- 1College of Horticulture and Plant Protection, Inner Mongolia Agricultural University, Hohhot, Inner Mongolia, China
- 2College of Agriculture, Guangxi University, Nanning, China
- 3Erdos Agricultural and Animal Husbandry Technology Promotion Center, Erdos, Inner Mongolia, China
- 4Bayannaoer Agriculture and Animal Husbandry Technology Promotion Center, Bayannaoer, Inner Mongolia, China
- 5Department of Plant Pathology, China Agricultural University, Beijing, China
Introduction: Pseudomonas fluorescens 2P24 was isolated from soil of natural decay associated with wheat take-all and it can effectively control soil-borne diseases caused by a variety of plant pathogens. 2,4-diacetylphloroglucinol (2,4-DAPG), is produced by P. fluorescens 2P24 and plays an important role in the prevention and control of plant diseases. To understand the resistant mechanism, in this study, we conducted experiments to explore the regulation role of rpoZ in the synthesis of the antibiotic 2,4-DAPG and regulation of QS system.
Methods: A random mini-Tn5 mutagenesis procedure was used to screen regulators for phlA transcription in stain PM901, which containing a phlA∷lacZ transcriptional fusion reporter plasmid. We identified 12 insertion mutants could significantly change phlA gene expression. By analyzing the amino acid sequences of the interrupted gene, we obtained a mutant strain Aa4-29 destroyed the rpoZ gene, which encodes the omiga subunit. We constructed the plasmid of rpoZ mutant (pBBR-△rpoZ) transformed into competent cells of P. fluorescens 2P24 by electro-transformation assay. The strains of P. fluorescens 2P24/pBBR, 2P24-△rpoZ/pBBR, 2P24-△rpoZ/pBBR-rpoZ were used to evaluate the regulation role of rpoZ in 2,4-DAPG production and quorum sensing system.
Results: According to β-galactosidase activity, we found that rpoZ positively regulated the expression of phlA (a synthesis gene of 2,4-DAPG) and PcoI (a synthesis gene of PcoI/PcoR QS signal system) at the transcriptional level. The production of 2,4-DAPG antibiotic and signal molecule AHL was influenced by rpoZ. Further, rpoZ was involved in regulating rsmA expression. RpoZ also has a certain regulatory effect on rpoS transcription, but no effect on the transcription of phlF, emhABC and emhR. According to the biocontrol assay, P. fluorescens 2P24 strains with rpoZ showed obvious antagonism ability against the Rhizoctonia solani in cotton, while the mutant strain of rpoZ lost the biocontrol effect. RpoZ had a significant effect on the swimming and biofilm formation in P. fluorescens 2P24.
Conclusion: Our data showed that rpoZ was an important regulator of QS system, 2,4-DAPG in P. fluorescens 2P24. This may imply that P. fluorescens 2P24 has evolved different regulatory features to adapt to different environmental threats.
1. Introduction
Plant growth-promoting rhizobacteria (PGPR) are closely related to plant roots, which can affect plant health and soil fertility. Pseudomonas fluorescens 2P24 generally colonizes in the root, inhibits plant soil borne diseases through producing 2,4-diacetylphloroglucinol (2,4-DAPG; Wei et al., 2004). The biosynthetic pathway of 2,4-DAPG has been clarified in several Pseudomonas strains (Vincent et al., 1991; Fenton et al., 1992). The 2,4-DAPG locus includes the four biosynthetic genes phlACBD that are transcribed as a single operon and is directly involved in the catalytic process of 2,4-DAPG production (Bangera and Thomashow, 1999). Among of them, PhlA, phlC and phlB are required for transacetylation of the monoacetylphloroglucinol (MAPG) precursor to generate DAPG and phlD is critical for the biosynthesis of MAPG (Bangera and Thomashow, 1999; Schnider-Keel et al., 2000). The phlF gene, which encodes a TetR-family transcriptional regulator, is located upstream of the phlA gene and blocks phlACBD transcription by binding to the phlA promoter region (Schnider-Keel et al., 2000; Li et al., 2018). Whereas, phlG, a gene located between the phlF and phlH genes (upstream of the phlACBD biosynthetic operon), mediates the conversion of DAPG to MAPG in P. fluorescens 2P24 (Zhao et al., 2020).
Except for the antibiotic production of P. fluorescens 2P24, another factor, quorum sensing (QS) regulation, should also pay attention in bacteria. QS system plays an important role in a diverse array of physiological activities, including symbiosis, virulence, competence, conjugation, antibiotic production, swarming, sporulation and biofilm formation (Kievit and Iglewski, 2000; Gonzalez and Keshavan, 2006; Wei and Zhang, 2006; Sakuragi and Kolter, 2007; Waters et al., 2008). The discovery of new regulators of QS system will help to further elucidate the signal transduction mechanism that bacteria survive under various environmental conditions. In Pseudomonas, regulatory elements of QS system, the stationary-phase sigma factor RpoS (Bertani and Venturi, 2004), the two-component regulatory system GacS/GacA (Reimmann et al., 1997), the small RNA-binding regulator RsmA (Pessi et al., 2001), the LuxR family member VqsR (Juhas et al., 2004) and the tetrahelical H-T-H superclass member RsaL (Rampioni et al., 2007) were identified. In P. fluorescens 2P24, the GacS-GacA system controls its target phlACBD by inducing four sRNAs (RsmX, RsmX1, RsmY, and RmZ) and repressing the levels of another sRNA, RgsA (Zhang et al., 2020a). The RsmA and RsmE proteins directly repress the translation of phlACBD mRNA, whereas four sRNAs (RsmX, RsmX1, RsmY, and RsmZ) depress the translation of phlACBD mRNA by sequestering the RsmA and RsmE proteins, thereby inducing the production of 2,4-DAPG (Zhang et al., 2020a,b). It was also reported that a quorum-sensing locus, pcoI/pcoR, which is involved in the regulation of root colonization and plant disease-suppressive ability in P. fluorescens 2P24, (Yan et al., 2009a).
DNA-dependent RNA polymerase (RNAP) is the central enzyme involved in gene expression and also constitutes a major target for genetic regulation (Darst et al., 1989, 1991; Schultz et al., 1993). The bacterial RNAP core enzyme consists of four subunits: alpha (α), beta (β), beta’ (β’) and omega (ω) subunit (Lonetto et al., 1992; Zhang et al., 1999). Among of them, the ω subunit encoded by the rpoZ gene was proposed to be an integral part of the core RNAP and is not essential for RNAP activity and cell survival, but can assist RNA polymerase assembly, help β ‘subunits fold and protect β’ subunits (Burgess, 1969; Dove and Hochschild, 1998; Ghosh et al., 2001; Mathew and Chatterji, 2006). Streptomyces kasugaensis produces an antibiotic called primathromycin (KSM), an aminoglycoside antibiotic, to control Pyricularia oryzae in rice. Kojima et al. showed that the production of primathromycin in the rpoZ mutant strain was reduced, and the formation of aerogenic mycelia was blocked, and these phenotypes could be restored to the wild type by the transfer of rpoZ complementary plasmid into the mutant strain (Kojima et al., 2002). This is indicating that rpoZ in S. primaviae regulated the production of primavithromycin. Transcription analysis of KSM synthesis genes showed that the expression of kasT (a specific transcriptional activator synthesized by KSM) was significantly decreased in rpoZ mutants, and the expression of kasT may require the participation of rpoZ or RNAP (including ω subunits). When the rpoZ gene of Mycobacterium smegmatis was mutated, the colony morphology of the rpoZ mutant strain was changed, and swimming ability, the biofilm formation, the strain cell growth were affected as well (Mathew et al., 2006).
As we have discussed above, P. fluorescens and its secondary metabolites play a very important role in biocontrol strategies. In this study, we identified a regulator of rpoZ in P. fluorescens 2P24, which is similar to rpoZ of several bacteria. The results showed that rpoZ regulates several genes including phlA, pcoI, rpoS, which indicated it might be an important upstream regulator of QS in P. fluorescens 2P24. We also found that strains with rpoZ showed obvious antagonism ability against the Rhizoctonia solani in cotton, and had significant effect on the swimming and biofilm formation in P. fluorescens 2P24.
2. Materials and methods
2.1. Bacterial strains and growth condition
Bacterial strains and plasmids used in this study are listed in Supplementary Table S1. Escherichia coli and P. fluorescens were cultured as described previously in (Wu et al., 2012; Zhao et al., 2020). E. coli was grown in Lysogenic broth (LB) medium at 37°C and P. fluorescens strains were grown at 28°C in LB medium, KB (King’s B medium; King et al., 1954) or ABM medium (Chilton et al., 1974).
2.2. Construction of rpoZ mutant and complementation strain
According to the flanking sequence of rpoZ gene of strain P. fluorescens 2P24, two pairs of primers, rpoZ 29,729/rpoZ 30,442 and rpoZ 30,518/rpoZ 31,424, were designed to amplify rpoZ gene (Supplementary Table S2). Using the genome of wild bacterium 2P24 as template, the upstream and downstream rpoZ genes were amplified with the length of 907 bp and 714 bp, respectively. The PCR products were treated with the restriction enzymes, EcoR I/Kpn I and KpnI/Hind III respectively, and then cloned into the vector pBLR digested with the corresponding EcoRI/Hind III restriction enzyme to obtain the suicide vector pBLR- △rpoZ. The construct was verified by diagnostic PCR by primer pair of G1/G2 and Ga/Gd using 2P24 genomic DNA and plasmid p299△G. A 378 bp fragment was lost in p299△G comparing with the wild type rpoZ gene according to the confirmation PCR and sequencing.
