Erratum: Case report: NUT carcinoma with MXI1::NUTM1 fusion characterized by abdominopelvic lesions and ovarian masses in a middle-aged female
- 1Department of Obstetrics and Gynecology, Peking University Third Hospital, Beijing, China
- 2National Clinical Research Center for Obstetrics and Gynecology, Peking University Third Hospital, Beijing, China
- 3Department of Pathology, School of Basic Medical Sciences, Third Hospital, Peking University Health Science Center, Beijing, China
- 4Beijing Advanced Innovation Center for Genomics, Peking University, Beijing, China
- 5Peking-Tsinghua Center for Life Sciences, Peking University, Beijing, China
Background: Nuclear protein of the testis (NUT) carcinoma is a rare subset of poorly differentiated, highly aggressive malignancy defined by NUTM1 gene rearrangements. Only three NUT cases of probable ovarian origin have been reported.
Case presentation: We report a case of NUT carcinoma in a 53-year-old female who presented with extensive abdominopelvic lesions and bilateral ovarian masses suggestive of advanced ovarian cancer. This patient was admitted to our hospital due to abdominal pain and distension for over two months. Imaging examinations suggested a possible malignancy of bilateral adnexal origin. This patient first underwent diagnostic laparoscopy. After receiving neoadjuvant chemotherapy, she underwent cytoreductive surgery. Surgical pathology showed infiltration of monotonous round tumor cells with no apparent differentiation characteristics. Immunohistochemistry (IHC) revealed nuclear expression of the NUT protein. And MXI1::NUTM1 fusion was identified by next-generation sequencing (NGS). Herein, we introduce an unusual NUT carcinoma and describe the clinical, imaging, and pathological features. In addition, we briefly reviewed the published literature and discussed the possibility of primary gynecological NUT carcinoma.
Conclusions: Identifying a NUT carcinoma arising from the abdominopelvic cavity is essential, and we underscore the need for NUT testing in undifferentiated malignant neoplasms that appear in this clinical setting. Although it is unclear from which origin this tumor arose, proper classification is essential for treatment planning.
Introduction
NUT carcinoma is a rare, aggressive, and relatively recently characterized subtype of poorly differentiated squamous cancer with remarkably unfavorable clinical outcomes (1). It was first described as thymic carcinoma in a young adult with the novel translocation t (15, 19) (q15; p13) in 1991 in Japan (2). NUT carcinoma most commonly harbors the BRD4-NUTM1 fusion oncoprotein. Other NUTM1-fusion partners include BRD3, NSD3, ZNF532, and ZNF592 (3). NUT carcinoma is an orphan disease with unknown origin. It can occur anywhere along the midline, with the typical sites being the upper aerodigestive tract (head, neck, thorax, and mediastinum), but also outside the midline (lung, salivary glands, pancreas, bladder, kidney, adrenal glands, ovary, and bone tissues.) (1, 4, 5). It most frequently occurs in adolescents and young adults but can occur at any age (0-81.7 years), with a median age varying from 16 to 24 years (6). The prognosis of NUT carcinoma is devastating; most patients progress rapidly and have hematologic or lymphatic metastases at diagnosis, with a median survival time of 6.7 months (7).
Although rare, the true incidence of NUT carcinoma is unknown because it is frequently misdiagnosed with other common malignancies, including poorly differentiated squamous cell carcinoma, sinonasal undifferentiated carcinoma, nasopharyngeal carcinoma, Ewing sarcoma, leukemia, thymic carcinoma, neuroblastoma and pancreatoblastoma, and even primary salivary gland carcinoma (1). It can be diagnosed with virtually 100% specificity and 87% sensitivity by positive (>50% of tumor nuclei) immunohistochemical (IHC) staining with the anti-NUT antibody, C52B1, which is normally restricted to expression in the testis (www.nmcregistry.org) (4, 6, 8). Characterization of the fusion gene by molecular analysis based on fluorescence in situ hybridization (FISH), reverse-transcriptase polymerase chain reaction (RT-PCR), cytogenetics, next-generation sequencing (NGS), or whole-exome sequencing (WES) can be an alternative to NUT IHC staining (6, 9).
In this study, we report a rare NUT carcinoma presenting with extensive abdominopelvic lesions and bilateral ovarian masses. This study was reported in agreement with the principles of the CARE guidelines (10).
Case report
A 53-year-old Chinese female presented to the Emergency Department of our hospital for persistent abdominal pain and distension for over two months. Two months ago, the patient went to a local hospital and obtained a transvaginal ultrasound that revealed thickened bilateral fallopian, suggesting adnexitis. She was treated with antibiotics, but the pain persisted. The serum CA125 and CA724 levels were elevated (469U/ml and 33.4U/ml, respectively). Abdomen and pelvis computed tomography (CT) showed heterogeneous stomach and bilateral adnexal masses, suggesting adnexal lesions or the Krukenberg tumor. She was then referred to the emergency room of our hospital.
In our hospital, she received thorough examinations. A gynecological examination revealed an abdominopelvic mass with an unclear boundary. The transvaginal ultrasound showed a 3.9×2.3cm solid pelvic mass with ascites (Figure 1A; Supplemental Figures S1A, B). The abdominal and pelvic enhanced CT, the pelvic magnetic resonance imaging (MRI), and the positron emission computed tomography (PET-CT) all revealed irregular, heterogeneous, and plump bilateral adnexal masses with diffusely thickened peritoneum, omentum, and mesangium, multiple soft-tissue nodules, slightly thickened intestinal wall, abdominal and pelvic effusion, and multiple enlarged lymph nodes (Figures 1B–E; Supplemental Figures S1C–F). The serum CA125 level was 439U/mL. The chest X-ray showed no abnormality. The patient underwent bilateral tubal sterilization more than 20 years ago and was found with a Helicobacter pylori infection two months ago. She went into natural menopause for one year and gave birth to two children. Personal and family history were unremarkable.
