Lower skeletal extension in Pleistocene Orbicella (Montastraea) corals than in their modern counterparts
- 1Coral Reefs Laboratory, Facultad de Ciencias Biológicas y Agropecuarias, Cuerpo Académico Ecosistemas Costeros, Universidad Veracruzana, Tuxpan, Mexico
- 2Unidad Académica de Sistemas Arrecifales, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Puerto Morelos, Mexico
Introduction: Despite warmer conditions during the Last Interglacial, coral colonies of Orbicella were abundant and reached large sizes on many Caribbean reefs, including the extinct O. nancyi.
Methods: To explore variation in growth rates, we examined the yearly mean linear extension of growth bands in two fossil Orbicella species and compared them with two modern species of the same genus from shallow waters of the wider Caribbean.We measured the linear extension of corals exposed in a fossil reef and their modern counterparts, from both in situ colonies and coral slab X-rays.
Results: Few coral colonies showed autocorrelation or a linear trend on their linear-growth time series. A Bayesian ANOVA showed lower linear-extension rates of fossils compared to modern colonies and similar or lower than other fossil corals from the Pleistocene. Growth rates and growth form contribute significantly to the amount of tissue and size of coral colonies and can be a decisive trait for inter and intra specific competition.
Discussion: It is unlikely that temperature or interspecific competition explain modern coral extension rates and the low rates of the fossils data, which seem to be controlled instead by past habitat conditions.
1 Introduction
Each year, corals of the Western Atlantic genus Orbicella (formerly Montastraea) deposit a skeletal band composed of high and low-density portions of calcium carbonate (Highsmith, 1979; Barns and Lough, 1993). The width of each band (linear extension) varies between years and is sensitive to natural and anthropogenic environmental changes (Buddemeier et al., 1974; Carilli et al., 2010; Rico-Esenaro et al., 2019). For example, changes in time series of the linear extension of Orbicella annularis at various sites in Puerto Rico correlated positively with water temperature (Dodge, 1981), and at the East Flower Gardens Bank, O. annularis extension rates varied inversely with annual discharge of the Atchafalaya river (Dodge and Lang, 1983). Mean linear extension value and variability depend on (i) the size of the coral colony and the length of the sampled time series, (ii) genetic differences between colonies (Osinga et al., 2011), and (iii) acclimation to the environment (Castillo et al., 2011; Groves et al., 2018). Furthermore, under moderately adverse conditions, species of Orbicella avoid a loss in linear extension by decreasing skeletal density (Dodge and Brass, 1984; Carricart-Ganivet and Merino, 2001). However, if conditions are severe enough, the mean linear extension is depressed (Dodge and Thomson, 1974; Dodge and Brass, 1984). The mean linear extension of Orbicella spp. can decrease with increasing temperature (Carricart-Ganivet, 2004), low light due to water turbidity and depth (Hubbard and Scaturo, 1985; Huston, 1985), sedimentation (Dodge et al., 1974; Torres, 2001; Carilli et al., 2009) and water quality (Dodge and Brass, 1984).
Several studies have examined the mean linear extension of fossil corals and made ecological and environmental inferences. For example, Gischler et al. (2009) reported a lower-than-present mean linear extension in massive Pleistocene Orbicella corals from lagoonal environments in Florida. They mentioned higher temperature as a probable cause during the Last Interglacial (LIG) which lasted ~10 ka (~130 to 120 ka). This interval was characterized by enhanced summer insolation that forced large polar deglaciation, leading to a reduced latitudinal temperature gradient and a higher sea level (Stirling et al., 1998; Denton et al., 2010). These conditions favored the extensive development of coral reefs as tropical waters expanded polewards and warm seas transgressed further across shallow shelves (Greenstein and Pandolfi, 2008; O’Lear et al., 2008; Blanchon, 2010; Kiessling et al., 2012). At that time, species richness in the Caribbean was slightly higher than present (Budd et al., 1994; Budd, 2000) and the Orbicella annularis species complex included three extant species, as well as a columnar form, and the organ-pipe Orbicella nancyi (Pandolfi, 2007). O. nancyi was widely distributed in shallow waters of San Andrés, Barbados, Lesser and the Greater Antilles, and Curaςao (Geister, 1977; Pandolfi and Jackson, 2001; Pandolfi et al., 2002). Pandolfi et al. (2002) proposed that the now-extinct O. nancyi had a high linear extension and that other species of Orbicella reduced their linear growth when this coral was present.
To investigate the extent to which environmental or ecological factors control on linear extension, we compared rates in massive and columnar species of Orbicella from a 125 kyr-old reef deposit at Xcaret, Quintana Roo, México (Jordán-Dahlgren, 1997; Blanchon et al., 2009; Blanchon, 2010), with two modern Orbicella species from shallow habitats of the wider Caribbean. We assess autocorrelation and linear trends of the coral’s time series and mean differences within and between fossil species in Xcaret and modern O. faveolata and O. annularis.
2 Materials and methods
2.1 Reef location
After the LIG high stand, sea level fell as glaciation proceeded, leaving fossil reefs elevated above the present sea level (Potter and Lambeck, 2004). Our study site is along the east coast of the Yucatán Peninsula, which is a karstic platform (Ward et al., 1985) where dissolution during sea-level lowstands has formed some of the largest known underground rivers (Steinich et al., 1996). At Xcaret, a tourist attraction located south of Playa del Carmen, México (Figure 1), one of these underground rivers has been artificially enlarged and provides unparalleled exposure of a fossil fringing reef (Jordán-Dahlgren, 1997; Blanchon, 2010). We worked on an area that corresponds to the proximal back-reef (Blanchon et al., 2009; Blanchon, 2010), which has a rich scleractinian assemblage that is mainly in growth position including, among others: Orbicella spp., Siderastrea spp., Pseudodiploria spp., Montastraea cavernosa and Pocillopora spp. (Figure 2). Fossil colonies of O. faveolata form the walls of Xcarets´tunnels and removing them would have caused significant damage; instead three large colonies were measured in situ. Elsewhere branches of O. nancyi could be easily detached and columns from three colonies were collected and examined by X-radiographs.
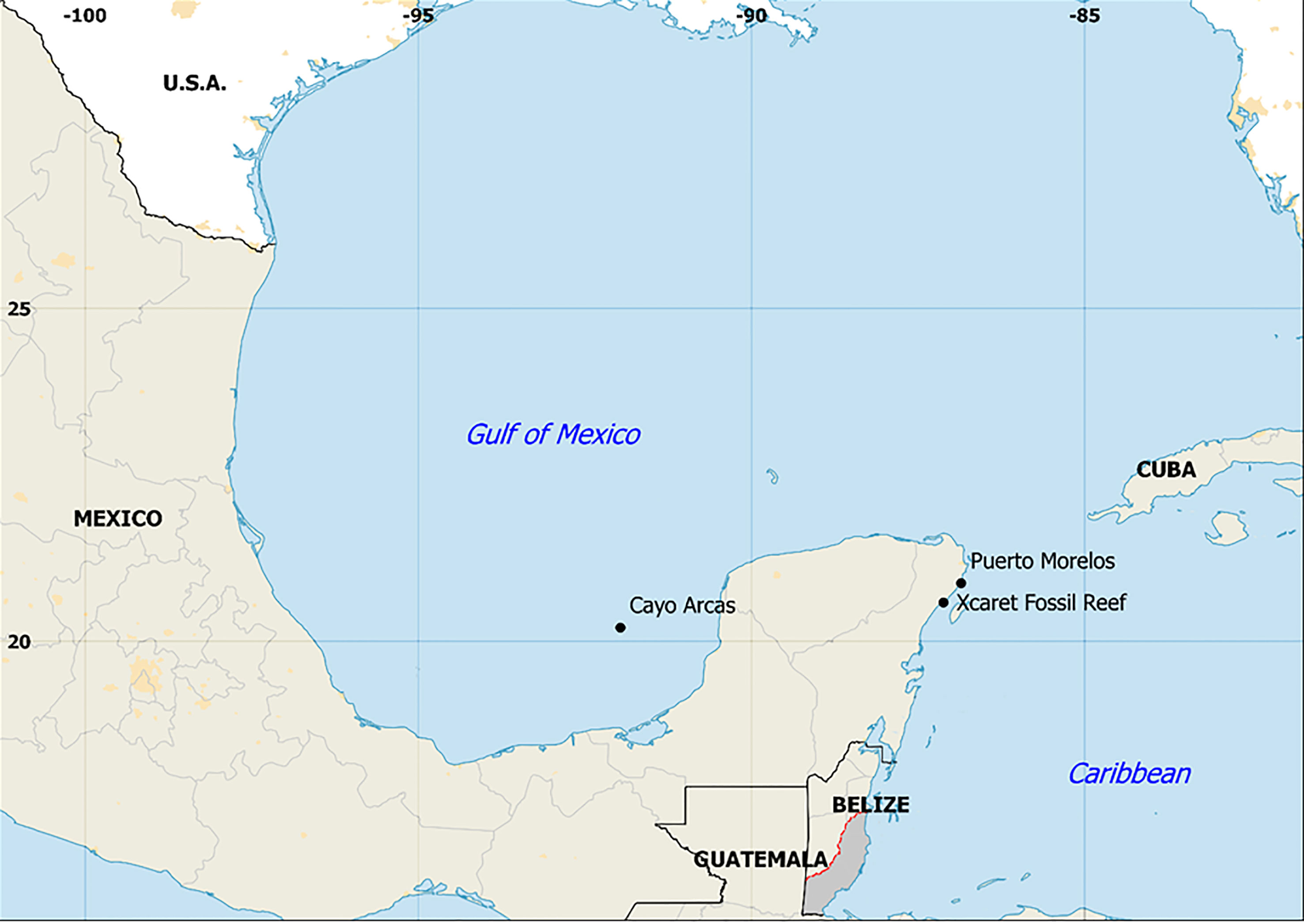
Figure 1 Location of the study sites (black circles). Fossil corals were located at Xcaret recreational park, Quintana Roo, México. The map was created in QGIS 2.4.0 (http://qgis.osgeo.org) using Natural Earth free vector and raster map data @naturalearthdata.com.