The plasmids of pBLR-△rpoZ were transformed into competent cells of P. fluorescens 2P24 by electro-transformation assay under screening of Km resistance in LB liquid medium. After 7 generations, bacteria grown on ABM medium containing Km and X-gal under the condition of 28°C for 24 h. The white clones were verified by PCR amplification.
The rpoZ gene was cloned into the shuttle vector pBBR, which was used for complementary experiments. Using the genome DNA of P. fluorescens 2P24 as template, primers rpoZ 30,059/rpoZ 30,712 were used to obtain the fragment of rpoZ gene. The fragment was digested by Hind III - KpnI and connected with pBBR to obtain the complementary vector, named as plasmid pBBR-rpoZ.
In order to amplify the complete rpoZ gene, PCR primers rpoZ 29,729/rpoZ 30,442 and rpoZ 30,518/rpoZ 31,424 were designed based on the gene sequence of Pseudomonas fluorescens 2P24. Using P. fluorescens 2P24 genome as a template, two amplified fragments were digested by EcoRI/KpnI and KpnI/HindIII restriction endonuclease enzyme, respectively, and then linked to the corresponding enzyme digested vector pBLR, and a suicidal deletion vector pBLR-△rpoZ was obtained. Using PCR primer rpoZ 30,059/rpoZ 30,712 and wild bacterium 2P24 as template, the 654 bp target fragment containing the complete rpoZ gene was amplified and then connected to the shuttle vector pBBR after being digested by Hind-III/Kpn I restriction enzyme. The complementary vector pBBR-rpoZ was obtained. The deletion vector and complementary vector were transferred into wild-type 2P24 strain to obtain the deletion mutant strain and complementary strain of rpoZ (Supplementary Figures S1A,B).
Each strain was cultured in ABM and LB liquid medium overnight, and the culture concentration was adjusted to the same OD600 = 0.8 with ABM and LB liquid medium, and then transferred to 40 ml ABM and LB liquid medium containing corresponding Amp and Gm antibiotics at a ratio of 1:1000, respectively. Then, placed in a 28°C incubator for shaking with120 r/min, and collected samples and measured OD600 every 3 h. Each sample was repeated 3 times.
2.3. Detection of QS signal molecule (AHL) in Pseudomonas fluorescens 2P24 and its derived strains
Strains of P. fluorescens 2P24/pBBR, 2P24-△rpoZ/pBBR, 2P24-△rpoZ/pBBR-rpoZ were inoculated into 5 ml LB medium containing Amp and Gm antibiotics, placed in a shaker with 120 r/min for 36 h at 28°C. 30 μl was inoculated into 30 ml LB medium containing Amp and Gm antibiotics and placed in a shaker for 120 r/min at 28°C. 800 μl of each bacterial solution to be tested was added into the same volume of ethyl acetate, and signals were extracted by extraction method. The organic phase was air-dried and dissolved in 100 μl methanol, diluted 10 times, and stored at −20°C. The reported strain A. tumefaciens NTL4 (pZLR4) was inoculated into ABM liquid medium, incubated in a shaker with 120 r/min for 24 h at 28°C, and stored at 4°C. 200 μl newly cultured reporter bacteria (A. tumefaciens NTL4) was measured after being cultured with 5 μl of each signal molecule for 4 h. Reporter bacteria added with 5 μl methanol were used as the control.
The QS signals were detected by β-galactosidase activities as described previously in (Miller, 1972). All experiments were performed in triplicate (Wu et al., 2012). Each strain was set 3 replicates.
2.4. Determination of 2,4 DAPG production
Quantification of 2,4-DAPG was done as described previously (Shanahan et al., 1992). P. fluorescens 2P24/pBBR, 2P24-△rpoZ/pBBR, 2P24-△rpoZ/pBBR-rpoZ was inoculated into 40 ml King’s B liquid medium and incubated in a shaker at 28°C with 120 r/min for 40 h to stationary stage. After centrifugation at 8,000 r/min for 10 min, the supernatant was taken out and acidified to pH 2.0 with 1 mol/l HC1. Equal volume of ethyl acetate was added for extraction, and the organic phase was extracted by rotary evaporation. The dry matter was dissolved with 50 ml methanol and determined by HPLC (UV2002 high performance Liquid chromatograph). C18 reverse column was used for HPLC: diameter: 150 × 4.6 mm; Detection wavelength: 270 nm; sample volume: 5 μl; mobile phase: water: ethyleye (V: V) = 45: 55, 0.1% H3P04; Flow rate: l.0 ml/min; retention time: 5.12 min. 2,4-DAPG chromatographic standard sample was purchased from Toronoto Research Chemicals Inc., (D365500).
2.5. Antagonism test of rpoZ in Pseudomonas fluorescens 2P24 against Rhizoctonia solani
Cultivation of Rhizoctonia solani and dual-culture confrontation assay was performed on PDA medium (Zhao et al., 2020). The fungus disk of strains with a diameter of 6 mm was placed in the center of the PDA plate, and the tested biocontrol bacteria were inoculated 2.5 cm away from the fungus disk. The plates were placed in an incubator at 25°C. The size of the inhibition zone was measured when the control fungus grew to the edge of the petri dish. Three replicates per treatment.
2.6. Motility tests
Motility tests were conducted as described by Rashid and Kornberg (2000), with slight modification (Wang et al., 2021). For swimming tests, we used water medium that contained 0.2% agar. Freshly cultured strains were dipped using pipette tips and inoculated on the surface of the center of plates. Then, plates were placed stably in the incubator and cultured at 28°C. After 24 h, the diameter of motility of clones was measured.
2.7. The biofilm formation of Pseudomonas fluorescens 2P24 and its derived strains
To test whether rpoZ affect the biofilm formation of P. fluorescens 2P24, the quantitative determination of biofilms is performed as described (Wei and Zhang, 2006) with slight modification. The strain of ropZ mutant was inoculated into LB liquid medium and cultured until saturated, then diluted into fresh LB liquid medium at the volume ratio of 1:1000. The formation of bioflim at the junction of solid and liquid was measured after 500 μl was added into a 2 ml centrifugation tube and incubated at 28°C for 24 h. Add 100 μl crystal violet with a concentration of 0.1% (W/V) to each tube. After 20–30 min staining at room temperature, rinse the centrifuge tube with strong distilled water. In the inside of the centrifuge tube, it can be observed to form a strong biofilm at the junction of the liquid level and the tube wall. The crystal violet combined with biofilm was fully dissolved by adding 1,200 μl of 95% ethanol, and the absorption value of OD570 was measured with 1,000 μl.
2.8. Statistical analysis
GraphPad Prism software version 5.01 (Graphpad Software, Inc.) was used for analysis of variance, followed by multiple comparisons using one way ANOVA, and p < 0.05 was considered statistically significant.
3. Results
3.1. Identification and characteristic of rpoZ gene in Pseudomonas fluorescens 2P24
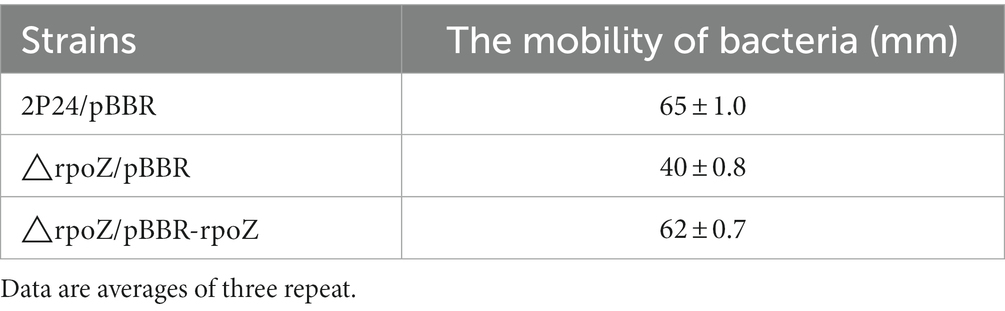
The random insertion method of Tn5 transposon was used to mutate the transcriptional fusion plasmid pGm-phlA contained in P. fluorecens 2P24 and screened the regulatory factors affecting the production of 2,4-DAPG. We identified 21 mutant strains that significantly reduced phlA expression (Supplementary Table S3). Because of Tn5 transposon is carrying Gm antibiotic resistance gene, the genes damaged by Tn5 transposons in mutant strains could be determined by analysis of the flanking sequence of Tn5. Restriction enzymes (SalI/EcoRI) were used for enzyme digestion and self-ligated. Plasmids were transformed into E. coli DH5α and inoculated on LB solid medium containing 30 μg/ml antibiotics Gm. The single colony containing the flanking sequence of Tn5 transposon was selected and examined by sequencing. The mutant Aa4-29 with the most reduced phlA gene expression was purified and further investigated. Sequence analysis showed that Tn5 was inserted into rpoZ gene of mutant strain Aa4-29, which was named as 2P24-△rpoZ (Figure 1A). We constructed the deletion mutant and complementary strain of rpoZ as shown in Supplementary Figures S1A,B.
