- 1Department of General Surgery, The First Affiliated Hospital of Nanchang University, Nanchang, China
- 2Department of General Surgery, The First Affiliated Hospital of Gannan Medical University, Ganzhou, China
With the development of immunotherapy, immune checkpoint inhibitors (ICIs) are widely used in clinical oncology and have achieved good results. ICIs could induce immune-related adverse events (irAEs) in cancer treatment, which warrant sufficient attention. Among them, immune myositis can manifest severe symptoms affecting the whole body, and immune myocarditis occurs with a low incidence but high fatality rate. Here we report a case of grade 3/4 adverse reactions in a patient with partial hepatectomy for malignancy after using ICIs and describe the clinical presentation, laboratory results, treatment, and prognosis. It emphasizes that clinicians should focus on being alert to irAEs in liver cancer patients who have received ICI therapy. The case we present is a 56-year-old male diagnosed with hepatocellular carcinoma. Right hepatic lobectomy was performed in April 2019. Postoperative follow-up showed that transcatheter arterial chemoembolization (TACE) combined with sorafenib (400 mg twice daily) failed to stop the recurrence of the tumor. In December 2020, the patient started to use Camrelizumab injections (200mg/injection every 21 days as a cycle). After 3 cycles, the patient had decreased muscle strength in both lower extremities with chest tightness, dyspnea, and expectoration (whitish sputum). The diagnosis was ICIs injection-induced immune myocarditis and myositis accompanied. The patient’s condition improved considerably by steroid pulse therapy timely. The case emphasizes that clinicians should focus on being alert to irAEs in liver cancer patients who have received ICI therapy.
Introduction
Primary liver cancer is among the most frequent solid tumor types that seriously threaten human health and is a leading cause of cancer-related death worldwide. According to GLOBOCAN 2020, there were approximately 910000 new cancer cases and around 830000 deaths in 2020 (1). Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer. Traditional treatment modalities include surgical resection, TACE, radiofrequency ablation (RFA), targeted therapy, etc. Although these methods can effectively address local lesions, they cannot eliminate all cancer cells. The residual cancer cells can lead to the recurrence and metastasis of malignant liver tumors. Recently, immunotherapy has been widely used to treat hepatocellular carcinoma (HCC). Many clinical practices or clinical studies are based on ICIs, including local treatment combined with immunotherapy, targeted therapy combined with immunotherapy, adjuvant immunotherapy following surgical resection, combination therapy of a double immune checkpoint, etc. Multiple studies have demonstrated that combining different treatment modalities with immunotherapies may represent an effective therapeutic strategy for HCC (2–5). The objective response rate (ORR) of combination therapy based on ICIs can reach approximately 30% (3, 4).
ICIs also known as immune checkpoint blockade (ICB). The primary reliable targets of HCC immunotherapy include programmed death receptor 1 (PD-1)/programmed death receptor ligand1 (PD-L1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) (6). In HCC immunotherapy, the anti-PD-1 monoclonal antibodies include Nivolumab, Pembrolizumab, Camrelizumab, and Tislelizumab; the anti-PD-L1 monoclonal antibodies include Atezolizumab, Durvalumab, and Sintilimab; the anti-CTLA-4 monoclonal antibodies include Ipilimumab and Tremelimumab. ICIs do not kill tumors directly but can activate the host’s immune system to generate effects in the immune microenvironment (TME), thereby decreasing the risk of local recurrence and distant metastasis (7). ICIs remove the standard inhibitory control that negatively regulates T-cell function in HCC treatment, but they may also induce T-cell hyper-activation and immune-related adverse events (irAEs) (8). These irAEs can affect all organs, including the skin, lungs, thyroid, digestive system, nervous system, musculoskeletal system, etc. Immune myocarditis and myositis are uncommon occurrences with potentially serious outcomes.
In 2016, Johnson et al. was first to report two cases of fulminant myocarditis following treatment with ICIs (9). They systematically described the incidence of myocarditis in a retrospective clinical trial population. The largest single-center series of immune myositis patients who received ICIs were also reported by Jeffrey Aldrich et al. in 2021 (10). Both immune myocarditis and immune myositis are uncommon occurrences with potentially serious outcomes. Compared with other tumors, the incidence of irAEs was not significantly different in HCC patients but tended to increase (11). Liver cancer, immune myocarditis and immune myositis co-occur in the same individual is rare. This case was reported immune myocarditis and myositis in a patient with partial hepatectomy for malignancy after using ICIs. We present the following case in accordance with the CARE reporting checklist.
Case description
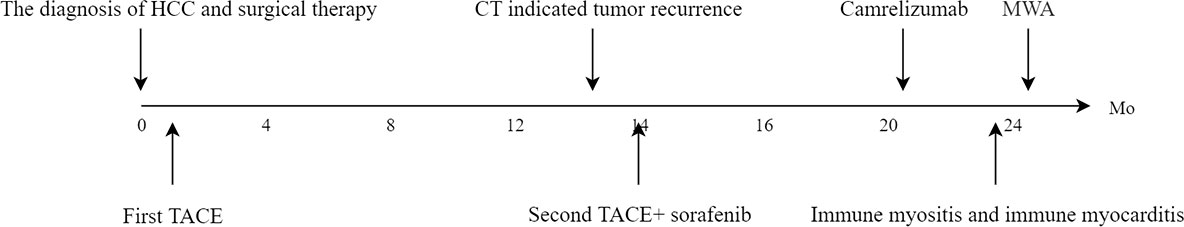
A 56-year-old male was admitted to the hospital because of dyspnea and chest tightness for 20 days (Figure 1). He had no previous history of autoimmune diseases or steroid medication. His personal history, and family history were negative. The vital signs of the patient were normal on admission. The patient with HCC in the right lobe of the liver was treated with a partial hepatectomy on April 2, 2019. The postoperative pathological results in poorly differentiated hepatocellular carcinoma and negative margins. On May 14, 2019, the patient underwent TACE because his microvascular infiltration (MVI) grade is M2. On May 26, 2020, abdominal contrast-enhanced computed tomography (CT) reexamination revealed multiple nodular enhancements, which could suggest tumor recurrence (Figure 2A). The patient received the second TACE combined with sorafenib (400 mg twice daily) on June 9, 2020. After six months, the enhanced CT scan revealed that the more enhanced nodules were bigger than before (Figure 2B). Consequently, he received treatment with the TACE again and started to use Camrelizumab injections (200mg/injection every 21 days as a cycle) on Dec 29, 2020. Following the previous two injections of Camrelizumab, the patient did not experience any discomfort and was discharged from the hospital. After two months of therapy, MRI was performed in the reexamination on February 16, 2021, revealing that the nodular enhancement slightly decreased in size. Good results were obtained in TACE combination with targeted agents and immunotherapy.
