- 1NeuroHIV Research Program, School of Medicine, University of Puerto Rico, San Juan, PR, United States
- 2Division of Neurology, Internal Medicine Department and NeuroHIV Research Program, School of Medicine, University of Puerto Rico, San Juan, PR, United States
- 3Department of General Social Sciences, University of Puerto Rico, San Juan, PR, United States
- 4Orthopaedic Surgery and Physical Medicine & Rehabilitation, Johns Hopkins University, Baltimore, MD, United States
- 5Department of Pharmacology and Toxicology, School of Medicine, NeuroHIV Research Program, Pharmacology Department, University of Puerto Rico, San Juan, PR, United States
Background: HIV-associated neurocognitive disorders (HAND) are one of the HIV-associated comorbidities affecting 20–50% of the people with HIV (PWH) infection. We found that the soluble insulin receptor (sIR) levels in plasma and cerebrospinal fluid (CSF) were significantly higher in HIV-infected women. The mechanism of sIR release into the plasma remains unknown, but the detection of the sIR in exosomes may uncover novel mechanisms of sIR secretion from HIV-infected cells and its contribution to HIV disease progression and HAND development. Quantification of sIR in urine may represent a less invasive and more accessible diagnostic tool. Our objective was to quantify sIR levels in plasma, plasma-derived exosomes, and urine, and evaluate their association with HAND and renal function.
Methods: We measured full-length sIR in the plasma and urine of 38 controls and 76 HIV-infected women by ELISA, and sIR, HIV-1 Tat, and reactive oxygen species (ROS) in exosomes by flow cytometry.
Results: Plasma and exosomes with sIR were significantly higher in HIV-infected women when compared with controls and HAND. Exosomal sIR positively correlated with exosomal ROS and exosomal HIV-1 Tat in HIV-infected women. Exosomal ROS was significantly higher in HIV-infected women with more symptomatic cognitive impairment. Plasma-derived exosomes exhibited significantly higher levels of astrocyte (GFAP) and neuronal (L1CAM) markers in HIV-infected women, confirming the presence of circulating CNS-derived exosomes in the blood of HIV-infected women. Urine sIR positively correlated with eGFR in controls, but not in HIV-infected women, regardless there was no significant difference in renal function as determined by the estimated glomerular filtration rate (eGFR, p = 0.762). In HIV-infected women, higher plasma sIR correlated with lower urine sIR that could suggest sIR retention in blood or decreased renal filtration.
Discussion: Higher plasma sIR levels and their correlation with ROS in plasma-derived exosomes with HAND suggest a combined role of metabolic disturbances, oxidative stress, exosome release, and cognitive decline. Communication between CNS and periphery is compromised in PWH, thus plasma-derived exosomes may shed light on disrupted cellular mechanisms in the brain of PWH. High plasma and low urine sIR levels could suggest sIR retention in blood or decreased renal filtration.
Introduction
The introduction of combined antiretroviral therapy (cART) to HIV treatment has improved the life quality and expectancy of people with HIV infection (PWH), transforming the disease into a chronic condition. However, this increased life expectancy combined with the long-term infection and cART, has increased the risk of comorbidities such as cardiovascular diseases, chronic inflammation, changes in body composition and metabolic activity, and insulin resistance (1–4). In the cART era, no clear evidence exists to understand the cellular and molecular basis of these comorbid conditions. Thus, there is a need to identify the factors that contribute to the disruption in insulin signaling and metabolic pathways and determine how these contribute to cognitive impairment in PWH. Furthermore, there are no biomarkers that could be used in routine clinical diagnosis of HAND, and the ones that have been identified show little correlation with the disease (5–11). There has been an urge to identify a biomarker since early diagnosis and interventions may help prevent the progression from mild to more severe forms of HAND or improve HAND (12).
Previously, we demonstrated that HIV-infected women with cognitive impairment have higher levels of soluble insulin receptor (sIR) in plasma and cerebrospinal fluid (CSF), when compared to HIV-negative women, as well as lower levels of insulin receptor substrate 1 (IRS-1) tyrosine phosphorylation in plasma (13–15). Metabolic enzymes are also altered in patients with HAND (16, 17). The mechanisms responsible for the secretion of the full-length sIR to the plasma of HIV-infected women with HAND remain unknown. The exosome-mediated release is one of the mechanisms responsible for the secretion of soluble full-length receptors and viral proteins from infected cells (18–20). These extracellular vesicles are single membrane organelles (~40–100 nm) produced by healthy and virus-infected cells and play different roles in normal and pathophysiological conditions (21, 22). They contain lipids, proteins, and nucleic acids (mRNAs and miRNAs). Some of these exosomal components have been explored as potential biomarkers to monitor cellular disease states. Extracellular vesicles have also been implicated in the processing of proteins associated with neurological diseases (18, 21, 23–25). Once the exosomes are released, they can exert regulatory influences on target cells such as the modulation of host immune responses and microbial pathogenesis (26). Their secretion from the HIV-infected cells may have different cargo and functions than those released from uninfected cells. In fact, HIV-1 infected monocyte-derived macrophages (MDM) release a higher number of exosomes compared to non-infected cells (20). In this study, we quantified sIR in exosomes derived from plasma obtained from HIV-infected women on cART and investigated if sIR levels were associated with the presence and severity of HAND. Since HIV infection can trigger oxidative stress (27), we measured the levels of reactive oxygen species (ROS) inside exosomes as well as the presence of the HIV-1 Tat protein. In addition, we measured astrocyte and neuronal markers in these plasma exosomes to determine their origin.
Although plasma, CSF, and exosomal sIR could serve as a biomarker for the presence and severity of HAND, there is a need for more accessible and less invasive diagnosis methods. Measuring full-length sIR in urine can reduce not only the risk of infection and pain in a blood collection method but also the sampling cost. For these reasons, in this study, we also investigated if sIR was present in the urine of HIV-infected women and their association with HAND and renal function. The quantification of sIR subunits in the urine has been previously explored as a good marker for evaluating diabetes risk (28, 29). It is likely that our findings will provide evidence to investigate other pathophysiological mechanisms involved in the secretion of sIR and viral proteins in these patients. This receptor may contribute to the cognitive decline observed in this population (13–15). The identification of novel mechanisms will serve to design innovative approaches and treatment regimens for the prevention and treatment of insulin resistance and cognitive impairment in HIV-infected patients.
Materials and Methods
Participants and Study Design
This was a cross-sectional study using patient-database information and the sample repository of the Hispanic-Latino Longitudinal Cohort of Women. This study was approved by the University of Puerto Rico Medical Sciences Campus (UPR-MSC) Institutional Review Board (#1330315), and all the participants signed written informed consent. This is a unique cohort of Hispanic HIV-infected women characterized by viral and immune profiles, neurological exams, and neuropsychological tests. A total of eighty-six (N = 86) HIV-infected women without a history of diabetes and thirty-five (N = 35) HIV-negative controls were evaluated, as previously described (13, 30). The HIV-infected women were grouped according to their cognitive performance as having normal cognition (N; n = 44), asymptomatic impairment (ANI; n = 10), and symptomatic impairment (SI, mild neurocognitive disorder [MND, n = 18] plus HIV-associated dementia [HAD, n = 14]; n = 32). The estimated glomerular filtration rate (eGFR) was calculated from serum creatinine (mg/dL) using a validated Chronic Kidney Disease Epidemiology Collaboration equation validated in 2009 by Levey et al. in a population of Caucasian and African–American study participants (31).
Characteristics of HIV-Infected Women
When the HIV-infected women were grouped by cognitive performance, no significant differences were observed between groups regarding age, body mass index (BMI), current CD4 cell count, Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), plasma viral load, blood urea nitrogen (BUN), creatinine, estimated glomerular filtration rate (eGFR), or toxicology (p > 0.05). No proteinuria or albuminuria was observed among the participants. Most HIV-infected women were using antiretroviral therapy (ART) (89.5%, or 77 of the 86 women) with protease inhibitors (51%, or 39 of the 77 women taking ART). There was no significant difference between groups of HAND severity in either use of ART, protease inhibitor, or CNS penetration effect [CPE, (32)] (p > 0.05; Table 1). However, twenty (n = 20) of the HIV-infected patients were coinfected with hepatitis C virus (HCV). HIV viral load was detectable in thirty-two (n = 32) women, which accounts for 37.2% of the HIV-infected subjects in this study.
