Nudix Hydroxylase 15 Mutations Strongly Predict Thiopurine-Induced Leukopenia Across Different Asian Ethnicities: Implications for Screening in a Diverse Population
- 1Division of Gastroenterology and Hepatology, Department of Medicine, University of Malaya Medical Centre, Kuala Lumpur, Malaysia
- 2Clinical Research Centre, Pantai Hospital Kuala Lumpur, Kuala Lumpur, Malaysia
- 3Department of Hepatology and Gastroenterology, Hospital Selayang, Selangor, Malaysia
- 4Department of Paediatrics, Faculty of Medicine, University Malaya, Kuala Lumpur, Malaysia
- 5Gastroenterology Unit, Department of Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 6Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia
Background and Aims: Thiopurines, which are immunosuppressive drugs for maintaining remission for inflammatory bowel disease, are known to cause myelotoxicity in patients with Nudix Hydroxylase 15 (NUDT15) genetic variants in some Asian countries with monoethnic populations. We aimed to investigate the association of NUDT15 variants with leukopenia in a multiethnic population in Southeast Asia.
Methods: Patients with a confirmed diagnosis of inflammatory bowel disease were recruited. We collected demographic and clinical characteristics and whole blood counts before and after initiating thiopurines. Thiopurine S-methyltransferase (TPMT) and NUDT15 genotypes were analyzed with the single nucleotide polymorphisms (SNPs) genotyping assay. Leukopenia was defined as a white blood cell (WBC) count < 3,000/μl.
Results: In this study, 19 (18.6%) of the 102 patients who had adequate thiopurine therapy experienced leukopenia, 11 patients (57.9%) had NUDT15 c.415C > T variants, 2 patients (10.5%) had NUDT15 c.52G > A variants while one (5.3%) had a TPMT variation. Individually, NUDT15 c.415C > T had a sensitivity and specificity of 57.9% and 94.0% (odds ratio [OR] = 21.45, 95% CI 5.94–77.41, p < 0.001), respectively, for predicting thiopurine-induced leukopenia, while NUDT15 c.52G > A was only observed in patients with leukopenia. As compared with patients with wild-type NUDT15, both NUDT15 variations had a combined sensitivity and specificity of 68.4% and 94%, respectively (OR = 33.80, 95% CI 8.99–127.05, p < 0.001), for predicting thiopurine-induced leukopenia as well as a shorter onset to leukopenia (median onset [months] 2.0 vs. 5.5; p = 0.045). Sub-group analysis showed that both NUDT15 variations were strongly associated with leukopenia among the Chinese and Indians but not among the Malays.
Conclusion: Nudix Hydroxylase 15 variants strongly predicted thiopurine-induced leukopenia across a multiethnic Southeast Asian population, particularly among the Chinese and Indians.
Introduction
Inflammatory bowel disease (IBD) is emerging rapidly in Asia, including Malaysia (1). The mean incidence of IBD in Malaysia increased from 0.07 to 0.69 per 100,000 person-years over the past two decades (2). Despite a paradigm shift in the management of IBD toward the early use of biologics in complicated cases or those with a severe phenotype, the Malaysian National IBD database from 2015 showed that biologics were only used in 2.5% of patients with IBD, whereas another 39.8% of patients were on thiopurines (unpublished data). This pattern of IBD therapy is likely to be similarly seen in many countries with limited resources where immunomodulators, such as thiopurines, remain an important therapy of choice for the maintenance of remission in patients with the steroid-dependent disease.
After oral administration, thiopurines undergo a complex series of metabolic pathways. Azathioprine (AZA), the most commonly used thiopurine, is converted to 6-mercaptopurine (6-MP), which is then enzymatically converted to its active metabolite, deoxy-6-thioguanosine 5′ triphosphate (6-TGN), through successive enzymatic conversion by hypoxanthine-guanine phosphoribosyl transferase (HGPRT) and inosine monophosphate dehydrogenase (IMPDH) (3, 4). The 6-TGN metabolite is the predominant active compound responsible for thiopurine therapeutic efficacy. During cell division, 6-TGN incorporates into the double-stranded DNA of the cellular nucleus, resulting in disruption of nucleic acid synthesis and cell apoptosis (4).
Tolerance to thiopurines varies widely among different populations based on their pharmacogenetic profile. Intolerance to thiopurines, such as potentially life-threatening myelotoxicity, has been reported in up to 28% of individuals exposed to AZA (5). Variations in the thiopurine S-methyltransferase (TPMT) gene are widely associated with an increased incidence of thiopurine-associated myelotoxicity (6). Despite that thiopurine-induced myelotoxicity was more commonly seen among Asians than Whites (7), the prevalence of TPMT gene variations is reportedly to be uncommon in Asian populations (8). This has led to the discovery of a novel predisposing gene, the Nudix Hydroxylase 15 (NUDT15), which was initially identified in a Korean genome-wide association study (9). Studies from China, Japan, Hong Kong, Singapore, and India have shown a significant association between NUDT15 variations (particularly c.415C > T) and thiopurine-induced myelotoxicity as well as confirming the lack of utility of TPMT variants in predicting thiopurine-induced myelosuppression (10–15).
