Fucoidan derived from Sargassum ilicifolium affects growth and hemato-immunological parameters and antioxidant defense in Oscar (Astronotus ocellatus)
- 1Animal Biological Product Research Group, Academic Center for Education, Culture and Research (ACECR), Tehran Organization, Tehran, Iran
- 2Department of Fisheries, Faculty of Fisheries and Environmental Sciences, Gorgan University of Agricultural Sciences and Natural Resources, Gorgan, Iran
- 3Department of Animal and Aquatic Sciences, Faculty of Agriculture, Chiang Mai University, Chiang Mai, Thailand
- 4Functional Feed Innovation Center (FuncFeed), Faculty of Agriculture, Chiang Mai University, Chiang Mai, Thailand
- 5Department of Biology, Islamic Azad University, Tehran, Iran
Fucoidan (Fuc) is a sulfated polysaccharide derived from brown algae and has various biological activities such as immune modulator, growth enhancer, antioxidant and bactericidal. However, there is no information about the effect of fucoidan on ornamental fish. This study investigated the effect of fucoidan from brown algae Sargassum ilicifolium on growth parameters, immunity, and biochemical, antioxidant, and bactericidal activity of serum in Oscar. Fish weighing 49.75 ± 0.96 g were fed fucoidan derived from S. ilicifolium algae at four levels (0%, 0.5%, 1%, and 2%) for 50 days. After 50 days of feeding with fucoidan, there was no significant increase in blood parameters except WBCs (Fuc 2%) compared to the control group (p < 0.05). The weight gain (WG), feed conversion ratio (FCR), and specific growth rate (SGR) after feeding with fucoidan had a significant increase compared to the control group (p < 0.05). Serum biochemical activity such as total protein (Tp) and globulin (Glb) in the groups fed with fucoidan had a significant increase compared to the control group (p < 0.05), but no significant difference was observed in the serum albumin (Alb) activity compared to the control group (p > 0.05). Antioxidant activity of serum, such as SOD and CAT, after feeding with fucoidan, had a significant increase compared to the control group (p < 0.05). In addition, immune parameters such as lysozyme and total complement in groups containing fucoidan significantly increased compared to the control group (p < 0.05). A significant increase was observed in NBT and serum bactericidal activity against Aeromonas hydrophila in the group containing Fuc 2% compared to the control group (p < 0.05). A significant increase was observed in total skin carotenoids in the groups containing 1% and 2% fucoidan compared to the control group (p < 0.05). Overall, it can be concluded that fucoidan of S. ilicifolium algae can increase growth and immune parameters. In addition, fucoidan derived from S. ilicifolium algae could increase skin pigmentation, antioxidant enzyme activity, and bactericidal activity against A. hydrophila.
1 Introduction
While a huge part of the global aquatic production is dedicated to food products, nowadays, the production of ornamental fish has become one of the most important poles of the aquaculture industry in many countries (Rahmati-Holasoo et al., 2022). In addition, these days, we see a significant increase in the expansion of ornamental fish stores, and ornamental fish aquariums are found more and more in offices, restaurants, homes, and hospitals (Hossain and Heo, 2021).
Ornamental fish encountered many stressful factors such as transportation, density, low water quality, and contaminated and inappropriate feeding, which cause a high prevalence of diseases in the field (Hossain and Heo, 2021). With the epidemic of infectious diseases, the aquaculture industry, especially ornamental fish, is threatened (Jaruboonyakorn et al., 2022). According to the findings of the researchers, antibiotics are not a sustainable and suitable solution for the treatment of diseases due to the reduction in immune system activity, irreversible environmental effects, and high cost (Thépot et al., 2022). As of the moment, the best and most appropriate alternative to antibiotics is the medicinal plants such as algae (Mariappan et al., 2023).
Algae are a potential source of medicinal properties and various biomedical applications due to the presence of numerous polysaccharide compounds in their structure (Carpena et al., 2022). Fucoidan (Fuc) is a sulfated polysaccharide consisting of fucose as the main monosaccharide along with some other monomers such as xylose, arabinose, glucose, galactose, mannose, and glucuronic acid (Martins et al., 2023). Fuc is mainly composed of fucose sugar with α-(1→3) and α-(1→4) glucosidic linkages (Zayed et al., 2020). Fucs with a wide range of molecular weight from 7 kDa to 2,379 kDa have been found in brown algae (Do-Amaral et al., 2020). This polysaccharide is found in the matrix of brown seaweed cells and is a sulfated homo- or hetero-polysaccharide (Do-Amaral et al., 2020). Fucs are composed of sulfate ester groups (Do-Amaral et al., 2020; Zayed et al., 2020) and increasing the sulfate content in fucoidan will lead to an increase in anticoagulant activity and antioxidant activities (Wang et al., 2010). Fuc has various biological activities such as immunomodulatory, anti-tumor, anti-coagulant, growth promotion, and antioxidant (Anisha et al., 2022; Haggag et al., 2023). However, in aquaculture, the properties of increasing immunity, growth, antioxidant, and combating bacteria and viruses have been proven (Salehpour et al., 2021; Li et al., 2023).
Cichlids are common ornamental fish due to their color diversity (Hoseinifar et al., 2016). Oscar (Astronotus ocellatus) belongs to the Cichlidae family and is native to the tropical regions of South America (Wang Q. et al., 2022). A. ocellatus is one of the most valuable, expensive, and popular aquarium ornamental fish species (Dopeikar et al., 2024). Oscars are bred worldwide for aquarium hobbyists, sport fishers, and food items. Many artificial Oscar varieties such as amlanism and common ones have been produced by selective breeding (Wang et al., 2023). A. ocellatus is a carnivorous, early maturing, relatively high fecundity, and intelligent species that is very popular among aquarists because of its colorful appearance (Zare et al., 2023). All these positive attributes make A. ocellatus a very good model for in vivo studies.
Previous studies have shown that the use of Fuc isolated from brown algae have a positive and significant effect in aquaculture (Traifalgar et al., 2010; Sivagnanavelmurugan et al., 2014; Tuller et al., 2014; Zeraatpisheh et al., 2018). For example, commercial Fuc caused a significant increase in growth, immunity, and antioxidant indices in Common carp (Cyprinus carpio) (Li et al., 2023). In addition, Fuc isolated from Cystoseira trinodis caused a significant increase in growth indicators, immune status, and relative percentage survival (RPS) after challenge with White Spot Syndrome Virus (WSSV) in Litopenaeus vannamei shrimp (Salehpour et al., 2021). However, research on the use of Fuc in ornamental fish has not been conducted. Furthermore, the positive effects of compounds derived from macro- and microalgae in ornamental fish are as follows. For example, Spirulina platensis algae powder had a significant effect on blood parameters such as hemoglobin, hematocrit, MCH, and MCHC in Oscar (Mohammadiazarm et al., 2021). Green macroalgae powder (Ulva intestinalis) increased the activity of antioxidant enzymes such as glutathione peroxidase (GPx) and superoxide dismutase (SOD) in Danio rerio (Rouhani et al., 2022). Considering the beneficial properties of Fuc obtained from brown algae, the purpose of this study was to investigate the effects of Fuc on growth indicators, immunity, and antioxidant enzyme activity on Oscar.
2 Material and methods
2.1 Ethics
All experiments were performed following the protocol approved by the Committee of Ethics of the Faculty of Sciences of the University of Tehran (357; 8 November 2000).
2.2 Algae collection
Sargassum ilicifolium was collected from the southern shores of the Persian Gulf in Delwar, Bushehr, Iran. S. ilicifolium was washed after collection and dried at room temperature for 48 h. Then, the desired algae were powdered using a grinder and kept in a cool place to extract Fuc.
2.3 Fucoidan extraction
Fuc was extracted from powdered S. ilicifolium by the method of Yang et al. (2008) with slight modifications (Yang et al., 2008). To remove pigments from algae, first, 125 g of algae powder was mixed with 85% ethanol (1:10), and the mixture was stirred for 1 day at room temperature. After 1 day, the supernatant (ethanol) was drained, and the residue (algae) was washed using acetone and centrifuged at 7,000 × g for 1 h. The supernatant was decanted, and the precipitated algae was collected. Then, in order to completely remove acetone, the algae was dried for 6 h at room temperature. The dried algae was mixed with distilled water (1:10) and then stirred at 65°C for 6 h. After 6 h, the solution was filtered with a Whatman filter paper, and the solid residue was mixed with distilled water (1:5). The solution was stirred at 65°C for 6 h. After 6 h, the solution was centrifuged at 7,000×g for 30 min, and the supernatant was collected and the precipitate eliminated. The supernatant was mixed with 1% CaCl2, and then, the solution was mechanically shaken for 10 min and kept at 4°C for 1 day. After 1 day, calcium alginate is precipitated and removed. The remaining solution was concentrated with 99% ethanol until the final concentration of ethanol reached 30%, and then, the solution was kept at 4°C for 6 h. Then, after centrifugation (7,000×g, 30 min), we brought the final concentration of the solution to 70% ethanol, and then, the solution was kept overnight at 4°C. Finally, the solution was filtered with Whatman paper and then washed with ethanol (99%) and acetone to obtain Fuc. After drying (overnight at room temperature), the obtained Fuc was stored at 4°C until the time of the experiments.
2.4 Chemical analysis
Total sugar content of Fuc was calculated by phenol-sulfuric acid method. The amount of total sugar was measured according to the method reported by Yang et al., and L-fucose was used as a standard reagent (Yang et al., 2017). The sulfate content in Fuc was measured by the standard protocol of Shao et al. (2014). Using the m-phenyl phenol method, total uronic acid in Fuc was determined (Chakraborti et al., 2004).
2.5 Analysis of monosaccharide
To analyze the composition of monosaccharides, 25 mg of Fuc was completely dissolved in 1 ml of distilled water and then mixed with 2 ml of trifluoroacetic acid (TFA). The intended solution was kept in an oven for 4 h at 110°C for hydrolysis. Then, the obtained samples with a volume of 30 µl were injected into the HPLC. The compounds of Fuc monosaccharides were measured by HPLC using the method of Farvin and Jacobsen (2013) (Farvin and Jacobsen, 2013).
2.6 Diet preparation and experimental design
Oscar (120 pieces) with an average weight of 49.75 ± 0.96 g were treated in an ornamental fish farm located in Pakdasht, Tehran, Iran. The fish were randomly divided into four treatments and three replications in 300-l aquariums with 10 fish in each tank. The culture system was a closed system with static water and constant aeration with 30% exchange every 2 days. The experimental treatments included the control group (without Fuc) and groups containing 0.5%, 1%, and 2% Fuc. Fuc was dissolved in 30 ml of distilled water and then added to a commercial diet containing 52% protein, 12.5% fat, 1.5% crude fiber, and 1% absorbable phosphorus (21 Beyza Mill Co., Shiraz, Iran). A total of 50 ml/kg of 3% gelatin solution was added to the diet after drying Fuc so that the active ingredients of Fuc do not dissolve in water. The fish were fed with a ratio of 2% of body weight three times daily, and the feeding rate was adjusted every 15 days after weighing. The breeding conditions during the experiment were 12:12 h (light:darkness) photoperiod, water temperature of 28 ± 1°C, dissolved oxygen of 6.86 ± 0.12 mg L−1, and pH 7.1 ± 0.11.
2.7 Calculation of growth parameters
After 50 days of rearing, growth parameters such as percentage of weight gain (%WG), specific growth rate (SGR), and food conversion ratio (FCR) were calculated using the following formula.
Weight gain (WG, %) = [final weight (Fw) − initial weight (Iw)] × 100/initial weight
Specific growth rate (SGR, % day−1) = [(Ln Fw – Ln Iw)/t (period of culture)] × 100
Food conversion ratio (FCR, g/g) = food intake (g)/(Fw − Iw)
2.8 Sample collection and hematological parameters
In order to take fish samples, feeding was stopped for 24 h (at the end of the 50th day). Three fish were randomly caught from each aquarium (nine fish from each treatment). and after anesthetization with clove powder (150 mg/l), blood sampling was done from the caudal vein (2 ml from each fish). The blood was divided into two parts: heparinized (to evaluate blood parameters) and non-heparinized (to obtain serum from blood). Non-heparinized blood was kept in the refrigerator for 12 h to clot. Clotted samples (3,000×g for 15 min at 4°C) were centrifuged to obtain serum, and serum was stored at −80°C for the analysis of biochemical, immunological, and antioxidant parameters (Khanzadeh et al., 2023; Khanzadeh et al., 2024). Blood parameters were measured using Grant methodology (Grant, 2015). White blood cells (WBCs) and red blood cells (RBCs) were counted after dilution with Dacie solution, using a Neubauer slide. Hematocrit (Hct) was measured by centrifuge method, and hemoglobin (Hb) was measured by cyano-met-hemoglobin method. By preparing blood slides stained by Giemsa, the differential count of leukocytes was done.
2.9 Serum bactericidal activity
The serum bactericidal activity was measured by the method described by Budino et al. with minor modifications (Budiño et al., 2006). Briefly, Aeromonas hydrophila (A. hydrophila), 2 × 108 (cfu ml−1) was added to the serum samples, and after pipetting (for 6 h at 20°C), they were incubated. After incubation, MTT (dimethylthiazole-diphenyltetrazolium bromide) was added to the suspensions and incubated for 30 min at room temperature. Finally, the suspensions were measured by the color change caused by the reduction of MTT by living bacteria (A. hydrophila) at a wavelength of 630 nm by an ELISA reader.
2.10 Biochemical parameters
Based on the method of Hosseinifar et al., serum biochemical parameters such as total protein (Tp) and albumin (Alb) were measured using commercial kit (Pars Azmoun, Tehran, Iran). The level of serum globulin (Glb) was calculated by subtracting the amount of Alb from the amount of Tp (Hoseinifar et al., 2015).
2.11 Antioxidant parameters
Serum catalase (CAT) activity was measured using a commercial kit (Navand, Urmia, Iran) according to the manufacturer’s instructions. The absorbance was measured by colorimetric method with the ELISA reader at a wavelength of 540 nm. In this assay, the unit of CAT activity was considered as the amount of sample that catalyzes the decomposition of 1 μmol of H2O2 into H2O and O2 in 1 min. Serum superoxide dismutase (SOD) activity was measured using a commercial kit (Navand, Urmia, Iran) according to the protocol of the commercial assay kit. The absorbance was measured using an ELISA reader at 540 nm. The unit of SOD activity is considered as the sample amount that catalyzes the decomposition of 1 μmol of O2− into H2O2 and O2 in 1 min.
