Unveiling the potential of linseed mucilage, its health benefits, and applications in food packaging
- 1Department of Food Science and Technology, National Institute of Food Technology Entrepreneurship and Management, Kundli, Haryana, India
- 2Department of Food and Nutrition and Food Technology, Institute of Home Economics, University of Delhi, New Delhi, India
Industrial waste products derived from the oil industry often contain valuable substances and elements with great potential. These by-products can be used for various purposes, including as nutrients, bioactive compounds, fuels, and polymers. Linseed mucilage (LM) is one such example of a beneficial by-product obtained from linseed. It possesses favorable chemical and functional properties, depending on its method of extraction. Different pretreatments, such as enzymatic extraction, microwave-assisted extraction, pulse electric field, and ultrasound-assisted extraction, have been explored by various researchers to enhance both the yield and quality of mucilage. Furthermore, LM has exhibited therapeutic effects in the treatment of obesity, diabetes, constipation, hyperlipidemia, cancer, and other lifestyle diseases. Additionally, it demonstrates favorable functional characteristics that make it suitable to be used in bioplastic production. These properties preserve food quality, prolong shelf life, and confer antimicrobial activity. It also has the potential to be used as a packaging material, especially considering the increasing demand for sustainable and biodegradable alternatives to plastics because of their detrimental impact on environmental health. This review primarily focuses on different extraction techniques used for linseed mucilage, its mechanism of action in terms of health benefits, and potential applications in food packaging.
1 Introduction
Mucilage is a thick, gel-like material generated by plants, mainly made up of complex carbohydrates such as arabinoxylans, pectins, cellulose, and other polysaccharide variations (1). It plays different important roles in the plant’s functioning, such as retaining water, promoting seed germination, and providing protection against harsh environmental conditions (2). This slimy substance creates a shield around seeds when it comes in contact with water and turns into a viscous slime (3). It is predominantly found in the seeds, roots, and outer coverings of pods or leaves in a variety of plants, including linseeds, chia seeds, okra pods, psyllium husks, Aloe vera leaves, and more (4–6). Mucilage’s free hydroxyl groups form hydrogen bonds with water molecules, creating a thick, viscous matrix (7). This adhesive and thickening property makes it invaluable in the food and packaging industries. Mucilage’s versatility comes from its ability to bind, thicken, and retain moisture, making it an eco-friendly, nutritious, and flexible alternative to many existing food synthetic additives. In food, it serves as a natural emulsifier, stabilizer, and thickening agent, enhancing texture and shelf life in products like sauces and dressings (8). Its ability to form gel aids in encapsulating flavors and nutrients (9). In food packaging, mucilage’s adhesive nature provides a basis for biodegradable adhesives and the production of edible food coatings and films. It has shown protection against the permeability of oxygen and moisture and also has favorable functional attributes such as tensile strength and durability (4, 10–13). Its moisture-retention properties help prolong the freshness of perishable foods. Since packaging is utilized for every food product, including water, oils, spices, and baked goods, mucilage plays a significant role in the food sector. Keeping in mind the severe environmental pollution of plastic packaging, there has been a shift from the use of petroleum-based plastics toward biodegradable plant-based edible packaging (14).
Linseed (Linum usitatissimum L.) is also known as flaxseed, depending on its use as seed, oil (linseed), or fiber (flaxseed) (15, 16). It is one of the traditional crops grown and utilized since ancient times. It belongs to the genus Linum and the family Linaceae (15). It is cultivated in approximately 47 nations for seed, fiber, and oil (17). Worldwide, the Asian continent holds the largest share of 35.4% of the total production (18). The appearance of linseed is flat-shiny, and it possesses an oval shape. There are mainly two known linseeds: brown and yellow. Many products of the linseed plant are readily available in the food market in their various forms: whole linseeds, linseed oil, milled linseed, and roasted linseed (19–21). The linseed plant’s most valuable product is its oil, commonly known as flaxseed oil. Linseed is extensively used due to its health benefits, as it contains many nutrients and bioactives like fatty acids, minerals, vitamins, phenolic compounds, dietary fiber, and protein (22, 23). Linseed mucilage is one of the by-products of linseed. On average, linseed can produce mucilage ranging from approximately 3–10% of its total seed weight (23–26); however, the amount of mucilage produced can vary based on many factors, including the variety of seed and its cultivation process, environmental conditions during mucilage production, or the processing stage of the seed. The functional properties, such as good water-holding, emulsifying, fat-replacing, textural, stabilizing, and interfacial properties, of linseed mucilage make it an invaluable ingredient in various applications across the food industry, including gluten-free bakery products, plant-based meat and dairy alternatives, salad dressings, edible gels, and emulsions. Additionally, its biodegradability makes it an attractive choice for creating edible coatings and films that contribute to a more environmentally friendly food packaging solution without compromising on physical properties. These properties contribute to the overall quality and shelf life of food products while also meeting consumers’ demands for healthier and more sustainable options (27–30). LM not only has good physical functionality but also acts as a functional food. It exhibits a number of nutritional benefits, including laxative, anti-obesity, hypolipidemic, anti-diabetic, hyperglycemic, anti-cancerous, and other health benefits, including prebiotic, anti-bacterial, and anti-inflammatory effects (31–34). The underlying mechanism behind the immense health benefits of LM is still unexplored due to the limited availability of reported literature. Exploring and exploiting the potential of underutilized LM holds promise for revolutionizing both the food industry and sustainable packaging. This review sheds light on the possible applications of LM and highlights it as an ingredient, additive, or substitute for food products, as well as an environmentally friendly alternative for packaging materials. Understanding the nutritive value of LM is vital for advancing toward healthier food options and their application in nutraceuticals as the public becomes more conscious of their dietary choices in terms of their long-term impact on health. Furthermore, embracing LM as a solution for packaging materials can greatly reduce the environmental footprint of the food industry, promoting greener and more sustainable practices.
2 Methodology
The articles collected and reviewed were obtained through a search of articles, both review and research, indexed in the Scopus database from 2000 to 2023. A bibliometric analysis was conducted through VOS viewer software (version 1.6.19). The keywords given as a prompt were “flaxseed” and “linseed mucilage” in the software. The VOSviewer analysis of articles on flaxseed and linseed mucilage revealed five distinct clusters, highlighting current trends in research. The pink color represents the terms diet therapy, clinical trial, functional food, glucose blood level, and nutritional value, whereas the red cluster consists of mucilage, solubility, encapsulation, hydrophobicity, polysaccharide, and gel. The green cluster covers terms related to biopolymers, adhesives, antioxidants, tensile strength, and physiochemical properties. The blue color represents the terms chemistry, extraction, temperature, pH, and ultrasound. The yellow color represents the adhesive agent, rheology, viscosity, and molecular weight (Figure 1).
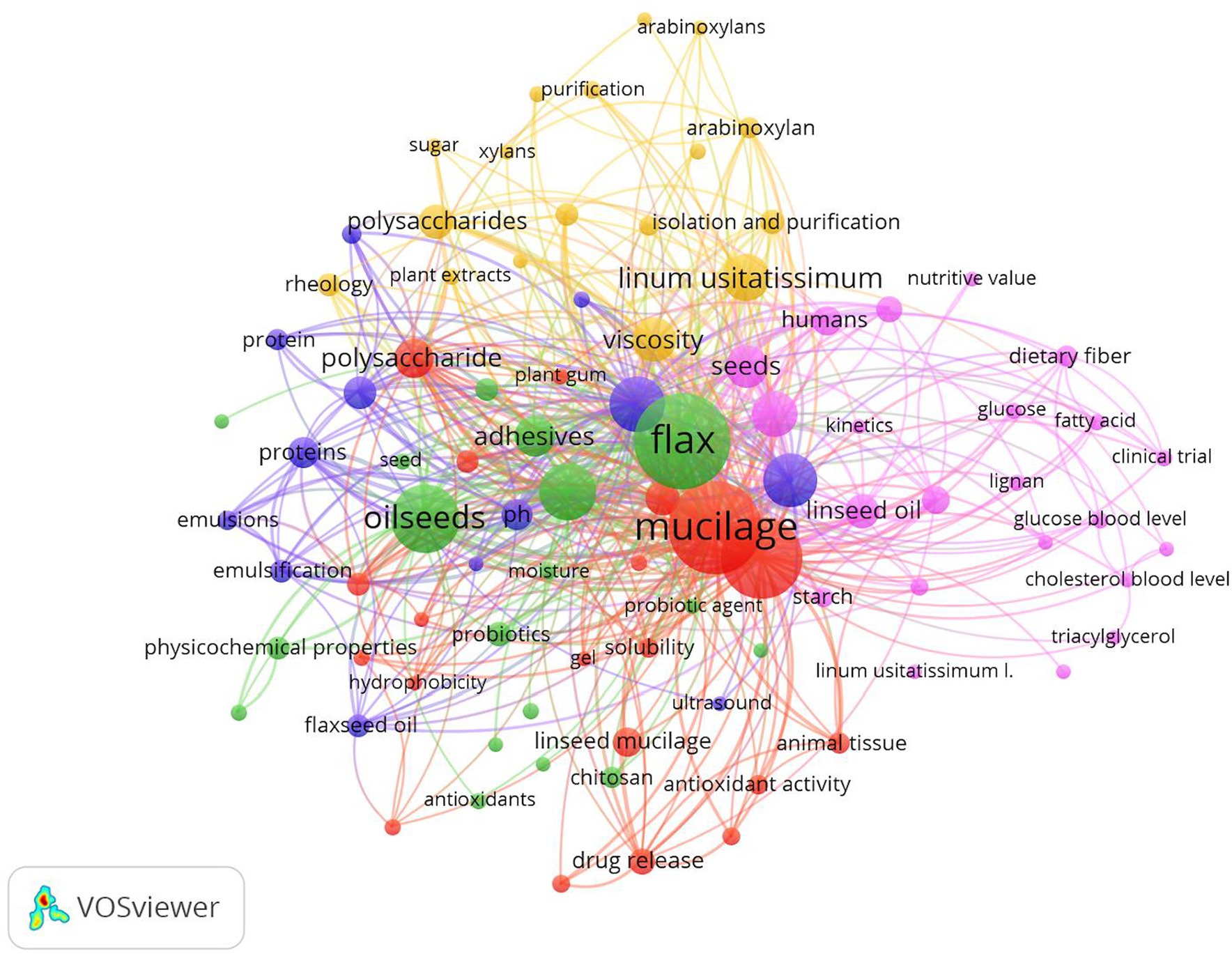
Figure 1. VOSviewer diagram with the analysis of co-occurrence of keywords indicating the publication trends on linseed mucilage (2000–December 2023).
3 Nutritional and chemical composition
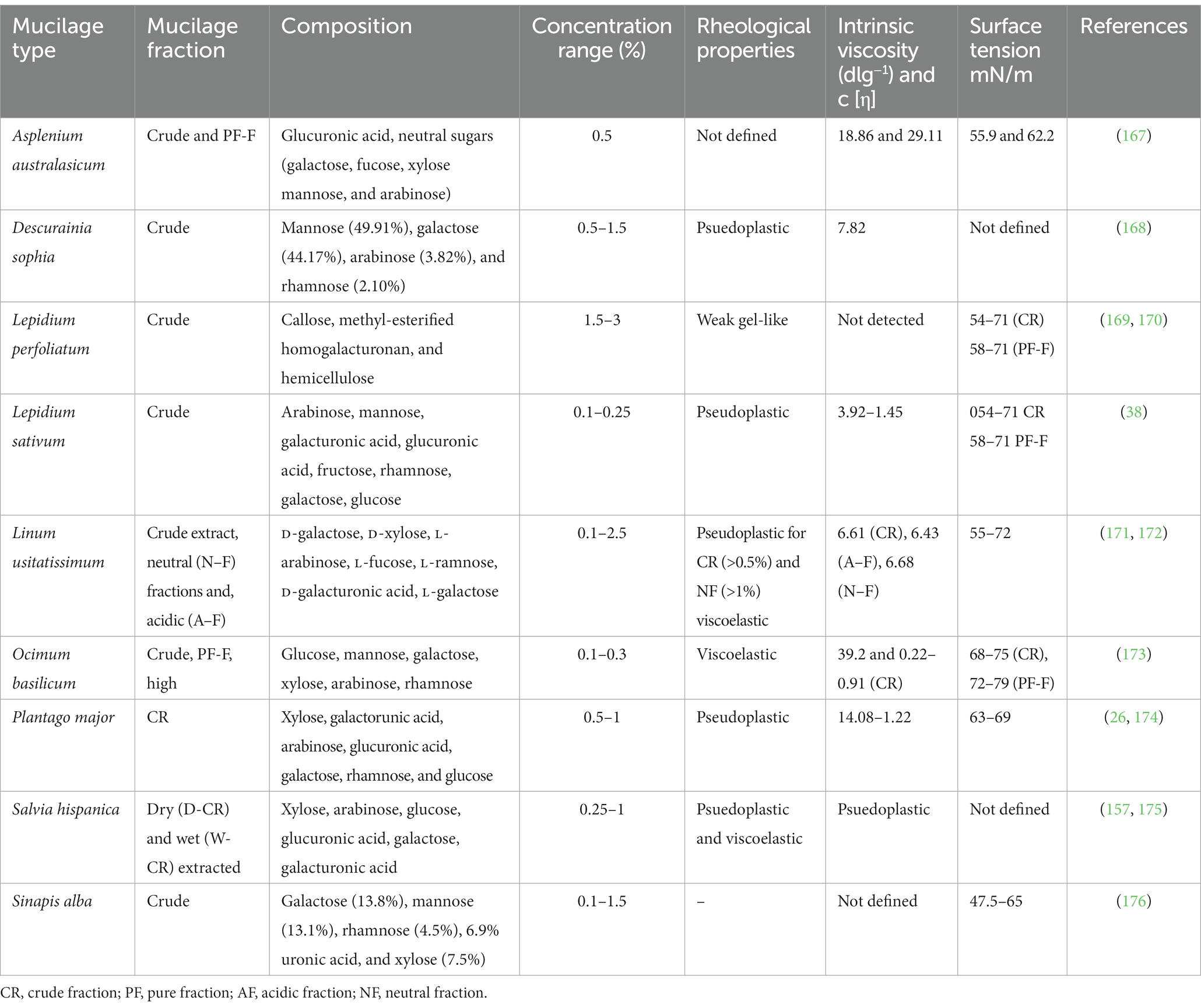
Linseed contains both soluble and insoluble fibers; its insoluble fraction contains lignin and cellulose, while the soluble fiber contains mucilage/gum, which is also well known as flaxseed gum (FG), linseed mucilage, or linseed gum (LG) (35). This soluble part of the seed is responsible for 6–10.2% of the mucilage content in linseed (21, 23). In comparison to other mucilage such as basil seed containing xylan (24.29%), glucan (2.31%), and glucomannan (43%), chia seed with glucose (19.6%), galactose (6.1%), arabinose (9.6%), xylose (38.5%), galacturonic acid (5.3%), and glucuronic acid (18.7%) (36, 37), and cress seed mucilage with glucose (1%), fructose (6.8%), arabinose (19.4%), rhamnose (1.9%), glucuronic acid (6.7%), and galactose (4.7%) (38). LM is composed of xylose (19–38%), galacturonic acid (21–36%), rhamnose (11–16%), arabinose (8–13%), galactose (12–16%), and glucose (4–6%). It is a heterogenic polysaccharide containing neutral and acidic parts, of which 75% is the neutral polymer, and has a molecular weight (MW) of approximately 1.2 × 106 g/mol. The acidic part has two fractions of polysaccharides designated as Acidic Fraction 1 (3.75%) with a MW of 6.5 × 105 g/mol and Acidic Fraction 2 (21.25%) with a MW of 1.7 × 104 g/mol (39). The acidic part consists of L-rhamnose, L-fucose, L-galactose, and D-galacturonic acid in the ratio of 2.6, 1:1.4:1.7, while the neutral fraction consists of D-xylose, L-arabinose, and D-galactose in the ratio of 3.5, 6.2:1 (40). The rhamnose-to-xylose ratio, representing the acidic to neutral polysaccharides, might vary from 0.3 to 2.2, but the ratio is often around 0.7 (25, 40). This acidic-to-neutral polysaccharide ratio of linseed varies substantially according to its origin and source of extraction. The acidic polysaccharides have a smaller molecular size and exhibit Newtonian flow-like behavior, whereas the neutral polymer has a larger molecular size and shows shear-thinning flow (41). Furthermore, LM is also rich in minerals such as zinc (15.43–53.43 mg/kg) and copper (18.87–148.08 mg/kg). However, it has a lower content of chromium, lead, and cadmium (42) (Table 1).
