Prevalence and determinants of opportunistic infections among HIV-infected adults receiving antiretroviral therapy in Ethiopia: A systematic review and meta-analysis
- 1School of Medicine, College of Health Sciences and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
- 2Wolaita Sodo University Comprehensive Specialized Hospital, Wolaita Sodo University, Wolaita Sodo, Ethiopia
- 3Department of Nursing, College of Health Sciences and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
- 4Department of Anesthesia, College of Health Sciences and Medicine, Arsi University, Assela, Ethiopia
- 5School of Public Health, College of Health Sciences and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
Background: Reliable data on the burden of opportunistic infections (OIs) after the initiation of antiretroviral therapy (ART) is critical for planning health services and reducing OI-related morbidity and mortality. Nevertheless, there has been no nationally representative information on the prevalence of OIs in our country. Therefore, we have undertaken this comprehensive systematic review and meta-analysis to estimate the pooled prevalence, and identify factors associated with the development of OIs in Human Immunodeficiency Virus (HIV)-infected adults receiving ART in Ethiopia.
Methods: Articles were searched in international electronic databases. A standardized Microsoft Excel spreadsheet and STATA software version 16 were used for data extraction and analysis, respectively. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist was used to write this report. The random-effect meta-analysis model was used to estimate the pooled effect. The statistical heterogeneity of the meta-analysis was checked. Subgroup and sensitivity analyses were also performed. Publication bias was examined in funnel plots and the nonparametric rank correlation test of Begg and the regression-based test of Egger. Association was expressed through a pooled odds ratio (OR) with a 95% Confidence Interval (CI).
Results: A total of 12 studies with 6,163 study participants were included. The pooled prevalence of OIs was 43.97% [95% CI (38.59, 49.34)]. Poor adherence to ART [OR, 5.90, 95% CI (3.05, 11.40)], under nutrition [OR, 3.70, 95% CI (2.01, 6.80)], CD4 T lymphocyte count <200 cells /μL [OR, 3.23 95% CI (2.06, 5.07)], and advanced World Health Organization (WHO) HIV clinical stages [OR, 4.84 95% CI (1.83, 12.82)] were determinants of OIs.
Conclusion: The pooled prevalence of OIs among adults taking ART is high. Poor adherence to ART, under nutrition, a CD4 T lymphocyte count <200 cells /μL, and advanced WHO HIV clinical stages were factors associated with the development of OIs.
Background
Opportunistic infections (OIs) are illnesses that occur more frequently and are more severe in people infected with the Human Immunodeficiency Virus (HIV) (1, 2). According to the Joint United Nations Programme on HIV/AIDS, 2022 Global HIV and AIDS Statistics fact sheet, 84.2 million people have become infected with HIV since the epidemic began, and 40.1 million have died from AIDS-related illnesses. Besides, around 650,000 people died from AIDS-related illnesses worldwide in 2021 (3).
People living with HIV are frequently exposed to co-infections during the disease (4) more frequently and severely, causing significant morbidity and mortality, and necessitating lifelong ART (2, 5). Thus, OIs remain the major driver of HIV-associated morbidity and mortality, accounting for the substantially higher mortality observed in Low and middle-income countries (LMICs) (6). High levels of early morbidity and mortality following ART initiation continue to be a distinctive feature of ART programs in Sub-Saharan Africa, although the introduction and scaling-up of ART have decreased overall mortality (7, 8).
Opportunistic infections are the leading cause of hospitalization and death in patients with HIV and still present formidable challenges for meager healthcare systems endeavoring to provide effective and efficient HIV care (9, 10). In resource-poor settings, between 20 and 52% of hospital beds are occupied by HIV-related OIs (11). Furthermore, 90% of HIV-related morbidity and mortality are attributed to OIs (1, 12). Between 2000 and 2019, 170.79 billion United States dollars in development assistance for health was spent on HIV globally and most of this aid went towards care and treatment for HIV (13).
Since the advent of ART, a drastic decrease in the magnitude OIs was observed, but still, the burden in countries in Africa differs markedly from those of industrialized countries (14). Though there have been no representative studies reporting the magnitude of OIs in adults in Africa, a systematic review and meta-analysis by MR, B.L., et al. revealed that bacterial pneumonia (32.51%), oral and esophageal candidiasis (24.77%), and bacteremia (23.18%) were the most common infections among HIV-infected children receiving ART in LMICs (15).
In Owerri, Imo State, South East Nigeria, among adults who were receiving ART, the overall prevalence of OIs was 22.4%, with candidiasis (8.6%), tuberculosis (7.7%), dermatitis (5.6%), and chronic diarrhea among the most prevalent cases (16). Similarly, research conducted in seven provinces in Indonesia revealed that OIs were prevalent in 33.51% of the PLHIV, with tuberculosis (48.6%), candidiasis (41.2%), and diarrhea (20%) being the most common OIs (17). Even though ART has been proven to be impactful in halting immune system impairment and preventing disease progression, studies showed that OIs have not gone (18, 19) either due to the unmasking of subclinical infection that occurs with immune recovery, drug toxicities and interactions, initial acquisition of a drug-resistant strain or high exposure to infectious agents (20, 21).
A study in the United Kingdom reported that late presentation to health facilities increases the odds of developing OIs and remains a significant problem in developed countries, with over 20% of patients in the United kingdom suffering from OIs (22). Besides, baseline Nevirapine-based regimens, higher viral load, treatment failure, and hemoglobin levels were factors associated with the development of OIs among adult patients taking ART (23–25). Furthermore, adherence to ART, nutritional status, isoniazid preventive therapy, and cotrimoxazole preventive therapy, place of residence, functional status, gender, age of the PLHIV, CD4 T lymphocytes count, and disclosure status have been associated with the development of OIs among adult PLHIV after initiation of ART (24, 26–28).
Reliable data on the burden of OIs after ART initiation is critical for planning health services and reducing OI-related morbidity and mortality. However, there has been no nationally representative information on the prevalence of OIs in our country. Therefore, we have undertaken this comprehensive systematic review and meta-analysis to estimate the pooled prevalence, and identify potential risk factors associated with the development of OIs in HIV-infected adults receiving ART in Ethiopia. Results obtained from this review are important for planning the delivery of HIV services in Ethiopia which includes the prevention and management of OIs, and the provision of comprehensive and high-quality care to HIV patients.
Methods
Study protocol registration and reporting
This systematic review and meta-analysis were undertaken to estimate the prevalence of OIs in HIV-infected adults receiving ART in Ethiopia and identify the associated factors. The study protocol for this review has been registered in an international database, the Prospective Register of Systematic Reviews, by the University of York Centre for Reviews and Dissemination with identification number CRD42022296126 (URL).1
Furthermore, modifications to the title and expected date of review finalization were made on October 17, 2022. A 17-item Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols 2015 checklist was used to guide protocol development (29). Besides, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 Checklist was used to report the review’s findings (30) (Additional file 1).
Research questions
We have undertaken this comprehensive systematic review and meta-analysis to seek an answer to the following questions: (1) What is the national burden of OIs in adult following the initiation of ART? (2) What are the potential risk factors that affect the occurrence of OIs in adults following the initiation of ART?
Inclusion criteria
The inclusion criteria for this review were based on the study characteristics and report characteristics determined by using the CoCoPop (Condition, Context, and Population) mnemonic (31). Thus, we included all observational studies (cross-sectional studies, case–control studies, and cohort studies) in this systematic review and meta-analysis.
Participants/Population: The individuals aged 15 and above years who participated in the studies that assessed the prevalence of OIs and/or associated factors were considered. According to Ethiopian national guidelines for HIV/AIDS treatment programs, adults include individuals aged greater or equal to 15 years (20).
Context: Limited to primary studies conducted in Ethiopia.
Language of publication: Articles reported in English were included.
Years of publication: Articles published between 2010 and 2022 were included.
Exclusion criteria
Studies reporting the outcome of interest in pre-ART PLHIV, without full-text access; articles that contained insufficient information; findings from personal opinions; articles reported outside the scope of the outcome of interest; qualitative study design; case reports; case series; letters; unpublished data; and previous systematic reviews were filtered out.
Information sources and search strategy
Literature search strategies were developed using medical subject headings (MeSH) and text words related to the outcomes of the study. The search typically included the following electronic bibliographic databases: Excerpta Medica database (EMBASE), PubMed, Web of Science, African Journal of Online (AJOL), Google Scholar, and Cochrane Library to ensure complete coverage of the topic by accounting for variability between the indexing in each database. The literature search was limited to studies published in the English language between 1st January 2010 and February 30th 2022 which explored prevalence, and/or factors associated with OIs among adults after initiation of ART. The reference lists of included studies identified through the search were scanned to ensure literature saturation. Where necessary, we also searched the authors’ files to ensure that all relevant materials had been captured.
We reviewed grey literature via Google. In addition, unpublished studies were sought from the official repositories of Ethiopian universities in particular Addis Ababa University and Jimma University. For the advanced search in PubMed, the following steps comprised the search process: Initially, the search statement was divided into main concepts: prevalence, Incidence, Opportunistic infections, associated factors, adults, antiretroviral therapy, and Ethiopia. Subsequently, we gathered keywords from Google scholar, Wikipedia, and Google for each concept, which was then searched independently in PubMed to find MeSH terms in the MeSH hierarchy tree and then combined in an advanced search.