Unlike the populations of China, Japan, Korea, and Hong Kong, which are largely monoethnic, Malaysia is unique, in that it is made up of three large ethnic groups: Malays, Chinese, and Indians (16). Indians from Malaysia are mainly from South India and Sri Lanka and are generally homogenous. Similarly, the Chinese in Malaysia, mainly from Southern China, are also homogeneous. The Malays, on other hand, are more heterogeneous due to interracial marriages with both the Chinese and Indian ethnic groups, despite being largely Austronesian in origin. Since thiopurines are commonly used as immunosuppressants in Malaysian patients with IBD, we aimed to investigate the association between NUDT15 variations (c.415C > T and c.52G > A) and leukopenia in this multiethnic population. We also ascertained whether there were any differences in NUDT15-associated leukopenia among the three ethnic groups.
Materials and Methods
Study Population
Patients with a confirmed diagnosis of IBD were recruited from the University of Malaya Medical Centre (UMMC), Hospital University Kebangsaan Malaysia (HUKM), and Hospital Selayang from March 2017 to February 2021. The diagnosis of IBD was made based on standard clinical, endoscopic, radiological, and histological criteria. Demographic and clinical characteristics of the patients, such as gender, age, age at diagnosis, IBD subtype (Crohn’s disease [CD], ulcerative colitis [UC], or IBD-unclassified [IBD-U]), duration, and dosage of thiopurine therapy, weight, concomitant medications, and blood results, were collected upon recruitment into the study. Blood was collected from the recruited patients during their routine blood check. DNA was extracted for genetic analysis. Informed consent was obtained from all patients prior to data and sample collection. This study was approved by the University of Malaya Medical Centre Ethics Committee (MREC-ID: 2017109-5662).
Patients who were never exposed to thiopurines and those with insufficient exposure to thiopurines (maximum dose < 1.0 mg/kg/day) without signs of myelotoxicity were excluded from the final analysis. Leukopenia was defined as a white blood cell (WBC) count < 3,000/μl.
Monitoring of Leukopenia
After thiopurine therapy was commenced, full blood counts were reviewed weekly in the first month and then every 3 months after dose escalation. Typically, the starting dose of AZA was 50 mg/day in our centers and was escalated subsequently to 1.5–2 mg/kg/day. Patients who developed nausea on AZA were switched to 6-MP at doses up to 1.5 mg/kg/day.
DNA Extraction
Genomic DNA was extracted from whole blood by using the DNeasy® Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. DNA quality and concentration were validated with the Nanodrop™ 2000 Spectrophotometer (Thermo Fisher, MA, United States) to ensure sufficient quantity and quality of the sample before gene amplification through polymerase chain reaction (PCR).
Single Nucleotide Polymorphisms Genotyping Analysis of Nudix Hydroxylase 15 and Thiopurine S-Methyltransferase Single Nucleotide Polymorphisms
Genotyping of NUDT15 c.415C > T (rs116855232), NUDT15 c.52G > A (rs147390019), TPMT c.719A > G (rs1142345), and TPMT c.460G > A 9(rs1800460) were performed with the Taqman® SNPs Genotyping Assay (Applied Biosystems, MA, United States) according to the manufacturer’s protocol. Briefly, 10 ng of genomic DNA was aliquoted into a Fast Optical 96-well Microplate and mixed with a PCR mixture containing TaqMan Genotyping Master Mix, TaqMan SNPs Genotyping Assay, and an appropriate amount of ultrapure water. Amplification of the sample was performed using the StepOne® Real-Time PCR machine (Applied Biosystem, MA, United States). The PCR thermal cycling was as followed: initial denaturing at 95°C for 10 s, followed by 95°C for 15 s for 50 cycles, and extension at 60°C. SNPs status was analyzed with Allelic Calling Function.
Statistical Analysis
A descriptive analysis was carried out on the demographics, clinical, and genetic characteristics. Continuous variables were expressed as median (interquartile range, IQR) and compared using a non-parametric test. Categorical variables were expressed as frequencies and compared using the X2 test or Fisher’s exact test. The value of p < 0.05 was considered significant. All analysis was carried out with SPSS Statistics v.26.0 (IBM, New York, NY, United States).
Results
Demographics, Clinical, and Genetic Characteristics
A total of 140 patients with a confirmed diagnosis of IBD were recruited. The demographic and clinical characteristics of the patients are shown in Table 1. The male:female ratio was 6:4, while the ethnicity composition was 40.7% Indian, 32.9% Chinese, and 26.4% Malay. Two-thirds (66.4%) of the patients have CD, 31.4% have UC, while the remaining 2.1% have IBD-U. All the 140 patients had TPMT/NUDT15 mutation analysis performed.
