Association between dietary factors and colorectal serrated polyps: a systematic review and meta-analysis
- 1Department of Big Data in Health Science, and Center for Clinical Big Data and Statistics, The Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
- 2Department of General Surgery, School of Medicine, Run Run Shaw Hospital, Zhejiang University, Hangzhou, Zhejiang, China
- 3Department of General Surgery, Three Gorges Hospital, Chongqing University, Chongqing, China
Background: Dietary factors may affect the incidence of colorectal serrated polyps (SP). However, its effects on SP are unclear as epidemiological studies on this topic have showed inconsistent results. The present systematic review and meta-analysis sought to evaluate the effects of dietary factors on SPs.
Methods: Studies regarding the association between dietary factors and SPs were identified by searching PubMed, Cochrane library, Embase and Chinese Biomedical Literature database from inception until 27 February 2023. Search terms include serrated, hyperplastic, adenoma, polyps, colorectal, rectal, rectum and risk. Heterogeneity was assessed using I2 statistics. The meta-analysis was conducted by using a random-effects model, and the pooled effects were expressed with odds ratios (OR) and 95% confidence intervals (95% CI). Probable sources of heterogeneity were identified through meta-regression. Subgroup analysis were based on lesion types, study designs, countries, and so on.
Results: 28 studies were ultimately eligible after scanning, and five dietary factors including vitamin D, calcium, folate, fiber and red or processed meat were excerpted. Higher intakes of vitamin D (OR = 0.95, 95%CI:0.90–1.02), calcium (OR = 0.97, 95%CI: 0.91–1.03) and folate (OR = 0.82, 95% CI: 0.6–1.13) were not significantly associated with SP. Fiber intake (OR = 0.90, 95% CI: 0.82–0.99) was a protective factor against SPs. Red meat intake increased the risk of SPs by 30% for the highest versus lowest intakes (OR = 1.30, 95% CI: 1.13–1.51). For different lesion types, higher folate intake was associated with a decreased risk of HPs (OR = 0.59, 95%CI: 0.44–0.79), and higher vitamin D intake decreased the risk of SPs including SSA/P (OR = 0.93, 95%CI: 0.88–0.98).
Conclusions: Higher dietary fiber intake plays an effective role in preventing SP, while red meat intake is associated with an increased risk of SP. This evidence provides guidance for us to prevent SP from a dietary perspective.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?, RecordID=340750.
1. Introduction
Colorectal cancer (CRC) is considered to be caused by the accumulation of various aberrant mutations in the epithelial cells lining the colorectal mucosa (1). In 2020, there were about 1.932 million new cases of CRC in the world, and the morbidity ranked third among all malignant tumors. There were about 935,000 deaths of colorectal cancer, and the mortality ranked the second among all malignant tumors, causing a serious disease burden to the world (2).
CRC mainly evolves from colorectal adenoma, that is, normal mucosa first appears with epithelial hyperplasia-like changes, and then can gradually transform into an adenoma, which can later develop into carcinoma in situ and invasive carcinoma (3, 4). Studies over the past decade have shown that colorectal serrated polyps (SP) also have the potential to progress to CRC, with 10–30% of CRC developing through serrated polyps (5, 6). SPs are most commonly classified as hyperplastic polyps (HP), sessile serrated adenomas/polyps (SSA/P) and traditional serrated adenomas.
The association of dietary factors with colorectal adenomas and CRC has been extensively explored, and the dose-response relationship has also been established. It is reported that the relative risk for developing CRC is 1.38 for a 50 g/day increase in alcohol intake (7), 0.90 for an increase of 10 g/day of dietary fiber (8), 1.24 for 120 a g/day increase of red meat, 1.36 for a 30 g/day increase of processed meat (9). A study showed that a healthy lifestyle can reduce the risk of morbidity and mortality of colorectal adenoma and CRC (10).
Gao et al. conducted a systematic review and meta-analysis, revealing a strong positive relationship between proximal SP and synchronous advanced neoplasia (11). However, HPs, the most prevalent type of SP, have a relatively lower chance to become cancerous (12). Many investigators have also investigated the relationship between different dietary factors and CRC precursor lesions, but the results remain controversial (13–18). The aim of this systematic review and meta-analysis, therefore, is to evaluate the association between dietary factors, including vitamin D, calcium, folate, fiber, and red meat, with the risk of SP.
2. Materials and methods
2.1. Search strategy
This meta-analysis was registered on PROSPERO (No. CRD42022340750) (19) and compliant with the main PRISMA statement (20, 21). Randomized controlled trials (RCT), cohort studies, case-control studies and cross-sectional studies published before 27 February 2023 were collected from electronic databases such as PubMed, Cochrane Library, Embase and China BioMedical Literature database.
The search terms were: (Serrated OR Hyperplastic) AND (Adenoma OR Polyps) AND (Colorectal OR Rectal OR Rectum) AND Risk (Supplementary material). Besides, the references of all the retrieved articles were checked to identify further relevant articles.
2.2. Selection criteria
The following inclusion characteristics based on “PICO(S)” criteria were agreed for screening papers: (1) Adults aged 18 years and over, undergoing endoscopic investigation of the colorectum. (2) Assessment of a dietary factor, for example, vitamin D, calcium, folate, fiber and red meat. (3) Comparisons of the risk of SPs between dietary factors exposure group and non-exposure (or lower exposure) group. (4) Risk of serrated colorectal polyps, encompassing HP, SSA/P and/or TSA. (5) Original studies in English or Chinese.
The exclusion criteria were as follows: (1) Study populations of other co-morbidities, for example, Crohn's Disease, ulcerative colitis, Barrett's esophagus, acromegaly; (2) Studies did not provide sufficient data for a meta-analysis; (3) The control group comprised a population with serrated polyps. (4) Reviews, comments, letters, and animal studies.
2.3. Data extraction and quality assessment
Two reviewers (ZX Zhu and XF Guan) used Endnote X9 software to screen titles and abstracts independently. All data were extracted by two researchers (NW Liu and XX Zhu) independently using a standardized form. If there was any disagreement between two reviewers, the report would be sent to a third researcher (XY Li) and fully discussed. Data extraction included: the name of the first author, published year, country, study design, age, cases, sample size, type of SPs, dietary factors and adjusted confounders. The Newcastle-Ottawa scale was used to assess the quality and bias risk of observational studies, and a study with 7 scores (or more) was defined as a high-quality study. The Cochrane collaboration tool were used to assess the quality of RCTs (22).