Boolean operators (AND and OR) were used to combine these concepts as follows: (Prevalence [Text Word]) OR {“Prevalence”[Mesh] AND (opportunistic infections [Text Word])} OR (“AIDS-Related Opportunistic Infections”[Mesh]) AND (HIV [Text Word]) OR (“HIV”[MeSH Terms]) AND Predictors [Text Word] OR (Risk Factors [Text Word]) OR {“Associated factors”[Text Word] OR (“Risk Factors”[Mesh])} AND (adults [Text Word]) OR (“adult”[MeSH Terms]) “anti-retroviral agents”[MeSH Terms] OR antiretroviral [Text Word] AND [Ethiopia (text word)] OR (“Ethiopia*”[Mesh]). Finally, we filtered the results to include just the most relevant ones. The search was double-blinded and conducted from February 30th to April 10, 2022, by three authors (BZ.W, AA. K, and MS.O). A separate file with the search details was supplied (Additional file 2).
Study selection procedures
The articles that were found through the electronic database searches were exported to the reference management software, EndNote X7, where duplicate studies were then eliminated. Two authors (BZ.W and MS.O) independently screened the titles and abstracts that were obtained by the search against the inclusion criteria. To describe the extent to which assessments by multiple authors are similar, inter-rater agreement was calculated after referring to the Cochrane handbook for systematic reviews.
In this case, kappa greater or equal to 75% was considered, indicating excellent agreement. The screened articles were then subjected to a full article review by two independent authors (ZZ and BG.A). A pre-defined eligibility criterion was used to determine which records were relevant and should be included in the review. Where more information was required to answer queries regarding eligibility, the remaining authors were involved. Disagreements were resolved through discussion. Moreover, the reasons for excluding the articles were recorded at each step.
Data extraction
Two authors (BZ.W and MS.O) abstracted the relevant data independently by using a standardized Microsoft Excel spreadsheet. For data extraction, Joana Briggs Institute (JBI)-adopted formats were employed (32). The first author’s name, sample characteristics, regions of study, year of publication, study design, study area, outcome measures, timing and procedures of data collection, response rates, knowledge of and attitudes toward epilepsy were collected.
The reliability agreement among the data extractors was evaluated and verified using Cohan’s kappa coefficient after data was recovered from 30 percent of the primary studies. As a consequence, the kappa coefficient’s strength of agreement was divided into four categories: low (0.20), fair (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80), and virtually perfect agreement (0.81–1) (33). A kappa statistic value of more than or equal to 0.5 was regarded as congruent and acceptable. In the case of disagreements between the two data extractors, a third author (BG.A) was involved in adjudicating unresolved disagreements through discussion and re-checking of the original articles.
Outcome measurement
The number of adult patients who had OIs was divided by the total number of adults taking ART and multiplied by one hundred to calculate the pooled prevalence of OIs among adults after the initiation of ART. The pooled effect for associated factors was investigated using the OR. Furthermore, variables identified as risk factors for OIs in at least three studies were taken into account.
Definition of terms
Authors of primary studies classified adherence to ART as good, fair, or poor using the definition from the national HIV care follow-up form. Good adherence was defined as taking more than 95% of prescribed doses, fair adherence as taking 85–94% of prescribed doses, and poor adherence as taking less than 85% of prescribed doses as documented in patient records. Nutritional status was also based on national HIV care and treatment guidelines, and a body mass index of less than 18.5 kg/m2 was a cut-off point for undernutrition irrespective of its severity. Furthermore, for adolescents and adults, advanced HIV diseases were defined as CD4 cell counts of 200 cells/mm3 or a WHO stage III or IV event (20).
Methodological quality (risk of bias) assessment
To assess the quality of the studies, the Joana Briggs Institute (JBI) critical appraisal checklists for descriptive cross-sectional studies comprised of 8 components (Additional file_ 3A), analytic cross-sectional comprised of 7 components (Additional file_3B), cohort studies comprised of 11 components (Additional file_3C), and case–control studies comprised of 10 components (Additional file_3D) were employed. Three authors (ZZ, BG. A, AA. K, and MS.O) independently assessed the methodological quality of each study. Disagreements were resolved through consultation with a third independent reviewer (BZ.W). Studies with a score of 7 or higher after being evaluated against these criteria were considered low risk and included in this systematic review and meta-analysis.
Data synthesis and meta-analysis
The extracted data were imported from a Microsoft Excel spreadsheet into STATA MP 16 statistical software (StataCorp LP, 4905 Lakeway Drive, College Station, TX 7845, United States) for analysis. The heterogeneity of the results was visually examined via the forest plots with pooled estimates. Thus, its presence was confirmed subjectively with a lack of overlap between the CI.
In addition, the statistical heterogeneity was explored more formally by using Cochran’s Q test (x2) at p-value <0.10 indicating significant heterogeneity. Another heterogeneity measure, Higgins and Thompson’s I2 statistics, was employed to estimate the percentages of the between-study variability where,0%,25 to50%,50 to 75%, and greater than or equal to75% indicated no heterogeneity, low heterogeneity, moderate heterogeneity, and high heterogeneity, respectively, (29).
The random-effect meta-analysis model was used to estimate Der Simonian and Laird’s pooled effect due to the presence of considerable statistical heterogeneity. Subgroup meta-analysis based on the study regions as covariates, meta-regression, and sensitivity analyses were also performed to investigate the source of statistical heterogeneity. Publication or dissemination bias was examined subjectively using funnel plots and objectively using the nonparametric rank correlation test of Begg (34) and the regression-based test of Egger for small study effects (35), with p < 0.05 being taken into consideration to declare potential publication bias. Results were presented in the form of tables, texts, and figures.
Result
Search and study selection
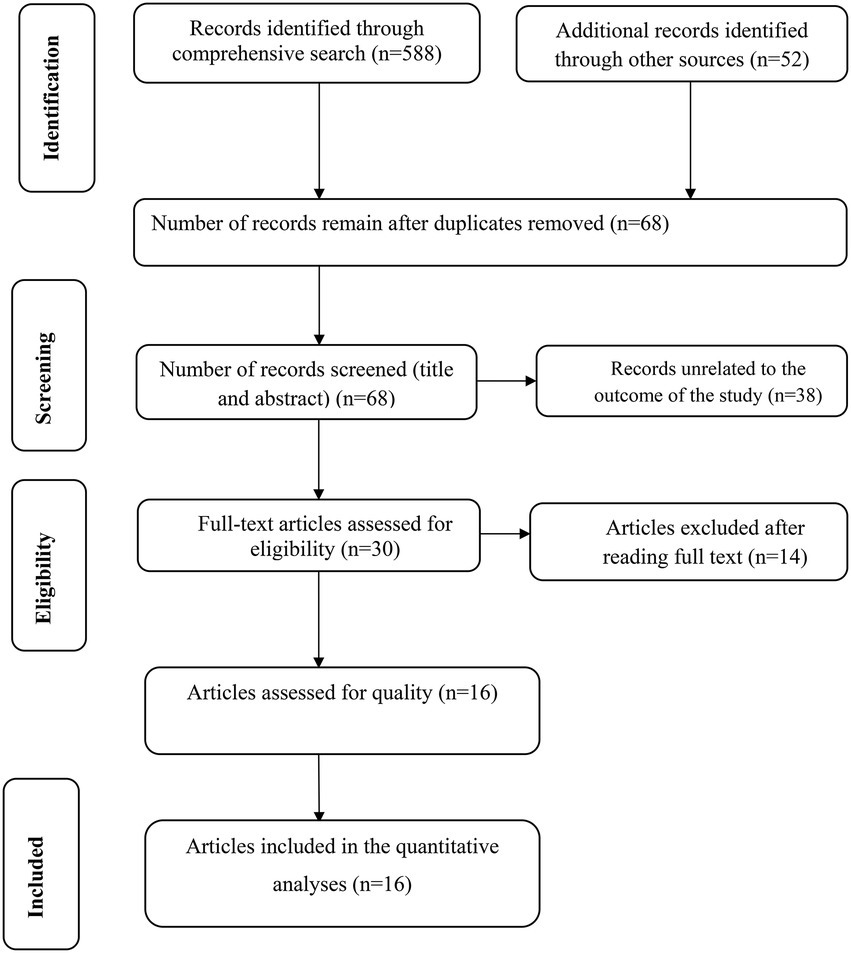
Our search was restricted to articles published in the English language between 1st January 2010 and February 30th 2022 in the electronic databases of PubMed, Web of Science, and EMBASE. In addition, Google, Google Scholar, and AJOL were searched. Through systematic and manual searching, 640 primary articles were found. Due to duplication, 572 articles were removed. The remaining 68 were screened based on their title and abstract, with 38 being eliminated as unrelated to our study. Finally, 30 full-text primary articles were evaluated against eligibility criteria, and 16 were selected for quantitative analysis (Figure 1).
