Association between the mental domain of the comprehensive geriatric assessment and prolonged length of stay in hospitalized older adults with mild to moderate frailty
- 1Department of Nursing, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 2Clinical Innovation and Research Center, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 3Department of Geriatrics and Gerontology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 4School of Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan
Introduction: Previous researches have shown the risk factors of prolonged length of stay (PLOS) in hospitalized older adults, but it is unclear what are the risk factors of PLOS in hospitalized older adults with mild to moderate frailty.
Objective: To identify the risk factors of PLOS in hospitalized older adults with mild to moderate frailty.
Methods: We recruited adults aged ≥65 years old with mild to moderate frailty admitted to a tertiary medical center in the southern Taiwan from June 2018 to September 2018. Each individual underwent a structural questionnaire interview within 72 h after admission and 72 h after discharge. The data were collected face-to-face, including demographic characteristics, comorbidities, length of stay (LOS), and multiple domains of the comprehensive geriatric assessment. The main outcome was PLOS.
Results: Individuals who had two or more drugs, were female, did not have cognitive impairment and had a Geriatric Depression Scale score ≥ 1 had a higher risk of PLOS (probability = 0.81), and these individuals accounted for 29% of the overall study population. Among male individuals younger than 87 years old, those with cognitive impairment had a higher risk of PLOS (probability = 0.76), and among male individuals without cognitive impairment, living alone was associated with a higher risk of PLOS (probability = 0.88).
Conclusion: Early detection and management of mood and cognition in older adults, together with comprehensive discharge planning and transition care, may be an important part of reducing LOS in hospitalized older adults with mild to moderate frailty.
1. Introduction
Due to the aging population, hospitals have had to address a continuous increase in the number of older adults, most of whom present with severe illness, multimorbidity, and frailty (1). Frailty is a condition, which can lead to a reduction in the physiological reserve, cumulative functional decline, and poor responses to stressors (2, 3). It is common in older adults with acute unplanned hospitalization, and its prevalence is 2–4 times higher in older adults with acute hospitalization than those in the community (4). It is also predictive of worse clinical outcomes, including prolonged length of stay (PLOS), discharge to a destination other than home, and mortality (4). The need for intensified medical care in older frail adults, as reflected by PLOS (5), contributes to the care burden among families and contributes to the economic burden among societies.
Previous studies have shown that risk factors of PLOS include individuals at risk for functional decline (5, 6), dependence in activities of daily living (5), dependence in instrumental activities of daily living (7), acute confusion state (8), cognition impairment (5, 6), malnutrition (7), falling history (7), the number of comorbidities (5), and frailty (8). However, the risk factors of PLOS in hospitalized older frail adults remain unclear.
The early detection of older frail adults at risk for adverse hospital outcomes would help the interdisciplinary team to provide a better management plan. For this goal, a complete assessment at hospital admission may be necessary; this kind of assessment in older frail adults is called a comprehensive geriatric assessment (CGA), and it is used to establish treatment strategies and interventions in frail older adults (9). CGA was first developed in the United Kingdom, and its concepts, indications, and applications evolved over time (10). It is a multidomain, multidisciplinary diagnostic and therapeutic process performed to assess the medical, mental, social, and functional problems of older adults, thereby contributing to a tailor-made and integrated plan for management and follow-up (11).
Therefore, we conducted a CGA-based prospective cohort study to identify the risk factors of PLOS in hospitalized older adults with mild to moderate frailty.
2. Methods
2.1. Participants
We recruited adults aged ≥65 years old with mild to moderate frailty who were admitted to medical wards in a tertiary medical center from June 2018 to September 2018. The exclusion criteria were delirium, critical or terminal illness, inability to communicate, and long-term bedridden state. Informed consent was obtained from all individuals, and the study was approved by the National Cheng Kung University Hospital Institutional Review Board (A-ER-106-261). Each individual underwent a structural questionnaire interview within 72 h after admission and 72 h after discharge. The data were collected face-to-face, including demographic characteristics, comorbidities, length of stay (LOS), and multiple domains of the CGA.
2.2. Comprehensive geriatric assessment
The CGA involves medical, mental, social, and functional domain assessment and management. The medical domain includes malnutrition, urine incontinence, falls, and the number of drugs; the mental domain includes mood and cognition; the social domain includes educational level, marital status, and living condition; and the functional domain includes frailty evaluation. All the components of CGA mentioned above were collected within 72 h after admission. These factors were selected because they are important in older adults, and previous reports have shown an association between these factors and PLOS (5–8). Frailty was assessed by the Clinical Frailty Scale (CFS) (12). The CFS is a simple and intuitive clinical examination with a nine-point scale, and it categorizes overall performance from very fit (CFS = 1), fit (CFS = 2), managing well (CFS = 3), living with very mild frailty (CFS = 4), living with mild frailty (CFS = 5), living with moderate frailty (CFS = 6), living with severe frailty (CFS = 7), living with very severe frailty (CFS = 8), and to terminally ill (CFS = 9). Older adults with CFS = 4–6 were classified to mild to moderate frailty within 72 h after admission. Mood state was evaluated by the Geriatric Depression Scale-5 (GDS-5), which is a five-item screening tool developed in 1999 (13). The GDS scores ranged from 0 to 5, with a sensitivity of 0.97 and a specificity of 0.85 using ≥2 as a cutoff point to define the presence of depressive symptoms (13). Cognition was measured by the Short Portable Mental Status Questionnaire (SPMSQ), which is 10-item screening tool developed in 1975 (14). Incorrect answers on more than two questions indicated impaired cognitive function. One more incorrect answer was allowed for the participants with a grade school education or lower, and one less incorrect answer was allowed for the participants with a high school education or higher (14). Malnutrition was assessed by the Malnutrition Universal Screening Tool (MUST) (15), which was developed by the British Association for Parenteral and Enteral Nutrition (BAPEN). It is a screening tool to categorize adults into low risk (MUST score = 0), medium risk (MUST score = 1), and high-risk categories for malnutrition (MUST score ≥ 2) (15). The participants with MUST scores ≥2 were defined as having malnutrition in the current study. Urine incontinence was defined if the individuals reported leakage of urine 6 days within the past year. A fall was indicated if fall episodes occurred ≥2 times in the past 6 months before admission. The number of drugs was reported by the individuals themselves.
