Comparison of Cervical Cytopathological Diagnosis Using Innovative Qi Brush and Traditional Cervex-Brush® Combi
- 1Department of Obstetrics and Gynecology, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
- 2Department of Obstetrics and Gynecology, Shaanxi Provincial People's Hospital, Xi'an, China
- 3Department of Pathology, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
- 4Aviation General Hospital of Beijing, Medical University and Beijing Institute of Translational Medicine, University of Chinese Academy of Sciences, Beijing, China
Objectives: To compare the effectiveness between Qi brush and Cervex-Brush® Combi for the diagnosis of cervical lesions.
Methods: After we registered a random-control clinical trial on the Chinese Clinical Trial Registry (No. XJTU1AF2017LSK-25), cervical cell samples were successively collected with both Qi brush and Cervex-Brush® Combi before undergoing colposcope. Colposcopy with biopsy was performed later. Histological diagnosis was regarded as the gold standard in this study. The following indices of the two brushes were compared: sampling degree of satisfaction and presence rate of metaplastic cells, together with sensitivity (Se), specificity (Sp), false positives (FP), false negatives (FN), positive predictive value (PPV), and negative predictive value (NPV). The kappa value was used to measure the inter-rater agreement of the Qi brush and Cervex-Brush® Combi in diagnosing cervical lesions.
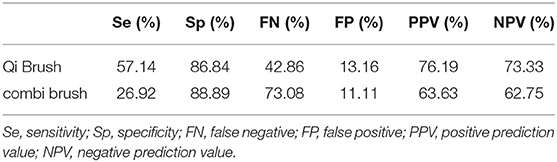
Results: In total, 74 patients were enrolled in this study. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the Qi brush were 57.14, 86.84, 76.19, and 73.33%, respectively. For the Cervex-Brush® Combi, they were 26.92, 88.89, 63.63, and 62.75%, respectively. In addition, the Qi brush had a higher satisfied sampling rate (89.19%) than the Cervex-Brush® Combi (83.78%), and the P-value was 0.336 using Chi-square test. The kappa value was 0.444, which indicated a medium agreement between these two brushes, and the sensitivity of the Qi brush was higher than that of the Cervex-Brush® Combi, with significant statistical difference (P = 0.039<0.05).
Conclusions: The Qi brush was more effective than the Cervex-Brush® Combi for sampling and also had a slightly higher accuracy in diagnosing in cytology. In terms of social and economic benefits, the Qi brush may be a better cervical cytology collector.
Introduction
Cervical cancer is the second-leading cause of cancer mortality among women aged 20–39 years worldwide, causing nine deaths per week in this age group, and the mortality among women in poor counties is twice that of women in affluent counties (1). In cervical cancer, squamous cell carcinoma is the most common type and accounts for almost 80% of cervical cancer. Squamous cell cancer generally begins with precancerous lesions, from low squamous intraepithelial lesions to high squamous intraepithelial lesions (2). Current cervical screening programs are used for detecting premalignant lesions to prevent invasive cervical cancer (3). Since the screening programs have been established, cervical cancer incidence rates have decreased by as much as 80% over the past 40 years in several Western countries (4, 5).
As screening with cervical cytology is considered to be an effective method for detecting cervical cancer and precancerous lesions, previous studies have illustrated that the sampling quality is associated with cervical sampling devices, which has resulted in around 60% false-negative results, especially with endogenous cervical cancer (6, 7). The collecting capacity of devices is largely affected by the shape of the device and its material, and a less-than-ideal device ultimately results in false results (8).
The sampling devices recommended by the European Guidelines for Quality Assurance are the combination of Cytobrush and Ayre spatula, the anatomical spatula, and the Cervex-Brush® Combi (9). A study of the Cervex-Brush Combi indicated that sampling with it resulted in a 2- to 3-fold harvest of endocervical cells when compared with the Cervex-Brush (10). Another study using the Cervex-Brush® Combi and Cytobrush + Ayres spatula, which included 1,235 patients, showed the specificities were 67.8 and 61.7%, respectively, and there was a better sensitivity for CIN2+ using Cervex-Brush® Combi when the cytology result was HSIL+ (6). However, the Cervex-Brush® Combi is relatively expensive for patients in developing countries.