Study characteristics
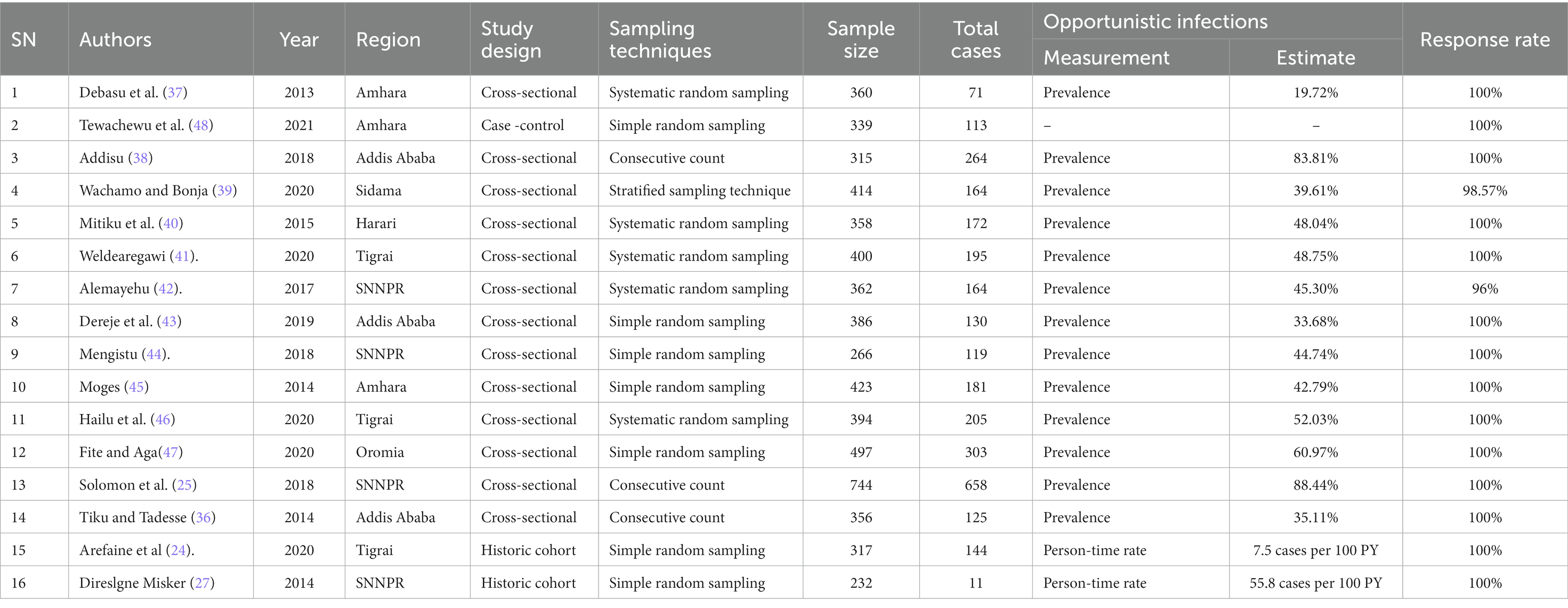
This systematic review and meta-analysis included 16 studies involving 6,163 participants, with a response rate ranging from 96 to 100%. More than 50% (n = 3,558) of the participants were female. Thirteen studies employed a cross-sectional study design (25, 36–47). The remaining two were cohort studies (24, 27), and one was a case–control study (48). All studies were conducted from 2013 to 2021 in different geographic locations in Ethiopia. Four of the primary studies included were based in Ethiopia’s Southern Nations Nationalities Peoples’ Regional States (SNNPRs) (25, 27, 42, 44), three in Amhara (37, 45, 48), three in Addis Ababa (36, 38, 43), three in Tigrai (24, 41, 46), one in Oromia (47), one in Sidama (39), and one in Harari (40). The highest (88.44%) (25) and the lowest (19.72%) (37) Prevalence of OIs was reported in studies conducted in Tercha Hospital, southern Ethiopia, and Gonder University Hospital, northern Ethiopia, respectively (Table 1).
Furthermore, about 13 primary studies assessed the spectrum of OIs (24, 25, 36–45, 47).
In terms of sampling techniques, systematic random sampling techniques (37, 40–42), simple random sampling techniques (24, 27, 43–45, 47, 48), combined stratified and simple sampling techniques (39), and consecutive count (25, 36, 38) were used to select the required study subjects. Furthermore, the authors of these studies employed a structured questionnaire, and or pre-tested and refined checklists to obtain necessary data. A complete summary of descriptive characteristics of all included primary studies is provided (see Additional file 4).
Prevalence of opportunistic infections
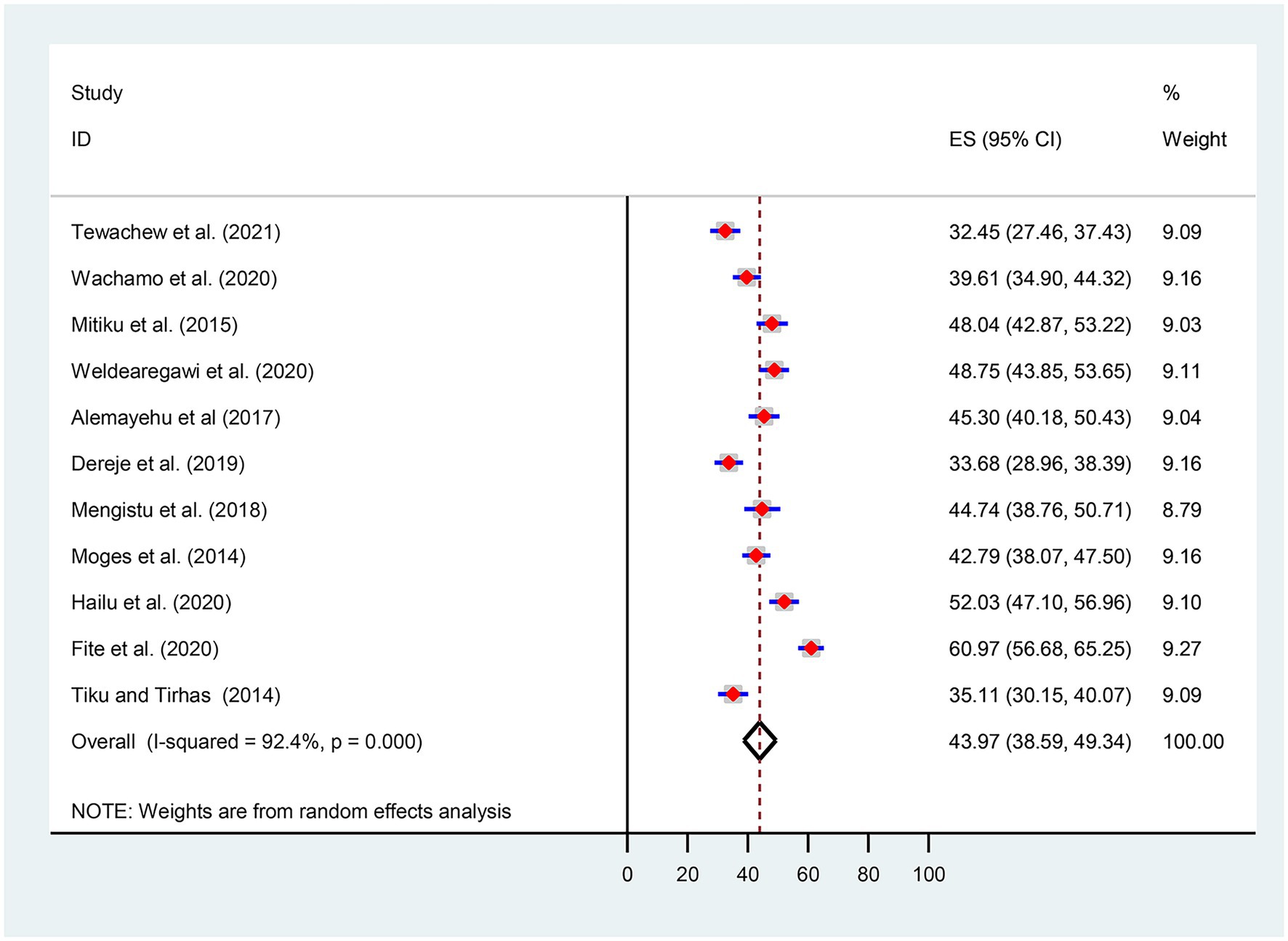
Given the substantial statistical heterogeneity in the fixed-effects model, the pooled estimate was determined using a random-effects model. Thus, an overall pooled prevalence of OIs among HIV-infected adult patients on ART in Ethiopia was found to be 43.97% [(95% CI, 38.59, 49.34), I2 = 92.4%, p < 0.001, n = 4,195] (Figure 2).
