Incidence of Parkinson’s disease and modifiable risk factors in Korean population: A longitudinal follow-up study of a nationwide cohort
- 1Department of Neurology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Republic of Korea
- 2Smart Healthcare Center, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Republic of Korea
- 3Department of Biomedical Research Center, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Republic of Korea
- 4Department of Neurology, Myongji Hospital, Hanyang University College of Medicine, Goyang, Republic of Korea
- 5Department of Obstetrics and Gynecology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Republic of Korea
Introduction: We aimed to investigate the incidence of Parkinson’s disease (PD) by age and year for each sex as well as the modifiable risk factors for PD. Using data from the Korean National Health Insurance Service, 938,635 PD and dementia-free participants aged ≥40 years who underwent general health examinations were followed to December 2019.
Methods: We analyzed the PD incidence rates according to age, year and sex. To investigate the modifiable risk factors for PD, we used the Cox regression model. Additionally, we calculated the population-attributable fraction to measure the impact of the risk factors on PD.
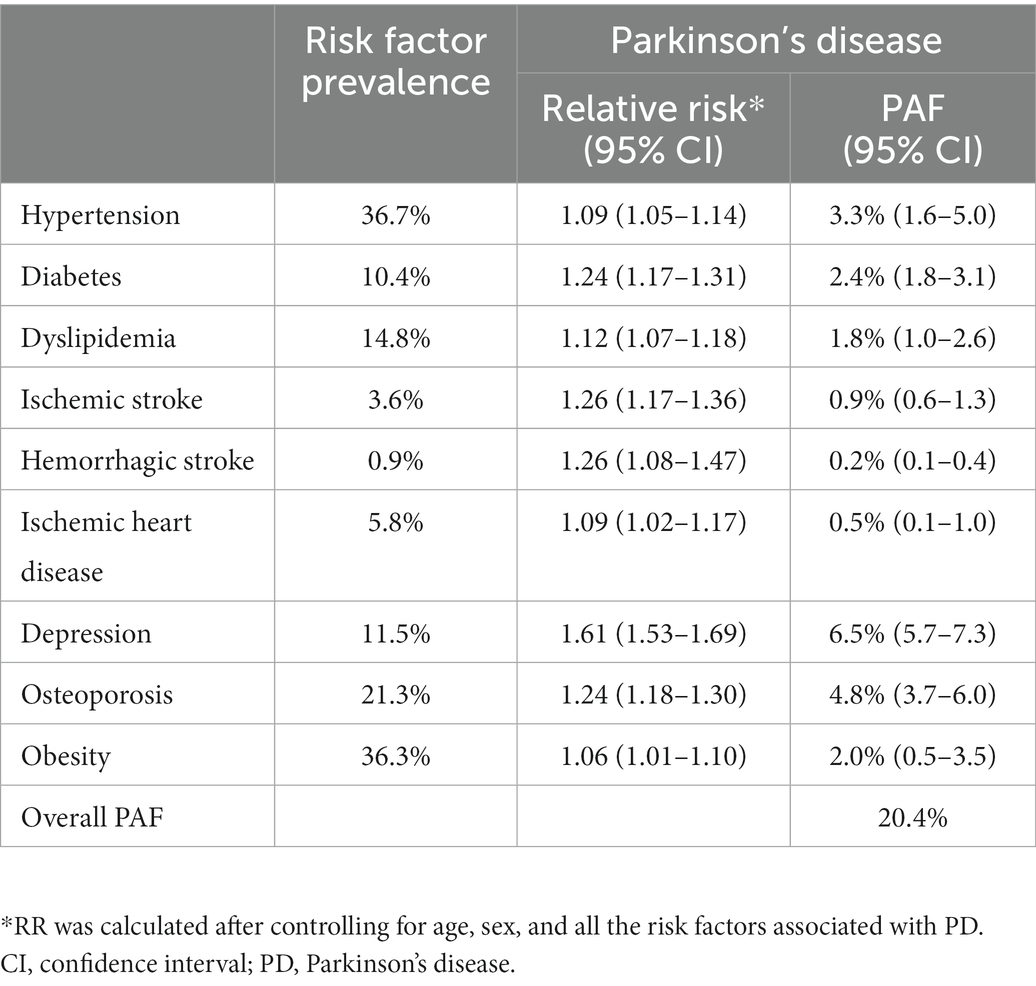
Results: During follow-up, 9,924 of the 938,635 (1.1%) participants developed PD. The incidence of PD increased continuously from 2007 to 2018, reaching 1.34 per 1,000 person-years in 2018. The incidence of PD also increases with age, up to 80 y. Presence of hypertension (SHR = 1.09, 95% CI 1.05 to 1.14), diabetes (SHR = 1.24, 95% CI 1.17 to 1.31), dyslipidemia (SHR = 1.12, 95% CI 1.07 to 1.18), ischemic stroke (SHR = 1.26, 95% CI 1.17 to 1.36), hemorrhagic stroke (SHR = 1.26, 95% CI 1.08 to 1.47), ischemic heart disease (SHR = 1.09, 95% CI 1.02 to 1.17), depression (SHR = 1.61, 95% CI 1.53 to 1.69), osteoporosis (SHR = 1.24, 95% CI 1.18 to 1.30), and obesity (SHR = 1.06, 95% CI 1.01 to 1.10) were independently associated with a higher risk for PD.
Discussion: Our results highlight the effect of modifiable risk factors for PD in the Korean population, which will help establish health care policies to prevent the development of PD.
1. Introduction
As the population ages, the number of patients with neurodegenerative diseases also rapidly increase, along with an increased socioeconomic burden (Bach et al., 2011). Parkinson’s disease (PD) is the second most common neurodegenerative disease and is characterized by progressive non-motor symptoms and motor deficits, including bradykinesia, tremors, and rigidity. Given that PD currently has no disease-modifying treatment, it is important to identify the incidence and modifiable risk factors of PD and to find effective strategies for preventing PD in public health care policies.
The incidence of PD varies across countries, ranging from 80.4 to 678 per 100,000 person-years (Baldereschi et al., 2000; Benito-León et al., 2004; de Lau et al., 2004; Taylor et al., 2006; Alves et al., 2009; Driver et al., 2009; Linder et al., 2010; Winter et al., 2010; Caslake et al., 2013). In the Korean population, the prevalence of PD has been steadily increasing, and the prevalence in people aged ≥50 y is approximately 0.4% (Park J. H. et al., 2019). However, a detailed information regarding age-specific PD incidence is lacking.
A variety of known modifiable risk factors exist for PD with varying degrees of impact on PD (Ascherio and Schwarzschild, 2016). However, the extent to which modifiable risk factors affect PD remains controversial. The prevention of PD has been the focus of research owing to the absence of disease-modifying medications. Risk factors that have a higher relative risk (RR) for PD and a higher prevalence in the elderly population may contribute more to PD incidence. Therefore, the RR and prevalence of each risk factor in the elderly population should be considered to establish public health care measures for PD prevention. In addition, among the risk factors, the effect of cardiometabolic syndrome on PD risk has not been established, although a growing body of evidence has shown that cardiometabolic syndrome is closely related to Alzheimer’s disease, included in neurodegenerative disease with PD.