2.3. Comorbidity
We reviewed the electronic medical records to collect data on comorbid conditions, and the Charlson Comorbidity Index (CCI) (16) was calculated. The CCI was developed in 1987, and it is often considered the gold-standard tool for assessing comorbidities in clinical research.
2.4. Demographic characteristics
The demographic characteristics included age, gender, marital status, education level, economic status, and institutionalization. Marital state was categorized into married or living with a partner and living without a partner (separated, divorced, widowed, or never married) (17). Educational level was categorized into illiteracy and literacy.
2.5. Prolonged length of stay
Prolonged length of stay is defined as a LOS greater than the 90th percentile of hospital days (18). In the current study, PLOS was defined as a LOS longer than 20 days. In contrast to PLOS, a LOS less than 20 days was defined as non-PLOS.
2.6. Statistical analysis
Descriptive statistics were used to summarize the baseline characteristics in the study cohort. The continuous variables were described by medians with 25 and 75th percentiles, and the categorical variables were described by numbers and proportions. The Wilcoxon rank sum test and Pearson’s chi-squared test were used to evaluate the differences in continuous and categorical variables, respectively, in individuals with PLOS and without PLOS.
The sample sizes of the PLOS group and non-PLOS group were imbalanced, which could influence the performance of a model with respect to accuracy and bias. To address imbalanced data, the synthetic minority oversampling technique (SMOTE) (19) was used to reduce the bias in this study. SMOTE is an oversampling technique that allows researchers to generate synthetic samples for minority patients (patients with PLOS). SMOTE is based on the k-nearest neighbor algorithm and works by selecting samples that are close in the feature space, thereby generating a regression line between the samples in feature space and synthesizing a new sample at a point along with the regression line. Table 1 shows the distribution of baseline characteristics with SMOTE, and they were highly similar to the distribution of characteristics without SMOTE (Supplementary Table S1). This result indicated that SMOTE would not distort the real distribution of baseline characteristics in the study cohort. The classification and regression tree (CART), a decision tree algorithm, was used to investigate the association between baseline characteristics (features) and PLOS in older adults. CART is a hierarchical structure consisting of branches and nodes. The leaf is the end of the branch, which indicates