The use of linseed oil cake in the diets of rohu, Labeo rohita (Hamilton), after solid-state fermentation with a fish gut bacterium, Bacillus pumilus (KF640221): an appraisal on growth, digestibility, body composition, and hematobiochemical profile
- 1Aquaculture Laboratory, Department of Zoology, The University of Burdwan, Burdwan, India
- 2Department of Agricultural Sciences, Faculty of Agro-Based Industry, University Malaysia Kelantan, Jeli, Malaysia
- 3Advanced Livestock and Aquaculture Research Group, Faculty of Agro-Based Industry, University Malaysia Kelantan, Jeli, Malaysia
- 4Department of Poultry Science, University of Arkansas, Fayetteville, AR, United States
Introduction: Linseed or flaxseed (Linum usitassimum L.) contains a prospective source of protein and energy to be utilized in animal feed. This study aimed at re-cycling and value-addition of Linseed Oil Cake (LOC) for formulation of non-conventional carp diets.
Methods: The LOC was bio-processed through solid state fermentation (SSF) with a fish gut bacterium, Bacillus pumilus (KF640221). Nine experimental sets of diets were formulated using raw (R1-R4) and SSF-processed (F1-F4) LOC at 10%, 20%, 30% and 40% levels substituting fishmeal as well as other ingredients in a reference diet, and rohu, Labeo rohita fingerlings (2.08±0.03 g) were fed for 70 days feeding trial. Growth, carcass composition, activities of digestive enzymes, digestibility and haemato-biochemical parameters were studied following standard methodologies.
Results: SSF significantly (P< 0.05) improved crude protein along with amino acids, whereas crude fibre and antinutritional factors were reduced considerably. Experimental diets were isocaloric (4.8 kcal) and isonitrogenous (36%). Diets with bio-processed LOC had significantly better performance than the raw LOC. Fish fed diet F3 with 30% fermented LOC resulted in the highest weight gain (6.25 ± 0.09 g), specific growth rate (% day -1) and carcass protein deposition (16.77±0.34%). Activities of the digestive enzymes (amylase, lipase and protease) were also significantly (P<0.05) higher in fish receiving diets containing fermented LOC. Analyses of blood parameters revealed that haemoglobin, erythrocytes, leukocytes, plasma lipid, total plasma protein, albumin and globulin contents were increased, while plasma glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) levels were decreased in fish fed bio-processed LOC supplemented diets.
Conclusion: The present study might propose substitution of fish meal along with other conventional ingredients by incorporation of 30% SSF-processed LOC in the diets of rohu with no negative effect to the growth performance, carcass composition and feed utilization.
1 Introduction
Finfish aquaculture has come out as one of the fastest and largest growing among the food production sectors. Aquaculture, a consistent source of employment generation and economic growth, depends mostly on the production and supply of the formulated fish feed that entails the majority of the production cost (Naylor et al., 2000). Fish meal (FM) is a vital component of feed for the majority of the farmed fish species and animals because of its excellent amino acid profile, essential fatty acid composition, high level of superior quality protein, high digestibility, and appreciable quantities of vitamins as well as minerals (Tacon, 1993; Zhou et al., 2004). Global aquaculture has continued to grow annually at the rate of approximately 6.7% during the last two decades (FAO, 2022), although the production of FM has remained more or less stable (Árnason et al., 2017). Price hike together with limitation in global supplies has necessitated the replacement of FM with alternative resources to reduce the aquafeed cost so as to sustain fish production at an affordable price. Therefore, replacing a portion of the FM along with other conventional feed ingredients with agro-industrial by-products has been the focus for the last few decades (Hardy, 2010). Oil cakes are produced as agro-industrial by-products after the extraction of oil from the seeds. Sustainable utilization of residual oil cakes seems to be important for the viability of the edible vegetable oil industry. Several oil cakes (e.g., groundnut, linseed, sesame, sunflower, soybean, etc.) as alternative protein sources of plant origin have been evaluated in formulated carp diets (Hossain et al., 2001; Mazurkiewicz, 2009; Das and Ghosh, 2015; Ghosh and Mandal, 2015; Banerjee and Ghosh, 2016). However, the inclusion of plant ingredients at elevated levels produced negative effects on the growth, digestibility, and overall health status of fish (Hardy, 2010; Kumar et al., 2010; Hansen and Hemre, 2013a; Wang et al., 2016; Mahmood et al., 2018; Magbanua and Ragaza, 2024). Aside from the insufficiency in some essential amino acids, dietary application of oil cakes for fish or farmed animals has been restricted owing to the occurrence of some antinutritional factors (ANFs), viz., polyphenols, phytic acid, trypsin inhibitors, and non-starch polysaccharides (NSPs) (Mandal and Ghosh, 2010; Ghosh and Mandal, 2015; Ghosh et al., 2019). It has been hypothesized that these ANFs may severely reduce the nutritional value of the diverse plant resources if not deactivated or destroyed (Ghosh et al., 2019).
The nutritive value of the plant-derived protein sources could be improved by adopting some processing strategies. In order to increase the bioavailability of the nutrients, diminution/elimination of the ANFs and supplementation of suitable additives to fill up known deficiencies might allow the incorporation of the plant feedstuffs in animal feeds at an elevated level (Saha and Ray, 2011). Preceding studies suggested that conventional biological or physical processing methods like seed germination, heat treatment, and/or water soaking were not amply capable of reducing the ANFs in the oil seeds/cakes (for review, see Ghosh et al., 2019). Nutritional losses in plant feedstuffs occurring through heat treatment or soaking in water were indicated (Mandal and Ghosh, 2020). From the evolutionary standpoint, colonization of gut microbiota somewhat enables herbivorous animals (e.g., ruminants) to prevail over the harmful effects of plant-derived ANFs (Xu et al., 2021). Likewise, the existence of gut-associated microbiota in diverse fish species has been recognized of late and their positive attributes related to the nutrition of the host fish have been established (Ray et al., 2012; Ghosh et al., 2019; Soltani et al., 2019; Ringø et al., 2022). Preceding studies have documented the occurrence of diverse exo-enzymes producer microorganisms within the fish gut capable of degrading complex polysaccharides and other plant-derived ANFs. For example, the presence of cellulose (Ray et al., 2007), tannin (Mandal and Ghosh, 2013; Talukdar et al., 2016), phytate (Khan and Ghosh, 2012; Das and Ghosh, 2014), and xylan (Banerjee et al., 2016)-degrading bacteria and yeasts was recorded in the guts of numerous freshwater fish species. Although fermentative nutrition has been less studied in aquatic animals, in-vitro degradation of complex plant-derived feed ingredients through solid-state fermentation (SSF) has been suggested as an effective strategy to diminish the ANFs therein. Owing to the activity of ANF-degrading enzymes secreted by the microorganisms, augmentation of the nutrient bioavailability in the SSF-processed substrate has been anticipated (Ghosh et al., 2019).
SSF is a biological process wherein microorganisms are cultivated on solid substrates with the least amount (≃60%) of water/moisture (Pandey, 1992; Van de Lagemaat and Pyle, 2001). As opposed to the physical processing techniques, value addition through the microbial synthesis of essential biomolecules along with deactivation of the ANFs might be expected during the SSF (Wee, 1991). Pretreatment as well as processing by microbial enzymes through SSF has been shown to enhance feed utilization by deactivation of ANFs (Ramachandran and Ray, 2007; Ghosh and Mandal, 2015; Mandal and Ghosh, 2019). Therefore, SSF with specific ANF-degrading microorganisms has received attention as a bioprocessing strategy in recent times. Biotransformations of crop remainders and detoxification of agricultural by-products are the cost-effective applications of SSF in view of agro-waste valorization and nutrient recycling (Pandey et al., 2001; Ghosh et al., 2019). In particular, SSF may be of interest wherein the crude bioprocessed substrate could be directly used for commercial applications (Tengerdy, 1998). Considering the above facts, this study aimed at bioprocessing of an agro-industrial by-product (linseed oil cake) through SSF by a specific ANF-degrading bacterium for likely utilization of the oil cakes as a component in formulated fish feed and to replace FM in part. In our preceding study, the complex polysaccharide-degrading ability of the gut-associated Bacillus pumilus (KF640221) isolated from rohu, Labeo rohita (Hamilton), was documented (Banerjee et al., 2016). Further study revealed the tannin- as well as the phytate-degrading capacity of the strain (Banerjee and Ghosh, 2016). Bioprocessing of the plant ingredients by autochthonous fish gut-associated microorganisms might ensure that neither the organism itself nor their metabolites would harm the fish (Mandal and Ghosh, 2019).
Linseed or flaxseed (Linum usitassimum L.) contains approximately 200–250 g kg−1 crude protein along with 400–430 g kg−1 oil (Lee et al., 1991), which constitutes a prospective source of protein and energy to be utilized in animal feed. In addition, it is an excellent source of ω-3 fatty acids (58.5%–59.7%), α-linolenic acid (ALA) in particular, which attracts attention for both human and animal nutrition (Doreau and Chilliard, 1997). The major producers of linseed are Tunisia, China, Argentina, and India although it is cultivated in almost all countries in the world. Aside from ANFs like tannins, trypsin inhibitor, or phytic acid, de-oiled linseed oil cake (LOC) contains a high amount of crude protein (≃34%; dry weight) and has the ability to be used as an FM replacer (Banerjee and Ghosh, 2016). Previous studies reported that the incorporation of LOC as a feed ingredient in the diets of walking catfish, Clarias batrachus fry (Hasan et al., 1989), and the Indian major carp, L. rohita (Hasan et al., 1991), achieved experimental success. This study evaluated the nutritive values of raw (dried and ground) and bioprocessed LOC along with value addition due to SSF. The potential use of the bioprocessed LOC has also been appraised in the formulated diets of L. rohita fingerlings.
2 Materials and methods
2.1 Microorganism used
The bacterium, B. pumilus LRF1X (KF640221), used in this study was collected from the proximal intestine of rohu, L. rohita, and described as an efficient NSP-degrading strain in a previous report (Banerjee et al., 2016). The culture was developed and maintained on tryptone soy broth (TSB) medium and stored at 4°C. Furthermore, cultures in TSB were preserved in 0.85% NaCl with 20% glycerol at −20°C for subsequent future use (Sugita et al., 1998).
2.2 Bioprocessing of linseed oil cake through solid-state fermentation
Dried and de-oiled LOC was procured from a local marketplace and utilized as a solid substrate. The LOC was dried in a hot air oven (80°C, 48 h), finely crushed in a mixer grinder, and passed through a fine mesh sieve (400 μm in diameter) to get uniform particle size. The SSF of the dried and de-oiled LOC was done under optimized parameters described in Banerjee and Ghosh (2016). An inoculum of B. pumilus (KF640221) was taken from a freshly raised culture. The total viable cells of the bacterium in TSB culture (37°C, 48 h) were determined, and the cell density was adjusted to 6.5 × 107 cells ml−1 for use as inoculums. Powdered LOC was moistened with liquid basal medium (60%, w/v; pH 7) containing (g L−1) MgSO4, 7H2O (0.3), K2HPO4 (0.15), and (NH4)2SO4 (2.0) and sterilized using an autoclave (121°C, 15 lb., 20 min). The sterilized substrate was supplemented with lactose (1%, v/w), ammonium sulfate (2.0%, w/v), NaCl (2%, w/w), and Tween 80 (1%, v/w). The entire mixture was inoculated with B. pumilus (6.0%, v/w; 6.5 × 107 cells ml−1), and fermentation was continued for 8 days at 35°C in a shaker-incubator (Banerjee and Ghosh, 2016).
