Associations of specific dietary unsaturated fatty acids with risk of overweight/obesity: population-based cohort study
- 1Lanxi Red Cross Hospital, Jinhua, Zhejiang, China
- 2Department of Nutrition, School of Public Health, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
- 3Lanxi People’s Hospital, Jinhua, Zhejiang, China
- 4Lanxi Center for Disease Control and Prevention, Jinhua, China
- 5Department of Food Science and Nutrition, Fuli Institute of Food Science, College of Biosystems Engineering and Food Science, Zhejiang University, Hangzhou, Zhejiang, China
- 6Lanxi Hospital of Traditional Chinese Medicine, Jinhua, Zhejiang, China
Background: The role of specific unsaturated fatty acids (FAs) in the development of overweight/obesity remains unclear in the general population. Here, we aimed to explore the associations of different types of unsaturated FAs with overweight/obesity risk among the Chinese population.
Methods: Eight thousand seven hundred forty-two subjects free of overweight/obesity at entry in the China Health and Nutrition Survey (CHNS) were followed up until 2015. Dietary unsaturated FAs were assessed by 3-day 24-h recalls with a weighing method in each wave. Cox regression models were used to obtain the hazard ratios (HRs) and 95% confidence intervals (CIs) for overweight/obesity risk associated with unsaturated FAs.
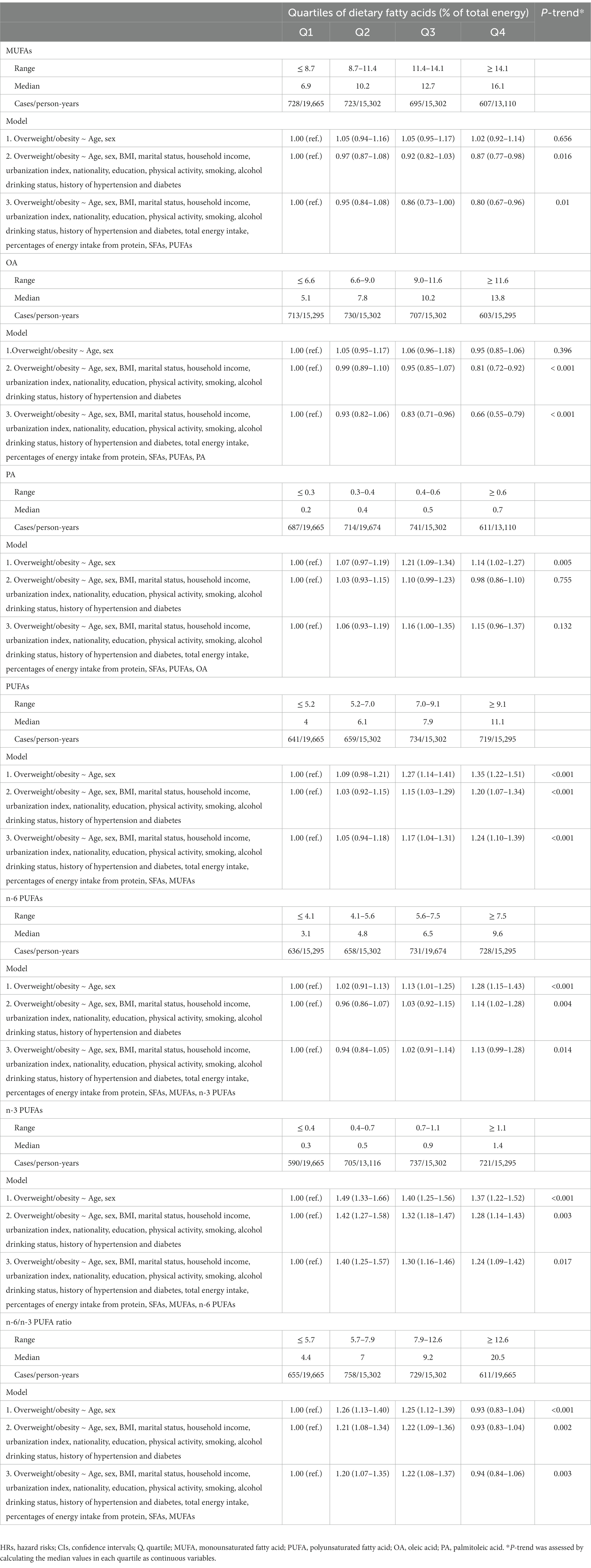
Results: During a median follow-up of 7 years, 2,753 subjects (1,350 males and 1,403 females) developed overweight/obesity. Consuming more monounsaturated FAs (MUFAs) was associated with a lower risk of overweight/obesity (highest vs. lowest quartile: HR 0.80, 95% CI 0.67–0.96; P-trend = 0.010). Similar inverse associations were observed for plant-MUFAs (HRQ4vsQ1 0.83, 95% CI: 0.73–0.94; P-trend = 0.003) and animal-MUFAs (HRQ4vsQ1 0.77, 95% CI: 0.64–0.94; P-trend = 0.004), total dietary oleic acid (OA) (HRQ4vsQ1 0.66, 95% CI: 0.55–0.79; P-trend <0.001), plant-OA (HRQ4vsQ1 0.73, 95% CI: 0.64–0.83; P-trend <0.001) and animal-OA (HRQ4vsQ1 0.68, 95% CI: 0.55–0.84; P-trend <0.001). In addition, the intakes of n-3 polyunsaturated FAs (PUFAs) (HRQ4vsQ1 1.24, 95% CI: 1.09–1.42; P-trend = 0.017) and α-linolenic acid (ALA) (HRQ4vsQ1 1.22, 95% CI: 1.07–1.39; P-trend = 0.039) but not marine n-3 PUFAs were positively linked to overweight/obesity risk. Consumption of n-6 PUFAs (HRQ4vsQ1 1.13, 95% CI: 0.99–1.28; P-trend = 0.014) and linoleic acid (LA) (HRQ4vsQ1 1.11, 95% CI: 0.98–1.26; P-trend = 0.020) had marginal and positive relationships with the incidence of overweight/obesity. N-6/n-3 PUFA ratio ranging from 5.7 to 12.6 was related to higher risk of overweight/obesity.