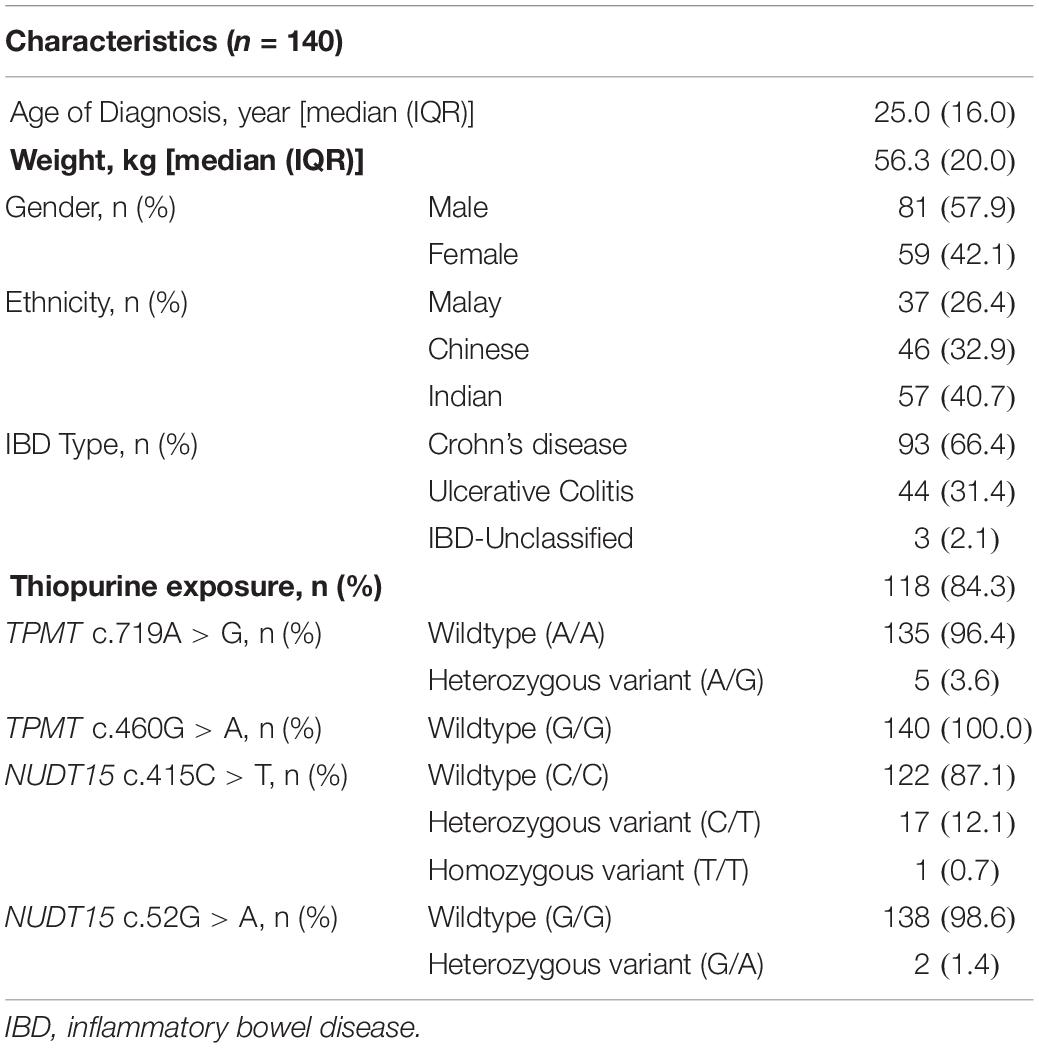
Table 1. Demographics, clinical, and the thiopurine S-methyltransferase (TPMT)/Nudix Hydroxylase 15 (NUDT15) genotypes in 140 patients with inflammatory bowel disease (IBD).
The overall frequency of TPMT c.719A > G (rs1142345) variation was 3.6% (n = 5), while TPMT c.460G > A was non-polymorphic in our study cohort. The overall frequency of NUDT15 c.415C > T and NUDT15 c.52G > A variations were 12.8% (n = 18) and 1.4% (n = 2), respectively.
Association Between Thiopurine S-Methyltransferase and Nudix Hydroxylase 15 Variants and Leukopenia
In this study, 119 of the 140 patients were treated with thiopurines. Of these, 17 patients had inadequate exposure (maximum dose < 1.0 mg/kg/day) during the time of recruitment. Thus, only 102 patients with sufficient exposure to thiopurine were included for the final analysis of the association between genetic variations and leukopenia.
Of the 102 patients, 19 (18.6%) patients experienced leukopenia during treatment with thiopurines. There were no significant differences in IBD subtype, age, body weight, gender, ethnicity, and thiopurine dosage (Table 2). Thiopurines were discontinued at a median of 5.0 months (IQR 8.0) in patients who developed leukopenia. Four of the five patients who were found to have TPMT c.719A > G (rs1142345) variation were analyzed for leukopenia. Only one patient (25%) developed leukopenia. The remaining 18 patients who developed leukopenia had wild-type TPMT variations.
In contrast, NUDT15 variations were significantly more common in individuals who developed leukopenia (odds ratio [OR] = 41.49, 95% CI, 9.55–180.28, p < 0.001) after thiopurine therapy. Ten of the 19 patients with leukopenia were heterozygous for NUDT15 c.415C > T while one patient was homozygous for NUDT15 c.415C > T variation. The patient who was homozygous for NUDT15 c.415C > T was a 25-year-old Chinese male (78 kg) who was started on 100 mg of AZA (1.3 mg/kg). The patient developed profound neutropenic sepsis (WBC count 6 × 109/L and neutrophil count unrecordable) and alopecia requiring antibiotics and granulocyte colony-stimulating factor (GCSF) for 2 weeks. Other two patients had NUDT15 c.52G > A (Table 2).
No co-occurrences of TPMT and NUDT15 variations were observed in our cohorts. No TPMT or NUDT15 variants were identified in five (26.3%) of the 19 patients who developed thiopurine-induced leukopenia.
