Temperature-Dependent Reproductive Success of Stickleback Lateral Plate Morphs: Implications for Population Polymorphism and Range Shifts Under Ocean Warming
- 1Coastal Ecology Section, Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung, Wadden Sea Station Sylt, List, Germany
- 2Department of Evolutionary Biology, University of Vienna, Vienna, Austria
- 3Messerli Research Institute, University of Veterinary Medicine Vienna, Medical University of Vienna, Vienna, Austria
- 4Division of Behavioural Ecology, Institute of Ecology and Evolution, University of Bern, Bern, Switzerland
- 5Natural History Museum in Vienna, First Zoological Department, Vienna, Austria
Changing environments associated with rapid climate change can shape direct measures of fitness such as reproductive success by altering mating behavior, fecundity and offspring development. Using a polymorphic oceanic population of threespine stickleback (Gasterosteus aculeatus), we investigated whether a 4°C increase in sea surface temperature influenced clutch siring success, reproductive output, and offspring growth among lateral plate morphs. Since low plated morphs are thought to have a selective advantage in warmer environments, we predicted that low plated males should have higher clutch siring success in +4°C environments, and that thermal plasticity of traits (e.g., egg size, offspring growth) should reflect different trait optima in different environments among plate morphs. Parentage analysis of egg clutches revealed temperature-specific clutch siring success, in that low plated males sired more clutches in +4°C environments and completely plated males sired more clutches at ambient (seasonal) temperature. Both completely and low plated females laid larger eggs when acclimated to +4°C, but only completely plated females had smaller clutches at +4°C. Offspring of low and partially plated females grew much less at +4°C compared to those of completely plated females. Taken together, our results demonstrate that ocean warming could impact reproductive success at various levels, with differential effects depending on phenotype, in this case, lateral plate morph. Some traits (clutch siring success, egg size) showed better performance for low plated fish at +4°C, whereas others (e.g., growth) did not. Higher clutch siring success of low plated males at elevated temperature might indicate a future shift in plate morph composition for polymorphic stickleback populations, with potential implications for colonization ability during range shifts under climate change.
Introduction
Rapid warming of the world’s oceans is a major threat to population persistence and biodiversity of marine ecosystems (Intergovernmental Panel on Climate Change [IPCC], 2014). Organisms can respond to fast changing environmental conditions either by moving to where conditions better match their thermal optima, or by remaining in place and coping via genetic adaptation and/or phenotypic plasticity (Gienapp et al., 2007). Thermal plasticity of fitness-related traits has been documented in numerous marine species, showing that different phenotypes will be adaptive in different environments (Munday et al., 2013). Importantly, changing environmental conditions may also influence reproductive success – a direct measure of fitness – and can affect different phenotypes at several levels: (1) changing environments can alter mating preferences (Candolin, 2019), which may lead to premating isolation, and thus, reduce the frequency of certain phenotypes in the population, (2) change the fecundity of particular phenotypes (Barneche et al., 2018), with some having higher reproductive output in changed conditions than others, and (3) have developmental effects on offspring (Monaghan, 2008), such that some phenotypes produce offspring that outperform others. Some of the general effects of climate warming on reproductive success include changes to mating behavior (Pilakouta and Alund, 2021), smaller egg sizes (Barneche et al., 2018), and smaller body sizes (Daufresne et al., 2009). Also, spawning adults and early developmental stages (e.g., embryos) were recently identified as the most vulnerable life stages of teleost fishes under climate change (Dahlke et al., 2020). Hence, ocean warming can influence how both natural selection and sexual selection shape phenotypes (Safran et al., 2013), with changes to reproductive success likely influenced by a combination of all of these factors.
The threespine stickleback (Gasterosteus aculeatus; hereafter referred to as stickleback), is a small teleost fish that is well known for its complex biology, with morphologically divergent populations distributed throughout the Northern hemisphere (Bell and Foster, 1994; Paepke, 2002). Nevertheless, most of this complexity is known only from freshwater populations (e.g., Wootton, 2009), which are derived from ancestral oceanic (anadromous and strictly marine) populations (Walker and Bell, 2000; Reusch et al., 2001; Raeymaekers et al., 2005; McGuigan et al., 2010; Spoljaric and Reimchen, 2011; Wund et al., 2012). Stickleback are polymorphic for the pattern of their lateral plates (bony armor plates lining the body) which aid against predation (Reimchen, 1983, 2000; Bergstrom, 2002; Barrett, 2010), but also influence swimming performance (Tudorache et al., 2007; Bjærke et al., 2010; Dalziel et al., 2011). Lateral plate morph is genetically determined (primarily by the Eda gene; Colosimo et al., 2005; Jones et al., 2006; Barrett, 2010), heritable (e.g., Hagen, 1973; Hermida et al., 2002; Loehr et al., 2012; Hansson et al., 2016; Østbye et al., 2018), and under strong selection from biotic and abiotic factors (Hagen and Moodie, 1982; Baumgartner and Bell, 1984; Bergstrom, 2002; Marchinko and Schluter, 2007; Barrett, 2010; Smith et al., 2014). Oceanic stickleback populations are predominantly comprised of the completely plated morph (plates extend from the head to the caudal fin) that is capable of migrating long distances (Wootton, 1984; Bell and Foster, 1994), whereas freshwater populations consist mostly of low plated fish (plates only on the anterior part of the trunk) with superior maneuverability. A partially plated morph expresses an intermediate but variable number of plates (plates absent in the middle of the body), and often occurs in brackish water (Wootton, 1976). Contrary to most oceanic populations, those of the North Sea (e.g., German Bight) are characterized by lateral plate polymorphism, comprised of mostly completely plated morphs (as well as some partially plated), but with a small proportion of low plated morphs present (Münzing, 1963; Wootton, 2009). Plate morph polymorphism in these coastal populations is thought to be maintained by a balance between divergent selection and gene flow (Raeymaekers et al., 2014).
The geographic distribution of plate morphs is also correlated with a number of habitat characteristics, one of which is temperature (Münzing, 1963; Wootton, 1976; Hagen and Moodie, 1982; Baumgartner and Bell, 1984; Reimchen, 2000; Des Roches et al., 2020; Smith et al., 2020). In freshwater populations, those with completely plated morphs predominantly occur in regions with cold winters and high annual temperature fluctuations, whereas the low plated morph is more common in regions with relatively warm winters and low annual temperature fluctuations (Hagen and Moodie, 1982; Paepke, 2002; Smith et al., 2020, 2021). Less is known for oceanic populations, but a temperature-dependent plate morph gradient also occurs in some marine and estuarine environments (Münzing, 1963; Baumgartner and Bell, 1984; Paepke, 2002; Des Roches et al., 2020). For instance, the frequency of low plated fish is generally higher at lower latitudes along Californian (Baumgartner and Bell, 1984; Des Roches et al., 2020) and European coasts (Münzing, 1963; Smith et al., 2020), but is shifting northward with climate change due to an increase in lentic estuarine habitats associated with climate warming and decreased precipitation (Des Roches et al., 2020), and/or correlated effects of smaller body size (Smith et al., 2020).
Studies investigating stickleback mating patterns suggest that mate choice is often strongly based on body size (Dieckmann and Doebeli, 1999; McKinnon and Rundle, 2002; Conte and Schluter, 2013; Berner et al., 2016), but also trophic traits, antipredator traits and male reproductive characters can contribute to non-random mating (Blouw and Hagen, 1990; Ziuganov, 1995; Nagel and Schluter, 1998; McKinnon and Rundle, 2002; Scott, 2004; Snowberg and Bolnick, 2008; Jenck et al., 2020). However, little is known about how changing environmental conditions may affect mating preferences for certain phenotypic traits. For instance, both increased water turbidity (Candolin et al., 2007; Wong et al., 2007) and temperature (Fuxjäger et al., 2019) have been shown to alter strong visual mate choice cues like body size. In turbid water, olfactory cues played a more important role when vision was impaired (Heuschele et al., 2009), whereas at higher water temperature, smaller males had increased reproductive success (regardless of the female’s body size) under the hypothesis that smaller males spent less energy on metabolism at higher temperature, and therefore, had more energy available for courtship and mating (Fuxjäger et al., 2019). Such environment-induced changes in mating success suggest that female preference may be influenced by environment-dependent male condition (see also Heuschele et al., 2009; Robinson et al., 2012), which will differ for specific trait-environment combinations. However, almost nothing is known about mating patterns among stickleback lateral plate morphs in general (but see Ziuganov, 1995), and whether mating success among plate morph phenotypes differs with environmental conditions has not yet been investigated.
Earlier studies that investigated fecundity and offspring growth differences among stickleback lateral plate morphs suggest morph-specific trait performance. Differences in reproductive output traits among plate morphs were investigated at the population level, and compared, for example, oceanic populations (completely plated) with freshwater populations (low plated fish; Baker, 1994; Baker et al., 1998). In a survey of 43 freshwater and oceanic stickleback populations from Haida Gwaii (Canada), Oravec and Reimchen (2013) found that these populations had larger eggs and smaller clutches than those in other geographical areas, which they attributed to low environmental temperature. They also found a negative association between egg size and (population mean) number of lateral plates, however, no data on egg sizes or clutch sizes for each lateral plate morph were reported (Oravec and Reimchen, 2013). For offspring growth, a handful of studies have compared growth among stickleback plate morphs. In one study, low plated fish had higher growth rates as juveniles than completely plated fish, but completely plated fish reached a similar size by the time of sexual maturity (Bell et al., 2010). In another study, partially and low plated offspring grew better than completely plated fish in freshwater, whereas all morphs grew similarly in salt water (Marchinko and Schluter, 2007). Nevertheless, little is known about reproductive output and offspring growth differences among lateral plate morphs within polymorphic oceanic populations, and even less with regard to thermal plasticity of these traits.
Due to fast changing climate conditions, oceanic stickleback populations are facing strong environmental pressure (Ramler et al., 2014; Shama and Wegner, 2014; Shama, 2015; Fuxjäger et al., 2019). Since plate morph distributions may be influenced by environmental temperature (Hagen and Moodie, 1982; Baumgartner and Bell, 1984; Reimchen, 2000), climate warming may affect the geographic distribution of polymorphic populations in the future. Importantly, if low plated stickleback cope seemingly better with warmer temperatures than completely plated fish (Münzing, 1963; Smith et al., 2021), adaptive benefits of mating with low plated morphs could arise, and might help to mediate some of the negative consequences of climate warming. In this study, we used large, outdoor mesocosms simulating ambient and ocean warming conditions to investigate the role of changing environments on reproductive success (clutch siring success, reproductive output and offspring growth) among stickleback lateral plate morphs. Specifically, we used a polymorphic oceanic population to test (1) if clutch siring success differs among lateral plate morphs, and also varies with environmental temperature, (2) if thermal plasticity for fecundity traits differs among plate morphs, and (3) if parental plate morph influences offspring growth trajectories under ocean warming.