Conclusion: Higher dietary intake of MUFAs was associated with lower overweight/obesity risk, which was mainly driven by dietary OA from either plant or animal sources. Intakes of ALA, n-6 PUFAs and LA were related to higher risk of overweight/obesity. These results support consuming more MUFAs for maintaining a healthy body weight among the Chinese population.
Introduction
Overweight and obesity are a great burden to the world, with the events of overweight and obesity tripling to 1.6 billion in 2016 compared to 1975s (1). Notably, overweight and obesity are vital risk factors for noncommunicable diseases including cardiovascular disease and cancer, which are leading causes of death (2, 3). China is also threatened by the epidemic of overweight and obesity. The latest data from the Chinese Residents Chronic Disease and Nutrition Surveillance (2020) highlighted that the prevalence of overweight and obesity has rapidly increased to approximately over 50% among Chinese adults (4).
Dietary habits are key modifiable factors to prevent a large fraction of overweight and obesity (5). Among them, different types of dietary fatty acids (FAs) have received great attention in human health (6). Dietary guidelines recommend reducing saturated FA (SFA) intake while increasing the intake of polyunsaturated FAs (PUFAs) and monounsaturated FAs (MUFAs) (7). However, these guidelines are based on cardiovascular benefits and fail to focus on specific FAs that could have divergent effects on body size. Previous studies summarized that the chain length, degree of unsaturation, and position and stereoisomeric configuration of the double bonds of FAs might affect FA oxidation rate thereby influencing body weight (8). Our previous study assessed the association of different chain-length SFA intake with overweight/obesity in the Chinese population, which indicated heterogeneous effects among SFAs with different chain lengths (9). In terms of unsaturated FAs, previous studies suggested that dietary oleic acid (OA) and long-chain n-3 PUFAs had a protective effect on body weight or composition (10, 11), while dietary intake of n-6 PUFAs including linoleic acid (LA) and arachidonic acid (AA) were supported to promote weight gain (12). Earlier studies have revealed different effects of MUFAs from animal and plant sources on human health (13–15). However, prospective studies assessing the effects on the development of overweight/obesity are lacking. Chinese adults have higher consumption of OA and LA, but lower intake of palmitoleic acid (PA), α-linolenic acid (ALA) and marine n-3 PUFAs including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (16). The current level of unsaturated FA consumption in relation to overweight/obesity development has not been assessed at a nationwide level in China.
To provide further evidence and advance the field, we investigated the diverse associations of different unsaturated FA intakes with the risk of overweight and obesity among 8,742 Chinese adults enrolled in the China Health and Nutrition Survey (CHNS).
Methods
Study population
CHNS is an ongoing cohort using multistage cluster random sampling to draw a sample of 30,000 individuals from 15 provinces and municipal cities in China. The CHNS was initiated in 1989. Subsequently, the surveys were conducted in 1991, 1993, 1997, 2000, 2004, 2006, 2009, 2011, and 2015. Detailed procedures have been described elsewhere (17, 18). Given that only adults aged 20–45 y were included in the 1989 round and the food codes from 1991 to 1993 round did not match the food codes in the Chinese Food Composition Table (FCT), participants in the current analysis were recruited from 1997 to 2011 round. We further excluded the participants aged under 20 years old (n = 8,706), without complete dietary data based on a 3-day 24-h dietary recall (n = 720), had a history of cardiovascular disease (CVD) or cancer at baseline (n = 550), with extreme energy intake (< 800 or > 4,200 kcal/day for men and < 600 or > 3,500 kcal/day for women, n = 181), without follow-up or with overweight/obesity at baseline (n = 8,005), and without BMI data during the follow-up (n = 2,572). Finally, 8,742 participants were selected in the present analyzes (Supplementary Figure S1).
Dietary assessment and covariates
In the CHNS, dietary assessments consist of a 3-day 24-h dietary recall for individuals and a weighing inventory for household food consumption at the same 3 days. Other details on dietary data collection have been described elsewhere (17). Nutrient intakes from various foods were calculated using FCT (19–21). The 1991 version of FCT was used in 1997 and 2000 to obtain dietary information, and the 2002 and 2004 versions were combined for other surveys. Cumulative mean values were computed for each nutrient to represent long-term consumption and reduce within-individual heterogeneity. In addition, demographic and lifestyle information was collected as well, including age, sex, physical activity, marital status, nationality, education level, household income, smoking, alcohol consumption, and history of hypertension and diabetes.
Ascertainment of overweight and obesity
The height and weight of each participant in each interview were measured by well-trained staffs with the use of standard protocol and instruments. BMI was calculated as body weight (kg) divided by height squared (m2). The ascertainment of overweight and obesity was according to the Chinese Criteria of Weight for Adults (WS/T 428–2013): participants with a range of 24 kg/m2 ≤ BMI < 28 kg/m2 were considered as overweight, while a BMI ≥ 28 kg/m2 was considered as obesity.
Statistical analyzes
Intakes of individual unsaturated FAs were expressed as percentages of total energy intake and then divided into quartiles (22). The baseline characteristics of participants were expressed as the means ± standard errors for continuous variables, while categorical variables were expressed as the percentages (%). To compare proportions or means of baseline characteristics among quartiles of MUFA or PUFA intake, chi-square test for categorical variables and analysis of variance (ANOVA) for continuous variables were applied. The follow-up duration of each participant was calculated from the baseline year to the year of developing overweight/obesity or the date of their last assessment. Multivariable Cox proportional hazards regression models were conducted to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for overweight/obesity risk with the first category of unsaturated FAs as the reference. Tests for trends were assessed by calculating the median values in each quartile as continuous variables. Three stepwise models were established with potential confounders considered as covariates: model 1 was a crude model adjusted for age and gender; model 2 was further adjusted for BMI (in kg/m2: < 18.5, 18.5–23.9, 24–27.9 or ≥ 28), nationality (Han or non-Han), education (less than high school, high school, some college or at least college), deprivation index (quartile), marital status (never married, married or living as married, widowed/divorced/separated, or unknown), household income (quartile), physical activity (no regular activity, low to moderate activity, or vigorous activity), smoking (never, former, current, or unknown), alcohol drinking status (non-drinker or drinker), history of hypertension (yes, no, or unknown) and diabetes (yes, no, or unknown); model 3 was additionally adjusted for the intake of total energy, percentages of energy from protein, SFAs and remaining FAs where appropriate. For the possible dose–response relationship between individual FAs and overweight/obesity, restricted cubic spline regression was performed with 4 knots at prespecified locations according to the percentiles of FAs.