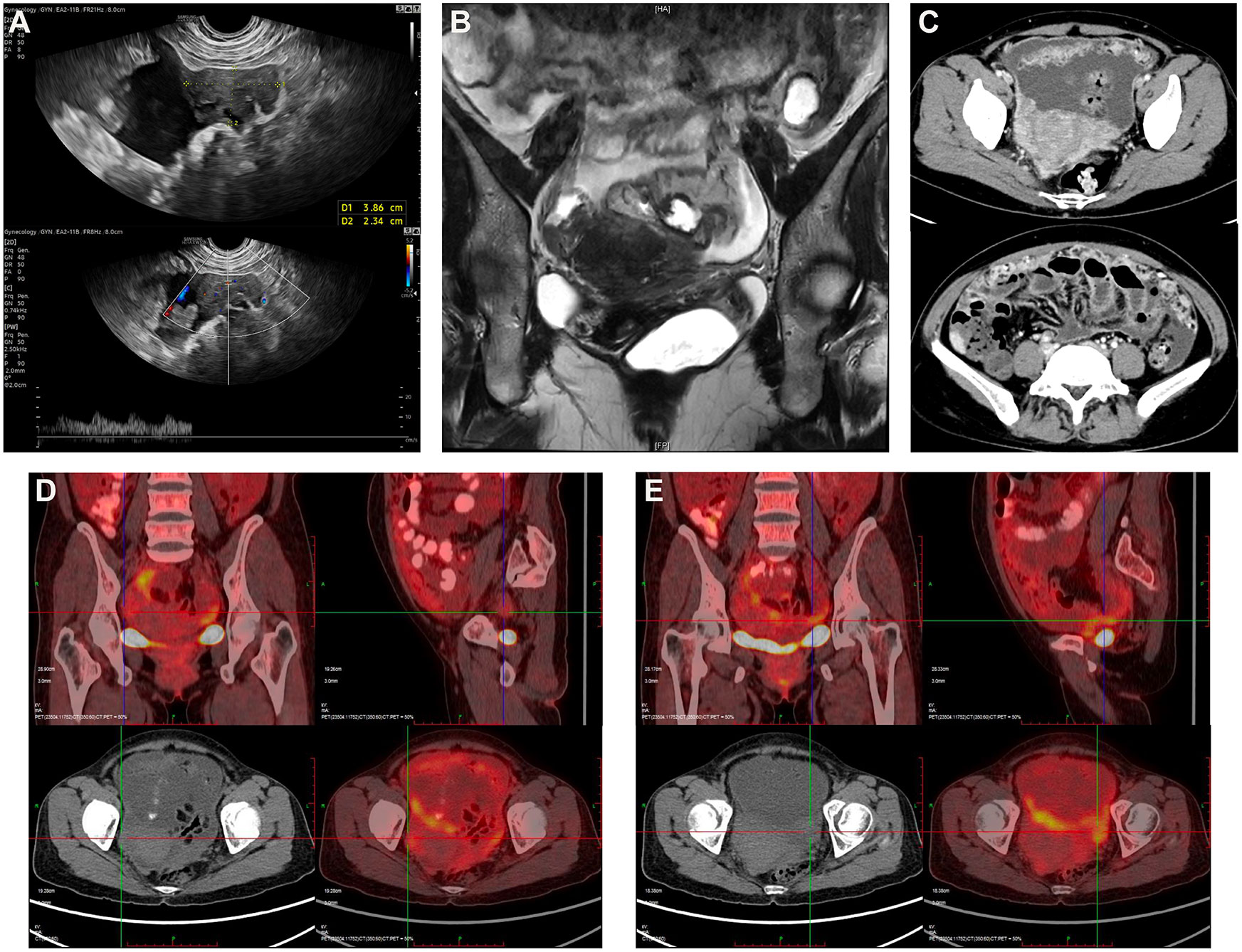
Figure 1 (A) Transvaginal ultrasound reveals a 3.9×2.3cm irregular hypoechoic mass in the right abdominal cavity with blood flow signals closely related to the peritoneum. (B) Pelvic MRI shows a suspicious mass in the right adnexal area and thickening of the omentum with pelvic effusion. (C) Abdominal and pelvic enhanced CT reveals plump bilateral adnexal masses, diffusely thickened peritoneum, omentum, and mesangium, slightly thickened intestinal wall, and abdominal and pelvic effusion. PET-CT displays plump bilateral adnexal areas, poorly demarcated from surrounding tissue, showing signs of hypermetabolism with (D) the SUVmax2.2 on the right and (E) the SUVmax3.1 on the left.
Treatment
Considering the possibility of ovarian or gastrointestinal malignancies, the patient was admitted to our hospital. After admission, the patient developed aggravated abdominal distension with an increase in body temperature to 39℃. After four days of intravenous anti-inflammatory, the patient underwent diagnostic laparoscopy with omentum and peritoneum biopsies in a stable condition, which suggested poorly differentiated carcinoma according to intraoperative consultation. About 2 liters of tawny ascites were drained for cytology confirmation during the operation, which revealed no tumor cells. The surgical findings showed multiple white granular tumor implants studding the omentum, peritoneum, and the surface of the diaphragm, liver, intestine, and uterus. A 9×9×3cm omental cake enclosed part of the intestine and was fixed. The omentum and intestine obscured bilateral ovaries and fallopian tubes. Combined with laboratory, imaging, and surgical findings, the possibility of advanced (stage IV) ovarian cancer was considered. Given the difficulty in performing satisfactory cytoreductive surgery, the patient was treated with three cycles of neoadjuvant chemotherapy with Paclitaxel-albumin, Carboplatin, and Bevacizumab.
After chemotherapy, the patient’s serum CA125 level was reduced to 107 U/mL. Imaging examination showed reduced abdominal and pelvic effusion, while abdominopelvic lesions were roughly the same as before (Supplemental Figure S2). The pathological findings of the previous operation excluded the common types of epithelial ovarian carcinoma, breast carcinoma, and neuroendocrine neoplasm. Still, the diagnosis could not be confirmed due to the poor differentiation characteristics of the tumor cells. Upper GI endoscopy and colonoscopy were performed to distinguish gastrointestinal tumors, but no mucosal lesions were identified (Supplemental Figure S3). The patient further underwent cytoreductive surgery, which showed a 20×10×4cm extensive gritty nodular omental cake densely adhered to the pelvic wall and part of the intestine and mesentery (Supplemental Figure S4A). The mesostenium and mesocolon were extensively thickened with contracture and stiff morphology. A heterogeneous mass about 2.5cm in diameter was found on the surface of the small intestine. The posterior wall of the uterus closely adhered to the rectum, and bilateral ovaries were enlarged with a solid nodular appearance (Supplemental Figure S4B). Total hysterectomy and bilateral salpingo-oophorectomy were performed. The omentum, small intestinal mass, and left pelvic lymph nodes were also removed. All specimen was submitted for pathological confirmation. It was an unsatisfactory cytoreductive surgery (R2), with the postoperative residuals being the diffuse thickened malignancy lesions in the mesentery. The patient recovered in a stable condition without any complications and was discharged home 13 days after surgery.
Pathological findings
Microscopic examination showed malignancy infiltration in bilateral ovaries involving the omentum, peritoneum, mass on the small intestine surface, and the serosa of the uterus and bilateral fallopian tubes. The tumor cells were uniform monotonous medium-sized round and oval with small-to-moderate eosinophilic cytoplasm, enlarged nuclei, high nuclear/cytoplasmic ratio, and uneven chromatin (Figure 2A). Lymphatic vascular involvement and lymph node metastases were frequently observed (Figure 2B). Solid sheets and nests of typically undifferentiated cells infiltrating surrounding normal omental tissue were present, with no abrupt keratinization (Figure 2C). Comparing the two surgical specimens, there was no significant regression of tumor cells after neoadjuvant chemotherapy (Figures 2C, D). Malignancy infiltration was found on the serosa of the uterus, while typical structures of the endometrium and myometrium remained, suggesting that it was not a primary uterine neoplasm (Figure 2E). It is noteworthy that although the malignancies significantly infiltrated the ovary, most of the ovarian cortical and corpus albicans were intact. The lesions were mainly close to the ovarian hilus and surrounded by large blood vessels (Figure 2F), so the possibility of secondary ovarian malignancy cannot be excluded.