Materials and Methods
Clutch Siring Success Experiment
Wild adult stickleback were caught by trawling from an oceanic population in the Sylt-Rømø Bight, Germany (55°05′ N, 8°39′ E) in February 2016. Adult fish (n = 215) were randomly divided between two large outdoor mesocosms (each 1800 l) set to two temperatures (ambient and +4°C), and were acclimated for 60 days during their reproductive conditioning phase. Mesocosms had permanent flow-through of seawater, and were heated using large heating elements connected to temperature sensors. Temperature was adjusted daily to match seasonal conditions in the Sylt-Rømø Bight, and was recorded hourly using HOBO Pendant ® Temperature/Light Data Loggers (Onset Computer Co., Bourne, MA, United States). Fish were fed daily with chironomid larvae ad libitum. After 2 months of temperature acclimation, all fish were caught and lateral plate morph determined by visual inspection. For low plated fish (L), lateral plates appear only on the anterior part of the trunk. The completely plated morph (C) is defined by a row of lateral plates from the head to the base of the caudal fin, and the partially plated morph (P) has a gap of at least two plates between the anterior plates and those on the caudal peduncle (Wootton, 2009). Fish were sorted by sex and plate morph, and transferred to 25 l aquaria (n = 20 fish per aquaria) in the laboratory set to the acclimation temperature.
At the start of the clutch siring success experiment, standard length (±1 mm), weight, sex and plate morph were determined for 96 fish. The first dorsal spine of these fish was clipped and stored at –20°C for later genotyping (see below). Fish were then assigned to one of eight large outdoor mesocosms (see Pansch et al., 2016 for mesocosm technical details) set to either ambient or +4°C in a full-factorial design. Specifically, each mesocosm consisted of six males (three from ambient acclimation temperature and three from +4°C, and with one male of each lateral plate morph per acclimation temperature), and six females following the same treatment combinations as males (Figure 1). Fish were size-matched within each mesocosm. On average, females were size-matched to ±5.63 mm and males to ±3.63 mm. The average size difference between males and females in each mesocosm was ±5.49 mm. Each mesocosm contained a mating arena comprised of a 97 × 97 cm frame enclosed with 5 mm mesh which was fixed to a platform positioned at 50 cm water depth. To allow each male to establish a possible territory and display courtship behaviors, six plastic trays were set within each mating arena to serve as nesting sites. Plastic trays (25 × 14 × 6 cm) contained a 3 cm layer of sand (1.25 kg) and 1.5 g nesting material (Wenco Nm 30/3 black sewing thread cut into 5-7 cm lengths conditioned for 2 days in seawater). Fish in the mesocosms were fed daily with chironomid larvae ad libitum for the duration of the experiment.
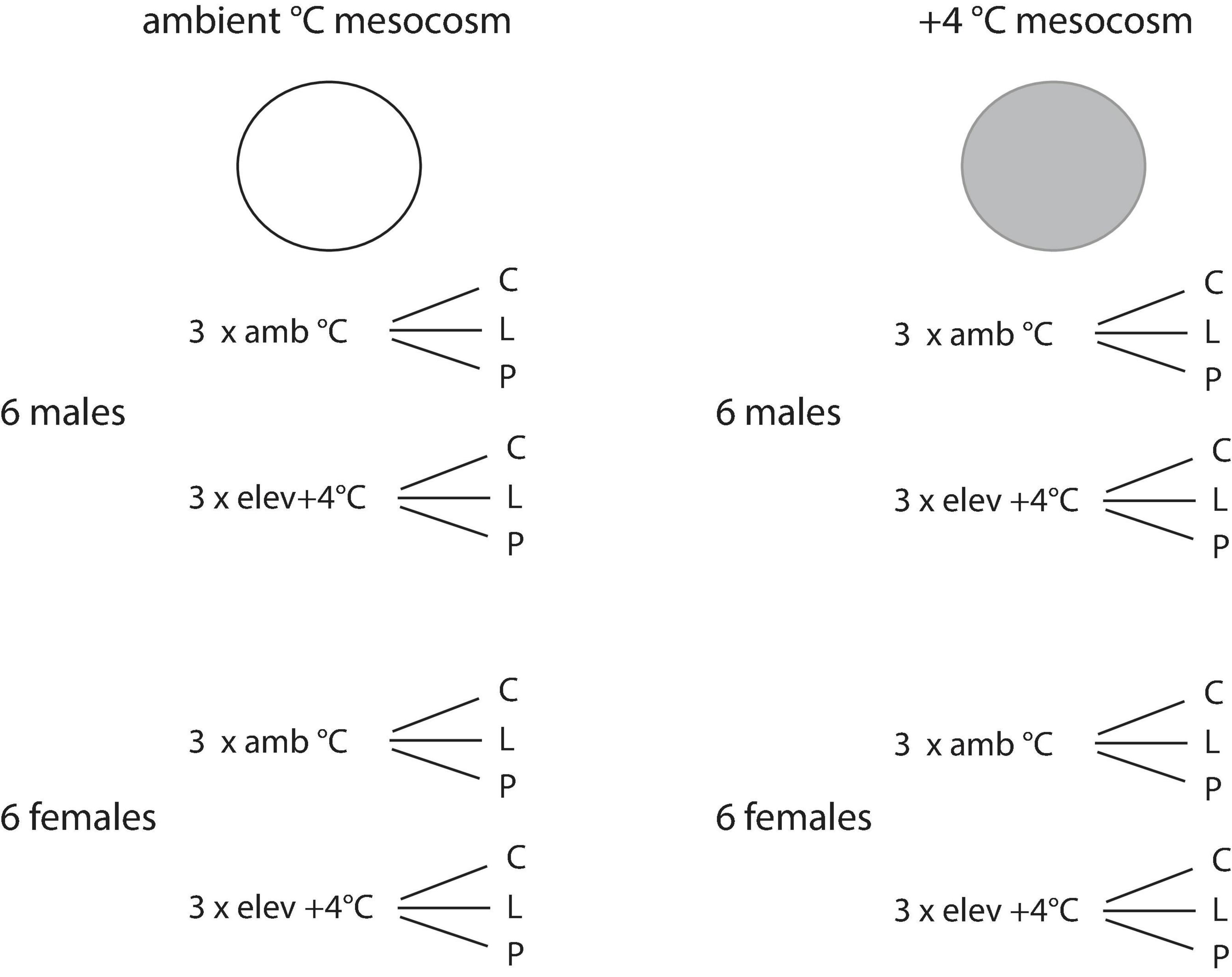
Figure 1. Experimental design schematic showing the combination of six males and six females from different temperature acclimation treatments (ambient, +4°C climate change scenario) and different plate morphs (c, complete; l, low plated; p, partial) in mesocosms set to either ambient or elevated (+4°C) temperature.
The experiment began on 28 April 2016 using water temperatures of 10°C (ambient) and 14°C (+4°C) and ended on 17 May 2016 with water temperatures of 16 and 20°C. Water temperature in the mesocosms was logged every 30 min using installed multi-sensor probes (Hydrolab DS5X Probe, OTT Messtechnik GmbH, Kempten, Germany). A nest report was taken every day to document the status and location of each nest in the experiment. Trays with active nests were removed every fourth day to carefully detach egg clutches (performed under a dissecting microscope to minimize disturbance of the nest). Removed trays were returned to the same position in the mating arena, and were supplemented with 0.2 g replacement nesting material. The experiment was stopped after at least seven clutches from each mesocosm had been collected (on average 9.75 clutches were collected from each mesocosm). At the end of the experiment, adult fish were recaptured, and weight (±1 mg), standard length (±1 mm), sex and plate morph was determined for each individual. Fish were then euthanized in an overdose of MS222 and stored at –20°C for later genetic analyses.
Fecundity Traits
All egg clutches were photographed under a dissecting microscope for later determination of egg size and clutch size using imaging software (LEICA QWIN, Leica Microsystems Imaging Solutions Ltd., Cambridge, United Kingdom). The diameter of 10 randomly chosen eggs per clutch was used to calculate the mean egg size of each clutch. Clutch size was determined by counting the number of eggs in each clutch. Egg clutches were transferred into 1 l glass beakers containing an air supply and filtered sea water set to the corresponding mesocosm temperature (ambient or +4°C). Water was changed in the beakers every third day until larvae began to hatch. Hatching success was estimated as the proportion of live larvae in relation to total larvae (live and dead) plus dead eggs/embryos after 2 days of hatching (i.e., unfertilized eggs were not included). Three days after hatching, 24 larvae from each clutch were randomly selected, euthanized in MS222 and frozen at –20°C for later determination of larval genotype.
Growth Experiment
For the growth experiment, ten randomly chosen larvae per clutch (n = 78 clutches) were photographed under a dissecting microscope at 2 days post-hatch for determination of larval size, and were further reared in 1 l beakers until 30 days post-hatch. At this point, the 10 larvae per clutch were transferred to a 2 l aquarium connected to a flow-through seawater system set at either ambient temperature or +4°C. All juvenile fish were photographed at 30, 60, and 90 days post-hatch under a dissecting microscope for analyses of offspring size (standard length ± 0.1 mm) using imaging software (LEICA QWIN V3.5.0; Leica Microsystems Imaging Solutions Ltd., Cambridge, United Kingdom). Throughout the growth experiment, juvenile fish were fed daily with live Artemia sp. nauplii ad libitum.
Genotyping and Parentage Analysis
All 96 adult fish and 16 larvae from each of 72 randomly chosen clutches (n = 1152 larvae in total) were genotyped at five microsatellite loci (see Kalbe et al., 2009 for loci details). DNA was extracted from spine clips for adults and whole 3 days old larvae using DNeasy Blood and Tissue Kits (Qiagen, Hilden, Germany) following the manufacturer’s protocol. The 20 μl multiplex PCR reactions consisted of 10 μl Multiplex Mastermix (Qiagen, Hilden, Germany), 8 μl primer mix (forward and reverse primer of 5196 HEX, 4170 6_FAM, 1125 6_FAM, 1097 NED and 7033 NED) and 2 μl DNA. Thermal cycling for PCR consisted of: 95°C for 5 min, 30 cycles of 94°C for 1 min, 58°C for 1 min and 72°C for 1 min, and 72°C for 10 min (as in Schade et al., 2014). Amplified fragments were diluted with water (1:20), and 1 μl of diluted PCR product was denatured in 15 μl Hi-Di Formamide containing an internal size standard (ROX500; Applied Biosystems, Foster City, CA, United States). Fragments were analyzed on an ABI 3130xl sequencer. Electropherograms were manually inspected using PeakScanner 1.0 (Applied Biosystems), and Tandem 1.01 (Matschiner and Salzburger, 2009) was used to bin final allele sizes.