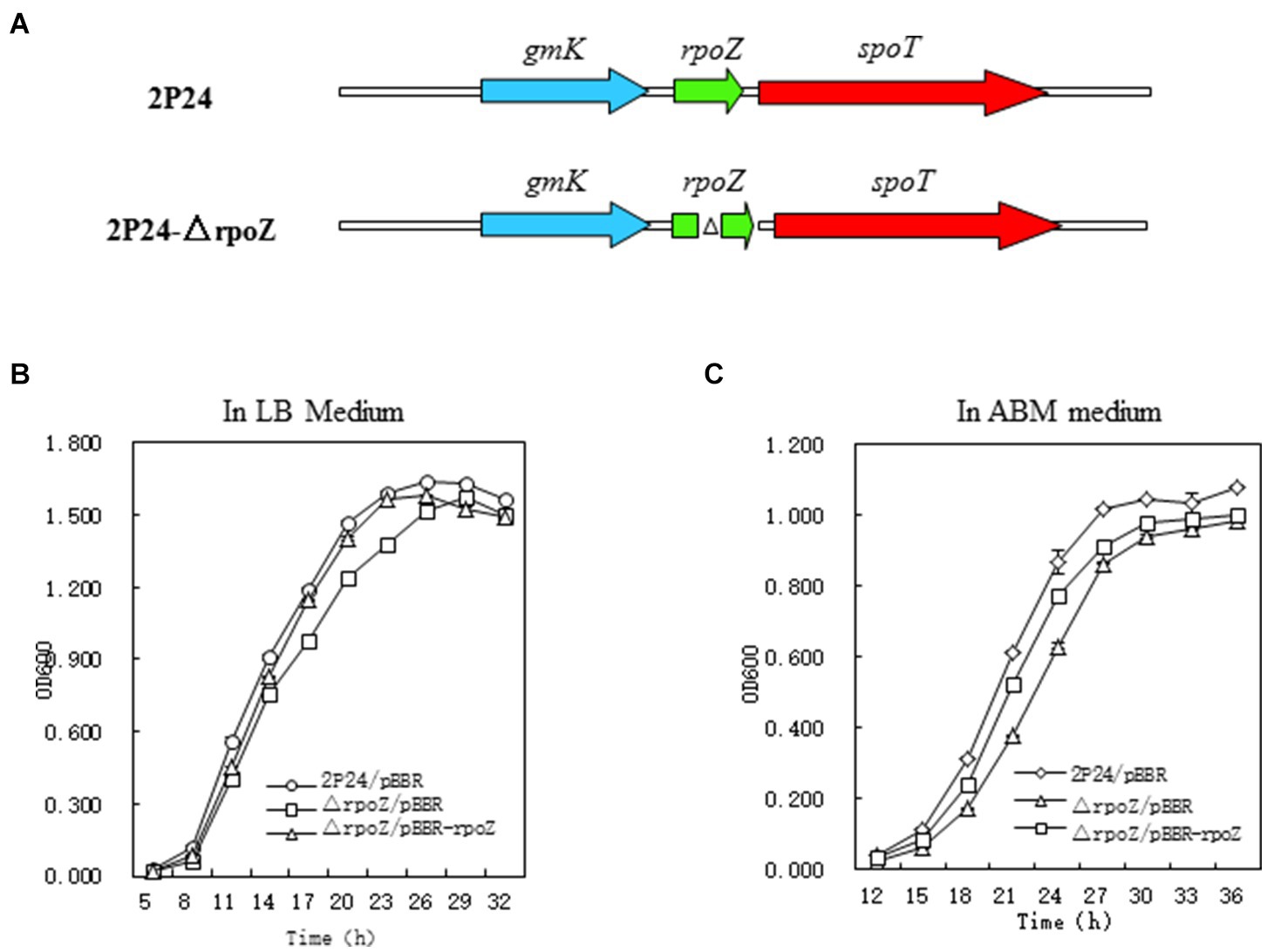
Figure 1. Structure and growth curve of rpoZ gene mutation. (A) Schematic diagram of the construction of the ropZ deletion mutant in Pseudomonas fluorescens 2P24. (B,C) Growth rate of wild-type (WT) P. fluorescens 2P24 and the ropZ deletion mutant in LB and ABM liquid medium. The error bars represent standard deviations and the statistical analysis was performed using a two-tailed t-test. Three replicates per treatment.
This gene is the ω-subunit of the encoding RNA polymerase, which encodes the synthesis of a 90 amino acid peptide chain with a molecular weight of about 10,105 Da. The RpoZ protein sequence in strain 2P24 is very similar to that of other RpoZ in Pseudomonas, among which has a similarity of 90% with the RpoZ sequence in Pseudomonas brassicacearum NFM421, has a similarity of 93% with the RpoZ sequence of Pseudomonas fluorescens PF0-1 and has a similarity of 86% with the RpoZ sequence of Pseudomonas syringae pv.tomato str.DC3000.
Analysis of the laterals of the Tn5 transposon revealed that there was a Gmk gene in the same transcriptional direction upstream of the rpoZ gene, which reportedly encodes the guanylate kinase (Supplementary Figure S1A). The downstream of the rpoZ gene is spoT gene, whose transcription direction is the same as rpoZ. SpoT gene encodes pyrophosphatase and is involved in regulating (p)ppGpp level in cells. The sequences of these three genes are also much conserved to upstream and downstream genes of rpoZ in E. coli and Streptomyces cerulosus (Santos-Beneit et al., 2011).
To test the effect of rpoZ mutant strain on the growth of P. fluoresens 2P24, we compared the wild-type strain P. fluoresens 2P24, rpoZ-deficient mutant strain △rpoZ/pBBR and complementary strain △rpoZ/PBBR-rpoZ in LB and ABM medium. The growth curve was drawn according to OD600 value at each time point (Figure 1C). The results showed that the growth curves of P. fluorescens 2P24/pBBR, △rpoZ/pBBR and △rpoZ/PBBR-rpoZ in LB and ABA medium were consistent (Figure 1B). The growth rate of the rpoZ mutant strain was significantly slower than that of the wild-type strain. This growth of the complementary strain carrying the rpoZ plasmid was recovered. These results indicated that the rpoZ gene played a role in the regulation of bacterial growth.
3.2. RpoZ regulates transcription of signal synthesis pcoI gene and production of signal molecule AHL
A promoter-free lacZ gene into the pcoI gene of the QS system on the genome of wild-type strain 2P24 was inserted and constructed pcoI∷lacZ as a marker gene for transcription fusion previously by (Yan et al., 2009b). The transcription of lacZ was driven by the pcoI gene promoter, and its schematic structure was shown in Figure 2A, by which we determined the level of pcoI gene transcription by detecting the β-galactosidase (LacZ) activity of the strain.
