- 1Division of Neurology, Children’s National Hospital, Washington, DC, United States
- 2Department of Neurology, George Washington University School of Medicine and Health Sciences, Washington, DC, United States
- 3Division of Neurogenetics and Neurodevelopmental Pediatrics, Children’s National Hospital, Washington DC, United States
Refractory movement disorders are a common feature of inborn errors of metabolism (IEMs), significantly impacting quality of life and potentially leading to life-threatening complications such as status dystonicus. Surgical techniques, including deep brain stimulation (DBS) and lesioning techniques, represent an additional treatment option. However, the application and benefits of these procedures in neurometabolic conditions is not well understood. This results in challenges selecting surgical candidates and counseling patients preoperatively. In this review, we explore the literature of surgical techniques for the treatment of movement disorders in IEMs. Globus pallidus internus DBS has emerged as a beneficial treatment option for dystonia in Panthotate-Kinase-associated Neurodegeneration. Additionally, several patients with Lesch–Nyhan Disease have shown improvement following pallidal stimulation, with more robust effects on self-injurious behavior than dystonia. Although there are numerous reports describing benefits of DBS for movement disorders in other IEMs, the sample sizes have generally been small, limiting meaningful conclusions. Currently, DBS is preferred to lesioning techniques. However, successful use of pallidotomy and thalamotomy in neurometabolic conditions has been reported and may have a role in selected patients. Surgical techniques have also been used successfully in patients with IEMs to treat status dystonicus. Advancing our knowledge of these treatment options could significantly improve the care for patients with neurometabolic conditions.
1. Introduction
Inborn errors of metabolism (IEMs) are a group of genetic disorders caused by deficiencies in a metabolic pathway, leading to the accumulation of toxic substrates or depletion of essential compounds. These conditions are characterized by multisystemic symptoms, with a high prevalence of central nervous system involvement (1, 2). Movement disorders are a common feature of IEMs, with dystonia being a frequently observed manifestation (2, 3). Dystonia is characterized by sustained or intermittent muscle contractions causing abnormal movements, postures, or both. These movements are typically patterned and twisting, and occasionally tremulous (4). Despite the emphasis on early diagnosis and prevention of neurologic injury, many IEMs do not have a specific treatment or patients are not identified until irreversible damage has occurred (3, 5). A significant proportion of these patients experience severe and pharmacologically resistant movement disorders that significantly impair their quality of life (QoL). Status dystonicus, a life-threating complication, can also present in this population (6, 7). As a result, effective treatments for movement disorders associated with IEMs represent a pressing clinical need.
Surgical interventions play a critical role in the management of refractory movement disorders. Techniques such as deep brain stimulation (DBS) and lesioning procedures, including pallidotomy and thalamotomy, have demonstrated considerable clinical benefit (8, 9). Currently, DBS has largely replaced lesioning techniques. Randomized controlled trials have shown DBS to be effective in treating tremor, Parkinson’s disease, and dystonia (10–12). Observational studies have also reported promising results of DBS in patients with tics, myoclonus, and chorea (13–15). Although less frequently than in Parkinson’s disease, DBS has also been used for other synucleopathies (16). Moreover, DBS has shown a positive effect in the treatment of some psychiatric conditions including self-injurious behaviors (SIB) and obsessive–compulsive disorder (OCD) (17, 18). Traditionally, DBS has been indicated based mainly on phenotype. However, with the advancement of genetic testing, it has become evident that genotype can play a significant role in predicting the effect of DBS. Consequently, studies have demonstrated differential effects of DBS in monogenetic dystonias and Parkinson’s disease, highlighting the importance of considering genetic factors in treatment outcomes (19, 20).
Deep brain stimulation presents a potential treatment option for patients with IEMs who commonly present with dystonia, other movement disorders, and comorbid psychiatric conditions. However, several factors have limited its use in this population. First, structural brain lesions, often located in the basal ganglia, are frequently observed in patients with IEMs. The efficacy of DBS for dystonia associated with structural lesions is more variable and less robust compared to so-called primary dystonia, leading to hesitation in patients and providers (19). Second, DBS does not affect the progression of the underlying disease. Therefore, a gradual loss of effect over time is expected due to the progressive nature of many of these conditions, questioning the long-term value of the intervention. Finally, anesthesia and surgery may trigger metabolic crises in susceptible patients, thus increasing the risk of DBS. Despite these limitations, an increased understanding of the indications, safety, and efficacy of surgical therapies for movement disorders in IEMs would be beneficial to improve QoL for this complex patient population.
In this review, we explore the existing literature on surgical interventions for movement disorders in IEMs, with a focus on DBS as the more prevalent technique. However, we also briefly discuss reports of lesioning procedures in this population. A PubMed search was performed using combinations of the following terms: “Deep Brain Stimulation”[Mesh], “Pallidotomy”[Mesh], “Neurosurgical Procedures”[Mesh], Deep Brain Stim*, Pallidotomy, Thalamotomy, “Metabolism, Inborn Errors”[Mesh], Neurometabol*, Inborn Errors of Metabolism, Neurodegeneration with brain iron accumulation, Lesch–Nyhan Disease, Mitochondrial, Pantothenate kinase-associated neurodegeneration, Wilson Disease. The references of selected articles were reviewed to identify additional relevant studies.
2. Neurodegeneration with brain iron accumulation
Neurodegeneration with Brain Iron Accumulation (NBIA) is a heterogenous group of inherited disorders characterized by anomalous iron deposition in the basal ganglia (21). The most prevalent form of NBIA is pantothenate kinase-associated neurodegeneration (PKAN), which results from biallelic variants in the PANK2 gene, encoding a crucial enzyme for coenzyme A synthesis (22). PKAN manifests as progressive dystonia, spasticity, and cognitive decline. It can be divided into classic PKAN, with onset in childhood and rapid progression, and atypical PKAN, with onset in adolescence or adulthood and a more gradual course. The characteristic eye-of-the-tiger sign is frequently observed on axial T2-weighted brain MRI scans (22). Two clinical trials of disease-specific therapies did not demonstrate significant clinical benefit relative to placebo (23, 24). Thus, current management focuses primarily on symptomatic treatment (25).
Globus pallidus internus (GPi) DBS has emerged as a promising therapeutic option for dystonia in PKAN (26, 27). A recent meta-analysis encompassing 99 patients reported an average 26% improvement in dystonia severity, as measured by the Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS) motor component (BFMDRS-M), at 12 months post-operatively (26). Notably, 30% of patients experienced a decrease in dystonia severity exceeding 50%, whereas 30% experienced an improvement of less than 10% (26–28). Patients with atypical PKAN tend to have a more favorable response to DBS than those with typical PKAN (26). Despite an observed dystonia progression 2–3 years post-surgery, many patients followed for up to 5 years still exhibited lower BFMDRS-M scores relative to pre-operative assessments (29–33). DBS has also been efficacious in treating pharmacologic-resistant status dystonicus in emergency scenarios (6, 34, 35).
The impact of DBS on the disability component of the BFMDRS (BFMDRS-D) is more limited, with no significant mean benefit in typical PKAN and a moderate effect in atypical PKAN (26). However, the BFMDRS-D has limitations in capturing critical aspects that adversely affect QoL, such as pain. Studies evaluating QoL have reported improvements of up to 80% 1 year post-surgery, with the greatest impact on pain and mobility (27, 36). Interestingly, improvements in cognitive tests after surgery have also been reported. This observation suggests that the cognitive decline observed in PKAN patients may be due in part to the challenges in accessing and completing cognitive tests caused by the severity of dystonia (33, 37, 38).