Parentage analysis was performed using the program COLONY 2.0 (Wang and Santure, 2009), a likelihood-based method that uses a group-wise approach to infer genealogies from multilocus genotype data (cf. Ursprung et al., 2011; Rausch et al., 2014). The full likelihood model was used with medium precision allowing for polygamous mating in both sexes. Each mesocosm was analyzed separately with males and females (six potential fathers and mothers each) representing the potential fathers and mothers for the respective clutches collected in that mesocosm (see also Fuxjäger et al., 2019). Only ‘Best (ML) Configuration’ assignments with the maximum likelihood obtained at the end of the computation in which COLONY found both parents (i.e., no simulated genotypes) were used for subsequent analyses. The COLONY analysis implements a full-pedigree likelihood approach which considers the likelihood of the entire pedigree structure and allows the simultaneous inference of parentage and sibship. However, it does not provide explicit p values for each of the pair assignments. To obtain high confidence in the parentage assignments, we investigated the discriminative power of the microsatellite data by conducting allele frequency analyses in Cervus (Kalinowski et al., 2007). We calculated the number of alleles per locus, polymorphic information content (PIC), as well as combined non-exclusion probabilities for parent pairs and combined non-exclusion probabilities for individual identity for each mesocosm separately using only parental genotypes.
Statistical Analysis
Clutch siring success, fecundity traits (mean egg size, clutch size) and offspring growth were analyzed as general linear mixed effect models using the MCMCglmm package in R (Hadfield, 2010). For all models, we ran Markov chains of 106 iterations, removed a burn-in of 105 iterations, and then kept every 1000th estimate after thinning. We fitted proper but uninformative priors covering half the variance of the trait for each random and fixed effect (i.e., V = 0.5, nu = 0.002) when fitting Gaussian or Poisson response variables, but fixed the variance to 1 when fitting Binomial response variables. We checked the resulting Markov chains for autocorrelation and stationarity, and only kept chains with an effective sampling size of >500 for each estimated parameter. Mesocosm was included as a random effect in all models, and family (clutch) was included as a random effect for the offspring growth models.
Clutch siring success was investigated by determining whether potential combinations of male and female plate morphs had any offspring (siring success) in the two reproductive environment (mesocosm) temperatures. The clutch siring success analysis included all males (nest owners, non-reproducing males and sneakers) and all possible male-female combinations within the mesocosms. The proportion of clutches sired was estimated as the number of realized siring events divided by the number of all potential siring events a given male could have achieved (see also Fuxjäger et al., 2019). We analyzed this as a binomial response variable using male and female size as covariates, male and female acclimation temperature, male and female plate morph and mesocosm temperature plus all of their interactions as fixed effects. Mean egg size was analyzed as a Gaussian response variable, and we fit female size, clutch size, female acclimation temperature, female plate morph and mesocosm temperature, plus the interactions between clutch size and female acclimation temperature and plate morph, and all two-way interactions between female acclimation temperature, plate morph and mesocosm temperature. Clutch size was modeled in the same way, but as a Poisson distributed response variable and using mean egg size as a covariate. Note: we did not fit the three-way interaction between female °C, female plate morph and mesocosm temperature for egg traits as there was only one clutch in one of the three-way combinations. Offspring growth over time (at 2, 30, 60, and 90 days post-hatch) was modeled as a Gaussian response variable as a function of male and female acclimation temperature, male and female plate morph, mesocosm temperature and all two-way interactions. The three-way interactions were not modeled due to three missing three-way combinations.
Results
Clutch Siring Success
We used parentage analysis to investigate whether clutch siring success differed among stickleback lateral plate morphs depending on environmental conditions, here, increased water temperature. For offspring assignments, all 96 parental fish were recovered at the end of the experiment, and their genotype could be reliably determined. During the course of the experiment (19 days), 78 clutches in total were collected from the eight mesocosms (39 clutches from ambient temperature and 39 clutches from +4°C mesocosms), and we randomly selected 72 clutches for genotyping. Of the 1152 larvae genotyped (16 larvae per clutch), 73 individuals (6.34%) could not be determined due to PCR failure. The microsatellite markers were highly informative, with a mean number of alleles per locus across mesocosms of 11.28 (SD = 0.48), an average PIC of 0.86 (SD = 0.01), an average combined non-exclusion probability per parent pair of 7.9*e–06, and a combined non-exclusion probability of identity of 9.0*e–07. Consequently, the power of our markers to reliably infer parentage within each mesocosm was very high.
Of the 72 clutches genotyped, 45 were single-male sirings by a nest-owner male (e.g., no extra-male fertilizations). The remaining 27 clutches were either sired by multiple males, contained eggs fertilized by sneaker males, and/or contained stolen eggs, resulting in 37.5% (27/72) of genotyped clutches reflecting some form of alternate reproductive strategy. The number of sneaking events (any siring success by a sneaker male) was similar in both mesocosm temperatures (16 events in ambient °C and 15 events in +4°C). The plate morph distribution of sneaking males was also similar between mesocosm temperatures, with the exception of fewer completely plated sneakers in +4°C mesocosms (4 complete/3 low/3 partially plated sneaker males in ambient mesocosms vs. 1 complete/3 low/4 partially plated sneaker males in +4°C mesocosms). Of the 48 males in the experiment (i.e., both mesocosm temperatures), 39 had some form of siring success, and the plate morph distribution of these males was 11 complete/13 low/15 partially plated. In total, 43 of the 48 females in the experiment managed to reproduce, with a plate morph distribution of 14 complete/15 low/14 partial.
Our GLMM analyses revealed that clutch siring success was significantly influenced by male size, female size, male acclimation temperature and the interaction between male plate morph and mesocosm (reproductive environment) temperature (Table 1). Overall, there was a positive relationship between body size (male and female) and clutch siring success. However, low plated nest-owner males were significantly smaller than both complete and partially plated nest-owner males (Figure 2), and had higher clutch siring success in +4°C mesocosms (interaction: low plate morph x + 4°C mesocosm; estimate = –0.137, p = 0.031, Supplementary Table 1). Sneaker males, on the other hand, were smaller than non-reproducing males (except partially plated males at +4°C), and low-plated sneaker males were smaller than the other plate morphs in both mesocosm temperatures (Figure 2). Overall, we found that low plated males had significantly higher clutch siring success in +4°C mesocosms when paired with females of all plate morphs, whereas completely plated males showed the opposite pattern and had higher siring success in ambient temperature mesocosms when mated with all female plate morph combinations (Figure 3). Partially plated males showed a mixed clutch siring success pattern, with higher success at +4°C when paired with completely plated females, lower success at +4°C when paired with partially plated females, and no difference in siring success between mesocosm temperatures when paired with low plated females (interaction: Meso x male plate P, Table 1 and Figure 3).
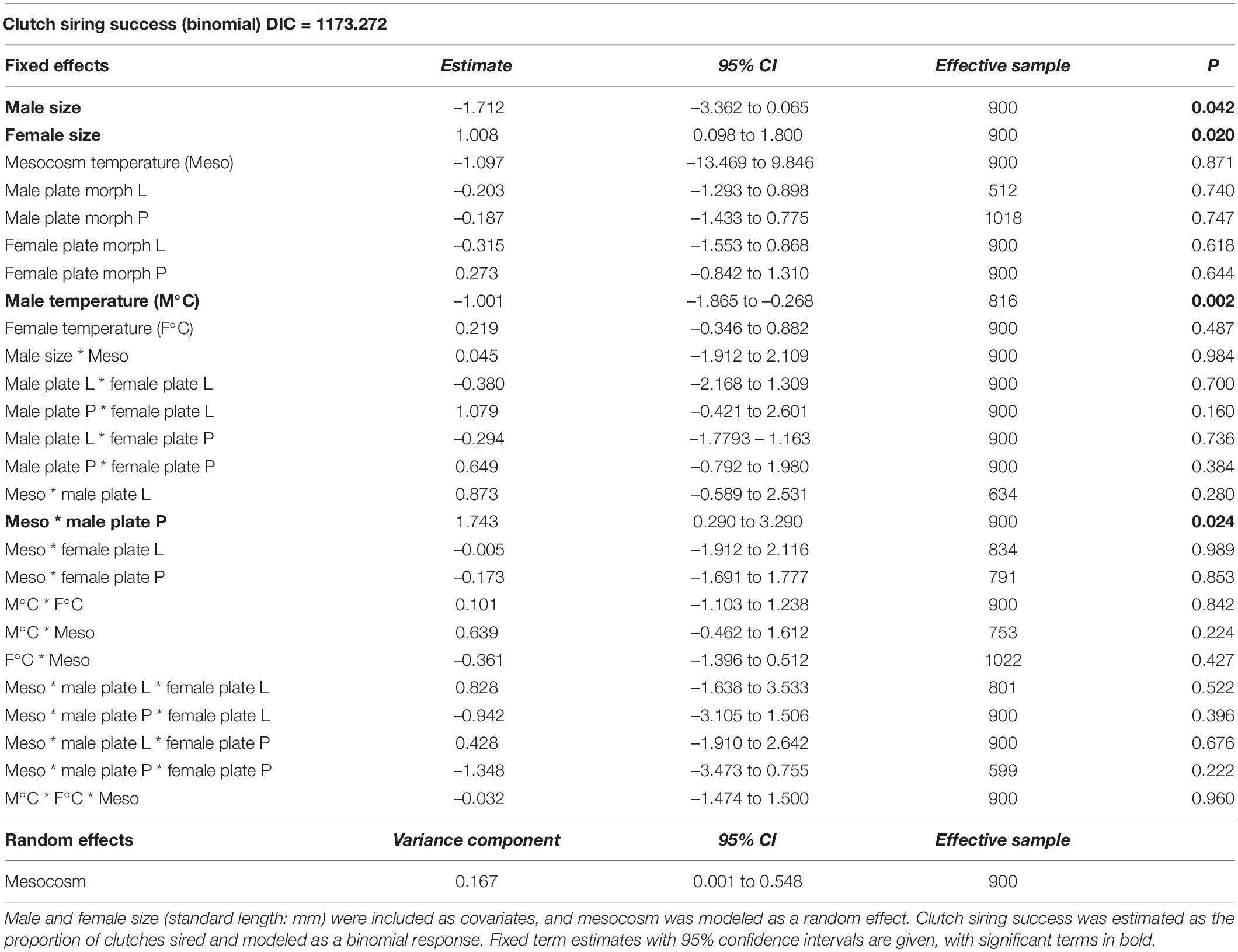
Table 1. Clutch siring success of stickleback (Gasterosteus aculeatus) depending on environmental (mesocosm) temperature, lateral plate morph (C, complete; L, low; P, partial), and acclimation temperature (°C) of males and females.

