Case report: Whole exome sequencing identified a novel mutation (p.Y301H) of MAF in a Chinese family with congenital cataracts
- 1Department of Anesthesiology, The Second Xiangya Hospital of Central South University, Changsha, China
- 2Department of Cell Biology, School of Life Sciences, Central South University, Changsha, China
- 3Department of Reproductive Genetics, Hebei General Hospital, Shijiazhuang, China
- 4Department of Obstetrics and Gynecology, Ordos Central Hospital, Ordos, China
Background: Congenital cataracts stand as the primary cause of childhood blindness globally, characterized by clouding of the eye’s lens at birth or shortly thereafter. Previous investigations have unveiled that a variant in the V-MAF avian musculoaponeurotic-fibrosarcoma oncogene homolog (MAF) gene can result in Ayme-Gripp syndrome and solitary cataract. Notably, MAF mutations have been infrequently reported in recent years.
Methods: In this investigation, we recruited a Chinese family with non-syndromic cataracts. Whole exome sequencing and Sanger sequencing were applied to scrutinize the genetic anomaly within the family.
Results: Through whole exome sequencing and subsequent data filtration, a new mutation (NM_005360, c.901T>C/p.Y301H) in the MAF gene was detected. Sanger sequencing validated the presence of this mutation in another affected individual. The p.Y301H mutation, situated in an evolutionarily preserved locus, was not detected in our 200 local control cohorts and various public databases. Additionally, multiple bioinformatic programs predicted that the mutation was deleterious and disrupted the bindings between MAF and its targets.
Conclusion: Hence, we have documented a new MAF mutation within a Chinese family exhibiting isolated congenital cataracts. Our study has the potential to broaden the spectrum of MAF mutations, offering insights into the mechanisms underlying cataract formation and facilitating genetic counseling and early diagnosis for congenital cataract patients.
Introduction