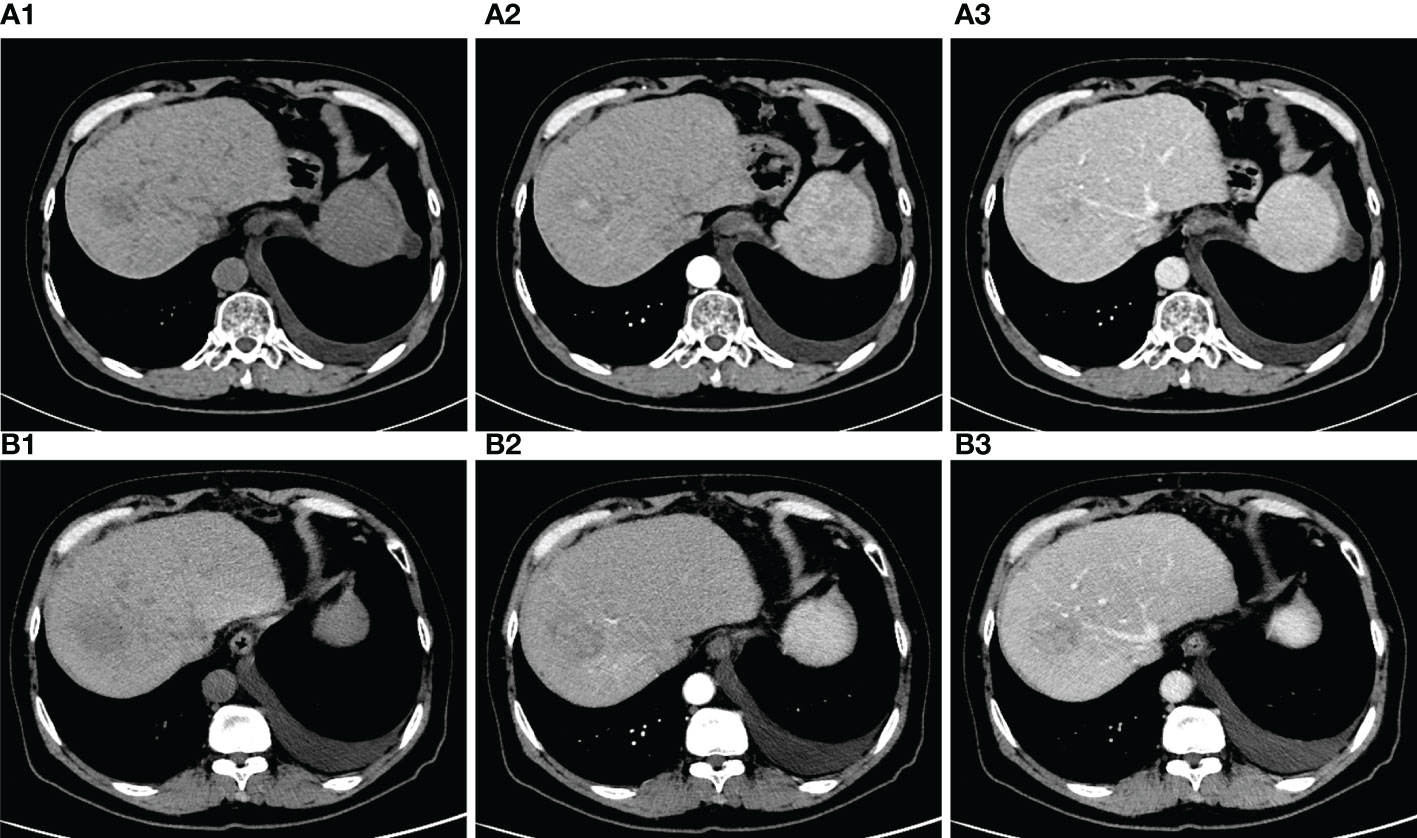
Figure 2 (A) (A1) plain phase (A2) arteria phase (A3) venous phase. Red arrows: tumor nodules. CT of the abdomen scan revealed that, nodules were observed with uneven density. On the enhanced scan, the nodules showed obvious enhancement, and no the venous and delayed scan showed slightly lower density. (B) (B1) plain phase (B2) arteria phase (B3) venous phase. Compared with the last CT, the nodules were bigger than before.