Alternative DBS targets may present a promising option for dystonia treatment in PKAN, particularly considering the frequent occurrence of pallidal iron accumulation (39). Although limited by a small sample size, reports have shown subthalamic nucleus (STN) DBS to result in BFMDRS-M improvements ranging from 46 to 87% at the 12-month postoperative mark (40–42). The posterior ventrolateral thalamus has also been infrequently utilized to treat PKAN dystonia (43). Outside PKAN, single cases of successful use of DBS for dystonia in mitochondrial membrane protein-associated degeneration, PLA2G6-associated neurodegeneration, and Woodhouse-Sakati syndrome have been reported (44–46). Furthermore, DBS has also been used to address severe OCD, bradykinesia, and tremor in NBIA patients with negative genetic testing (47, 48).
3. Lesch–Nyhan disease
Lesch–Nyhan disease (LND) is caused by loss of the purine salvage enzyme hypoxanthine-guanine phosphoribosyltransferase (HPRT), leading to hyperuricemia, dystonia, spasticity, cognitive impairment, and SIB. The efficacy of GPi-DBS in LND has been studied in 12 patients with a follow-up period ranging from 6 months to 12 years, with a median of 2 years and 5 months (49–57). In 9 of these patients pre- and post-operative BFMDRS-M scores were available, with a mean improvement of 20%. One patient improved by > 50% and 4 patients showed less than 10% improvement (51, 56, 58). The remaining 3 patients without BFMDRS reported a significant subjective improvement of dystonia (50, 52). Remarkably, all patients experienced an improvement in SIB, with complete resolution in some cases (55, 56, 58). Adverse events were reported in half the patients (57).
The therapeutic effect of DBS on SIB is influenced by the neuroanatomical location of stimulation. One patient with bilateral GPi-DBS that suffered a unilateral lead fracture had a recurrence of SIB limited to the contralateral side of the body. In another patient with bilateral anterior and posterior GPi electrodes, it was noted that SIB, but not dystonia, recurred when only the anterior stimulation was turned off (53, 54). Despite the posterior GPi being the primary target for dystonia, the anterior GPi, which has stronger limbic connections, may play a more critical role in managing SIB. Consequently, dual GPi stimulation has been suggested to be more effective for managing both dystonia and SIB. However, due to the lack of empirical evidence showing advantages over single GPi stimulation and the increased risk of adverse events associated with additional leads, it has not been widely adopted (51, 52, 54, 57).
While the efficacy of DBS in LND seems promising, there are indications that publication bias may be inflating the benefits. A survey of caregivers of 14 LND patients that received DBS, recruited from different patient groups, revealed more modest benefits (58). The mean time since surgery was 5.5 years and 6 patients had discontinued DBS. The responses leaned slightly towards improvement in abnormal movement, self-injurious behaviors, oppositional behavior, apathy, and agitation, but with wide variability (58). Thirteen patients reported adverse effects from DBS (58). Additionally, a recent large cohort of French and Italian HPRT-deficient patients briefly mentioned four patients with disappointing DBS results, but no specific information on surgery or outcomes was provided (59). Due to the limited information, these patients are not generally included in systematic reviews (57, 58).
4. Mitochondrial diseases
Mitochondrial diseases are a group of conditions that are genetically and clinically diverse, often resulting in complex movement disorders that are difficult to treat. Bilateral GPi-DBS has been reported as an effective management strategy for dystonia in patients with variants in SLCA25A42, TWNK, TIMM8A, and multiple mitochondrial DNA deletions (60–63). When reported, improvements in the BFMDRS-M ranged from 36 to 64%, with a follow-up of 1–2 years. Two cases with TWNK mutation also noted mild improvement of parkinsonian symptoms, one of which also had bilateral STN-DBS (61). It is worth noting that none of the patients treated with GPi-DBS had pallidal lesions on brain imaging. Additionally, DBS with a thalamic target has been employed to manage severe postural-kinetic tremor in a patient with biopsy-confirmed mitochondrial disease after multiple stroke-like episodes (64).
Despite reports showing benefits from DBS, there have also been cases with disappointing outcomes. For instance, in one patient with biopsy-confirmed complex I deficiency presenting with dystonia and myoclonus, an initial reduction in myoclonus was observed, but symptoms recurred by 12 months after bilateral GPi-DBS. Only minimal effect on dystonia was noted (65). Another patient with multiple mitochondrial deletions presenting with rapidly progressive dystonia-parkinsonism also had only a brief and temporary response after bilateral GPi-DBS. Additional nucleus ventralis oralis anterior stimulation was attempted but also failed to have a sustained response (66).
5. Wilson disease
Wilson Disease (WD) is a recessive genetic disorder caused by pathogenic variants of ATP7B that leads to copper accumulation, resulting in hepatic, neurologic, and psychiatric problems when left untreated. Movement disorders, such as tremor, dystonia, chorea, and parkinsonism, are common among patients (67). Treatment is focused on timely diagnosis and initiation of chelation therapy to prevent permanent neurologic and hepatic injury. Unfortunately, persistent refractory movement disorders are not uncommon among patients with WD (67, 68).
Reports of DBS in WD are scarce. GPi-DBS for dystonia has shown limited benefits in a few patients, with improvements of up to 14% in the BFMDRS-M, while several patients had no response (45, 69–71). Despite these disappointing results, one report showed a much higher impact of DBS in improving caregiver burden, reflecting the limitations of the BFMDRS (69). DBS have also been used for treating tremor in WD by targeting the ventral intermediate thalamic nucleus (Vim). Reports of Vim-DBS with objective outcomes are rare and most evidence has been extrapolated from other secondary tremor literature (68). One patient who had both tremor and dystonia experienced a reduction in the Fahn-Tolosa-Marin tremor score of 97% and in the BFMDRS-M of 65% 2 years after bilateral posterior subthalamic area stimulation (PSA) (72). The PSA comprises the caudal zona incerta and the prelemniscal radiation, both of which have been found to be effective targets for tremor and bradykinesia control. Stimulation of the prelemniscal radiation has also shown promise in treating dystonia. Considering the high incidence of dystonia and tremor and associated lenticular injury in WD, targeting the PSA is an intriguing option for this patient population (72–74).
6. Glutaric aciduria type I
Glutaric aciduria type I (GA1) is caused by biallelic mutations of glutaryl CoA dehydrogenase. Most patients are asymptomatic or present with unspecific symptoms like hypotonia, delayed motor development, and macrocephaly early in life. If left untreated, 80–90% of infants develop irreversible striatal damage with severe generalized dystonia following an acute encephalopathic crisis (75). Treatment is focused on prevention of irreversible injury with metabolic interventions. Due to the crucial role of timely intervention before metabolic decompensation, GA1 is included in the newborn screening programs of several countries (3). GPi-DBS has been reported in a handful of patients, sometimes as a palliative measure (28, 49, 76–79). Some patients showed a modest improvement with reductions of up to 18% in the BFMDRS-M (49, 76, 77). However, a similar number of patients have failed to respond to DBS (28, 78). One report suggests that improvement in pain may be higher than in dystonia scales, but pain scales are rarely reported (80).
