Cancer Related Subarachnoid Hemorrhage: A Multicenter Retrospective Study Using Propensity Score Matching Analysis
- 1Department of Neurology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2Department of Neurology, Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou, China
- 3Department of Neurology, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, China
- 4Department of Neurology, The Second Affiliated Hospital of Guangxi Medical University, Nanning, China
Objective: To investigate the clinical features, risk factors and underlying pathogenesis of cancer related subarachnoid hemorrhage (SAH).
Methods: Clinical data of SAH in patients with active cancer from January 2010 to December 2020 at four centers were retrospectively reviewed. Patients with active cancer without SAH were matched to SAH patients with active cancer group. Logistic regression was applied to investigate the independent risk factors of SAH in patients with active cancer, after a 1:1 propensity score matching (PSM). A receiver operator characteristic curve was configured to calculate the optimal cut-off value of the joint predictive factor for cancer related SAH.
Results: A total of 82 SAH patients with active cancer and 309 patients with active cancer alone were included. Most SAH patients with cancer had poor outcomes, with 30-day mortality of 41.5%, and with 90-day mortality of 52.0%. The PSM yielded 75 pairs of study participants. Logistic regression revealed that a decrease in platelet and prolonged prothrombin time were the independent risk factors of cancer related SAH. In addition, receiver operator characteristic curve of the joint predictive factor showed the largest AUC of 0.8131, with cut-off value equaling to 11.719, with a sensitivity of 65.3% and specificity of 89.3%.
Conclusion: Patients with cancer related SAH often have poor outcomes. The decrease in platelet and prolonged prothrombin time are the independent risk factors of cancer related SAH, and the joint predictive factor with cutoff value equal to 11.719 should hence serve as a novel biomarker of cancer related SAH.
Introduction
Cancer related stroke has recently received increasing attention from clinicians. Although the risk of hemorrhagic stroke and the risk of ischemic stroke are almost equal in patients with cancer (Zöller et al., 2012), hemorrhagic stroke seems to receive less attention than ischemic stroke. This is because clinicians often view hemorrhagic stroke as a catastrophic and terminal event (Bitoh et al., 1984). As a result, data about cancer patients with hemorrhagic stroke, especially on SAH are not much. Previous study with small samples shows that SAH may occur in patients with various kind of cancer, including primary intracranial tumors, spinal tumors, somatic solid tumors, and hematological tumors. It was also found that the pathogenesis of SAH in patients with cancer is associated with intratumoral hemorrhage (ITH) in intracranial primary tumors or metastatic tumor. Pathogenesis of SAH is also associated with the blood coagulation dysfunction in no intracranial cancer. It has also been reported that most of SAH in patients with cancer tend to have poor prognosis (Spetzger et al., 1995; Nagai et al., 2008; Kukreja et al., 2014). In 2010, systematic retrospective clinical research on cancer with intracranial hemorrhage (ICH) found that among 181 active cancer patients with ICH, 48 (25.4%) patients had SAH. The study revealed that ITH (61%) and coagulopathy (46%) accounted for the majority of hemorrhages, whereas hypertension (5%) was rare, with median survival of 3 months [95% confidence interval (CI) 2–4], and its 30-day mortality of 31% (Navi et al., 2010). In 2016, a different study reported that previous cancer history was a risk factor for the poor functional outcome of SAH (Shibahara et al., 2016). However, the risk factors of SAH in patients with active cancer and the relationship between active cancer and SAH have not been well elucidated.
Therefore, the present study aimed to investigate the clinical features, risk factors and underlying pathogenesis of cancer related SAH by conducting a multicenter retrospective study using propensity score matching (PSM) analysis. It was hypothesized that active cancer may play an important role in the development of SAH in patients with active cancer. Eligible patients with active cancer related SAH were enrolled and age and sex matched patients with active cancer alone were also enrolled in the ratio of nearly 1:4. The univariate and multivariate binary logistic regression were applied before and after PSM to investigate independent risk factors and underlying pathogenesis of cancer related SAH. Thus, this study may contribute to establishing the relationship between SAH and the cancer. Additionally, this study may help in detecting the patients at high risk of SAH and help clinicians to take effective preventive and therapeutic measures for such patients.
Materials and Methods
Patients
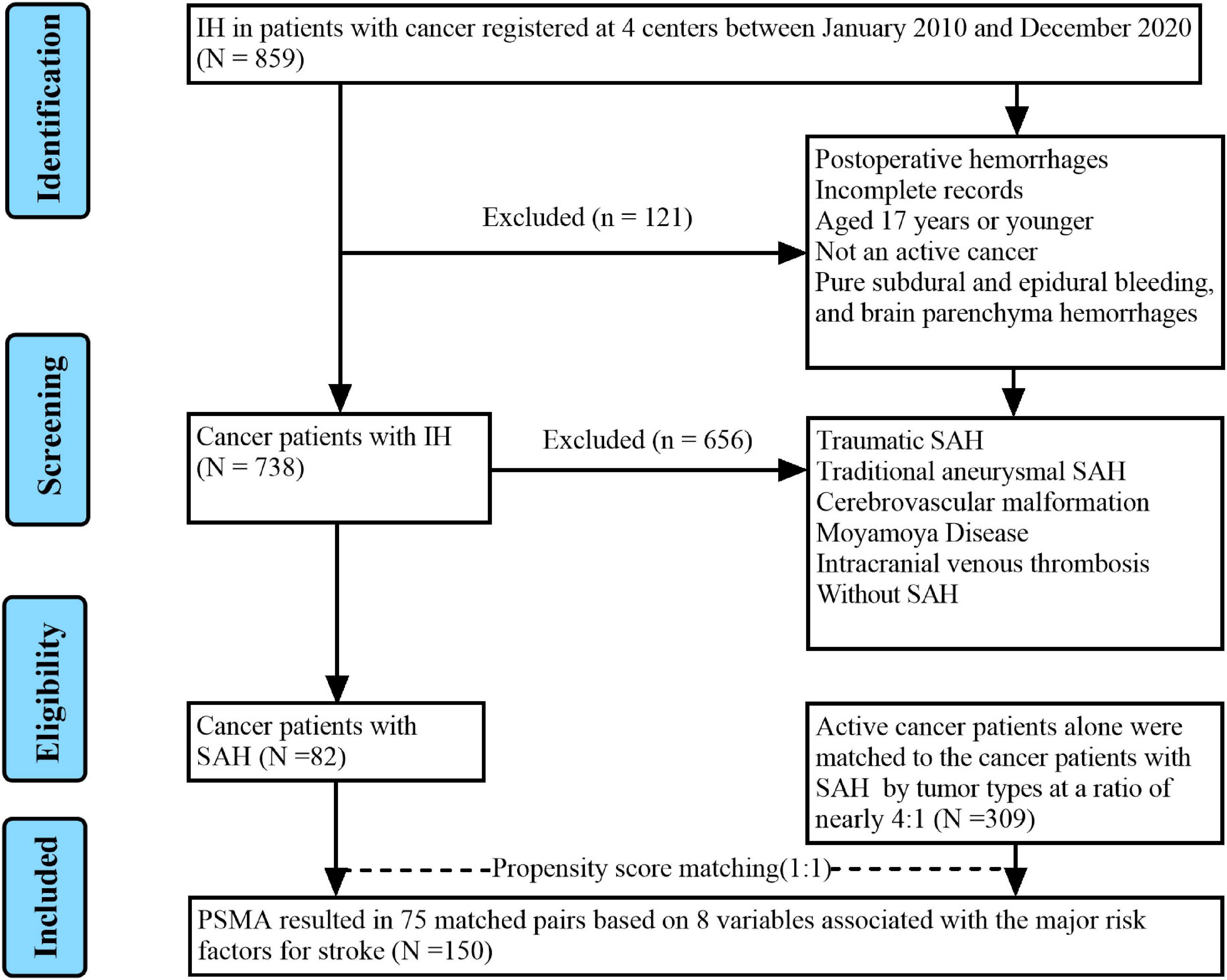
We retrospectively analyzed the records of all SAH patients with active cancer from Guangxi medical university affiliated tumor hospital, the first, second and fourth affiliated hospitals of Guangxi medical university between January 2010 and December 2020. Patients met the following recruitment criteria were enrolled in SAH with active cancer group: (1) Age ≥ 18 years; (2) spontaneous SAH was confirmed by CT, MRI, or lumbar puncture; (3) cancer was demonstrated by pathological examination; and (4) active cancer was defined as a diagnosis of cancer within 6 months before enrollment, any treatment for cancer within the previous 6 months, or recurrent or metastatic cancer. The exclusion criteria of SAH in patients with active cancer group included: (1) Age < 18 years; (2) patients had postoperative hemorrhages; (3) cancer patients with SAH due to trauma, traditional aneurysm, cerebrovascular malformations, moyamoya disease, intracranial venous thrombosis; (4) incomplete medical records. Patients with active cancer alone were matched to SAH in patients with active cancer group by tumor types were recruited at a ratio of nearly 4:1 as control group. Our records search was as outlined in Figure 1.