Using the Korean National Health Insurance Service (KNHIS) data, the first goal of our study was to investigate PD incidence by age and year for each sex. The second goal was to explore the hazard ratio (HR) of each modifiable risk factor for PD. The third goal was to evaluate the RR of each modifiable risk factor for PD and estimate the attributable fraction of the risk factors in elderly Koreans.
2. Materials and methods
This study was approved by the Institutional Review Board of the Korea University Guro Hospital and adhered to the principles of the Declaration of Helsinki.
2.1. Data source
We used a customized dataset from the KNHIS, which includes more than 99% of the Korean population (approximately 50 million).1 The KNHIS database includes personal information; health insurance claim codes (procedures and prescriptions); diagnostic codes from the Korean Standard Classification of Diseases, 7th Revision, which is based on the International Classification of Diseases, 10th Revision (ICD-10); death records from the Korean National Statistical Office; and general medical examination data for each participant from 2002 to 2019. Data on body mass index (BMI) and behavioral characteristics, including frequency of physical activity, smoking, and alcohol consumption, were obtained from the general health examinations in the KNHIS database.
2.2. PD and dementia-free cohort
To exclude participants with PD and dementia, PD was defined according to the ICD-10 code (G20) and prescriptions of PD medication. Dementia was defined according to the ICD-10 codes (F00, F01, F02, F03, F05, G30, or G31) and dementia medication prescription.
In the NHIS dataset, 6,257,567 PD and dementia-free participants aged 45 y or older who underwent general health examinations were identified. We randomly selected 15% (938,635) of the participants and enrolled them in the present study.
2.3. Definition of modifiable risk factors
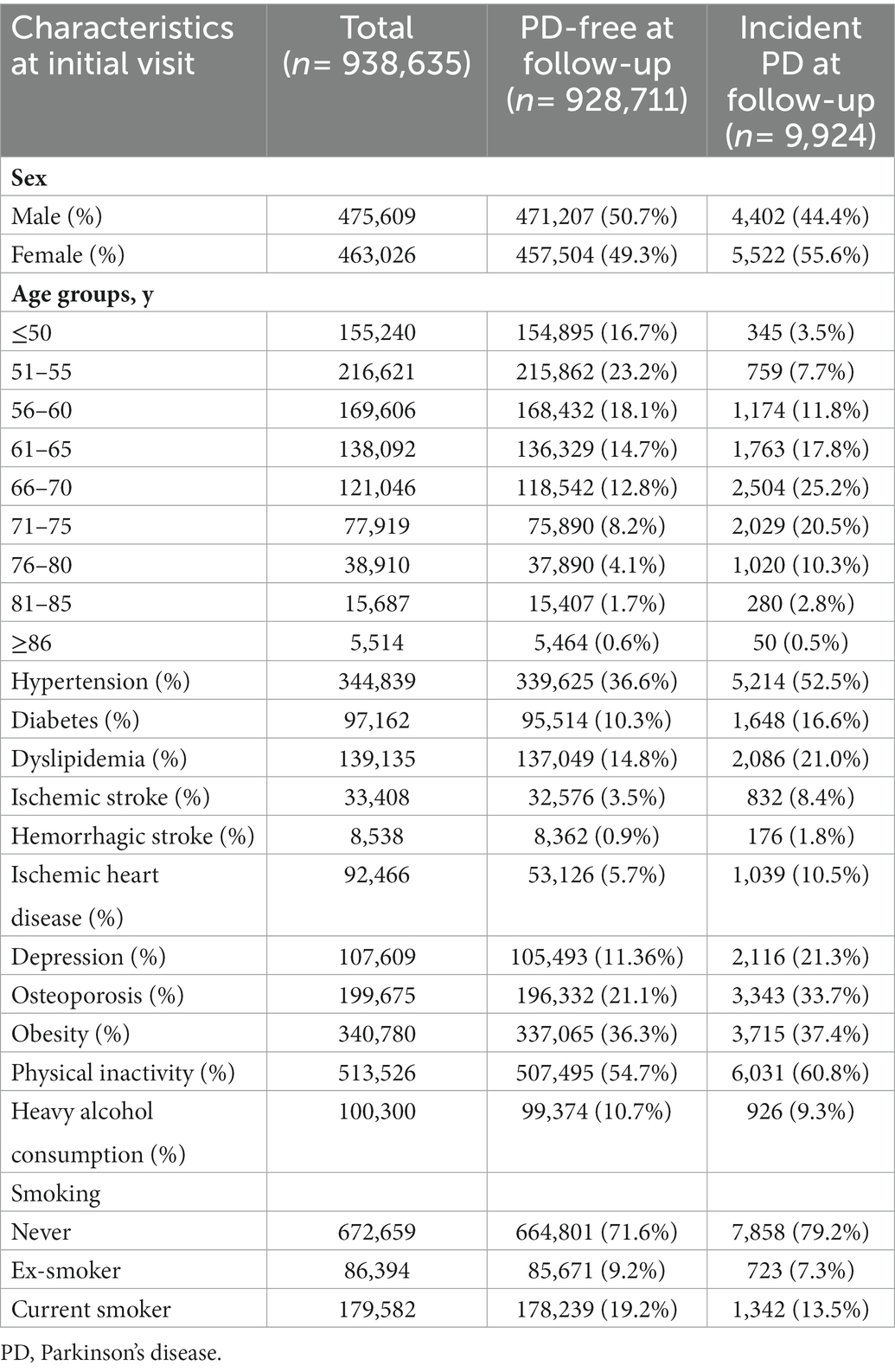
With respect to the modifiable risk factors for PD, we considered hypertension, diabetes, hyperlipidemia, ischemic stroke, hemorrhagic stroke, ischemic heart disease, depression, osteoporosis, obesity, physical inactivity, smoking status, and heavy alcohol consumption. The presence of hypertension was defined according to ICD-10 code (I10-15) and prescription of antihypertensive medication. The presence of diabetes was defined according to the ICD-10 code (E8-14) and prescription of antidiabetic medication. The presence of hyperlipidemia was defined according to the ICD-10 code (E78) and the prescription of lipid-lowering medication. The presence of ischemic stroke was defined according to the ICD-10 code (I63-66) and prescription of antiplatelet or anticoagulation agents. Hemorrhagic stroke was defined according to the ICD-10 code (I60-62). The presence of ischemic heart disease was defined according to the ICD-10 code (I20-25) and the prescription of antiplatelet or anticoagulation agents. Depression was defined according to the ICD-10 code (F32-34). Osteoporosis was defined according to the ICD-10 code (M80-82). Obesity was defined as a BMI ≥ 25 kg/m2. Physical inactivity was defined as the absence of physical activity, even once a week. Smoking status was grouped into three levels: never smoked, ex-smoker, and current smoker. Heavy alcohol consumption was defined as alcohol consumption more than three times per week.
2.4. Definition of outcome and follow-Up
The outcome of the study was the development of PD, which was defined according to the ICD-10 code (G20) and prescription of PD medication for ≥3 months. Furthermore, to exclude secondary parkinsonism and atypical parkinsonism, such as progressive supranuclear palsy and multiple system atrophy, we excluded participants who additionally had ICD-10 code (G21-23) after diagnosis of PD from the outcome. Participants without PD during follow-up were considered to have completed the study on the date of death or at the end of follow-up. The patients were followed up from the date of the general health examinations (baseline) to the date of PD diagnosis, date of death, or until December 2019.
