direct targeting of Sp1. Exp Ther Med (2019) 17(3):1509–16. doi: 10.3892/etm.2018.7106
28. Kong KL, Kwong DL, Chan TH, Law SY, Chen L, Li Y, et al. Microrna-375 inhibits tumour growth and metastasis in oesophageal squamous cell carcinoma through repressing insulin-like growth factor 1 receptor. Gut (2012) 61(1):33–42. doi: 10.1136/gutjnl-2011-300178
29. Komatsu S, Ichikawa D, Takeshita H, Konishi H, Nagata H, Hirajima S, et al. Prognostic impact of circulating mir-21 and mir-375 in plasma of patients with esophageal squamous cell carcinoma. Expert Opin Biol Ther (2012) 12 Suppl 1:S53–9. doi: 10.1517/14712598.2012.681373
30. Isozaki Y, Hoshino I, Akutsu Y, Hanari N, Mori M, Nishimori T, et al. Usefulness of Microrna−375 as a prognostic and therapeutic tool in esophageal squamous cell carcinoma. Int J Oncol (2015) 46(3):1059–66. doi: 10.3892/ijo.2014.2789
31. Li BX, Yu Q, Shi ZL, Li P, Fu S. Circulating micrornas in esophageal squamous cell carcinoma: Association with locoregional staging and survival. Int J Clin Exp Med (2015) 8(5):7241–50.
32. Lin C, Zhang S, Wang Y, Wang Y, Nice E, Guo C, et al. Functional role of a novel long noncoding rna ttn-As1 in esophageal squamous cell carcinoma progression and metastasis. Clin Cancer research: an Off J Am Assoc Cancer Res (2018) 24(2):486–98. doi: 10.1158/1078-0432.Ccr-17-1851
33. Gao SH, Liu J, Zhang HJ, Zhao N, Zhang J. Low mir-133a expression is a predictor of outcome in patients with esophageal squamous cell cancer. Eur Rev Med Pharmacol Sci (2016) 20(18):3788–92.
34. Akanuma N, Hoshino I, Akutsu Y, Murakami K, Isozaki Y, Maruyama T, et al. Microrna-133a regulates the mrnas of two invadopodia-related proteins, Fscn1 and Mmp14, in esophageal cancer. Br J cancer (2014) 110(1):189–98. doi: 10.1038/bjc.2013.676
35. Liu H, Zheng M, Zhao Y, Zhang S. Mir-143 inhibits migration and invasion through regulating Lasp1 in human esophageal cancer. Int J Clin Exp pathology (2019) 12(2):466–76.
36. He Z, Yi J, Liu X, Chen J, Han S, Jin L, et al. Mir-143-3p functions as a tumor suppressor by regulating cell proliferation, invasion and epithelial-mesenchymal transition by targeting qki-5 in esophageal squamous cell carcinoma. Mol cancer (2016) 15(1):51. doi: 10.1186/s12943-016-0533-3
37. Zhang B, Li R, Chang CX, Han Y, Shi SB, Tian J. Pemetrexed plus dendritic cells as third-line therapy for metastatic esophageal squamous cell carcinoma. OncoTar Ther (2016) 9:3901–6. doi: 10.2147/ott.S107319
38. Tanaka K, Miyata H, Yamasaki M, Sugimura K, Takahashi T, Kurokawa Y, et al. Circulating mir-200c levels significantly predict response to chemotherapy and prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. Ann Surg Oncol (2013) 20 Suppl 3:S607–15. doi: 10.1245/s10434-013-3093-4
39. Ko MA, Zehong G, Virtanen C, Guindi M, Waddell TK, Keshavjee S, et al. Microrna expression profiling of esophageal cancer before and after induction chemoradiotherapy. Ann Thorac surg (2012) 94(4):1094–102. doi: 10.1016/j.athoracsur.2012.04.145
40. Feber A, Xi L, Pennathur A, Gooding WE, Bandla S, Wu M, et al. Microrna prognostic signature for nodal metastases and survival in esophageal adenocarcinoma. Ann Thorac Surg (2011) 91(5):1523–30. doi: 10.1016/j.athoracsur.2011.01.056
41. Jin W, Luo W, Fang W, Wang Y, Wang L, Shen Q, et al. Mir-145 expression level in tissue predicts prognosis of patients with esophageal squamous cell carcinoma. Pathol Res Practice (2019) 215(6):152401. doi: 10.1016/j.prp.2019.03.029
42. Shimonosono M, Idichi T, Seki N, Yamada Y, Arai T, Arigami T, et al. Molecular pathogenesis of esophageal squamous cell carcinoma: Identification of the antitumor effects of Mir−145−3p on gene regulation. Int J Oncol (2019) 54(2):673–88. doi: 10.3892/ijo.2018.4657
43. Hamano R, Miyata H, Yamasaki M, Kurokawa Y, Hara J, Moon JH, et al. Overexpression of mir-200c induces chemoresistance in esophageal cancers mediated through activation of the akt signaling pathway. Clin Cancer Research: An Off J Am Assoc Cancer Res (2011) 17(9):3029–38. doi: 10.1158/1078-0432.Ccr-10-2532