7. Other neurometabolic conditions
DBS has been used rarely as a treatment option in several other IEMs, with dystonia being the most common indication. However, there have also been reports of successful management of other movement disorders such as parkinsonism (7, 45, 70, 81–99). Table 1 provides a summary of these cases. The GBA gene deserves additional discussion. Biallelic pathogenic variants of GBA cause Gaucher’s disease, a lysosomal storage disorder. Traditionally, Gaucher’s disease has been classified based on the presence (type 2 and 3) or absence (type 1) of neurologic symptoms. However, Parkinson’s disease has been reported more frequently in patients with type 1 Gaucher’s disease (100). Reported cases of DBS this population are included in Table 1. Additionally, Parkinson’s disease patients who carry a single GBA variant present with earlier onset of symptoms and a more aggressive disease course. Certain variants of the GBA gene carry a higher risk in this context (101). While STN-DBS has been associated with good motor outcomes in this population, it has been suggested that it may have negative effects on cognitive outcomes. It remains unclear whether this observation is attributed to the DBS treatment itself, the GBA carrier status, or a combination of both factors. For these reasons, GPi-DBS has been proposed as a potentially cognitively safer option, although the evidence supporting this target for the GBA-associated Parkinson’s disease population is still limited (101).
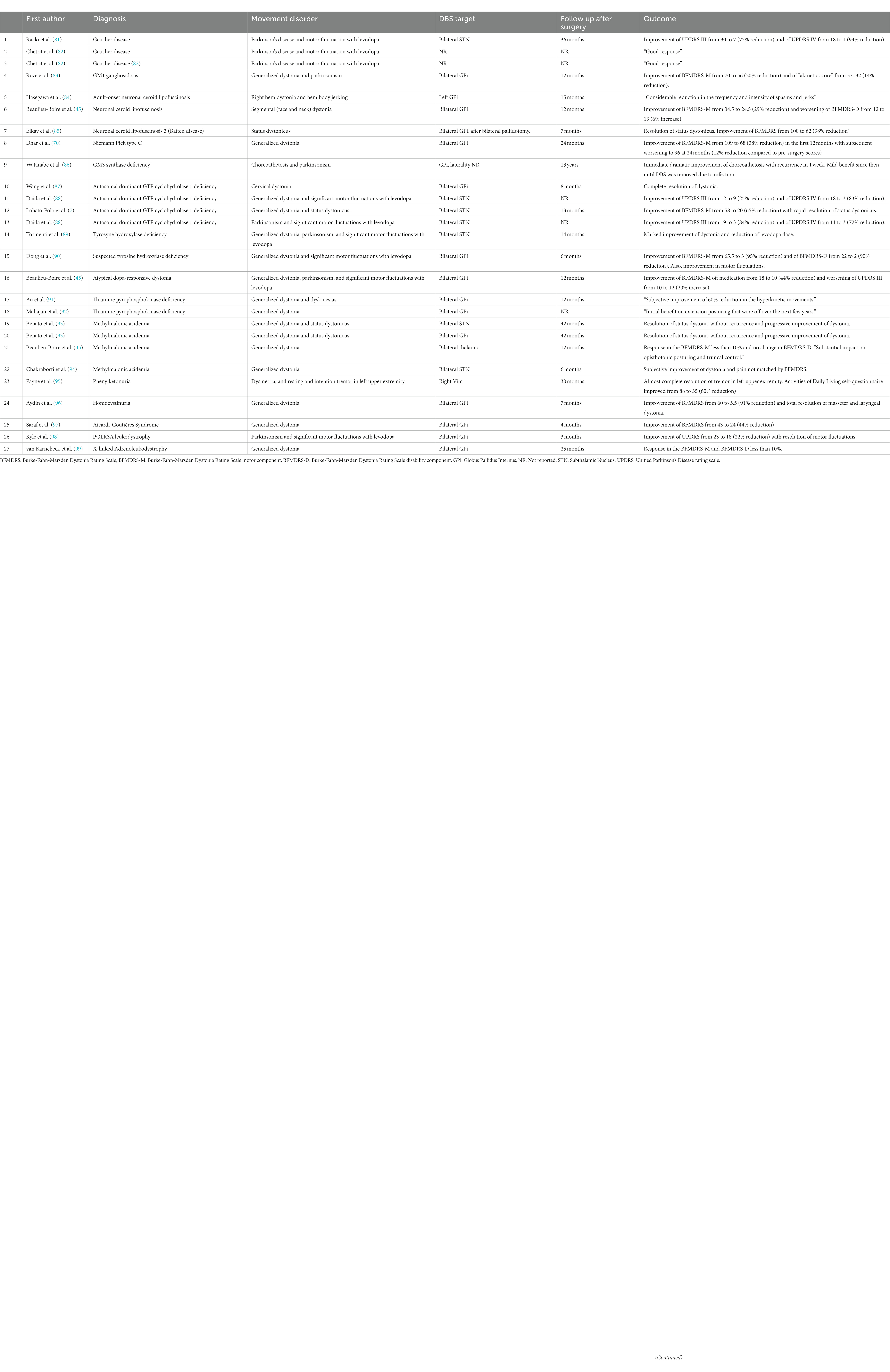
Table 1. Summary of reported patients with rare neurometabolic conditions treated with deep brain stimulation.
8. Lesioning techniques
Clinical trials in patients with essential tremor and Parkinson’s disease have shown the benefits of thalamotomy (102, 103). A recent systematic review also found benefits of pallidotomy for dystonia (104). In recent years, there has been a resurgence of interest in lesioning techniques, in part due to the development of new technologies such as magnetic resonance–guided high-intensity focused ultrasound (105). While DBS offers advantages such as reversibility, adjustability of stimulation parameters, and a better safety profile for bilateral procedures, it also comes with the potential risks of infections, skin erosion, and hardware malfunction (8, 79, 104, 106). Additionally, DBS programming is time-consuming, expensive, and requires frequent clinic visits. DBS devices also require periodic surgical procedures for battery replacement (9, 104).
Certain characteristics of patients with IEMs may favor lesioning techniques over DBS. Device infections, skin erosion, and hardware malfunction are more common in children and patients with dystonia (79, 104). Dystonia treatment often requires higher stimulation, leading to faster depletion of batteries (107). Finally, many of these patients have limited mobility and are already having frequent clinic visits with multiple specialties. DBS may add excessively to the burden on patients and their families. Reports of lesioning techniques in IEMs are rare and with short follow-up periods. In patients with PKAN, thalamotomy has been useful for treating dystonia and tremor, while pallidotomy has been effective for dystonia and status dystonicus (70, 108–113). Several cases of successful treatment of tremor in WD with Vim thalamotomy have also been reported (70, 111, 114). Pallidotomy has rarely been used in patients with Batten Disease and GA1 (80, 85).
9. Discussion
We conducted a literature review to assess the use of surgical treatments for managing movement disorders in patients with IEMs. Not surprisingly, the available evidence is primarily limited to single cases or case series of selected patients. In most cases, studies have focused on the use of pallidal stimulation for treating dystonia. Overall, GPi-DBS appears to provide benefit for dystonia in PKAN, with sustained benefit several years after the procedure (26, 29). GPi-DBS for LND is also promising, with more notable effects observed in SIB than in dystonia (58). Although there are multiple reports of successful DBS use in other IEM and for other movement disorders, the sample sizes have generally been small, precluding meaningful conclusions. Notably, other surgical interventions, like baclofen pumps, were beyond the scope of this review but may be beneficial in specific patients. Similarly, novel surgical interventions, like neural bypasses, represent intriguing future approaches for patients with movement disorders (115).