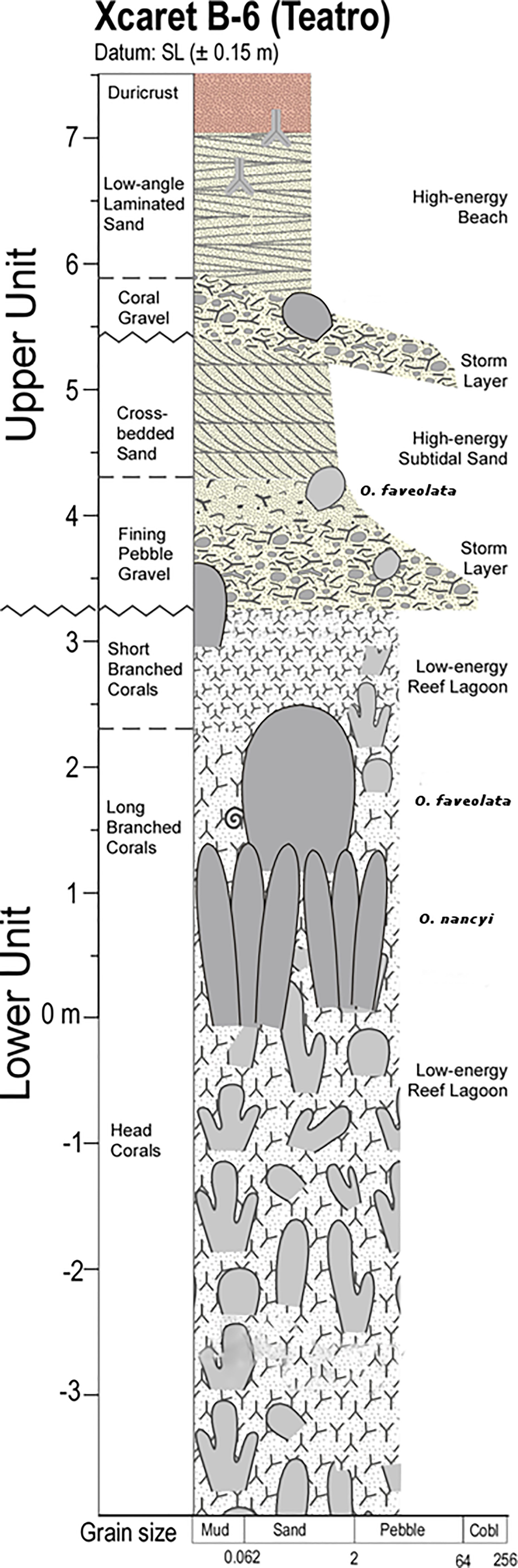
Figure 2 Stratigraphic columns from Xcaret´s fossil reef showing the strata of fossil corals with reference to present sea level.
To compare these fossils with modern corals, we used core slabs from colonies of Orbicella faveolata and O. annularis collected from the Gulf of Mexico and the Caribbean. Extension rates came from 14 colonies of O. annularis and 16 colonies of O. faveolata from Cayo Arcas, collected by Jordán-Dahlgren and Jordán-Garza in 2008. In addition, we used data from five colonies of O. annularis and three colonies of O. faveolata from Puerto Morelos (Carricart-Ganivet et al., 1994; Carricart-Ganivet et al., 2000 and Carricart-Ganivet and Merino, 2001). All modern coral colonies were living in shallow (2 to 10 m) environments, which are comparable to the paleo-water depth (2-7 m) of fossil corals in the proximal back-reef zone of Xcaret (Blanchon, 2010).
2.2 Linear extension rates of fossil and modern corals
Linear extension data of fossil corals where collected from three organ-pipe colonies (Orbicella nancyi, Pandolfi, 2007) and three colonies with massive morphotypes (most closely resembling modern O. faveolata) found in growth position in the back-reef zone behind the upper reef tract at Xcaret, México (Figures 2, 3). Although the fossil corals were not dated, they came from the same site and stratigraphic level (0 to +2.3 m above mean sea level) as the corals that Blanchon et al. (2009) used for dating. According to these authors, the approximate age of this reef ranges between ~117 and ~127 ky ago, which corresponds to the last interglaciation and marine isotope stage 5e (Blanchon, 2010; Dahl-Jensen et al., 2013).