Subgroup analyzes were conducted stratified by gender, age, smoking status, alcohol consumption, physical activity, education level, household income, and history of hypertension and diabetes. In sensitivity analysis, to test the robustness of models, we further adjusted for cholesterol intake, occupation and alternative healthy eating index (AHEI) (23), excluded participants with extreme BMI (< 18.5 kg/m2), and excluded participants with hypertension or diabetes at baseline.
All these analyzes were performed with the use of SAS version 9.4 (SAS Institute, Cary, NC, United States). Two-sided probability values <0.05 were considered statistically significant.
Results
Baseline characteristics
The baseline characteristics of 8,742 participants according to quartiles of total MUFA and PUFA intakes are shown in Table 1. Participants who consumed more MUFA or PUFA were older (p value <0.001) and women (p value <0.001). Furthermore, they had higher household income (p value <0.001), higher education levels (p value <0.001), higher intake of SFAs (p value <0.001) and cholesterol (p value <0.001), and higher prevalence of diabetes (p value = 0.005 for MUFA, p value <0.001 for PUFA). On the contrary, they smoked (p value <0.001) and drank alcohol (p value <0.001) less frequently, and had less physical activity (p value <0.001) and total energy intake (p value <0.001).
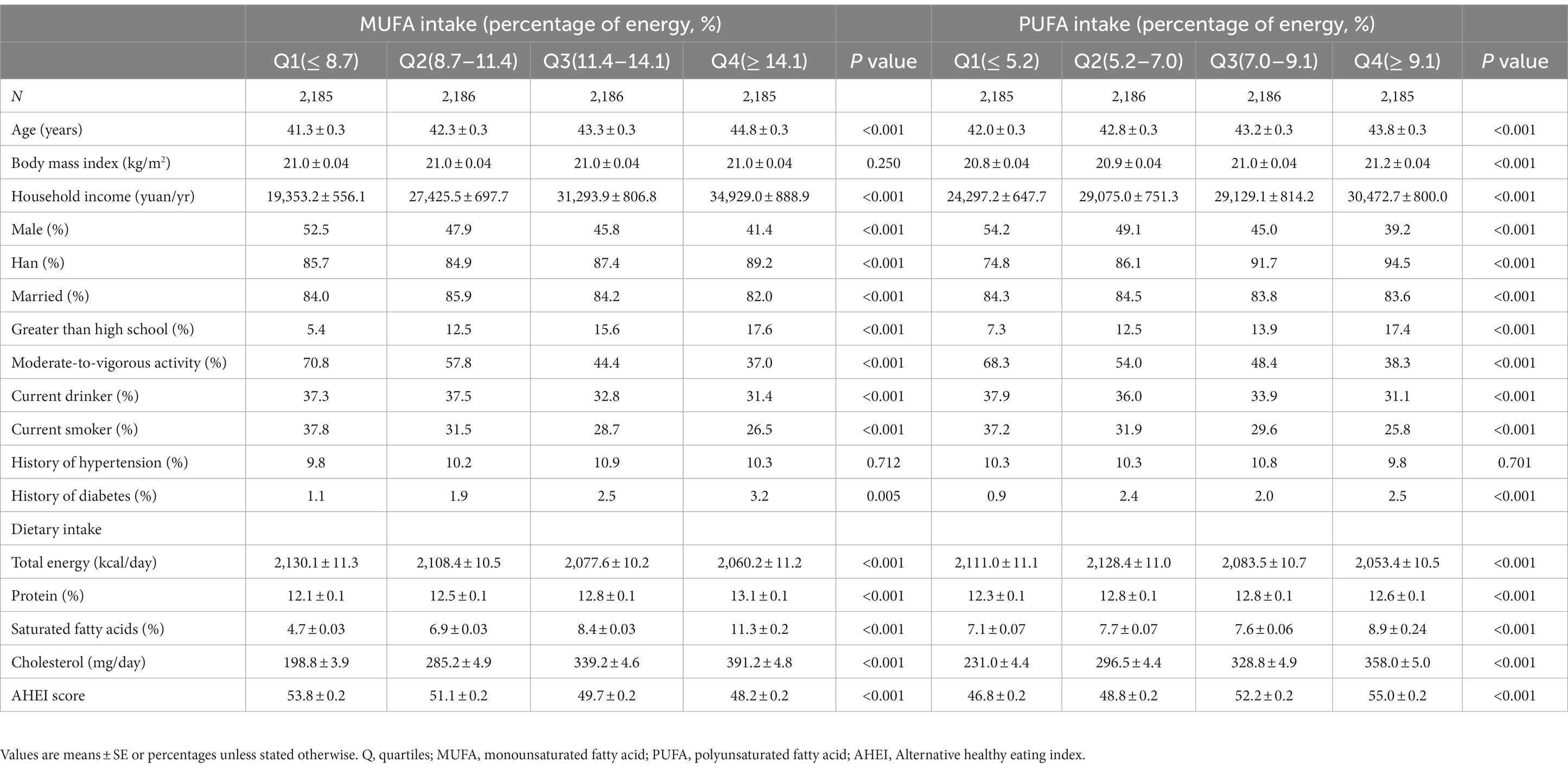
Table 1. Characteristics of the participants at baseline classified by the quartiles of MUFA and PUFA intakes.
MUFA intake and risk of overweight and obesity
Over a median of 7-year follow-up, 1,350 males and 1,403 females developed overweight/obesity. In model 3 with potential confounders fully adjusted, participants in the highest quartile of MUFA intake presented a significant reduction of 20% in the risk of overweight/obesity (HRQ4vsQ1 0.80, 95% CI: 0.67–0.96; P-trend = 0.010; Table 2). The inverse association was also observed for OA intake (HRQ4vsQ1 0.66, 95% CI: 0.55–0.79; P-trend <0.001), whereas PA intake was not associated with the risk of overweight/obesity (HRQ4vsQ1 1.15, 95% CI: 0.96–1.37; P-trend = 0.132; Table 2). In terms of dietary source of MUFAs, both animal-derived (HRQ4vsQ1 0.77, 95% CI: 0.64–0.94; P-trend = 0.004) and plant-derived MUFAs (HRQ4vsQ1 0.83, 95% CI: 0.73–0.94; P-trend = 0.003) had inverse associations with overweight/obesity development (Table 3). The results of OA derived from animal (HRQ4vsQ1 0.68, 95% CI: 0.55–0.84; P-trend <0.001) and plant sources (HRQ4vsQ1 0.73, 95% CI: 0.64–0.83; P-trend <0.001) exhibited similar association patterns (Supplementary Table S1). However, the adjusted HRs and 95% CIs suggested a detrimental association for plant-PA (HRQ4vsQ1 1.29, 95% CI: 1.14–1.47; P-trend = 0.002) but not animal-PA consumption (HRQ4vsQ1 0.86, 95% CI: 0.71–1.05; P-trend = 0.132; Supplementary Table S1). Restricted cubic spline regression produced similar findings for these MUFAs (Figure 1; Supplementary Figure S2).