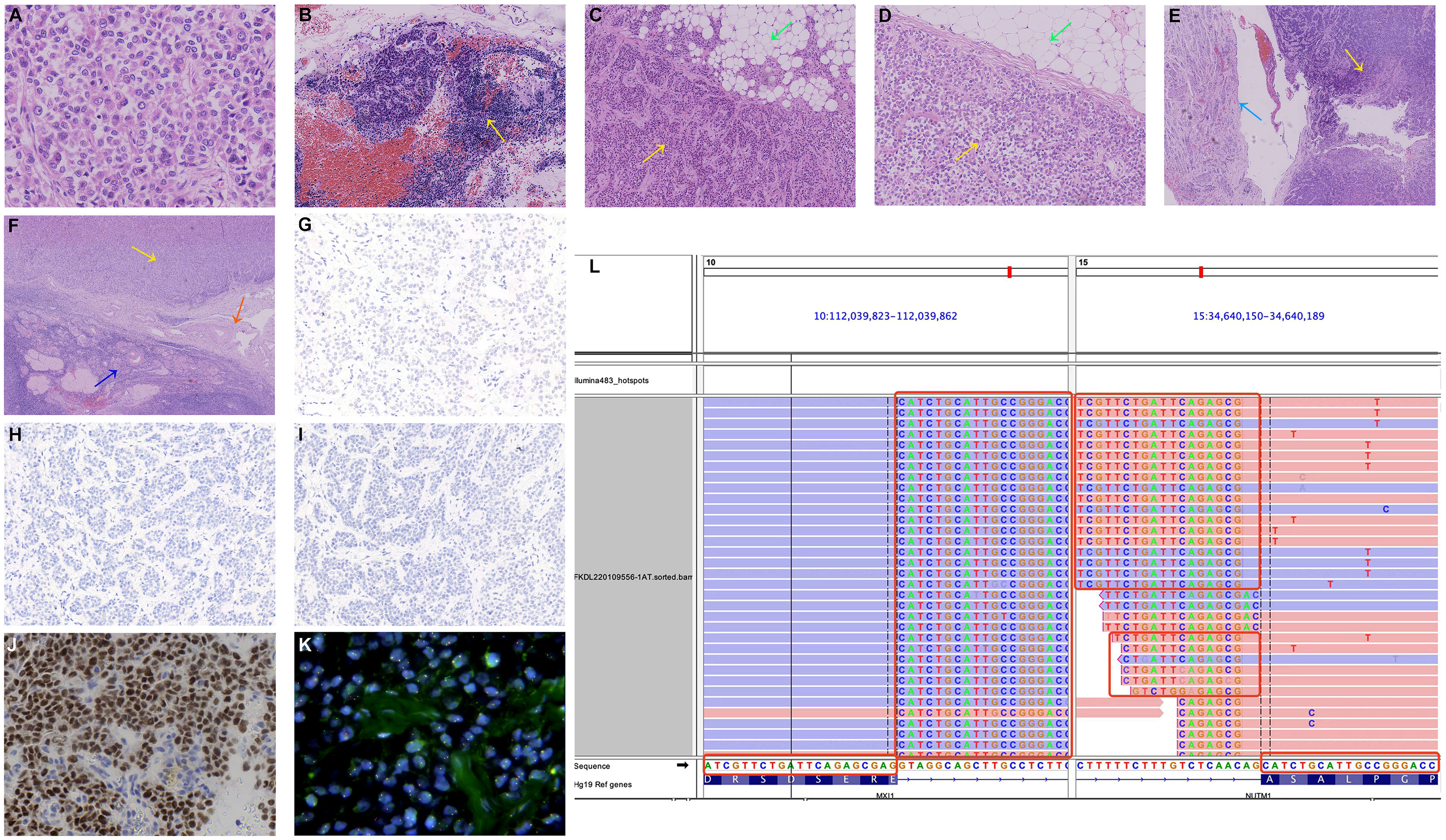
Figure 2 Microscopic findings. (A) Uniform monotonous medium-sized round and oval tumor cells with moderate eosinophilic cytoplasm, enlarged nuclei, high nuclear/cytoplasmic ratio, and uneven chromatin (hematoxylin and eosin (HE)). (B) Tumor cells infiltrate the pelvic lymph nodes (HE, yellow arrow). (C) A section of the omental biopsy specimen with the omental tissue at the upper right (HE, green arrow) and the tumor cells at the lower left (HE, yellow arrow). (D) A section of the omental specimen from cytoreductive surgery with no significant regression of tumor cells after chemotherapy (HE, omental tissue: green arrow, tumor cells: yellow arrow). (E) Tumor cells (HE, yellow arrow) infiltrate the serosa of the uterus (HE, azure arrow). (F) The tumor mass is close to the ovarian hilus (HE, yellow arrow), surrounded by large blood vessels (HE, orange arrow), with most of the typical stricture of ovarian cortical and corpus albicans preserved (HE, sapphire arrow). (G) Negative CK pan IHC staining. (H) Negative p40 IHC staining. (I) Negative p63 IHC staining. (J) Strong and diffuse nuclear reactivity for NUT in the tumor cells (NUT IHC staining). (K) Negative NUT FISH test. No significant red-green signal separation was observed. (L) NGS identified an MXI1::NUTM1 fusion consisting of MXI1 exon 5 and NUTM1 exon 3 obtained for the tumor specimens of the patient.