Blood and Urine Sample Preparation
Fresh blood samples were drawn from each patient and collected in acid citrate dextrose (ACD) tubes. Plasma samples were obtained after centrifuging the blood samples twice at room temperature for 10 min at 355 × g. A plasma aliquot of 700 μl was used for viral load determination, aliquots of 500 μl were used to determine sIR full-length levels, and aliquots of 1 ml were used to determine sIR full-length levels in exosomes. Urine samples (10 ml) were collected from each patient, centrifuged at 2,000 × g for 10 min to remove debris, and analyzed for sIR levels as described below.
Soluble Insulin Receptor Full-Length (sIR-αβ, Intact) Assay
Plasma and urine sIR full-length levels were determined as previously published (13). Briefly, the levels were determined by sandwich ELISA using anti-IR antibodies (Abcam, Cambridge, MA) and a FITC-secondary antibody (Abcam, Cambridge, MA). The samples were analyzed in a Cytofluor 4000 (Applied Biosystems, CA) using 485/530 nm excitation/emission filters.
Determination of sIR, HIV-1 Tat, ROS, GFAP, and L1CAM Levels in Exosomes
Exosomes were isolated from plasma by ultracentrifugation (100,000 x g) and then incubated with aldehyde/sulfate beads 4%w/v (1.4 × 109/ml beads; Life Technologies) overnight at 4°C (24, 33, 34). Exosome-coated beads (4 μg/μl) were incubated with CD63-Alexa 647 and Rab-5b- PE antibodies (Abcam, MA) for 1 h at 4°C (35). Then, the exosomes were permeabilized using the BD Cytofix Cytoperm Kit (BD biosciences, CA) for 20 min at 4°C and incubated with corresponding primary and secondary antibodies. For insulin receptors, exosomes were incubated with anti-IR β-subunit antibody (Abcam, MA), followed by a FITC-conjugated anti-mouse secondary antibody as previously (13). For ROS, exosomes were stained using the Oxidative Stress Detection Reagent (Green Fluorescent) as described by the manufacturer (ENZO Life Sciences, Farmingdale, NY). For HIV-1 Tat protein, exosomes were also permeabilized as described above and labeled using anti-HIV-1 Tat- FITC fluorescent antibody (Abcam, MA). Glial fibrillary acidic protein (GFAP) is an astrocyte marker, which was detected using Recombinant Alexa Fluor® 488 anti-GFAP rabbit monoclonal antibody, EPR1034Y clone (Abcam, MA). L1 cell adhesion molecule protein (L1CAM) is a protein associated with neurons, which was detected using PE-conjugated anti-L1CAM mouse monoclonal antibody, clone 5G3 (Abcam, MA). Anti-GFAP and anti-L1CAM both were used at dilutions of 5 μl per 106 cells/ml suspensions. The samples were analyzed using a FACSAria flow cytometer (BD Biosciences, CA). Two measurements were performed in the exosomes, sIR, HIV-1 Tat, and ROS levels per exosome and the percentage of exosomes with sIR, HIV-1 Tat, and ROS.
Plasma CD163 ELISA
CD163 was measured in plasma samples collected in ACD blood collection tubes using solid-phase sandwich ELISA assay (R&D systems Human CD163 Quantikine ELISA Kit #DC1630) following the manufacturer's instructions. The minimum sample dilution was 1:10, and the maximum was 1:40. Data were reported as mean (ng/ml) and standard deviation.
Flow Cytometry
Flow cytometric analyses were carried out using a FACSCelesta cytometer (BD Biosciences, CA). Fluorescent exosomes labeled with Anti-CD63-Alexa647 (FL4 channel, 661/16 nm), Anti-Rab5b-PE (FL2 channel, 585 nm), and Anti-IRβ-FITC, Anti-Tat-FITC or ROS fluorescent reagent (FL1 channel, 525 nm) were identified first in the FL2 vs. FL4 dot plots, and then the levels of sIR, HIV-1 Tat, and ROS in the exosomes were determined from the median peak channel of the FL1-histograms. The FACSDIVA software (BD Biosciences, CA) was used for data acquisition and multivariate analysis. Cells were gated in forward/side scatter dot plots. Data on scatter parameters and histograms were acquired in log mode. A total of fifty thousand events were evaluated for each sample and the median peak channel obtained from the histograms was used to determine the levels of sIR in exosomes.
Statistical Analyses
Age, CD4 count, viral load, BMI, toxicology, cART treatment, and use of protease inhibitor in cART regimen (yes or no), Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), and CNS penetrance effectiveness (CPE) score variables were compared among the three HIV-infected groups stratified by cognitive impairment as having normal cognition (N), asymptomatic impairment (ANI), or symptomatic impairment (SI), and reported as the median and interquartile range (Table 1). D'Agostino and Pearson omnibus normality test was performed on all variables and the outcomes were measured. Because the clinical data and parameters measured in the samples were not always normally distributed, comparisons were made using the Mann–Whitney tests for two samples and Kruskal–Wallis with Dunn's multiple comparisons test for more than two sample groups. Correlations were performed using Spearman's rank test. All statistical analyses were performed with IBM SPSS Statistics v24.0 and GraphPad Prism v6.0 softwares. Statistical significance was considered at p < 0.05.
Results
Full-Length sIR Levels Were Increased in Plasma of HIV-Infected Women
As previously published (13–15), full-length sIR levels in the plasma of HIV-infected women were significantly increased relative to controls (p < 0.001, Figure 1A), with significant differences among the control group and HIV-infected women stratified by cognitive impairment (C vs. N, p = 0.003, C vs. SI, p = 0.009) (Figure 1B). No differences in plasma sIR were observed among HIV patients.
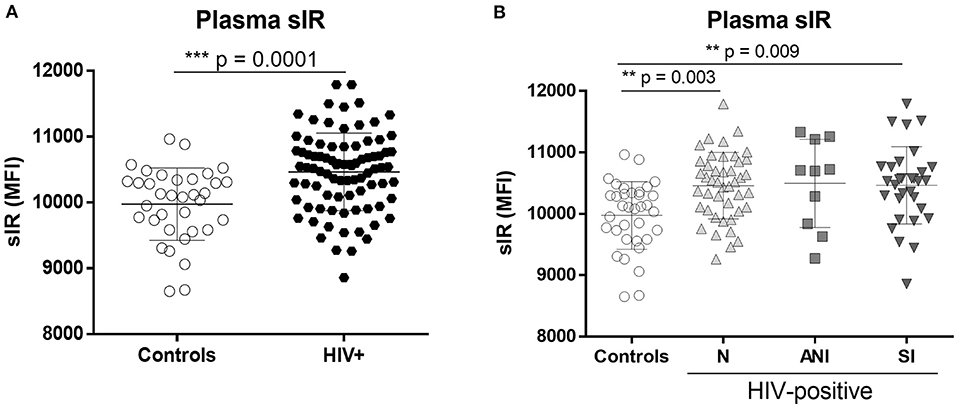
Figure 1. Levels of the soluble insulin receptor in plasma of control and HIV-infected women. sIR was measured in the plasma of HIV-negative (controls n = 35) and HIV-infected (n = 86) women by flow cytometry (A). HIV-infected women were stratified by HAND status as normal cognition (N) (n = 44), asymptomatic cognitive impaired (ANI) (n = 10), and symptomatic impaired (SI; MND and HAD) (n = 32) (B). Graphs show mean and standard deviation [Controls (9,975 ± 549.2), N (10,459 ± 541.6), ANI (10,497 ± 718.7) and SI (10,464 ± 630.8)] results were graphed as mean fluorescence intensity (MFI) units. Analysis was performed using the Unpaired t-test and Kruskal–Wallis test with Dunn's multiple comparisons.
Exosomes With sIR Were Detected in HIV-Infected Women and Associated With HAND
HIV-infected women had significantly higher levels of exosomes with sIR than did controls (p = 0.001) as well as exosomes containing ROS (p = 0.035; Figure 2A). We observed a significant association between exosomes with sIR and HIV-1 infection, where N and SI showed a higher percentage of exosomes with sIR when compared with controls (N vs. C, p = 0.032 and SI vs. C, p = 0.038, Figure 2B). The SI group presented higher levels of ROS containing exosomes than controls (SI vs. C, p = 0.023, Figure 2C), but no significant differences were observed among the HAND groups. No significant differences were observed in the percentage of exosomes containing HIV-1 Tat between the HAND groups (Figure 2D). However, in HIV-infected patients, a higher percentage of HIV-1 Tat+ exosomes correlated with lower levels of nadir CD4+ T cells (Figure 2E, p < 0.001). The levels of ROS and sIR inside exosomes isolated from controls showed no correlation (Figure 2F; p = 0.477). However, in HIV-infected women, the levels of sIR inside exosomes correlated with exosomal ROS (p = 0.019) and HIV-1 Tat levels (p < 0.0001; Figure 2G). When stratified by normal cognition and cognitive impairment, the correlation between sIR and ROS inside exosomes was maintained among normal cognitive HIV-infected women (Figure 2H; p = 0.021) but not in cognitive-impaired women (Figures 2I,J). The infected correlation between sIR and HIV-1 Tat was significant in both normal cognitive (Figure 2H) and cognitive-impaired women (Figures 2I,J; p < 0.0001).