44. Zhao Q, Liu Y, Wang T, Yang Y, Ni H, Liu H, et al. Mir-375 inhibits the stemness of breast cancer cells by blocking the Jak2/Stat3 signaling. Eur J Pharmacol (2020) 884:173359. doi: 10.1016/j.ejphar.2020.173359
45. Xie D, Yuan P, Wang D, Jin H, Chen H. Expression and prognostic significance of mir-375 and mir-221 in liver cancer. Oncol Letters (2017) 14(2):2305–9. doi: 10.3892/ol.2017.6423
46. Zhang G, Wang J, Zheng R, Song B, Huang L, Liu Y, et al. Mir-133 targets Yes1 and inhibits the growth of triple-negative breast cancer cells. Technol Cancer Res Treat (2020) 19:1533033820927011. doi: 10.1177/1533033820927011
47. Chen Q, Zhou L, Ye X, Tao M, Wu J. Mir-145-5p suppresses proliferation, metastasis and emt of colorectal cancer by targeting Cdca3. Pathol Res practice (2020) 216(4):152872. doi: 10.1016/j.prp.2020.152872
48. Boubaker NS, Spagnuolo M, Trabelsi N, Said R, Gurtner A, Regazzo G, et al. Mir-143 expression profiles in urinary bladder cancer: Correlation with clinical and epidemiological parameters. Mol Biol Rep (2020) 47(2):1283–92. doi: 10.1007/s11033-019-05228-1
49. Yi J, Jin L, Chen J, Feng B, He Z, Chen L, et al. Mir-375 suppresses invasion and metastasis by direct targeting of Shox2 in esophageal squamous cell carcinoma. Acta Biochim Biophys Sinica (2017) 49(2):159–69. doi: 10.1093/abbs/gmw131
50. Osako Y, Seki N, Kita Y, Yonemori K, Koshizuka K, Kurozumi A, et al. Regulation of Mmp13 by antitumor microrna-375 markedly inhibits cancer cell migration and invasion in esophageal squamous cell carcinoma. Int J Oncol (2016) 49(6):2255–64. doi: 10.3892/ijo.2016.3745
51. Gao S, Zhao ZY, Zhang ZY, Zhang Y, Wu R. Prognostic value of micrornas in esophageal carcinoma: A meta-analysis. Clin Trans Gastroenterol (2018) 9(11):203. doi: 10.1038/s41424-018-0070-z
52. Campayo M, Navarro A, Benítez JC, Santasusagna S, Ferrer C, Monzó M, et al. Mir-21, mir-99b and mir-375 combination as predictive response signature for preoperative chemoradiotherapy in rectal cancer. PLoS One (2018) 13(11):e0206542. doi: 10.1371/journal.pone.0206542
53. Xu F, Ye ML, Zhang YP, Li WJ, Li MT, Wang HZ, et al. Microrna-375-3p enhances chemosensitivity to 5-fluorouracil by targeting thymidylate synthase in colorectal cancer. Cancer Science (2020) 111(5):1528–41. doi: 10.1111/cas.14356
54. Fu H, Fu L, Xie C, Zuo WS, Liu YS, Zheng MZ, et al. Mir-375 inhibits cancer stem cell phenotype and tamoxifen resistance by degrading Hoxb3 in human er-positive breast cancer. Oncol Rep (2017) 37(2):1093–9. doi: 10.3892/or.2017.5360
55. Weidle UH, Birzele F, Auslaender S, Brinkmann U. Down-regulated micrornas in gastric carcinoma may be targets for therapeutic intervention and replacement therapy. Anticancer Res (2021) 41(9):4185–202. doi: 10.21873/anticanres.15223
56. Záveský L, Jandáková E, Weinberger V, Hanzíková V, Slanař O, Kohoutová M. Ascites in ovarian cancer: Microrna deregulations and their potential roles in ovarian carcinogenesis. Cancer Biomarkers: Section A Dis Markers (2022) 33(1):1–16. doi: 10.3233/cbm-210219
Keywords: micro-RNA, esophageal carcinoma, prognosis, meta-analysis, biomaker
Citation: Fang P, Zhou J, Li X, Luan S, Xiao X, Shang Q, Zhang H, Yang Y, Zeng X and Yuan Y (2022) Prognostic value of micro-RNA 375, 133, 143, 145 in esophageal carcinoma: A systematic review and meta-analysis. Front. Oncol. 12:828339. doi: 10.3389/fonc.2022.828339
Received: 03 December 2021; Accepted: 24 August 2022;
Published: 13 September 2022.
Edited by:
Kanjoormana Aryan Manu, Amala Cancer Research Centre, IndiaReviewed by:
Amit Gupta, All India Institute of Medical Sciences, IndiaKin Israel Notarte, Johns Hopkins Medicinee, United States
Priyanka Sharma, University of Texas MD Anderson Cancer Center, United States
Copyright © 2022 Fang, Zhou, Li, Luan, Xiao, Shang, Zhang, Yang, Zeng and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Yuan, yongyuan@scu.edu.cn
†These authors have contributed equally to this work
 Pinhao Fang
Pinhao Fang Jianfeng Zhou
Jianfeng Zhou Xiaokun Li1†
Xiaokun Li1† Siyuan Luan
Siyuan Luan Xin Xiao
Xin Xiao Hanlu Zhang
Hanlu Zhang Yong Yuan
Yong Yuan