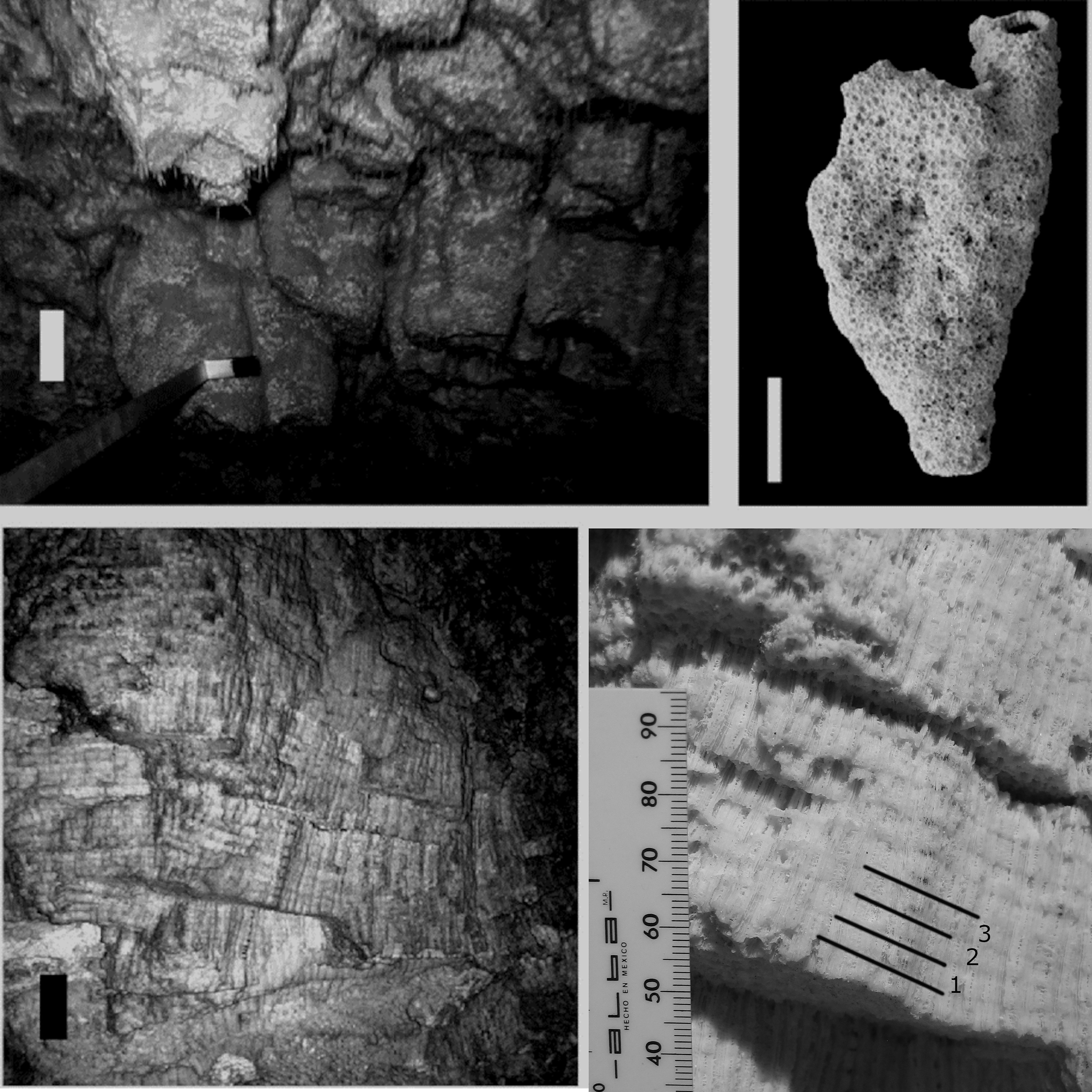
Figure 3 Organ-pipe colonies (Orbicella nancyi) were reachable via the underground river (top left, scale 10 cm) and columns were detached by hand. The columns were club-shaped and all covered by intact corallites (top right, scale 1.5 cm); the massive colonies (O. faveolata) were exposed on cave walls (bottom left, scale 5 cm) and growth characteristics were measurable in situ with the width of 3 bands shown in black (bottom right).
Samples from the organ pipe corals were sectioned with a rock saw for X-ray analysis (Knutson et al., 1972), and the slabs were digitized using a flat-bed scanner (Figure 3). The width of the growth bands was measured with the program Coral XDS (Helmle et al., 2002). Growth analysis was feasible in situ for the massive morphotype, as colonies had been sectioned during cave excavations for tourism. The colonies were found encased in the fossil-reef framework in apparent growth positions and showed corallites with vertical orientations typical of living corals. In these colonies, the growth bands could be clearly distinguished due to diagenetic alteration (Weil and Knowlton, 1993). We measured linear extension directly on these colonies using a vernier caliper (minimum scale 0. 05 cm, Figure 3). We then compared the Xcaret fossil corals with modern members of the same species complex from shallow water habitats (Figure 1). The methods for obtaining coral-band widths were similar in all modern cases: X-radiographs from core slices from different coral colonies (Figure 4). Although the vernier and X-radiographs methods differ, both are directly measuring the width of the grow bands with a precision.
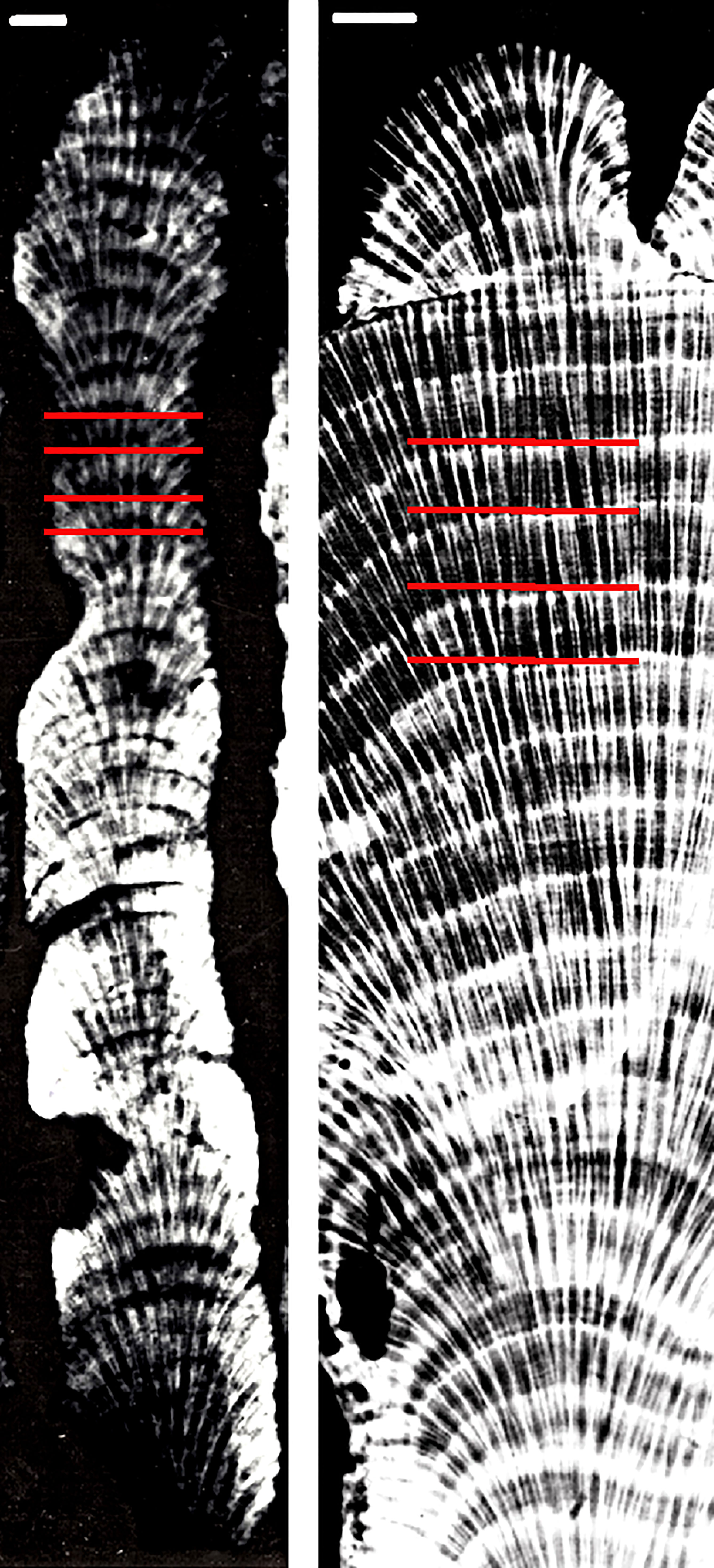
Figure 4 X-radiographs of fossil O. nancyi (left) and modern O. faveolata (right). White bars represent 1 cm. Red lines delimit the location of growth bands.
2.3 Data analysis
For each coral’s time series, we estimated lag autocorrelation using a function that compares the covariance sequence at time t (ct) to the original’s time series covariance (c0) using the autocorrelation function (acf) in R (R Core Team, 2022). In addition, a linear trend of each coral’s time series was established by estimating the slope of a generalized least squares model using package “nlme” in R (Pinheiro et al., 2022). Linear extension measurements from all colonies were grouped by species (O. nancyi, O. faveolata, and O. annularis) and location (Gulf of Mexico, the Caribbean, and Pleistocene Caribbean). To test for mean differences in linear extension for each species and location as fixed factors and with the coral colony as a random factor, we used a Bayesian ANOVA (Supplementary Data) employing a normal prior and accounting for heteroscedasticity between groups (McCarthy, 2008). Comparisons were made between all groups, and differences were assessed with the 95% credible intervals of the unstandardized effect sizes (the difference between group means), not including 0 (Nakagawa and Cuthill, 2007).