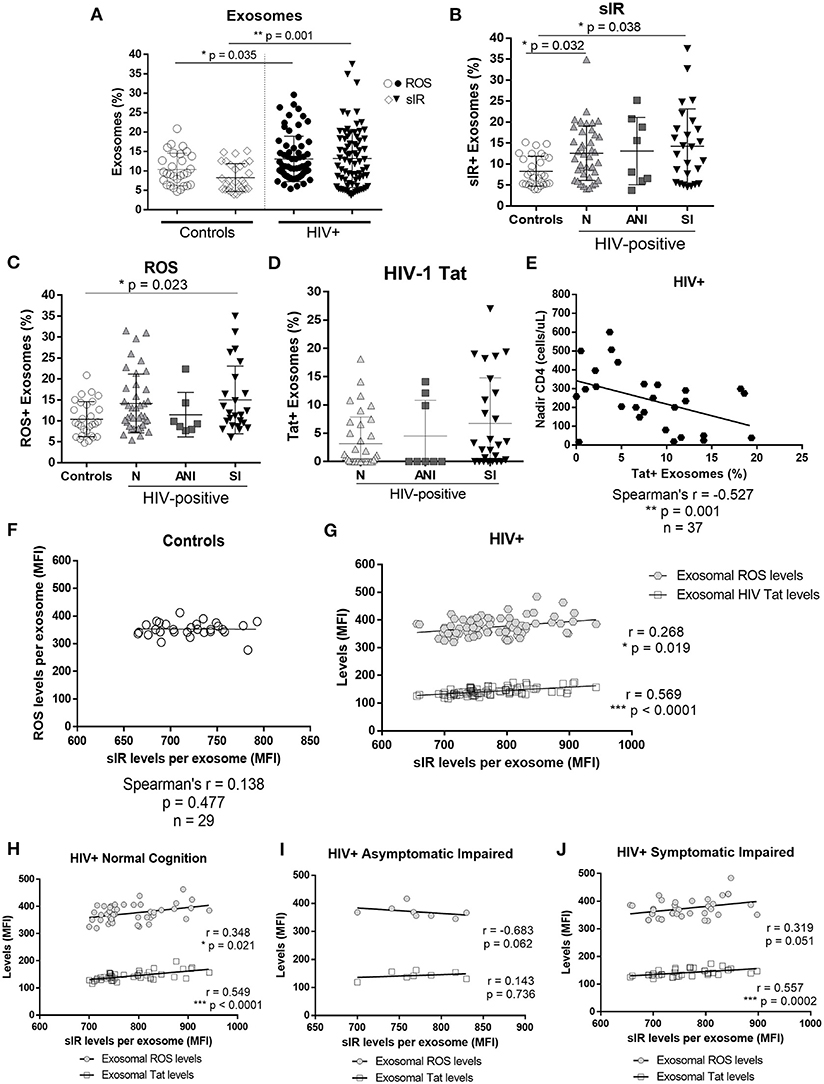
Figure 2. Exosomal sIR, HIV-1 Tat, and ROS in control and HIV-infected women. Levels of sIR+, HIV-1 Tat+, and ROS+ exosomes (exosome %) as well as the levels inside the exosomes were measured in exosomes isolated from the plasma of HIV-negative controls and HIV-infected women by flow cytometry (A). HIV-infected women were stratified by HAND status as normal cognition (N), asymptomatic cognitive-impaired (ANI), and symptomatic impaired (SI; MND and HAD) (B–D). Flow cytometry results were graphed as mean fluorescence intensity (MFI). Graphs show the mean and standard deviation. For percentage of sIR+ exosomes (B): [Controls (8.29 ± 3.58), N (12.59 ± 6.48), ANI (13.11 ± 8.01), and SI (14.25 ± 8.89)]; for ROS+ exosomes (C): [Controls (10.40 ± 4.18), N (14.15 ± 7.00), ANI (11.46 ± 5.31), and SI (15.01 ± 8.08)]; and for HIV-1 Tat+ exosomes (D): [N (3.16 ± 4.74), ANI (4.51 ± 6.33), and SI (6.75 ± 8.06)]. Kruskal–Wallis and Dunn's multiple comparisons were used. Spearman's correlation was also conducted between HIV-1 Tat+ exosomes (%) and nadir CD4+ levels in HIV-infected women in samples where HIV-1 Tat+ exosomes were detectable (E). Spearman's correlations between exosomal levels of sIR, ROS, and HIV-1Tat (HIV-infected samples only) were conducted in controls (F) and HIV-infected exosomes (G). Stratified by cognitive performance, correlations of exosomal sIR with exosomal ROS and HIV-1 Tat were determined in HIV-infected women with normal cognition (H), asymptomatic impairment (I), and symptomatic impairment (J) using a Spearman's correlation.
Plasma sIR Levels and Exosomal sIR Levels Were Not Associated
Since the exosomes were purified from the plasma, we performed Spearman's correlation tests to determine if the levels of sIR in exosomes are associated with levels of sIR in plasma. There was no correlation between plasma and exosomal sIR in controls or HIV-infected women stratified by HAND (p >0.05; Figures 3A–D). HIV-1 Tat and ROS levels inside exosomes did not correlate with plasma sIR in controls nor HIV-infected women. Among the HIV-infected women with normal cognition, the levels of sIR in plasma positively correlated with the levels of HIV-1 Tat (Figure 3B; p = 0.019). This was not observed in those with cognitive impairment (Figures 3C,D).
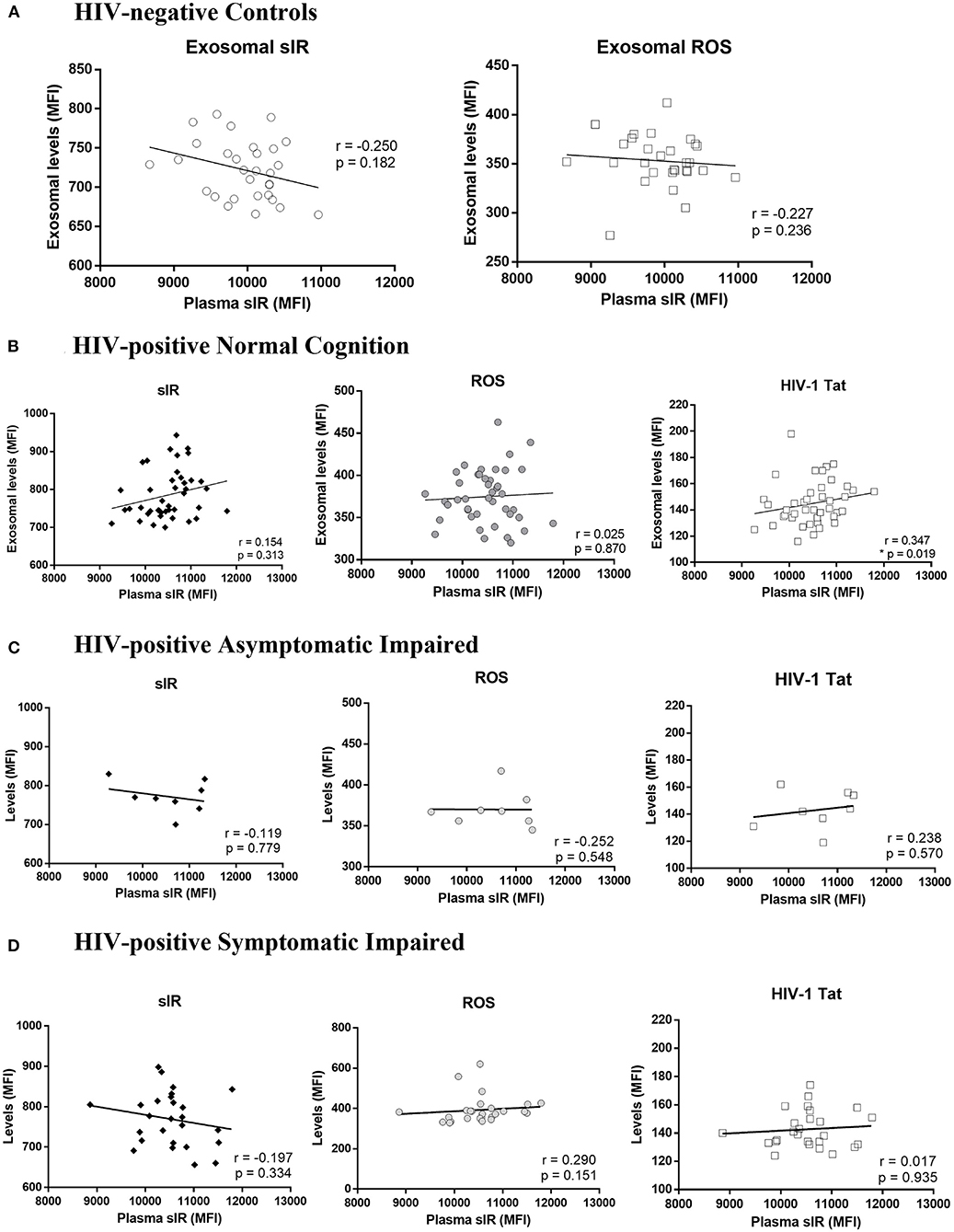
Figure 3. Association between soluble insulin receptor levels in exosomes and plasma. Associations between plasma sIR and exosomal levels of sIR, ROS, and HIV-1 Tat (levels per exosome) were determined by Spearman's correlations tests in HIV-negative controls (A) and HIV-infected women with normal cognition (B), asymptomatic impairment (C), and symptomatic impairment (D).
