Upper airway disease diagnosis as a predictive biomarker of therapeutic response to biologics in severe asthma
- 1Department of Pulmonary Medicine, Cliniques Universitaires Saint-Luc, Brussels, Belgium
- 2Department of Pulmonary Medicine, Centre Hospitalier Universitaire UCL Namur, Université catholique de Louvain, Yvoir, Belgium
- 3Pole of Pulmonology, ENT and Dermatology, Institute of Experimental and Cliniqal Research, Université catholique de Louvain, Brussels, Belgium
Asthma is a heterogeneous disease sharing airway instability but with different biology, risk factors, and response-to-therapy patterns. Biologics have revolutionized the one-size-fits-to-all approach to personalized medicine in severe asthma (SA), which relies on the identification of biomarkers that define distinct endotypes. Thus, blood eosinophils and, to some extent, exhaled nitric oxide (FeNO) can predict the response to approved anti-type 2 (T2) biologics (anti-IgE, anti–IL-5, and anti–IL-4R alpha), whereas age at onset and comorbidities such as anxiety/depression, obesity, reflux, and upper airway disease (UAD) also influence therapeutic responses in SA. In this article, focusing on the predictive value of biomarkers for the therapeutic response to biologics in SA, we first summarize the level of prediction achieved by T2 biomarkers (blood eosinophils, FeNO) and then review whether data support the predictive value of upper airway diagnosis on such outcomes. Post hoc analysis of most studies with T2 biologics suggests that chronic rhinosinusitis with nasal polyps (CRSwNP) and, to a lower extent, allergic rhinitis may help in predicting clinical response. Considering that T2 biologics are now also approved for the treatment of severe CRSwNP, diagnosis of upper airway disease is a key step in determining eligibility for such therapy.
1. Introduction: Theragnostic biomarkers in severe asthma
Asthma is a heterogeneous disease sharing common features (e.g., airway hyperresponsiveness) but with different underlying biological (hence referred to as endotypes), risk factors, and response to treatment patterns. Most patients with asthma may be well controlled by the ICS-LABA therapy, but 5–10% of patients have a more difficult, sometimes severe disease (severe asthma, SA). The therapeutic paradigm has recently (beginning of the 21st century) evolved from a one-size-fits-all approach to a phenotype-based approach that relies on the expression of specific biomarkers that are now targetable by biological therapies (1–5).
The National Institutes of Health (NIH) Biomarkers Definitions Working Group defines a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (6). The WHO suggested a broader definition as “almost any measurement reflecting an interaction between a biological system and a potential hazard, which may be chemical, physical, or biological. The measured response may be functional and physiological, biochemical at the cellular level, or a molecular interaction”.1 There are different types of biomarkers based on their main clinical application, namely diagnosis, monitoring, pharmacodynamic/response, prediction and prognostic, safety, and susceptibility/risk assessment.2
The biomarkers that are currently validated in asthma relate to type 2 (T2) immunity, namely blood (or sputum) eosinophils and exhaled nitric oxide (FeNO), which are elevated in more than half of patients with severe asthma (7–9). In addition, those T2 biomarkers may overlap with allergy/atopic sensitization, which is also present in approximately half of the patients with SA, resulting in a large overlap between allergic and non-allergic patients with T2/eosinophilic asthma. Global Initiative for Asthma (GINA) guidelines recommend biologics as an add-on therapy for patients with severe T2 asthma who remain uncontrolled (including with severe exacerbations) despite step 4 therapy. Following the large overlap between T2 asthma subsets and the absence of head-to-head trials currently published with biologics that could demonstrate better efficacy in certain groups of patients (10), biomarkers that could help discriminate patients with T2 severe asthma who should be preferentially treated by an anti-T2 biologic rather than another is valuable and could partially substitute for the current practice, which is primarily based on a try-and-error approach (11–14).
Theragnostic is an emerging field of medicine that combines therapeutic and diagnostic purposes with the intention to simultaneously or sequentially diagnose and treat medical conditions. In SA, blood eosinophils and exhaled nitric oxide (FeNO) can predict the response to approved anti-T2 biologics (anti-IgE, anti–IL-5, and anti–IL-4Rα). In addition to biology, age at the onset and comorbidities, such as anxiety/depression, obesity, reflux, and upper airway disease (UAD), also influence therapeutic responses to this disease.
In this article, after summarizing the predictive value of validated T2 biomarkers, we review clinical data on the potential predictive value of upper airway disease (UAD) diagnosis on the response to biologics in SA, as well as discuss its potential positioning in the current landscape of asthma-related biomarkers.
2. Biomarkers predicting the response to anti-T2 therapies
Several biomarkers are available to phenotype asthma, some of which may predict clinical response to corticosteroids and T2 biologics; these include blood (or sputum) eosinophils, FeNO, serum total IgE (tIgE) levels, and periostin (15, 16).
2.1. Eosinophils
The prevalence of increased eosinophils is observed in ~ 50–60% of patients with mild-to-moderate asthma, and probably a larger proportion in SA (17, 18), as well as up to 80% in patients with corticosteroid-naive asthma (19).
First, airway eosinophilia is a prognostic biomarker as it correlates with the degree of airway hyperreactivity, exacerbation rate, poor symptom control, as well as small airway dysfunction (20–24). In addition, sputum eosinophilia predicts corticosteroid response in asthma, as first described by Brown in 1958 (25). This finding was confirmed 40 years later by Pavord et al. (26) and others since then (27), also showing that a treatment strategy guided by the sputum eosinophil count did reduce SA exacerbations compared to standard management (28, 29). Sputum eosinophils were considered to be more stable than blood eosinophils (30) and provided the best ROC curve among T2 biomarkers for predicting the response to a short steroid burst (31), which was confirmed by others (32). However, in the “Dose Ranging Efficacy And safety with Mepolizumab” (DREAM) study, the response to mepolizumab was poorly predicted by sputum eosinophilia ≥3% (33), in contrast to blood eosinophils, where patients with eosinophils >300/ul responded better than those with <300/ul (34, 35). It should also be noted that in the Phase I study with benralizumab, an anti–IL-5 receptor mAb, the effect of the therapy on sputum eosinophils was more variable than on blood eosinophils (36). Thus, in patients with naïve asthma, blood eosinophils correlate with sputum eosinophils with a precision ranging from 59 to 92% sensitivity and 65 to 91% specificity (37). The use of induced sputum has some limitations. First, not all patients can produce good quality sputum, and it is usually accepted that in trained teams, the success rate of the procedure (combining a successful induction and quality criteria) is approximately 70–80%. Second, sputum induction may induce bronchoconstriction, and it is recommended to administer per or pre-procedure short-acting bronchodilators as well as in case of high-risk patients (i.e., subjects with FEV1/forced vital capacity <0.7 post-salbutamol, unstable asthmatic patients or for a patient with post-bronchodilator FEV1 ≤65% predicted) to use isotonic solution instead of hypertonic saline. However, when performed according to recommendations, induced sputum is safe in subjects with moderate-to-severe asthma (32, 38, 39). Third, the procedure of sputum induction itself may influence the composition of airway inflammatory mediators for a few hours; thus, for this reason, it is advised to leave 24 h between sputum inductions to obtain reproducible results (40). Fourth, sputum eosinophils may vary over time (36, 41), and with the disease control (42), but acceptable reproducibility (Ri values > 0.8) has been reported in patients with eosinophilic inflammation (43). The predictive value of blood eosinophils was confirmed in clinical trials with reslizumab, another anti–IL-5 monoclonal antibody, with significant clinical effects observed in patients with blood eosinophils higher than 200/μl at baseline (44, 45). Accordingly, patients treated with benralizumab, an anti–IL-5 receptor mAb, and with baseline blood eosinophils ≥300/μl had significantly lower exacerbation rates (46) and a cutoff of ≥300 eosinophils/μl was used in subsequent phase III studies (47, 48). The greatest clinical benefit was observed in patients with blood eosinophil ≥150/μL and baseline FeNO of ≥25 ppb in trials with dupilumab, a mAb directed against the alpha subunit of the interleukin (IL)-4 receptor, thereby blocking both IL-4 and IL-13 (14, 49). In contrast, blood eosinophils were less predictive of clinical response to tezepelumab, an anti-epithelial TSLP antibody (50).