2.4. Statistical analysis
If studies reported results from the same population and their investigated factors overlapped, the most recent publications or studies with the biggest sample size were used for meta-analysis. Pooled ORs of dietary factors with 95 % CI for the risk of SPs were calculated using random effects models for the highest v. the lowest categories. The between-study heterogeneity was assessed by Cochran's Q-test and I2 statistic, and heterogeneity was considered to be significant if I2 > 50% and P < 0.05. The summary OR was calculated through the random-effects model, which takes into account the presence of heterogeneity in their calculations (23). Forest plots were drawn to show each study's result and estimate the pooled effect sizes. A sensitivity analysis was performed to evaluate the stability of the results. Each time one study was omitted to evaluate the risk estimate. Subgroup analyses were further conducted according to lesion type (HP or SP/SSA/P), study design (cross-sectional, case-control, cohort or RCT), country (USA or others) and adjustments including smoking, alcohol intake or body mass index (BMI), to explore the potential source of heterogeneity, if data were permitted. Besides, we conducted sensitivity analyses to assess the stability, and reliability of the pooled estimates by omitting one estimate at a time sequentially and recalculating the pooled results. The stability and reliability were confirmed if no single study altered the significance of the pooled estimate. Furthermore, publication bias was assessed through funnel plot and Egger's linear regression test (24). All statistical analysis was performed with STATA software (Version 17.0; Stata Corporation, College Station, TX, USA). P ≤ 0.05 were considered statistically significant.
3. Results
3.1. Search results and study characteristics
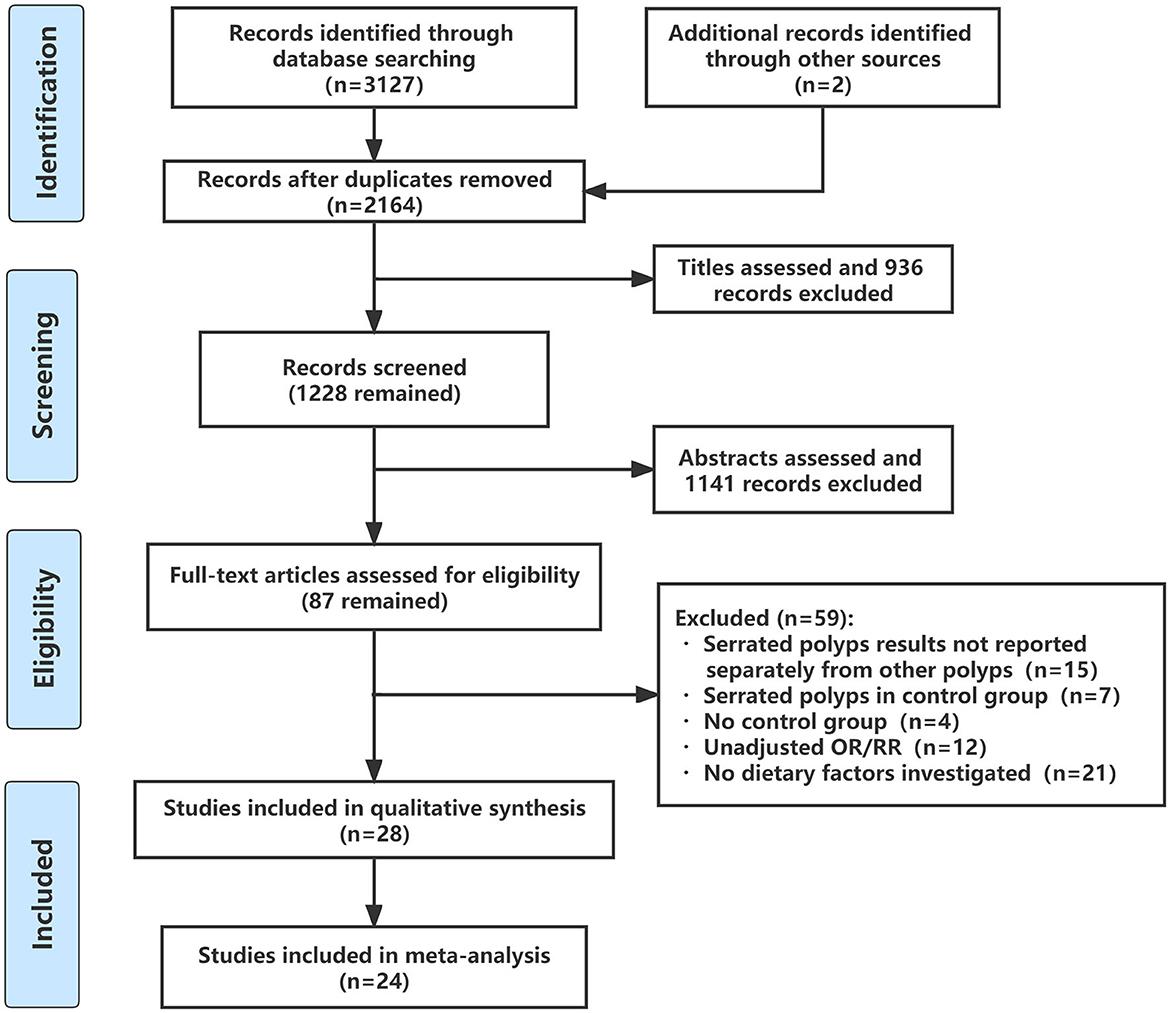
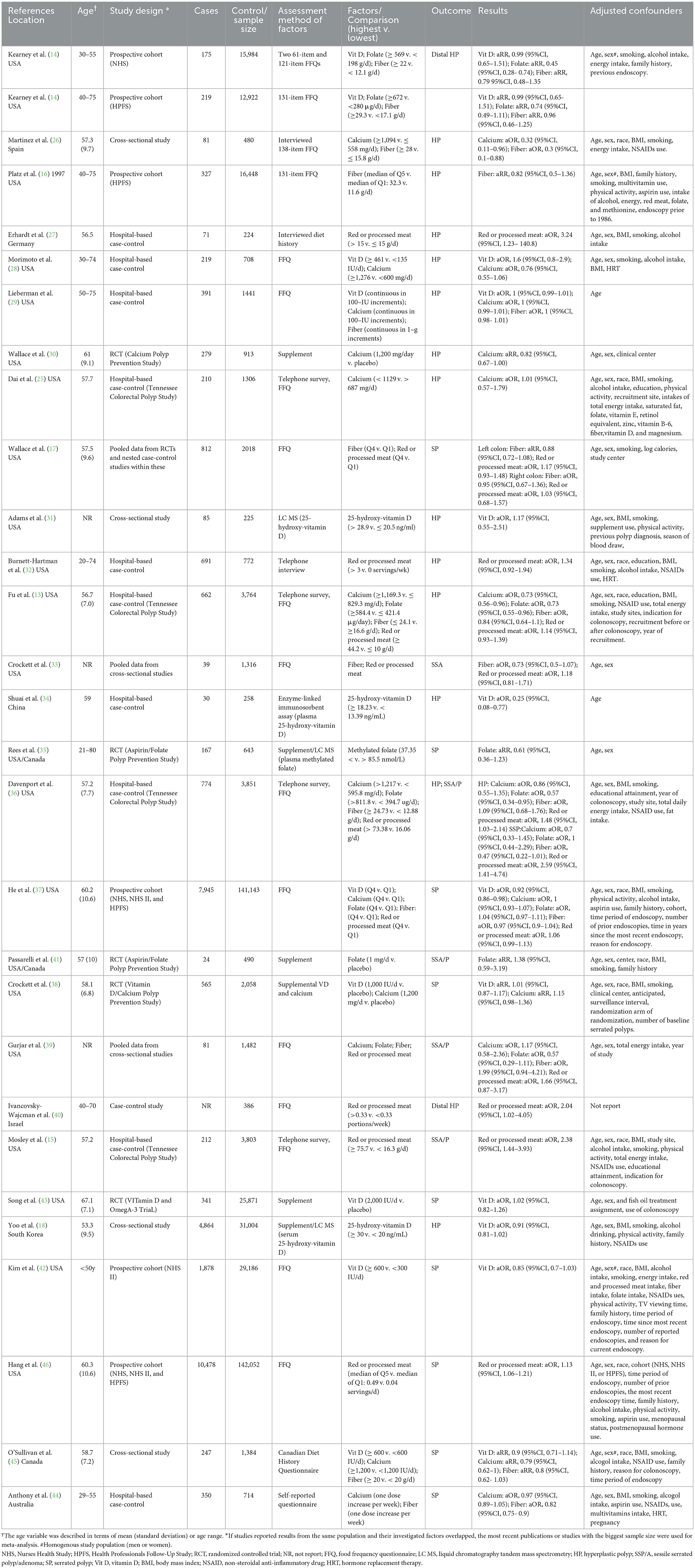
The flow through the selection process was described in a modified PRISMA diagram (Figure 1). A total of 3,129 articles were retrieved from the databases and reference lists during the initial screening process. After a full-text review, 28 studies were included for qualitative analysis, among which four studies reported the same population (13, 14, 16, 25), so 24 studies were left for meta-analysis (15, 17, 18, 26–46). Table 1 summarized the basic characteristics of each study. There were six cross-sectional studies, 12 case-control studies, five cohort studies, and five RCTs. Assessments for risk bias of included observational studies and RCTs were shown in Supplementary Table 1, Supplementary Figure 1, respectively. According to the Newcastle-Ottawa scale and Cochrane collaboration tool, 18 observational studies were rated as high-quality studies. The included RCTs were not at appreciable risk of bias.