Given that vital rates (like survival and reproduction) in modular organisms like corals are linked to colony size (Hughes and Connell, 1987; Hughes et al., 1992; Rodríguez-Martínez et al., 2011) and ultimately to the surface of living tissue (Weil et al., 2009), we highlight the importance of even small differences in growth rates using a simple growth model of massive (like O. faveolta or O. annularis) and columnar (like O. nancyi, see Pandolfi, 2007):
with SA = surface area of living tissue in cm2 for massive and columnar growth forms; r = initial radius (0.5 cm) of the coral colony or column in cm; t= time in years and GR = growth rate in cm/y, h = initial column height (0.5 cm), NC = number of columns, and dr= damping rate (0.5) to account for a columnar form that increases in diameter at half the rate than in height.
3 Results
Neither modern nor fossil corals show consistent lag-1 autocorrelation on their linear extension time series. As shown in Table S1, of the 44 colonies sampled, only 11% showed lag-1 autocorrelation. Similarly, no consistent linear trends were found. Only 25% of the coral colonies showed significant trends, with three modern corals showing a positive trend, five showing a negative trend, and two fossil corals showing positive linear trends (Table S1). More interestingly, results show that fossil colonies of Orbicella have linear extension rates that are ~40% lower than modern corals (Figure 5, Table 1). The mean linear extension rate in fossil corals from Pleistocene Xcaret vary between 0.52 (0.48 to 0.56) for the fossil O. faveolta and 0.66 (0.58 to 0.74) cm/year for O. nancyi (mean and 95% credible intervals, Figure 4). In contrast, the mean linear extension rates in modern corals are similar for O. annularis and O. faveolata and vary between 0.87 (0.84 to 0.89) at the Gulf of Mexico and 0.99 (0.94 to 1.06) cm/year at the Mexican Caribbean. The mean extension rate is slightly higher in the Caribbean (Car) than the Gulf of Mexico (GoM) (mean effect size: GoM vs Car. -0.099 (-0.15 to -0.04) cm/year) and higher in both modern seas compared to Pleistocene Caribbean (PCar) (mean effect size: GoM vs PCar 0.377 (0.32 to 0.42) cm/year and Car vs PCar 0.47 (0.4 to 0.54) cm/year).
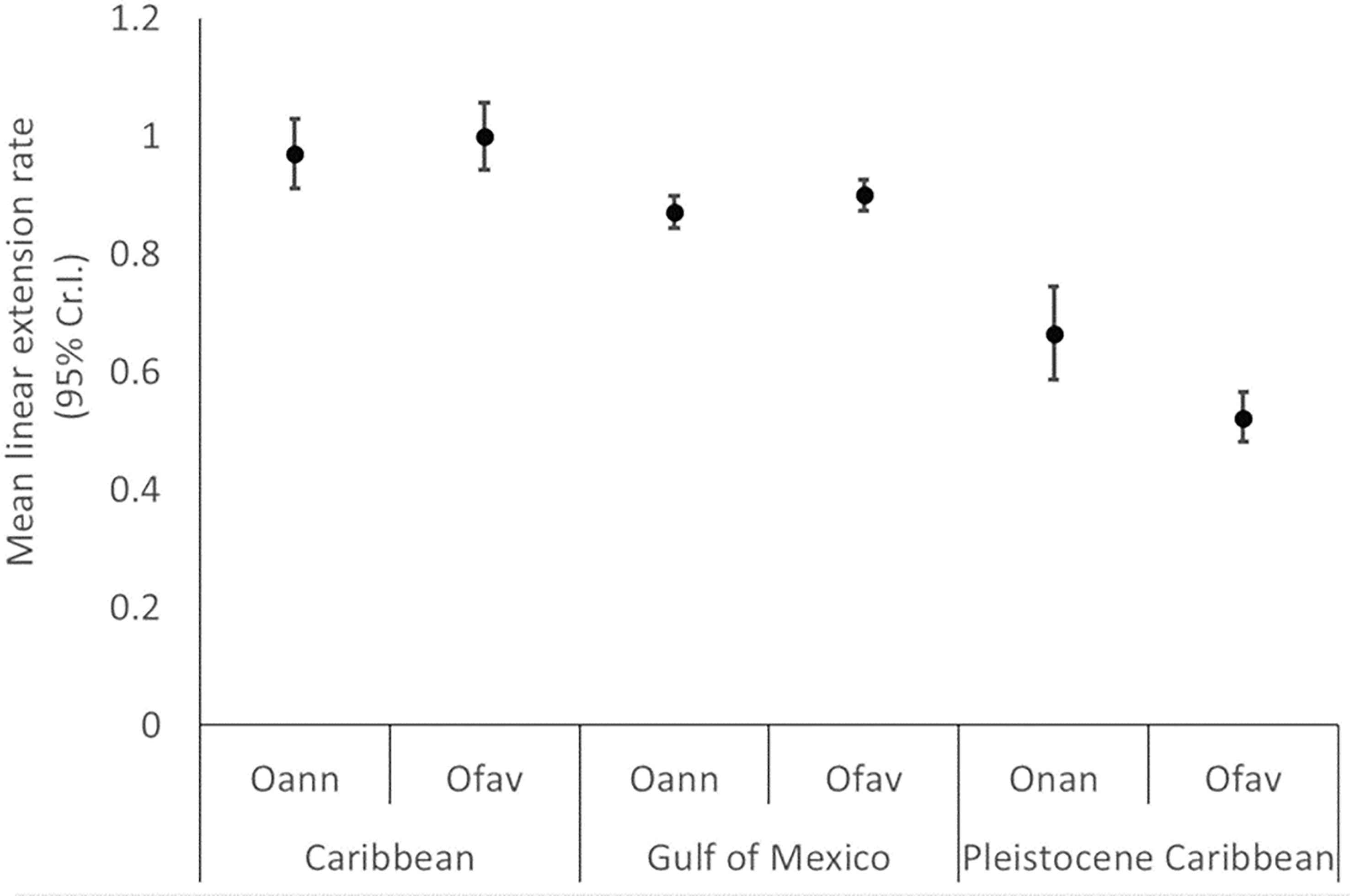
Figure 5 Mean linear extension rates (cm/year) and 95% credible intervals of modern and fossil corals (Oann = Orbicella annularis, Ofav= O. faveolata and Onan = O. nancyi). The number of measured skeletal bands is shown (n). Horizontal lines depict the highest and lowest confidence interval observed at each sampled region: Gulf of Mexico, Mexican Caribbean and Pleistocene Xcaret.
Differences in the mean linear extension exist between the fossil corals with O. faveolata having credibly lower linear extension than O. nancyi with a mean effect size of 0.14 (0.05 to 0.23) cm/year. Compared to their modern counterparts, the corals O. nancyi and fossil O. faveolata have lower extension rates (Figure 5). In general, modern coral species showed no significant differences in their growth rates or had small effect sizes (Table 2). However, the effect sizes of the differences in growth rates are large between modern and fossil corals and intermediate between the two fossil species at Xcaret (Table 1). Compared to other Atlantic coral fossils of the same genus or species, Xcaret´s O. nancyi shows a lower mean linear extension and massive forms show a range from 0.5 to 0.9 cm/year (Table 3).