2.5. Statistical analyses
Baseline characteristics are presented as mean ± standard deviation or median (interquartile range) and frequency (%). First, in the PD-free cohort, we calculated the PD incidence rates and confidence intervals of the incidence rates under the assumption that the number of outcomes follows a Poisson distribution. Second, to investigate the modifiable risk factors for PD, we calculated HR using the Cox regression model, including each modifiable risk factor as a separate predictor after controlling for age and sex (model 1). We further performed the Cox regression model including modifiable risk factors as predictors that showed statistical significance in model 1 after controlling for age and sex (model 2). Third, we calculated the RR using log-binomial regression to adjust for age, sex, and modifiable risk factors (McNutt et al., 2003). These models included the risk factors that showed statistical significance in Cox regression model 1 and were controlled for age and sex. Finally, the population-attributable fraction (PAF) was calculated using Levin’s formula:
With respect to the prevalence of modifiable risk factors, we considered the prevalence in our cohort as presented in Table 1. To identify the combined effects of risk factors, we obtained the overall PAFs using the following formula:
Sensitivity analyses were used to exclude participants with stroke to eliminate the mediating or confounding effects of stroke on the relationship between the modifiable risk factors and the development of PD.
All reported p-values were two-sided and the significance level was set at 0.05. All analyses were performed using SAS (version 9.3; SAS Institute Inc., Cary, NC, United States).
3. Results
3.1. Clinical characteristics of the study participants at baseline
Among the 938,635 participants in the PD and dementia-free cohorts, 463,026 (49.3%) were women. The most prevalent modifiable risk factor was physical inactivity (54.7%), followed by hypertension (36.7%), obesity (36.3%), osteoporosis (21.3%), and current smoking (19.1%, Table 1). Subjects who developed PD were more likely to be and have hypertension, diabetes, dyslipidemia, ischemic heart disease, ischemic stroke, hemorrhagic stroke, depression, and osteoporosis than those who were PD-free at follow-up.
