The functional connectivity of the basal ganglia subregions changed in mid-aged and young males with chronic prostatitis/chronic pelvic pain syndrome
- 1Department of Rehabilitation Medicine, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2Department of Medical Imaging, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 3Department of Medical Imaging, Xi’an No.3 Hospital, Xi’an, China
- 4Department of Urology, Xi’an No.3 Hospital, Xi’an, China
Background: The Basal ganglia (BG) played a crucial role in the brain-level mechanisms of chronic pain disorders. However, the functional changes of BG in chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) are still poorly understood. This study investigated the BG subregions’ resting-state functional connectivity (rs-FC) in CP/CPPS patients compared with healthy controls.
Methods: Twenty eight patients with CP/CPPS and 28 age- and education-matched healthy males underwent clinical measurements and 3T brain MR imaging, including T1-weighted structural images and resting-state functional imaging. The data were analyzed by the seeded-based rs-FC analysis. Then, a machine learning method was applied to assess the feasibility of detecting CP/CPPS patients through the changed rs-FC.
Results: Compared with healthy males, patients presented decreased rs-FC between the BG subregions and right middle cingulate cortex, and correlated with pain (r = 0.51, p-uncorrected = 0.005) and urinary symptoms (r = –0.4, p-uncorrected = 0.034). The left superior temporal gyrus and right supramarginal gyrus showed decreased rs-FC with the BG subregions as well. The area under the receiver operating characteristic curve of 0.943 (accuracy = 80%, F1-score = 80.6%) was achieved for the classification of CP/CPPS patients and healthy males with support vector machine (SVM) based on the changed rs-FC.
Conclusion: These findings provide evidence of altered BG subregions’ rs-FC in CP/CPPS, which may contribute to our understanding of the BG’s role in CP/CPPS.
Introduction
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is one of the most common urologic diseases, with the prevalence rate ranging from 3 to 16% all over the world (Engeler et al., 2013). CP/CPPS affects 8.4% of men in China, especially those between 31 and 40 years old (Liang et al., 2009). The persistent pain and urinary symptoms severely bother the patients and urologists. The key to breaking this situation is a better understanding of the ambiguous pathophysiological mechanisms (Pontari, 2013). Nowadays, neuroimaging studies have led to advances in comprehending CP/CPPS mechanisms at the brain level beyond the prostate. Previous studies assessed alterations in brain structure or function in the patients with CP/CPPS, such as medial areas of the motor-sensory cortex, the posterior insula, and the periaqueductal gray, which indicated the abnormal sensorimotor processing of the pelvic area and descending modulation of pain in this chronic disorder (Clemens et al., 2019).
In chronic pain conditions, the basal ganglia (BG) were supposed to integrate many aspects of pain. These include the integration of motor, emotional, autonomic, and cognitive responses to pain (Borsook et al., 2010). The multiple painful stimuli produced increased or decreased activation in the subregions of BG (Becerra et al., 2006, 2015; Lebel et al., 2008), which implicated that BG were involved in nociceptive signal processing. The increase of BG gray matter has been reported in chronic back pain (Tagliaferri et al., 2021), fibromyalgia (Schmidt-Wilcke et al., 2007), and chronic vulval pain (Schweinhardt et al., 2008), suggesting these structural changes might be associated with some underlying functional alterations in chronic pain conditions. In complex region pain syndrome (CRPS) (Becerra et al., 2014; Azqueta-Gavaldon et al., 2020), the decreased functional connectivity (FC) of BG with sensorimotor network and Default Mode Network (DMN) were involved a maintained fear of pain and movement avoidance, and the information integration and environment perception in pain processing. In contrast, females with chronic pelvic pain presented increased FC between BG and posterior cingulate cortex, another hub of the DMN (Martucci et al., 2015). In clinical practice, BG were a target region for chronic pain treatment. The deep brain stimulation at the globus pallidus could improve the chronic pain symptoms in Parkinson’s disease (Gong et al., 2020). These results highlighted the important role of BG in pain processing. In particular, A whole-brain analysis between CP/CPPS and healthy controls (HCs) compared FC effect size directly and found the difference in the BG subregion greater than 1 in magnitude (Kutch et al., 2015). However, it remains unclear concerning the functional properties of BG and subregions in males with CP/CPPS.
Herein, in this MRI-based research, we hypothesized that the resting-state functional connectivity (rs-FC) of the BG subregions was altered in patients with CP/CPPS and investigated the relationship between the altered rs-FC and clinical measures of CP/CPPS. And, we explored the feasibility of detecting CP/CPPS by the support vector machine (SVM) algorithm based on the altered rs-FC.
Materials and methods
This study was performed from April 2020 to August 2021. The patients with CP/CPPS were recruited from the urology outpatient department, and age- and education- matched healthy males were recruited as healthy controls in this study. The written informed consents were obtained from all participants and conducted following the Declaration of Helsinki.
Participants
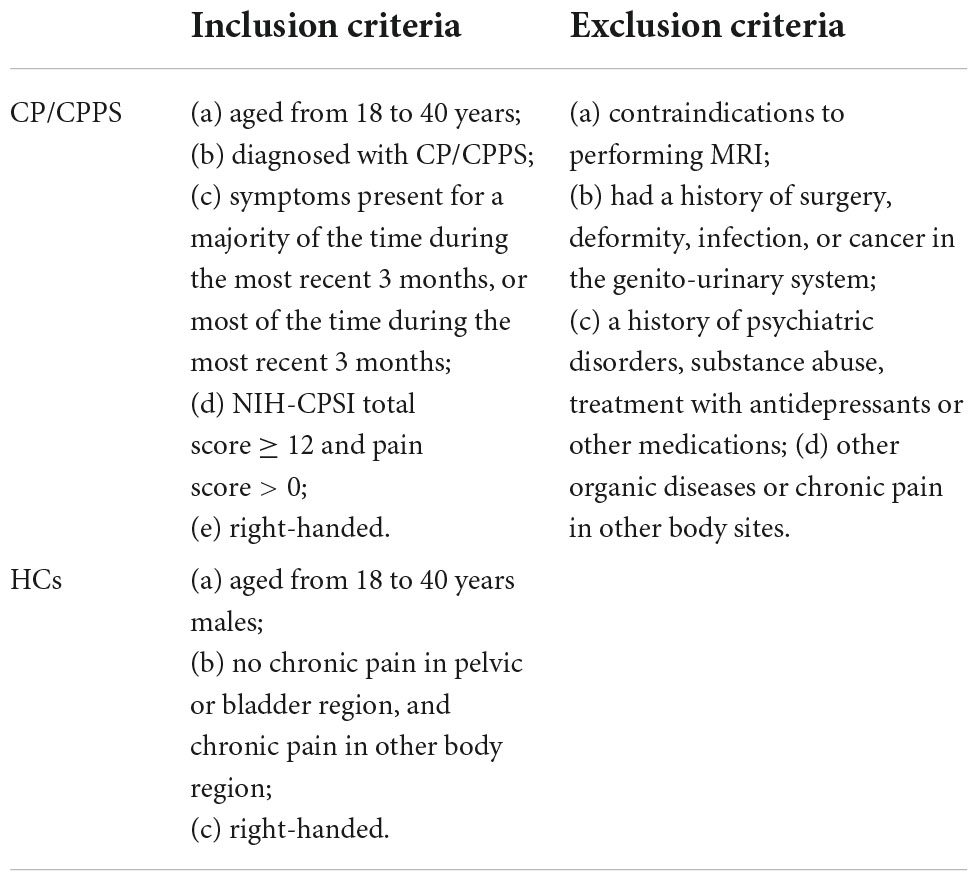
Twenty-eight patients with CP/CPPS (31.18 ± 4.33 years, mean ± SD) and 28 demographically similar HCs (30.11 ± 2.91 years, mean ± SD) were included in this study. The inclusion and exclusion criteria of all subjects was shown in Table 1. The diagnosis of CP/CPPS was established after history-taking, physical examination, laboratory examination, and kidney, ureter, bladder, and prostate ultrasound, and consistent with the National Institutes of Health definition of CP/CPPS. The clinical profiles of patients with CP/CPPS were shown in Supplementary Table 1.