2.3 Formulation and preparation of experimental diets
The raw and SSF-processed LOC was dried out and evaluated for proximate composition, ANFs, and amino acid contents before its inclusion into the experimental diets (Table 1). Other ingredients and formulated diets were also analyzed for proximate composition (dry weight basis), a brief description of which has been incorporated in a later section. A diet with FM as the key protein source was considered as the reference diet (RD). Altogether, nine sets of experimental diets were formulated with raw (R1–R4) and SSF-processed (F1–F4) LOC replacing FM at 10%, 20%, 30%, and 40% levels (w/w) in the RD (Table 2). Each of the experimental feed was formulated independently using WinFeed 2.8 software. Feed ingredients were finely pulverized, sieved to get a homogeneous particle size (diameter,<400 μm), and mixed carefully. Cod liver oil (1.0% each) was added and the mixture was made into a dough with lukewarm water. To each of the formulated diets, carboxymethylcellulose (CMC, 1%) and chromic oxide (1%) were added as the binder and external digestibility marker, respectively. A commercial vitamin–mineral premix (Supradyn, Bayer Consumer Care AG, Basel, Switzerland) was added to the diets prior to pelletization. The dough was made to pass through a hand pelletizer (1.5 mm pellets). The pellets were initially sun-dried and further dried in a hot air oven (60°C, 96 h). The dried pellets were crushed, packed in airtight plastic bags, and stored in a refrigerator (4°C) until use.
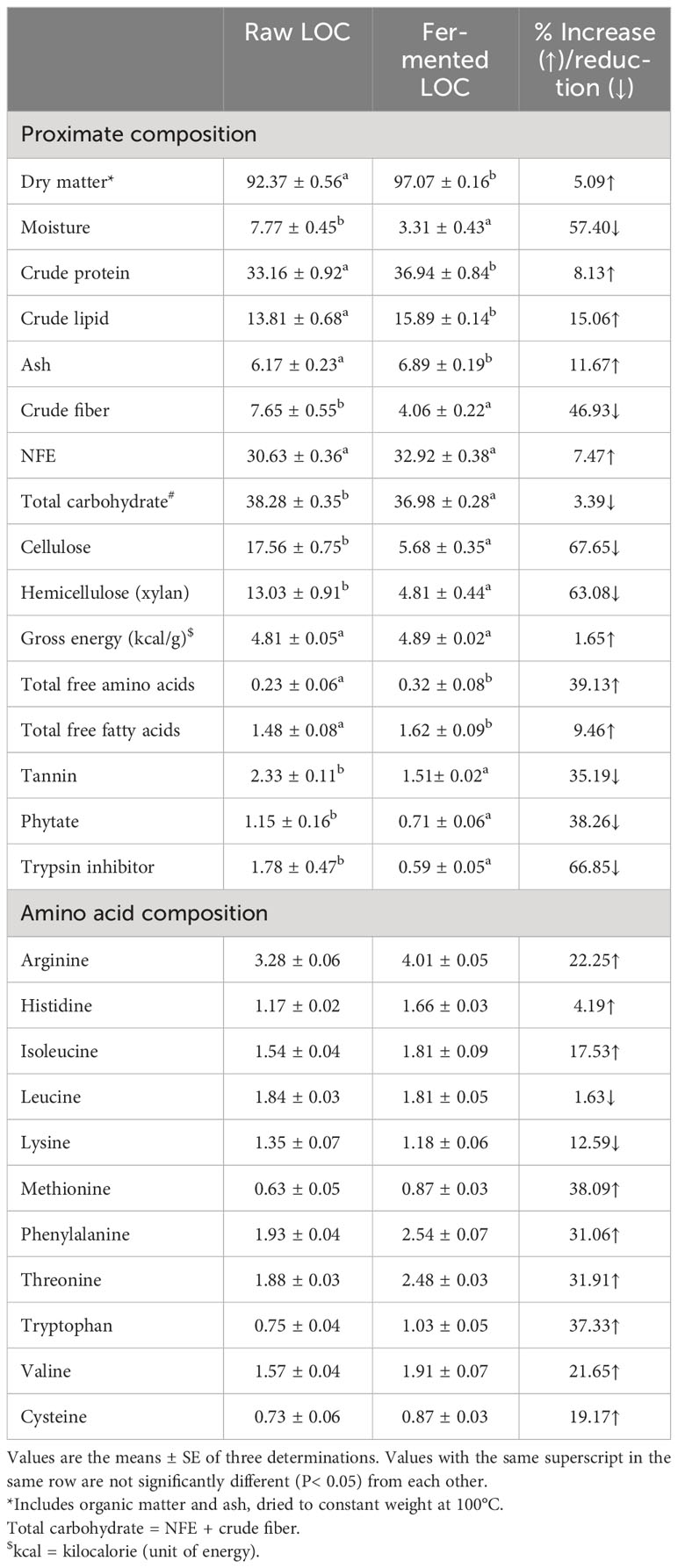
Table 1 Proximate composition and amino acid composition (on % dry matter basis) of the raw and SSF-processed (fermented) linseed oil cake (LOC).
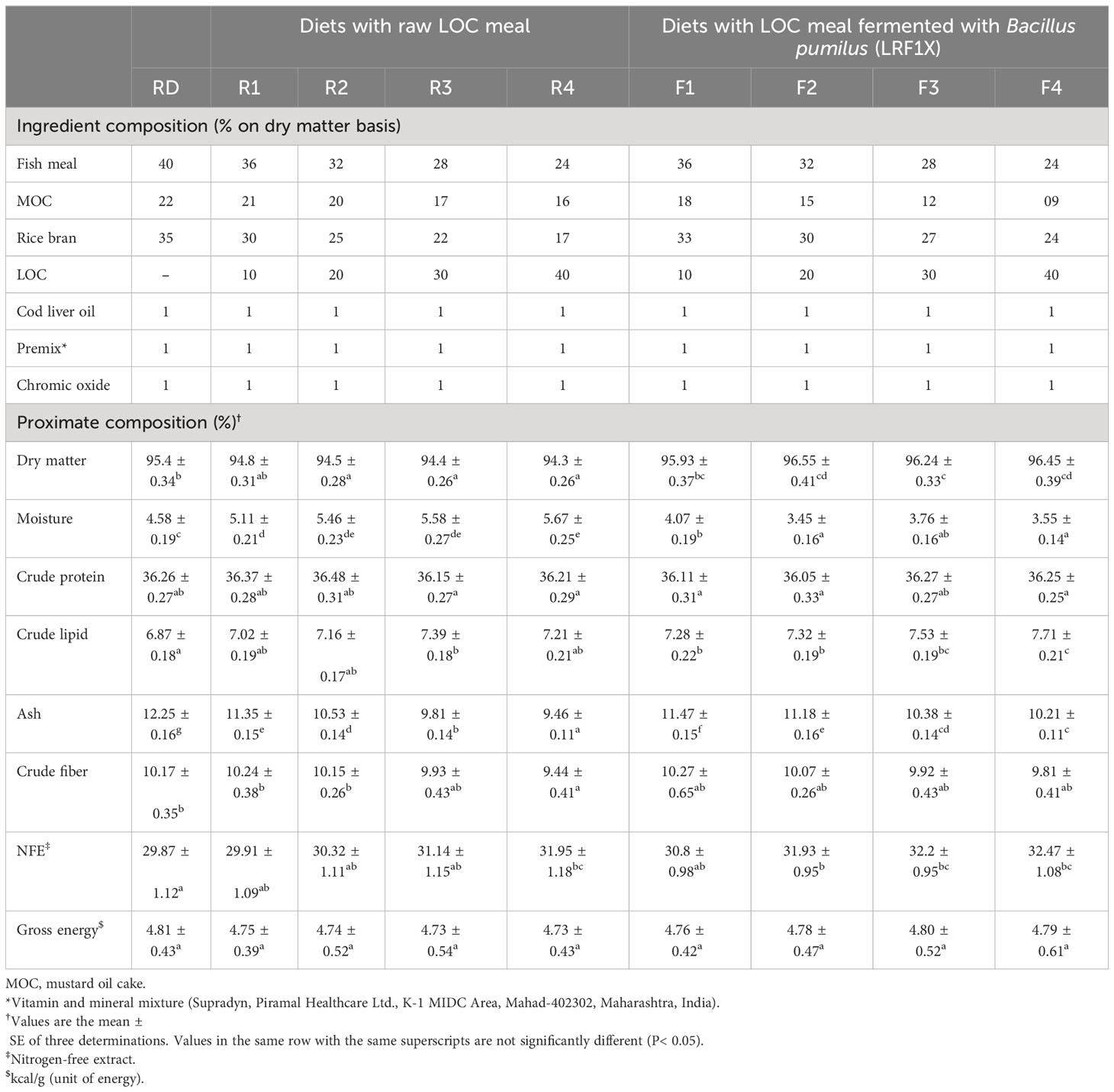
Table 2 Ingredient composition (% dry weight) and proximate composition of the experimental diets (on a dry matter basis).
2.4 Experimental design
Healthy fingerlings of rohu, L. rohita, produced by induced breeding and raised in a local fish farm (22.8929°N, 88.4220°E) were procured and acclimatized for 15 days in fiber-reinforced plastic (FRP) tanks (350 L of water). The experimental fish were handled and the feeding trial was designed following the institutional ethics committee guidelines. The experimental protocol also complied with the ARRIVE guidelines. The feeding trial was carried out in 27 FRP tanks (9 sets × 3 replicates) for 70 days and under laboratory condition with continuous aeration. The fingerlings (2.05 ± 0.05 g) were distributed in the FRP tanks at random with a stocking density of 20 fish per tank. The experimental fish were fed twice daily (08.00 h and 13.00 h) at the rate of 3% (w/w) of the total live body weight per day. Fish from each replicate was weighed every 10th day to adjust the daily ration of the experimental fish. The uneaten feed was collected after 5 h of each feeding and oven-dried (100°C) to calculate the feed conversion ratio (FCR). The fecal matter released by the fish was collected daily from each tank by pipetting following Spyridakis et al. (1989). Pooled fecal samples from each replicate were dried (55°C) and stored at −20°C for subsequent use in digestibility determination (Mohanta et al., 2009). After termination of the feeding trial, the fish were weighed and five fish from each tank were sampled, anesthetized, homogenized, and analyzed for whole-body (carcass) composition. Diverse parameters of the ambient water, viz., temperature (°C), dissolved oxygen (mg L−1), and pH, were monitored during the experimental period at weekly intervals after the American Public Health Association (APHA, 2012). The water quality parameters fluctuated within a narrow range in all experimental groups: temperature (28°C–30°C), dissolved oxygen (6.7–7.1 mg L–1), and pH (7.1–7.3).
2.5 Analyses of feed ingredients, diets, and fish carcass
Feed ingredients, experimental diets, fecal samples, and fish carcass were analyzed for proximate composition following the methods described by the Association of Official Analytical Chemists (AOAC, 2005). Moisture level was measured by drying the samples initially at 100°C ± 5°C (30 min) and thereafter at 60°C until a stable weight was attained. The crude protein content (N × 6.25) was estimated by the Kjeldahl system consisting of micro-Kjeldahl digestion along with distillation units (KjelTRON, Tulin Equipments, Chennai, India). Crude lipid (ether extract) and crude fiber contents were determined using Socsplus and Fibraplus systems (Pelican Equipments, Chennai, India), respectively. Ash content was estimated by ignition in a muffle furnace (550°C ± 5°C). Nitrogen-free extract (NFE) was calculated by subtracting the sum of crude fiber, crude protein, crude lipid, ash, and moisture contents from 100 (Maynard et al., 1979). A bomb calorimeter (Lab-X, Kolkata, India) was used to measure the gross energy. Total free amino acids were extracted with ethanol (80%) and quantified with ninhydrin reagent using a calibration curve of glycine (Moore and Stein, 1948). Free fatty acids were extracted in a neutral solvent (ether and alcohol 95%, 1:1 v/v), titrated against KOH (0.1 N), and determined as oleic acid equivalents (Cox and Pearson, 1962). The amino acid profile of the raw and SSF-processed LOC was established using a high-performance liquid chromatography (HPLC) system (Agilent Technologies-1260 Infinity, California, United States) fitted with an ion-exchange/reversed-phase column (Zorbax Eclipse XDB-C18, Agilent Technologies). Essential amino acids were determined following Ishida et al. (1981) and Henderson et al. (2000). Tryptophan content was determined using a spectrophotometer following Sastry and Tammuru (1985).