the probability of the outcome of interest in the final set of decision rules of the tree. The Gini index is a criterion for optimal splitting of nodes in developing a decision tree model. To avoid overfitting the data, we tuned the complexity parameter (CP) to make the relative error of the decision tree smaller. Supplementary Figure S1 shows the CP for the size of the decision tree and indicates that 10 nodes were optimal.
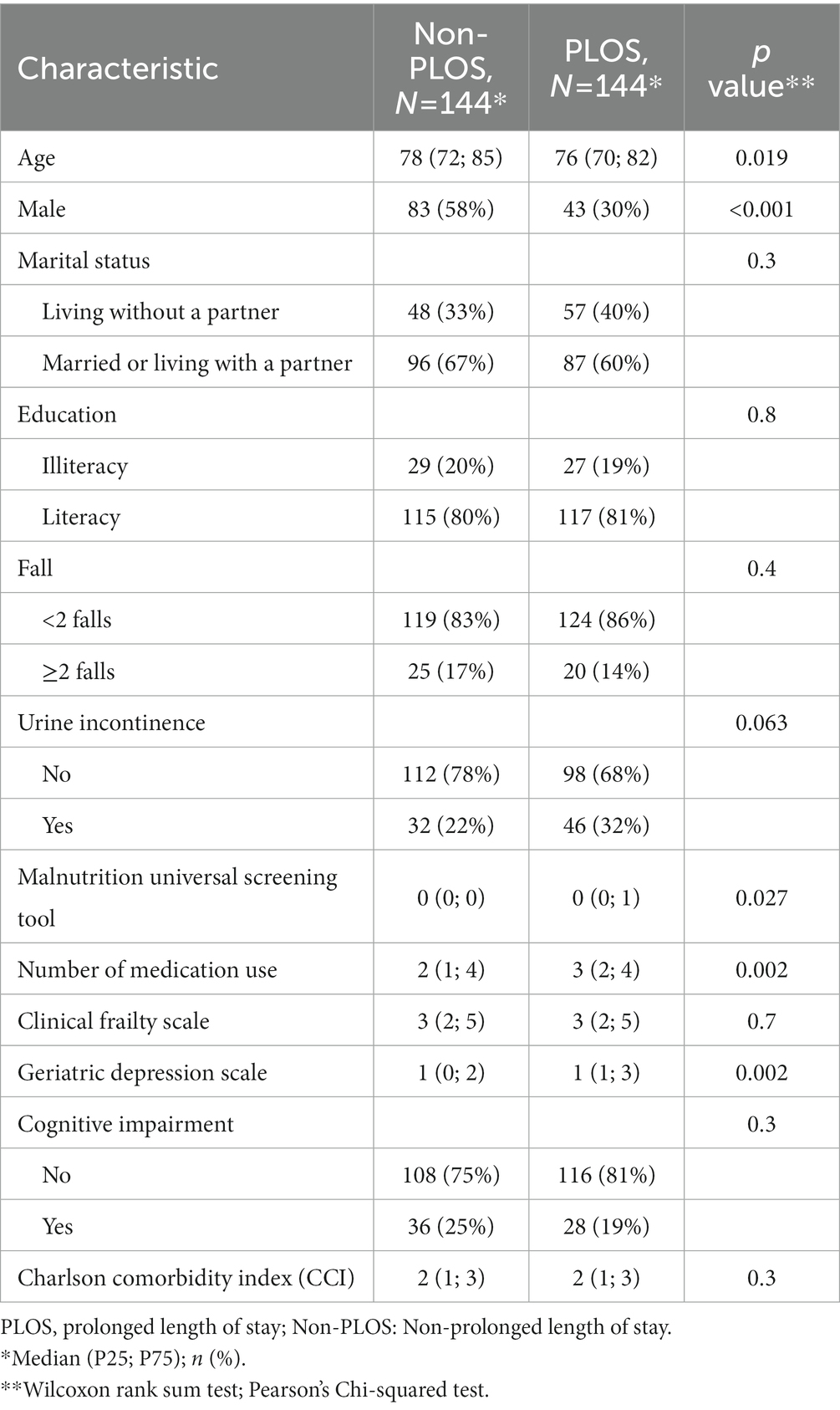
Table 1. The baseline characteristics of the study population using the synthetic minority oversampling technique (SMOTE).
The entire sample was randomly split into a 70% training set containing 202 patients and a 30% testing set containing 86 patients. The training set was used to construct models, and the testing set was used to evaluate the generalizability of models independently. The model performance was evaluated in terms of accuracy, receiver operating characteristic (ROC) curves, area under the curve (AUC), sensitivity, specificity, and predictive positive value (PPV). Statistical analyses were carried out using R (version 4.2.1) software. “rpart,” “rpart.plot,” and “pROC” were used in the current study.
3. Results
Two hundred eighty-eight older adults were recruited in the study cohort by using SMOTE. There were 144 individuals with PLOS and 144 individuals with non-PLOS. The mean age was 76 years old in individuals with PLOS and 78 years old in individuals with non-PLOS. The proportion of male individuals with PLOS (30%) was significantly lower than that of male individuals with non-PLOS (58%). The number of drugs reported among individuals with PLOS was higher than that in individuals without PLOS. Detailed baseline characteristics are presented in Table 1.
The performance in the training set was used to evaluate the internal prediction capacity for constructing the decision tree model. The accuracy was 0.81 (0.76, 0.87), the area under the receiver operating curve (AUROC) was 0.86 (0.81, 0.92), the sensitivity was 0.85 (0.79, 0.90), the specificity was 0.77 (0.72, 0.83), and the positive predictive value (PPV) was 0.80 (0.75, 0.86). The performance in the testing set was used to evaluate generalization capacity. The accuracy was 0.73 (0.64, 0.83), the AUROC was 0.78 (0.69, 0.87), the sensitivity was 0.82 (0.73, 0.91), the specificity was 0.66 (0.57, 0.75), and the PPV was 0.67 (0.57, 0.76). Overall, the performance in the testing set was lower than that in the training set (Table 2).
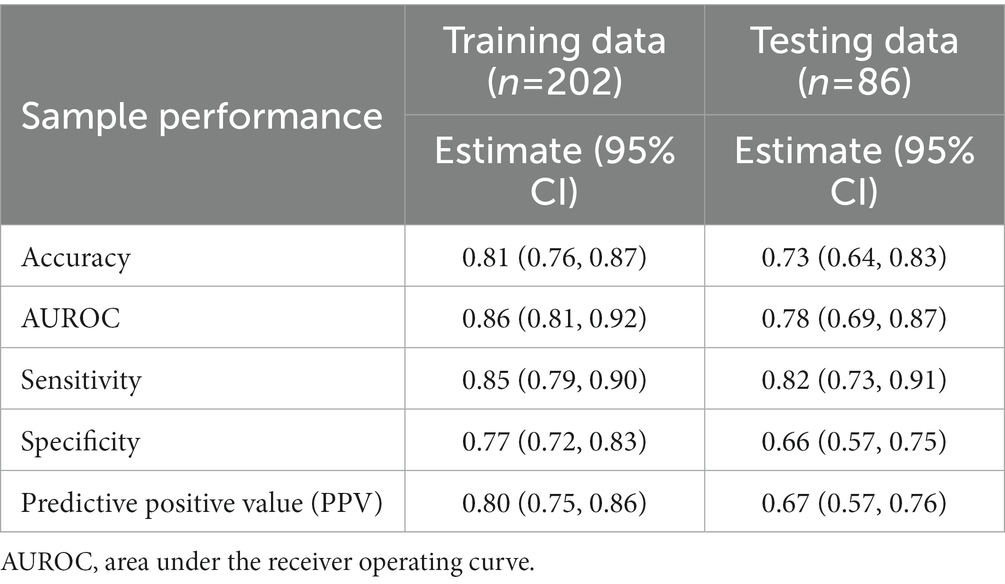
Table 2. The performance of classification and regression tree (CART) for classification of prolonged length of stay in training and testing datasets.