Congenital cataracts, an ophthalmic disease presented at birth or developing in infancy, can lead to a clouding in the lens of the eye that can cause blurry vision or blindness (1–3). As the predominant cause of visual impairment and blindness among children, congenital cataracts affect approximately 200,000 children worldwide, with an estimated prevalence ranging from three to six per 10,000 live births (4, 5). The current studies believe that multiple factors including genetics, infections, radiation exposure, toxic agents, and metabolic disturbance underlie the occurrence of congenital cataracts (3). To date, over 40 genes responsible for the molecular etiology of isolated or syndromic congenital cataracts, featuring autosomal dominant or autosomal recessive inheritance patterns, have been identified (1, 6).
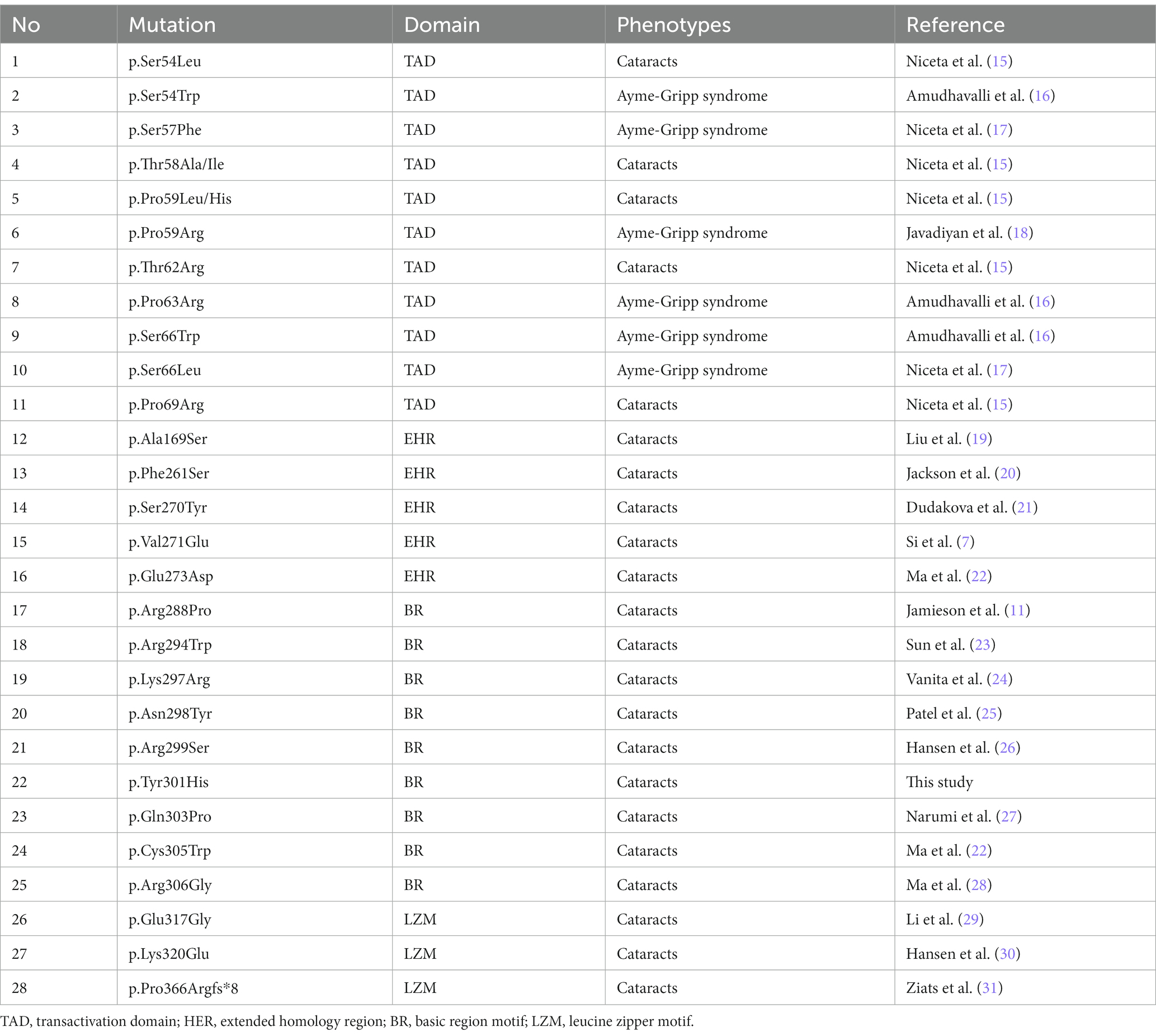
The V-MAF avian musculoaponeurotic-fibrosarcoma oncogene homolog (MAF) gene, also known as MAF bZIP transcription factor (OMIM#177075), is situated on chromosome 16q23.2, spanning approximately 6.9 kilobases (7). As a DNA-binding transcription factor with a leucine zipper motif that can control the activity of target genes, MAF can serve as a transcriptional activator or repressor, regulating diverse cellular processes, including embryonic lens fiber cell development, heightened T-cell susceptibility to apoptosis, and chondrocyte terminal differentiation (8–10). In 2002, Jamieson et al. (11) initially reported the association of MAF with cataracts, ocular anterior segment dysgenesis, and coloboma. Subsequently, approximately 30 MAF mutations have been detected in patients with Ayme-Gripp syndrome and isolated cataracts (7).
Here, we recruited a Chinese family affected by isolated congenital cataracts. Utilizing whole exome sequencing and Sanger sequencing, we sought to uncover the genetic abnormalities in the affected individuals.
Case presentation
Here, we enrolled the family from Hebei province, China (Figure 1A). The affected proband, a four-year-old boy was admitted to Hebei General Hospital and diagnosed as a total congenital cataract of the right eye (Figure 1B). A medical history survey suggested that the patient suffered from poor vision in dim light at approximately 3 years old but did not arouse parents’ attention, and the symptoms of the child developed to lens complete opacification from pupil gray appearance quickly during this year. Finally, the patient agreed to undergo surgery for cataract extraction and implantation for the posterior chamber intraocular lens, and the eye vision is gradually recovering. A study of the family history indicated that his father also suffered from cataracts and underwent cataract extraction in infancy. The proband’s grandmother was blind and died several years ago.
