The Global Pregnancy Collaboration (CoLab) symposium on short- and long-term outcomes in offspring whose mothers had preeclampsia: A scoping review of clinical evidence
- 1Department of Family Medicine and Population Health Sciences, Wayne State University School of Medicine, Detroit, MI, United States
- 2Magee-Womens Research Institute, Pittsburgh, PA, United States
- 3Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh, Pittsburgh, PA, United States
- 4Group of Research and Innovation in Vascular Health, Chillán, Chile
- 5Vascular Physiology Laboratory, Department of Basic Sciences, Faculty of Sciences, University of Bío-Bío, Chillán, Chile
- 6Department of Obstetrics Gynecology and Reproductive Sciences, Epidemiology and Clinical and Translational Research, Magee-Womens Research Institute, University of Pittsburgh, Pittsburgh, PA, United States
Preeclampsia is a maternal syndrome characterized by the new onset of hypertension after 20 weeks of gestation associated with multisystemic complications leading to high maternal and fetal/neonatal morbidity and mortality. However, sequelae of preeclampsia may extend years after pregnancy in both mothers and their children. In addition to the long-term adverse cardiovascular effects of preeclampsia in the mother, observational studies have reported elevated risk of cardiovascular, metabolic, cerebral and cognitive complications in children born from women with preeclampsia. Less clear is whether the association between maternal preeclampsia and offspring sequelae are causal, or to what degree the associations might be driven by fetal factors including impaired growth and the health of its placenta. Our discussion of these complexities in the 2018 Global Pregnancy Collaboration annual meeting prompted us to write this review. We aimed to summarize the evidence of an association between maternal preeclampsia and neurobehavioral developmental disorders in offspring in hopes of generating greater research interest in this important topic.
Introduction
It has been recognized for many years that pregnancy complications are associated with acute and chronic morbidities in offspring. Historically this has been attributed to early delivery and the failure of the infant to reach its growth potential [i.e., fetal growth restriction (FGR)]. Both conditions can occur with preeclampsia, a maternal multisystemic disorder diagnosed by new onset hypertension with other systemic involvement. Therefore, it is difficult to unravel whether preeclampsia directly effects offspring (Figure 1). This is of more than theoretical importance. Determining a direct effect of preeclampsia on offspring could provide insight into the pathogenesis of developmental disorders and hopefully inform the development of directed therapy. This review describes the historical context and modern clinical evidence of adverse outcomes in offspring whose mothers had preeclampsia or other hypertensive disorders. We conclude by discussing the challenges to discriminate an independent effect.
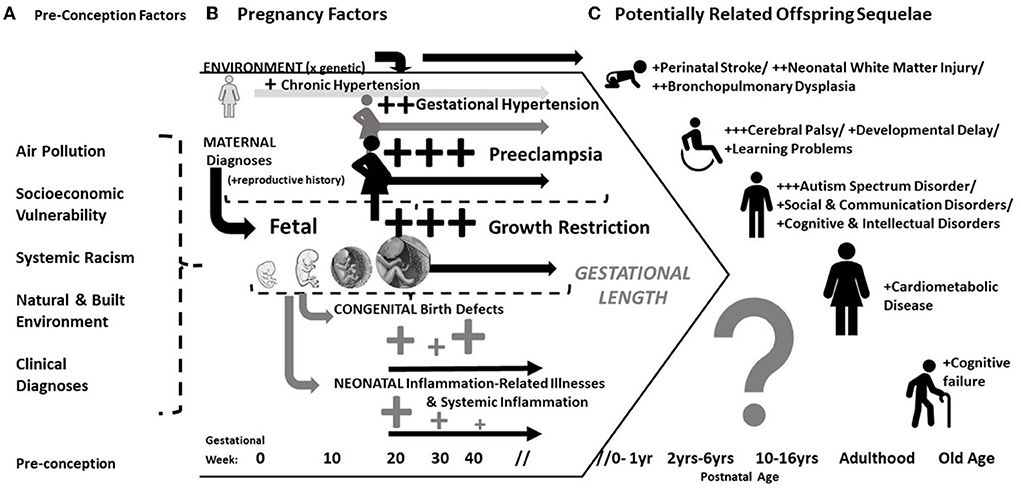
Figure 1. Conceptual model of the antecedents, correlates and potential sequelae in offspring of mothers with preeclampsia. (A) Pre-conception/environmental factors are associated hypertensive pregnancy disorders and developmental disorders; (B) Either by shared underlying mechanism or confounding, preeclampsia is often associated with fetal/placental diseases, congenital anomalies detected at birth, and downstream inflammation-related illnesses after birth; (C) Largely depending on the length of gestation (and therefore co-occurrence with fetal growth problems), preeclampsia is associated with heightened risks of multiple developmental disorders and also chronic cardiovascular disease.
Historical context of research linking pregnancy and developmental disorders
Prematurity (a.k.a., “dysmaturity”, “immaturity” and preterm delivery) and placental dysfunction
“Prematurity” was recognized as a key risk factor for perinatal mortality and developmental disorders by mid-19th century, along with birth complications and hypoxia (1–3). The earliest definitions were imprecise [e.g., gestation beyond 6 months but before “term” (4, 5)]. The 37 week cutoff was proposed in 1902 (6), but it was not widely used until decades later (5, 7). Early obstetricians defined “dysmaturity” as a lesser amount of subcutaneous fat (1907) or weight (1915) than expected at term (8). These criteria were quickly supplanted by the more easily measured 2,500 g birth weight criterion proposed in 1919 (9). In subsequent decades a reduced birth weight for gestation was linked with developmental disability. By 1940, a review concluded that “...children born preterm demonstrate developmental retardation and that the lower the birth weight the greater the retardation (10, 11)”. Thereafter, “dysmaturity” was recognized in children born preterm and it was interpreted as a sign of “placental insufficiency” or “placental dysfunction syndrome” (8, 12, 13).
Early suspected etiologic factors
Infection and inflammation
Pregnancy infections were identified as a potential cause of developmental disorders in the mid-nineteenth century. However, as early as 1895 Putnam remarked that “…the direct effects of infection are not to be feared more than the prostrating influences of the original struggle (p. 254)”. He proposed that infection and related processes (e.g., “edema”) can cause nervous system and cardiometabolic disorders because it “…joins hands with developmental or inherited tendencies, or accentuate(s) the habitual strains of life… (p. 255) (14)”. Pregnancy infection took on new meaning after 1941 when “German measles” was linked to congenital cataracts in children (15). During the 1950s−60s amniotic fluid infection syndrome was described (16) and there was speculation about microbial roots of intellectual disability (17). In 1973, evidence from autopsies prompted the inference that non-infectious inflammatory stimuli can also damage the developing brain (18). However, skepticism persisted prior to the arrival of modern microbiologic techniques.
Socioeconomic status, the importance of labor events and “other factors”
Social disadvantage was also implicated as an important risk factor for pregnancy and developmental disorders in children (11). In 1959, investigators surmised “…that there are positive and probably etiologic relationships between low socioeconomic status and prenatal and paranatal (sic) abnormalities which may in turn serve as precursors to retarded behavioral development, and to certain neuropsychiatric disorders of childhood such as cerebral palsy, epilepsy, mental deficiency and behavior disorder (19).” Social disadvantage was said to provide only a hint toward some yet unidentified mechanism. Therefore, in the late 1950s the Collaborative Perinatal Project (CPP) began to investigate: “(1) the conditions of the pregnancy itself, such as infection, trauma, drug reactions or the progress of labor. These include normal and abnormal physiology of pregnancy, labor and delivery, (2) the environmental factors—social and economic conditions, emotional stress, or medical care—influencing parents, (3) the biological factors—age, medical and reproductive history, immunologic characteristics—in parents, and (4) the genetic background of the parents (20)”.
Modern era
The CPP extended the focus of the causes of adverse outcomes for offspring from birth complications and toward intrauterine inflammation. Complexities arose as neonatal care improved in subsequent decades. As survival improved among successively earlier deliveries, postnatal morbidities were increasingly prevalent. The chronic disease era also emerged in epidemiology; with it came recognition of the implications of multicausality (21). That is, “Cause and effect no longer seem to bear a straight lineal relationship to each other. Circular mechanisms of positive and negative feedbacks have taken over in the operational depths of homeostasis. The chain of causation is fast dissolving before our eyes to be replaced by some form of invariable association that does not lend itself readily to a graphic, mathematical, or any other representation (22)”. Consequently, we are now tasked with determining whether preeclampsia and other pregnancy disorders are causally related to a mix of neonatal and long-term morbidities whose risk profiles overlap and often depend on gestational length. Unfortunately, while modern research has made great strides in describing underlying pathophysiology, this has not yet resulted in much progress developing interventions that substantially improve maternal and child health (23).
Association of preeclampsia with adverse offspring outcomes
Congenital abnormalities
Cardiovascular defects
Multiple studies report increased risk of birth defects with preeclampsia. However, most are not large enough to provide reliable information about specific diagnoses. In addition, criteria for preeclampsia diagnosis had changed over the years. Results also differ with early- or late-onset and pre-existing hypertension.