The presence of ANFs in raw and SSF-processed LOC was estimated following standard methodologies, a brief account of which has been described in previous reports (Ghosh and Mandal, 2015; Banerjee and Ghosh, 2016). Briefly, tannin was extracted in boiled distilled water and determined with Folin Denis reagent (Schanderi, 1970). Cellulose content was estimated with anthrone reagent (Updegraff, 1969). Xylan/hemicellulose content was determined by deducting the acid detergent fiber from the neutral detergent fiber (Goering and Van Soest, 1975). Phytic acid was extracted in HCl (2.4%) and estimated using a modified Wade reagent (0.03% FeCl3, 6H2O + 0.3% sulfosalicylic acid) (Vaintraub and Lapteva, 1988). Benzoyl-dl-arginine-p-nitroanilide (BAPNA) was used as a substrate to determine the trypsin inhibitor (Smith et al., 1980).
Analyses of the proximate composition of the fish carcass (wet weight) were done at the commencement and termination of the feeding trial as per the methods stated above.
2.6 Analyses of growth performance and feed utilization
Body mass gain (BMG, %), specific growth rate (SGR, % day−1), protein efficiency ratio (PER), FCR, and apparent net protein utilization (ANPU, %) were measured to determine the growth performance of the experimental fish (Steffens, 1989). For determination of digestibility, chromic oxide (Cr2O3) in the diets and fecal matter were measured spectrophotometrically following the perchloric acid digestion method (Bolin et al., 1952). The apparent dry matter digestibility (ADD, %) and apparent nutrient digestibility (%) were calculated following standard formulae (Cho et al., 1982). The formulae used for the evaluation of growth performance and digestibility parameters are detailed in Ghosh and Mandal (2015).
(Where, ln = Natural logarithm).
2.7 Analyses of digestive enzymes
Three fish were taken from each replicate and anesthetized with 0.03% tricaine methane-sulfonate (MS-222), gastrointestinal (GI) tracts were removed, and the pooled samples were used to determine the activities of digestive enzymes prior to commencement and after completion of the experimental feeding. GI tract homogenates (10%) prepared with phosphate buffered saline (PBS; 0.1 M, pH 7.4, 0.89% NaCl) were centrifuged (10,000×g, 4°C, 30 min), and the resultant supernatants were used as the source of enzyme extracts. The protein content of the enzyme extract was determined using the standard solution of bovine serum albumin (BSA) (Lowry et al., 1951). Amylase activity was analyzed by the dinitro-salicylic acid method using soluble starch as the substrate (Bernfeld, 1955). Unit activity (U) of amylase was expressed as the μg of maltose liberated ml−1 of enzyme extract min−1. Caseinase assay method involving Hammarsten casein as the substrate was followed for the determination of the protease activity (Walter, 1984). Unit activity (U) of protease was presented as μg of tyrosine liberated ml−1 of enzyme extract min−1 under standard assay conditions. Lipase activity was determined using olive oil substrate (Bier, 1955). Lipase activity unit (U) was depicted as μmole of free fatty acid liberated ml−1 of enzyme extract h−1.
2.8 Microbial culture
Enumeration of total heterotrophic as well as diverse enzymes producing microbiota within the GI tract of experimental fish was carried out at the beginning and termination of the feeding trial. The homogenate of the pooled GI tracts of three fish was taken (for each replicate), serial dilutions (1:10; 10−5) were made (Beveridge et al., 1991), and the diluted samples were used as inoculums. To enumerate cellulase-, xylanase-, amylase-, protease-, and lipase-producing microbiota, inoculums (0.1 ml) were added into carboxymethylcellulose (CMC), birchwood xylan (XA), starch (SA), peptone gelatine (PG), and tributyrin (TA)-supplemented agar plates, respectively. Likewise, sterile tryptone soy agar media (TSA; HiMedia, India) plates were used to evaluate the culturable heterotrophic microbial community. Inoculated culture plates were incubated (30°C ± 1°C; 24–48 h), and the number of colonies per unit volume of inoculums (gut homogenate) was determined by multiplying the colony numbers on each plate by the reciprocal dilution (Rahmatullah and Beveridge, 1993). The data were transformed as log viable counts g−1 intestinal tissue (LVCs). Compositions of the culture media used in the study were depicted in previous reports (Banerjee et al., 2016; Mandal and Ghosh, 2019).
2.9 Analyses of hematobiochemical profile
Feeding was suspended for 24 h prior to the collection of blood samples from the experimental fish. Fish were anesthetized with MS-222 and blood from the caudal vein was collected in heparinized Eppendorf tubes (Khan et al., 2015). Total erythrocyte and leucocyte counts were determined with the heparinized blood (Johnson et al., 2002). The heparinized blood was diluted with PBS, and erythrocytes (red blood cells, RBC) were counted in a hemocytometer. Blood samples diluted with Natt–Herrick solution were stained with gentian violet (1%), and a Neubauer hemocytometer was used to carry out leucocyte (white blood cells, WBC) count. The cyanmethemoglobin method was followed to determine the hemoglobin (Hb) concentration (Blaxhall and Daisley, 1973). For the plasma profile, the samples were centrifuged (1,500×g, 15 min, 4°C) to obtain blood plasma and stored at −20°C until use. Blood biochemical parameters, i.e., plasma protein, lipid, glutamic-oxaloacetic transaminase (GOT), glutamic-pyruvic transaminase (GPT), glucose, albumin, and globulin were estimated using a fully automated clinical biochemical analyzer (EM Destiny 180, Transasia Bio-Medicals Ltd., Mumbai, India) using standard biochemical kits.
2.10 Statistical analysis
Statistical analysis of the experimental data was accomplished by analysis of variance (ANOVA). The mean difference between the experimental groups was tested for significance (P< 0.05) and compared by Tukey’s test after Zar (2010). The statistical analyses were done using SPSS ver.19 software (Kinnear and Gray, 2009).
3 Results
3.1 Processing of LOC and experimental diets
Proximate compositions as well as amino acid contents (% dry matter) of the raw and bioprocessed LOC under SSF are given in Table 1. Bioprocessing with SSF brought about changes in the proximate composition of the LOC substrate. Minor improvements in crude protein, crude lipid, ash, total free amino acids, and fatty acids were noticed in the SSF-processed LOC. Meanwhile, crude fiber and ANFs (phytic acid, tannin, and trypsin inhibitor) were significantly (P< 0.05) reduced in the fermented LOC as compared with the raw LOC. The NSPs, cellulose, and xylan were also decreased in the fermented LOC. The amino acid profile exhibited a significant increase in some of the amino acids, e.g., arginine, cysteine, histidine, isoleucine, phenylalanine, methionine, threonine, tryptophan, and valine. However, lysine and leucine were decreased due to SSF. Ingredient composition along with proximate analysis of the experimental diets is depicted in Table 2. The experimental diets were isocaloric (≈4.8 kcal/g) and isonitrogenous (≈36% crude protein).
3.2 Growth performance and nutrient utilization
The results on growth parameters and nutrient utilization in rohu fingerlings fed experimental diets are presented in Table 3. Overall, significant increases (P< 0.05) in BMG (%) and SGR (% day−1) were recorded for the fish receiving diets containing SSF-processed LOC compared with the raw LOC-incorporated diets. Rohu fingerlings reared on diet F3 containing 30% SSF-processed LOC had the highest weight gain (207.88 ± 4.05) and SGR (1.61 ± 0.05), which were significantly different (P< 0.05) from the other feeding groups. Similar results were obtained for PER and ANPU. FCR was the lowest for the fish fed diet F3 (1.88 ± 0.09) and the highest for the fish fed diet R4 (3.15 ± 0.06) containing 40% raw LOC.
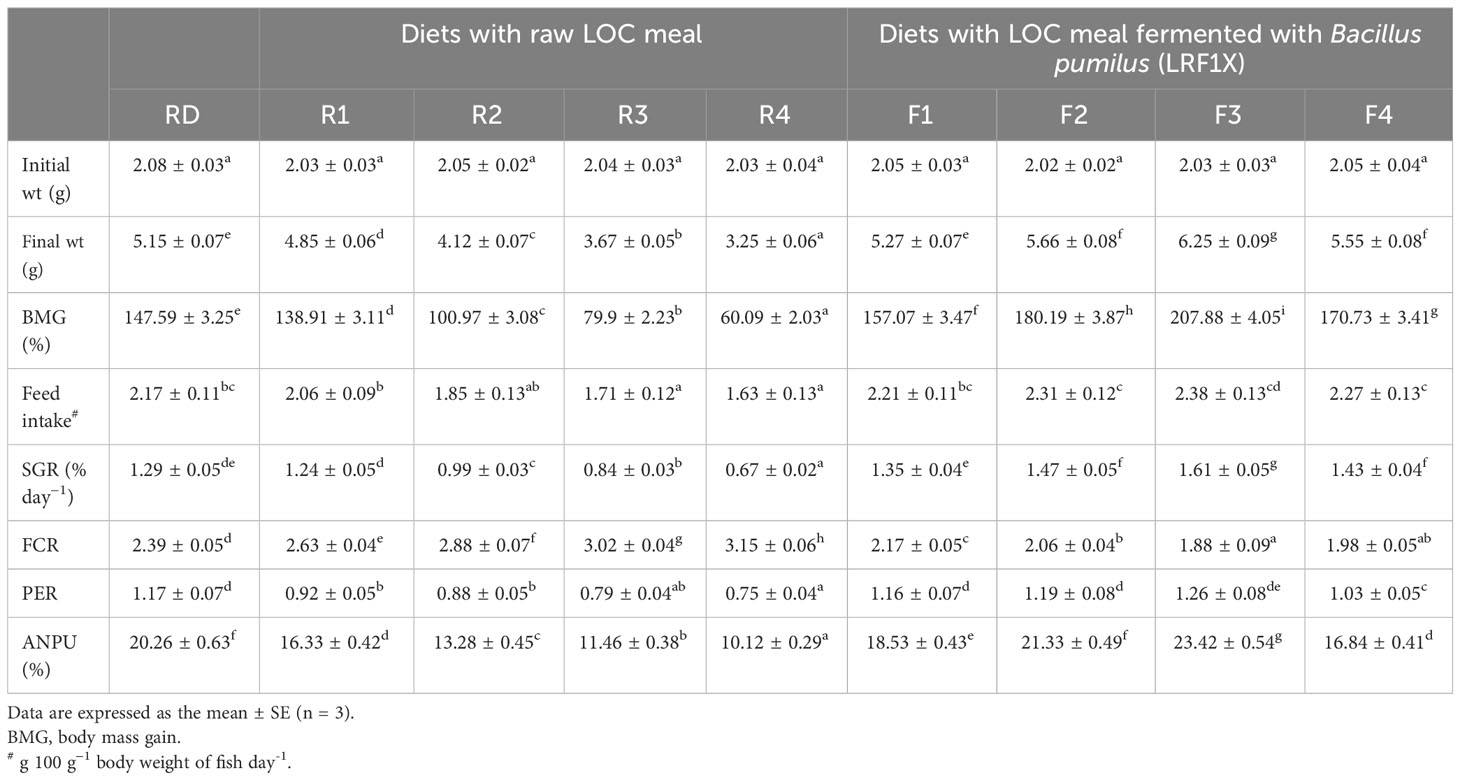
Table 3 Growth performances and feed utilization efficiency in Labeo rohita fingerlings fed experimental diets for 70 days.