Astrocyte and Neuron-Derived Exosomes Are Increased in the Plasma of HIV-Infected Patients
Intrigued by the cells of origin of these exosomes isolated from plasma, we measured the levels of GFAP and L1CAM in the purified exosome samples. GFAP is a marker of astrocytes and L1CAM is a marker of neurons. Both GFAP and L1CAM-positive exosomes were significantly increased in the plasma of HIV-infected women when compared with controls (p < 0.001 and p = 0.015, Figures 4A,B, respectively). Among the HIV-infected group, GFAP-infected exosomes are significantly higher in those with normal cognitive infection when compared with those with cognitive impairment (SI, Figure 4C; p = 0.029). No differences were observed in the L1CAM levels between the HAND groups (Figure 4D). No correlation was observed between exosomal GFAP levels and ROS-positive exosomes in controls or HIV-infected women with normal cognition (Figures 4E,F; p = 0.107 and p = 0.118, respectively), or asymptomatic impaired (Figure 4G; p = 0.115). However, a positive correlation was observed between exosomal GFAP levels and ROS-positive exosomes in HIV-infected women with symptomatic impairment (Figure 4H; p = 0.034).

Figure 4. Astrocyte and neuronal markers in plasma-derived exosomes from HIV-infected women. Glial fibrillary acidic protein (GFAP), an astrocyte marker, and L1 cell adhesion molecule protein (L1CAM), a neuronal marker, were measured in plasma-derived exosomes by flow cytometry. Mann–Whitney tests were used to compare GFAP (A) and L1CAM (B) in HIV-negative control subjects and HIV-infected women. There were significant difference in GFAP-infected exosomes (C) levels between N vs. SI groups [N (58.8 ± 15.5), ANI (57.8 ± 4.4), and SI (47.8 ± 18.7)] by Kruskal–Wallis test (statistic 7.276; p = 0.0263) with Dunn's multiple comparisons (N vs. SI p = 0.029). No significant differences were detected in L1CAM-infected exosome levels (D) between the HAND groups by the Kruskal–Wallis test with Dunn's multiple comparisons (statistic 2.408; p = 0.300), [N (7.7 ± 4.9), ANI (6.5 ± 4.1), SI (6.3 ± 5.5)]. Spearman's correlations were used to determine associations between the percentage of ROS+ exosomes and the levels of GFAP immunoreactivity per exosome in HIV-negative control subjects (E) and HIV-infected patients stratified by HAND (F–H).
sIR in the Urine and eGFR Levels of HIV-Infected Women
sIR levels were successfully quantified in the urine of HIV-infected women and controls. There were no differences in urine sIR levels between controls and HIV-infected women (Figure 5A) or when the samples were stratified by HAND (Figure 5B). The estimated glomerular filtration rate (eGFR), an indicator of kidney function, was similar in controls and HIV-infected women (p = 0.115, Figures 5C,D). No differences in urine sIR were observed among HIV-infected women stratified by protease inhibitors use in their cART regimen (p = 0.46, data not shown). A positive correlation between sIR in urine and eGFR was observed in controls (Figure 5E; p = 0.031). However, this correlation is not present in HIV-infected women (Figure 5F; p = 0.944). Urine sIR levels negatively correlate with the ART CPE score in cognitively impaired HIV-infected women (Figure 5H; p = 0.038) but not in normal cognitive HIV-infected women (Figure 5G).
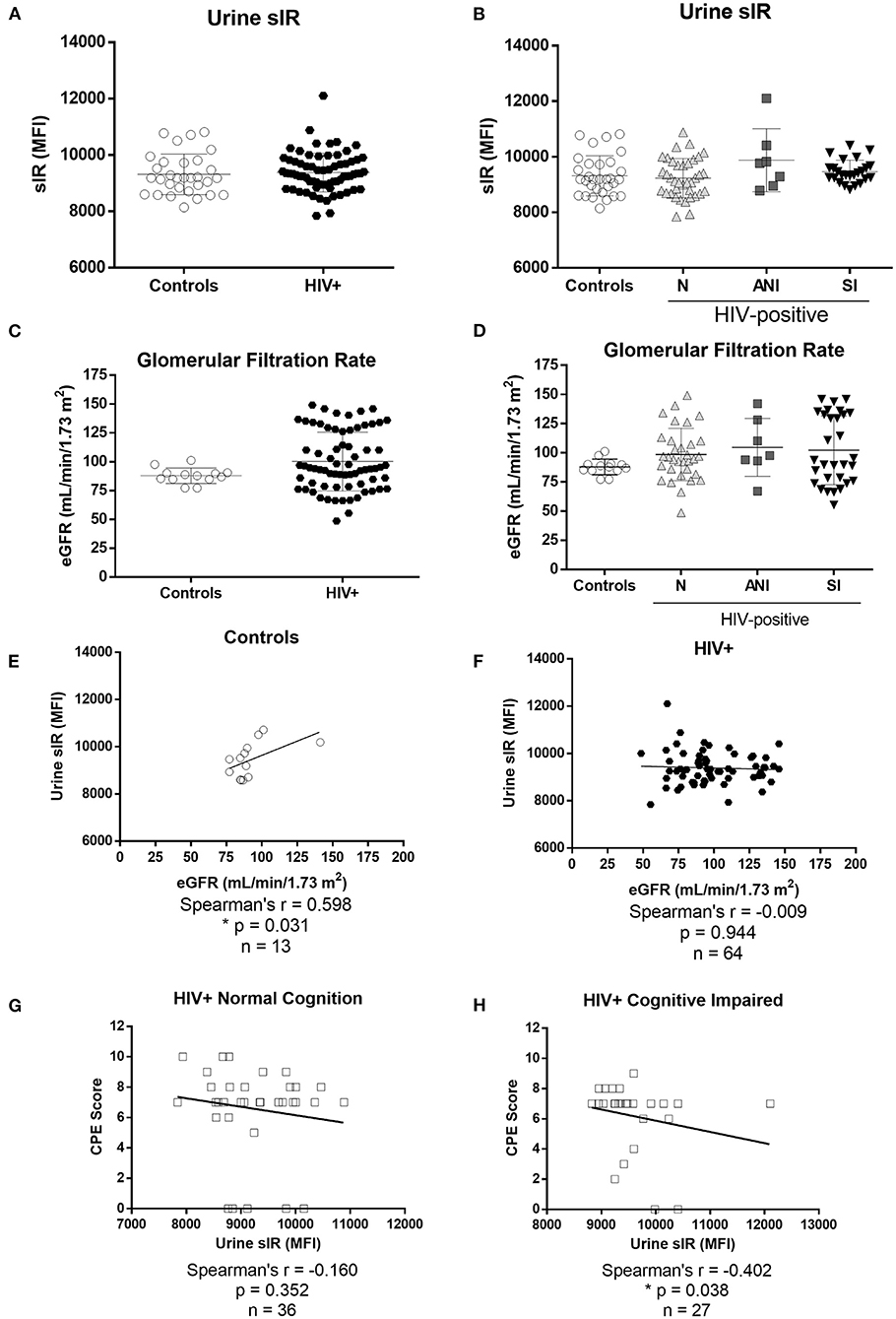
Figure 5. Levels of soluble insulin receptor in urine and estimated glomerular filtration rate of HIV-negative control and HIV-infected women. sIR was measured in the urine of HIV-negative (controls n = 31) and HIV-infected (n = 76) by flow cytometry (A). HIV-infected women were stratified by HAND status as normal cognition (N), asymptomatic cognitive-impaired (ANI), and symptomatic impaired (SI; MND and HAD) (B). eGFR (ml/min/1.73 m2) was measured in HIV-negative controls (n = 13) and HIV-infected women (n = 71) grouped (C) and stratified by cognitive function (D). Flow cytometry results were graphed as mean fluorescence intensity (MFI). Graphs show mean and standard deviation [Controls (9,316 ± 719.8), N (9,230 ± 703.8), ANI (9,876 ± 1131), and SI (9,465 ± 412.2). Spearman's correlations between levels of sIR (MFI) and eGFR were conducted in urine samples from controls (E) and HIV-infected women (F). Spearman's correlations between levels of urine sIR and CPE score were conducted in samples from HIV-infected women without and with cognitive impairment (G,H).