A large case-control study reported an elevated rate of preeclampsia in mothers of infants with cardiac defects (24). In Quebec, the risk of non-critical heart defects was elevated with preeclampsia [prevalence ratio (PR); 95% confidence interval (CI), 1.6; 1.5–1.7], after excluding stillbirths and children with chromosomal anomalies (25). Compared to late-onset, early-onset preeclampsia (<34 weeks) was associated with higher risks of both non-critical and critical heart defects (PR; 95%CI, 2.8; 1.7–4.5 and 5.6; 5–6, respectively). A Norwegian population-based study that excluded women with chronic hypertension also found children of preeclamptic pregnancies were two-fold more likely to have severe congenital heart defects than their peers whose mothers did not have preeclampsia (26). With early onset (<34 weeks), mild and severe preeclampsia were associated with two- and six-fold increased risks, respectively. By contrast, the risk of severe cardiac defects was elevated by 70% only with the severe form of late-onset preeclampsia.
At least two large studies found different risks of cardiac defects between chronic hypertension and preeclampsia superimposed onto chronic hypertension. The WHO Multi-Country Survey of 359 facilities across 29 countries reported two-fold elevated risk of cardiac defects with preeclampsia. By contrast, the risks of central nervous system, renal, limb, lip and cleft palate defects were elevated with preeclampsia superimposed onto chronic hypertension [odds ratios (ORs) > 4] (27). The Slone Birth Defects Study found four-to-six-fold increased risks of ventricular septal defects and atrial septal defects with preeclampsia superimposed on chronic hypertension (28).
Microcephaly
The relationship between preeclampsia or gestational hypertension and microcephaly is not clear. Some studies report an increased risk with preeclampsia (29). While the Helsinki birth cohort study found a reduced mean head circumference in children of preeclamptic mothers, children of mothers with gestational hypertension had increased head circumference relative to body length (30). A large extremely preterm birth cohort study found that children born severely fetal growth restricted, most of whose mothers had preeclampsia, had more than ten-fold higher risk of microcephaly than their unexposed peers (31). However, preeclampsia was not examined separately due to extensive overlap with FGR.
Neonatal and early childhood outcomes
Mortality
The risk of perinatal mortality is increased with preeclampsia and severe gestational hypertension. Yet, a reduced risk was reported with mild hypertension. Gestational age and birth weight appear to moderate these relationships.
The risks of neonatal mortality (32) and infant death (33) were nearly doubled in recent studies of children of preeclamptic mothers. However, a so-called “protective” relationship was observed among the subset born preterm or with very low birth weight (34). A US population-based study of 57 million singleton live births and stillbirths at 24–46 weeks' gestation found that gestational hypertension was associated with a 30% increased risk of neonatal death. By contrast, a recent systematic review reported reduced odds of mortality in infants exposed to gestational hypertension (35). However, mortality risk doubled with severe hypertension and it was increased by 40% with preeclampsia based on two studies of very preterm births.
The WHO Antenatal Care Trial examined early childhood outcomes separately for preeclampsia, gestational hypertension and “unexplained FGR” (i.e., birth weight < 10th centile for gestation not associated with maternal smoking, maternal undernutrition, preeclampsia, gestational hypertension, or congenital malformations) (36). Unadjusted models showed nearly five-fold increased risk of neonatal death with preeclampsia, but no relationship with gestational hypertension. However, adjustment for study site, treatment group, socioeconomic status and non-linear birth weight resulted in non-significant associations. By contrast, adjusting only for study site and socioeconomic status, both associations were statistically significant [adjusted (a) OR; 95% CI, 4.1; 2.5–6.6 and 1.8; 1.2–2.8, respectively]. Hence, gestational age and birth weight were mediators. The risks of neonatal mortality were also increased five-fold with FGR secondary to preeclampsia and three-fold with FGR secondary to gestational hypertension, adjusting for study site, treatment group and socioeconomic status.
Perinatal stroke
The rate of perinatal stroke is elevated with preeclampsia, gestational hypertension and FGR. A meta-analysis of 11 studies associated preeclampsia with two-fold increased risk of perinatal stroke (37), consistent with findings from subsequent studies of term gestations (38). Larger studies reported a stronger magnitude of association. In a case-control study nested within a cohort of 199,176 births, mothers of children who suffered perinatal stroke had preeclampsia five times more often than mothers of children who without stroke. (39). In a separate multivariable model, preeclampsia and FGR were each linked with three-to-five-fold increased odds (40). Ischemic stroke also occurs more frequently in infants exposed to gestational hypertension (41).
Neonatal encephalopathy and white matter irregularities in children born preterm
The rate of neonatal encephalopathy, an important risk factor for perinatal stroke (42), is increased six-fold with preeclampsia (43). One study of children born extremely preterm examined maternal and fetal conditions separately. The risk of neurosonographic ventriculomegaly or echolucent lesion was nearly three-fold higher among children delivered for fetal indication without preeclampsia compared to their peers whose mothers had preeclampsia (44). Recent preliminary evidence hints at altered cerebral vessel calibers and increased regional brain volumes in children of preeclamptic mothers compared to a control group whose mothers had normal pregnancies that was matched on gestational age at delivery, sex and age at examination (45).
Bronchopulmonary dysplasia
BPD occurs more frequently in children of preeclamptic mothers only with preterm delivery. FGR and pre-existing hypertension seem to moderate the relationship.
The risk of BPD was increased with exposure to preeclampsia in multiple studies of different intervals of preterm delivery (e.g., <28 or <32 weeks) (46–49). By contrast, a very low birth weight cohort study reported BPD odds were decreased by 20% with preeclampsia. However, post-hoc analysis indicated the protective association occurred only for more physiologically mature children (50). A more recent study of 247 children born of preeclamptic pregnancies at 24– <32 weeks of gestation found four-fold increased BPD risk with preeclampsia, adjusting for nulliparity, prolonged preterm pre-labor ruptured membranes, gestational age at birth and fetal sex (51). Yet, the relationship was no longer statistically significant with additional adjustment for other intermediates: antenatal corticosteroids, birth weight Z-score, mode of delivery, respiratory distress syndrome, invasive ventilation, admission of postnatal corticosteroids, clinical or proven sepsis, treatment of a persistent ductus arteriosus.
A 2018 meta-analysis reported increased BPD risk among children born prior to 29 weeks whose mothers had gestational hypertension (35). This is consistent with an international cohort study that associated gestational hypertension with BPD risk increased by 20%, adjusting for maternal age, multiple births, sex, gestational age, birth weight z-score, antenatal steroids, mode of delivery and network (52).
In contrast to the above studies, at least one showed a protective association between chronic hypertension and BPD among births at 30–34 weeks (46). However, another study of children born at 30–34 weeks whose mothers had preeclampsia found six-fold increased BPD risk with birth weight below the 10th centile for gestation, adjusting for gestational age and other potential confounders (53).
Neurodevelopmental and mental health disorders
Cerebral palsy
Preeclampsia and gestational hypertension are associated with increased CP risk, but gestational age at delivery and FGR moderate these relationships. A US study of 122,476 mother child pairs attempted to disentangle the complex relationships between preeclampsia, preterm birth and CP (54). Children of preeclamptic mothers had two-fold increased risk of CP; the earlier the diagnosis the stronger the association. However, there was no relationship between CP and term preeclampsia. By contrast, another study of children born at term associated preeclampsia with increased CP risk, but only when the child was small for gestational age (SGA) (55). A subsequent Israeli population-based study associated preeclampsia with two-fold increased risk of CP (56). Both early onset preeclampsia (<34 weeks) and SGA were “independently” linked with nine-fold and three-fold increased CP risk, respectively. However, a more recent analysis from the same regional hospital associated severe but not mild preeclampsia with elevated CP risk (57).
An Australian reconstructed total population-cohort study reported that new onset hypertension during pregnancy irrespective of proteinuria was associated with two-to-nine-fold increased risk of CP among all deliveries on each week after 27 weeks of gestation (58). A recent analysis of the Taiwan National Health Insurance Research Database further showed that a combination of preeclampsia with maternal diabetes, fetal growth restriction or preterm delivery was associated with even higher risk than preeclampsia alone (59).
Autism spectrum disorder
Preeclampsia and gestational hypertension are associated with a small but statistically significant increase in ASD risk. Several meta-analyses report 30%-to-50% higher ASD risk in children of mothers with preeclampsia than their unexposed peers (60–63). The relationship is not specific because chronic and gestational hypertension were each associated with similarly elevated risks (OR range 1.37–1.48) (60). Another meta-analysis showed that multivariable adjustment rendered the association between gestational hypertension and ASD non-significant; however, this should be interpreted with caution because there was no statistical difference in the relationship between ASD and preeclampsia or gestational hypertension (p = 0.33), and thus the justification for separating the two disorders is unclear (64). In one of the largest studies to date, combined exposure to preeclampsia and birthweight two or more standard deviations below expected was associated with higher ASD risk than preeclampsia among children with higher birthweights (65). More recently, a combined cohort study of more than four million live-born singletons in Denmark during 1978–2012 and Sweden during 1987–2010 found the strongest associations between early-/severe-preeclampsia and ASD (hazard ratios ranged from 2-to-4) (66). A recent analysis of national health insurance data additionally associated the combination of preeclampsia with maternal diabetes, fetal growth restriction or preterm delivery with even higher risk than preeclampsia alone (59). The possibly that placental dysfunction and impaired fetal growth sensitizes offspring toward even higher risks of ASD that with exposure to preeclampsia alone is further supported by a Swedish population-based study of all singleton live births (65). A separate analysis further suggests that intergenerational exposure to preeclampsia conveys heightened risk of ASD (67).