3.2. PD incidence by year, age, and sex
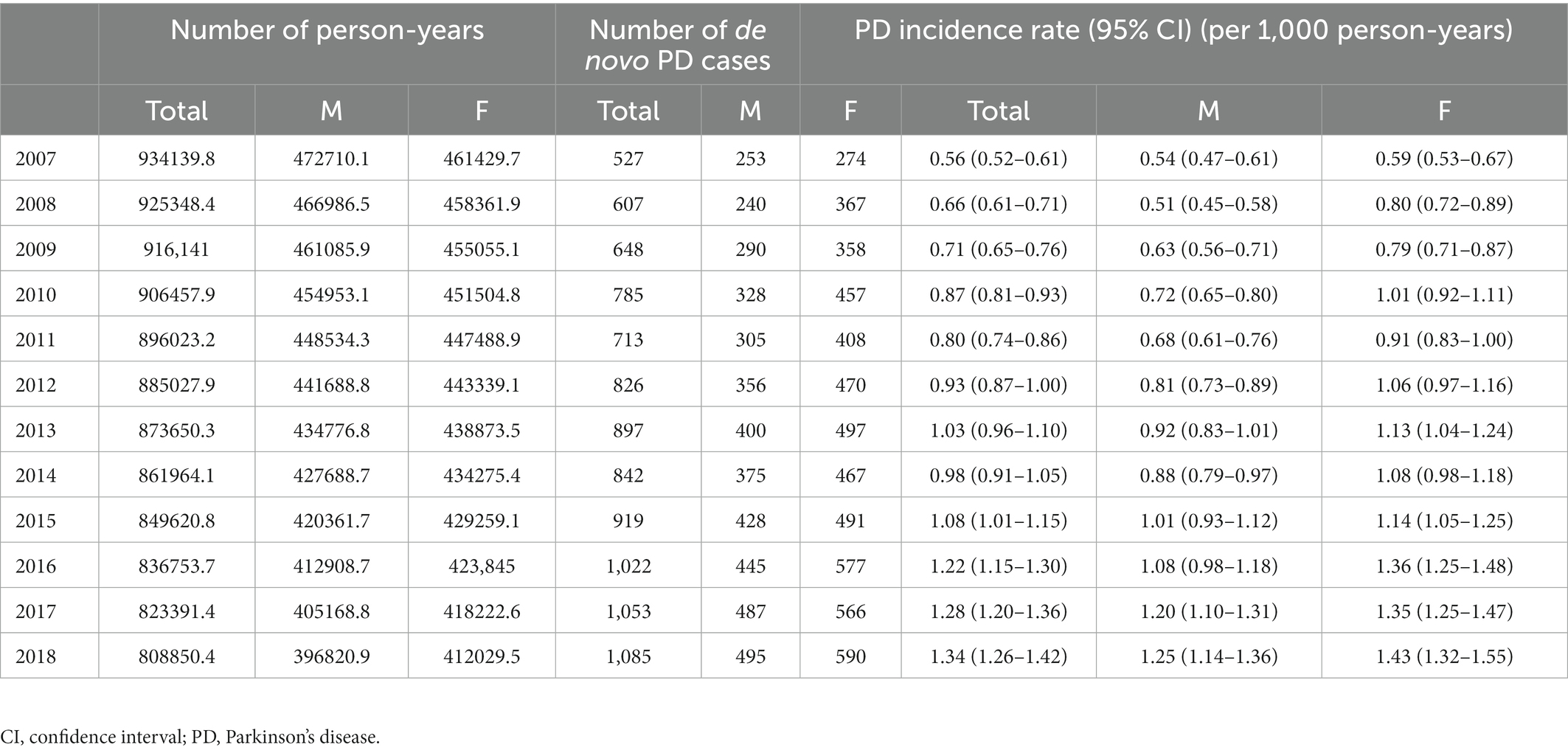
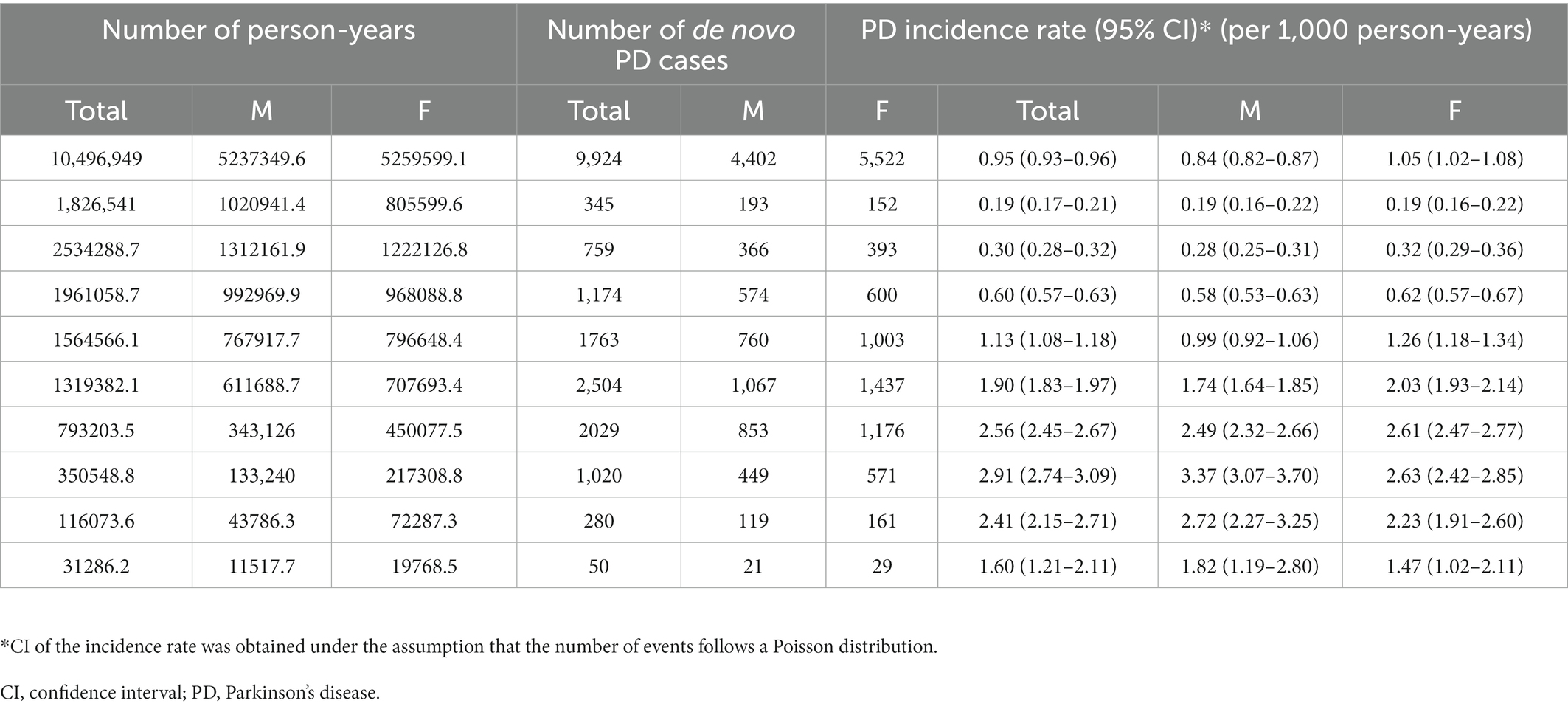
During follow-up, 9,924 of the 938,635 (1.1%) participants developed PD. The incidence rate of PD showed annual growth, increasing from 0.56 per 1,000 person-years in 2006 to 1.34 per 1,000 person-years in 2018 (Table 2). The incidence rate of PD also increases with age, up to 80 y. Specifically, the incidence rate of PD was only 0.19 per 1,000 person-years among participants who were 50 y and less, while that of PD increased to 2.91 per 1,000 person-years among participants who were 76 to 80 y old (Table 3). In terms of sex, women (9,447; 54.4%) were more likely to develop PD than men (7,910; 45.57%). The incidence rate of PD in women (1.05 per 1,000 person-years) was higher than that in men (0.84 per 1,000 person-years, Table 3).
3.3. Modifiable risk factors for PD
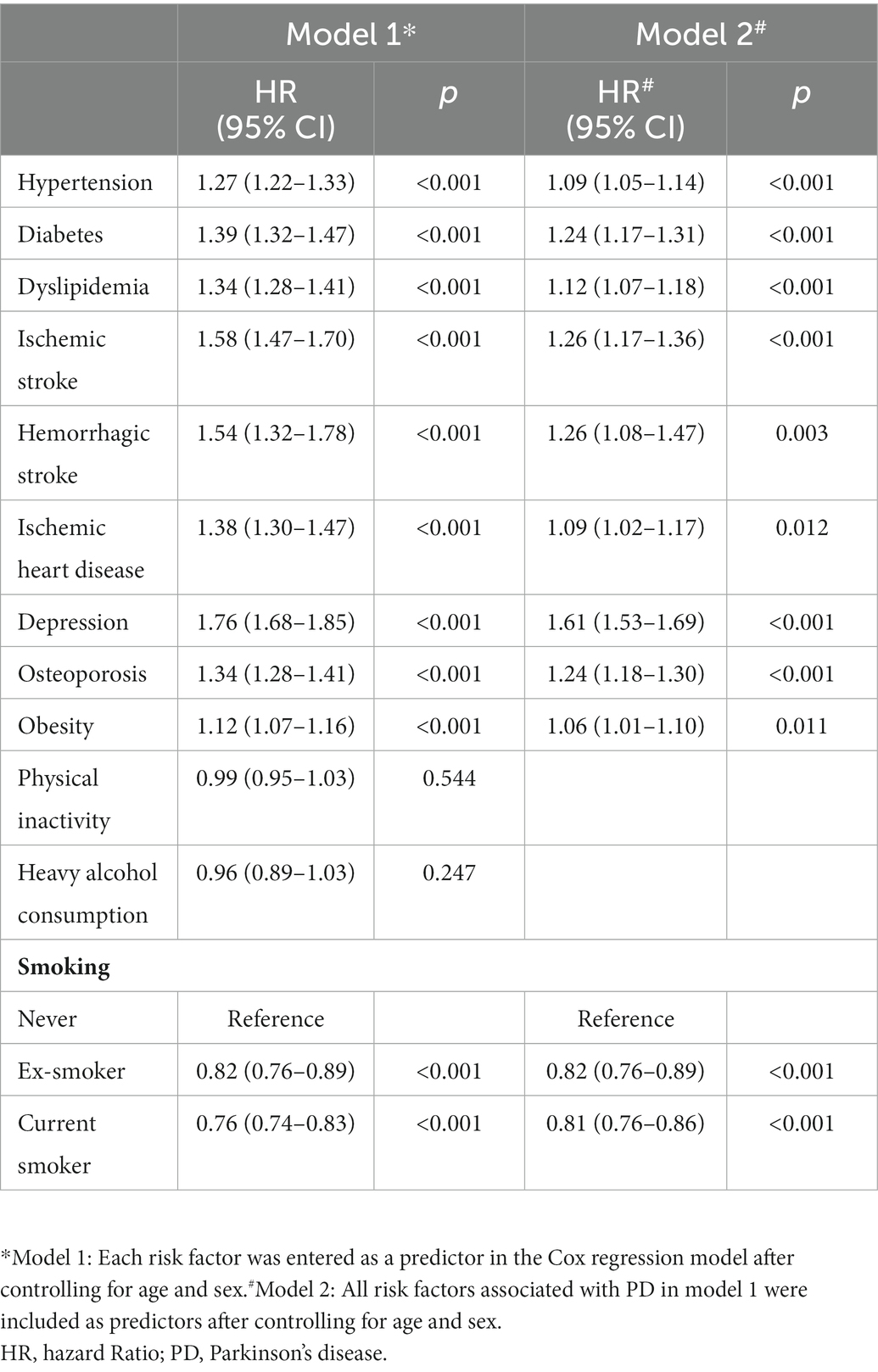
In model 1, presence of hypertension (subdistribution hazard ratio [SHR] = 1.27, 95% confidence interval [CI] 1.22 to 1.33), diabetes (SHR = 1.39, 95% CI 1.32 to 1.47), dyslipidemia (SHR = 1.34, 95% CI 1.28 to 1.41), ischemic stroke (SHR = 1.58, 95% CI 1.47 to 1.70), hemorrhagic stroke (SHR = 1.54, 95% CI 1.32 to 1.78), ischemic heart disease (SHR = 1.38, 95% CI 1.30 to 1.47), depression (SHR = 1.76, 95% CI 1.68 to 1.85), osteoporosis (SHR = 1.34, 95% CI 1.28 to 1.41), and obesity (SHR = 1.12, 95% CI 1.07 to 1.16) increased the risk of PD (Table 4). In model 2, presence of hypertension (SHR = 1.09, 95% CI 1.05 to 1.14), diabetes (SHR = 1.24, 95% CI 1.17 to 1.31), dyslipidemia (SHR = 1.12, 95% CI 1.07 to 1.18), ischemic stroke (SHR = 1.26, 95% CI 1.17 to 1.36), hemorrhagic stroke (SHR = 1.26, 95% CI 1.08 to 1.47), ischemic heart disease (SHR = 1.09, 95% CI 1.02 to 1.17), depression (SHR = 1.61, 95% CI 1.53 to 1.69), osteoporosis (SHR = 1.24, 95% CI 1.18 to 1.30), and obesity (SHR = 1.06, 95% CI 1.01 to 1.10) remained independently associated with a higher risk for PD (Table 4).