3.2. Dietary factors and risk of colorectal serrated polyps
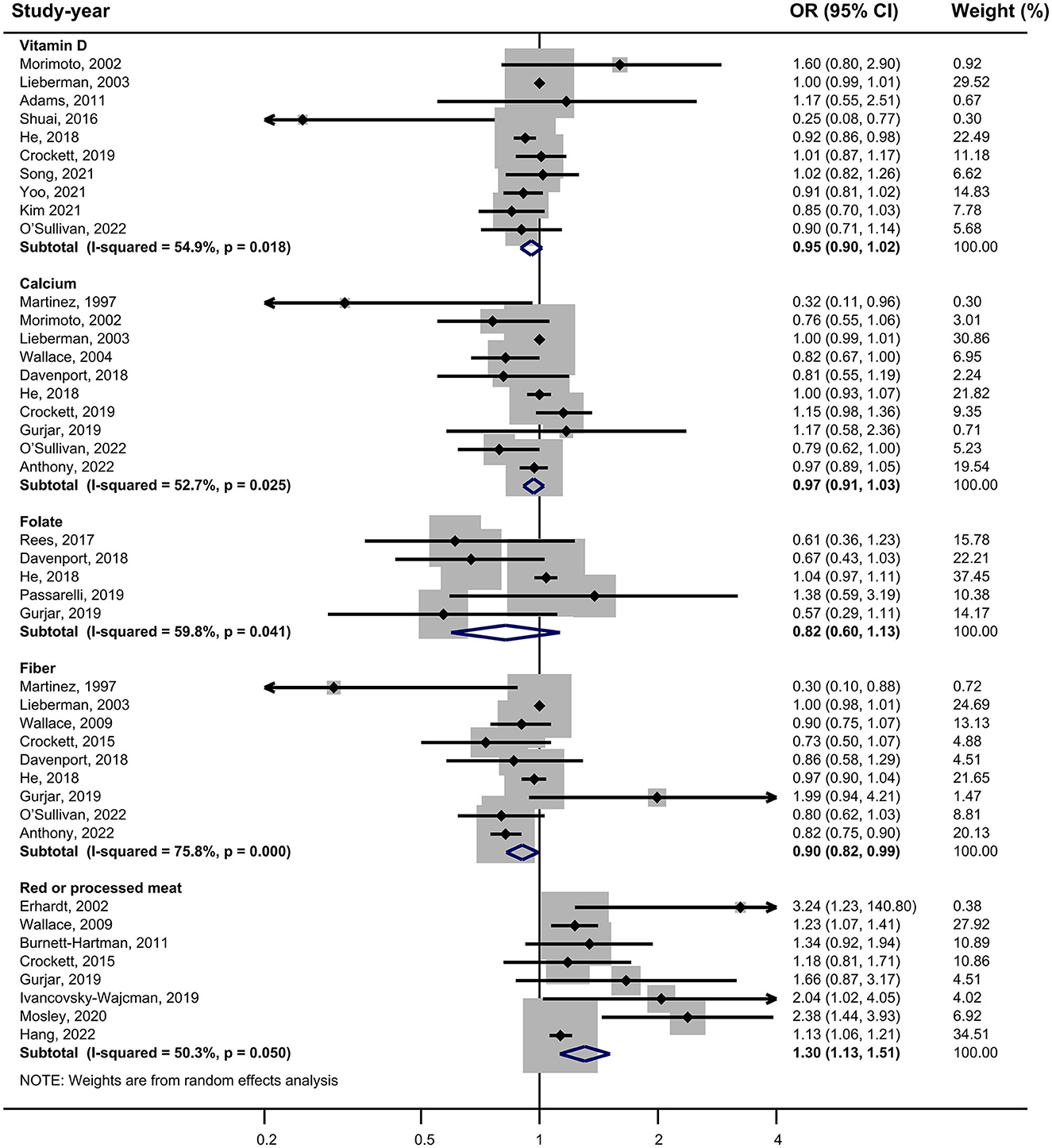
Vitamin D: 10 studies investigated the association between vitamin D and the risk of SPs (18, 28, 29, 31, 34, 37, 38, 42, 43, 45). The forest plot illustrated a close-to-significant decreased risk of SPs with increased levels of vitamin D intake (OR = 0.95, 95%CI:0.90–1.02), and statistically significant heterogeneity was found (I2 = 54.9%; P = 0.018) (Figure 2). Subgroup analysis showed that vitamin D may be related to a statistically significant decrease in SP risk in cohort studies (OR = 0.91, 0.86–0.97) (Table 2). Besides, similar associations were observed when studies adjusted for smoking (OR = 0.93, 95%CI:0.88–0.97), alcohol intake (OR = 0.91, 95%CI: 0.87–0.96) or BMI (OR = 0.93, 95%CI:0.88–0.97). Also, these adjustments were identified as sources of heterogeneity by meta-regression analyses.