3.3 Apparent dry matter and nutrient digestibility
Data pertaining to apparent dry matter or total digestibility (ADD) and digestibility of the nutrients (protein, lipid) for rohu fed reference and test diets are presented in Table 4. Overall, fish fed diets with SSF-processed LOC were recorded with improved digestibility parameters. The fish reared on diet F3 exhibited the highest ADD (65.87 ± 0.13) and apparent protein digestibility (APD; 89.15 ± 0.32), which were significantly (P< 0.05) higher than the other feeding groups. Apparent lipid digestibility (ALD) was also maximum (92.51 ± 0.38) for diet F3 containing 30% SSF-processed LOC, although it did not differ significantly (P< 0.05) from diets F2 and F4.
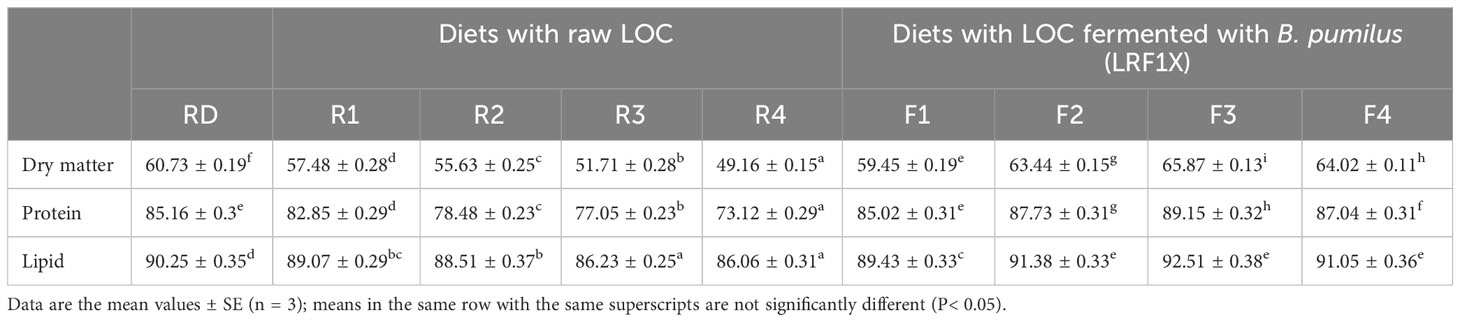
Table 4 Apparent digestibilities of dry matter and nutrients in Labeo rohita fingerlings fed experimental diets for 70 days.
3.4 Proximate carcass composition
Proximate carcass compositions of the experimental fish are depicted in Table 5. Carcass protein and lipid depositions were significantly (P< 0.05) higher in fish fed fermented LOC-incorporated diets, as compared with the other experimental diets as well as the initial value. The maximum carcass protein accumulation was recorded in fish fed diet F3 (16.77 ± 0.34), although it did not vary significantly (P< 0.05) from the dietary group F2. The highest carcass lipid content was also exhibited by the fish fed diet F3. Moisture content declined over the initial value, although the difference was not significant (P< 0.05) for the groups R2, R3, and R4 fed diets containing raw LOC at different levels. The lowest moisture in carcass was recorded in the fish fed diet F3 (74.49 ± 1.34), although it did not differ significantly from the fish reared on diets F2 and F4. The maximum carcass ash content (1.86 ± 0.11) was recorded with the fish fed diet F3. Although carcass ash content decreased from the initial value, it did not vary significantly (P< 0.05) in fish fed ≧20% fermented LOC-incorporated diets (F2, F3, and F4).
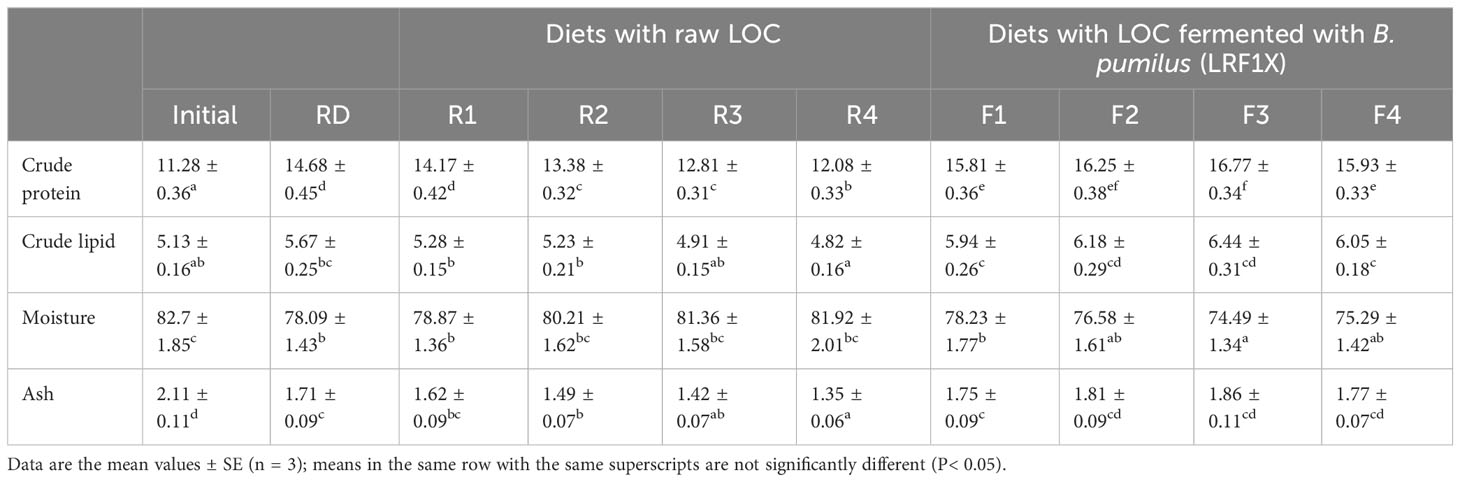
Table 5 Proximate carcass composition (% wet weight) of Labeo rohita fingerlings fed experimental diets for 70 days.
3.5 Digestive enzyme activity
Amylase, protease, and lipase activities in the GI tracts of L. rohita fingerlings belonging to different experimental groups are presented in Table 6. Overall, the activities of the studied digestive enzymes increased over the initial values in all dietary groups, except amylase and lipase activities in the group R4 fed diet with 40% raw LOC. At ≧20% inclusion level, the fish fed diets with SSF-processed LOC demonstrated significantly (P< 0.05) higher activities of all the three enzymes as compared with the groups reared on raw LOC-incorporated diets. The maximum amylase, protease, and lipase activities were noted in the fish that received diet F3 containing 30% SSF-processed LOC, and it was significantly (P< 0.05) different from the other experimental groups including the group fed RD.
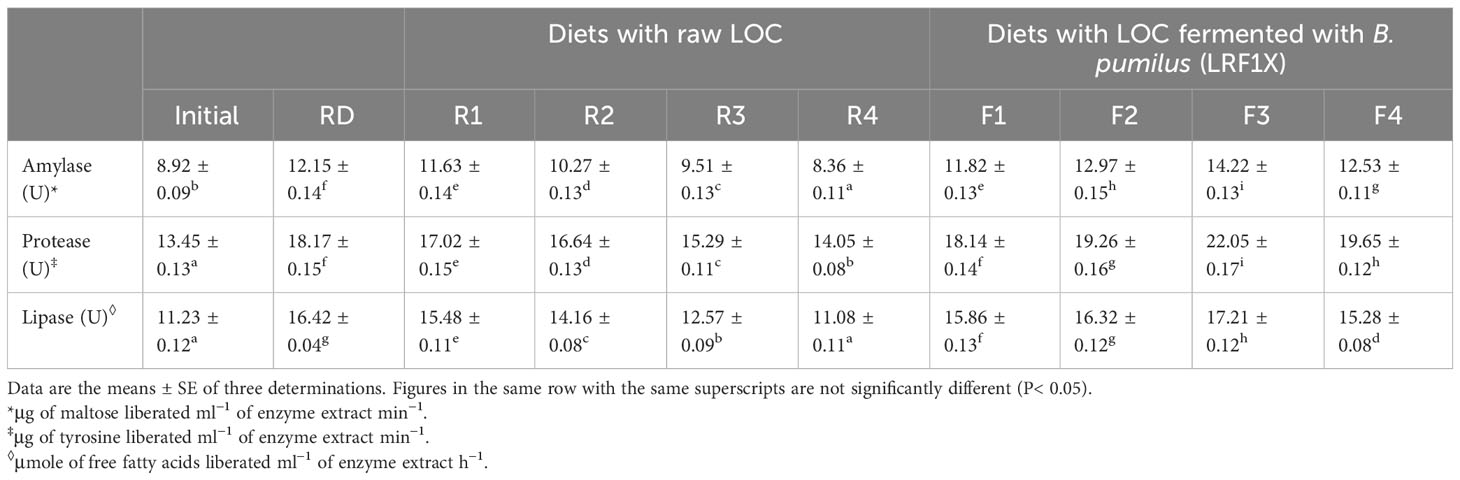
Table 6 Intestinal amylase, protease, and lipase activities in Labeo rohita fingerlings fed experimental diets for 70 days.
3.6 Gastrointestinal microbiota
An account of culturable heterotrophic and different exo-enzymes producing microorganisms (viz., cellulolytic, xylanolytic, amylolytic, proteolytic, and lipolytic) in the GI tracts of fish fed experimental diets revealed that feeding raw LOC-included diets resulted in considerable loss of gut microbiota, while increased LVCs were detected in fish fed diets containing SSF-processed LOC (Table 7). The maximum LVCs of culturable heterotrophic, cellulolytic, xylanolytic, amylolytic, proteolytic, and lipolytic microbiota were documented in the GI tracts of fish fed diet F4.
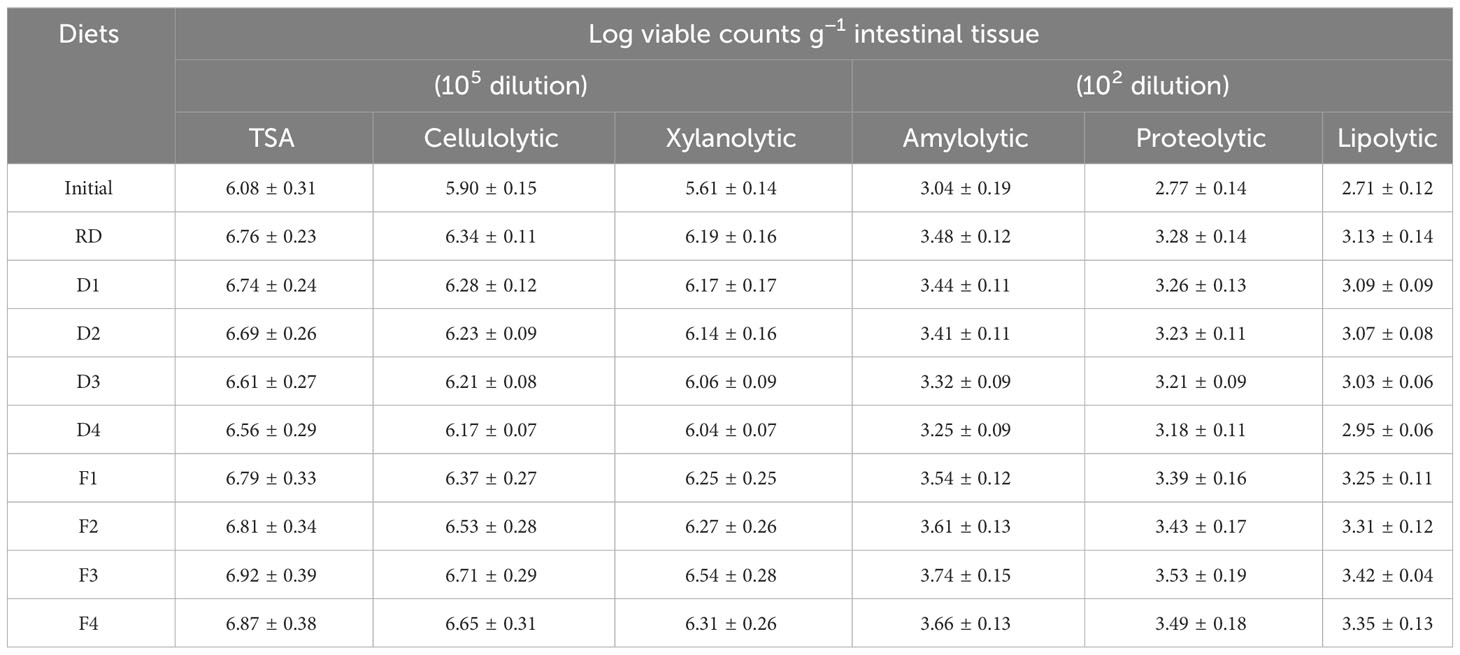
Table 7 Microbial counts (log viable counts g−1 intestinal tissue) in the intestine of Labeo rohita fingerlings fed experimental diets for 70 days.