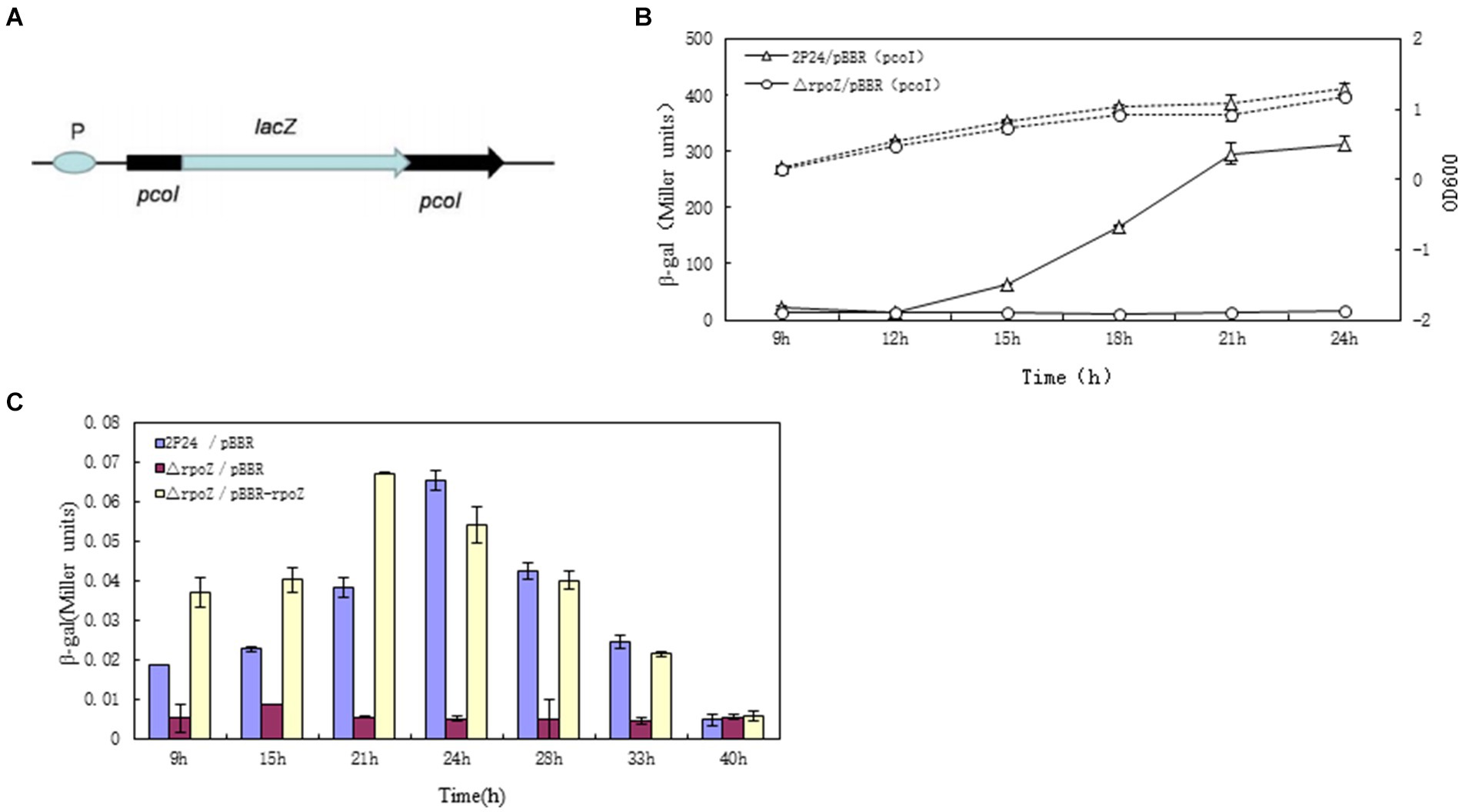
Figure 2. RopZ regulates pcoI expression in P. fluorescens 2P24. (A) The graphic presentation of constructed E. coli reporter plasmids. pSB-pPcoI contained the promoter region of pcol fused to the promoterless lacZ gene. (B) The β-galactosidase activity of wild type 2P24/pBBR (pcoI) and ropZ mutant △rpoZ/pBBR (pcoI) was detected and shown as the solid lines with Miller units. The dotted lines were growth curve. (C) Detection of the signal accumulation of ropZ mutants in A. tumefaciens NTL4 (pZLR4). The signal molecules were extracted from wild type 2P24/pBBR, ropZ mutant △rpoZ/pBBR and complementary strain △rpoZ/pBBR-rpoZ cultures and incubated with A. tumefaciens NTL4 (pZLR4). Then, the β-gal activities were detected at different time points as shown in horizontal coordinate. The ordinate represents the ratio of β-galactosidase activity to OD600 value of each detected strain. Each value was calculated by 3 replicates. The values were from at least three independent assays. Three independent experiments were performed and the error bars were calculated standard deviations of experimental data.
The β-galactosidase activity of wild-type strain of P. fluorescens 2P24/pBBR and rpoZ gene mutant △rpoZ/pBBR was measured and the growth curve was drawn by the value of OD600 in the culture medium (Figure 2B). The growth curve showed that the mutant rpoZ gene delayed the growth of the bacteria compared with the wild-type strain. The β-galactosidase activity of △rpoZ/pBBR was significantly lower than that of wild-type strain 2P24/pBBR during the whole growth process, which indicated that the transcription activity of pcoI gene was greatly reduced after rpoZ gene mutation. We suggested that rpoZ positively regulated the expression of pcoI gene in strain P. fluoresens 2P24 at the transcriptional level.
In P. fluoresens 2P24, the pcoI gene is a synthase that is responsible for synthesizing quorum sensing signalling molecules. We also confirmed rpoZ gene also affects the synthesis of signal molecules in the QS system through regulation of pcoI expression. The signal molecules produced by the wild strains and the derived strains were extracted and interacted with the reported strain A. tumefaciens NTL4 (pZLR4). NTL4 is a modified engineering strain that cannot generate signal molecule itself, but it carries TraG∷LacZ fusion gene to detect exogenous QS signal molecule and promote the expression of LacZ (Chai et al., 2001). Therefore, the LacZ activity level of strain A. tumefaciens NTL4 can be reported to compare the content of signal molecules in each sample to be tested.
Compared with the wild strain 2P24, the β-galactosidase activity of the reported strain was significantly decreased by the extract of the signal molecule of △rpoZ/pBBR, which meant that the production of signal molecule of △rpoZ/pBBR was smaller than that of wild type 2P24. And this change can be restored by the intact rpoZ gene carried by the plasmid PBBR-rpoZ. Therefore, we believed that rpoZ gene positively regulated the synthesis of signal molecules in P. fluoresens 2P24. This was consistent with the positive regulation of pcoI gene transcription by rpoZ, which was proved that rpoZ played a positive role in quorum sensing system.
In addition, the signal molecules produced by wild-type strain 2P24 have obvious characteristics on different culture time points (Figure 2C). In the early stage of culture, the synthesis amount of signal molecules was very low. However, with the increase of culture time, the accumulation of signal molecules reached the peak at 24 h, and after the highest accumulation maintained for a period of time, the content of signal molecules in the culture medium began to decline again.
3.3. Effects of rpoZ gene on transcription of antibiotic synthesis gene phlA and 2,4-DAPG production
It was reported that extracellular secondary metabolite 2,4-DAPG from P. fluoresens 2P24 played an important role in controlling effects on soil borne disease in wheat (Zhou et al., 2005). The 2,4-DAPG production was regulated by antibiotic synthesis gene phlA. To investigate whether rpoZ gene ws involved in transcription of phlA and 2,4-DAPG production, the activity of β-galactosidase in PhlA-Lacz transcription fusion was detected in wild-type strain 2P24 and △rpoZ/pBBR. The β-galactosidase activity of each strain was measured and the growth curve was drawn by the value of OD600. The results showed that the growth of the bacteria containing the mutant rpoZ gene significantly delayed compared with that of the wild-type strain (Figure 3A). The expression of phlA gene in △rpoZ/pBBR was significantly lower than that of wild-type strain 2P24/pBBR during the whole growth process, which indicated that the transcription activity of phlA gene was greatly reduced in △rpoZ/pBBR. This was suggested that rpoZ positively regulated the expression of phlA gene in strain P. fluoresens 2P24 at the transcriptional level.
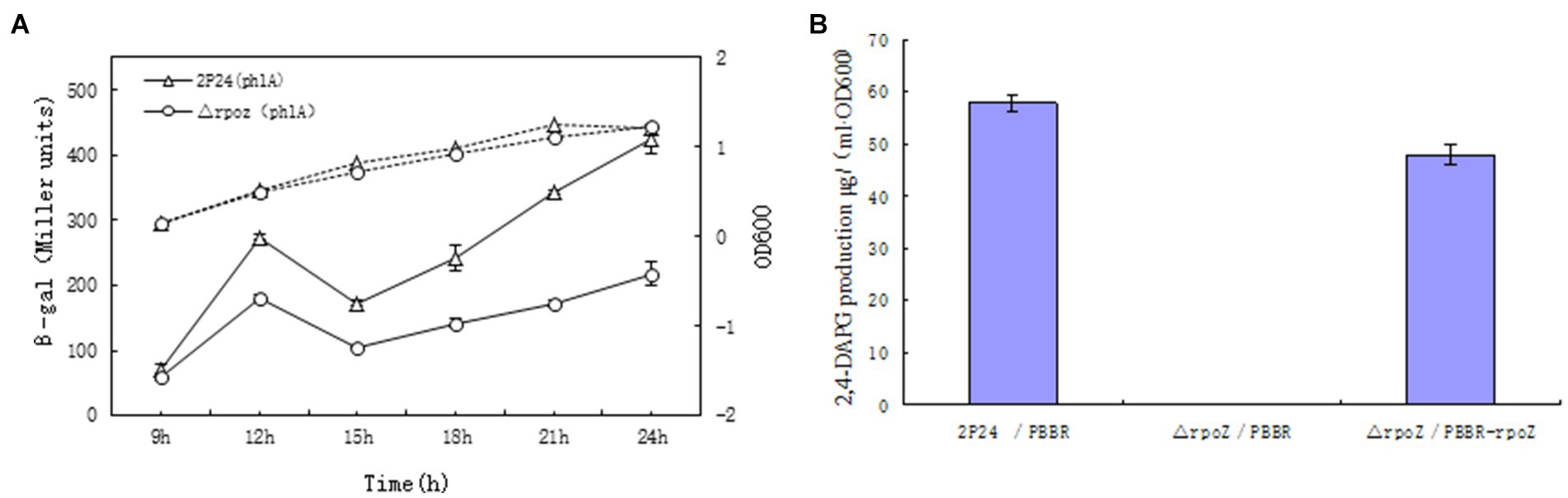
Figure 3. RopZ regulates phlA expression and 2,4-DAPG production in P. fluorescens 2P24. (A) β-galactosidase activity of wild type 2P24 and △rpoZ and ropZ mutant was detected and shown. (B) 2,4-DAPG production was detected in from wild type 2P24/pBBR, ropZ mutant △rpoZ/pBBR and complementary strain △rpoZ/pBBR-rpoZ.