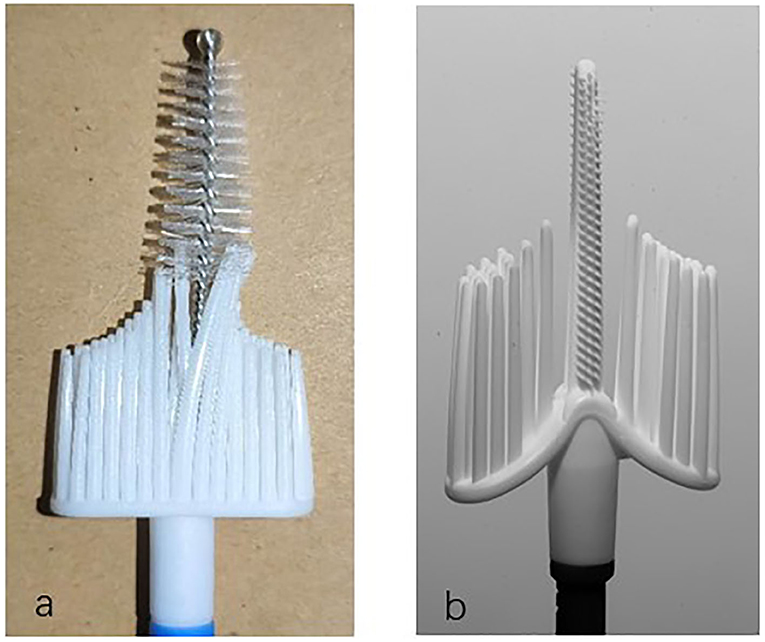
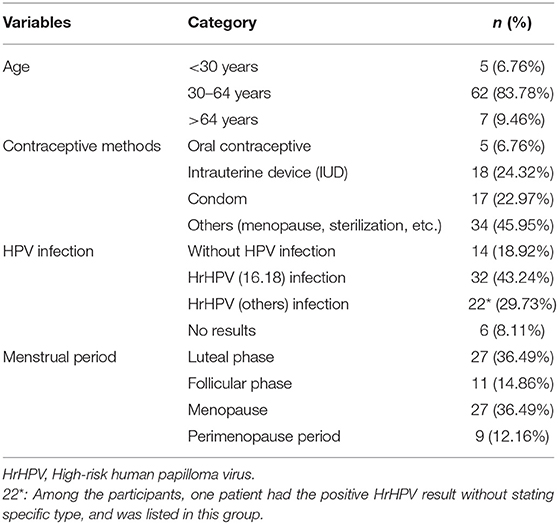
Based on previous studies, our research group developed a patented cervical brush named the Qi brush (patent ZL 201420720356.8). The Qi brush (Figure 1) consists of endocervical and ectocervical arms with plastic bristles, sheath, and handle. The length of the endocervical arm is 2.0 cm, and the length of the bristles is 0.25–0.4 cm from top to near the ectocervical arm. The design of the endocervical arm allows the brush to fit the cervical canal and enable maximum sampling of cells. The ectocervical arm is semilunar and 1.0 cm long, and the length of the bristles is 1.5–2.5 cm from side to middle. As for the Cervex-Brush® Combi, the endocervical bristle is 1.0 cm long and is composed of short semicircular soft, flexible fibers, and its length is isometric. However, the length of cervical canal is 2.5–3.0 cm, which implies that an endocervical bristle of right length might result in more satisfied sampling.
In this study, we aimed to evaluate the accuracy of liquid-based cytology using the Qi brush vs. the Cervex-Brush® Combi for the diagnosis of cervical cytology.
