Evaluation of the effect of Loigolactobacillus coryniformis K8 CECT 5711 consumption in health care workers exposed to COVID-19
- 1Hospital Universitario San Cecilio, Granada, Spain
- 2Department of Nursing, Faculty of Health Sciences, University of Granada, Granada, Spain
- 3Hospital Virgen de las Nieves, Granada, Spain
- 4Department of Research and Development, Biosearch Life, a Kerry Company, Granada, Spain
Following the spread of the SARS-CoV-2 coronavirus, an unprecedented burden has been placed on health care systems, with health care workers (HCWs) being most at risk of COVID-19 infection. The effect of the probiotic Loigolactobacillus coryniformis K8 CECT 5711 on frontline HCWs exposed to the virus was studied in a randomized, double-blind, placebo controlled trial. Parameters related to the incidence and severity of COVID-19 as well as the immune response and the side effects of the COVID-19 vaccine were evaluated. For 2 months, a group of 250 front-line HCWs over the age of 20 was randomly allocated to receive either L. coryniformis K8 or a placebo daily. SARS-CoV-2 infection incidence was verified via PCR or antigen test. In those volunteers who were vaccinated during the intervention, serum levels of specific IgG were analyzed at the end of the study. The incidence of COVID-19 infection was very low [IR (SD) = 0.016 (0.011)], and no significant difference was found between the groups [IRR (95% CI): 1.008 (0.140–7.268), p = 0.994]. For immune response analysis, the total sample was divided according to the days between the first dose and the antibody analysis (cutoff points were set at ≤ 56, 57–80 and ≥ 81 days). The specific IgG level decreased over time (p > 0.001). However, in the subgroup of subjects for whom more than 81 days had passed since they received the first dose, the specific IgG levels were significantly higher in the those that took the L. coryniformis K8 [7.12 (0.21)] than in the control group [6.48 (0.19)] (P = 0.040). Interestingly, the subjects who started probiotic consumption before the first dose reported significantly fewer side effects (of any kind) at the 1st dose of the vaccine (OR: 0.524, p = 0.043), specifically less arm pain (OR: 0.467, p = 0.017). In conclusion, the administration of L. coryniformis K8 CECT 5711 to HCWs helps to extend the immune protection generated by the COVID-19 vaccine over time.
Introduction
The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has quickly spread throughout the world, leading to an enormous strain on health-care systems and inflicting millions of infections and deaths worldwide (1).
Health care workers (HCWs) are at high risk for COVID-19 infection, not only due to close contact with highly infectious patients, but also due to undiagnosed or subclinical infectious case exposure. Moreover, the stress and physical overexertion caused by the pandemic may affect the immune response of HCWs (2). Indeed, a meta-analysis of ninety-seven studies estimated that the prevalence of SARS-CoV-2 infection in HCWs was 11% up to July 2020 (3), which constituted a significant proportion of all COVID-19 patients (4). Although the severity and mortality among HCWs were relatively low compared to those of other population groups (3), these COVID-19 events have led in a shortage of health staff adding a burden in the pandemic fighting.
Because of their immunomodulatory, anti-inflammatory, antioxidant, and antiviral properties, some authors have suggested that probiotics may have a role in the prevention and/or moderation of COVID-19 severity (5–7). However, to date, only one study that has been performed in COVID-19 patients showed that a probiotic formula may help to reduce duration of digestive and non-digestive symptoms compare to placebo (8), so more studies were needed to determine the role of probiotics in this disease. In this sense, the Loigolactobacillus coryniformis K8 CECT 5711 strain has been demonstrated to present immunomodulatory activity in adults (9) and children (10, 11). Furthermore, when given orally to healthy individuals in the context of hepatitis A vaccination, this strain was demonstrated to boost specific antibody levels against the hepatitis A virus (12). Additionally, in another trial carried out in elderly participants, L. coryniformis K8 increased the immunological response to influenza vaccination and reduced the symptoms associated with respiratory infections (13). Very recently, a randomized clinical trial performed in COVID-vaccinated nursing home residents, evidenced the usefulness of L. coryniformis K8 in the context of the COVID-19 pandemic by increasing the specific immune response after infection with COVID-19 and by helping the vaccine-specific responses in the elderly (14).
Here, we report the findings of a randomized, placebo-controlled, double-blind trial to assess the effect of the consumption of the probiotic strain L. coryniformis K8 CECT 5711 on the incidence and severity of COVID-19 in frontline HCWs exposed to the virus. Additionally, the immune response and the side effects of the COVID-19 vaccine were evaluated in a subgroup of these HCWs.
Materials and methods
Study design and subjects
A randomized, double-blinded, placebo-controlled multicenter study was performed. The study was carried out in two of the reference hospitals treating COVID-19 patients (Hospital San Cecilio and Hospital Virgen de las Nieves) in the province of Granada (Andalusia, Spain) in two time periods: from 24 April to 20 July 2020 and from 9 December 2020 to 11 May 2021 (Supplementary Figure 1).
The inclusion criteria were active HCWs older than 20 years who were caring for COVID-19 patients, including those in all professional categories. Having a COVID-19 medical history (confirmed by PCR or serology tests) prior to the beginning of the intervention, presenting symptomology compatible with COVID-19 at the start of the intervention, being diagnosed with an immunocompromising condition, or being pregnant or planning to become pregnant in the next few months were all exclusion criteria. The study was conducted according to the Declaration of Helsinki, and the protocol was approved by the Regional Ethical Committee (Granada, Spain). Informed consent was obtained from all subjects. The trial was registered with the United States Library of Medicine under the number NCT04366180.1
The incidence of COVID-19 in HCWs was the primary outcome used to calculate the sample size. Few data for SARS-CoV-2 infection among HCWs were available when the protocol was set up (March 2020), so a COVID-19 incidence of 10–15% was estimated for our sample. The sample size was defined for the comparison of two independent proportions using the chi-square test. For an alpha of 5% and a power of 80%, taking 12.5% as the predicted COVID-19 incidence and 10% as the minimal difference of interest to be found between the groups and considering a probable loss of 15% of the subjects, a sample of 125 participants per group (total n = 250) was required.