Data Collection and Follow-Up
Clinical data of all patients, including age, gender, vascular risk factors, type of cancer, etiology of subarachnoid hemorrhage (SAH) in patients with cancer, presentation, current cancer treatment, vascular risk factors, and outcome were noted. In addition, laboratory data at diagnosis were also recorded including routine blood tests, blood biochemistry, coagulation function and levels of plasma D-dimer as well as tumor markers. Findings of imaging examinations such as echocardiography, transcranial Doppler ultrasound (TCD), cranial CT, computed tomography angiography (CTA), MRI, magnetic resonance angiography (MRA), and digital subtraction angiography (DSA) were also collected. In the present study, the following criteria were employed to diagnose coagulopathy: platelets < 100/mm3, prothrombin time > 13 s, international normalized ratio > 1.5, activated partial thromboplastin time > 45 s and disseminated intravascular coagulation (DIC) (fibrinogen < 200 mg/dL and D-dimer > 290 ng/dL). The severity of clinical status was assessed by Hunt and Hess grade.
Statistical Analysis
Statistical analysis was performed using SPSS version 25.0 software (IBM). Primarily, each variable was tested for normal distribution using the Kolmogorov-Smirnov test. The data was then expressed as mean ± standard deviation for normally distributed variables or median (25–75%) for the variables without normal distribution and the classified variable was represented by count (percentage). The Mann-Whitney u-test was used for non-normally distributed variables; unpaired comparisons were made with the student’s unpaired t-test, while Pearson’s χ2 or Fisher’s exact test was used to compare categorical variables. Besides, the Kaplan–Meier method was used to estimate the rate of cumulative events, and differences between groups were assessed using the log-rank test. A Cox regression model was constructed to evaluate the hazard ratio for 30- and 90-day mortality in cancer with SAH group and predictors of mortality univariate. Variables with P < 0.05 in univariate analyses were considered explanatory variables and were entered to multivariate models with entry and exit levels set at 0.05 to produce the final multivariate analyses.
To eliminate the impacts of conventional vascular risks on SAH in cancer patients, a PSM, using a multivariable logistic regression model based on: age, gender, previous strokes, coronary heart disease, hypertension, diabetes, current smoker, and drinking was performed. As a result, pairs of patients receiving cancer with SAH group or active cancer group alone were derived using 1:1 greedy nearest neighbor matching within propensity score of 0.02. This strategy resulted in 75 matched pairs in each group. The balance of variables between groups after propensity score-matching was analyzed using a paired t-test for continuous measures and the McNemar test (for categorical variables). Moreover, for non-normal data, the Wilcoxon sum rank test was used (Wilcoxon signed rank test for paired within-group comparisons).