Correspondingly, HPLC was used to detect whether the yield of 2,4-DAPG was consistent with the positive regulatory effect of rpoZ gene on the expression of phlA gene. The 2,4-DAPG production of △rpoZ/pBBR was minimal compared with that of wild strain 2P24, and this change was recovered by the intact rpoZ gene carried by the plasmid △rpoZ/pBBR (Figure 3B). Therefore, we believed that rpoZ gene in P. fluoresens 2P24 has an effect on the production of 2,4-DAPG antibiotics.
3.4. RpoZ affects 2,4 DAPG production through positive regulation of rsmA
In P. fluorescens 2P24, the RsmA and RsmE proteins directly repress the translation of phlACBD mRNA, whereas four sRNAs RsmX, RsmX1, RsmY, and RsmZ derepress the translation of phlACBD by sequestering the RsmA and RsmE proteins, thereby inducing the production of 2,4-DAPG (Zhang et al., 2020b). To examine how influence of rpoZ on those genes of rsmA,rsmE, three sRNAs rsmX, rsmY, and rsmZ, the different transcription LacZ fusion reporter plasmids of small RNA molecules (rsmX-lacZ, rsmY-LacZ, rsmZ-lacZ) were constructed and electrocuted into wild bacteria P. fluorescens 2P24/pBBR and △rpoZ/pBBR. The β-galactosidase activity of each bacterium was measured and profiled as shown in Figure 4.
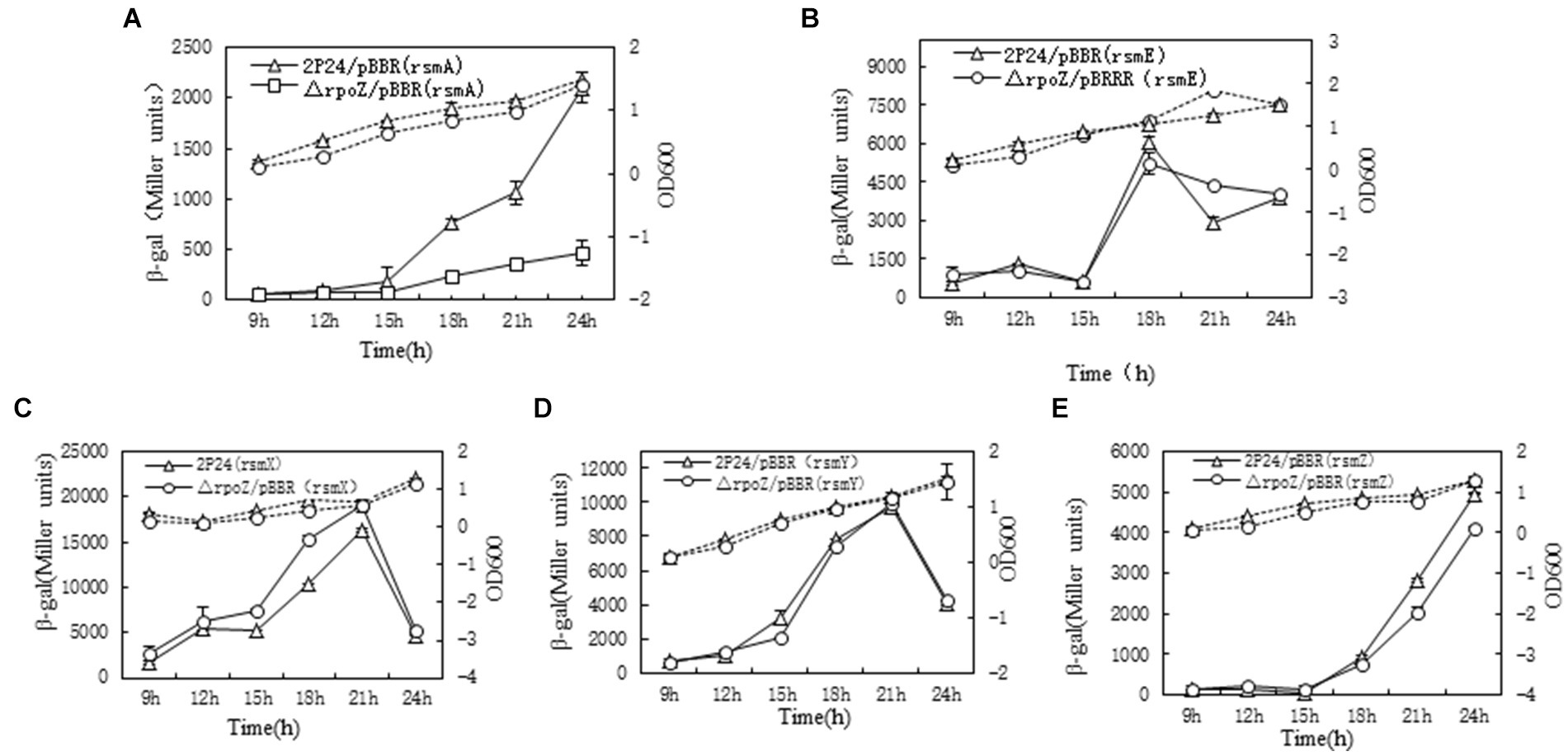
Figure 4. Determination of rpoZ gene influence gene related with 2,4 DAPG production. To determine ropZ regulation effect on expression of rsmA, rsmE, rsmX, rsmY and rsmZ, the β-gal activities in different strains were detected at different time points as shown in A–E. The β-galactosidase activity to OD600 value of each detected strain were shown as the solid lines with Miller units. The dotted lines were growth curve. Each value was calculated by 3 replicates. The values were from at least three independent assays. Three independent experiments were performed and the error bars were calculated.
The results showed that rpoZ gene has no effect on the transcription expression of rsmE gene, but rpoZ gene has a significant effect on the transcription expression of rsmA (about 4 times) (Figures 4A,B). rpoZ gene mutation had no effect on the transcription of rsmX, rsmY and rsmZ genes, which indicated that rpoZ was not involved in the expression of rsmX, rsmY and rsmZ genes at the transcription level in strain 2P24 (Figures 4C–E). RsmX, rsmY and rsmZ genes are regulated by the GacS/GacA two-factor regulatory system, which positively regulates the expression of small RNA molecules. RpoZ gene did not regulate the transcription of rsmX, rsmY and rsmZ genes. This was indicating that the effect of rpoZ gene on antibiotic 2,4-DAPG production was not regulated by the expression of rsmX, rsmY and rsmZ gene at transcription level, but was regulated by the rsmA, which directly repressing the translation of phlACBD mRNA and inducing the reduction the production of 2,4-DAPG.
3.5. RpoZ regulates transcription of rpoS gene
PhlF is an inhibitor of 2,4-DAPG synthesis. By binding to the operon of PhO, PhlF protein can inhibit the binding of its RNA synthase and the region of the phlA gene promoter, thus inhibiting the transcription of phlACBD gene (Schnider-Keel et al., 2000; Li et al., 2018). However, with the growth of bacteria, a small amount of 2,4-DAPG can bind PhlF protein to counter this inhibition effect, which resulted to synthesize a large amount of 2.4-DAPG. As can be seen from Figure 5A, theβ-galactosidase activity of △rpoZ/pBBR and wild type 2P24/pBBR showed no difference, indicated that rpoZ gene had no effect on phlF gene transcription. Therefore, it was believed that rpoZ did not regulate the expression of phlF gene at the transcriptional level in strain 2P24.