Materials and Methods
Patients
From March 2018 to August 2019, a total of 74 patients were enrolled in this trial in the First Affiliated Hospital of Xi'an Jiao Tong University. The inclusion criterion of the study was as follows: patients who were detected with abnormal ThinPrep cytology test (TCT) results and who needed to undergo colposcopy with biopsy, the histological diagnosis being used as the gold standard. The exclusion criteria included total hysterectomy, cervical surgery including conization, loop electrosurgical excision procedure (LEEP), radiation therapy, acute inflammation, and confirmation of pregnancy. The study was approved by the Institutional Review Board of the First Affiliated Hospital of Xi'an Jiaotong University. Informed consent was obtained from all patients.
Sample Collection
All participants were numbered according to the registration order. Before the colposcopy, the patients in the odd-numbered group were sampled with the Qi brush (Xi'an Meijiajia Bio-Technologies Co. Ltd., China) (Figure 1a) first and then the Cervex-Brush® Combi (Rovers® Medical Devices, Netherlands, 89171-022) (Figure 1b) (6), while the patients in the even-numbered group were sampled in the reverse order. The instructions for use of these two brushes were as follows: expose the cervix, wipe off the secretion with a cotton swab, gingerly insert the brush into the endocervical canal until the lateral bristles touch the ectocervix, rotate t turns clockwise, and finally insert the brush head into the preservation solution bottle (6). The two bottles were labeled clearly and only the operating doctor knew the tags and their corresponding variables. Finally, the samples were delivered to the Department of Pathology for further staining procedures and cytological diagnosis. Participants then underwent colposcopy with biopsy after collection of cytological samples.
Cytological and Histological Diagnosis
Both cytological and histological diagnoses were made by two experienced pathologists using conventional optical microscopy. The pathologists evaluated the quantity of columnar cells, squamous cells, and metaplastic squamous cells from both endo- and exocervical samples. Samples containing one cellular group with >15 cells were defined as poor samples. In other words, the presence of two or more cellular groups of at least 15 cells was regarded as acceptable (11).
The Bethesda 3-tier system was used as the cervical cytological diagnostic criteria. And positive results included: (1) atypical squamous cells of unknown significance (ASC-US); (2) lesion that cannot exclude high-grade squamous intraepithelial lesion (ASC-H); (3) low-grade squamous intraepithelial lesion (LSIL); (4) low-grade squamous intraepithelial lesion (LSIL); (5) high-grade squamous intraepithelial lesion (HSIL); (6) squamous cell carcinoma (SCC) (12). As for the histological diagnosis, CIN1-3 (Cervical Intraepithelial Neoplasia) and squamous carcinoma were regarded as positive results according to the WHO classification (13).
Statistical Analysis
The data of all the participants were obtained from their medical records. In this study, we compared the sampling degree of satisfaction and presence rate of metaplastic cells by using the Qi brush and the Cervex-Brush® Combi. Sensitivity (Se), specificity (Sp), false positives (FP), false negatives (FN), positive predictive values (PPV), and negative predictive values (NPV) were analyzed for the comparison of the diagnostic accuracy of the two brushes. Chi-square test was used for numerical variables, and P < 0.05 indicated significant statistical difference. The inter-rater agreement of the Qi brush and the Cervex-Brush® Combi in diagnosing cervical lesions was measured by Cohen's kappa coefficient. Analysis was processed by SPSS22.0 statistical software.
Results
Characterization of Patients Included
Overall, 74 patients from the Inpatient Department of the First Affiliated Hospital of Xi'an Jiao Tong University were enrolled in this study, and all of the participants ultimately underwent colposcopy and biopsy. The characteristics of the patients are shown in Table 1. The age of the patients included ranged from 21 to 68 years, with an average age of 45.39 years. Most of the patients were between 30 and 64 years of age (n = 62,83.78%), which was the age recommended for screening. As for the choice of contraceptive method, oral contraceptives (n = 5, 6.76%) were the least common choice, while IUD (intrauterine device) (n = 18, 24.32%) and condoms (n = 17, 22.97%) were more commonly used. Since 27 of the patients were menopausal women and 9 of them were at perimenopausal stage, natural menopause and sterilization were the most common choices. Among the patients enrolled, 54 of them were infected with HrHPV (high-risk papilloma virus), and 32 of them were positive for HPV16, HPV18, or both.