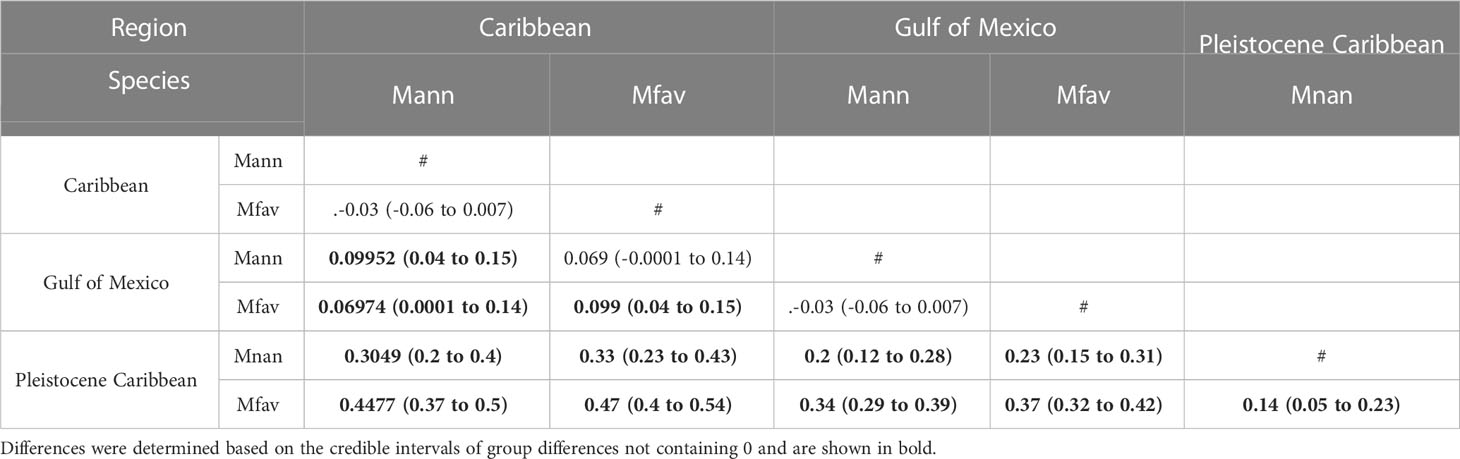
Table 2 Multiple comparisons of mean linear extension rate between modern and fossil corals from a Bayesian ANOVA.
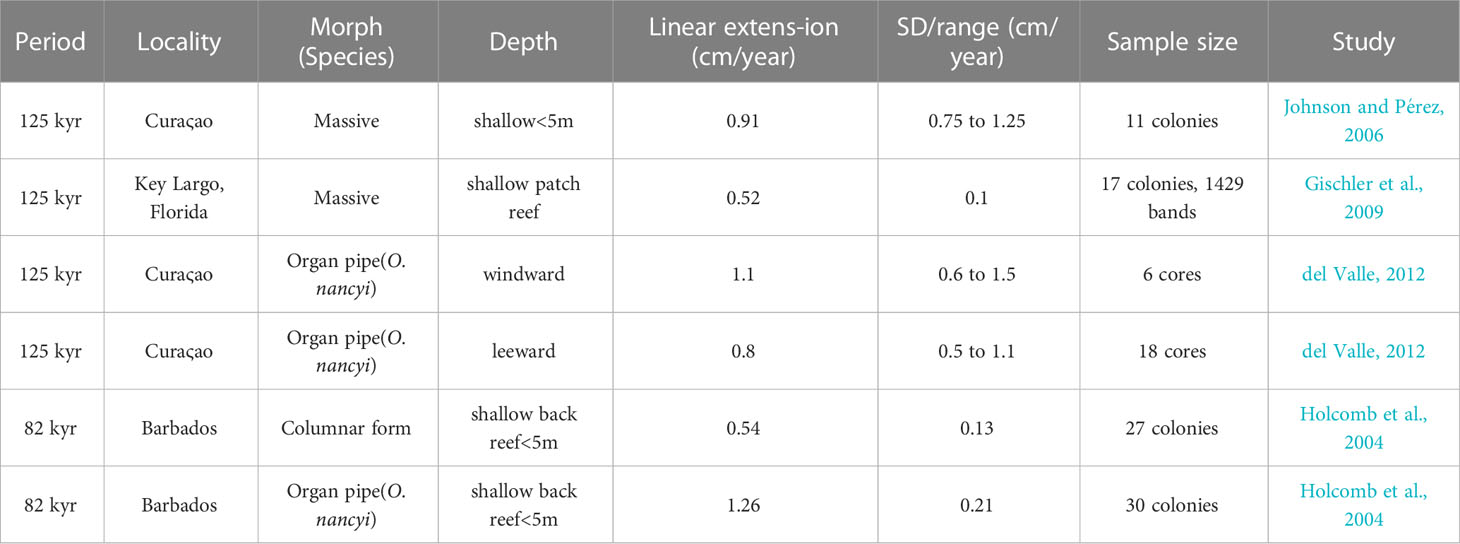
Table 3 Mean linear extension rates for fossil Orbicella spp. Corals for 125kyr and 82kyr Pleistocene at different localities in the Caribbean.
Small differences in mean linear extension can, in a few years, have a significant effect on the colony size in terms of the amount of tissue grown; in addition, a columnar growth form like O. nancyi’s has an even more significant effect on the rapid growth of living tissue (Figure 6).
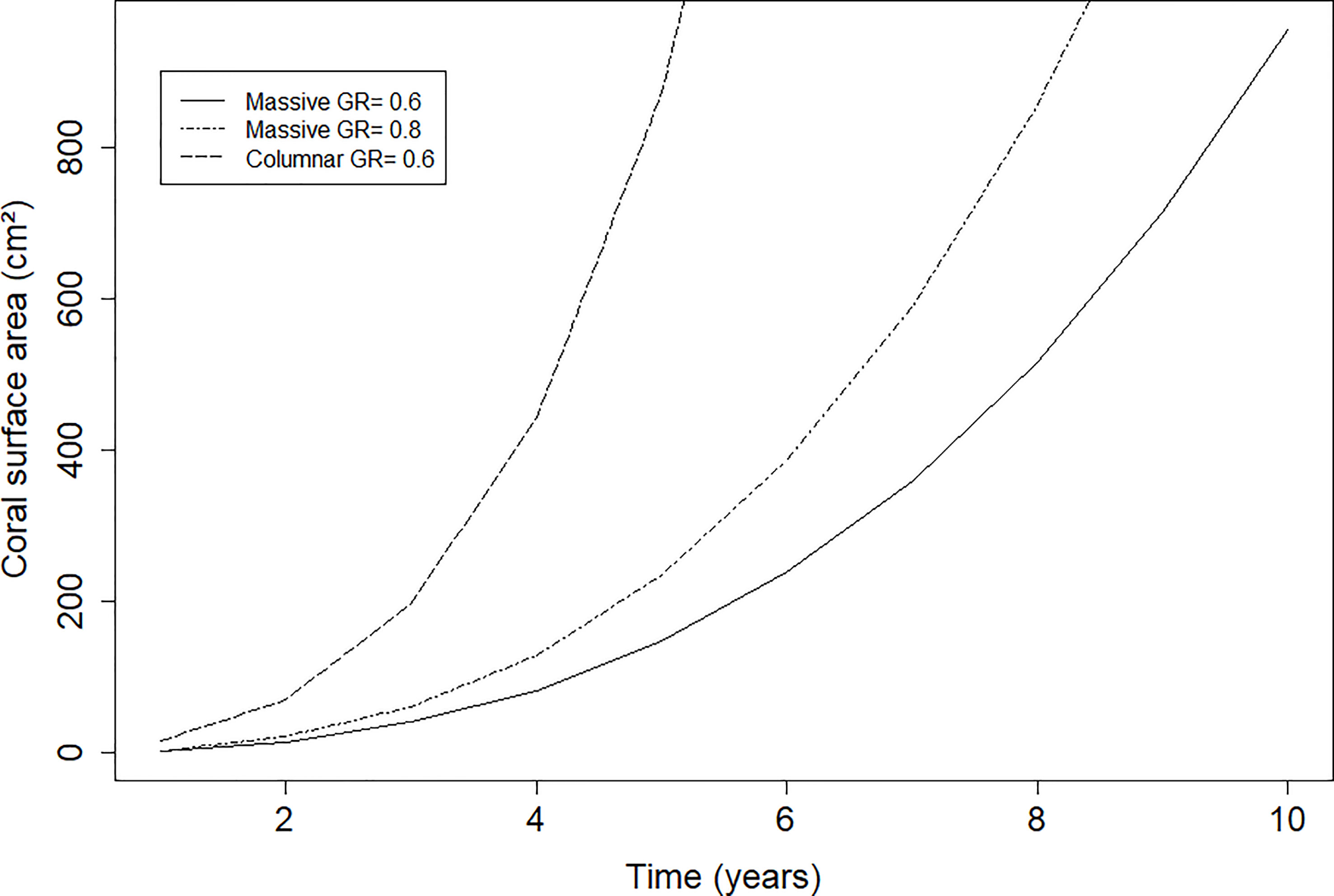
Figure 6 Modelled growth of colony surface (cm2) of massive growth forms with linear extension rates of 0.6 cm/year and 0.8 cm/year and a columnar growth form with a linear extension of 0.6 and 6 columns.