2.12 Measurement of non-specific immune parameters
To measure respiratory burst activity, first, 0.1 ml of blood (heparinized) was mixed well with 0.1 ml of 0.2% nitro blue tetrazolium (NBT) solution and incubated for 30 min at room temperature. Then, 0.1 ml of the sample (mixture of blood and NBT) was added to 1 ml of dimethylformamide solution and mixed well. Finally, the samples were centrifuged, and the supernatant was read at 540 nm (Kondera et al., 2020). Micrococcus luteus was used as serum lysozyme assay and suspended in phosphate buffer (pH 6.2). A volume of 10 µl of the serum sample was mixed with 90 µl of this suspension (bacteria + PBS), and the decrease in optical density was measured in 5 min at 450 nm. Each 0.001 decrease in optical density per minute was considered as one unit of lysozyme activity (Ross et al., 2000). To evaluate serum complement activity, the method of Erfanmanesh et al. was used. Briefly, first, 230 μl of complement buffer (pH = 2.5, containing 0.5 mmol of magnesium chloride and 1.5 mmol of calcium chloride) was mixed with 20 μl of serum sample, and then, 50 μl of washed rabbit erythrocytes was added to each well of the ELISA plate. The samples were subjected to mechanical shaking for 2 min to mix well; then, they were incubated at 4°C for 12 h. Eventually, the absorbance of the supernatant was measured with a spectrophotometer at a wavelength of 430 nm (Erfanmanesh et al., 2023).
2.13 Total skin carotenoid
Total skin carotenoids were measured using the method of Ramamoorthy et al. Briefly, first, Na2SO4 (1.5 g) was mixed with acetone (5 ml). Then, the fish skin was collected (300 mg) and well homogenized with a homogenizer for 2 min. Acetone was added to the fish skin until the volume of the solution reached 10 ml. The samples were kept at 4°C for 3 days and then centrifuged at 3,500×g for 5 min. The absorbance of the samples was recorded at 475 nm (Ramamoorthy et al., 2010). Total carotenoids (TCs) were calculated through the following formula:
TCs (mg kg−1) = ml of solution × dilution factor absorb (OD)/sample weight (g) × 2,500
2.14 Statistical analysis
GraphPad PRISM software (version 9.5.1.733) was used for data analysis. First, the homogeneity and normality of the standard deviation (SD) of the data were checked using Shapiro–Wilk’s statistical test. Then, one-way ANOVA and Tukey’s analysis of variance were performed to compare the groups. Data are presented as mean ± SD.
3 Results
3.1 Analysis of polysaccharides
After removing lipids, protein, and alginate, Fuc was obtained from S. ilicifolium. Fuc had 64.32% total sugar, 18.1% sulfate, and 11.5% uronic acid, respectively. After HPLC, the percentage of Fuc monosaccharides were as follows: Fucose was 58.7%, galactose was 16.3%, mannose was 6.1%, xylose was 4.5%, and glucose was 3.1%.
3.2 Hematological parameters
At the end of the feeding period, the groups fed with Fuc-supplement had no significant effect on blood parameters such as RBCs, % Hct, % neutrophils (Net), % lymphocytes (Lym), and Hb (Figures 1–3, p > 0.05). Fish fed with 2% Fuc supplement had a significant increase in the number of WBCs compared to the control group (Figure 4, p = 0.0036), while other groups fed with Fuc (0.5 and 1%) did not have a significant difference with the control group (Figure 4, p > 0.05). Meanwhile, there was a significant difference between the 2% and 1% Fuc groups in the number of WBCs in Oscar (Figure 4, p = 0.0414).
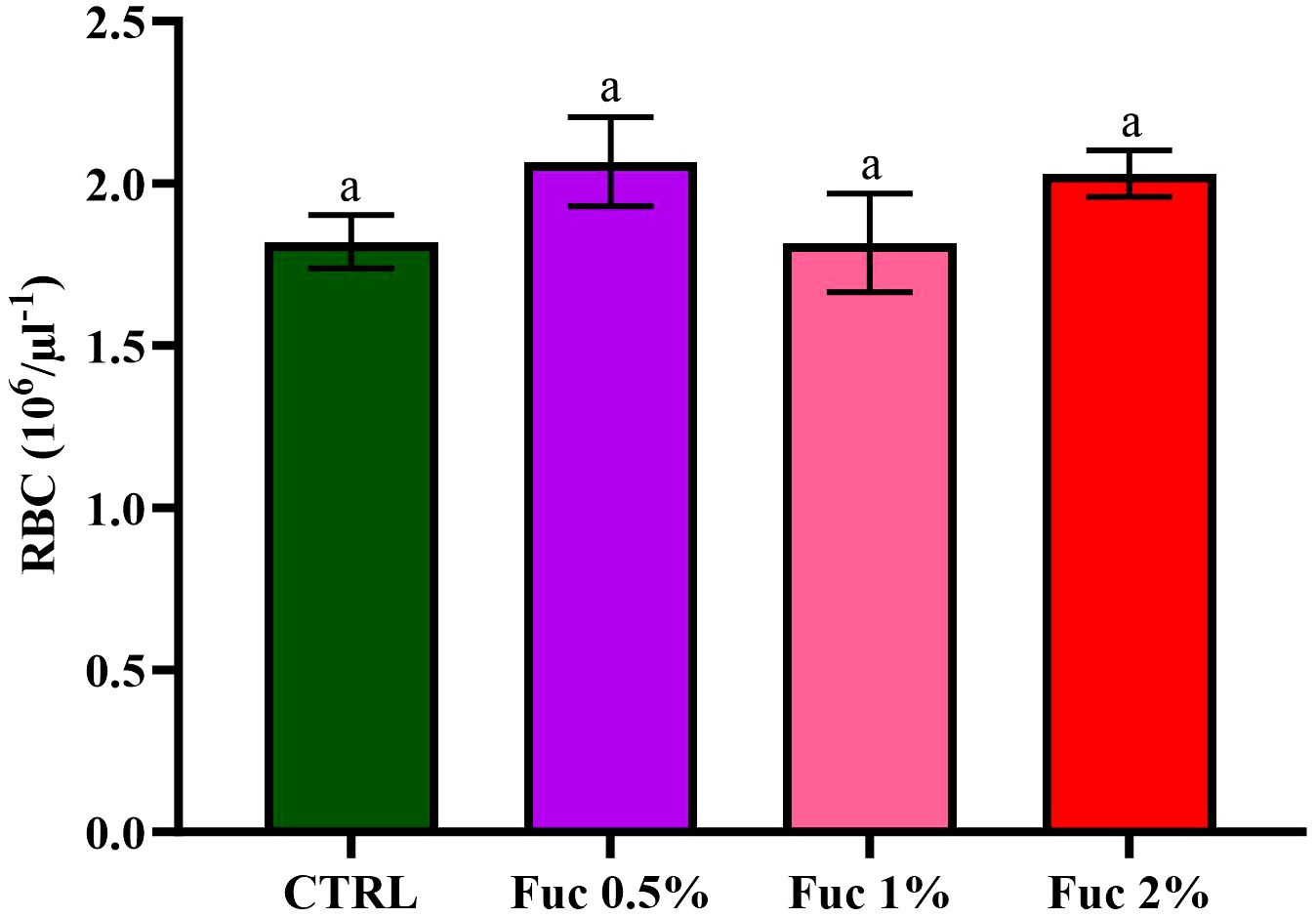
Figure 1 The amount of RBCs in the groups fed with 0.5%, 1%, and 2% Fuc supplements in the Oscar. Values are mean ± SD (n = 9 fish per treatment). Heterogeneous lowercase letters indicate significant differences in different levels of Fuc compared to the control group. Statistical analysis was performed using one-way ANOVA and Tukey’s test to show the difference between the groups. RBC, red blood cell; CTRL, control; Fuc, fucoidan.
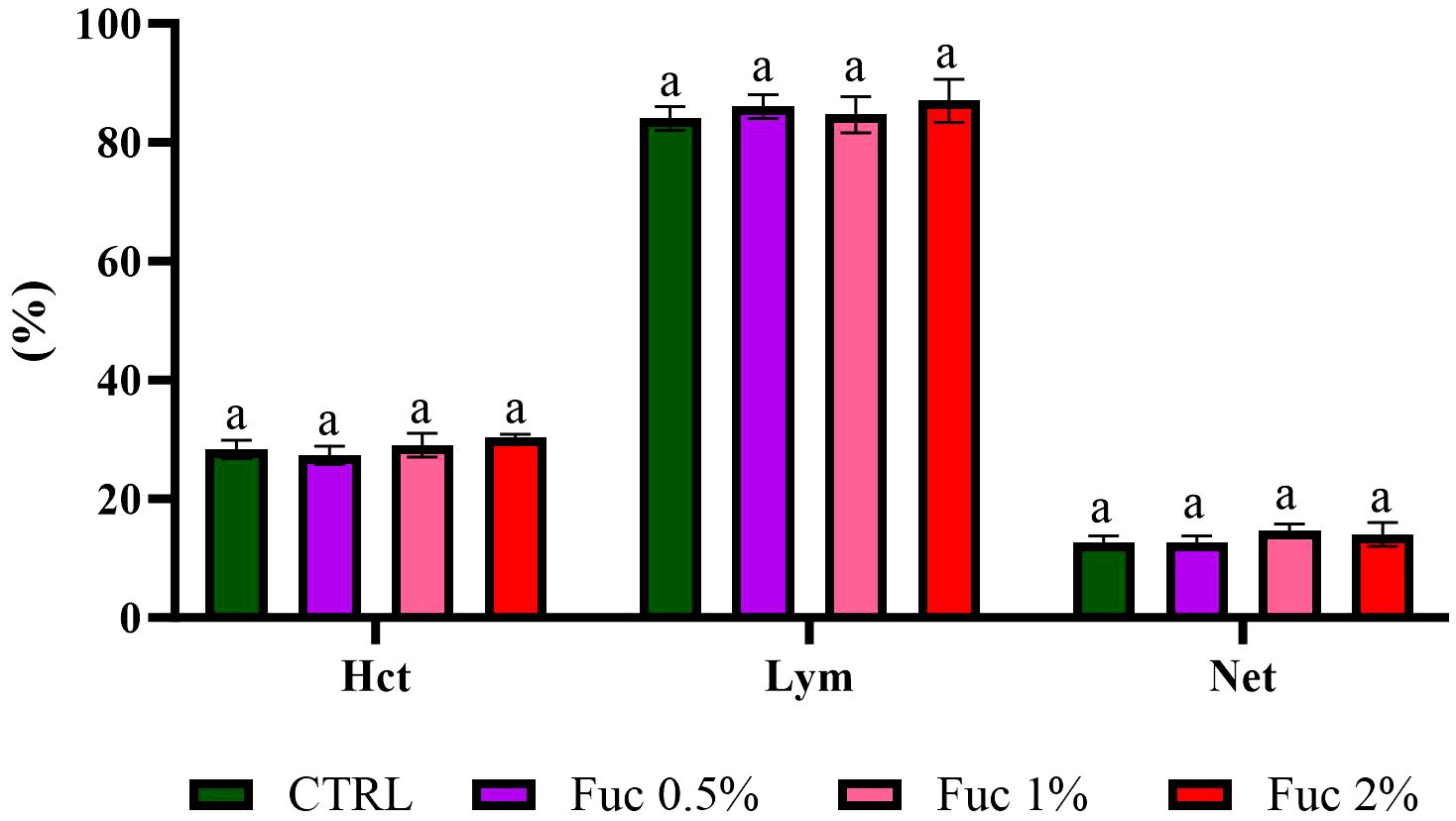
Figure 2 The percentage of Hct, Lym, and Net in the groups fed with 0.5%, 1%, and 2% Fuc supplements in the Oscar. Values are mean ± SD (n = 9 fish per treatment). Heterogeneous lowercase letters indicate significant differences in different levels of Fuc compared to the control group. Statistical analysis was performed using one-way ANOVA and Tukey’s test to show the difference between the groups. Hct, hematocrit; Lym, lymphocyte; Net, neutrophil; CTRL, control; Fuc, fucoidan.