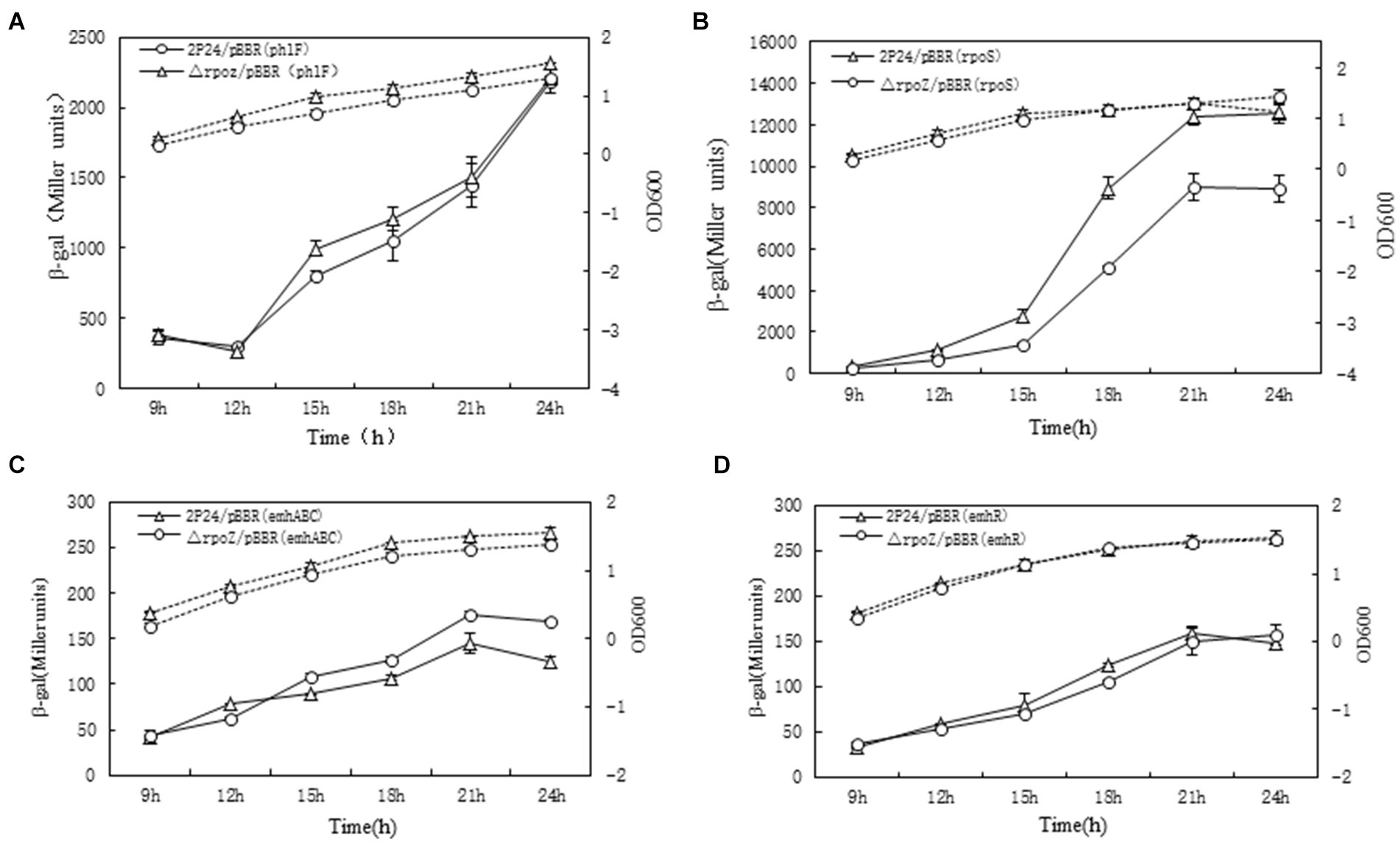
Figure 5. RpoZ regulates phlF, rpoS, emhABC and emhR expression in P. fluorescens 2P24. the β-gal activities in different strains were detected at different time points as shown in (A–D). The β-galactosidase activity to OD600 value of each detected strain was shown as the solid lines with Miller units. The dotted lines were growth curve. Each value was calculated by 3 replicates. The values were from at least three independent assays. Three independent experiments were performed and the error bars were calculated.
RpoS is an important regulatory factor in the quiescent phase of bacteria (Hengge-Aronis, 2002), which is regulated by bacteria growth state. And rpoS affects the synthesis of secondary metabolites. The rpoS gene promoter fusion plasmid rpoS-LacZ was transferred into the wild strain 2P24 and the mutant strain respectively, and the β-galactosidase activity was detected. The results showed that the β-galactosidase activity of the wild-type strain was 1,189 Miller units, and the mutant strain was 800 Miller units. This indicated that rpoZ gene positively regulated the expression of rpoS at the transcriptional level (Figure 5B). From Figure 5B, we can also see that rpoS gene plays a certain role in the growth of bacteria. Before the stable period, the growth of rpoZ-deficient strain was slower than that of wild-type strain, but after the stable period, the growth of rpoZ-deficient strains was faster than that of wild-type strain.
The multidrug-resistant pump with active bacterial efflux is a system that bacteria can resist starting under adverse environment, by which harmful substances can be excluded from the cell. EmhR-emhABC pumps in P. fluorescens 2P24 are typical multi-drug resistant pumps. EmhR is the regulator of EmhABC pump, and the transcription level is negatively regulated by EmhABC. As shown in Figures 5C,D, there was no difference in β-galactosidase activity between mutant △rpoZ/pBBR and wild-type 2P24/pBBR, suggested that rpoZ did not regulate the expression of emhABC and emhR genes at the transcriptional level in strain 2P24. The growth curve of culture medium showed that emhR gene had no obvious influence on the bacteria growth.
3.6. Determination of biocontrol characteristic of rpoZ in Pseudomonas fluorescens 2P24
Previous studies have shown that 2,4-DAPG is the main biocontrol factor in many biocontrol bacteria (Wei and Zhang, 2005; Tian et al., 2010; Zhao et al., 2020). HPLC results showed that the wild strain could produce 2,4-DAPG, while the mutant strain △rpoZ/pBBR produced very little 2,4-DAPG (Figure 6A; Table 1). The antagonism experiment also proved that the mutant strain of rpoZ lost the antagonism ability against the Rhizoctonia solani in cotton. These results indicated that rpoZ had an important antagonistic effect on 2,4-DAPG pathogens. RpoZ gene had a significant effect on the swimming of the strain. The swimming of rpoZ deletion mutant was significantly slower than that of the wild type (Figure 6B; Table 2).

Figure 6. Determination of biocontrol characteristic of rpoZ in P. fluorescens 2P24. (A) Antagonistic ability of rpoZ in P. fluorescens 2P24 and its derivates against Rhizoctonia solani are shown. (B) The mobilities of P. fluorescens 2P24 and its derivates on medium are shown. (C) Regulation of rpoZ on biofilm formation. *p < 0.05 by Student’s t test.
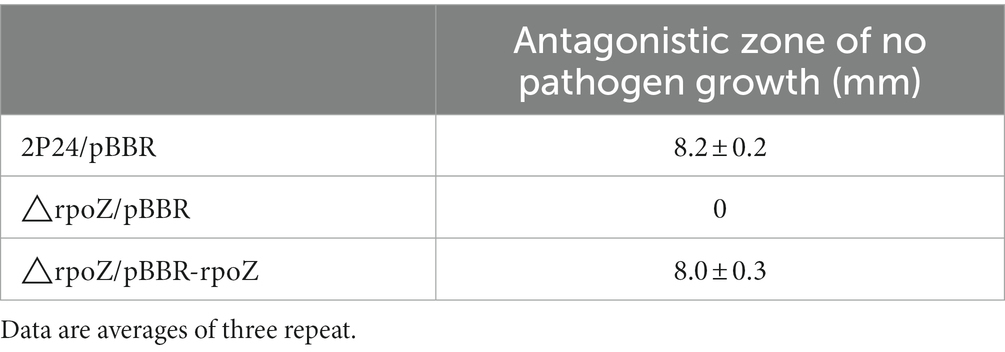
Table 1. Antagonistic ability of rpoZ in Pseudomonas fluorescens 2P24 and its derivates against Rhizoctonia solani.
Biofilm is a solid structure similar to membrane formed by extracellular polysaccharides and microorganisms on the solid surface. Bacteria in the biofilm form an interactive community and are not free planktic cells (Cvitkvitch et al., 2003). It has been reported that pcoI positively regulated the formation of biofilm in P. fluorescens 2P24, and rpoZ positively regulated the transcription expression of pcoI (Wang et al., 2021). Therefore, we tested the regulatory effect of rpoZ gene on biofilm. We found that the formation of biofilm in rpoZ mutant strains decreased significantly compared with wild-type strain, and the complementary strain also basically recovered the level of wild-type strain (Figure 6C). This indicated that rpoZ gene had an effect on biofilm formation.
4. Discussion
It was well known that the widely conserved omega subunit encoded by RpoZ is the smallest subunit of Escherichia coli RNA polymerase but is dispensable for bacterial growth (Sarkar et al., 2013). RpoZ is located upstream of spoT and shared a promoter with spoT. SpoT and rpoZ mutant strains showed slower growth. The researchers concluded that the slower growth phenotype of rpoZ mutant could be inhibited by relA, suggesting that the slower growth was not caused by the loss of rpoZ, but was the result of increased (P)ppGpp levels due to changes in polarity (Wendrich et al., 2000). To mimic the strict response, RNAP (with or without ω subunit) was analysed using an in vitro fusion transcription system. ω subunit was involved in the regulation of intracellular ppGpp. ω subunit was involved in the regulation of relA gene expression. When ω subunit was absent, relA transcriptional expression was decreased, thus ppGpp level and mRNA expression were decreased. The insertion mutation of rpoZ conferred a slow-growth phenotype when it was introduced into most strains (Gentry and Burgess, 1989). These results suggested that rpoZ indirectly regulates the intracellular levels of (P)ppGpp (Gentry and Burgess, 1989). The slower growth phenotype of rpoZ mutant wasn’t attributed to the polar of spoT in the downstream. The researchers concluded that the slower growth phenotype of rpoZ mutant could be inhibited by relA, suggesting that the slower growth was not caused by the loss of rpoZ. And deletion of ω subunit does not affect the synthesis of (p)ppGpp, and any difference in phenotype observed is possibly due to the reduced binding of (p)ppGpp to RNAP in ∆rpoZ strain (Bhardwaj et al., 2018). But other scientists reported that deletion of ω does not affect the synthesis of (p)ppGpp, and any difference in phenotype observed is possibly due to the reduced binding of (p)ppGpp to RNAP in ∆rpoZ strain (Bhardwaj et al., 2018), So far, ∆rpoZ stain showed slow growth because of the influence of (p)ppGpp, rather than the polar of the spoT gene.