Evaluation of Sampling Degree of Satisfaction
There were eight poor samples using the Qi brush and 12 poor samples using the Cervex-Brush® Combi. Among the eight samples using the Qi brush, five were sampled after using the Cervex-Brush® Combi. As for the 12 samples using the Cervex-Brush® Combi, seven were sampled after using the Qi brush. In addition, two patients' samples were poor samples no matter which brush was used for sampling. Ultimately, the satisfied sampling rate of the Qi brush and the Cervex-Brush® Combi were 89.19 and 83.78%, respectively. However, P > 0.05 (P = 0.336) using Chi-square test indicated there was no statistical difference using those two brushes.
As for obtaining squamous metaplastic cells using different instruments, there were 46/66 samples (69.7%) and 44/62 samples (70.97%) that used the Qi brush and the Cervex-Brush® Combi, respectively. P > 0.05 (P = 0.875) using Chi-square test indicated there was no statistical difference using those two brushes in obtaining metaplastic cells.
Cytological and Histological Diagnosis
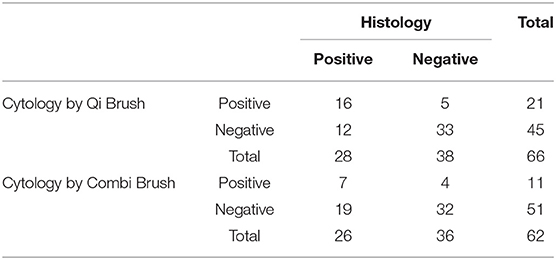
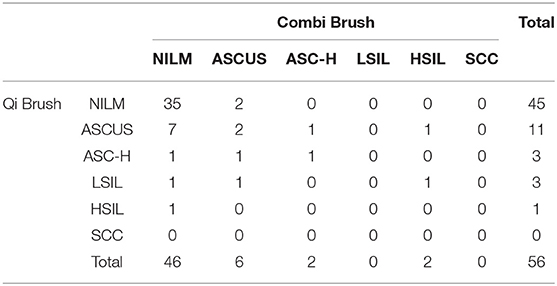
Tables 2, 3 show the comparison of cytological and histological diagnoses and the diagnostic accuracy between the Qi brush and the Cervex-Brush® Combi, respectively. The diagnostic sensitivity of the Qi brush and the Cervex-Brush® Combi was 57.14 and 26.92%, respectively, with significant statistical difference (P = 0.039 <0.05). The specificity was 86.84 and 88.89%, respectively. The Qi brush had a diagnostic accuracy in PPV of 76.19% and in NPV of 73.33%, while the Cervex-Brush® Combi was 63.63 and 62.75%, respectively. The comparison of cytological diagnosis between the two brushes is represented in Table 4. The kappa value was 0.444, which indicates a medium agreement between these two brushes, and the diagnostic sensitivity of the Qi brush was significantly higher than that of the Cervex-Brush® Combi.