Finally, 255 participants were found to meet the inclusion criteria and were randomly allocated to one of two groups using a computer-generated randomization procedure. The placebo group received a daily capsule containing 220 mg of maltodextrin, whereas the probiotic group received a daily capsule containing 3 × 109 colony forming units of the L. coryniformis K8 strain in a matrix of the same maltodextrin combination in a quantity to achieve the same capsule weight (220 mg). This dose has been proven to be effective and safe in previous studies performed in adult population (12–14). The probiotic and placebo were packed in similar gelatin capsules in plastic containers, with just the randomization code distinguishing them. The individuals were given treatments for 2 months.
Study outcomes and sample collection
The primary outcome of the study was to evaluate the incidence of SARS-CoV-2 infection confirmed by PCR or antigen testing. The secondary outcomes included determining the severity and duration of SARS-CoV-2 infection. Additionally, the immune response and side effects of the COVID-19 vaccine were evaluated in a subgroup of these HCWs.
At the baseline visit, the HCWs were informed about the study details, asked to sign the informed consent form, and received a rapid serology test for COVID-19. The subjects who were negative for this test and met the rest of the inclusion/exclusion criteria were asked about their baseline data and medical history, and the corresponding treatment was dispensed for the total duration of the study (Supplementary Figure 1). Likewise, the subjects received a data collection booklet to record their symptoms and its duration in case they had a COVID-19 infection confirmed by test. For volunteers with compatible symptomatology or in the case of close contact with a COVID-19-positive patient, a PCR or antigen test to determine SARS-CoV-2 infection was done. All COVID-19 patients continued taking the study product. The follow-up visits were conducted monthly, at which data recorded in the diary were reviewed and adverse events, defined as any unfavorable or unintended effect, were collected.
The subgroup of volunteers who received the COVID-19 vaccine during the intervention were asked for a blood sample at the end of the study. Blood was collected in Vacutainer tubes (BD Biosciences) and allowed to clot. The serum was separated within an hour by centrifugation at 1.000–1.500 × g for 10 min, and serum aliquots were stored at −20°C. The Liaison SARS-CoV-2 S1/S2 IgG test (DiaSorin, Antony, France), a chemiluminescent microparticle immunoassay that utilizes a mix of SARS-CoV-2 recombinant S1 and S2 proteins as capture antigens, was used to obtain quantitative measures of human SARS-CoV-2 specific IgG levels. Analyses were performed according to the instructions of the manufacturer. During the follow-up visits, information about the side effects suffered after the first and the second doses of the COVID-19 vaccine were also recorded for this subgroup of volunteers.
Statistical analysis
Normal probability plots and the Shapiro–Wilk test were used to determine the normality of the distribution for all observed variables. Data for continuous variables are reported as the mean (standard deviation, SD) and categorical variables as n (%). Continuous variables were examined with the Student’s t test or the non-parametric Kruskal–Wallis technique, as applicable, for comparisons between groups at the start of the trial (probiotic group vs. control group), and categorical variables were evaluated with chi-square tests. The occurrence of SARS-CoV-2 infection was described using the incidence ratio (IR) and incidence rate ratio (IRR), with the 95% CI and p value for the IRR calculated by a logistic regression model adjusted by the corresponding covariates.
In the subgroup of volunteers who received the COVID-19 vaccine, data from the immunogenicity analysis are presented as the mean (SE) of the log transformed data. The sample was divided into tertiles according to the days between the first dose and the antibody analysis (cutoff points were set at ≤ 56, 57–80 and ≥ 81 days). Differences between the groups were evaluated by univariate model analysis, adjusted by the corresponding covariates. Side effects are presented as counts, percentages, and odds ratios (ORs), with the 95% CIs and p values for the ORs calculated by a logistic regression model adjusted by the corresponding covariates.
A general alpha level of 0.05 was used as the cutoff point for statistical significance. SPSS software version 27.0 for Windows (SPSS, Chicago, IL, United States) was used for statistical analysis.