Subgroup meta-analysis
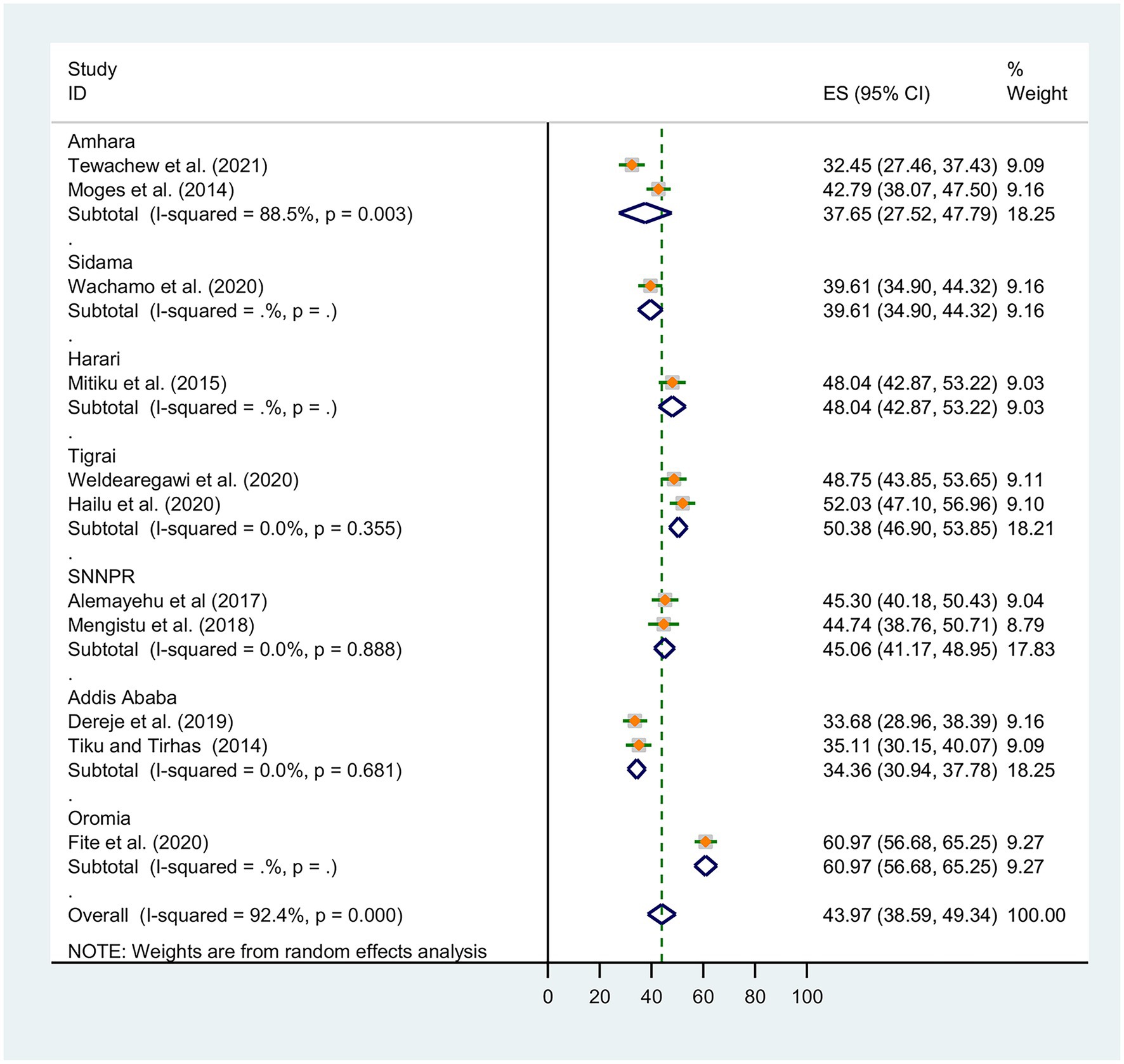
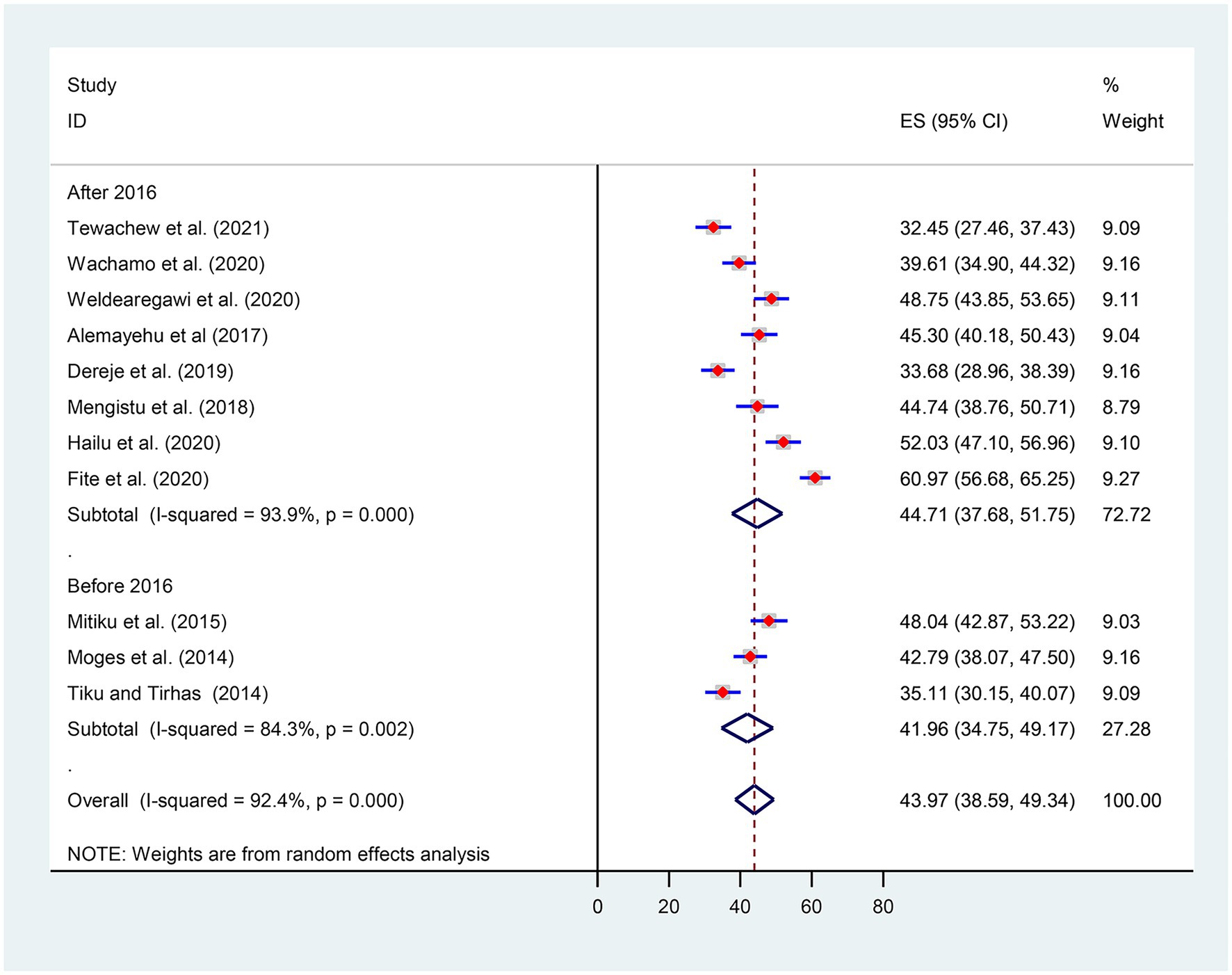
Subgroup analysis was conducted based on Ethiopia’s administrative regions where primary studies were based, and the year of publication. Thus, we observed regional variations in the prevalence of OIs in this review. The prevalence of OIs was found to range between 31.36% [(95% CI: 30.94, 37.78), I2 = 0.0%, p < 0.0681, n = 762] in random effects pooled meta-analysis in Amhara region and 60.97% (n = 497) in Ethiopia’s Oromia region with single study (Figure 3).
Considering the introduction of the WHO test and treatment recommendation, we also conducted a subgroup analysis by categorizing it before and after 2016. Thus, the pooled prevalence of OIs after the test and treatment recommendation was found to be 44.71% [(95% CI: 37.68, 51.75), I2 = 93.9%, p < 0.001, n = 3,058] (Figure 4).
Meta-regression
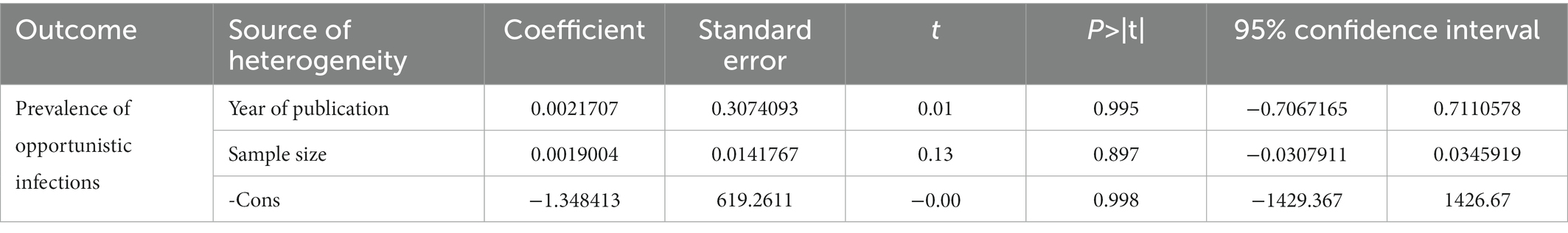
Random-effects meta-regression using sample size and year of publication as covariates was performed to explore the source of heterogeneity at a 5% significance level. As shown in Table 2, these covariates were not found to be the source of heterogeneity.