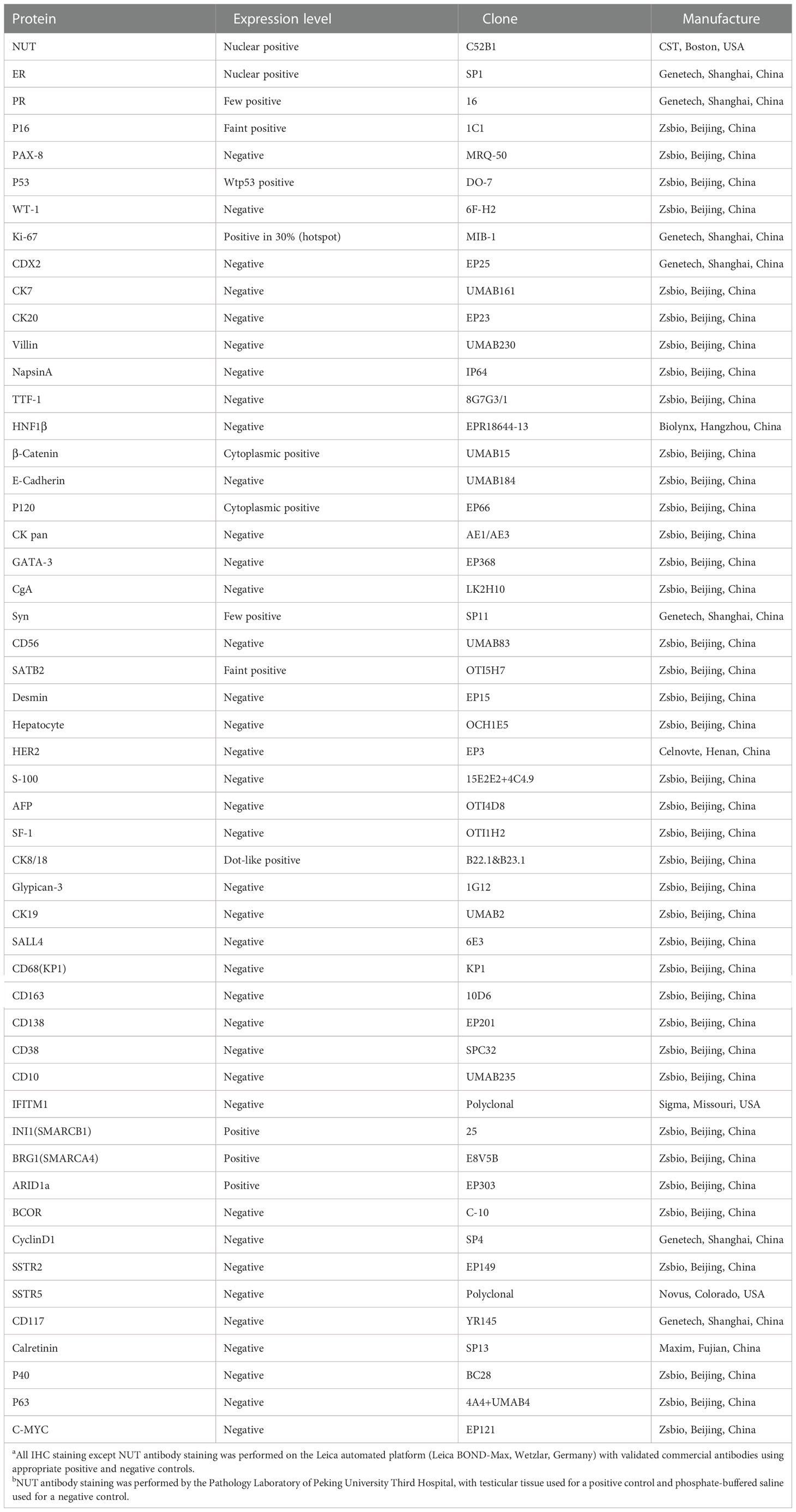
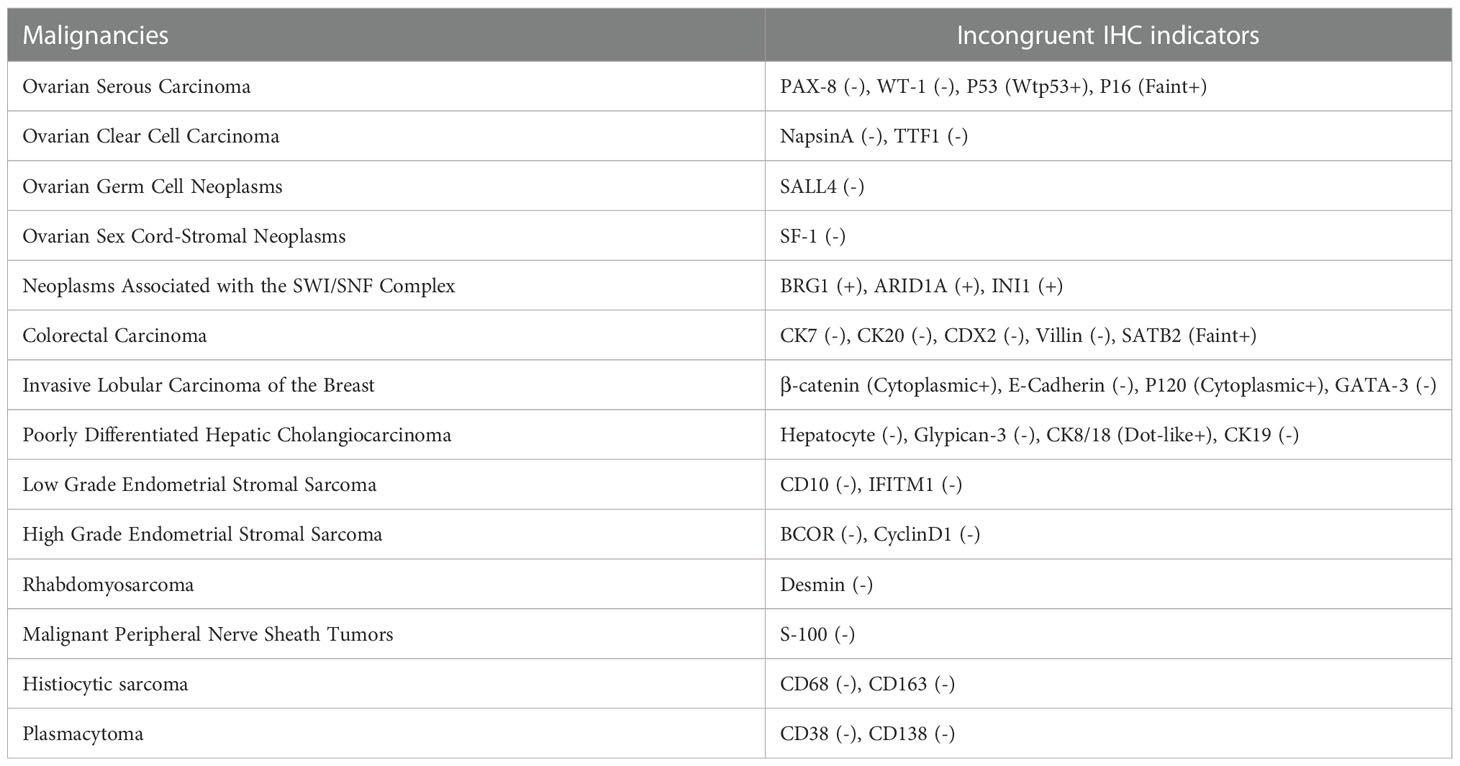
IHC was conducted to help confirm the diagnosis (Table 1). Tumor cells positively expressed monoclonal ER, INI1(SMARCB1), BRG1(SMARCA4), and ARID1a, with patchy expressions of monoclonal PR, P16, Syn, SATB2, and CK8/18. The β-Catenin and P120 were positive in the cytoplasm. The Ki-67 labeling index was approximately 30%. There was no expression of CK pan (Figure 2G), p40 (Figure 2H), p63 (Figure 2I), and other IHC markers (i.e., CD56, CK7, WT-1, TTF-1, Desmin, C-myc, CgA, CDX2, INSM1, SSTR2, SSTR5). Details of all the IHC results were listed in Table 1. Based on the morphological and IHC features, 14 malignancies with similar morphology were compared, including ovarian serous carcinoma, ovarian clear cell carcinoma, ovarian germ cell neoplasms, ovarian sex cord-stromal neoplasms, neoplasms associated with the SWI/SNF complex, colorectal carcinoma, invasive lobular carcinoma of the breast, poorly differentiated hepatic cholangiocarcinoma, low grade endometrial stromal sarcoma, high grade endometrial stromal sarcoma, rhabdomyosarcoma, malignant peripheral nerve sheath tumors, histiocytic sarcoma, and plasmacytoma (Table 2). Additionally, to distinguish it from Ewing sarcoma, dual-color break-apart FISH was conducted to test for EWSR1 gene rearrangements, which revealed a negative result.