Questionnaires
The National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) was applied to assess the severity of CP/CPPS symptoms (Litwin et al., 1999). The NIH-CPSI consists of nine items divided into three domains, (1) the severity of pain or discomfort include the location, intensity, and frequency (4 items, 0–21 points); (2) the severity of urinary symptoms focus on irritative and obstructive urinary symptoms (2 items, 0–10 points); and (3) the quality-of-life (QoL) evaluate the symptoms effect on ordinary life (3 items, 0–12 points). The higher three sub-scores (pain, urinary symptoms, and QoL) and the total score (0–43 points) indicate worse symptoms.
All the subjects were assessed for catastrophic thoughts through the Pain catastrophizing Scale (PCS) (Xu et al., 2015). The PCS is a 13-item self-report measure designed to assess catastrophic thoughts or feelings accompanying the experience of pain. Which can be divided into the three components of pain catastrophizing: rumination (e.g., “I can’t seem to keep it out of my mind”); magnification (e.g., “I wonder whether something serious may happen”); and helplessness (e.g., “There is nothing I can do to reduce the intensity of pain”). This scale has been translated and standardized in Chinese population. Participants rated in reference to a previous pain event on a 5-point Likert scale ranging from 0 (not at all) to 4 (always).
Neuroimaging data acquisition
All participants underwent MR scans on a 3T MR scanner (Ingenia, Philips Healthcare, Best, Netherlands) equipped with a 32-channel head coil. All participants were asked to empty their bladder and lie still in a relaxed position with their eyes closed while remaining awake and avoiding specific thoughts. Headphones and foam pads were used to reduce noise interference and minimize head motion. Routine sequence scanning was first performed to exclude obvious structural damage. High-resolution brain structural images were acquired from each subject [repetition time (TR) = 8.2 ms, echo time (TE) = 3.8 ms, flip angle = 8°, slice thickness = 1 mm, field of view (FOV) = 24 × 24 cm2, matrix size = 240 × 240, voxel size = 1 × 1 × 1 mm3]. The rs-fMRI images were obtained from gradient-echo-planar imaging sequence (TR = 2,500 ms, TE = 30 ms, flip angle = 90°, voxel size = 3 × 3 × 3 mm3, slice thickness = 3 mm with no gap, slices = 50, matrix size = 80 × 80, FOV = 24 × 24 cm2, volumes = 180).
Image preprocessing
All neuroimaging data were processed by using CONN connectivity toolbox V20.a1 (Whitfield-Gabrieli and Nieto-Castanon, 2012), based on MATLAB 2019. The structural and functional data underwent the preprocessing pipeline for volume-based analyses, including (1) removing the first four volumes to avoid the potential noise related to the participants’ adaptation to the scanner, (2) functional realignment and unwarping, the subjects with head motion exceeding 3 mm or 3° were excluded, and no participants met this exclusion criterion. (3) functional slice-timing correction, (4) functional outlier identification (ART-based scrubbing), (5) functional segmentation and normalization to Montreal Neurological Institute (MNI) space, (6) structural segmentation and normalization in the MNI -space, (7) functional smoothing by using a Gaussian kernel with full-width at half-maximum of 6 mm. For the scrubbing, outliers were defined as composite movement greater than 0.5 mm or more than 3 standard deviations away from the mean image intensity. Denoising was processed by the usual covariates (white-matter and cerebrospinal fluid signals) and band-pass filtering with the default CONN values (0.008–0.09 Hz). The comparison of the imaging quality and head motion showed no significant difference between two groups (Supplementary Figure 1). Finally, head motion-related artifacts were reduced by the component-based noise correction method (CompCor).
Seed-based functional connectivity analysis
A seed-based analysis was performed to explore the whole-brain voxel-wise rs-FC alteration in the BG subregions between two groups. The bilateral BG subregions were defined based on the Harvard-Oxford Brain Atlas per CONN Toolbox protocol (Whitfield-Gabrieli and Nieto-Castanon, 2012). A total of 8 BG subregions were defined as the region of interest (ROIs) for the following analysis, including the bilateral caudate nucleus (NC), globus pallidus (GP), nucleus accumbens (NAc), and putamen (PU). The time course of the average blood oxygen level-dependent (BOLD) signal was extracted from each ROI. Then, Pearson correlation coefficients were calculated between the mean time series of each ROI and that of each voxel of the whole brain. Fisher z transformation was performed to improve the normal distribution of the data. The two-sample t-tests were used to group comparisons of seed-based rs-FC. The results were considered significant at a threshold of voxel-wise p < 0.001 and cluster-level p < 0.05, with the false positive rate corrected (FDR-corrected) for between-group comparisons. The resulting maps were overlaid on the rendered views using MRIcron,2 and the location of the surviving brain regions was reported by the automated anatomical labeling (AAL) template (Tzourio-Mazoyer et al., 2002).
Statistical analysis
Two-sample t-tests or Mann–Whitney U-tests were used to evaluate the differences in age, and PCS scores according to the normality and homogeneity of variances assessed by Kolmogorov–Smirnov and Levene’s tests, respectively. The significant level of non-imaging data was set to p = 0.05. Statistical analyses were performed with R (R version 4.0.2, The R Foundation for Statistical Computing).
Correlation analysis
Compared to HCs, the rs-FC with significant CP/CPPS group changes was extracted to explore the relationship with clinical features and the score of questionnaires, respectively. Then, the Spearman correlation analysis was performed by using GraphPad Prism 9 to evaluate the correlation between abnormal rs-FC and changed measurements in patients with CP/CPPS. The FDR method was used to correct the results of the correlation analysis for multiple comparisons.
Machine learning
Based on the altered rs-FC, classification models of CP/CPPS were established by using the SVM. We adopted nested resampling and stratification sampling strategies to reduce selection bias and avoid overfitting or underfitting (Supplementary Figure 2). Fivefold cross-validation (CV) were adopted to get different training and testing data sets (outer resampling). As for the training data, we used the 10-fold CV to get different inner training and testing data sets (inner resampling). Then, a grid search was performed to determine the optimal parameter for the model tuning within the inner resampling. The learners with the tuned hyperparameter configuration obtained in the inner resampling were applied to the outer training data set. The classification performance of the machine learning algorithms was evaluated by the accuracy, F1-Score, and area under the receiver operating characteristic curve (AUC-ROC). The classification measurement of outer training data was calculated and taken as the average to evaluate the generalization ability of the classification model.