Results
Study data, compliance and baseline characteristics of the subjects
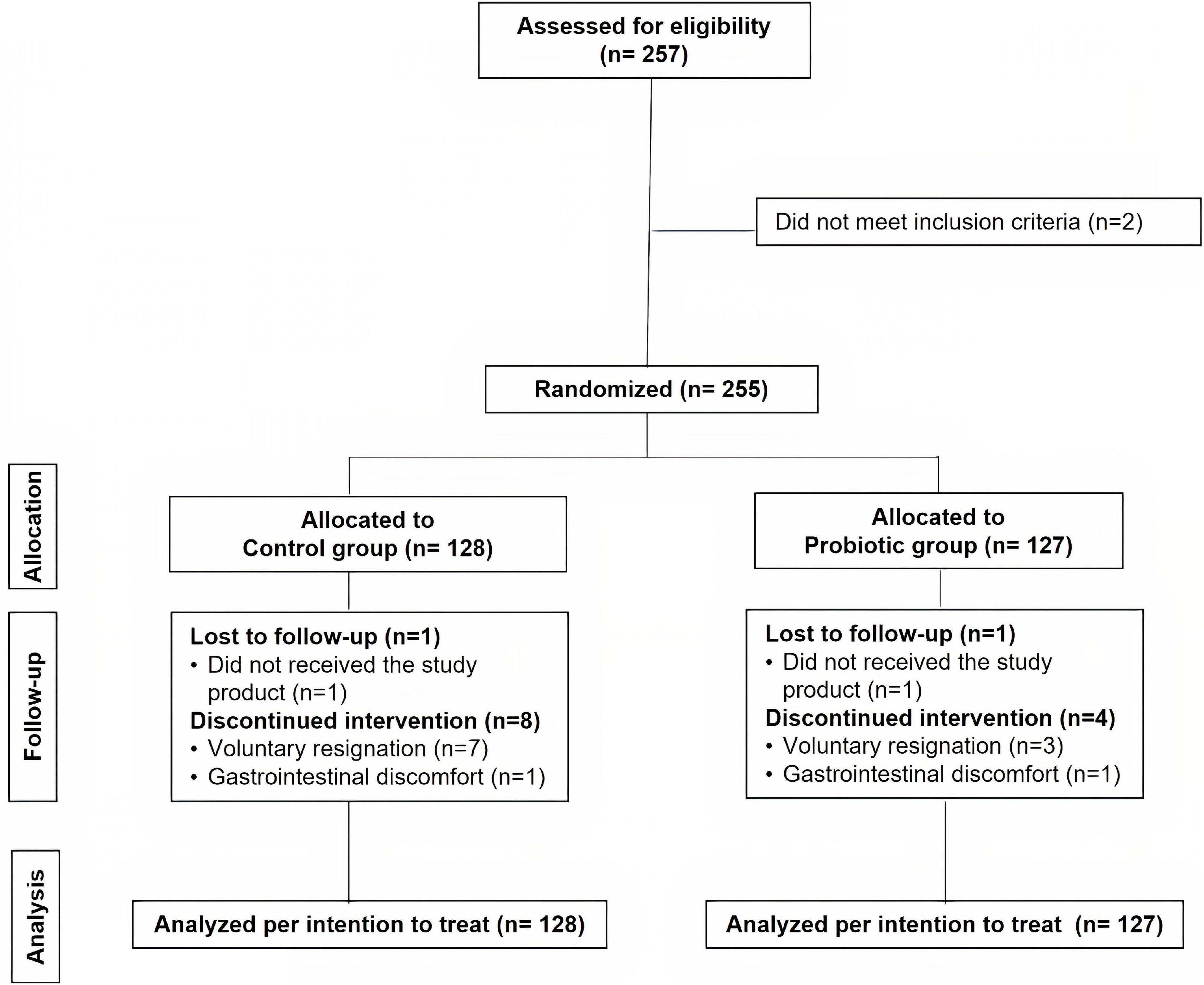
A total of 257 HCWs were evaluated for eligibility, of whom 2 were excluded because they did not match the inclusion criteria (Figure 1). Finally, 255 subjects were recruited and randomly assigned to two groups: the probiotic group (n = 127) and the control group (n = 128). For the reasons indicated in the study flow chart (Figure 1), nine participants in the control group and five volunteers in the probiotic group ceased the intervention and dropped out of the study before the end of the 2-month intervention period. There were no differences in the number or causes of withdrawals across the groups. The compliance percentage was corroborated to be very high (≈100%). Data were analyzed for all the subjects randomized in the study [analysis per intention to treat (ITT), n = 128 in the control group and n = 127 in the probiotic group]. No adverse events resulting from the intake of either type of treatment were reported.
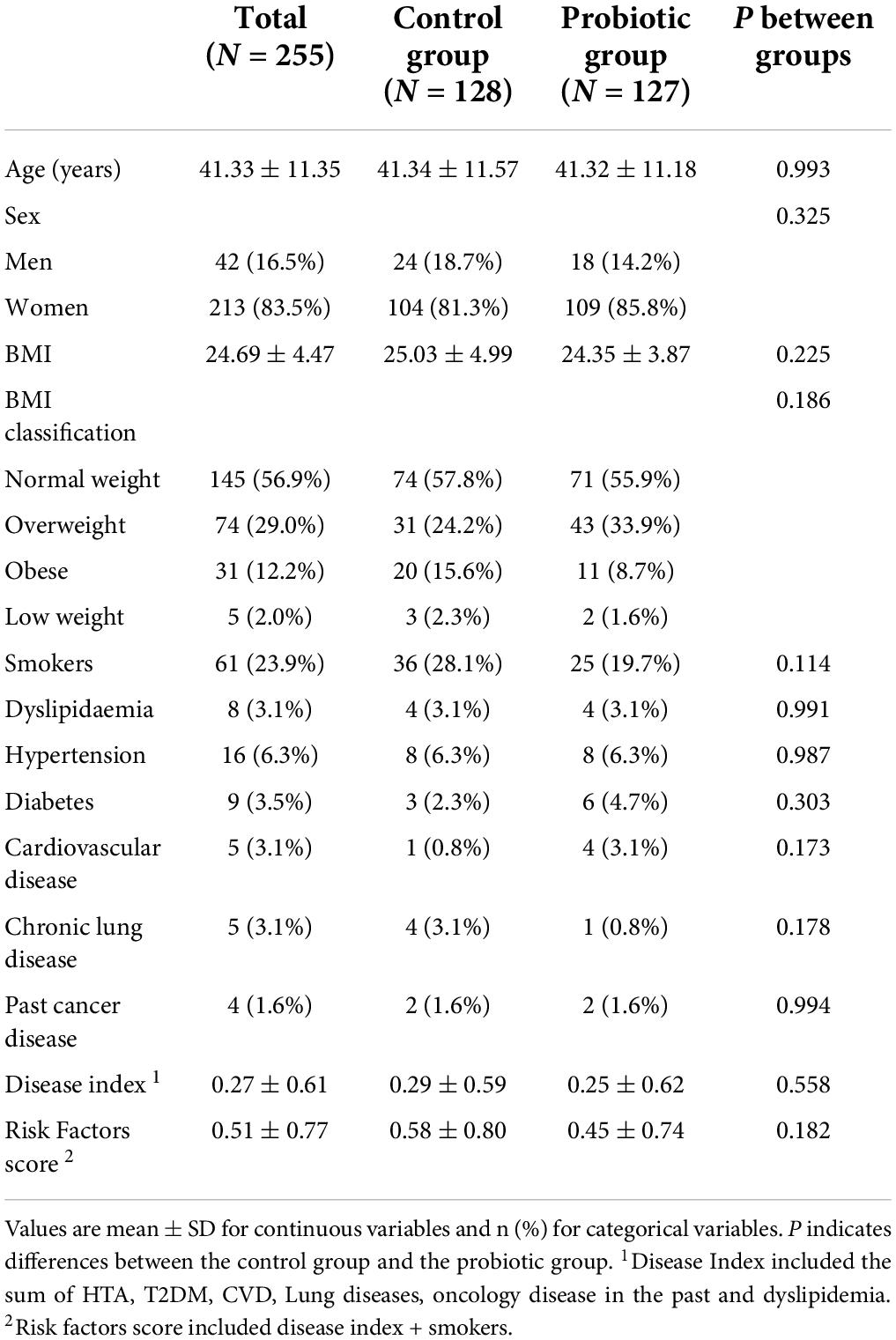
Table 1 shows the baseline characteristics of the 255 HCWs included in the ITT analysis. There were no significant differences between the study groups.
COVID-19 infection incidence, severity, and duration
During the intervention, four HCWs were infected with the SARS-CoV-2 virus; two cases occurred in the control group [incidence rate (IR) (SD) = 0.016 (0.011)], and two cases occurred in the probiotic group [IR (SD) = 0.016 (0.011)]. No significant difference between the groups in the incidence of COVID-19 infection was detected [IRR (95% CI): 1.008 (0.140–7.268), p = 0.994]. When the model was adjusted by sex, age, hospital and the disease index, the IR (SD) in the control group was 0.0006 (1.2148) and