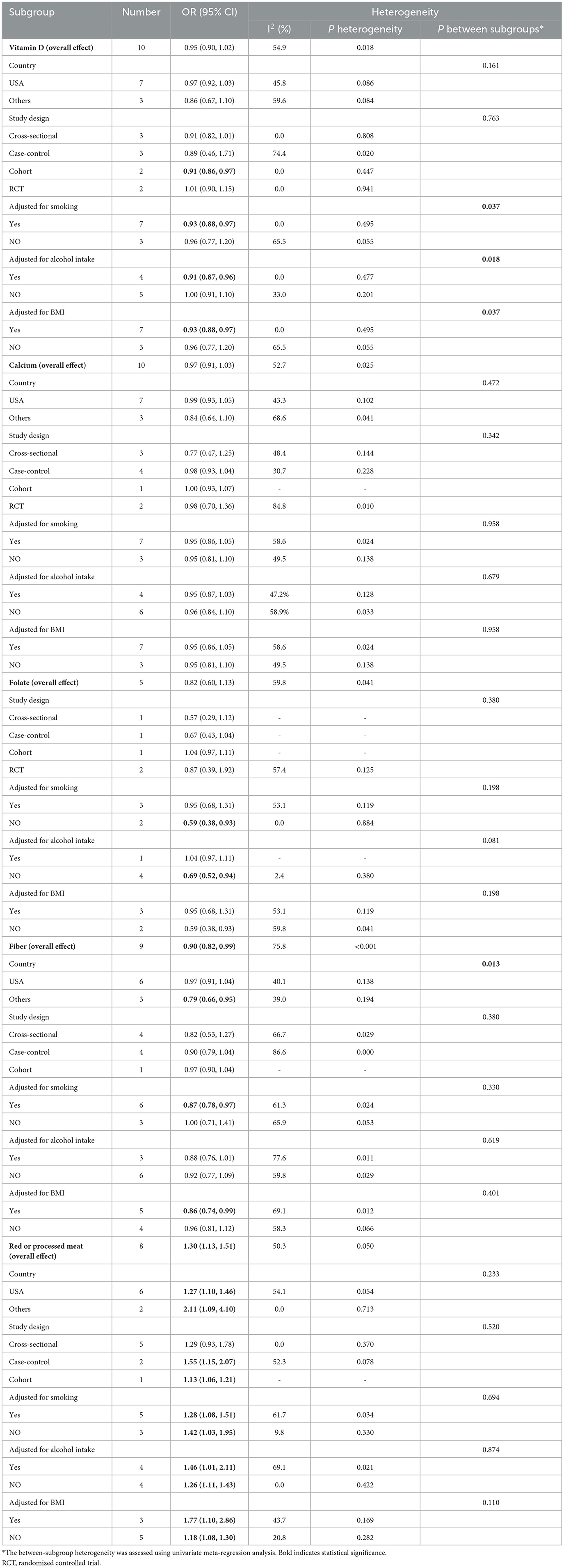
Table 2. Subgroup analysis of vitamin D, calcium, folate, fiber and red or processed meat and gastric cancer risk.
Calcium: based on 10 studies (26, 28–30, 36–39, 44, 45), the pooled OR (95% CI) for higher intake of calcium compared to lower intake was 0.97 (0.91–1.03) (Figure 2), and moderate heterogeneity was found (I2 = 52.7%; P = 0.025). In all subgroups, calcium intake was not associated with risk of SPs, and countries, study designs and adjustments did not influence the heterogeneity of included studies (Table 2).
Folate: for folate, five studies were included in the pooled analysis (35–37, 39, 41), which indicated that folate intake was not statistically associated with SPs (OR = 0.82, 95% CI: 0.60–1.13) (Figure 2), and we identified moderate heterogeneity (I 2 = 59.8%; P = 0.041). Protective effects were detected in studies not adjusting smoking (OR = 0.59, 95% CI:0.38–0.93) or alcohol intake (OR=0.69, 95% CI:0.52–0.94) (Table 2).
Fiber: Nine studies investigating the risk of SPs with regard to the level of fiber intake were involved in the meta-analysis (17, 26, 29, 33, 36, 37, 39, 44, 45). The result showed that the risk of SPs in the highest fiber intake group decreased by 10% compared with the lowest fiber intake group (OR = 0.90, 95% CI: 0.82–0.99) (Figure 2), and large heterogeneity was discovered (I2= 75.8%; P < 0.001). Meta-regression showed statistically significant differences in the heterogeneity of subgroups with different countries (Table 2). Studies conducted in the USA did not reveal a statistical correlation between fiber intake and SP risk (OR = 0.97, 95% CI:0.91–1.04), while studies in other counties found fiber intake had a protective effect against SPs (OR = 0.79, 95% CI:0.66–0.95).
Red or processed meat: the pooled result of eight studies (15, 17, 27, 32, 33, 39, 40, 46) showed that higher red or processed meat intake was associated with an increased risk of SP (OR = 1.30, 95% CI: 1.13–1.51), and moderate heterogeneity was observed (I2= 50.3%; P = 0.050). This association was found in almost all subgroups (Table 2).
For different lesion types, as was shown in Figure 3, higher folate intake was associated with a decreased risk of HP (OR = 0.59, 95%CI: 0.44–0.79), and higher vitamin D intake decreased the risk of SP including SSA/P (OR = 0.93, 95%CI: 0.88–0.98). Fiber was perhaps a protective factor for SP and SSA/P (OR = 0.87, 95%CI: 0.77, 0.99), but not for HP (OR = 0.91, 95%CI: 0.74–1.13).