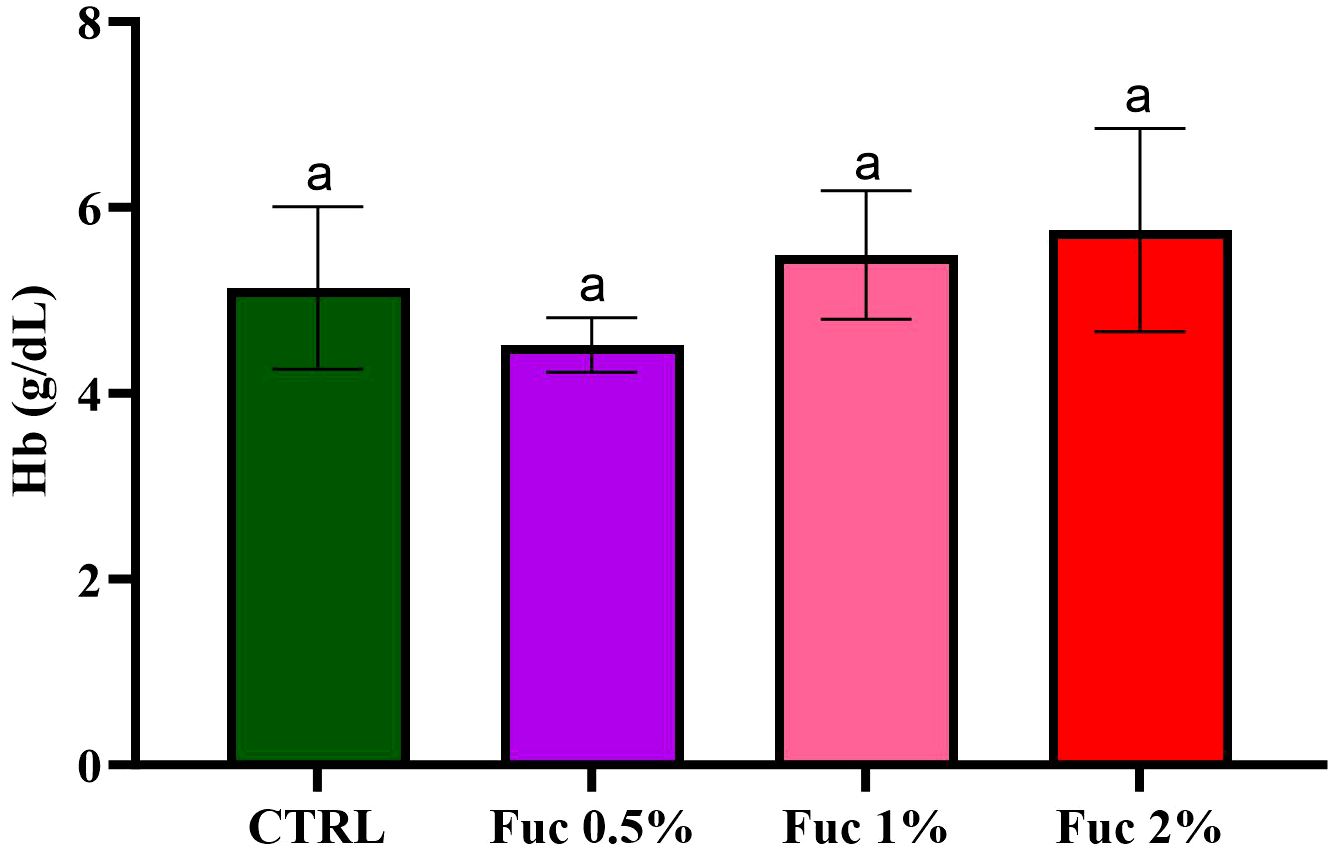
Figure 3 The amount of Hb in the groups fed with 0.5%, 1%, and 2% Fuc supplements in the Oscar. Values are mean ± SD (n = 9 fish per treatment). Heterogeneous lowercase letters indicate significant differences in different levels of Fuc compared to the control group. Statistical analysis was performed using one-way ANOVA and Tukey’s test to show the difference between the groups. Hb, hemoglobin; CTRL, control; Fuc, fucoidan.
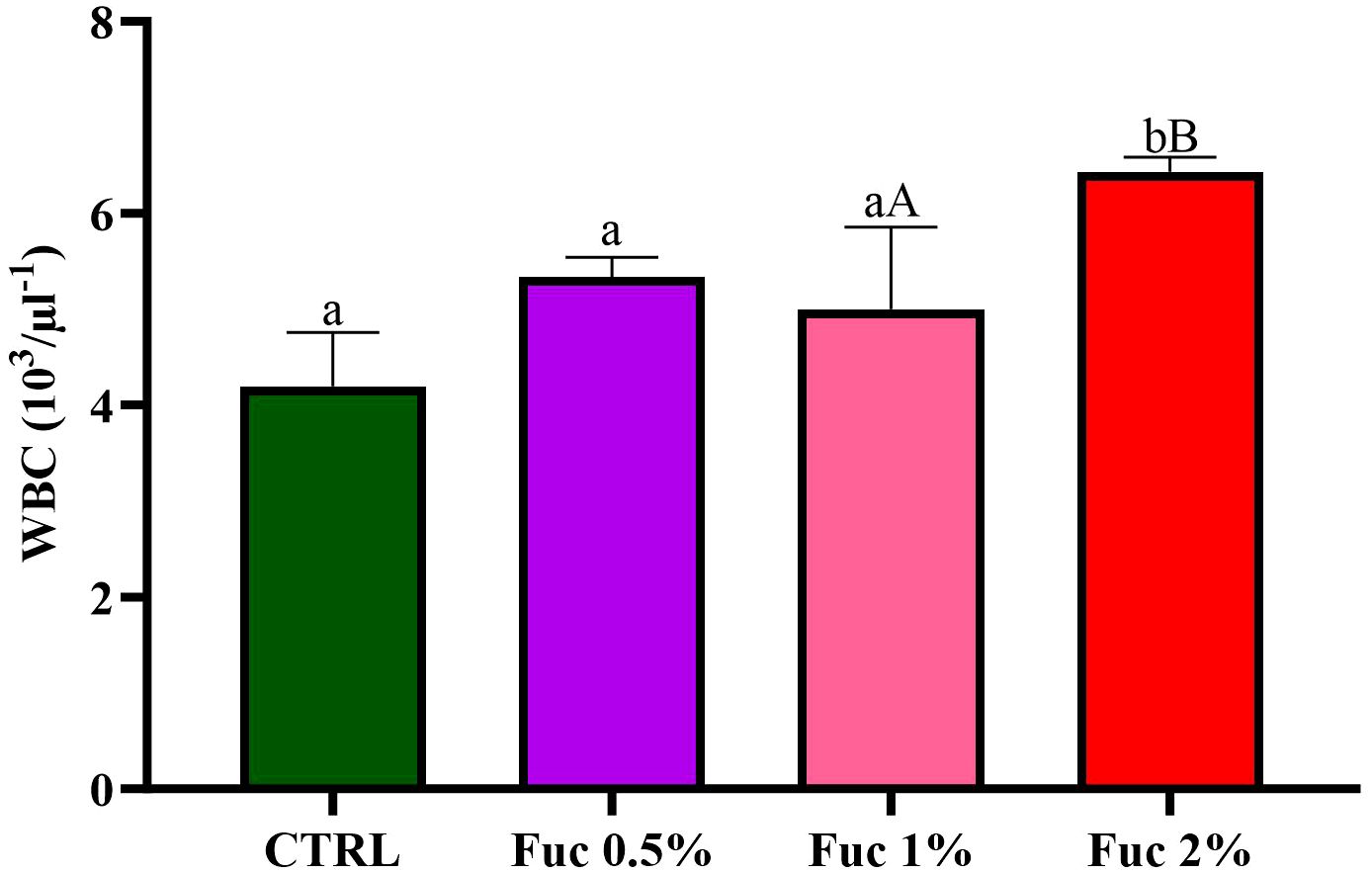
Figure 4 The number of WBCs in the groups fed with 0.5%, 1%, and 2% Fuc supplements in the Oscar. Values are mean ± SD (n = 9 fish per treatment). Heterogeneous lowercase letters indicate significant differences in different levels of Fuc compared to the control group. Heterogeneous capital letters indicate significant differences between different Fuc levels. Statistical analysis was performed using one-way ANOVA and Tukey’s test to show the difference between the groups. WBC, white blood cell; CTRL, control; Fuc, fucoidan.
3.3 Growth parameters
Fish fed with Fuc supplements (1% and 2%) showed a positive growth response compared to the control group and the 0.5% Fuc group (Figures 5–7, p < 0.05). The percentage of WG in fish fed with Fuc supplements (1% and 2%) had a significant increase compared to the control group and the 0.5% Fuc group (Figure 5, p < 0.05). The highest percentage of WG was observed in the 2% Fuc supplement group (77.72%). The SGR in fish fed with Fuc supplements (1% and 2%) had a significant increase compared to the control group and 0.5% Fuc group (Figure 6, p < 0.05). The highest SGR of 2% Fuc supplement group was observed (1.15). In addition, the FCR in fish fed with Fuc supplements (1% and 2%) had a significant decrease compared to the control group and 0.5% Fuc group (Figure 7, p < 0.05). The lowest FCR was observed in Fuc 2% (1.29) and Fuc 1% (1.41) groups.
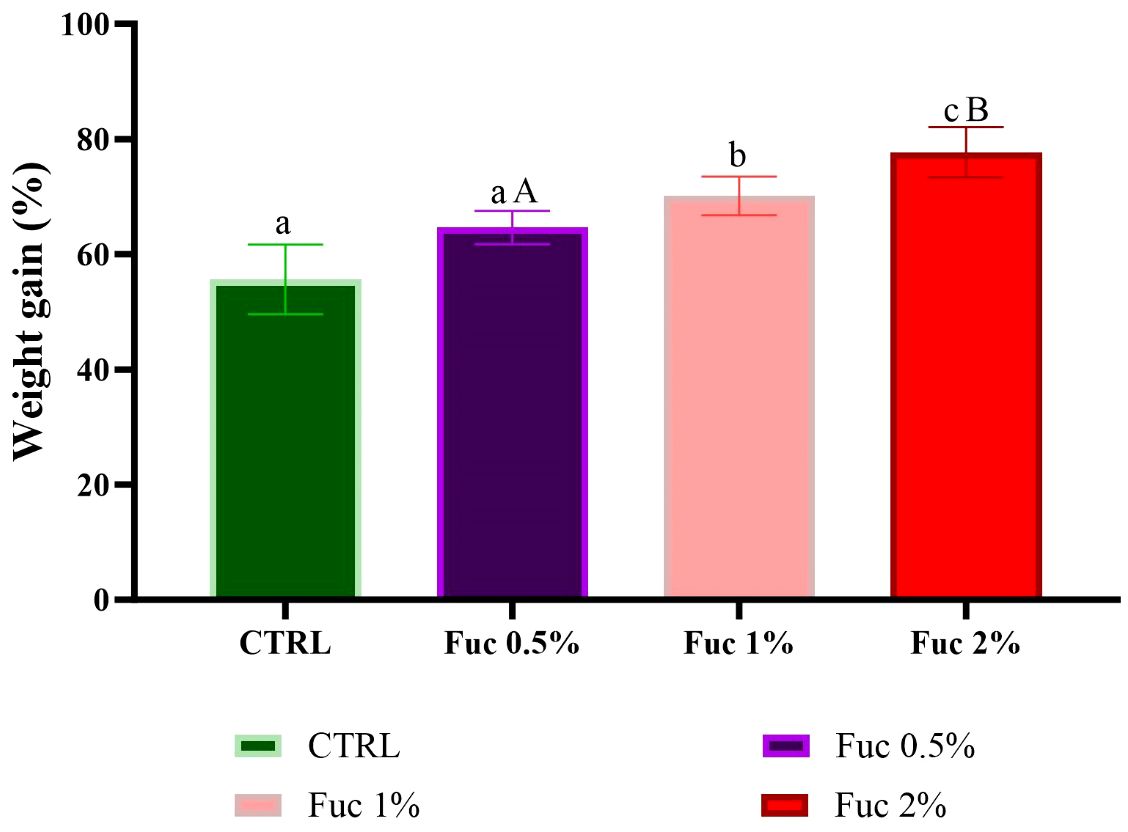
Figure 5 The percentage of WG in the groups fed with 0.5, 1%, and 2% Fuc supplements in the Oscar. Values are mean ± SD (n = 30 fish per treatment). Heterogeneous lowercase letters indicate significant differences in different levels of Fuc compared to the control group. Heterogeneous capital letters indicate significant differences between different Fuc levels. Statistical analysis was performed using one-way ANOVA and Tukey’s test to show the difference between the groups. WG, weight gain; CTRL, control; Fuc, fucoidan; Ns, not significant.
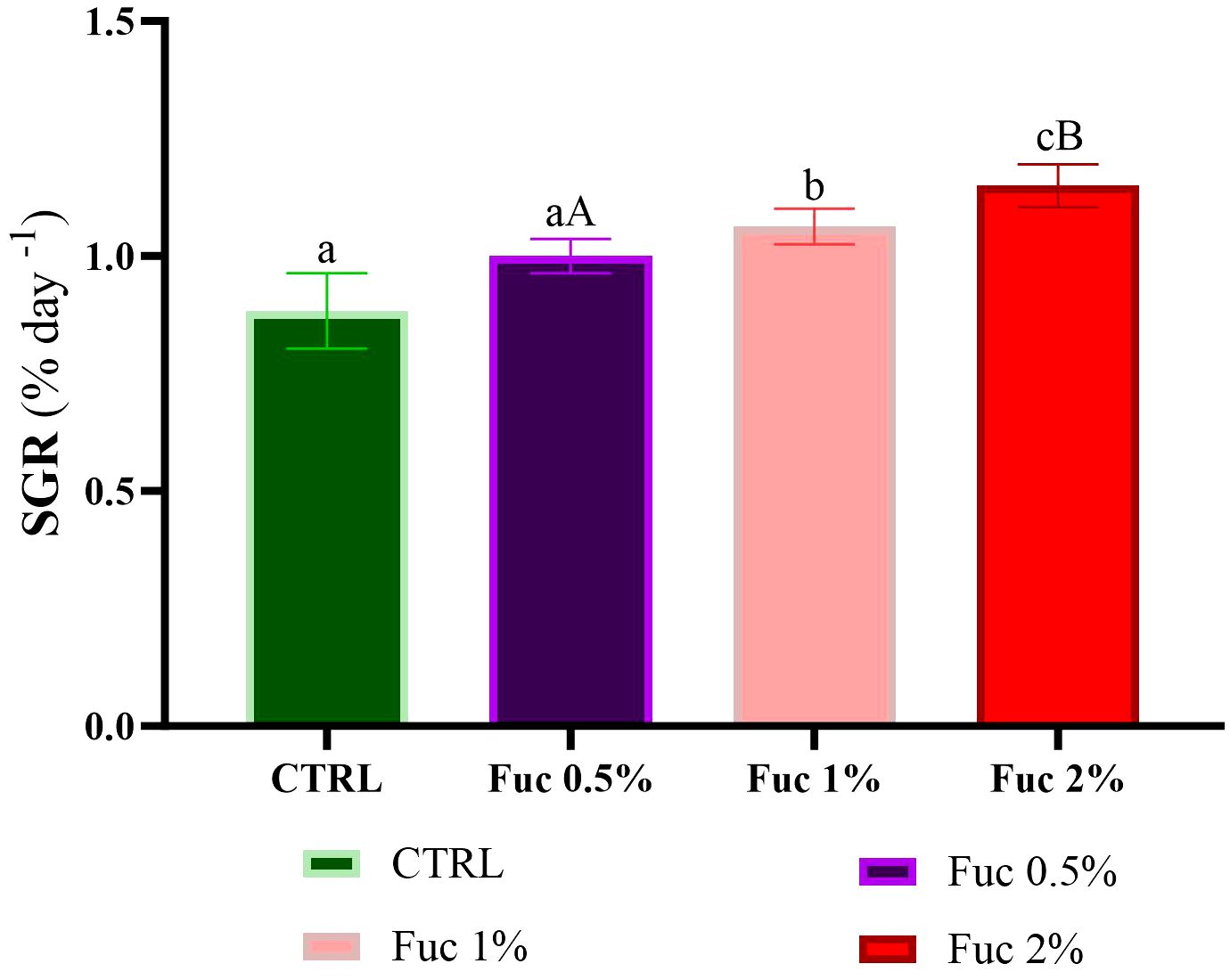
Figure 6 SGR in the groups fed with 0.5%, 1%, and 2% Fuc supplements in the Oscar. Values are mean ± SD (n = 30 fish per treatment). Heterogeneous lowercase letters indicate significant differences in different levels of Fuc compared to the control group. Heterogeneous capital letters indicate significant differences between different Fuc levels. Statistical analysis was performed using one-way ANOVA and Tukey’s test to show the difference between the groups. SGR, specific growth rate; CTRL, control; Fuc, fucoidan; Ns, not significant.