3.4. Population-attributable fraction for PD
As presented in Table 5, among the modifiable risk factors, depression had the greatest impact on PD (PAF, 6.5%), followed by osteoporosis (PAF, 4.8%), and hypertension (PAF, 3.3%). The overall PAF of the modifiable risk factors was 20.4%.
3.5. Sensitivity analyses
Among the participants without stroke, hypertension (SHR = 1.09, 95% CI 1.03 to 1.13), diabetes (SHR = 1.24, 95% CI 1.17 to 1.32), dyslipidemia (SHR = 1.14, 95% CI 1.08 to 1.21), ischemic heart disease (SHR = 1.13, 95% CI 1.04 to 1.22), depression (SHR = 1.68, 95% CI 1.59 to 1.77), osteoporosis (SHR = 1.25, 95% CI 1.19 to 1.31), and obesity (SHR = 1.05, 95% CI 1.00 to 1.10) were independently associated with a higher risk for PD (Supplementary Table 1).
4. Discussion
In the present study, we identified the incidence and modifiable risk factors of PD using the Korean nationwide cohort data. The major findings of this study are as follows. First, the incidence of PD increased continuously from 2007 to 2018, reaching 1.34 per 1,000 person-years in 2018. Second, the incidence of PD increases with age, up to 80 y. Third, cardiometabolic syndromes, depression, and osteoporosis are associated with a higher incidence of PD, independent of stroke. Overall, our results will help in the design of public health policies for PD prevention.
Our first major finding was the increasing trend in the incidence of PD in South Korea from 2007 to 2018. Trends in PD incidence vary depending on the study design, population, and period. Stable or slightly decreasing trends have been reported in Western countries, such as the United States, the United Kingdom, France, and the Netherlands during the 2010s (Akushevich et al., 2013; Horsfall et al., 2013; Blin et al., 2015; Darweesh et al., 2016; Evans et al., 2016). Conversely, several studies have reported an annual increase in PD incidence (Liu et al., 2016; Savica et al., 2016). In the Minnesota population aged ≥70 years old and older, the incidence of PD increased from 0.80 per 1,000 person-years to 1.37 per 1,000 person-years over 30 y (Savica et al., 2016). An increasing trend has also been identified in Taiwan, which is included in the far-eastern Asian countries along with Korea (Liu et al., 2016). This increasing trend may be attributed to better recognition of PD in older patients with comorbidities. In recent years, physicians have begun to diagnose elderly individuals with cancer, cardiovascular diseases, or other conditions as having PD, because parkinsonism symptoms have become important in the overall clinical outcome and are considered one of the major causes of disability and mortality. In addition, the KNHIS started to cover dopamine transporter images in 2016, and consequently, the diagnosis of PD became relatively simplified, which could contribute to an increasing point of PD incidence in 2016. The increasing incidence of PD may also be explained by the increase in the prevalence of modifiable risk factors for PD, such as hypertension and dyslipidemia, and the dramatic decrease in the rate of smoking, a protective factor for PD, in Korea (Korea Health Statistics 2019, Korea National Health and Nutrition Examination Survey,2).
Our second major finding was that the incidence of PD increased with age, up to 80 y. It is well known that PD prevalence is low (0.13–1.6%) in populations aged less than 60 y, after which there is a sharp increase in incidence (Kis et al., 2002; Benito-León et al., 2003; Chan et al., 2005; Blin et al., 2015). These results are consistent with our findings that PD incidence was only 0.36 per 1,000 person-years among participants aged 60 y or less, whereas PD incidence increased to 2.91 per 1,000 person-years among those aged 76–80 y. Although age may be an important risk factor for PD, peak age-specific incidence varies among studies. Several studies have reported that PD incidence uniformly increases up to the ninth decade (Allyson Jones et al., 2012; Caslake et al., 2013; Blin et al., 2015), whereas others have found a decline in PD incidence among the oldest old population. We also found that the age-specific incidence peaked in the population aged 76–80 y and declined beyond this age. However, a direct comparison between the studies is difficult because of the small sample sizes in the oldest old group and varying definitions (von Campenhausen et al., 2005). The decline in the oldest old population may be due to the following reasons. First, the high burden of comorbidities, such as dementia and musculoskeletal disease, in the oldest old group increases the diagnostic uncertainty for PD (Meara et al., 1999). Second, individuals with PD at the oldest old age may not use a medical institution (Bowling et al., 1991). Third, mortality selection may cause unobserved heterogeneity within the oldest old group, which in turn determines the ratio of individuals with and without PD in favor of the latter group (Vaupel et al., 1979).
Contrary to our expectations, we observed that PD incidence in women was significantly higher than that in men. A growing body of evidence shows a predominance of PD incidence in the male population (Baldereschi et al., 2000; Clavería et al., 2002; Benito-León et al., 2003; Alves et al., 2009; Nerius et al., 2017) or no sex difference in PD incidence (Linder et al., 2010; Winter et al., 2010). However, a few Asian studies have reported female predominance in PD (Kimura et al., 2002; Park J. H. et al., 2019). Although the underlying mechanisms for this discrepancy remain unclear, genetic, hormonal, cultural, and environmental factors may mediate such outcomes. First, Asian women may have different genetic susceptibilities to PD. Second, substantial differences in cultural and environmental factors during childhood in the elderly population (in the mid 1900s) in Korea may cause sex-related disparities in educational levels and literacy rates associated with brain reserves. Furthermore, Korean women may encounter more risk factors, including dietary deficiencies, agricultural occupations, pesticide use, and head trauma. Finally, the greater average longevity of women in Korea may lead to a quicker increase in the elderly female population, which in turn causes female predominance. In fact, the sex-specific difference in life expectancy was higher in South Korea (men, 79.7 y and women, 85.7 y) than in European countries (men, 79.7 y and women, 82.8 y) according to the Office for National Statistics.