One important issue in clinical practice relates to the variability of blood eosinophils. Blood eosinophils may also vary due to disease activity as well as intrinsic day-to-day (and even within-day) changes. In contrast to oral steroids, which influence blood eosinophil counts, inhaled corticosteroids (ICS) impact airway eosinophils but only slightly influence blood eosinophils (51, 52). It renders mandatory repeated measurements before “labeling” a patient as eosinophilic or not (46, 53).
2.2. Exhaled nitric oxide (FeNO)
Nitric oxide (NO) is a gas that can be measured in exhaled breath and is increased upon the activation of the airway epithelium by IL-4/IL-13, which upregulates iNOS expression, whereas eosinophilia is primarily increased following IL-5 upregulation (54, 55). Recommendation on the exhaled biomarkers has been published by Horvath et al. (56). According to GINA and recent ERS guidelines, FeNO > 50 ppb (adults) and >35 ppb (children) are indicative of eosinophilic inflammation and the diagnosis of asthma (57). FeNO (≤20 or ≥50 ppb) may also discriminate the inflammatory phenotype (T2-low or T2-high, respectively) during asthma exacerbation (58). Similar to eosinophils, it is also a validated biomarker that predicts the response to inhaled corticosteroids (ICS) (57, 59). In addition, as iNOS is suppressed by ICS irrespectively of their effect on airway inflammation, it may help to identify non-adherence to maintenance therapy (60), as well as a persistent T2 phenotype in SA (61), and to guide the use of biological therapies (62).
Accordingly, with its regulation by IL-4/IL-13, anti–IL-5(R) biologics (mepolizumab, benralizumab) do not alter significantly FeNO. High FeNO may, however, predict a better response to mepolizumab, as “super-responders” (defined by upper 25% of ACQ-5 improvement, corresponding to 24% of patients) have a higher FeNO (41 vs. 23 ppb) at baseline (63). A stronger predictive effect was consistently observed with dupilumab, which achieved a greater benefit in patients with FeNO ≥25 ppb. In patients with a FeNO ≥50 ppb, the improvement in FEV1 reached 390 ml in the dupilumab-treated group compared to placebo (14, 64), whereas FeNO ≥25 ppb was also associated with a higher reduction of exacerbations and maintenance oral corticosteroids (OCSs) dose (64–66), as confirmed in real-life studies (67). A post hoc analysis found the greatest treatment response to dupilumab in patients with FeNO ≥25 ppb and blood eosinophils ≥150/μl (64). For omalizumab, the ATS/ERS guidelines recommend (conditionally and with a low quality of evidence) using a FeNO cutoff of ≥19.5 ppb to identify adolescents and adults with allergic SA who could be more likely to benefit from anti-IgE treatment (68), based on a subgroup analysis that showed a reduction of the exacerbation rate, a longer time to first asthma exacerbation and a larger improvement of mean QLQ in the ≥19.5 ppb subgroup (69). In contrast, the benefit of tezepelumab on exacerbations was observed irrespectively of baseline FeNO (50).
2.3. Total serum IgE
The first biologic that was developed and demonstrated efficacy in severe asthma is omalizumab (1–4), which is a humanized anti-IgE mAb that binds the C3 region of the IgE-Fc fragment and captures circulating IgE, preventing interaction with the FcεRI and thus interrupting the allergic cascade (70). Omalizumab reduced asthma exacerbations, inhaled corticosteroid dose, rescued medication use, as well as the rate of serious asthma exacerbations and the need for unscheduled outpatient visits, emergency room treatment, and hospitalization in patients with moderate-to-severe allergic asthma (71). Baseline tIgE is a poor predictor of anti-IgE efficacy, the only impact being observed in the pivotal INNOVATE study (72) is that patients in the lowest quartile (i.e., <76 kU/L) had a significantly lower benefit. Pooled analysis showed that the benefits were globally irrespective of total IgE levels and that pre-treatment baseline characteristics cannot reliably predict which patients will benefit the most from omalizumab (11, 73). However, a more recent study showed that T2 biomarkers (FeNO, eosinophils, and periostin) could slightly but significantly predict a better response to omalizumab (69).
2.4. Periostin and other biomarkers
Periostin is an extracellular matrix protein produced by airway epithelial cells and fibroblasts upon activation by IL-4 or IL-13, which is implicated in tissue remodeling (74) and correlated with lung function decline (75) as well as asthma exacerbations (despite high-dose ICS therapy). Patients with high serum levels of periostin had a greater improvement in lung function and reduced asthma exacerbations following treatment with the anti–IL-13 lebrikizumab (76–78). Other biomarkers, such as eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), galectin-10, or bromotyrosine (BrTyr), have also been studied, as well as volatile organic compounds (VOCs) but are not yet validated and/or implemented for use in clinical practice (79–91).
In addition to markers related to airway immunobiology, late (adult) vs. early (childhood) onset of the disease is associated with non-allergic, eosinophilic vs. allergic asthma, respectively (5). Subsequently, adult disease onset in eosinophilic SA may be considered a clinical biomarker that could predict an enhanced role of eosinophilic, rather than allergic, inflammation.
3. Upper airway disease as a theragnostic biomarker in severe asthma
Upper airway diseases (UADs) are mainly represented by allergic rhinitis (and rhinoconjunctivitis), non-allergic rhinitis, and chronic rhinosinusitis with (CRSwNP) or without nasal polyps (CRSsNP), all of which are highly prevalent comorbidities that impact disease control in asthma. Thus, it is estimated that 80% of patients with asthma have allergic rhinitis, while 22–42% have CRS (92). Similarly to asthma, different phenotypes of CRSwNP have been described. Most patients with CRSwNP show a T2 inflammation, as shown by a recent study in which 87% of patients with CRSwNP had T2 inflammation, while only a few are characterized by T1 or T3 inflammation or a mixed phenotype (17, 18, and 26%, respectively) (93). The currently approved biologics for asthma target the T2 immune pathway. Pathogens and environmental factors may induce, following interactions with the airway epithelium and antigen-presenting cells, the differentiation of naïve CD4+ T cells into Th2 cells that release IL-4 and IL-13 interacting with B cells to produce IgE that may activate mast cells. IL-5 is also released by Th2 and innate lymphoid cell (ILC)-type 2, acting as a pivotal factor in the differentiation, survival, and activation of eosinophils, basophils, and mast cells (94).