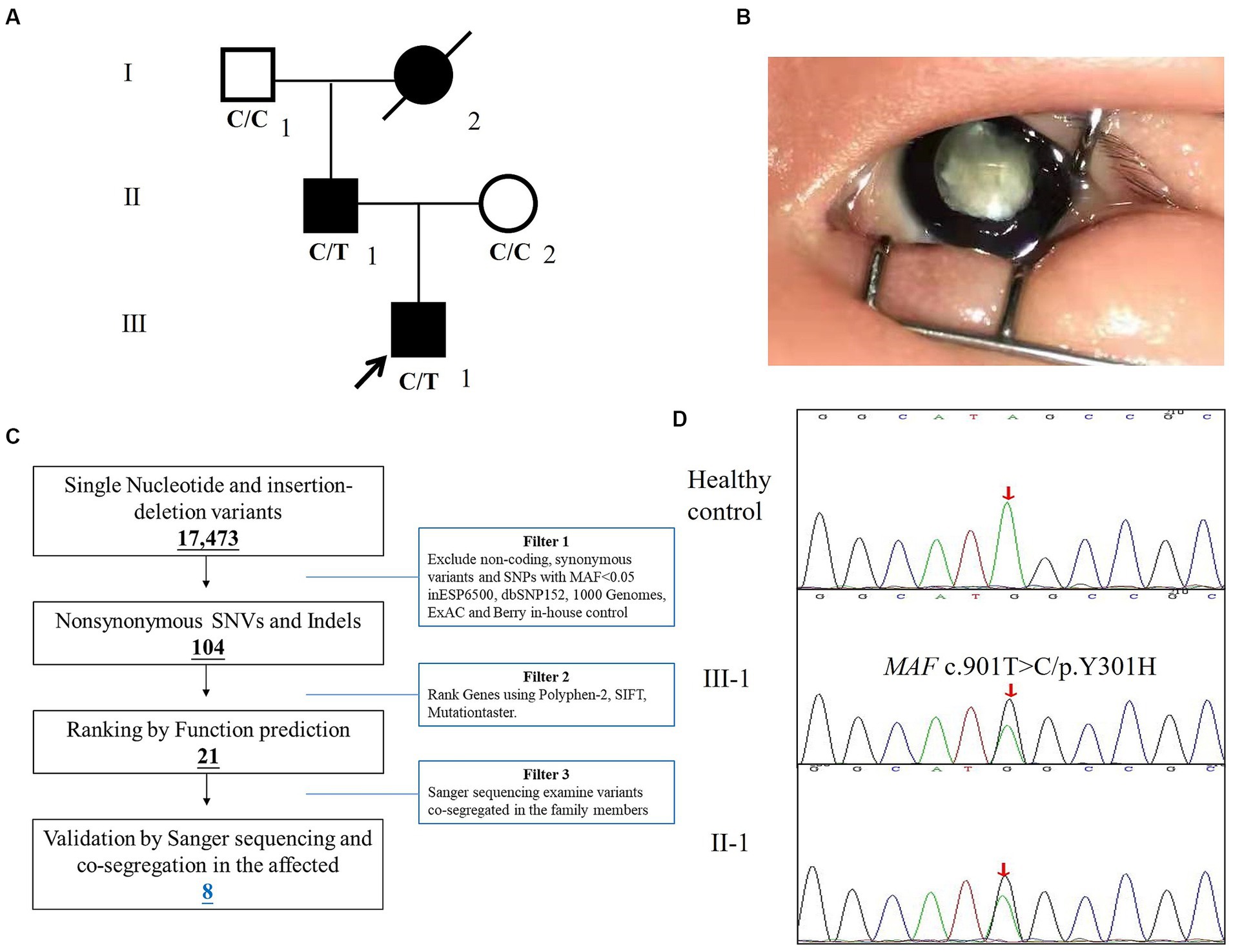
Figure 1. The clinical and genetic analysis of the family with congenital cataracts. (A) Pedigree of the family. White circles/squares are unaffected family members, and the arrow indicates the proband. (B) The right eye of the proband. (C) Schematic representation of the filter strategies employed in our study. (D) Sanger DNA sequencing chromatogram demonstrates the heterozygosity for an MAF missense mutation (c.901T>C/p.Y301H) in the family.
Laboratory investigations
Whole peripheral blood samples of the patient and his family were obtained and stored in EDTA/citrate tubes. Genomic DNA was extracted by GenElute blood genomic DNA extraction kit (Sigma-Aldrich, NA 2010). Whole Exome sequencing (WES) was mainly conducted by Berry Genomics (Beijing, China) and Agilent SureSelect Human All Exon V6 kits and Illumina HiSeq2500 (Illumina Inc., San Diego, United States) and matched to Human Reference Genome (hg19). GATK online software1 was used to detect SNP, Indel, and variants, and the Annovar (Annotate Variation) tool was used to functionally annotate genetic variant results in detail.
The strategies of data filtering are as follows (12, 13): (a) non-coding and synonymous variants, SNPs, or frameshift-causing INDELs with an alternative allele frequency >0.05 in the NHLBI Exome Sequencing Project Exome Variant Server (ESP6500), dbSNP152,2 the 1,000 Genomes project,3 the ExAC database,4 or in-house exome databases of BerryGenomics (2000 exomes) were excluded; (b) the filtered SNVs and INDELs, predicted by SIFT,5 Polyphen2,6 and MutationTaster7 to be causing damage remained; (c) co-segregation analysis was conducted in each family.
The filtered mutations validation and co-segregation analysis were performed by Sanger sequencing. The primer pairs (the sequence of primers will be provided upon request) were designed by Primer 5. The sequences of the primers were: 5′-3′ TCAGCAAGGAGGAGGTGAT and 3′-5′ CTGCTCACCAACTTCTCGTATT. The sequence of the polymerase chain reaction products was determined using the ABI 3100 Genetic Analyzer (ABI, United States).