Individually, NUDT15 c.415C > T had a sensitivity and specificity of 57.9 and 94.0%, respectively, (OR = 21.45, 95% CI 5.94–77.41, p < 0.001) in predicting thiopurine-induced leukopenia while NUDT15 c.52G > A were only observed in patients with leukopenia. In combination, both NUDT15 variations had a sensitivity and specificity of 68.4 and 94%, respectively, of predicting thiopurine-induced leukopenia (OR = 33.80, 95% CI 8.99–127.05, p < 0.001) (Table 3).
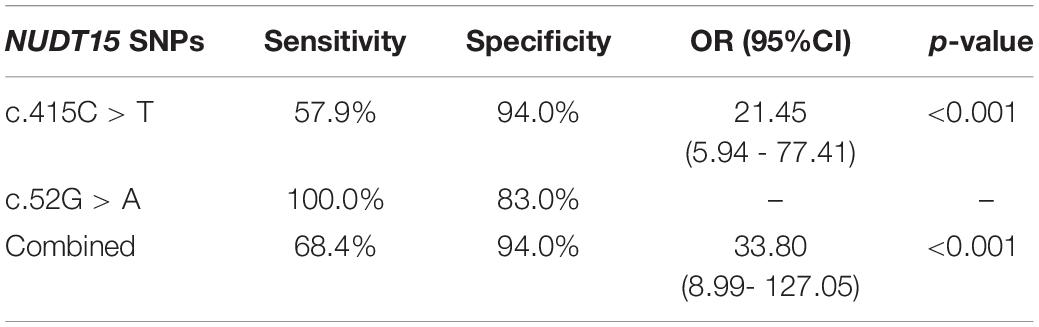
Table 3. Sensitivity and specificity of combined NUDT15 SNPs c415.C > T and c.52G > A in predicting thiopurine-induced leukopenia.
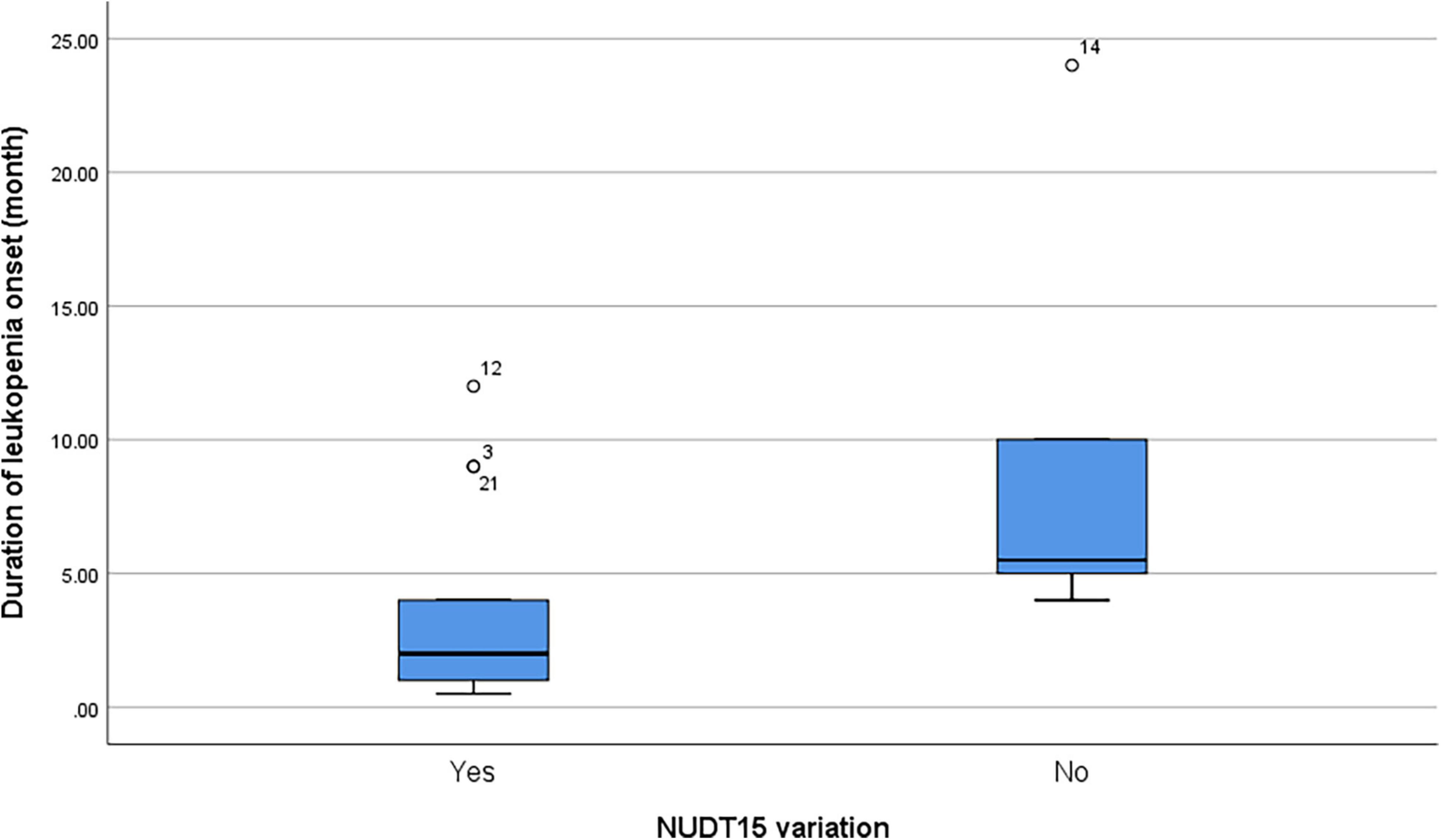
Among patients with leukopenia (n = 19), the presence of NUDT15 variations (n = 13 including both NUDT15 c.415C > T and NUDT15 c.52G > A) also predicted a shorter onset to leukopenia (median = 2 months and range = 0.5–12 months) compared to patients with wild-type NUDT15 (median = 5.5 months, range = 4–24 months; p = 0.045) (Figure 1).