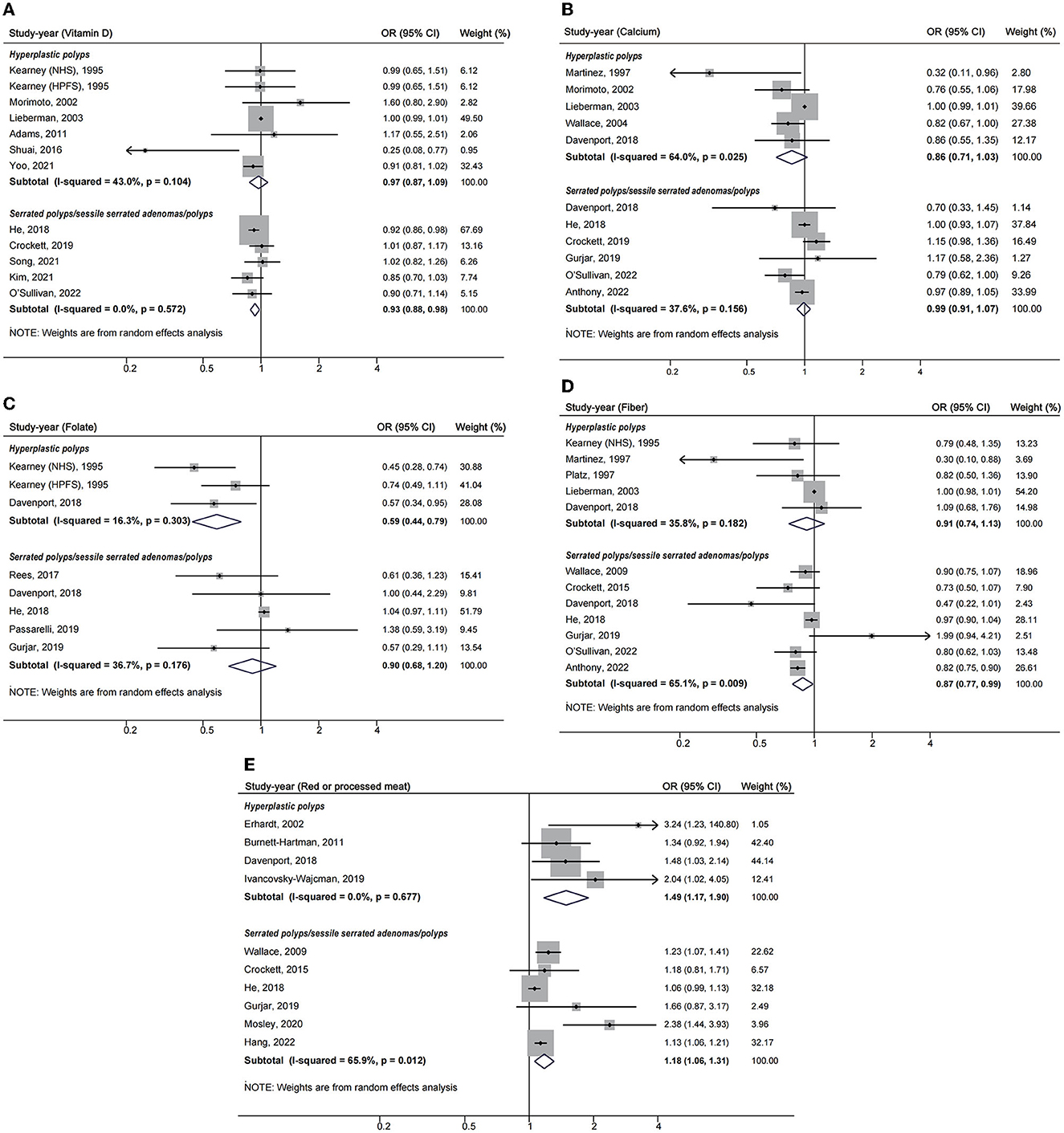
Figure 3. Forest plots of highest v. lowest category of dietary factors and serrated polyp risk for different lesion types. (A) Vitamin D; (B) calcium; (C) folate; (D) fiber; (E) red or processed meat.
3.3. Publication bias and sensitivity analysis
After visual inspection, we found asymmetries in funnel plots (Supplementary Figure 2). However, Egger's regression tests showed that a significant publication bias was indicated only for red or processed meat (t = 3.67, P = 0.010) (Supplementary Table 2). Following sensitivity analysis, the exclusion of any single study did not affect overall estimates for the influence of vitamin D, calcium and red or processed meat on SP risk, but the significance of pooled estimates of folate and fiber was changed when some studies were removed (Figure 4).
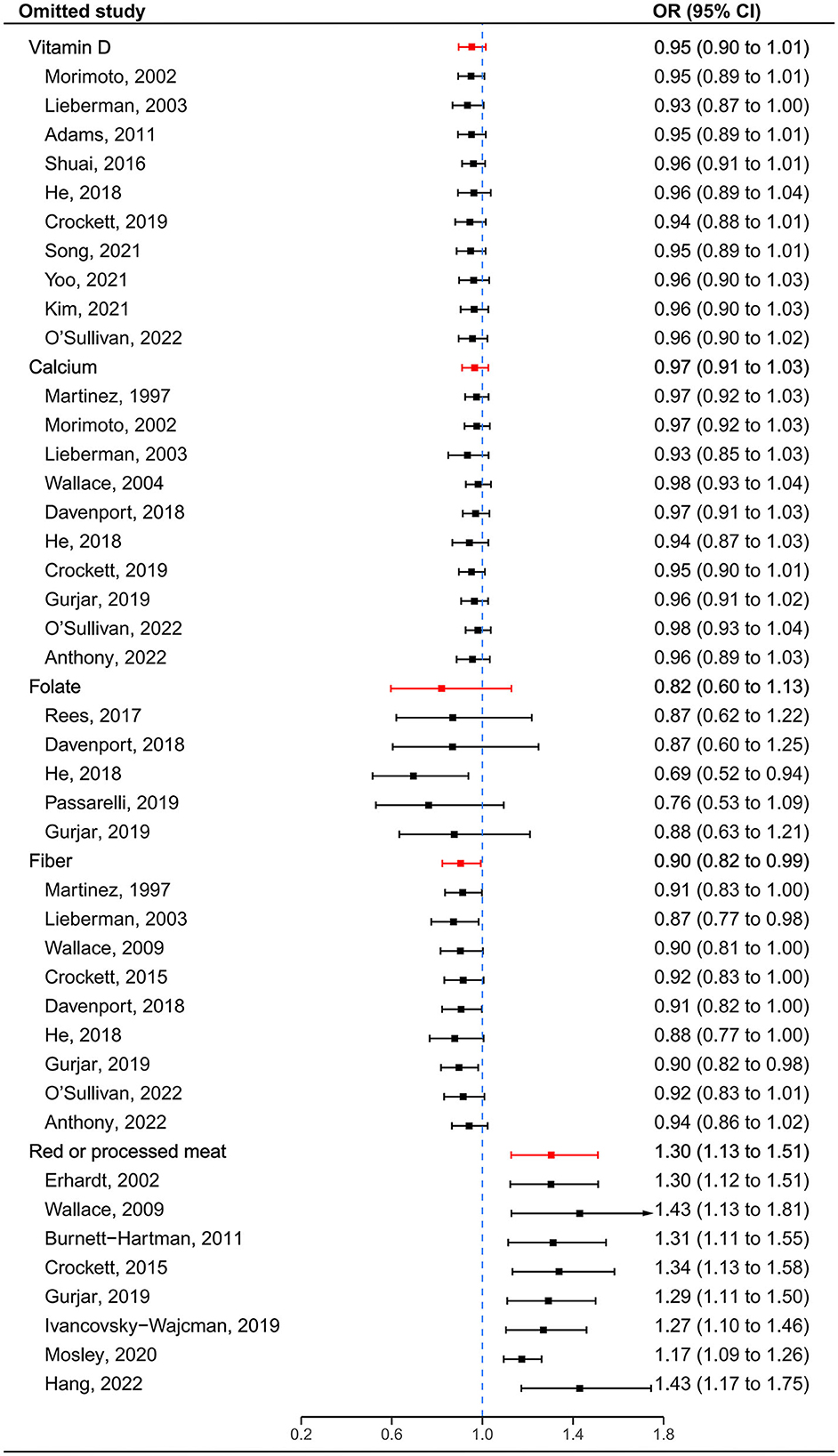
Figure 4. Sensitivity analyses by omitting one study at a time. The red lines represent the confidence intervals when all estimates are pooled for each dietary factor. The blue dashed line represents the null.