To find independent risk factors of SAH in patients with cancer, univariate and multivariate binary logistic regression was applied. Univariate significant factors at P < 0.05 were included in the stepwise logistic regression model, with entry and exit levels set at 0.05 for the final multivariate analysis. Receiver operating characteristic (ROC) curve analysis was used to evaluate the diagnostic performance of the resulting regression model based on its AUC, sensitivity, and specificity values. Optimal cut-off value was determined by the Youden index. All p-values were two-sided, and P < 0.05 was considered statistically significant. No formal calculation of sample size was performed because of the characteristics of cancer with SAH.
Ethics
Ethical approval of this study was provided by the Guangxi Medical University Review Board. The written informed consent was waivered because of the retrospective nature of our study.
Results
Patient Profiles
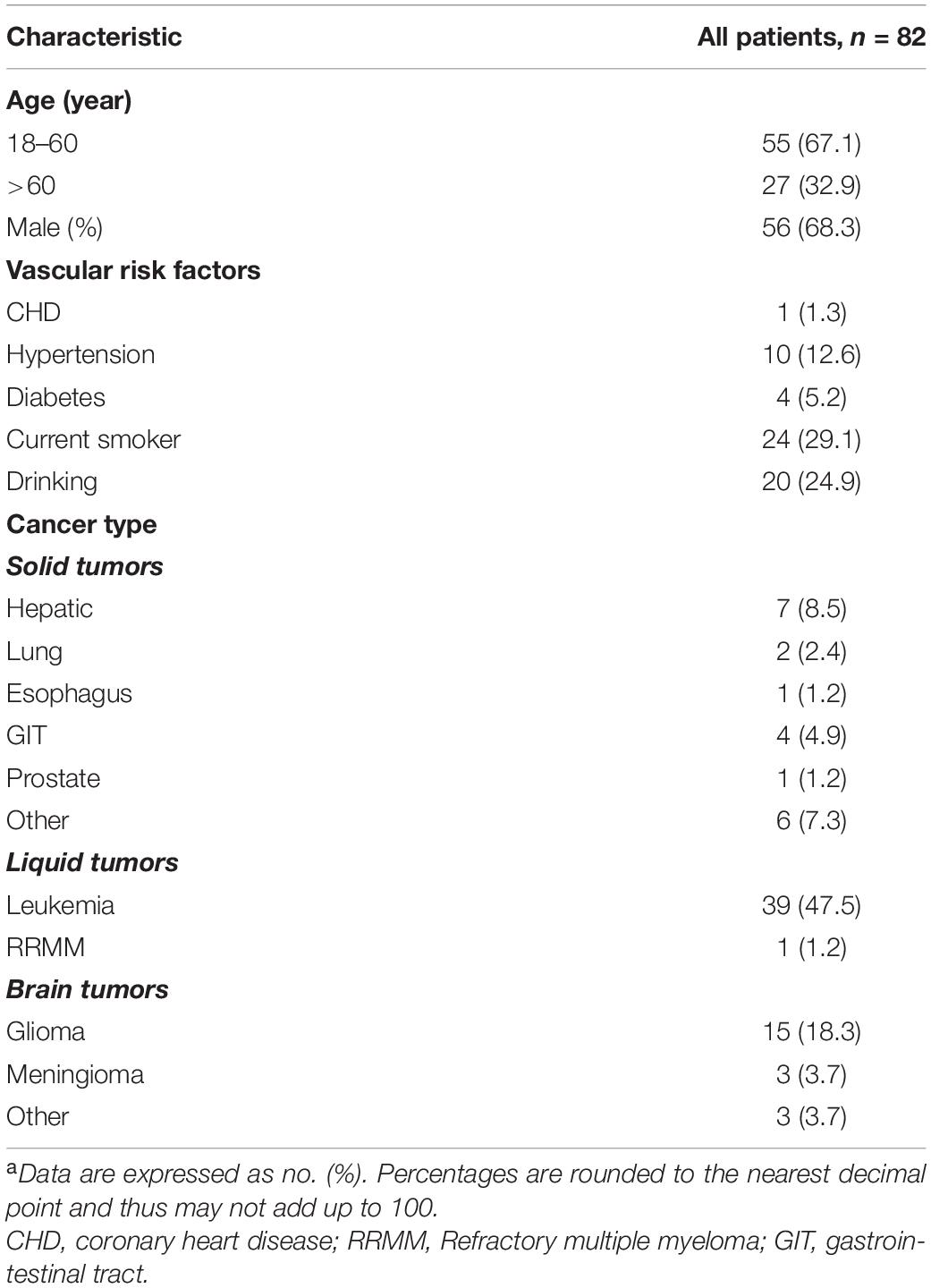
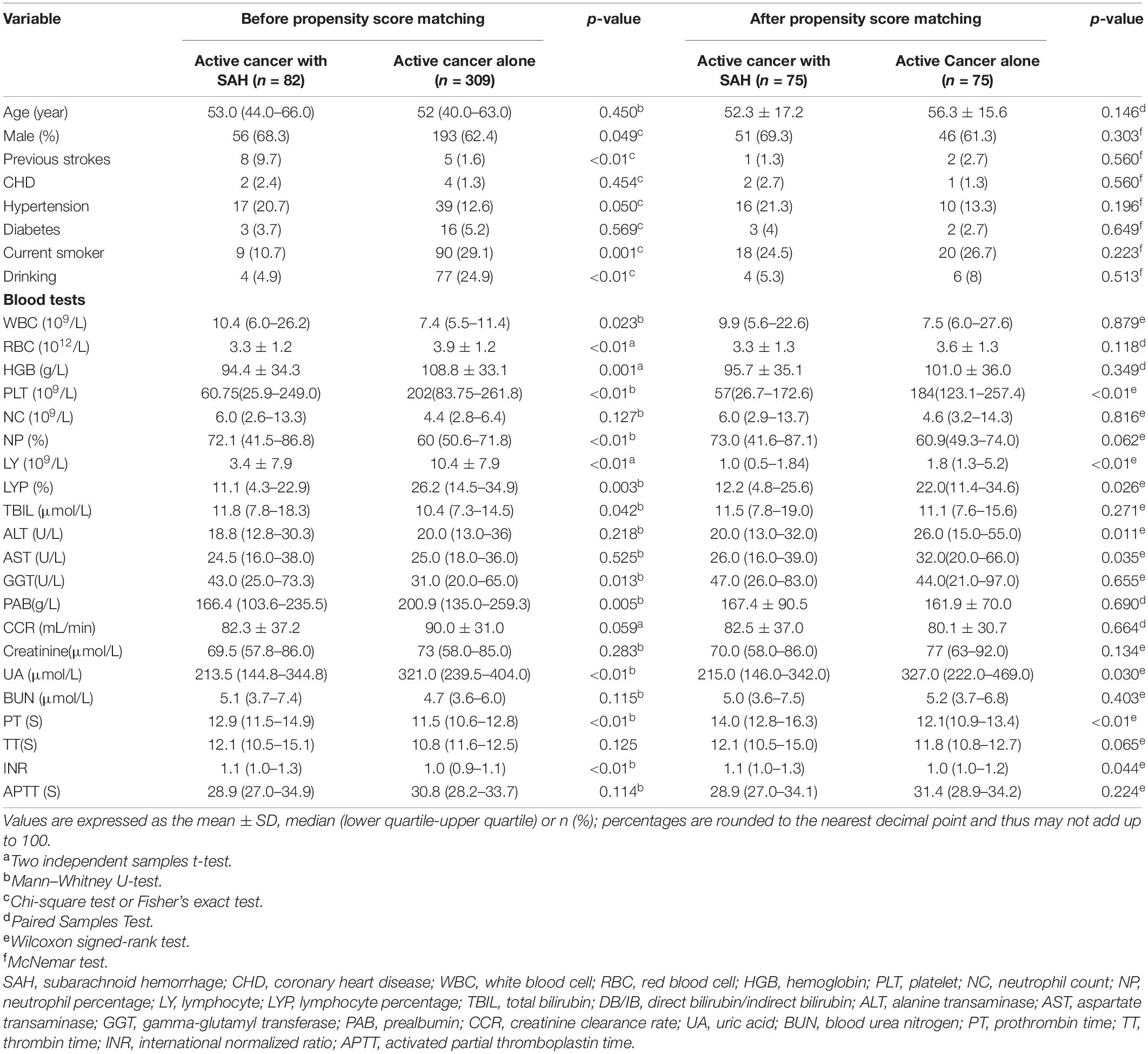
Among the 859 cancer patients with ICH admitted to the four centers during the study period, 82 active cancer patients with SAH meeting all eligibility criteria and were included in the final analysis. Concurrently, regarding the control group, 309 patients with active cancer alone were matched to the active cancer patients with SAH by tumor types at a ratio of nearly 4:1. Moreover, after propensity matching, a total of 150 patients were included in the study population. The PSM yielded 75 pairs of study participants in an unbiased database. Before the propensity score-matching was performed, cancer patients with SAH group contained 56 (68.3%) men and 26 (31.7%) women (Table 1). The median age was 53 (range 44–66) years.
Cancer Type
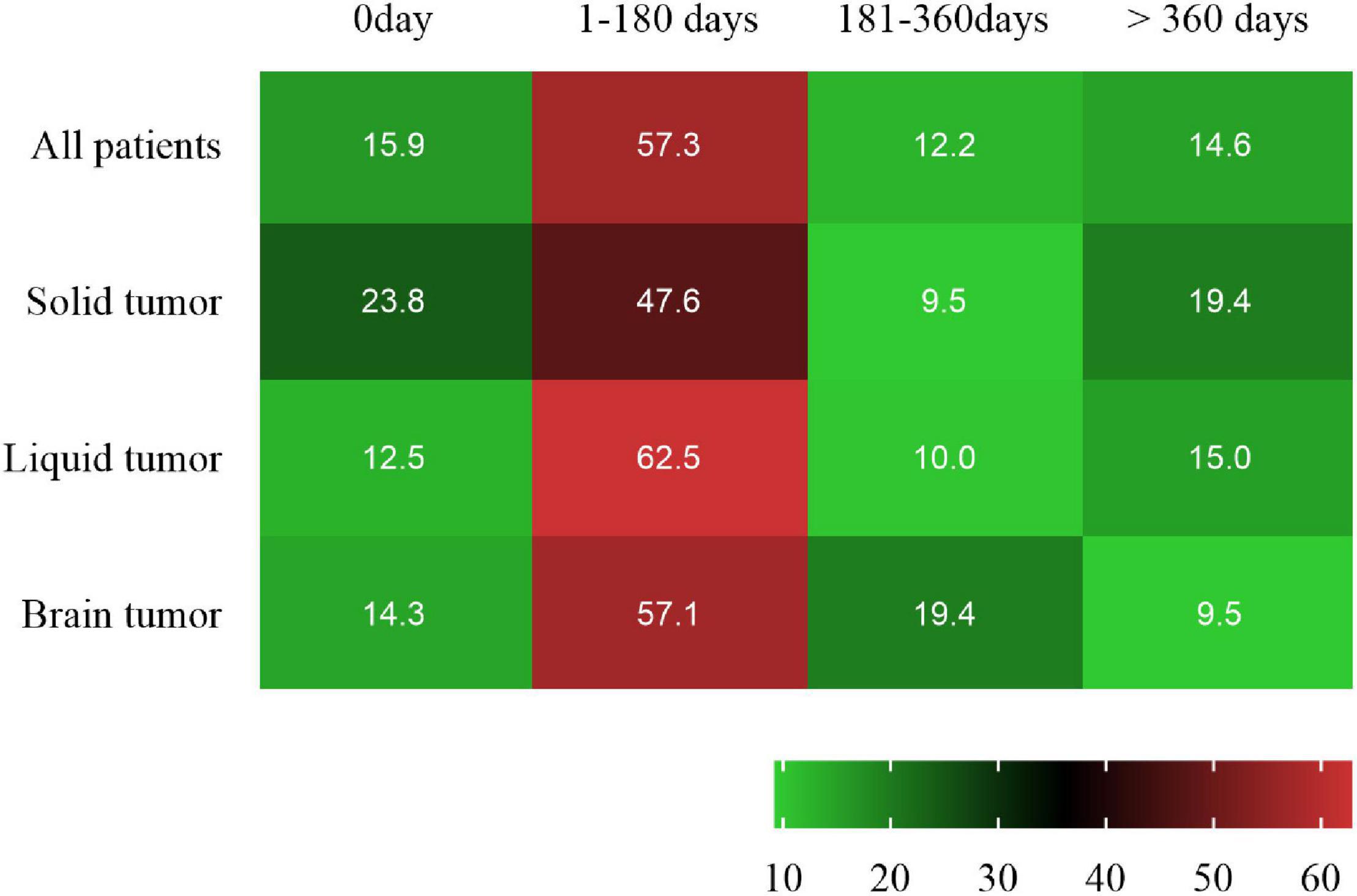
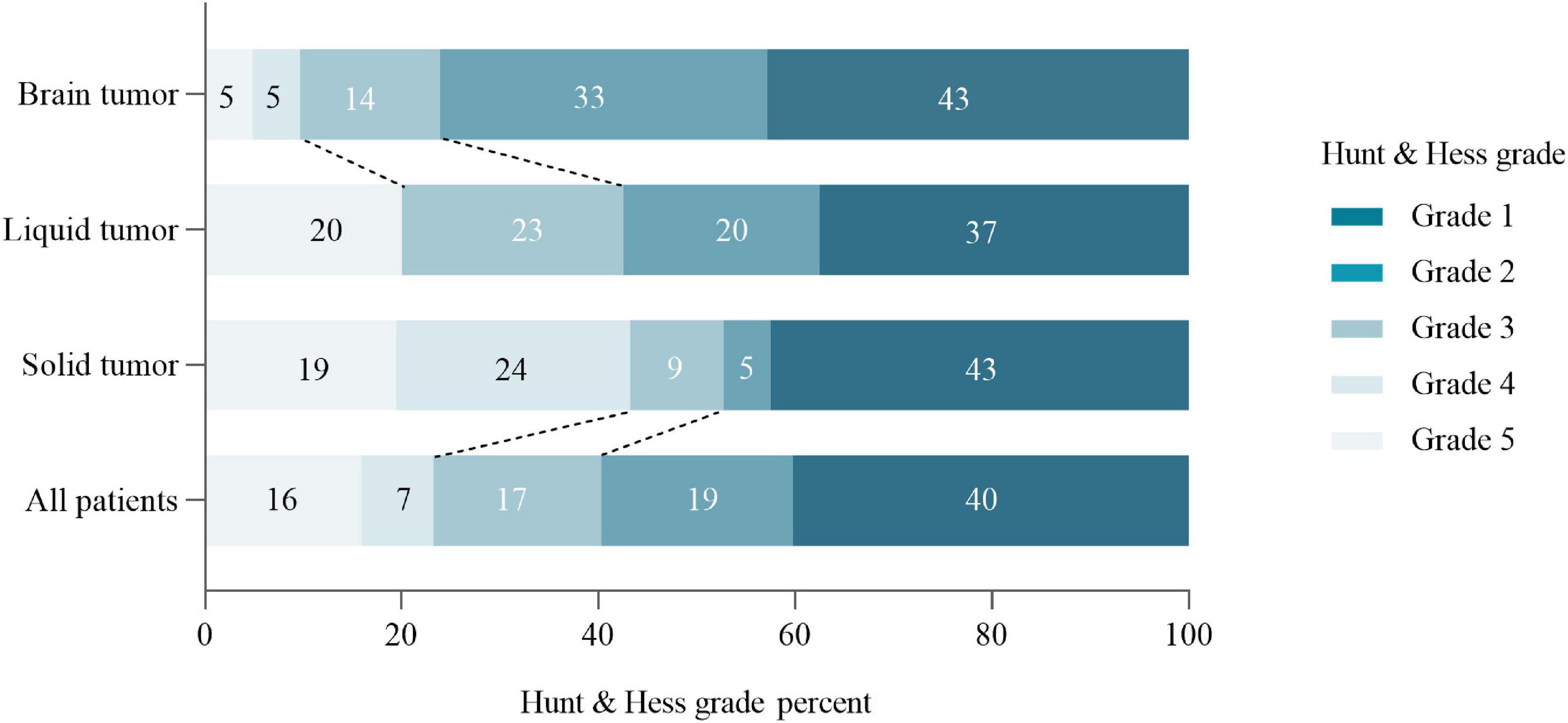
The cancer contributing to SAH was a solid tumor in 21 patients (25.6%), a primary brain tumor in 21 (25.6%) and a hematologic tumor in 40 (48.8%). The most common pathological type of cancer was Leukemia (47.5%), followed by glioma (18.3%) and hepatoma (8.5%) (Table 1). After diagnosis of cancer, 47 (57.3%), 10 (12.2%), and 12 (14.6%) patients experienced SAH in the first 6 months, 6 months to 1 year and > 1 year, respectively. Additionally, 13 (15.9%) cancer patients have SAH as the first presentation (Figure 2 and Supplementary Appendix Table 1).