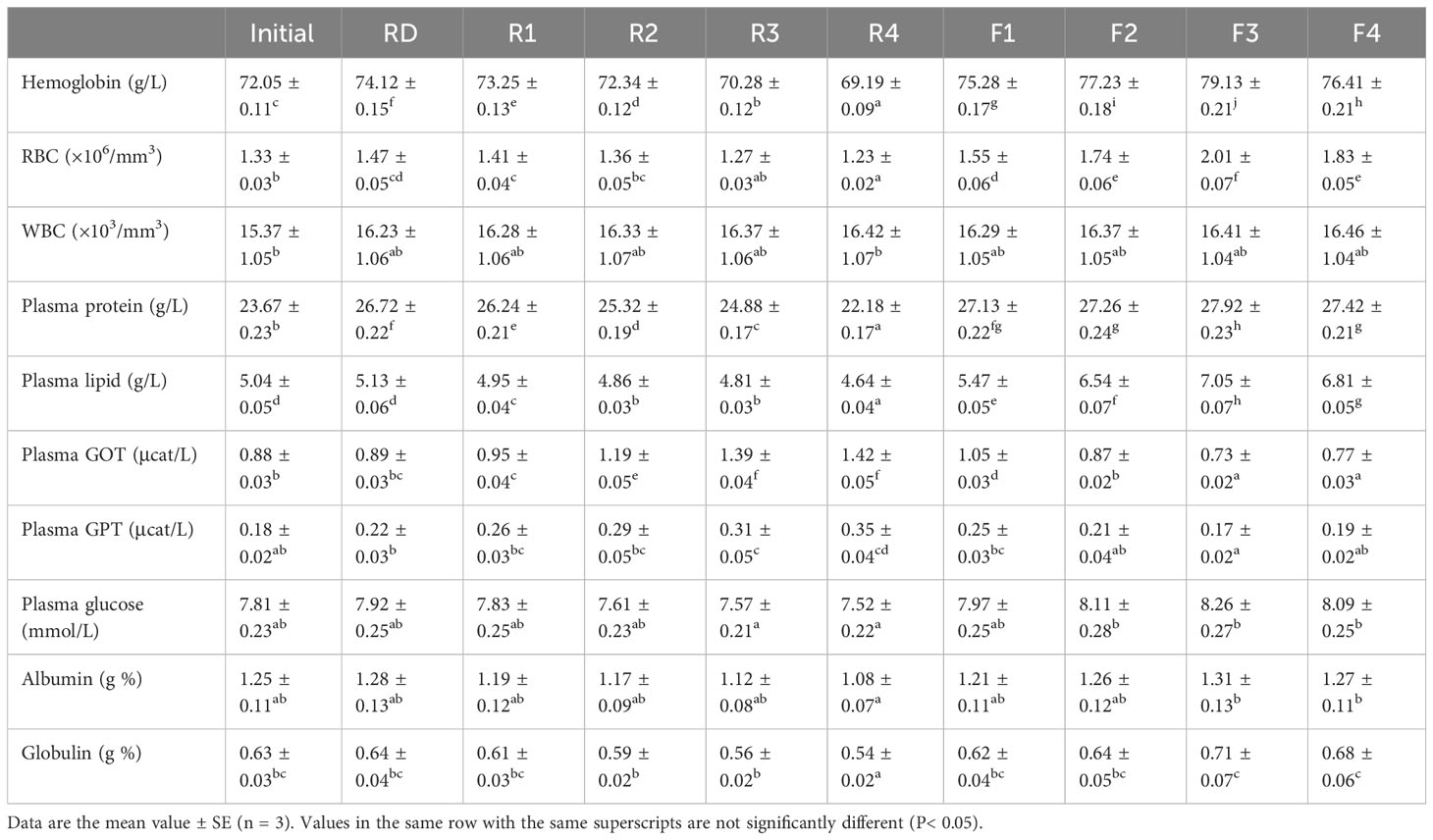
3.7 Hematobiochemical profile
The hematobiochemical profiles in L. rohita fingerlings fed experimental diets are presented in Table 8. Overall, hematobiochemical profiles improved in fish fed diets comprising SSF-processed LOC as compared with the groups fed RD and raw LOC-included diets. RBC count and hemoglobin concentration in the blood were noticed to increase significantly (P< 0.05) in fish that received diets with SSF-processed LOC when compared with the other groups. The highest values for RBC and hemoglobin were detected with the group fed diet F3. In contrast, the WBC count did not vary significantly (P< 0.05) among the dietary groups. Plasma protein, lipid, and glucose levels in the blood were also significantly (P< 0.05) improved with feeding fermented LOC in the diets than the other dietary groups, and the maximum levels were detected in fish fed diet F3 containing 30% SSF-processed LOC. However, GOT and GPT were decreased significantly (P< 0.05) in the groups fed diets with SSF-processed LOC. The results revealed that plasma GOT and GPT levels were the highest in fish fed diet F3, even if they did not vary significantly from the groups raised with diet F4 for GOT and diets F2 along with F4 for GPT.
4 Discussion
Substitution of FM with alternative sources of plant-derived protein attained success at different extents depending on the nature and composition of the ingredients, the level of inclusion, and the processing methods (Ghosh et al., 2019). This study exhibited the potential application of the SSF-processed LOC as an alternative as well as a non-conventional protein source in the formulation of carp diets. Linseed meal was evaluated previously as a source of energy-donating nutrients (e.g., protein and lipid), essential fatty acids, and amino acids (Hossain and Jauncey, 1989; Lee et al., 1991). The composition of the linseed meal or oil cake depends on the growing environment, crop variety, and processing conditions (Morris, 2007). An appraisal of the major nutrients and amino acid profile of the LOC obtained after oil extraction is given in the present report. Despite its potentially rich nutrient composition, LOC is an underutilized agro-industrial by-product. In the presently reported study, the fish receiving bioprocessed LOC-incorporated diets exhibited better performance in terms of average live weight gain (%), SGR, PER, carcass composition, and activity of the digestive enzymes than the groups fed similar levels of raw (unprocessed) LOC-containing diets. The results achieved in this study evidently indicated that bioprocessed (fermented) LOC may substitute up to 30% FM (w/w) in the diets for rohu fingerlings without compromising their growth as well as nutrient utilization. Furthermore, it was noticed that the effectiveness of the diet decreased at 40% substitution level of the SSF-processed LOC. Our study complied with the findings of Roy et al. (2014), where 30% incorporation of fermented sesame oilseed meal was optimum for the maximum growth of L. rohita fingerlings. In their study, sesame oilseed meal was processed under SSF with two phytase-producing strains of Bacillus licheniformis. On account of fermentation, improvement of the protein quality in solid substrates due to microbial synthesis has been indicated elsewhere (Mukhopadhyay and Ray, 2005; Roy et al., 2014; Ghosh and Mandal, 2015). In the present report, SSF of LOC resulted in an increase in the crude protein content from 33% to nearly 37% and a decline in the crude fiber content from 7.65% to 4.06%, which were in accordance with previous reports (Roy et al., 2014; Ghosh and Mandal, 2015; Mandal and Ghosh, 2019). In addition, processing of the raw LOC to reduce/deactivate the ANFs seemed to be essential intending its effective utilization as an alternative protein source in formulated animal feed (Jackson et al., 1982). Generally, tannins, phytate compounds, trypsin inhibitors, and NSPs (cellulose and xylan) are considered the major ANFs limiting the bioavailability of energy and nutrients in oilseed cakes (Ghosh et al., 2019). Tannins hinder the digestibility of nutrients by inhibiting digestive enzymes and producing indigestible complexes with feed protein eventually leading to growth impediment (Krogdahl, 1989). The protease inhibitors form stable complexes with proteolytic enzymes like trypsin or chymotrypsin and, thus, restrain access to the active site of the enzymes (Maitra et al., 2007). Phytic acid chelates with protein and/or mineral components forming phytate complexes and thereby reduces protein as well as mineral bioavailability (Hossain and Jauncey, 1989). The most significant adverse effects associated with NSPs are their viscous nature, morphological or physiological effects on the digestive tract, and interactions with gut epithelium, mucus, and microbiota (Angkanaporn et al., 1994; Choct et al., 1996). In the present study, SSF (fermentation) by fish gut-associated B. pumilus LRF1X (KF640221) improved the nutritional potential of the LOC by increasing its crude protein and lipid contents, along with decreasing the contents of cellulose, xylan, and other ANFs (e.g., tannin, phytate, and trypsin inhibitors). The availability of total free amino acids and free fatty acids was higher in the SSF-processed LOC than that in the raw LOC. Moreover, bioprocessing through SSF brought about an increase in the majority of the amino acids including two important sulfur-containing amino acids, viz., methionine and cysteine. Thus, SSF most likely led to the enhancement of the nutritive value of the LOC. As revealed in previous reports, value addition of the solid substrate through microbial synthesis during the SSF was expected (Wee, 1991). Our study was in harmony with the preceding reports on SSF of sesame oilseed meal (Roy et al., 2014), sesame oil cake (Das and Ghosh, 2015), groundnut oil cake (Ghosh and Mandal, 2015), and aquatic weeds (Mandal and Ghosh, 2019). Very recently, the application of fermented Moringa oleifera leaf meal in the diets of Megalobrama amblycephala juveniles was reported to exhibit significant improvement in muscle crude protein, crude lipid, and total free amino acids (Jiang et al., 2023).
Carps are susceptible to the elevated level of plant proteins in their diets due to low palatability and deficient amino acids and ANF contents (Kumar et al., 2011). The presently reported study noticed a decrease in the growth of the L. rohita fingerlings with increasing levels of raw LOC in the diets. Fish fed diets having SSF-processed LOC attained higher weight gain, SGR (% day−1), PER, and ANPU compared with the fish fed diets with the same level of raw LOC. The efficiency of the LOC was noticed to decline at a higher inclusion level (i.e., 40%), even if it was bioprocessed through SSF. Thus, the importance of the optimum incorporation level might be realized for the intended plant feed ingredients to be used in feed formulation. Comparable trends were recorded in L. rohita fed high levels of dietary oilseed meals after processing through SSF (Roy et al., 2014; Ghosh and Mandal, 2015). Growth improvement of the rohu fingerlings fed SSF-processed LOC-incorporated diets noticed in this study could be attributed to the enhanced release of nutrients by fermentative degradation of the complex biomolecules (Roy et al., 2014). In contrast, the poor growth of fish associated with raw oil cake-incorporated diets was perhaps due to amino acid imbalance and reduced bioavailability of the nutrients as a consequence of ANFs and high fiber therein (Ghosh and Mandal, 2015).