that in the probiotic group was 0.0006 (1.1016), with no significant difference between the groups [IRR (95% CI): 0.907 (0.123–6.695), p = 0.923]. Of the four HCWs who were infected, one was asymptomatic, and three presented mild symptoms (cough, fever, headache, malaise, and diarrhea). The mean duration of COVID-19 symptoms was 4 ± 1 days (significant differences between the groups were not performed due to low incidence).
Immune response to and side effects from the COVID-19 vaccine
A subgroup of 95 volunteers received the COVID-19 vaccine during the intervention (Table 2 and Supplementary Table 1). There were no significant differences in the baseline parameters between the study groups (Supplementary Table 1). Table 2 shows data related to the COVID-19 vaccine in this subgroup of subjects. All these subjects received a complete vaccination schedule of a mRNA vaccine, either the Comirnaty (BNT162b2 mRNA, BioNTech-Pfizer) or Spikevax (mRNA-1273, Moderna) vaccine, with no differences between groups (p = 0.127). Most of the volunteers started the intervention before receiving the first dose of the vaccine (51.6%); some started the intervention between the first and the second dose (33.7%) of the vaccine; and the least started the intervention after the second dose (12.5%) of the vaccine, with no differences between groups (p = 0.672). Of the 95 subjects, 85 underwent blood sample collection for specific IgG antibody analysis at the end of the study. The mean time between the first dose and the antibody analysis was 68.81 ± 23.56 days in the control group and 67.64 ± 19.78 days in the probiotic group (p = 0.803).
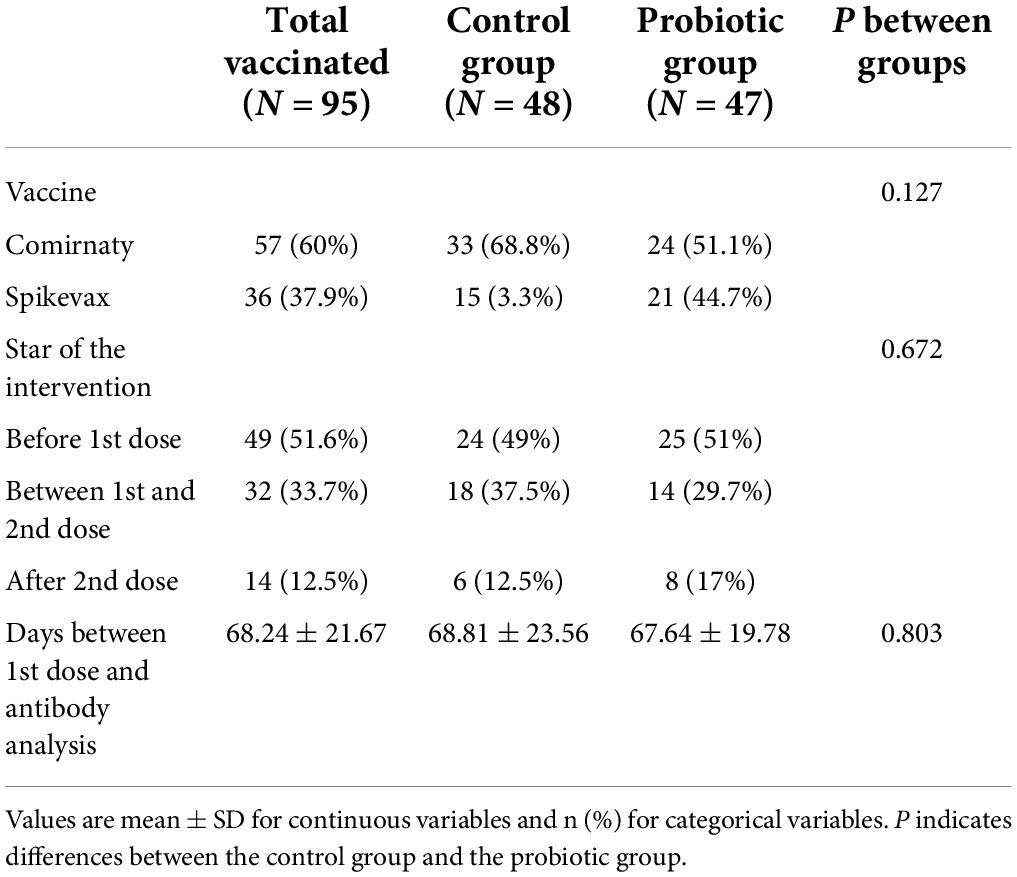
Table 2. Data related to the COVID-19 vaccine of the subgroup of subjects receiving the vaccine during intervention.