4 Extraction methods
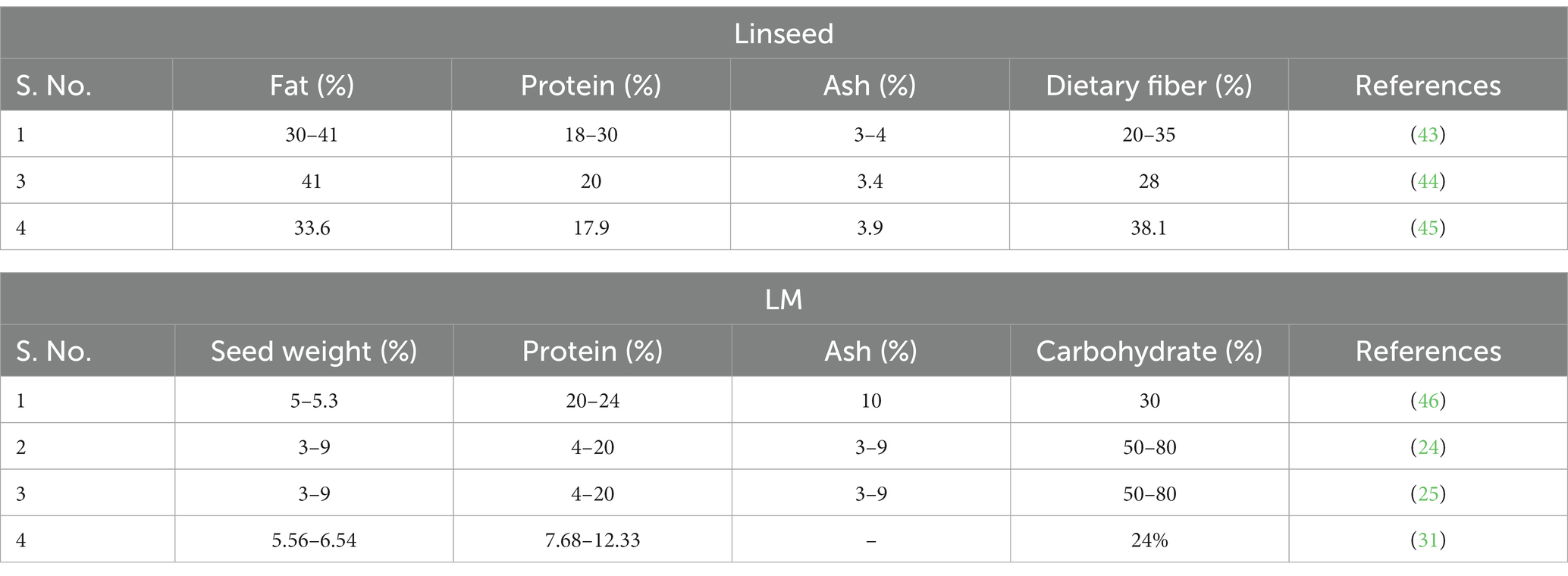
Extraction of LM through aqueous extraction methods, such as hot extraction and solvent extraction, is affected by a number of factors, such as temperature, pH, mucilage content, seed-to-water ratio, ionic strength, and extraction method, which affect mucilage composition (47). The yield and purity of LM can be increased by different techniques, such as enzymatic extraction, microwave-assisted extraction (MAE), pulse electric field (PEF), and ultrasound-assisted extraction (UAE). Extraction process of LM is an important factor that substantially affects the final yield and functional, chemical, and rheological characteristics (48). Mucilage extraction involves two primary stages: maceration and precipitation. During maceration, the raw materials are immersed in a solvent or water at room temperature for a specific duration under controlled conditions. Both the duration and temperature of maceration have a positive impact on the proteins and molecular weight of the mucilage. Additionally, the use of solvents like acid, alkali, and EDTA has been found to enhance both the yield and quality of mucilage (49). However, it is worth noting that excessively high maceration temperatures and prolonged stirring can develop mucilage with an undesirable color, making it less suitable for commercial purposes. This can be prevented by acid pretreatments (5). Precipitation comprises drying the extracted mucilage for further use. Whereas, from a technological standpoint, extracting LM can be broken down into three crucial phases: raw material preparation, extraction, and recovery. Raw material preparation encompasses mechanical procedures such as removing impurities, husking, and screening. The extraction phase involves the release of mucilage, which occurs in two distinct stages: hydration and swelling. In hydration, the initial steps involve soaking seeds in the solvent/water. Once the linseed is hydrated, the mucilage undergoes rapid expansion, causing the external cell walls to rupture and form a thick mucilage capsule that separates the seed surface from its surroundings. This critical phase is influenced by agitation and thermal exposure, as these factors can affect the chemical bonds and facilitate the release of mucilage (2, 50). In the last recovery phase, the hydrated LM is separated through various methods, including filtration, scraping, alcoholic precipitation, or high-speed centrifugation (Figure 2). To improve the final yield, solvents such as isopropyl alcohol, ethanol, ethylene diamine tetra-acetic acid (EDTA) (49), sodium hydroxide (51), and hydrochloric acid have also been employed (7, 52).
A number of techniques have been used to extract mucilage, for which linseeds can be used as whole seed, crushed, or in dehulled form. Whole seed is a quick, effective water extraction procedure designed to enhance the removal of mucilage from whole seeds. This method is generally used before grinding and oil separation (39, 53–56). Crushed linseeds offer the main advantage of utilizing industrial by-products effectively, although they might reduce the quality of extracted mucilage due to the mixing of proteins. Earlier studies on crushed linseed included kefir fermented beverage yogurt and sourdough linseed polysaccharides (24, 39, 54, 55). Mucilage extraction from linseed hull is a more efficient method, but this method needs technical help as dehulling is extra laborious work and the transfer of oil during dehulling is a challenge (57, 58).
4.1 Aqueous extraction method
Aqueous extraction method, also known as wet extraction, involves well-established techniques used for generations due to its good yield and easy mechanism. Raw linseeds are soaked in water or a suitable solvent, followed by various mechanical and thermal treatments to facilitate mucilage release. Various solvents can be employed for extracting mucilage, including cold water, hot water, mild acidic, and alkali extraction methods. The acid and alkali extraction method has a higher mucilage yield compared to water extraction, attributed to the solubilization of insoluble polysaccharides within cell walls through de-esterification and subsequent elimination processes (25, 52, 59, 60). Aqueous extraction method, which considers the use of organic solvents, drastic temperature, and energy requirements during extraction, limits its application in the food industry; therefore, various pretreatments are employed as part of the extraction method, which gives high-value mucilage for edible use.
4.2 Role of pretreatments
These treatments are used for the extraction/recovery of functional compounds from the cell because of their unique mechanisms to disrupt cells, which include enzymatic extraction (EE), microwave-assisted extraction (MAE), pulse electric field (PEF), and ultrasound-assisted extraction (UAE). Among them, the highest yield was found in hot water extraction (HWE) (8.96%), followed by UAE (7.84%), MAE (7.01%), and at last, AAE (6.44%) (61). Whereas, the efficiency of UAE is better than magnetic stirring and microwave (52). The major challenges associated with these technologies are mainly running costs and good capital investment in their implementation and application.
4.2.1 Enzymes
The underlying mechanism is based on the natural ability (specificity and regioselectivity) of enzymes to catalyze the hydrolysis of those components that are resistant to mass transfer, such as cell walls or binding to the target components, for example, pectin in the material matrix. When certain enzymes, such as cellulases, pectinases, and hemicellulases, are added to the extraction process, the structural integrity of the cell membranes and the wall is disrupted and degraded, enhancing the recovery of the target substances (62, 63). The enzymes used are specific to the cell wall composition. The effect of enzymes on cell wall breakdown and release of bioactive substances varied due to a number of factors, such as enzyme concentration and composition, solid-to-liquid ratio, type of solvent used during extraction, pH, enzyme/substrate ratio, time, and extraction temperature. For the extraction of LM, enzymes such as Pectinex Smash XXL, cellulase, β-glucosidase, and sulfatase are frequently utilized. The increase in extraction rate from 59 to 82% was accompanied by an increase in Pectinex Smash XXL from 50 to 200 μL kg−1 (64). This method has been widely applied in extracting bioactive compounds from linseed and yam (64–66).
4.2.2 Microwave-assisted extraction
The microwave treatment works on the principles of dipole rotation and ionic polarization. The vibrational moments in dipoles and ions are responsible for their kinetic energy, which converts to heat energy due to frictional effects. The volumetric heating raises the extraction temperature and accelerates the mass transfer rates, thereby improving the extraction yield. The permissible operating frequencies of microwave systems are 2,450 MHz and 915 MHz, which are most frequently used for heating in industrial and residential settings (67, 68). Factors such as frequency, microwave power, irradiation period, particle size, moisture content, solid-to-liquid ratio, solvent composition and type, extraction pressure, extraction temperature, and number of extraction cycles affect MAE (69, 70). However, in contrast, other studies reported that linseed carbohydrates are not sufficiently agitated to allow for any noticeable improvement in their extraction, and a large amount of energy is wasted in heating the water molecules, making microwave-aided extraction the least efficient (51).
4.2.3 Pulsed electric field
It works on the principle of electro-permeabilization, which forms pores and membrane breakdowns, leading to enhanced bioactive compound extraction (71–73). This technique enhances the solution’s solvent extraction and dehydration processes and increases the extraction rate by 50–80%. It was observed that there was an increase in yield up to 0.5 kV/cm, but higher power outputs ranging from 1 to 5 kV/cm did not result in a further increase in mucilage yield (74). Additionally, removing moisture from the material resulted in increased electrical conductivity, which may be due to the PEF-induced effect. Factors such as dehulling, milling, fractioning, the material’s electrical conductivity, the solvent used, frequency, pulse width, time, temperature, and wave shape affects PEF. The PEF requires more time to boost the effectiveness of the extraction, or a higher-energy PEF treatment may be necessary. However, this may have a negative impact on the structural integrity of bioactive materials since temperature increases throughout the treatment process are linked to bioactive materials (74).
4.2.4 Ultrasonication alternative extraction
UAE is an alternative extraction technique with more benefits than traditional extraction and is usually exercised for the extraction of bioactive compounds, volatile compounds, polysaccharides, and essential oils from different sources, including spices, herbs, roots, and seeds (75–77). UAE aims to provide efficient energy consumption, better antioxidant properties, and reduced extraction time. Some researchers have adopted hurdle technology, like in grapefruit, to extract pectin but found that extended temperature and time might lead to these polysaccharides and protein degradation (78). Several process parameters affecting ultrasonification extractions include extraction time and cycle, the solvent’s nature, sample characteristics like matrix characteristics, particle size, solid-to-liquid ratio, chemical parameters acidity, pH, alkalinity, and temperature that varied the mucilage yield from 7.24 to 11.04% (79). UAE employed for LM extraction shows minimal impact on protein and monosaccharide composition and reduces LM’s intrinsic viscosity (61). The ultrasonic waves tend to improve the extraction efficiency, solubility, and foam stability of mucilage (80). Furthermore, this technique proved to be more effective than magnetic stirring and even microwaves, possibly due to its higher mass transfer coefficient and higher order kinetics (51) (Table 2).
4.3 Factors affecting the extraction of linseed mucilage
Various technological parameters affect the yield of LM during extraction, such as pH, temperature, seed-to-water ratio, apparent viscosity, and protein content (82). With the increase in temperature, a reduction in water absorption and emulsifying capacity were observed (83). However, higher temperatures were associated with higher yield, ash, and protein content, which hampers the quality of the final mucilage (41). Therefore, moderate temperatures below 75°C were recommended to minimize protein denaturation (84). Additionally, pH significantly affects mucilage’s extraction yield and its fiber content. The maximum yield is obtained at the isoelectric point, and excessive use of acidic medium during the extraction of LM leads to the deterioration of mucilage. Acidic precipitation causes the protein residual matter in LM to decrease (e.g., acetic, trichloroacetic, and others). Although neither the sugar content nor the proximity of mucilage have changed during acidic precipitation, the LM extracted showed high thermal and mild acidic pH stability, lower surface charge density, and better solvation affinity, showing that LM is technically feasible for use in food product applications (84). However, the seed-to-water ratio is considered the other significant factor affecting mucilage yield (52). Seed-to-water ratio may lead to an increase or decrease in the viscosity of the medium and hence make it difficult to recover, which necessitates an optimal system dilution. Overall, temperature, pH, and seed-to-water ratio are the important factors that need to be considered for the extraction of mucilage (Table 3).