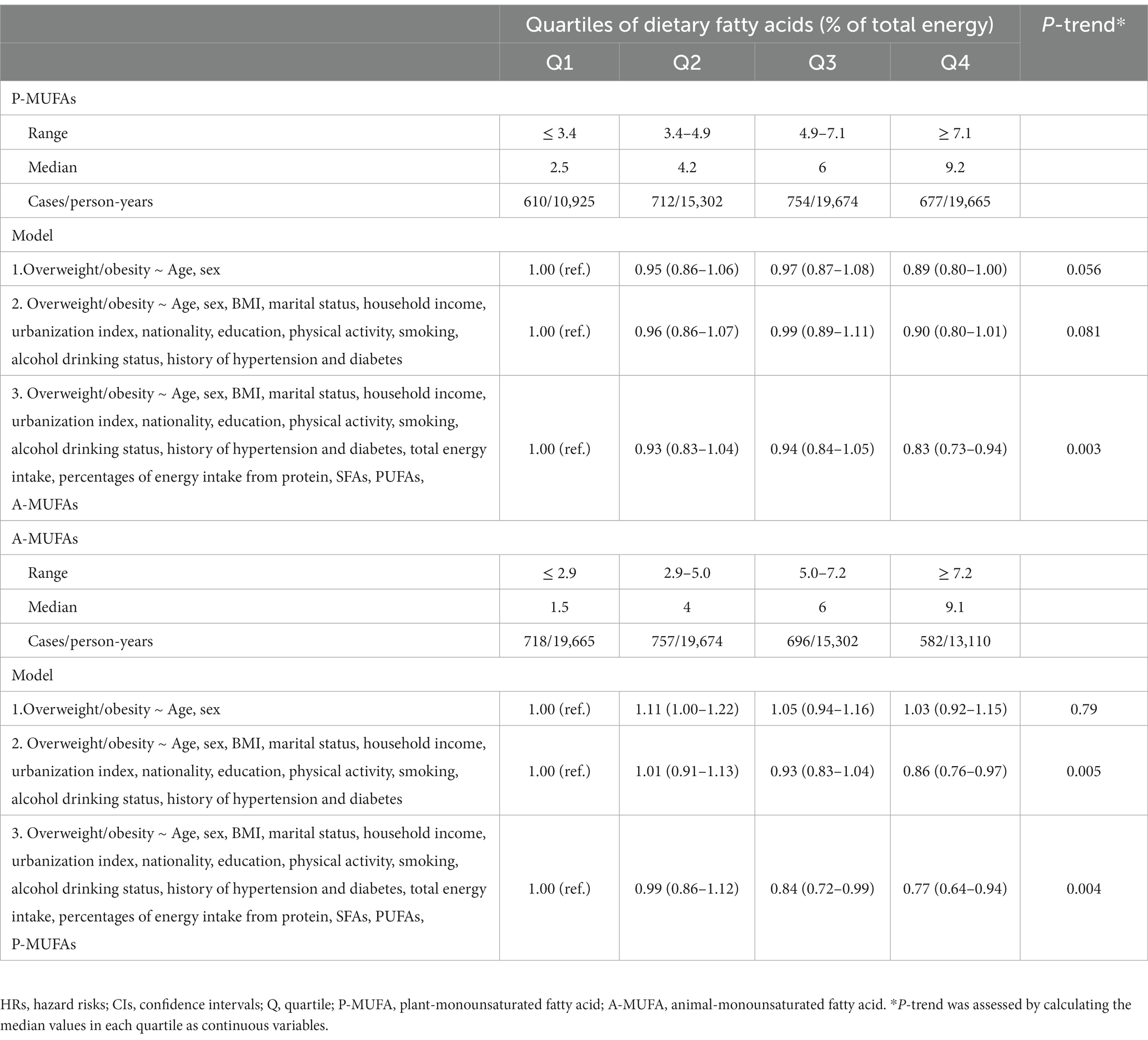
Table 3. HRs (95% CIs) for the overweight/obesity risk according to MUFAs from plant and animal sources.