Sensitivity meta-analyses
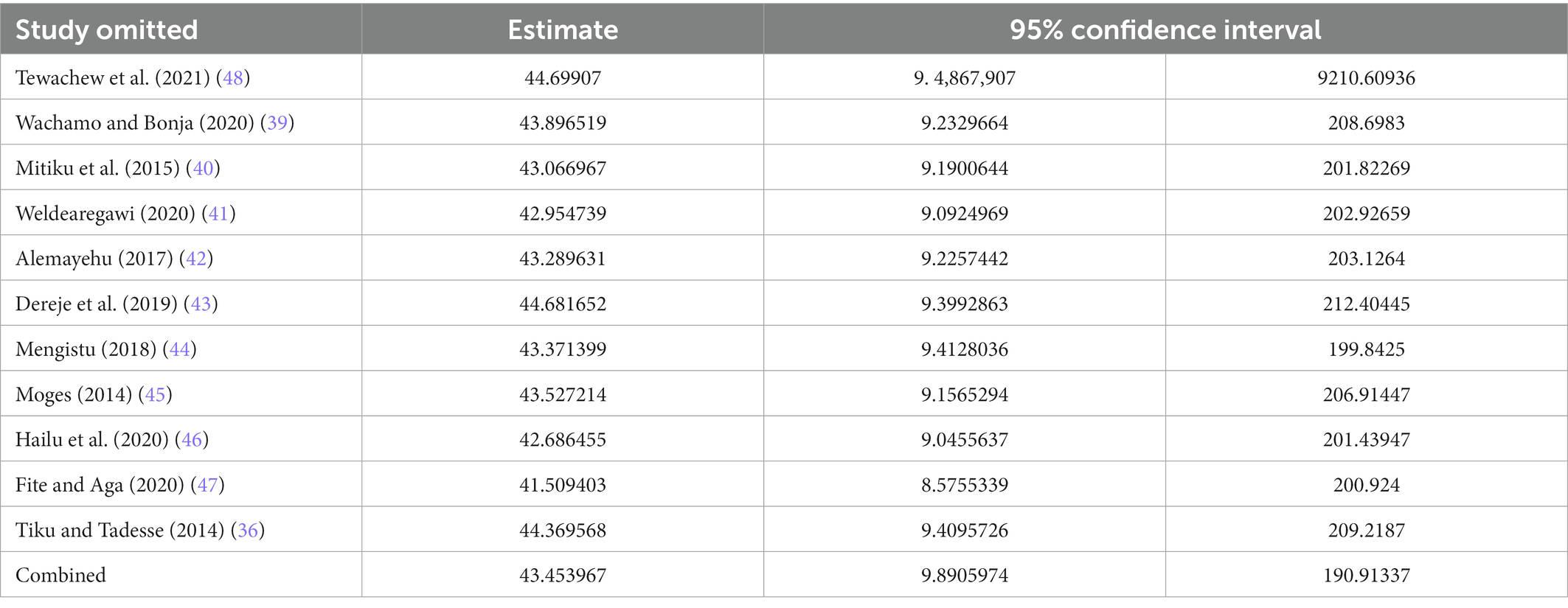
We conducted a leave-out-one meta-analysis to explore the effect of each study on the overall prevalence of OIs while gradually excluding each study. Results showed that the combined prevalence of OIs did not significantly change as a result of the excluded study (Table 3).
Publication bias (reporting bias)
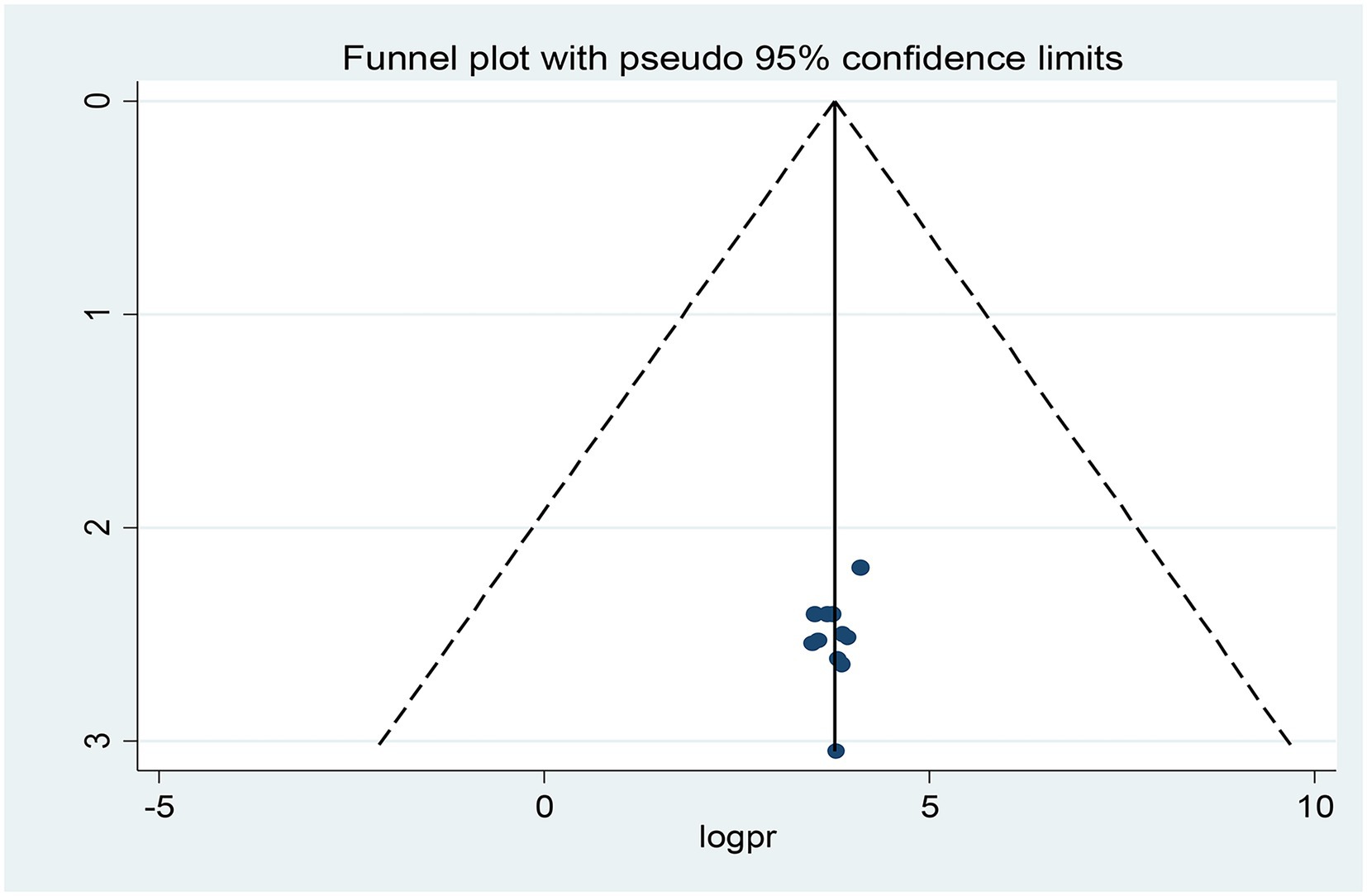
Publication bias was assessed subjectively using a funnel plot and objectively by the regression-based test of Egger and the nonparametric rank correlation test of Begg at p < 0.05. Looking at the funnel plot (Figure 5), the number of studies on the left and right sides of the plot is the same; thus, the plot is symmetrical, indicating that no publication bias was detected.
Moreover, neither Egger’s linear regression test (t = −0.67, p = 0.517) nor Begg’s rank correlation test (z = 0.16, p = 0.876) was statistically significant for a pooled prevalence of opportunistic infections, corroborating that there is no evidence of small study effects.