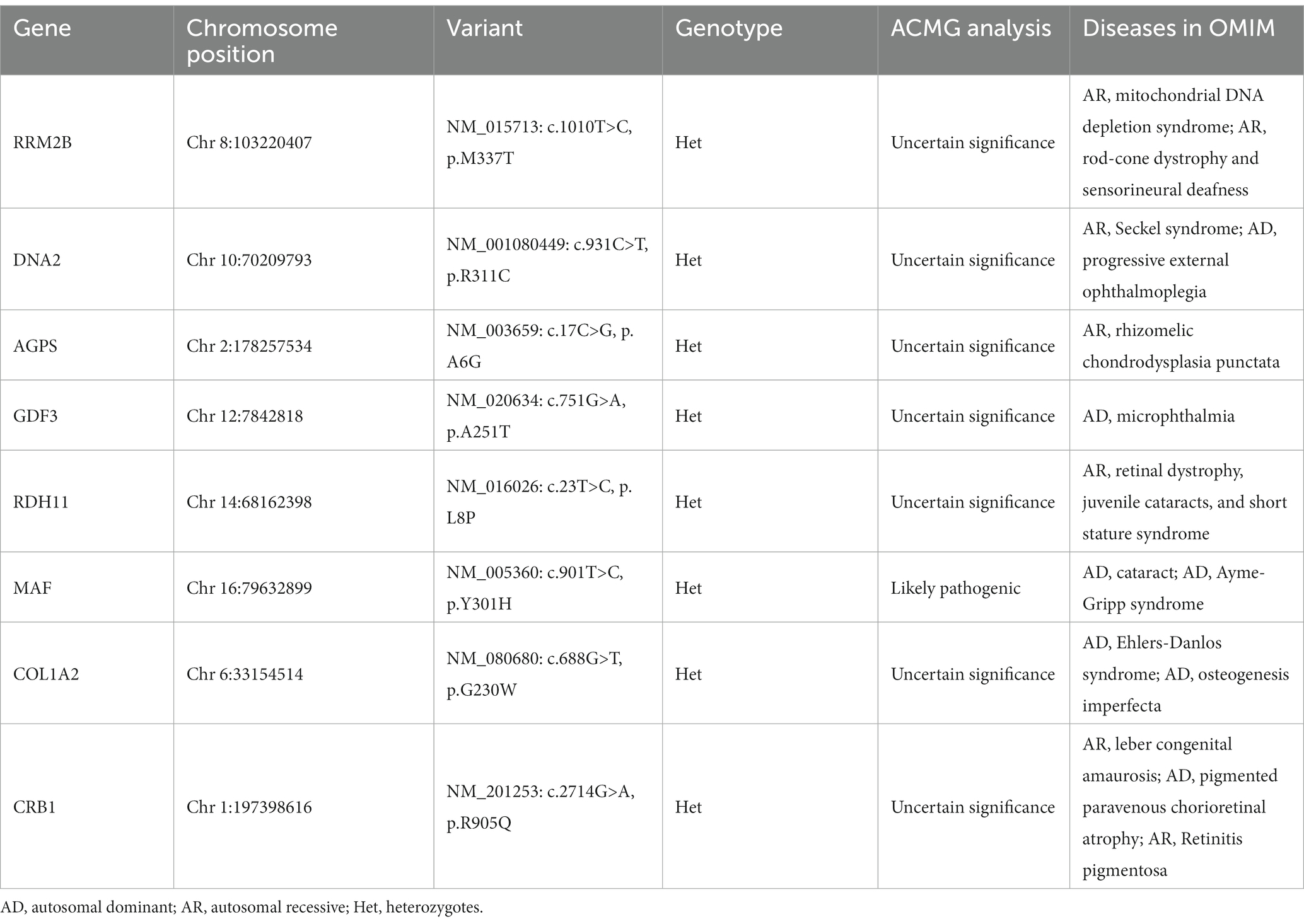
After the abovementioned data filtering and Sanger sequencing validation (Figure 1C), a new mutation (NM_005360, c.901T>C/p.Y301H) of MAF was discerned in the proband (Table 1). No other meaningful mutations related to cataracts were detected. Co-segregation analysis indicated that the new mutation was present in the affected family members and was not detected in unaffected family members and healthy controls as well as 200 local control cohorts (Figure 1D). Three programs for analyzing protein functions, polyphen2, SIFT, and MutationTaster, predicted that the p.Y301H variants are probably damaging (1.0), deleterious (0.00), and disease-causing (0.99), respectively. Cross-species alignment analysis of MAF amino acid sequences revealed that this mutated site was highly evolutionarily conserved (Figure 2A). Swiss-Model and alphafold2 online software (14) found that the p.Y301H mutation changed the surface hydrophobic area and surface charge of MAF (Figure 2B).