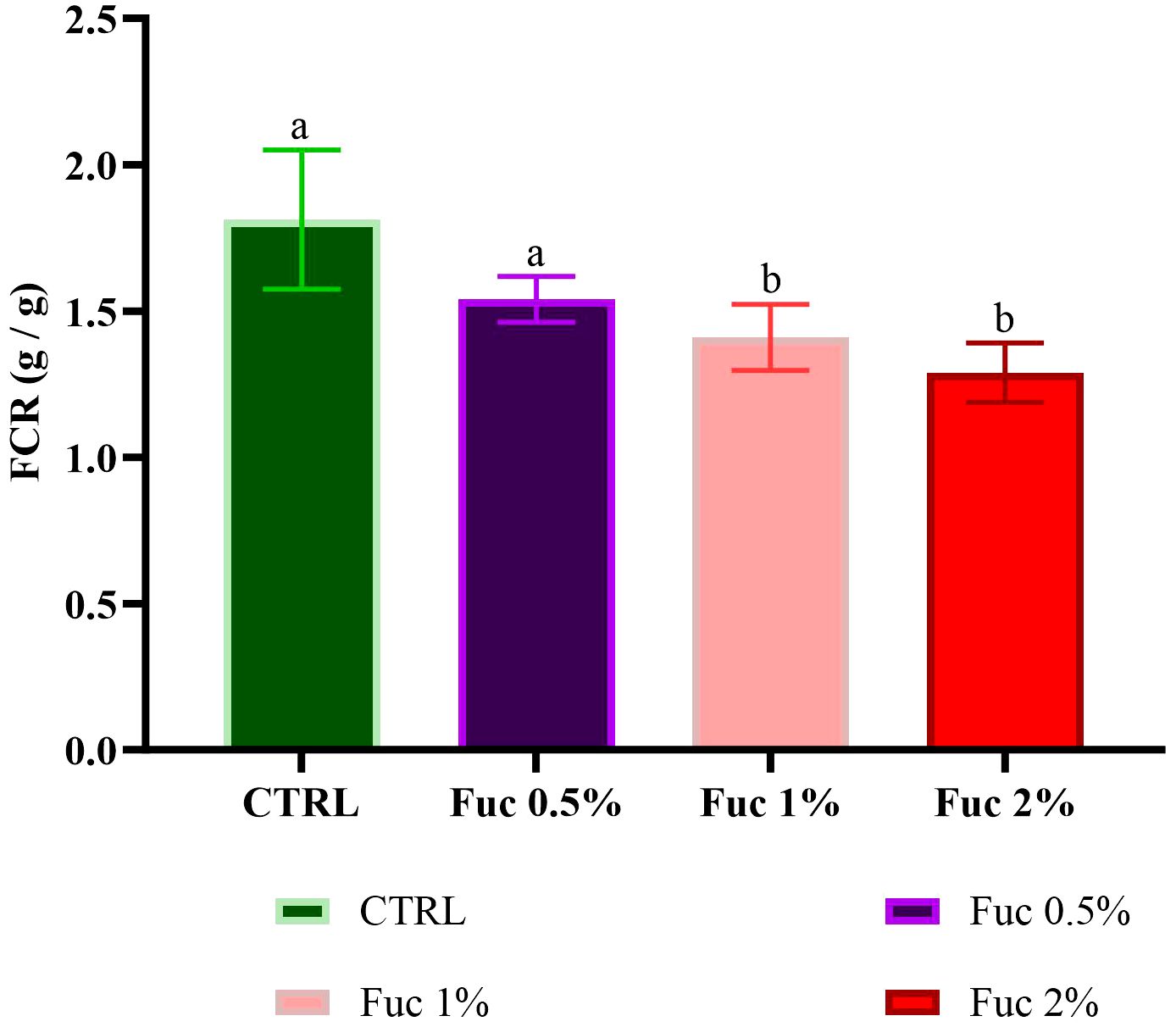
Figure 7 FCR in the groups fed with 0.5%, 1%, and 2% Fuc supplements in the Oscar. Values are mean ± SD (n = 30 fish per treatment). Heterogeneous lowercase letters indicate significant differences in different levels of Fuc compared to the control group. Statistical analysis was performed using one-way ANOVA and Tukey’s test to show the difference between the groups. FCR, feed conversion ratio; CTRL, control; Fuc, fucoidan; Ns, not significant.
3.4 Biochemical parameters
Serum biochemical parameters including Tp in all groups and Glb in 1% and 2% Fuc supplement groups had a significant increase compared to the control group (Figure 8, p < 0.05). No significant difference in serum Alb activity was observed in the groups fed with Fuc supplement compared to the control group (Figure 8, p > 0.05).
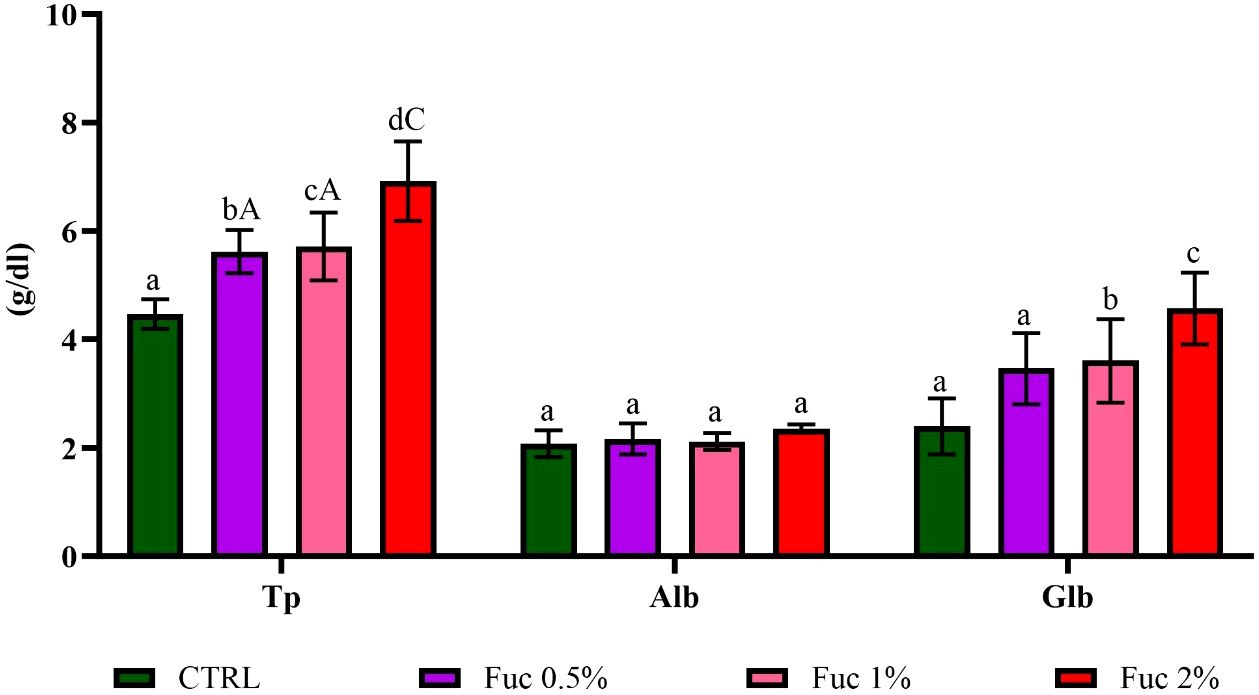
Figure 8 The activity of Tp, Alb, and Glb in the groups fed with 0.5%, 1%, and 2% Fuc supplements in the Oscar. Values are mean ± SD (n = 9 fish per treatment). Heterogeneous lowercase letters indicate significant differences in different levels of Fuc compared to the control group. Heterogeneous capital letters indicate significant differences between different Fuc levels. Statistical analysis was performed using one-way ANOVA and Tukey’s test to show the difference between the groups. Tp, total protein; Alb, albumin; Glb, globulin; CTRL, control; Fuc, fucoidan.
3.5 Antioxidant parameters
The activity of antioxidant enzymes, including SOD (Fuc 1% and 2%) and CAT (Fuc 2%) increased significantly compared to the control group (Figure 9, p< 0.05). In addition, a significant difference in the activity of SOD was observed between the groups fed with Fuc supplement (Figure 9, p < 0.05). It should be noted that a significant difference between the Fuc 0.5% and Fuc 2% groups was observed (Figure 9, p < 0.05).
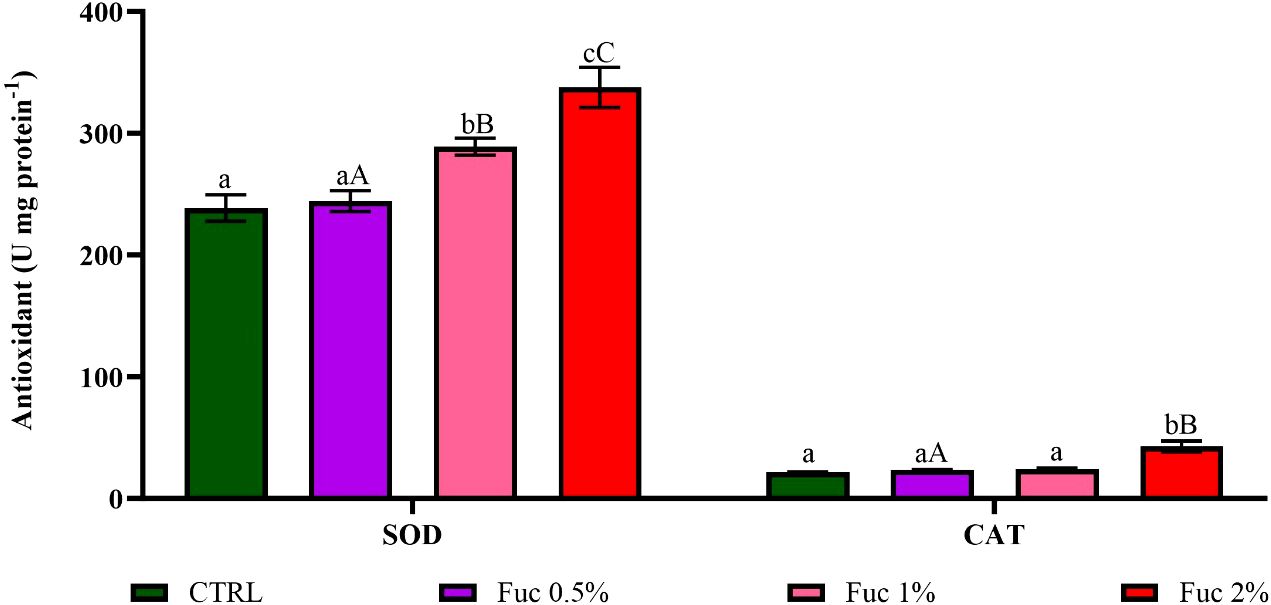
Figure 9 The activity of serum SOD and CAT in the groups fed with 0.5%, 1%, and 2% Fuc supplements in the Oscar. Values are mean ± SD (n = 9 fish per treatment). Heterogeneous lowercase letters indicate significant differences in different levels of Fuc compared to the control group. Heterogeneous capital letters indicate significant differences between different Fuc levels. Statistical analysis was performed using one-way ANOVA and Tukey’s test to show the difference between the groups. SOD, superoxide dismutase; CAT, catalase; CTRL, control; Fuc, fucoidan.
3.6 Immunological parameters
Serum complement activity in all groups fed with Fuc supplement had a significant increase compared to the control group (Figure 10, p < 0.05). The highest serum complement activity was observed in 2% Fuc (8.33). Serum lysozyme activity in the groups fed with 1% and 2% Fuc supplements was significant compared to the control group and the 0.5% Fuc group (Figure 11, p < 0.05). The highest lysozyme activity was observed in the group fed with 2% Fuc supplement (1,133.3).