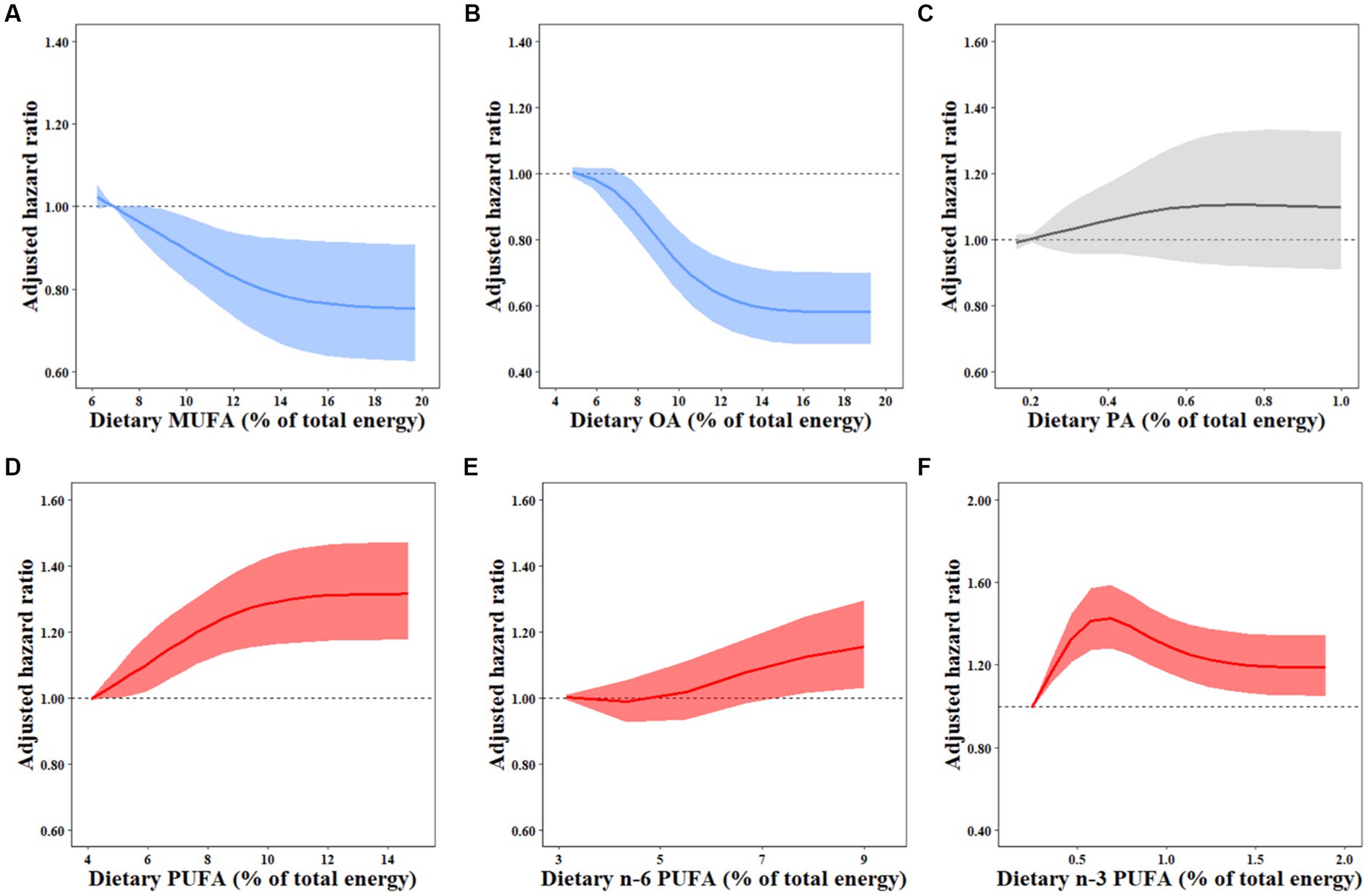
Figure 1. Dose–response relationships between dietary FAs and overweight/obesity risk. HRs for the overweight/obesity risk associated with dietary MUFAs (A), OA (B), PA (C), PUFAs (D), N-6 PUFAs (E), and N-3 PUFAs (F) were estimated by restricted cubic-spline regression adjusted for age and sex, marital status, BMI, household income, urbanization index, nationality, education, physical activity, smoking, alcohol drinking status, history of hypertension and diabetes, total energy intake, percentages of energy intake from protein, SFAs, and remaining fatty acids where appropriate. MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; OA, oleic acid; PA, palmitoleic acid.
PUFA intake and risk of overweight and obesity
Total dietary PUFA intake was related to an increased risk of overweight/obesity in fully adjusted model 3 (HRQ4vsQ1 1.24, 95% CI: 1.10–1.39; P-trend <0.001; Table 2). Participants in the highest quartiles of n-3 PUFAs (HRQ4vsQ1 1.24, 95% CI: 1.09–1.42; P-trend = 0.017) and ALA (HRQ4vsQ1 1.22, 95% CI: 1.07–1.39; P−trend = 0.039) but not marine n-3 PUFAs (HRQ4vsQ1 0.83, 95% CI: 0.68–1.02; P-trend = 0.176) had increased overweight/obesity risk compared to the lowest quartiles (Supplementary Table S2). N-6 PUFA intake was marginally and positively correlated with the risk of overweight/obesity (HRQ4vsQ1 1.13, 95% CI: 0.99–1.28; P-trend = 0.014), which was primarily owing to LA (HR Q4vsQ1 1.11, 95% CI: 0.98–1.26; P-trend = 0.020) but not AA (HR Q4vsQ1 0.96, 95% CI: 0.82–1.13; P-trend = 0.515) intake (Supplementary Table S2). Compared with the lowest quartile, the highest quartile of the n-6/n-3 PUFA ratio was not significantly associated with the incidence of overweight/obesity (HRQ4vsQ1 0.94, 95% CI: 0.84–1.06), but higher risk was observed for the n-6/n-3 PUFA ratio ranging from 5.7 to 12.6 (Table 2). Similar results for these PUFAs were demonstrated by restricted cubic spline regressions (Figure 1; Supplementary Figure S3).
Subgroup and sensitivity analyzes
Subgroup analyzes showed that the inverse associations of overweight/obesity incidence with total dietary MUFA intake were only significant in women (P-interaction = 0.041), non-drinkers (P-interaction <0.001), and those with higher education level (P-interaction = 0.012) and lower physical activity level (P-interaction = 0.040). Moreover, the positive associations of PUFA intake with the risk of overweight/obesity only appeared in participants with lower household income (P-interaction = 0.007; Supplementary Table S3). In sensitivity analyzes, the associations between unsaturated FA intake and overweight/obesity incidence were not materially changed after further adjustment for dietary cholesterol intake, occupation and AHEI, excluding participants with extremely lower BMI (BMI < 18.5 kg/m2) or those with hypertension or diabetes at baseline (Supplementary Tables S4, S5).
Discussion
To our knowledge, this prospective study is the first to assess the associations of specific dietary unsaturated FA intake with overweight/obesity development among the Chinese population. After adjustment for major potential risk factors, we found that total MUFA, plant-MUFA, animal-MUFA, plant-OA and animal-OA intake was consistently and inversely associated with the risk of overweight/obesity, while ALA, n-6 PUFA and LA intake was positively related to overweight/obesity risk.