4 Discussion
The fossil coral at Xcaret with the highest linear extension was O. nancyi, followed by O. faveolata (Figure 5). Although O. nancyi has a higher linear extension than fossil O. faveolata, both species have a mean linear extension that is ~ 0.5 cm lower than expected (~ 50% less) when compared to modern corals and the fossil O. nancyi from Barbados (Tomascik, 1990; Holcomb et al., 2004). Slow growing fossil corals have also been reported elsewhere, including massive forms in Florida (Gischler et al., 2009), columnar forms in Barbados (Holcomb et al., 2004) and organ pipe forms in Curaςao (del Valle, 2012). By contrast some Orbicella spp. fossils show high growth rates (> 0.8 cm/year), including massive forms from Curaςao (Johnson and Pérez, 2006), and organ pipe forms from Curaςao and Barbados (Holcomb et al., 2004; del Valle, 2012). Both fast and slow-growing fossil corals come from paleo-habitats that were less than 6 m deep.
Two possible explanations have been given for low extension rates on fossil corals. First, Gischler et al. (2009), working with massive Orbicella from the LIG in Florida, considered temperature as a possible environmental factor inhibiting linear extension. Second, Pandolfi et al. (2002) and Pandolfi (2007) proposed that the linear extension of Pleistocene Orbicella corals sympatric with O. nancyi was depressed through competition for space and light. According to this competition hypothesis, the extinction of O. nancyi at ~82 kyr caused a character release in the O. annularis species complex, partly because O. nancyi was a fast grower and, through competition, suppressed the growth of sympatric Orbicella spp. Upon extinction, O. nancyi released the extant species from the competition, and linear extension rates increased presumably to modern values.
Neither of these hypotheses, however, sufficiently explains differences in the linear extension data between Xcaret and modern corals. Our modern O. faveolata and O. annularis extension data occur in two regions with different temperature regimes: the Mexican Caribbean Sea, which has a higher (~1.3° C) mean sea surface temperature than the Gulf of Mexico (Carricart-Ganivet, 2004). This temperature difference is like the estimated higher sea temperature of the LIG (Otto-Bliesner et al., 2006), and yet differences in linear extension between modern corals in both regions are not as large compared to fossil and modern corals. Modern temperature differences between the regions are likely within the acclimation capacities of these corals (Dodge and Brass, 1984; Carricart-Ganivet and Merino, 2001; Carricart-Ganivet, 2004). In addition, Helmle et al. (2011) showed a recent increase in linear extension for O. faveolata that made coral growth tolerant to the ongoing increase in temperature due to climate change. In fact, Orbicella corals can exhibit a positive correlation between linear extension and temperature (Dodge, 1981), yet higher water temperatures can lead to reduced rates of skeletal extension (Gischler et al., 2009). This suggests that there may be a temperature threshold beyond which coral extension is negatively impacted. The exact mechanisms behind the threshold are not fully understood but might be related to the physiology of the coral. Under moderate stress Orbicella avoids a loss in linear extension by decreasing skeletal density (Carricart-Ganivet and Merino, 2001), but Mendes and Woodley (2002) showed a decrease in extension rate of O. annularis after a bleaching event possibly related to an impairment in reproduction, the effect was harsher the longer the extreme water temperature lasted. Maintaining high linear extension rates might be advantageous to the corals to compete for light and space but at high physiological cost (Rinkevich, 1996).
Nevertheless, competition could have the opposite effect. Corals compete using various mechanisms (Richardson et al., 1979) and their growth rates have been shown to decrease in the presence of macroalgae (Lirman, 2001), turf algae (Quan-Young and Espinoza-Avalos, 2006) and other corals (Tanner, 1997; Lapid and Chadwick, 2006). As a consequence, colony growth rates can influence community structure and development through competition (Maguire and Porter, 1977). Comparing the extant Orbicella species, interspecific ecological interactions show that O. annularis is the least aggressive, followed by O. faveolata and O. franksi (Weil and Knowlton, 1993). This aggressiveness between competing species can result in tissue mortality and sometimes the suppression of mean linear growth rates, with faster-growing species escaping competition by size (Sebens, 1982; Tanner, 1997; Cruz-Piñon et al., 2003). For our modern corals, which share the same habitat, we found no evidence that the stronger competitor, O. faveolata, had systematically higher linear extension rates (Figure 5, Table 1). This finding is consistent with Cruz-Piñon et al. (2003), who reported higher skeletal extension rates in O. faveolata than in O. annularis, whereas Tomascik (1990) reported a reverse trend in the same corals from Barbados, and Van Veghel and Bosscher (1993) found no significant differences between the two species. This lack of consistency in species linear extension differences is likely due to interspecific competition being rare on modern reefs (van Woesik, 2002), and the large variability in reef environments and growth plasticity in species of Orbicella (Foster, 1979). Given that Xcaret’s O. nancyi shows a lower extension rate than modern and fossil corals in other locations, it is possible that other characteristics, such as growth form (Figure 5), made O. nancyi a stronger competitor (Pandolfi et al., 2002). Despite being in the same stratigraphic unit and level, it is uncertain if these coral species were cohorts that lived together. However, the clear facies on the deposit point to similar environmental conditions during the time the corals grew (Figure 2). Also, members of the Orbicella complex seem to have coexisted at different locations and periods of the Pleistocene (see Pandolfi et al., 2002) and it is likely that this also occurred at Xcaret.
The lack of a consistent temperature or species effect on extension rates implies that the lower linear extension rates of fossil Orbicella at both Xcaret and Florida (Gischler et al., 2009) is more likely to have resulted from deleterious habitat conditions. Data from O. nancyi samples collected by del Valle (2012), showed lower extension rates on the leeward side of Curaçao. Lower extension rates in back-reef corals from Xcaret are consistent with those of lagoonal corals reported by Gischler et al. (2009). Corals at both of these sites grew in or adjacent to large lagoons that resulted from extensive coastal inundation during a sea-level highstand at least 6 m higher than present (Blanchon et al., 2009). As a result, the lagoons and adjacent back reef environments must have been subject to high sediment flux and were associated with the development of sediment-tolerant biota (Blanchon, 2010). If coral growth in and near these lagoons was indeed depressed by sedimentation, then the same species from different habitats should show higher extension rates, which seems to be the case. More growth data from Pleistocene corals in different paleo-habitats are required to test this hypothesis and better understand their response to changing environments.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
AJ-G, EJ-D collected data. AJ-G, EJ-D performed the statistical analysis. AJ-G, PB, EJ-D, JC-G analysed the results and wrote the manuscript. All authors contributed to reviewing and editing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by CONACyT grant 1043508 to PB.
Acknowledgments
We want to thank the Xcaret administration, who allowed unrestricted access to the park to work on the fossil reef. We also thank J.M. Pandolfi for his help in identifying the fossil species O. nancyi, R.E. Rodríguez-Martínez for her editorial comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GHP declared a shared affiliation with the authors PB, JPCG and EJD to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1098430/full#supplementary-material
References
Barns D. J., Lough J. M. (1993). On the nature and causes of density banding in massive coral skeletons. J. Exp. Mar. Biol. Ecol. 167, 91–108. doi: 10.1016/0022-0981(93)90186-R
Blanchon P. (2010). Reef demise and back-stepping during the last interglacial, northeast Yucatan. Coral Reefs 29, 481–498. doi: 10.1007/s00338-010-0599-0
Blanchon P., Eisenhauer A., Fietzke J., Liebetrau V. (2009). Rapid sea-level rise and reef back-stepping at the close of the last interglacial highstand. Nature 458, 881–885. doi: 10.1038/nature07933
Budd A. F. (2000). Diversity and extinction in the Cenozoic history of Caribbean reefs. Coral Reefs 19, 25–35. doi: 10.1007/s003380050222
Budd A. F., Stemann T. A., Johnson K. G. (1994). Stratigraphic distributions of genera and species of neogene to recent Caribbean reef corals. J. Paleontol. 68, 951–977. doi: 10.1017/S0022336000026585
Buddemeier R. W., Maragos J. E., Knutson D. W. (1974). Radiographic studies of reef coral exoskeletons: Rates and patterns of coral growth. J. Exp. Mar. Biol. Ecol. 14, 179–199. doi: 10.1016/0022-0981(74)90024-0
Carilli J. E., Norris R. D., Black B., Walsh S. M., McField M. (2010). Century-scale records of coral growth rates indicate that local stressors reduce coral thermal tolerance threshold. Glob. Change Biol. 16, 1247–1257. doi: 10.1111/j.1365-2486.2009.02043.x
Carilli J. E., Prouty N. G., Hughen K. A., Norris R. D. (2009). Century-scale records of land-based activities recorded in mesoamerican coral cores. Mar. pollut. Bull. 58, 1835–1842. doi: 10.1016/j.marpolbul.2009.07.024
Carricart-Ganivet J. P. (2004). Sea Surface temperature and the growth of the West Atlantic reef-building coral Montastraea annularis. J. Exp. Mar. Biol. Ecol. 302, 249–260. doi: 10.1016/j.jembe.2003.10.015
Carricart-Ganivet J. P., Beltrán-Torres A. U., Merino M., Ruiz-Zárate M. A. (2000). Skeletal extensión, density and calcification rate of the reef building coral Montastraea annularis (Ellis and solander) in the Mexican Caribbean. Bull. Mar. Sci. 66, 215–224.