We have trained the machine learning model for significant rs-FC of each seed independently. And then, to see whether we can get better performance with all these seeds, we trained the model again by combing the rs-FC of all seeds. The machine learning was performed in R version 4.0.2 and the Machine Learning in R package (MLR3) (Lang et al., 2019).
Results
Demographic and clinical data
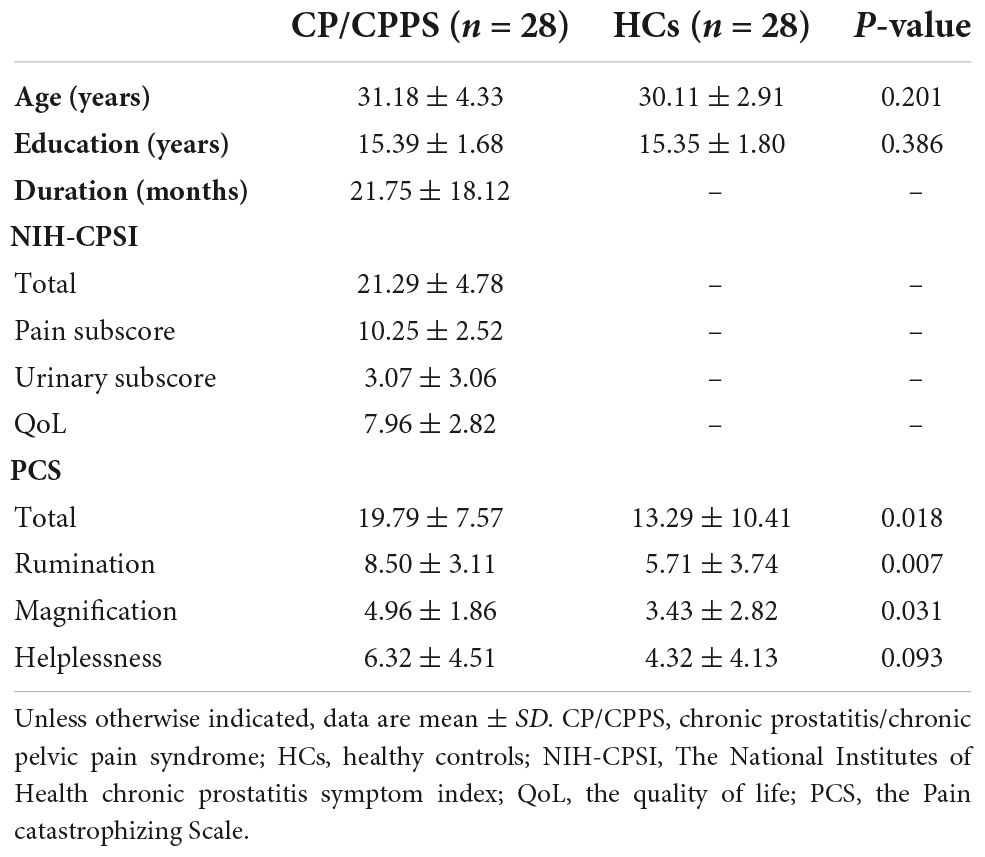
Compared to HCs, no significant differences were found for age (p = 0.201), and education (p = 0.386). The PCS total scores (p = 0.018) and three sub-scores (Rumination p = 0.007, Magnification p = 0.031, Helplessness p = 0.093) were higher in CP/CPPS group. In the CP/CPPS group, the total score, pain sub-score, urinary symptoms sub-score, and QoL sub-score of NIH-CPSI were 21.29 ± 4.78, 10.25 ± 2.52, 3.07 ± 3.06, and 7.96 ± 2.82 (mean ± SD), respectively. The disease duration was 21.75 ± 18.12 months (mean ± SD). The detailed information is listed in Table 2.
Seed-based functional connectivity analysis
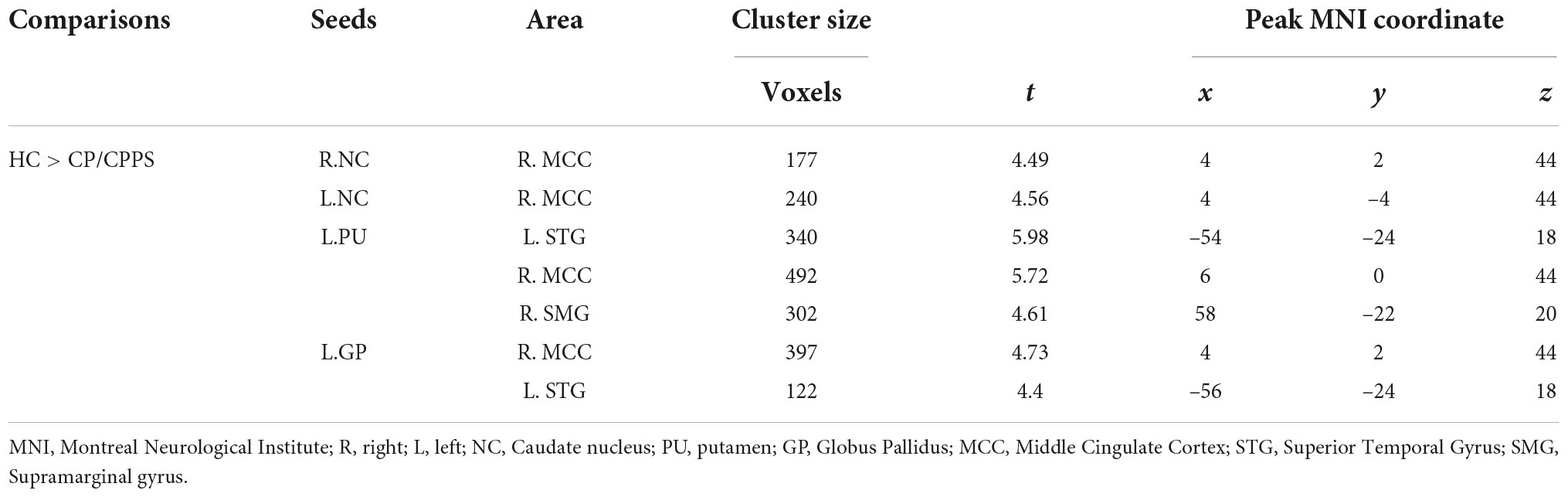
Compared with HCs, patients with CP/CPPS exhibited significantly decreased rs-FC between the bilateral NC and the right middle cingulate cortex (R.MCC) (Figures 1A,B). Meanwhile, the left GP (L.GP)-R.MCC/left superior temporal gyrus (L.STG) was presented with decreased rs-FC in CP/CPPS group (Figure 1C). The rs-FC of L.PU- L.STG/R.MCC/right supramarginal gyrus (R.SMG) was reduced in the patients with CP/CPPS (Figure 1D and Table 3). There was no significant change in rs-FC of other seeds (R.PU, R.GP, and bilateral NAc).