Data on the potential predictive value of upper airway disease (UAD) diagnosis on the biologic response in severe asthma emerged from post hoc analyses of randomized, placebo-controlled trials (RCTs) by stratifying a posteriori enrolled patients according to the presence (vs. absence) of UAD, specifically allergic rhinitis or CRSwNP. This will be discussed separately for each biologic that is currently approved for the treatment of SA. Interestingly, the same biologics—namely anti-IgE, anti–IL-5, and anti–IL-4Rα–have been recently approved for the treatment of severe CRSwNP (while dupilumab was approved first in severe CRSwNP, before asthma), further increasing the relevance of integrating UAD in the management of (severe) asthma.
3.1. Omalizumab
Omalizumab is the first monoclonal antibody registered (in 2003) for asthma treatment, before other indications such as chronic rhinosinusitis with nasal polyposis (CRSwNS) and chronic urticaria. Few studies evaluated the impact of allergic rhinitis as a biomarker of response to omalizumab. One post hoc analysis of a phase 3 RCT conducted in Chinese patients with moderate-to-severe persistent allergic asthma showed that asthma symptoms (ACQ score) and asthma-related quality of life (QLQ score) were significantly improved after 24 weeks of treatment with omalizumab compared to placebo in patients with perennial allergic rhinitis, in contrast with those without rhinitis. Unfortunately, the level of blood eosinophils according to the presence (or not) of rhinitis was not reported; the mean level is 296/μl in the total cohort (95) (Table 1). In another retrospective study of the reversal of airway obstruction (defined by FEV1 normalization, vs. persistent airflow limitation, PAL) upon omalizumab in severe allergic asthma (up to 4 years of treatment), patients with FEV1 normalization had a significantly higher proportion of rhinitis than patients with PAL (83 vs. 43%; p = 0.027). The same finding was seen when considering CRSwNP, 72% of patients with FEV1 normalization had CRSwNP compared to 29% in the PAL group (p = 0.031). It is important to notice that the mean levels of blood eosinophils and FeNO were higher in the group of patients with reversal of airway obstruction than in those with persistent obstruction (754/μL and 66.8 ppb vs. 351/μL and 23 ppb, respectively) (97).
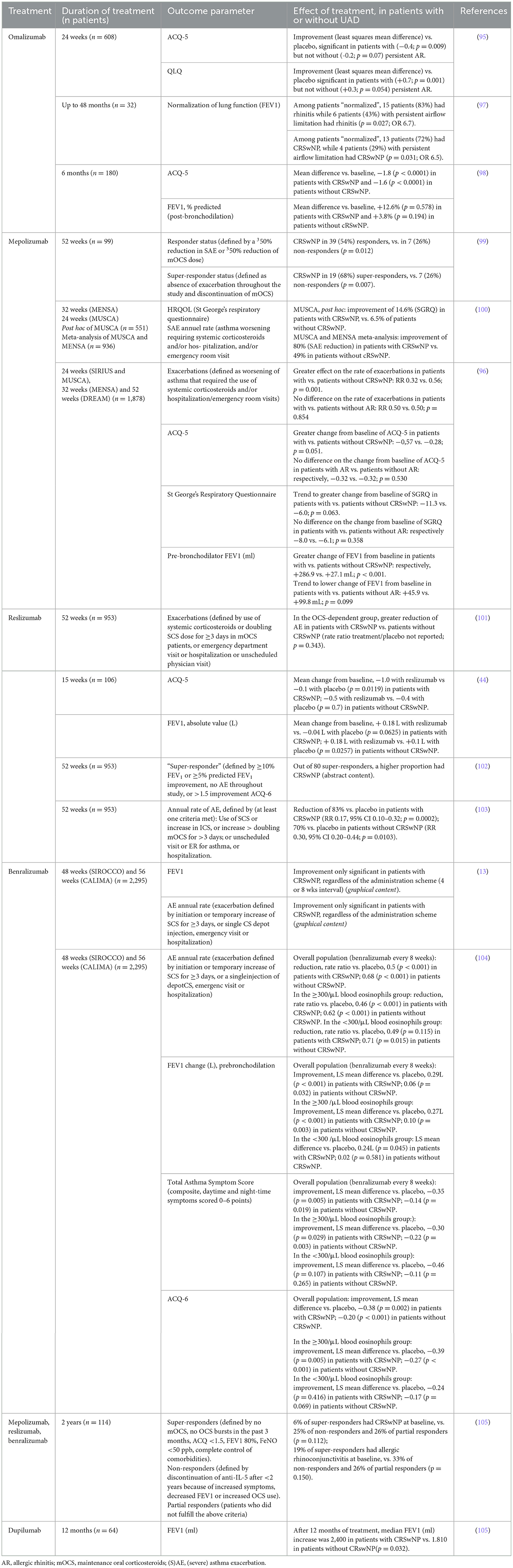
Table 1. Predictive value of UAD diagnosis on asthma-related outcomes in post hoc analysis of randomized controlled trials [except ref. (88–90, 96)] with biologics.
In an Australian prospective observational study of patients with allergic SA, 15% had CRSwNP, and the benefit of omalizumab on asthma symptoms (ACQ-5 score) and lung function (FEV1% predicted) after 6 months of treatment was similar whether or not patients had NP (ACQ-5 mean difference −1.6 without vs. −1.8 with CRSwNP; FEV1 change +3.8% without vs. +12.6% with CRSwNP) (98) (Table 1).
3.2. Mepolizumab
Mepolizumab was the first registered (in 2004) anti–IL-5 biologic for the treatment of severe eosinophilic asthma (SEA), before validation in other conditions including CRSwNP, hypereosinophilic syndrome (HES), and eosinophilic granulomatosis with polyangiitis (EGPA). A few studies showed that CRSwNP may predict the response to mepolizumab in SA. A first retrospective single-center study of patients who received mepolizumab for SEA (>300/μL in the preceding 12 months) and classified as responders [defined by a ≥50% reduction in exacerbations, or ≥50% reduction of the prednisolone dose if requiring maintenance oral corticosteroids (mOCS)], super-responders (absence of exacerbation for the 52 weeks of follow-up and discontinuation of mOCS), or non-responders showed that CRSwNP at baseline was associated with the responder and super-responder status. Indeed, 54.2% of responders and 67.9% of super-responders had CRSwNP compared to 25.9% of non-responders (p = 0.012 and p = 0.007, respectively) (99) (Table 1).
In a post hoc analysis of the MUSCA trial, mepolizumab had a greater benefit on health-related quality of life-related to lower airway symptoms in patients with SEA and CRSwNP compared to patients without NP, whereas, in a meta-analysis of MUSCA and MENSA trials, the benefit of mepolizumab on the annual exacerbation rate was also greater in patients with CRSwNP compared to patients without CRSwNP (80% reduction in patients with, vs. 49% in patients without CRSwNP). In this last meta-analysis, patients with CRSwNP had a higher mean blood eosinophil count than those without CRSwNP (440/μl vs. 209/μl) (100). A post hoc meta-analysis of the DREAM, MENSA, SIRIUS, and MUSCA studies also reports a superior effect of mepolizumab in SEA patients with UAD. Indeed, the presence of nasal polyps (compared to the absence of CRSwNP) was associated with a significantly higher benefit in terms of exacerbations (RR 0.32 vs. 0.56; p = 0,001) and change in FEV1 from baseline (+286.9 ml vs. + 27.1; p = 0,001). A trend favoring mepolizumab (vs. placebo) in patients with CRSwNP was shown when evaluating effects on ACQ-5 and St George's Respiratory Questionnaire. The presence of allergic rhinitis was also evaluated as a predictor of response but showed no beneficial effect in terms of exacerbations, FEV1, ACQ-5, or on the St George's Respiratory Questionnaire (Table 1). Mean levels of blood eosinophils across the four studies were from 250 to 340/μl (96).