Schizophrenia
Preeclampsia is associated with increased risk of schizophrenia in the offspring, but FGR might mediate or moderate the relationship. A meta-analysis of 11 studies reported 40% increased risk of schizophrenia in the children of mothers with hypertensive pregnancy disorders (68). An analysis of registry data from Norway similarly reported 30% elevated odds of schizophrenia with preeclampsia (69). By contrast, a separate population-based study linked preeclampsia with more than two-fold increased risk of schizophrenia (RR; 95% CI, 2.5; 1.4–4.5), independent of congenital anomalies (70). A Danish population-based case-control study also found three-fold higher incidence of preeclampsia in mothers of children with schizophrenia than among controls, adjusting for other pregnancy complications (e.g., maternal anemia, threatened preterm delivery, hemorrhage, sepsis), family psychiatric history, urbanicity of place of birth, maternal citizenship, paternal age, and parental wealth (71). Unadjusted models also showed that lowest quintile birth weight for gestation was associated with 60% greater incidence, unlike highest quintile birth weight, when each group is compared to their middle quintile birth weight peers. However, birth weight for gestation did not convey significant risk information in the full multivariable model.
Attention deficit hyperactivity disorder
ADHD risk is elevated in children of women with preeclampsia, but the magnitude of association differs after multivariable adjustment for potential confounders. A recent meta-analysis of ten studies found that preeclampsia and gestational hypertension were each significantly associated with 30% increased risk of ADHD in children (64). This is consistent with a large cohort and a nested case-control study that reported significant ORs ranging from 1.2-to-1.34 for ADHD (72, 73). Stronger associations were reported in the Avon Longitudinal Study of Parents and Children. Generalized estimating equation models that adjusted for multiple sets of potential confounders consistently found approximately three-fold higher risk of ADHD in children of preeclamptic mothers compared to children not exposed (74). By contrast, a recent study of combined singleton births from Denmark and Sweden reported a smaller but significant association that was driven by two-fold elevated risk of ADHD in children whose mothers had early-/severe-preeclampsia (66). Additional evidence supports the possibility that maternal diabetes, preterm delivery or FGR might modify the association between preeclampsia and ADHD in offspring (59), although exposure continues to convey increased risk at term gestation (75). Intriguingly, intergenerational exposure to preeclampsia might play a role in ADHD was well (67).
Epilepsy
The incidence of epilepsy in children was increased with exposure to maternal preeclampsia at term in a large US study; by 16% with mild preeclampsia, by 68% with post term preeclampsia, by 41% with severe term preeclampsia, and by nearly three-fold with severe post term preeclampsia (76). A study of 95,450 mother-child pairs also associated preeclampsia with 60% increased risk of epilepsy only when diagnosed at term (77); a subsequent population-based cohort study of term births adds further support (75).
Mental health problems
Findings about the relationship between preeclampsia and offspring mental health problems are mixed (68). One cohort study linked preeclampsia with significantly increased risk of offspring psychosis (78), whereas two case-control studies found no association (79, 80). The frequency of social problems was also increased in offspring of preeclamptic mothers who were born after 34 weeks (81).
In the Helsinki birth cohort, people whose primiparous mothers had preeclampsia had higher depressive symptom scores, but this was not the case with children of multiparous women (82). Interestingly when analyzed by fetal sex, preeclampsia was associated with 55% reduced risk of mental disorder among males (83). By contrast, gestational hypertension was associated with increased risk of depression and mood disorders, but not anxiety (78, 82, 83). Gestational hypertension has also been associated with internalizing morbidity, whereas preeclampsia was linked to reduced internalizing symptoms when examined separately (84).
Cognitive and intellectual disorders
Preeclampsia and gestational hypertension may be associated with mild cognitive and intellectual deficits, but FGR and pre-pregnancy body mass index might moderate these relationships.
Population-based studies have reported two-fold increased odds of mild cognitive limitations in offspring of mothers with gestational hypertension, adjusting for high pre-pregnancy body mass index, multiparity, low education, and male sex (85). Similar relationships were also observed in adults. A study of 17,457 Danish conscripts found a 34% higher prevalence of low cognitive function among men whose mothers had gestational hypertension compared to their peers whose mothers were normotensive (86). Yet, the adjusted prevalence ratios were elevated with mild preeclampsia and not with severe preeclampsia or eclampsia. On the other hand, the corresponding adjusted mean IQ differences were −2.0 (95% CI, −4.0 to 0.0), −3.2 (95% CI, −4.7, −1.8), and −2.0 (95% CI, −7.2 to 3.2), respectively (86). A more recent and larger study from Norway and Sweden reported four-fold higher risk of intellectual disability in the children of mothers with early-/severe- preeclampsia compared to their unexposed peers (double the magnitude of association between any hypertensive disorder low IQ) (66).
Even at age 70 years, people whose mothers had hypertensive disorders more frequently report subjective complaints of cognitive failures (87).
Adult men in the Helsinki Birth Cohort Study whose mothers were hypertensive had slightly reduced intellectual abilities compared to their unexposed peers; parity, socioeconomic status and preterm delivery modulated these relationships (88). An Icelandic population-based study recently reported reduced mathematics scores at ages 9, 12, and 15 years in children exposed to preeclampsia or eclampsia (89). The corresponding differences were 0.44 points (95% CI, <0.01–0.89), 0.59 points (95% CI, 0.13–1.06) and 0.59 points (95% CI, 0.08–1.10), respectively, adjusting for maternal citizenship, maternal age, maternal marital status, maternal occupation, parity, singleton birth, birth year, birth place, ADHD diagnosis, place of test administration, 5-min Apgar score, SGA, gestational age at birth and whether the child was ahead or behind a grade relative to peers of the same age. By contrast, an Australian study found no relationship between gestational hypertension and offspring cognitive development when accounting for pre-pregnancy maternal body mass index (90).
Small studies have reported worse verbal and full-scale intelligence scores at 3-to-8 years in FGR children whose mothers had preeclampsia (91, 92). In contrast to these findings, at least one study found no significant differences in IQ, school achievements, or neurodevelopmental scores at age 9–10 years among fetal growth restricted children exposed to maternal hypertensive disorders (n = 17 with maternal preeclampsia and n = 25 after gestational hypertension) compared to growth restricted children of normotensive mothers (n = 78) (93).
Developmental origins of adult disease
Many of the adverse outcomes recognized neonatally in infants of mothers with preeclampsia have lifelong ramifications. In addition, there are later life adverse outcomes not readily recognized at birth. Again, in many instances these have not been untangled from preterm birth and FGR.
Cardiometabolic and endocrine disorders
Children born at term whose mother had preeclampsia have 50%-to-60% increased incidence of endocrine, nutritional and metabolic diseases, and diseases of the blood and blood-forming organs (94). Several studies also linked preeclampsia with changes in offspring blood pressure, vascular function and body mass (95, 96). Indeed, evidence from a recent case-control study (97) supports the possibility that preeclampsia, even without co-occurring FGR, is associated with cardiac remodeling and dysfunction akin to what has been described in studies of late-onset FGR (98) in the pre- (99, 100)/neonatal (101, 102) periods and beyond (103).
A recent meta-analysis of data on 53,029 individuals in 36 studies associated preeclampsia with a ~5 mm Hg greater mean systolic, ~4 mm Hg greater mean diastolic blood pressure, and ~0.4 kg/m2 greater mean body mass index during childhood or young adulthood (104). There was no difference in offspring blood levels of cholesterol, triglycerides, glucose or fasting insulin. It is unclear if there are differences by subtype; e.g., a small Australian study found that only preeclampsia resulting in preterm birth was associated with three-fold increased risk of hypertension by 20 years of age (105).
An Israeli population-based cohort study of singletons that excluded mothers with chronic hypertension found a significant linear increase in the proportion of offspring who developed cardiovascular disease among women with no preeclampsia (0.24%), mild preeclampsia (0.33%), severe preeclampsia (0.51%) and eclampsia (2.73%) (106). Incidence rates were increased specifically for hypertension, arrhythmias and heart failure, unlike for cardiomyopathy and pulmonary heart disease. A similar trend was observed for offspring obesity (0.2, 0.4, 0.4, 1.4%; p < 0.001). Severe preeclampsia was associated with two-fold elevated risk of cardiovascular morbidity at term. However, in offspring born preterm, neither severe preeclampsia nor mild preeclampsia was associated with cardiovascular morbidity. In the long term follow up of the Helsinki study, individuals whose mothers had preeclampsia or gestational hypertension had 40%-to-90% increased risk of stroke, an association that persisted after adjustment for birthweight (30).
Several studies have found sex-differences in the relationship between preeclampsia and offspring blood pressure (107, 108). A large case-control study (145 girls and 283 boys whose mothers had preeclampsia and 12,701 girls and 20,416 boys whose mothers did not have preeclampsia) found that the risk of a systolic blood pressure > 140 mmHg at 17 years was increased by two-fold for girls, but there was no such relationship among boys adjusting for body mass index, weight and birthweight (109). Another study similarly found that adolescents exposed to preeclampsia had higher systolic blood pressure than children of normotensive mothers (115 vs. 113 mmHg, p = 0.03), but there was no difference in diastolic blood pressure (66 vs. 65 mmHg, p = 0.10); adjustment for maternal body mass and blood pressure attenuated the associations (110).