Our third major finding was that cardiometabolic syndrome, depression, and osteoporosis were associated with a higher incidence of PD, independent of stroke. In particular, among cardiometabolic syndromes, diabetes contributes the most to the development of PD, which is consistent with previous findings that diabetes is associated with a higher PD risk (Hu et al., 2007; Driver et al., 2008; Schernhammer et al., 2011; Xu et al., 2011; Sun et al., 2012). However, the association between hypertension, dyslipidemia, obesity, and PD remains controversial. Many previous studies in Western countries have shown that hypertension, dyslipidemia, and obesity are not associated with a higher risk of PD (Abbott et al., 2002; Logroscino et al., 2007; Simon et al., 2007; Kyrozis et al., 2013; Ascherio and Schwarzschild, 2016), whereas a few studies have reported that dyslipidemia and obesity may be risk factors for PD (Hu et al., 2006, 2008). These discordant results may be related to the modest effect of cardiometabolic syndrome on incident PD, or undiscerned confounding or modifying factors that modulate the relationship between cardiometabolic syndrome and PD risk. Another explanation for this discrepancy is ethnic differences in the effects of cardiometabolic syndromes on PD. Compared to European populations, Asian populations have a higher incidence of cardiometabolic syndrome (Yoon et al., 2006) and associated complications, including coronary artery disease (McKeigue et al., 1989), stroke (Eastwood et al., 2015), dementia (Niu et al., 2017; Park J. E. et al., 2019; Jang et al., 2021), and high mortality rates (Wild et al., 2007). Additionally, studies have shown that Asian populations tend to have higher visceral fat and lower subcutaneous fat than European populations with similar BMI (Nazare et al., 2012). Such unequal fat distribution may be associated with more severe cardiometabolic complications commonly seen in Asian populations, given that visceral fat is associated with arteriosclerosis and compromised brain health (Debette et al., 2010; Isaac et al., 2011; Kato et al., 2011; Widya et al., 2015).
We also found that depression and osteoporosis increased PD risk, suggesting that depression and osteoporosis preceded the diagnosis of PD. Although depression is a well-known premotor symptom (Tolosa et al., 2007), the temporal relationship between osteoporosis and PD remains poorly understood. Contrary to previous findings of a greater risk of osteoporosis in patients with PD (Torsney et al., 2014), the present study revealed that patients with osteoporosis were at a high risk of PD. Although further studies should be necessary to identify the mechanism, our findings suggested that osteoporosis and PD might have an interactive relationship or that osteoporosis might be a veiled premotor symptom of PD.
Our study has several limitations that should be addressed. First, the discordance between the diagnosis of PD in clinical practice and that recorded in the KNHIS may have led to inaccurate results. However, these issues could be mitigated by the fact that the diagnostic code of PD was classified into the registration code in the program for rare intractable diseases to increase diagnostic accuracy. Additionally, we added the prescription of PD medication for ≥3 months as the outcome definition. Second, we could not define cardiometabolic syndrome using blood pressure measurements and laboratory data such as fasting glucose and total cholesterol levels. Third, we could not assess the exposure time and changes in the risk factors and risk factors that occurred after 2006. Fourth, we could not consider the potential effect of antihypertensive, antidiabetic, and lipid-lowering medication. Fifth, due to a lack of information on a positive family history, traumatic brain injury, exposure to pesticides, dietary patterns, air pollution, and social isolation, we did not identify the PAF of these factors.
Despite the aforementioned limitations, our study aimed to identify age-and sex-specific PD incidence based on a nationwide cohort that included a larger number of elderly participants. In addition, our results highlight the effect of modifiable risk factors for PD in the Korean population, which will help establish health care policies to prevent the development of PD.
Data availability statement
The datasets presented in this article are not readily available because The Korean NHIS database is confidential and approved for use by researchers who meet the criteria for access through the Korea National Health Insurance Sharing Service (NHISS) Institutional Data Access Committee (https://nhiss.nhis.or.kr/bd/ay/bdaya001iv.do). If data are requested for additional analysis, the corresponding author would consider it deliberately to offer after passing the review process of the Korea NHISS Institutional Data Access Committee and after payment of the data access fee charged to the requester. Requests to access the datasets should be directed to S-BK, parkinson@korea.ac.kr.
Ethics statement
The studies involving human participants were reviewed and approved by this study was approved by the Institutional Review Board of the Korea University Guro Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
SK analyzed and interpreted the data and drafted the manuscript for intellectual content. S-JM and MK analyzed and interpreted the data. SC and GC played major roles in data acquisition. S-BK acquired the data, designed and conceptualized the study, and revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: 2022R1I1A1A01056956) and Korea University Guro Hospital (KOREA RESEARCH-DRIVEN HOSPITAL; grant number: O2208241).
Acknowledgments
This study was performed using the database from the National Health Insurance System, and the results do not necessarily represent the opinions of the National Health Insurance Corporation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1094778/full#supplementary-material
Footnotes
References
Abbott, R. D., Ross, G. W., White, L. R., Nelson, J. S., Masaki, K. H., Tanner, C. M., et al. (2002). Midlife adiposity and the future risk of Parkinson's disease. Neurology 59, 1051–1057. doi: 10.1212/wnl.59.7.1051
Akushevich, I., Kravchenko, J., Ukraintseva, S., Arbeev, K., and Yashin, A. I. (2013). Time trends of incidence of age-associated diseases in the US elderly population: medicare-based analysis. Age Ageing 42, 494–500. doi: 10.1093/ageing/aft032