Figure 2. Size of male nest owners and sneakers (mm ± SE) relative to non-reproducing males for each plate morph (C, complete; L, low; P, partial) in ambient and +4°C mesocosms.

Figure 3. Clutch siring success (measured as the proportion of clutches sired) for each male plate morph in combination with female plate morphs (c, complete; l, low; p, partially plated) in ambient and elevated (+4°C) temperature mesocosms.
Egg Size, Clutch Size, and Hatching Success
To investigate if thermal plasticity of fecundity traits differed among lateral plate morphs, we estimated mean egg size, clutch size and hatching success of egg clutches collected from nests within the mesocosms, with both female acclimation temperature (during reproductive conditioning) and mesocosm temperature (during egg laying) set to either ambient or +4°C. Of the 72 clutches genotyped, 39 contained eggs with single-female parentage. Only these clutches were used in the analyses of mean egg size and clutch size, since multiple-female parentage clutches may have included females from different acclimation temperatures and of different plate morph. Mean egg size was significantly influenced by female size, female acclimation temperature (female °C), the interaction between clutch size and female °C, and the interaction between female °C and plate morph (Table 2). Overall, there was a positive relationship between female size and egg size, but there was no difference in female size among plate morphs (ANOVA F2,90 = 0.447; p = 0.641) or acclimation temperatures (ANOVA F1,92 = 0.838; p = 0.363) at the start of the experiment. Partially and low plated females laid slightly larger eggs (1.71 ± 0.06 mm and 1.70 ± 0.06 mm, respectively) than completely plated females (1.69 ± 0.07 mm). However, completely and low plated females produced larger eggs when acclimated at +4°C, whereas partially plated females produced larger eggs when acclimated at ambient temperature (Figure 4A).

Table 2. Mean egg size (A) and clutch size (B) of stickleback (Gasterosteus aculeatus) females of differing lateral plate morph (C, complete; L, low; P, partial) depending on acclimation temperature (F°C) and environmental (mesocosm) temperature.
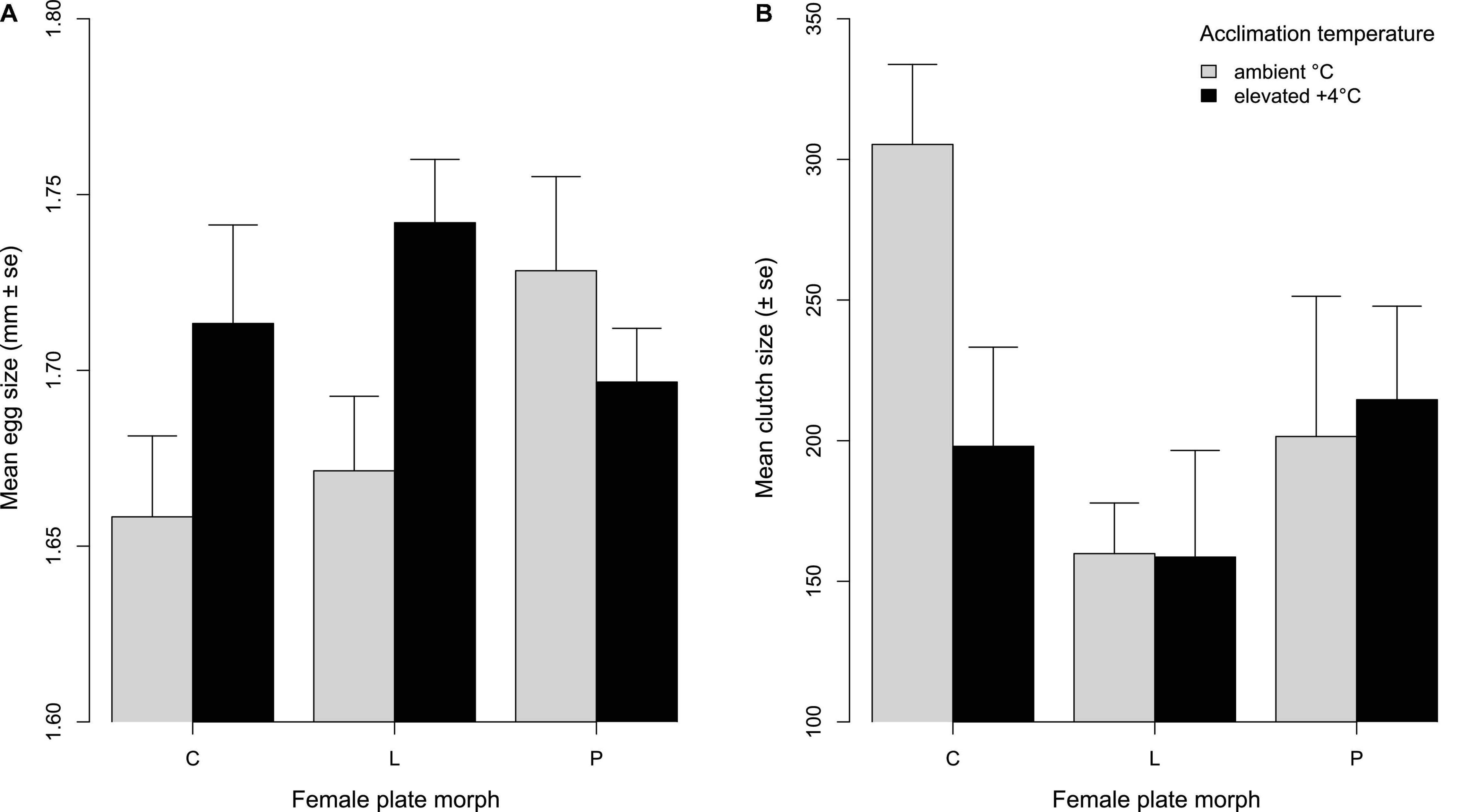
Figure 4. (A) Mean egg size (mm ± SE) of complete (C), low (L), and partially (P) plated females acclimated to ambient or elevated (+4°C) temperature. (B) Mean clutch size (±SE) depending on female plate morph and acclimation temperature (ambient and +4°C).
Clutch size was significantly influenced by female acclimation °C, the interaction between egg size and female °C, and the interaction between female °C and plate morph (Table 2). The number of eggs per clutch ranged from 39 to 397, and on average, completely plated females laid the largest clutches (251.67 ± 93.5), low plated females laid the smallest clutches (159.33 ± 62.0), and partially plated females were intermediate (209.33 ± 105.2). However, completely plated females laid smaller clutches when acclimated to +4°C, whereas low and partially plated females had similar sized clutches in both acclimation temperatures (Figure 4B). There was a tradeoff between egg size and clutch size (bigger eggs but smaller clutches), driven by females acclimated to +4°C (Supplementary Figure 1a), and the tradeoff was most apparent for low plated females, resulting from larger eggs laid at +4°C (Supplementary Figure 1b). Hatching success of the 78 clutches collected from all mesocosms was high (74.98% in ambient and 82.30% in +4°C mesocosms), and did not differ significantly between mesocosm temperatures (ANOVA F1,76 = 1.814; p = 0.227). For the 39 single-female parentage clutches, overall hatching success was also high (81.30%), and there were no significant effects of female °C (ANOVA F1,23 = 0.572; p = 0.457), female plate morph (F2,23 = 2.083; p = 0.147), or mesocosm temperature (F1,6 = 0.402; p = 0.550) on hatching success.
Offspring Growth
Since climate warming has been shown to influence fish body size (Daufresne et al., 2009), we investigated whether parental thermal history as well as lateral plate morph might modulate temperature-induced changes to offspring body size. For offspring growth (body size over time) analyses, only clutches with single-couple parentage (i.e., no extra-pair fertilizations) were used (n = 27) so that potential effects of paternal or maternal acclimation temperature and plate morph could be tested. Offspring growth was significantly influenced by parental acclimation temperature, parental plate morph combination, and interactions between parental plate morph and mesocosm temperature (Table 3). Mesocosm temperature (offspring growth environment) interacted with parental plate morph to influence offspring size at various time points (Figures 5A–D). At 30 days post-hatch, offspring of completely and low plated females were significantly smaller in +4°C mesocosms, whereas offspring of partially plated females were similarly sized in both mesocosm temperatures (Figure 5B). However, at 60 and 90 days, offspring of low and partially plated females were markedly smaller in +4°C mesocosms, whereas offspring of completely plated females were similarly sized in both mesocosm temperatures (Table 3 and Figures 5C,D). Parental plate morph combination also influenced offspring size (averaged over mesocosm temperatures due to three missing three-way interaction combinations). At 2 days post-hatch, effects of LxP (male × female plate morph) parents on offspring size were only marginally significant (p = 0.058; Table 3 and Supplementary Figure 2a), whereas at 30 and 90 days post-hatch, a number of parental plate morph combinations had significant effects on offspring size (Table 3). At 30 days, offspring with a parental plate morph combination of LxP and PxP were significantly larger than those from CxC (Supplementary Figure 2b), and at 90 days, offspring of PxP were still significantly larger than those from CxC (Supplementary Figure 2d).