Potential sources of variation in prior studies relating preeclampsia to adverse offspring outcomes
Are preeclampsia and gestational hypertension different disorders?
There is overlap between preeclampsia and gestational hypertension that cannot currently be resolved. It is likely that some proportion of gestational hypertension has benign fetal outcomes. In other cases, gestational hypertension is unrecognized chronic hypertension masked by reduced blood pressure in early pregnancy (111, 112). Nonetheless, up to half of women with gestational hypertension eventually develop proteinuria or other end organ dysfunction and progression is most likely with onset prior to 32 gestational weeks (113, 114). Because gestational hypertension may raise the risk of some adverse outcomes [e.g., perinatal mortality (111, 115)], studies that fail to remove affected women from reference groups can derive spurious null associations. Early investigations tended to compare children of preeclamptic mothers to their unexposed peers whose mothers may have been hypertensive. More recently, outcomes of children whose mothers had different types of hypertensive disorders are compared to children of normotensive mothers (64). However, this is not yet universal.
Bias in small, single-center, and referral hospital studies
Women lumped together under the banner of preeclampsia in small studies can have appreciably different characteristics that affect their progeny's risk for adverse outcomes. Investigations performed in referral hospitals are especially susceptible to bias [i.e., Berkson's bias; see (116)]. For example, a study conducted in two NICUs in Northern and Southern China reported that children of preeclamptic mothers are at three-fold increased risk of retinopathy of prematurity and multivariable adjustment for potential confounders strengthened the relationship (117). Yet, separate analyses in each hospital revealed opposite relationships, adjusting for gestational age and birth weight. Residual bias presumably from unaccounted confounders probably explains the discrepancy.
Bias in low birth weight cohort studies
Because birth weight is tightly linked with physiologic maturation, low birth weight cohort studies tend to over-select growth restricted newborns who are more mature than their peers (118). This can distort relationships between preeclampsia and offspring outcomes that are associated with preterm delivery. For example, a post-hoc analysis found that a protective association between preeclampsia and BPD in a very low birth weight cohort occurred only in children who were more physiologically mature than their peers (50). Additional support comes from a study of gestational hypertension and CP performed in a low birthweight cohort and a preterm birth cohort both sampled from a Western Australian register (119). A strong protective association was observed when infants were separated by birthweight that was not present with sampling by gestational age.
Multi-center studies that enroll participants based on gestational age rather than birthweight are preferable, but this alone may not fully resolve potential bias (120, 121). Additional co-factors typically need to be considered as potential confounders. That is, the earlier the preterm delivery, the greater the number of developmental disorder risk factors. Therefore, investigators typically need to account for contributions from these other risk factors to estimate an association between preeclampsia and developmental disorders in preterm birth cohorts.
The so-called protective relationship between preeclampsia and adverse outcomes
Protective relationships between preeclampsia and adverse offspring outcomes typically occur in studies of high-risk populations. This is probably because there is no truly “normal” or “healthy” reference group for comparison (121). Therefore, preeclampsia exposure is compared to a mixed profile of risk factors among non-preeclamptic pregnancies [e.g., intrauterine inflammation or infection (122)]. In other words, high-risk is the product of multiple exposures and preeclampsia is associated with less morbidity than others in some studies (48, 121, 123, 124). Thus, it is inappropriate to claim that preeclampsia is truly protective under such circumstances. It is more appropriate to infer that preeclampsia is associated with less risk than other pathologies in high-risk infants not exposed to preeclampsia.
Limitations of multivariable regression
The association between preeclampsia and offspring sequelae is typically estimated using multiple logistic regression (125). These models assume no important variables are omitted, no extraneous variables are included, independent variables are measured without error, and that there are no interactions among independent variables (126). Yet, in studies of preeclampsia independent variables often violate assumptions of independence (e.g., gestational age and birth weight are correlated), measurement error is common (e.g., in gestational age estimation), and most are too small to examine interactions. Nor is it understood which variables are important or extraneous.
A major concern in recent years focuses on the pitfalls of adjusting for intermediaries (i.e., entities that lie along a causal pathway between an exposure and outcome). If unmeasured factors cause pregnancy complications and adverse offspring outcomes, then adjustment for intermediaries can bias or spuriously reverse the direction of associations (127–132). Consequently, some investigators claim that adjustment for gestational age seldom provides trustworthy answers about causation (133). Others propose a fetuses-at-risk (FAR) approach [i.e., calculating risk with all fetuses in the denominator rather than live births; (134–137)]. The FAR approach is useful for justifying indicated preterm delivery by demonstrating higher risk of stillbirth with ongoing gestation (137). However, there are important critiques when this is applied to postnatal outcomes [e.g., fetuses are unable to be diagnosed with CP, therefore FAR rates are difficult to interpret. (138–141)].
Perhaps the most cogent advice on handling gestational length in models of developmental disorders is, “…when information about delivery-associated exposures, vulnerabilities, response modifiers, and maternal characteristics is unavailable, adjusting for gestational age at delivery (category) may be the only, if suboptimal, strategy (p. 126)” (142). That said, judgments about causal inference in high-risk populations like extremely preterm births must consider competing risks among unexposed infants, findings among later preterm and term births and evidence from pre-clinical studies.
Difficulties separating preeclampsia, fetal growth restriction and preterm delivery
Preeclampsia is a common indication for preterm delivery. No adjustment method can fully resolve confounding by indication in observational studies (143). Complexities arise with preeclampsia because the earlier the delivery, the more the overlap among FGR, placental malperfusion and inflammation (144–149). For example, half of all pregnancies with early-onset preeclampsia prior to 34 weeks deliver fetal growth restricted newborns (144, 146). By contrast, about three of four newborns from preeclamptic pregnancies born prior to the 28th week of gestation are fetal growth restricted (150). Indeed, recent evidence suggests that fetal growth is impaired only in severe preeclampsia and not in mild pregnancy-associated hypertensive disorders compared to normotensive pregnancy (151). Thus, to examine sequelae of FGR separate from those of preeclampsia at periviable gestational lengths requires massive sample size. Alternatively, case-control studies have contrasted preeclampsia and FGR without preeclampsia. However, if the design fails to account for gestational length differences, then preeclampsia with growth restriction and early preterm delivery is typically compared to FGR without preeclampsia and later delivery (152).
Conclusion
Very large, carefully designed, multi-center longitudinal studies with high quality data and follow-up far beyond birth hospital discharge are needed to determine whether preeclampsia causes developmental disorders in offspring. These studies will need to consider the complex interrelationships among preconceptual and early pregnancy factors (e.g., social disadvantage, infection, obesity), fetal and placental characteristics (e.g., sex, malperfusion), maternal factors (e.g., weight gain, substance use), birth and postnatal exposures (e.g., illnesses). Iterative integration of evidence from observational studies and pre-clinical models is probably necessary to make progress considering the complexity (153, 154). If such a discrimination is achieved, then emerging data on the pathogenesis of preeclampsia can inform relevant pathways for adverse fetal outcomes and perhaps guide interventions.
Author contributions
SK conceived the writing project, wrote the first draft of the manuscript, critically reviewed/revised iterations, and approved the final submission. ES, CE, and JR critically reviewed/revised iterations of the manuscript and approved the submitted version. All authors contributed to the article and approved the submitted version.
Funding
CE was funded by Fondecyt 1200250 (Chile).
Acknowledgments
The authors would like to thank the researchers belonging to CoLab for their valuable input during the 2018 CoLab annual meeting and specifically the speakers whose presentations guided this discussion: Ernesto Figueiró-Filho, Laura Benschop Tom McElrath, Gordon Smith, Chris Redman, Dino Giussani, Jason O'Connor, Justin Dean, and Pierre Gressens.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Little WJ. On the Incidence of Abnormal Parturition, Difficult Labour, Premature Birth Asphyxia Neonatorum on the Mental and Physical Condition of the Child Especially in Relation to Deformities. Trnasactions of the Obstetrical Society of London (1862).
3. Statistics V. A Memorial Volume of Selections From the Reports and Writings of William Farr. Ed Noel A Humphreys. London: Offices of the Sanitary Institute and Edward Stanford (1885).
5. Wilcox AJ. Fertility and Pregnancy: An Epidemiologic Perspective. Oxford: Oxford University Press (2010).
7. Anctil AO, Joshi GB, Lucas WE, Little WA, Callagan DA. Prematurity: a more precise approach to identification. Obstet Gynecol. (1964) 24:716–21.