3.3. Reslizumab
Reslizumab, a humanized IgG4 monoclonal antibody that targets IL-5, was the second anti–IL-5 biologic registered for SEA (in 2017). A post hoc analysis of two duplicate RCTs in which patients with eosinophilic (≥400 cells/μL) asthma and who remained inadequately controlled on at least medium-dose ICS were randomized to placebo or reslizumab (3 mg/kg every 4 weeks) for 52 weeks and categorized by use of maintenance OCS. As a secondary outcome, the post hoc analysis assessed biomarkers that predicted response in terms of exacerbations (defined by the use of systemic corticosteroids in steroid-naïve patients or a doubling of systemic corticosteroids dose for ≥3 days in OCS-dependent patients, emergency department visit, or hospitalization, or unscheduled physician visit) in OCS-dependent patients and showed that the presence of CRSwNP did not impact the benefit on asthma exacerbations, although there was a trend toward higher reduction in patients with CRSwNP. The mean blood eosinophil count was 607/μl in those patients at baseline, but blood eosinophil counts in patients with or without CRSwNP were not reported (101) (Table 1).
The benefit of reslizumab on asthma symptoms (ACQ-5 score) was significant in patients with CRSwNP (−1.0 vs. −0.1 in the placebo group, p = 0.012), whereas only a trend was observed for the overall population (−0.7 vs. −0.3, p = 0.054). In contrast, the benefit of lung function was not increased in patients with CRSwNP. The median blood eosinophil count was 600/μl in the CRSwNP group and 400/μl in the group without CRSwNP (44). Another post hoc analysis of the same phase 3 trials, classified patients as non-responders, moderate-responders, high-responders, or super-responders based on the number of the following criteria at week 52 (0, 1, 2, or 3, respectively): ≥10% FEV1 improvement, or ≥5% predicted FEV1 improvement, no exacerbation during the 52 weeks of study, or >1.5 improvements of ACQ-6. They found that compared to non-responders, super-responders to reslizumab were more likely to have CRSwNP (102).
A recent post hoc study showed that the reduction in the annual exacerbation rate was almost similar between groups, with a slightly higher percentage in patients with CRSwNP (83 vs. 70%) (103).
3.4. Benralizumab
Benralizumab, the only approved humanized anti–IL-5 receptor antibody, was also approved for SEA treatment in 2017 following SIROCCO and CALIMA phase 3 trials in which patients with severe, uncontrolled asthma receiving high-dose ICS plus LABA received either benralizumab every 4 or 8 weeks, or placebo. Evaluation of treatment was made up to 48 weeks in the SIROCCO study and up to 56 weeks in the CALIMA study (47, 48). A first study showed that a greater improvement in FEV1 and asthma exacerbation rate was reached in patients with CRSwNP, in addition to those with blood eosinophils ≥300 cells/μl, and regardless of the administration scheme of benralizumab (every 4 or 8 weeks) (13) (Table 1). In the second post hoc analysis of the SIROCCO and CALIMA trials on annual exacerbation rate in the overall population and in the ≥300/μl blood eosinophil population, the presence of CRSwNP increased the benefit of benralizumab every 8 weeks vs. placebo compared to the overall population, with CRSwNP ranking second as a predictive factor after OCS use at baseline. In patients with <300 eosinophils/μl, the effect of CRSwNP was not significant although a trend to a higher reduction of AER was observed. Similar findings were observed for lung function (pre-bronchodilation FEV1), with the greatest impact of CRSwNP observed with benralizumab vs. placebo. Similar results were also achieved in the ≥300 eosinophils/μl population and for asthma symptoms, compared to the overall population (104) (Table 1).
Finally, one single-center, real-life study reported that patients with CRSwNP or allergic rhinoconjunctivitis had a lower chance of being super-responders to anti–IL-5(R) biologics (mepolizumab, reslizumab, or benralizumab), but this was probably biased by the fact that complete control of comorbidities (chronic rhinosinusitis and NP, as well as chronic otitis, allergic rhinoconjunctivitis, and atopic dermatitis) was required to define such response (Table 1). In this population, FeNO levels at baseline were similar across the groups of patients (non-responders, partial responders, and super-responders), and all groups showed a blood eosinophil count >300/μl, with the highest level in partial responders (570/μl) (105).
3.5. Dupilumab
Dupilumab is a monoclonal antibody targeting IL-4Rα and subsequently inhibiting IL-4 and IL-13 activities. It was recently approved (in 2019) for asthma treatment, and it is also indicated in severe CRSwNP and severe atopic dermatitis. However, only a few data are available to assess whether UAD may predict response to dupilumab in asthma. A French study reported a greater improvement in lung function in patients with CRSwNP than those without (Table 1). In contrast, blood eosinophils did not significantly predict the effect on lung function, OCS use, or ACT score (using 100/μ or 300/μ cutoffs) (105).
4. Conclusion
Blood eosinophils and FeNO have emerged as biomarkers capable of predicting the response of patients with asthma to ICS and T2 biologics. They are easy to measure, in both in- and out-patient settings and are non-invasive, but they suffer from variability (mainly for eosinophils, e.g., between-sample or nyctemeral) and/or multiple interfering factors such as environmental exposures, e.g., to cigarette smoke or allergens (mainly for FeNO). This article provides a comprehensive review of the data collectively indicating that UAD, in particular, CRSwNP and, to a lower extent, allergic rhinitis, predict the response to T2 biologics in severe asthma, as well as being improved by such therapies. Moreover, CRSwNP could be viewed as a stable biomarker that does not suffer from intrinsic variability, at least in Western countries, as CRSwNP displays a different inflammatory phenotype in Eastern countries (106). Since most studies did not specifically address this point, it is unclear to what extent the prediction by CRSwNP status is independent (or not) of eosinophils or FeNO. In contrast, the efficacy of anti–IL-5 biologics on CRSwNP outcomes (such as the polyp size) does not seem to differ whether patients had comorbid asthma or not (107). This observation is consistent with the fact that CRSwNP is most often eosinophilic/T2 in nature (in Western populations), whereas the underlying biology of SA may be related more frequently to different endotypes.
Altogether the data reviewed in this article support that the diagnosis of UAD in patients with SA with the indication of biotherapy is a key step in refining the clinical profile and has added value to T2 biomarkers (blood eosinophils and FeNO) to predict the clinical response. As there is no published head-to-head trial comparing biologics for CRSwNP or for severe asthma and, although some studies are ongoing (10), only indirect comparisons are available, we propose to refer to the acronym “ABC,” standing for age (at the onset of the disease), biology (eosinophils, FeNO), and comorbidities, as the three key sets of biomarkers that impact the therapeutic response to biologics in SA (Figure 1) and that could help clinicians to decide the first most appropriate biologic in a given patient. In particular, UAD represents a strong factor with comorbid CRSwNP-SA, defining a particular eosinophilic condition for which anti–IL-5 biologics have increased efficacy and should be managed through a collaborative and multidisciplinary approach (108).