Presentation and Etiology
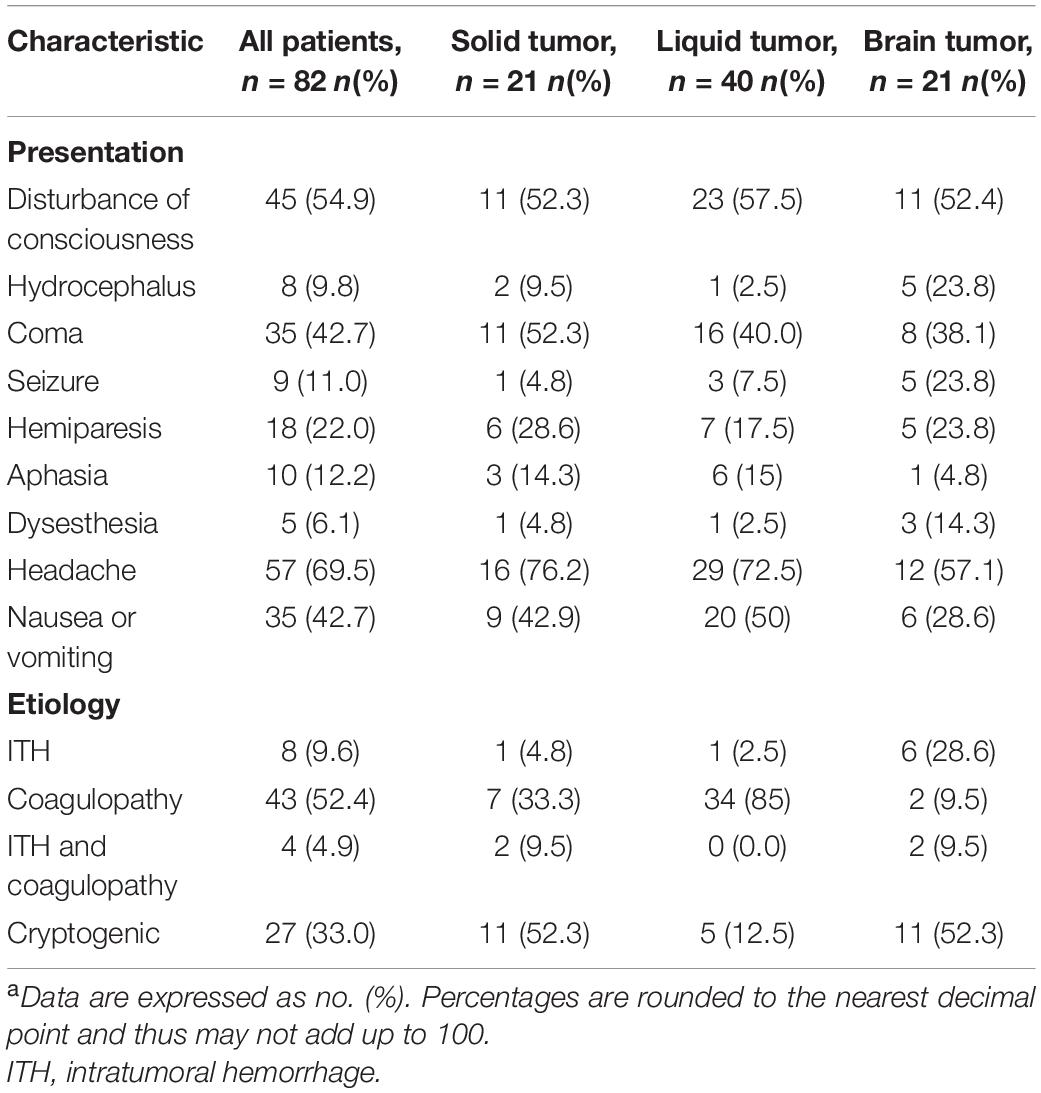
The presentation and pathogenesis of SAH in cancer group was as summarized in Table 2. Patients with active cancer and SAH present with the usual symptoms of SAH such as headache and vomiting. However, the SAH in patients with active cancer showed unique pathogenesis. In the present study, it was found that the possible etiology of SAH was mostly related to coagulopathy (52.4%), followed by ITH (9.6%), and was rarely related to coagulopathy combined with ITH. However, the pathogenesis of SAH in 27 (33.0%) patients was found to be indefinite. For patients with liquid tumors such as Leukemia, SAH was mostly due to coagulation dysfunction, whereas for patients with brain tumors, SAH was commonly caused by ITH.
Imaging Features
Radiologic investigation with computed tomography of the brain and computed tomography angiography revealed typical manifestations of cancer with SAH (Figures 3A–F).