Ethnicity and Nudix Hydroxylase 15 Variations
The sub-group analysis was conducted for the three different ethnic groups in Malaysia separately. The NUDT15 variations (combination of c.415C > T and c.52G > A) were shown to be strongly associated with leukopenia among Chinese and Indians. In Malay patients, however, only 1 patient (25%) with leukopenia had NUDT15 mutations. This was not statistically significant due to the small number of patients (Table 4).
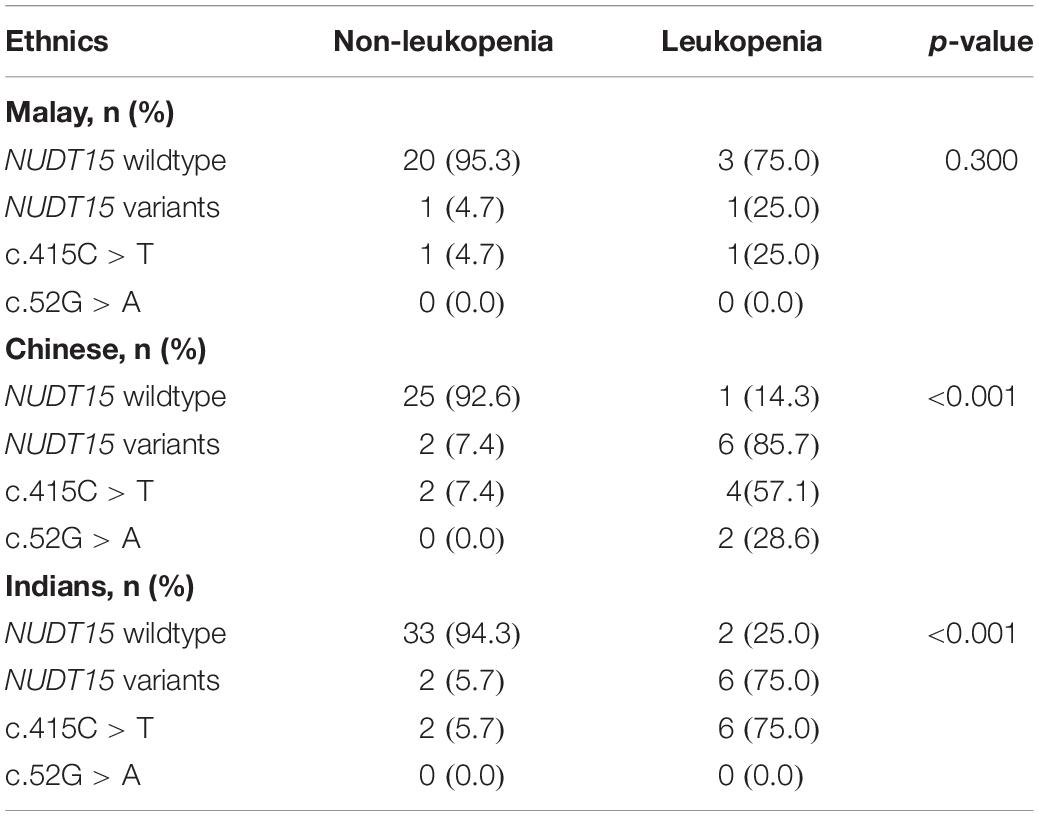
Table 4. The NUDT15 variations in patients with IBD with and without leukopenia among the three major ethnic groups in Malaysia.
Discussion
Thiopurine is an important therapeutic agent in the armamentarium of autoimmune conditions in countries where reimbursement for expensive therapies, such as biologics, remains limited. Unfortunately, they are associated with a significant risk of complications, and among the most life-threatening is leukopenia, which is closely linked with the individual’s pharmacogenetic profile. TPMT, the first identified gene to be strongly associated with thiopurine-induced myelotoxicity, was discovered mostly among White populations. Studies have shown that TPMT genotyping prior to thiopurine therapy reduces the risk of adverse effects without compromising treatment efficacy (17–20). However, the low prevalence of TPMT variations in Asians limited the utility of TPMT genotyping. Many recent studies from Asia have shown that the frequency of TPMT mutation is about 1.2–2.0% in the Asian population and was not predictive of the occurrence of leukopenia in this population (9–11, 21–23). In contrast, most studies from this region have shown that the incidences of allelic mutation in NUDT15, mainly c.415C > T, range between 7.4 and 25.7% in Asian populations and strongly predict leukopenia (11–15, 21, 22).