Figure 1 indicates that if an individual reported using two or more drugs, was female, did not have cognitive impairment and had GDS ≥ 1, he or she had a higher risk of PLOS (probability = 0.81), and these individuals accounted for 29% of the overall study population. Among the male individuals, those aged less than 87 years old and with cognitive impairment had a higher risk of PLOS (probability = 0.76). Although there were two situations [P(y = PLOS|drugs used ≧ 2, female, without cognitive impairment, GDS < 1, urine incontinence) = 1.0, and P(y = PLOS|drugs used ≧ 2, male, age < 87, without cognitive impairment, live alone) = 0.88] that were associated with a higher risk of PLOS, the sample sizes were small in the leaves (3 and 4%).
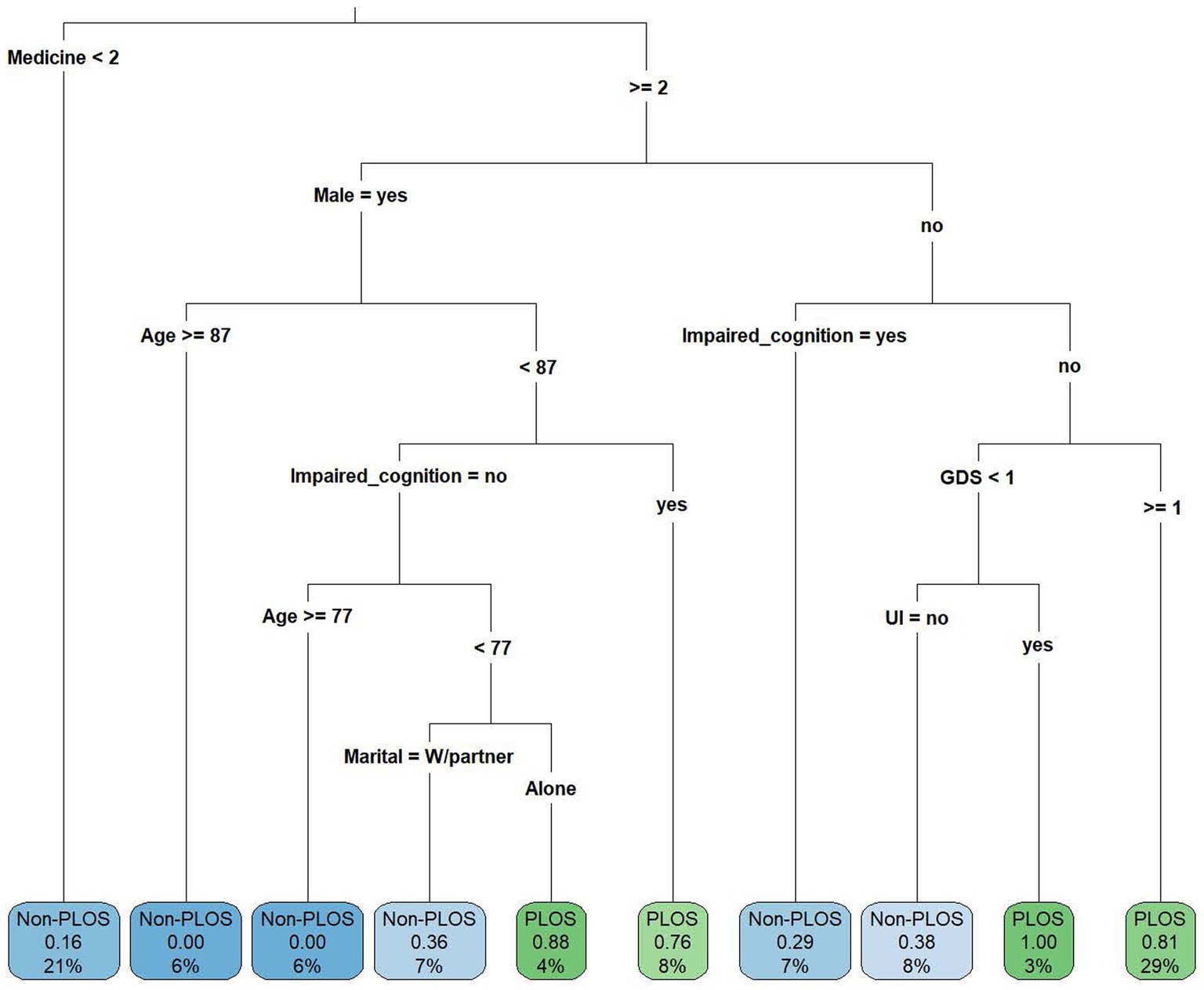
Figure 1. The classification and regression tree (CART) showing the decision criteria for predicting prolonged length of stay. GDS, geriatric depression scale; PLOS, prolonged length of stay; Non-PLOS, Non-prolonged length of stay; UI, urine incontinence; and W/partner, with partner.