5 Health benefits associated with linseed mucilage
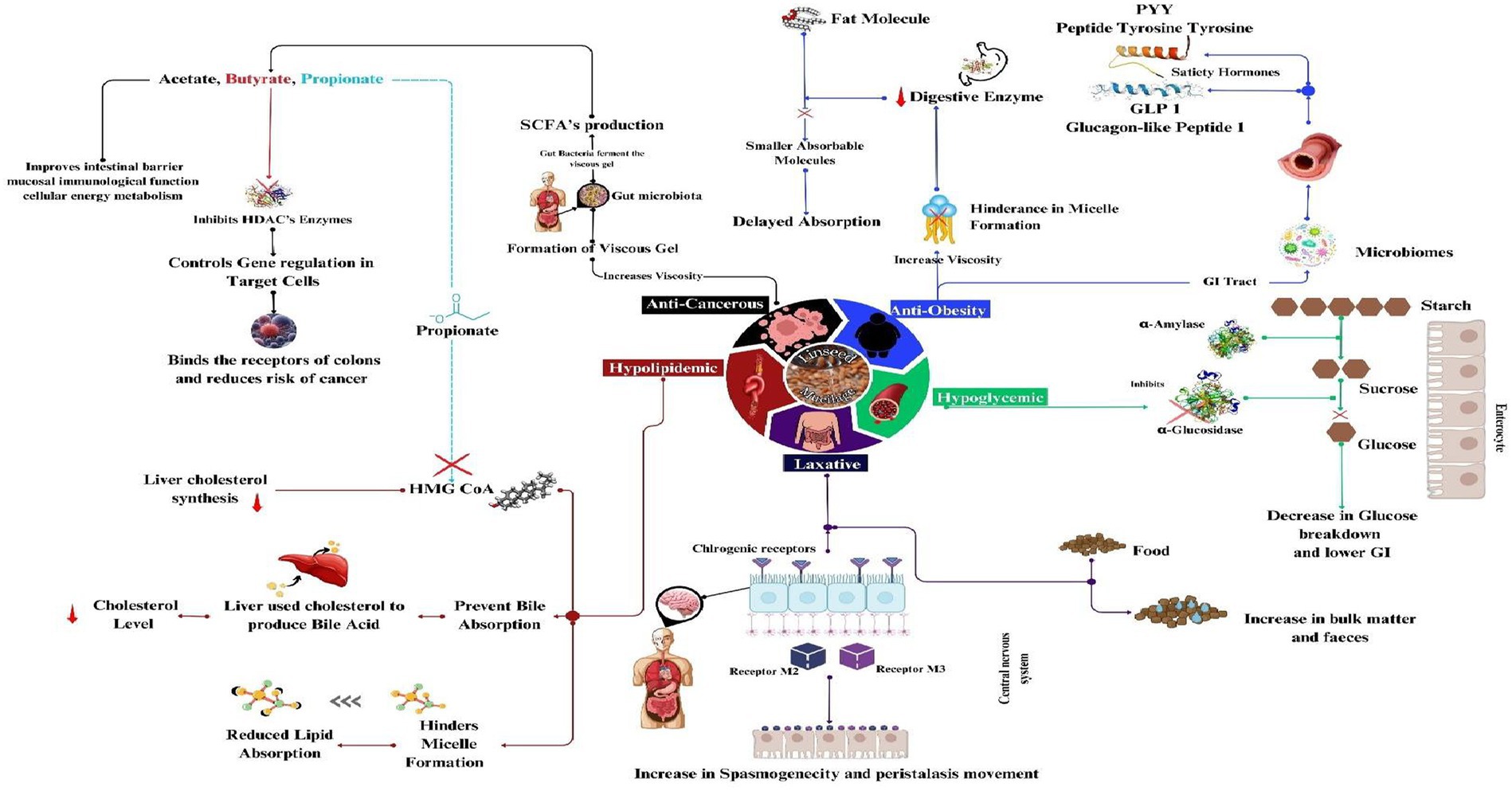
LM is a therapeutic by-product that addresses a number of medical issues. Studies have shown remarkable health advantages of LM, including delaying gastric emptying, controlling glycemic load, anti-cancer effects, anti-ulcer effects, laxative effects, and reducing constipation (31–34). LM has a deep mechanism of action in the prevention of diseases through the modulation of various metabolites, as shown in Figure 3 (Table 4).
5.1 Hypolipidemic
Cardiovascular conditions are a wide variety of diseases that affect the heart and blood vessels. One of its conditions is hyperlipidemia, which refers to a class of either inherited or acquired conditions characterized by high lipid levels in the human body. Globally, and especially in the Western hemisphere, elevated cholesterol is responsible for one-third of ischemic heart disease, resulting in an estimated 2.6 million deaths worldwide (99). Various studies have reported hypolipidemic properties of LM, and there could be a number of plausible mechanisms. LM hinders the formation of micelles and reduces lipid absorption, which may be related to the decline in hepatocyte production of VLDL (100). LM also prevents the reuptake of bile acids; more bile acids are produced in the liver, which diverts cholesterol from being used to make lipoproteins, lowering blood cholesterol. Another reason could be that LM speeds up bile excretion from the body and thus reduces LDL cholesterol (3, 101). LM surpasses through the small intestine’s digestive process and gets quickly fermented by the large intestine’s microbiota, leading to the production of short-chain fatty acids (SCFAs)—especially propionate, which inhibits the synthesis of cholesterol by reducing HMG CoA reductase activity (key enzyme synthesis of cholesterol) (102). Propionate can activate particular GPCRs, including GPR41 and GPR43, which are present in many types of organs, including adipose tissue and the liver. The regulation of lipid metabolism and cholesterol homeostasis have both been linked to the positive metabolic consequences of activating these receptors (103, 104). Linseed dietary fibers have been proven to be strongly fermentable in rats and humans, and results reported that the intervention of 5 g of dietary fibers from linseeds every day for a week significantly decreased total and LDL cholesterol by significantly increasing fecal fat extraction. Another possible mechanism of the hypolipidemic property of LM could be the effect of LM on bile acid metabolism (105). LM dissolves in water, resulting in the formation of viscous gels and, thus, increasing intraluminal viscosity. This modification of the gut lumen’s rheology could be one of the reasons that suggests the mechanical- or physical-based action of LM (104). Viscous fibers from Aloe vera leaf mucilage, psyllium husk, fruit, and vegetable fiber have also exhibited a hypolipidemic effect (106).
5.2 Hypoglycemic effect
Over the last several years, type 2 diabetes cases have soared, and the number of cases increased from 108 million in 1980 to 422 million, especially in low- and middle-income nations compared to high-income nations (107). There could be a number of factors, such as obesity, lack of physical activity, and smoking, responsible for diabetes. Since type 2 diabetes is characterized by hyperglycemia and impaired insulin sensitivity, carbohydrates have become a special dietary component of special importance.
Dietary fibers are well known to decrease the incidence of type 2 diabetes through glycemic management or reduced calorie consumption. LM is a polysaccharide compound that plays an important role in controlling diabetes. Studies have shown mucilage positively affects blood sugars in animals and humans (108). Soluble fibers delayed gastric emptying and decreased macronutrient absorption, resulting in lower insulin levels and postprandial blood glucose (109). Arabinoxylan (AX) is one of the soluble fibers found in LM; it quickly gets fermented by the colon’s bacteria in the GI tract. There is an inverse correlation between the amount of AX-rich bread consumed and the postprandial glucose response (103). The peculiar structure and rheological characteristics of LM, which have increased viscosity and improved swelling properties and help reduce blood glucose levels through a trapping mechanism, which is the main underlying mechanism for this impact, may be further explained by the inhibition of intestinal-glycosidases by mucilage (110). Also, this gelation process tends to retard enzyme mobilization for starch hydrolysis and glucose resorption (111). One such study, where adding 5 g of LM daily for 3 months lowered total and LDL cholesterol in type 2 diabetes by 10 and 16%, respectively (34) proves the importance of LM as an anti-diabetic agent. Different mucilage, like fenugreek seed mucilage and okra, have been attributed to the reduction in glucose levels (108, 112).
5.3 Anticancer
According to the WHO, cancer is the major cause of mortality worldwide, with more than 10 million deaths worldwide in 2020, and the most prevalent were found to be breast, lung, and colon cancer (113). Linseed is a rich source of both fibers (28%), such as soluble (1/3) and insoluble (2/3) fibers, whereas LM is a rich source of soluble fiber (114). Increased dietary fiber intake is linked to a lower likelihood of cancer. Soluble fibers in LM form viscous gel-like gooey substances in the colon that are easily fermented by gut microbiomes into short-chain fatty acids. LM can prevent cancer through several mechanisms, including bulking stool, speeding up transit time, and fermentation into SCFAs. Short-chain fatty acids enhance cell proliferation of the colonic mucosa, reducing the risk of colon cancer (104). Animal studies provide the link between SCFA and soluble fibers such as in mice and pigs, who were fed on a variety of linseed fibers and wheat. After incubation, gas production, and SCFA profiles were assessed, the amount of SCFA produced was highest in fecal samples obtained from animals fed with soluble LM compared to other fibers (115). Acetate, propionate, and butyrate are three SCFAs that are generated through the bacterial fermentation of soluble fiber in the intestinal lumen. SCFAs have several positive effects on the gut and general health, including acting as energy substrates for the gut. The most well-studied SCFA, butyrate, is responsible for many of the positive health benefits brought on by the intestinal fermentation of the mucilage. The SCFA butyrate improves its intestinal barrier, mucosal immunological function, and cellular energy metabolism and has some rather strong anti-inflammatory characteristics.
Butyrate can help prevent uncontrolled cell development and oxidative stress by inhibiting the enzyme histone deacetylase HDACs, which control particular genes (i.e., epigenetic regulation) in target cells (116, 117). BOHB (β-hydroxy butyrate) is an HDAC inhibitor; butyrate has comparable tumor-suppressing properties because it attaches to the same cell surface receptor in the colon. This reason could probably be one of the underlying processes through which the consumption of LM is thought to help lower the risk of colon cancer. In a supporting study compared to β-glucan control, linseed fiber treatment boosted SCFA synthesis and showed increased bacterial selection pressure (118). One clinical study proved that including 50 g LM/day for 4 weeks in adults could increase bowel movements per week by 30% compared to baseline (109). Other fibers, like psyllium husk, were also used for the same purpose. Both LM and psyllium products increase fecal bulk weight and are strongly linked with beneficial mechanisms that might improve colon health (119).
5.4 Anti-obesity
Over 1 billion individuals, including 340 million teenagers, 39 million children, and 650 million adults, are obese, which is still rising. According to the WHO, 167 million individuals, both children and adults, will be less healthy by 2025 due to being overweight or obese (120). This increases their risk of developing cardiovascular disease, diabetes, and cancer. One primary factor leading to obesity other than genetics and medical conditions is an increased energy intake-to-energy output ratio (121).
Dietary fiber can control calorie intake, facilitate weight loss, or maintain a healthy body weight (113). LM is a rich source of soluble dietary fiber and could serve as an anti-obesity agent. There could be a few proposed hidden mechanisms behind this. The viscous linseed fibers help lower body fat and control appetite satisfaction. LM is a soluble fiber that, on entering the large intestine, gets fermented and produces two hormones, glucagon-like peptide (GLP-1) and peptide YY (PYY); both of these gut hormones have a crucial role in inducing satiety (96, 122). Soluble fiber expands in the GI tract and transforms into a viscous substance that prolongs intestinal transit time, enabling thorough digestion and absorption. Food remains in the tract for longer, decreasing the next meal consumption (123).
Clinical trials provide evidence supporting that linseed mucilage, being abundant in soluble fiber, effectively contributes to the reduction of body weight (124, 125). Another possible mechanism responsible for this is the significant decrease in energy intake (126). The diets’ ME (metabolizable energy) content dropped as fiber intake rose. It should be noted that increasing fiber intake often lowers fat and protein digestibility (127). There could be a loss in weight with the consumption of LM, but this is accompanied by the type of diet (low or high fat) consumed (128, 129).
5.5 Other health benefits
5.5.1 Laxative effect
Constipation is one of the gastrointestinal motility disorders associated with severe complications such as hemorrhoids, fecal impaction, rectal prolapse, perforation, anal fissures, and overflow diarrhea. These are some of the consequences of constipation. Constipation is a condition that affects 16% of individuals globally (130). Factors affecting constipation include type of diet, colonic motility, genetic predisposition, absorption, daily behaviors, socioeconomic status, pharmaceuticals, and biological factors, of which diet is the most important factor.
LM exhibits laxative properties, which are among its most well-known health advantages in treating constipation. The possible mechanism for this may be that an increase in the viscosity of the gastrointestinal content plays a vital role in defecation; due to its high water-binding capacity, it enhances the moisture level in feces, which causes a change in the quality, resulting in increased peristalsis (bowel movement). The other possible mechanism is via a cholinergic pathway that makes cell lines spasmogenic in nature (131). LM activates the chlorogenic receptors in the central nervous system (CNS) that stimulate the muscarinic receptors (M2 and M3) to produce spasmogenic action, enhancing peristaltic movement in the GIT (132). LM is a soluble fiber that helps delay stomach emptying and has a moderate laxative (133). Furthermore, LM helps reduce the porosity of ulcers as it is viscous in nature, which causes increased viscosity in GIT. This significantly decreases the length and number of gastric ulcers and acts as an anti-ulcerative agent (31).
5.5.2 Prebiotics
Linseed mucilage acts as a prebiotic functional food, which might positively impact the human intestinal microbiota. This behavior facilitates changing bowel habits associated with the prevention of various illnesses like intestinal cancers due to its rich polysaccharide composition and high concentration of soluble heteropolysaccharides, which are the primary source of SCFAs. LM acts as a potential prebiotic and is known to confer health benefits to the host (8). However, it is noteworthy that the acidic fraction did not exhibit prebiotic activity, potentially due to the extensive branching of the xylose units. This branching structure may limit the accessibility of probiotic strains for fermentation. In contrast, the neutral polysaccharide fraction demonstrated significant potential for enhancing the growth of probiotic bacteria. Similar effects have been observed in mucilage derived from chia seeds (Hyptis suaveolens L.), psyllium seed, Aloe vera, basil, and P. ovata seed (134).
5.5.3 Antioxidant effect
Antioxidants are the chemicals that eliminate/scavenge free radicals from the system (135). These have preventive effects on heart diseases, diabetes, cancer, and other severe complications. These antioxidants directly combat free radicals to prevent or minimize cell oxidative damage. Mucilage is a polysaccharide that exhibits natural antioxidant properties directly proportional to the mucilage dose (136). LM shows good antioxidant quantity as it is rich in phenolic compounds such as ellagic acid, cinnamic acid, caffeic acid, epicatechin, and vanillic acid (137–139). This behavior of LM positively impacts the gut as it helps synthesize α-glucosidase inhibitors and lipase, which in turn regulate the metabolism of the GIT tract (91). Other supporting studies associated with mucilage antioxidant activity were recorded in okra, quince seed mucilage, and Opuntia ficus-indica (140, 141).