Finally, we conducted NUT immunostaining (clone C52B1) and revealed diffusely positive expression in the nucleus of tumor cells (Figure 2J). However, the FISH experiment found no disruption or translocation of the NUTM1 gene locus (Figure 2K). We subsequently identified the gene fusion of MXI1 exon 5 (NM_130439.3) to NUTM1 exon 3 (NM_175741.3) via a targeted RNA-based NGS platform (DA8600, Daan Gene, Guangzhou, China) on tissue samples, which finally confirmed NUT carcinoma two months after cytoreductive surgery (Figure 2L). Besides, we also identified the gene fusion of MTMR3 exon 5 (NM_021090.4) to SFI1 exon 3 (NM_001007467.3).
Follow-Up
One week after diagnosis, the patient developed fever and an increased burden of malignancy, with imaging of advanced diffusely thickened peritoneum, omentum, and mesangium, progressed multiple metastatic lymph nodes, and newly developed abdomino pelvic effusion. Unfortunately, although the patient was adequately informed and the potential feasibility of antitumor therapy was introduced, the patient refused further treatment due to financial difficulties and decided to be discharged to a local hospital for symptomatic relief and supportive treatment. She developed systemic symptoms and passed away four months and 18 days after cytoreductive surgery (Figure 3).
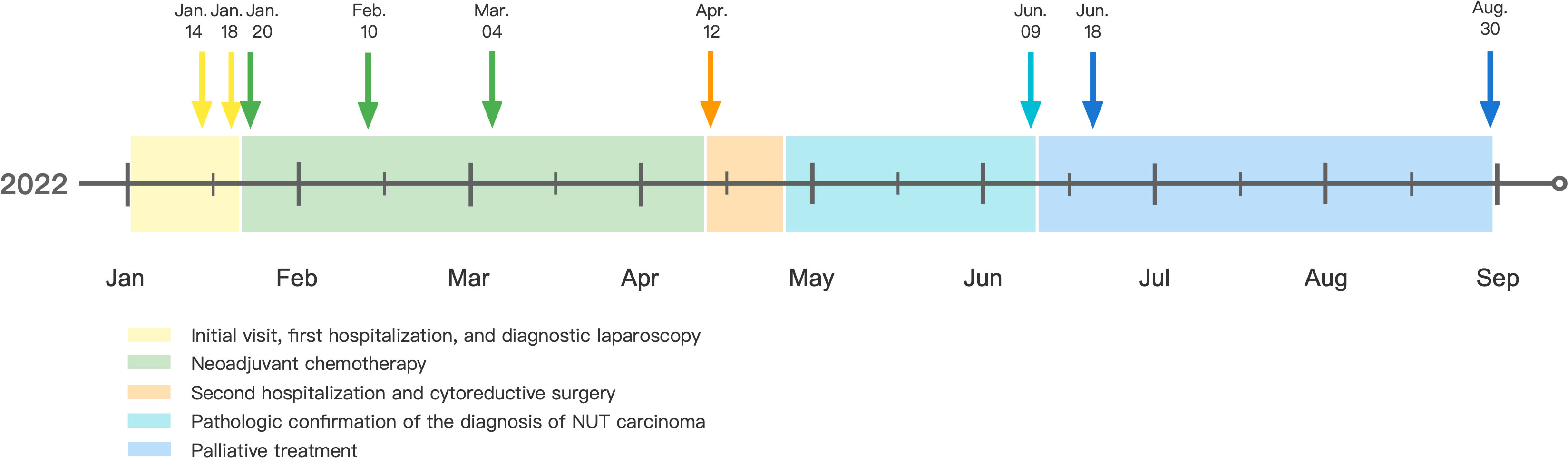
Figure 3 Time diagram from Jan 2022 to Sep 2022 of the patient: on Jan 14, 2022, abdominopelvic lesions and ovarian masses were identified by imaging examination; on Jan 18, 2022, diagnostic laparoscopy was performed. Pathological findings of the omentum and peritoneum biopsies suggested a poorly differentiated carcinoma. On Jan 20, 2022, the patient began the first course of neoadjuvant chemotherapy with Paclitaxel-albumin, Carboplatin, and Bevacizumab; on Feb 10, 2022, the patient received the second course of neoadjuvant chemotherapy; on Mar 4, 2022, the patient received the third course of neoadjuvant chemotherapy; on Apr 12, 2022, cytoreductive surgery was performed; on Jun 9, 2022, NUT carcinoma was confirmed by morphology, immunohistochemistry, and genetic alterations. On Jun 18, 2022, imaging examinations revealed a progressed malignancy burden. The patient refused antitumor therapy and passed away on Aug 30, 2022.
Discussion
NUT, a protein product of the NUTM1 gene with highly specific physiologic expression in post-meiotic spermatids, is an emerging neoplastic driver when fused with genes related to transcription regulation (3). Although initially found to form fusion oncoproteins with bromodomain proteins in a series of fatal midline carcinomas, NUT has been identified with non-bromodomain partners in some non-midline neoplasms with varied histological morphology, including sarcoma, poroma, porocarcinoma, and acute lymphoblastic leukemias (11–14). Herein, we report a rare case of NUT carcinoma in a middle-aged woman presenting extensive abdominopelvic lesions and bilateral ovarian masses and initially considered advanced ovarian cancer.
Although increasingly reported outside midline sites, NUT carcinoma rarely presents ovarian lesions. As in our case, bilateral ovarian masses may raise concern for a gynecologic tract primary site of NUT carcinoma. Ovarian lesions are uncommon for NUT carcinoma, with only three case reports identified in the previous literature (5, 9, 11). The first patient was a 38-year-old white female presenting with mediastinal and bilateral ovarian masses; the possibility of primary ovarian lesions cannot be excluded (9). The second patient was a young female with ovarian and lung lesions, and the primary focus was uncertain (11). The third patient, similar to ours, was a middle-aged woman harboring a massive mass in the pelvic cavity with diffusely thickened peritoneum and multiple enlarged distant lymph nodes, indicating advanced ovarian cancer (5). In general, these patients and ours presented with extensive lesions involving the ovaries, so the possibility of the ovary origin should be considered. Of concern is that our patient’s ovarian lesions were mainly distributed near the vessels of the ovarian hilus, with most of the typical structures of the ovary intact. Therefore, the possibility of secondary ovarian malignancy cannot be ruled out. Given that this patient’s lesions were predominantly in the abdominal and pelvic presenting with extensive thickened peritoneum, omentum, and mesangium, the possibility of primary NUT carcinoma in the peritoneum, omentum, or mesangium also cannot be ignored. These have not been previously reported or considered in the literature.
NUT carcinoma usually displays evidence of squamous differentiation either by histologic findings or immunohistochemically expression of p63, p40, CK-pan, and other markers associated with squamous differentiation (15). Similar to Jung et al., we observed strong ER expression, no expression of p63 or p40, and no abrupt squamous foci (5), and we also found weakly positive expression of PR and weak and dot-like CK8/18 expression, which is not common in classic NUT carcinoma (11). However, the IHC profiles of our study excluded 14 other morphologically similar malignancies and secured the diagnosis of a poorly differentiated NUT carcinoma. Recently, similar high ER reactivity was described in one report of the NUTM1-rearranged neoplasia with primary foot lesions, which was diagnosed as NUT sarcoma due to morphological consistency with extraskeletal myxoid chondrosarcoma (11). However, sarcomatoid differentiation is not remarkable in this case. The unique ER and PR expression may indicate that this tumor arose from a primary gynecologic site. Some reports of NUT carcinoma have shown some focal features of their possible origins. A recent rare report of an NSD3:NUTM1 fusion carcinoma arising in the thyroid showed retention of focal thyroglobulin production (16). In brief, the exact value of ER and PR expression in NUT carcinoma remains unclear, which may indicate a gynecologic origin and requires further investigation.