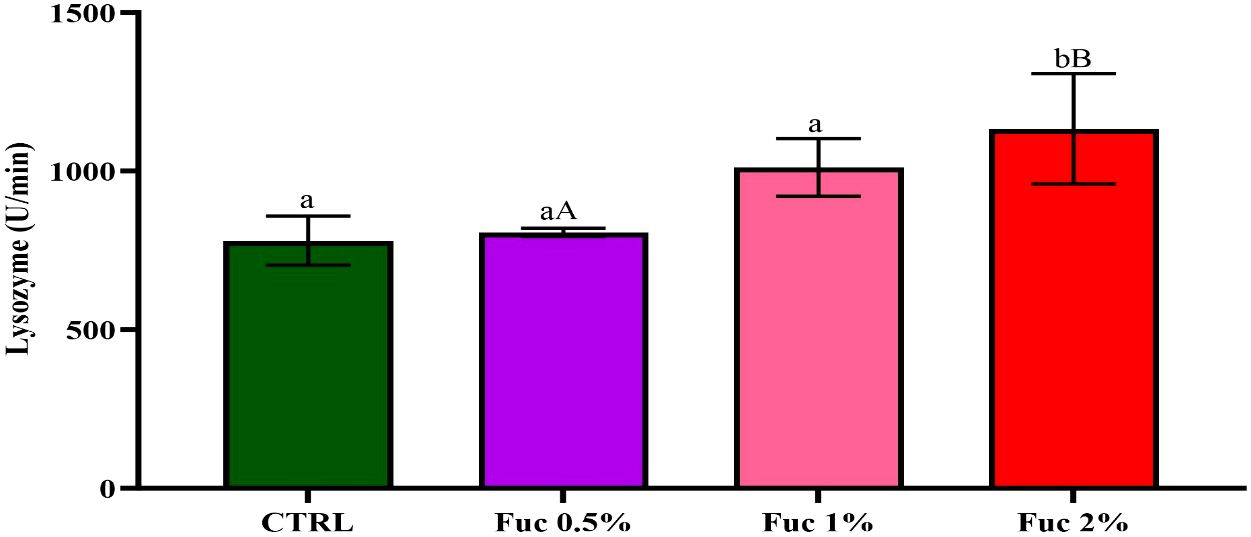
Figure 10 The activity of serum lysozyme in the groups fed with 0.5%, 1%, and 2% Fuc supplements in the Oscar. Values are mean ± SD (n = 9 fish per treatment). Heterogeneous lowercase letters indicate significant differences in different levels of Fuc compared to the control group. Heterogeneous capital letters indicate significant differences between different Fuc levels. Statistical analysis was performed using one-way ANOVA and Tukey’s test to show the difference between the groups. CTRL, control; Fuc, fucoidan.
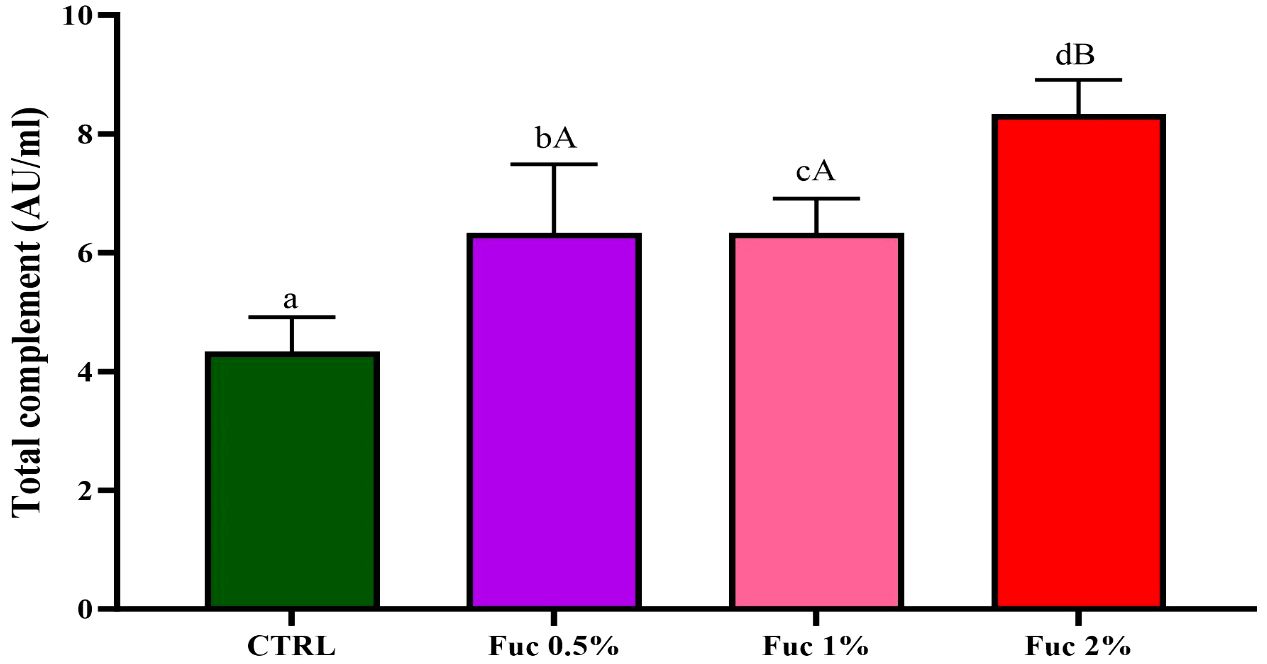
Figure 11 The activity of serum total complement in the groups fed with 0.5%, 1%, and 2% Fuc supplements in the Oscar. Values are mean ± SD (n = 9 fish per treatment). Heterogeneous lowercase letters indicate significant differences in different levels of Fuc compared to the control group. Heterogeneous capital letters indicate significant differences between different Fuc levels. Statistical analysis was performed using one-way ANOVA and Tukey’s test to show the difference between the groups. CTRL, control; Fuc, fucoidan.
3.7 Respiratory burst and bactericidal activity
Respiratory burst and serum bactericidal activity against A. hydrophila in the feeding group with 2% Fuc supplement had a significant increase compared to the control group (Figure 12, p < 0.05).
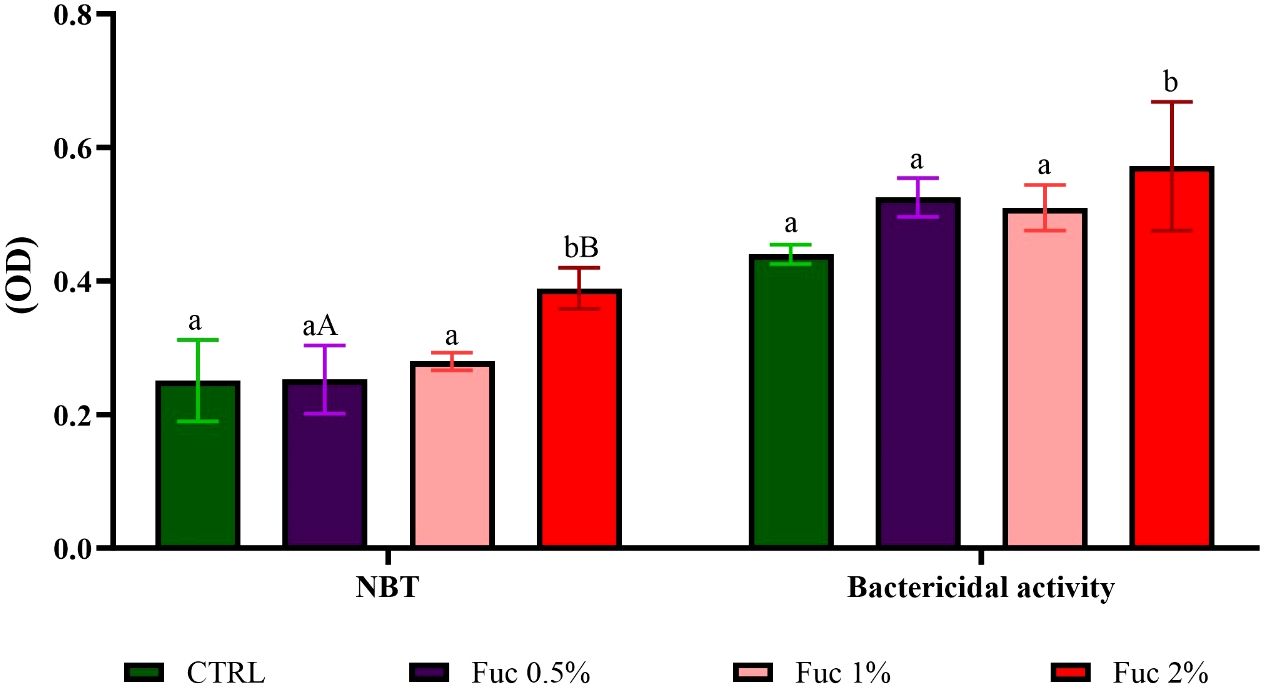
Figure 12 The respiratory burst and bactericidal activity in the groups fed with 0.5%, 1%, and 2% Fuc supplements in the Oscar. Values are mean ± SD (n = 9 fish per treatment). Heterogeneous lowercase letters indicate significant differences in different levels of Fuc compared to the control group. Heterogeneous capital letters indicate significant differences between different Fuc levels. Statistical analysis was performed using one-way ANOVA and Tukey’s test to show the difference between the groups. NBT, nitroblue tetrazolium; CTRL, control; Fuc, fucoidan.
3.8 Total skin carotenoid
After 50 days of feeding with Fuc supplement, the carotenoid content of the whole skin increased significantly in the Fuc 1% and 2% groups compared to the control group (Figure 13, p< 0.05). In addition, a significant difference was observed between the groups (0.5% vs. 1%, 0.5% vs. 2%, and 1% vs. 2%) fed with Fuc supplement.
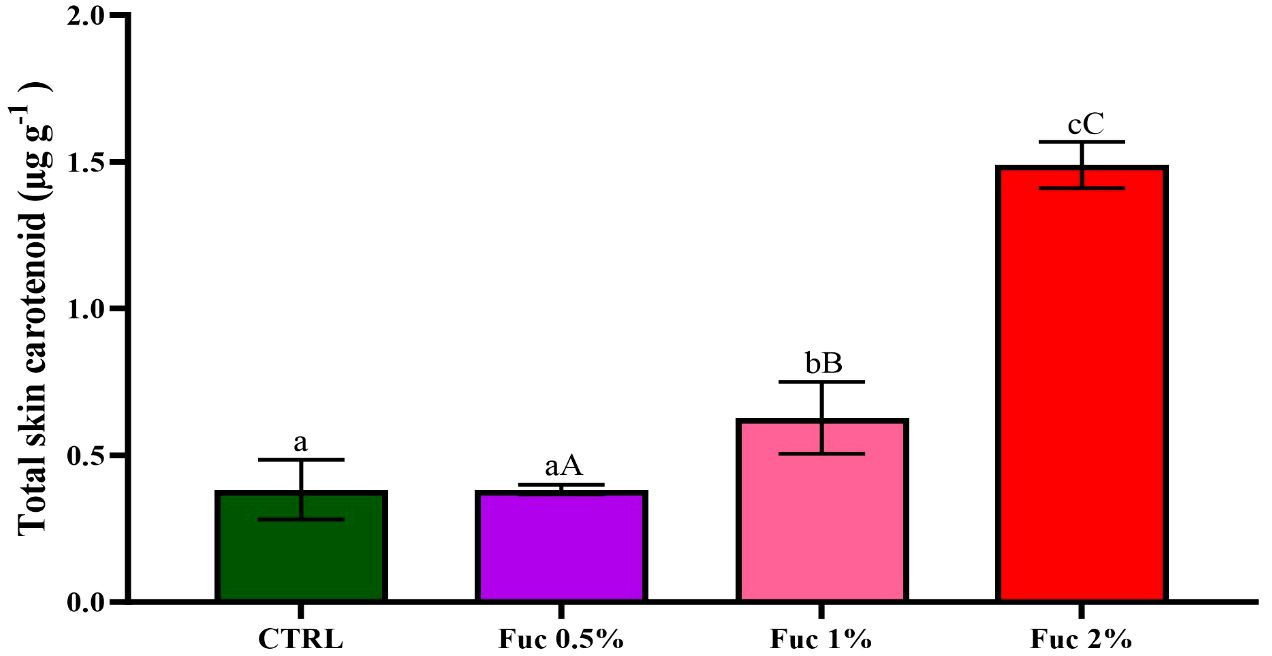
Figure 13 Total skin carotenoid in the groups fed with 0.5, 1%, and 2% Fuc supplements in the Oscar. Values are mean ± SD (n = 9 fish per treatment). Heterogeneous lowercase letters indicate significant differences in different levels of Fuc compared to the control group. Heterogeneous capital letters indicate significant differences between different Fuc levels. Statistical analysis was performed using one-way ANOVA and Tukey’s test to show the difference between the groups. CTRL, control; Fuc, fucoidan.
4 Discussion
Marine seaweeds, especially brown seaweeds, are rich in bioactive substances such as carotenoids, dietary fibers, vitamins, minerals, trace elements, iodine and bromine, and nutrients such as amino acids, protein, fats, and carbohydrates (Lakshmanan et al., 2022). Seaweeds are rich in sulfated polysaccharides such as Fuc, alginates, and laminarins (Liu et al., 2020).
Fucoidan, a complex sulfated polysaccharide, constitutes the primary component of seaweed cell walls (Zargari et al., 2023). Demonstrating notable biological activities, Fuc exhibits antioxidative, antiviral, and antibacterial properties (Jayawardena et al., 2022). These attributes stem from the considerable structural diversity inherent in various species of brown algae, coupled with the presence of sulfate moieties. Factors influencing Fuc’s chemical composition and structural characteristics encompass seaweed species, geographical location, growth conditions, and extraction methodologies (Kazemi et al., 2023). These variables significantly impact parameters such as the composition and proportion of monosaccharides, molecular weight, branching patterns, and glycosidic bond features. Consequently, variations in these attributes lead to discernible distinctions in the functional properties and biological activities of sulfated polysaccharides.
4.1 Analysis of polysaccharides
The analysis of relative chemical compounds in Fuc extracted from S. ilicifolium reveals a notable presence of sulfate. At the stage of full maturation, the carbohydrate content reaches its peak value. Thus, the elevated concentration of carbohydrates (total sugar) obtained may serve as an indicator of the seaweed’s complete maturity at the time of sampling. The identification of monosaccharide units within the Fuc extracted from S. ilicifolium algae delineates the composition as follows: 58.8% fucose, 4.5% xylose, 3.1% glucose, 16.3% galactose, 6.1% mannose, and 11.5% glucuronic acid.
In a study conducted by Raguraman et al. (2019), the monosaccharide composition of sulfated polysaccharides extracted from brown algae S. tenerrimum was elucidated, indicating 52.3% fucose, 1.7% galactose, 3.8% mannose, and 4.1% arabinose (Raguraman et al., 2019). Likewise, a review by Lee et al. (2013) on the monosaccharide composition of Fuc from Ecklonia cava reported percentages of 61.1% fucose, 3.9% rhamnose, 27.2% galactose, and 7% xylose (Lee et al., 2013). In a broader context, it is evident that monosaccharide compounds in algae vary significantly based on the species, geographical location, and sampling time.