Allyson Jones, C., Wayne Martin, W. R., Wieler, M., King-Jesso, P., and Voaklander, D. C. (2012). Incidence and mortality of Parkinson's disease in older Canadians. Parkinsonism Relat. Disord. 18, 327–331. doi: 10.1016/j.parkreldis.2011.11.018
Alves, G., Müller, B., Herlofson, K., HogenEsch, I., Telstad, W., Aarsland, D., et al. (2009). Incidence of Parkinson's disease in Norway: the Norwegian ParkWest study. J. Neurol. Neurosurg. Psychiatry 80, 851–857. doi: 10.1136/jnnp.2008.168211
Ascherio, A., and Schwarzschild, M. A. (2016). The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol. 15, 1257–1272. doi: 10.1016/s1474-4422(16)30230-7
Bach, J. P., Ziegler, U., Deuschl, G., Dodel, R., and Doblhammer-Reiter, G. (2011). Projected numbers of people with movement disorders in the years 2030 and 2050. Move. disorders Off. J. Move. Disord. Soc. 26, 2286–2290. doi: 10.1002/mds.23878
Baldereschi, M., Di Carlo, A., Rocca, W. A., Vanni, P., Maggi, S., Perissinotto, E., et al. (2000). Parkinson's disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA working group Italian Longitudinal Study on Aging Neurology 55, 1358–1363. doi: 10.1212/wnl.55.9.1358
Benito-León, J., Bermejo-Pareja, F., Morales-González, J. M., Porta-Etessam, J., Trincado, R., Vega, S., et al. (2004). Incidence of Parkinson disease and parkinsonism in three elderly populations of Central Spain. Neurology 62, 734–741. doi: 10.1212/01.wnl.0000113727.73153.68
Benito-León, J., Bermejo-Pareja, F., Rodríguez, J., Molina, J. A., Gabriel, R., and Morales, J. M. (2003). Prevalence of PD and other types of parkinsonism in three elderly populations of Central Spain. Move. Disord. Off. J. Move. Disord. Soc. 18, 267–274. doi: 10.1002/mds.10362
Blin, P., Dureau-Pournin, C., Foubert-Samier, A., Grolleau, A., Corbillon, E., Jové, J., et al. (2015). Parkinson's disease incidence and prevalence assessment in France using the national healthcare insurance database. Eur. J. Neurol. 22, 464–471. doi: 10.1111/ene.12592
Bowling, A., Farquhar, M., and Browne, P. (1991). Use of services in old age: data from three surveys of elderly people. Soc. Sci. Med. 33, 689–700. doi: 10.1016/0277-9536(91)90023-6
Caslake, R., Taylor, K., Scott, N., Gordon, J., Harris, C., Wilde, K., et al. (2013). Age-, gender-, and socioeconomic status-specific incidence of Parkinson's disease and parkinsonism in Northeast Scotland: the PINE study. Parkinsonism Relat. Disord. 19, 515–521. doi: 10.1016/j.parkreldis.2013.01.014
Chan, D. K., Cordato, D., Karr, M., Ong, B., Lei, H., Liu, J., et al. (2005). Prevalence of Parkinson's disease in Sydney. Acta Neurol. Scand. 111, 7–11. doi: 10.1111/j.1600-0404.2004.00348.x
Clavería, L. E., Duarte, J., Sevillano, M. D., Pérez-Sempere, A., Cabezas, C., Rodríguez, F., et al. (2002). Prevalence of Parkinson's disease in Cantalejo, Spain: a door-to-door survey. Move. Disord. Off. J. Move. Disord. Soc. 17, 242–249. doi: 10.1002/mds.10087
Darweesh, S. K., Koudstaal, P. J., Stricker, B. H., Hofman, A., and Ikram, M. A. (2016). Trends in the incidence of Parkinson disease in the general population: the Rotterdam study. Am. J. Epidemiol. 183, 1018–1026. doi: 10.1093/aje/kwv271
de Lau, L. M., Giesbergen, P. C., de Rijk, M. C., Hofman, A., Koudstaal, P. J., and Breteler, M. M. (2004). Incidence of Parkinsonism and Parkinson disease in a general population: the Rotterdam study. Neurology 63, 1240–1244. doi: 10.1212/01.wnl.0000140706.52798.be
Debette, S., Beiser, A., Hoffmann, U., DeCarli, C., O'Donnell, C. J., Massaro, J. M., et al. (2010). Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann. Neurol. 68, 136–144. doi: 10.1002/ana.22062
Driver, J. A., Logroscino, G., Gaziano, J. M., and Kurth, T. (2009). Incidence and remaining lifetime risk of Parkinson disease in advanced age. Neurology 72, 432–438. doi: 10.1212/01.wnl.0000341769.50075.bb
Driver, J. A., Smith, A., Buring, J. E., Gaziano, J. M., Kurth, T., and Logroscino, G. (2008). Prospective cohort study of type 2 diabetes and the risk of Parkinson's disease. Diabetes Care 31, 2003–2005. doi: 10.2337/dc08-0688
Eastwood, S. V., Tillin, T., Chaturvedi, N., and Hughes, A. D. (2015). Ethnic differences in associations between blood pressure and stroke in south Asian and European men. Hypertension 66, 481–488. doi: 10.1161/hypertensionaha.115.05672
Evans, J. R., Cummins, G., Breen, D. P., Foltynie, T., Mason, S. L., Brayne, C. E., et al. (2016). Comparative epidemiology of incident Parkinson's disease in Cambridgeshire, UK. J. Neurol. Neurosurg. Psychiatry 87, 1034–1036. doi: 10.1136/jnnp-2015-312581
Horsfall, L., Petersen, I., Walters, K., and Schrag, A. (2013). Time trends in incidence of Parkinson's disease diagnosis in UK primary care. J. Neurol. 260, 1351–1357. doi: 10.1007/s00415-012-6804-z
Hu, G., Antikainen, R., Jousilahti, P., Kivipelto, M., and Tuomilehto, J. (2008). Total cholesterol and the risk of Parkinson disease. Neurology 70, 1972–1979. doi: 10.1212/01.wnl.0000312511.62699.a8
Hu, G., Jousilahti, P., Bidel, S., Antikainen, R., and Tuomilehto, J. (2007). Type 2 diabetes and the risk of Parkinson's disease. Diabetes Care 30, 842–847. doi: 10.2337/dc06-2011
Hu, G., Jousilahti, P., Nissinen, A., Antikainen, R., Kivipelto, M., and Tuomilehto, J. (2006). Body mass index and the risk of Parkinson disease. Neurology 67, 1955–1959. doi: 10.1212/01.wnl.0000247052.18422.e5
Isaac, V., Sim, S., Zheng, H., Zagorodnov, V., Tai, E. S., and Chee, M. (2011). Adverse associations between visceral adiposity, brain structure, and cognitive performance in healthy elderly. Front. Aging Neurosci. 3:12. doi: 10.3389/fnagi.2011.00012
Jang, J.-W., Park, J. H., Kim, S., Lee, S.-H., Lee, S.-H., and Kim, Y.-J. (2021). Prevalence and incidence of dementia in South Korea: a Nationwide analysis of the National Health Insurance Service senior cohort. J. Clin. Neurol. 17, 249–256. doi: 10.3988/jcn.2021.17.2.249
Kato, A., Ishida, J., Endo, Y., Takita, T., Furuhashi, M., Maruyama, Y., et al. (2011). Association of abdominal visceral adiposity and thigh sarcopenia with changes of arteriosclerosis in haemodialysis patients. Nephrol. Dialysis Transpl. 26, 1967–1976. doi: 10.1093/ndt/gfq652
Kimura, H., Kurimura, M., Wada, M., Kawanami, T., Kurita, K., Suzuki, Y., et al. (2002). Female preponderance of Parkinson's disease in Japan. Neuroepidemiology 21, 292–296. doi: 10.1159/000065527