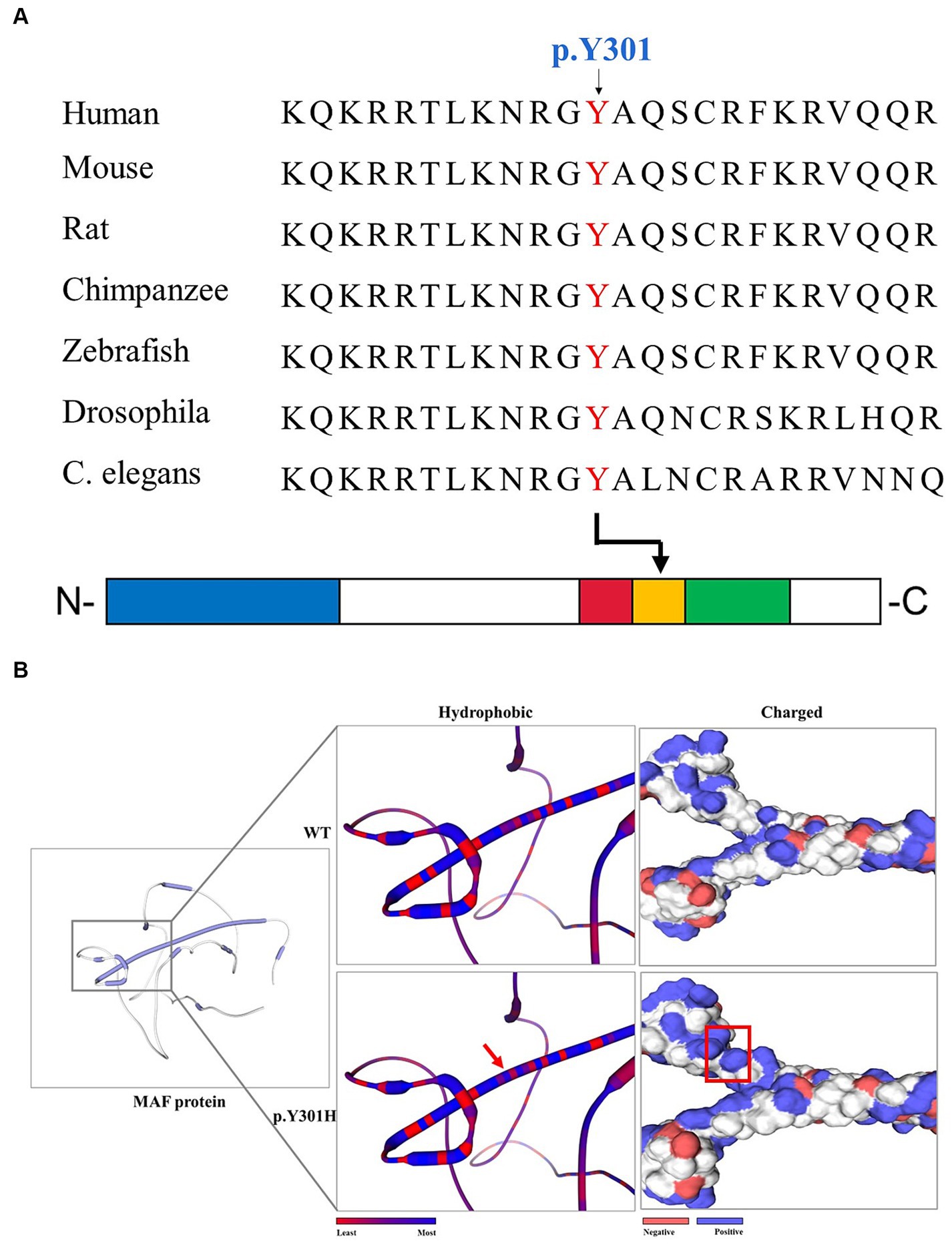
Figure 2. The bioinformatics analysis of the MAF p.Y301H mutation. (A) Alignment of multiple MAF protein sequences across species. The Y301-affected amino acid is located in the highly conserved amino acid region in different mammals (from Ensembl). The red column shows the Y301 site. Blue square: transactivation domain. Red square: extended homology region. Orange square: basic region motif. Green square: leucine zipper motif. (B) The hydrophobic surface area and surface charge of the WT and mutated MAF were predicted by SWISS-MODEL and alphafold2. The red arrow and red square indicate the differences between WT and mutated protein.
Discussion
Congenital cataracts represent a highly diverse ocular disorder both clinically and genetically (3). Mutations in MAF have been linked to various types and severities of human congenital cataracts (Table 2) (32). MAF proteins play a pivotal role in eyes and lens development, regulating the expression of crystallin genes, MIP (major intrinsic protein of the ocular lens fiber membrane), and other genes expressed in lens fiber cells from the formation of the lens pre-placode to the development of lens fiber cells and lens epithelium (8–10). In C57BL/6J mice, homozygous deletion of MAF results in embryonic lethality, but the lens fiber cell MAF condition knockout mice (MAFΔTAM) develops abnormal lens structure, and the expression of crystallin genes in MAFΔTAM mice eyes are reduced compared to WT mice (33). The mice data were consistent with human genetic studies, highlighting the essential role of MAF in the differentiation and cell cycle arrest of lens fiber cells. Although the first mutation of MAF was reported in 2002 (11), the MAF novel mutation was rarely reported in recent years. Here, we identified a new variant (NM_005360, c.901T>C/p.Y301H) of MAF in a Chinese family with congenital cataracts via whole exome sequencing and Sanger sequencing. Our study expanded the mutation spectrum of MAF and further proved that mutations in MAF may lead to congenital cataracts.
The MAF protein consists of an N-terminal transactivation domain with a regulatory function and a C-terminal DNA binding domain (18). The C-terminal domain is further divided into three conserved regions (Figure 2A): extended homology region, basic region motif, and leucine zipper motif (15, 34). Earlier studies have indicated that the N-terminal variants may result in Ayme-Gripp syndrome which presents with cataracts, hearing loss, epilepsy, intellectual disability, etc., while most of the C-terminal mutation carriers only showed ocular diseases such as cataracts (Table 2) (7). Here, the mutation (p.Y301H) is also situated in the C-terminal of MAF, which provides more evidence that C-terminal mutations are responsible for isolated cataracts.