Myotubularin-related protein 3 (MTMR3) is an inositol lipid 3-phosphatase with extensive homology to myotubularin (17). It has been found to be related to inflammatory bowel disease (18), X-linked myotubular myopathy (17, 19), and type 4B Charcot-Marie-Tooth disease (20). SFI1 (SFI1 Centrin Binding Protein), essential for proper stability of centrioles and ciliogenesis regulation (21), is associated with diabetic nephropathy (22), neuroblastoma (23), and microcephaly (24). However, MTMR3:: SFI1 fusion has not been previously reported. MAX interactor 1 (MXI1), the reported NUTM1 companion gene, belongs to the MAD family and encodes a transcription factor with a bHLH-Zip motif (25). MXI1 interacts specifically with transcription cofactor Max (25), regulates Myc activity via transcriptional inhibition, and plays a role in the regulation of cell proliferation (26, 27). So far, Three cases of MXI1::NUTM1 fusion neoplasm have been reported in published literature (28–30). Agreeing with previous cases, our case shows the appearance of a primitive small round cell morphology and is diagnosed based on the positive staining of the NUT protein. Although all four cases (including this case) generally show no expression of typical NUT carcinoma markers, the IHC profile of this case is slightly different with negative expression of Desmin and positive expressions of ER, PR, and CK8/18. Despite the negative expression of myc in MXI1::NUTM1 fusion neoplasm, evidence has shown that MXI1, which are normally repressors of MYC activity, can be converted into MYC-like mimics by fusion to NUTM1 (28). This may potentially be instructive in the therapeutic options.
NUT carcinoma was highly aggressive, remarkably refractory to chemotherapy and radiotherapy, and rapidly fatal. Most patients succumb to rapid disease progression with early locoregional invasion and distant metastases (7, 31). The prognosis is related to the fusion partners and malignancy sites. Analysis of 124 patients from the NUT Midline Carcinoma Registry (www.nmcregistry.org) found that the median overall survival (OS) of non-thoracic NUT carcinoma is longer than thoracic NUT carcinoma, so as non-BRD4-NUTM1 fusions compared with BRD4-NUTM1 fusion (32). In about 75% of cases, NUTM1 is fused with BRD4, a member of the dual bromodomain and extra terminal domain (BET) family proteins (15, 33). NUT-mediated genome-wide histone modification is vital in the pathogenesis of NUT carcinoma via activating the histone acetyltransferase (HAT) p300, a factor required for enhancer function achievement and the transcription of oncogenes or tumor suppressor genes like MYC and SOX2 (15).
Some cases have reported excellent tumor stabilization effects of Ewing sarcoma chemotherapy, concurrent Chemoradiotherapy (CCRT), and immunovirotherapy (34–37). Nasal NUT carcinoma has remarkably responded to Ewing sarcoma-based chemotherapy regimen and concurrent radiation in several cases (34, 35). And CCRT has been found helpful in achieving complete remission in several patients suffering from head, neck, and thoracic NUT carcinoma (36, 38). Recently, a case demonstrated the feasibility of an add-on immunovirotherapy regimen in a patent with thoracic NUT carcinoma, which includes an oncolytic virus (talimogene laherparepvec (T-VEC), IMLYGIC®) together with the immune checkpoint inhibitor pembrolizumab as an add-on to a basic therapy (cytostatic chemotherapy, radiation therapy, and epigenetic therapy) and shows a significant improvement of tumor stabilization and the patient’s quality of life (37). And it has been reported that positive PD-L1 expression may be associated with better survival and may indicate the potential of immune checkpoint inhibitors in treating NUT carcinoma (15, 38).
Although the standard therapy for NUT carcinoma has not been well established, therapeutic epigenetic modifiers are emerging potential regimens for NUT carcinoma treatment. Promising epigenetic therapies include the inhibition of DNA binding by BET bromodomain inhibitors (BETis) and the modification of downstream histones by histone deacetylase inhibitors (HDACis) (15, 39, 40). Several epigenetic therapies are under development and have shown some promising preclinical findings, including BETis (i.e., ABBV-075 (mivebresib), ABBV-744, and BI 894999), p300 BDi + BETi (i.e., GNE-781 + birabresib), CDK9 inhibition, CDK4/6i + BETi, DNA-damaging agents, and HDACis (15, 39–41). To date, several phase I/IIa clinical trials focused on BETi monotherapy have been completed and showed preliminary antitumor activity, including birabresib (aka MK-8628/OTX015, OncoEthix) (42), molibresib (aka iBET-762, GlaxoSmithKline) (43), ODM-207 (Orion Pharma) (44), RO6870810 (aka TEN-010, Roche) (45), and BMS-986158 (Bristol-Myers Squibb) (46). But more clinical trials are needed to confirm the safety and feasibility of these regimens for widespread use. Some ongoing phase I/II clinical trials include BETi combined with chemotherapy treatment (ZEN3694 + cisplatin/etoposide, CTEP 10507 and NCT05019716), dual BET and CBP/p300 inhibitor (EP31670, NCT05488548).
Of the three cases that reported NUT carcinoma with gynecological lesions, two described the treatment and prognosis. The first patient developed multiple diffuse progressive metastases throughout the body within three weeks of diagnosis, including liver, lung, brain, adrenal glands, pelvic cavity, bone, and lymph nodes. Although receiving chemoradiotherapy and targeted therapy, she died of a severe infection two months and 19 days after diagnosis (9). Another patient was a 54-year-old Korean woman who was treated with chemotherapy with bleomycin, etoposide, and cisplatin (BEP) but showed significant aggravated tumor burden with chest and lymph node metastases (5). Regardless of the therapies, conditions of NUT carcinoma patients with gynecologic tract lesions presented uncontrolled. Remarkably, the 38-year-old white female with mediastinal and ovarian masses received systemic chemotherapy with an HDACi (Romidepsin), but the tumor significantly progressed (9). Of the three cases that reported MXI1::NUTM1 fusion, two were not eligible for clinical trials and were too unwell to receive other basic therapies (28, 30), and one received an off-label use of Romidepsin but got progressively worse (29). Therefore, more experimental and clinical studies are required to characterize the drug resistance mechanisms and to screen the targeted therapies.
Conclusion
We report the clinical and pathological findings of a rare NUT carcinoma with MXI1::NUTM1 fusion characterized by extensive abdominopelvic lesions and bilateral ovarian masses and share the difficulties and pitfalls of the diagnostic process. We emphasize the importance of NUT testing in undifferentiated malignant neoplasms with extensive abdominopelvic and ovarian involvement and put forward the possible gynecologic origin of NUT carcinoma, which is essential for proper classification and treatment planning.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HL and JQ conceived, conducted, and participated in the whole process of this study. HJ carried out the entire procedure, including the medical records collection, manuscript drafting, and manuscript revision. CW and ZH revised the manuscript. YW provided figures and clinical pathological analysis. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the National Natural Science Foundation of China (Grant No. 82201839).