5.5.4 Anti-bacterial effect
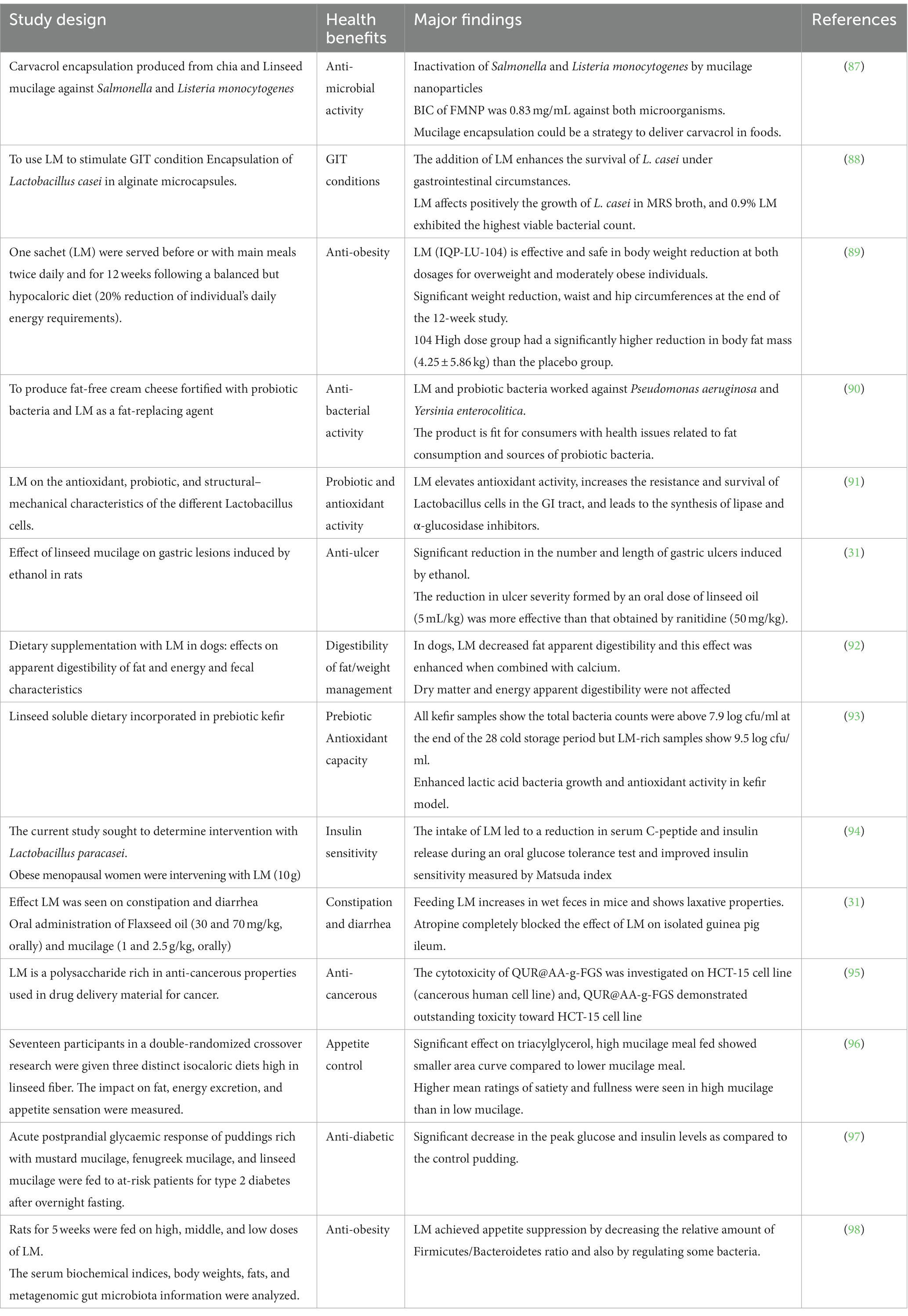
Linseed mucilage may have anti-bacterial properties against many bacterial strains. This might be due to its specific bioactive compounds and complex composition. It is effective against Escherichia coli, followed by Pseudomonas aeruginosa and Staphylococcus aureus (142). Other mucilage, such as those obtained from chia seed mucilage-based films, act against P. aeruginosa, E. coli, and S. aureus (140). LM is a rich source of polysaccharides like arabinoxylan and rhamnogalacturonans, which might interact with bacterial cell walls and disturb membrane integrity. Moreover, the good water-holding capacity might trap bacteria and prevent adherence to the surface, decreasing the chances of colonization and growth. LM provides evidence and successfully protects L. rhamnosus GG from the harsh environment (87).
5.5.5 Anti-inflammatory
Linseed mucilage has long been used as a nutritional supplement and in cosmetics. One of the reasons it is regarded as a useful component in the pharmaceutical and food sectors is due to its excellent anti-inflammatory action. A number of studies support that increasing dietary fiber, like LM intake, can decrease circulating levels of C-reactive protein (CRP), which is an inflammation marker in the body. CRP is also a predictor for CHD (coronary heart disease); the same relationship was established between dietary fiber and CRP (143). Furthermore, the DPPH scavenging activity and beta-carotene bleaching inhibition show LM to have strong antioxidant activity. These antioxidant properties of LM are thought to contribute to its healing or anti-inflammatory effect and are not dependent on the form of administration, whether it is applied topically or consumed orally (144).
6 Application in food
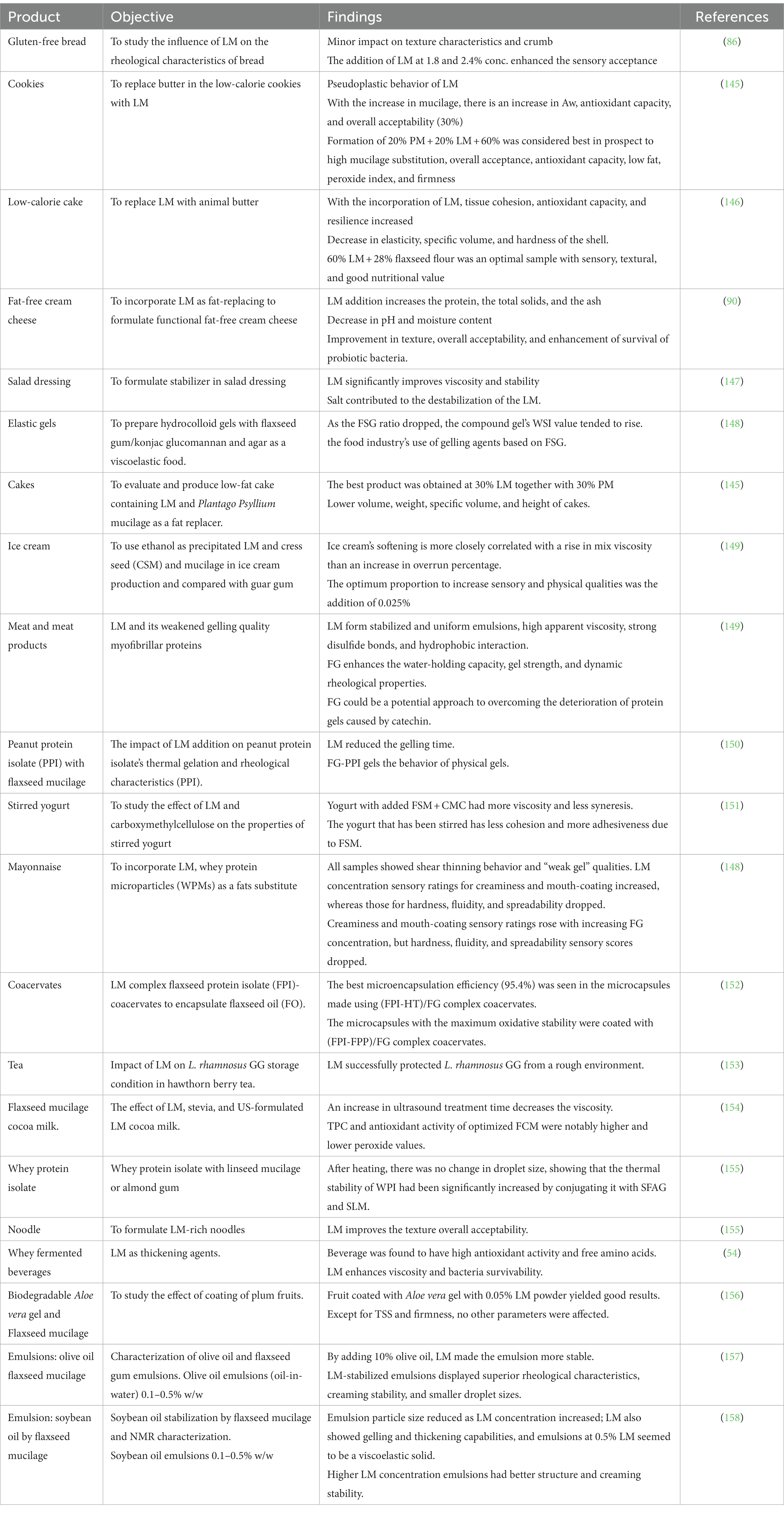
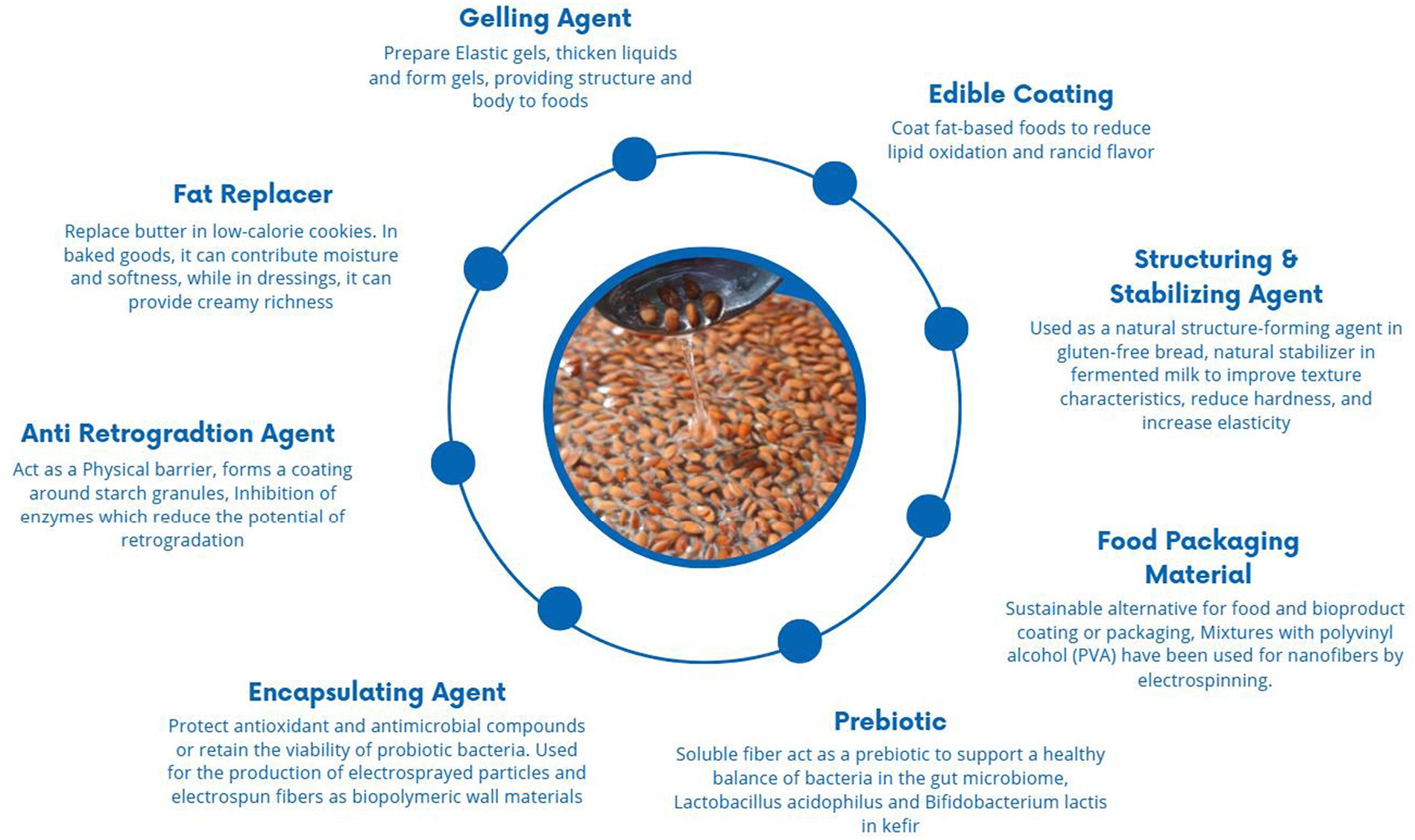
Due to its chemical structure and compositional features, LM acts as a gelling, thickening, binding, emulsifying, structuring, and fat-replacing agent (Table 5). Additionally, it shows good antioxidant potential and bioactive compounds, which makes it a valuable ingredient in functional foods and nutraceutical products. LM is considered a hydrocolloid with significant gelling properties; its addition improves the dynamic properties of the material, makes it stronger, and increases its ability to retain water due to increased hydrophobic interactions and cross-linking, which help in forming stable and consistent emulsions with a higher viscosity (25, 159). Mixing LM with other hydrocolloids increases the viscosity and has major benefits, such as reducing gel syneresis as observed in yogurt (154). Various factors, such as temperature, pH, and salt concentration, could affect gel strength. The dissolving temperature increased along with the gelling and melting points, and the addition of salt reduced the gel’s strength by lowering the Zeta potential. Various gluten replacers/structure-forming agents are used, such as hydrocolloids such as methylcellulose (MC), hydroxypropyl methylcellulose (HPMC), pectin, guar gum, xanthan, konjac gum, carrageenan, locust bean gum, agar, and psyllium gum, which are often thickeners and used as replacers in bread.
LM incorporated in gluten-free bread enhances the bread quality of the dough as its arabinoxylan contents maintain gas cells during the initial baking stage, expanding the oven rise and enhancing the properties of the bread as its structure, loaf volume, crumb firmness, and texture (86, 160). Furthermore, incorporating LM exhibits comparable rheological characteristics to starch, pectin, and guar gum and contributes to enhancing the sensory attributes of bread (86). LM incorporated at high concentrations increases the viscosity of an aqueous dispersion and exerts a positive effect on surface-active proteins, but at higher concentrations, the increased viscosity can also reduce the free expansion of foam (161). Additionally, it enhances the meat product quality, and incorporating LM into starch can inhibit retrogradation by forming hydrogen bonds with starch molecules, which leads to increased water absorption, which helps to maintain the texture of starch gels and improve overall quality. LM is also used as a fat replacer to develop fat-free cheese; its incorporation reduces moisture content and increases viscosity (90). A number of mucilage-based products are commonly found in diets, including supplements, natural thickeners, pharmaceuticals, and personal care products (Figure 4).