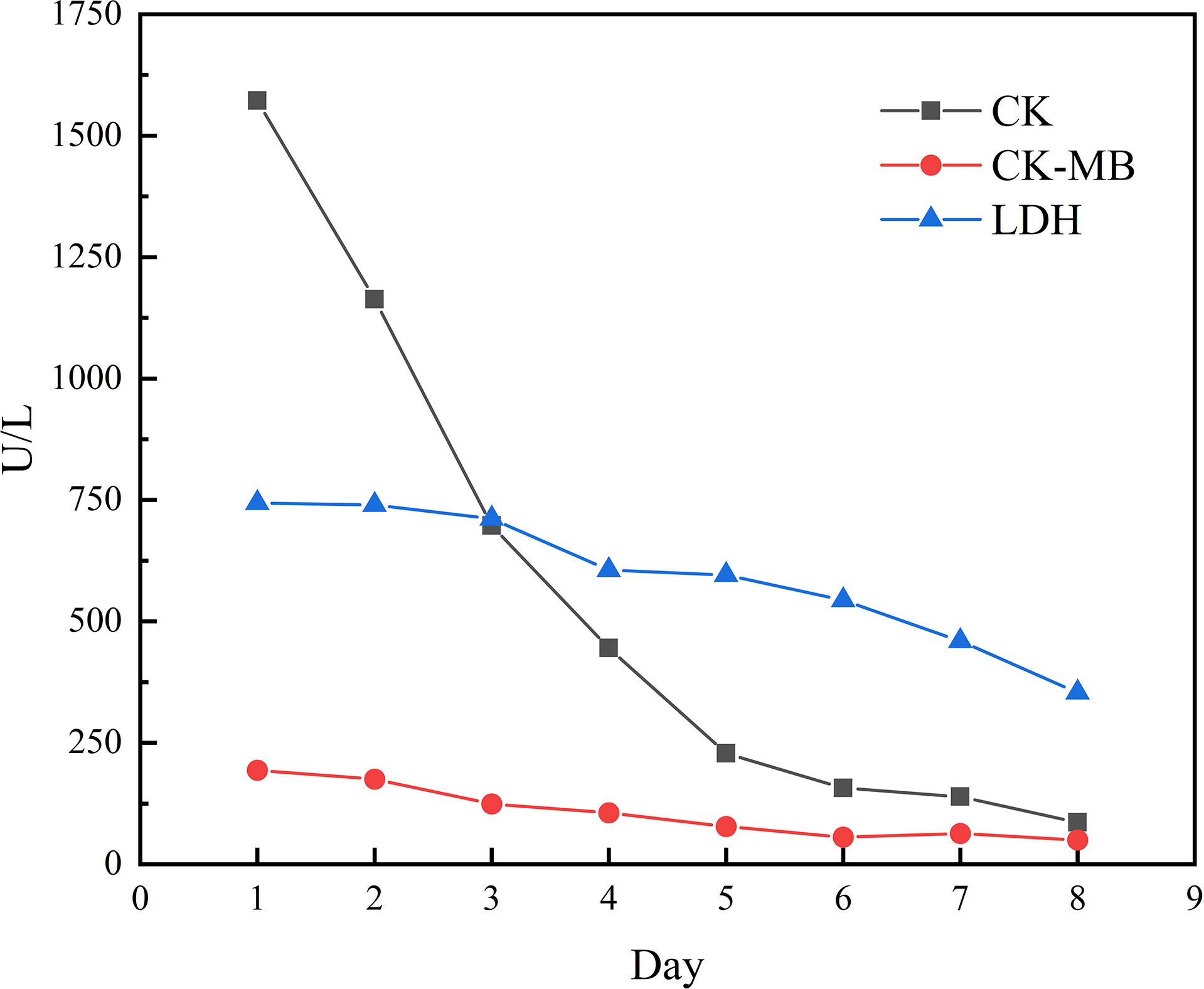
However, the patient had decreased muscle strength in both lower extremities with chest tightness, dyspnea, and expectoration (whitish sputum) on Feb 09, 2021. His performance status (PS) according to the Eastern Cooperative Oncology Group (ECOG) score was 2. Physical examination revealed left eyelid ptosis and grade IV muscle strength in both lower limbs. His cardiac color Doppler ultrasound and electrocardiography showed no obvious abnormalities. Biochemical parameters showed elevated cardiac biomarkers, creatine kinase (CK)1572.1 U/L (normal value 50-310 U/L), creatine kinase isoenzyme (CK-MB) 193.6 U/L (normal value 0-24.0 U/L), lactate dehydrogenase (LDH) 744.0 U/L (normal value 120-250 U/L), myoglobin >900 µg/L (normal value 23-11 µg/L), cardiac troponin (cTnI) level and brain natriuretic peptide (BNP) is normal. The following results evidenced abnormal liver function: alanine aminotransferase (ALT) 108.6 U/L (normal value 9-50 U/L) and AST 146.3 U/L (normal value 15-40 U/L). A muscle biopsy was taken from the left anterior tibialis muscle, and surgical findings showed inflammatory muscle (Figure 3). Based on his disease courses, clinical presentations, laboratory test results, and muscle biopsy findings, the diagnosis was ICIs injection-induced immune myocarditis and myositis accompanied by elevated transaminases, which is considered a grade 3/4 ICIs-induced adverse reaction. The patient discontinued immunotherapy and received a proper treatment immediately, including methylprednisolone sodium succinate intravenously for 7 days, with gradually decreasing doses. Polyene phosphatidylcholine and omeprazole sodium were also administered via an intravenous drip for supportive treatment. After 7 days of therapy, the patient stated that dyspnea and chest tightness significantly improved than before. His blood biochemical parameters, such as CK, CK-MB, and LDH have also gradually decreased (Figure 4). On Mar 12, 2021, re-examination via bio-chemistry indicated the following results: creatine kinase (CK) 82.6 U/L (normal value 50-310 U/L), creatine kinase isoenzyme (CK-MB) 50.0 U/L (normal value 0-24.0 U/L), lactate dehydrogenase (LDH) 353.8 U/L (normal value 120-250 U/L). The patient’s ECOG PS increasingly improved to 0, and no more episodes of the above symptoms. Because patient’s symptoms had improved significantly and the myocardial injury markers showed a significant decline. The patient was discharged from the hospital on day 9 and given oral methylprednisolone tablets (40mg daily, gradually decreasing doses) and proton pump inhibitor PPI.