Although prevention of neurological dysfunction and pharmacological treatment of movement disorders remain the primary focus for IEMs, the use of DBS should be considered on an individual basis and after open discussions with families of patients with severe refractory movement disorders. Objective measures of movement disorder severity, such as the BFMDRS, should be obtained pre- and post-operatively. However, the extent of DBS benefit may be missed by purely motor scales. Therefore, it is also important to track patient-specific goals, non-motor symptoms, caregiver burden, and QoL. Surgical management should be strongly considered for refractory status dystonicus, which can be life-threatening, as multiple reports have shown symptom resolution in various patients with IEMs (7, 26, 93).
An increased understanding of the effect of surgical techniques for treatment of movement disorders in IEMs would provide additional guidance for providers and families considering these procedures. Given the rarity and heterogeneity of these conditions, clinical trials are unlikely. However, relying solely on case reports and series of selected patients significantly increases the risk of publication bias and may overestimate the effectiveness of DBS. Consistent with this, the first large case series of consecutive PKAN patients showed a lower effect of GPi-DBS than initial reports, although still significant (27). The literature on DBS in LND also suggests that cases with less favorable outcomes are not being reported (58, 59). Encouraging the publication of negative results and large series of consecutive patients is crucial to increase knowledge in this area. This is likely to require multi-institutional collaboration, as it is improbable that a single center will have more than a handful of these patients. Another limitation is that reported follow-up periods are often short. Due to the progressive nature of many of these conditions, long-term outcome reports are needed. Finally, the exploration of the metabolic effects of DBS may be of unique interest in IEMs. DBS can alter regional glucose uptake and neurotransmitter production. A better understanding of these changes could provide valuable guidance for the use of DBS in IEMs that impair energy production or monoamine synthesis (116).
While most of the literature has concentrated on pallidal stimulation for dystonia, it would be valuable to explore further alternative surgical options. First, given the high frequency of basal ganglia injury, the effect of stimulation of other targets such as the STN is an intriguing question. Second, it is important to examine the effect of DBS on other movement disorders, either presenting alone or in combination with dystonia, as many patients with IEMs present with complex movement disorders. Third, the effect of DBS on comorbid neuropsychiatric symptoms should also be studied. Fourth, assessing the utility of new DBS technologies, such as local field potential recording and directional leads, may provide additional treatment options for this population. Finally, it is important to assess the potential indications of lesioning techniques.
Author contributions
AZ participated in the conception, literature review, and first draft of this manuscript. AG participated in the conception, and review and critique of the manuscript. Both authors have reviewed and approved the final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ferreira, CR, van Karnebeek, CDM, Vockley, J, and Blau, N. A proposed nosology of inborn errors of metabolism. Genet Med. (2019) 21:102–6. doi: 10.1038/s41436-018-0022-8
2. Ortigoza-Escobar, JD. A proposed diagnostic algorithm for inborn errors of metabolism presenting with movements disorders. Front Neurol. (2020) 11:582160. doi: 10.3389/fneur.2020.582160
3. Ebrahimi-Fakhari, D, Van Karnebeek, C, and Munchau, A. Movement disorders in treatable inborn errors of metabolism. Mov Disord. (2019) 34:598–613. doi: 10.1002/mds.27568
4. Albanese, A, Bhatia, K, Bressman, SB, DeLong, MR, Fahn, S, Fung, VSC, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. (2013) 28:863–73. doi: 10.1002/mds.25475
5. Galosi, S, Nardecchia, F, and Leuzzi, V. Treatable inherited movement disorders in children: spotlight on clinical and biochemical features. Mov Disord Clin Pract. (2020) 7:154–66. doi: 10.1002/mdc3.12897
6. Franzini, A, Cordella, R, Rizzi, M, Marras, CE, Messina, G, Zorzi, G, et al. Deep brain stimulation in critical care conditions. J Neural Transm (Vienna). (2014) 121:391–8. doi: 10.1007/s00702-013-1122-x
7. Lobato-Polo, J, Ospina-Delgado, D, Orrego-Gonzalez, E, Gomez-Castro, JF, Orozco, JL, and Enriquez-Marulanda, A. Deep brain stimulation surgery for status Dystonicus: a single-center experience and literature review. World Neurosurg. (2018) 114:e992–e1001. doi: 10.1016/j.wneu.2018.03.129
8. Kluger, BM, Klepitskaya, O, and Okun, MS. Surgical treatment of movement disorders. Neurol Clin. (2009) 27:633–77. doi: 10.1016/j.ncl.2009.04.006
9. Gelineau-Morel, R, Kruer, MC, Garris, JF, Abu Libdeh, A, Barbosa, DAN, Coffman, KA, et al. Deep brain stimulation for pediatric dystonia: a review of the literature and suggested programming algorithm. J Child Neurol. (2022) 37:813–24. doi: 10.1177/08830738221115248
10. Kupsch, A, Benecke, R, Müller, J, Trottenberg, T, Schneider, GH, Poewe, W, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. (2006) 355:1978–90. doi: 10.1056/NEJMoa063618
11. Deuschl, G, Schade-Brittinger, C, Krack, P, Volkmann, J, Schäfer, H, Bötzel, K, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. (2006) 355:896–908. doi: 10.1056/NEJMoa060281
12. Rehncrona, S, Johnels, B, Widner, H, Törnqvist, AL, Hariz, M, and Sydow, O. Long-term efficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Mov Disord. (2003) 18:163–70. doi: 10.1002/mds.10309
13. Martinez-Ramirez, D, Jimenez-Shahed, J, Leckman, JF, Porta, M, Servello, D, Meng, FG, et al. Efficacy and safety of deep brain stimulation in Tourette syndrome: the international Tourette syndrome deep brain stimulation public database and registry. JAMA Neurol. (2018) 75:353–9. doi: 10.1001/jamaneurol.2017.4317