Subjects were divided into tertiles according to the days between the first dose and the antibody analysis (cutoff points were set at ≤ 56, 57–80 and ≥ 81 days). In general, a significant difference was observed among the tertiles (p < 0.001), with the specific IgG levels being lower as more time passed between the first dose and the antibody analysis. However, in the subgroup of volunteers in which more than 81 days had elapsed since they received the first dose, the specific IgG levels were significantly higher in the subjects that received L. coryniformis K8 than in the control group (p = 0.040) (Figure 2).
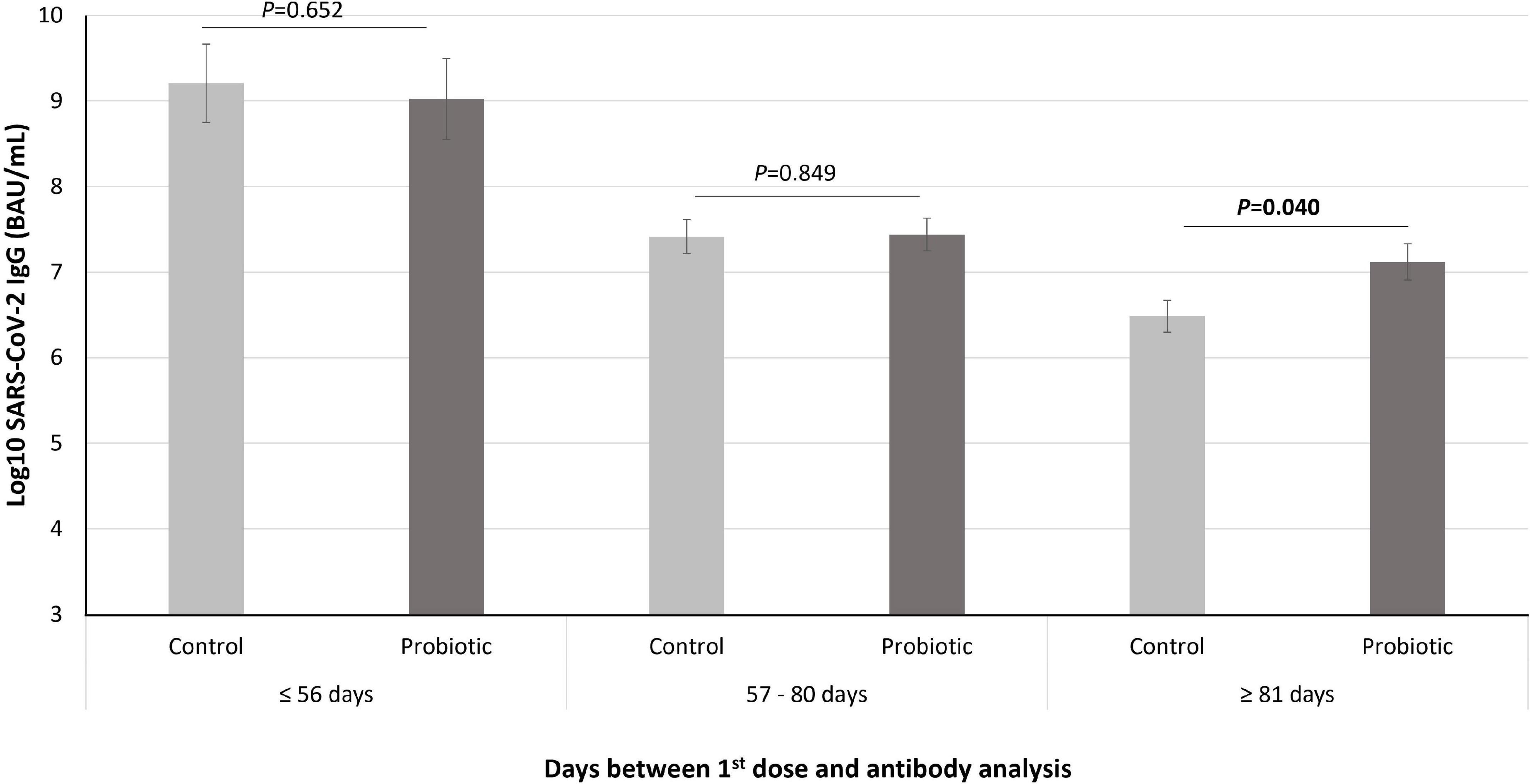
Figure 2. Levels of SARS-CoV-2 IgG (represented in Log10 of BAU/mL) in the subjects that received the COVID-19 vaccine during intervention (n = 85) divided into tertiles according to the days between the first dose and the antibody analysis. Data are represented as mean (bars) and SE (vertical lines). P value indicated differences between probiotic group (dark gray bars) and control (light gray bars) groups (univariate models adjusted by age, sex, age, type of vaccine, and start of the intervention).