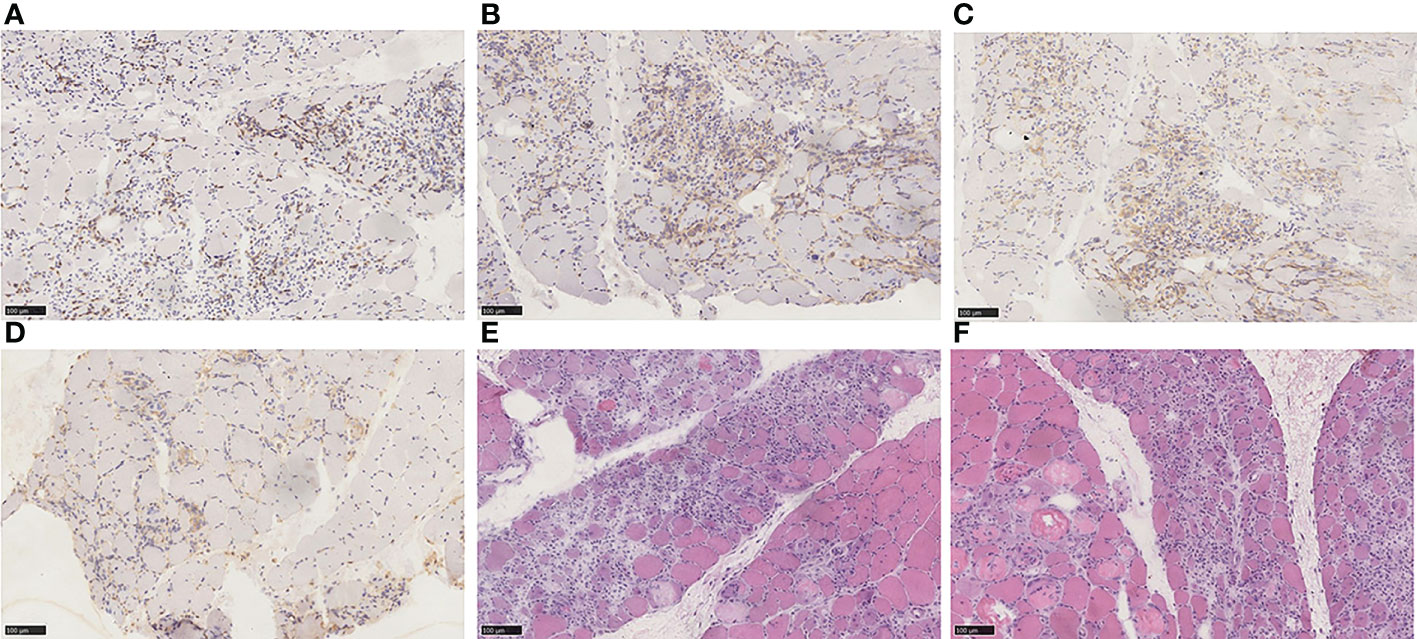
Figure 3 (A) Immunohistochemical staining for CD3. (B) Immunohistochemical staining for CD4. (C) Immunohistochemical staining for CD8. (D) Immunohistochemical staining for CD68. (E) HE staining. (F) HE staining. Endomysium expressed an abundance of CD68-positive cells and was also positive for CD3, CD4, and CD8 expression. The main pathological characteristics of skeletal muscle included necrosis of myofibers, regeneration, and inflammatory cell infiltration. Diagnosis: PD-1 mediated immune myositis with the medical history.
One week after hospital discharge, reexamination showed that the patient’s CK-MB and LDH were all within the normal range. The patient had no obvious discomfort in the subsequent follow-up. At a follow-up examination one year later, magnetic resonance imaging (MRI) showed a subcapsular focus of the left lobe of the liver. The 1.7cm sized nodules on the hepatobiliary phase of MRI were performed with hypointense. Subsequently, the patient was treated by percutaneous microwave ablation. The stage of the operation was uneventful without complications. Rechallenges of targeted drugs or immunotherapy were not implemented in the subsequent therapy. To date, the patient generally fair well, and his liver have no new lesions.
Discussion
Unlike other organs, the liver is an important component of the immune system that has a dual blood supply from the portal vein and the hepatic artery. It links the portal vasculature to the entire circulatory system. Local and systemic immune responses were associated with liver immune cells, such as hepatic stellate cells (HSCs), kupffer cells (KCs), dendritic cells (DC), myeloid cells, lymphocytes, and others (12). When varieties of antigens pass through, the liver can avoid systemic immune response and induce immune tolerance because of tight regulation derived from numbers of immunosuppressive cells, cytokines, and ligands (13). Liver tumor cells more easily escape immune surveillance due to the immune tolerance mechanism. On the other hand, the carcinogenesis of HCC is a multi-factor process resulting from the excess accumulation of aflatoxin and chronic infection of the hepatitis virus. Chronic hepatitis and liver fibrosis provide an immunosuppressive environment that benefits hepatocytes transforming and cancer cells reproducing. These mechanisms established the theoretical foundation for the development and clinical immunotherapy of HCC. Clinical studies with checkpoint-blocking antibodies targeting PD-1/PD-L1 and CTLA-4 are currently initiating. For advanced HCC patients, ICIs exhibit therapeutic benefits by enhancing T cells–mediated immunity. It is important to note that uncontrolled activation of cytotoxic T-cells has brought about many side effects in clinical immunotherapy. Because ICIs activate immunity throughout the body instead of just in the tumor, irAEs involve almost all organ systems. IrAEs of antibodies targeting PD-1 and/or PD-L1 include hypothyroidism and interstitial pneumonia, whereas irAEs of antibodies targeting CTLA-4 encompass mainly colitis and hypophysitis (14–16). However, most HCC patients have liver cirrhosis and portal hypertension. Their symptoms also overlap with the toxicity of ICIs. Likewise, the results of irAEs can increase liver and/or extrahepatic organ manifestations caused by cirrhosis.