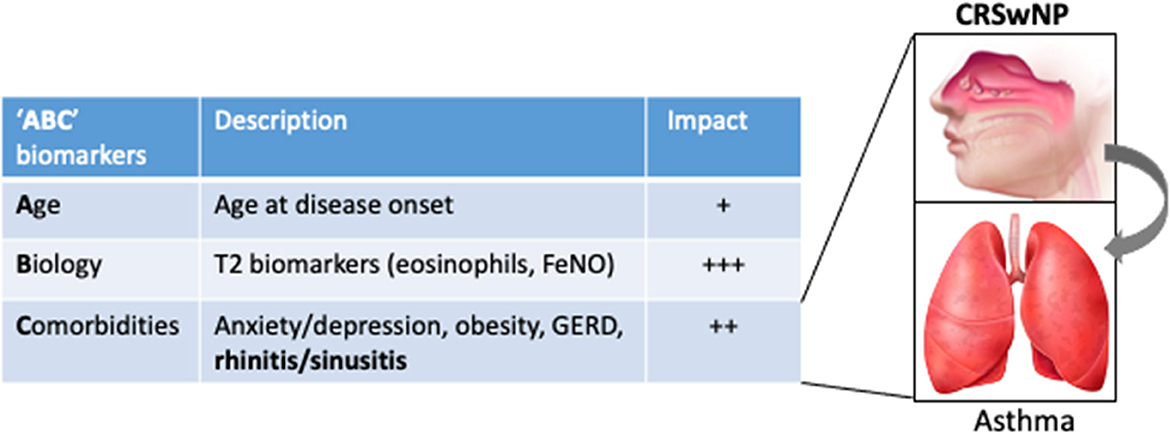
Figure 1. The “ABC” sets of biomarkers that influence the response to biologics in severe asthma, illustrated by their respective estimated impact on the response to anti–IL-5 therapies. For instance, the impact calculated (odds ratio, OR) for adult onset and CRSwNP on the probability to be defined as a responder to mepolizumab in a “real-life” series (88) were 1.20 and 1.36 (vs. the absence of CRSwNP), while it was not significant for eosinophils. Inset, illustrating the impact of UAD (in particular CRSwNP) on asthma outcomes upon biotherapy.
Author contributions
SC and VD drafted the manuscript. CP designed and revised the manuscript and made the figure. All authors contributed to the article and approved the submitted version.
Funding
CP is fellow of the Fonds National de la Recherche Scientifique (FNRS grant 1.R016.20), Belgium.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^Inchem. Available online at: http://www.inchem.org/documents/ehc/ehc/ehc155.htm.
2. ^Manzanares. Available online at: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/biomarkers.
References
1. Milgrom H, Fick RB, Su JQ, Reimann JD, Bush RK, Watrous ML, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb-E25 Study Group. N Engl J Med. (1999) 341:1966–73. doi: 10.1056/NEJM199912233412603
2. Holgate S, Bousquet J, Wenzel S, Fox H, Liu J, Castellsague J. Efficacy of omalizumab, an anti-immunoglobulin E antibody, in patients with allergic asthma at high risk of serious asthma-related morbidity and mortality. Curr Med Res Opin. (2001) 17:233–40. doi: 10.1185/030079901753403126
3. Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. (2001) 108:184–90. doi: 10.1067/mai.2001.117880
4. Soler M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. (2001) 18:254–61. doi: 10.1183/09031936.01.00092101
5. Brusselle GG, Koppelman GH. Biologic Therapies for Severe Asthma. N Engl J Med. (2022) 386:157–71. doi: 10.1056/NEJMra2032506
6. Biomarkers Definitions Working G. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. (2001) 69:89–95. doi: 10.1067/mcp.2001.113989
7. Ray A, Raundhal M, Oriss TB, Ray P, Wenzel SE. Current concepts of severe asthma. J Clin Invest. (2016) 126:2394–403. doi: 10.1172/JCI84144
8. McDowell PJ, Heaney LG. Different endotypes and phenotypes drive the heterogeneity in severe asthma. Allergy. (2020) 75:302–10. doi: 10.1111/all.13966
9. Georas SN, Wright RJ, Ivanova A, Israel E, LaVange LM, Akuthota P, et al. The Precision Interventions for Severe and/or Exacerbation-Prone (PrecISE) Asthma Network: An overview of Network organization, procedures, and interventions. J Allergy Clin Immunol. (2022) 149:488–516.e9. doi: 10.1016/j.jaci.2021.10.035
10. Pilette C, Brightling C, Lacombe D, Brusselle G. Urgent need for pragmatic trial platforms in severe asthma. Lancet Respir Med. (2018) 6:581–3. doi: 10.1016/S2213-2600(18)30291-1
11. Bousquet J, Rabe K, Humbert M, Chung KF, Berger W, Fox H, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med. (2007) 101:1483–92. doi: 10.1016/j.rmed.2007.01.011
12. Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. (2014) 371:1198–207. doi: 10.1056/NEJMoa1403290
13. FitzGerald JM, Bleecker ER, Menzies-Gow A, Zangrilli JG, Hirsch I, Metcalfe P, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med. (2018) 6:51–64. doi: 10.1016/S2213-2600(17)30344-2
14. Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med. (2018) 378:2486–96. doi: 10.1056/NEJMoa1804092
15. Porpodis K, Tsiouprou I, Apostolopoulos A, Ntontsi P, Fouka E, Papakosta D, et al. Eosinophilic asthma, phenotypes-endotypes and current biomarkers of choice. J Pers Med. (2022) 12:1093. doi: 10.3390/jpm12071093
16. Custovic A, Siddiqui S, Saglani S. Considering biomarkers in asthma disease severity. J Allergy Clin Immunol. (2022) 149:480–7. doi: 10.1016/j.jaci.2021.11.021
17. Rupani H, Fong WCG, Kyyaly A, Kurukulaaratchy RJ. Recent insights into the management of inflammation in asthma. J Inflamm Res. (2021) 14:4371–97. doi: 10.2147/JIR.S295038
18. Frossing L, Silberbrandt A, Von Bulow A, Backer V, Porsbjerg C. The prevalence of subtypes of type 2 inflammation in an unselected population of patients with severe asthma. J Allergy Clin Immunol Pract. (2021) 9:1267–75. doi: 10.1016/j.jaip.2020.09.051
19. Schleich F, Brusselle G, Louis R, Vandenplas O, Michils A, Pilette C, et al. Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR). Respir Med. (2014) 108:1723–32. doi: 10.1016/j.rmed.2014.10.007
20. Bradley BL, Azzawi M, Jacobson M, Assoufi B, Collins JV, Irani AM, et al. Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. (1991) 88:661–74. doi: 10.1016/0091-6749(91)90160-P
21. Vignola AM, Chanez P, Chiappara G, Siena L, Merendino A, Reina C, et al. Evaluation of apoptosis of eosinophils, macrophages, and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol. (1999) 103:563–73. doi: 10.1016/S0091-6749(99)70225-3
22. Louis R, Sele J, Henket M, Cataldo D, Bettiol J, Seiden L, et al. Sputum eosinophil count in a large population of patients with mild to moderate steroid-naive asthma: distribution and relationship with methacholine bronchial hyperresponsiveness. Allergy. (2002) 57:907–12. doi: 10.1034/j.1398-9995.2002.23608.x
23. Schleich FN, Louis R. Importance of concomitant local and systemic eosinophilia in uncontrolled asthma. Eur Respir J. (2014) 44:1098–9. doi: 10.1183/09031936.00118014
24. Abdo M, Pedersen F, Kirsten AM, Veith V, Biller H, Trinkmann F, et al. Longitudinal Impact of Sputum Inflammatory Phenotypes on Small Airway Dysfunction and Disease Outcomes in Asthma. J Allergy Clin Immunol Pract. (2022) 10:1545–53.e2. doi: 10.1016/j.jaip.2022.02.020
25. Brown HM. Treatment of chronic asthma with prednisolone; significance of eosinophils in the sputum. Lancet. (1958) 2:1245–7. doi: 10.1016/S0140-6736(58)91385-0
26. Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet. (1999) 353:2213–4. doi: 10.1016/S0140-6736(99)01813-9
27. Rhyou HI, Nam YH. Predictive factors of response to inhaled corticosteroids in newly diagnosed asthma: A real-world observational study. Ann Allergy Asthma Immunol. (2020) 125:177–81. doi: 10.1016/j.anai.2020.04.025
28. Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. (2002) 360:1715–21. doi: 10.1016/S0140-6736(02)11679-5
29. Jayaram L, Pizzichini MM, Cook RJ, Boulet LP, Lemiere C, Pizzichini E, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. (2006) 27:483–94. doi: 10.1183/09031936.06.00137704
30. Pizzichini E, Pizzichini MM, Efthimiadis A, Dolovich J, Hargreave FE. Measuring airway inflammation in asthma: eosinophils and eosinophilic cationic protein in induced sputum compared with peripheral blood. J Allergy Clin Immunol. (1997) 99:539–44. doi: 10.1016/S0091-6749(97)70082-4
31. Peters MC, Kerr S, Dunican EM, Woodruff PG, Fajt ML, Levy BD, et al. Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J Allergy Clin Immunol. (2019) 143:104–13.e14. doi: 10.1016/j.jaci.2017.12.1009
32. Paggiaro PL, Chanez P, Holz O, Ind PW, Djukanovic R, Maestrelli P, et al. Sputum induction. Eur Respir J Suppl. (2002) 37:3s–8s. doi: 10.1183/09031936.02.00000302
33. Katz LE, Gleich GJ, Hartley BF, Yancey SW, Ortega HG. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc. (2014) 11:531–6. doi: 10.1513/AnnalsATS.201310-354OC
34. Pavord ID, Buhl R, Kraft M, Prazma CM, Price RG, Howarth PH, et al. Evaluation of sputum eosinophil count as a predictor of treatment response to mepolizumab. ERJ Open Res. (2022) 8:560. doi: 10.1183/23120541.00560-2021
35. Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. (2012) 380:651–9. doi: 10.1016/S0140-6736(12)60988-X
36. Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. (2013) 132:1086–96 e5. doi: 10.1016/j.jaci.2013.05.020
37. Guida G, Bagnasco D, Carriero V, Bertolini F, Ricciardolo FLM, Nicola S, et al. Critical evaluation of asthma biomarkers in clinical practice. Front Med (Lausanne). (2022) 9:969243. doi: 10.3389/fmed.2022.969243
38. Guiot J, Demarche S, Henket M, Paulus V, Graff S, Schleich F, et al. Methodology for sputum induction and laboratory processing. J Vis Exp. (2017) 130:56612. doi: 10.3791/56612-v
39. Fahy JV, Boushey HA, Lazarus SC, Mauger EA, Cherniack RM, Chinchilli VM, et al. Safety and reproducibility of sputum induction in asthmatic subjects in a multicenter study. Am J Respir Crit Care Med. (2001) 163:1470–5. doi: 10.1164/ajrccm.163.6.9901105
40. Weiszhar Z, Horvath I. Induced sputum analysis: step by step. Breathe. (2013) 9:300-306. doi: 10.1183/20734735.042912
41. Rossall MR, Cadden PA, Molphy SD, Plumb J, Singh D. Repeatability of induced sputum measurements in moderate to severe asthma. Respir Med. (2014) 108:1566–8. doi: 10.1016/j.rmed.2014.08.004
42. Demarche SF, Schleich FN, Paulus VA, Henket MA, Van Hees TJ, Louis RE. Asthma control and sputum eosinophils: a longitudinal study in daily practice. J Allergy Clin Immunol Pract. (2017) 5:1335–1343.e5. doi: 10.1016/j.jaip.2017.01.026
43. Bacci E, Cianchetti S, Carnevali S, Bartoli ML, Dente FL, Di Franco A, et al. Induced sputum is a reproducible method to assess airway inflammation in asthma. Mediators Inflamm. (2002) 11:293–8. doi: 10.1080/09629350210000015692
44. Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. (2011) 184:1125–32. doi: 10.1164/rccm.201103-0396OC
45. Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 Study of Reslizumab in Patients With Poorly Controlled Asthma: Effects Across a Broad Range of Eosinophil Counts. Chest. (2016) 150:799–810. doi: 10.1016/j.chest.2016.03.018
46. Chipps BE, Jarjour N, Calhoun WJ, Iqbal A, Haselkorn T, Yang M, et al. A Comprehensive Analysis of the Stability of Blood Eosinophil Levels. Ann Am Thorac Soc. (2021) 18:1978–87. doi: 10.1513/AnnalsATS.202010-1249OC
47. Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. (2016) 388:2115–27. doi: 10.1016/S0140-6736(16)31324-1
48. FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. (2016) 388:2128–41. doi: 10.1016/S0140-6736(16)31322-8
49. Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. (2016) 388:31–44. doi: 10.1016/S0140-6736(16)30307-5
50. Corren J, Pham TH, Garcia Gil E, Salapa K, Ren P, Parnes JR, et al. Baseline type 2 biomarker levels and response to tezepelumab in severe asthma. Allergy. (2022) 77:1786–96. doi: 10.1111/all.15197
51. Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, Hamilton RG, Hunt JF, Kita H, Liu AH, Panettieri RA Jr, Schleimer RP, Minnicozzi M. Asthma outcomes: biomarkers. J Allergy Clin Immunol. (2012) 129:S9–S23. doi: 10.1016/j.jaci.2011.12.979
52. Demarche SF, Schleich FN, Henket MA, Paulus VA, Van Hees TJ, Louis RE. Effectiveness of inhaled corticosteroids in real life on clinical outcomes, sputum cells and systemic inflammation in asthmatics: a retrospective cohort study in a secondary care centre. BMJ Open. (2017) 7:e018186. doi: 10.1136/bmjopen-2017-018186
53. Barber C, Azim A, Newell C, Kyyaly A, Rupani H, Haitchi HM, et al. Validation and further insight into the International Severe Asthma Registry (ISAR) eosinophil gradient algorithm in the Wessex AsThma CoHort of difficult asthma (WATCH) using historical blood eosinophil counts and induced sputum. Clin Exp Allergy. (2022) 52:792–6. doi: 10.1111/cea.14109
54. Wagener AH, de Nijs SB, Lutter R, Sousa AR, Weersink EJ, Bel EH, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. (2015) 70:115–20. doi: 10.1136/thoraxjnl-2014-205634
55. Robinson D, Humbert M, Buhl R, Cruz AA, Inoue H, Korom S, et al. Revisiting Type 2-high and Type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy. (2017) 47:161–75. doi: 10.1111/cea.12880
56. Horváth I, Barnes PJ, Loukides S, et al. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur Respir J. (2017) 49:1600965. doi: 10.1183/13993003.E4904-2017