Our study shows that the prevalence of NUDT15 c.415C > T is 12.8% in Malaysian patients with IBD, similar to a study conducted in Singapore with similar major ethnicities (14). In our multiethnic population, the predictive sensitivity of NUDT15 c.415C > T for leukopenia was 57.9%, which was within the range of other studies with homogeneous ethnicity including Chinese (49–75%) (10, 11, 24), Koreans (40–90%) (9, 22, 25), and Japanese (44–60%) (12, 13). In addition, NUDT15 c.415C > T was also reported to be associated with thiopurine-induced myelotoxicity in European and native American population (20, 26, 27), although the prevalence was much lower compared with Asian populations. The predictive sensitivity of NUDT15 c.415C > T was 3.27% in general Europeans (26) and 4.0% in non-Finnish Europeans (27). Other variants of NUDT15 were shown to have a higher association with the European population. Walker et al. (27) reported that 9.5% of non-Finnish European patients with IBD with thiopurine-induced myelotoxicity had NUDT15 variants, with the majority having p.Gly17_Val18del (NUDT15 c.37_42delGGAGTC) variant (19/35) (27). Schaeffeler et al. (26) on the other hand reported a higher prevalence of NUDT15 c.*7G > A (7.48%) in their general European population (26). In both studies, TPMT variants were still mainly responsible for their thiopurine-induced myelotoxicity.
The other variant, NUDT15 c.52G > A, had a much lower prevalence and was only found among the Chinese ethnic group. Both cases were leukopenic, which resulted in a sensitivity of 100%, but due to the very small numbers, this should be interpreted with caution. Sutiman et al. (14) reported that two heterozygous c.52G > A mutations in the Malays and Chinese populations were shown to have trends of the lower nadir of white cell count and absolute neutrophil count; however, no statistical significance was seen due to the low prevalence and small sample size (14). A large cohort study conducted involving the Han Chinese population reported a prevalence of 4.5% and a sensitivity of 47.9% in predicting leukopenia for this variant (28).
Combining these two, NUDT15 variations strongly predicted leukopenia in our population, where individuals with the variants were 41 times more likely to develop leukopenia than those without. Patients with NUDT15 variations were also shown to correlate with a shorter onset of leukopenia compared with patients with wild-type NUDT15. Yang et al. (9) reported that NUDT15 C > T was found to have a higher correlation with the onset of leukopenia at less than 8 weeks. Other studies concluded that NUDT15 c.415C > T variation was associated with early onset leukopenia (29, 30). Interestingly, NUDT15 variants were not identified in about one-third of patients who developed leukopenia in the current study. Schaeffeler et al. (26) also showed that about 38% of thiopurine-induced myelotixicty patients did not have TPMT or NUDT15 variations and comedication toxicity was also excluded (26). We postulate that this may be due to other novel mutations within the NUDT15 gene or other genes that have not yet been discovered.
In view of our multiethnic population, we also ascertained specifically whether the NUDT15 variants predicted leukopenia in all the major ethnic groups in Malaysia or only in certain ethnic groups. The present study showed clearly that NUDT15 variants strongly predicted leukopenia not only in the Chinese ethnic group but also in the Indian ethnic group. This is somewhat surprising as the ethnic Indians in Malaysia are thought to be genetically diverse when compared with other homogeneous East Asian populations (Japanese, Koreans, and Chinese). Nevertheless, the finding is completely consistent with studies from India, which showed that the NUDT15 variants also strongly predicted leukopenia (15). Numerically, NUDT15 variations did not seem to be highly predictive in Malays (only 1 out of the 4 patients with leukopenia had the variant), and only 2 out of 25 Malay individuals in this study had the variants. Compared with the Chinese and Indians, which had a predictive percentage of 87.5 and 75%, respectively, NUDT15 variants did not have a significant contribution to the leukopenia in Malays. However, this could also be due to the small sample size and the lower number of patients with leukopenia of Malay ethnicity.
Inflammatory bowel disease is emerging rapidly in Malaysia. The highest prevalence is among the Indian ethnic group, but it is reported in all the major ethnicities (31). As mentioned previously, thiopurines remain an important therapeutic option in our population and will probably continue to be one as they are relatively cheap, efficacious, and familiar with physicians as compared with other immunomodulators, such as methotrexate, tacrolimus, and mycophenolate. Therefore, optimizing its use will remain a relevant issue in the near future in Asia. Recently, the Clinical Pharmacogenetics Implementation Consortium Guidelines also recommended NUDT15 genotyping in the Asian population if available (32). Unlike TPMT, which was widely recognized, currently, there is no regulation or enforced screening of NUDT15 despite much research having proven the relevance of these genetic factors prior to starting thiopurine therapy. From our study, we encourage all patients regardless of ethnicity to be screened for the identified NUDT15 mutations in view of the high sensitivity and specificity (14, 15, 30). Life-threatening side effects in our patient who was homozygous for the NUDT15 variant could have been avoided had screening been carried out prior to commencing therapy.
The main limitation of our study is the relatively small sample size. Future studies involving larger cohorts, particularly among the Malay ethnic group, are needed. Another limitation is that this study focused only on two of each NUDT15 variant mainly due to the high prevalence reported in previous studies. Other NUDT15 variations, such as c.457C > T, c.37_42delGGAGTC, and c.55_56insGAGTCG, were not included in this study, owing to the low frequency of these variants reported previously in Singapore (14, 20). Therefore, potential contribution of these variants may be missed out in our cohort.