The spectrum of opportunistic infections
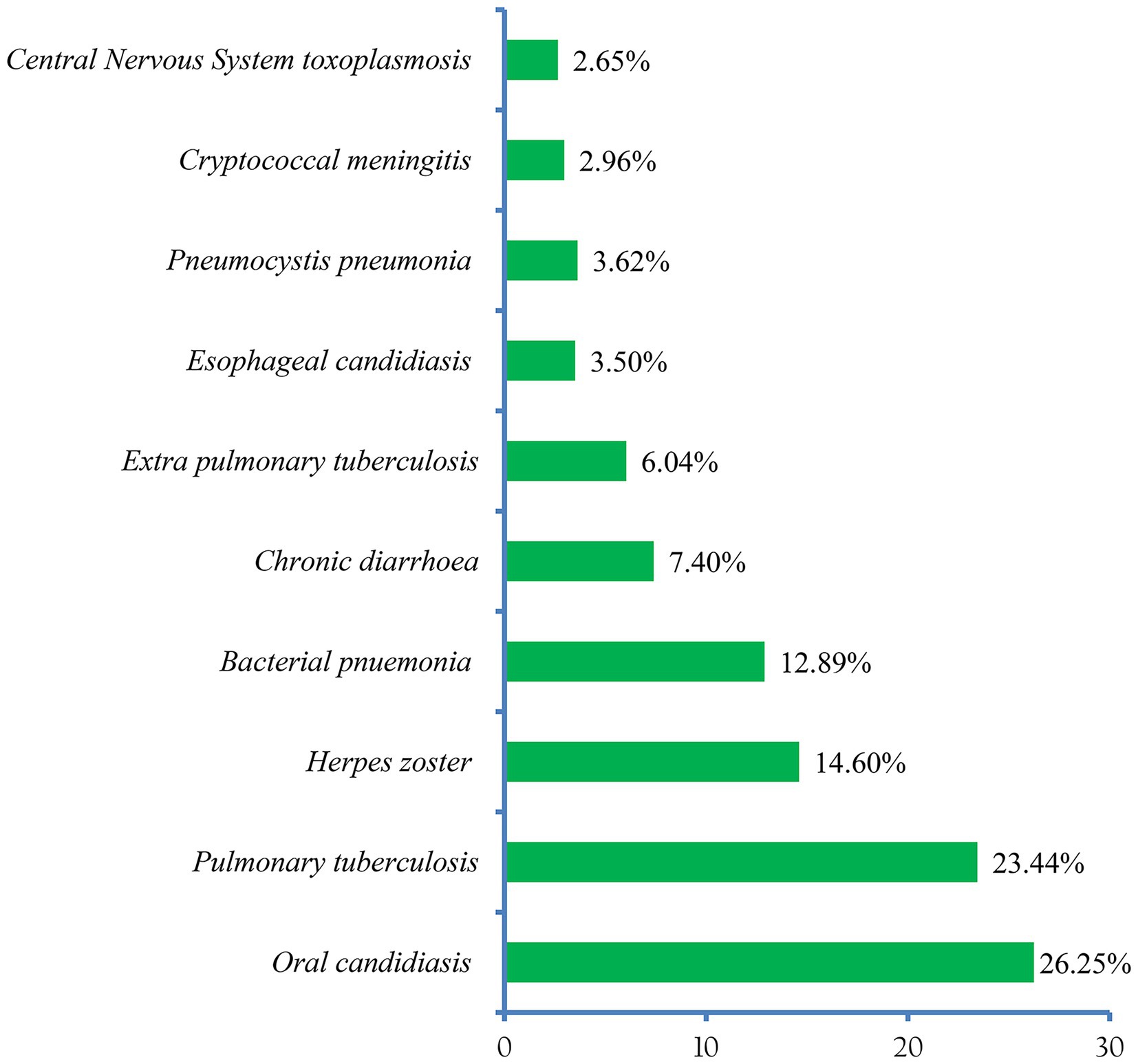
About 13 (81.25% of the included studies) in this review reported the patterns of OIs (24, 25, 36–45, 47). Accordingly, oral candidiasis (25.31%) was the most prevalent OI among HIV-infected adults after the initiation of ART. Pulmonary tuberculosis (22.04%), and herpes zoster (14.95%) ranked second and third, respectively (Figure 6).
Factors associated with the development of opportunistic infections
About 15 primary studies included in this meta-analysis have reported various factors as determinants of OIs among HIV-infected adult patients who were taking antiretroviral therapy in Ethiopia (24, 25, 27, 36–41, 43–48). We identified five predictor variables out of seven extracted variables, each reported by at least three primary studies, that were found to be associated with the development of OIs. These include level of adherence to ART, body mass index, baseline CD4 T lymphocytes count, and WHO HIV clinical stage. The pooled OR was used to estimate the association between these variables and the occurrence of OIs, and a statistically significant association between the outcome and predictor variables was declared at two-sided p < 0.05 with 95% CI.
To begin with, the odds of developing OIs were approximately 6 times {OR, 5.90 [95% CI (3.05, 11.40), p < 0.001, I2 = 76.4%]} higher in adult PLHIV who had a good level of adherence to ART compared to fair and good adherents. Additionally, the odds of developing OIs were 3.7 times higher in adult PLHIV with under nutrition than in adult PLHIV with normal body mass index {OR, 3.70 [95% CI (2.01, 6.80), p < 0.001, I2 = 71.4%]}. In this meta-analysis, it was also found that the odds of OIs occurring was 3.23 times [OR, 3.23 95% CI (2.06, 5.07), p < 0.001, I2 = 79.8%] more likely in PLHIV who had baseline CD4 T lymphocytes less than 200 cells per microliter compared to their counterparts. Moreover, the odds of exhibiting OIs were approximately 5 times [OR, 4.84 95% CI (1.83, 12.82), p = 0.002, I2 = 89.5%] more likely in adult PLHIV who had presented with advanced WHO HIV clinical stage at enrollment to the program (Table 4).
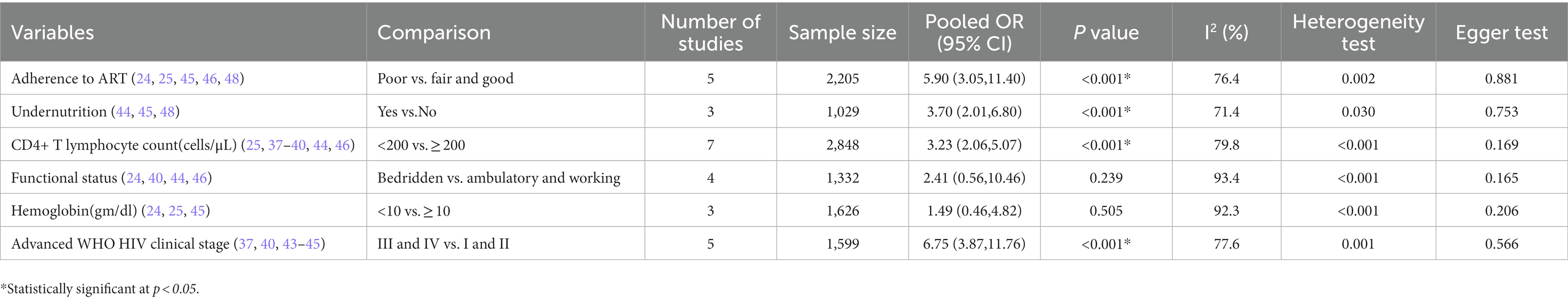
Table 4. Factors associated with the development of OIs among adults after initiation of ART in Ethiopia.
Discussion
In this systematic review and meta-analysis, we sought to estimate the pooled prevalence of OIs and identify potential predictors among adult PLHIV after initiation of ART in Ethiopia. The overall random effects meta-analysis revealed that the pooled prevalence of OIs was 43.97% (95% CI: 38.59, 49.34). This finding was by far higher than OIs prevalence in Owerri, Imo State, South East Nigeria(22.4%) (16), Indonesia(33.51%) (17), and Uganda (6.7%) (49). This can be explained by the study design, and sample size. Another possible explanation could be due to a disparity in the level of engagement in HIV care in these countries.
In terms of spectrum and magnitude, the six most common OIs identified in this review were: oral candidiasis (25.31%), pulmonary tuberculosis (22.04%), herpes zoster (14.95%), bacterial pneumonia (12.50%), chronic diarrhea (6.66%), and extrapulmonary tuberculosis (5.49%). The pattern differs from a previous national cross-sectional study that examined HIV patients’ medical records to determine the prevalence of OIs in selected Ethiopian health facilities over 1 year (2003–2004) (50). This could be due to the latter involving both pre-ART and PLHIV receiving ART.
Furthermore, the study period may have an impact on the apparent findings. However, it is congruent with the study in Indonesia (51), where tuberculosis, candidiasis, and chronic diarrhea were the major causes of morbidity. In addition, the pattern is not different from a review finding in LMIC, where Oral candidiasis, unspecified tuberculosis, herpes zoster, pulmonary tuberculosis, and bacterial pneumonia were the most common OIs in ART-naive participants (52).
In this analysis, we also found that poor adherence to ART, under nutrition, CD4 T lymphocyte counts of less than 200 cells per microliter, and presentation with advanced WHO HIV clinical stages were potential determinants of OIs among adult PLHIV after the commencement of ART. To begin with, adult PLHIV who were poorly adherent to ART had a strong risk of developing OIs. This finding was congruent with other study findings from a west African country, Nigeria (53). This is because poor adherence to ART reduces the effectiveness of ARV drugs while also accelerating viral replication and immune suppression, creating a favorable environment for the development of OIs.
Being under nourished was another determinant factor that affects the accusation of OIs among adult PLHIV. This finding was in agreement with previous studies in KwaZulu-Natal, South Africa, where low body mass index was associated with morbidity and mortality among HIV patients (54). This could be due to a complex and mutually reinforcing relationship between malnutrition and HIV infection. Furthermore, malnutrition can result from HIV-induced immune impairment and the resulting OIs can cause poor appetite and nutrition absorption from the gastrointestinal.
Moreover, PLHIV whose baseline CD4 T lymphocytes measured less than 200 cells per microliter had three times higher odds of developing OIs compared to their counterparts. This finding is concordant with a study in India, which was conducted among AIDS patients in Mangalore, Karnataka, and found that patients with CD4 counts of 200 cells/mm3 were at a high risk of developing an advanced form of OIs such as tuberculosis, Pneumocystis jiroveci pneumo-nia, and cryptococcal meningitis (55). Another study by Ghate et al., carried out to identify OIs among HIV-infected individuals by stages of immunodeficiency, revealed that PLHIV with baseline CD4 counts of less than 200 cells per microliter were six times more likely to develop OIs (56). This finding appears to be correct because CD4 T lymphocytes play a detrimental role in the induction of both humoral and cellular immunity to combat OIs.
In this review, baseline advanced (III and IV) WHO HIV clinical stage was another potential determinant of OIs after the initiation of ART than stages I and II. Our finding was consistent with a report from Mulago-Mbarara teaching hospitals’ joint AIDS program, in Kampala, Uganda (49), Nigeria (57), and the South African cohort (58). This can be explained by the fact that the progression of HIV from infection through subtle symptoms to advanced HIV disease is correlated with prolonged immune suppression, which is partly a reflection of late presentation to health facilities. Besides, individuals with advanced HIV diseases are prone to acquiring multiple other OIs.