Carricart-Ganivet J. P., Horta-Puga G., Ruiz-Zárate M. A., Ruiz-Zárate E. (1994). Tasas retrospectivas de crecimiento del coral hermatípico Montastraea annularis (Scleractinia: Faviidae) en arrecifes al sur del golfo de méxico. Rev. Biol. Trop. 42, 515–521. Available at : https://revistas.ucr.ac.cr/index.php/rbt/article/view/23253
Carricart-Ganivet J. P., Merino M. (2001). Growth responses of the reef-building coral Montastraea annularis along a gradient of continental influence in the southern gulf of méxico. Bull. Mar. Sci. 68, 133–146.
Castillo K. D., Ries J. B., Weiss J. M. (2011). Declining coral skeletal extension for forereef colonies of Siderastrea siderea on the mesoamerican barrier reef system, southern Belize. PloS One 6, e14615. doi: 10.1371/journal.pone.0014615
Cruz-Piñon G., Carricart-Ganivet J. P., Espinoza-Avalos J. (2003). Monthly skeletal extensión rates of the hermatypic corals Montastraea annularis and Montastraea faveolata: biological and environmental controls. Mar. Biol. 143, 491–500. doi: 10.1007/s00227-003-1127-3
Dahl-Jensen D., Gogineni P., White J. W. C. (2013). Reconstruction of the last interglacial period from the NEEM ice core. Inside Pages 21, 22–23. doi: 10.22498/pages.21.1.22
del Valle T. M. (2012). Comparative growth rates of the extinct coral montastraea nancyi: a dominant framework builder in the pleistocene (MIS 5e) reefs of curacao, netherland antilles Vol. 30 (University of Cincinnati). Master Thesis.
Denton G. H., Anderson R. F., Toggweiler J. R., Edwards R. L., Schaefer J. M., Putnam A. E. (2010). The last glacial termination. Science 328, 1652–1656. doi: 10.1126/science.1184119
Dodge R. E. (1981). “Growth characteristics of reef-building corals within and external to a naval ordnance range: Vieques, Puerto Rico,” in Proc. 4th int. coral reef symp, vol. 2 . Eds. Gomez E. D., Birkeland C. E., Buddemeier R. W., Johannes R. E., Marsh J. A. Jr., Tsuda R. T. (Manila, Philippines: Marine Sciences Center, University of the PHillipines), 241–248.
Dodge R. E., Aller R. C., Thomson J. (1974). Coral growth related to resuspension of bottom sediments. Nature 247, 574–577. doi: 10.1038/247574a0
Dodge R. E., Brass G. W. (1984). Skeletal extension, density and calcification of the reef coral, Montastraea annularis: St. crois, US virgin islands. Bull. Mar. Sci. 34, 288–307.
Dodge R. E., Lang J. C. (1983). Environmental correlates of hermatypic coral (Montastrea annularis) growth on the East flower gardens bank, northwest gulf of Mexico. Limnol. Oceanogr. 28, 228–240. doi: 10.4319/lo.1983.28.2.0228
Dodge R. E., Thomson J. (1974). The natural radiochemical and growth records in contemporary hermatypic corals from the Atlantic and Caribbean. Earth Planet. Sc. Lett. 23, 313–322. doi: 10.1016/0012-821X(74)90121-6
Foster A. B. (1979). Phenotypic plasticity in the reef corals Montastraea annularis (Ellis & Solander) and Siderastrea siderea (Ellis & Solander). J. E. M. Biol. Ecol. 39, 25–54.
Geister J. (1977). Occurrence of Pocillopora in late pleistocene Caribbean coral reefs. Mémoires du Bureau Recherches Géologiques Minieres 89, 378–388.
Gischler E., Hudson J. H., Storz D. (2009). Growth of pleistocene massive corals in south Florida: low skeletal extension-rates and possible ENSO, decadal, and multi-decadal cyclicities. Coral Reefs 28, 823–830. doi: 10.1007/s00338-009-0537-1
Greenstein B. J., Pandolfi J. M. (2008). Escaping the heat: range shifts of reef coral taxa in coastal Western Australia. Global Change Biol. 14, 513–528. doi: 10.1111/j.1365-2486.2007.01506.x
Groves S. H., Holstein D. M., Enochs I. C., Kolodzeij G., Manzello D. P., Brandt M. E., et al. (2018). Growth rates of porites astreoides and orbicella franksi in mesophotic habitats surrounding st. Thomas, US virgin islands. Coral Reefs 37, 345–354. doi: 10.1007/s00338-018-1660-7
Helmle K. P., Dodge R. E., Swart P. K., Gledhill D. K., Eakin C. M. (2011). Growth rates of Florida corals from 1937 to 1996 and their response to climate change. Nat. Commun. 2, 215. doi: 10.1038/ncomms1222
Helmle K. P., Kohler K. E., Dodge R. E. (2002) The coral X-radiograph densitometry system: CoralCDS., (2002) (Nova Southeastern University). Available at: http://www.nova.edu/ocean/coralxds/index.html (Accessed 2nd December 2014).
Highsmith R. C. (1979). Coral growth rates and environmental control of density banding. J. Exp. Mar. Biol. Ecol. 37, 105–125. doi: 10.1016/0022-0981(79)90089-3
Holcomb M., Pandolfi J. M., Macintyre I. G., Budd A. F. (2004). Use of X-radiographs to distinguish members of the Montastraea annularis reef-coral species complex. Hydrobiologia 530, 211–222. doi: 10.1007/s10750-004-2652-x
Hubbard D. K., Scaturo D. (1985). Growth rates of seven species of scleractinean corals from cane bay and salt river, st. Croix, USVI. Bull. Mar. Sci. 36, 325–338.
Hughes T. P., Ayre D., Connell J. H. (1992). The evolutionary ecology of corals. Trends Ecol. Evol. 7, 292–295. doi: 10.1016/0169-5347(92)90225-Z
Hughes T. P., Connell J. H. (1987). Population dynamics based on size or age? a reef-coral analysis. Am. Nat. 129, 818–829. doi: 10.1086/284677
Huston M. (1985). Variation in coral growth rates with depth at discovery bay, Jamaica. Coral Reefs 4, 19–25. doi: 10.1007/BF00302200
Johnson K. G., Pérez M. E. (2006). Skeletal extension rates of Cenozoic Caribbean reef corals. Palaios 21, 262–271. doi: 10.2110/palo.2004.p04-52
Jordán-Dahlgren E. (1997). “A Caribbean coral reef community of the pleistocene,” in Proc. 8th int. coral reef symp, vol. 2 . Eds. Lessios H. A., Macintyre I. G. (Balboa, Panama: Smithsonian Tropical Research Institute), 1681–1686.