14. Gonzalez, V, Cif, L, Biolsi, B, Garcia-Ptacek, S, Seychelles, A, Sanrey, E, et al. Deep brain stimulation for Huntington’s disease: long-term results of a prospective open-label study. J Neurosurg. (2014) 121:114–22. doi: 10.3171/2014.2.JNS131722
15. Krause, P, Koch, K, Gruber, D, Kupsch, A, Gharabaghi, A, Schneider, GH, et al. Long-term effects of pallidal and thalamic deep brain stimulation in myoclonus dystonia. Eur J Neurol. (2021) 28:1566–73. doi: 10.1111/ene.14737
16. Sriram, S, Root, K, Chacko, K, Patel, A, and Lucke-Wold, B. Surgical Management of Synucleinopathies. Biomedicine. (2022) 10:10. doi: 10.3390/biomedicines10102657
17. Peeters, S, Skoch, J, Holt, H, Mubita, L, Choudhary, EA, Vadivelu, KP, et al. Functional Neuroanatomy of secondary self-injurious behavior. Pediatr Neurosurg. (2018) 53:71–80. doi: 10.1159/000485385
18. Ooms, P, Mantione, M, Figee, M, Schuurman, PR, van den Munckhof, P, and Denys, D. Deep brain stimulation for obsessive-compulsive disorders: long-term analysis of quality of life. J Neurol Neurosurg Psychiatry. (2014) 85:153–8. doi: 10.1136/jnnp-2012-302550
19. Artusi, CA, Dwivedi, A, Romagnolo, A, Bortolani, S, Marsili, L, Imbalzano, G, et al. Differential response to pallidal deep brain stimulation among monogenic dystonias: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. (2020) 91:426–33. doi: 10.1136/jnnp-2019-322169
20. Artusi, CA, Dwivedi, AK, Romagnolo, A, Pal, G, Kauffman, M, Mata, I, et al. Association of subthalamic deep brain stimulation with motor, functional, and pharmacologic outcomes in patients with monogenic Parkinson disease: a systematic review and meta-analysis. JAMA Netw Open. (2019) 2:e187800. doi: 10.1001/jamanetworkopen.2018.7800
21. Lee, JH, Yun, JY, Gregory, A, Hogarth, P, and Hayflick, SJ. Brain MRI pattern recognition in Neurodegeneration with brain Iron accumulation. Front Neurol. (2020) 11:1024. doi: 10.3389/fneur.2020.01024
22. Hayflick, SJ, Kurian, MA, and Hogarth, P. Neurodegeneration with brain iron accumulation. Handb Clin Neurol. (2018) 147:293–305. doi: 10.1016/B978-0-444-63233-3.00019-1
23. Klopstock, T, Tricta, F, Neumayr, L, Karin, I, Zorzi, G, Fradette, C, et al. Safety and efficacy of deferiprone for pantothenate kinase-associated neurodegeneration: a randomised, double-blind, controlled trial and an open-label extension study. Lancet Neurol. (2019) 18:631–42. doi: 10.1016/S1474-4422(19)30142-5
24. Klopstock, T, Videnovic, A, Bischoff, AT, Bonnet, C, Cif, L, Comella, C, et al. Fosmetpantotenate randomized controlled trial in Pantothenate kinase-associated Neurodegeneration. Mov Disord. (2021) 36:1342–52. doi: 10.1002/mds.28392
25. Hogarth, P, Kurian, MA, Gregory, A, Csányi, B, Zagustin, T, Kmiec, T, et al. Consensus clinical management guideline for pantothenate kinase-associated neurodegeneration (PKAN). Mol Genet Metab. (2017) 120:278–87. doi: 10.1016/j.ymgme.2016.11.004
26. De Vloo, P, Lee, DJ, Dallapiazza, RF, Rohani, M, Fasano, A, Munhoz, RP, et al. Deep brain stimulation for pantothenate kinase-associated neurodegeneration: a meta-analysis. Mov Disord. (2019) 34:264–73. doi: 10.1002/mds.27563
27. Timmermann, L, Pauls, KA, Wieland, K, Jech, R, Kurlemann, G, Sharma, N, et al. Dystonia in neurodegeneration with brain iron accumulation: outcome of bilateral pallidal stimulation. Brain. (2010) 133:701–12. doi: 10.1093/brain/awq022
28. Tsering, D, Tochen, L, Lavenstein, B, Reddy, SK, Granader, Y, Keating, RF, et al. Considerations in deep brain stimulation (DBS) for pediatric secondary dystonia. Childs Nerv Syst. (2017) 33:631–7. doi: 10.1007/s00381-017-3361-x
29. Woo, KA, Kim, HJ, Jeon, SH, Park, HR, Park, KW, Lee, SH, et al. Long-term outcomes of deep brain stimulation in Pantothenate kinase-associated Neurodegeneration-related dystonia. J Mov Disord. (2022) 15:241–8. doi: 10.14802/jmd.22002
30. Picillo, M, Pellecchia, MT, Vitale, C, Barone, P, and Amboni, M. Pallidal stimulation in atypical pantothenate kinase-associated neurodegeneration: six-year follow-up. Mov Disord. (2014) 29:276–7. doi: 10.1002/mds.25709
31. Meoni, S, Fraix, V, Castrioto, A, Benabid, AL, Seigneuret, E, Vercueil, L, et al. Pallidal deep brain stimulation for dystonia: a long term study. J Neurol Neurosurg Psychiatry. (2017) 88:960–7. doi: 10.1136/jnnp-2016-315504
32. Krause, M, Fogel, W, Tronnier, V, Pohle, S, Hörtnagel, K, Thyen, U, et al. Long-term benefit to pallidal deep brain stimulation in a case of dystonia secondary to pantothenate kinase-associated neurodegeneration. Mov Disord. (2006) 21:2255–7. doi: 10.1002/mds.21166
33. Adamovicová, M, Jech, R, Urgošík, D, Spacková, N, and Krepelová, A. Pallidal stimulation in siblings with pantothenate kinase-associated neurodegeneration: four-year follow-up. Mov Disord. (2011) 26:184–7. doi: 10.1002/mds.23349
34. Grandas, F, Fernandez-Carballal, C, Guzman-de-Villoria, J, and Ampuero, I. Treatment of a dystonic storm with pallidal stimulation in a patient with PANK2 mutation. Mov Disord. (2011) 26:921–2. doi: 10.1002/mds.23586
35. Tanrıkulu, B, Özen, A, Günal, DI, Türkdoğan, D, Bayraklı, F, Bayri, Y, et al. Deep brain stimulation as treatment for dystonic storm in pantothenate kinase-associated neurodegeneration syndrome: case report of a patient with homozygous C.628 2 T > G mutation of the PANK2 gene. Acta Neurochir. (2015) 157:1513–7. doi: 10.1007/s00701-015-2514-5
36. Svetel, M, Tomić, A, Dragašević, N, Petrović, I, Kresojević, N, Jech, R, et al. Clinical course of patients with pantothenate kinase-associated neurodegeneration (PKAN) before and after DBS surgery. J Neurol. (2019) 266:2962–9. doi: 10.1007/s00415-019-09499-3
37. Mahoney, R, Selway, R, and Lin, JP. Cognitive functioning in children with pantothenate-kinase-associated neurodegeneration undergoing deep brain stimulation. Dev Med Child Neurol. (2011) 53:275–9. doi: 10.1111/j.1469-8749.2010.03815.x
38. Isaac, C, Wright, I, Bhattacharyya, D, Baxter, P, and Rowe, J. Pallidal stimulation for pantothenate kinase-associated neurodegeneration dystonia. Arch Dis Child. (2008) 93:239–40. doi: 10.1136/adc.2007.118968
39. Larsh, T, Wu, SW, Vadivelu, S, Grant, GA, and O’Malley, JA. Deep brain stimulation for pediatric dystonia. Semin Pediatr Neurol. (2021) 38:100896. doi: 10.1016/j.spen.2021.100896
40. Li, H, Li, D, Yang, W, Yan, H, Zhao, Z, and Yang, H. Deep brain stimulation (DBS) with subthalamic nucleus (STN) as target for pediatric patients with PKAN. World Neurosurg. (2022) 163:e317–22. doi: 10.1016/j.wneu.2022.03.130