Correlation Between Urine sIR, Plasma sIR, and Exosomal sIR
No correlation was observed between urine and plasma sIR in controls (p = 0.981; Figure 6A). On the contrary, these parameters had a significant negative correlation in HIV-infected women (p = 0.002; Figure 6B).
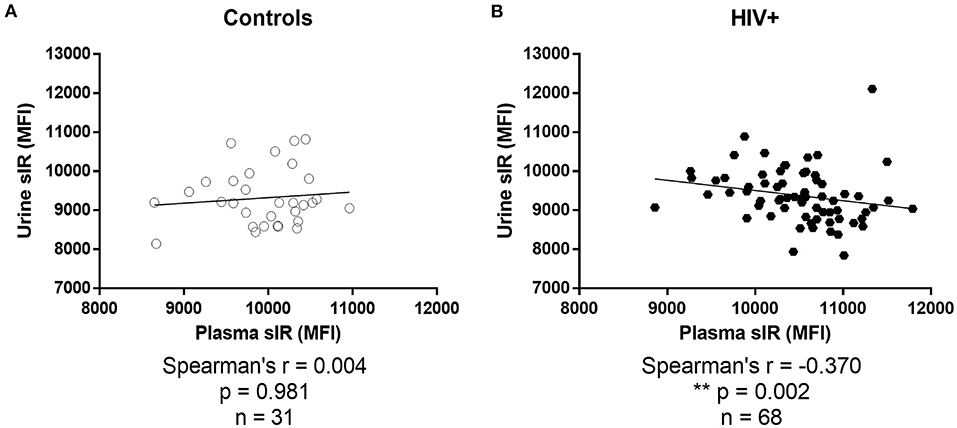
Figure 6. Association between soluble insulin receptor levels in urine and plasma. Associations between sIR levels in plasma and urine were determined by Spearman's correlations tests in controls (A) and HIV-infected women (B).
HCV Coinfection Increased Urine sIR but Not Plasma sIR Levels
A secondary analysis evaluating HIV/HCV coinfected patients was performed to determine the differences in sIR levels. The plasma levels were similar in HIV-infected and HIV/HCV coinfected women (p = 0.419; Supplementary Figure 1A). Urine levels were significantly higher in HIV/HCV coinfection (Supplementary Figure 1B, p = 0.020). sIR, HIV-1 Tat, ROS, and GFAP and L1CAM-positive exosomes were similar in both groups (Supplementary Figures 1C–G). There were no differences when stratified by the HAND groups (data not shown). CD163 was measured as a marker of macrophage activity and inflammation in the plasma of HIV-infected women only. Plasma CD163 did not differ between HIV-infected women stratified by cognitive impairment (p = 0.051; data not shown). However, HIV/HCV coinfected women had higher CD163 levels in plasma (p = 0.002) when compared to HIV-infected women negative for HCV (Supplementary Figure 1H). HIV/HCV coinfected patients performed similarly in neuropsychological tests (Supplementary Figure 2).
HIV-Infected Women With Positive Toxicology Test Results Did Not Alter sIR Findings
Table 1 reports a borderline significantly higher percentage of HIV-infected women having infected toxicology results. The drugs reported as being used were cocaine (n = 7), marijuana (n = 7), or a combination of the two (n = 3) [3 participants did not provide a report of the drugs used].
When we compared plasma sIR levels across groups defined by HIV-infected and infected toxicology status, the levels were significantly increased in HIV-infected women without infected toxicology results [median (IQR): 10,556 (10,040, 10,889), p < 0.05] and with toxicology infected results [10,442 (10,085, 10,721), p < 0.05] compared to controls (all of whom had negative toxicology results). Differences were not seen with urine sIR between these three groups, however, similar elevated levels of ROS per exosome were observed (p < 0.05).
Given that no controls had infected the toxicology results, we did not include an interaction term between toxicology and HAND status. While our sample size was not sufficient to perform a non-parametric regression of plasma sIR as a function of HAND status and toxicology, we did use a log transformation of plasma sIR and conduct a linear regression with these main effects and an interaction term. Controlling for the influence of infected toxicology results, log plasma sIR was significantly elevated in HIV-infected women with symptomatic cognitive impairment.
Discussion
PWH using cART suffer metabolic alterations that could contribute to the development of HAND (1, 2, 36). As previously observed, plasma sIR levels were significantly increased in HIV-infected women when compared with controls, especially those with symptomatic cognitive impairment (Figure 1). HIV-infected women with cognitive impairment have higher levels of sIR in plasma and CSF when compared to HIV-negative women, as well as higher insulin binding to sIR and lower levels of IRS-1 tyrosine phosphorylation in plasma (13, 14). It is not clear how sIR is released from the cells to the blood. One of the possible mechanisms of secretion is exosomes.
There are extensive data on viral proteins contained in exosomes derived from HIV-infected cells and secreted to the plasma (18, 37–41). These exosomes are potentially involved in indirect exosome-mediated neurotoxicity associated with HIV-1 infection (42, 43). Cell-derived receptors can also be secreted to the extracellular compartment via exosome release (44, 45) and their generation is observed during HIV infection (13–15, 46–48). Most of these receptors are dysfunctional and lack a transmembrane domain region (44, 45, 49). Our results show that sIR, ROS, and HIV-1 Tat are secreted from cells via exosomes in HIV-infected women and that HIV infection significantly increases the number of sIR, ROS, and HIV-1 Tat-containing exosomes in the blood of these patients. Moreover, exosomal sIR levels positively correlate with exosomal ROS and HIV-1 Tat levels in HIV-infected patients but not in controls (Figures 2F,G). Exosome levels of sIR, ROS, and HIV-1 Tat are significantly increased in HIV-infected women mainly those with symptomatic cognitive impairment. These findings suggest that metabolic derangements, oxidative stress, and HIV neurotoxins contribute to the presence and severity of HAND. The fact that exosomal sIR, ROS, and HIV-1 Tat levels did not correlate with total sIR levels in plasma, suggests that the exosomal fraction reflects changes that are not observed when analyzing the whole plasma, and may contain different biomarkers associated with HIV infection worth exploring in future studies.
An interesting finding worth discussing is the negative correlation between the percentage of exosomes containing HIV-1 Tat and the nadir CD4 T-cell count, which suggests that T-cell deficiency may predispose other cells to the secretion of HIV-1 Tat in exosomes, which may eventually lead to neuronal injury. Exosomal HIV-1 Tat may also represent a marker of HIV-associated immune dysfunction.