An understanding of the nutrient digestibility of feed as well as feed ingredients brings out the interchangeability of the ingredients without hampering animal performance. The results obtained in the present study indicated a gradual decline of the apparent dry matter digestibility (ADD) and nutrient digestibility parameters (apparent protein digestibility, APD, and apparent lipid digestibility, ALD) with the elevated level of raw LOC in the experimental feed. Analogous trends of lowering down the APD values were reported with elevated inclusion levels of raw mustard oil cake (Hossain and Jauncey, 1989), sesame seed meal (Mukhopadhyay and Ray, 1999a; Roy et al., 2014), sesame oil cake (Das and Ghosh, 2015), groundnut oil cake (Ghosh and Mandal, 2015), copra meal (Mukhopadhyay and Ray, 1999b), grass pea (Ramachandran and Ray, 2004; Ramachandran et al., 2005), leaf meals (Ray and Das, 1994; Bairagi et al., 2004; Mandal and Ghosh, 2019; Jiang et al., 2023), black gram (Ramachandran and Ray, 2007), and linseed meals (Hasan et al., 1991; Mukhopadhyay and Ray, 2005) in the carp diets. Values of the evaluated digestibility parameters were increased up to 30% inclusion level of SSF-processed LOC replacing 30% FM (w/w) and thereafter declined. In compliance with the present report, previous studies established that fermentation improved the protein digestibility of plant feedstuffs (Ghosh and Mandal, 2015). The presence of ANFs might affect the digestibility of diverse nutrients in the diets explicating erroneous results (Lall, 1991). For digestibility determination, the fecal samples were pulled together by immediate pipetting (Spyridakis et al., 1989). Thus, leaching out of some nutrients and the possibility for overestimation of the digestibility parameters may not be ruled out. However, an indigestible binder (CMC) was added to increase the water stability of the diets, which could assist in binding the fecal particles as well as minimizing the effect of leaching (De la Noüe and Choubert, 1986).
The proximate whole body/carcass composition (i.e., moisture, crude protein, crude lipid, and ash) of the experimental fish was considerably influenced by the incorporation level of raw as well as fermented LOC in the diets replacing FM. Although all of the experimental fish (groups) were fed isoproteinaceous diets, the carcass protein deposition was significantly higher in fish fed SSF-processed LOC-incorporated diets than the RD, and a rising level of raw LOC was linked with a decline in carcass protein, lipid, and ash contents. In conformity with the present study, a similar pattern for carcass protein and lipid contents was noted in the previous reports wherein elevated levels of fermented seed or oil cake meals were incorporated into the carp diets (Roy et al., 2014; Das and Ghosh, 2015; Ghosh and Mandal, 2015).
Studies on the digestive enzymes associated with the GI tract might suggest adaptation to the composition of food in fishes (Kuz’mina, 1991). Overall, significantly reduced activities of protease, amylase, and lipase were noticed in the GI tract of L. rohita on the inclusion of raw LOC in the diets. A decrease in digestive enzyme activities with the inclusion of fermented groundnut oil cake in the diets of rohu fingerlings was recorded (Ghosh and Mandal, 2015), which was in concurrence with the present report. However, the groups fed bioprocessed LOC and RD were observed to have a considerable increase in digestive enzyme activity as compared with the fish fed raw LOC-incorporated diets. Thus, most likely, the fish were able to utilize the nutrients from the RD and diets containing SSF-processed LOC more efficiently than the diets with raw LOC. The decrease in protease activities with elevated levels of raw LOC in the diets might be correlated with the decrease in protein bioavailability from LOC, poor growth, and decline in protein digestibility. The presently reported study was in compliance with previous reports, where the decline in the activities of trypsin or other proteases was recorded due to the inclusion of enhanced levels of plant protein in the diets suggesting that proteases might be extremely susceptible to plant-derived ANFs (Krogdahl et al., 1994; Escaffre et al., 1997; Santigosa et al., 2008; Kumar et al., 2011; Ghosh and Mandal, 2015). In fact, the progressive decline in enzyme activity with a gradual increase in the incorporation levels of raw LOC in the diets might be linked with the endogenous ANFs in the oil cakes, e.g., tannins, phytic acid, and trypsin inhibitors (Ghosh et al., 2019). Nevertheless, processing of the LOC by heat treatment (autoclaving) followed by SSF using B. pumilus could reduce ANFs, improve the activity of the digestive enzymes, and enhance the nutrient availability in fish fed SSF-processed LOC in the diets.
The intestinal microbiota in fish reflects the microbial content of ingested food and of the surrounding environment that influences various host functions including growth, development, digestion, nutrition, immune function, and disease resistance (Mohapatra et al., 2012; Ghosh et al., 2019). In the present study, an increase in the culturable heterotrophic as well as diverse exo-enzymes producing microbial populations within the gut of fish fed fermented LOC-incorporated diets was recorded. Meanwhile, an opposite trend was noticed in fish fed diets with raw LOC. A decline in the microbial population in fish fed diets containing raw LOC could be associated with the detrimental effects of the plant-derived secondary metabolites in feed, which have been accounted to restrain gastrointestinal microflora by inhibition of enzyme activity and/or substrate deprivation (Spinelli et al., 1983). Moreover, several of the endogenous secondary metabolites in plants are known to be bacteriostatic or toxic to the microorganisms, limiting the growth and propagation of the microbiota (Scalbert, 1991). An increase in microbial population within the gut of fish fed fermented LOC in the diets might be due to the fact that the LOC was bioprocessed through SSF using a bacterium, and the fermented substrate was included in the diet as a whole. Although the diet-related change in the gut microbiome of the experimental fish was not appraised in this study, the results of the study might be indicative of the probiotic potential of the spore-forming bacterium used in SSF (Hong et al., 2005; Ghosh et al., 2019).
Hematobiochemical parameters are symptomatic of the fish health status like other animals. Leucocytes (WBC) are known to play a key role in innate or non-specific immunity, and their number appears to be increased in stressful situations, e.g., infection and dietary imbalance (Roberts, 1978). In this study, even if dietary groups had no significant effect (P > 0.05) on WBC counts, the apparent increase in the groups receiving fermented LOC-incorporated diets could be attributable to the increased microbial load, as evident from the microbiological examination. An increase in the hemoglobin level in the groups fed SSF-processed LOC-incorporated diets might imply improved bioavailability of Fe than raw LOC as a consequence of bioprocessing, which was in harmony with a previous report in L. rohita (Baruah et al., 2009). Likewise, the SSF-processed LOC-incorporated dietary groups had significantly increased (P > 0.05) RBC. In addition, elevated blood glucose levels in the fish fed formulated diets might signify that the inclusion of bioprocessed LOC, replacing 30% FM, in the diet did not induce metabolic stress among the experimental fish. Plasma GOT and plasma GPT levels decreased with the incorporation of a higher proportion of SSF-processed LOC in feed. The GOT and GPT levels can help to diagnose any damage or injury of the heart or liver, where an elevated level could be associated with severe stress (Huang et al., 2006). In the present experiment, low GOT and GPT levels detected in the fish fed SSF-processed LOC-incorporated diets might indicate that processed LOC did not induce tissue damage or stress in the experimental fish.
5 Conclusion
This study established the acceptable nutritive value of the SSF-processed LOC as an alternative ingredient in the carp diet. In addition, the study highlighted the efficacy of a fish gut-associated bacterium in improving the nutritional value of the oil cake when used as a substrate for bioprocessing through SSF. An inclusion level of up to 30% SSF-processed LOC (replacing 30% of FM, w/w) in the practical diet of rohu fingerlings did not interfere with growth performance, nutrient utilization, and body composition when compared with the FM-based reference diet or raw LOC at the same level of inclusion. Therefore, the inclusion of bioprocessed LOC may be practiced for partial substitution of FM and/or other conventional feed ingredients in the formulation of carp diets. Incorporation of fermented LOC would be cost-effective since it is not expensive and much more economical than FM and involved a simple processing technique. However, further experimentation in the field condition with a large number of fish and replication is needed prior to recommending it to the aquaculture industry.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Dissection Monitoring Committee, The University of Burdwan. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SB: Data curation, Validation, Writing – original draft, Formal Analysis, Investigation, Methodology, Software, Visualization. ZK: Formal Analysis, Validation, Writing – review & editing. GT: Writing – review & editing, Funding acquisition. KG: Funding acquisition, Writing – review & editing, Conceptualization, Data curation, Project administration, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Department of Science and Technology and Biotechnology, Government of West Bengal, India (Project No. No. ST/P/S&T/2G-33/2017), and the USDA-NIFA Sustainable Agriculture Systems (Grant No. 2019-69012-29905).
Acknowledgments
The first author is obliged to The University Grants Commission, New Delhi, India, for the research fellowship (UGC-NET-JRF). The authors are grateful to the Head, Department of Zoology (DST-FIST sponsored), The University of Burdwan, West Bengal, India; Department of Science and Technology, New Delhi, India (PURSE Program); and Department of Science and Technology and Biotechnology, Government of West Bengal, India (Project No. ST/P/S&T/2G-33/2017), for providing the research facilities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Angkanaporn K., Choct M., Bryden W. L., Annison E. F., Annison G. (1994). Effects of wheat pentosans on endogenous amino acid losses in chickens. J. Sci. Food Agric. 66, 399–404. doi: 10.1002/jsfa.2740660319
AOAC (2005). “Official method of analysis,” in Association of Officiating Analytical Chemists, 18th ed. (Arlington, VA, USA: Association of the Official Analytical Chemists).
APHA (American Public Health Association, American Water Works Association, Water Environment Federation) (2012). Standard Methods for the Examination of Water and Wastewater, 22nd ed. (New York, USA: American Public Health Association).
Árnason J., Imsland A. K. D., Helmig T., Gunnarsson S., Kristjánsson G.Ö. (2017). Fishmeal replacement by mixed plant proteins and effect on growth and sensory attributes in on-growing turbot. Aquac. Nutr. 24, 1041–1047. doi: 10.1111/anu.12642
Bairagi A., Sarkar Ghosh K., Sen S. K., Ray A. K. (2004). Evaluation of nutritive value of Leucaena leucocephala leaf meal inoculated with fish intestinal bacteria Bacillus subtilis and Bacillus circulans in formulated diets for rohu, Labeo rohita (Hamilton) fingerlings. Aquac. Res. 35, 436–446. doi: 10.1111/j.1365-2109.2004.01028.x
Banerjee S., Ghosh K. (2016). Bio-processing of linseed oil-cakethrough solid state fermentation by non-starch polysaccharide degrading fish gut bacteria. Ferment. Technol. 5, 1–10. doi: 10.4172/2167-7972.1000127
Banerjee S., Mukherjee A., Dutta D., Ghosh K. (2016). Non-starch polysaccharide degrading gut bacteria in Indian major carps and exotic carps. Jordan J. Biol. Sci. 9, 69–78. doi: 10.12816/0027010
Baruah K., Pal A. K., Sahu N. P., Debnath D., Yengkokpam S. (2009). Dietary crude protein, citric acid and microbial phytase interacts to influence the hemato-immunological parameters of Rohu, Labeo rohita, Juveniles. J. World Aquac. Soc 40, 824–831. doi: 10.1111/j.1749-7345.2009.00304.x
Bernfeld P. (1955). “Amylase (alpha) and (beta),” in Methods in Enzymology. Eds. Colowick S. P., Kaplan N. O. (New York: Academic Press) 1, 149–150.
Beveridge M. C. M., Sikdar P. K., Frerichs G. N., Millar S. (1991). The ingestion of bacteria in suspension by the common carp Cyprinus carpio L. J. Fish. Biol. 39, 825–831. doi: 10.1111/j.1095-8649.1991.tb04412.x
Bier M. (1955). Methods in enzymology. Eds. Colowick S. P., Kaplan N. O. (New York: Academic Press), 627–642.