7 Linseed mucilage in food packaging
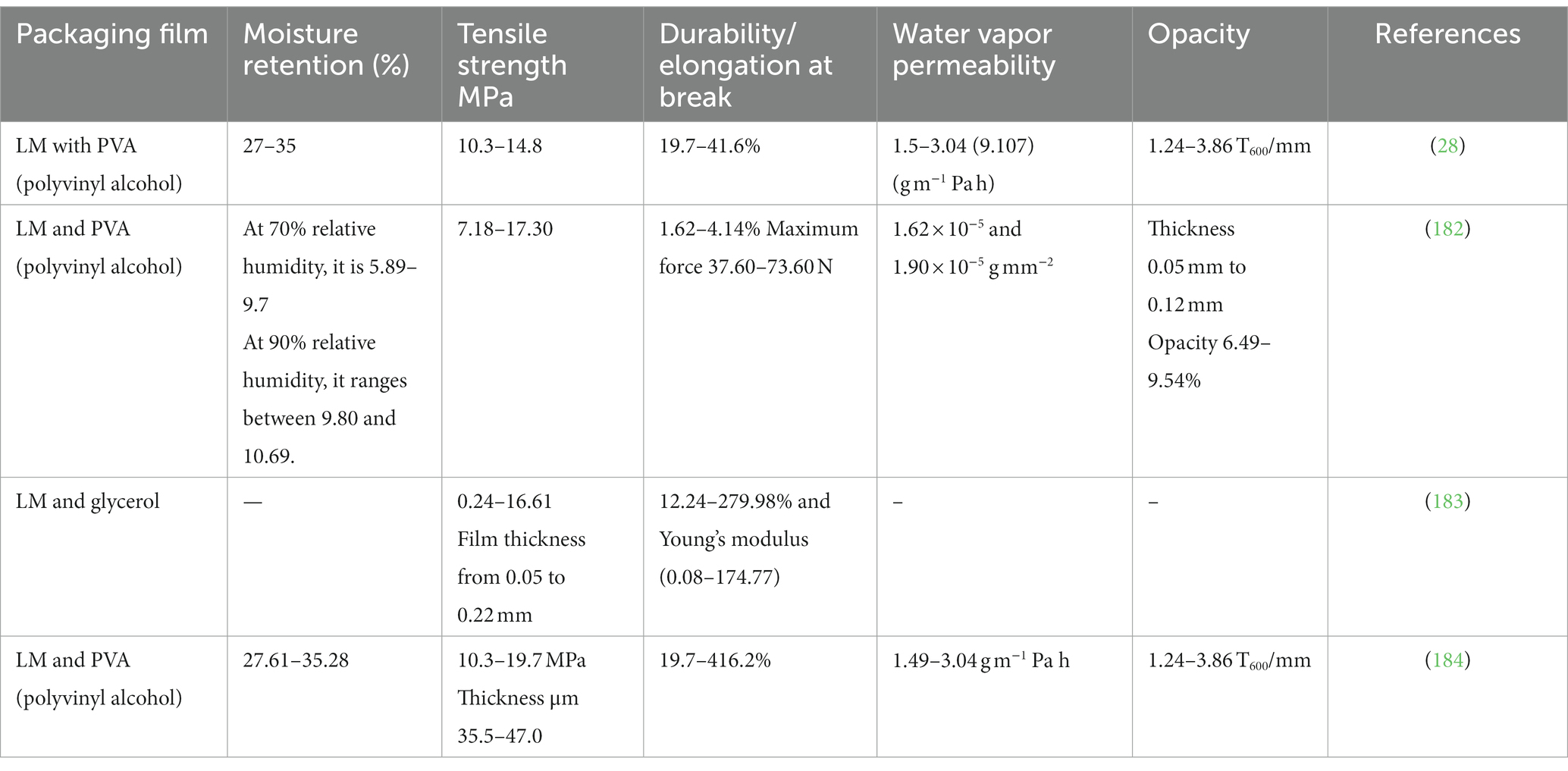
Due to escalating environmental concerns and the global pollution crisis, the demand for biodegradable plant-based polymers (gums, mucilage, cellulose, and glucans) has garnered growing research attention. This shift is owed to their benefits, which include biodegradability, cost-effectiveness, and ease of production (162). The enhanced solubility, emulsion, hydrophobicity, barrier, and mechanical properties of polysaccharides, such as carboxymethyl cellulose, Aloe vera, chitosan, alginate, and pectin, successfully reduce the problem of quality loss of food products during storage (163–166). The food packaging industry is a huge sector, with its primary significance stemming from its ability to prevent the loss of nutrients and minimize microbial growth and other environmental contaminants, ultimately leading to product shelf-life extension. Edible packaging should possess certain mechanical and barrier qualities. An ideal edible package is generally colorless/tasteless, strong, and possesses low moisture and gas permeability. Some factors that make up the mechanical properties are tensile strength, elongation at break, deformability, and elastic modulus, as mentioned in Table 6 (177, 178). The mechanical properties depend on different environmental factors and the composition of the film. These properties are important to maintain the integrity of food products during processing and storage.
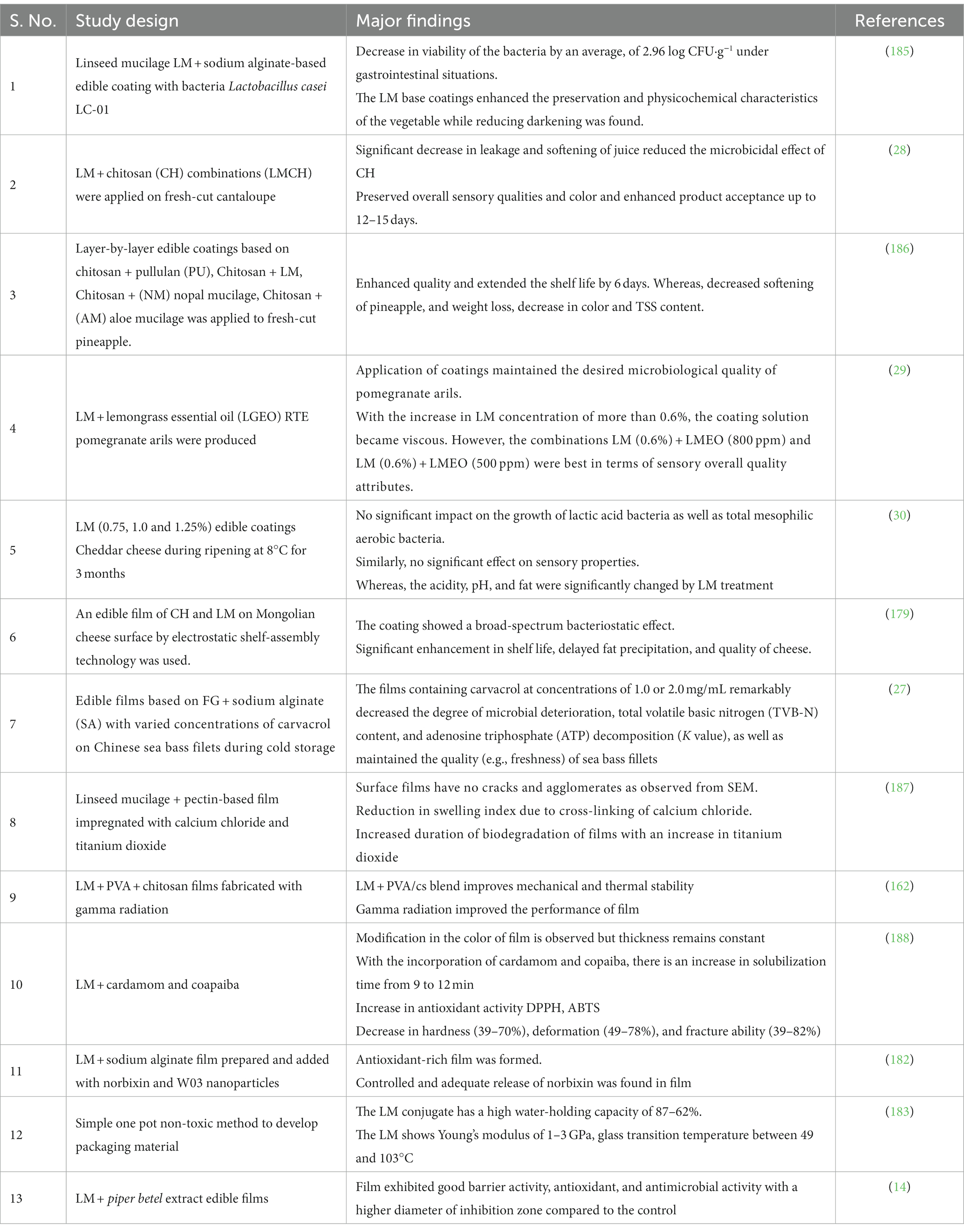
LM holds potential as an edible coating in food products (27, 28, 179). This application is based on its excellent functional properties, such as film-forming ability, moisture retention, biodegradability, water vapor permeability, adhesiveness, natural resistance to microbes, and various health benefits in the body (180). Moreover, LM shows excellent antioxidant, anti-bacterial, and antimicrobial properties that lead to enhanced packaging functionality. LM-based films not only increase shelf life from UV light during storage but also effectively maintain the viability of probiotics, reducing leakage and fruit softening and preserving sensorial properties (28, 31). The chemical reaction between the intermolecular bonds in the polymer chain (181), such as tertiary conjugate, was formulated using LM, gelatin, and oxidized tannic acid and showed the use of LM as wall material (9) (Table 7).
However, there are certain constraints because the films produced from pure mucilage lack the requisite mechanical properties to meet the food packaging requirements. Additional challenges associated with the use of LM in food packaging include high water solubility, high viscosity, and high swelling capacity. An effective way to ameliorate the physicochemical characteristics of LM coating is through blending modification (182). Numerous research endeavors focused on exploring the capacity of LM as an application in bioplastics, film formation, and edible coatings, as shown in Table 8.
8 Conclusion and future perspectives
Linseed mucilage (LM) studies have unveiled an opportunity to explore it as an abundant source of dietary fibers and bioactive compounds. The application of LM as a functional food is supported by its remarkable health-promoting properties. The conventional extraction methods are harsh, leading to deterioration of the quality of LM and thus a reduction in its health advantages. This can be overcome by non-conventional pretreatment approaches prior to extraction that aim to improve the mucilage quality, thus promoting their industrial utilization. The addition of LM to food products has been made possible owing to its excellent functional properties, namely water-holding, gelling, thickening, binding, texturing, foaming, emulsion formation, and stabilizing characteristics. The protective properties exhibited by LM aid in increasing the shelf life of food products, thus suggesting its potential application as a food packaging film and coating while maintaining its sensory properties. The use of LM opens opportunities for greener, innovative prospects and emerging developments in the field of food packaging by providing a potential solution for plastic packaging waste. Subsequent research efforts could be directed toward enhancing the mechanical strength of LM films and improving their microbial resistance to provide a complete solution as biodegradable packaging on a commercial scale.
Author contributions
MC: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Resources, Visualization. RC: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Supervision, Validation. BT: Writing – original draft, Writing – review & editing. SH: Writing – original draft, Writing – review & editing. PS: Writing – original draft, Writing – review & editing. AD: Writing – review & editing. AP: Writing – review & editing, Software.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Procacci, S , Bojórquez-Quintal, E , Platamone, G , Maccioni, O , Vecchio, VL , Morreale, V, et al. Opuntia ficus-indica pruning waste recycling: recovery and characterization of mucilage from cladodes. Nat Resour. (2021) 12:91–107. doi: 10.4236/nr.2021.124008
2. Tsai, AYL , McGee, R , Dean, GH , Haughn, GW , and Sawa, S . Seed mucilage: biological functions and potential applications in biotechnology. Plant Cell Physiol. (2021) 62:1847–57. doi: 10.1093/pcp/pcab099
3. Fukumitsu, S , Aida, K , Shimizu, H , and Toyoda, K . Flaxseed lignan lowers blood cholesterol and decreases liver disease risk factors in moderately hypercholesterolemic men. Nutr Res. (2010) 30:441–6. doi: 10.1016/j.nutres.2010.06.004
4. Goksen, G , Demir, D , Dhama, K , Kumar, M , Shao, P , Xie, F, et al. Mucilage polysaccharide as a plant secretion: potential trends in food and biomedical applications. Int J Biol Macromol. (2023) 230:123146. doi: 10.1016/j.ijbiomac.2023.123146
5. Kamel, R , Afifi, SM , Kassem, IAA , Elkasabgy, NA , and Farag, MA . Arabinoxylan and rhamnogalacturonan mucilage: outgoing and potential trends of pharmaceutical, environmental, and medicinal merits. Int J Biol Macromol. (2020) 165:2550–64. doi: 10.1016/j.ijbiomac.2020.10.175
6. Galloway, AF , Knox, P , and Krause, K . Sticky mucilages and exudates of plants: putative microenvironmental design elements with biotechnological value. New Phytol. (2020) 225:1461–9. doi: 10.1111/nph.16144
7. Tosif, MM , Najda, A , Bains, A , Kaushik, R , Dhull, SB , Chawla, P, et al. A comprehensive review on plant-derived mucilage: characterization, functional properties, applications, and its utilization for nanocarrier fabrication. Polymers. (2021) 13:1066. doi: 10.3390/polym13071066
8. Puligundla, P , and Lim, S . A review of extraction techniques and food applications of flaxseed mucilage. Foods. (2022) 11:1677. doi: 10.3390/foods11121677
9. Mohseni, F , and Goli, SAH . Encapsulation of flaxseed oil in the tertiary conjugate of oxidized tannic acid-gelatin and flaxseed (Linum usitatissimum) mucilage. Int J Biol Macromol. (2019) 140:959–64. doi: 10.1016/j.ijbiomac.2019.08.197
10. Alhssan, E , Ercan, SŞ , and Bozkurt, H . Effect of flaxseed mucilage and gum Arabic on probiotic survival and quality of kefir during cold storage. Foods. (2023) 12:662. doi: 10.3390/foods12030662
11. Mishra, A , Mohite, AM , and Sharma, N . Influence of particle size on physical, mechanical, thermal, and morphological properties of tamarind- fenugreek mucilage biodegradable films. Polym Bull. (2023) 80:3119–33. doi: 10.1007/s00289-022-04214-1
12. Seid Mohammadifard, SZ , Zariinghalami, S , Zandi, M , and Pakpour, M . Influence of the mucilage and chia seed (Salvia hispanica L.) oil addition on the physicochemical and sensory properties of yoghurt during storage time. J Food Sci Technol [Internet]. (2023) 19:237–49. doi: 10.22034/FSCT.19.132.237
13. Karami, N , Kamkar, A , Shahbazi, Y , and Misaghi, A . Effects of active chitosan-flaxseed mucilage-based films on the preservation of minced trout fillets: a comparison among aerobic, vacuum, and modified atmosphere packaging. Packag Technol Sci. (2020) 33:469–84. doi: 10.1002/pts.2530
14. Manzoor, A , Ahmad, S , and Yousuf, B . Development and characterization of edible films based on flaxseed gum incorporated with Piper betle extract. Int J Biol Macromol. (2023) 245:125562. doi: 10.1016/j.ijbiomac.2023.125562
15. Jhala, A , and Hall, LM . Flax (Linum usitatissimum L.): current uses and future applications. Aust J Basic Appl Sci. (2010) 4:4304–12.
16. Singh, PK , Chopra, P , Singh, PK , and Chopra, P . Double purpose linseed: a viable option for doubling farmers’ income in the north-western Himalyan region. Indian Farming. (2018) 68:6
17. ICAR (2018). Available at: https://aicrp.icar.gov.in/linseed/pc-massage/
18. Bahadorian, A , Sadrameli, SM , Pahlavanzadeh, H , and Ilani Kashkouli, MN . Optimization study of linseed biodiesel production via in-situ transesterification and slow pyrolysis of obtained linseed residue. Renew Energy. (2023) 203:10–9. doi: 10.1016/j.renene.2022.12.043
19. Nykter, M , Kymäläinen, HR , and Gates, F . Quality characteristics of edible linseed oil. Agric Food Sci. (2008) 15:402. doi: 10.2137/145960606780061443
20. Shim, YY , Gui, B , Wang, Y , and Reaney, MJT . Flaxseed (Linum usitatissimum L.) oil processing and selected products. Trends Food Sci Technol. (2015) 43:162–77. doi: 10.1016/j.tifs.2015.03.001
21. Singh, KK , Mridula, D , Rehal, J , and Barnwal, P . Flaxseed: a potential source of food, feed and Fiber. Crit Rev Food Sci Nutr. (2011) 51:210–22. doi: 10.1080/10408390903537241
22. Goyal, A , Sharma, V , Upadhyay, N , Gill, S , and Sihag, M . Flax and flaxseed oil: an ancient medicine & modern functional food. J Food Sci Technol. (2014) 51:1633–53. doi: 10.1007/s13197-013-1247-9
23. Gros, C , Lanoisellé, JL , and Vorobiev, E . Towards an alternative extraction process for linseed oil. Chem Eng Res Des. (2003) 81:1059–65. doi: 10.1205/026387603770866182
24. Fedeniuk, RW , and Biliaderis, CG . Composition and physicochemical properties of linseed (Linum usitatissimum L.) mucilage. J Agric Food Chem. (1994) 42:240–7. doi: 10.1021/jf00038a003
25. Cui, W , and Mazza, G . Physicochemical characteristics of flaxseed gum. Food Res Int. (1996) 29:397–402. doi: 10.1016/0963-9969(96)00005-1
26. Samuelsen, AB . The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J Ethnopharmacol. (2000) 71:1–21. doi: 10.1016/S0378-8741(00)00212-9
27. Fang, S , Zhou, Q , Hu, Y , Liu, F , Mei, J , and Xie, J . Antimicrobial carvacrol incorporated in flaxseed gum-sodium alginate active films to improve the quality attributes of Chinese Sea bass (Lateolabrax maculatus) during cold storage. Molecules. (2019) 24:3292. doi: 10.3390/molecules24183292
28. Treviño-Garza, M , Correa-Cerón, R , Ortiz-Lechuga, E , Solís-Arévalo, K , Castillo-Hernández, S , Gallardo-Rivera, C, et al. Effect of linseed (Linum usitatissimum) mucilage and chitosan edible coatings on quality and shelf-life of fresh-cut cantaloupe (Cucumis melo). Coatings. (2019) 9:368. doi: 10.3390/coatings9060368
29. Yousuf, B , and Srivastava, AK . Flaxseed gum in combination with lemongrass essential oil as an effective edible coating for ready-to-eat pomegranate arils. Int J Biol Macromol. (2017) 104:1030–8. doi: 10.1016/j.ijbiomac.2017.07.025
30. Soleimani-Rambod, A , Zomorodi, S , Naghizadeh Raeisi, S , Khosrowshahi Asl, A , and Shahidi, SA . The effect of xanthan gum and flaxseed mucilage as edible coatings in Cheddar cheese during ripening. Coatings. (2018) 8:80. doi: 10.3390/coatings8020080
31. Dugani, A , Auzzi, A , Naas, F , and Megwez, S . Effects of the oil and mucilage from flaxseed (Linum Usitatissimum) on gastric lesions induced by ethanol in rats. Libyan J Med. (2008) 3:166–9. doi: 10.3402/ljm.v3i4.4787
32. DeLuca, JAA , Garcia-Villatoro, EL , and Allred, CD . Flaxseed bioactive compounds and colorectal Cancer prevention. Curr Oncol Rep. (2018) 20:59. doi: 10.1007/s11912-018-0704-z
33. Moghaddasi, MS . Linseed and usages in humanlife. In: Advances in Environmental Biology. (2011) 1380–93. Gale Academic OneFile. Available at: https://link.gale.com/apps/doc/ (Accessed January 19, 2024).