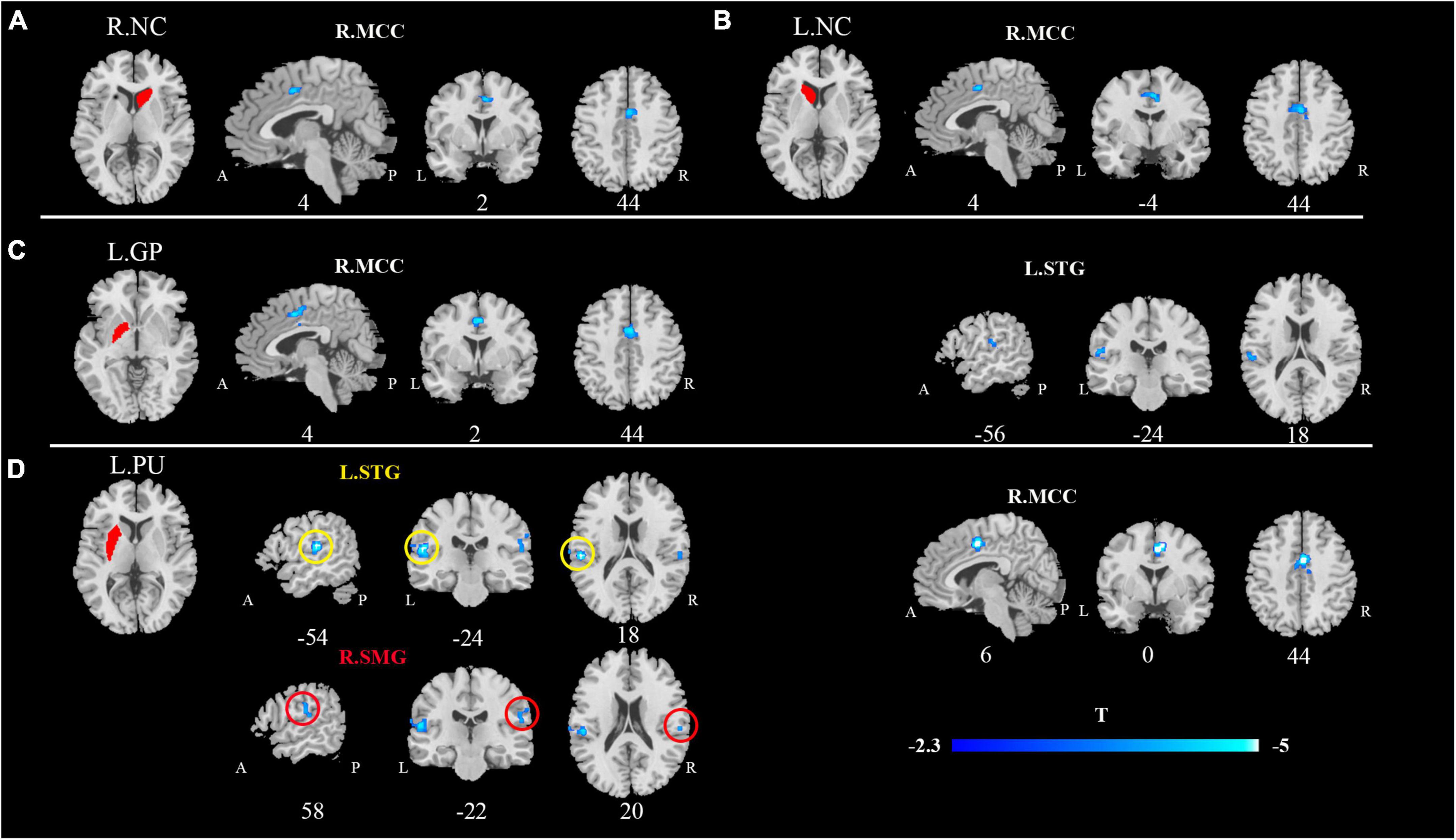
Figure 1. Decreased functional connectivity of CP/CPPS in contrast to healthy controls between the seed regions. (A) R.NC; (B) L.NC; (C) L.GP; (D) L.PU and affected brain regions. All images were shown with an FDR correction of P < 0.05. R, right; L, left; NC, Caudate nucleus; PU, putamen; GP, Globus Pallidus.
Correlation analysis
In CP/CPPS group, L.GP-R.MCC rs-FC were positively correlated with pain sub-score (Figure 2A, R = 0.51, p = 0.005) and negatively correlated with urinary symptoms sub-score (Figure 2B, R = –0.4, p = 0.034). However, these correlations were statistically insignificant after multiple FDR comparisons. In addition, there were no significant correlations between altered rs-FC and other measurement values in the CP/CPPS group.
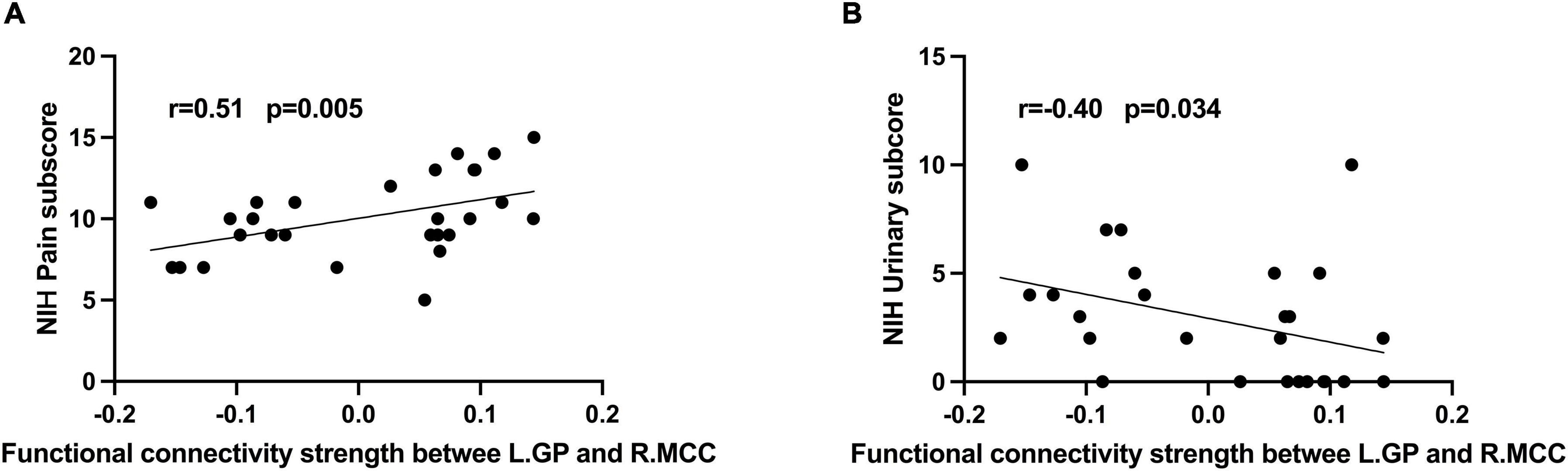
Figure 2. Correlation analysis between (A) rs-FC of L.GP- R.MCC and NIH-CPSI pain score (r = 0.51, P = 0.005); (B) rs-FC of L.GP- R.MCC and NIH-CPSI urinary score (r = –0.40, P = 0.034).