4. Discussion
The current study demonstrated that two items in the mental domain and one item in the social domain of the CGA are associated with the PLOS in hospitalized older adults with mild to moderate frailty. If an individual reported using two or more drugs, was female, had no cognitive impairment and had GDS ≥ 1, she had a higher risk of PLOS (probability = 0.81), and these individuals accounted for 29% of the overall study population. Among male individuals aged less than 87 years old, those with cognitive impairment had a higher risk of PLOS (probability = 0.76), and among male individuals without cognitive impairment, living alone was associated with a higher risk of PLOS (probability = 0.88).
We showed that older adults with depressive symptoms were associated with a higher risk of PLOS, compatible with previous studies (20–23). GDS ≥ 2 as a cut-off point to define depressive symptoms was based on a sample from the general population (13). However, our CART results showed a different split node of GDS ≥ 1 based on female individuals using multiple medications. This split node implied that these individuals were sensitive to the risk of PLOS with mild depressive symptoms. In addition, a validation study in Taiwan (24) suggested that the cut-off point could be further lowered to 0/1 to increase the sensitivity to 100% at the screening stage.
Previous studies reported that older adults with depressive disorders had higher risks of PLOS, and those with depressive disorders showed 2-fold odds compared to those with non-depressive disorders (20–23). In addition, our results indicated that older females with depressive symptoms had higher risks of PLOS, consistent with a previous report showing that females were at risk for mental disorders compared to males (25). The sex differences in mental health could be explained by biological factors (e.g., gene and sex hormones), psychological factors (e.g., intrapersonal and interpersonal traits), and environmental factors (e.g., early life severe adversity) (26).
Depression is a common psychiatric disorder in older adults and is associated with higher complications and dependence (27). Depression in older adults is not normal aging (28), and late-life depression is characterized by atypical presentation of symptoms, including more somatic symptoms than mood symptoms, making it difficult to detect and treat (27). The association between depressed mood and PLOS in older adults could be explained by higher physical comorbidity (21), functional dependence (21), the direct impact of depressive symptoms on the immune system (29), the sympathetic nervous system (29), and the hypothalamic–pituitary–adrenal axis (29). It is also well-known that depressive individuals often do not follow medical advice for underlying medical conditions and have poorer treatment adherence (30). Older adults with depressed mood may have difficulties in effective communication with health professionals (20), and depressed mood may also affect one’s motivation toward recovery (31), both of which could result in a delay in diagnosis, treatment, and PLOS. Although it is unclear whether improving depression care will reduce LOS in acute hospitalized older adults, it could be investigated as a potential strategy to improve hospital outcomes (31).
We revealed that older adults with cognitive impairment were associated with a higher risk of PLOS, consistent with previous reports (32–36). Previous studies showed that older adults with cognitive impairment had higher risks of PLOS, and those with cognitive impairment showed a 0.8–15.3-day longer LOS than those without cognitive impairment (32–36). CART is a method that can be used for both classification and regression problems. It divides the data into subsets based on the values of the predictor variables and assigns an output value to each leaf node. In this study, we used CART to analyze the risk of PLOS in hospitalized individuals with different characteristics. We found that there were two different situations for the risk of PLOS between male and female individuals. In male individuals, cognitive impairment was a significant risk factor for PLOS, which could lead to a 0.76 probability of having PLOS, but in female individuals, cognitive impairment was not a significant risk factor for PLOS. This suggests that there are sex differences in the effect of cognitive impairment on LOS. Cognitive impairment remains a risk factor for PLOS, more so in male individuals than in female individuals.
Although we showed that older males with cognitive impairment had higher risks of PLOS, sex differences in dementia risk are unclear. One recent study suggested that females may have greater cognitive reserve but a faster decline in cognitive function than men, which could contribute to sex differences in late-life dementia (37). The difference might be due to socioeconomic, life stress or geographic factors, but further studies are needed in the future (37).
Cognitive impairment is not uncommon among older adults with acute hospitalization, but it is under recognized by health professionals with adverse outcomes (35), including disorientation (32), irritability (32), restlessness (32), falls (32, 36), decubitus ulcers (36), incontinence (32, 36), indwelling catheters (36), and medication error (36). Individuals with cognitive impairment are at a significantly greater risk of PLOS than those without cognitive impairment, possibly due to differences in effective care for individuals with cognitive impairment in hospitals and intrinsic mechanisms making these individuals at higher risk of deterioration (34). In addition, higher rates of potentially preventable events in individuals with cognitive impairment, including delirium, pneumonia, urinary tract infections, and decubitus ulcers, may also lead to PLOS (38). While individuals with dementia are mostly recognized and managed, those with cognitive impairment are mostly undetected even under routine screening (34). With comprehensive and enhanced recognition of cognitive impairment, interventions to improve care for these vulnerable groups of older adults would be possible (34).