Findings suggest that the odds of immune myocarditis range from 0.04% to 1.14%, but 25-50% lethal is high (17). Emma Matzen et al. (18). systematically reviewed the literature on 87 ICI-induced myocarditis patients in PubMed. They found that most cases were melanoma (n = 39), lung cancer (n = 19), renal cell cancer (n = 10), and thymoma cancer patients (n = 4). The median number of cycles of manifestation of cardiotoxic symptoms was 2 (range 1 to 13 cycles). The mechanisms underlying the association between ICIs treatment and myocarditis are still unclear. It suggested that ICIs treatment causes immune activation in HCC patients, concomitant with reducing T cell tolerance to normal tissues. The PD-1 knockout mice have an increased incidence of autoimmune dilated cardiomyopathy and mortality rates, with the deposition of diffuse antibodies (immunoglobulin G) in cardiomyocytes (19). PD-1 pathway plays an essential role in the regulation of the immune response of cardiac (19). In necropsy and histological analysis from ICIs-related fatal myocarditis patients, Cytotoxic T lymphocyte (CTL) infiltration and PD-L1 Inhibition are present in cardiomyocytes (9, 20). PD-L1 protein can be found in normal human cells. When they are integrated with the CTL receptors, the ability to protect overactivated cells can be activated (17, 20). Similar to what has been observed in humans, the infiltrated lymphocytes in monkey hearts include mainly CD4+ and CD8+ T cells, and immunohistochemical stains for PD-1 and PD-L1 are positive (21). Clinical signs and symptoms of immune myocarditis include non-specific ones of fever, shortness of breath, and fatigue. Typical symptoms include palpitations, chest pain, arrhythmias, heart failure, and even cardiogenic shock. Clinical signs and symptoms are not enough for a diagnosis of immune myocarditis. The clinical diagnosis should be concluded based on medication history, clinical manifestations, and laboratory results (cardiac injury marker, e. g.) rather than only based on clinical signs and symptoms. Troponin levels have been reported to be elevated in 94% of ICI-related myocarditis patients, and BNP/NT-proBNP levels can be raised in 66% of cases (22, 23). Whether troponin, BNP, or NT-proBNP, these biological indicators are not specific to the diagnosis of immune myocarditis. Echocardiography, cardiac MRI, or even cardiac biopsy may be necessary for some situations. We should also clearly diagnose any other cardiac diseases presented with the above symptoms. ICIs therapy should be immediately discontinued if patients are diagnosed with immune myocarditis. Depending on the severity of irAEs, patients should rapidly administer corticosteroids (1 to 2mg/kg) (24). Infliximab/anti-thymocyte globulin (ATG) is the attemptable treatment when high-dose corticosteroid therapy fails (24).
Jeffrey Aldrich et al. systematically reviewed the 9,088 cases receiving ICIs at the University of the Texas MD Anderson Cancer Center (10). The results demonstrated that the probability of immune myositis was 0.04% (36 cases). Other clinical symptoms, such as myocardial injury, myasthenia gravis, and respiratory failure, have been associated with half of the patient. The prognosis of single myositis patients is better than multiple symptoms patients. The mechanism of immune myositis may be similar to that of immune myocarditis. Because of shared antigens among myocardium and skeletal muscle, the loss of immunological tolerance induced by ICIs, and afterward caused irAEs. We performed a pathologic examination of the left anterior tibialis muscle biopsy samples. There were necrosis and regeneration of muscle fibers, and inflammatory cell infiltration was visible inside the muscle fibers. Mehdi Touat et al. (25) investigated the pathological results of 9 patients with immune myositis. Necrotic myofibers of varying degrees were noted in all patients; large amounts of macrophages were located in perimysial and endomysial tissue; CD4+, CD8+, and CD68+cells were infiltrated in endomysial tissue. Our pathological outcomes are in accordance with this finding (Figure 3). Patients with immune myositis could develop muscle aches and exhaustion as the initial symptom. However, it should be noted that the history of other neurologic disorders plays a crucial role in clinical work. Understanding muscle strength by physical examination and assessing the extent of muscle inflammation by laboratory tests also provide evidence for diagnosis. CK elevation occurs in almost all cases (26). T cells are stimulated by immune checkpoint suppression and manifest an aggressive response that results in irAEs (9). Skeletal muscle pathology of immune myositis included the infiltration of mononuclear cells, especially CD8+ T cells CD4+ T cells and B cells (27). These results in CD4+ T cell-mediated B cell activation and synthesis of pathogenic high-affinity autoantibodies (IgG1 and 3 or IgG4 subclass) (28). These antibodies were strongly associated with postsynaptic membrane clustering and structure maintenance of neuromuscular synapses by binding to the nicotinic acetylcholine receptor (AchR) or muscle-specific tyrosine-kinase (MuSK), etc (28). Ultimately, the whole process cause muscle strength decreased. Most cases of overlapping MG were positive in antibodies against acetylcholine receptors (AChR), but a small number of AChR-negative patients have symptoms of drooping eyelids and double vision (29, 30). Clinical unclear diagnosis of immune myositis should be confirmed with EMG or muscular biopsy. ICIs should be withdrawn once immune myositis is diagnosed. According to the severity of Adverse effects, patients should be treated with oral or intravenous corticosteroids. We could further use nonsteroidal anti-inflammatory drugs (NSAIDs) to treat muscle soreness after excluding some contraindications. If there is no appreciable benefit after 4-6 weeks, additional immunosuppression should also be taken into consideration (24).