Classification performance by machine learning
The performance of SVM classifiers based on different features (rs-FC of L.GP, L.NC, L.PU, R.NC, and all seeds) at each outer resampling was shown in Figures 3A–C. CP/CPPS patients were classified with mean accuracy of 82% (L.GP), 71.7% (L.NC), 79% (L.PU), 61.3% (R.NC), and 80% (all seeds). The mean F1-Score of the classifiers were 82.5% (L.GP), 71.7% (L.NC), 76.7% (L.PU), 67.1% (R.NC), and 80.6% (all seeds). The area under the AUC-ROC was 0.884 (L.GP), 0.792 (L.NC), 0.917 (L.PU), 0.729 (R.NC), and 0.943 (all seeds) (Figure 3D). The SVM based on the combination of all the seeds achieved a satisfying performance, especially the largest AUC-ROC.
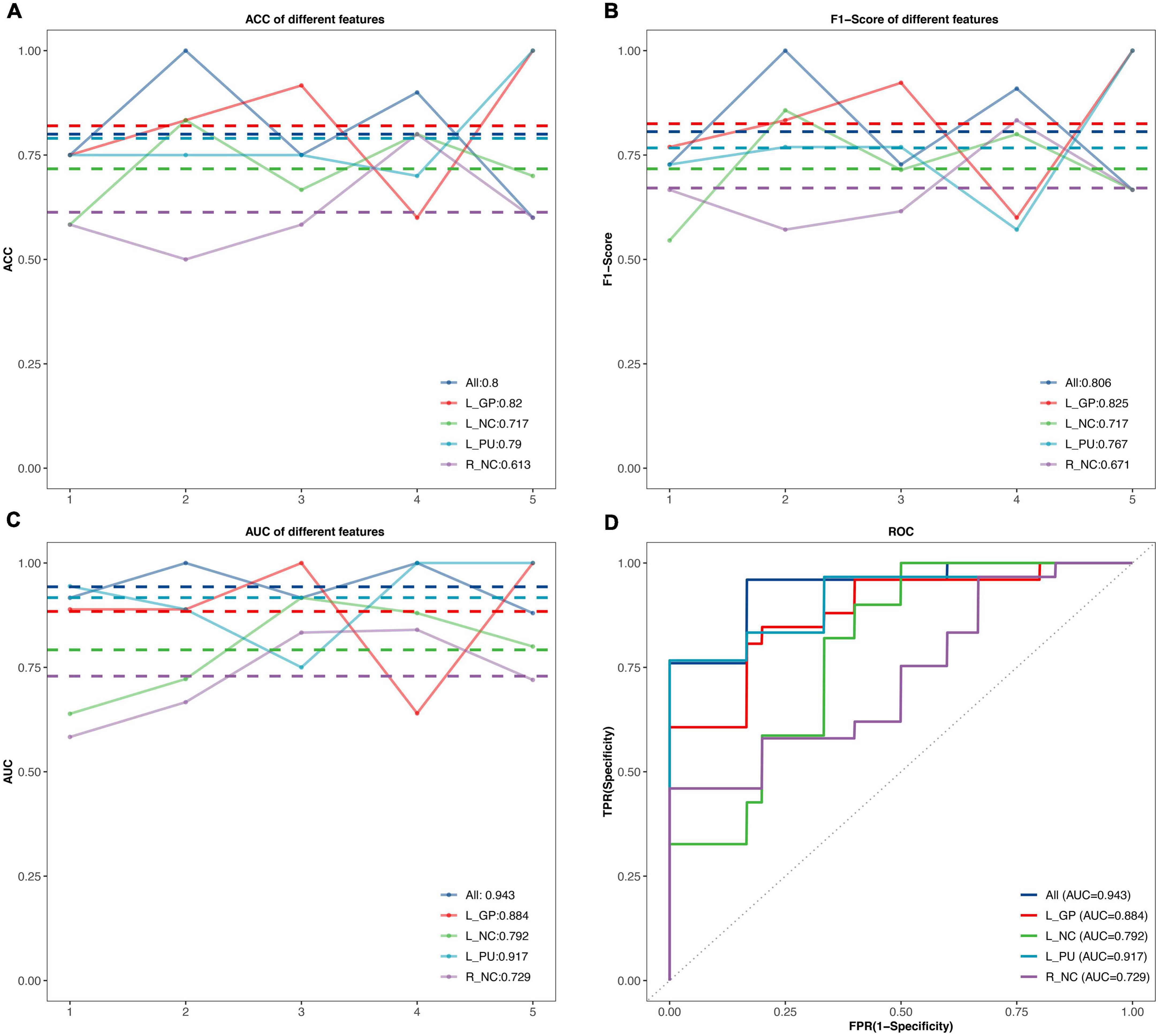
Figure 3. The classification performance of the SVM model. (A–C) The accuracy, F1-Score, and the area under curves (AUC) values in the testing set for different features. The horizontal axis represents five outer resampling, the vertical axis represents the values of measurement. The mean value of each measurement is marked with the dotted line of the same color as the broken line. (D) The receiver operating characteristic (ROC) curves. ACC, accuracy; AUC, area under the curve; R, right; L, left; NC, Caudate nucleus; PU, putamen; GP, Globus Pallidus.
Discussion
This study investigated the rs-FC of BG subregions and their relationship with clinical features in mid-aged and young CP/CPPS patients. Compared to the HCs, CP/CPPS patients presented decreased connectivity of BG subregions (bilateral NC, L.PU, L.GP) and MCC. Meanwhile, L.PU and L.GP showed reduced connectivity with the left STG. We also found lowered rs-FC of L.PU with R.SMG in CP/CPPS patients. Furthermore, correlations analysis indicated the decreased rs-FC of L.GP-R.MCC was positive with pain and negative with urinary symptoms. Based on these alterations, we established SVM classifiers for distinguishing CP/CPPS patients from HCs. The SVM based on the all altered rs-FC of BG subregions to identify CP/CPPS achieved the largest ROC-AUC.
As we know, this is the first study to examine the rs-FC of BG subregions in young and mid-age males with CP/CPPS. We found the reduced rs-FC of NC, L.PU, and L.GP with MCC in CP/CPPS. As a central hub in pain processing, MCC was connected with other brain areas involved in the processing of pain (Vogt, 2005; Eisenberger, 2012). In chronic pain conditions, such as refractory neuropathic pain (Maarrawi et al., 2007), fibromyalgia syndrome (Pérez-Aranda et al., 2019), and chronic low back pain (Ivo et al., 2013), MCC presented structural and functional abnormalities. The MCC was associated with pain intensity and motor responses toward nociceptive (Shackman et al., 2011). A graph theory study found that patients with CP/CPPS presented disrupted topological organizations of MCC, the higher the global and local efficiency of MCC, the more serious chronic pain (Huang et al., 2021a). In our study, the correlation analysis showed decreased rs-FC of L.GP-MCC was a positive correlation with pain symptoms in CP/CPPS patients. In addition to being involved in pain processing, MCC generated the desire to void and urinary urgency (Griffiths, 2015). We also found the urinary symptoms of CP/CPPS were negatively correlated with rs-FC of L.GP-MCC. These results may implicate that the detaching between BG and MCC affects the clinical symptoms of CP/CPPS.