We found that older adults living alone were associated with PLOS. One study identified caregiver stress and nursing home placement as potential modifiable risk factors for PLOS (39). Another study proposed that new formal social care requirements in survivors of acute illness and unmeasured variables of informal care requirements would be factors related to delayed discharge (40). Delays in the provision of social and therapy requirements (41) and awaiting a downstream bed (42) were also associated with PLOS in older adults. The most appropriate strategies to avoid PLOS included integrated systems between the hospitals and community care, interdisciplinary service provision, tailor-made services, and discharge planning initiated during hospitalization with regular follow-up after discharge (43).
We designed a CGA-based prospective cohort to evaluate the association between risk factors and LOS in hospitalized older adults with mild to moderate frailty. Since the approach for older adults is totally different from that for their younger counterparts, a CGA-based model, including physiological, psychological, social, and functional domain assessment and management, was used for a more thorough and detailed data collection process. We identified that the psychiatric components of geriatric syndromes, older adults with depressive symptoms, cognitive impairment, and living alone, are associated with PLOS in hospitalized older adults with mild to moderate frailty.
To our knowledge, two studies have used the CGA-based model to analyze the risk factors of PLOS in older adults. One study (5) showed that individuals at risk for functional decline, the number of comorbidities, reduced activities of daily living, cognition impairment, and signs of depression were important predictors of LOS. Another study (7) revealed that dependence in instrumental activities of daily living, malnutrition, and history of falls were associated with a longer LOS. Our results and two other studies underline the necessity of a CGA-based model for older adult care since the risk factors of PLOS belong to different domains. Although one recent systemic review and meta-analysis showed that the CGA had no significant effect on LOS, due to the presence of high heterogeneity and controversial results, the strength of evidence for the results was limited (44). Further studies are necessary to clarify the influence of the CGA-based model on LOS in hospitalized older adults with mild to moderate frailty.
Our study had several limitations. First, the number of drugs was self-reported or proxy-reported, and there could be record bias. Second, it was a single-center study with a small sample size, and the data should be interpreted with caution. Third, we only recruited older adults hospitalized with medical illness, and extrapolation of the results to other specialties should be very careful. Fourth, we used SMOTE to deal with imbalanced data, which might cause overfitting of modeling owing to synthesizing new samples with nearest neighbor selection. Thus, we tuned the complexity parameter in the decision model to reduce the bias of overfitting.
5. Conclusion
The mental and social domains in the CGA, including depressive symptoms, cognitive impairment, and living alone, were associated with PLOS in hospitalized older adults with mild to moderate frailty. Early detection and management of mood and cognition in older adults, together with comprehensive discharge planning and transition care, may be an important part of reducing LOS in acute hospitalized older adults with mild to moderate frailty.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by National Cheng Kung University Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
D-CY and C-CS were responsible for the study design, interpretation of data, and writing, reviewing, and editing the manuscript. Y-CY was responsible for the study design, collection of data, and writing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Funding for this study was provided by the National Cheng Kung University Hospital (Intramural grant: NCKUH-10709003). The sponsors had no involvements in the study results.
Acknowledgments
We would like to show appreciation to American Journal Experts for English editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1191940/full#supplementary-material
References
1. Rowe, JW, Fulmer, T, and Fried, L. Preparing for better health and health Care for an Aging Population. JAMA. (2016) 316:1643–4. doi: 10.1001/jama.2016.12335