34. Thakur, G , Mitra, A , Pal, K , and Rousseau, D . Effect of flaxseed gum on reduction of blood glucose and cholesterol in type 2 diabetic patients. Int J Food Sci Nutr. (2009) 60:126–36. doi: 10.1080/09637480903022735
35. Kaur, M , Kaur, R , and Punia, S . Characterization of mucilages extracted from different flaxseed (Linum usitatissiumum L.) cultivars: a heteropolysaccharide with desirable functional and rheological properties. Int J Biol Macromol. (2018) 117:919–27. doi: 10.1016/j.ijbiomac.2018.06.010
36. Hosseini, MS , and Nabid, MR . Synthesis of chemically cross-linked hydrogel films based on basil seed (Ocimum basilicum L.) mucilage for wound dressing drug delivery applications. Int J Biol Macromol. (2020) 163:336–47. doi: 10.1016/j.ijbiomac.2020.06.252
37. Avlani, D , Agarwal, V , Khattry, V , Biswas, GR , and Majee, SB . Exploring properties of sweet basil seed mucilage in development of pharmaceutical suspensions and surfactant-free stable emulsions. Int J Appl Pharm. (2019) 11:124. doi: 10.22159/ijap.2019v11i1.29877
38. Behrouzian, F , Razavi, SMA , and Phillips, GO . Cress seed (Lepidium sativum) mucilage, an overview. Bioact Carbohydr Diet Fibre. (2014) 3:17–28. doi: 10.1016/j.bcdf.2014.01.001
39. Warr, J , Michaud, P , Picton, L , Muller, G , Courtois, B , Ralainirina, R, et al. Large-scale purification of water-soluble polysaccharides from flaxseed mucilage, and isolation of a new anionic polymer. Chromatographia. (2003) 58:331–5. doi: 10.1365/s10337-003-0060-4
40. Muralikrishna, G , Salimath, PV , and Tharanathan, RN . Structural features of an arabinoxylan and a rhamno-galacturonan derived from linseed mucilage. Carbohydr Res. (1987) 161:265–71. doi: 10.1016/S0008-6215(00)90083-1
41. Barbary, OM , El-Sohaimy, S , El-Saadani, MA , and Zeitoun, A . Extraction, composition and physicochemical properties of flaxseed mucilage. J Adv Agric Res. (2009) 14:605–22.
42. Guilloux, K , Gaillard, I , Courtois, J , Courtois, B , and Petit, E . Production of arabinoxylan-oligosaccharides from flaxseed (Linum usitatissimum). J Agric Food Chem. (2009) 57:11308–13. doi: 10.1021/jf902212z
43. Bekhit, AEDA , Shavandi, A , Jodjaja, T , Birch, J , Teh, S , Mohamed Ahmed, IA, et al. Flaxseed: composition, detoxification, utilization, and opportunities. Biocatal Agric Biotechnol. (2018) 13:129–52. doi: 10.1016/j.bcab.2017.11.017
44. Morris, DH , and Vaisey-Genser, M . Availability and labeling of flaxseed food products and supplements In: LU Thompson and SC Cunnane, editors. Flaxseed in human nutrition. Champaign, IL: AOCS Press (2003)
45. Bozan, B , and Temelli, F . Chemical composition and oxidative stability of flax, safflower and poppy seed and seed oils. Bioresour Technol. (2008) 99:6354–9. doi: 10.1016/j.biortech.2007.12.009
46. Bhatty, R. S . Nutrient composition of whole flaxseed and flaxseed meal. (1995). Available at: https://cir.nii.ac.jp/crid/1570854175364920192
47. Farahnaky, A , Shanesazzadeh, E , Mesbahi, G , and Majzoobi, M . Effect of various salts and pH condition on rheological properties of Salvia macrosiphon hydrocolloid solutions. J Food Eng. (2013) 116:782–8. doi: 10.1016/j.jfoodeng.2013.01.036
48. Nayak, AK , Pal, D , Pradhan, J , and Hasnain, MS . Fenugreek seed mucilage-alginate mucoadhesive beads of metformin HCl: design, optimization and evaluation. Int J Biol Macromol. (2013) 54:144–54. doi: 10.1016/j.ijbiomac.2012.12.008
49. Rashid, F , Ahmed, Z , Hussain, S , Huang, JY , and Ahmad, A . Linum usitatissimum L. seeds: flax gum extraction, physicochemical and functional characterization. Carbohydr Polym. (2019) 215:29–38. doi: 10.1016/j.carbpol.2019.03.054
50. Naran, R , Chen, G , and Carpita, NC . Novel Rhamnogalacturonan I and Arabinoxylan polysaccharides of flax seed mucilage. Plant Physiol. (2008) 148:132–41. doi: 10.1104/pp.108.123513
51. Fabre, JF , Lacroux, E , Valentin, R , and Mouloungui, Z . Ultrasonication as a highly efficient method of flaxseed mucilage extraction. Ind Crop Prod. (2015) 65:354–60. doi: 10.1016/j.indcrop.2014.11.015
52. Ziolkovska, A . Laws of flaxseed mucilage extraction. Food Hydrocoll. (2012) 26:197–204. doi: 10.1016/j.foodhyd.2011.04.022
53. Diederichsen, A , Raney, JP , and Duguid, SD . Variation of mucilage in flax seed and its relationship with other seed characters. Crop Sci. (2006) 46:365–71. doi: 10.2135/cropsci2005.0146
54. Łopusiewicz, Ł , Drozłowska, E , Siedlecka, P , Mężyńska, M , Bartkowiak, A , Sienkiewicz, M, et al. Development, characterization, and bioactivity of non-dairy kefir-like fermented beverage based on flaxseed oil cake. Foods. (2019) 8:544. doi: 10.3390/foods8110544
55. Ray, S , Paynel, F , Morvan, C , Lerouge, P , Driouich, A , and Ray, B . Characterization of mucilage polysaccharides, arabinogalactanproteins and cell-wall hemicellulosic polysaccharides isolated from flax seed meal: a wealth of structural moieties. Carbohydr Polym. (2013) 93:651–60. doi: 10.1016/j.carbpol.2012.12.034
56. Rocha, MS , Rocha, LCS , Feijó, MBDS , Marotta, PLLDS , and Mourão, SC . Multiobjective optimization of the flaxseed mucilage extraction process using normal-boundary intersection approach. Br Food J. (2021) 123:3805–23. doi: 10.1108/BFJ-06-2020-0501
57. Ding, HH , Cui, SW , Goff, HD , Wang, Q , Chen, J , and Han, NF . Soluble polysaccharides from flaxseed kernel as a new source of dietary fibres: extraction and physicochemical characterization. Food Res Int. (2014) 56:166–73. doi: 10.1016/j.foodres.2013.12.005
58. Qian, QY . Structure-function relationship of flaxseed gum from flaxseed hulls. The University of Guelph. (2014). Available at: https://atrium.lib.uoguelph.ca/server/api/core/bitstreams/61d80509-2e39-4771-9ffa-2360307acdf9/content
59. Koocheki, A , Mortazavi, SA , Shahidi, F , Razavi, SMA , Kadkhodaee, R , and Milani, JM . Optimization of mucilage extraction from qodume shirazi seed (Alyssum homolocarpum) using response surface methodology. J Food Process Eng. (2009) 33:861–82. doi: 10.1111/j.1745-4530.2008.00312.x
60. Nazir, S , Wani, IA , and Masoodi, FA . Extraction optimization of mucilage from basil (Ocimum basilicum L.) seeds using response surface methodology. J Adv Res. (2017) 8:235–44. doi: 10.1016/j.jare.2017.01.003
61. Safdar, B , Zhihua, P , Xinqi, L , Jatoi, MA , and Rashid, MT . Influence of different extraction techniques on recovery, purity, antioxidant activities, and microstructure of flaxseed gum. J Food Sci. (2020) 85:3168–82. doi: 10.1111/1750-3841.15426
62. De Moura, JMLN , Campbell, K , Mahfuz, A , Jung, S , Glatz, CE , and Johnson, L . Enzyme-assisted aqueous extraction of oil and protein from soybeans and cream De-emulsification. J Am Oil Chem Soc. (2008) 85:985–95. doi: 10.1007/s11746-008-1282-2
63. Puri, M , Sharma, D , and Barrow, CJ . Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. (2012) 30:37–44. doi: 10.1016/j.tibtech.2011.06.014
64. Ribeiro, BD , Barreto, DW , and Coelho, MAZ . Enzyme-enhanced extraction of phenolic compounds and proteins from flaxseed meal. ISRN Biotechnol. (2013) 2013:1–6. doi: 10.5402/2013/521067
65. Ma, F , Wang, D , Zhang, Y , Li, M , Qing, W , Tikkanen-Kaukanen, C, et al. Characterisation of the mucilage polysaccharides from Dioscorea opposita Thunb. With enzymatic hydrolysis. Food Chem. (2018) 245:13–21. doi: 10.1016/j.foodchem.2017.10.080
66. Wanasundara, PKJPD , and Shahidi, F . Removal of flaxseed mucilage by chemical and enzymatic treatments. Food Chem. (1997) 59:47–55. doi: 10.1016/S0308-8146(96)00093-3
67. Azmir, J , Zaidul, ISM , Rahman, MM , Sharif, KM , Mohamed, A , Sahena, F, et al. Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng. (2013) 117:426–36. doi: 10.1016/j.jfoodeng.2013.01.014
68. Kaderides, K , Papaoikonomou, L , Serafim, M , and Goula, AM . Microwave-assisted extraction of phenolics from pomegranate peels: optimization, kinetics, and comparison with ultrasounds extraction. Chem Eng Process. (2019) 137:1–11. doi: 10.1016/j.cep.2019.01.006
69. Chen, XQ , Liu, Q , Jiang, XY , and Zeng, F . Microwave-assisted extraction of polysaccharides from solanum nigrum. J Cent S Univ Technol. (2005) 12:556–60. doi: 10.1007/s11771-005-0122-x
70. Felkai-Haddache, L , Remini, H , Dulong, V , Mamou-Belhabib, K , Picton, L , Madani, K, et al. Conventional and microwave-assisted extraction of mucilage from Opuntia ficus-indica Cladodes: physico-chemical and rheological properties. Food Bioprocess Technol. (2016) 9:481–92. doi: 10.1007/s11947-015-1640-7
71. Boussetta, N , Soichi, E , Lanoisellé, JL , and Vorobiev, E . Valorization of oilseed residues: extraction of polyphenols from flaxseed hulls by pulsed electric fields. Ind Crop Prod. (2014) 52:347–53. doi: 10.1016/j.indcrop.2013.10.048
72. Kumari, B , Tiwari, BK , Hossain, MB , Brunton, NP , and Rai, DK . Recent advances on application of ultrasound and pulsed electric field Technologies in the extraction of bioactives from agro-industrial by-products. Food Bioprocess Technol. (2018) 11:223–41. doi: 10.1007/s11947-017-1961-9
73. Savova, M , Bart, H , and Seikova, I . Enhancement of mass transfer in solid-liquid extraction by pulsed electric field. J Chem Technol Metall. (2005) 40:329–334.