4.2 Hematological parameters
The hematopoietic system serves as a highly responsive mechanism for addressing compounds introduced into the body, functioning as a crucial indicator of physiological and pathological conditions in animals (Pietras and DeGregori, 2019). Consequently, the analysis of blood parameters proves effective in evaluating the organism’s condition. Monitoring alterations in hematological indicators emerges as an efficient technique for discerning the impact of food compounds and supplements on animal physiology.
WBCs constitute vital components of the immune system, playing a pivotal role in the synthesis of antimicrobial compounds, enzymes, complement proteins, and immunoglobulins (Wang D. et al., 2022). Antioxidants, such as Fuc, may contribute to the regulation of hematopoiesis and leukopoiesis by their capacity to eliminate reactive oxygen species (ROS) and alleviate oxidative stress (Kumar et al., 2022). Noteworthy, antioxidant supplements like vitamin C, E, and beta-carotene have demonstrated the potential to enhance hematopoiesis and leukopoiesis by mitigating oxidative stress and inflammation (El Basuini et al., 2020; El Basuini et al., 2021). For instance, vitamin C supplementation has been associated with increased WBC production and improved immune system functionality (Khadim and Al-Fartusie, 2021).
Macroalgae, rich in polyphenols crucial for preventing oxidation, play a significant role in influencing hematological parameters. Among these, WBCs are identified as crucial contributors to non-specific immunological reactions in fish. Elevation of WBCs has been observed in both natural and experimental diseases, and following the administration of various vaccinations and immunostimulants. In the current investigation, the addition of Fuc extracted from S. ilicifolium algae did not result in a significant alteration in the number of RBCs, Hb, and Hct. However, it led to an increase in the count of WBCs.
In a parallel study, the extract of S. angustifolium algae demonstrated a similar effect by elevating WBCs in Oncorhynchus mykiss (Zeraatpisheh et al., 2018). A combination of three algae Ulva lactuca, Jania rubens, and Pterocladia capillacea significantly augmented the levels of RBCs, Hb, Hct, and WBCs in catfish compared to the control group (Abdelhamid et al., 2021). Yang et al. (2014) reported that Fuc derived from S. horneri had no significant effect on blood parameters in yellow catfish (Pelteobagrus fulvidraco) (Yang et al., 2014).
The observed increase in WBCs may be attributed to the presence of sulfate groups in the composition of Fuc and the existence of carotenoids, polyphenols, and carbohydrates, including fucose, xylose, glucose, galactose, mannose, and glucuronic acid in algae. These compounds not only enhance non-specific immunity and the antioxidant defense system but also contribute to the improvement of color in fish muscle tissue and skin, thereby positively influencing organoleptic properties.
4.3 Growth parameters
Fats and carbohydrates constitute the primary sources of dietary energy. The current investigation demonstrates a significant enhancement in the growth performance of A. ocellatus through the consumption of a 2% Fuc supplement, compared to the control group. Several studies, including those conducted by (Zargari et al., 2023) (Sivagnanavelmurugan et al., 2014), and (Traifalgar et al., 2010), have reported a dose-dependent improvement in growth performance with oral Fuc.
Tuller et al. (2014) specifically noted that a 52-day dietary Fuc supplementation significantly influenced the growth of juvenile L. calcarifer (Tuller et al., 2014). Fish provided with a 1% Fuc food supplement exhibited significantly greater length and weight compared to the control group, as supported by histological studies indicating muscle fiber hypertrophy stimulated by oral Fuc. Traifalgar et al. (2010) observed that oral Fuc addition significantly enhanced both growth and immune responses (Traifalgar et al., 2010).
Ramazanov et al. (2003) demonstrated that sulfated polysaccharides, such as Fuc, modulate growth by influencing the hypertrophy and hyperplasia of muscle cells through inhibition of myostatin protein production, a key factor in reducing muscle growth (Ramazanov et al., 2003). Their findings suggest that inactivation of myostatin protein could be a possible mechanism contributing to the improved performance observed in Fuc-containing diets. Additionally, the increased growth associated with Fuc-containing diets may be attributed to enhanced feed utilization, as evidenced by an augmented secretion of digestive enzymes due to the consumption of sulfated polysaccharides (Fuc) (Ozorio et al., 2015).
In the present study, both weight gain (WG) and specific growth rate (SGR) exhibited increases with Fuc supplementation, with the Fuc 2% treatment yielding the highest values. Similar positive effects on growth improvement, feed efficiency, and protein utilization were also observed in sea bream fish fed diets containing Ascophyllum nodosum and Undaria pinnatifida (Yone et al., 1986). In line with our results, Fuc had a significant effect on growth indices such as SGR and WG in Nile tilapia (Mahgoub et al., 2020). In addition, in another study, the significant effect of Mozuku Fuc on growth indicators such as SGR and WG in Pagrus major was reported (Sony et al., 2019). Sajina et al. (2019) reported that Fuc derived from S. wightii had a significant effect on growth parameters in Labeo rohita (Sajina et al., 2019).
4.4 Biochemical parameters
The total protein, albumin, and plasma globulin indicators serve as crucial markers for the indirect assessment of health and stress status. In the context of this investigation, noteworthy increases were observed in both total protein and globulin levels among fish subjects that were administered a diet enriched with 2% Fuc extracted from S. ilicifolium. This significant elevation implies a positive impact of the aforementioned compound on the health of A. ocellatus. The findings of this study align with prior research conducted by Akbary et al. in 2018. They reported analogous outcomes wherein the inclusion of varying concentrations of methanolic extract of Ulva rigida, as a dietary supplement, led to heightened levels of total serum protein, albumin, and globulin in Mugil cephalus (Akbary and Aminikhoei, 2018). Meanwhile, no significant difference was observed in the amount of serum albumin in the fish that received the Fuc diet. Khanzadeh et al. (2020) reported that Fuc has no significant effect on serum biochemical parameters such as albumin, total protein, and globulin in Nile tilapia (Khanzadeh et al., 2020). Consistent with our findings, a significant effect of Fuc derived from S. wightii on biochemical parameters such as total protein and globulin was reported in Pangasianodon hypophthalmus (Prabu et al., 2016).
Seaweeds emerge as noteworthy contributors to the piscine immune system, presenting a rich source of immunostimulatory compounds. As evidenced by Thepot et al. in 2021, these compounds confer potential advantages by augmenting fish immune responses and bolstering resistance to pathogens (Thepot et al., 2021). The sulfated polysaccharides found in seaweeds demonstrate particular promise in modulating the non-specific immune system of aquatic organisms, as highlighted by studies conducted by (Valente et al., 2016). Consequently, seaweeds may be regarded as pivotal agents in fortifying immunity in aquatic species.
4.5 Antioxidant parameters
Reactive oxygen species (ROS) are generated during the metabolic processes of aerobic cells. Free radicals, such as ROS, play a crucial role in regulating cell growth and development within the body, preventing the proliferation of abnormal cells and bacterial and viral infections in living organisms. However, an excessive accumulation of radicals in the body may lead to symptoms of cytotoxicity and contribute to a range of acute and chronic diseases, tissue damage, and mutations in living animals (Martemucci et al., 2022).
Antioxidants are compounds that delay or prevent the oxidation process by neutralizing free radicals in body cells. Although numerous commercial synthetic antioxidants have been developed to mitigate the harmful effects of free radicals, these synthetic compounds may induce secondary harmful side effects (Abo-Shady et al., 2023). Consequently, the exploration of natural antioxidants produced by plants and various algal species as secondary metabolites has become a pivotal focus in research (Abo-Shady et al., 2023).
ROS can cause various disorders by attacking macromolecules. Superoxide dismutase (SOD) and catalase (CAT) are common antioxidant enzymes in most live organisms that can protect cells from damage induced by ROS. Some studies have demonstrated that Fucose (Fuc) extracted from brown seaweed exhibits significant inhibitory effects on DPPH free radicals, superoxide anions, hydroxyl radicals, and hydrogen peroxide (Zargari et al., 2023) (Kang et al., 2008). and (Song et al., 2017) observed that Fuc extracted from Saccharina japonica could reduce serum malondialdehyde content and significantly increase plasma and tissue SOD activities in rats with liver injury. In the present study, serum CAT and SOD enzymes were significantly influenced by the specific dietary level of Fuc. Therefore, it can be concluded that this compound enhances the antioxidant system capacity in A. ocellatus by elevating the activity of serum CAT and SOD enzymes. The antioxidant properties of Fuc can be attributed to the total phenol content. Phenolic compounds may act as antioxidants through various mechanisms, including their role as reducing agents and hydrogen donors, neutralization of oxygen radicals, and metal chelating ability (Zargari et al., 2023). The antioxidant activity of phenolic compounds is closely linked to their structure, particularly the number and position of hydroxyl groups (Kazemi et al., 2023). Despite the antioxidant properties of fucoidan, it can be said that the addition of fucoidan to the diet with an effect at the molecular level and improvement of the relative expression of SOD and CAT genes has led to an increase in their levels in the body. Consistent with our results, Fuc extracted from Padina boryana significantly reduced intracellular ROS levels in zebrafish and protected against apoptosis in this fish (Jayawardena et al., 2020). In addition, in another study, sulfated polysaccharides derived from Gracilaria lemaneiformis had a significant effect on antioxidant defense such as CAT, and SOD in rabbitfish (Siganus canaliculatus) (Bakky et al., 2023).
4.6 Immunological parameters
Lysozyme, a cationic enzyme, acts on the peptidoglycan present in the bacterial cell wall, leading to the lysis of certain Gram-positive bacteria. Moreover, lysozyme, in conjunction with complement system proteins, exhibits bactericidal activity against certain Gram-negative bacteria. In the current investigation, the introduction of Fuc extracted from S. ilicifolium algae into the diet resulted in the modulation of serum lysozyme activity in Oscar.
Mir et al. (2017) explored the synergistic impact of oral Fuc and methionine on the innate immune response of L. rohita fish, revealing that this polysaccharide enhances lysozyme activity (Mir et al., 2017). As lysozyme in fish is produced by Net and macrophages (Sakai, 1999), the observed elevation in lysozyme activity and phagocytosis in macrophages due to Fuc-induced macrophage activation suggests a potential correlation with an increase in WBC count.
Consistent with our findings, Yin et al. (2014) observed a significant rise in serum lysozyme activity after 48 days of administering diets containing laminarin to Epinephelus coioides (Yin et al., 2014). Complement proteins constitute a crucial component of humoral immunity (innate immunity) and play a pivotal role in immune system signaling in response to pathogenic presence. Complement component 3 (C3) and complement component 4 (C4) serve as key components in the classical and lectin pathways of the complement system. While complement activation is typically advantageous to the host, prolonged activation can lead to adverse effects and immunosuppression. In contrast to warm-blooded vertebrates, fish predominantly activate the complement system through the alternative pathway rather than the classical pathway (Tissot et al., 2003). The C3 isoform, a functional and gene-derived component, is a key element in the complement system of bony fishes. Emadi et al. (2010) introduced Dunaliella salina algae into the diet of rainbow trout, revealing a significant increase in the levels of C3 and C4 with escalating algae concentrations, indicating a notable difference from the control treatment (Emadi et al., 2010).
The results of the present study showed that the number of WBCs had a direct relationship with the dose of Fuc in the diet. The addition of different levels of Fuc in the diet of Oscar significantly leads to an increase in the number of WBCs.
4.7 Respiratory burst and bactericidal activity
In the current investigation, the fish subjected to a diet incorporating 2% Fucose (Fuc) exhibited the highest levels of serum bactericidal activity. Those fish nourished with a diet containing 2% Fuc demonstrated elevated levels of serum lysozyme and complement activity. The identification of protective proteins within fish bloodstream, such as complement proteins and lysozyme, can be accomplished through the assessment of serum bactericidal activity, which constitutes a non-specific defense mechanism against the proliferation of infectious microorganisms. Polysaccharides, in part, can serve as an energy source for intestinal epithelial cells and the gut microbiome. Previous studies have suggested that Fuc may stimulate the immune system through sulfated polysaccharides (Zargari et al., 2023).
The findings of this study revealed a 2% increase in respiratory burst activity in Oscar fed with a diet containing Fuc extracted from S. ilicifolium algae compared to the control group. Respiratory burst, a robust antibacterial mechanism in fish, is associated with cytokine release and an inflammatory response. During the respiratory burst, phagocytes generate various oxygen radicals in direct proportion to the intensity of the respiratory burst (Thomas, 2017). Specifically in fish, phagocytic cells, including monocytes, macrophages, and Net, can generate superoxide anions to eliminate pathogens (Liu et al., 2014). Moreover, these cells can augment the bactericidal activity of lysozyme as an opsonin and activate the complement system along with other phagocytes. In the research of Sony et al. (2018), Mozuku Fuc had no significant effect on respiratory burst activity in P. major (Sony et al., 2019).
4.8 Total skin carotenoid
Seaweeds contain diverse pigments, with carotenoids being prominent compounds frequently encountered in marine algae, manifested as ester compounds wherein hydroxyl groups undergo acylation with fatty acids (Mariutti and Mercadante, 2018). Following absorption, transport, and metabolic conversion, carotenoids designated for body coloration are deposited within chromatophore cells. These pigments play a pivotal role in determining the coloration of animal skin and muscle tissue, contributing to the remarkable color pattern variations observed in fish (Price et al., 2008). Like other animals, fish predominantly acquire requisite carotenoid compounds from their diet. Carotenoid esters in food undergo hydrolysis before absorption, facilitated by lipoproteins during transportation into the bloodstream. The skin of fish comprises a blend of dietary carotenoids and converted carotenoids, often esterified with fatty acids (Ninwichian et al., 2020; Tan et al., 2022).
Despite the absence of conclusive evidence regarding the direct impact of oral Fuc (Fucose) supplementation on fish skin carotenoids, this study suggests that the observed increase in skin carotenoid levels may be attributed to the presence of this compound in Fuc extracted from S. ilicifolium algae. Moreover, the modulation of carotenoid levels might be associated with the main chain characterized by a sugary nature in Fuc. Polysaccharides, constituting sugars, are integral for lipid metabolism stored in adipose tissue (Fernandez-Quintela et al., 2007; Bonet et al., 2020). Consequently, heightened dietary polysaccharides prompt increased metabolism of adipose tissue fats, potentially influencing the absorption and resynthesis of dietary carotenoids (Bonet et al., 2020).