Acknowledgments
The authors sincerely thank all doctors and nurses in the Gynecology Ward One of Peking University Third Hospital. We gratefully acknowledge Dr. Bo Yu, Dr. Yingxi Wang, Dr. Jiaqi Huang, Dr. Shuxian Ren, Dr. Linlin Zhang, and Dr. Qizheng Wang for participating in the patient’s clinical work. And we sincerely thank Prof. Bo Zhang and Dr. Zixiu Zhao of the Department of Pathology of Peking University Third Hospital for their advice on clinical pathological analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1091877/full#supplementary-material
References
1. French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol (2012) 7:247–65. doi: 10.1146/annurev-pathol-011811-132438
2. Kubonishi I, Takehara N, Iwata J, Sonobe H, Ohtsuki Y, Abe T, et al. Novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res (1991) 51(12):3327–8.
3. Eagen KP, French CA. Supercharging BRD4 with NUT in carcinoma. Oncogene. (2021) 40(8):1396–408. doi: 10.1038/s41388-020-01625-0
5. Jung M, Kim SI, Kim JW, Jeon YK, Lee C. NUT carcinoma in the pelvic cavity with unusual pathologic features. Int J Gynecol Pathol (2022) 41(3):292–7. doi: 10.1097/PGP.0000000000000801
6. Moreno V, Saluja K, Pina-Oviedo S. NUT carcinoma: Clinicopathologic features, molecular genetics and epigenetics. Front Oncol (2022) 12:860830. doi: 10.3389/fonc.2022.860830
7. Bauer DE, Mitchell CM, Strait KM, Lathan CS, Stelow EB, Lüer SC, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res (2012) 18(20):5773–9. doi: 10.1158/1078-0432.CCR-12-1153
8. Haack H, Johnson LA, Fry CJ, Crosby K, Polakiewicz RD, Stelow EB, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol (2009) 33(7):984–91. doi: 10.1097/PAS.0b013e318198d666
9. Dragoescu E, French C, Cassano A, Baker S Jr., Chafe W. NUT midline carcinoma presenting with bilateral ovarian metastases: a case report. Int J Gynecol Pathol (2015) 34(2):136–42. doi: 10.1097/PGP.0000000000000129
10. Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. (2017) 89:218–35. doi: 10.1016/j.jclinepi.2017.04.026
11. Stevens TM, Morlote D, Xiu J, Swensen J, Brandwein-Weber M, Miettinen MM, et al. NUTM1-rearranged neoplasia: a multi-institution experience yields novel fusion partners and expands the histologic spectrum. Mod Pathol (2019) 32(6):764–73. doi: 10.1038/s41379-019-0206-z
12. Khabirova E, Jardine L, Coorens THH, Webb S, Treger TD, Engelbert J, et al. Single-cell transcriptomics reveals a distinct developmental state of KMT2A-rearranged infant b-cell acute lymphoblastic leukemia. Nat Med (2022) 28(4):743–51. doi: 10.1038/s41591-022-01720-7
13. Tamura R, Nakaoka H, Yoshihara K, Mori Y, Yachida N, Nishikawa N, et al. Novel MXD4-NUTM1 fusion transcript identified in primary ovarian undifferentiated small round cell sarcoma. Genes Chromosomes Cancer. (2018) 57(11):557–63. doi: 10.1002/gcc.22668
14. McEvoy CR, Fox SB, Prall OWJ. Emerging entities in NUTM1-rearranged neoplasms. Genes Chromosomes Cancer. (2020) 59(6):375–85. doi: 10.1002/gcc.22838
15. French CA, Cheng ML, Hanna GJ, DuBois SG, Chau NG, Hann CL, et al. Report of the first international symposium on NUT carcinoma. Clin Cancer Res (2022) 28(12):2493–505. doi: 10.1158/1078-0432.CCR-22-0591
16. Allison DB, Rueckert J, Cornea V, Lee CY, Dueber J, Bocklage T. Thyroid carcinoma with NSD3::NUTM1 fusion: a case with thyrocyte differentiation and colloid production. Endocr Pathol (2022) 33(2):315–26. doi: 10.1007/s12022-021-09700-2
17. Walker DM, Urbé S, Dove SK, Tenza D, Raposo G, Clague MJ. Characterization of MTMR3. an inositol lipid 3-phosphatase with novel substrate specificity. Curr Biol (2001) 11(20):1600–5. doi: 10.1016/S0960-9822(01)00501-2
18. Hoefkens E, Nys K, John JM, Van Steen K, Arijs I, van der Goten J, et al. Genetic association and functional role of crohn disease risk alleles involved in microbial sensing, autophagy, and endoplasmic reticulum (ER) stress. Autophagy. (2013) 9(12):2046–55. doi: 10.4161/auto.26337
19. Schaletzky J, Dove SK, Short B, Lorenzo O, Clague MJ, Barr FA. Phosphatidylinositol-5-phosphate activation and conserved substrate specificity of the myotubularin phosphatidylinositol 3-phosphatases. Curr Biol (2003) 13(6):504–9. doi: 10.1016/S0960-9822(03)00132-5
20. Kim SA, Taylor GS, Torgersen KM, Dixon JE. Myotubularin and MTMR2, phosphatidylinositol 3-phosphatases mutated in myotubular myopathy and type 4B charcot-Marie-Tooth disease. J Biol Chem (2002) 277(6):4526–31. doi: 10.1074/jbc.M111087200
21. Laporte MH, Bouhlel IB, Bertiaux E, Morrison CG, Giroud A, Borgers S, et al. Human SFI1 and centrin form a complex critical for centriole architecture and ciliogenesis. EMBO J (2022) 41(21):e112107. doi: 10.15252/embj.2022112107
22. McDonough CW, Palmer ND, Hicks PJ, Roh BH, An SS, Cooke JN, et al. A genome-wide association study for diabetic nephropathy genes in African americans. Kidney Int (2011) 79(5):563–72. doi: 10.1038/ki.2010.467
23. Esposito MR, Binatti A, Pantile M, Coppe A, Mazzocco K, Longo L, et al. Somatic mutations in specific and connected subpathways are associated with short neuroblastoma patients’ survival and indicate proteins targetable at onset of disease. Int J Cancer. (2018) 143(10):2525–36. doi: 10.1002/ijc.31748
24. Kodani A, Moyer T, Chen A, Holland A, Walsh CA, Reiter JF. SFI1 promotes centriole duplication by recruiting USP9X to stabilize the microcephaly protein STIL. J Cell Biol (2019) 218(7):2185–97. doi: 10.1083/jcb.201803041
25. Zervos AS, Gyuris J, Brent R. Mxi1, a protein that specifically interacts with max to bind myc-max recognition sites. Cell. (1993) 72(2):223–32. doi: 10.1016/0092-8674(93)90662-A
26. Dugast-Darzacq C, Grange T, Schreiber-Agus NB. Differential effects of Mxi1-SRalpha and Mxi1-SRbeta in myc antagonism. FEBS J (2007) 274(17):4643–53. doi: 10.1111/j.1742-4658.2007.05992.x
27. Wu W, Hu Z, Wang F, Gu H, Jiang X, Xu J, et al. Mxi1-0 regulates the growth of human umbilical vein endothelial cells through extracellular signal-regulated kinase 1/2 (ERK1/2) and interleukin-8 (IL-8)-dependent pathways. PloS One (2017) 12(6):e0178831. doi: 10.1371/journal.pone.0178831
28. McEvoy CR, Holliday H, Thio N, Mitchell C, Choong DY, Yellapu B, et al. A MXI1-NUTM1 fusion protein with MYC-like activity suggests a novel oncogenic mechanism in a subset of NUTM1-rearranged tumors. Lab Invest. (2021) 101(1):26–37. doi: 10.1038/s41374-020-00484-3
29. Chen L, Larsen B, Dermawan JK, Zarka MA. Cytomorphology of NUTM1-rearranged sarcoma involving pleural fluid. Diagn Cytopathol. (2022) 50(9):E244–e7. doi: 10.1002/dc.24968
30. Duan FL, Hou J, Guo P, Tan H, Dai J, Tang Z, et al. Clinicopathologic features of an MXI1::NUTM1 fusion neoplasm; a new molecular variant of the family of NUTM1-rearranged neoplasm. Histopathology. (2022) 81(4):536–9. doi: 10.1111/his.14720
31. Chau NG, Hurwitz S, Mitchell CM, Aserlind A, Grunfeld N, Kaplan L, et al. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer. (2016) 122(23):3632–40. doi: 10.1002/cncr.30242
32. Chau NG, Ma C, Danga K, Al-Sayegh H, Nardi V, Barrette R, et al. An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) midline carcinoma: Analysis of 124 patients. JNCI Cancer Spectr (2020) 4(2):pkz094. doi: 10.1093/jncics/pkz094
33. French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR, Fletcher JA. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res (2003) 63(2):304–7.