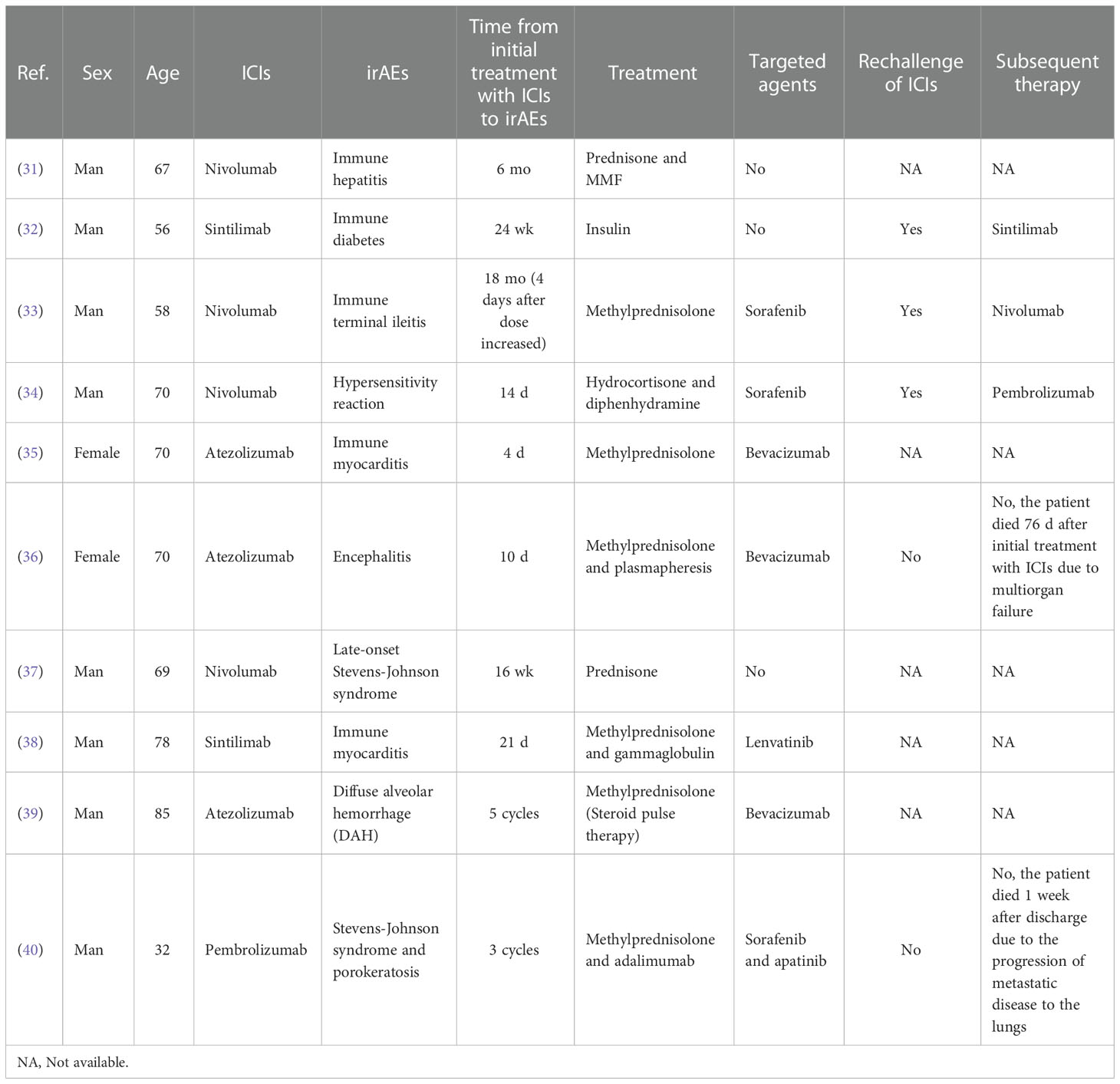
Immune myositis and immune myocarditis could either occur alone or together. Clinicians should be vigilant of signs of other irAEs when one irAE is detected early. We summarized the characteristics of irAEs of HCC patients from case reports on PubMed (Table 1). We observed that the time from initial treatment with ICIs to irAEs was not long. Constantin et al. reported a case of Immune terminal ileitis due to an increasing dose of Nivolumab in a 58-year-old HCC man (33). Many patients were treated with targeted agents during immunotherapy (Table 1). This patient took antiangiogenic drugs (sorafenib) because of positive MVI and disease progression. Unlike ICIs, adverse targeted drug reaction was mostly found to be associated with the inhibition of vascular endothelial cell. The half-life of ICIs was 21 days, and antiangiogenic drugs’ half-life was 7 to 45h. The adverse targeted drug reaction occurs relatively early (1–2 weeks), while irAEs occur relatively late (within 3 months). The combination of Tyrosine kinase inhibitors (TKi) and ICIs expand the incidence of irAEs compared to ICIs monotherapy (41). Therefore, we discontinued sorafenib and Camrelizumab when our patient presented severe irAEs. The irAEs of liver cancer do not exhibit evident specificity compared to other tumor types. Offending drugs timely and steroid pulse therapy is an effective strategy for the treatment of most HCC patients. This patient was taken under an intravenous high-dose methylprednisolone sodium succinate therapy first. His CK-MB and LDH are still out of range after 7 days of intravenous steroid treatment. This suggests that mild inflammation and necrosis could exist in cardiac and skeletal muscles. But all myocardial injury markers decreased more than before. So we used a lower dose of methylprednisolone tablets and administered it orally. Treating with steroids until the cardiac function returns to baseline, then dose tape at least 4 weeks (42). Most irAEs were mild to moderate in severity and were reversible, but severe irAEs were still life-threatening. When the symptoms are relieved, the rechallenge of ICIs can be taken into account, but the decision to rechallenge must be interpreted with caution. Replacement of ICI is one of the optional schemes for immunosuppressant maintenance therapy (Table 1). When irAEs reach grade 3/4, rechallenge of ICIs is not generally recommended. Although rechallenge of ICIs after irAEs showed similar efficacy outcomes compared with initial ICI treatment (43). The reoccurrence odds of irAEs could be enhanced, and the reoccurrence time of irAEs could be advanced. For such cases, a closer long-term follow-up seems crucial. Patients could have permanent discontinuation once the irAEs have recurred (42).