Conclusion
In conclusion, NUDT15 variants strongly predicted thiopurine-induced leukopenia across a multiethnic Asian population, particularly among the Chinese and Indians. Screening for these variants should be mandatory prior to commencing thiopurine therapy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical Research Ethics Committee, University of Malaya Medical Centre. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
IH, WL, and RR: study conception. NI, SS, RN, KC, ZW, and AL: acquisition of data. X-HK and SW: analysis and interpretation of data and drafting the manuscript. IH, WL, RR, NI, SS, RN, KC, ZW, and AL: revision for important intellectual content. All authors approved the final version of the manuscript.
Funding
This study was supported by the Malaysian Society of Gastroenterology & Hepatology (MSGH) Research Award.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.880937/full#supplementary-material
References
1. Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: east meets west. J Gastroenterol Hepatol. (2020) 35:380–9. doi: 10.1111/jgh.14872
2. Hilmi I, Jaya F, Chua A, Heng WC, Singh H, Goh KL. A first study on the incidence and prevalence of IBD in Malaysia–results from the Kinta Valley IBD epidemiology study. J Crohns Colitis. (2015) 9:404–9. doi: 10.1093/ecco-jcc/jjv039
3. Dewit O, Starkel P, Roblin X. Thiopurine metabolism monitoring: implications in inflammatory bowel diseases. Eur J Clin Invest. (2010) 40:1037–47. doi: 10.1111/j.1365-2362.2010.02346.x
4. Bradford K, Shih DQ. Optimizing 6-mercaptopurine and azathioprine therapy in the management of inflammatory bowel disease. World J Gastroenterol. (2011) 17:4166–73. doi: 10.3748/wjg.v17.i37.4166
5. Asada A, Nishida A, Shioya M, Imaeda H, Inatomi O, Bamba S, et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol. (2016) 51:22–9. doi: 10.1007/s00535-015-1142-4
6. Dean L. Azathioprine Therapy and TPMT and NUDT15 Genotype. Bethesda, MD: National Center for Biotechnology Information (2012).
7. Luber RP, Honap S, Cunningham G, Irving PM. Can we predict the toxicity and response to thiopurines in inflammatory bowel diseases? Front Med. (2019) 6:279. doi: 10.3389/fmed.2019.00279
8. Asadov C, Aliyeva G, Mustafayeva K. Thiopurine S-Methyltransferase as a pharmacogenetic biomarker: significance of testing and review of major methods. Cardiovasc Hematol Agents Med Chem. (2017) 15:23–30. doi: 10.2174/1871525715666170529091921
9. Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. (2014) 46:1017–20. doi: 10.1038/ng.3060
10. Wang HH, He Y, Wang HX, Liao CL, Peng Y, Tao LJ, et al. Comparison of TPMT and NUDT15 polymorphisms in Chinese patients with inflammatory bowel disease. World J Gastroenterol. (2018) 24:941–8. doi: 10.3748/wjg.v24.i8.941
11. Zhu X, Wang X-D, Chao K, Zhi M, Zheng H, Ruan H-L, et al. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn’s disease. Aliment Pharmacol Ther. (2016) 44:967–75. doi: 10.1111/apt.13796
12. Kakuta Y, Naito T, Onodera M, Kuroha M, Kimura T, Shiga H, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. (2016) 16:280–5. doi: 10.1038/tpj.2015.43
13. Sato T, Takagawa T, Kakuta Y, Nishio A, Kawai M, Kamikozuru K, et al. NUDT15, FTO, and RUNX1 genetic variants and thiopurine intolerance among Japanese patients with inflammatory bowel diseases. Intest Res. (2017) 15:328–37. doi: 10.5217/ir.2017.15.3.328
14. Sutiman N, Chen S, Ling KL, Chuah SW, Leong WF, Nadiger V, et al. Predictive role of NUDT15 variants on thiopurine-induced myelotoxicity in Asian inflammatory bowel disease patients. Pharmacogenomics. (2018) 19:31–43. doi: 10.2217/pgs-2017-0147
15. Banerjee R, Ravikanth VV, Pal P, Bale G, Avanthi US, Goren I, et al. NUDT15 C415T variant compared with TPMT genotyping in predicting azathioprine-induced leucopenia: prospective analysis of 1014 inflammatory bowel disease patients in India. Aliment Pharmacol Ther. (2020) 52:1683–94. doi: 10.1111/apt.16137
16. MyGovernment. Demography of Population, Malaysia. (2016). Available online at: https://www.malaysia.gov.my/portal/content/30114 (accessed October 9, 2021).