Limitations and strengths of this study
The limitations of this systematic review and meta-analysis need to be acknowledged. First, because there has been no previous nationally representative study, our findings make comparing trends in OIs prevalence difficult. Besides, some of the OIs were diagnosed only clinically due to limited diagnostic capacity in under-resourced country, which may overestimate or underestimate or introduce potential misclassification for some morbidity. Finally, due to the scarcity of primary studies, articles published prior to 5 years were included. Therefore, the results need to be interpreted with caution.
On the other hand, the current systematic review and meta-analysis has also strengths. The protocol for this study has been registered. More than seven online databases were searched to avoid missing published studies, including articles published in African journals. In addition, a manual search was performed to retrieve the article using Google Scholar. During the selection of articles, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 Checklist was strictly followed, and the articles were closely assessed for their quality using the newly amended JBI critical appraisal tool. Furthermore, we used broader inclusion criteria to ensure we did not miss articles.
Conclusion and recommendations
In summary, the pooled random effect meta-analysis revealed that the percentage of OIs among adult PLHIV after the commencement of ART is high. In the subgroup meta-analysis, regional variations in the prevalence were observed, with the highest in the Oromia region and the lowest in the Amhara region. Oral candidiasis, pulmonary tuberculosis, herpes zoster, bacterial pneumonia, chronic diarrhea, and extrapulmonary tuberculosis are the six major OIs. Moreover, poor adherence to ART, under nutrition, CD4 T lymphocyte counts of less than 200 cells per microliter, and presentation with advanced WHO HIV clinical stages were associated with the development of OIs.
Therefore, it is critical to provide intensified care for patients presenting with advanced HIV disease, lower immunologic status, and under nutrition which includes screening and prompt provision of chemoprophylaxis to reduce or avoid the risk of developing OIs after ART initiation. Furthermore, enhanced adherence counseling should be provided based on the patient’s adherence track records.
Author contributions
BW conceptualized the study, and prepared and registered the study protocol. MO, BW, BA, AK, and ZZ searched, screened articles, and were involved in the risk of bias assessment. MO and BW were involved in data abstraction, statistical analysis results interpretation, and writing the initial and final drafts of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank the authors of the primary studies included in this systematic review and meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1087086/full#supplementary-material
Abbreviations
AIDS, Acquired Immunodeficiency Syndrome; ART, Antiretroviral Therapy; CI, Confidence Interval; HIV, Human Immunodeficiency Virus; JBI, Johanna Briggs Institute; LMICs, Low-and Middle-Income countries; OIs, Opportunistic Infections; OR, Odds ratio; PLHIV, People Living with HIV; SNNPRs, Southern Nations Nationalities Peoples’ Regional State; WHO, World Health Organization.
Footnotes
References
1.Benson, C.A. Guidelines for prevention and treatment opportunistic infections in HIV-infected adults and adolescents; recommendations from CDC, the National Institutes of Health, and the HIV medicine association/Infectious Diseases Society of America. (2009) Centers for Disease Control (CDC).
2.Masur, H. Recurring and emerging questions related to management of HIV-related opportunistic infections. Topics Antiviral Med. (2018) 26:79–84.
3.The Joint United Nations Programme on HIV/AIDS (UNAIDS). (2022). Global HIV & AIDS statistics — Fact sheet. Available at: https://www.unaids.org/en/resources/fact-sheet.
4.Losina, E, and Freedberg, KA. Life expectancy in HIV. British Med J Publishing Group. (2011) 343:d6015. doi: 10.1136/bmj.d6015
5.Goud, T, and Ramesh, K. Opportunistic infections among HIV patients attending tertiary care hospital, Karnataka, India. Int J Curr Microbiol App Sci. (2014) 3:824–9.
6.IeDEA, T. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-and high-income countries. J Acquir Immune Defic Syndr. (2014) 65:e8:–e16. doi: 10.1097/QAI.0b013e3182a39979
7.Marshall, CS, Curtis, AJ, Spelman, T, O’Brien, DP, Greig, J, Shanks, L, et al. Impact of HIV-associated conditions on mortality in people commencing anti-retroviral therapy in resource limited settings. PLoS One. (2013) 8:e68445. doi: 10.1371/journal.pone.0068445
8.Collaboration, A.T.i.L.I.C. and A.C. Collaboration. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. (2006) 367:817–24. doi: 10.1016/S0140-6736(06)68337-2
9.Mirani, G, Williams, PL, Chernoff, M, Abzug, MJ, Levin, MJ, Seage GR 3rd,, et al. Changing trends in complications and mortality rates among US youth and young adults with HIV infection in the era of combination antiretroviral therapy. Clin Infect Dis. (2015) 61:1850–61. doi: 10.1093/cid/civ687
10.Palella, FJ Jr, Baker, RK, Moorman, AC, Chmiel, JS, Wood, KC, Brooks, JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. JAIDS J Acquired Immune Deficiency Syndromes. (2006) 43:27–34. doi: 10.1097/01.qai.0000233310.90484.16
11.Krentz, H, Dean, S, and Gill, M. Longitudinal assessment (1995–2003) of hospitalizations of HIV-infected patients within a geographical population in Canada. HIV Med. (2006) 7:457–66. doi: 10.1111/j.1468-1293.2006.00408.x
12.Rubaihayo, J, Tumwesigye, NM, Konde-Lule, J, Wamani, H, Nakku-Joloba, E, and Makumbi, F. Frequency and distribution patterns of opportunistic infections associated with HIV/AIDS in Uganda. BMC Res Notes. (2016) 9:501. doi: 10.1186/s13104-016-2317-7
13.Micah, AE, Cogswell, IE, Cunningham, B, Ezoe, S, Harle, AC, Maddison, ER, et al. Tracking development assistance for health and for COVID-19: a review of development assistance, government, out-of-pocket, and other private spending on health for 204 countries and territories, 1990–2050. Lancet. (2021) 398:1317–43. doi: 10.1016/S0140-6736(21)01258-7
14.Maartens, G. Opportunistic infections associated with HIV infection in Africa. Oral Dis. (2002) 8:76–9. doi: 10.1034/j.1601-0825.2002.00016.x
15.B-Lajoie, MR, Drouin, O, Bartlett, G, Nguyen, Q, Low, A, Gavriilidis, G, et al. Incidence and prevalence of opportunistic and other infections and the impact of antiretroviral therapy among HIV-infected children in Low- and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. (2016) 62:1586–94. doi: 10.1093/cid/ciw139
16.Iroezindu, MO. Prevalence and risk factors for opportunistic infections in HIV patients receiving antiretroviral therapy in a resource-limited setting in Nigeria. J AIDS Clinical Res. (2013) 01:S3. doi: 10.4172/2155-6113.S3-002
17.Asmarawati, TP. Opportunistic infection manifestation of HIV-AIDS patients in Airlangga university hospital Surabaya. IOP Conference Series: Earth and Environmental Science. (2018) 125:012061. doi: 10.1088/1755-1315/125/1/012061
18.MoH. Guidelines formanagement of opportunistic infections and anti retroviral treatment in adolescents and adults in Ethiopia. Federal HIV/AIDS prevention and control office. (2008) The Federal Democratic Republic of Ethiopia (FDRE), Ministry of Health of Ethiopia (MoHE).
19.Brooks, JT, Kaplan, JE, Holmes, KK, Benson, C, Pau, A, and Masur, H. HIV-associated opportunistic infections—going, going, but not gone: the continued need for prevention and treatment guidelines. Clin Infect Dis. (2009) 48:609–11. doi: 10.1086/596756
20.FMOH. National Guidelines for Comprehensive HIV prevention, care and treatment. (2018) The Federal Democratic Republic of Ethiopia (FDRE), Ministry of Health of Ethiopia (MoHE).