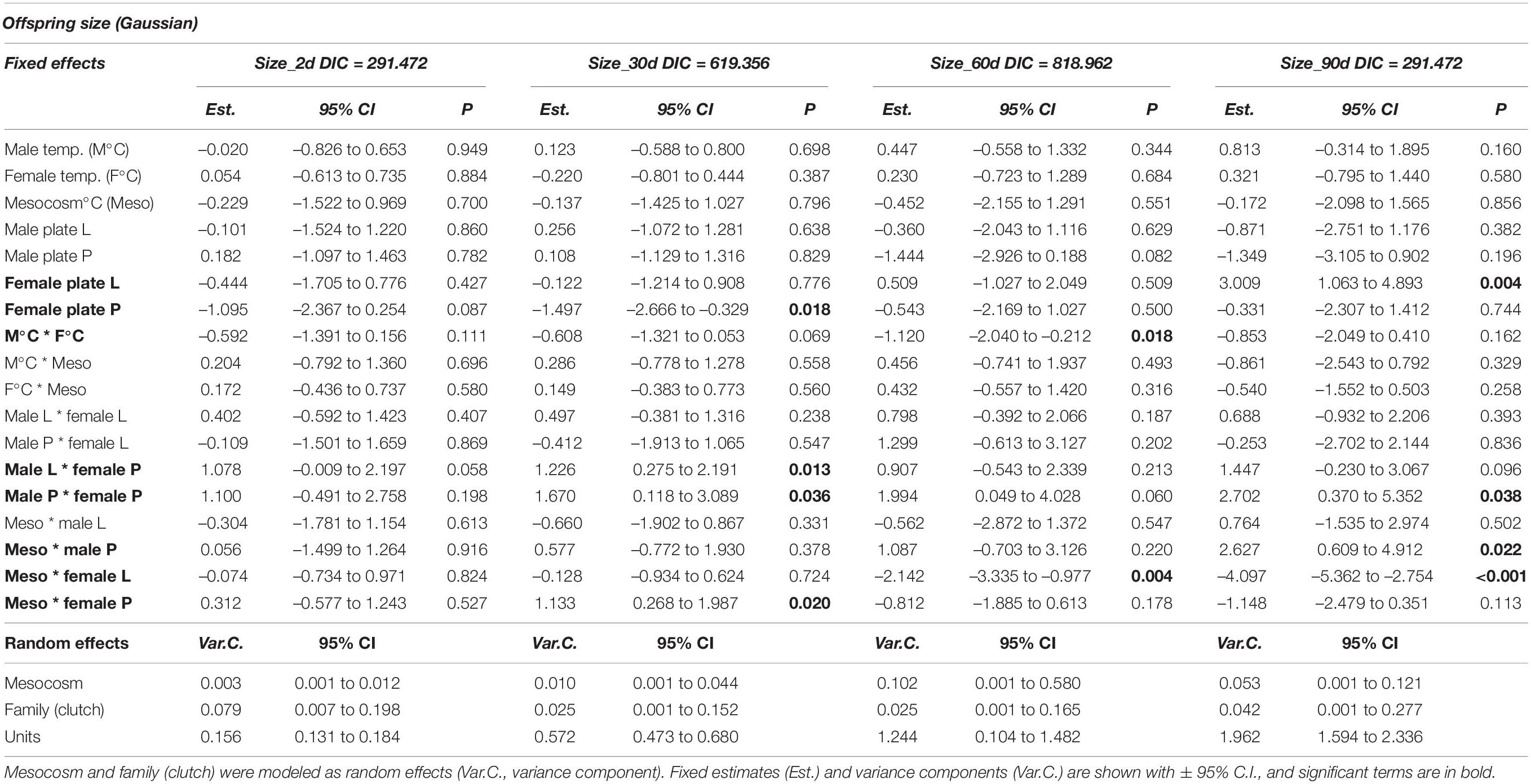
Table 3. Offspring size (standard length: mm) over time (at 2 days to 90 days post-hatch) of stickleback (Gasterosteus aculeatus) depending on parental acclimation temperature (M°C, F°C), offspring environment (mesocosm) temperature, and parental plate morph (C, complete; L, low; P, partial).
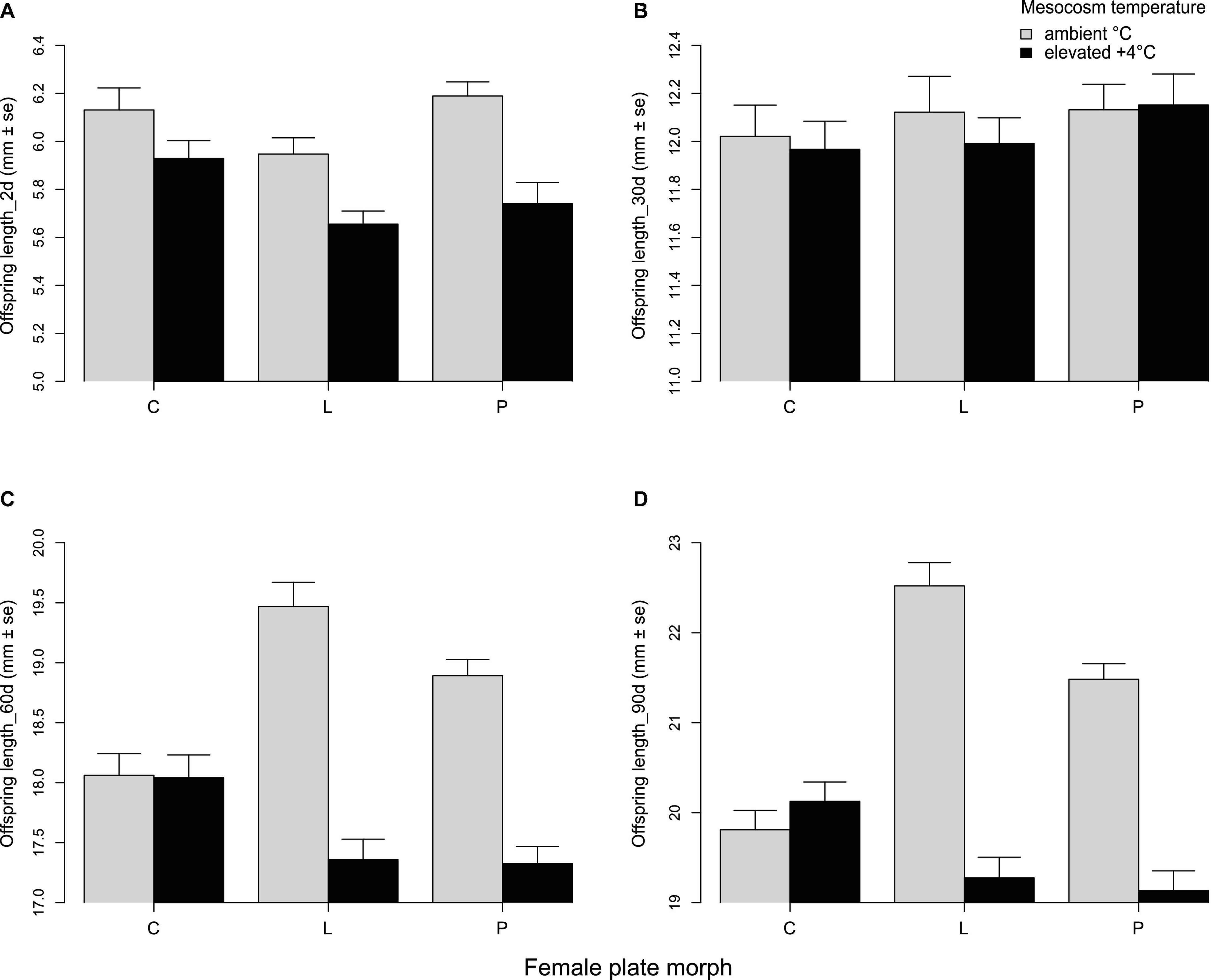
Figure 5. Mean length (mm ± SE) of stickleback offspring depending on maternal plate morph (C, complete; L, low; P, partial) and environmental (mesocosm) temperature (ambient or +4°C): (A) 2 days post-hatch, (B) 30 days post-hatch, (C) 60 days post-hatch, and (D) 90 days post-hatch.
Discussion
Our study revealed that stickleback lateral plate morphs showed different plastic responses to simulated ocean warming for several traits related to reproductive success, a direct measure of fitness. Most interesting, we detected environment-specific clutch siring success, in that low plated males sired more clutches in elevated temperature (+4°C) environments and completely plated males sired more clutches at ambient temperature, possibly reflecting energy tradeoffs between male morphology, metabolism, courtship behavior, and thermal environment. Fecundity traits (egg size and clutch size) differed depending on female plate morph and acclimation temperature experienced during the last phases of oogenesis, and offspring growth varied depending on the interaction between parental plate morph and offspring thermal environment. Taken together, our results demonstrate that reproductive success measured at multiple levels (clutch siring success, fecundity, offspring growth) differed among lateral plate morphs depending on environmental temperature, which could, thus, lead to shifts in plate morph polymorphism and colonization ability during range shifts of oceanic stickleback populations under climate change.
Male Plate Morph and Reproductive Environment Temperature Shape Clutch Siring Success
In our siring success experiment, alternate reproductive strategies (e.g., sneaking or egg theft) were found in 37.5% of genotyped clutches. This relatively high proportion is in line with other studies (e.g., Largiadèr et al., 2001; Fuxjäger et al., 2019), demonstrating that alternate strategies may be common in stickleback, with potentially large influences on male reproductive success. Stickleback males not only steal foreign eggs, but occasionally, the sneaker returns and steals the eggs he fertilized to place them in his own nest (Largiadèr et al., 2001; Le Comber et al., 2003). For males with empty nests, egg thievery can be the first step to a successful breeding season since females prefer to spawn in nests already containing eggs (Bell and Foster, 1994; Largiadèr et al., 2001; Le Comber et al., 2003). In our experiment, sneaking events were equally distributed between mesocosm temperatures and there were no large differences among plate morphs. That is, we did not detect environment-dependent sneaking success that differed by lateral plate morph. We did find a clear pattern that (almost) all sneaking males were smaller than non-reproducing males, consistent with other studies showing that small size is often advantageous, and may even be a prerequisite for successful alternate reproductive strategies (Taborsky, 2008).
Clutch siring success of nest owners, on the other hand, differed depending on male plate morph, size and reproductive environment (mesocosm) temperature. Most interestingly, low plated nest owners were smaller than non-reproducing males, and had higher siring success in +4°C mesocosms compared to ambient temperature. Completely plated males showed the opposite pattern (higher siring success in ambient mesocosms; no size difference compared to non-reproducing males), and partially plated males showed a mixed siring success pattern in terms of body size and mesocosm temperature. Rather than assortative mating where particular morphs preferentially choose the same morph phenotype, we predicted that increasing water temperature should lead to higher reproductive success for low plated males irrespective of the plate morph of the female. Indeed, this is what we found, in that low plated males sired the most clutches in +4°C mesocosms and completely plated males sired the most at ambient °C (in both cases with all female plate morphs), suggestive of context-dependent or environment-dependent mate choice (Heuschele et al., 2009; Robinson et al., 2012; McGhee et al., 2015). However, further studies including behavioral assays and controlled choice experiments are needed to determine if female preference is the mechanism underlying the differences in mating success among lateral plate morphs found in our study.