74. Puértolas, E , Luengo, E , Álvarez, I , and Raso, J . Improving mass transfer to soften tissues by pulsed electric fields: fundamentals and applications. Annu Rev Food Sci Technol. (2012) 3:263–82. doi: 10.1146/annurev-food-022811-101208
75. Hromádková, Z , Ebringerová, A , and Valachovič, P . Ultrasound-assisted extraction of water-soluble polysaccharides from the roots of valerian (Valeriana officinalis L.). Ultrason Sonochem. (2002) 9:37–44. doi: 10.1016/S1350-4177(01)00093-1
76. Jacotet-Navarro, M , Rombaut, N , Fabiano-Tixier, AS , Danguien, M , Bily, A , and Chemat, F . Ultrasound versus microwave as green processes for extraction of rosmarinic, carnosic and ursolic acids from rosemary. Ultrason Sonochem. (2015) 27:102–9. doi: 10.1016/j.ultsonch.2015.05.006
77. Sereshti, H , Heidari, R , and Samadi, S . Determination of volatile components of saffron by optimised ultrasound-assisted extraction in tandem with dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry. Food Chem. (2014) 143:499–505. doi: 10.1016/j.foodchem.2013.08.024
78. Medina-Meza, IG , and Barbosa-Cánovas, GV . Assisted extraction of bioactive compounds from plum and grape peels by ultrasonics and pulsed electric fields. J Food Eng. (2015) 166:268–75. doi: 10.1016/j.jfoodeng.2015.06.012
79. Akhtar, M , Mushtaq, Z , Ahmad, N , Khan, M , Ahmad, M , Hussain, A, et al. Optimal ultrasound-assisted process extraction, characterization, and functional product development from flaxseed meal derived polysaccharide gum. PRO. (2019) 7:189. doi: 10.3390/pr7040189
80. Emadzadeh, MK , Aarabi, A , Najvani, FA , Chiani, M , and Mehrabi, MR . The effect of extraction method on physicochemical properties of mucilage extracted from yellow and brown flaxseeds. Jundishapur J Nat Pharm Prod. (2022) 17. doi: 10.5812/jjnpp-123952
81. Yu, X , Huang, S , Yang, F , Qin, X , Nie, C , Deng, Q, et al. Effect of microwave exposure to flaxseed on the composition, structure and techno-functionality of gum polysaccharides. Food Hydrocoll. (2022) 125:107447. doi: 10.1016/j.foodhyd.2021.107447
82. Oomah, BD , Kenaschuk, EO , Cui, W , and Mazza, G . Variation in the composition of water-soluble polysaccharides in flaxseed. J Agric Food Chem. (1995) 43:1484–8. doi: 10.1021/jf00054a013
83. Kaushik, P , Dowling, K , Adhikari, R , Barrow, CJ , and Adhikari, B . Effect of extraction temperature on composition, structure and functional properties of flaxseed gum. Food Chem. (2017) 215:333–40. doi: 10.1016/j.foodchem.2016.07.137
84. Hellebois, T , Fortuin, J , Xu, X , Shaplov, AS , Gaiani, C , and Soukoulis, C . Structure conformation, physicochemical and rheological properties of flaxseed gums extracted under alkaline and acidic conditions. Int J Biol Macromol. (2021) 192:1217–30. doi: 10.1016/j.ijbiomac.2021.10.087
85. Zhang, W , Xu, S , Wang, Z , Yang, R , and Lu, R . Demucilaging and dehulling flaxseed with a wet process. LWT Food Sci Technol. (2009) 42:1193–8. doi: 10.1016/j.lwt.2009.01.001
86. Korus, J , Witczak, T , Ziobro, R , and Juszczak, L . Linseed (Linum usitatissimum L.) mucilage as a novel structure forming agent in gluten-free bread. LWT Food Sci Technol. (2015) 62:257–64. doi: 10.1016/j.lwt.2015.01.040
87. Cacciatore, FA , Maders, C , Alexandre, B , Barreto Pinilla, CM , Brandelli, A , and Da Silva, MP . Carvacrol encapsulation into nanoparticles produced from chia and flaxseed mucilage: characterization, stability and antimicrobial activity against Salmonella and Listeria monocytogenes. Food Microbiol. (2022) 108:104116. doi: 10.1016/j.fm.2022.104116
88. Shafizadeh, A , Golestan, L , Ahmadi, M , Darjani, P , and Ghorbani, HA . Enrichment of set yoghurt with flaxseed oil, flaxseed mucilage and free or encapsulated Lacticaseibacillus casei: effect on probiotic survival and yoghurt quality attributes. Food Sci Technol Int. (2022). doi: 10.1177/10820132221136303
89. Bongartz, U , Hochmann, U , Grube, B , Uebelhack, R , Alt, F , Erlenbeck, C, et al. Flaxseed mucilage (IQP-LU-104) reduces body weight in overweight and moderately obese individuals in a 12-week, three-arm, double-blind, randomized, and placebo-controlled clinical study. Obes Facts. (2022) 15:395–404. doi: 10.1159/000522082
90. Akl, EM , Abdelhamid, SM , Wagdy, SM , and Salama, HH . Manufacture of functional fat-free cream cheese fortified with probiotic Bacteria and flaxseed mucilage as a fat replacing agent. Curr Nutr Food Sci. (2020) 16:1393–403. doi: 10.2174/1573401316666200227112157
91. Sungatullina, A , Petrova, T , Kharina, M , Mikshina, P , and Nikitina, E . Effect of flaxseed mucilage on the probiotic, antioxidant, and structural-mechanical properties of the different Lactobacillus cells. Fermentation. (2023) 9:486. doi: 10.3390/fermentation9050486
92. Nybroe, S , Astrup, A , and Bjørnvad, CR . Dietary supplementation with flaxseed mucilage alone or in combination with calcium in dogs: effects on apparent digestibility of fat and energy and fecal characteristics. Int J Obes. (2016) 40:1884–90. doi: 10.1038/ijo.2016.139
93. HadiNezhad, M , Duc, C , Han, NF , and Hosseinian, F . Flaxseed soluble dietary fibre enhances lactic acid bacterial survival and growth in kefir and possesses high antioxidant capacity. J Food Res. (2013) 2:152. doi: 10.5539/jfr.v2n5p152
94. Brahe, LK , Le Chatelier, E , Prifti, E , Pons, N , Kennedy, S , Blædel, T, et al. Dietary modulation of the gut microbiota - a randomised controlled trial in obese postmenopausal women. Br J Nutr. (2015) 114:406–17. doi: 10.1017/S0007114515001786
95. Kumar, B , Panday, SK , and Kumar, P . Synthesis of pH-sensitive nanocarrier-based acrylic acid-grafted-flaxseed gum for quercetin delivery for anti-cancer application. Bioact Carbohydr Diet Fibre. (2023) 30:100370. doi: 10.1016/j.bcdf.2023.100370
96. Kristensen, M , Jensen, MG , Aarestrup, J , Petersen, KE , Søndergaard, L , Mikkelsen, MS, et al. Flaxseed dietary fibers lower cholesterol and increase fecal fat excretion, but magnitude of effect depend on food type. Nutr Metab. (2012) 9:8. doi: 10.1186/1743-7075-9-8
97. Kay, BA , Trigatti, K , MacNeil, MB , Klingel, SL , Repin, N , Douglas Goff, H, et al. Pudding products enriched with yellow mustard mucilage, fenugreek gum or flaxseed mucilage and matched for simulated intestinal viscosity significantly reduce postprandial peak glucose and insulin in adults at risk for type 2 diabetes. J Funct Foods. (2017) 37:603–11. doi: 10.1016/j.jff.2017.08.017
98. Luo, J , Li, Y , Mai, Y , Gao, L , Ou, S , Wang, Y, et al. Flaxseed gum reduces body weight by regulating gut microbiota. J Funct Foods. (2018) 47:136–42. doi: 10.1016/j.jff.2018.05.042
99. WHO . (2022). Available at: https://www.who.int/data/gho/indicator-metadataregistry/imrdetails
100. Boban, PT , Nambisan, B , and Sudhakaran, PR . Hypolipidaemic effect of chemically different mucilages in rats: a comparative study. Br J Nutr. (2006) 96:1021–9. doi: 10.1017/BJN20061944
101. Surampudi, P , Enkhmaa, B , Anuurad, E , and Berglund, L . Lipid lowering with soluble dietary fiber. Curr Atheroscler Rep. (2016) 18:75. doi: 10.1007/s11883-016-0624-z
102. Amaral, L , Morgan, D , Stephen, A , and Whiting, S . Effect of propionate on lipid-metabolism in healthy-human subjects. FASEB J. (1992) 6:1655.
103. Lu, Y , Fan, C , Li, P , Lu, Y , Chang, X , and Qi, K . Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci Rep. (2016) 6:37589. doi: 10.1038/srep37589
104. Singh, R , De, S , and Belkheir, A . Avena sativa (oat), a potential Neutraceutical and therapeutic agent: an overview. Crit Rev Food Sci Nutr. (2013) 53:126–44. doi: 10.1080/10408398.2010.526725
105. Berggren, AM , Björck, IME , Nyman, EMGL , and Eggum, BO . Short-chain fatty acid content and pH in caecum of rats given various sources of carbohydrates. J Sci Food Agric. (1993) 63:397–406. doi: 10.1002/jsfa.2740630405
106. Ganji, V , and Kies, CV . Psyllium husk fibre supplementation to soybean and coconut oil diets of humans: effect on fat digestibility and faecal fatty acid excretion. Eur J Clin Nutr. (1994) 48:595–7.
107. WHO . (2023) Available at: https://www.who.int/news-room/fact-sheets/detail/diabetes (Accessed April 5, 2023).
108. Kumar, GS , Shetty, AK , and Salimath, PV . Modulatory effect of fenugreek seed mucilage and spent turmeric on intestinal and renal Disaccharidases in Streptozotocin induced diabetic rats. Plant Foods Hum Nutr. (2005) 60:87–91. doi: 10.1007/s11130-005-5104-5
109. Jenkins, DJ , Wolever, TM , Leeds, AR , Gassull, MA , Haisman, P , Dilawari, J, et al. Dietary fibres, fibre analogues, and glucose tolerance: importance of viscosity. BMJ. (1978) 1:1392–4. doi: 10.1136/bmj.1.6124.1392
110. Nuñez-López, MA , Paredes-López, O , and Reynoso-Camacho, R . Functional and hypoglycemic properties of nopal cladodes (O. ficus-indica) at different maturity stages using in vitro and in vivo tests. J Agric Food Chem. (2013) 61:10981–6. doi: 10.1021/jf403834x
111. Juárez-Reyes, K , Brindis, F , Medina-Campos, ON , Pedraza-Chaverri, J , Bye, R , Linares, E, et al. Hypoglycemic, antihyperglycemic, and antioxidant effects of the edible plant Anoda cristata. J Ethnopharmacol. (2015) 161:36–45. doi: 10.1016/j.jep.2014.11.052
112. Uddin Zim, AFMI , Khatun, J , Khan, MF , Hossain, MA , and Haque, MM . Evaluation of in vitro antioxidant activity of okra mucilage and its antidiabetic and antihyperlipidemic effect in alloxan-induced diabetic mice. Food Sci Nutr. (2021) 9:6854–65. doi: 10.1002/fsn3.2641
113. WHO (2023). Available at: https://www.who.int/health-topics/cancer#tab=tab_1
114. Kajla, P , Sharma, A , and Sood, DR . Flaxseed—a potential functional food source. J Food Sci Technol. (2015) 52:1857–71. doi: 10.1007/s13197-014-1293-y
115. Xu, J , Zhou, X , Chen, C , Deng, Q , Huang, Q , Yang, J, et al. Laxative effects of partially defatted flaxseed meal on normal and experimental constipated mice. BMC Complement Altern Med. (2012) 12:14. doi: 10.1186/1472-6882-12-14
116. Chang, PV , Hao, L , Offermanns, S , and Medzhitov, R . The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci. (2014) 111:2247–52. doi: 10.1073/pnas.1322269111
117. Kootte, RS , Vrieze, A , Holleman, F , Dallinga-Thie, GM , Zoetendal, EG , De Vos, WM, et al. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obes Metab. (2012) 14:112–20. doi: 10.1111/j.1463-1326.2011.01483.x
118. Lin, B , Gong, J , Wang, Q , Cui, S , Yu, H , and Huang, B . In-vitro assessment of the effects of dietary fibers on microbial fermentation and communities from large intestinal digesta of pigs. Food Hydrocoll. (2011) 25:180–8. doi: 10.1016/j.foodhyd.2010.02.006
119. Dahl, WJ , Lockert, EA , Cammer, AL , and Whiting, SJ . Effects of flax Fiber on laxation and glycemic response in healthy volunteers. J Med Food. (2005) 8:508–11. doi: 10.1089/jmf.2005.8.508
120. WHO (2023). Available at: https://Www.Who.Int/News/Item/04-03-2022-World-Obesity-Day-2022-Accelerating-Action-to-Stop-Obesity
121. Singh, P , and Rai, SN . Factors affecting obesity and its treatment. Obes Med. (2019) 16:100140. doi: 10.1016/j.obmed.2019.100140
122. Keenan, Hillary A. Positivity of C-peptide, GADA and IA2 antibodies in type 1 diabetic patients with extreme duration. (2006) 55:A65.
123. Schneeman, BO . Dietary fibre and gastrointestinal function In: BV McCleary and L Prosky, editors. Advanced dietary fibre technology. 1st ed. Oxford: Wiley (2000). 168–76.