17. Rocha JC, Cheng C, Liu W, Kishi S, Das S, Cook EH, et al. Pharmacogenetics of outcome in children with acute lymphoblastic leukemia. Blood. (2005) 105:4752–8. doi: 10.1182/blood-2004-11-4544
18. Relling MV, Pui CH, Cheng C, Evans WE. Thiopurine methyltransferase in acute lymphoblastic leukemia. Blood. (2006) 107:843–4. doi: 10.1182/blood-2005-08-3379
19. Pui CH, Pei D, Sandlund JT, Ribeiro RC, Rubnitz JE, Raimondi SC, et al. Long-term results of St Jude total therapy studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. (2010) 24:371–82. doi: 10.1038/leu.2009.252
20. Moriyama T, Relling MV, Yang JJ. Inherited genetic variation in childhood acute lymphoblastic leukemia. Blood. (2015) 125:3988–95. doi: 10.1182/blood-2014-12-580001
21. Liang DC, Yang CP, Liu HC, Jaing TH, Chen SH, Hung IJ, et al. NUDT15 gene polymorphism related to mercaptopurine intolerance in Taiwan Chinese children with acute lymphoblastic leukemia. Pharmacogenomics J. (2016) 16:536–9. doi: 10.1038/tpj.2015.75
22. Lee YJ, Hwang EH, Park JH, Shin JH, Kang B, Kim SY. NUDT15 variant is the most common variant associated with thiopurine-induced early leukopenia and alopecia in Korean pediatric patients with Crohn’s disease. Eur J Gastroenterol Hepatol. (2016) 28:475–8. doi: 10.1097/MEG.0000000000000564
23. Takatsu N, Matsui T, Murakami Y, Ishihara H, Hisabe T, Nagahama T, et al. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol. (2009) 24:1258–64. doi: 10.1111/j.1440-1746.2009.05917.x
24. Huang P-W, Tseng Y-H, Tsai T-F. Predictive value of NUDT15 variants on neutropenia among Han Chinese patients with dermatologic diseases: a single-center observational study. Dermatol Ther (Heidelb). (2020) 10:263–71. doi: 10.1007/s13555-020-00360-4
25. Kim SY, Shin JH, Park JS, Kang SY, Nam TS, Kim JK, et al. NUDT15 p.R139C variant is common and strongly associated with azathioprine-induced early leukopenia and severe alopecia in Korean patients with various neurological diseases. J Neurol Sci. (2017) 378:64–8. doi: 10.1016/j.jns.2017.04.041
26. Schaeffeler E, Jaeger SU, Klumpp V, Yang JJ, Igel S, Hinze L, et al. Impact of NUDT15 genetics on severe thiopurine-related hematotoxicity in patients with European ancestry. Genet Med. (2019) 21:2145–50. doi: 10.1038/s41436-019-0448-7
27. Walker GJ, Harrison JW, Heap GA, Voskuil MD, Andersen V, Anderson CA, et al. Association of genetic variants in NUDT15 with thiopurine-induced myelosuppression in patients with inflammatory bowel disease. JAMA. (2019) 321:773–85. doi: 10.1001/jama.2019.0709
28. Chao K, Wang X, Cao Q, Qian J, Wu K, Zhu X, et al. Combined detection of NUDT15 variants could highly predict thiopurine-induced leukopenia in Chinese patients with inflammatory bowel disease: a multicenter analysis. Inflamm Bowel Dis. (2017) 23:1592–9. doi: 10.1097/MIB.0000000000001148
29. Fei X, Shu Q, Zhu H, Hua B, Wang S, Guo L, et al. NUDT15 R139C variants increase the risk of azathioprine-induced leukopenia in Chinese autoimmune patients. Front Pharmacol. (2018) 9:460. doi: 10.3389/fphar.2018.00460
30. Miao Q, Yan L, Zhou Y, Li Y, Zou Y, Wang L, et al. Association of genetic variants in TPMT, ITPA, and NUDT15 with azathioprine-induced myelosuppression in southwest china patients with autoimmune hepatitis. Sci Rep. (2021) 11:7984. doi: 10.1038/s41598-021-87095-0
31. Mokhtar NM, Nawawi KNM, Verasingam J, Zhiqin W, Sagap I, Azman ZAM, et al. A four-decade analysis of the incidence trends, sociodemographic and clinical characteristics of inflammatory bowel disease patients at single tertiary centre, Kuala Lumpur, Malaysia. BMC Public Health. (2019) 19:550. doi: 10.1186/s12889-019-6858-2
Keywords: NUDT15, inflammatory bowel disease, leukopenia, genetic polymorphism, thiopurines
Citation: Khoo X-H, Wong SY, Ibrahim NRW, Ng RT, Chew KS, Lee WS, Wong ZQ, Raja Ali RA, Shahrani S, Leow AH-R and Hilmi IN (2022) Nudix Hydroxylase 15 Mutations Strongly Predict Thiopurine-Induced Leukopenia Across Different Asian Ethnicities: Implications for Screening in a Diverse Population. Front. Med. 9:880937. doi: 10.3389/fmed.2022.880937
Received: 22 February 2022; Accepted: 28 April 2022;
Published: 05 August 2022.
Edited by:
Mitsuhiro Takeno, Nippon Medical School Musashi Kosugi Hospital, JapanReviewed by:
Masaaki Mori, Tokyo Medical and Dental University, JapanPrateek Bhatia, Postgraduate Institute of Medical Education and Research, India
Copyright © 2022 Khoo, Wong, Ibrahim, Ng, Chew, Lee, Wong, Raja Ali, Shahrani, Leow and Hilmi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ida Normiha Hilmi, ida@ummc.edu.my
 Xin-Hui Khoo1
Xin-Hui Khoo1  Ruey Terng Ng
Ruey Terng Ng Way Seah Lee
Way Seah Lee Raja Affend Raja Ali
Raja Affend Raja Ali Alex Hwong-Ruey Leow
Alex Hwong-Ruey Leow Ida Normiha Hilmi
Ida Normiha Hilmi