The BG-cingulate circuit is one of the BG-thalamocortical circuits (Alexander et al., 1986). A Diffusion Tensor Imaging (DTI) study revealed that patients with CP/CPPS presented microstructural differences within the BG-thalamocortical circuits (Woodworth et al., 2015). The striatum (NC and PU) is the input nucleus and receives signal inputs from the multiple cortexes, the GP is the output nucleus and from there back to the associated cortical area (Marchand, 2010). The sensory and motor control progressively integrated with their subsequent passage through BG (Borsook et al., 2010). Thus, the BG is considered to be involved in the modulatory system of pain (Barceló et al., 2012; Becerra et al., 2015). Similar to our finding, the temporomandibular disorder patients showed reduced rs-FC of BG-cingulate, which was positively correlated with the pain intensity (He et al., 2018). BG is densely populated with opiate receptors in humans (Blackburn et al., 1988). Especially in the BG-cingulate circuit, the inhibition of the cingulate cortex or BG could relieve pain in the rodent model of chronic pain (Zhuang et al., 2021). In the present study, we found decreased L.GP-MCC rs-FC was a positive correlation with pain symptoms in CP/CPPS patients. The reduced rs-FC of BG-cingulate might be a compensatory coping strategy in chronic pain conditions. The compensation strategy seems to lose efficacy for individuals with more serious pain. So, in this study, the reduced rs-FC within the BG-cingulate circuit may be associated with aberrant central control in pain processing in patients in CP/CPPS.
We also found that L.STG and R.SMG presented decreased rs-FC with L.PU and L.GP. The R.SMG belongs to the ventral attention network (VAN), which supports bottom-up attention to important, behaviorally relevant stimuli, even if they are not salient or distinctive (Power et al., 2011). The STG is generally considered to be important as auditory perception and emotional regulatory part of the human brain, which is essential for individual stressful experiences, cognitive processes, and adaptive behavior (Huang et al., 2021b). A few neuroimaging studies about pain found that the function or structure of SMG (Yu et al., 2019; Ionta et al., 2020; Naor et al., 2020; Yang et al., 2021) and STG (Cleve et al., 2017; Zhao et al., 2017; van Ettinger-Veenstra et al., 2019; Zhang Y. et al., 2019; Zhang Y. N. et al., 2019; Spisak et al., 2020; Yang et al., 2020) was changed. The pain experience results from the integration of pain processing in a given individual (Wiech et al., 2008), our finding might partly reflect this integration. However, how these brain regions affect pain is incompletely understood, it needs to be further investigated.
Machine learning has been widely used to identify various chronic pain as an auxiliary method for diagnosis and prediction in neuroimaging studies. Based on the amplitude of low-frequency fluctuation within the region of interest (ROI), the researchers trained machine learning models that could discriminate chronic low back pain patients from HCs and reach the highest accuracy of 71.1% (Zhang Y. et al., 2019). The rs-FC was selected as an input feature of SVM that could differentiate irritable bowel syndrome patients with a ROC-AUC of 0.71 (Mao et al., 2020). An SVM classifier based on the changed brain morphology to identify patients with chronic pelvic pain achieved an accuracy of 73% (Bagarinao et al., 2014). In the present study, we combined all altered rs-FC of BG subregions to build the SVM classifier, which achieved the highest ROC-AUC and satisfactory performance. The altered rs-FC of BG subregions had an advanced ability to objectively classify individuals with CP/CPPS and played an important role in CP/CPPS mechanisms at the brain level.
Our study had several limitations. First, our research has a cross-sectional design with a relatively modest size. Second, this study did not examine other precise structures of BG, such as nucleus subthalamic and substantia nigra pars. Finally, the nested resampling approach was used to construct and tune the SVM model due to the small sample size, which could cause the overfitting issue. Further studies with larger sample sizes should be extended to these subdivisions to investigate the FC patterns of CP/CPPS patients. The explorations of the functional changes within longitudinal observation and after treatments could provide more information about the role of BG on the pathogenesis of CP/CPPS.
Conclusion
In conclusion, this study directly supported the hypothesis that CP/CPPS was associated with abnormal function in the BG. Specifically, we found reduced rs-FC in the cortico-BG in CP/CPPS patients compared to healthy controls. The abnormal connectivity was associated with deficits in pain processing and micturition control in CP/CPPS. Based on the rs-FC of BG subregions, machine learning could be a reliable classifier in differentiating CP/CPPS patients and HCs. This current work provided neuroimaging evidence to improve our understanding of the neuropathological mechanisms of CP/CPPS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Ethics Review Board of Xi’an No.3 Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XL, JH, and MZ contributed to the conception and design of the study. W-JM, J-LL, JH, and W-XB contributed to the acquisition of the data. XN, H-NL, W-HD, and XL contributed to the analysis and interpretation of the data. X-YZ, XN, and XL contributed to the drafting of the article and critical revisions. All authors approved the submission of the manuscript for consideration.
Funding
This research was supported by the National Natural Science Foundation of China (grant no. 82202121), Natural Science Foundation of Shaanxi Province (grant no. 2022JQ-778), and Key Research and Development Projects of Shaanxi Province (grant no. 2022SF-584).