OA is the most common type of MUFAs and is mainly consumed from vegetable oil and pork by the Chinese people (14). The beneficial effect of OA on preventing overweight/obesity was supported by several mechanistic studies. First, an OA-rich diet could increase the fat oxidation rate compared to a high SFA diet (24). In addition, the derivative of OA, oleoylethanolamide (OEA) plays a role in appetite modulation and energy intake (25). Although studies on OA biomarkers came to the contrary conclusion that serum OA concertation was positively linked with incident obesity (26). The difference may be due to the fact that serum OA is not an appropriate biomarker for dietary intake but for de novo lipogenesis in humans. The accessible regulator of lipogenic gene expression of endogenously synthesized OA and dietary OA was not similar (27). Due to accumulating epidemiology evidence highlighting the importance of food sources of MUFAs on health (13–15), we further assessed the associations of animal and plant sources of MUFAs/OA with the risk of overweight/obesity and found that both sources in CHNS were consistently associated with a reduced risk of overweight/obesity. Moreover, plant-MUFAs seemed less protective than animal-MUFAs in our study, which could be explained by the common cooking method such as stir-frying and griddling applied to vegetable oils in the daily life of the Chinese. Frying vegetable oil may increase the energy density and trans-FA (TFA) formation (28).
PA is another type of MUFAs and has been evidenced to be beneficial for weight maintenance (29). However, the low intake level of PA consumed in the current analyzes resulted in a null association of total PA or animal-PA with incident overweight/obesity, whereas dietary plant-PA intake was linked to overweight/obesity development. The main source of plant-PA in China was soybean oil (14), which was considered more obesogenic than coconut oil and fructose in mice (30). In addition, the cooking methods of the Chinese may also explain this detected adverse association.
Previous epidemiological studies have confirmed the positive correlation between LA intake and incident overweight/obesity that we identified. A cohort including 20,049 participants with a median of 6.5 years of follow-up concluded that dietary LA intake was positively related to weight gain (31). Similarly, another prospective study conducted in Germany found that the baseline level of erythrocyte LA was associated with a higher overweight/obesity risk in middle-aged and older women during a mean of 10.4-y follow-up (32). Besides, several animal experiments also validated the current findings that dietary n-6 PUFA intake was adipogenic (12, 33). The metabolites of dietary LA, such as anandamide and 2-arachidonyl glycerol, which promoted energy intake and weight gain by reducing hypothalamic satiety signaling and skeletal muscle glucose uptake, and increasing accumulation of lipid droplets in the liver, may be responsible for the effect of LA on body size (12). Moreover, prostacyclin converted from dietary LA could stimulate adipocyte differentiation through several pathways, including activating the peroxisome proliferator-activated receptor (PPAR) family and the CCAAT-enhancer binding protein family (CEBPβ and CEBPδ) (12). For AA, a previous study reported that AA promoted adipogenesis (33), which contradicted the existing conclusion that AA intake was not significantly associated with overweight/obesity development. This discrepancy may mainly be due to the overall low consumption of AA (mean intake: 0.02% kcal/d) in the Chinese population.
ALA is an essential n-3 PUFA and mostly accounted for the positive association of n-3 PUFAs in the current analysis. A cross-sectional study based on the National Health and Nutrition Examination Survey and the What We Eat in America found that the relationship of ALA intake was stratified by some sociodemographic groups as a positive association of ALA with BMI was detected among non-Hispanic black individuals (34). Furthermore, ALA in vegetable oils can be transformed into harmful trans-ALA during stir-frying (35), which may account for the adverse relationship as stir-frying was commonly used for ALA-rich vegetable oils among Chinese people. In addition, ALA-enriched diacylglycerol (ALA-DAG) was more prone to weight gaining compared to ALA-enriched triacylglycerol (ALA-TAG) (36). However, we did not divide ALA into ALA-DAG and ALA-TAG, which may also to some extent explain the harmful association for ALA intake. Although we failed to detect an association between marine n-3 PUFA intake and overweight/obesity risk, the in vivo studies have demonstrated that fish oil supplementation, which contained high concentrations of EPA and DHA, could offset weight gain induced by a high-fat diet (11, 37). The mechanism of long-chain n-3 PUFAs could be briefly proposed as stimulating lipid oxidation (38), enhancing satiety (39), and inducing browning of white adipose tissue (40). The extremely low consumption of long-chain n-3 PUFAs in our study has a large gap to the 250 mg/d as international dietary guideline recommends, which probably biased the associations toward the null.
The competition on the same enzyme of LA and ALA during the production of AA and EPA/DHA provided a basic theory of the n-6/n-3 PUFA ratio (41). Previous studies focusing on the ratio of n-6 to n-3 PUFAs summarized a positive association between dietary n-6/n-3 PUFA ratio and overweight/obesity incidence (42), which was generally consistent with our results. However, we found divergent associations between dietary LA and AA, and the difference was also observed between ALA and marine n-3 PUFAs. Our findings indicate that the n-6/n-3 PUFA ratio may not be a proper measurement linking unsaturated FA intake to incident overweight/obesity, whereas specific types of unsaturated FAs should be considered when increasing the intake of unsaturated FAs.
In subgroup analyzes, the association of overweight/obesity with total dietary MUFA intake was only significant in women, non-drinkers, and those with higher education levels and lower physical activity levels. These interactions may due to the higher intake levels of MUFAs among persons with the above characteristics. Other detected interactions remain to be elucidated in future studies.
The current study has some strengths. First, the large population and long-term follow-up could reduce the probability of reverse causation. In addition, cumulative intake of unsaturated FAs was used to represent a long-term diet, and within-individual heterogeneity could be reduced as well. Furthermore, this study systematically assessed the associations of different types of unsaturated FAs from diet with overweight/obesity risk. Despite these strengths, some limitations should also be recognized in the current study. First, measurement bias could not be controlled completely, but using the cumulative average intake of nutrients helped to reduce measurement errors. Second, although we adjusted for many potential confounders in models, unmeasured factors still remained and may influence observed results. Third, dietary TFAs was not adjusted in models due to unavailable data. However, the consumption of dietary TFAs was very low in China (43), which may not significantly change our documented results. Fourth, the findings may not apply to other populations because the cooking style and dietary patterns were unique to the Chinese population. Finally, it was not sufficient to establish causality due to the observational nature of this study.