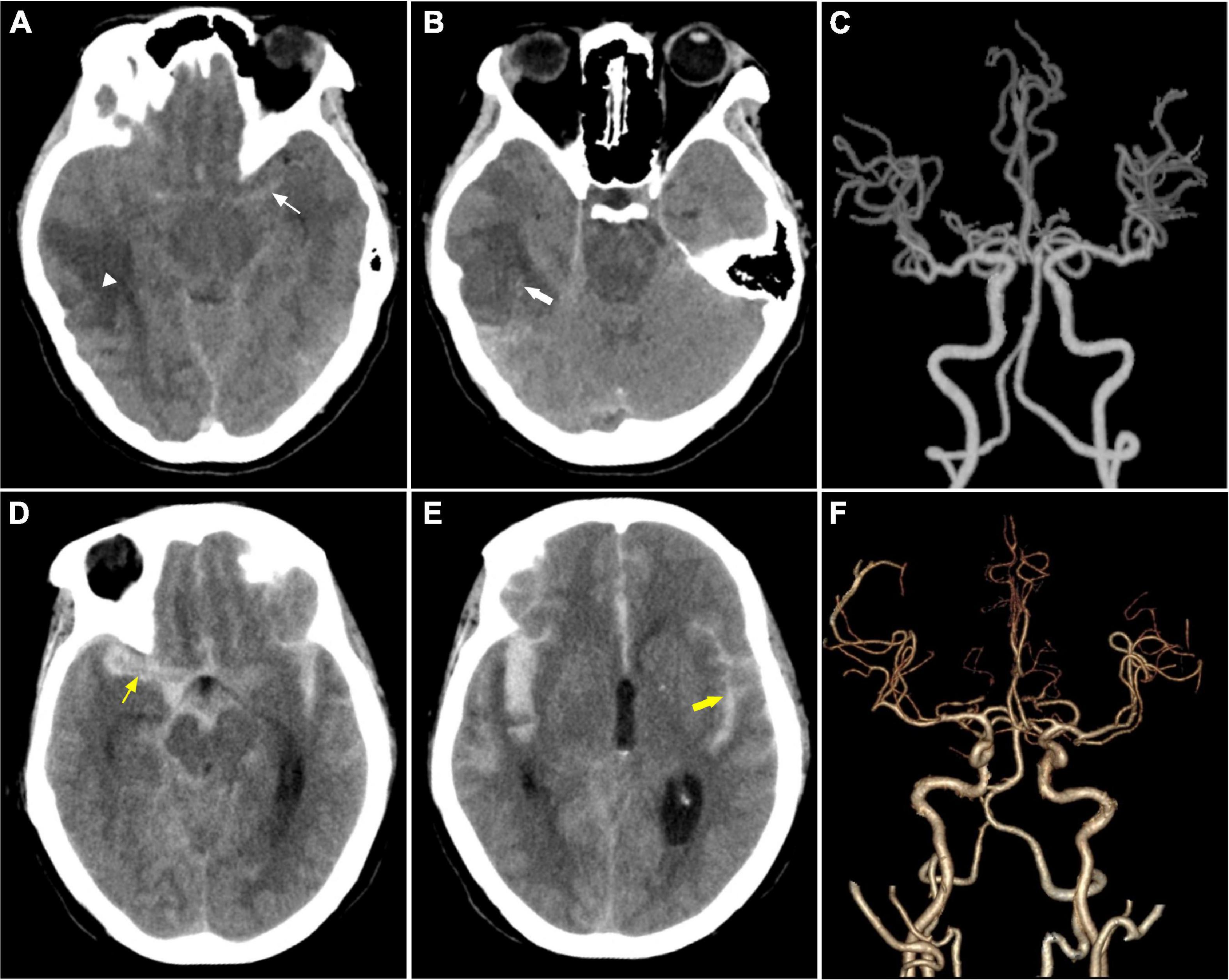
Figure 3. Imaging features. (A–C) Were from a 28-year- female with astrocytic glioma experienced SAH due to ITH in the first 11 days after the diagnosis of astrocytic glioma. It shows a tumor in the brain (white triangle) and subarachnoid hemorrhage (thin white arrow) in (A), intratumoral bleeding in (B) (thick white arrow), normal cerebral arteries in (C). (D–F) Were from a middle-aged female patient with acute monocytic leukemia combined with coagulopathy suffered from SAH. (D,E) Showed massive subarachnoid hemorrhage with ventricle hematoma. (F) Shows normal cerebral arteries.
Severity Score of Subarachnoid Hemorrhage
Results of the severity score of cancer patients with SAH were as shown in Figure 4. Generally, 12 (16%) cancer patients with SAH were found with 5 scores of Hunt and Hess grade.
Treatment
In our study, cancer patients who developed SAH were treated as follows: Lowering intracranial pressure therapy was administered to 72 patients (87.8%). Antibiotics are used to control bacterial or fungal infections (73.1%). Reversal of coagulopathy was attempted with platelet transfusion, and/or fresh-frozen plasma (48.8%), whereas emergency surgery for SAH is relatively rare (12.2%). In the present study, SAH in patients with active cancer received stander treatment for SAH and cancer according to guidelines for SAH (Connolly et al., 2012; Meurer et al., 2016).
Survival Analysis
Survival analysis using Kaplan–Meier curves showed that most SAH patients with cancer had poor outcomes, with 30-day mortality 41.5%, and with 90-day mortality 52.0%. Moreover, the survival time of cancer patients with SAH differed (p = 0.041) depending on the type of cancer, with median survival of 22 days for patients with liquid tumors and 27 days for patients with solid tumors. However, due to the relatively long survival time of brain tumors and the limitation of follow-up time, the median survival data for the time could not be obtained (Figure 5A). Furthermore, the survival time of patients with cancer related SAH based on etiology showed difference (p = 0.038), with median survival of 21 days for patients with ITH, 19 days for patients with coagulopathy and 8 days for patients with both ITH and coagulopathy (Figure 5B).
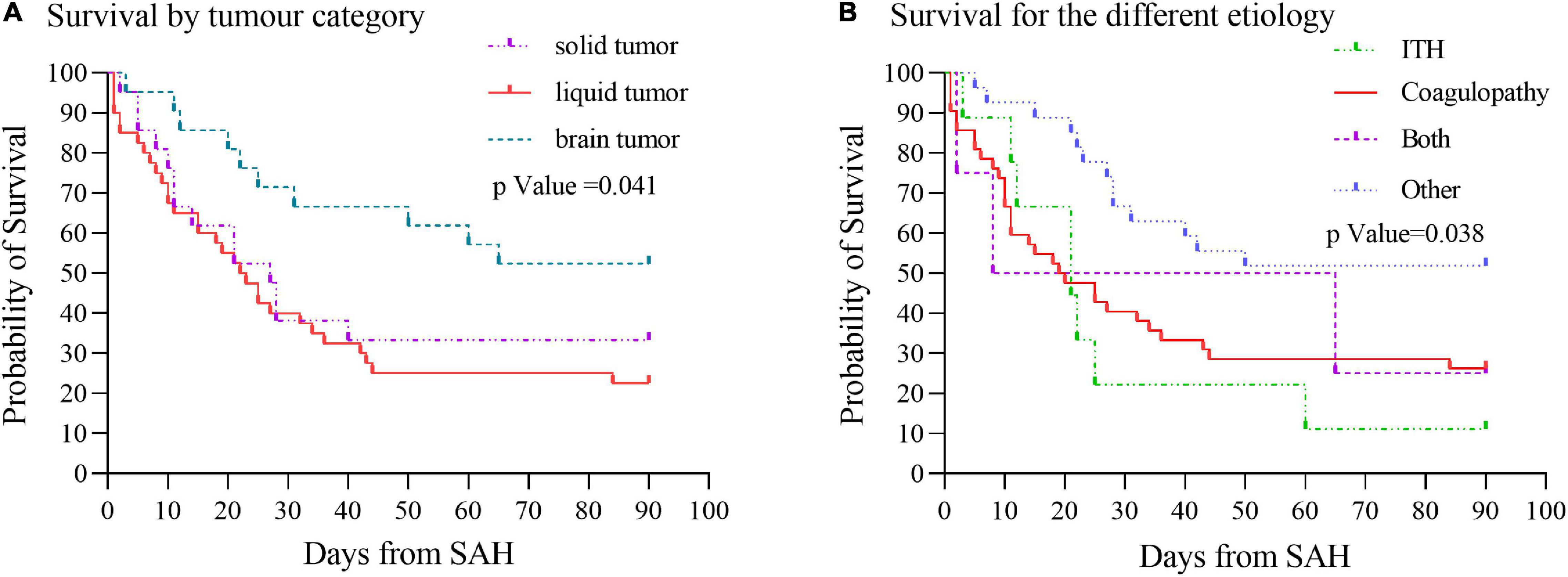
Figure 5. Survival curves by cancer type and etiology of SAH. (A) Cancers were categorized as solid, liquid, or brain tumor. Kaplan–Meier curves showed survival time of cancer patients with SAH differed (p = 0.041) by cancer type. (B) Etiology of SAH was grouped as intratumoral hemorrhage, coagulopathy, both intratumoral hemorrhage and coagulopathy, or other diagnoses. Survival analysis based on the etiology of SAH showed survival time difference (p = 0.038). ITH, intratumoral hemorrhage.
Survival analysis of Cox regression model showed that 7 variables were significant predictors of 30-day mortality: having coagulopathy, aphasia, activated partial thromboplastin time, gamma-glutamyl transferase, atrial fibrillation, prealbumin and cryptogenic SAH. On the other hand, 5 variables were found to be significant predictors of 90-day mortality: having liquid tumor, activated partial thromboplastin time, prealbumin, cryptogenic SAH and coagulopathy. The results of univariate analysis of continuous and categorical variables relating to 30-day and 90-day mortality survival rate in original 82 cancer patients with SAH were as presented in Supplementary Appendix Tables 2–5. In the multivariate Cox regression models, the predictors of 30-and 90-day mortality in the whole cohort were summarized in Figure 6 and Supplementary Appendix Table 6.