Acknowledgments
We would like to thank all patients and healthy volunteers that participated in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor LB declared a shared parent affiliation with the authors at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnhum.2022.1013425/full#supplementary-material
Footnotes
References
Alexander, G. E., DeLong, M. R., and Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381. doi: 10.1146/annurev.ne.09.030186.002041
Azqueta-Gavaldon, M., Youssef, A. M., Storz, C., Lemme, J., Schulte-Göcking, H., Becerra, L., et al. (2020). Implications of the putamen in pain and motor deficits in complex regional pain syndrome. Pain 161, 595–608. doi: 10.1097/j.pain.0000000000001745
Bagarinao, E., Johnson, K. A., Martucci, K. T., Ichesco, E., Farmer, M. A., Labus, J., et al. (2014). Preliminary structural MRI based brain classification of chronic pelvic pain: A MAPP network study. Pain 155, 2502–2509. doi: 10.1016/j.pain.2014.09.002
Barceló, A. C., Filippini, B., and Pazo, J. H. (2012). The striatum and pain modulation. Cell. Mol. Neurobiol. 32, 1–12. doi: 10.1007/s10571-011-9737-7
Becerra, L., Harter, K., Gonzalez, R. G., and Borsook, D. (2006). Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth. Analg. 103, 208–216. doi: 10.1213/01.ane.0000221457.71536.e0
Becerra, L., Sava, S., Simons, L. E., Drosos, A. M., Sethna, N., Berde, C., et al. (2014). Intrinsic brain networks normalize with treatment in pediatric complex regional pain syndrome. Neuroimage Clin. 6, 347–369. doi: 10.1016/j.nicl.2014.07.012
Becerra, L., Schwartzman, R. J., Kiefer, R. T., Rohr, P., Moulton, E. A., Wallin, D., et al. (2015). CNS measures of pain responses pre- and post-anesthetic ketamine in a patient with complex regional pain syndrome. Pain Med. 16, 2368–2385. doi: 10.1111/pme.12939
Blackburn, T. P., Cross, A. J., Hille, C., and Slater, P. (1988). Autoradiographic localization of delta opiate receptors in rat and human brain. Neuroscience 27, 497–506. doi: 10.1016/0306-4522(88)90283-7
Borsook, D., Upadhyay, J., Chudler, E. H., and Becerra, L. (2010). A key role of the basal ganglia in pain and analgesia–Insights gained through human functional imaging. Mol. Pain 6:27. doi: 10.1186/1744-8069-6-27
Clemens, J. Q., Mullins, C., Ackerman, A. L., Bavendam, T., van Bokhoven, A., Ellingson, B. M., et al. (2019). Urologic chronic pelvic pain syndrome: Insights from the MAPP research network. Nat. Rev. Urol. 16, 187–200. doi: 10.1038/s41585-018-0135-5
Cleve, M., Gussew, A., Wagner, G., Bär, K. J., and Reichenbach, J. R. (2017). Assessment of intra- and inter-regional interrelations between GABA+, Glx and BOLD during pain perception in the human brain – A combined (1)H fMRS and fMRI study. Neuroscience 365, 125–136. doi: 10.1016/j.neuroscience.2017.09.037
Eisenberger, N. I. (2012). The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nat. Rev. Neurosci. 13, 421–434. doi: 10.1038/nrn3231
Engeler, D. S., Baranowski, A. P., Dinis-Oliveira, P., Elneil, S., Hughes, J., Messelink, E. J., et al. (2013). The 2013 EAU guidelines on chronic pelvic pain: Is management of chronic pelvic pain a habit, a philosophy, or a science? 10 years of development. Eur. Urol. 64, 431–439. doi: 10.1016/j.eururo.2013.04.035
Gong, S., Xu, M., Tao, Y., Jin, H., Liu, Y., Sun, X., et al. (2020). Comparison of subthalamic nucleus and globus pallidus internus deep brain stimulation surgery on Parkinson disease-related pain. World Neurosurg. 135, e94–e99. doi: 10.1016/j.wneu.2019.11.026
Griffiths, D. (2015). Neural control of micturition in humans: A working model. Nat. Rev. Urol. 12, 695–705. doi: 10.1038/nrurol.2015.266
He, S., Li, F., Gu, T., Ma, H., Li, X., Zou, S., et al. (2018). Reduced corticostriatal functional connectivity in temporomandibular disorders. Hum. Brain Mapp. 39, 2563–2572. doi: 10.1002/hbm.24023
Huang, X., Chen, J., Liu, S., Gong, Q., Liu, T., Lu, C., et al. (2021a). Impaired frontal-parietal control network in chronic prostatitis/chronic pelvic pain syndrome revealed by graph theoretical analysis: A DTI study. Eur. J. Neurosci. 53, 1060–1071. doi: 10.1111/ejn.14962
Huang, X., Zhang, D., Wang, P., Mao, C., Miao, Z., Liu, C., et al. (2021b). Altered amygdala effective connectivity in migraine without aura: Evidence from resting-state fMRI with Granger causality analysis. J. Headache Pain 22:25. doi: 10.1186/s10194-021-01240-8
Ionta, S., Costantini, M., Ferretti, A., Galati, G., Romani, G. L., and Aglioti, S. M. (2020). Visual similarity and psychological closeness are neurally dissociable in the brain response to vicarious pain. Cortex 133, 295–308. doi: 10.1016/j.cortex.2020.09.028
Ivo, R., Nicklas, A., Dargel, J., Sobottke, R., Delank, K. S., Eysel, P., et al. (2013). Brain structural and psychometric alterations in chronic low back pain. Eur. Spine J. 22, 1958–1964. doi: 10.1007/s00586-013-2692-x
Kutch, J. J., Yani, M. S., Asavasopon, S., Kirages, D. J., Rana, M., Cosand, L., et al. (2015). Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP: Research network neuroimaging study. Neuroimage Clin. 8, 493–502. doi: 10.1016/j.nicl.2015.05.013
Lang, M., Binder, M., Richter, J., Schratz, P., Pfisterer, F., Coors, S., et al. (2019). Mlr3: A modern object-oriented machine learning framework in R. J. Open Source Softw. 4:1903.
Lebel, A., Becerra, L., Wallin, D., Moulton, E. A., Morris, S., Pendse, G., et al. (2008). FMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain 131(Pt 7), 1854–1879. doi: 10.1093/brain/awn123
Liang, C. Z., Li, H. J., Wang, Z. P., Xing, J. P., Hu, W. L., Zhang, T. F., et al. (2009). The prevalence of prostatitis-like symptoms in China. J. Urol. 182, 558–563. doi: 10.1016/j.juro.2009.04.011
Litwin, M. S., McNaughton-Collins, M., Fowler, F. J. Jr., Nickel, J. C., Calhoun, E. A., Pontari, M. A., et al. (1999). The national institutes of health chronic prostatitis symptom index: Development and validation of a new outcome measure. Chronic prostatitis collaborative research network. J. Urol. 162, 369–375. doi: 10.1016/s0022-5347(05)68562-x
Maarrawi, J., Peyron, R., Mertens, P., Costes, N., Magnin, M., Sindou, M., et al. (2007). Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology 69, 827–834. doi: 10.1212/01.wnl.0000269783.86997.37
Mao, C. P., Chen, F. R., Huo, J. H., Zhang, L., Zhang, G. R., Zhang, B., et al. (2020). Altered resting-state functional connectivity and effective connectivity of the habenula in irritable bowel syndrome: A cross-sectional and machine learning study. Hum. Brain Mapp. 41, 3655–3666. doi: 10.1002/hbm.25038
Marchand, W. R. (2010). Cortico-basal ganglia circuitry: A review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Struct. Funct. 215, 73–96. doi: 10.1007/s00429-010-0280-y