Furthermore, the p.Y301H mutation is in the BR motif of the C-terminal, which is the mutational hot spot region of MAF (7). Previous studies have suggested that the BR motif is responsible for binding the specific target promoters of crystallin genes including CRYGA, CRYAA, CRYBA1, and CRYBA4, which are associated with inherited cataracts (32, 35). Additionally, ChIP-seq studies have identified several non-crystallin genes crucial for maintaining lens transparency as direct targets of MAF (36, 37). Hence, the p.Y301H mutation may disrupt the bindings between MAF and the target genes such as crystallin genes, and finally disrupt the expression of promoters of crystallin genes, leading to congenital cataracts. Following the ACMG guideline (38), the p.Y301H mutation is likely pathogenic (PM1 + PM2 + PP1 + PP2 + PP3).
Congenital cataracts persist as a leading cause of global blindness, and early surgical intervention, prolonged postoperative amblyopia training, and visual reconstruction constitute the primary therapeutic approaches for the disease (39, 40). Early diagnosis is paramount, particularly in the era of precision medicine, where technologies such as PCR, Sanger sequencing, high-throughput sequencing, and gene editing have facilitated the discovery of numerous pathogenic genes linked to congenital cataract development. This advancement enhances our understanding of the disease’s pathogenesis and lays the groundwork for genetics-based treatments (6, 28). In the future, we can develop a genetic detection panel that contains all the reported mutations of MAF including the p.Y301H, and the panel may contribute to the genetic counseling and early diagnosis of congenital cataract patients.
Conclusion
Hence, we detected a new mutation (NM_005360, c.901T>C/p.Y301H) of MAF in a Chinese family with congenital cataracts by employing whole exome sequencing and Sanger sequencing. Our study not only explores the genetic lesion of the family and broadens the spectrum of MAF mutations but also confirms that the MAF mutation was linked to non-syndromic total congenital cataracts and facilitates genetic counseling and early diagnosis for congenital cataract patients. Certainly, there were still some limitations in this study, for example, the sample size was small and population specificity was not excluded.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Hebei General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
Z-JL: Data curation, Formal analysis, Writing – original draft. J-YL: Formal analysis, Writing – review & editing. JL: Resources, Writing – review & editing. F-NW: Resources, Writing – review & editing. WC: Resources, Writing – review & editing. LZ: Formal analysis, Funding acquisition, Writing – original draft. Y-LL: Funding acquisition, Resources, Writing – original draft. L-LF: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Hunan Province (2023JJ20078), the Clinical Medical Personnel Training Program of Hebei Provincial Health Commission (Y-LL), and the Inner Mongolia Science and Technology Innovation Guidance Project (NM-KJCXYD-018).
Acknowledgments
The authors thank all the subjects for participating in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^www.broadinstitute.org/gatk
2. ^http://www.ncbi.nlm.nih.gov/projects/SNP/index.html
3. ^http://www.1000genomes.org/
4. ^http://exac.broadinstitute.org
References
1. Berry, V, Georgiou, M, Fujinami, K, Quinlan, R, Moore, A, and Michaelides, M. Inherited cataracts: molecular genetics, clinical features, disease mechanisms and novel therapeutic approaches. Br J Ophthalmol. (2020) 104:1331–7. doi: 10.1136/bjophthalmol-2019-315282
2. Gali, HE, Sella, R, and Afshari, NA. Cataract grading systems: a review of past and present. Curr Opin Ophthalmol. (2019) 30:13–8. doi: 10.1097/ICU.0000000000000542
3. Liu, YC, Wilkins, M, Kim, T, Malyugin, B, and Mehta, JS. Cataracts. Lancet. (2017) 390:600–12. doi: 10.1016/S0140-6736(17)30544-5
4. Hashemi, H, Pakzad, R, Yekta, A, Aghamirsalim, M, Pakbin, M, Ramin, S, et al. Global and regional prevalence of age-related cataract: a comprehensive systematic review and meta-analysis. Eye. (2020) 34:1357–70. doi: 10.1038/s41433-020-0806-3
5. Medsinge, A, and Nischal, KK. Pediatric cataract: challenges and future directions. Clin Ophthalmol. (2015) 9:77–90. doi: 10.2147/OPTH.S59009