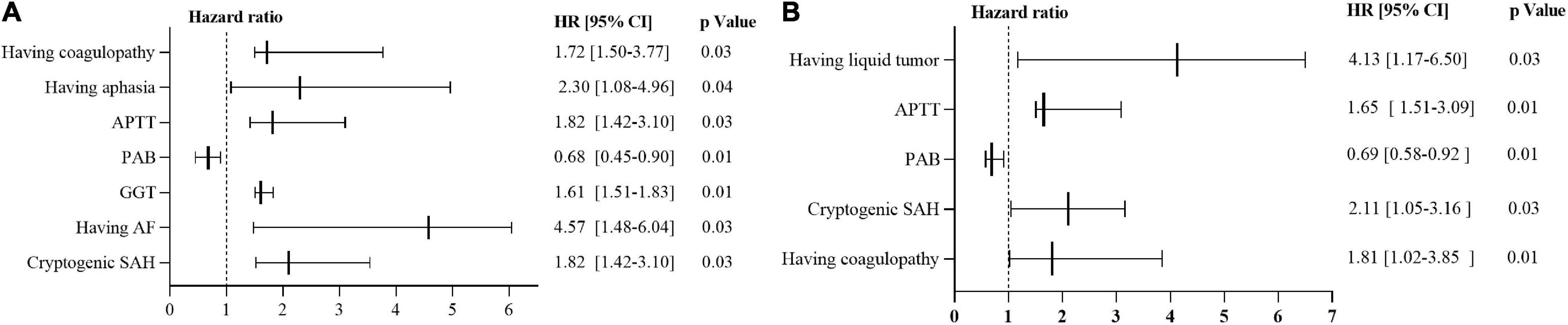
Figure 6. Predictors of mortality via Cox regression models. (A) Predictors of 30-day mortality via Cox regression models. (B) Predictors of 90-day mortality via Cox regression models. HR > 1 implies unfavorable prognosis for SAH in patients with active cancer. HR of 1 corresponds to no effect. HR, hazard ratio; 95% CI = 95% confidence interval; SAH, subarachnoid hemorrhage; APTT, activated partial thromboplastin time; PAB, prealbumin; GGT, gamma-glutamyl transferase; AF, Atrial fibrillation.
Univariate Analysis and Multivariate Analysis
Before PSM was performed, a univariate analysis between the 82 SAH in patients with active cancer and 309 patients with active cancer alone showed that nineteen variables were significantly related to the onset of SAH. The variables were: sex; having a previous stroke; hypertension; smoker; drinking; having a brain tumor; the level of white blood cell, red blood cell, hemoglobin, platelet, neutrophil percentage, lymphocyte, lymphocyte percentage, total bilirubin, gamma-glutamyl transferase, prealbumin, uric acid, prothrombin time and international normalized ratio (Table 3).
A multivariate logistic regression analysis demonstrated that two variables were significantly relating to the onset of SAH: having previous stroke [p = 0.006; odds ratio (OR), 3.85; 95% CI, 1.73–5.06] and prothrombin time [p = 0.028; odds ratio (OR), 1.14; 95% CI, 1.02–1.29] (Supplementary Appendix Table 7).
Nevertheless, the results of a univariate analysis of the propensity score-matched groups revealed that eight variables were significantly related to the onset of SAH: The level of platelet, lymphocyte, lymphocyte percentage, alanine transaminase, aspartate transaminase, uric acid, prothrombin time, and international normalized ratio (Table 3). Multivariate analysis revealed that only two variable was significantly related to the onset of SAH: the level of platelet [p < 0.001; odds ratio (OR), 0.87; 95% CI, 0.69–0.98]; prothrombin time (p < 0.001; OR, 1.559; 95% CI, 1.229–1.976) (Figure 7 and Supplementary Appendix Table 8).
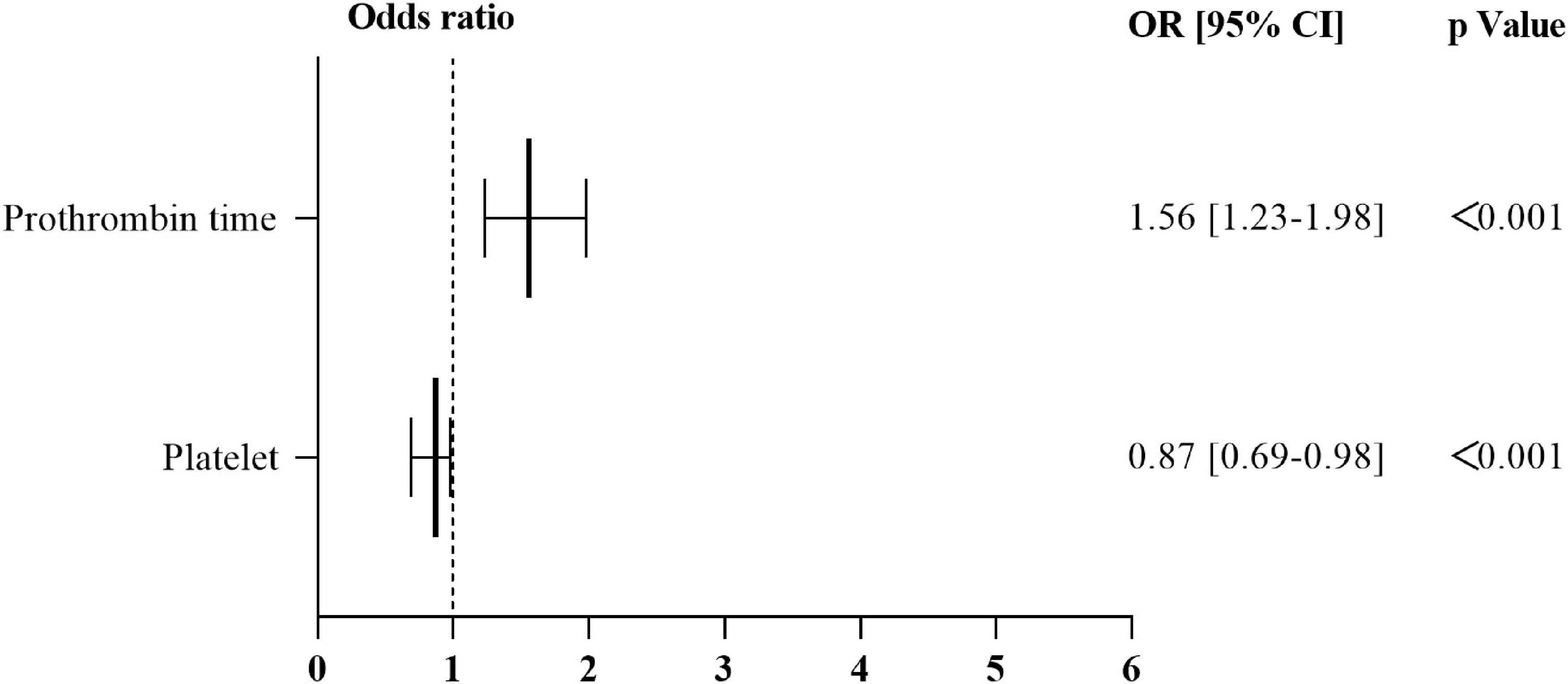
Figure 7. Results of multivariate logistic regression analysis relating to the onset of SAH in the propensity score–matched group. OR > 1 independent risk factor for the onset of SAH. OR of 1 corresponds to no effect. OR, odds ratio; 95% CI, 95% confidence interval; SAH, subarachnoid hemorrhage.
Receiver Operating Characteristic Curves
The ROC curves for identifying the onset of SAH in patients with active cancer from prothrombin time, platelet, and joint predictor (combing prothrombin time and platelet) were shown in Figure 8. Using prothrombin time and platelets as covariates X1 and X2, respectively, the joint predictive factor expression is obtained: Logit(P) = β1/β1*X1 + β2/β1*X2 (Supplementary Appendix Table 6). ROC curve of the joint predictive factor showed the largest AUC of 0.8131 (95% CI 0.745–0.881, P < 0.001), indicating good overall accuracy of the test. The optimum diagnostic cut-off value for the joint predictive factor calculated from the ROC was 11.719, with a sensitivity of 65.3% and specificity of 89.3% (Figure 8 and Supplementary Appendix Table 9).