Kis, B., Schrag, A., Ben-Shlomo, Y., Klein, C., Gasperi, A., Spoegler, F., et al. (2002). Novel three-stage ascertainment method: prevalence of PD and Parkinsonism in South Tyrol, Italy. Neurology 58, 1820–1825. doi: 10.1212/wnl.58.12.1820
Kyrozis, A., Ghika, A., Stathopoulos, P., Vassilopoulos, D., Trichopoulos, D., and Trichopoulou, A. (2013). Dietary and lifestyle variables in relation to incidence of Parkinson's disease in Greece. Eur. J. Epidemiol. 28, 67–77. doi: 10.1007/s10654-012-9760-0
Linder, J., Stenlund, H., and Forsgren, L. (2010). Incidence of Parkinson's disease and parkinsonism in northern Sweden: a population-based study. Move. Disord. Off. J. Move. Disord. Soc. 25, 341–348. doi: 10.1002/mds.22987
Liu, C. C., Li, C. Y., Lee, P. C., and Sun, Y. (2016). Variations in incidence and prevalence of Parkinson's disease in Taiwan: a population-based Nationwide study. Park. Dis. 2016:8756359. doi: 10.1155/2016/8756359
Logroscino, G., Sesso, H. D., Paffenbarger, R. S., and Lee, I. M. (2007). Body mass index and risk of Parkinson's disease: a prospective cohort study. Am. J. Epidemiol. 166, 1186–1190. doi: 10.1093/aje/kwm211
McKeigue, P. M., Miller, G. J., and Marmot, M. G. (1989). Coronary heart disease in south Asians overseas: a review. J. Clin. Epidemiol. 42, 597–609. doi: 10.1016/0895-4356(89)90002-4
McNutt, L. A., Wu, C., Xue, X., and Hafner, J. P. (2003). Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am. J. Epidemiol. 157, 940–943. doi: 10.1093/aje/kwg074
Meara, J., Bhowmick, B. K., and Hobson, P. (1999). Accuracy of diagnosis in patients with presumed Parkinson's disease. Age Ageing 28, 99–102. doi: 10.1093/ageing/28.2.99
Nazare, J. A., Smith, J. D., Borel, A. L., Haffner, S. M., Balkau, B., Ross, R., et al. (2012). Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the international study of prediction of intra-abdominal adiposity and its relationship with Cardiometabolic risk/intra-abdominal adiposity. Am. J. Clin. Nutr. 96, 714–726. doi: 10.3945/ajcn.112.035758
Nerius, M., Fink, A., and Doblhammer, G. (2017). Parkinson's disease in Germany: prevalence and incidence based on health claims data. Acta Neurol. Scand. 136, 386–392. doi: 10.1111/ane.12694
Niu, H., Álvarez-Álvarez, I., Guillén-Grima, F., and Aguinaga-Ontoso, I. (2017). Prevalence and incidence of Alzheimer's disease in Europe: a meta-analysis. Neurologia (Barcelona, Spain) 32, 523–532. doi: 10.1016/j.nrl.2016.02.016
Park, J. E., Kim, B. S., Kim, K. W., Hahm, B. J., Sohn, J. H., and Suk, H. W. (2019). Decline in the incidence of all-cause and Alzheimer's disease dementia: a 12-year-later rural cohort study in Korea. J. Korean Med. Sci. 34:e293. doi: 10.3346/jkms.2019.34.e293
Park, J. H., Kim, D. H., Kwon, D. Y., Choi, M., Kim, S., Jung, J. H., et al. (2019). Trends in the incidence and prevalence of Parkinson's disease in Korea: a nationwide, population-based study. BMC Geriatr. 19:320. doi: 10.1186/s12877-019-1332-7
Savica, R., Grossardt, B. R., Bower, J. H., Ahlskog, J. E., and Rocca, W. A. (2016). Time trends in the incidence of Parkinson disease. JAMA Neurol. 73, 981–989. doi: 10.1001/jamaneurol.2016.0947
Schernhammer, E., Hansen, J., Rugbjerg, K., Wermuth, L., and Ritz, B. (2011). Diabetes and the risk of developing Parkinson's disease in Denmark. Diabetes Care 34, 1102–1108. doi: 10.2337/dc10-1333
Simon, K. C., Chen, H., Schwarzschild, M., and Ascherio, A. (2007). Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology 69, 1688–1695. doi: 10.1212/01.wnl.0000271883.45010.8a
Sun, Y., Chang, Y. H., Chen, H. F., Su, Y. H., Su, H. F., and Li, C. Y. (2012). Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care 35, 1047–1049. doi: 10.2337/dc11-1511
Taylor, K. S., Counsell, C. E., Harris, C. E., Gordon, J. C., and Smith, W. C. (2006). Pilot study of the incidence and prognosis of degenerative parkinsonian disorders in Aberdeen, United Kingdom: methods and preliminary results. Move. Disord. Off. J. Move. Disord. Soc. 21, 976–982. doi: 10.1002/mds.20866
Tolosa, E., Compta, Y., and Gaig, C. (2007). The premotor phase of Parkinson's disease. Parkinsonism Relat. Disord. 13, S2–S7. doi: 10.1016/j.parkreldis.2007.06.007
Torsney, K. M., Noyce, A. J., Doherty, K. M., Bestwick, J. P., Dobson, R., and Lees, A. J. (2014). Bone health in Parkinson's disease: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 85, 1159–1166. doi: 10.1136/jnnp-2013-307307
Vaupel, J. W., Manton, K. G., and Stallard, E. (1979). The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography 16, 439–454. doi: 10.2307/2061224
von Campenhausen, S., Bornschein, B., Wick, R., Bötzel, K., Sampaio, C., Poewe, W., et al. (2005). Prevalence and incidence of Parkinson's disease in Europe. Euro. Neuropsychopharmacol. J. Euro. Coll. Neuropsychopharmacol. 15, 473–490. doi: 10.1016/j.euroneuro.2005.04.007
Widya, R. L., Kroft, L. J., Altmann-Schneider, I., van den Berg-Huysmans, A. A., van der Bijl, N., de Roos, A., et al. (2015). Visceral adipose tissue is associated with microstructural brain tissue damage. Obesity 23, 1092–1096. doi: 10.1002/oby.21048
Wild, S. H., Fischbacher, C., Brock, A., Griffiths, C., and Bhopal, R. (2007). Mortality from all causes and circulatory disease by country of birth in England and Wales 2001-2003. J. Public Health 29, 191–198. doi: 10.1093/pubmed/fdm010
Winter, Y., Bezdolnyy, Y., Katunina, E., Avakjan, G., Reese, J. P., Klotsche, J., et al. (2010). Incidence of Parkinson's disease and atypical Parkinsonism: Russian population-based study. Move. Disord. Off. J. Move. Disord. Soc. 25, 349–356. doi: 10.1002/mds.22966
Xu, Q., Park, Y., Huang, X., Hollenbeck, A., Blair, A., Schatzkin, A., et al. (2011). Diabetes and risk of Parkinson's disease. Diabetes Care 34, 910–915. doi: 10.2337/dc10-1922
Keywords: Parkinson’s disease, incidence, modifiable risk factor, cardiometabolic syndrome, osteoporosis, depression
Citation: Kang SH, Moon S-J, Kang M, Chung SJ, Cho GJ and Koh S-B (2023) Incidence of Parkinson’s disease and modifiable risk factors in Korean population: A longitudinal follow-up study of a nationwide cohort. Front. Aging Neurosci. 15:1094778. doi: 10.3389/fnagi.2023.1094778
Edited by:
Claire Xi Zhang, Xi'an Jiaotong-Liverpool University, ChinaReviewed by:
Wooyoung Jang, Gangneung Asan Hospital, Republic of KoreaDavid James Brooks, Newcastle University, United Kingdom
Copyright © 2023 Kang, Moon, Kang, Chung, Cho and Koh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seong-Beom Koh, ✉ parkinson@korea.ac.kr
 Sung Hoon Kang
Sung Hoon Kang Seok-Joo Moon2
Seok-Joo Moon2