4. Discussion
This systematic review and meta-analysis is the latest and most comprehensive study investigating dietary factors and their influence on risk of serrated colorectal polyps. Meta-analyses revealed a statistically significant increased risk of SP associated with red or processed meat intake, and fiber intake was protective against SP. Higher intakes of vitamin D, calcium and folate were not significantly associated with SP risk.
Together, vitamin D and calcium are involved in regulating inflammation, differentiation, apoptosis and carcinogenesis in various cells, and have been widely studied as protective factors against colorectal cancer (47). Lopez-Caleya et al. conducted a meta-analysis based on case-control studies, showing that dietary intake of vitamin D can reduce the risk of CRC by 4% (48). Another more comprehensive meta-analysis done by Huang et al. showed that vitamin D intake and calcium intake were protective factors for colorectal adenomas, and a significant dose-response was observed between the intake of vitamin D or calcium and colorectal tumor incidence (49). According to our results, though vitamin D intake did not reduce the risk of HP, it significantly reduced risk of SP including SSA/P by 7%. Besides, inverse associations between vitamin D intake and SP risk were also observed when studies adjusted for smoking, alcohol intake or BMI. Therefore, we assumed that vitamin D had a certain protective effect on SPs, but more high-quality studies were still needed to confirm this conclusion. Calcium intake did not play a protective role against SP. In an RCT, Crockett et al. revealed that supplementary calcium and the combination of calcium and vitamin D3 even increased the risk of SSA/Ps (38).
Folate is abundant in leafy green vegetables, legumes, and cereals. It has been a focus of CRC chemo-prevention research for several decades because of its role in DNA methylation, repair, and nucleotide synthesis (50). In a randomized controlled trial and a cohort study, both folate supplementation and dietary folate are beneficial for the primary prevention of colorectal adenomas (51, 52). Moazzen et al. conducted a meta-analysis about folate and CRC risk, the results showed higher folic acid intake significantly reduced CRC risk by 23% and 29% in case-control studies and cohort studies, respectively, whereas folic acid supplementation had no significant effect (53). However, in the meta-analysis, neither observational studies nor RCTs suggested folate intake decreased the risk of SP (41). Ree et al. investigated the concentration of serum folates and risk of SPs, finding that people with methylated serum folate concentrations between 37.35 and 60.44 nmol/L had lower SP risk compared with those with concentrations lower than 37.35 nmol/L, but much higher serum folate concentration produced non-significant effect statistically, which might indicate a U-shaped dose-response relationship between folate and SP risk and provided more direct evidence (35). Overall, differences in subject baseline characteristics, methods of folate status assessment, and the biologically effective dose of folic acid in the body were likely to account for the above heterogeneity.
The current meta-analysis suggested fiber intake decreased the risk of SPs by 10% for the highest vs. lowest intakes. Dietary fiber affects colorectal cancer risk by increasing fecal volume, diluting fecal carcinogens in the colonic lumen, and shortening fecal transit time through the gut (54). Recent studies have shown that dietary fiber increases microbiota diversity in the gut (55) and gut microbiota is associated with CRC risk (56). A dose-response relationship meta-analysis found that each 10 g/d increase in the intake of cereal fiber was associated with a 9% lower risk of colorectal cancer (8), which supported our results.
The WHO has classified red meat as a class 2A carcinogen. A recent expert panel report also supported the role of red and processed meats in CRC risk (57). Processed red meat contains nitroso compounds that can cause DNA alkylation, which can lead to cellular carcinogenesis (58). Two meta-analyses performed by Zhao et al. manifested that compared with the lowest red meat intake, the highest red meat intake increased the risk of colonic adenomas and colorectal cancer (59, 60). In our study, red meat intake increased the risk of SP by 30%, which was consistent with the previous studies and added strong risk factor evidence.
The study provided a comprehensive analysis of associations between the above dietary factors and SP risk, which remain controversial today. Although most previous studies reported that calcium and folate intake could protect people from colorectal adenomas and cancer, the current meta-analyses found they had no such protective effect on SP.
This work was subjected to some shortcomings. First, as most studies were conducted based on U.S. populations, it is not appropriate to generalize our findings to African and Asian populations. Second, the results of folate and fiber intake may not be very stable according to sensitivity analyses. Besides, the included studies varied in many ways such as study design, adjustment for confounders, and different ascertainment methods for factor exposure, which might have an impact on the results. To minimize the effect of this limitation, we adopted random-effect models to pool the estimates. Subgroup analyses and meta-regression were also conducted to detect probable sources of heterogeneity, which enabled us to account for these differences.
5. Conclusions
The results of this meta-analysis indicate that higher dietary calcium, folate and fiber intake can reduce the risk of SP, and vitamin D intake may have the effect of preventing SPs, which needs to be determined by more evidence. Red meat intake is associated with an increased risk of SP. This evidence provides guidance for us to prevent SPs from a dietary perspective, such as moderately increasing fiber intake and reducing red meat intake. Further high-quality research is needed to clarify the role of vitamin D, folate, and calcium intake in SPs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
XL, DX, and ZZ conceived and designed the meta-analysis. ZZ, XZ, XG, and NL searched the literature. ZZ, XZ, XG, NL, and SD extracted the data. ZZ analyzed the data, contributed analysis tools, and wrote the manuscript. XL revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1187539/full#supplementary-material
Supplementary figure 1. Risk of bias summary of RCTs.
Supplementary figure 2. Funnel plots with pseudo 95% confidence intervals.