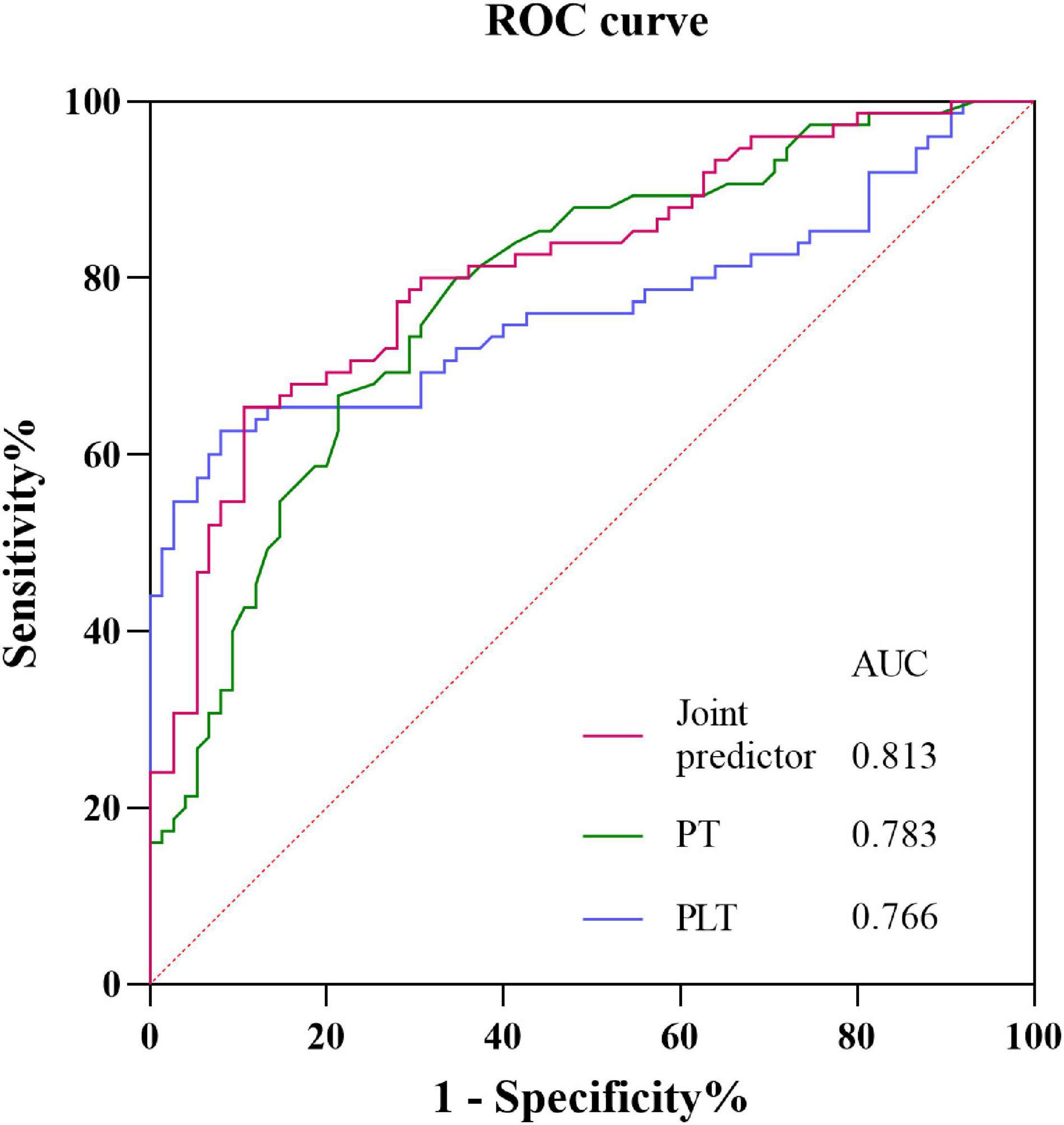
Figure 8. Receiver operating characteristic curves obtained with different discriminatory models for predicting the onset of SAH in patients with active cancer. The joint predictive factor showed the largest AUC of 0.8131 (95% CI 0.745–0.881, P < 0.001). The optimum cut-off point was 11.719. At this cut-off value, the sensitivity was 65.3% and the specificity was 89.3%. CI, Confidence interval; ROC, Receiver operator characteristic; AUC, Area under the curve.
Discussion
SAH in patients with active cancer has received insufficient attention (Schrader et al., 2000; Harold, 2019). The clinical features of SAH in patients with active cancer are noticeable (Velander et al., 2012; Harold, 2019), and may be an initial feature of the malignancy, complication of active malignancy or delayed consequence of cancer and its management (Kim et al., 2013). In the present study, most SAH in patients with active cancer had common clinical manifestations, such as headache, nausea and vomiting which correlated with the findings of the previous studies (Navi et al., 2010; Shibahara et al., 2016; Matsuda et al., 2018; Tan et al., 2019). Further, this study represented the largest clinical series of SAH in patients with cancer. It was found that most SAH in patients with active cancer developed SAH within the first 6 months after diagnosis of cancer, suggesting that as soon as cancer diagnosis is established, measures should be taken to prevent SAH. Besides, some cancer patients have SAH as the first presentation. SAH had been reported in 1956 as the first presentation (Peterson and Heros, 2014), which indicate that when the pathogenesis of SAH was unexplained, the measures should be taken to determine the causes including aneurysms negative in first examination and occult cancer (Giombini et al., 1988; Kaim et al., 2010).
On the other hand, the ICH in patients with cancer were considered catastrophic and terminal event (Bitoh et al., 1984). As a result, the clinical feature of cerebral hemorrhage and SAH in patients with cancer remained not fully understood. In the present research, the severity of SAH was assessed with Hunt and Hess grade. It was revealed that nearly one in six patients have severe Hunt and Hess grade score. Among them, liquid tumors were rated as the most severe Hunt and Hess grade, which corroborates with the findings of two previous studies (Mocco et al., 2006; Pegoli et al., 2015). This suggests that SAH in patients with cancer is serious and requires prompt and aggressive rescue measures. For the sake of exploring 30 and 90 days mortality of SAH patients with active cancer, Kaplan–Meier curves based on cancer type and etiology were established. Subsequently, it was found that SAH patients with active cancer had poor outcomes, causing 30-day mortality of 41.5%, and 90-day mortality 52.0%. Moreover, it was also found that tumor types and the causes of SAH were related to the median survival time of SAH in patients with cancer, which was also similar to previous studies (Navi et al., 2010). Nevertheless, the present study added new insight about SAH in patients with active cancer.
ICH, including SAH in patients with cancer have been found with poor prognosis (Navi et al., 2010; Shibahara et al., 2016). However, predictors of mortality of SAH in patients with active cancer have not been investigated. Here, cox regression models were configured to explore the predictors of mortality. It was that liquid tumor, activated partial thromboplastin time, prealbumin, coagulopathy, gamma-glutamyl transferase, atrial fibrillation, and cryptogenic SAH were independent factors of survival in SAH patients with active cancer. This provided useful information facilitating clinicians to take up appropriate therapeutic measures.
Further, univariate analysis and multivariate analysis were also applied to reveal the independent risk factors of occurrence of SAH in patients with active cancer. It was noted that previous stroke, prothrombin time were the independent risk factors of occurrence of SAH in patients with active cancer. Additionally, to reveal the independent risk for cancer related SAH, a PSM eliminating the impacts of conventional vascular risks on SAH in cancer patients was performed. The PSM found that the decrease of platelet and prolonged PT time were the independent risk factors of cancer related SAH. In 2014, two studies provided a link between thrombocytopenia and prognosis or development of stroke (ischemic or hemorrhagic) (Park and Lee, 2014; Pósfaié et al., 2014). Therefore, the present study confirmed that decrease of platelet and prolonged PT associated with cancer related SAH.
However, decreased platelet and prolonged PT are also found in cancers and other diseases as a common coagulation marker (Pósfaié et al., 2014; Zhang et al., 2015; Schiffer et al., 2018). Considering that development of SAH in active cancer patients may be caused by the combined effects of the decrease of platelet and prolonged PT, the joint predictive factor was calculated. That the area under the ROC curve of the joint predictive factor was larger than that of each decreased platelet and prolonged PT and the sensitivity and specificity of the joint predictive factor were highest. This suggests that that the joint predictive factor could serve as a potential biomarker of cancer related SAH. Furthermore, by having the cutoff value of the joint predictive factor equal to 11.719, clinicians can discover the patient at high risk of SAH for cancer patients and identify the cancer related SAH from other etiologic SAH.
This study had some limitations which should also be mentioned before generalizing the present findings. Generally, this study was a retrospective comparison with propensity score-matching to minimize the bias in patient selection, but unobserved confounders remained. On the other hand, further research is needed because previous studies have suggested that factors affecting the occurrence of SAH include histological characteristics of the tumor, tumorous position, cancerous aneurysm and direct invasion of meningeal blood vessels, selective serotonin reuptake inhibitor as well as estrogen replacement therapy (Liwnicz et al., 1987; Navi et al., 2010; Qureshi et al., 2015; Renoux et al., 2017; Andrew et al., 2018; Yuichi et al., 2018; Tan et al., 2019).