57. Louis R, Satia I, Ojanguren I, Schleich F, Bonini M, Tonia T, et al. European respiratory society guidelines for the diagnosis of asthma in adults. Eur Respir J. (2022) 60:2101585. doi: 10.1183/13993003.01585-2021
58. McDowell PJ, Diver S, Yang F, Borg C, Busby J, Brown V, et al. The inflammatory profile of exacerbations in patients with severe refractory eosinophilic asthma receiving mepolizumab (the MEX study): a prospective observational study. Lancet Respir Med. (2021) 9:1174–84. doi: 10.1016/S2213-2600(21)00004-7
59. Smith AD, Cowan JO, Brassett KP, Filsell S, McLachlan C, Monti-Sheehan G, et al. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med. (2005) 172:453–9. doi: 10.1164/rccm.200411-1498OC
60. McNicholl DM, Stevenson M, McGarvey LP, Heaney LG. The utility of fractional exhaled nitric oxide suppression in the identification of nonadherence in difficult asthma. Am J Respir Crit Care Med. (2012) 186:1102–8. doi: 10.1164/rccm.201204-0587OC
61. Silkoff PE, Lent AM, Busacker AA, Katial RK, Balzar S, Strand M, et al. Exhaled nitric oxide identifies the persistent eosinophilic phenotype in severe refractory asthma. J Allergy Clin Immunol. (2005) 116:1249–55. doi: 10.1016/j.jaci.2005.09.029
62. Alving K, Diamant Z, Lucas S, Magnussen H, Pavord ID, Piacentini G, et al. Point-of-care biomarkers in asthma management: Time to move forward. Allergy. (2020) 75:995–7. doi: 10.1111/all.14045
63. Harvey ES, Langton D, Katelaris C, Stevens S, Farah CS, Gillman A, et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur Respir J. (2020) 55:1902420. doi: 10.1183/13993003.02420-2019
64. Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N Engl J Med. (2018) 378:2475–85. doi: 10.1056/NEJMoa1804093
65. Busse WW, Maspero JF, Rabe KF, Papi A, Wenzel SE, Ford LB, et al. Liberty asthma QUEST: phase 3 randomized, double-blind, placebo-controlled, parallel-group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate-to-severe asthma. Adv Ther. (2018) 35:737–48. doi: 10.1007/s12325-018-0702-4
66. Corren J, Castro M, O'Riordan T, Hanania NA, Pavord ID, Quirce S, et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-severe allergic asthma. J Allergy Clin Immunol Pract. (2020) 8:516–26. doi: 10.1016/j.jaip.2019.08.050
67. Carpagnano GE, Scioscia G, Buonamico E, Lacedonia D, Diaferia F, Capozza E, et al. Early effectiveness of type-2 severe asthma treatment with dupilumab in a real-life setting; a FeNO-driven choice that leads to winning management. Multidiscip Respir Med. (2022) 17:797. doi: 10.4081/mrm.2022.797
68. Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. (2020) 55:1900588. doi: 10.1183/13993003.00588-2019
69. Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. (2013) 187:804–11. doi: 10.1164/rccm.201208-1414OC
70. Lowe PJ, Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPD analysis of a biomarker, total IgE. Br J Clin Pharmacol. (2011) 72:306–20. doi: 10.1111/j.1365-2125.2011.03962.x
71. Corren J, Casale T, Deniz Y, Ashby M. Omalizumab, a recombinant humanized anti-IgE antibody, reduces asthma-related emergency room visits and hospitalizations in patients with allergic asthma. J Allergy Clin Immunol. (2003) 111:87–90. doi: 10.1067/mai.2003.49
72. Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. (2005) 60:309–16. doi: 10.1111/j.1398-9995.2004.00772.x
73. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. (2004) 125:1378–86. doi: 10.1378/chest.125.4.1378
74. Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. (2006) 118:98–104. doi: 10.1016/j.jaci.2006.02.046
75. James A, Janson C, Malinovschi A, Holweg C, Alving K, Ono J, et al. Serum periostin relates to type-2 inflammation and lung function in asthma: Data from the large population-based cohort Swedish GA(2)LEN. Allergy. (2017) 72:1753–60. doi: 10.1111/all.13181
76. Brightling CE, Chanez P, Leigh R, O'Byrne PM, Korn S, She D, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. (2015) 3:692–701. doi: 10.1016/S2213-2600(15)00197-6
77. Hanania NA, Noonan M, Corren J, Korenblat P, Zheng Y, Fischer SK, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. (2015) 70:748–56. doi: 10.1136/thoraxjnl-2014-206719
78. Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. (2011) 365:1088–98. doi: 10.1056/NEJMoa1106469
79. Hamann KJ, Barker RL, Ten RM, Gleich GJ. The molecular biology of eosinophil granule proteins. Int Arch Allergy Appl Immunol. (1991) 94:202–9. doi: 10.1159/000235362
80. Morioka J, Kurosawa M, Inamura H, Nakagami R, Mizushima Y, Omura Y, et al. Increased END/EPX in ongoing asthma. Allergy. (2000) 55:1203–4. doi: 10.1034/j.1398-9995.2000.00858.x
81. An J, Lee JH, Sim JH, Song WJ, Kwon HS, Cho YS, et al. Serum eosinophil-derived neurotoxin better reflect asthma control status than blood eosinophil counts. J Allergy Clin Immunol Pract. (2020) 8:2681–8 e1. doi: 10.1016/j.jaip.2020.03.035
82. Lee Y, Lee JH, Yang EM, Kwon E, Jung CG, Kim SC, et al. Serum levels of eosinophil-derived neurotoxin: a biomarker for asthma severity in adult asthmatics. Allergy Asthma Immunol Res. (2019) 11:394–405. doi: 10.4168/aair.2019.11.3.394
83. Grootendorst DC, Dahlen SE, Van Den Bos JW, Duiverman EJ, Veselic-Charvat M, Vrijlandt EJ, et al. Benefits of high altitude allergen avoidance in atopic adolescents with moderate to severe asthma, over and above treatment with high dose inhaled steroids. Clin Exp Allergy. (2001) 31:400–8. doi: 10.1046/j.1365-2222.2001.01022.x
84. Kristjansson S, Strannegard IL, Strannegard O, Peterson C, Enander I, Wennergren G. Urinary eosinophil protein X in children with atopic asthma: a useful marker of antiinflammatory treatment. J Allergy Clin Immunol. (1996) 97:1179–87. doi: 10.1016/S0091-6749(96)70182-3
85. Pham TH, Damera G, Newbold P, Ranade K. Reductions in eosinophil biomarkers by benralizumab in patients with asthma. Respir Med. (2016) 111:21–9. doi: 10.1016/j.rmed.2016.01.003