8. Sjostedt S, Engleson G, Rooth G. Dysmaturity. Arch Dis Child. (1958) 33:123–30. doi: 10.1136/adc.33.168.123
9. Ylppö A. Das Wachstum der Frühgeborenen von der Geburt bis zum Schulalter. Eur J Pediatr. (1919) 24:111–78. doi: 10.1007/BF02222073
10. Hack M, Klein NK, Taylor HG. Long-term developmental outcomes of low birth weight infants. Future Child. (1995) 5:176–96. doi: 10.2307/1602514
11. Benton AL. Mental development of prematurely born children: a critical review of the literature. Am J Orthopsychiatry. (1940) 10:719–46. doi: 10.1111/j.1939-0025.1940.tb05738.x
12. Clifford SH. Pediatric aspects of the placental dysfunction syndrome in postmaturity. J Am Med Assoc. (1957) 165:1663–5. doi: 10.1001/jama.1957.02980310015005
13. Selander P. Postmature infants. Acta Paediatr. (1954) 43:587–91. doi: 10.1111/j.1651-2227.1954.tb04069.x
14. Putnam. Putnam on the relation of infectious processes to diseases of the nervous system. Pathology and Etiology. Brain. (1895) 18:435–57. doi: 10.1093/brain/18.2-3.435
15. Gregg NM. Congenital cataract following German measles in the mother. 1941. Epidemiol Infect. (1991) 107:iii–xiv. doi: 10.1017/S0950268800048627
16. Blanc WA. Amniotic infection syndrome; pathogenesis, morphology, and significance in circumnatal mortality. Clin Obstet Gynecol. (1959) 2:705–34. doi: 10.1097/00003081-195902030-00010
17. Stern H, Booth JC, Elek SD, Fleck DG. Microbial causes of mental retardation. The role of prenatal infections with cytomegalovirus, rubella virus, and toxoplasma. Lancet. (1969) 2:443–8. doi: 10.1016/S0140-6736(69)90162-7
18. Leviton A, Gilles FH. Epidemiologic study of perinatal telencephalic leuco-encephalopathy in an autopsy population. J Neurol Sci. (1973) 18:53–66. doi: 10.1016/0022-510X(73)90020-8
19. Pasamanick B, Knobloch H, Lilienfeld AM. Socioeconomic status and some precursors of neuropsychiatric disorder. Am J Orthopsychiatry. (1956) 26:594–601. doi: 10.1111/j.1939-0025.1956.tb06205.x
20. Klebanoff MA. The collaborative perinatal project: a 50-year retrospective. Paediatr Perinat Epidemiol. (2009) 23:2–8. doi: 10.1111/j.1365-3016.2008.00984.x
21. Susser M, Stein Z. Eras in Epidemiology: The Evolution of Ideas. Oxford: Oxford University Press (2009).
23. Roberts JM. The perplexing pregnancy disorder preeclampsia: what next? Physiol Genomics. (2018) 50:459–67. doi: 10.1152/physiolgenomics.00017.2018
24. Ruiz A, Ferrer Q, Sanchez O, Ribera I, Arevalo S, Alomar O, et al. Placenta-related complications in women carrying a foetus with congenital heart disease. J Matern Fetal Neonatal Med. (2016) 29:3271–5. doi: 10.3109/14767058.2015.1121480
25. Auger N, Fraser WD, Healy-Profitos J, Arbour L. Association between preeclampsia and congenital heart defects. JAMA. (2015) 314:1588–98. doi: 10.1001/jama.2015.12505
26. Brodwall K, Leirgul E, Greve G, Vollset SE, Holmstrom H, Tell GS, et al. Possible common aetiology behind maternal preeclampsia and congenital heart defects in the child: a cardiovascular diseases in norway project study. Paediatr Perinat Epidemiol. (2016) 30:76–85. doi: 10.1111/ppe.12252
27. Bellizzi S, Ali MM, Abalos E, Betran AP, Kapila J, Pileggi-Castro C, et al. Are hypertensive disorders in pregnancy associated with congenital malformations in offspring? Evidence from the WHO Multicountry cross sectional survey on maternal and newborn health. BMC Pregnancy Childbirth. (2016) 16:198. doi: 10.1186/s12884-016-0987-8
28. van Gelder MM, Van Bennekom CM, Louik C, Werler MM, Roeleveld N, Mitchell AA. Maternal hypertensive disorders, antihypertensive medication use, and the risk of birth defects: a case-control study. BJOG. (2015) 122:1002–9. doi: 10.1111/1471-0528.13138
29. Nelson DB, Chalak LF, McIntire DD, Leveno KJ. Is preeclampsia associated with fetal malformation? A review and report of original research. J Matern Fetal Neonatal Med. (2015) 28:2135–40. doi: 10.3109/14767058.2014.980808
30. Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. (2009) 40:1176–80. doi: 10.1161/STROKEAHA.108.538025
31. McElrath TF, Allred EN, Kuban K, Hecht JL, Onderdonk A, O'Shea TM, et al. Factors associated with small head circumference at birth among infants born before the 28th week. Am J Obstet Gynecol. (2010) 203:138.e1–8. doi: 10.1016/j.ajog.2010.05.006
32. Basso O, Rasmussen S, Weinberg CR, Wilcox AJ, Irgens LM, Skjaerven R. Trends in fetal and infant survival following preeclampsia. JAMA. (2006) 296:1357–62. doi: 10.1001/jama.296.11.1357
33. Greene T, Hartnett ME. Programming error led to underestimate of effect sizes in study of association of maternal preeclampsia and risk of infant retinopathy of prematurity. JAMA Ophthalmol. (2019) 137:119. doi: 10.1001/jamaophthalmol.2018.5055
34. Shulman JP, Weng C, Wilkes J, Greene T, Hartnett ME. Association of maternal preeclampsia with infant risk of premature birth and retinopathy of prematurity. JAMA Ophthalmol. (2017) 135:947–53. doi: 10.1001/jamaophthalmol.2017.2697
35. Razak A, Florendo-Chin A, Banfield L, Abdul Wahab MG, McDonald S, Shah PS, et al. Pregnancy-induced hypertension and neonatal outcomes: a systematic review and meta-analysis. J Perinatol. (2018) 38:46–53. doi: 10.1038/jp.2017.162
36. Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba'aqeel H, et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. (2006) 194:921–31. doi: 10.1016/j.ajog.2005.10.813
37. Li C, Miao JK, Xu Y, Hua YY, Ma Q, Zhou LL, et al. Prenatal, perinatal and neonatal risk factors for perinatal arterial ischaemic stroke: a systematic review and meta-analysis. Eur J Neurol. (2017) 24:1006–15. doi: 10.1111/ene.13337
38. Srivastava R, Dunbar M, Shevell M, Oskoui M, Basu A, Rivkin MJ, et al. Development and validation of a prediction model for perinatal arterial ischemic stroke in term neonates. JAMA Netw Open. (2022) 5:e2219203. doi: 10.1001/jamanetworkopen.2022.19203
39. Lee J, Croen LA, Backstrand KH, Yoshida CK, Henning LH, Lindan C, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. (2005) 293:723–9. doi: 10.1001/jama.293.6.723
40. Wu YW, March WM, Croen LA, Grether JK, Escobar GJ, Newman TB. Perinatal stroke in children with motor impairment: a population-based study. Pediatrics. (2004) 114:612–9. doi: 10.1542/peds.2004-0385
41. Mann JR, McDermott S, Pan C, Hardin JW. Maternal hypertension and intrapartum fever are associated with increased risk of ischemic stroke during infancy. Dev Med Child Neurol. (2013) 55:58–64. doi: 10.1111/j.1469-8749.2012.04409.x
42. Cole L, Dewey D, Letourneau N, Kaplan BJ, Chaput K, Gallagher C, et al. Clinical characteristics, risk factors, and outcomes associated with neonatal hemorrhagic stroke: a population-based case-control study. JAMA Pediatr. (2017) 171:230–8. doi: 10.1001/jamapediatrics.2016.4151
43. Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O'Sullivan F, Burton PR, et al. Antepartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. (1998) 317:1549–53. doi: 10.1136/bmj.317.7172.1549
44. McElrath TF, Allred EN, Boggess KA, Kuban K, O'Shea TM, Paneth N, et al. Maternal antenatal complications and the risk of neonatal cerebral white matter damage and later cerebral palsy in children born at an extremely low gestational age. Am J Epidemiol. (2009) 170:819–28. doi: 10.1093/aje/kwp206
45. Figueiro-Filho EA, Croy BA, Reynolds JN, Dang F, Piro D, Ratsep MT, et al. Diffusion tensor imaging of white matter in children born from preeclamptic gestations. AJNR Am J Neuroradiol. (2017) 38:801–6. doi: 10.3174/ajnr.A5064
46. Rocha GM, de Lima FF, Machado AP, Guimaraes HA, Hypertensive Hypertensive Disorders of Pregnancy Study G. Hypertensive disorders during pregnancy and risk of bronchopulmonary dysplasia in very preterm infants. Am J Perinatol. (2019) 36:176–83. doi: 10.1055/s-0038-1660865
47. Hansen AR, Barnes CM, Folkman J, McElrath TF. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr. (2010) 156:532–6. doi: 10.1016/j.jpeds.2009.10.018
48. Gagliardi L, Rusconi F, Da Fre M, Mello G, Carnielli V, Di Lallo D, et al. Pregnancy disorders leading to very preterm birth influence neonatal outcomes: results of the population-based ACTION cohort study. Pediatr Res. (2013) 73:794–801. doi: 10.1038/pr.2013.52
49. Rocha G, de Lima FF, Machado AP, Guimaraes H, Collaborators Collaborators of the Hypertensive Disorders of Pregnancy Study G. Preeclampsia predicts higher incidence of bronchopulmonary dysplasia. J Perinatol. (2018) 38:1165–73. doi: 10.1038/s41372-018-0133-8
50. Yen TA, Yang HI, Hsieh WS, Chou HC, Chen CY, Tsou KI, et al. Preeclampsia and the risk of bronchopulmonary dysplasia in VLBW infants: a population based study. PLoS ONE. (2013) 8:e75168. doi: 10.1371/journal.pone.0075168