Before starting ICIs treatment, a comprehensive assessment is needed for judging the possibility of developing irAEs. This includes the patient’s general condition, the previous history of immune disease, laboratory tests and radiographic examinations. HCC patients were usually accompanied by liver cirrhosis or fatty liver which can cause abnormal liver function (44). Therefore, the etiology should be investigated promptly once abnormal liver function has occurred. Whether acute hepatitis or compression by tumors, active treatment is required before immunotherapy (45). Abnormal liver function may also cause endocrine/metabolic disorders (46). It is mandatory to evaluate thyroid function periodically. Chemotherapy is one of the most important treatment modalities for advanced HCC. But the combination of ICIs and chemotherapy can also expand the incidence of acute kidney injury (47, 48). Regular checkups, such as renal function tests or urine routines, need to be performed. Cutaneous adverse reactions can be detected by frequent and detailed physical examination. Further examination is needed once the diagnosis is suspected, such as enzyme-linked immunosorbent assays and immunofluorescence tests (49). In conclusion, early detection and early treatment are extremely important in the treatment of irAEs. Any new symptoms after ICI initiation could be irAEs. Presently, various therapeutic approaches combined with ICI therapy have become an essential treatment for HCC, and immunotherapy should not be abandoned easily because of potential irAEs. Adequate judgment, close monitoring, and early detection are needed clinically. Only in this way can we obtain the ideal clinical outcome for each individual.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. The participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: HM and RW. Data curation: YX, WL, and JW. Writing-original draft: HM and WW. Writing-review & editing: KF and RW. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J hepatol (2017) 66(3):545–51. doi: 10.1016/j.jhep.2016.10.029
3. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol (2020) 38(26):2960–70. doi: 10.1200/JCO.20.00808
4. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
5. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol (2020) 6(11):e204564. doi: 10.1001/jamaoncol.2020.4564
6. Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol cancer (2019) 18(1):155. doi: 10.1186/s12943-019-1091-2
7. Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res (2020) 30(6):507–19. doi: 10.1038/s41422-020-0337-2
8. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med (2018) 378(2):158–68. doi: 10.1056/NEJMra1703481
9. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med (2016) 375(18):1749–55. doi: 10.1056/NEJMoa1609214
10. Aldrich J, Pundole X, Tummala S, Palaskas N, Andersen CR, Shoukier M, et al. Inflammatory myositis in cancer patients receiving immune checkpoint inhibitors. Arthritis Rheumatol (Hoboken NJ) (2021) 73(5):866–74. doi: 10.1002/art.41604
11. Sangro B, Chan SL, Meyer T, Reig M, El-Khoueiry A, Galle PR. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J hepatol (2020) 72(2):320–41. doi: 10.1016/j.jhep.2019.10.021
12. Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol hepatol (2021) 18(8):525–43. doi: 10.1038/s41575-021-00438-0
13. Crispe IN. Immune tolerance in liver disease. Hepatol (Baltimore Md). (2014) 60(6):2109–17. doi: 10.1002/hep.27254
14. Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta-analysis. JAMA Oncol (2018) 4(2):173–82. doi: 10.1001/jamaoncol.2017.3064
15. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. New Engl J Med (2015) 372(26):2521–32. doi: 10.1056/NEJMoa1503093
16. Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet (London England) (2017) 390(10105):1853–62. doi: 10.1016/S0140-6736(17)31601-X
17. Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: Pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc (2020) 9(2):e013757. doi: 10.1161/JAHA.119.013757
18. Matzen E, Bartels LE, Løgstrup B, Horskær S, Stilling C, Donskov F. Immune checkpoint inhibitor-induced myocarditis in cancer patients: a case report and review of reported cases. Cardio-oncol (London England) (2021) 7(1):27. doi: 10.1186/s40959-021-00114-x
19. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Sci (New York NY) (2001) 291(5502):319–22. doi: 10.1126/science.291.5502.319
20. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest (2015) 125(9):3335–7. doi: 10.1172/JCI83871
21. Ji C, Roy MD, Golas J, Vitsky A, Ram S, Kumpf SW, et al. Myocarditis in cynomolgus monkeys following treatment with immune checkpoint inhibitors. Clin Cancer Res (2019) 25(15):4735–48. doi: 10.1158/1078-0432.CCR-18-4083
22. Lee Chuy K, Oikonomou EK, Postow MA, Callahan MK, Chapman PB, Shoushtari AN, et al. Myocarditis surveillance in patients with advanced melanoma on combination immune checkpoint inhibitor therapy: The memorial Sloan Kettering cancer center experience. Oncologist (2019) 24(5):e196–e7. doi: 10.1634/theoncologist.2019-0040
23. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol (2018) 71(16):1755–64. doi: 10.1016/j.jacc.2018.02.037
24. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol (2018) 36(17):1714–68. doi: 10.1200/JCO.2017.77.6385
25. Touat M, Maisonobe T, Knauss S, Ben Hadj Salem O, Hervier B, Auré K, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology (2018) 91(10):e985–e94. doi: 10.1212/WNL.0000000000006124
26. Safa H, Johnson DH, Trinh VA, Rodgers TE, Lin H, Suarez-Almazor ME, et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J immunother cancer (2019) 7(1):319. doi: 10.1186/s40425-019-0774-y