86. Tomizawa H, Yamada Y, Arima M, Miyabe Y, Fukuchi M, Hikichi H, et al. Galectin-10 as a Potential Biomarker for Eosinophilic Diseases. Biomolecules. (2022) 12:1385. doi: 10.3390/biom12101385
87. Gelardi M, Netti GS, Giancaspro R, Spadaccino F, Pennella A, Fiore V, et al. Chronic rhinosinusitis with nasal polyposis (CRSwNP): the correlation between expression of Galectin-10 and Clinical-Cytological Grading (CCG). Am J Rhinol Allergy. (2022) 36:229–37. doi: 10.1177/19458924211049867
88. Kobayashi K, Nagase H, Sugimoto N, Yamamoto S, Tanaka A, Fukunaga K, et al. Mepolizumab decreased the levels of serum galectin-10 and eosinophil cationic protein in asthma. Asia Pac Allergy. (2021) 11:e31. doi: 10.5415/apallergy.2021.11.e31
89. Wang Z, Xu W, Comhair SAA, Fu X, Shao Z, Bearden R, et al. Urinary total conjugated 3-bromotyrosine, asthma severity, and exacerbation risk. Am J Physiol Lung Cell Mol Physiol. (2022) 323:L548–L57. doi: 10.1152/ajplung.00141.2022
90. Cowan DC, Taylor DR, Peterson LE, Cowan JO, Palmay R, Williamson A, et al. Biomarker-based asthma phenotypes of corticosteroid response. J Allergy Clin Immunol. (2015) 135:877–83 e1. doi: 10.1016/j.jaci.2014.10.026
91. Mogensen I, James A, Malinovschi A. Systemic and breath biomarkers for asthma: an update. Curr Opin Allergy Clin Immunol. (2020) 20:71–9. doi: 10.1097/ACI.0000000000000599
92. Tiotiu A, Plavec D, Novakova S, Mihaicuta S, Novakova P, Labor M, et al. Current opinions for the management of asthma associated with ear, nose and throat comorbidities. Eur Respir Rev. (2018) 27:180056. doi: 10.1183/16000617.0056-2018
93. Stevens WW, Peters AT, Tan BK, Klingler AI, Poposki JA, Hulse KE, et al. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J Allergy Clin Immunol Pract. (2019) 7:2812–20.e3. doi: 10.1016/j.jaip.2019.05.009
94. Kato A, Schleimer RP, Bleier BS. Mechanisms and pathogenesis of chronic rhinosinusitis. J Allergy Clin Immunol. (2022) 149:1491–503. doi: 10.1016/j.jaci.2022.02.016
95. Li J, Wang C, Liu C, Kang J, Kong L, Huang Y, et al. Efficacy predictors of omalizumab in Chinese patients with moderate-to-severe allergic asthma: Findings from a post-hoc analysis of a randomised phase III study. World Allergy Organ J. (2020) 13:100469. doi: 10.1016/j.waojou.2020.100469
96. Gibson PG, Prazma CM, Chupp GL, Bradford ES, Forshag M, Mallett SA, et al. Mepolizumab improves clinical outcomes in patients with severe asthma and comorbid conditions. Respir Res. (2021) 22:171. doi: 10.1186/s12931-021-01746-4
97. Solidoro P, Patrucco F, de Blasio F, Brussino L, Bellocchia M, Dassetto D, et al. Predictors of reversible airway obstruction with omalizumab in severe asthma: a real-life study. Ther Adv Respir Dis. (2019) 13:1753466619841274. doi: 10.1177/1753466619841274
98. Gibson PG, Reddel H, McDonald VM, Marks G, Jenkins C, Gillman A, et al. Effectiveness and response predictors of omalizumab in a severe allergic asthma population with a high prevalence of comorbidities: the Australian Xolair Registry. Intern Med J. (2016) 46:1054–62. doi: 10.1111/imj.13166
99. Kavanagh JE, d'Ancona G, Elstad M, Green L, Fernandes M, Thomson L, et al. Real-world effectiveness and the characteristics of a “super-responder” to mepolizumab in severe eosinophilic asthma. Chest. (2020) 158:491–500. doi: 10.1016/j.chest.2020.03.042
100. Howarth P, Chupp G, Nelsen LM, Bradford ES, Bratton DJ, Smith SG, et al. Severe eosinophilic asthma with nasal polyposis: A phenotype for improved sinonasal and asthma outcomes with mepolizumab therapy. J Allergy Clin Immunol. (2020) 145:1713–5. doi: 10.1016/j.jaci.2020.02.002
101. Nair P, Bardin P, Humbert M, Murphy KR, Hickey L, Garin M, et al. Efficacy of Intravenous Reslizumab in Oral Corticosteroid-Dependent Asthma. J Allergy Clin Immunol Pract. (2020) 8:555–64. doi: 10.1016/j.jaip.2019.09.036
102. Wechsler M, McDonald M, Garin MC. Reslizumab high-responder and super-responder asthma patients. Am J Respir Crit Care Med. (2018) 197:A1375.
103. Weinstein SF, Germinaro M, Bardin P, Korn S, Bateman ED. Efficacy of reslizumab with asthma, chronic sinusitis with nasal polyps and elevated blood eosinophils. J Aller Clin Immunol. (2016) 137:AB86. doi: 10.1016/j.jaci.2015.12.409
104. Bleecker ER, Wechsler ME, FitzGerald JM, Menzies-Gow A, Wu Y, Hirsch I, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. (2018) 52:1800936. doi: 10.1183/13993003.00936-2018
105. Eger K, Kroes JA, Ten Brinke A, Bel EH. Long-Term Therapy Response to Anti-IL-5 Biologics in Severe Asthma-A Real-Life Evaluation. J Allergy Clin Immunol Pract. (2021) 9:1194–200. doi: 10.1016/j.jaip.2020.10.010
106. Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. (2016) 138:1344–53. doi: 10.1016/j.jaci.2016.05.041
107. Bachert C, Sousa AR, Han JK, Schlosser RJ, Sowerby LJ, Hopkins C, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps: Treatment efficacy by comorbidity and blood eosinophil count. J Allergy Clin Immunol. (2022) 149:1711–21. doi: 10.1016/j.jaci.2021.10.040
Keywords: upper airway disease, severe asthma, nasal polyposis, predictive biomarker, theragnostics
Citation: Cottin S, Doyen V and Pilette C (2023) Upper airway disease diagnosis as a predictive biomarker of therapeutic response to biologics in severe asthma. Front. Med. 10:1129300. doi: 10.3389/fmed.2023.1129300
Received: 21 December 2022; Accepted: 27 February 2023;
Published: 22 March 2023.
Edited by:
Konstantinos Porpodis, Aristotle University of Thessaloniki, GreeceReviewed by:
András Bikov, The University of Manchester, United KingdomPatrizia Pignatti, Scientific Clinical Institute Maugeri (ICS Maugeri), Italy
Copyright © 2023 Cottin, Doyen and Pilette. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charles Pilette, charles.pilette@uclouvain.be
 Sophie Cottin
Sophie Cottin Virginie Doyen
Virginie Doyen Charles Pilette
Charles Pilette