51. Wilmink FA, Reijnierse J, Reiss IKM, Steegers EAP, de Jonge RCJ, Research Research Consortium Neonatology South-West of the N. Preeclampsia and risk of developing bronchopulmonary dysplasia in very preterm neonates. Pregnancy Hypertens. (2019) 15:57–63. doi: 10.1016/j.preghy.2018.11.001
52. Gemmell L, Martin L, Murphy KE, Modi N, Hakansson S, Reichman B, et al. Hypertensive disorders of pregnancy and outcomes of preterm infants of 24 to 28 weeks' gestation. J Perinatol. (2016) 36:1067–72. doi: 10.1038/jp.2016.133
53. Dravet-Gounot P, Torchin H, Goffinet F, Aubelle MS, El Ayoubi M, Lefevre C, et al. Bronchopulmonary dysplasia in neonates born to mothers with preeclampsia: impact of small for gestational age. PLoS ONE. (2018) 13:e0204498. doi: 10.1371/journal.pone.0204498
54. Mann JR, McDermott S, Griffith MI, Hardin J, Gregg A. Uncovering the complex relationship between pre-eclampsia, preterm birth and cerebral palsy. Paediatr Perinat Epidemiol. (2011) 25:100–10. doi: 10.1111/j.1365-3016.2010.01157.x
55. Strand KM, Heimstad R, Iversen AC, Austgulen R, Lydersen S, Andersen GL, et al. Mediators of the association between pre-eclampsia and cerebral palsy: population based cohort study. BMJ. (2013) 347:f4089. doi: 10.1136/bmj.f4089
56. Mor O, Stavsky M, Yitshak-Sade M, Mastrolia SA, Beer-Weisel R, Rafaeli-Yehudai T, et al. Early onset preeclampsia and cerebral palsy: a double hit model? Am J Obstet Gynecol. (2016) 214:105 e1–9. doi: 10.1016/j.ajog.2015.08.020
57. Nahum Sacks K, Friger M, Shoham-Vardi I, Sergienko R, Spiegel E, Landau D, et al. Long-term neuropsychiatric morbidity in children exposed prenatally to preeclampsia. Early Hum Dev. (2019) 130:96–100. doi: 10.1016/j.earlhumdev.2019.01.016
58. Blair E, Watson L, Australian Cerebral Palsy Register G. Cerebral palsy and perinatal mortality after pregnancy-induced hypertension across the gestational age spectrum: observations of a reconstructed total population cohort. Dev Med Child Neurol. (2016) 58:76–81. doi: 10.1111/dmcn.13014
59. Chen KR, Yu T, Kang L, Lien YJ, Kuo PL. Childhood neurodevelopmental disorders and maternal hypertensive disorder of pregnancy. Dev Med Child Neurol. (2021) 63:1107–13. doi: 10.1111/dmcn.14893
60. Xu RT, Chang QX, Wang QQ, Zhang J, Xia LX, Zhong N, et al. Association between hypertensive disorders of pregnancy and risk of autism in offspring: a systematic review and meta-analysis of observational studies. Oncotarget. (2018) 9:1291–301. doi: 10.18632/oncotarget.23030
61. Dachew BA, Mamun A, Maravilla JC, Alati R. Pre-eclampsia and the risk of autism-spectrum disorder in offspring: meta-analysis. Br J Psychiatry. (2018) 212:142–7. doi: 10.1192/bjp.2017.27
62. Nomura Y, John RM, Janssen AB, Davey C, Finik J, Buthmann J, et al. Neurodevelopmental consequences in offspring of mothers with preeclampsia during pregnancy: underlying biological mechanism via imprinting genes. Arch Gynecol Obstet. (2017) 295:1319–29. doi: 10.1007/s00404-017-4347-3
63. Figueiro-Filho EA, Mak LE, Reynolds JN, Stroman PW, Smith GN, Forkert ND, et al. Neurological function in children born to preeclamptic and hypertensive mothers - A systematic review. Pregnancy Hypertens. (2017) 10:1–6. doi: 10.1016/j.preghy.2017.07.144
64. Maher GM, O'Keeffe GW, Kearney PM, Kenny LC, Dinan TG, Mattsson M, et al. Association of hypertensive disorders of pregnancy with risk of neurodevelopmental disorders in offspring: a systematic review and meta-analysis. JAMA Psychiatry. (2018) 75:809–19. doi: 10.1001/jamapsychiatry.2018.0854
65. Maher GM, O'Keeffe GW, Dalman C, Kearney PM, McCarthy FP, Kenny LC, et al. Association between preeclampsia and autism spectrum disorder: a population-based study. J Child Psychol Psychiatry. (2019) 61:131–9. doi: 10.1111/jcpp.13127
66. Wang H, László KD, Gissler M, Li F, Zhang J, Yu Y, et al. Maternal hypertensive disorders and neurodevelopmental disorders in offspring: a population-based cohort in two Nordic countries. Eur J Epidemiol. (2021) 36:519–30. doi: 10.1007/s10654-021-00756-2
67. Maher GM, Dalman C, O'Keeffe GW, Kearney PM, McCarthy FP, Kenny LC, et al. Association between preeclampsia and autism spectrum disorder and attention deficit hyperactivity disorder: an intergenerational analysis. Acta Psychiatr Scand. (2020) 142:348–50. doi: 10.1111/acps.13180
68. Dachew BA, Mamun A, Maravilla JC, Alati R. Association between hypertensive disorders of pregnancy and the development of offspring mental and behavioural problems: A systematic review and meta-analysis. Psychiatry Res. (2018) 260:458–67. doi: 10.1016/j.psychres.2017.12.027
69. Eide MG, Moster D, Irgens LM, Reichborn-Kjennerud T, Stoltenberg C, Skjaerven R, et al. Degree of fetal growth restriction associated with schizophrenia risk in a national cohort. Psychol Med. (2013) 43:2057–66. doi: 10.1017/S003329171200267X
70. Dalman C, Allebeck P, Cullberg J, Grunewald C, Koster M. Obstetric complications and the risk of schizophrenia: a longitudinal study of a national birth cohort. Arch Gen Psychiatry. (1999) 56:234–40. doi: 10.1001/archpsyc.56.3.234
71. Byrne M, Agerbo E, Bennedsen B, Eaton WW, Mortensen PB. Obstetric conditions and risk of first admission with schizophrenia: a Danish national register based study. Schizophr Res. (2007) 97:51–9. doi: 10.1016/j.schres.2007.07.018
72. Getahun D, Rhoads GG, Demissie K, Lu SE, Quinn VP, Fassett MJ, et al. In utero exposure to ischemic-hypoxic conditions and attention-deficit/hyperactivity disorder. Pediatrics. (2013) 131:e53–61. doi: 10.1542/peds.2012-1298
73. Mann JR, McDermott S. Are maternal genitourinary infection and pre-eclampsia associated with ADHD in school-aged children? J Atten Disord. (2011) 15:667–73. doi: 10.1177/1087054710370566
74. Dachew BA, Scott JG, Mamun A, Alati R. Pre-eclampsia and the risk of attention-deficit/hyperactivity disorder in offspring: findings from the ALSPAC birth cohort study. Psychiatry Res. (2019) 272:392–7. doi: 10.1016/j.psychres.2018.12.123
75. Sun BZ, Moster D, Harmon QE, Wilcox AJ. Association of preeclampsia in term births with neurodevelopmental disorders in offspring. JAMA Psychiatry. (2020) 77:823–9. doi: 10.1001/jamapsychiatry.2020.0306
76. Wu CS, Sun Y, Vestergaard M, Christensen J, Ness RB, Haggerty CL, et al. Preeclampsia and risk for epilepsy in offspring. Pediatrics. (2008) 122:1072–8. doi: 10.1542/peds.2007-3666
77. Mann JR, McDermott S. Maternal pre-eclampsia is associated with childhood epilepsy in South Carolina children insured by Medicaid. Epilepsy Behav. (2011) 20:506–11. doi: 10.1016/j.yebeh.2011.01.006
78. Tuovinen S, Aalto-Viljakainen T, Eriksson JG, Kajantie E, Lahti J, Pesonen AK, et al. Maternal hypertensive disorders during pregnancy: adaptive functioning and psychiatric and psychological problems of the older offspring. BJOG. (2014) 121:1482–91. doi: 10.1111/1471-0528.12753
79. Bain M, Juszczak E, McInneny K, Kendell RE. Obstetric complications and affective psychoses. Two case-control studies based on structured obstetric records. Br J Psychiatry. (2000) 176:523–6. doi: 10.1192/bjp.176.6.523
80. Zammit S, Odd D, Horwood J, Thompson A, Thomas K, Menezes P, et al. Investigating whether adverse prenatal and perinatal events are associated with non-clinical psychotic symptoms at age 12 years in the ALSPAC birth cohort. Psychol Med. (2009) 39:1457–67. doi: 10.1017/S0033291708005126
81. Beukers F, Aarnoudse-Moens CSH, van Weissenbruch MM, Ganzevoort W, van Goudoever JB, van Wassenaer-Leemhuis AG. Fetal growth restriction with brain sparing: neurocognitive and behavioral outcomes at 12 years of age. J Pediatr. (2017) 188:103–9.e2. doi: 10.1016/j.jpeds.2017.06.003
82. Tuovinen S, Raikkonen K, Kajantie E, Pesonen AK, Heinonen K, Osmond C, et al. Depressive symptoms in adulthood and intrauterine exposure to pre-eclampsia: the Helsinki Birth Cohort Study. BJOG. (2010) 117:1236–42. doi: 10.1111/j.1471-0528.2010.02634.x
83. Tuovinen S, Raikkonen K, Pesonen AK, Lahti M, Heinonen K, Wahlbeck K, et al. Hypertensive disorders in pregnancy and risk of severe mental disorders in the offspring in adulthood: the Helsinki Birth Cohort Study. J Psychiatr Res. (2012) 46:303–10. doi: 10.1016/j.jpsychires.2011.11.015
84. Robinson M, Mattes E, Oddy WH, de Klerk NH, Li J, McLean NJ, et al. Hypertensive diseases of pregnancy and the development of behavioral problems in childhood and adolescence: the Western Australian Pregnancy Cohort Study. J Pediatr. (2009) 154:218–24. doi: 10.1016/j.jpeds.2008.07.061