Additionally, the elevation in skin carotenoid levels in fish fed with a diet containing 2% Fuc could be linked to the upregulation of genes involved in carotenoid synthesis. Although the genes orchestrating skin coloration processes remain largely unidentified, this study draws parallels from a cichlid and betta fish analysis. The investigation revealed genes associated with xanthophore formation (e.g., pax7 and sox10), stimulation of intracellular pigment synthesis (e.g., tubb, vim, kif5b), and absorption (e.g., oscarb1) and storage (e.g., plin6) of carotenoids in relation to carotenoid-based skin coloration (Ahi et al., 2020; Zhang et al., 2023).
5 Conclusion
Further investigations are imperative to derive conclusive insights into the impact of Fuc extracted from S. ilicifolium algae when employed as a dietary supplement on a commercial scale within the aquaculture industry. Nonetheless, the current study reveals promising outcomes, including heightened growth, reduced feed conversion ratio, and an augmented expression of innate immunity parameters. This is substantiated by the assessment of bactericidal activity, indicating that the utilization of Fuc extracted from S. ilicifolium algae, with its inherent antioxidant properties, possesses the capability to modulate the host’s immune system and antioxidant defense mechanisms. These findings underscore the considerable potential of Fuc for application in the aquaculture industry.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
All experiments were performed following the protocol approved by the ethics committee of the faculty of sciences of the University of Tehran (357; 8 November 2000). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MK: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Data curation, Conceptualization. SH: Writing – review & editing, Visualization, Validation, Software, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Supervision, Resources, Conceptualization. AZ: Visualization, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Validation, Supervision, Software, Resources, Project administration, Methodology. HT: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation. HD: Writing – review & editing, Writing – original draft, Validation, Conceptualization. NR: Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded personally.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelhamid A. F., Ayoub H. F., Abd El-Gawad E. A., Abdelghany M. F., Abdel-Tawwab M. (2021). Potential effects of dietary seaweeds mixture on the growth performance, antioxidant status, immunity response, and resistance of striped catfish (Pangasianodon hypophthalmus) against Aeromonas hydrophila infection. Fish Shellfish Immunol. 119, 76–83. doi: 10.1016/j.fsi.2021.09.043
Abo-Shady A. M., Gheda S. F., Ismail G. A., Cotas J., Pereira L., Abdel-Karim O. H. (2023). Antioxidant and antidiabetic activity of algae. Life 13, 460. doi: 10.3390/life13020460
Ahi E. P., Lecaudey L. A., Ziegelbecker A., Steiner O., Glabonjat R., Goessler W., et al. (2020). Comparative transcriptomics reveals candidate carotenoid color genes in an East African cichlid fish. BMC Genomics 21, 1–15. doi: 10.1186/s12864-020-6473-8
Akbary P., Aminikhoei Z. (2018). Effect of water-soluble polysaccharide extract from the green alga Ulva rigida on growth performance, antioxidant enzyme activity, and immune stimulation of grey mullet Mugil cephalus. J. Appl. Phycol. 30, 1345–1353. doi: 10.1007/s10811-017-1299-8
Anisha G. S., Padmakumari S., Patel A. K., Pandey A., Singhania R. R. (2022). Fucoidan from marine macroalgae: Biological actions and applications in regenerative medicine, drug delivery systems and food industry. Bioengineering. 9, 472. doi: 10.3390/bioengineering9090472
Bakky M. A. H., Tran N. T., Zhang Y., Hu H., Lin H., Zhang M., et al. (2023). Effects of dietary supplementation of Gracilaria lemaneiformis-derived sulfated polysaccharides on the growth, antioxidant capacity, and innate immunity of rabbitfish (Siganus canaliculatus). Fish Shellfish Immunol. 139, 108933. doi: 10.1016/j.fsi.2023.108933
Bonet M. L., Ribot J., Galmés S., Serra F., Palou A. (2020). Carotenoids and carotenoid conversion products in adipose tissue biology and obesity: Pre-clinical and human studies. Biochim. Biophys. Acta (BBA)-Molecular Cell Biol. Lipids 1865, 158676. doi: 10.1016/j.bbalip.2020.158676
Budiño B., Cal R. M., Piazzon M. C., Lamas J. (2006). The activity of several components of the innate immune system in diploid and triploid turbot. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 145, 108–113. doi: 10.1016/j.cbpa.2006.05.007
Carpena M., García-Pérez P., García-Oliveira P., Chamorro F., Otero P., Lourenço-Lopes C., et al. (2022). Biological properties and potential of compounds extracted from red seaweeds. Phytochem. Rev. 22, 1–32. doi: 10.1007/s11101-022-09826-z
Chakraborti S., Michael J. R., Chakraborti T. (2004). Role of an aprotinin-sensitive protease in protein kinase Cα-mediated activation of cytosolic phospholipase A2 by calcium ionophore (A23187) in pulmonary endothelium. Cell Signal. 16, 751–762. doi: 10.1016/j.cellsig.2003.12.006
Do-Amaral C. C. F., Pacheco B. S., Seixas F. K., Pereira C. M. P., Collares T. (2020). Antitumoral effects of fucoidan on bladder cancer. Algal Res. 47, 101884. doi: 10.1016/j.algal.2020.101884
Dopeikar H., Khoshkholgh M., Ghasemi S. A., Morshedi V. (2024). Effects of background color on growth, stress, biochemical, hematological, and immunological responses, and expression of growth-related genes in oscar fish (Astronotus ocellatus). Aquaculture Res. 2024. doi: 10.1155/2024/6957201
El Basuini M. F., Shahin S. A., Teiba I. I., Zaki M. A., El-Hais A. M., Sewilam H., et al. (2021). The influence of dietary coenzyme Q10 and vitamin C on the growth rate, immunity, oxidative-related genes, and the resistance against Streptococcus agalactiae of Nile tilapia (Oreochromis niloticus). Aquaculture 531, 735862. doi: 10.1016/j.aquaculture.2020.735862
El Basuini M. F., Teiba I. I., Zaki M. A., Alabssawy A. N., El-Hais A. M., Gabr A. A., et al. (2020). Assessing the effectiveness of CoQ10 dietary supplementation on growth performance, digestive enzymes, blood health, immune response, and oxidative-related genes expression of Nile tilapia (Oreochromis niloticus). Fish shellfish Immunol. 98, 420–428. doi: 10.1016/j.fsi.2020.01.052
Emadi H., Amaninejad P., Emtiazjoo M., Sahhafi H. H. (2010). Effects of Dunaliella microalgae (Dunaliellasalina) on different levels of complement [C. sub. 3],[C. sub. 4] and antioxidant capacity in rainbow trout (Oncorhynchusmykiss). Adv. Environ. Biol. 4, 456–464.
Erfanmanesh A., Beikzadeh B., Khanzadeh M. (2023). Efficacy of polyvalent vaccine on immune response and disease resistance against streptococcosis/lactococcosis and yersiniosis in rainbow trout (Oncorhynchus mykiss). Vet. Res. Commun. 4, 1347–1355. doi: 10.1007/s11259-023-10081-6
Farvin K. H. S., Jacobsen C. (2013). Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 138, 1670–1681. doi: 10.1016/j.foodchem.2012.10.078
Fernandez-Quintela A., Churruca I., Portillo M. P. (2007). The role of dietary fat in adipose tissue metabolism. Public Health Nutr. 10, 1126–1131. doi: 10.1017/S1368980007000602
Grant K. R. (2015). Fish hematology and associated disorders. Vet. Clin. Exot Anim. Pract. 18, 83–103. doi: 10.1016/j.cvex.2014.09.007
Haggag Y. A., Abd Elrahman A. A., Ulber R., Zayed A. (2023). Fucoidan in pharmaceutical formulations: A comprehensive review for smart drug delivery systems. Mar. Drugs 21, 112. doi: 10.3390/md21020112
Hoseinifar S. H., Khalili M., Rufchaei R., Raeisi M., Attar M., Cordero H., et al. (2015). Effects of date palm fruit extracts on skin mucosal immunity, immune related genes expression and growth performance of common carp (Cyprinus carpio) fry. Fish Shellfish Immunol. 47, 706–711. doi: 10.1016/j.fsi.2015.09.046
Hoseinifar S. H., Khalili M., Sun Y. Z. (2016). Intestinal histomorphology, autochthonous microbiota and growth performance of the oscar (Astronotus ocellatus Agassiz, 1831) following dietary administration of xylooligosaccharide. J. Appl. Ichthyol. 32, 1137–1141. doi: 10.1111/jai.13118
Hossain S., Heo G. (2021). Ornamental fish: a potential source of pathogenic and multidrug-resistant motile Aeromonas spp. Lett. Appl. Microbiol. 72, 2–12. doi: 10.1111/lam.13373
Jaruboonyakorn P., Tejangkura T., Chontananarth T. (2022). Multiplex PCR development for the simultaneous and rapid detection of two pathogenic flukes, Dactylogyrus spp. and Centrocestus formosanus, in ornamental fishes. Aquaculture. 548, 737660. doi: 10.1016/j.aquaculture.2021.737660
Jayawardena T. U., Nagahawatta D. P., Fernando I. P. S., Kim Y. T., Kim J. S., Kim W. S., et al. (2022). A Review on fucoidan structure, extraction techniques, and its role as an immunomodulatory agent. Mar. Drugs 20, 755. doi: 10.3390/md20120755
Jayawardena T. U., Wang L., Sanjeewa K. A., Kang S. I., Lee J. S., Jeon Y. J. (2020). Antioxidant potential of sulfated polysaccharides from Padina boryana; Protective effect against oxidative stress in in vitro and in vivo zebrafish model. Mar. Drugs 18, 212. doi: 10.3390/md18040212
Kang K. S., Kim I. D., Kwon R. H., Lee J. Y., Kang J. S., Ha B. J. (2008). The effects of fucoidan extracts on CCl 4-induced liver injury. Arch. pharmacal Res. 31, 622–627. doi: 10.1007/s12272-001-1203-8
Kazemi M., Fathi M., Jahanbin K., Taghdir M., Abbaszadeh S. (2023). Optimization of ultrasonic-assisted hot acidic solvent extraction of ulvan from Ulva intestinalis of the Persian Gulf: Evaluation of structural, techno-functional, and bioactivity properties. Food Hydrocolloids 142, 108837. doi: 10.1016/j.foodhyd.2023.108837
Khadim R. M., Al-Fartusie F. S. (2021). Antioxidant vitamins and their effect on immune system. J. Physics: Conf. Ser. 1853, 012065. IOP Publishing. doi: 10.1088/1742-6596/1853/1/012065
Khanzadeh M., Beikzadeh B., Hoseinifar S. H. (2023). The Effects of Laurencia caspica Algae Extract on Hemato-Immunological Parameters, Antioxidant Defense, and Resistance against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Aquac Nutr. 2023. doi: 10.1155/2023/8882736
Khanzadeh M., Hoseinifar S. H., Beikzadeh B. (2024). Investigating the effect of hydroalcoholic extract of red algae (Laurencia caspica) on growth performance, mucosal immunity, digestive enzyme activity and resistance to Streptococcus iniae and Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus). Aquaculture Rep. 35, 101984. doi: 10.1016/j.aqrep.2024.101984
Khanzadeh M., Vazirzadeh A., Farhadi A. (2020). Effect of Extract and Fucoidan of Sargassum sp. on Growth, biochemical, Immunity and antioxidant Parameters of Nile Tilapia (Oreochromis niloticus). Iranian Sci. Fisheries J. 29, 97–108. doi: 10.22092/ISFJ.2020.122997
Kondera E., Bojarski B., Ługowska K., Kot B., Witeska M. (2020). Effects of oxytetracycline and gentamicin therapeutic doses on hematological, biochemical and hematopoietic parameters in Cyprinus carpio juveniles. Animals. 10, 2278. doi: 10.3390/ani10122278
Kumar S., Saxena J., Srivastava V. K., Kaushik S., Singh H., Abo-EL-Sooud K., et al. (2022). The interplay of oxidative stress and ROS scavenging: Antioxidants as a therapeutic potential in sepsis. Vaccines 10, 1575. doi: 10.3390/vaccines10101575
Lakshmanan A., Balasubramanian B., Maluventhen V., Malaisamy A., Baskaran R., Liu W. C., et al. (2022). Extraction and characterization of fucoidan derived from sargassum ilicifolium and its biomedical potential with in silico molecular docking. Appl. Sci. 12, 13010. doi: 10.3390/app122413010
Lee S. H., Kang M. C., Moon S. H., Jeon B. T., Jeon Y. J. (2013). Potential use of ultrasound in antioxidant extraction from Ecklonia cava. Algae 28, 371–378. doi: 10.4490/algae.2013.28.4.371
Li F., Sun H., Li Y., He D., Ren C., Zhu C., et al. (2023). Effects of fucoidan on growth performance, immunity, antioxidant ability, digestive enzyme activity, and hepatic morphology in juvenile common carp (Cyprinus carpio). Front. Mar. Sci. 10, 1167400. doi: 10.3389/fmars.2023.1167400
Liu J., Wu S. Y., Chen L., Li Q. J., Shen Y. Z., Jin L., et al. (2020). Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. Int. J. Biol. Macromol. 155, 1385–1392. doi: 10.1016/j.ijbiomac.2019.11.113
Liu L., Zhou Y., Zhao X., Wang H., Wang L., Yuan G., et al. (2014). Oligochitosan stimulated phagocytic activity of macrophages from blunt snout bream (Megalobrama amblycephala) associated with respiratory burst coupled with nitric oxide production. Dev. Comp. Immunol. 47, 17–24. doi: 10.1016/j.dci.2014.06.005
Mahgoub H. A., El-Adl M. A., Ghanem H. M., Martyniuk C. J. (2020). The effect of fucoidan or potassium permanganate on growth performance, intestinal pathology, and antioxidant status in Nile tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 46, 2109–2131. doi: 10.1007/s10695-020-00858-w
Mariappan B., Kaliyamurthi V., Binesh A. (2023). “Medicinal plants or plant derived compounds used in aquaculture,” in Recent advances in aquaculture microbial technology (Academic Press: Elsevier), 153–207. doi: 10.1016/B978-0-323-90261-8.00003-1
Mariutti L. R., Mercadante A. Z. (2018). Carotenoid esters analysis and occurrence: What do we know so far? Arch. Biochem. Biophys. 648, 36–43. doi: 10.1016/j.abb.2018.04.005
Martemucci G., Costagliola C., Mariano M., D’andrea L., Napolitano P., D’Alessandro A. G. (2022). Free radical properties, source and targets, antioxidant consumption and health. Oxygen 2, 48–78. doi: 10.3390/oxygen2020006
Martins A., Alves C., Silva J., Pinteus S., Gaspar H., Pedrosa R. (2023). Sulfated polysaccharides from macroalgae—A simple roadmap for chemical characterization. Polymers (Basel). 15, 399. doi: 10.3390/polym15020399
Mir I. N., Sahu N. P., Pal A. K., Makesh M. (2017). Synergistic effect of l-methionine and fucoidan rich extract in eliciting growth and non-specific immune response of Labeo rohita fingerlings against Aeromonas hydrophila. Aquaculture 479, 396–403. doi: 10.1016/j.aquaculture.2017.06.001
Mohammadiazarm H., Maniat M., Ghorbanijezeh K., Ghotbeddin N. (2021). Effects of spirulina powder (Spirulina platensis) as a dietary additive on Oscar fish, Astronotus ocellatus: Assessing growth performance, body composition, digestive enzyme activity, immune-biochemical parameters, blood indices and total pigmentation. Aquaculture Nutr. 27, 252–260. doi: 10.1111/anu.13182
Ninwichian P., Chookird D., Phuwan N. (2020). Effects of dietary supplementation with natural carotenoid sources on growth performance and skin coloration of fancy carp, Cyprinus carpio L. Iranian J. Fisheries Sci. 19, 167–181. doi: 10.22092/ijfs.2019.118784
Ozorio R. A., Lopes R. G., Goes B. S., da SILVA C. P., Derner R. B., Fracalossi D. M. (2015). Growth and enzymatic profile of the pacific white shrimp fed with Porphyridium cruentum extract. Boletim do Instituto Pesca 41, 123–131.