The sexual cue underlying higher clutch siring success of low plated males at elevated temperature is unclear. One candidate trait is metabolic performance: small, low plated males may have a metabolic advantage at elevated temperature allowing them to spend more energy on courtship, mating and/or brood care (Kraak et al., 1999; Fuxjäger et al., 2019). Nevertheless, studies of metabolic rates of stickleback plate morphs have revealed contradicting results. Comparing completely plated anadromous with low plated freshwater populations, Tudorache et al. (2007) found higher metabolic rates in completely plated fish, whereas Morozov et al. (2018) did not find any differences in metabolic rate between both eco- and morphotypes. Also, Schaarschmidt and Jürss (2003) did not detect differences in metabolic performance related to migratory behavior between non-migrating freshwater and migrating anadromous stickleback. However, to the best of our knowledge, there is currently no data on metabolic performance of low plated oceanic stickleback, especially with regard to reproductive environment temperature.
Thermal Plasticity of Fecundity Traits Differs With Female Plate Morph
Temperature-mediated variation in egg size has been shown in numerous fish species, with a common pattern of larger eggs produced at lower temperature (Barneche et al., 2018). In line with this, previous studies of the stickleback population investigated here found that mothers produced larger eggs when acclimated to ambient temperature, and smaller eggs when acclimated to a +4°C ocean warming scenario (Shama and Wegner, 2014; Shama, 2015, 2017). In the current study, we found a contrasting overall pattern of larger eggs produced at +4°C (driven by completely and low plated females, see below). However, previous studies compared 17°C (ambient) with 21°C (+4°C), and 21°C was shown to have negative effects on several traits, e.g., development (Ramler et al., 2014), growth (Shama et al., 2014; Shama and Wegner, 2014; Shama, 2015, 2017), and survival (Schade et al., 2014). In the current study, mothers were acclimated during their reproductive conditioning phase at temperatures starting at either 10 or 14°C, and laid eggs in mesocosms reaching either 16 or 20°C. It is likely that the optimum temperature range for late oogenesis and egg ripening for this population lies above 13°C but below 21°C (see Ramler et al., 2014), suggesting that 10°C may be too cold to promote high energy states necessary to induce reproduction and/or large egg size (Baker et al., 2015).
Interestingly, females allocated resources to eggs differently depending on plate morph and the temperature experienced during acclimation. Completely and low plated females produced larger eggs when acclimated at +4°C, whereas partially plated females produced larger eggs when acclimated at ambient temperature. Moreover, tradeoffs between egg size and clutch size differed not only between acclimation temperatures (as shown in many studies e.g., Bownds et al., 2010; Liefting et al., 2010), but also among plate morphs. Completely plated females laid larger eggs but smaller clutches at +4°C (and smaller eggs but larger clutches at ambient °C), whereas low and partially plated females laid similar sized clutches under both acclimation temperatures. Overall, the slope of the egg size-clutch size tradeoff was only negative when females were acclimated to +4°C, and low plated females showed the steepest slope. Several studies have found that freshwater, low plated females tend to produce large but few eggs (Baker, 1994), but thermal plasticity of egg allocation was not investigated. Our results indicate that the strong negative correlation between egg size and clutch size for low plated females is not likely due to large eggs being more energetically costly to produce (since their clutch sizes did not differ between acclimation temperatures), but may reflect different morphology – physiology – fitness relationships among plate morphs (Morozov et al., 2018). Differing tradeoffs between fecundity and egg size were also found among morphs within a polymorphic population of Arctic charr, driven by different adaptations to feeding and habitat utilization (Smalås et al., 2017), but this remains to be investigated in oceanic stickleback.
Offspring Size Varies With Parental Plate Morph and Growth Environment Temperature
Our growth experiment showed that both the lateral plate morph of parents and offspring environmental temperature influenced body size. Although we could not test all parental plate morph combinations in both temperature environments (due to three missing combinations), we found that offspring size varied depending on the interaction between maternal plate morph and mesocosm temperature at a number of time points. Here, we found that temperature-mediated differences among plate morphs became most apparent after 60 days, when offspring of low and partially plated mothers were much smaller in +4°C mesocosms, whereas offspring of completely plated mothers were less influenced by environmental temperature. We also found that offspring of low and partially plated parents grew better than those from completely plated parents (averaged over mesocosm temperature), at least up to 90 days post-hatch. Low plated morphs have been shown to differ from completely plated morphs for a number of traits important for colonization of freshwater (growth rate, foraging behavior, immune response; Østbye et al., 2018). Our results concur with these, and suggest that morph-specific growth rates of oceanic stickleback could play a role in colonization during range shifts under ocean warming.
Intriguingly, although low plated females acclimated to +4°C produced the largest eggs, these offspring did not grow best at elevated temperature. Previous studies of this population investigating the relationship between egg size and offspring size found contrasting results. In some cases, large eggs become small offspring (as in the current study), whereas in others, small eggs went on to become large offspring (e.g., Shama, 2015), suggesting that other maternal inputs to eggs (e.g., hormones, epigenetic modifications) that are unrelated to egg size play a role in shaping offspring size (Shama et al., 2016). Specifically, we previously found maternally mediated transgenerational plasticity effects on early offspring growth (0–30 days), with offspring growing best at the same temperature their mother was acclimated to Shama et al. (2014). However, growth in later stages (60–90 days) was primarily influenced by offspring environmental temperature, and offspring grew better at ambient (17°C) than at elevated temperature (21°C; see also Shama and Wegner, 2014; Shama, 2015, 2017). In the current study, we did not detect any transgenerational plasticity effects on offspring size [e.g., no parent environment (acclimation °C) by offspring environment (mesocosm °C) interaction], and perhaps, none should be expected since parents and offspring experienced different temperatures. As stated above, mothers exposed to the +4°C scenario experienced 14°C at the start of the experiment (potentially leading to larger egg size), whereas offspring growth would have occurred at 20°C (which is above their growth thermal optimum). The ambient temperature scenario, on the other hand, was likely too cold at the start of the experiment for large egg production (10°C), but the optimum temperature for offspring growth (16°C), essentially decoupling parent and offspring environments.
Our results also showed that higher clutch siring success of low plated males in +4°C mesocosms did not translate to larger size of low plated offspring at +4°C (up to 90 days). Nevertheless, there may be other positive effects of this siring success in the next generation. Smaller offspring at elevated temperature is a common finding in climate change experiments, and is one of the universal ecological responses to global warming in aquatic systems (Daufresne et al., 2009; Shama, 2017; Fuxjäger et al., 2019). Despite some potentially negative effects of reduced body size for various aspects of their life history (e.g., male–male competition), advantages of a smaller body size should not be ignored. For instance, if smaller individuals are able to allocate resources from growth into gonad development earlier, they might be able to reach sexual maturity faster than their conspecifics with prolonged growth (Daufresne et al., 2009). Moreover, as shown here, small size may be beneficial in warmer environments due to lower metabolic demand, leaving more energy available for courtship behavior, potentially leading to higher mating success (see also Fuxjäger et al., 2019).
Conclusion
Our study found that a temperature increase of 4°C as predicted by several climate models (Intergovernmental Panel on Climate Change [IPCC], 2014) will influence a number of reproductive success-related traits of oceanic stickleback, and that thermal plasticity of these traits differed with lateral plate morph. Some traits (clutch siring success, egg size) showed better performance for low plated fish at elevated temperature, whereas others (e.g., growth) did not. Higher clutch siring success of low plated males at elevated temperature might indicate a future shift in plate morph composition for polymorphic populations. One key trait coupled to such a shift is sustained swimming ability, since low plated fish lack a keel on the caudal peduncle, putting them at a hydrodynamic disadvantage (Wootton, 1984). Energetic tradeoffs between sustained swimming, growth and reproduction (McKinnon et al., 2004), together with environment-dependent mate choice for low plated males could shift the morph distribution in polymorphic populations, with potential consequences for long migrations and range shifts under ocean warming.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.org/10.1594/PANGAEA.937325, PANGAEA.
Ethics Statement
The animal study was reviewed and approved by Ministerium für Energiewende, Landwirtschaft, Umwelt, Natur und Digitalisierung.
Author Contributions
LS and HA conceived the study. SW and LF conducted the experiment, collected the genotype, and phenotypic data. SW and ER conducted the parentage analyses. LS carried out the statistical analyses. SW wrote the first draft of the manuscript. All authors contributed to the final version.
Funding
This study was funded by the Coastal Ecology Section of the Alfred-Wegener-Institute Wadden Sea Station Sylt. The authors acknowledge support by the Open Access Publication Funds of Alfred-Wegener-Institut Helmholtz- Zentrum für Polar- und Meeresforschung.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Many thanks to Petra Kadel for setting up and maintaining the mesocosms, Kaibil Escobar Wolf for taking care of the fish, Nancy Kuehne for help with the genotyping, and Mathias Wegner for help with MCMC GLMM.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.759450/full#supplementary-material
References
Baker, J. A. (1994). “Life history variation in female threespine stickleback,” in The Evolutionary Biology of the Threespine Stickleback, eds M. A. Bell and S. A. Foster (New York, NY: Oxford University Press), 144–187.
Baker, J. A., Foster, S. A., Heins, D. C., Bell, M. A., and King, R. W. (1998). Variation in female life-history traits among Alaskan populations of the threespine stickleback, Gasterosteus aculeatus L. (Pisces: gasterosteidae). Biol. J. Linnean Soc. 63, 141–159. doi: 10.1006/bijl.1997.0187
Baker, J. A., Wund, M., Heins, D., King, R. W., Reyes, M. L., and Foster, S. A. (2015). Life-history plasticity in female threespine stickleback. Heredity 115, 322–334. doi: 10.1038/hdy.2015.65
Barneche, D. R., Burgess, S. C., and Marshell, D. J. (2018). Global environmental drivers of marine fish egg size. Glob. Ecol. Biogeogr. 27, 890–898. doi: 10.1016/j.envpol.2020.114711
Barrett, R. D. H. (2010). Review paper: adaptive evolution of lateral plates in three-spined stickleback Gasterosteus aculeatus: a case study in functional analysis of natural variation. J. Fish Biol. 77, 311–328. doi: 10.1111/j.1095-8649.2010.02640.x
Baumgartner, J. V., and Bell, M. A. (1984). Lateral plate morph variation in California populations of the threespine stickleback, Gasterosteus aculeatus. Evolution 38, 665–674. doi: 10.1111/j.1558-5646.1984.tb00333.x
Bell, M. A., and Foster, S. A. (1994). “Introduction to the evolutionary biology of the threespine stickleback,” in The Evolutionary Biology of the Threespine Stickleback, eds M. A. Bell and S. A. Foster (New York, NY: Oxford University Press), 1–27.