To further explore the regulation role of rpoZ, in this study, we identified rpoZ gene from P. fluorescens 2P24 and demonstrated it had positive regulation role in the expression of phlA at the transcriptional level, which affected the production of antibiotic 2,4-DAPG, and it resulted in the decreased biocontrol ability in ∆rpoZ stain (Zhou et al., 2005). It has been showed that the rpoZ gene was required for antibiotic production and morphological differentiation but is not essential for growth in Streptomyces kasugaensis (Kojima et al., 2002; Santos-Beneit et al., 2011). Deletion of the gene rpoZ in Mycobacterium smegmatis results in reducing growth rate, a change in colony morphology and fragmentation of the beta’ subunit in the enzyme assembly (Mathew et al., 2005). In rpoZ mutant, production of actinorhodin,undecylprodigiosin and gray pigment closely associated with spores decreased, especially the expression of actinorhodin and gray pigment was completely inhibited (Santos-Beneit et al., 2011).
Previous studies have shown that PcoI/PcoR QS system in strain P. fluorescens 2P24 positively regulates biofilm formation and colonization of root circumference (Yan et al., 2009a,b). RpoZ gene positive regulation role in the expression pcoI gene at the transcriptional level, which affected the production of the signalling molecule AHL, and RpoZ gene positively regulated QS system. Therefore, we proposed that rpoZ could also affect colonization ability of root circumference and biofilm formation through QS system, thus affecting biocontrol ability. It reported that ΔrpoZ strain of E. coli showed defective biofilm formation only in minimal media and this indicated that ω subunit plays an important role in biofilm formation under stress conditions (Weiss et al., 2017). ΔrpoZ strain in M. smegmatis and S. aureus is known to be defective in biofilm formation (Mathew et al., 2006; Weiss et al., 2017).
The absence of RpoZ leads to a different set of genes being transcribed as seen in E. coli. It had an effect on the expression of rsmA, but it had no effect on the expression and transcription of untranslated other small RNA (Weiss et al., 2017). RsmA and rsmE are carbon storage regulatory factors, which can bind to the ribosome binding site RBS of mRNA transcribed by secondary metabolites HCN, 2,4-DAPG and Plt synthesis genes, thus preventing the initiation of translation and regulating the synthesis of these secondary metabolites.
5. Conclusion
Using Tn5 mutagenesis, we obtained rpoZ mutant in P. fluorescens 2P24. This facilitated us to study the rpoZ function. Our data showed that rpoZ gene was an important regulator of antibiotic 2,4-DAPG and QS system, which is important for investigating the mechanism of biocontrol activity in P. fluorescens 2P24.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
MZ, HZ, and BD wrote the manuscript. LZ, XW, and HZ designed the experiments. YW and XW performed the experiments. BD, DW, NL, and QZ analyzed the sequencing data. BD and DW advised on the English language editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 30860166), Science and Technology Xingmeng project of Inner Mongolia in China (grant no. KJXM-EEDS-2020008), and China Agriculture Research System of MOF and MARA (grant no. CARS-07-C-3).
Acknowledgments
We thank Gail P. Ferguson, Martin Schuster, Zhaoqing Luo and Rui Zhou for plasmids and reagents preparation. We thank Jianying Yue and Zhiying Wang for critical discussions on the project design and technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1160913/full#supplementary-material
References
Bangera, M. G., and Thomashow, L. S. (1999). Identification and characterization of a gene cluster for synthesis of the polyketide antibiotic 2,4-diacetylphloroglucinol from Pseudomonas fluorescens Q2–87. J. Bacteriol. 181, 3155–3163. doi: 10.1128/JB.181.10.3155-3163.1999
Bertani, I., and Venturi, V. (2004). Regulation of the N-acyl homoserine lactone-dependent quorum-sensing system in rhizosphere Pseudomonas putida WCS358 and cross-talk with the stationaryphase RpoS sigma factor and the global regulator GacA. Appl. Environ. Microbiol. 70, 5493–5502. doi: 10.1128/AEM.70.9.5493-5502.2004
Bhardwaj, N., Syal, K., and Chatterji, D. (2018). The role of ω-subunit of Escherichia coli RNA polymerase in stress response. Genes Cells 23, 357–369. doi: 10.1111/gtc.12577
Burgess, R. R. (1969). Separation and characterization of the subunits of ribonucleic acid polymerase. J. Biol. Chem. 244, 6168–6176. doi: 10.1016/S0021-9258(18)63521-5
Chai, Y., Zhu, J., and Winans, S. C. (2001). And TrlR, a defective TraR-like protein of Agrobacterium tumefaciens, blocks TraR function in vitro by forming inactive TraR: Tra R dimer. Mol. Microbiol. 40, 414–421. doi: 10.1046/j.1365-2958.2001.02385.x
Chilton, M. D., Currier, T. C., Farrand, S. K., Bendich, A. J., Gordon, M. P., and Nester, E. W. (1974). Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc. Natl. Acad. Sci. U. S. A. 71, 3672–3676. doi: 10.1073/pnas.71.9.3672
Cvitkvitch, D. G., Li, Y. H., and Ellen, R. P. (2003). Quorum sensing and bioflim formation in streptococcal infections. Microbiology 112, 1626–1632. doi: 10.1172/JCI20430
Darst, S. A., Edwards, A. M., Kubalek, E. W., and Kornberg, R. D. (1991). Three-dimensional structure of yeast RNA polymerase II at 16 a resolution. Cells 66, 121–128. doi: 10.1016/0092-8674(91)90144-n
Darst, S. A., Kubalek, E. W., and Kornberg, R. D. (1989). Three-dimensional structure of Escherichia coli RNA polymerase holoenzyme determined by electron crystallography. Nature 340, 730–732. doi: 10.1038/340730a0
Dove, S. L., and Hochschild, A. (1998). Conversion of the omega subunit of Escherichia coli RNA polymerase into a transcriptional activator or an activation target. Genes Dev 12, 745–754. doi: 10.1101/gad.12.5.745
Fenton, A. M., Stphens, P. M., and Crowley, J. (1992). Exploitation of gene (s) involved in 2,4-diacetylphloroglucinol biosynthesis to confer a new biocontrol capability to a Pseudomonas strain. Appl. Environ. Microbiol. 58, 3873–3878. doi: 10.1002/bit.260401020
Gentry, D. R., and Burgess, R. (1989). Rpo Z, encoding the omega Subuni t of Escherichia coli RNA polymerase, is in the same operon as spot. J. Bacteriol. 171, 1271–1277. doi: 10.1128/jb.171.3.1271-1277.1989
Ghosh, P., Ishihama, A., and Chatterji, D. (2001). Escherichia coli RNA polymerase subunit omega and its N-terminal domain bind full-length β′to facilitate incorporation into the α2β sub-assembly. FEBS J. 268, 4621–4627. doi: 10.1046/j.1432-1327.2001.02381.x
Gonzalez, J. E., and Keshavan, N. D. (2006). Messing with bacterial quorum sensing. Microbiol. Mol. Biol. Rev. 70, 859–875. doi: 10.1128/MMBR.00002-06
Hengge-Aronis, R. (2002). Signal transduction and regulatory mechanisms involve in control of the σs (RpoS) subnit of RNA polymerase. Microbiol Mol Biol R. 66:373. doi: 10.1128/MMBR.66.3.373-395.2002
Juhas, M., Wiehlmann, L., Huber, B., Jordan, D., Lauber, J., Salunkhe, P., et al. (2004). Global regulation of quorum sensing and virulence by Vqs R in Pseudomonas aeruginosa. Microbiology 150, 831–841. doi: 10.1099/mic.0.26906-0
Kievit, T. R., and Iglewski, B. H. (2000). Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68, 4839–4849. doi: 10.1128/IAI.68.9.4839-4849.2000
King, E. O., Ward, M. K., and Raney, D. E. (1954). Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 44, 301–307.