Our study also revealed that these exosomes have astrocyte and neuron markers on their surface, suggesting there is communication between the CNS and periphery, and that exosomes derived from astrocytes and neurons may also serve as blood biomarkers of HIV infection since they were significantly increased in exosomes from HIV-infected women. Astrocyte-derived exosomes significantly correlated with ROS-infected exosomes, pointing to an association between astrocyte activation and oxidative stress (Figure 4H). The fact that approximately 50% of the exosomes isolated from plasma are positive for astrocyte markers in the HIV-infected group confirms that HIV-1 infection activates astrocyte activity. The high levels of GFAP-positive exosomes in the plasma of PWH suggest astrogliosis and/or immune processes ongoing in the CNS despite cART treatment. It has been recently reported that human astrocytes are productively infected in vivo and support the spread of HIV-1 from the brain to the periphery, in a chimeric mouse model of HIV-1, which continued even after cART injection (50). Together, these results suggest that astrocytes play a significant role in neuroHIV despite cART treatment. Brain-derived exosomes have a biomarker potential for all neurodegenerative diseases and may serve as drug delivery vehicles to the CNS as well (51–59). It has been reported that neuronal-derived extracellular vesicles are present in CSF and serum of HIV-1 transgenic rats (60). In PWH, the content cargo of neuronal-derived exosomes is altered, with higher proteins associated with neurotoxicity and neurocognitive impairment, such as neurofilament light chain (NF-L) and amyloid-beta (61). Therefore, we need mechanistic studies to determine the sources, proportions, and cargo of plasma exosomes, and what is their role in HIV disease progression and/or cognitive decline.
The detection and quantification of the sIR ectodomain in urine have been previously evaluated for the early identification of patients at risk of developing diabetes mellitus (28). We were able to measure the sIR full-length levels in the urine of HIV-infected women and controls. The urine sIR levels were similar in HIV-infected women when compared to controls (Figure 5A). A significant positive correlation was observed between sIR in urine and eGFR only in controls (Figure 5E), suggesting that in control patients with normal kidney function, sIR is excreted in the urine.
Kidney function may be affected by age, hypertension, diabetes, medications including ARTs, and infections such as HIV and HCV (62). It is known that HIV can infect and replicate within the renal epithelial cells and dysregulate the epithelial cell function (63). Renal diseases secondary to HIV infection are considered a glomerular-dominant diseases and may present as HIV-associated nephropathy, acute renal failure, and chronic renal failure (63). Risk factors for kidney disease in PWH are older age, black race, higher viral loads, low CD4 cell counts, chronic use of ART, coinfection with HCV, and comorbidities such as diabetes, hypertension, and increased life expectancy—all may be contributing to the renal injury and dysfunction (62, 64–66). However, the interaction between HIV infection and the renal cell is unclarified (62). The kidney has a role in glucose regulation [reviewed in (67)]. Renal IRs are mostly in the proximal and distal convoluted tubules and insulin binding occurs in the glomeruli, renal cortex, and medulla. The kidney regulates the overall glucose homeostasis by the uptake and utilization of glucose and glucogenesis. The kidney function in our cohort was determined by the eGFR, creatine, and BUN levels, and the presence of proteinuria or albuminuria in the urinalysis, all found to be normal, and no significant differences were observed between the HIV-infected and control women suggesting that the renal filtration function is adequate. Also, most of the HIV-infected women were virally suppressed and the CD4 cell amount was higher than 200 cells/mm3, suggesting a low possibility of having proteinuria and renal failure as determined by Szczech et al. (68). Therefore, our finding of urine sIR may not be affected by the HIV infection or ART, although mild dysfunction cannot be completely discarded since specific biomarkers for renal injury were not tested (69, 70). As we know, high sIR levels in the plasma bind significant percentages of free plasma insulin and are associated with HAND status (14). Thus, the removal of the receptor from the circulation and subsequent excretion from the body may be beneficial for cognitive function in PWH. It is known that other peptides are removed from the circulation by glomerular filtration and account for a significant percentage of overall systemic removal from the plasma (71). On the other hand, glomerular hyperfiltration has been associated with insulin resistance in other study cohorts (72). We did not find differences in eGFR between HIV-infected and control women, nor among HAND stratified groups. However, we did find that in the HIV-infected women, the urine sIR levels did not correlate with the eGFR as it did in the control group. We also observed a negative correlation between plasma sIR and urine sIR in HIV-infected women, suggesting that at higher plasma sIR levels, there is a decrease urine secretion of sIR, an observation not present in the control group. The mechanism for removal of the insulin receptor isoforms from blood is still poorly understood. In PWH, possible explanations may include direct or combined effect of HIV infection and cART on kidney function, kidney insulin resistance, or that the IR may be trapped at other organs or tissues, therefore, longitudinal studies may be useful.
We are still not clear on the mechanism by which sIR is generated and then secreted in exosomes to the plasma of PWH and excreted in the urine. However, our findings suggest that HIV-1 infection may trigger the release of sIR to plasma, urine, and exosomes, subtly affecting neurocognitive functions in PWH. Exosome-mediated release of both sIR and HIV-1 Tat may uncover novel mechanisms to explain the pathogenic processes associated with insulin resistance and cognitive impairment in the PWH. The identification of these molecular mechanisms could assist in finding novel targets and pathways that may lead to early diagnosis, identifying risk factors, evaluating treatment effects in clinical trials, and understanding the mechanisms leading to HAND.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Oficina Para la Protección de Participantes Humanos en Investigación (IRB/OPPHI) University of Puerto Rico, Medical Sciences Campus. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
VW and YG were responsible for the conception and design of the study. VW, YC-R, YG, MM, RR, BD, RR-B, and ER contributed to the data acquisition. VW, YC-R, RS, and YG were involved in the analysis and interpretation of data. VW, YC-R, and YG drafted the first version of the article. All authors critically revised the manuscript for important intellectual content. All authors gave final approval of the version to be submitted.
Funding
This work was supported by R01NS099036, R21MH095524, UPR-MSC Center for Collaborative Research in Health Disparities U54MD007600, NYU COMRADE Program R25NS094093, K22NS118975, and The Hispanic Alliance for Clinical and Translational Research (Alliance) U54GM133807.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.809956/full#supplementary-material
Supplementary Figure 1. Comparison of sIR levels measured in study participants segregated as HIV-infected and HIV/HCV coinfected women. Measures from HIV-infected women samples were divided by hepatitis C (HCV) coinfection to compare the levels of sIR in plasma (A), sIR in urine (B), sIR in exosomes (C), HIV-1 Tat in exosomes (D), ROS in exosomes (E), percentage of GFAP+ exosomes (F), percentage of L1CAM+ exosomes (G), and CD163 in plasma (H). Analyses were conducted using the Mann–Whitney test.
Supplementary Figure 2. Global and individual neuropsychological tests z-scores for study participants segregated as HIV-infected and HIV/HCV coinfected women. Z-scores from HIV-infected women were divided by hepatitis C (HCV) coinfection to compare the cognitive function (A) and the eight cognitive domains tested individually (B–I), using an unpaired t-test.