Discussion
Currently, there are two types of screening terms for cervical cancer, the Papanicolaou test and the HPV test. As for cytology screening, since liquid-based cytology was introduced in the 1990s, it has seemed to be a better tool for processing cervical samples (14–16). Sampling devices recommended by the European Guidelines for Quality Assurance include the cervical brush (Cervex-Brush, Rovers), a combination of a spatula for ectocervical sampling and an endocervical brush for endocervical sampling, and an extended-tip spatula alone (9). The combination of the Cytobrush and the Ayre spatula was once the most commonly used sampling device for the reason that the Ayre spatula alone failed to collect cells in the cervical canal. However, the use of two sampling devices generated more blood contamination on the background of the slides (17). The Aylesbury spatula was the most commonly used extended-tip spatula used alone and was reported to obtain more endocervical cells than other spatulas (18). Since the spatula can't cannot be applied to patients with cervical stenosis, the Cervex-Brush—which had the advantages of painless operation, low bleeding volume in patients, and the sampling of cervical cells—was later more commonly used. The Cervex-Brush® Combi used in this research combined the important features of the original Cervex-Brush® with the benefits of the EndoCervex-Brush® and resulted in a 2- to 3-fold increase in the number of sampled endocervical cells compared to the Cervex-Brush (10). As depicted in the product brochure and Figure 1b, the endocervical bristle of the Cervex-Brush® Combi is 1.0 cm long, it is composed of short semicircular soft, flexible fibers, and its length is isometric. Comparatively, the endocervical bristle of the Qi brush is 2.0 cm long and is spirally formed with short burrs, which gradually become longer and denser from top to bottom according to the anatomical structure of the cervical canal.
Although in theory the design of the Qi brush could lead to higher sampling satisfaction rate and more chances for obtaining metaplastic cells, the results in this study showed there is no statistical difference between the two brushes. Moreover, the sampling satisfaction rate of the Qi brush and the Cervex-Brush® Combi is 89.19 and 83.78%, respectively, which is lower than De Palo's 94.68% and Abdali's 92.1% (19, 20). This might be because the two types of cervical brushes were sampled alternately. When one of the cervical brushes was sampled first and rotated 2–3 turns clockwise, there was a risk of bleeding caused by cervical columnar epithelial injury. Subsequently, the background of the cervical slides was contaminated with red blood cells, resulting in decreased sampling satisfaction rate.
As Woodman and Mitchell noted, it is actually the presence of metaplastic cells that predicts squamous dysplasia, and lumping the columnar cells and metaplastic cells together weakens the association (21, 22). In this research, the metaplastic cells presence rate of the Qi brush and the Cervex-Brush® Combi is 69.7 and 70.97% (P = 0.875>0.05), respectively, with no significant difference between them. It could also result in low sensitivity and false-negative results, in addition to the sampling satisfaction rate.
In this study, taking the histological results as the gold standard, we compared the diagnostic accuracy using the Qi brush and the Cervex-Brush® Combi. Compared with the Cervex-Brush® Combi, the Qi brush had a higher sensitivity (57.14%) and PPV (76.19%). The specificity and NPV of the Qi brush were 86.84 and 73.33%, respectively, while those of the Cervex-Brush® Combi were 88.89 and 62.75%. However, the sensitivity of the Cervex-Brush® Combi in this study was lower than in the previous study, which might have been due to the professionalism of the operators and the relatively small number of patients enrolled in this study (6, 10, 23, 24). In addition, the price of the Qi brush is less than that of the Cervex-Brush® Combi, which is around 20 yuan. That makes the Qi brush an economical and practical choice for screening when combined with the economic social benefits.
The limitations of this study are due to the small number of participants and the failure to strictly control operational normatives. Consequently, further study based on larger study populations is under way to verify the use of the Qi brush in cervical cytology sampling.