Conclusion
In conclusion, cancer patients with SAH often have poor prognosis. The decrease in platelet and prolonged PT are the independent risk factor of cancer related SAH. Further, the joint predictive factor with cutoff value equal to 11.719 should serves as a novel biomarker to facilitate clinicians in establish the patients at high risk of SAH and identify cancer related SAH from other etiologic SAH. However, further studies should be conducted to confirm the present findings.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Guangxi Medical University Review Board approval number: 2021 (KY-E-185). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This study was supported by the Foundation of Prevention and Control of Chronic Diseases in Central-South China (Guangxi), (No. 2018YFC1311305) and the Foundation of Science and Technology Plan Projects of Qingxiu District of Nanning, (No. 2020043).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2022.813084/full#supplementary-material
References
Andrew, N., Johnson, M. D., Guan, W., and Michael, L. Y. (2018). Metastatic prostate cancer mimicking a subdural hematoma: a case report and literature review. J. Clin. Neuro. 55, 109–112. doi: 10.1016/j.jocn.2018.06.035
Bitoh, S., Hasegawa, H., Ohtsuki, H., Obashi, J., Fujiwara, M., and Sakurai, M. (1984). Cerebral neoplasms initially presenting with massive intracerebral hemorrhage. Surg. Neurol. 22, 57–62. doi: 10.1016/0090-3019(84)90230-1
Connolly, E. S., Rabinstein, A. A., Carhuapoma, J. R., Colin, P., Derdeyn, C. P., Dion, J., et al. (2012). Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 43, 1711–1737. doi: 10.1161/STR.0b013e3182587839
Giombini, S., Bruzzone, M. G., and Pluchino, F. (1988). Subarachnoid hemorrhage of unexplained cause. Neurosurgery 22, 313–316. doi: 10.1227/00006123-198802000-00006
Harold, P. (2019). Adams Jr.cancer and cerebrovascular Disease. Curr. Neurol. Neurosci. Rep. 19:73. doi: 10.1007/s11910-019-0985-0
Kaim, A., Proske, M., Kirsch, E., Weymarn, A., Rad, E. W., and Steinbrich, W. (2010). Value of repeat-angiography in cases of unexplained subarachnoid hemorrhage (SAH). Acta Neurol. Scand. 93, 366–373. doi: 10.1111/j.1600-0404.1996.tb00011.x
Kim, J. M., Jung, K. H., Park, K. H., Lee, S. T., Chu, K., and Roh, J. K. (2013). Clinical manifestation of cancer related stroke: retrospective case–control study. J. Neuro-Oncol. 111, 295–301. doi: 10.1007/s11060-012-1011-4
Kukreja, S., Ambekar, S., Sharma, M., and Nanda, A. (2014). Cauda equina schwannoma presenting with intratumoral hemorrhage and intracranial subarachnoid hemorrhage. J. Neurosurg. Spine 21, 357–360. doi: 10.3171/2014.5.SPINE131014
Liwnicz, B. H., Wu, S. Z., and Tew, J. M. (1987). The relationship between the capillary structure and hemorrhage in gliomas. J. Neurosurg. 66, 536–541. doi: 10.3171/jns.1987.66.4.0536 25.C
Matsuda, R., Nakamura, M., Tanaka, Y., Takamura, Y., Nakagawa, I., and Motoyama, Y. (2018). Glioblastoma mimicking subarachnoid hemorrhage of unknown etiology: a case Report. World Neurosurg. 120, 54–58. doi: 10.1016/j.wneu.2018.08.149
Meurer, W. J., Walsh, B., Vilke, G. M., and Coyne, C. J. (2016). Clinical guidelines for the emergency department evaluation of subarachnoid hemorrhage. J. Emerg. Med. 50, 696–701. doi: 10.1016/j.jemermed.2015.07.048
Mocco, J., Ransom, E. R., Komotar, R. J., Schmidt, J. M., and Connolly, E. S. (2006). Preoperative prediction of long-term outcome in poor-grade aneurysmal subarachnoid hemorrhage. Neurosurgery 59, 529–538. doi: 10.1227/01.NEU.0000228680.22550.A2
Nagai, S., Asai, T., Watanabe, T., Oshima, K., Hangaishi, A., Kanda, Y., et al. (2008). Simultaneous appearance of central nervous system relapse and subarachnoid hemorrhage during the treatment for acute promyelocytic leukemia. Ann. Hematol. 87, 593–595. doi: 10.1007/s00277-008-0436-5
Navi, B. B., Reichman, J. S., Berlin, D., Reiner, A. S., Panageas, K. S., Segal, A. Z., et al. (2010). Intracerebral and subarachnoid hemorrhage in patients with cancer. Neurology 74, 494–501. doi: 10.1212/WNL.0b013e3181cef837
Park, H. K., and Lee, S. H. (2014). Ischemic stroke associated with immune thrombocytopenia: lesion patterns and characteristics. Neurolog. Sci. 35, 1801–1806. doi: 10.1007/s10072-014-1843-0
Pegoli, M., Mandrekar, J., Rabinstein, A. A., and Lanzino, G. (2015). Predictors of excellent functional outcome in aneurysmal subarachnoid hemorrhage. J. Neurosurg. 122, 414–418. doi: 10.3171/2014.10.JNS14290
Peterson, E., and Heros, R. C. (2014). Don’t lose the lungs for the brain: pulmonary complications after subarachnoid hemorrhage. World Neurosurg. 82, 167–168. doi: 10.1016/j.wneu.2014.04.069
Pósfaié, E., Marton, I., Szõke, A., Borbényi, Z., Vécsei, L., Csomor, A., et al. (2014). Stroke in essential thrombocythemia. J. Neurol. Sci. 336, 260–262. doi: 10.1016/j.jns.2013.10.016
Qureshi, A. I., Malik, A. A., Saeed, O., Defillo, A., Sherr, G. T., and Suri, M. F. K. (2015). Hormone replacement therapy and the risk of subarachnoid hemorrhage in postmenopausal women. J. Neurosurg. 124, 45–50. doi: 10.3171/2014.12.JNS142329
Renoux, C., Vahey, S., Dell’Aniello, S., and Boivin, J. F. O. (2017). association of selective serotonin reuptake inhibitors with the risk for spontaneous intracranial hemorrhage. Jama Neurol. 74, 173–180. doi: 10.1001/jamaneurol.2016.4529
Schiffer, C. A., Bohlke, K., Delaney, M., Hume, H., Magdalinski, A. J., and Mccullough, J. J. (2018). platelet transfusion for patients with cancer: american society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 36, 283–299. doi: 10.1200/JCO.2017.76.1734
Schrader, B., Barth, H., Lang, E. W., Buhl, R., Hugo, H. H., Biederer, J., et al. (2000). Spontaneous intracranial haematomas caused by neoplasms. Acta Neurochirurgica. 142, 979–985. doi: 10.1007/s007010070052
Shibahara, I., Watanabe, T., Ezura, M., Inoue, T., Fujimura, M., Kimura, N., et al. (2016). Clinical features of subarachnoid hemorrhage in patients with positive cancer history. J. Neurooncol. 128, 129–136. doi: 10.1007/s11060-016-2085-1
Spetzger, U., Mull, M., Sure, U., and Gilsbach, J. (1995). Subarachnoid and intraventricular hemorrhage causedby hypernephroma metastasis, accompanied by innocent bilateral posterior communicating artery aneurysms. Surg. Neurol. 44, 275–278. doi: 10.1016/0090-3019(95)00170-0
Tan, D., Daly, C., Xenos, C., Lai, L. T., and Chandra, R. V. (2019). Glioblastoma presenting as spontaneous subarachnoid hemorrhage: technical case note of combined endovascular and microsurgical vision-sparing treatment. World Neurosurg. 128, 426–430. doi: 10.1016/j.wneu.2019.05.090
Velander, A. J., DeAngelis, L. M., and Navi, B. B. (2012). Intracranial hemorrhage in patients with cancer. Curr. Atheroscler. Rep. 14, 373–381. doi: 10.1007/s11883-012-0250-3
Yuichi, I., Yoshio, A., Takashi, I., Sho, O., Masaaki, K., and Toshihiko, W. (2018). A case of subarachnoid hemorrhage from ruptured oncotic fusiform aneurysms from choriocarcinoma metastasis treated with aneurysmectomy and vessel reconstruction. World Neurosurg. 113, 98–102. doi: 10.1016/j.wneu.2018.02.049
Zhang, D., Gong, S., Jin, H., Wang, J., Ping, S., Zou, W., et al. (2015). Coagulation parameters and risk of progressive hemorrhagic injury after traumatic brain injury: a systematic review and Meta-Analysis. Biomed. Res. Internat. 2015:261825. doi: 10.1155/2015/261825
Keywords: stroke, subarachnoid hemorrhage, neoplasms, propensity score, cerebral hemorrhage
Citation: Chen S, Zhang J, Lu X, Cen G, Song Y, Deng X, Xie Y, Liu L, Liu Q, Huang J, Li J, Yang H, Shi S, Pan L and Liang Z (2022) Cancer Related Subarachnoid Hemorrhage: A Multicenter Retrospective Study Using Propensity Score Matching Analysis. Front. Cell. Neurosci. 16:813084. doi: 10.3389/fncel.2022.813084
Received: 27 November 2021; Accepted: 11 January 2022;
Published: 07 February 2022.
Edited by:
Walace Gomes-Leal, Federal University of Western Pará, BrazilReviewed by:
Edmundo Luís Rodrigues Pereira, Federal University of Pará, BrazilCarlos Danilo Cardoso Matos Silva, Salvador University, Brazil
Copyright © 2022 Chen, Zhang, Lu, Cen, Song, Deng, Xie, Liu, Liu, Huang, Li, Yang, Shi, Pan and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhijian Liang, liangzhijian@gxmu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Shijian Chen1†
Shijian Chen1†  Gengyu Cen
Gengyu Cen Zhijian Liang
Zhijian Liang