6. Li, J, Chen, X, Yan, Y, and Yao, K. Molecular genetics of congenital cataracts. Exp Eye Res. (2020) 191:107872. doi: 10.1016/j.exer.2019.107872
7. Si, N, Song, Z, Meng, X, Li, X, Xiao, W, and Zhang, X. A novel MAF missense mutation leads to congenital nuclear cataract by impacting the transactivation of crystallin and noncrystallin genes. Gene. (2019) 692:113–8. doi: 10.1016/j.gene.2019.01.011
8. Cvekl, A, Mcgreal, R, and Liu, W. Lens development and crystallin gene expression. Prog Mol Biol Transl Sci. (2015) 134:129–67. doi: 10.1016/bs.pmbts.2015.05.001
9. Ring, BZ, Cordes, SP, Overbeek, PA, and Barsh, GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development. (2000) 127:307–17. doi: 10.1242/dev.127.2.307
10. Xie, Q, Mcgreal, R, Harris, R, Gao, CY, Liu, W, Reneker, LW, et al. Regulation of c-Maf and alphaA-Crystallin in ocular lens by fibroblast growth factor Signaling. J Biol Chem. (2016) 291:3947–58. doi: 10.1074/jbc.M115.705103
11. Jamieson, RV, Perveen, R, Kerr, B, Carette, M, Yardley, J, Heon, E, et al. Domain disruption and mutation of the bZIP transcription factor, MAF, associated with cataract, ocular anterior segment dysgenesis and coloboma. Hum Mol Genet. (2002) 11:33–42. doi: 10.1093/hmg/11.1.33
12. Fang, GM, Miao, JX, Peng, Y, Zhai, YF, Wang, CC, Zhao, XY, et al. Identification of three FBN1 mutations in Chinese patients with typical or incomplete Marfan syndrome by whole-exome sequencing. Cardiovasc Innov Appl. (2020) 5:19–26. doi: 10.15212/CVIA.2019.0576
13. Yu, R, Liu, L, Li, YL, and Fan, LL. MITF p.Arg217Thr variant identified in a Han Chinese family with Tietz/Waardenburg syndrome. Biomed Res Int. (2021) 2021:4381272. doi: 10.1155/2021/4381272
14. Lu, G, Zhang, Y, Xia, H, He, X, Xu, P, Wu, L, et al. Identification of a de novo mutation of the FOXG1 gene and comprehensive analysis for molecular factors in Chinese FOXG1-related encephalopathies. Front Mol Neurosci. (2022) 15:1039990. doi: 10.3389/fnmol.2022.1039990
15. Niceta, M, Stellacci, E, Gripp, KW, Zampino, G, Kousi, M, Anselmi, M, et al. Mutations impairing GSK3-mediated MAF phosphorylation cause cataract, deafness, intellectual disability, seizures, and a down syndrome-like Facies. Am J Hum Genet. (2015) 96:816–25. doi: 10.1016/j.ajhg.2015.03.001
16. Amudhavalli, SM, Hanson, R, Angle, B, Bontempo, K, and Gripp, KW. Further delineation of Aymé-Gripp syndrome and use of automated facial analysis tool. Am J Med Genet A. (2018) 176:1648–56. doi: 10.1002/ajmg.a.38832
17. Niceta, M, Barbuti, D, Gupta, N, Ruggiero, C, Tizzano, EF, Graul-Neumann, L, et al. Skeletal abnormalities are common features in Aymé-Gripp syndrome. Clin Genet. (2020) 97:362–9. doi: 10.1111/cge.13651
18. Javadiyan, S, Craig, JE, Sharma, S, Lower, KM, Casey, T, Haan, E, et al. Novel missense mutation in the bZIP transcription factor, MAF, associated with congenital cataract, developmental delay, seizures and hearing loss (Ayme-Gripp syndrome). BMC Med Genet. (2017) 18:52. doi: 10.1186/s12881-017-0414-7
19. Liu, Y, Liu, X, Qin, D, Zhao, Y, Cao, X, Deng, X, et al. Clinical Utility of Next-Generation Sequencing for Developmental Disorders in the Rehabilitation Department: Experiences from a Single Chinese Center. J Mol Neurosci. (2021) 71:845–53. doi: 10.1007/s12031-020-01707-4
20. Jackson, D, Malka, S, Harding, P, Palma, J, Dunbar, H, and Moosajee, M. Molecular diagnostic challenges for non-retinal developmental eye disorders in the United Kingdom. Am J Med Genet C Semin Med Genet. (2020) 184:578–89. doi: 10.1002/ajmg.c.31837
21. Dudakova, L, Stranecky, V, Ulmanova, O, Hlavova, E, Trková, M, Vincent, AL, et al. Segregation of a novel p.(Ser270Tyr) MAF mutation and p.(Tyr56∗) CRYGD variant in a family with dominantly inherited congenital cataracts. Mol Biol Rep. (2017) 44:435–40. doi: 10.1007/s11033-017-4121-4
22. Ma, AS, Grigg, JR, Ho, G, Prokudin, I, Farnsworth, E, Holman, K, et al. Sporadic and Familial Congenital Cataracts: Mutational Spectrum and New Diagnoses Using Next-Generation Sequencing. Hum Mutat. (2016) 37:371–84. doi: 10.1002/humu.22948
23. Sun, W, Xiao, X, Li, S, Guo, X, and Zhang, Q. Exome sequencing of 18 Chinese families with congenital cataracts: a new sight of the NHS gene. PLoS One. (2014) 9:e100455. doi: 10.1371/journal.pone.0100455