85. Heikura U, Hartikainen AL, Nordstrom T, Pouta A, Taanila A, Jarvelin MR. Maternal hypertensive disorders during pregnancy and mild cognitive limitations in the offspring. Paediatr Perinat Epidemiol. (2013) 27:188–98. doi: 10.1111/ppe.12028
86. Ehrenstein V, Rothman KJ, Pedersen L, Hatch EE, Sorensen HT. Pregnancy-associated hypertensive disorders and adult cognitive function among Danish conscripts. Am J Epidemiol. (2009) 170:1025–31. doi: 10.1093/aje/kwp223
87. Tuovinen S, Eriksson JG, Kajantie E, Lahti J, Pesonen AK, Heinonen K, et al. Maternal hypertensive disorders in pregnancy and self-reported cognitive impairment of the offspring 70 years later: the Helsinki Birth Cohort Study. Am J Obstet Gynecol. (2013) 208:200.e1–9. doi: 10.1016/j.ajog.2012.12.017
88. Tuovinen S, Raikkonen K, Kajantie E, Leskinen JT, Henriksson M, Pesonen AK, et al. Hypertensive disorders in pregnancy and intellectual abilities in the offspring in young adulthood: the Helsinki Birth Cohort Study. Ann Med. (2012) 44:394–403. doi: 10.3109/07853890.2011.573497
89. Sverrisson FA, Bateman BT, Aspelund T, Skulason S, Zoega H. Preeclampsia and academic performance in children: a nationwide study from Iceland. PLoS ONE. (2018) 13:e0207884. doi: 10.1371/journal.pone.0207884
90. Adane AA, Mishra GD, Tooth LR. Maternal preconception weight trajectories, pregnancy complications and offspring's childhood physical and cognitive development. J Dev Orig Health Dis. (2018) 9:653–60. doi: 10.1017/S2040174418000570
91. Many A, Fattal A, Leitner Y, Kupferminc MJ, Harel S, Jaffa A. Neurodevelopmental and cognitive assessment of children born growth restricted to mothers with and without preeclampsia. Hypertens Pregnancy. (2003) 22:25–9. doi: 10.1081/PRG-120016791
92. Morsing E, Marsal K. Pre-eclampsia- an additional risk factor for cognitive impairment at school age after intrauterine growth restriction and very preterm birth. Early Hum Dev. (2014) 90:99–101. doi: 10.1016/j.earlhumdev.2013.12.002
93. Leitner Y, Harel S, Geva R, Eshel R, Yaffo A, Many A. The neurocognitive outcome of IUGR children born to mothers with and without preeclampsia. J Matern Fetal Neonatal Med. (2012) 25:2206–8. doi: 10.3109/14767058.2012.684164
94. Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J. Health of children born to mothers who had preeclampsia: a population-based cohort study. Am J Obstet Gynecol. (2009) 201:269.e1–e10. doi: 10.1016/j.ajog.2009.06.060
95. Goffin SM, Derraik JGB, Groom KM, Cutfield WS. Maternal pre-eclampsia and long-term offspring health: is there a shadow cast? Pregnancy Hypertens. (2018) 12:11–5. doi: 10.1016/j.preghy.2018.02.003
96. Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. (2010) 122:488–94. doi: 10.1161/CIRCULATIONAHA.110.941203
97. Youssef L, Miranda J, Paules C, Garcia-Otero L, Vellvé K, Kalapotharakos G, et al. Fetal cardiac remodeling and dysfunction is associated with both preeclampsia and fetal growth restriction. Am J Obstet Gynecol. (2020) 222:79.e1–9. doi: 10.1016/j.ajog.2019.07.025
98. Crispi F, Crovetto F, Gratacos E. Intrauterine growth restriction and later cardiovascular function. Early Hum Dev. (2018) 126:23–27. doi: 10.1016/j.earlhumdev.2018.08.013
99. Rizzo G, Mattioli C, Mappa I, Bitsadze V, Khizroeva J, Slodki M, et al. Hemodynamic factors associated with fetal cardiac remodeling in late fetal growth restriction: a prospective study. J Perinatal Med. (2019) 47:683–8. doi: 10.1515/jpm-2019-0217
100. Turkyilmaz G, Turkyilmaz S, Uygur L. Fetal cardiac alterations in the late-onset growth-restricted fetuses: a prospective case-control study. J Obstet Gynaecol Res. (2022) 48:373–8. doi: 10.1111/jog.15114
101. Rodriguez-Guerineau L, Perez-Cruz M, Gomez Roig MD, Cambra FJ, Carretero J, Prada F, et al. Cardiovascular adaptation to extrauterine life after intrauterine growth restriction. Cardiol Young. (2018) 28:284–91. doi: 10.1017/S1047951117001949
102. Le N, Phad N, de Waal K. Cardiac remodeling during the neonatal intensive care period; a window of opportunity for early prevention of heart failure? Early Hum Dev. (2020) 151:105168. doi: 10.1016/j.earlhumdev.2020.105168
103. Sarvari SI, Rodriguez-Lopez M, Nunez-Garcia M, Sitges M, Sepulveda-Martinez A, Camara O, et al. Persistence of cardiac remodeling in preadolescents with fetal growth restriction. Circ Cardiovasc Imaging. (2017) 10:e005270. doi: 10.1161/CIRCIMAGING.116.005270
104. Andraweera PH, Lassi ZS. Cardiovascular risk factors in offspring of preeclamptic pregnancies-systematic review and meta-analysis. J Pediatr. (2019) 208:104–13.e6. doi: 10.1016/j.jpeds.2018.12.008
105. Davis EF, Lewandowski AJ, Aye C, Williamson W, Boardman H, Huang RC, et al. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: insights from a 20-year prospective follow-up birth cohort. BMJ Open. (2015) 5:e008136. doi: 10.1136/bmjopen-2015-008136
106. Nahum Sacks K, Friger M, Shoham-Vardi I, Spiegel E, Sergienko R, Landau D, et al. Prenatal exposure to preeclampsia as an independent risk factor for long-term cardiovascular morbidity of the offspring. Pregnancy Hypertens. (2018) 13:181–6. doi: 10.1016/j.preghy.2018.06.013
107. Tenhola S, Rahiala E, Halonen P, Vanninen E, Voutilainen R. Maternal preeclampsia predicts elevated blood pressure in 12-year-old children: evaluation by ambulatory blood pressure monitoring. Pediatr Res. (2006) 59:320–4. doi: 10.1203/01.pdr.0000196734.54473.e3
108. Langford HG, Watson RL. Prepregnant blood pressure, hypertension during pregnancy, and later blood pressure of mothers and offspring. Hypertension. (1980) 2:130–3. doi: 10.1161/01.HYP.2.4.130
109. Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Pre-eclampsia and offspring's blood pressure, cognitive ability and physical development at 17-years-of-age. Br J Obstetr Gynaecol. (1991) 98:1009–14. doi: 10.1111/j.1471-0528.1991.tb15339.x
110. Oglaend B, Forman MR, Romundstad PR, Nilsen ST, Vatten LJ. Blood pressure in early adolescence in the offspring of preeclamptic and normotensive pregnancies. J Hypertens. (2009) 27:2051–4. doi: 10.1097/HJH.0b013e328330052a
111. Homer CS, Brown MA, Mangos G, Davis GK. Non-proteinuric pre-eclampsia: a novel risk indicator in women with gestational hypertension. J Hypertens. (2008) 26:295–302. doi: 10.1097/HJH.0b013e3282f1a953
112. Melamed N, Ray JG, Hladunewich M, Cox B, Kingdom JC. Gestational hypertension and preeclampsia: are they the same disease? J Obstet Gynaecol Can. (2014) 36:642–7. doi: 10.1016/S1701-2163(15)30545-4
113. Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. (2009) 200:481 e1–7. doi: 10.1016/j.ajog.2008.07.048
114. Magee LA, von Dadelszen P, Bohun CM, Rey E, El-Zibdeh M, Stalker S, et al. Serious perinatal complications of non-proteinuric hypertension: an international, multicentre, retrospective cohort study. J Obstet Gynaecol Can. (2003) 25:372–82. doi: 10.1016/S1701-2163(16)30579-5
115. Thornton CE, Makris A, Ogle RF, Tooher JM, Hennessy A. Role of proteinuria in defining pre-eclampsia: clinical outcomes for women and babies. Clin Exp Pharmacol Physiol. (2010) 37:466–70. doi: 10.1111/j.1440-1681.2009.05334.x
116. Westreich D. Berkson's bias, selection bias, and missing data. Epidemiology. (2012) 23:159–64. doi: 10.1097/EDE.0b013e31823b6296
117. Chen Y, Xun D, Wang YC, Wang B, Geng SH, Chen H, et al. Incidence and risk factors of retinopathy of prematurity in two neonatal intensive care units in North and South China. Chin Med J. (2015) 128:914–8. doi: 10.4103/0366-6999.154294
118. Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol. (1991) 134:604–13. doi: 10.1093/oxfordjournals.aje.a116133
119. Blair E. The undesirable consequences of controlling for birth weight in perinatal epidemiological studies. J Epidemiol Commun Health. (1996) 50:559–63. doi: 10.1136/jech.50.5.559
120. Kramer MS. Gestational age, birthweight, and their influence on neonatal outcome. Acta Paediatr. (2015) 104:5–6. doi: 10.1111/apa.12818
121. Snowden JM, Basso O. Causal inference in studies of preterm babies: a simulation study. BJOG. (2018) 125:686–92. doi: 10.1111/1471-0528.14942
122. Greenwood C, Yudkin P, Sellers S, Impey L, Doyle P. Why is there a modifying effect of gestational age on risk factors for cerebral palsy? Arch Dis Childhood Fetal Neonatal Ed. (2005) 90:141–6. doi: 10.1136/adc.2004.052860
123. Collins M, Paneth N. Preeclampsia and cerebral palsy: are they related? Dev Med Child Neurol. (1998) 40:207–11. doi: 10.1111/j.1469-8749.1998.tb15449.x
124. Snowden JM, Bovbjerg ML, Dissanayake M, Basso O. The curse of the perinatal epidemiologist: inferring causation amidst selection. Curr Epidemiol Rep. (2018) 5:379–87. doi: 10.1007/s40471-018-0172-x