Kojima, I., Kasuga, K., Kobayashi, M., Fukasawa, A., Mizuno, S., Arisawa, A., et al. (2002). The rpo Z gene, encoding the RNA polymerase omega subunit, is required for antibiotic production and morphological differentiation in Streptomyces kasugaensis. J. Bacteriol. 184, 6417–6423. doi: 10.1128/JB.185.1.386.2003
Li, X., Gu, G. Q., Chen, W., Gao, L. J., Wu, X. H., and Zhang, L. Q. (2018). The outer membrane protein Opr F and the sigma factor sig X regulate antibiotic production in Pseudomonas fluorescens 2P24. Microbiol Res. 206, 159–167. doi: 10.1016/j.micres.2017.10.006
Lonetto, M., Gribskov, M., and Gross, C. A. (1992). The 70 family: sequence conservation and evolutionary relationships. J. Bacteriol. 174, 3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992
Mathew, R., and Chatterji, D. (2006). The evolving story of the omega subunit of bacterial RNA polymerase. Trends Microbiol. 14, 450–455. doi: 10.1016/j.tim.2006.08.002
Mathew, R., Chatterji, D., Balachandar, R., and Chatterji, D. (2006). Deletion of the rpoZ gene, encoding the ω subunit of RNA polymerase, results in pleiotropic surface-related phenotypes in Mycobacterium smegmatis. Microbiology 152, 1741–1750. doi: 10.1099/mic.0.28879-0
Mathew, R., Ramakanth, M., and Chatterji, D. (2005). Deletion of the gene rpo Z, encoding the omega subunit of RNA polymerase, in Mycobacterium smegmatis results in fragmentation of the beta' subunit in the enzyme assembly. J. Bacteriol. 187, 6565–6570. doi: 10.1128/JB.187.18.6565-6570.2005
Miller, J. H. (1972). Experiments in molecular genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
Pessi, G., Williams, F., Hindle, Z., Heurlier, K., Holden, M. T., Camara, M., et al. (2001). The global posttranscriptional regulator Rsm a modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J. Bacteriol. 183, 6676–6683. doi: 10.1128/JB.183.22.6676-6683.2001
Rampioni, G., Polticelli, F., Bertani, I., Righetti, K., Venturi, V., Zennaro, E., et al. (2007). The Pseudomonas quorum-sensing regulator Rsa L belongs to the tetrahelical superclass of H-T-H proteins. J. Bacteriol. 189, 1922–1930. doi: 10.1128/JB.01552-06
Rashid, M. H., and Kornberg, A. (2000). Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 97, 4885–4890. doi: 10.1073/pnas.060030097
Reimmann, C., Beyeler, M., Latifi, A., Winteler, H., Foglino, M., Lazdunski, A., et al. (1997). The global activator GacA of Pseudomonas aeruginosa PAO1 positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 24, 309–319. doi: 10.1046/j.1365-2958.1997.3291701.x
Sakuragi, Y., and Kolter, R. (2007). Quorum-sensing regulation of the biofilm matrix genes (pel) of Pseudomonas aeruginosa. J. Bacteriol. 189, 5383–5386. doi: 10.1128/JB.00137-07
Santos-Beneit, F., Barriuso-Iglesias, M., Fernández-Martínez, L. T., Martínez-Castro, M., Sola-Landa, A., Rodríguez-García, A., et al. (2011). The RNA polymerase omega factor Rpo Z is regulated by pho P and has an important role in antibiotic biosynthesis and morphological differentiation in Streptomyces coelicolor. Appl. Environ. Microbiol. 77, 7586–7594. doi: 10.1128/AEM.00465-11
Sarkar, P., Sardesai, A. A., Murakami, K. S., and Chatterji, D. (2013). Inactivation of the bacterial RNA polymerase due to acquisition of secondary structure by the ω subunit. J. Biol. Chem. 288, 25076–25087. doi: 10.1074/jbc.M113.468520
Schnider-Keel, U., Seematter, A., Maurhofer, M., Blumer, C., Duffy, B., Gigot-Bonnefoy, C., et al. (2000). Autoinduction of 2,4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescens CHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J. Bacteriol. 182, 1215–1225. doi: 10.1128/JB.182.5.1215-1225.2000
Schultz, P., Célia, H., Riva, M., Sentenac, A., and Oudet, P. (1993). Three-dimensional model of yeast RNA polymerase I determined by electron microscopy of two-dimensional crystals. EMBO J. 12, 2601–2607. doi: 10.1002/j.1460-2075.1993.tb05920.x
Shanahan, P., O’Sullivan, D. J., Simpson, P., Glennon, J. D., and O’Gara, F. (1992). Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl. Environ. Microbiol. 58, 353–358. doi: 10.1016/S0065-2164(08)70256-9
Tian, T., Wu, X. G., Duan, H. M., and Zhang, L. Q. (2010). The resistance-nodulation- division efflux pump Emh ABC influences the production of 2,4-diacetylphloroglucinol in Pseudomonas fluorescens 2P24. Microbiology 156, 39–48. doi: 10.1099/mic.0.031161-0
Vincent, M. N., Harrison, L. A., Brackin, J. M., Kovacevich, P. A., Mukerji, P., Weller, D. M., et al. (1991). Genetic analysis of the antifungal activity of a soilborne Pseudomonas aureofaciens strain. Appl. Environ. Microbiol. 57, 2928–2934. doi: 10.1128/aem.57.10.2928-2934.1991
Wang, J., Luo, Y., Gu, Y., and Wei, H. L. (2021). Characterization of the SPI-1 type III secretion system in Pseudomonas fluorescens 2P24. Front. Microbiol. 12:749037. doi: 10.3389/fmicb.2021.749037
Waters, C. M., Lu, W., Rabinowitz, J. D., and Bassler, B. L. (2008). Quorum sensing controls biofilm formation in Vibrio cholera through modulation of cyclic di-GMP levels and repression of vps T. J. Bacteriol. 190, 2527–2536. doi: 10.1128/JB.01756-07
Wei, H. L., Wang, Y., Zhang, L. Q., and Tang, W. H. (2004). Identification and characterization of biocontrol bacterial strain 2P24 and CPF-10. Acta Phytopathol Sin. 34, 80–85. doi: 10.13926/j.cnki.apps.2004.01.014
Wei, H. L., and Zhang, L. Q. (2005). Cloning and functional characterization of the gacS gene of the biocontrol strain Pseuodomonas fluorescens 2P24. Acta Microbiol Sin. 45, 368–372. doi: 10.13343/j.cnki.wsxb.2005.03.011
Wei, H. L., and Zhang, L. Q. (2006). Quorum-sensing system influences root colonization and biological control ability in Pseudomonas fluorescens 2P24. Antonie Van Leeuwenhoek 89, 267–280. doi: 10.1007/s10482-005-9028-8
Weiss, A., Moore, B. D., Tremblay, M. H., Chaput, D., Kremer, A., and Shaw, L. N. (2017). The omega subunit governs RNA polymerase stability and transcriptional specificity in Staphylococcus aureus. J. Bacteriol. 199:e00459-16. doi: 10.1128/JB.00459-16
Wendrich, T. M., Beckering, C. L., and Marahiel, M. (2000). Charecter ization of the Rel a/Spol T gene from Bacillus Stearothermophilus. FEMS Mirobiologv Letter 190, 195–201. doi: 10.1016/s0378-1097(00)00335-9
Wu, X., Liu, J., Zhang, W., and Zhang, L. (2012). Multiple-level regulation of 2,4-diacetylphloroglucinol production by the sigma regulator Psr a in Pseudomonas fluorescens 2P24. PLoS One 7:e50149. doi: 10.1371/journal.pone.0050149
Yan, Q., Gao, W., Wu, X. G., and Zhang, L. Q. (2009a). Regulation of the Pco I/Pco R quorum-sensing system in Pseudomonas fluorescens 2P24 by the pho P/pho Q two-component system. Microbiology 155, 124–133. doi: 10.1099/mic.0.020750-0
Yan, Q., Wu, X. G., Wei, H. L., Wang, H. M., and Zhang, L. Q. (2009b). Differential control of the Pco I/Pco R quorum-sensing system in Pseudomonas fluorescens 2P24 by sigma factor RpoS and the Gac S/GacA two-component regulatory system. Microbiol. Res. 164, 18–26. doi: 10.1016/j.micres.2008.02.001
Zhang, G., Campbell, E. A., Minakhin, L., Richter, C., Severinov, K., and Darst, S. A. (1999). Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 a resolution. Cells 98, 811–824. doi: 10.1016/s0092-8674(00)81515-9
Zhang, Y., Zhang, B., Wu, H., Wu, X., Yan, Q., and Zhang, L. Q. (2020b). Pleiotropic effects of Rsm a and Rsm E proteins in Pseudomonas fluorescens 2P24. BMC Microbiol. 20, 20:191. doi: 10.1186/s12866-020-01880-x
Zhang, Y., Zhang, B., Wu, X., and Zhang, L. Q. (2020a). Characterization the role of GacA-dependent small RNAs and Rsm a family proteins on 2,4-diacetylphloroglucinol production in Pseudomonas fluorescens 2P24. Microbiol Res. Mar 233:126391. doi: 10.1016/j.micres.2019.126391
Zhao, M. M., Lyu, N., Wang, D., Wu, X. G., Zhao, Y. Z., Zhang, L. Q., et al. (2020). Phl G mediates the conversion of DAPG to MAPG in Pseudomonas fluorescens 2P24. Sci. Rep. 10, 10:4296. doi: 10.1038/s41598-020-60555-9
Keywords: Pseudomonas fluorescen 2P24, rpoZ, 2,4-DAPG, quorum sensing, biocontrol
Citation: Wei Y, Dong B, Wu X, Zhao M, Wang D, Li N, Zhang Q, Zhang L and Zhou H (2023) RpoZ regulates 2,4-DAPG production and quorum sensing system in Pseudomonas fluorescens 2P24. Front. Microbiol. 14:1160913. doi: 10.3389/fmicb.2023.1160913
Edited by:
Ernesto Perez-Rueda, Universidad Nacional Autónoma de México, MexicoReviewed by:
Yantao Jia, Chinese Academy of Sciences (CAS), ChinaRikky Rai, University of Allahabad, India
Copyright © 2023 Wei, Dong, Wu, Zhao, Wang, Li, Zhang, Zhang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyou Zhou, hongyouzhou2002@aliyun.com.cn; Liqun Zhang, zhanglq@cau.edu.cn
†These authors have contributed equally to this work
 Yarui Wei1†
Yarui Wei1† Baozhu Dong
Baozhu Dong Mingmin Zhao
Mingmin Zhao Liqun Zhang
Liqun Zhang Hongyou Zhou
Hongyou Zhou