Our study showed that cervical cytology sampled with the Qi brush has a higher accuracy with histological diagnosis in detecting cervical cytological results. In addition, the Qi brush has a lower insufficient sampling rate. After the social and economic benefits are factored in, the Qi brush may be a better cervical cytology collector.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation, to any qualified researcher.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the First Affiliated Hospital of Xi'an Jiaotong University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
QL, YZ, and XT devised the conceptual idea. LWu, YL, XF, LZ, HH, and GS executed the work. XT wrote the manuscript. LH, LWa, and YW critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Shaanxi Provincial Collaborative Technology Innovation Project (2018XT-002 and 2017XT-026), the Clinical Research Award of the First Affiliated Hospital of Xi'an Jiaotong University, China (XJTU1AF-2018-017 and XJTU1AF-CRF-2019-002), the Major Basic Research Project of Natural Science of Shaanxi Provincial Science and Technology Department (2018JM7073 and 2017ZDJC-11), the Key Research and Development Project of Shaanxi Provincial Science and Technology Department (2017ZDXM-SF-068 and 2019QYPY-138), and the Medical Research Project of Xi'an Social Development Guidance Plan (2017117SF/YX011-3). The sponsors have no involvement in study design, data collection, analysis, and interpretation, or in writing of the manuscript. The authors thank the patient for their support and cooperation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank the participating women as well as all doctors, nurses, and laboratory staff employed at The First Affiliated Hospital of Xi'an Jiaotong University.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA A Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Ramírez A, Vera E, Gamboa-Domínguez A, Lambert P, Gariglio P, Camacho J. Calcium-activated potassium channels as potential early markers of human cervical cancer. Oncol Lett. (2018) 15:7249–54. doi: 10.3892/ol.2018.8187
3. Kremer WW, Berkhof J, Bleeker MC, Heideman DA, van Trommel NE, van Baal MW, et al. Role of FAM19A4/miR124-2 methylation analysis in predicting regression or non-regression of CIN2/3 lesions: a protocol of an observational longitudinal cohort study. BMJ Open. (2019) 9:e029017. doi: 10.1136/bmjopen-2019-029017
4. Toliman PJ, Kaldor JM, Tabrizi SN, Vallely AJ. Innovative approaches to cervical cancer screening in low- and middle-income countries. Climacteric. (2018) 21:235–8. doi: 10.1080/13697137.2018.1439917
5. Wood B, Lofters A, Vahabi M. Strategies to reach marginalized women for cervical cancer screening: A qualitative study of stakeholder perspectives. Curr Oncol. (2018) 25:e8–16. doi: 10.3747/co.25.3851
6. Simonsen M, Tavares Guerreiro Fregnani JH, Possati Resende JC, Antoniazzi M, Longatto-Filho A, Scapulatempo-Neto C. Comparison of the cervex-brush® combi and the cytobrush+ayres spatula combination for cervical sampling in liquid-based cytology. PLoS ONE. (2016) 11:e0164077. doi: 10.1371/journal.pone.0164077
7. Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, et al. Screening for cervical cancer: US preventive services task force recommendation statement. JAMA. (2018) 320:674–86. doi: 10.1001/jama.2018.10897
8. Hoda RS, Loukeris K, Abdul-Karim FW. Gynecologic cytology on conventional and liquid-based preparations: a comprehensive review of similarities and differences. Diagn Cytopathol. (2013) 41:257–78. doi: 10.1002/dc.22842
9. Lukic A, Iannaccio S, Heyn R, Villani S, Nobili F, Giarnieri E, et al. Satisfactory sampling in cytological cervical diagnosis: comparison between a conventional and a new sampling device. Anticancer Res. (2013) 33:917–22.