References
1. Valcour V, Rubin LH, Tien P, Anastos K, Young M, Mack W, et al. Human immunodeficiency virus (HIV) modulates the associations between insulin resistance and cognition in the current combination antiretroviral therapy (cART) era: a study of the Women's Interagency HIV Study (WIHS). J Neurovirol. (2015) 21:415–21. doi: 10.1007/s13365-015-0330-6
2. Non LR, Escota G V, Powderly WG. HIV and its relationship to insulin resistance and lipid abnormalities. Transl Res. (2017) 183:41–56. doi: 10.1016/j.trsl.2016.12.007
3. Marcus JL, Leyden WA, Alexeeff SE, Anderson AN, Hechter RC, Hu H, et al. Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000-2016. JAMA Netw open. (2020) 3:e207954. doi: 10.1001/jamanetworkopen.2020.7954
4. Negin J, Martiniuk A, Cumming RG, Naidoo N, Phaswana-Mafuya N, Madurai L, et al. Prevalence of HIV and chronic comorbidities among older adults. AIDS. (2012) 26:S55–63. doi: 10.1097/QAD.0b013e3283558459
5. McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: mind the gap. Ann Neurol. (2010) 67:699–714. doi: 10.1002/ana.22053
6. McArthur JC, HIV. dementia: an evolving disease. J Neuroimmunol. (2004) 157:3–10. doi: 10.1016/j.jneuroim.2004.08.042
7. Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. (2007) 8:33–44. doi: 10.1038/nrn2040
8. Schutzer SE, Berger JR, Brunner M. Identification of potential antibody markers in HIV-associated dementia. J Neuroimmunol. (2004) 157:120–5. doi: 10.1016/j.jneuroim.2004.08.024
9. Wojna V, Skolasky RL, Hechavarría R, Mayo R, Selnes O, McArthur JC, et al. Prevalence of human immunodeficiency virus–associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol. (2006) 12:356–64. doi: 10.1080/13550280600964576
10. McArthur JC, Brew BJ. HIV-associated neurocognitive disorders: is there a hidden epidemic? AIDS. (2010) 24:1367–70. doi: 10.1097/QAD.0b013e3283391d56
11. Price RW, Epstein LG, Becker JT, Cinque P, Gisslen M, Pulliam L, et al. Biomarkers of HIV-1 CNS infection and injury. Neurology. (2007) 69:1781–8. doi: 10.1212/01.wnl.0000278457.55877.eb
12. Grant I, Franklin DR, Deutsch R, Woods SP, Vaida F, Ellis RJ, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. (2014) 82:2055–62. doi: 10.1212/WNL.0000000000000492
13. Gerena Y, Skolasky RL, Velez JM, Toro-Nieves D, Mayo R, Nath A, et al. Soluble and cell-associated insulin receptor dysfunction correlates with severity of HAND in HIV-infected women. PLoS ONE. (2012) 7:1–8. doi: 10.1371/journal.pone.0037358
14. Gerena Y, Menéndez-Delmestre R, Skolasky RL, Hechavarria RM, Pérez S, Hilera C, et al. Soluble insulin receptor as a source of insulin resistance and cognitive impairment in HIV-seropositive women. J Neurovirol. (2015) 21:113–9. doi: 10.1007/s13365-014-0310-2
15. Gerena Y, Menéndez-Delmestre R, Delgado-Nieves A, Vélez J, Méndez-Álvarez J, Sierra-Pagan JE, et al. Release of soluble insulin receptor from neurons by cerebrospinal fluid from patients with neurocognitive dysfunction and HIV infection. Front Neurol. (2019) 10:285. doi: 10.3389/fneur.2019.00285
16. Cantres-Rosario YM, Acevedo-Mariani FM, Pérez-Laspiur J, Haskins WE, Plaud M, Cantres-Rosario YM, et al. Microwave & magnetic proteomics of macrophages from patients with HIV-associated cognitive impairment. PLoS One. (2017) 12:1–18. doi: 10.1371/journal.pone.0181779
17. Cotto B, Natarajanseenivasan K, Langford D. HIV-1 infection alters energy metabolism in the brain: Contributions to HIV-associated neurocognitive disorders. Prog Neurobiol. (2019) 181:101616. doi: 10.1016/j.pneurobio.2019.101616
18. Lenassi M, Cagney G, Liao M, Vaupotič T, Bartholomeeusen K, Cheng Y, et al. Nef is Secreted in Exosomes and Triggers Apoptosis in Bystander CD4+ T Cells. Traffic. (2010) 11:110–22. doi: 10.1111/j.1600-0854.2009.01006.x
19. Barclay RA, Khatkar P, Mensah G, DeMarino C, Chu JSC, Lepene B, et al. An Omics Approach to Extracellular Vesicles from HIV-1 Infected Cells. Cells. (2019) 8:787. doi: 10.3390/cells8080787
20. Kadiu I, Narayanasamy P, Dash PK, Zhang W, Gendelman HE. Biochemical and Biologic Characterization of Exosomes and Microvesicles as Facilitators of HIV-1 Infection in Macrophages. J Immunol. (2012) 189:744–54. doi: 10.4049/jimmunol.1102244
21. András IE, Leda A, Contreras MG, Bertrand L, Park M, Skowronska M, et al. Extracellular vesicles of the blood-brain barrier: Role in the HIV-1 associated amyloid beta pathology. Mol Cell Neurosci. (2017) 79:12–22. doi: 10.1016/j.mcn.2016.12.006
22. Nigro A, Colombo F, Casella G, Finardi A, Verderio C, Furlan R. Myeloid extracellular vesicles: Messengers from the Demented Brain. Front Immunol. (2016) 7:1–5. doi: 10.3389/fimmu.2016.00017
23. Zanotti S, Gibertini S, Blasevich F, Bragato C, Ruggieri A, Saredi S, et al. Exosomes and exosomal miRNAs from muscle-derived fibroblasts promote skeletal muscle fibrosis. Matrix Biol. (2018) 74:77–100. doi: 10.1016/j.matbio.2018.07.003
24. Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. (2007) 110:3234–44. doi: 10.1182/blood-2007-03-079152
25. Sun B, Fernandes N, Pulliam L. Blood neuron-derived exosomes identify HIV cognitive impairment and gender differences. AIDS. (2019) 31:F9. doi: 10.1097/QAD.0000000000001595
26. Bellingham S a, Guo BB, Coleman BM, Hill AF. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol. (2012) 3:124. doi: 10.3389/fphys.2012.00124
27. Ivanov AV, Valuev-Elliston VT, Ivanova ON, Kochetkov SN, Starodubova ES, Bartosch B, et al. Oxidative stress during HIV infection: mechanisms and consequences. Oxid Med Cell Longev. (2016) 2016:1–18. doi: 10.1155/2016/8910396
28. Umehara A, Nishioka M, Obata T, Ebina Y, Shiota H, Hashida S, et al. novel ultra-sensitive enzyme immunoassay for soluble human insulin receptor ectodomain and its measurement in urine from healthy subjects and patients with diabetes mellitus. Clin Biochem. (2009) 42:1468–75. doi: 10.1016/j.clinbiochem.2009.06.014
29. Soluble Insulin Receptor Study Group. Soluble insulin receptor ectodomain is elevated in the plasma of patients with diabetes. Diabetes. (2007) 56:2028–35. doi: 10.2337/db07-0394
30. Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. (2007) 69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b
31. Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604. doi: 10.7326/0003-4819-150-9-200905050-00006
32. Letendre S. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. (2008) 65:65. doi: 10.1001/archneurol.2007.31
33. Saunderson SC, Schuberth PC, Dunn AC, Miller L, Hock BD, MacKay PA, et al. Induction of exosome release in primary B cells stimulated via CD40 and the IL-4 receptor. J Immunol. (2008) 180:8146–52. doi: 10.4049/jimmunol.180.12.8146
34. Keller S, Ridinger J, Rupp A-K, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. (2011) 9:86. doi: 10.1186/1479-5876-9-86
35. Logozzi M, De Milito A, Lugini L, Borghi M, Calabrò L, Spada M, et al. High levels of exosomes expressing CD63 and Caveolin-1 in plasma of melanoma patients. PLoS ONE. (2009) 4:e5219. doi: 10.1371/journal.pone.0005219
36. Araujo S, Bañón S, Machuca I, Moreno A, Pérez-Elías MJ, Casado JL. Prevalence of insulin resistance and risk of diabetes mellitus in HIV-infected patients receiving current antiretroviral drugs. Eur J Endocrinol. (2014) 171:545–54. doi: 10.1530/EJE-14-0337
37. Campbell TD, Khan M, Huang M-B, Bond VC, Powell MD. HIV-1 Nef protein is secreted into vesicles that can fuse with target cells and virions. Ethn Dis. (2008) 18:S2-14–9.