References
1. Binefa G, Rodriguez-Moranta F, Teule A, Medina-Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol. (2014) 20:6786–808. doi: 10.3748/wjg.v20.i22.6786
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clini. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Hale VL, Chen J, Johnson S, Harrington SC, Yab TC, Smyrk TC, et al. Shifts in the fecal microbiota associated with adenomatous polyps. Cancer Epidemiol Biomarkers Prevent. (2017) 26:85–94. doi: 10.1158/1055-9965.EPI-16-0337
4. Peters BA, Dominianni C, Shapiro JA, Church TR, Wu J, Miller G, et al. The gut microbiota in conventional and serrated precursors of colorectal cancer. Microbiome. (2016) 4:69. doi: 10.1186/s40168-016-0218-6
5. Yamane L, Scapulatempo-Neto C, Reis RM, Guimaraes DP. Serrated pathway in colorectal carcinogenesis. World J Gastroenterol. (2014) 20:2634–40. doi: 10.3748/wjg.v20.i10.2634
6. Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. (2010) 138:2088–100. doi: 10.1053/j.gastro.2009.12.066
7. Fedirko V, Tramacere I, Bagnardi V, Rota M, Scotti L, Islami F, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. (2011) 22:1958–72. doi: 10.1093/annonc/mdq653
8. Aune D, Chan DSM, Lau R, Vieira R, Greenwood DC, Kampman E, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. (2011). 343:d6617. doi: 10.1136/bmj.d6617
9. Norat T, Lukanova A, Ferrari P, Riboli E. Meat consumption and colorectal cancer risk: Dose-response meta-analysis of epidemiological studies. Int J Cancer. (2002) 98:241–56. doi: 10.1002/ijc.10126
10. Yu J, Feng Q, Kim JH, Zhu Y. Combined effect of healthy lifestyle factors and risks of colorectal adenoma, colorectal cancer, and colorectal cancer mortality: systematic review and meta-analysis. Front Oncol. (2022) 12:827019. doi: 10.3389/fonc.2022.827019
11. Gao Q, Tsoi KKF, Hirai HW, Wong MCS, Chan FKL, Wu JCY, et al. Serrated polyps and the risk of synchronous colorectal advanced neoplasia: a systematic review and meta-analysis. Am J Gastroenterol. (2015) 110:501–10. doi: 10.1038/ajg.2015.49
13. Fu Z, Shrubsole MJ, Smalley WE, Wu H, Chen Z, Shyr Y, et al. Lifestyle factors and their combined impact on the risk of colorectal polyps. Am J Epidemiol. (2012) 176:766–76. doi: 10.1093/aje/kws157
14. Kearney J, Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, et al. Diet, alcohol, and smoking and the occurrence of hyperplastic polyps of the colon and rectum (United-States). Cancer Causes Control. (1995) 6:45–56. doi: 10.1007/BF00051680
15. Mosley D, Su T, Murff HJ, Smalley WE, Ness RM, Zheng W, et al. Meat intake, meat cooking methods, and meat-derived mutagen exposure and risk of sessile serrated lesions. Am J Clini Nutrit. (2020) 111:1244–51. doi: 10.1093/ajcn/nqaa030
16. Platz EA, Giovannucci E, Rimm EB, Rockett HRH, Stampfer MJ, Colditz GA, et al. Dietary fiber and distal colorectal adenoma in men. Cancer Epidemiol Biomarkers Prev. (1997) 6:661–70.
17. Wallace K, Grau MV, Ahnen D, Snover DC, Robertson DJ, Mahnke D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev. (2009) 18:2310–7. doi: 10.1158/1055-9965.EPI-09-0211
18. Yoo MY, Lee J, Chung JI, Yeo Y, Cho IY. The association between serum vitamin d concentration and colon polyp: a cross-sectional study using health care screening database in a tertiary hospital in Korea. Korean J Fam Med. (2021) 42:303–9. doi: 10.4082/kjfm.20.0181
19. Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Systemat Rev. (2012) 1:2. doi: 10.1186/2046-4053-2-4
20. Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data the PRISMA-IPD statement. JAMA. (2015) 313:1657–65. doi: 10.1001/jama.2015.3656
21. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
22. Zhu Z, Zhu X, Gu L, Zhan Y, Chen L, Li X. Association between vitamin D and influenza: meta-analysis and systematic review of randomized controlled trials. Front Nutrit. (2022) 8:799709. doi: 10.3389/fnut.2021.799709
23. Dersimonian R, Laird N. Meta analysis in. clinical-trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
24. Harbord RM, Egger M, Sterne JAC. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. (2006) 25:3443–57. doi: 10.1002/sim.2380
25. Dai Q, Shrubsole MJ, Ness RM, Schlundt D, Cai Q, Smalley WE, et al. The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am J Clin Nutr. (2007) 86:743–51. doi: 10.1093/ajcn/86.3.743
26. Martinez ME, McPherson RS, Levin B, Glober GA. A case-control study of dietary intake and other lifestyle risk factors for hyperplastic polyps. Gastroenterology. (1997) 113:423–9. doi: 10.1053/gast.1997.v113.pm9247459
27. Erhardt JG, Kreichgauer HP, Meisner C, Bode JC, Bode C. Alcohol, cigarette smoking, dietary factors and the risk of colorectal adenomas and hyperplastic polyps—a case control study. Eur J Nutr. (2002) 41:35–43. doi: 10.1007/s003940200004
28. Morimoto LM, Newcomb PA, Ulrich CM, Bostick RM, Lais CJ, Potter JD. Risk factors for hyperplastic and adenomatous polyps: Evidence for malignant potential? Cancer Epidemiol Biomarkers Prev. (2002) 11:1012–8.
29. Lieberman DA, Prindiville S, Weiss DG, Willett W, Grp VACS. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA-JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION. (2003) 290:2959–67. doi: 10.1001/jama.290.22.2959
30. Wallace K, Baron JA, Cole BF, Sandler RS, Karagas MR, Beach MA, et al. Effect of calcium supplementation on the risk of large bowel polyps. J Natl Cancer Inst. (2004) 96:921–5. doi: 10.1093/jnci/djh165
31. Adams SV, Newcomb PA, Burnett-Hartman AN, White E, Mandelson MT, Potter JD. Circulating 25-hydroxyvitamin-D and risk of colorectal adenomas and hyperplastic polyps. Nutr Cancer. (2011) 63:319–26. doi: 10.1080/01635581.2011.535960
32. Burnett-Hartman AN, Newcomb PA, Mandelson MT, Adams SV, Wernli KJ, Shadman M, et al. Colorectal polyp type and the association with charred meat consumption, smoking, and microsomal epoxide hydrolase polymorphisms. Nutr Cancer. (2011) 63:583–92. doi: 10.1080/01635581.2011.553021
33. Crockett SD, Martin CF, Baron JA, Sandler RS. Obesity, waist-hip-ratio, diet, and physical activity and risk of serrated polyps and sessile serrated adenomas: A cross-sectional study. Gastroenterology. (2015) 148:S670–S1. doi: 10.1016/S0016-5085(15)32263-0
34. Shuai Q, Zhang YZ, Wang H, Yu ED, Li GX, Li ZS, et al. Association between plasma 25-hydroxy vitamin D levels and risk of colorectal neoplasms: a case-control study. Acad J Second Military Med Univ. (2016) 37:536–43.
35. Rees JR, Morris CB, Peacock JL, Ueland PM, Barry EL, McKeown-Eyssen GE, et al. Unmetabolized folic acid, tetrahydrofolate, and colorectal adenoma risk. Cancer Prev Res. (2017) 10:451–8. doi: 10.1158/1940-6207.CAPR-16-0278
36. Davenport JR, Su T, Zhao Z, Coleman HG, Smalley WE, Ness RM, et al. Modifiable lifestyle factors associated with risk of sessile serrated polyps, conventional adenomas and hyperplastic polyps. Gut. (2018) 67:456–65. doi: 10.1136/gutjnl-2016-312893
37. He X, Wu K, Ogino S, Giovannucci EL, Chan AT, Song M. Association between risk factors for colorectal cancer and risk of serrated polyps and conventional adenomas. Gastroenterology. (2018) 155:355–73.e18. doi: 10.1053/j.gastro.2018.04.019
38. Crockett S, Barry E, Mott L, Ahnen D, Robertson D, Anderson J, et al. Calcium and vitamin D supplementation and increased risk of serrated polyps: results from a randomised clinical trial. Gut. (2019) 68:475–86. doi: 10.1136/gutjnl-2017-315242
39. Gurjar MS, Galanko J, Martin C, Baron JA, Sandler R, Crockett S. High riboflavin intake protects against sessile serrated adenomas/polyps: a pooled study of dietary risk factors for serrated polyps. Gastroenterology. (2019) 156:S676. doi: 10.1016/S0016-5085(19)38601-9
40. Ivancovsky-Wajcman D, Zelber-Sagi S, Fliss-Isakov N, Webb M, Zemel M, Shibolet O, et al. High meat intake and low sRAGE are associated with colonicpolyps mainly in smokers in a case control study. United Eur Gastroenterol J. (2019) 7:655–6.