Blaxhall P. C., Daisley K. W. (1973). Routine haematological methods for use with fish blood. J. Fish. Biol. 5, 771–781. doi: 10.1111/j.1095-8649.1973.tb04510.x
Bolin D. W., King R. P., Klosterman E. W. (1952). A simplified method for the determination of chromic oxide (Cr2O3) when used as an index substance. Science 116, 634–635. doi: 10.1126/science.116.3023.634
Cho C. Y., Slinger S. J., Bayley H. S. (1982). Bioenergetics of salmonid fishes: energy intake, expenditure and productivity. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 73, 25–41. doi: 10.1016/0305-0491(82)90198-5
Choct M., Hughes R. J., Wang J., Bedford M. R., Morgan A. J., Annison G. (1996). Increased small intestinal fermentation is partly responsible for the anti-nutritive activity of non-starch polysaccharides in chickens. Br. Poult. Sci. 37, 609–621. doi: 10.1080/00071669608417891
Cox H. E., Pearson D. (1962). The Chemical Analysis of Foods (New York: Chemical Publishing Co. Inc).
Das P., Ghosh K. (2014). The presence of phytase in yeasts isolated from the gastrointestinal tract of four major carps [Labeo rohita (Hamilton 1822), catla catla (Hamilton 1822), cirrhinus mrigala (Hamilton 1822), hypophthalmichthys molitrix (Valenciennes 1844)], climbing perch [Anabas testudineus(Bloch 1792)] and Mozambique tilapia [Oreochromis mossambicus (Linnaeus 1758)]. J. Appl. Ichthyol. 30, 403–407. doi: 10.1111/jai.12274
Das P., Ghosh K. (2015). Improvement of nutritive value of sesame oil cake in formulated diets for rohu, Labeo rohita (Hamilton) after bio-processing through solid state fermentation by a phytase-producing fish gut bacterium. Int. J. Aquat. Biol. 3, 89–101. doi: 10.22034/ijab.v3i2.52
De la Noüe J., Choubert G. (1986). Digestibility in rainbow trout: comparison of the direct and indirect methods of measurement. The Progressive Fish-Culturist 48, 190–195. doi: 10.1577/1548-8640(1986)48<190:DIRT>2.0.CO;2
Doreau M., Chilliard Y. (1997). Effects of ruminal or postruminal fish oil supplementation on intake and digestion in dairy cows. Reprod. Nutr. Dev. 37, 113–124. doi: 10.1051/rnd:19970112
Escaffre A. M., ZamboninoInfante J. L., Cahu C. L., Mambrini M., Bergot P., Kaushik S. J. (1997). Nutritional value of soy protein concentrate for larvae of common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Aquac 153, 63–80. doi: 10.1016/S0044-8486(97)00010-0
FAO (2022). The State of World Fisheries and Aquaculture 2022. (Rome, Italy: Food and Agriculture Organization of the United Nations).
Ghosh K., Mandal S. (2015). Nutritional evaluation of groundnutoil cake in formulated diets for rohu, Labeo rohita (Hamilton) fingerlings after solid state fermentation with a tannase producing yeast, Pichia kudriavzevii (GU939629) isolated fromfish gut. Aquac. Rep. 2, 82–90. doi: 10.1016/j.aqrep.2015.08.006
Ghosh K., Ray A. K., Ringø E. (2019). Applications of plant ingredients for tropical and sub-tropical finfish: possibilities and challenges. Rev. Aquacult. 11, 793–815. doi: 10.1111/raq.12258
Goering H. K., Van Soest P. J. (1975). Forage fiber analysis-Agr. Res. Ser. Handbook: Vol. 379 (Washington, DC, USA: Agricultural Research Service - United States Department of Agriculture).
Hansen A. C., Hemre G. I. (2013). Effect of replacing fish meal and oil with plant resources in on-growing diets for Atlantic cod Gadus morhua L. Aquacult. Nutr. 19, 641–650. doi: 10.1111/anu.12078
Hardy R. W. (2010). Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquac. Res. 41, 770–776. doi: 10.1111/j.1365-2109.2009.02349.x
Hasan M. R., Alam M. G. M., Islam M. A. (1989). Aquacultural research” in Asia: management techniques and nutrition. Evaluation of some indigenous ingredients as dietary protein sources for the catfish (Clarias batrachus Linnaeus) fry. Eds. Huisman E. A., Zonniveld N., Beuwmangs A. H. M. (Wageningen: Center for Agricultural Publishing and Documentations, Pudoc Scientific Publishers), 125–137.
Hasan M. R., Azad A. K., Omar, Farooque A. M. O., Akand A. M., Das P. M. (1991). Fish nutrition research in Asia, Special Publication No. 5. Ed. De Silva S.S. (Manila, Philippines: Asian Fisheries Society), 107–117.
Henderson J. W., Ricker R. D., Bidlingermeyer B. A., Woodward C. (2000). Rapid, accurate, sensitive, and reproducible HPLC analysis of amino acids. Agilent. Technol. 1100, 1–10. doi: 10.3168/jds.2009-3017
Hong H. A., Duc le H., Cutting S. M. (2005). The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 29, 813–835. doi: 10.1016/j.femsre.2004.12.001
Hossain M. A., Focken U., Becker K. (2001). Evaluation of an unconventional legume seed, Sesbania aculeata, as a dietary protein source for common carp, Cyprinus carpio L. Aquac 198, 129–140. doi: 10.1016/S0044-8486(00)00574-3
Hossain M. A., Jauncey K. (1989). Nutritional evaluation of some Bangladeshi oilseeds meals as partial substitutes for fish meal in the diet of common carp, Cyprinus carpio L. Aquac. Res. 20, 225–268. doi: 10.1111/j.1365-2109.1989.tb00351.x
Huang X. J., Choi Y. K., Im H. S., Yarimaga O., Yoon E., Kim H. S. (2006). Aspartate Amino transferase (AST/GOT) and Alanine Amino transferase (ALT/GPT) Detection Techniques. Sensors 6, 756–782. doi: 10.3390/s6070756
Ishida Y., Fujita T., Asai K. (1981). New detection and separation method for amino acids by high-performance liquid chromatography. J. Chromatogr. 204, 143–148. doi: 10.1016/S0021-9673(00)81650-7
Jackson A. J., Capper B. S., Matty A. J. (1982). Evaluation of some plant protein incomplete diets for the tilapia Sarotherodonmos sambicus. Aquac 27, 97–109. doi: 10.1016/0044-8486(82)90129-6
Jiang W., Qian L., Zhao Y., Lin Y., Yang Y., Shen H., et al. (2023). The Application of Moringa oleifera Leaf Meal and Its Fermentation Products in the Diet of Megalobrama amblycephala Juveniles. Fermentation 9, 577. doi: 10.3390/fermentation9060577
Johnson C. W., Timmons D. L., Hall P. E., Kuby P. (2002). Essential laboratory mathematics: Concepts and applications for the chemical and clinical laboratory technician. 2nd ed., Delmar Cengage. (USA: Skidmore-Roth Pub.). 268
Khan A., Ghosh K. (2012). Characterization and identification of gut-associated phytase-producing bacteria in some freshwater fish cultured in ponds. Acta Icth. Piscat. 42, 37–45. doi: 10.3750/AIP2011.42.1.05
Khan K. U., Zuberi A., Ullah I., Shami S. A. (2015). Effects of graded level of dietary L- ascorbyl- 2- polyphosphate on growth performance and some hematological indices of Juvenile Mahseer (Tor putitora). Int. J. Agric. Biol. 17, 821–827. doi: 10.17957/IJAB/14.0023
Krogdahl A. (1989). “Alternative protein sources from plants contain anti-nutrients affecting digestion in salmonids,” in The Current Status of Fish Nutrition in Aqua-culture. Proceedings of the Third International Symposium on Feeding and Nutrition in Fish. Eds. Takeda M., Watanabe T. Comp. Biochem. Physiol. (Part A: Physiology) (Tokyo: Tokyo University of Fisheries), 253–261.
Krogdahl A., Lea T. B., Olli J. L. (1994). Soybean proteinase inhibitors affect intestinal trypsin activities and amino acid digestibilities in rainbow trout (Oncorhynchus mykiss) 107, 215–219. doi: 10.1016/0300-9629(94)90296-8
Kumar V., Makkar H. P. S., Becker K. (2010). Dietary inclusion of detoxified Jatropha curcas kernel meal, effects on growth performance and metabolic efficiency in common carp, Cyprinus carpio (Linnaeus). Fish. Physiol. Biochem. 36, 1159–1170. doi: 10.1007/s10695-010-9394-7
Kumar V., Makkar H. P. S., Becker K. (2011). Detoxified Jatropha curcas kernel meal as a dietary protein source: Growth performance, nutrient utilization and digestive enzymes in common carp (Cyprinus carpio L.) fingerlings. Aquac. Nutr. 17, 313–326. doi: 10.1111/j.1365-2095.2010.00777.x
Kuz’mina V. V. (1991). Evolutionary features of the digestive-transport function in fish. Plenun Publishing Corporation, Bloomberg. Zhurnal Évolyutsionnoi Biokhimiii Fiziol. 27, 167–175.
Lall S. P. (1991). Fish Nutrition Research in Asia. Ed. De Silva S.S. (Manila, Philippines: Asian Fisheries Society), 1–12.
Lee K., Olomu J. M., Sim J. S. (1991). Live performance, carcass yield, protein and energy retention of broiler chickens fed canola and flax full-fat seeds and the restored mixtures of meal and oil. Can. J. Anim. Sci. 71, 897–903. doi: 10.4141/cjas91-105
Lowry O. H., Rosebrough N. J., Farr A. L., Randell R. J. (1951). Protein measurement with folin reagent. J. Biol. Chem. 193, 265–273. doi: 10.1016/S0021-9258(19)52451-6
Magbanua T. O., Ragaza A. R. (2024). Selected dietary plant-based proteins for growth and health response of Nile tilapia Oreochromis niloticus. Aquac. Fish 9 (1), 3–19. doi: 10.1016/j.aaf.2022.04.001
Mahmood S., Khan N., Iqbal K. J., Ashraf M., Khalique A. (2018). Evaluation of water hyacinth (Eichhornia crassipes) supplemented diets on the growth, digestibility and histology of grass carp (Ctenopharyngodon idella) fingerlings. J. Appl. Anim. Res. 46, 24–28. doi: 10.1080/09712119.2016.1256291
Maitra S., Ramachandran S., Ray A. K. (2007). In vitro assay of plant protease inhibitors from four different sources on digestive proteases of rohu, Labeo rohita (Hamilton), fingerlings. Aquac. Res. 38, 156–165. doi: 10.1111/j.1365-2109.2006.01640.x
Mandal S., Ghosh K. (2010). Accumulation of tannin in different tissues of Indian major carps and exotic carps. Aquac. Res. 41, 945–948. doi: 10.1111/j.1365-2109.2009.02371.x
Mandal S., Ghosh K. (2013). Optimization of tannase production and improvement of nutritional quality of two potential low-priced plant feedstuffs under solid state fermentation by Pichia kudriavzevii isolated from fish gut. Food Biotechnol. 27, 86–103. doi: 10.1080/08905436.2012.755929
Mandal S., Ghosh K. (2019). Utilization of fermented Pistia leafs in the diet of Rohu, Labeo rohita (Hamilton): effects on growth, digestibility and whole body composition. Waste Biomass Valorization 10, 3331–3342. doi: 10.1007/s12649-018-0336-4
Mandal S., Ghosh K. (2020). Effect of different processing techniques on nutrient and anti-nutrient compositions of plant feedstuffs for their probable use as aqua-feed ingredients. J. Inland Fish. Soc India. 52 (2), 173–182. doi: 10.47780/jifsi.52.2.2020.109943
Maynard L., Loosil J., Hintz H., Warner R. (1979). Animal Nutrition. 7th. Ed. Zappa C. R. (New York, USA: McGraw-Hill), 13–14.