2. Fried, LP, Tangen, CM, Walston, J, Newman, AB, Hirsch, C, Gottdiener, J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–57. doi: 10.1093/gerona/56.3.m146
3. Hoogendijk, EO, Afilalo, J, Ensrud, KE, Kowal, P, Onder, G, and Fried, LP. Frailty: implications for clinical practice and public health. Lancet. (2019) 394:1365–75. doi: 10.1016/s0140-6736(19)31786-6
4. Boucher, EL, Gan, JM, Rothwell, PM, Shepperd, S, and Pendlebury, ST. Prevalence and outcomes of frailty in unplanned hospital admissions: a systematic review and meta-analysis of hospital-wide and general (internal) medicine cohorts. EClinicalMedicine. (2023) 59:101947. doi: 10.1016/j.eclinm.2023.101947
5. Scharf, AC, Gronewold, J, Dahlmann, C, Schlitzer, J, Kribben, A, Gerken, G, et al. Clinical and functional patient characteristics predict medical needs in older patients at risk of functional decline. BMC Geriatr. (2020) 20:75. doi: 10.1186/s12877-020-1443-1
6. Bo, M, Fonte, G, Pivaro, F, Bonetto, M, Comi, C, Giorgis, V, et al. Prevalence of and factors associated with prolonged length of stay in older hospitalized medical patients. Geriatr Gerontol Int. (2016) 16:314–21. doi: 10.1111/ggi.12471
7. Avelino-Silva, TJ, Farfel, JM, Curiati, JA, Amaral, JR, Campora, F, and Jacob-Filho, W. Comprehensive geriatric assessment predicts mortality and adverse outcomes in hospitalized older adults. BMC Geriatr. (2014) 14:129. doi: 10.1186/1471-2318-14-129
8. Romero-Ortuno, R, Forsyth, DR, Wilson, KJ, Cameron, E, Wallis, S, Biram, R, et al. The Association of Geriatric Syndromes with hospital outcomes. J Hosp Med. (2017) 12:83–9. doi: 10.12788/jhm.2685
9. Lee, H, Lee, E, and Jang, IY. Frailty and comprehensive geriatric assessment. J Korean Med Sci. (2020) 35:e16. doi: 10.3346/jkms.2020.35.e16
10. Matthews, DA . Dr. Marjory Warren and the origin of British geriatrics. J Am Geriatr Soc. (1984) 32:253–8. doi: 10.1111/j.1532-5415.1984.tb02017.x
11. Ellis, G, Gardner, M, Tsiachristas, A, Langhorne, P, Burke, O, Harwood, RH, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. (2017) 2017:CD006211. doi: 10.1002/14651858.CD006211.pub3
12. Rockwood, K, and Theou, O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J. (2020) 23:254–9. doi: 10.5770/cgj.23.463
13. Hoyl, MT, Alessi, CA, Harker, JO, Josephson, KR, Pietruszka, FM, Koelfgen, M, et al. Development and testing of a five-item version of the geriatric depression scale. J Am Geriatr Soc. (1999) 47:873–8. doi: 10.1111/j.1532-5415.1999.tb03848.x
14. Pfeiffer, E . A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. (1975) 23:433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x
15. Stratton, RJ, Hackston, A, Longmore, D, Dixon, R, Price, S, Stroud, M, et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the 'malnutrition universal screening tool' ('MUST') for adults. Br J Nutr. (2004) 92:799–808. doi: 10.1079/bjn20041258
16. Charlson, ME, Pompei, P, Ales, KL, and MacKenzie, CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
17. Hsu, HC . Trajectories and covariates of life satisfaction among older adults in Taiwan. Arch Gerontol Geriatr. (2012) 55:210–6. doi: 10.1016/j.archger.2011.08.011
18. Yeh, ST, and Wu, SC. Implication of different definitions of overstay for acute inpatients. Taiwan J Public Health. (2008) 27:301–8. doi: 10.6288/TJPH2008-27-04-04
19. Chawla, NV, Bowyer, KW, Hall, LO, and Kegelmeyer, WP. SMOTE: synthetic minority over-sampling technique. J Artif Intell Res. (2002) 16:321–57. doi: 10.1613/jair.953
20. Prina, AM, Huisman, M, Yeap, BB, Hankey, GJ, Flicker, L, Brayne, C, et al. Association between depression and hospital outcomes among older men. CMAJ. (2013) 185:117–23. doi: 10.1503/cmaj.121171
21. Prina, AM, Deeg, D, Brayne, C, Beekman, A, and Huisman, M. The association between depressive symptoms and non-psychiatric hospitalisation in older adults. PLoS One. (2012) 7:e34821. doi: 10.1371/journal.pone.0034821
22. Wong, SY, Mercer, SM, Leung, J, and Woo, J. The relationship between clinically relevant depressive symptoms and episodes and duration of all cause hospitalization in southern Chinese elderly. J Affect Disord. (2009) 113:272–8. doi: 10.1016/j.jad.2008.06.008
23. Rowan, PJ, Davidson, K, Campbell, JA, Dobrez, DG, and MacLean, DR. Depressive symptoms predict medical care utilization in a population-based sample. Psychol Med. (2002) 32:903–8. doi: 10.1017/s0033291702005767
24. Chin, W-C, Liu, C-Y, Lee, C-P, and Chu, C-L. Validation of five short versions of the geriatric depression scale in the elder population in Taiwan. Taiwan J Psychiatry. (2014) 28:156–63.