24. Vanita, V, Singh, D, Robinson, PN, Sperling, K, and Singh, JR. A novel mutation in the DNA-binding domain of MAF at 16q23.1 associated with autosomal dominant “cerulean cataract” in an Indian family. Am J Med Genet A. (2006) 140:558–66. doi: 10.1002/ajmg.a.31126
25. Patel, A, Hayward, JD, Tailor, V, Nyanhete, R, Ahlfors, H, Gabriel, C, et al. The Oculome Panel Test: Next-Generation Sequencing to Diagnose a Diverse Range of Genetic Developmental Eye Disorders. Ophthalmology. (2019) 126:888–907. doi: 10.1016/j.ophtha.2018.12.050
26. Hansen, L, Eiberg, H, and Rosenberg, T. Novel MAF mutation in a family with congenital cataract-microcornea syndrome. Mol Vis. (2007) 13:2019–22
27. Narumi, Y, Nishina, S, Tokimitsu, M, Aoki, Y, Kosaki, R, Wakui, K, et al. Identification of a novel missense mutation of MAF in a Japanese family with congenital cataract by whole exome sequencing: a clinical report and review of literature. Am J Med Genet A. (2014) 164:1272–6. doi: 10.1002/ajmg.a.36433
28. Ma, A, Grigg, JR, Flaherty, M, Smith, J, Minoche, AE, Cowley, MJ, et al. Genome sequencing in congenital cataracts improves diagnostic yield. Hum Mutat. (2021) 42:1173–83. doi: 10.1002/humu.24240
29. Li, J, Leng, Y, Han, S, Yan, L, Lu, C, Luo, Y, et al. Clinical and genetic characteristics of Chinese patients with familial or sporadic pediatric cataract. Orphanet J Rare Dis. (2018) 13:94. doi: 10.1186/s13023-018-0828-0
30. Hansen, L, Mikkelsen, A, Nürnberg, P, Nürnberg, G, Anjum, I, Eiberg, H, et al. Comprehensive mutational screening in a cohort of Danish families with hereditary congenital cataract. Invest Ophthalmol Vis Sci. (2009) 50:3291–303. doi: 10.1167/iovs.08-3149
31. Ziats, MN, Ahmad, A, Bernat, JA, Fisher, R, Glassford, M, Hannibal, MC, et al. Genotype-phenotype analysis of 523 patients by genetics evaluation and clinical exome sequencing. Pediatr Res. (2020) 87:735–9. doi: 10.1038/s41390-019-0611-5
32. Vanita, V, Guo, G, Singh, D, Ott, CE, and Robinson, PN. Differential effect of cataract-associated mutations in MAF on transactivation of MAF target genes. Mol Cell Biochem. (2014) 396:137–45. doi: 10.1007/s11010-014-2150-z
33. Fujino, M, Tagami, A, Ojima, M, Mizuno, S, Abdellatif, AM, Kuno, A, et al. C-MAF deletion in adult C57BL/6J mice induces cataract formation and abnormal differentiation of lens fiber cells. Exp Anim. (2020) 69:242–9. doi: 10.1538/expanim.19-0137
34. Imbratta, C, Hussein, H, Andris, F, and Verdeil, G. C-MAF, a Swiss army knife for tolerance in lymphocytes. Front Immunol. (2020) 11:206. doi: 10.3389/fimmu.2020.00206
35. Li, L, Yue, JF, Kong, DQ, Sun, MM, Li, K, and Zheng, GY. Novel cataract-causing variant c.177dupC in c-MAF regulates the expression of crystallin genes for cell apoptosis via a mitochondria-dependent pathway. Mol Gen Genomics. (2023) 298:495–506. doi: 10.1007/s00438-022-01982-3
36. Anand, D, and Lachke, SA. Systems biology of lens development: a paradigm for disease gene discovery in the eye. Exp Eye Res. (2017) 156:22–33. doi: 10.1016/j.exer.2016.03.010
37. Reza, HM, Urano, A, Shimada, N, and Yasuda, K. Sequential and combinatorial roles of maf family genes define proper lens development. Mol Vis. (2007) 13:18–30.
38. Richards, S, Aziz, N, Bale, S, Bick, D, Das, S, Gastier-Foster, J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. (2015) 17:405–24. doi: 10.1038/gim.2015.30
39. Lenhart, PD, and Lambert, SR. Current management of infantile cataracts. Surv Ophthalmol. (2022) 67:1476–505. doi: 10.1016/j.survophthal.2022.03.005
Keywords: congenital cataract, inherited cataract, MAF, whole exome sequencing, missense mutation
Citation: Lin Z-J, Long J-Y, Li J, Wang F-N, Chu W, Zhu L, Li Y-L and Fan L-L (2024) Case report: Whole exome sequencing identified a novel mutation (p.Y301H) of MAF in a Chinese family with congenital cataracts. Front. Med. 11:1332992. doi: 10.3389/fmed.2024.1332992
Edited by:
Huiliang Wen, Nanchang University, ChinaReviewed by:
Haotian Zheng, University of Pennsylvania, United StatesJin Wu, Roswell Park Comprehensive Cancer Center, United States
Chuanyi Zhang, Regeneron Genetic Center, United States
Copyright © 2024 Lin, Long, Li, Wang, Chu, Zhu, Li and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhu, zl1480@qq.com; Ya-Li Li, Lyl8703@sina.com; Liang-Liang Fan, swfanliangliang@csu.edu.cn
 Zhao-Jing Lin1
Zhao-Jing Lin1  Liang-Liang Fan
Liang-Liang Fan