Finally, subjects who started the consumption of the probiotic before the first dose of the vaccine reported significantly fewer side effects (any type) to the 1st dose of the vaccine (OR: 0.524, p = 0.043), specifically less pain at the site of inoculation (OR: 0.467, p = 0.017), than those who received the placebo. No significant effects were found regarding side effects to the second dose of the vaccine.
Discussion
Health care workers are still on the front line of the battle against COVID-19 and, therefore, constituted one of the groups at the highest risk of infection during this raging pandemic (15). Vaccination has been proven to be the key for the reduction of the risk of COVID-19 infection in HCWs (16), but different studies have observed a drop in antibody levels over time (17, 18), which could have clinical consequences. Therefore, several authors have called for action to develop effective therapies and preventive measures to reduce infection and flatten the COVID-19 curve, and, in this context, probiotics could have a role (5). Our research group recently showed the usefulness of the probiotic L. coryniformis K8 in enhancing the immunological response of elderly people in the context of the COVID-19 vaccination (14). In the present study, we corroborated the role of L. coryniformis K8 as an adjuvant for boosting immunity by helping to extend the immune protection over time after COVID-19 vaccination in a group of HCWs.
Several randomized clinical trials have shown the immunomodulatory activity of the probiotic strain L. coryniformis K8 (12–14) and support the working hypothesis. Indeed, in one of the studies performed in the context of influenza vaccination, it was observed that the incidence of local symptoms associated with respiratory infections (cough, sore throat, and nasal congestion) was 48% lower in the group that received the probiotic strain compared to the control group in a follow-up period of 6 months (13). Interestingly, in a very recent study we performed in an elderly population in the context of the COVID-19 pandemic, we observed that the percentage of asymptomatic patients was three times higher in the group that consumed L. coryniformis K8 than in the control group, although we did not observe significant differences due to the low number of observed cases (IR = 0.095). However, a very low incidence of COVID-19 was detected in HCWs during the present study (IR = 0.016), so the hypothesized beneficial effect of the consumption of L. coryniformis K8 on the incidence and severity of COVID-19 could not be evaluated and, therefore, no conclusions can be drawn to this matter. This low COVID-19 incidence could mainly be explained by the timing of the study: the first recruitment wave (from the end of April to July 2020) was a period with a very low infection rate in Granada (Spain) (19); whereas the second recruitment wave (from December 2020 to May 2021), although coincided with the third wave of COVID-19 in Granada (Spain) (19), also concurred with the HCWs COVID-19 vaccination first campaign which was recognized and determined to be very effective (20). Therefore, further studies should be performed to reach a conclusion about the effect of L. coryniformis K8 on the incidence and severity of COVID-19.
We surveyed the impact of the L. coryniformis K8 on the immunological response elicited by the COVID-19 vaccine, observing in the vaccinated subjects a significantly lower level of specific IgG in those who had been vaccinated for the longest time. This observation is in agreement with several follow-up studies that observed a significant decline in the immune humoral response over time after a full mRNA COVID-19 vaccine schedule (18, 21–23). Interestingly, we found in the subgroup of subjects for whom more than 81 days had elapsed since they received the first dose, that the IgG-specific levels were significantly higher in the volunteers who received L. coryniformis K8 than in those who received the placebo. This finding is in line with the results obtained in our previous study performed with vaccinated nursing home residents. In the subset of volunteers who were diagnosed with COVID-19 during the intervention, those who took L. coryniformis K8 had greater IgG-specific levels than those who took the placebo (14). Although the possible mechanisms involved in the activation of IgG production must be elucidated, the obtained results may have important clinical applications, since some studies found an association between RBD-IgG levels and protection against SARS-CoV-2 in different populations (24, 25). Moreover, a very recent case–control study carried out in Sweden with more than 1.3 million people showed that vaccine efficacy waned markedly 6 months after the last dose of the COVID-19 vaccine, increasing the risk of infection, hospitalization, and severe disease (26). Additional studies with a longer follow-up time should be performed to determine the effect of L. coryniformis K8 on humoral immune response sustainability over time after COVID-19 vaccination.
After inoculation with any vaccine, temporary side effects caused by tissue trauma at the site of inoculation and by the activation of the immune response may appear. A study by Pormohammad et al. (27) indicated that RNA-based vaccines had a more robust immune response, exhibiting greater frequencies of reactogenicity side effects, such as site pain, redness, swelling, headache, fever, tiredness, induration, myalgia, chills, vomiting, and itching. Kadali et al. (28) also reported that after the administration of the first dose of the COVID-19 vaccine, HCWs communicated a wide range of symptoms, which, although not life-threatening, did lead to the interruption of their work activities, requiring sick leave in a percentage close to 28%. Therefore, the mitigation of the side effects after the first dose of the vaccine reported in the group that took L. coryniformis K8 may have repercussions both for the economy and in the quality of life of the vaccinated subjects.
Some limitations of this study must be acknowledged. First, the low COVID-19 incidence detected during the study did not allow us to fulfill the main aim of the study. Second, the sample size in the vaccinated sample subgroup was relatively small. Moreover, the study was performed in a very homogeneous population of healthy Caucasian HCWs, which limits the generalization of our results to other age groups, non-healthy population groups or ethnicities. Therefore, further studies with other population groups should be performed.