Pietras E. M., DeGregori J. (2019). Haematopoietic system. (Oxford Academic), 358–409. doi: 10.1093/oxfordhb/9780198789666.013.9
Prabu D. L., Sahu N. P., Pal A. K., Dasgupta S., Narendra A. (2016). Immunomodulation and interferon gamma gene expression in sutchi cat fish, Pangasianodon hypophthalmus: effect of dietary fucoidan rich seaweed extract (FRSE) on pre and post challenge period. Aquaculture Res. 47, 199–218. doi: 10.1111/are.12482
Price A. C., Weadick C. J., Shim J., Rodd F. H. (2008). Pigments, patterns, and fish behavior. Zebrafish 5, 297–307. doi: 10.1089/zeb.2008.0551
Raguraman V., Jyotsna J., Palaniappan S., Gopal S., Thirugnanasambandam R., Kirubagaran R. (2019). Sulfated polysaccharide from Sargassum tenerrimum attenuates oxidative stress induced reactive oxygen species production in in vitro and in zebrafish model. Carbohydr. polymers 203, 441–449. doi: 10.1016/j.carbpol.2018.09.056
Rahmati-Holasoo H., Marandi A., Ebrahimzadeh Mousavi H., Taheri Mirghaed A. (2022). Parasitic fauna of farmed freshwater ornamental fish in the northwest of Iran. Aquac Int. 30, 633–652. doi: 10.1007/s10499-021-00832-0
Ramamoorthy K., Bhuvaneswari S., Sankar G., Sakkaravarthi K. (2010). Proximate composition and carotenoid content of natural carotenoid sources and its colour enhancement on marine ornamental fish Amphiprion ocellaris (Cuveir, 1880). World J. Fish Mar. Sci. 2, 545–550.
Ramazanov Z., Jimenez del Rio M., Ziegenfuss T. (2003). Sulfated polysaccharides of brown seaweed Cystoseira canariensis bind to serum myostatin protein. Acta physiologica pharmacologica Bulgarica 27, 101–106.
Ross N. W., Firth K. J., Wang A., Burka J. F., Johnson S. C. (2000). Changes in hydrolytic enzyme activities of naive Atlantic salmon Salmo salar skin mucus due to infection with the salmon louse Lepeophtheirus salmonis and cortisol implantation. Dis. Aquat Organ. 41, 43–51. doi: 10.3354/dao041043
Rouhani E., Safari R., Imanpour M. R., Hoseinifar S. H., Yazici M., El-Haroun E. (2022). Effect of dietary administration of green macroalgae (Ulva intestinalis) on mucosal and systemic immune parameters, antioxidant defence, and related gene expression in zebrafish (Danio rerio). Aquaculture Nutr. 2022, 1–11. doi: 10.1155/2022/7693468
Sajina K. A., Sahu N. P., Varghese T., Jain K. K. (2019). Fucoidan-rich Sargassum wightii extract supplemented with α-amylase improve growth and immune responses of Labeo rohita (Hamilton, 1822) fingerlings. J. Appl. Phycol. 31, 2469–2480. doi: 10.1007/s10811-019-1742-0
Sakai M. (1999). Current research status of fish immunostimulants. Aquaculture 172, 63–92. doi: 10.1016/S0044-8486(98)00436-0
Salehpour R., Biuki N. A., Mohammadi M., Dashtiannasab A., Ebrahimnejad P. (2021). The dietary effect of fucoidan extracted from brown seaweed, Cystoseira trinodis (C. Agardh) on growth and disease resistance to WSSV in shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 119, 84–95. doi: 10.1016/j.fsi.2021.09.005
Shao P., Pei Y., Fang Z., Sun P. (2014). Effects of partial desulfation on antioxidant and inhibition of DLD cancer cell of Ulva fasciata polysaccharide. Int. J. Biol. Macromol. 65, 307–313. doi: 10.1016/j.ijbiomac.2014.01.043
Sivagnanavelmurugan M., Thaddaeus B. J., Palavesam A., Immanuel G. (2014). Dietary effect of Sargassum wightii fucoidan to enhance growth, prophenoloxidase gene expression of Penaeus monodon and immune resistance to Vibrio parahaemolyticus. Fish Shellfish Immunol. 39, 439–449. doi: 10.1016/j.fsi.2014.05.037
Song Y., Wang Q., He Y., Ren D., Kow F., Li J., et al. (2017). The positive effects of fucoidans extracted from the brown seaweed Saccharina japonica on protection against CCl 4-induced liver injury. J. Appl. Phycol. 29, 2077–2087. doi: 10.1007/s10811-017-1097-3
Sony N. M., Ishikawa M., Hossain M. S., Koshio S., Yokoyama S. (2019). The effect of dietary fucoidan on growth, immune functions, blood characteristics and oxidative stress resistance of juvenile red sea bream, Pagrus major. Fish Physiol. Biochem. 45, 439–454. doi: 10.1007/s10695-018-0575-0
Tan K., Zhang H., Zheng H. (2022). Carotenoid content and composition: A special focus on commercially important fish and shellfish. Crit. Rev. Food Sci. Nutr. 64, 544–561. doi: 10.1080/10408398.2022.2106937
Thepot V., Campbell A. H., Paul N. A., Rimmer M. A. (2021). Seaweed dietary supplements enhance the innate immune response of the mottled rabbitfish, Siganus fuscescens. Fish Shellfish Immunol. 113, 176–184. doi: 10.1016/j.fsi.2021.03.018
Thépot V., Campbell A. H., Rimmer M. A., Jelocnik M., Johnston C., Evans B., et al. (2022). Dietary inclusion of the red seaweed Asparagopsis taxiformis boosts production, stimulates immune response and modulates gut microbiota in Atlantic salmon, Salmo salar. Aquaculture 546, 737286. doi: 10.1016/j.aquaculture.2021.737286
Thomas D. C. (2017). The phagocyte respiratory burst: Historical perspectives and recent advances. Immunol. Lett. 192, 88–96. doi: 10.1016/j.imlet.2017.08.016
Tissot B., Montdargent B., Chevolot L., Varenne A., Descroix S., Gareil P., et al. (2003). Interaction of fucoidan with the proteins of the complement classical pathway. Biochim. Biophys. Acta (BBA)-Proteins Proteomics 1651, 5–16. doi: 10.1016/S1570-9639(03)00230-9
Traifalgar R. F., Kira H., Thanh Tung H. A., Raafat Michael F., Laining A., Yokoyama S., et al. (2010). Influence of dietary fucoidan supplementation on growth and immunological response of juvenile Marsupenaeus japonicus. J. World Aquaculture Soc. 41, 235–244. doi: 10.1111/j.1749-7345.2010.00363.x
Tuller J., De Santis C., Jerry D. R. (2014). Dietary influence of Fucoidan supplementation on growth of Lates calcarifer (Bloch). Aquaculture Res. 45, 749–754. doi: 10.1111/are.2014.45.issue-4
Valente L. M., Araújo M., Batista S., Peixoto M. J., Sousa-Pinto I., Brotas V., et al. (2016). Carotenoid deposition, flesh quality and immunological response of Nile tilapia fed increasing levels of IMTA-cultivated Ulva spp. J. Appl. Phycol. 28, 691–701. doi: 10.1007/s10811-015-0590-9
Wang D., Wang S., Zhou Z., Bai D., Zhang Q., Ai X., et al. (2022). White blood cell membrane-coated nanoparticles: recent development and medical applications. Adv. Healthc. Mater. 11, 2101349. doi: 10.1002/adhm.202101349
Wang Q., Wen B., Micah A. D., Gao J. Z., Chen Z. Z. (2023). Integration of transcriptomics and metabolomics reveals amelanism mechanism of Oscar Astronotus ocellatus (Agassiz, 1831). Hydrobiologia 850, 2275–2298. doi: 10.1007/s10750-022-04921-w
Wang Q., Zhang Y. S., Peng Q. L., Wen B., Gao J. Z., Chen Z. Z. (2022). Distinct skin morphological and transcriptomic profiles between wild and albino Oscar Astronotus ocellatus. Comp. Biochem. Physiol. Part D Genomics Proteomics 41, 100944. doi: 10.1016/j.cbd.2021.100944
Wang J., Zhang Q., Zhang Z., Song H., Li P. (2010). Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 46, 6–12. doi: 10.1016/j.ijbiomac.2009.10.015
Yang W. N., Chen P. W., Huang C. Y. (2017). Compositional characteristics and in vitro evaluations of antioxidant and neuroprotective properties of crude extracts of fucoidan prepared from compressional puffing-pretreated Sargassum crassifolium. Mar. Drugs 15, 183. doi: 10.3390/md15060183
Yang C., Chung D., You S. (2008). Determination of physicochemical properties of sulphated fucans from sporophyll of Undaria pinnatifida using light scattering technique. Food Chem. 111, 503–507. doi: 10.1016/j.foodchem.2008.03.085
Yang Q., Yang R., Li M., Zhou Q., Liang X., Elmada Z. C. (2014). Effects of dietary fucoidan on the blood constituents, anti-oxidation and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco). Fish shellfish Immunol. 41, 264–270. doi: 10.1016/j.fsi.2014.09.003
Yin G., Li W., Lin Q., Lin X. I., Lin J., Zhu Q., et al. (2014). Dietary administration of laminarin improves the growth performance and immune responses in Epinephelus coioides. Fish shellfish Immunol. 41, 402–406. doi: 10.1016/j.fsi.2014.09.027
Yone Y., Furuichi M., Urano K. (1986). Effects of dietary wakame Undaria pinnatifida and Ascophyllum nodosum supplements on growth, feed efficiency, and proximate compositions of liver and muscle of red sea bream. Nippon Suisan Gakkaishi 52, 1465–1468. doi: 10.2331/suisan.52.1465
Zare M., Heidari E., Hosseini Choupani S. M., Akhavan S. R., Rombenso A., Esmaeili N. (2023). The recovery time between early mild stress and final acute stress affects survival rate, immunity, health, and physiology of oscar (Astronotus ocellatus). Animals. 13, 1606. doi: 10.3390/ani13101606
Zargari A., Nejatian M., Abbaszadeh S., Jahanbin K., Bagheri T., Hedayati A., et al. (2023). Modulation of toxicity effects of CuSO4 by sulfated polysaccharides extracted from brown algae (Sargassum tenerrimum) in Danio rerio as a model. Sci. Rep. 13, 11429. doi: 10.1038/s41598-023-38549-0
Zayed A., El-Aasr M., Ibrahim A. R. S., Ulber R. (2020). Fucoidan characterization: Determination of purity and physicochemical and chemical properties. Mar. Drugs 18, 571. doi: 10.3390/md18110571
Zeraatpisheh F., Firouzbakhsh F., Khalili K. J. (2018). Effects of the macroalga Sargassum angustifolium hot water extract on hematological parameters and immune responses in rainbow trout (Oncohrynchus mykiss) infected with Yersinia rukeri. J. Appl. Phycol. 30, 2029–2037. doi: 10.1007/s10811-018-1395-4
Keywords: Sargassum ilicifolium, fucoidan, growth, antioxidant, immunity, total skin pigment
Citation: Khanzadeh M, Hoseinifar SH, Zargari A, Tabibi H, Van Doan H and Rabetimarghezar N (2024) Fucoidan derived from Sargassum ilicifolium affects growth and hemato-immunological parameters and antioxidant defense in Oscar (Astronotus ocellatus). Front. Mar. Sci. 11:1370871. doi: 10.3389/fmars.2024.1370871
Received: 17 January 2024; Accepted: 02 April 2024;
Published: 24 April 2024.
Edited by:
Yafei Duan, South China Sea Fisheries Research Institute, ChinaReviewed by:
Ahmad Gharaei, University of Zabol, IranBasanta Kumar Das, Central Inland Fisheries Research Institute (ICAR), India
Copyright © 2024 Khanzadeh, Hoseinifar, Zargari, Tabibi, Van Doan and Rabetimarghezar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Majid Khanzadeh, khanzade@acecr.ac.ir
 Majid Khanzadeh
Majid Khanzadeh Seyed Hossein Hoseinifar
Seyed Hossein Hoseinifar Ashkan Zargari2
Ashkan Zargari2  Hamidreza Tabibi
Hamidreza Tabibi Hien Van Doan
Hien Van Doan