27. Dalakas MC. Pathophysiology of inflammatory and autoimmune myopathies. Presse med (Paris France 1983) (2011) 40(4 Pt 2):e237–47. doi: 10.1016/j.lpm.2011.01.005
28. Melzer N, Ruck T, Fuhr P, Gold R, Hohlfeld R, Marx A, et al. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the guidelines of the German neurological society. J neurol (2016) 263(8):1473–94. doi: 10.1007/s00415-016-8045-z
29. Fan Z, Li Z, Shen F, Zhang X, Lei L, Su S, et al. Favorable effects of tacrolimus monotherapy on myasthenia gravis patients. Front neurol (2020) 11:594152. doi: 10.3389/fneur.2020.594152
30. Tzartos SJ, Barkas T, Cung MT, Mamalaki A, Marraud M, Orlewski P, et al. Anatomy of the antigenic structure of a large membrane autoantigen, the muscle-type nicotinic acetylcholine receptor. Immunol Rev (1998) 163:89–120. doi: 10.1111/j.1600-065X.1998.tb01190.x
31. Hsu C, Marshall JL, He AR. Workup and management of immune-mediated hepatobiliary pancreatic toxicities that develop during immune checkpoint inhibitor treatment. oncol (2020) 25(2):105–11. doi: 10.1634/theoncologist.2018-0162
32. Wen L, Zou X, Chen Y, Bai X, Liang T. Sintilimab-induced autoimmune diabetes in a patient with the anti-tumor effect of partial regression. Front Immunol (2020) 11:2076. doi: 10.3389/fimmu.2020.02076
33. Dasanu CA, Plaxe SC, Gupta V, Popescu IM, Grover M, Alvarez-Argote J. Severe terminal ileitis induced by single-agent nivolumab administered every four weeks. J Oncol Pharm Pract (2020) 26(6):1516–9. doi: 10.1177/1078155220903367
34. Choi B, McBride A, Scott AJ. Treatment with pembrolizumab after hypersensitivity reaction to nivolumab in a patient with hepatocellular carcinoma. Am J health-syst Pharm (2019) 76(21):1749–52. doi: 10.1093/ajhp/zxz189
35. Iwasaki S, Hidaka H, Uojima H, Hashimura M, Nabeta T, Sanoyama I, et al. A case of immune checkpoint inhibitor-associated myocarditis after initiation of atezolizumab plus bevacizumab therapy for advanced hepatocellular carcinoma. Clin J gastroenterol (2021) 14(4):1233–9. doi: 10.1007/s12328-021-01442-2
36. Özdirik B, Jost-Brinkmann F, Savic LJ, Mohr R, Tacke F, Ploner CJ, et al. Atezolizumab and bevacizumab-induced encephalitis in advanced hepatocellular carcinoma: Case report and literature review. Medicine (2021) 100(24):e26377. doi: 10.1097/MD.0000000000026377
37. Dasanu CA. Late-onset stevens-Johnson syndrome due to nivolumab use for hepatocellular carcinoma. J Oncol Pharm Pract Off Publ Int Soc Oncol Pharm Pract (2019) 25(8):2052–5. doi: 10.1177/1078155219830166
38. Ji H, Wen Z, Liu B, Chen H, Lin Q, Chen Z. Sintilimab induced ICIAM in the treatment of advanced HCC: A case report and analysis of research progress. Front Immunol (2022) 13:995121. doi: 10.3389/fimmu.2022.995121
39. Shijubou N, Sawai T, Hatakeyama T, Munakata S, Yamazoe M. Alveolar hemorrhage caused by the combination of immune checkpoint inhibitors (ICIs) and angiogenesis inhibitors: The underlying long-term vascular endothelial growth factor (VEGF) inhibition. Cureus (2022) 14(3):e23272. doi: 10.7759/cureus.23272
40. Zhang J, Zhang P, Xu QY, Zhu YT, Chen W, Ji C. Pembrolizumab associated stevens-Johnson syndrome with porokeratosis in a patient in the setting of primary hepatocellular carcinoma. Australas J Dermatol (2022) 63(1):e71–e4. doi: 10.1111/ajd.13704
41. Mo DC, Luo PH, Huang SX, Wang HL, Huang JF. Safety and efficacy of pembrolizumab plus lenvatinib versus pembrolizumab and lenvatinib monotherapies in cancers: A systematic review. Int immunopharmacol (2021) 91:107281. doi: 10.1016/j.intimp.2020.107281
42. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Cancer Netw JNCCN (2020) 18(3):230–41. doi: 10.6004/jnccn.2020.0012
43. Ratanasrimetha P, Reddy VD, Kala J, Tchakarov A, Glass WF, Msaouel P, et al. Case report: Successful treatment of late-onset immune checkpoint inhibitor-associated membranous nephropathy in a patient with advanced renal cell carcinoma. Front Immunol (2022) 13:898811. doi: 10.3389/fimmu.2022.898811
44. Marengo A, Rosso C, Bugianesi E. Liver cancer: Connections with obesity, fatty liver, and cirrhosis. Annu Rev Med (2016) 67:103–17. doi: 10.1146/annurev-med-090514-013832
45. Spillane S, Baxi S, Torres AZ, Lenis D, Freedman AN, Mariotto AB, et al. Organ dysfunction in patients with advanced melanoma treated with immune checkpoint inhibitors. oncol (2020) 25(11):e1753–e62. doi: 10.1634/theoncologist.2020-0055
46. Malespin M, Nassri A. Endocrine diseases and the liver: An update. Clinics liver disease. (2019) 23(2):233–46. doi: 10.1016/j.cld.2018.12.006
47. Murakami N, Motwani S, Riella LV. Renal complications of immune checkpoint blockade. Curr problems cancer (2017) 41(2):100–10. doi: 10.1016/j.currproblcancer.2016.12.004
48. Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35(34):3815–22. doi: 10.1200/JCO.2016.72.1167
Keywords: hepatocellular carcinoma, immune checkpoint inhibitors, irAEs (immune-related adverse events), immune myositis, immune myocarditis
Citation: Mei H, Wen W, Fang K, Xiong Y, Liu W, Wang J and Wan R (2023) Immune checkpoint inhibitor-induced myocarditis and myositis in liver cancer patients: A case report and literature review. Front. Oncol. 12:1088659. doi: 10.3389/fonc.2022.1088659
Received: 03 November 2022; Accepted: 23 December 2022;
Published: 11 January 2023.
Edited by:
Jinhui Liu, Nanjing Medical University, ChinaReviewed by:
Zhiping Hu, University of Pittsburgh, United StatesMing-Hsien Chan, Academia Sinica, Taiwan
Emil Bulatov, Kazan Federal University, Russia
Huiwu Ouyang, Cold Spring Harbor Laboratory, United States
Copyright © 2023 Mei, Wen, Fang, Xiong, Liu, Wang and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renhua Wan, zww726696@sina.com
†These authors share first authorship
 Haoran Mei
Haoran Mei Wu Wen
Wu Wen Kang Fang1
Kang Fang1 Renhua Wan
Renhua Wan