41. Liu, Z, Liu, Y, Yang, Y, Wang, L, Dou, W, Guo, J, et al. Subthalamic nuclei stimulation in patients with Pantothenate kinase-associated Neurodegeneration (PKAN). Neuromodulation. (2017) 20:484–91. doi: 10.1111/ner.12549
42. Ge, M, Zhang, K, Ma, Y, Meng, FG, Hu, WH, Yang, AC, et al. Bilateral subthalamic nucleus stimulation in the treatment of neurodegeneration with brain iron accumulation type 1. Stereotact Funct Neurosurg. (2011) 89:162–6. doi: 10.1159/000323374
43. Vercueil, L, Pollak, P, Fraix, V, Caputo, E, Moro, E, Benazzouz, A, et al. Deep brain stimulation in the treatment of severe dystonia. J Neurol. (2001) 248:695–700. doi: 10.1007/s004150170116
44. Canaz, H, Karalok, I, Topcular, B, Agaoglu, M, Yapici, Z, and Aydin, S. DBS in pediatric patients: institutional experience. Childs Nerv Syst. (2018) 34:1771–6. doi: 10.1007/s00381-018-3839-1
45. Beaulieu-Boire, I, Aquino, CC, Fasano, A, Poon, YY, Fallis, M, Lang, AE, et al. Deep brain stimulation in rare inherited Dystonias. Brain Stimul. (2016) 9:905–10. doi: 10.1016/j.brs.2016.07.009
46. Cif, L, Kurian, MA, Gonzalez, V, Garcia-Ptacek, S, Roujeau, T, Gelisse, P, et al. Atypical PLA2G6-associated Neurodegeneration: social communication impairment, dystonia and response to deep brain stimulation. Mov Disord Clin Pract. (2014) 1:128–31. doi: 10.1002/mdc3.12030
47. Rowe, J, Khan, A, Romanowski, C, Isaac, C, Khan, S, Mair, R, et al. Clinical experience with Pedunculopontine nucleus stimulation in conditions with Nigrostriatal disconnection. World Neurosurg. (2016) 89:9–18. doi: 10.1016/j.wneu.2015.11.054
48. Senova, S, Mallet, L, Gurruchaga, JM, Rabu, C, Derosin, M, Yelnik, J, et al. Severe obsessive-compulsive disorder secondary to Neurodegeneration with brain Iron accumulation: complete remission after subthalamic nuclei deep brain stimulation. Biol Psychiatry. (2020) 87:e39–41. doi: 10.1016/j.biopsych.2019.07.006
49. Air, EL, Ostrem, JL, Sanger, TD, and Starr, PA. Deep brain stimulation in children: experience and technical pearls. J Neurosurg Pediatr. (2011) 8:566–74. doi: 10.3171/2011.8.PEDS11153
50. Deon, LL, Kalichman, MA, Booth, CL, Slavin, KV, and Gaebler-Spira, DJ. Pallidal deep-brain stimulation associated with complete remission of self-injurious behaviors in a patient with Lesch-Nyhan syndrome: a case report. J Child Neurol. (2012) 27:117–20. doi: 10.1177/0883073811415853
51. Tambirajoo, K, Furlanetti, L, Hasegawa, H, Raslan, A, Gimeno, H, Lin, JP, et al. Deep brain stimulation of the internal Pallidum in Lesch-Nyhan syndrome: clinical outcomes and connectivity analysis. Neuromodulation. (2021) 24:380–91. doi: 10.1111/ner.13217
52. Pralong, E, Pollo, C, Coubes, P, Bloch, J, Roulet, E, Tétreault, MH, et al. Electrophysiological characteristics of limbic and motor globus pallidus internus (GPI) neurons in two cases of Lesch-Nyhan syndrome. Neurophysiol Clin. (2005) 35:168–73. doi: 10.1016/j.neucli.2005.12.004
53. Abel, TJ, Dalm, BD, Grossbach, AJ, Jackson, AW, Thomsen, T, and Greenlee, JD. Lateralized effect of pallidal stimulation on self-mutilation in Lesch-Nyhan disease. J Neurosurg Pediatr. (2014) 14:594–7. doi: 10.3171/2014.8.PEDS1451
54. Cif, L, Biolsi, B, Gavarini, S, Saux, A, Robles, SG, Tancu, C, et al. Antero-ventral internal pallidum stimulation improves behavioral disorders in Lesch-Nyhan disease. Mov Disord. (2007) 22:2126–9. doi: 10.1002/mds.21723
55. Taira, T, Kobayashi, T, and Hori, T. Disappearance of self-mutilating behavior in a patient with lesch-nyhan syndrome after bilateral chronic stimulation of the globus pallidus internus. Case Report J Neurosurg. (2003) 98:414–6. doi: 10.3171/jns.2003.98.2.0414
56. Piedimonte, F, Andreani, JC, Piedimonte, L, Micheli, F, Graff, P, and Bacaro, V. Remarkable clinical improvement with bilateral globus pallidus internus deep brain stimulation in a case of Lesch-Nyhan disease: five-year follow-up. Neuromodulation. (2015) 18:118–22. doi: 10.1111/ner.12261
57. Deng, H, Xiong, BT, Wu, Y, and Wang, W. Deep brain stimulation in Lesch-Nyhan syndrome: a systematic review. Neurosurg Rev. (2023) 46:40. doi: 10.1007/s10143-023-01950-4
58. Visser, JE, Cotton, AC, Schretlen, DJ, Bloch, J, Tedroff, K, Schechtmann, G, et al. Deep brain stimulation in Lesch-Nyhan disease: outcomes from the patient’s perspective. Dev Med Child Neurol. (2021) 63:963–8. doi: 10.1111/dmcn.14852
59. Madeo, A, Di Rocco, M, Brassier, A, Bahi-Buisson, N, De Lonlay, P, and Ceballos-Picot, I. Clinical, biochemical and genetic characteristics of a cohort of 101 French and Italian patients with HPRT deficiency. Mol Genet Metab. (2019) 127:147–57. doi: 10.1016/j.ymgme.2019.06.001
60. Aldosary, M, Baselm, S, Abdulrahim, M, Almass, R, Alsagob, M, AlMasseri, Z, et al. SLC25A42-associated mitochondrial encephalomyopathy: report of additional founder cases and functional characterization of a novel deletion. JIMD Rep. (2021) 60:75–87. doi: 10.1002/jmd2.12218
61. Zhu, GY, Zhang, RL, Chen, YC, Liu, YY, Liu, DF, Wang, SY, et al. Characteristics of globus pallidus internus local field potentials in generalized dystonia patients with TWNK mutation. Clin Neurophysiol. (2020) 131:1453–61. doi: 10.1016/j.clinph.2020.03.023
62. Cif, L, Gonzalez, V, Garcia-Ptacek, S, James, S, Boetto, J, Seychelles, A, et al. Progressive dystonia in Mohr-Tranebjaerg syndrome with cochlear implant and deep brain stimulation. Mov Disord. (2013) 28:737–8. doi: 10.1002/mds.25519
63. Aniello, MS, Martino, D, Petruzzella, V, Eleopra, R, Mancuso, M, Dell’Aglio, R, et al. Bilateral striatal necrosis, dystonia and multiple mitochondrial DNA deletions: case study and effect of deep brain stimulation. Mov Disord. (2008) 23:114–8. doi: 10.1002/mds.21760
64. Kovacs, N, Pal, E, Balas, I, Janszky, J, Nagy, F, and Merkli, H. Neurosurgical treatment of tremor in mitochondrial encephalopathy. Mov Disord. (2006) 21:2227–30. doi: 10.1002/mds.21128
65. Martinez-Ramirez, D, Hack, N, Vasquez, ML, Morita, H, Giugni, JC, Wolf, JM, et al. Deep brain stimulation in a case of mitochondrial disease. Mov Disord Clin Pract. (2016) 3:139–45. doi: 10.1002/mdc3.12241