Martucci, K. T., Shirer, W. R., Bagarinao, E., Johnson, K. A., Farmer, M. A., Labus, J. S., et al. (2015). The posterior medial cortex in urologic chronic pelvic pain syndrome: Detachment from default mode network-a resting-state study from the MAPP research network. Pain 156, 1755–1764. doi: 10.1097/j.pain.0000000000000238
Naor, N., Rohr, C., Schaare, L. H., Limbachia, C., Shamay-Tsoory, S., and Okon-Singer, H. (2020). The neural networks underlying reappraisal of empathy for pain. Soc. Cogn. Affect. Neurosci. 15, 733–744. doi: 10.1093/scan/nsaa094
Pérez-Aranda, A., Andrés-Rodríguez, L., Feliu-Soler, A., Núñez, C., Stephan-Otto, C., Pastor-Mira, M. A., et al. (2019). Clustering a large Spanish sample of patients with fibromyalgia using the fibromyalgia impact questionnaire-revised: Differences in clinical outcomes, economic costs, inflammatory markers, and gray matter volumes. Pain 160, 908–921. doi: 10.1097/j.pain.0000000000001468
Pontari, M. A. (2013). Etiology of chronic prostatitis/chronic pelvic pain syndrome: Psychoimmunoneurendocrine dysfunction (PINE syndrome) or just a really bad infection? World J. Urol. 31, 725–732. doi: 10.1007/s00345-013-1061-z
Power, J. D., Cohen, A. L., Nelson, S. M., Wig, G. S., Barnes, K. A., Church, J. A., et al. (2011). Functional network organization of the human brain. Neuron 72, 665–678. doi: 10.1016/j.neuron.2011.09.006
Schmidt-Wilcke, T., Luerding, R., Weigand, T., Jürgens, T., Schuierer, G., Leinisch, E., et al. (2007). Striatal grey matter increase in patients suffering from fibromyalgia–A voxel-based morphometry study. Pain 132(Suppl. 1) S109–S116. doi: 10.1016/j.pain.2007.05.010
Schweinhardt, P., Kuchinad, A., Pukall, C. F., and Bushnell, M. C. (2008). Increased gray matter density in young women with chronic vulvar pain. Pain 140, 411–419. doi: 10.1016/j.pain.2008.09.014
Shackman, A. J., Salomons, T. V., Slagter, H. A., Fox, A. S., Winter, J. J., and Davidson, R. J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 12, 154–167. doi: 10.1038/nrn2994
Spisak, T., Kincses, B., Schlitt, F., Zunhammer, M., Schmidt-Wilcke, T., Kincses, Z. T., et al. (2020). Pain-free resting-state functional brain connectivity predicts individual pain sensitivity. Nat. Commun. 11:187. doi: 10.1038/s41467-019-13785-z
Tagliaferri, S. D., Fitzgibbon, B. M., Owen, P. J., Miller, C. T., Bowe, S. J., and Belavy, D. L. (2021). Brain structure, psychosocial, and physical health in acute and chronic back pain: A UK BioBank study. Pain 163, 1277–1290. doi: 10.1097/j.pain.0000000000002524
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. doi: 10.1006/nimg.2001.0978
van Ettinger-Veenstra, H., Lundberg, P., Alföldi, P., Södermark, M., Graven-Nielsen, T., Sjörs, A., et al. (2019). Chronic widespread pain patients show disrupted cortical connectivity in default mode and salience networks, modulated by pain sensitivity. J. Pain Res. 12, 1743–1755. doi: 10.2147/jpr.S189443
Vogt, B. A. (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 6, 533–544. doi: 10.1038/nrn1704
Whitfield-Gabrieli, S., and Nieto-Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141. doi: 10.1089/brain.2012.0073
Wiech, K., Ploner, M., and Tracey, I. (2008). Neurocognitive aspects of pain perception. Trends Cogn. Sci. 12, 306–313. doi: 10.1016/j.tics.2008.05.005
Woodworth, D., Mayer, E., Leu, K., Ashe-McNalley, C., Naliboff, B. D., Labus, J. S., et al. (2015). Unique microstructural changes in the brain associated with Urological Chronic Pelvic Pain Syndrome (UCPPS) revealed by diffusion tensor MRI, super-resolution track density imaging, and statistical parameter mapping: A MAPP network neuroimaging study. PLoS One 10:e0140250. doi: 10.1371/journal.pone.0140250
Xu, X., Wei, X., Wang, F., Liu, J., Chen, H., Xiong, Y., et al. (2015). Validation of a simplified Chinese version of the pain catastrophizing scale and an exploration of the factors predicting catastrophizing in pain clinic patients. Pain Physician 18, E1059–E1072.
Yang, H., Yang, X., Liu, H., Long, H., Hu, H., Wang, Q., et al. (2021). Placebo modulation in orthodontic pain: A single-blind functional magnetic resonance study. Radiol. Med. 126, 1356–1365. doi: 10.1007/s11547-021-01374-4
Yang, Q., Xu, H., Zhang, M., Wang, Y., and Li, D. (2020). Volumetric and functional connectivity alterations in patients with chronic cervical spondylotic pain. Neuroradiology 62, 995–1001. doi: 10.1007/s00234-020-02413-z
Yu, S. W., Lin, S. H., Tsai, C. C., Chaudhuri, K. R., Huang, Y. C., Chen, Y. S., et al. (2019). Acupuncture effect and mechanism for treating pain in patients with Parkinson’s disease. Front. Neurol. 10:1114. doi: 10.3389/fneur.2019.01114
Zhang, Y., Zhu, Y., Pei, Y., Zhao, Y., Zhou, F., Huang, M., et al. (2019). Disrupted interhemispheric functional coordination in patients with chronic low back-related leg pain: A multiscale frequency-related homotopic connectivity study. J. Pain Res. 12, 2615–2626. doi: 10.2147/jpr.S213526
Zhang, Y. N., Huo, J. W., Huang, Y. R., Hao, Y., and Chen, Z. Y. (2019). Altered amplitude of low-frequency fluctuation and regional cerebral blood flow in females with primary dysmenorrhea: A resting-state fMRI and arterial spin labeling study. J. Pain Res. 12, 1243–1250. doi: 10.2147/jpr.S177502
Zhang, B., Jung, M., Tu, Y., Gollub, R., Lang, C., Ortiz, A., et al. (2019). Identifying brain regions associated with the neuropathology of chronic low back pain: A resting-state amplitude of low-frequency fluctuation study. Br. J. Anaesth. 123, e303–e311. doi: 10.1016/j.bja.2019.02.021
Zhao, Z., Huang, T., Tang, C., Ni, K., Pan, X., Yan, C., et al. (2017). Altered resting-state intra- and inter- network functional connectivity in patients with persistent somatoform pain disorder. PLoS One 12:e0176494. doi: 10.1371/journal.pone.0176494
Keywords: basal ganglia, resting-state functional MRI, functional connectivity, machine learning, chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS)
Citation: Lan X, Niu X, Bai W-X, Li H-N, Zhu X-Y, Ma W-J, Li J-L, Dun W-H, Zhang M and He J (2022) The functional connectivity of the basal ganglia subregions changed in mid-aged and young males with chronic prostatitis/chronic pelvic pain syndrome. Front. Hum. Neurosci. 16:1013425. doi: 10.3389/fnhum.2022.1013425
Received: 07 August 2022; Accepted: 13 September 2022;
Published: 30 September 2022.
Edited by:
Lijun Bai, Xi’an Jiaotong University, ChinaReviewed by:
Lei Gao, Zhongnan Hospital of Wuhan University, ChinaBochao Cheng, Sichuan University, China
Copyright © 2022 Lan, Niu, Bai, Li, Zhu, Ma, Li, Dun, Zhang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Zhang, zhangming01@xjtu.edu.cn; Juan He, hejuan198553@126.com
†These authors have contributed equally to this work and share the first authorship
 Xi Lan
Xi Lan Xuan Niu2†
Xuan Niu2†  Jian-Long Li
Jian-Long Li Wang-Huan Dun
Wang-Huan Dun Ming Zhang
Ming Zhang