124. Abd El-Wahab, A , Chuppava, B , Siebert, DC , Visscher, C , and Kamphues, J . Digestibility of a lignocellulose supplemented diet and fecal quality in beagle dogs. Animals. (2022) 12:1965. doi: 10.3390/ani12151965
125. Adam, CL , Williams, PA , Dalby, MJ , Garden, K , Thomson, LM , Richardson, AJ, et al. Different types of soluble fermentable dietary fibre decrease food intake, body weight gain and adiposity in young adult male rats. Nutr Metab. (2014) 11:36. doi: 10.1186/1743-7075-11-36
126. Tucker, LA , and Thomas, KS . Increasing Total Fiber intake reduces risk of weight and fat gains in women. J Nutr. (2009) 139:576–81. doi: 10.3945/jn.108.096685
127. Baer, DJ , Rumpler, WV , Miles, CW , and Fahey, GC . Dietary fiber decreases the metabolizable energy content and nutrient digestibility of mixed diets fed to humans. J Nutr. (1997) 127:579–86. doi: 10.1093/jn/127.4.579
128. Isken, F , Klaus, S , Osterhoff, M , Pfeiffer, AFH , and Weickert, MO . Effects of long-term soluble vs. insoluble dietary fiber intake on high-fat diet-induced obesity in C57BL/6J mice. J Nutr Biochem. (2010) 21:278–84. doi: 10.1016/j.jnutbio.2008.12.012
129. Lattimer, JM , and Haub, MD . Effects of dietary fiber and its components on metabolic health. Nutrients. (2010) 2:1266–89. doi: 10.3390/nu2121266
130. Forootan, M , Bagheri, N , and Darvishi, M . Chronic constipation: a review of literature. Medicine. (2018) 97:e10631. doi: 10.1097/MD.0000000000010631
131. Kučka, M , Ražná, K , Harenčár, Ľ , and Kolarovičová, T . Plant seed mucilage—great potential for sticky matter. Forum Nutr. (2022) 2:253–69. doi: 10.3390/nutraceuticals2040019
132. Hanif Palla, A , and Gilani, AH . Dual effectiveness of flaxseed in constipation and diarrhea: possible mechanism. J Ethnopharmacol. (2015) 169:60–8. doi: 10.1016/j.jep.2015.03.064
133. Tarpila, Anneli and, Wennberg, Tero , and Tarpila, Simo . Flaxseed as a functional food. Current Topics in Nutraceutical Research. (2005). Available at: https://researchportal.helsinki.fi/en/publications/flaxseed-as-a-functional-food
134. Gullón, B , Gullón, P , Tavaria, F , Alonso, JL , and Pintado, M . In vitro assessment of the prebiotic potential of Aloe vera mucilage and its impact on the human microbiota. Food Funct. (2015) 6:525–31. doi: 10.1039/C4FO00857J
135. Desmarchelier, C , Coussio, J , and Ciccia, G . Antioxidant and free radical scavenging effects in extracts of the medicinal herb Achyrocline satureioides (Lam.) DC. (“marcela”). Braz J Med Biol Res. (1998) 31:1163–70. doi: 10.1590/S0100-879X1998000900010
136. Keshani-Dokht, S , Emam-Djomeh, Z , Yarmand, MS , and Fathi, M . Extraction, chemical composition, rheological behavior, antioxidant activity and functional properties of Cordia myxa mucilage. Int J Biol Macromol. (2018) 118:485–93. doi: 10.1016/j.ijbiomac.2018.06.069
137. Hadad, S , and Goli, SAH . Fabrication and characterization of electrospun nanofibers using flaxseed (Linum usitatissimum) mucilage. Int J Biol Macromol. (2018) 114:408–14. doi: 10.1016/j.ijbiomac.2018.03.154
138. Karami, N , Kamkar, A , Shahbazi, Y , and Misaghi, A . Edible films based on chitosan-flaxseed mucilage: in vitro antimicrobial and antioxidant properties and their application on survival of food-borne pathogenic bacteria in raw minced trout fillets. Pharm Biomed Res. (2019) 5. doi: 10.18502/pbr.v5i2.1580
139. Vieira, JM , Mantovani, RA , Raposo, MFJ , Coimbra, MA , Vicente, AA , and Cunha, RL . Effect of extraction temperature on rheological behavior and antioxidant capacity of flaxseed gum. Carbohydr Polym. (2019) 213:217–27. doi: 10.1016/j.carbpol.2019.02.078
140. Jouki, M , Khazaei, N , Ghasemlou, M , and HadiNezhad, M . Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydr Polym. (2013) 96:39–46. doi: 10.1016/j.carbpol.2013.03.077
141. Nampuak, C , and Tongkhao, K . Okra mucilage powder: a novel functional ingredient with antioxidant activity and antibacterial mode of action revealed by scanning and transmission electron microscopy. Int J Food Sci Technol. (2020) 55:569–77. doi: 10.1111/ijfs.14308
142. MaG, G-R , Coria-Caballero, V , Tranquilino-Rodríguez, E , Dasgupta-Schubert, N , Villicaña-Méndez, M , Agarwal, V, et al. Ecological method for the synthesis, characterization and antimicrobial effect of silver nanoparticles produced and stabilized with a mixture of mucilage/proteins extracted from flaxseed. J Inorg Organomet Polym Mater. (2021) 31:3406–15. doi: 10.1007/s10904-021-01968-5
143. Ma, Y , Griffith, JA , Chasan-Taber, L , Olendzki, BC , Jackson, E , Stanek, EJ, et al. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. (2006) 83:760–6. doi: 10.1093/ajcn/83.4.760
144. Bouaziz, W , Lang, PO , Schmitt, E , Kaltenbach, G , Geny, B , and Vogel, T . Health benefits of multicomponent training programmes in seniors: a systematic review. Int J Clin Pract. (2016) 70:520–36. doi: 10.1111/ijcp.12822
145. Mohtarami, F , Rashidi, Z , and Pirsa, S . Extraction of flaxseed and Plantago Psyllium mucilage: investigation of rheological properties and efficiency as a fat substitute for the production of low-calorie cookies. J Food Process Preserv [Internet]. (2022) 46:e16964. doi: 10.1111/jfpp.16964
146. Ahmadinia, F , Mohtarami, F , Esmaili, M , and Pirsa, S . Production of low-calorie cake by partial replacement of flaxseed mucilage and flaxseed flour and investigation of its physicochemical, textural and sensory characteristics. (2022). Available at: https://www.researchsquare.com/article/rs-2243938/v1
147. Stewart, S , and Mazza, G . Effect of flaxseed gum on quality and stability of a model salad dressing. J Food Qual. (2000) 23:373–90. doi: 10.1111/j.1745-4557.2000.tb00565.x
148. Yang, J , Choi, YJ , and Hahn, J . Development of flaxseed gum/konjac glucomannan with agar as gelling agents with enhanced elastic properties. Food Sci Biotechnol. (2023) 32:181–92. doi: 10.1007/s10068-022-01179-9
149. Jia, N , Lin, S , Zhang, F , Zheng, D , and Liu, D . Improved effect of flaxseed gum on the weakened gelling properties of myofibrillar protein induced by catechin. Food Chem. (2022) 372:131136. doi: 10.1016/j.foodchem.2021.131136
150. Chen, C , Huang, X , Wang, L , Li, D , and Adhikari, B . Effect of flaxseed gum on the rheological properties of peanut protein isolate dispersions and gels. LWT. (2016) 74:528–33. doi: 10.1016/j.lwt.2016.08.013
151. Basiri, S , Tajbakhsh, S , and Shekarforoush, SS . Fortification of stirred yoghurt with mucilage-free flaxseed and its physicochemical, microbial, textural and sensory properties. Int Dairy J. (2022) 131:105384. doi: 10.1016/j.idairyj.2022.105384
152. Pham, LB , Wang, B , Zisu, B , Truong, T , and Adhikari, B . Microencapsulation of flaxseed oil using polyphenol-adducted flaxseed protein isolate-flaxseed gum complex coacervates. Food Hydrocoll. (2020) 107:105944. doi: 10.1016/j.foodhyd.2020.105944
153. Lai, K , How, Y , and Pui, L . Storage stability of microencapsulated Lactobacillus rhamnosus GG in hawthorn berry tea with flaxseed mucilage. J Food Process Preserv [Internet]. (2020) 44:e14965. doi: 10.1111/jfpp.14965
154. Azarpazhooh, E , Rashidi, H , Sharayei, P , Behmadi, H , and Ramaswamy, HS . Effect of flaxseed-mucilage and Stevia on physico-chemical, antioxidant and sensorial properties of formulated cocoa milk. Food Hydrocoll Health. (2021) 1:100017. doi: 10.1016/j.fhfh.2021.100017
155. Kishk, YFM , Elsheshetawy, HE , and Mahmoud, EAM . Influence of isolated flaxseed mucilage as a non-starch polysaccharide on noodle quality: flaxseed mucilage and noodle quality. Int J Food Sci Technol. (2011) 46:661–8. doi: 10.1111/j.1365-2621.2010.02547.x
156. Ali, MR , Yousef, A , and Ali, A . Application of biodegradable Aloe vera gel and linseed mucilage for extending the shelf life of plums. Res J Pharm Technol. (2021) 14:1579–85. doi: 10.5958/0974-360X.2021.00279.1
157. Guo, Q , Zhu, X , Zhen, W , Li, Z , Kang, J , Sun, X, et al. Rheological properties and stabilizing effects of high-temperature extracted flaxseed gum on oil/water emulsion systems. Food Hydrocoll. (2021) 112:106289. doi: 10.1016/j.foodhyd.2020.106289
158. Wang, B , Li, D , Wang, LJ , and Özkan, N . Effect of concentrated flaxseed protein on the stability and rheological properties of soybean oil-in-water emulsions. J Food Eng. (2010) 96:555–61. doi: 10.1016/j.jfoodeng.2009.09.001
159. Chen, HH , Xu, SY , and Wang, Z . Gelation properties of flaxseed gum. J Food Eng. (2006) 77:295–303. doi: 10.1016/j.jfoodeng.2005.06.033
160. Goesaert, H , Brijs, K , Veraverbeke, WS , Courtin, CM , Gebruers, K , and Delcour, JA . Wheat flour constituents: how they impact bread quality, and how to impact their functionality. Trends Food Sci Technol. (2005) 16:12–30. doi: 10.1016/j.tifs.2004.02.011
161. Kadivar, M. Studies on integrated processes for the recovery of mucilage, hull, oil and protein from Solin (low linolenic acid flax). (2001). Available at: https://harvest.usask.ca/handle/10388/etd-10212004-002848
162. Raslan, HA , and Sokary, R . Eco-friendly flaxseed mucilage biofilms fabricated by gamma irradiation. Radiochim Acta. (2023) 111:481–93. doi: 10.1515/ract-2022-0090
163. Otoni, CG , Avena-Bustillos, RJ , Azeredo, HMC , Lorevice, MV , Moura, MR , Mattoso, LHC, et al. Recent advances on edible films based on fruits and vegetables—a review. Compr Rev Food Sci Food Saf. (2017) 16:1151–69. doi: 10.1111/1541-4337.12281
164. Abdalrazeq, M , Giosafatto, CVL , Esposito, M , Fenderico, M , Di Pierro, P , and Porta, R . Glycerol-plasticized films obtained from whey proteins denatured at alkaline pH. Coatings. (2019) 9:322. doi: 10.3390/coatings9050322
165. Cakmak, H , Ilyasoglu-Buyukkestelli, H , Sogut, E , Ozyurt, VH , Gumus-Bonacina, CE , and Simsek, S . A review on recent advances of plant mucilages and their applications in food industry: extraction, functional properties and health benefits. Food Hydrocoll Health. (2023) 3:100131. doi: 10.1016/j.fhfh.2023.100131
166. Sagnelli, D , Hooshmand, K , Kemmer, G , Kirkensgaard, J , Mortensen, K , Giosafatto, C, et al. Cross-linked amylose bio-plastic: a transgenic-based compostable plastic alternative. Int J Mol Sci. (2017) 18:2075. doi: 10.3390/ijms18102075
167. Zeng, WW , and Lai, LS . Characterization of the mucilage extracted from the edible fronds of bird’s nest fern (Asplenium australasicum) with enzymatic modifications. Food Hydrocoll. (2016) 53:84–92. doi: 10.1016/j.foodhyd.2015.03.026
168. Hamidabadi Sherahi, M , Fathi, M , Zhandari, F , Hashemi, SMB , and Rashidi, A . Structural characterization and physicochemical properties of Descurainia sophia seed gum. Food Hydrocoll. (2017) 66:82–9. doi: 10.1016/j.foodhyd.2016.12.010
169. Hesarinejad, MA , Koocheki, A , and Razavi, SMA . Dynamic rheological properties of Lepidium perfoliatum seed gum: effect of concentration, temperature and heating/cooling rate. Food Hydrocoll. (2014) 35:583–9. doi: 10.1016/j.foodhyd.2013.07.017
170. Huang, D , Wang, C , Yuan, J , Cao, J , and Lan, H . Differentiation of the seed coat and composition of the mucilage of Lepidium perfoliatum L.: a desert annual with typical myxospermy. Acta Biochim Biophys Sin. (2015) 47:775–87. doi: 10.1093/abbs/gmv078
171. Qian, KY , Cui, SW , Wu, Y , and Goff, HD . Flaxseed gum from flaxseed hulls: extraction, fractionation, and characterization. Food Hydrocoll. (2012) 28:275–83. doi: 10.1016/j.foodhyd.2011.12.019
172. Karazhiyan, H , Razavi, SMA , Phillips, GO , Fang, Y , Al-Assaf, S , Nishinari, K, et al. Rheological properties of Lepidium sativum seed extract as a function of concentration, temperature and time. Food Hydrocoll. (2009) 23:2062–8. doi: 10.1016/j.foodhyd.2009.03.019
173. Naji-Tabasi, S , and Razavi, SMA . Functional properties and applications of basil seed gum: an overview. Food Hydrocoll. (2017) 73:313–25. doi: 10.1016/j.foodhyd.2017.07.007
174. Alizadeh Behbahani, B , Tabatabaei Yazdi, F , Shahidi, F , Hesarinejad, MA , Mortazavi, SA , and Mohebbi, M . Plantago major seed mucilage: optimization of extraction and some physicochemical and rheological aspects. Carbohydr Polym. (2017) 155:68–77. doi: 10.1016/j.carbpol.2016.08.051
175. Timilsena, YP , Adhikari, R , Kasapis, S , and Adhikari, B . Molecular and functional characteristics of purified gum from Australian chia seeds. Carbohydr Polym. (2016) 136:128–36. doi: 10.1016/j.carbpol.2015.09.035
176. Wu, Y , Cui, W , Eskin, NAM , and Goff, HD . Fractionation and partial characterization of non-pectic polysaccharides from yellow mustard mucilage. Food Hydrocoll. (2009) 23:1535–41. doi: 10.1016/j.foodhyd.2008.10.010
177. Popović, S , Hromiš, N , Šuput, D , Bulut, S , Romanić, R , and Lazić, V . Valorization of by-products from the production of pressed edible oils to produce biopolymer films In: MF Ramadan , editor. Cold pressed oils. Amsterdam: Elsevier (2020). 15–30.
178. Kandasamy, S , Yoo, J , Yun, J , Kang, HB , Seol, KH , Kim, HW, et al. Application of whey protein-based edible films and coatings in food industries: an updated overview. Coatings. (2021) 11:1056. doi: 10.3390/coatings11091056
179. Lu, Z , Saldaña, MDA , Jin, Z , Sun, W , Gao, P , Bilige, M, et al. Layer-by-layer electrostatic self-assembled coatings based on flaxseed gum and chitosan for Mongolian cheese preservation. Innov Food Sci Emerg Technol. (2021) 73:102785. doi: 10.1016/j.ifset.2021.102785
180. Li, X , Wang, Y , and Li, D . Effects of flaxseed gum addition and drying conditions on creep-recovery properties and water vapour transmission rate of starch-based films. Int J Food Eng. (2009) 5:10. doi: 10.2202/1556-3758.1729/html
181. Hager, AS , Vallons, KJR , and Arendt, EK . Influence of Gallic acid and tannic acid on the mechanical and barrier properties of wheat gluten films. J Agric Food Chem. (2012) 60:6157–63. doi: 10.1021/jf300983m
182. Dadkhah, H , Pirsa, S , Javadi, A , and Mohtarami, F . Biodegradable film based on sodium alginate/flax seed mucilage modified with norbixin and WO3 nanoparticles: investigation of physicochemical properties and release of active substances. Biomass Conv Bioref. (2023). doi: 10.1007/s13399-023-03780-2
183. Tallawi, M , Amrein, D , Gemmecker, G , Aifantis, KE , and Drechsler, K . A novel polysaccharide/zein conjugate as an alternative green plastic. Sci Rep. (2023) 13:13161. doi: 10.1038/s41598-023-40293-4
184. Wu, M , Li, D , Wang, LJ , Zhou, YG , and Mao, ZH . Rheological property of extruded and enzyme treated flaxseed mucilage. Carbohydr Polym. (2010) 80:460–6. doi: 10.1016/j.carbpol.2009.12.003
185. Rodrigues, FJ , Cedran, MF , and Garcia, S . Influence of linseed mucilage incorporated into an alginate-base edible coating containing probiotic bacteria on shelf-life of fresh-cut Yacon (Smallanthus sonchifolius). Food Bioprocess Technol. (2018) 11:1605–14. doi: 10.1007/s11947-018-2128-z
186. Treviño-Garza, MZ , García, S , Heredia, N , MaG, A-G , and Arévalo-Niño, K . Layer-by-layer edible coatings based on mucilages, pullulan and chitosan and its effect on quality and preservation of fresh-cut pineapple (Ananas comosus). Postharvest Biol Technol. (2017) 128:63–75. doi: 10.1016/j.postharvbio.2017.01.007
187. Akhila, K , Ramakanth, D , Rao, LL , and Gaikwad, KK . UV-blocking biodegradable film based on flaxseed mucilage/pectin impregnated with titanium dioxide and calcium chloride for food packaging applications. Int J Biol Macromol [Internet]. (2023) 239:124335. doi: 10.1016/j.ijbiomac.2023.124335
188. Treviño-Garza, MZ , Saldívar-Vázquez, AK , López-Villarreal, SM , Lara-Banda, MDR , Elizondo-Luevano, JH , Chávez-Montes, A, et al. Production and preliminary characterization of linseed mucilage-based films loaded with cardamom (Elettaria cardamomum) and copaiba (Copaifera officinalis). Coatings. (2023) 13:1574. doi: 10.3390/coatings13091574
Keywords: linseed mucilage, extraction, edible films, coatings, health, by-product
Citation: Chand M, Chopra R, Talwar B, Homroy S, Singh PK, Dhiman A and Payyunni AW (2024) Unveiling the potential of linseed mucilage, its health benefits, and applications in food packaging. Front. Nutr. 11:1334247. doi: 10.3389/fnut.2024.1334247
Edited by:
Abhishek Dutt Tripathi, Banaras Hindu University, IndiaReviewed by:
Abhishek Gupta, Banaras Hindu University, IndiaPrasad Rasane, Lovely Professional University, India
Copyright © 2024 Chand, Chopra, Talwar, Homroy, Singh, Dhiman and Payyunni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajni Chopra, rajnichopra.niftem@gmail.com
 Monika Chand1
Monika Chand1  Rajni Chopra
Rajni Chopra Aishwarya Dhiman
Aishwarya Dhiman