Bell, M. A., Gangavalli, A. K., Bewick, A., and Aguirre, W. E. (2010). Frequency of Ectodysplasin alleles and limited introgression between sympatric threespine stickleback populations. Environ. Biol. Fishes 89, 189–198. doi: 10.1007/s10641-010-9712-z
Bergstrom, C. A. (2002). Fast-start swimming performance and reduction in lateral plate number in threespine stickleback. Canadian J. Zool. 80, 207–213. doi: 10.1139/z01-226
Berner, D., Ammann, M., Spencer, E., Rüegg, A., Lüscher, D., and Moser, D. (2016). Sexual isolation promotes divergence between parapatric lake and stream stickleback. J. Evol. Biol. 30, 401–411. doi: 10.1111/jeb.13016
Bjærke, O., Østbye, K., Lampe, H. M., and Vøllestad, L. A. (2010). Covariation in shape and foraging behaviour in lateral plate morphs in the three-spined stickleback. Ecol. Freshw. Fish 19, 249–256. doi: 10.1111/j.1600-0633.2010.00409.x
Blouw, D. M., and Hagen, D. W. (1990). Breeding ecology and evidence of reproductive isolation of a widespread stickleback fish (Gasterosteidae) in Nova Scotia, Canada. Biol. J. Linnean Soc. 39, 195–217. doi: 10.1111/j.1095-8312.1990.tb00512.x
Bownds, C., Wilson, R., and Marshall, D. J. (2010). Why do colder mothers produce larger eggs? An optimality approach. J. Exp. Biol. 213, 3796–3801. doi: 10.1242/jeb.043356
Candolin, U. (2019). Mate choice in a changing world. Biol. Rev. 94, 1246–1260. doi: 10.1111/brv.12501
Candolin, U., Salesto, T., and Evers, M. (2007). Changed environmental conditions weaken sexual selection in sticklebacks. Eur. Soc. Evol. Biol. 20, 233–239. doi: 10.1111/j.1420-9101.2006.01207.x
Colosimo, P. F., Hosemann, K. E., Balabhadra, S., Villarreal, G. Jr., Dickson, M., Grimwood, J., et al. (2005). Widespread parallel evolution in sticklebacks by repeated fixation of Ectodysplasin alleles. Science 307, 1928–1933. doi: 10.1126/science.1107239
Conte, G. L., and Schluter, D. (2013). Experimental confirmation that body size determines mate preference via phenotype matching in a stickleback species pair. Evolution 67, 1477–1484. doi: 10.1111/evo.12041
Dahlke, F. T., Wohlrab, S., Butzin, M., and Pö, H.-O. (2020). Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 369, 65–70.
Dalziel, A. C., Vines, T. H., and Schulte, P. M. (2011). Reductions in prolonged swimming capacity following freshwater colonization in multiple threespine stickleback populations. Evolution 66, 1226–1239. doi: 10.1111/j.1558-5646.2011.01498.x
Daufresne, M., Lengfellner, K., and Sommer, U. (2009). Global warming benefits the small in aquatic ecosystems. Proc. Natl. Acad. Sci. U.S.A. 106, 12788–12793. doi: 10.1073/pnas.0902080106
Des Roches, S., Bell, M. A., and Palkovacs, E. (2020). Climate-driven habitat change causes evolution in Threespine Stickleback. Glob. Change Biol. 26, 597–606. doi: 10.1111/gcb.14892
Dieckmann, U., and Doebeli, M. (1999). On the origin of species by sympatric speciation. Nature 400, 354–357. doi: 10.1038/22521
Fuxjäger, L., Wanzenböck, S., Ringler, E., Wegner, M. K., Ahnelt, H., and Shama, L. N. S. (2019). Within-generation and transgenerational plasticity of mate choice in oceanic stickleback under climate change. Philos. Trans. R. Soc. B 374, 1–12. doi: 10.1098/rstb.2018.0183
Gienapp, P., Teplitsky, C., Alho, J. S., Mills, J. A., and Merliä, J. (2007). Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 1–12. doi: 10.1111/j.1365-294X.2007.03413.x
Hadfield, J. D. (2010). MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22.
Hagen, D. (1973). Inheritance of numbers of lateral plates and gill rakers in Gasterosteus aculeatus. Heredity 30, 303–312. doi: 10.1038/hdy.1973.40
Hagen, D. W., and Moodie, G. E. E. (1982). Polymorphism for plate morphs in Gasterosteus aculeatus on the east coast of Canada and an hypothesis for their global distribution. Canadian J. Zool. 60, 1032–1042.
Hansson, T. H., Fischer, B., Mazzarella, A. B., Voje, K. L., and Vøllestad, L. A. (2016). Lateral plate number in low-plated threespine stickleback: a study of plasticity and heritability. Ecol. Evol. 6, 3154–3160. doi: 10.1002/ece3.2020
Hermida, M., Fernández, C., Amaro, R., and San Miguel, E. (2002). Heritability and “evolvability” of meristic characters in a natural population of Gasterosteus aculeatus. Canadian J. Zool. 80, 532–541.
Heuschele, J., Mannerla, M., Gienapp, P., and Candolin, U. (2009). Environment-dependent use of mate choice cues in sticklebacks. Behav. Ecol. 20, 1223–1227. doi: 10.1093/beheco/arp123
Intergovernmental Panel on Climate Change [IPCC] (2014). “Topic 1: observed changes and their causes,” in Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds Core Writing Team, R. K. Pachauri and L. A. Meyer (Geneva: IPCC), 151.
Jenck, C. S., Lehto, W. R., Ketterman, B. T., Sloan, L. F., Sexton, A. N., and Tinghitella, R. M. (2020). Phenotypic divergence among threespine stickleback that differ in nuptial coloration. Ecol. Evol. 10, 2900–2916. doi: 10.1002/ece3.6105
Jones, F. C., Brown, C., Pemberton, J. M., and Braithwaite, V. A. (2006). Reproductive isolation in a threespine stickleback hybrid zone. J. Evol. Biol. 19, 1531–1544. doi: 10.1111/j.1420-9101.2006.01122.x
Kalbe, M., Eizaguirre, C., Dankert, I., Reusch, T. B. H., Wegner, M. K., and Milinski, M. (2009). Lifetime reproductive success is maximized with optimal histocompatibility complex diversity. Proc. R. Soc. B 276, 925–934. doi: 10.1098/rspb.2008.1466
Kalinowski, S. T., Taper, M. L., and Marshall, T. C. (2007). Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106. doi: 10.1111/j.1365-294X.2007.03089.x
Kraak, S. B. M., Bakker, T. C. M., and Mundwiler, B. (1999). Sexual selection in sticklebacks in the field: correlates of reproductive, mating, and paternal success. Behav. Ecol. 10, 696–706.
Largiadèr, C. R., Fries, V., and Bakker, T. C. M. (2001). Genetic analysis of sneaking and egg-thievery in a natural population of the three-spined stickleback (Gasterosteus aculeatus L.). Heredity 86, 459–468. doi: 10.1046/j.1365-2540.2001.00850.x
Le Comber, S., Faulkes, C., Formosinho, J., and Smith, C. (2003). Response of territorial males to the threat of sneaking in the three-spined stickleback (Gasterosteus aculeatus): a field study. J. Zool. 261, 15–20. doi: 10.1017/s0952836903003911
Liefting, M., Weerenbeck, M., van Dooremalen, C., and Ellers, J. (2010). Temperature-induced plasticity in egg size and resistance of eggs to temperature stress in a soil arthropod. Funct. Ecol. 24, 1291–1298.
Loehr, J., Leinonen, T., Herczeg, G., O’Hara, R. B., and Merilä, J. (2012). Heritability of asymmetry and lateral plate number in the threespine stickleback. PLoS One 7:e39843. doi: 10.1371/journal.pone.0039843
Marchinko, K. B., and Schluter, D. (2007). Parallel evolution by correlated response: lateral plate reduction in threespine stickleback. Evolution 61, 1084–1090. doi: 10.1111/j.1558-5646.2007.00103.x
Matschiner, M., and Salzburger, W. (2009). TANDEM: integrating automated allele binning into genetics and genomics workflows. Bioinformatics 25, 1982–1983. doi: 10.1093/bioinformatics/btp303
McGhee, K. E., Feng, S., Leasure, S., and Bell, A. M. (2015). A female’s past experience with predators affects male courtship and the care her offspring will receive from their father. Proc. R. Soc. B 282:20151840.
McGuigan, K., Nishimura, N., Currey, M., Hurwit, D., and Cresko, W. A. (2010). Cryptic genetic variation and body size evolution in threespine stickleback. Evolution 65, 1203–1211. doi: 10.1111/j.1558-5646.2010.01195.x
McKinnon, J. S., Mori, S., Blackman, B. K., David, L., Kingsley, D. M., Jamieson, L., et al. (2004). Evidence for ecology;s role in speciation. Nature 429, 294–298.
McKinnon, J. S., and Rundle, H. D. (2002). Speciation in nature: the threespine stickleback model systems. Trends Ecol. Evol. 17, 480–488. doi: 10.1016/s0169-5347(02)02579-x
Monaghan, P. (2008). Early growth conditions, phenotypic development and environmental change. Philos. Trans. R. Soc. 363, 1635–1645. doi: 10.1098/rstb.2007.0011
Morozov, S., Leinonen, T., Merilä, J., and McCairns, S. R. J. (2018). Selection on the morphology–physiology-performance nexus: lessons from freshwater stickleback morphs. Ecol. Evol. 8, 1286–1299. doi: 10.1002/ece3.3644
Munday, P. L., Warner, R. R., Monro, K., Pandolfi, J. M., and Marshall, D. J. (2013). Predicting evolutionary responses to climate change in the sea. Ecol. Lett. 16, 1488–1500. doi: 10.1111/ele.12185
Münzing, J. (1963). The evolution of variation and distributional patterns in European populations of the three-spined stickleback, Gasterosteus aculeatus. Evolution 17, 320–332. doi: 10.2307/2406161
Nagel, L., and Schluter, D. (1998). Body size, natural selection, and speciation in sticklebacks. Evolution 52, 209–218. doi: 10.1111/j.1558-5646.1998.tb05154.x
Oravec, T. J., and Reimchen, T. E. (2013). Divergent reproductive life histories in Haida Gwaii stickleback (Gasterosteus spp.). Canadian J. Zool. 91, 17–24. doi: 10.1139/cjz-2012-0175
Østbye, K., Taugbøl, A., Ravinet, M., Harrod, C., Pettersen, R. A., Bernatchez, L., et al. (2018). Ongoing niche differentiation under high gene flow in a polymorphic brackish water threespine stickleback (Gasterosteus aculeatus) population. BMC Evol. Biol. 18:14. doi: 10.1186/s12862-018-1128-y
Paepke, H. J. (2002). “Gasterosteus aculeatus Linneaus, 1758,” in The Freshwater Fishes of Europe, Vol. 5, eds P. M. Banarescu and H. J. Paepke (Wiebelsheim: Aula-Verlag), 209–256.