25. Sialino, LD, van Oostrom, SH, Wijnhoven, HAH, Picavet, S, Verschuren, WMM, Visser, M, et al. Sex differences in mental health among older adults: investigating time trends and possible risk groups with regard to age, educational level and ethnicity. Aging Ment Health. (2021) 25:2355–64. doi: 10.1080/13607863.2020.1847248
26. Kuehner, C . Why is depression more common among women than among men? Lancet Psychiatry. (2017) 4:146–58. doi: 10.1016/s2215-0366(16)30263-2
27. Devita, M, de Salvo, R, Ravelli, A, de Rui, M, Coin, A, Sergi, G Snr, et al. Recognizing depression in the elderly: practical guidance and challenges for clinical management. Neuropsychiatr Dis Treat. (2022) 18:2867–80. doi: 10.2147/ndt.S347356
28. Casey, DA . Depression in older adults: a treatable medical condition. Prim Care. (2017) 44:499–510. doi: 10.1016/j.pop.2017.04.007
29. Kiecolt-Glaser, JK, and Glaser, R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. (2002) 53:873–6. doi: 10.1016/s0022-3999(02)00309-4
30. Katon, WJ . Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. (2011) 13:7–23. doi: 10.31887/DCNS.2011.13.1/wkaton
31. Prina, AM, Cosco, TD, Dening, T, Beekman, A, Brayne, C, and Huisman, M. The association between depressive symptoms in the community, non-psychiatric hospital admission and hospital outcomes: a systematic review. J Psychosom Res. (2015) 78:25–33. doi: 10.1016/j.jpsychores.2014.11.002
32. Wolf, D, Rhein, C, Geschke, K, and Fellgiebel, A. Preventable hospitalizations among older patients with cognitive impairments and dementia. Int Psychogeriatr. (2019) 31:383–91. doi: 10.1017/s1041610218000960
33. Möllers, T, Perna, L, Ihle, P, Schubert, I, Bauer, J, and Brenner, H. Factors associated with length of stay in hospital patients with and without dementia. J Alzheimers Dis. (2019) 67:1055–65. doi: 10.3233/jad-180593
34. Fogg, C, Meredith, P, Culliford, D, Bridges, J, Spice, C, and Griffiths, P. Cognitive impairment is independently associated with mortality, extended hospital stays and early readmission of older people with emergency hospital admissions: a retrospective cohort study. Int J Nurs Stud. (2019) 96:1–8. doi: 10.1016/j.ijnurstu.2019.02.005
35. Power, C, Duffy, R, Bates, H, Healy, M, Gleeson, P, Lawlor, BA, et al. The detection, diagnosis, and impact of cognitive impairment among inpatients aged 65 years and over in an Irish general hospital—a prospective observational study. Int Psychogeriatr. (2017) 29:1879–88. doi: 10.1017/s1041610217001326
36. Tropea, J, LoGiudice, D, Liew, D, Gorelik, A, and Brand, C. Poorer outcomes and greater healthcare costs for hospitalised older people with dementia and delirium: a retrospective cohort study. Int J Geriatr Psychiatry. (2017) 32:539–47. doi: 10.1002/gps.4491
37. Levine, DA, Gross, AL, Briceño, EM, Tilton, N, Giordani, BJ, Sussman, JB, et al. Sex differences in cognitive decline among US adults. JAMA Netw Open. (2021) 4:e210169. doi: 10.1001/jamanetworkopen.2021.0169
38. Bail, K, Berry, H, Grealish, L, Draper, B, Karmel, R, Gibson, D, et al. Potentially preventable complications of urinary tract infections, pressure areas, pneumonia, and delirium in hospitalised dementia patients: retrospective cohort study. BMJ Open. (2013) 3:e002770. doi: 10.1136/bmjopen-2013-002770
39. Toh, HJ, Lim, ZY, Yap, P, and Tang, T. Factors associated with prolonged length of stay in older patients. Singap Med J. (2017) 58:134–8. doi: 10.11622/smedj.2016158
40. Moore, G, Hartley, P, and Romero-Ortuno, R. Health and social factors associated with a delayed discharge amongst inpatients in acute geriatric wards: a retrospective observational study. Geriatr Gerontol Int. (2018) 18:530–7. doi: 10.1111/ggi.13212
41. Jasinarachchi, KH, Ibrahim, IR, Keegan, BC, Mathialagan, R, McGourty, JC, Phillips, JRN, et al. Delayed transfer of care from NHS secondary care to primary care in England: its determinants, effect on hospital bed days, prevalence of acute medical conditions and deaths during delay, in older adults aged 65 years and over. BMC Geriatr. (2009) 9:4. doi: 10.1186/1471-2318-9-4
42. Hendy, P, Patel, JH, Kordbacheh, T, Laskar, N, and Harbord, M. In-depth analysis of delays to patient discharge: a metropolitan teaching hospital experience. Clin Med. (2012) 12:320–3. doi: 10.7861/clinmedicine.12-4-320
43. Coffey, A, Leahy-Warren, P, Savage, E, Hegarty, J, Cornally, N, Day, MR, et al. Interventions to promote early discharge and avoid inappropriate hospital (re)admission: a systematic review. Int J Environ Res Public Health. (2019) 16:2457. doi: 10.3390/ijerph16142457
44. Chen, Z, Ding, Z, Chen, C, Sun, Y, Jiang, Y, Liu, F, et al. Effectiveness of comprehensive geriatric assessment intervention on quality of life, caregiver burden and length of hospital stay: a systematic review and meta-analysis of randomised controlled trials. BMC Geriatr. (2021) 21:377. doi: 10.1186/s12877-021-02319-2
Keywords: older adults, frailty, mental domain, comprehensive geriatric assessment, prolonged length of stay
Citation: Yu Y-C, Su C-C and Yang D-C (2023) Association between the mental domain of the comprehensive geriatric assessment and prolonged length of stay in hospitalized older adults with mild to moderate frailty. Front. Med. 10:1191940. doi: 10.3389/fmed.2023.1191940
Edited by:
Robbert Gobbens, Inholland University of Applied Sciences, NetherlandsReviewed by:
Sumru Savas, Ege University, TürkiyeSónia Martins, Instituto Superior de Serviço Social do Porto, Portugal
Copyright © 2023 Yu, Su and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deng-Chi Yang, stethoscope@hotmail.com
†These authors have contributed equally to this work
 Yung-Chen Yu
Yung-Chen Yu Chien-Chou Su
Chien-Chou Su Deng-Chi Yang
Deng-Chi Yang