34. Davis BN, Karabakhtsian RG, Pettigrew AL, Arnold SM, French CA, Brill YM. Nuclear protein in testis midline carcinomas: a lethal and underrecognized entity. Arch Pathol Lab Med (2011) 135(11):1494–8. doi: 10.5858/arpa.2010-0389-CR
35. Arimizu K, Hirano G, Makiyama C, Matsuo M, Sasaguri T, Makiyama A. NUT carcinoma of the nasal cavity that responded to a chemotherapy regimen for ewing’s sarcoma family of tumors: a case report. BMC Cancer (2018) 18(1):1134. doi: 10.1186/s12885-018-5087-x
36. Muramatsu J, Takada K, Sugita S, Tsuchiya T, Yamamoto K, Takagi M, et al. Complete response induced by concurrent chemoradiotherapy in a patient with NUT carcinoma. Intern Med (2022) 61(8):1299–304. doi: 10.2169/internalmedicine.7741-21
37. Kloker LD, Calukovic B, Benzler K, Golf A, Böhm S, Günther S, et al. Case report: Immunovirotherapy as a novel add-on treatment in a patient with thoracic NUT carcinoma. Front Oncol (2022) 12:995744. doi: 10.3389/fonc.2022.995744
38. Pan M, Chang JS. Durable complete remission of PD-L1 positive NUT carcinoma treated with concurrent chemotherapy and radiation. Perm J (2020) 25:1–3. doi: 10.7812/TPP/20.251
39. Jung M, Kim S, Lee JK, Yoon SO, Park HS, Hong SW, et al. Clinicopathological and preclinical findings of NUT carcinoma: A multicenter study. Oncologist. (2019) 24(8):e740–e8. doi: 10.1634/theoncologist.2018-0477
40. Shiota H, Alekseyenko AA, Wang ZA, Filic I, Knox TM, Luong NM, et al. Chemical screen identifies diverse and novel histone deacetylase inhibitors as repressors of NUT function: Implications for NUT carcinoma pathogenesis and treatment. Mol Cancer Res (2021) 19(11):1818–30. doi: 10.1158/1541-7786.MCR-21-0259
41. Sun K, Atoyan R, Borek MA, Dellarocca S, Samson ME, Ma AW, et al. Dual HDAC and PI3K inhibitor CUDC-907 downregulates MYC and suppresses growth of MYC-dependent cancers. Mol Cancer Ther (2017) 16(2):285–99. doi: 10.1158/1535-7163.MCT-16-0390
42. Lewin J, Soria JC, Stathis A, Delord JP, Peters S, Awada A, et al. Phase ib trial with birabresib, a small-molecule inhibitor of bromodomain and extraterminal proteins, in patients with selected advanced solid tumors. J Clin Oncol (2018) 36(30):3007–14. doi: 10.1200/JCO.2018.78.2292
43. Piha-Paul SA, Hann CL, French CA, Cousin S, Braña I, Cassier PA, et al. Phase 1 study of molibresib (GSK525762), a bromodomain and extra-terminal domain protein inhibitor, in NUT carcinoma and other solid tumors. JNCI Cancer Spectr (2020) 4(2):pkz093. doi: 10.1093/jncics/pkz093
44. Ameratunga M, Braña I, Bono P, Postel-Vinay S, Plummer R, Aspegren J, et al. First-in-human phase 1 open label study of the BET inhibitor ODM-207 in patients with selected solid tumours. Br J Cancer. (2020) 123(12):1730–6. doi: 10.1038/s41416-020-01077-z
45. Shapiro GI, LoRusso P, Dowlati A, TD K, CA J, Vaishampayan U, et al. A phase 1 study of RO6870810, a novel bromodomain and extra-terminal protein inhibitor, in patients with NUT carcinoma, other solid tumours, or diffuse large b-cell lymphoma. Br J Cancer (2021) 124(4):744–53. doi: 10.1038/s41416-020-01180-1
46. Hilton J, Cristea M, Postel-Vinay S, Baldini C, Voskoboynik M, Edenfield W, et al. BMS-986158, a small molecule inhibitor of the bromodomain and extraterminal domain proteins, in patients with selected advanced solid tumors: Results from a phase 1/2a trial. Cancers (Basel) (2022) 14(17). doi: 10.3390/cancers14174079
Keywords: NUT carcinoma, ovarian neoplasms, undifferentiated pelvic neoplasms, case report, NUT rearrangement
Citation: Jiang H, Wang C, Hou Z, Wang Y, Qiao J and Li H (2023) Case report: NUT carcinoma with MXI1::NUTM1 fusion characterized by abdominopelvic lesions and ovarian masses in a middle-aged female. Front. Oncol. 12:1091877. doi: 10.3389/fonc.2022.1091877
Received: 07 November 2022; Accepted: 08 December 2022;
Published: 11 January 2023.
Edited by:
Zhaolun Cai, Sichuan University, ChinaReviewed by:
Minggui Pan, Kaiser Permanente, United StatesDerek Allison, University of Kentucky, United States
Jorge León, University of São Paulo, Brazil
Copyright © 2023 Jiang, Wang, Hou, Wang, Qiao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huajun Li, chinalhj1967@126.com; Jie Qiao, jie.qiao@263.net
 Huahua Jiang
Huahua Jiang Chao Wang1,2
Chao Wang1,2 Zheng Hou
Zheng Hou Jie Qiao
Jie Qiao Huajun Li
Huajun Li