Mazurkiewicz J. (2009). Utilization of domestic plant components in diets for common carp Cyprinus carpio L. Arch. Pol. Fish. 17, 5–39. doi: 10.2478/v10086-009-0001-4
Mohanta K. N., Mohanty S. N., Jena J., Narottam Prasad Sahu N. P., Patro B. (2009). Carbohydrate level in the diet of silver barb, Puntius gonionotus (Bleeker) fingerlings: effect on growth, nutrient utilization and whole body composition. Aquac. Res. 40, 927–937. doi: 10.1111/j.1365-2109.2009.02186.x
Mohapatra S., Chakraborty T., Prusty A. K., Das P., Paniprasad K., Mohanta K. N. (2012). Use different microbial probiotic in the diet of silver barb, Puntius gonionotus (Bleeker) fingerlings: effect on growth, nutrient digestibility and retention, digestive enzyme activities and intestinal microflora. Aquacult. Nutr. 18, 1–11. doi: 10.1111/j.1365-2095.2011.00866.x
Moore S., Stein W. W. (1948). Photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 176, 367–388. doi: 10.1016/S0021-9258(18)51034-6
Morris D. H. (2007). Flax—a health and nutrition primer. Available at: www.flaxcouncil.ca.
Mukhopadhyay N., Ray A. K. (1999a). Utilization of copra meal in the formulation of compound diets for rohu, Labeo rohita fingerlings. J. Appl. Ichthyol. 15, 127–131. doi: 10.1046/j.1439-0426.1999.00132.x
Mukhopadhyay N., Ray A. K. (1999b). Effect of fermentation on the nutritive value of sesame seed meal in the diets for rohu, Labeo rohita (Hamilton), fingerlings. Aquac. Nutr. 5, 229–236. doi: 10.1046/j.1365-2095.1999.00101.x
Mukhopadhyay N., Ray A. K. (2005). Effect of fermentation on apparent total and nutrient digestibility of linseed, Linum usitatissimum, meal in rohu Labeo rohita, fingerlings. Acta Ichthyol. Piscat. 35, 73–78. doi: 10.3750/AIP2005.35.2.02
Naylor R. L., Goldburg R. J., Primavera J. H., Kautsky N., Beveridge M. C. M., Clay J., et al. (2000). Effect of aquaculture on world fish supplies. Nature 405, 1017–1024. doi: 10.1038/35016500
Pandey A. (1992). Recent development in solid-state fermentation. Process Biochem. 27, 109–116. doi: 10.1016/0032-9592(92)80017-W
Pandey A., Soccol C. R., Rodriguez-Leon J. A., Nigam P. (2001). Solid-State fermentation in Biotechnology (New Delhi: Asia tech Publishers).
Rahmatullah S. M., Beveridge M. C. M. (1993). Ingestion of bacteria in suspension by Indian major carps (Catla catla, Labeo rohita) and Chinese carps (Hypophthalmichthys molitrix, Aristichthys nobilis). Hydrobiologia 264, 79–84. doi: 10.1007/BF00014095
Ramachandran S., Bairagi A., Ray A. K. (2005). Improvement of nutritive value of grass pea (Lathyrus sativus) seed meal in the formulated diets for rohu, Labeo rohita (Hamilton) fingerlings after fermentation with a fish gut bacterium. Bioresour. Technol. 96, 1465–1472. doi: 10.1016/j.biortech.2004.12.002
Ramachandran S., Ray A. K. (2004). Inclusion of extruded grass pea, Lathyrus sativus seed meal in compound diets for rohu, Labeo rohita (Hamilton 1822) fingerlings. Acta Ichthyol. Et. Piscat. 34, 205–218. doi: 10.3750/AIP2004.34.2.08
Ramachandran S., Ray A. K. (2007). Nutritional evaluation of fermented black gram (Phaseolus mungo) seed meal in compound diets for rohu, Labeo rohita (Hamilton), fingerlings. J. Appl. Ichthyol. 23, 74–79. doi: 10.1111/j.1439-0426.2006.00772.x
Ray A. K., Bairagi A., Sarkar Ghosh K., Sen S. K. (2007). Optimization of fermentation conditions for cellulase production by Bacillus subtilis CY5 and Bacillus circulans TP3 isolated from fish gut. Acta Ichthyol. Piscat. 37, 47–53. doi: 10.3750/AIP2007.37.1.07
Ray A. K., Das I. (1994). Apparent digestibility of some aquatic macrophytes in rohu, Labeo rohita (Ham.), fingerlings. J. Aquacult. Trop. 9, 335–342.
Ray A. K., Ghosh K., Ringø E. (2012). Enzyme-producing bacteria isolated from fish gut: a review. Aquacult. Nutr. 18, 465–492. doi: 10.1111/j.1365-2095.2012.00943.x
Ringø E., Ramasamy H., Soltani M., Ghosh K. (2022). The effect of gut microbiota and probiotics on metabolism in fish and shrimp. Animals 12, 3016. doi: 10.3390/ani12213016
Roberts R. J. (1978). “The pathophysiology and systematic pathology of teleost,” in Fish pathology. Ed. Roberts R. J. (London: Baillière Tindall), 55–91.
Roy T., Banerjee G., Dan S. K., Ghosh P., Ray A. K. (2014). Improvement of nutritive value of sesame oilseed meal informulated diets for rohu, Labeo rohita (Hamilton), fingerlings after fermentation with two phytase-producing bacterial strains isolated from fish gut. Aquac. Int. 22, 633–652. doi: 10.1007/s10499-013-9691-0
Saha S., Ray A. K. (2011). Evaluation of nutritive value of water hyacinth (Eichhornia crassipes) leaf meal in compound diets for rohu, Labeo rohita (Hamilton,1822) fingerlings after fermentation with two bacterial strains isolated from fish gut. Turkish J. Fish. Aquat. Sci. 11, 199–209. doi: 10.4194/trjfas.2011.0204
Santigosa E., Sánchez J., Médale F., Kaushik S., Pérez-Sánchez J., Gallardo M. A. (2008). Modifications of digestive enzymes in trout (Oncorhynchus mykiss) and sea bream (Sparus aurata) in response to dietary fish meal replacement by plant protein sources. Aquaculture 282, 68–74. doi: 10.1016/j.aquaculture.2008.06.007
Sastry C. S. P., Tammuru M. K. (1985). Spectrophotometric determination of tryptophan in protein. J. Food Sci. 22, 146–147.
Scalbert A. (1991). Antimicrobial properties of tannins. Phytochemistry 30, 3875–3883. doi: 10.1016/0031-9422(91)83426-L
Smith C., Van Megen W., Twaalhoven L., Hitchcock C. (1980). The determination of trypsin inhibitor levels in foodstuffs. J. Sci. Food Agric. 3, 341–350. doi: 10.1002/jsfa.2740310403
Soltani M., Ghosh K., Hoseinifar S. M., Kumar V., Lymbery A. J., Roy S., et al. (2019). Genus bacillus, promising probiotics in aquaculture: Aquatic animal origin, bio-active components, bioremediation and efficacy in fish and shellfish. Rev. Fish. Sci. Aquac. 27, 331–379. doi: 10.1080/23308249.2019.1597010
Spinelli J., Houle C. R., Wekell J. C. (1983). The effect of phytate on the growth of rainbow trout (Salmo gairdneri) fed purified diets containing varying quantities of calcium and magnesium. Aquacult 30, 71–83. doi: 10.1016/0044-8486(83)90153-9
Spyridakis P., Metailler R., Gabaudan J., Riaza A. (1989). Studies on nutrient digestibility in European sea bass (Dicendrarchus labrax) 1. Methodological aspects concerning faeces collection. Aquaculture 77, 61–70. doi: 10.1016/0044-8486(89)90021-5
Sugita H., Hirose Y., Matsuo N., Deguchi Y. (1998). Production of the antibacterial substance by Bacillus sp. strain NM 12, an intestinal bacterium of Japanese coastal fish. Aquac 165, 269–280. doi: 10.1016/S0044-8486(98)00267-1
Tacon A. G. J. (1993). Feed ingredients for crustaceans natural foods and processed feedstuffs (FAO, Rome: FAO Fisheries Circular No. 866).
Talukdar S., Ringø E., Ghosh K. (2016). Extracellular tannase-producing bacteria detected in the digestive tracts of seven freshwater fishes. Acta Ichthyol. Piscat. 46, 201–210. doi: 10.3750/AIP2016.46.3.04
Tengerdy R. P. (1998). Advances in Biotechnology. Ed. Pandey A. (New Delhi: Educational Publishers and Distributors), 13–16.
Updegraff D. M. (1969). Semimicro determination of cellulose in biological material. Anal. Biochem. 32, 420–424. doi: 10.1016/S0003-2697(69)80009-6
Vaintraub I. A., Lapteva N. A. (1988). Colorimetric determination of phytate in unpurified extracts of seeds and the products of their processing. Anal. Biochem. 175, 227–230. doi: 10.1016/0003-2697(88)90382-X
Van de Lagemaat J., Pyle D. L. (2001). Solid-state fermentation and bioremediation: development of a continuous process for the production of fungal tannase. Chem. Eng. J. 84, 115–123. doi: 10.1016/S1385-8947(01)00196-6
Walter H. E. (1984). “Proteinases: methods with hemoglobin, casein and azocoll as substrates,” Methods of Enzymatic Analysis Eds. Bergmeyer H. U. (Germany: Verlag Chemie, Weinheim) 5, 270–277.
Wang Y., Yu S., Wang Y., Che J., Zhao L., Bu X., et al. (2016). Effect of replacing fish meal with soybean meal on growth, feed utilization and nitrogen and phosphorus excretion of juvenile Pseudobagrus ussuriensis. Aquac. Res. 47, 3145–3155. doi: 10.1111/are.12765
Wee K. L. (1991). “Use of non-conventional feedstuff of plant origin as fish feeds–is it practical and economically feasible?,” in Fish Nutrition Research in Asia. Ed. De Silva S.S. (Manila, Philippines: Asian Fisheries Society), 13–22.
Xu Q., Qiao Q., Gao Y., Hou J., Hu M., Du Y., et al. (2021). Gut microbiota and their role in health and metabolic disease of dairy cow. Front. Nutr. 8, 701511. doi: 10.3389/fnut.2021.701511
Zar J. H. (2010). Biostatistical analysis. 5th ed. (New Delhi, India: Pearson Education Singapore Ptd. Ltd., Indian Branch).
Keywords: cellulose, xylan, growth performance, amino acid, digestive enzymes, haematological parameters
Citation: Banerjee S, Kari ZA, Téllez-Isaías G and Ghosh K (2023) The use of linseed oil cake in the diets of rohu, Labeo rohita (Hamilton), after solid-state fermentation with a fish gut bacterium, Bacillus pumilus (KF640221): an appraisal on growth, digestibility, body composition, and hematobiochemical profile. Front. Mar. Sci. 10:1278704. doi: 10.3389/fmars.2023.1278704
Received: 16 August 2023; Accepted: 04 October 2023;
Published: 27 October 2023.
Edited by:
Amit Ranjan, Tamil Nadu Fisheries University, IndiaReviewed by:
Sheena K Baby, Tamil Nadu Fisheries University, IndiaSarvendra Kumar, College of Fisheries Kishanganj, India
Copyright © 2023 Banerjee, Kari, Téllez-Isaías and Ghosh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Koushik Ghosh, kghosh@zoo.buruniv.ac.in; kghoshbu@gmail.com
 Sudeshna Banerjee1
Sudeshna Banerjee1  Zulhisyam Abdul Kari
Zulhisyam Abdul Kari Guillermo Téllez-Isaías
Guillermo Téllez-Isaías Koushik Ghosh
Koushik Ghosh