125. Box GE, Draper NR. Empirical Model-Building and Response Surfaces. New York, NY: John Wiley & Sons (1987).
126. Steyerberg EW, Eijkemans MJC, Harrell FE, Habbema JDF. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. (2000) 19:1059–79. doi: 10.1002/(SICI)1097-0258(20000430)19:8<1059::AID-SIM412>3.0.CO;2-0
127. Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol. (2017) 217:167–75. doi: 10.1016/j.ajog.2017.04.016
128. Ananth CV, Schisterman EF. Hidden biases in observational epidemiology: the case of unmeasured confounding. BJOG. (2017) 125:644–6. doi: 10.1111/1471-0528.14960
129. Cole SR, Platt RW, Schisterman EF, Chu H, Westreich D, Richardson D, et al. Illustrating bias due to conditioning on a collider. Int J Epidemiol. (2010) 39:417–20. doi: 10.1093/ije/dyp334
130. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. (2009) 20:488–95. doi: 10.1097/EDE.0b013e3181a819a1
131. VanderWeele TJ, Robins JM. Directed acyclic graphs, sufficient causes, and the properties of conditioning on a common effect. Am J Epidemiol. (2007) 166:1096–104. doi: 10.1093/aje/kwm179
132. Hernandez-Diaz S, Schisterman EF, Hernan MA. The birth weight “paradox” uncovered? Am J Epidemiol. (2006) 164:1115–20. doi: 10.1093/aje/kwj275
133. Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. (2011) 174:1062–8. doi: 10.1093/aje/kwr230
134. Joseph KS, Kramer MS. The fetuses-at-risk approach: survival analysis from a fetal perspective. Acta Obstet Gynecol Scand. (2018) 97:454–65. doi: 10.1111/aogs.13194
135. Joseph KS. The fetuses-at-risk approach: clarification of semantic and conceptual misapprehension. BMC Pregnancy Childbirth. (2008) 8:11. doi: 10.1186/1471-2393-8-11
136. Joseph KS. Theory of obstetrics: an epidemiologic framework for justifying medically indicated early delivery. BMC Pregnancy Childbirth. (2007) 7:4. doi: 10.1186/1471-2393-7-4
137. Joseph KS. Incidence-based measures of birth, growth restriction, and death can free perinatal epidemiology from erroneous concepts of risk. J Clin Epidemiol. (2004) 57:889–97. doi: 10.1016/j.jclinepi.2003.11.018
138. Basso O. Implications of using a fetuses-at-risk approach when fetuses are not at risk. Paediatr Perinat Epidemiol. (2016) 30:3–10. doi: 10.1111/ppe.12254
139. Caughey AB, Snowden JM. Measuring perinatal complications: different approaches depending on who is at risk. Paediatr Perinat Epidemiol. (2016) 30:23–4. doi: 10.1111/ppe.12257
140. Werler MM, Parker SE. Bias from conditioning on live-births in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants (Liew et al. 2015). Int J Epidemiol. (2015) 44:1079–80. doi: 10.1093/ije/dyv139
141. Smith GC. Quantifying the risk of different types of perinatal death in relation to gestational age: researchers at risk of causing confusion. Paediatr Perinat Epidemiol. (2016) 30:18–9. doi: 10.1111/ppe.12259
142. Leviton A, Blair E, Dammann O, Allred E. The wealth of information conveyed by gestational age. J Pediatr. (2005) 146:123–7. doi: 10.1016/j.jpeds.2004.09.028
143. Bosco JL, Silliman RA, Thwin SS, Geiger AM, Buist DS, Prout MN, et al. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. (2010) 63:64–74. doi: 10.1016/j.jclinepi.2009.03.001
144. Hung TH, Hsieh TT, Chen SF. Risk of abnormal fetal growth in women with early- and late-onset preeclampsia. Pregnancy Hypertens. (2018) 12:201–6. doi: 10.1016/j.preghy.2017.09.003
145. Rasmussen S, Irgens LM. Fetal growth and body proportion in preeclampsia. Obstet Gynecol. (2003) 101:575–83. doi: 10.1097/00006250-200303000-00026
146. Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Preeclampsia and fetal growth. Obstet Gynecol. (2000) 96:950–5. doi: 10.1097/00006250-200012000-00016
147. Korzeniewski SJ, Romero R, Chaiworapongsa T, Chaemsaithong P, Kim CJ, Kim YM, et al. Maternal plasma angiogenic index-1 (placental growth factor/soluble vascular endothelial growth factor receptor-1) is a biomarker for the burden of placental lesions consistent with uteroplacental underperfusion: a longitudinal case-cohort study. Am J Obstet Gynecol. (2016) 214:629.e1–17. doi: 10.1016/j.ajog.2015.11.015
148. Srinivas SK, Edlow AG, Neff PM, Sammel MD, Andrela CM, Elovitz MA. Rethinking IUGR in preeclampsia: dependent or independent of maternal hypertension? J Perinatol. (2009) 29:680–4. doi: 10.1038/jp.2009.83
149. Geyl C, Clouqueur E, Lambert J, Subtil D, Debarge V, Deruelle P. Links between preeclampsia and intrauterine growth restriction. Gynecol Obstet Fertil. (2014) 42:229–33. doi: 10.1016/j.gyobfe.2014.01.008
150. Korzeniewski SJ, Allred EN, Joseph RM, Heeren T, Kuban KCK, O'Shea TM, et al. Neurodevelopment at age 10 years of children born <28 weeks with fetal growth restriction. Pediatrics. (2017) 140:e20170697. doi: 10.1542/peds.2017-0697
151. Mateus J, Newman RB, Zhang C, Pugh SJ, Grewal J, Kim S, et al. Fetal growth patterns in pregnancy-associated hypertensive disorders: NICHD fetal growth studies. Am J Obstet Gynecol. (2019) 221:635 e1–e16. doi: 10.1016/j.ajog.2019.06.028
152. Erez O, Romero R, Vaisbuch E, Kusanovic JP, Mazaki-Tovi S, Chaiworapongsa T, et al. The pattern and magnitude of “in vivo thrombin generation” differ in women with preeclampsia and in those with SGA fetuses without preeclampsia. J Matern Fetal Neonatal Med. (2018) 31:1671–80. doi: 10.1080/14767058.2017.1323327
153. Dammann O, Gray P, Gressens P, Wolkenhauer O, Leviton A. Systems epidemiology: what's in a name? Online J Public Health Inform. (2014) 6:e198. doi: 10.5210/ojphi.v6i3.5571
Keywords: fetal growth restriction, intrauterine growth restriction, preterm delivery, autism, cerebral palsy, neurodevelopmental disorder
Citation: Korzeniewski SJ, Sutton E, Escudero C and Roberts JM (2022) The Global Pregnancy Collaboration (CoLab) symposium on short- and long-term outcomes in offspring whose mothers had preeclampsia: A scoping review of clinical evidence. Front. Med. 9:984291. doi: 10.3389/fmed.2022.984291
Received: 04 July 2022; Accepted: 16 August 2022;
Published: 30 August 2022.
Edited by:
Nandor Gabor Than, Hungarian Academy of Sciences (MTA), HungaryReviewed by:
Shali Mazaki Tovi, Sheba Medical Center, IsraelAlvaro Sepúlveda-Martínez, University of Chile, Chile
Copyright © 2022 Korzeniewski, Sutton, Escudero and Roberts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven J. Korzeniewski, sKorzeni@med.wayne.edu
 Steven J. Korzeniewski
Steven J. Korzeniewski Elizabeth Sutton2,3
Elizabeth Sutton2,3  Carlos Escudero
Carlos Escudero James M. Roberts
James M. Roberts