21.Bariha, P, Mohapatra, MK, Kullu, BK, Karua, PC, Biswal, SB, and Thakur, A. Prospective study of opportunistic infections among HIV infected patients in VSS Institute of Medical Science and Research, Burla, Sambalpur, Odisha, India. Int J Advances in Med. (2018) 5:566. doi: 10.18203/2349-3933.ijam20181980
22.UK Collaborative HIV Cohort (UK CHIC) Steering CommitteeSabin, CA, Schwenk, A, Johnson, MA, Gazzard, B, Fisher, M, et al. Late diagnosis in the HAART era: proposed common definitions and associations with mortality. AIDS (London, England). (2010) 24:723–7. doi: 10.1097/QAD.0b013e328333fa0f
23.Weissberg, D, Mubiru, F, Kambugu, A, Fehr, J, Kiragga, A, von Braun, A, et al. Ten years of antiretroviral therapy: incidences, patterns and risk factors of opportunistic infections in an urban Ugandan cohort. PLoS One. (2018) 13:e0206796. doi: 10.1371/journal.pone.0206796
24.Arefaine, ZG, Abebe, S, Bekele, E, Adem, A, Adama, Y, H. Brockmeyer, N, et al. Incidence and predictors of HIV related opportunistic infections after initiation of highly active antiretroviral therapy at Ayder referral hospital, Mekelle, Ethiopia: a retrospective single centered cohort study. PLoS One. (2020) 15:e0229757. doi: 10.1371/journal.pone.0229757
25.Solomon, FB, Angore, BN, Koyra, HC, Tufa, EG, Berheto, TM, and Admasu, M. Spectrum of opportunistic infections and associated factors among people living with HIV/AIDS in the era of highly active anti-retroviral treatment in Dawro zone hospital: a retrospective study. BMC Res Notes. (2018) 11:604. doi: 10.1186/s13104-018-3707-9
26.Dagnaw Tegegne, K, Cherie, N, Tadesse, F, Tilahun, L, Kassaw, MW, and Biset, G. Incidence and predictors of opportunistic infections among adult HIV infected patients on anti-retroviral therapy at Dessie comprehensive specialized hospital, Ethiopia: a retrospective follow-up study. HIV AIDS (Auckl). (2022) 14:195–206. doi: 10.2147/HIV.S346182
27.Direslgne Misker, MD. Habtamu Mellie effect of highly active antiretroviral therapy on incidence of opportunistic infections among HIV positive adults in public health facilities of Arba Minch town, South Ethiopia: retrospective cohort study. J AIDS Clinical Res. (2014) 05:1–6. doi: 10.4172/2155-6113.1000330
28.Zhou, J, Paton, NI, and Ditangco, R, The TREAT Asia HIV Observational Database. AIDS-defining illness diagnosed within 90 days after starting highly active antiretroviral therapy among patients from the TREAT Asia HIV observational database. Int J STD AIDS. (2007) 18:446–52. doi: 10.1258/095646207781147283
29.Shamseer, L, Moher, D, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 349:647. doi: 10.1136/bmj.g7647
30.Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10:1–11. doi: 10.1186/s13643-021-01626-4
31.Munn, Z, Moola, S, Lisy, K, Riitano, D, and Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. (2015) 13:147–53. doi: 10.1097/XEB.0000000000000054
32.Aromataris, E., and Munn, Z. (Eds.). JBI manual for evidence synthesis. JBI. (2020). Available at: https://synthesismanual.jbi.global.
33.Sim, J, and Wright, CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. (2005) 85:257–68. doi: 10.1093/ptj/85.3.257
34.Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
35.Egger, M, Smith, GD, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
36.Tiku, S, and Tadesse, T. Assessment of trends of HIV and its opportunistic infections in Ethiopian police settings. Ethiop J Heal Dev. (2014) 28:163–68.
37.Debasu Damtie, GY, Woldeyohannes, D, and Anagaw, B. Common opportunistic infections and their CD4 cell correlates among HIV-infected patients attending at antiretroviral therapy clinic of Gondar University hospital, Northwest Ethiopia. BMC Health Serv Res. (2013) 6:1–7. doi: 10.1186/1756-0500-6-534
38.Addisu Deribe, WE. Magnitude and determinants of opportunistic infections among HIV/AIDSPatients in Sphmmc, Addis Ababa. Ethiopia: Retrospective Study (2018).
39.Wachamo, D, and Bonja, F. Magnitude of opportunistic infections and associated factors among HIV-positive adults on ART at selected public hospitals in Sidama National Regional State. Southern Ethiopia HIV AIDS (Auckl). (2020) 12:479–87. doi: 10.2147/HIV.S265274
40.Mitiku, H, Weldegebreal, F, and Teklemariam, Z. Magnitude of opportunistic infections and associated factors in HIV-infected adults on antiretroviral therapy in eastern Ethiopia. HIV AIDS (Auckl). (2015) 7:137–44. doi: 10.2147/HIV.S79545
41.Weldearegawi, TZ. The magnitude of opportunistic infections and associated factors in HIV-infected adults on antiretroviral therapy in southern zone Tigray, Ethiopia: a cross-sectional study. Pan Afr Med J. (2020) 35:126. doi: 10.11604/pamj.2020.35.126.17839
42.Alemayehu, M. Opportunistic infections among HIV/AIDS patients taking anti-retroviral therapy at tertiary Care Hospital in Wolaita Zone, southern Ethiopia. J AIDS Clinical Res. (2017) 08:2–4. doi: 10.4172/2155-6113.1000665
43.Dereje, N, Moges, K, Nigatu, Y, and Holland, R. Prevalence and predictors of opportunistic infections among HIV positive adults on antiretroviral therapy (on-ART) versus pre-ART in Addis Ababa, Ethiopia: a comparative cross-sectional study. HIV AIDS (Auckl). (2019) 11:229–37. doi: 10.2147/HIV.S218213
44.Mengistu, D. Prevalence of opportunistic infections and associated factors among adult HIV Positivepatients on the new anti-retroviral treatment protocol at the health institutions offering art in Gedeo zone, southern, Ethiopia, 2018. (2018). AAU Institutional Repository School of Allied Health Science.
45.Moges, NA. Prevalence of opportunistic infections and associated factors among HIV positive patients taking anti-retroviral therapy in DebreMarkos referral hospital, Northwest Ethiopia. J AIDS Clinical Res. (2014) 05:1–6. doi: 10.4172/2155-6113.1000301
46.Hailu, T. H., Hailu, T., Hagos, H., Gemechu, K., Tesfay, H., and Tadesse, B. Prevalence of opportunistic infections and associated factors among HIV-infected patients onAntiretroviral therapy in eastern zone of Tigray, Ethiopia:A Cross-sectional Study. (2020).
47.Fite, MB, and Aga, DJ. Spectrum of opportunistic disease and associated factors among patients attending ART Clinic. Western Ethiopia: Nekemte Specialized Hospital (2020).
48.Tewachew, AS, Mekonnen, WN, Mekuria, AD, and Amare, YE. Determinants of opportunistic infections among HIV-positive patients on HAART in Debre Berhan referral hospital, north Shoa zone, Ethiopia, 2020: a case-control study. HIV AIDS (Auckl). (2021) 13:337–47. doi: 10.2147/HIV.S298661
49.Muhumuza, S, Ouma, J, and Semitala, F. Factors associated with development of opportunistic infections among patients on ART at a Ugandan program-MJAP. Retrovirology. (2010) 7:1. doi: 10.1186/1742-4690-7-S1-P77
50.Weldegebreal, T, Ahmed, I, Muhiye, A, Belete, S, Bekele, A, and Kaba, M. Magnitude of opportunistic diseases and their predictors among adult people living with HIV enrolled in care: national level cross sectional study, Ethiopia. BMC Public Health. (2018) 18:820. doi: 10.1186/s12889-018-5733-x
51.Rusli, R, and Setiawaty, V. The cases with opportunistic infections among HIV patients in Indonesia 2011. BMC Infect Dis. (2014) 14:14(S2). doi: 10.1186/1471-2334-14-S2-P45
52.Low, A, Gavriilidis, G, Larke, N, B-Lajoie, MR, Drouin, O, Stover, J, et al. Incidence of opportunistic infections and the impact of antiretroviral therapy among HIV-infected adults in Low- and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. (2016) 62:1595–603. doi: 10.1093/cid/ciw125
53.Salami, A, Olatunji, P, and Oluboyo, P. Spectrum and prognostic significance of opportunistic diseases in HIV/AIDS patients in Ilorin, Nigeria. West Afr J Med. (2006) 25:52–6. doi: 10.4314/wajm.v25i1.28245
54.Naidoo, K, Yende-Zuma, N, and Augustine, S. A retrospective cohort study of body mass index and survival in HIV infected patients with and without TB co-infection. Infect Dis Poverty. (2018) 7:35. doi: 10.1186/s40249-018-0418-3
55.Saldanha, D, Gupta, N, Shenoy, S, and Saralaya, V. Prevalence of opportunistic infections in AIDS patients in Mangalore. Karnataka Tropical doctor. (2008) 38:172–3. doi: 10.1258/td.2007.070171
56.Ghate, M, Deshpande, S, Tripathy, S, Nene, M, Gedam, P, Godbole, S, et al. Incidence of common opportunistic infections in HIV-infected individuals in Pune, India: analysis by stages of immunosuppression represented by CD4 counts. Int J Infect Dis. (2009) 13:e1–8. doi: 10.1016/j.ijid.2008.03.029
57.Iroezindu, M. Prevalence and risk factors for opportunistic infections in HIV patients receiving antiretroviral therapy in a resource-limited setting in Nigeria. J AIDs Clin Res. (2013) 3:002. doi: 10.4172/2155-6113.S3-002
Keywords: opportunistic infections, prevalence, HIV, Ethiopia, antiretroviral therapy, associated factors
Citation: Woldegeorgis BZ, Zekarias Z, Adem BG, Obsa MS and Kerbo AA (2023) Prevalence and determinants of opportunistic infections among HIV-infected adults receiving antiretroviral therapy in Ethiopia: A systematic review and meta-analysis. Front. Med. 10:1087086. doi: 10.3389/fmed.2023.1087086
Edited by:
Xiaogang Xiang, Shanghai Jiao Tong University, ChinaReviewed by:
Wondwossen Amogne Degu, Addis Ababa University, EthiopiaFikadu Tadesse Nigusso, World Food Programme, Italy
Copyright © 2023 Woldegeorgis, Zekarias, Adem, Obsa and Kerbo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beshada Zerfu Woldegeorgis, ✉ beshadazerfu@gmail.com
 Beshada Zerfu Woldegeorgis
Beshada Zerfu Woldegeorgis Zewdineh Zekarias2
Zewdineh Zekarias2  Bulcha Guye Adem
Bulcha Guye Adem Mohammed Suleiman Obsa
Mohammed Suleiman Obsa Amene Abebe Kerbo
Amene Abebe Kerbo