66. Pelzer, E, Pauls, AK, Binder, E, Brunn, A, Fink, GR, and Timmermann, L. Deep brain stimulation in rapidly progressive Parkinson-dystonia syndrome due to mitochondrial disorder. Parkinsonism Relat Disord. (2012) 18:672–4. doi: 10.1016/j.parkreldis.2011.10.012
67. Bandmann, O, Weiss, KH, and Kaler, SG. Wilson’s disease and other neurological copper disorders. Lancet Neurol. (2015) 14:103–13. doi: 10.1016/S1474-4422(14)70190-5
68. Hedera, P. Treatment of Wilson’s disease motor complications with deep brain stimulation. Ann N Y Acad Sci. (2014) 1315:16–23. doi: 10.1111/nyas.12372
69. Sidiropoulos, C, Hutchison, W, Mestre, T, Moro, E, Prescott, IA, Mizrachi, AV, et al. Bilateral pallidal stimulation for Wilson’s disease. Mov Disord. (2013) 28:1292–5. doi: 10.1002/mds.25446
70. Dhar, D, Holla, VV, Kamble, N, Yadav, R, Srinivas, D, and Pal, PK. Surgical outcomes in rare movement disorders: a report of seventeen patients from India and review of literature. Tremor Other Hyperkinet Mov (NY). (2022) 12:22. doi: 10.5334/tohm.693
71. Manjunath, M, Yadav, R, Dwarakanath, S, Jhunjhunwala, K, Jafar, A, Surathi, P, et al. Experience of pallidal deep brain stimulation in dystonia at a tertiary care Centre in India: an initial experience. Neurol India. (2017) 65:1322–9. doi: 10.4103/0028-3886.217957
72. Low, HL, Alexander, SK, Misbahuddin, A, and Gillett, GT. Posterior subthalamic area deep brain stimulation for treatment of tremor and dystonia in Wilson’s disease. Brain Stimul. (2019) 12:1304–6. doi: 10.1016/j.brs.2019.05.014
73. Plaha, P, Khan, S, and Gill, SS. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry. (2008) 79:504–13. doi: 10.1136/jnnp.2006.112334
74. Baumgartner, AJ, Thompson, JA, Kern, DS, and Ojemann, SG. Novel targets in deep brain stimulation for movement disorders. Neurosurg Rev. (2022) 45:2593–613. doi: 10.1007/s10143-022-01770-y
75. Boy, N, Muhlhausen, C, Maier, EM, Ballhausen, D, Baumgartner, MR, Beblor, S, et al. Recommendations for diagnosing and managing individuals with glutaric aciduria type 1: third revision. J Inherit Metab Dis. (2022) 46:482–519. doi: 10.1002/jimd.12566
76. Lipsman, N, Ellis, M, and Lozano, AM. Current and future indications for deep brain stimulation in pediatric populations. Neurosurg Focus. (2010) 29:E2. doi: 10.3171/2010.5.FOCUS1095
77. Lumsden, DE, Kaminska, M, Gimeno, H, Tustin, K, Baker, L, Perides, S, et al. Proportion of life lived with dystonia inversely correlates with response to pallidal deep brain stimulation in both primary and secondary childhood dystonia. Dev Med Child Neurol. (2013) 55:567–74. doi: 10.1111/dmcn.12117
78. FitzGerald, JJ, Rosendal, F, de Pennington, N, Joint, C, Forrow, B, Fletcher, C, et al. Long-term outcome of deep brain stimulation in generalised dystonia: a series of 60 cases. J Neurol Neurosurg Psychiatry. (2014) 85:1371–6. doi: 10.1136/jnnp-2013-306833
79. Kaminska, M, Perides, S, Lumsden, DE, Nakou, V, Selway, R, Ashkan, K, et al. Complications of deep brain stimulation (DBS) for dystonia in children—the challenges and 10 year experience in a large paediatric cohort. Eur J Paediatr Neurol. (2017) 21:168–75. doi: 10.1016/j.ejpn.2016.07.024
80. Rakocevic, G, Lyons, KE, Wilkinson, SB, Overman, JW, and Pahwa, R. Bilateral pallidotomy for severe dystonia in an 18-month-old child with glutaric aciduria. Stereotact Funct Neurosurg. (2004) 82:80–3. doi: 10.1159/000077405
81. Racki, V, Papic, E, Almahariq, F, Chudy, D, and Vuletic, V. The successful three-year outcome of deep brain stimulation in Gaucher disease type 1 associated Parkinson’s disease: a case report. Mov Disord Clin Pract. (2021) 8:604–6. doi: 10.1002/mdc3.13185
82. Chetrit, EB, Alcalay, RN, Steiner-Birmanns, B, Altarescu, G, Phillips, M, Elstein, D, et al. Phenotype in patients with Gaucher disease and Parkinson disease. Blood Cells Mol Dis. (2013) 50:218–21. doi: 10.1016/j.bcmd.2012.11.011
83. Roze, E, Navarro, S, Cornu, P, Welter, ML, and Vidailhet, M. Deep brain stimulation of the globus pallidus for generalized dystonia in GM1 type 3 gangliosidosis: technical case report. Neurosurgery. (2006) 59:E1340. doi: 10.1227/01.NEU.0000245620.24603.1B
84. Hasegawa, H, Alkufri, F, Munro, N, Zebian, B, Hulse, N, King, A, et al. GPi deep brain stimulation for palliation of hemidystonia and hemibody jerking in a patient with suspected adult onset neuronal ceroid lipofuscinosis. J Neurol Sci. (2016) 362:228–9. doi: 10.1016/j.jns.2016.01.042
85. Elkay, M, Silver, K, Penn, RD, and Dalvi, A. Dystonic storm due to Batten’s disease treated with pallidotomy and deep brain stimulation. Mov Disord. (2009) 24:1048–53. doi: 10.1002/mds.22515
86. Watanabe, S, Lei, M, Nakagawa, E, Takeshita, E, Inamori, KI, Shishido, F, et al. Neurological insights on two siblings with GM3 synthase deficiency due to novel compound heterozygous ST3GAL5 variants. Brain Dev. (2023) 45:270–7. doi: 10.1016/j.braindev.2023.01.002
87. Wang, X, Mei, S, Tian, Z, Wang, L, Hao, G, Zhu, X, et al. Case report: clinical outcome from Pallidal stimulation in a patient with levodopa-resistant Dopa-responsive dystonia. Front Neurol. (2022) 13:921577. doi: 10.3389/fneur.2022.921577
88. Daida, K, Nishioka, K, Shimo, Y, Umemura, A, Yoshino, H, and Hattori, N. Deep brain stimulation shows high efficacy in two patients with GCH1 variants. Parkinsonism Relat Disord. (2019) 65:277–8. doi: 10.1016/j.parkreldis.2019.06.002
89. Tormenti, MJ, Tomycz, ND, Coffman, KA, Kondziolka, D, Crammond, DJ, and Tyler-Kabara, EC. Bilateral subthalamic nucleus deep brain stimulation for dopa-responsive dystonia in a 6-year-old child. J Neurosurg Pediatr. (2011) 7:650–3. doi: 10.3171/2011.3.PEDS10402
90. Dong, W, Luo, B, Qiu, C, Jiang, X, Qu, X, Zhang, L, et al. Deep brain stimulation for the treatment of Dopa-responsive dystonia: a case report and literature review. World Neurosurg. (2020) 136:394–398.e5. doi: 10.1016/j.wneu.2020.01.032
91. Au, LWC, Lee, HHC, Sheng, B, Chan, KY, Yau, EKC, Mak, CM, et al. Movement disorders associated with thiamine pyrophosphokinase deficiency: Intrafamilial variability in the phenotype. Clin Neurol Neurosurg. (2020) 199:106258. doi: 10.1016/j.clineuro.2020.106258
92. Mahajan, A, and Sidiropoulos, C. TPK1 mutation induced childhood onset idiopathic generalized dystonia: report of a rare mutation and effect of deep brain stimulation. J Neurol Sci. (2017) 376:42–3. doi: 10.1016/j.jns.2017.02.063