In conclusion, the administration of L. coryniformis K8 CECT 5711 to a group of HCW helps to sustain the immune humoral response generated by the COVID-19 vaccine over time. These results support the capacity of the probiotic strain L. coryniformis K8 to boost the immunological response, as demonstrated in several clinical trials (12, 13), including a previous trial that was also performed in the context of the COVID-19 pandemic (14). Probiotics may be a natural and safe alternative for improving vaccination effectiveness, particularly in critical groups such as HCWs. The effect of this probiotic in the prevention and mitigation of COVID-19 should be further investigated.
Data availability statement
The datasets presented in this article are not readily available because participants of this study did not authorize the transfer of their data to a third party or to be used for purposes other than those established in the project. Requests to access the datasets should be directed to ruth.blanco@kerry.com.
Ethics statement
The studies involving human participants were reviewed and approved by the Regional Ethical Committee (Granada, Spain). The patients/participants provided their written informed consent to participate in this study.
Author contributions
RR-B and MO participated in the conception of the study, designed the methodology, and contributed to the manuscript writing. JS-G participated in the design of the study and critically revised the manuscript. ÁC-V and AA recruited and followed-up the volunteers. JM-L provided study materials and performed the data curation. RB-R participated in the study design, analyzed the data, interpreted the results, and wrote the draft of the manuscript. All authors have read and approved the final manuscript.
Funding
This research was funded by the Regional Ministry of Economic Transformation, Industry, Knowledge and Universities and FEDER Funds (Project CV20_075443), Junta de Andalucia, Spain.
Acknowledgments
We wish to thank Noemi Garcia for technical assistance and all the volunteers who participated in this study.
Conflict of interest
JM-L, MO, and RB-R are workers of Biosearch Life, a Kerry Company, owner of the patent of Loigolactobacillus coryniformis CECT 5711.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.962566/full#supplementary-material
Footnotes
References
1. WHO. Coronavirus (COVID-19) Dashboard. (2020). Available online at: https://covid19.who.int/ (Accessed April 15, 2022).
2. Chew NWS, Lee GKH, Tan BYQ, Jing M, Goh Y, Ngiam NJH, et al. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain Behav Immun. (2020) 88:559–65. doi: 10.1016/j.bbi.2020.04.049
3. Gómez-Ochoa SA, Franco OH, Rojas LZ, Raguindin PF, Roa-Díaz ZM, Wyssmann BM, et al. COVID-19 in healthcare workers: a living systematic review and meta-analysis of prevalence risk factors clinical characteristics and outcomes. Am J Epidemiol. (2020) 190:161–75. doi: 10.1093/aje/kwaa191
4. Sahu AK, Amrithanand VT, Mathew R, Aggarwal P, Nayer J, Bhoi S. COVID-19 in health care workers – a systematic review and meta-analysis. Am J Emerg Med. (2020) 38:1727–31. doi: 10.1016/j.ajem.2020.05.113
5. Kurian SJ, Unnikrishnan MK, Miraj SS, Bagchi D, Banerjee M, Reddy BS, et al. Probiotics in prevention and treatment of covid-19: current perspective and future prospects. Arch Med Res. (2021) 52:582–94. doi: 10.1016/j.arcmed.2021.03.002
6. Singh K, Rao A. Probiotics: a potential immunomodulator in COVID-19 infection management. Nutr Res. (2021) 87:1–12. doi: 10.1016/j.nutres.2020.12.014
7. Mahooti M, Miri SM, Abdolalipour E, Ghaemi A. The immunomodulatory effects of probiotics on respiratory viral infections: a hint for COVID-19 treatment? Microb Pathog. (2020) 148:104452. doi: 10.1016/j.micpath.2020.104452
8. Gutiérrez-Castrellón P, Gandara-Martí T, Abreu Y, Abreu AT, Nieto-Rufino CD, López-Orduña E, et al. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes. (2022) 14:2018899. doi: 10.1080/19490976.2021.2018899
9. Olivares M, Díaz-Ropero MP, Gómez N, Lara-Villoslada F, Sierra S, Maldonado JA, et al. The consumption of two new probiotic strains Lactobacillus Gasseri CECT 5714 and Lactobacillus Coryniformis CECT 5711 boosts the immune system of healthy humans. Int Microbiol. (2006) 9:47–52.
10. Lara-Villoslada F, Sierra S, Boza J. Efectos beneficiosos en niños sanos del consumo de un producto lácteo que contiene dos cepas probióticas. Lactobacillus coryniformis CECT5711 y Lactobacillus gasseri CECT5714. Nutr Hosp. (2007) 22:496–502.