In conclusion, the current findings supported that total dietary MUFA intake was inversely associated with the risk of overweight/obesity, which was mainly driven by dietary OA from both plant and animal sources. ALA and LA had strong positive associations with overweight/obesity risk. Our findings emphasize the importance of increasing the consumption of MUFAs, especially OA, in overweight/obesity prevention among the Chinese population.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cpc.unc.edu/projects/china/data/. The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review boards at the University of North Carolina, Chapel Hill and the National Institute of Nutrition and Food Safety from the Chinese Center for Disease Control and Prevention. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YZ, WZ, and JJ conceived and designed the study. YA, XHL, and PZ did the data cleaning, analysis and interpretation. YA wrote the manuscript. WC, YA, XCL, YL, HY, PZ, YZ, and JJ were involved in data acquisition. JJ is the guarantor. All authors contributed to the interpretation of the data and critical revision of the manuscript for important intellectual content and approved the final draft.
Funding
The study was supported by the Zhejiang Provincial Natural Science Foundation of China (Grant Number: LZ20C200001). The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1150709/full#supplementary-material
References
1. World Health Organization. Cardiovascular diseases (CVDs). (2021). Available at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (Accessed June 11, 2021).
2. Powell-Wiley, TM, Poirier, P, Burke, LE, Despres, JP, Gordon-Larsen, P, Lavie, CJ, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. (2021) 143:e984–e1010. doi: 10.1161/CIR.0000000000000973
3. Avgerinos, KI, Spyrou, N, Mantzoros, CS, and Dalamaga, M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. (2019) 92:121–35. doi: 10.1016/j.metabol.2018.11.001
4. Pan, X-F, Wang, L, and Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. (2021) 9:373–92. doi: 10.1016/s2213-8587(21)00045-0
5. Chao, AM, Quigley, KM, and Wadden, TA. Dietary interventions for obesity: clinical and mechanistic findings. J Clin Invest. (2021) 131:131. doi: 10.1172/JCI140065
6. Calder, PC. Functional roles of fatty acids and their effects on human health. JPEN J Parenter Enteral Nutr. (2015) 39:18S–32S. doi: 10.1177/0148607115595980
7. U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary guidelines for Americans. 8th Edn. (2015). Available at: https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines/previous-dietary-guidelines/2015 (Accessed December 2015).
8. DeLany, JP, Windhauser, MM, Champagne, CM, and Bray, GA. Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr. (2000) 72:905–11. doi: 10.1093/ajcn/72.4.905
9. Wu, F, Mao, L, Zhang, Y, Chen, X, Zhuang, P, Wang, W, et al. Individual SFA intake and risk of overweight/obesity: findings from a population-based nationwide cohort study. Br J Nutr. (2021) 128:75–83. doi: 10.1017/S0007114521002890
10. Tutunchi, H, Ostadrahimi, A, and Saghafi-Asl, M. The effects of diets enriched in monounsaturated oleic acid on the management and prevention of obesity: a systematic review of human intervention studies. Adv Nutr. (2020) 11:864–77. doi: 10.1093/advances/nmaa013
11. Sharma, P, and Agnihotri, N. Fish oil and corn oil induced differential effect on beiging of visceral and subcutaneous white adipose tissue in high-fat-diet-induced obesity. J Nutr Biochem. (2020) 84:108458. doi: 10.1016/j.jnutbio.2020.108458
12. Naughton, SS, Mathai, ML, Hryciw, DH, and McAinch, AJ. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. (2016) 125:90–9. doi: 10.1016/j.prostaglandins.2016.06.003
13. Mao, L, Zhang, Y, Wang, W, Zhuang, P, Wu, F, and Jiao, J. Plant-sourced and animal-sourced monounsaturated fatty acid intakes in relation to mortality: a prospective nationwide cohort study. Eur J Nutr. (2020) 59:1989–98. doi: 10.1007/s00394-019-02048-8
14. Zhuang, P, Zhang, Y, Mao, L, Wang, L, Wu, F, Cheng, L, et al. The association between consumption of monounsaturated fats from animal-v. plant-based foods and the risk of type 2 diabetes: a prospective nationwide cohort study. Br J Nutr. (2020) 124:102–11. doi: 10.1017/S0007114520000677
15. Zong, G, Li, Y, Sampson, L, Dougherty, LW, Willett, WC, Wanders, AJ, et al. Monounsaturated fats from plant and animal sources in relation to risk of coronary heart disease among US men and women. Am J Clin Nutr. (2018) 107:445–53. doi: 10.1093/ajcn/nqx0004
16. Shen, X, Fang, A, He, J, Liu, Z, Guo, M, Gao, R, et al. Trends in dietary fat and fatty acid intakes and related food sources among Chinese adults: a longitudinal study from the China health and nutrition survey (1997-2011). Public Health Nutr. (2017) 20:2927–36. doi: 10.1017/S1368980017001781
17. Zhang, B, Zhai, FY, Du, SF, and Popkin, BM. The China health and nutrition survey, 1989-2011. Obes Rev. (2014) 15:2–7. doi: 10.1111/obr.12119
18. Popkin, BM, Du, S, Zhai, F, and Zhang, B. Cohort profile: the China health and nutrition survey--monitoring and understanding socio-economic and health change in China, 1989-2011. Int J Epidemiol. (2010) 39:1435–40. doi: 10.1093/ije/dyp322
20. Institute of Nutrition and Food Safety. China CDC: China food composition 2002. Beijing: Peking University Medical Press (2002).
21. Institute of Nutrition and Food Safety. China CDC: China food composition 2004. Beijing: Peking University Medical Press (2005).