Kiessling W., Simpson C., Beck B., Mewis H., Pandolfi J. M. (2012). Equatorial decline of reef corals during the last pleistocene interglacial. P. Natl. Acad. Sci. 109, 21378–21383. doi: 10.1073/pnas.1214037110
Knutson D. W., Buddemeier R., Smith S. V. (1972). Coral chronometers: Seasonal growth band in reef corals. Science 177, 270–272. doi: 10.1126/science.177.4045.270
Lapid E. D., Chadwick N. E. (2006). Long-term effects of competition on coral growth and sweeper tentacle development. Mar. Ecol. Prog. Ser. 313, 115–123. doi: 10.3354/meps313115
Lirman D. (2001). Competition between macroalgae and corals: effects of herbivore exclusion and increased algal biomass on coral survivorship and growth. Coral reefs 19, 392–399. doi: 10.1007/s003380000125
Maguire L. A., Porter J. W. (1977). A spatial model of growth and competition strategies in coral communities. Ecol. Model. 3, 249–271. doi: 10.1016/0304-3800(77)90007-2
McCarthy M. A. (2008). Bayesian Methods for ecology (Cambridge University Press), 295. doi: 10.1017/CBO9780511802454
Mendes J. M., Woodley J. D. (2002). Effect of the 1995-1996 bleaching event on polyp tissue depth, growth, reproduction and skeletal band formation in montastraea annularis. Mar. Ecol. Prog. Ser. 235, 93–102. doi: 10.3354/meps235093
Nakagawa S., Cuthill I. C. (2007). Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605. doi: 10.1111/j.1469-185X.2007.00027.x
O’Lear M. J., Hearty P. J., McCulloch M. T. (2008). U-Series evidence for widespread reef development in shark bay during the last interglacial. Palaeogeogr. Palaeoclimatol. Palaeoecol. 259, 424–435. doi: 10.1016/j.palaeo.2007.10.022
Osinga R., Schutter M., Griffioen B., Wijffels R. H., Verreth J. A., Shafir S., et al. (2011). The biology and economics of coral growth. Mar. Biotechnol. 13, 658–671. doi: 10.1007/s10126-011-9382-7
Otto-Bliesner B. L., Marshall S. J., Overpeck J. T., Miller G. H., Hu A. (2006). Simulating Arctic climate warmth and icefield retreat in the last interglaciation. Science 311, 1751–1753. doi: 10.1126/science.1120808
Pandolfi J. M. (2007). A new, extinct pleistocene reef coral from the Montastraea “annularis”. species complex J. Paleont. 8, 472–482. doi: 10.1666/pleo05046.1
Pandolfi J. M., Jackson J. B. (2001). Community structure of pleistocene coral reefs of curaçao, Netherlands antilles. Ecol. Monog. 71, 49–67. doi: 10.1890/0012-9615(2001)071[0049:CSOPCR]2.0.CO;2
Pandolfi J. M., Lovelock C. E., Budd A. F. (2002). Character release following extinction in a Caribbean reef coral species complex. Evolution 56, 479–501. doi: 10.1111/j.0014-3820.2002.tb01360.x
Pinheiro J., Bates D., R Core Team (2022) Nlme: Linear and nonlinear mixed effects models. r package version 3. Available at: https://CRAN.R-project.org/package=nlme.
Potter E. K., Lambeck K. (2004). Reconciliation of sea-level observations in the Western north Atlantic during the last glacial cycle. Earth Planet. Sc. Lett. 217, 171–181. doi: 10.1016/S0012-821X(03)00587-9
Quan-Young L. I., Espinoza-Avalos J. (2006). Reduction of zooxanthellae density, chlorophyll a concentration, and tissue thickness of the coral Montastraea faveolata (Scleractinia) when competing with mixed turf algae. Limnol. Oceanogr. 51, 1159–1166. doi: 10.4319/lo.2006.51.2.1159
R Core Team. (2022). A language and environment for statistical computing. (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Richardson C. A., Dustan P., Lang J. C. (1979). Maintenance of living space by sweeper tentacles of Montastrea cavernosa, a Caribbean reef coral. Mar. Biol. 55, 181–186. doi: 10.1007/BF00396816
Rico-Esenaro S. D., Sanchez-Cabeza J. A., Carricart-Ganivet J. P., Montagna P., Ruiz-Fernández A. C. (2019). Uncertainty and variability of extension rate, density and calcification rate of a hermatypic coral (Orbicella faveolata). Sci. Total Environ. 650, 1576–1581. doi: 10.1016/j.scitotenv.2018.08.397
Rinkevich B. (1996). Do reproduction and regeneration in damaged corals compete for energy allocation? Mar. Ecol. Prog. Ser. 143, 297–302. doi: 10.3354/meps143297
Rodríguez-Martínez R. E., Jordan-Garza A. G., Maldonado M. A., Blanchon P. (2011). Controls on coral-ground development along the northern mesoamerican reef tract. PloS One 6, 28461. doi: 10.1371/journal.pone.0028461
Sebens K. P. (1982). Competition for space: growth rate, reproductive output, and escape in size. Am. Nat. 120, 189–197. doi: 10.1086/283982
Steinich B., Velázquez-Olimán G., Marín L. E., Perry E. (1996). Determination of the ground water divide in the karst aquifer of yucatán, méxico, combining geochemical and hydrogeological data. Geofísica Internacional 35, 153–159. doi: 10.22201/igeof.00167169p.1996.35.2.857
Stirling C. H., Esat T. M., Lambeck K., McCulloch M. T. (1998). Timing and duration of the last interglacial: evidence for a restricted interval of widespread coral reef growth. Earth Planet. Sc. Lett. 160, 745–762. doi: 10.1016/S0012-821X(98)00125-3
Tanner J. E. (1997). Interspecific competition reduces fitness in scleractinian corals. J. Exp. Mar. Biol. Ecol. 214, 19–34. doi: 10.1016/S0022-0981(97)00024-5
Tomascik T. (1990). Growth rates of two morphotypes of Montastraea annularis along a eutrophication gradient, Barbados, WI. Mar. pollut. Bull. 21, 376–381. doi: 10.1016/0025-326X(90)90645-O
Torres J. L. (2001). Impacts of sedimentation on the growth rates of Montastraea annularis in southwest Puerto Rico. Bull. Mar. Sci. 69, 631–637.
Van Veghel M. L., Bosscher H. (1993). Variation in linear growth and skeletal density within the polymorphic reef building coral Montastraea annularis. bull. Mar. Sci. 56, 902–908.
van Woesik R. (2002). Processes regulating coral communities. Comments Theor. Biol. 7, 201–214. doi: 10.1080/08948550214054
Ward W. C., Weidie A. E., Back W. (1985). Geology and hydrogeology of the Yucatan and quaternary geology of northeastern Yucatan peninsula (New Orleans Geological Society), 160. Available at : https://searchworks.stanford.edu/view/1606188
Weil E., Cróquer A., Urreiztieta I. (2009). Yellow band disease compromises the reproductive output of the Caribbean reef-building coral Montastraea faveolata (Anthozoa, scleractinia). Dis. Aquat. organisms 87, 45–55. doi: 10.3354/dao02103
Keywords: Pleistocene, coral reef, fossil corals, Orbicella nancyi, coral extension rates
Citation: Jordán-Garza AG, Blanchon P, Carricart-Ganivet JP and Jordán-Dahlgren E (2023) Lower skeletal extension in Pleistocene Orbicella (Montastraea) corals than in their modern counterparts. Front. Mar. Sci. 10:1098430. doi: 10.3389/fmars.2023.1098430
Received: 14 November 2022; Accepted: 07 April 2023;
Published: 25 April 2023.
Edited by:
Stefano Goffredo, University of Bologna, ItalyReviewed by:
Antonietta Rosso, University of Catania, ItalyGuillermo Horta-Puga, Universidad Nacional Autónoma de México, Mexico
Clark Sherman, University of Puerto Rico at Mayagüez, Puerto Rico
Copyright © 2023 Jordán-Garza, Blanchon, Carricart-Ganivet and Jordán-Dahlgren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adán Guillermo Jordán-Garza, ajordan@uv.mx
 Adán Guillermo Jordán-Garza
Adán Guillermo Jordán-Garza Paul Blanchon
Paul Blanchon Juan P. Carricart-Ganivet
Juan P. Carricart-Ganivet Eric Jordán-Dahlgren
Eric Jordán-Dahlgren