41. Passarelli M, Barry E, Rees J, Mott L, Zhang D, Ahnen D, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutrit. (2019). 110:903–11. doi: 10.1093/ajcn/nqz160
42. Kim H, Lipsyc-Sharf M, Zong X, Wang X, Hur J, Song M, et al. Total vitamin D intake and risks of early-onset colorectal cancer and precursors. Gastroenterology. (2021) 161:1208. doi: 10.1053/j.gastro.2021.07.002
43. Song M, Lee I, Manson J, Buring J, Dushkes R, Gordon D, et al. No association between vitamin D supplementation and risk of colorectal adenomas or serrated polyps in a randomized trial. Clin Gastroenterol Hepatol. (2021) 19:128–35.e6. doi: 10.1016/j.cgh.2020.02.013
44. Anthony E, Reece JC, Milanzi E, Joo JE, Joseland S, Clendenning M, et al. Body Mass Index, sex, non-steroidal anti-inflammatory drug medications, smoking and alcohol are differentially associated with World Health Organisation criteria and colorectal cancer risk in people with Serrated Polyposis Syndrome: an Australian case-control study. BMC Gastroenterol. (2022) 22:489. doi: 10.1186/s12876-022-02557-7
45. O'Sullivan DE, Ruan Y, Forbes N, Heitman SJ, Hilsden RJ, Pader J, et al. Long-term use of hormone replacement therapy is associated with a lower risk of developing high-risk serrated polyps in women. J Clin Gastroenterol. (2022) 56:697–704. doi: 10.1097/MCG.0000000000001606
46. Hang D, Wang L, Fang Z, Du M, Wang K, He X, et al. Ultra-processed food consumption and risk of colorectal cancer precursors: results from 3 prospective cohorts. J Natl Cancer Inst. (2023) 115:155–64. doi: 10.1093/jnci/djac221
47. Nana K, Li L, Hamada T, Zhi Rong Q, Nowak JA, Yin C, et al. Calcium intake and colon cancer risk subtypes by tumor molecular characteristics. Cancer Causes Control. (2019) 30:637–49. doi: 10.1007/s10552-019-01165-3
48. Lopez-Caleya JF, Ortega-Valin L, Fernandez-Villa T, Delgado-Rodriguez M, Martin-Sanchez V, Molina AJ. The role of calcium and vitamin D dietary intake on risk of colorectal cancer: systematic review and meta-analysis of case-control studies. Cancer Causes Control. (2022) 33:167–82. doi: 10.1007/s10552-021-01512-3
49. Huang DD, Lei SQ, Wu YH, Weng MH, Zhou YW, Xu JW, et al. Additively protective effects of vitamin D and calcium against colorectal adenoma incidence, malignant transformation and progression: A systematic review and meta-analysis. Clin Nutr. (2020) 39:2525–38. doi: 10.1016/j.clnu.2019.11.012
50. Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr. (2002) 132:2350s−5s. doi: 10.1093/jn/132.8.2350S
51. Gibson TM, Weinstein SJ, Pfeiffer RM, Hollenbeck AR, Subar AF, Schatzkin A, et al. Pre- and postfortification intake of folate and risk of colorectal cancer in a large prospective cohort study in the United States. Am J Clin Nutrit. (2011) 94:1053–62. doi: 10.3945/ajcn.110.002659
52. Gao Q-Y, Chen H-M, Chen Y-X, Wang Y-C, Wang Z-H, Tang J-T, et al. Folic acid prevents the initial occurrence of sporadic colorectal adenoma in chinese older than 50 years of age: a randomized clinical trial. Cancer Prev Res. (2013) 6:744–52. doi: 10.1158/1940-6207.CAPR-13-0013
53. Moazzen S, Dolatkhah R, Tabrizi JS, Shaarbafi J, Alizadeh BZ, de Bock GH, et al. Folic acid intake and folate status and colorectal cancer risk: A systematic review and meta-analysis. Clin Nutr. (2018) 37:1926–34. doi: 10.1016/j.clnu.2017.10.010
54. Lupton JR, Turner ND. Potential protective mechanisms of wheat bran fiber. Am J Med. (1999) 106:24s−7s. doi: 10.1016/S0002-9343(98)00343-X
55. Segata N. Gut microbiome: westernization and the disappearance of intestinal diversity. Curr Biol. (2015) 25:R611–3. doi: 10.1016/j.cub.2015.05.040
56. Flemer B, Lynch DB, Brown JM, Jeffery IB, Ryan FJ, Claesson MJ, et al. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. (2017) 66:633–43. doi: 10.1136/gutjnl-2015-309595
57. Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. (2015) 16:1599–600. doi: 10.1016/S1470-2045(15)00444-1
58. Van Hecke T, Vossen E, Hemeryck LY, Vanden Bussche J, Vanhaecke L, De Smet S. Increased oxidative and nitrosative reactions during digestion could contribute to the association between well-done red meat consumption and colorectal cancer. Food Chem. (2015) 187:29–36. doi: 10.1016/j.foodchem.2015.04.029
59. Zhao Z, Yin Z, Hang Z, Zhang C, Zhao Q. Association between red and processed meat intake and colorectal adenoma incidence and recurrence: a systematic review and meta-analysis. Oncotarget. (2018) 9:32373–82. doi: 10.18632/oncotarget.23561
Keywords: colorectal serrated polyps, colorectal cancer, dietary factors, meta-analysis, systematic review
Citation: Zhu Z, Guan X, Liu N, Zhu X, Dai S, Xiong D and Li X (2023) Association between dietary factors and colorectal serrated polyps: a systematic review and meta-analysis. Front. Nutr. 10:1187539. doi: 10.3389/fnut.2023.1187539
Received: 30 March 2023; Accepted: 27 June 2023;
Published: 27 July 2023.
Edited by:
Raul Zamora-Ros, Institut d'Investigacio Biomedica de Bellvitge (IDIBELL), SpainReviewed by:
Venkataraghavan Ramamoorthy, Baptist Health South Florida, United StatesXianlei Cai, Ningbo University, China
Copyright © 2023 Zhu, Guan, Liu, Zhu, Dai, Xiong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuyang Li, lixiuyang@zju.edu.cn; Dehai Xiong, 1208505185@qq.com
 Zhixin Zhu
Zhixin Zhu Xifei Guan
Xifei Guan Nawen Liu
Nawen Liu Xiaoxia Zhu
Xiaoxia Zhu Sheng Dai
Sheng Dai Dehai Xiong
Dehai Xiong Xiuyang Li
Xiuyang Li