10. Depuydt CE, Benoy IH, Bailleul EJ, Vandepitte J, Vereecken AJ, Bogers JJ. Improved endocervical sampling and HPV viral load detection by Cervex-Brush Combi. Cytopathology. (2006) 17:374–81. doi: 10.1111/j.1365-2303.2006.00386.x
11. Valenzuela P, Martinez P, Santana A, Garrido N, Cano A, Arnanz F. Comparison of cervical smears secured with different instruments. Acta Obstetr Gynecol Scand. (2001) 80:262–6. doi: 10.1034/j.1600-0412.2001.080003262.x
12. Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. (2002) 287:2114–9. doi: 10.1001/jama.287.16.2114
13. Rohner E, Rahangdale L, Sanusi B, Knittel AK, Vaughan L, Chesko K, et al. Test accuracy of human papillomavirus in urine for detection of cervical intraepithelial neoplasia. J Clin Microbiol. (2020) 58:e01443-19. doi: 10.1128/JCM.01443-19
14. Klinkhamer PJJM, Meerding WJ, Rosier PFWM, Hanselaar AGJM. Liquid-based cervical cytology. Cancer. (2003) 99:263–71. doi: 10.1002/cncr.11673
15. Nishio H, Iwata T, Nomura H, Morisada T, Takeshima N, Takano H, et al. Liquid-based cytology versus conventional cytology for detection of uterine cervical lesions: a prospective observational study. Jpn J Clin Oncol. (2018) 48:522–8. doi: 10.1093/jjco/hyy050
16. Pankaj S, Nazneen S, Kumari S, Kumari A, Kumari A, Kumari J, et al. Comparison of conventional Pap smear and liquid-based cytology: a study of cervical cancer screening at a tertiary care center in Bihar. Indian J Cancer. (2018) 55:80–3. doi: 10.4103/ijc.IJC_352_17
17. Rahnama P, Faghihzadeh S, Ziaei S. Effect of the sampling sequence on the quality of Papanicolaou smear. Int J Gynecol Cancer. (2005) 15:66–9. doi: 10.1136/ijgc-00009577-200501000-00011
18. Goorney BP, Lacey CJ, Sutton J. Ayre v Aylesbury cervical spatulas. Genitourin Med. (1989) 65:161–2. doi: 10.1136/sti.65.3.161
19. de Palo G, Stefanon B, Alasio L, Pilotti S. Exact Touch: a new device for cervical cytology. Comparison with Ayre spatula plus. Cytobrush Cytopathol. (2000) 11:322–5. doi: 10.1046/j.1365-2303.2000.00219.x
20. Abdali K, Soleimani M, Khajehei M, Tabatabaee HR, Komar PV, Montazer NR. Comparison of Pap smear quality with anatomical spatula and convenience (spatula-cytobrush) methods: a single blind clinical trial. Asian Pac J Cancer Prev. (2010) 11:1769–72.
21. Woodman CB, Williams D, Yates M, Tomlinson K, Ward K, Luesley D. Indicators of effective cytological sampling of the uterine cervix. Lancet. (1989) 2:88–90. doi: 10.1016/S0140-6736(89)90324-3
22. Leung KM, Lam M, Lee JWY, Yeoh GPS, Chan KW. The significance of endocervical cells and metaplastic squamous cells in liquid-based cervical cytology. Diagn Cytopathol. (2009) 37:241–3. doi: 10.1002/dc.20981
23. Ferris DG, Heidemann NL, Litaker MS, Crosby JH, Macfee MS. The efficacy of liquid-based cervical cytology using direct-to-vial sample collection. J Fam Pract. (2000) 49:1005–11.
Keywords: Qi brush, Cervex-Brush® Combi, cervical cancer, cervical cytology, screening
Citation: Zou Y, Tuo X, Wu L, Liu Y, Feng X, Zhao L, Han L, Wang L, Wang Y, Hou H, Shi G and Li Q (2020) Comparison of Cervical Cytopathological Diagnosis Using Innovative Qi Brush and Traditional Cervex-Brush® Combi. Front. Med. 7:369. doi: 10.3389/fmed.2020.00369
Received: 15 April 2020; Accepted: 16 June 2020;
Published: 24 July 2020.
Edited by:
Renato Franco, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Gustavo Rubino de Azevedo Focchi, Federal University of São Paulo, BrazilMaria Contaldo, University of Campania Luigi Vanvitelli, Italy
Copyright © 2020 Zou, Tuo, Wu, Liu, Feng, Zhao, Han, Wang, Wang, Hou, Shi and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiling Li, liqiling@mail.xjtu.edu.cn
†These authors have contributed equally to this work
 Yuliang Zou1†
Yuliang Zou1†  Xiaoqian Tuo
Xiaoqian Tuo Xue Feng
Xue Feng Lanbo Zhao
Lanbo Zhao Yiran Wang
Yiran Wang Qiling Li
Qiling Li