Pansch, A., Winde, V., Asmus, R., and Asmus, H. (2016). Tidal benthic mesocosms simulating future climate change scenarios in the field of marine ecology. Limnol. Oceanogr.: Methods 14, 257–267. doi: 10.1002/lom3.10086
Pilakouta, N., and Alund, M. (2021). Sexual selection and environmental change: what do we know and what comes next? Curr. Zool. 67, 293–298. doi: 10.1093/cz/zoab021
Raeymaekers, J. A. M., Konijnendijk, N., Larmuseau, M. H. D., Hellemans, B., De Meester, L., and Volkaert, F. A. M. (2014). A gene with major phenotypic effects as a target for selection vs. homogenizing gene flow. Mol. Ecol. 23, 162–181. doi: 10.1111/mec.12582
Raeymaekers, J. A. M., Maes, G. E., Audenaert, E., and Volckaert, F. A. M. (2005). Detecting Holocene divergence in the anadromous–freshwater three-spined stickleback (Gasterosteus aculeatus) system. Mol. Ecol. 14, 1001–1014. doi: 10.1111/j.1365-294X.2005.02456.x
Ramler, D., Mitteroecker, P., Shama, L. N. S., Wegner, M. K., and Ahnelt, H. (2014). Nonlinear effects of temperature on body form and developmental canalization in the threespine stickleback. J. Evol. Biol. 27, 497–507. doi: 10.1111/jeb.12311
Rausch, A. M., Hödl, W., Ringler, E., Jehle, R., and Sztatecsny, M. (2014). Male body size and parental relatedness but not nuptial colouration influence paternity success during scramble competition in Rana arvalis. Behaviour 151, 1869–1884.
Reimchen, T. E. (1983). Structural relationships between spines and lateral plates in threespine stickleback (Gasterosteus aculeatus). Evolution 37, 931–946. doi: 10.1111/j.1558-5646.1983.tb05622.x
Reimchen, T. E. (2000). Predator handling failures of lateral plate morphs in Gasterosteus aculeatus: functional implications for the ancestral plate condition. Behaviour 137, 1081–1096.
Reusch, T. B. H., Wegner, K. M., and Kalbe, M. (2001). Rapid genetic divergence in postglacial populations of threespine stickleback (Gasterosteus aculeatus): the role of habitat type, drainage and geographical proximity. Mol. Ecol. 10, 2435–2445. doi: 10.1046/j.0962-1083.2001.01366.x
Robinson, M. R., van Doorn, S. G., Gustafsson, L., and Qvarnström, A. (2012). Environment-dependent selection on mate choice in a natural population of birds. Ecol. Lett. 15, 611–618. doi: 10.1111/j.1461-0248.2012.01780.x
Safran, R. J., Scordato, E. S. C., Symes, L. B., Rodriguez, R. L., and Mendelson, T. C. (2013). Contributions of natural and sexual selection to the evolution of premating reproductive isolation: a research agenda. Trends Ecol. Evol. 28, 643–650. doi: 10.1016/j.tree.2013.08.004
Schaarschmidt, T., and Jürss, K. (2003). Locomotory capacity of Baltic Sea and freshwater populations of the threespine stickleback (Gasterosteus aculeatus). Comparat. Biochem. Physiol. Part A: Mol. Integrat. Physiol. 135, 411–424. doi: 10.1016/s1095-6433(03)00109-0
Schade, F. M., Shama, L. N. S., and Wegner, M. K. (2014). Impact of thermal stress on evolutionary trajectories of pathogen resistance in three-spined stickleback (Gasterosteus aculeatus). BMC Evol. Biol. 14:164. doi: 10.1186/s12862-014-0164-5
Scott, R. J. (2004). Assortative mating between adjacent populations of threespine stickleback (Gasterosteus aculeatus). Ecol. Freshw. Fish 13, 1–7.
Shama, L. N. S. (2015). Bet hedging in a warming ocean: predictability of maternal environment shapes offspring size variation in marine sticklebacks. Global Change Biol. 21, 4387–4400. doi: 10.1111/gcb.13041
Shama, L. N. S. (2017). The mean and variance of climate change in the oceans: hidden evolutionary potential under stochastic environmental variability in marine sticklebacks. Nat. Sci. Rep. 7, 1–14. doi: 10.1038/s41598-017-07140-9
Shama, L. N. S., Mark, F. C., Strobel, A., Lokmer, A., John, U., and Wegner, M. K. (2016). Transgenerational effects persist down the maternal line in marine sticklebacks: gene expression matches physiology in a warming ocean. Evol. Appl. 9, 1096–1111. doi: 10.1111/eva.12370
Shama, L. N. S., Strobel, A., Mark, F. C., and Wegner, M. K. (2014). Transgenerational plasticity in marine sticklebacks: maternal effects mediate impacts of a warming ocean. Funct. Ecol. 28, 1–11. doi: 10.1111/jeb.12490
Shama, L. N. S., and Wegner, M. K. (2014). Grandparental effects in marine sticklebacks: transgenerational plasticity across multiple generations. J. Evol. Biol. 27, 2297–2307.
Smalås, A., Amundsen, P., and Knudsen, R. (2017). The trade-off between fecundity and egg size in a polymorphic population of Arctic charr (Salvelinus alpinus (L.)) in Skogsfjordvatn, subarctic Norway. Ecol. Evol. 7, 2018–2024. doi: 10.1002/ece3.2669
Smith, C., Spence, R., Barber, I., Przybylski, M., and Wootton, R. J. (2014). The role of calcium and predation on plate morph evolution in the three-spined stickleback (Gasterosteus aculeatus). Ecol. Evol. 4, 3550–3554. doi: 10.1002/ece3.1180
Smith, C., Zieba, G., and Prybylski, M. (2021). Elevated temperatures drive the evolution of armour loss in the threespine stickleback Gasterosteus aculeatus. Funct. Ecol. 35, 1735–1744. doi: 10.1111/1365-2435.13846
Smith, C., Zieba, G., Spence, R., Klepaker, T., and Przybylski, M. (2020). Three-spined stickleback armour predicted by body size, minimum winter temperature and pH. J. Zool. 311, 13–22.
Snowberg, L. K., and Bolnick, D. I. (2008). Assortative Mating by diet in a phenotypically unimodal but ecologically variable population of stickleback. Am. Naturalist 172, 733–739. doi: 10.1086/591692
Spoljaric, M. A., and Reimchen, T. E. (2011). Habitat-specific trends in ontogeny of body shape in stickleback from coastal archipelago: potential for rapid shifts in colonizing populations. J. Morphol. 272, 590–597. doi: 10.1002/jmor.10939
Taborsky, M. (2008). “Alternative reproductive tactics in fish,” in Alternative Reproductive Tactics: an Integrative Approach, eds R. Oliveira, M. Taborsky, and H. J. Brookman (New York, NY: Cambridge University Press), 251–299.
Tudorache, C., Blust, R., and De Boeck, G. (2007). Swimming capacity and energetics of migrating and non-migrating morphs of three-spined stickleback Gasterosteus aculeatus L. and their ecological implications. J. Fish Biol. 71, 1448–1456. doi: 10.1111/j.1095-8649.2007.01612.x
Ursprung, E., Ringler, M., Jehle, R., and Hödl, W. (2011). Strong male/male competition allows for nonchoosy females: high levels of polygynandry in a territorial frog with paternal care. Mol. Ecol. 20, 1759–1771. doi: 10.1111/j.1365-294X.2011.05056.x
Walker, J. A., and Bell, M. A. (2000). Net evolutionary trajectories of body shape evolution within a microgeographic radiation of threespine sticklebacks (Gasterosteus aculeatus). J. Zool. 252, 293–302. doi: 10.1111/j.1469-7998.2000.tb00624.x
Wang, J., and Santure, A. W. (2009). Parentage and sibship inference from multilocus genotype data under polygamy. Genetics 181, 1579–1594. doi: 10.1534/genetics.108.100214
Wong, B. B. M., Candolin, U., and Lindström, K. (2007). Environmental deterioration compromises socially enforced signals of male quality in three-spined sticklebacks. Am. Nat. 170, 184–189.
Wootton, R. J. (2009). The Darwinian stickleback Gasterosteus aculeatus: a history of evolutionary studies. J. Fish Biol. 75, 1919–1942. doi: 10.1111/j.1095-8649.2009.02412.x
Wund, M. A., Valena, S., Wood, S., and Baker, J. A. (2012). Ancestral plasticity and allometry in threespine stickleback reveal phenotypes associated with derived, freshwater ecotypes. Biol. J. Linnean Soc. 105, 573–583. doi: 10.5061/dryad.hb824gd4
Keywords: climate change, environment-dependent reproductive success, phenotypic plasticity, egg size, offspring growth, parentage analysis, lateral plate morph, Gasterosteus aculeatus
Citation: Wanzenböck S, Fuxjäger L, Ringler E, Ahnelt H and Shama LNS (2022) Temperature-Dependent Reproductive Success of Stickleback Lateral Plate Morphs: Implications for Population Polymorphism and Range Shifts Under Ocean Warming. Front. Mar. Sci. 9:759450. doi: 10.3389/fmars.2022.759450
Received: 16 August 2021; Accepted: 11 January 2022;
Published: 01 February 2022.
Edited by:
Chih-hao Hsieh, National Taiwan University, TaiwanReviewed by:
Dian-Han Kuo, National Taiwan University, TaiwanLengxob Yong, University of Exeter, United Kingdom
Copyright © 2022 Wanzenböck, Fuxjäger, Ringler, Ahnelt and Shama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisa N. S. Shama, lisa.shama@awi.de; orcid.org/0000-0002-9017-9950
 Sylvia Wanzenböck
Sylvia Wanzenböck Lukas Fuxjäger1,2
Lukas Fuxjäger1,2  Eva Ringler
Eva Ringler Harald Ahnelt
Harald Ahnelt Lisa N. S. Shama
Lisa N. S. Shama