11. Martínez-Cañavate A, Sierra S, Lara-Villoslada F, Romero J, Maldonado J, Boza J, et al. Probiotic dairy product containing L. Gasseri CECT5714 and L. Coryniformis CECT5711 induces immunological changes in children suffering from allergy. Pediatr Allergy Immunol. (2009) 20:592–600. doi: 10.1111/j.1399-3038.2008.00833.x
12. Redondo N, Nova E, Gheorghe A, Díaz LE, Hernández A, Marcos A. Evaluation of Lactobacillus Coryniformis CECT5711 strain as a coadjuvant in a vaccination process: a randomised clinical trial in healthy adults. Nutr Metab. (2017) 14:2. doi: 10.1186/s12986-016-0154-2
13. Fonollá J, Gracián C, Maldonado-Lobón JA, Romero C, Bédmar A, Carrillo JC, et al. Effects of Lactobacillus Coryniformis K8 CECT5711 on the immune response to influenza vaccination and the assessment of common respiratory symptoms in elderly subjects: a randomized controlled trial. Eur J Nutr. (2019) 58:83–90. doi: 10.1007/s00394-017-1573-1
14. Fernández-Ferreiro A, Formigo-Couceiro FJ, Veiga-Gutierrez R, Maldonado-Lobón JA, Hermida-Cao AM, Rodriguez C, et al. Effects of Loigolactobacillus Coryniformis K8 CECT 5711 on the immune response of elderly subjects to covid-19 vaccination: a randomized controlled trial. Nutrients. (2022) 14:228. doi: 10.3390/nu14010228
15. Bandyopadhyay S, Baticulon RE, Kadhum M, Alser M, Ojuka DK, Badereddin Y, et al. Infection and mortality of healthcare workers worldwide from COVID-19: a systematic review. BMJ Glob Health. (2020) 5:e003097. doi: 10.1136/bmjgh-2020-003097
16. Chandan S, Khan SR, Deliwala S, Mohan BP, Ramai D, Chandan OC, et al. Postvaccination SARS-CoV-2 infection among healthcare workers: a systematic review and meta-analysis. J Med Virol. (2021) 94:1428–41. doi: 10.1002/jmv.27457
17. Canaday DH, Oyebanji OA, Keresztesy D, Payne M, Wilk D, Carias L, et al. Significant reduction in vaccine-induced antibody levels and neutralization activity among healthcare workers and nursing home residents 6 months following COVID-19 BNT162b2 MRNA vaccination. Clin Infect Dis. (2021) [Online ahead of print]. doi: 10.1093/cid/ciab963.
18. Salvagno GL, Henry BM, Pighi L, De Nitto S, Gianfilippi GL, Lippi G. Three-month analysis of total humoral response to pfizer BNT162b2 MRNA COVID-19 vaccination in healthcare workers. J Infect. (2021) 83:e4–5. doi: 10.1016/j.jinf.2021.06.024
19. Centro Nacional de Epidemiología. COVID-19 en España. (2022). Available online at: https://cnecovid.isciii.es/covid19/#provincias (accessed May 10, 2022).
20. Soriano V, de Mendoza C, Gómez-Gallego F, Corral O, Barreiro P. Third Wave of COVID-19 in Madrid Spain. Int J Infect Dis. (2021) 107:212–4. doi: 10.1016/j.ijid.2021.04.074
21. Zee JS, Lai KT, Ho MK, Leung AC, Fung LH, Luk WP, et al. Serological response to MRNA and inactivated COVID-19 vaccine in healthcare workers in Hong Kong: decline in antibodies 12 weeks after two doses. Hong Kong Med J. (2021) 27:380–3. doi: 10.12809/hkmj219744
22. Favresse J, Bayart JL, Mullier F, Elsen M, Eucher C, Van Eeckhoudt S, et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg Microbes Infect. (2021) 10:1495–8. doi: 10.1080/22221751.2021.1953403
23. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. New Engl J Med. (2021) 385:e84. doi: 10.1056/NEJMoa2114583
24. Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the MRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. (2022) 375:43–50. doi: 10.1126/science.abm3425
25. Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. (2021) 385:1474–84. doi: 10.1056/NEJMoa2109072
26. Björk J, Bonander C, Moghaddassi M, Rasmussen M, Malmqvist U, Kahn F, et al. Surveillance of COVID-19 vaccine effectiveness - a real-time case-control study in Southern Sweden. Epidemiol Infect. (2022) 150:1–15. doi: 10.1017/S0950268822000425
27. Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Razizadeh MH, Turner DL, et al. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines. (2021) 9:467. doi: 10.3390/vaccines9050467
28. Kadali RAK, Janagama R, Peruru S, Gajula V, Madathala RR, Chennaiahgari N, et al. Non-life-threatening adverse effects with COVID-19 MRNA-1273 vaccine: a randomized cross-sectional study on healthcare workers with detailed self-reported symptoms. J Med Virol. (2021) 93:4420–9. doi: 10.1002/jmv.26996
Keywords: probiotic, immune response, COVID-19, health care workers, randomized clinical trial
Citation: Rodriguez-Blanque R, Sánchez-García JC, Cobos-Vargas Á, Aguilar Quesada A, Maldonado-Lobón JA, Olivares M and Blanco-Rojo R (2022) Evaluation of the effect of Loigolactobacillus coryniformis K8 CECT 5711 consumption in health care workers exposed to COVID-19. Front. Nutr. 9:962566. doi: 10.3389/fnut.2022.962566
Received: 15 June 2022; Accepted: 18 July 2022;
Published: 03 August 2022.
Edited by:
Julio Villena, Centro de Referencia para Lactobacilos (CONICET), ArgentinaReviewed by:
Susana Salva, Centro de Referencia para Lactobacilos (CONICET), ArgentinaMaja Šikić Pogačar, University of Maribor, Slovenia
Copyright © 2022 Rodriguez-Blanque, Sánchez-García, Cobos-Vargas, Aguilar Quesada, Maldonado-Lobón, Olivares and Blanco-Rojo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruth Blanco-Rojo, ruth.blanco@kerry.com
 Raquel Rodriguez-Blanque
Raquel Rodriguez-Blanque Juan Carlos Sánchez-García
Juan Carlos Sánchez-García Ángel Cobos-Vargas1
Ángel Cobos-Vargas1  Ana Aguilar Quesada
Ana Aguilar Quesada Mónica Olivares
Mónica Olivares Ruth Blanco-Rojo
Ruth Blanco-Rojo