38. Muratori C, Cavallin LE, Krätzel K, Tinari A, De Milito A, Fais S, et al. Massive secretion by T cells is caused by HIV nef in infected cells and by nef transfer to bystander cells. Cell Host Microbe. (2009) 6:218–30. doi: 10.1016/j.chom.2009.06.009
39. Ali SA, Huang M-B, Campbell PE, Roth WW, Campbell T, Khan M, et al. Genetic characterization of HIV type 1 Nef-induced vesicle secretion. AIDS Res Hum Retroviruses. (2010) 26:173–92. doi: 10.1089/aid.2009.0068
40. Shelton MN, Huang M-B, Ali SA, Powell MD, Bond VC. Secretion modification region-derived peptide disrupts HIV-1 Nef's interaction with mortalin and blocks virus and nef exosome release. J Virol. (2012) 86:406–19. doi: 10.1128/JVI.05720-11
41. Aqil M, Mallik S, Bandyopadhyay S, Maulik U, Jameel S. Transcriptomic analysis of mrnas in human monocytic cells expressing the HIV-1 nef protein and their exosomes. Biomed Res Int. (2015) 2015:1–10. doi: 10.1155/2015/492395
42. Hu G, Yang L, Cai Y, Niu F, Mezzacappa F, Callen S, et al. Emerging roles of extracellular vesicles in neurodegenerative disorders: focus on HIV-associated neurological complications. Cell Death Dis. (2016) 7:e2481. doi: 10.1038/cddis.2016.336
43. Hu G, Drescher KM, Chen X-M. Exosomal miRNAs: biological properties and therapeutic potential. Front Genet. (2012) 3:56. doi: 10.3389/fgene.2012.00056
44. Levine SJ. Mechanisms of soluble cytokine receptor generation. J Immunol. (2004) 173:5343–8. doi: 10.4049/jimmunol.173.9.5343
45. Levine SJ. Molecular mechanisms of soluble cytokine receptor generation. J Biol Chem. (2008) 283:14177–81. doi: 10.1074/jbc.R700052200
46. Stone S, Price P, Keane N, Murray R, French M. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med. (2002) 3:21–7. doi: 10.1046/j.1464-2662.2001.00096.x
47. Ray A, Ndugwa C, Mmiro F, Ricks MO, Semba RD. Soluble transferrin receptor as an indicator of iron deficiency in HIV-infected infants. Ann Trop Paediatr. (2007) 27:11–6. doi: 10.1179/146532807X170457
48. Uzasci L, Nath A, Cotter R. Oxidative stress and the HIV-infected brain proteome. J Neuroimmune Pharmacol. (2013) 8:1167–80. doi: 10.1007/s11481-013-9444-x
49. Hawari FI, Rouhani FN, Cui X, Yu Z-X, Buckley C, Kaler M, et al. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc Natl Acad Sci. (2004) 101:1297–302. doi: 10.1073/pnas.0307981100
50. Lutgen V, Narasipura SD, Barbian HJ, Richards M, Wallace J, Razmpour R, et al. HIV infects astrocytes in vivo and egresses from the brain to the periphery. PLoS Pathogens. (2020) 16:e1008381. doi: 10.1371/journal.ppat.1008381
51. Song Z, Xu Y, Deng W, Zhang L, Zhu H, Yu P, Qu Y, Zhao W, Han Y, Qin C. Brain derived exosomes are a double-edged sword in Alzheimer's disease. Front Mol Neurosci. (2020) 13:79. doi: 10.3389/fnmol.2020.00079
52. Hornung S, Dutta S, Bitan G. CNS-Derived Blood Exosomes as a Promising Source of Biomarkers: Opportunities and Challenges. Front Mol Neurosci. (2020) 13:38. doi: 10.3389/fnmol.2020.00038
53. Watson LS, Hamlett ED, Stone TD, Sims-Robinson C. Neuronally derived extracellular vesicles: an emerging tool for understanding Alzheimer's disease. Mol Neurodegener. (2019) 14:22. doi: 10.1186/s13024-019-0317-5
54. Kumar A, Kim S, Su Y, Sharma M, Kumar P, Singh S, et al. Brain cell-derived exosomes in plasma serve as neurodegeneration biomarkers in male cynomolgus monkeys self-administrating oxycodone. EBioMedicine. (2021) 63:103192. doi: 10.1016/j.ebiom.2020.103192
55. Arioz BI, Tufekci KU, Olcum M, Durur DY, Akarlar BA, Ozlu N, et al. Proteome profiling of neuron-derived exosomes in Alzheimer's disease reveals hemoglobin as a potential biomarker. Neurosci Lett. (2021) 755:135914. doi: 10.1016/j.neulet.2021.135914
56. Saeedi S, Israel S, Nagy C, Turecki G. The emerging role of exosomes in mental disorders. Transl Psychiatry. (2019) 9:122. doi: 10.1038/s41398-019-0459-9
57. Frühbeis C, Fröhlich D, Krämer-Albers E-M. Emerging roles of exosomes in neuron–glia communication. Front Physiol. (2012) 3:119. doi: 10.3389/fphys.2012.00119
58. Sun B, Dalvi P, Abadjian L, Tang N, Pulliam L. Blood neuron-derived exosomes as biomarkers of cognitive impairment in HIV. Aids. (2017) 31:F9–F17.
59. Mustapic M, Eitan E, Werner JK, Berkowitz ST, Lazaropoulos MP, Tran J, Goetzl EJ, Kapogiannis D. Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front Neurosci. (2017) 11:278. doi: 10.3389/fnins.2017.00278
60. Dagur RS, Liao K, Sil S, Niu F, Sun Z, Lyubchenko YL, et al. Neuronal-derived extracellular vesicles are enriched in the brain and serum of HIV-1 transgenic rats. J Extracell Vesicles. (2020) 9:1703249. doi: 10.1080/20013078.2019.1703249
61. Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease. J Neurovirol. (2019) 25:702–9. doi: 10.1007/s13365-018-0695-4
62. Phair J, Palella F. Renal disease in HIV-infected individuals. Curr Opin HIV AIDS. (2011) 6:285–9. doi: 10.1097/COH.0b013e3283476bc3
63. Melendez Rivera JG, Hashmi MF. HIV Nephropathy. (2021). Available online at: http://www.ncbi.nlm.nih.gov/pubmed/32644560
65. Winston J, Deray G, Hawkins T, Szczech L, Wyatt C, Young B. Kidney disease in patients with HIV infection and AIDS. Clin Infect Dis. (2008) 47:1449–57. doi: 10.1086/593099
66. Jotwani V, Scherzer R, Abraham A, Estrella MM, Bennett M, Devarajan P, et al. Does HIV infection promote early kidney injury in women? Antivir Ther. (2013) 19:79–87. doi: 10.3851/IMP2677
67. Singh S, Sharma R, Kumari M, Tiwari S. Insulin receptors in the kidneys in health and disease. World J Nephrol. (2019) 8:11–22. doi: 10.5527/wjn.v8.i1.11
68. Szczech LA, Gange SJ, van der Horst C, Bartlett JA, Young M, Cohen MH, et al. Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int. (2002) 61:195–202. doi: 10.1046/j.1523-1755.2002.00094.x
69. Jotwani V, Scherzer R, Estrella MM, Jacobson LP, Witt MD, Palella F, et al. Association of HIV infection with biomarkers of kidney injury and fibrosis in the Multicenter AIDS cohort study. Antivir Ther. (2017) 22:421–9. doi: 10.3851/IMP3124
70. Driver TH, Scherzer R, Peralta CA, Tien PC, Estrella MM, Parikh CR, et al. Comparisons of creatinine and cystatin C for detection of kidney disease and prediction of all-cause mortality in HIV-infected women. AIDS. (2013) 27:2291–9. doi: 10.1097/QAD.0b013e328362e874
71. Shrestha S, Sunaga H, Hanaoka H, Yamaguchi A, Kuwahara S, Umbarawan Y, et al. Circulating FABP4 is eliminated by the kidney via glomerular filtration followed by megalin-mediated reabsorption. Sci Rep. (2018) 8:16451. doi: 10.1038/s41598-018-34902-w
Keywords: insulin receptor, exosome, urine, plasma, cognitive dysfunction, biomarker, women
Citation: Cantres-Rosario YM, Wojna V, Ruiz R, Diaz B, Matos M, Rodriguez-Benitez RJ, Rodriguez E, Skolasky RL and Gerena Y (2022) Soluble Insulin Receptor Levels in Plasma, Exosomes, and Urine and Its Association With HIV-Associated Neurocognitive Disorders. Front. Neurol. 13:809956. doi: 10.3389/fneur.2022.809956
Received: 05 November 2021; Accepted: 06 April 2022;
Published: 02 June 2022.
Edited by:
Avindra Nath, National Institute of Neurological Disorders and Stroke (NIH), United StatesReviewed by:
Pamela Knapp, Virginia Commonwealth University, United StatesTory P. Johnson, Johns Hopkins Medicine, United States
Copyright © 2022 Cantres-Rosario, Wojna, Ruiz, Diaz, Matos, Rodriguez-Benitez, Rodriguez, Skolasky and Gerena. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valerie Wojna, valerie.wojna1@upr.edu; Yamil Gerena, yamil.gerena@upr.edu
†These authors share first authorship
 Yisel M. Cantres-Rosario
Yisel M. Cantres-Rosario Valerie Wojna
Valerie Wojna Rafael Ruiz1
Rafael Ruiz1 Bexaida Diaz
Bexaida Diaz Rosa J. Rodriguez-Benitez
Rosa J. Rodriguez-Benitez Richard L. Skolasky
Richard L. Skolasky Yamil Gerena
Yamil Gerena