22. McCullough, LE, and Byrd, DA. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. (2022). doi: 10.1093/aje/kwac071
23. Wang, ZH, Adair, SR, Cai, JW, Gordon-Larsen, P, Siega-Riz, AM, Zhang, B, et al. Diet quality is linked to insulin resistance among adults in China. J Nutr. (2017) 147:2102–8. doi: 10.3945/jn.117.256180
24. Kien, CL, Bunn, JY, Stevens, R, Bain, J, Ikayeva, O, Crain, K, et al. Dietary intake of palmitate and oleate has broad impact on systemic and tissue lipid profiles in humans. Am J Clin Nutr. (2014) 99:436–45. doi: 10.3945/ajcn.113.070557
25. Mennella, I, Savarese, M, Ferracane, R, Sacchi, R, and Vitaglione, P. Oleic acid content of a meal promotes oleoylethanolamide response and reduces subsequent energy intake in humans. Food Funct. (2015) 6:203–9. doi: 10.1039/c4fo00697f
26. Kaikkonen, JE, Jula, A, Viikari, JSA, Juonala, M, Hutri-Kahonen, N, Kahonen, M, et al. Associations of serum fatty acid proportions with obesity, insulin resistance, blood pressure, and fatty liver: the cardiovascular risk in young Finns study. J Nutr. (2021) 151:970–8. doi: 10.1093/jn/nxaa409
27. Lounis, MA, Bergeron, KF, Burhans, MS, Ntambi, JM, and Mounier, C. Oleate activates SREBP-1 signaling activity in SCD1-deficient hepatocytes. Am J Physiol Endocrinol Metab. (2017) 313:E710–20. doi: 10.1152/ajpendo.00151.2017
28. Sayon-Orea, C, Carlos, S, and Martinez-Gonzalez, MA. Does cooking with vegetable oils increase the risk of chronic diseases? A systematic review. Br J Nutr. (2015) 113:S36–48. doi: 10.1017/S0007114514002931
29. Yang, ZH, Takeo, J, and Katayama, M. Oral administration of omega-7 palmitoleic acid induces satiety and the release of appetite-related hormones in male rats. Appetite. (2013) 65:1–7. doi: 10.1016/j.appet.2013.01.009
30. Deol, P, Evans, JR, Dhahbi, J, Chellappa, K, Han, DS, Spindler, S, et al. Soybean oil is more obesogenic and diabetogenic than coconut oil and fructose in mouse: potential role for the liver. PLoS One. (2015) 10:e0132672. doi: 10.1371/journal.pone.0132672
31. Nimptsch, K, Berg-Beckhoff, G, and Linseisen, J. Effect of dietary fatty acid intake on prospective weight change in the Heidelberg cohort of the European prospective investigation into Cancer and nutrition. Public Health Nutr. (2010) 13:1636–46. doi: 10.1017/S1368980009993041
32. Wang, L, Manson, JE, Rautiainen, S, Gaziano, JM, Buring, JE, Tsai, MY, et al. A prospective study of erythrocyte polyunsaturated fatty acid, weight gain, and risk of becoming overweight or obese in middle-aged and older women. Eur J Nutr. (2016) 55:687–97. doi: 10.1007/s00394-015-0889-y
33. Mak, IL, Lavery, P, Agellon, S, Rauch, F, Murshed, M, and Weiler, HA. Arachidonic acid exacerbates diet-induced obesity and reduces bone mineral content without impacting bone strength in growing male rats. J Nutr Biochem. (2019) 73:108226. doi: 10.1016/j.jnutbio.2019.108226
34. Raatz, SK, Conrad, Z, Johnson, LK, Picklo, MJ, and Jahns, L. Relationship of the reported intakes of fat and fatty acids to body weight in US adults. Nutrients. (2017) 9:9. doi: 10.3390/nu9050438
35. Guo, Q, Li, T, Qu, Y, Wang, X, Liu, L, Liu, H, et al. Molecular formation mechanism of trans linolenic acid in thermally induced α-linolenic acid. LWT. (2020) 130:109595. doi: 10.1016/j.lwt.2020.109595
36. Ando, Y, Saito, S, Miura, H, Osaki, N, and Katsuragi, Y. Consumption of alpha-linolenic acid-enriched diacylglycerol induces increase in dietary fat oxidation compared with alpha-linolenic acid-enriched triacylglycerol: a randomized, double-blind trial. Nutr Res. (2017) 48:85–92. doi: 10.1016/j.nutres.2017.10.012
37. Qin, N, Song, G, Ren, X, Zhang, L, Gao, J, Xia, X, et al. Fish oil extracted from Coregonus peled improves obese phenotype and changes gut microbiota in a high-fat diet-induced mouse model of recurrent obesity. Food Funct. (2020) 11:6158–69. doi: 10.1039/d0fo00911c
38. Delarue, J, Allain-Jeannic, G, Guillerm, S, Cruciani-Guglielmacci, C, Magnan, C, Moineau, MP, et al. Interaction of low dose of fish oil and glucocorticoids on insulin sensitivity and lipolysis in healthy humans: a randomized controlled study. Mol Nutr Food Res. (2016) 60:886–96. doi: 10.1002/mnfr.201500469
39. Maher, T, and Clegg, ME. Dietary lipids with potential to affect satiety: mechanisms and evidence. Crit Rev Food Sci Nutr. (2019) 59:1619–44. doi: 10.1080/10408398.2017.1423277
40. Zhuang, P, Lu, Y, Shou, Q, Mao, L, He, L, Wang, J, et al. Differential anti-Adipogenic effects of eicosapentaenoic and docosahexaenoic acids in obesity. Mol Nutr Food Res. (2019) 63:e1801135. doi: 10.1002/mnfr.201801135
41. Harris, WS. The omega-6: omega-3 ratio: A critical appraisal and possible successor. Prostaglandins Leukot Essent Fatty Acids. (2018) 132:34–40. doi: 10.1016/j.plefa.2018.03.003
42. Simopoulos, AP. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. (2016) 8:128. doi: 10.3390/nu8030128
Keywords: unsaturated fatty acids, obesity, overweight, prospective study, China health and nutrition survey
Citation: Chen W, Ao Y, Lan X, Tong W, Liu X, Zhang X, Ye Q, Li Y, Liu L, Ye H, Zhuang P, Zhang Y, Zheng W and Jiao J (2023) Associations of specific dietary unsaturated fatty acids with risk of overweight/obesity: population-based cohort study. Front. Nutr. 10:1150709. doi: 10.3389/fnut.2023.1150709
Edited by:
Kelly Johnson, Coastal Carolina University, United StatesReviewed by:
Maharshi Bhaswant, Tohoku University, JapanSumei Hu, Beijing Technology and Business University, China
Copyright © 2023 Chen, Ao, Lan, Tong, Liu, Zhang, Ye, Li, Liu, Ye, Zhuang, Zhang, Zheng and Jiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weifang Zheng, zhengweifang1972@163.com; Jingjing Jiao, jingjingjiao@zju.edu.cn
†These authors have contributed equally to this work
 Weiming Chen1†
Weiming Chen1†  Yang Ao
Yang Ao Xiaohui Liu
Xiaohui Liu Yin Li
Yin Li Pan Zhuang
Pan Zhuang Yu Zhang
Yu Zhang Weifang Zheng
Weifang Zheng Jingjing Jiao
Jingjing Jiao