93. Benato, A, Carecchio, M, Burlina, A, Paoloni, F, Sartori, S, Nosadini, M, et al. Long-term effect of subthalamic and pallidal deep brain stimulation for status dystonicus in children with methylmalonic acidemia and GNAO1 mutation. J Neural Transm (Vienna). (2019) 126:739–57. doi: 10.1007/s00702-019-02010-2
94. Chakraborti, S, Hasegawa, H, Lumsden, DE, Ali, W, Kaminska, M, Lin, JP, et al. Bilateral subthalamic nucleus deep brain stimulation for refractory total body dystonia secondary to metabolic autopallidotomy in a 4-year-old boy with infantile methylmalonic acidemia: case report. J Neurosurg Pediatr. (2013) 12:374–9. doi: 10.3171/2013.7.PEDS1350
95. Payne, MS, Brown, BL, Rao, J, and Payne, BR. Treatment of phenylketonuria-associated tremor with deep brain stimulation: case report. Neurosurgery. (2005) 56:E868. doi: 10.1227/01.NEU.0000156492.99035.EE
96. Aydin, S, Abuzayed, B, Varlibas, F, Apaydin, H, Mengi, M, Kucukyuruk, B, et al. Treatment of homocystinuria-related dystonia with deep brain stimulation: a case report. Stereotact Funct Neurosurg. (2011) 89:210–3. doi: 10.1159/000325703
97. Saraf, U, Chandarana, M, Puthenveedu, DK, Kesavapisharady, K, Krishnan, S, and Kishore, A. Childhood-onset dystonia attributed to Aicardi-Goutieres syndrome and responsive to deep brain stimulation. Mov Disord Clin Pract. (2021) 8:613–5. doi: 10.1002/mdc3.13205
98. Kyle, K, Mason, X, Bordelon, Y, Pouratian, N, and Bronstein, J. Adult onset POLR3A leukodystrophy presenting with parkinsonism treated with pallidal deep brain stimulation. Parkinsonism Relat Disord. (2021) 85:23–5. doi: 10.1016/j.parkreldis.2021.02.018
99. van Karnebeek, C, Horvath, G, Murphy, T, Purtzki, J, Bowden, K, Sirrs, S, et al. Deep brain stimulation and dantrolene for secondary dystonia in x-linked adrenoleukodystrophy. JIMD Rep. (2015) 15:113–6. doi: 10.1007/8904_2014_305
100. Bultron, G, Kacena, K, Pearson, D, Boxer, M, Yang, R, Sathe, S, et al. The risk of Parkinson’s disease in type 1 Gaucher disease. J Inherit Metab Dis. (2010) 33:167–73. doi: 10.1007/s10545-010-9055-0
101. Artusi, CA, and Lopiano, L. Should we offer deep brain stimulation to Parkinson’s disease patients with GBA mutations? Front Neurol. (2023) 14:1158977. doi: 10.3389/fneur.2023.1158977
102. Bond, AE, Shah, BB, Huss, DS, Dallapiazza, RF, Warren, A, Harrison, MB, et al. Safety and efficacy of focused ultrasound Thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease: a randomized clinical trial. JAMA Neurol. (2017) 74:1412–8. doi: 10.1001/jamaneurol.2017.3098
103. Elias, WJ, Lipsman, N, Ondo, WG, Ghanouni, P, Kim, YG, Lee, W, et al. A randomized trial of focused ultrasound Thalamotomy for essential tremor. N Engl J Med. (2016) 375:730–9. doi: 10.1056/NEJMoa1600159
104. Centen, LM, Oterdoom, DLM, Tijssen, MAJ, Lesman-Leegte, I, van Egmond, ME, and van Dijk, JMC. Bilateral Pallidotomy for dystonia: a systematic review. Mov Disord. (2021) 36:547–57. doi: 10.1002/mds.28384
105. Walters, H, and Shah, BB. Focused ultrasound and other Lesioning therapies in movement disorders. Curr Neurol Neurosci Rep. (2019) 19:66. doi: 10.1007/s11910-019-0975-2
106. Hooper, AK, Okun, MS, Foote, KD, Fernandez, HH, Jacobson, C, Zeilman, P, et al. Clinical cases where lesion therapy was chosen over deep brain stimulation. Stereotact Funct Neurosurg. (2008) 86:147–52. doi: 10.1159/000120426
107. Blahak, C, Capelle, HH, Baezner, H, Kinfe, TM, Hennerici, MG, and Krauss, JK. Battery lifetime in pallidal deep brain stimulation for dystonia. Eur J Neurol. (2011) 18:872–5. doi: 10.1111/j.1468-1331.2010.03290.x
108. Balas, I, Kovacs, N, and Hollody, K. Staged bilateral stereotactic pallidothalamotomy for life-threatening dystonia in a child with Hallervorden-Spatz disease. Mov Disord. (2006) 21:82–5. doi: 10.1002/mds.20655
109. Kyriagis, M, Grattan-Smith, P, Scheinberg, A, Teo, C, Nakaji, N, and Waugh, M. Status dystonicus and Hallervorden-Spatz disease: treatment with intrathecal baclofen and pallidotomy. J Paediatr Child Health. (2004) 40:322–5. doi: 10.1111/j.1440-1754.2004.00374.x
110. Justesen, CR, Penn, RD, Kroin, JS, and Egel, RT. Stereotactic pallidotomy in a child with Hallervorden-Spatz disease. Case report. J Neurosurg. (1999) 90:551–4. doi: 10.3171/jns.1999.90.3.0551
111. Dwarakanath, S, Zafar, A, Yadav, R, Arivazhagan, A, Netravathi, M, Sampath, S, et al. Does lesioning surgery have a role in the management of multietiological tremor in the era of deep brain stimulation? Clin Neurol Neurosurg. (2014) 125:131–6. doi: 10.1016/j.clineuro.2014.07.016
112. Keegan, MT, Flick, RP, Matsumoto, JY, Davis, DH, and Lanier, WL. Anesthetic management for two-stage computer-assisted, stereotactic thalamotomy in a child with Hallervorden-Spatz disease. J Neurosurg Anesthesiol. (2000) 12:107–11. doi: 10.1097/00008506-200004000-00006
113. Tsukamoto, H, Inui, K, Taniike, M, Nishimoto, J, Midorikawa, M, Yoshimine, T, et al. A case of Hallervorden-Spatz disease: progressive and intractable dystonia controlled by bilateral thalamotomy. Brain and Development. (1992) 14:269–72. doi: 10.1016/S0387-7604(12)80246-4
114. Pal, PK, Sinha, S, Pillai, S, Taly, AB, and Abraham, RG. Successful treatment of tremor in Wilson’s disease by thalamotomy: a case report. Mov Disord. (2007) 22:2287–90. doi: 10.1002/mds.21750
115. Zuccaroli, I, Lucke-Wold, B, Palla, A, Eremiev, A, Sorrentino, Z, Zakare-Fagbamila, R, et al. Neural bypasses: literature review and future directions in developing artificial neural connections. OBM Neurobiol. (2023) 07:1–24. doi: 10.21926/obm.neurobiol.2301158
Keywords: movement disorder, dystonia, neurometabolic disorder, deep brain stimulation, pallidotomy, thalamotomy
Citation: Zea Vera A and Gropman AL (2023) Surgical treatment of movement disorders in neurometabolic conditions. Front. Neurol. 14:1205339. doi: 10.3389/fneur.2023.1205339
Edited by:
Divyani Garg, Vardhman Mahavir Medical College and Safdarjung Hospital, IndiaReviewed by:
Brandon Peter Lucke-Wold, University of Florida, United StatesLuca Marsili, University of Cincinnati, United States
Copyright © 2023 Zea Vera and Gropman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea L. Gropman, agropman@childrensnational.org
 Alonso Zea Vera
Alonso Zea Vera Andrea L. Gropman
Andrea L. Gropman